Abstract
Subgroup analyses from clinical studies have suggested that among metastatic non-small cell lung cancer (NSCLC) patients receiving chemotherapy, females may derive less benefit from the addition of the vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab than males. This has raised the question of whether estrogen may impact the response to anti-angiogenic therapy. To address this, we investigated the effects of estrogen on tumor growth, angiogenesis, and the response to bevacizumab in human xenograft models of NSCLC. We observed that estrogen induced marked resistance to bevacizumab, which was accompanied by a 2.3 fold increase in tumor vascular pericyte coverage (p=0.01) and an upregulation of pro-angiogenic factors VEGF and platelet derived growth factor-BB (PDGF-BB). We also investigated the role of infiltrating myeloid cells, a population which has been associated with resistance to anti-VEGF therapies. We observed that estrogen induced a greater than 2-fold increase (p = 0.001) in the recruitment of tumor infiltrating myeloid cells as well as concomitant increases in the myeloid recruitment factors granulocyte colony-stimulating factor (G-CSF) and chemokine ligand 1 (CXCL1). Blockade of the estrogen receptor pathway using fulvestrant re-sensitized tumors to VEGF targeting as evidenced by reduced tumor vasculature and an increase in overall survival in our NSCLC xenograft models. Collectively, these data provide evidence that estrogen may promote resistance to VEGF-targeted therapies, potentially by enhancing pericyte coverage and myeloid recruitment, and suggest that estrogen receptor blockade merits further investigation as an approach to enhance the effects of antiangiogenic therapy.
Keywords: Non-small cell lung cancer, estrogen, tumor endothelium, pericytes, bevacizumab, fulvestrant
Introduction
Lung cancer is the leading cause of cancer related deaths with over 130,000 patient deaths estimated in 2020 alone [1]. While the rate of lung cancer mortality in the United States has declined among male patients, lung cancer related deaths have remained stable in female patients [2]. Female never-smokers are more likely to develop lung cancer compared to their male counterparts [3, 4], and females experience an increased risk in lung cancer development compared to men who have the same low levels of cigarette exposure [5]. One possible explanation for this disparity is that sex-related hormones may impact lung cancer development. Multiple studies have reported that female patients with lung cancer who had prior menopausal hormone replacement therapy had worse survival outcome compared to female patients who did not undergo menopausal hormone therapy, although the effect of estrogen on lung cancer outcomes remains controversial [6–8]. Other preclinical and clinical studies have suggested that estrogen signaling may negatively impact response to therapeutic regimens such as epidermal growth factor receptor (EGFR) inhibitors [9–11].
Anti-angiogenic agents have been extensively studied in patients with NSCLC [12]. The VEGF-targeting antibody, bevacizumab, is approved for the treatment of locally advanced, recurrent, or metastatic non-squamous NSCLC [13, 14]. Unfortunately, not all patients receive benefit from anti-angiogenic agents, and resistance remains a clinical challenge [15, 16]. Sex-related differences have been observed in the therapeutic response of patients with NSCLC to anti-angiogenic agents. The Eastern Cooperative Oncology Group (ECOG) 4599 trial showed that male but not female patients with NSCLC received clinical benefit from bevacizumab treatment when combined with carboplatin plus paclitaxel [17]. Estrogen expression and availability are higher in female patients compared to male patients, which may shape a sex-biased gene expression pattern and supports lung cell neoplasia [18]. Moreover, elevated expression of aromatase, the enzyme which converts testosterone into estrogen, correlates with a poorer prognosis in female patients with NSCLC [19].
Additional evidence supports the interaction between estrogen and angiogenic regulators such as VEGF. The combination of estrogen and progesterone has been shown to enhance expression of VEGF in primary embryonic lung cells [20], and estradiol can enhance the activity of endothelial cells both in vitro [21] and in vivo [22]. Endothelial cell proliferation, migration, and tube formation are important in neovascularization and tumorigenesis, and angiogenic tube formation and wounding healing were suppressed by estrogen receptor inhibition in vitro, supporting a role for estrogen in regulation of angiogenesis [23].
In this study, we investigated the impact of estrogen on the in vivo growth of NSCLC tumors. We found that estrogen promoted tumor growth, enhanced pericyte coverage of the tumor vasculature, and increased infiltration of myeloid cells. Moreover, estrogen was sufficient to induce resistance to bevacizumab, while estrogen receptor pathway blockade using fulvestrant was able to abrogate these changes. Our findings suggest that targeting the estrogen receptor (ER) pathway merits further investigation as a strategy to enhance the efficacy of VEGF inhibitors in female patients with NSCLC.
Materials and Methods
Cell culture:
NSCLC cell lines HCC827, A549, and Calu-6 were obtained from ATCC and maintained as previously described [24]. All cell lines were genotyped to confirm their identity.
Cell Proliferation:
Cells were serum-starved for 48 hours in phenol red–free MEM with 5% charcoal-stripped FBS before plating. Cells (3,000 per well) were plated into each well of a 96-well plate and allowed to adhere for 24 hours before the addition of estrogen (E2) (10−9 M) and fulvestrant (10−8 M). Ninety-six hours later, 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT; thiazolyl blue) was added to each well and incubated at 37°C and 5% CO2 for 2 hours followed by medium removal and solubilization in 100 μL DMSO. The resulting color change was read at 570 nm and calculated as absorbance above background. Each point condition was performed in quadruplicate.
Luciferase activity assays:
Cells (3×105/well) were seeded in 24-well plate with 5% charcoal stripped serum in phenol red free DMEM, transfected with 100 ng of pGL2-VEGF-luciferase, or 100ng of ERE-Luciferase [25] and 2.5ng of pRL-TK (Promega, Madison, WI). Estrogen (10−9 M) was added 24 hours after transfection. Cells were incubated at 37°C and 5% CO2 for 24 hours, lysed, and luciferase activity was determined using the dual-luciferase assay kit according to the manufacturer’s instructions (Promega). Relative firefly luciferase activity was normalized to renilla luciferase driven by the thymidine kinase minimal promoter.
Animals and tumor xenografts:
Female (ovariectomized) athymic nude mice (NCI-nu, 4–8 weeks old) were obtained from the Animal Production Area of the National Cancer Institute (Frederick Cancer Center, Frederick, MD) and housed in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. Exogenous E2 (Estradiol 17-β) was supplied with estrogen tubing with approximately 80 pg/ml release [26, 27] was placed under the skin, above the shoulder, and the wound was closed with a wound clip [28]. A549 cells (1×106) or HCC827 (3.5×106) cells were injected subcutanteously into the dorsal flank. When the tumor reached 250–300 mm3, animals were randomized into treatment groups. Mice were treated with a monoclonal antibody against VEGF, bevacizumab (BV) (10mg/kg), intra-peritoneally, twice a week. Tumors were harvested when volumes reached 1000 mm3 and divided into 3 sections; one was fixed in formalin and embedded in paraffin, another was frozen in OCT compound, and the third was snap frozen in liquid nitrogen. Serum was extracted and stored in −80˚C for further analysis.
Immunohistochemistry:
Frozen tissue sections were used to evaluate CD31, desmin, and CD11b expression. Specimens were sectioned (8–10 μM thickness). Frozen sections were stained with anti-Ki-67 antibody (BD Biosciences, 1:500) at 4˚C overnight for cell proliferation analysis. For vasculature staining, frozen sections were stained with anti-CD31 antibody (BD Biosciences, 1:500) and anti-desmin antibody (BD Biosciences, 1:400) at 4˚C overnight. Immunofluorescence microscopy was performed using a Zeiss Axioplot 2 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY). Representative images were obtained using a AxioCam MRC5 camera and Axio vision software 4.6. For CD11b staining, frozen sections were stained with anti-CD11b antibody (BD Biosciences, 1:50) at 4˚C overnight. Secondary goat anti-rat HRP antibodies (BD Biosciences, 1:200) were diluted and incubated for 1 hour. For VEGF and PDGFFB staining, frozen sections were stained with anti-VEGF antibody (BD Biosciences, 1:50) or anti-PDGFBB antibodiy (BD Biosciences, 1:100) at 4˚C overnight. Secondary goat anti-rat HRP antibodies (BD Biosciences, 1:200) were diluted and incubated for 1 hour.
Determination of Microvessel Density (MVD) and Pericyte Coverage:
Tumor MVD was determined as described previously [29]. In brief, tumors were examined microscopically to identify hot spots by low magnification (10X), and the mean MVD was quantified as the total number of CD31+ structures observed in a minimum of five microscopic fields at higher power of vision per tumor (200X). For each group, tumors from 4 mice from each group receiving short and long-term treatment were used. To determine the extent of pericyte coverage on the tumor vasculature, tumor sections were stained for CD31 (red) and desmin (green) as described above. Five fields per tumor were randomly identified at original magnification 200X, and blood vessels that were at least 50% covered by green desmin+ cells were considered to be positive for pericyte coverage.
Determination of CD11b, VEGF, or PDGFBB positive cells:
In brief, we examined tumors microscopically to identify tumor positive area and tumor negative necrotic area. The number of CD11b, VEGF, or PDGFBB positive cells (both cytoplasmic and nuclear staining) were counted in a minimum of eight high power microscopic fields (400X) from periphery to the center/core of the tumor. For each group, tumors from 4 mice were used as indicated in the respective figure.
Determination of Serum Levels of G-CSF, PDGFBB, VEGF, and CXCL1:
Serum levels of angiogenic factors were measured in tumor-bearing mice by multiplex bead assay (BioRad, Hercules, CA; Millipore, Billerica, MA) in a 96-well plate according to the manufacturer’s protocol. Serum samples were tested in duplicate, and the mean value used for analysis.
Human studies:
ESR1 (encodes ERα), ESR2 (encodes ERβ), ITGAM, PECAM, and CXCL1 gene expression levels were determined by microarray analysis (Illumina v3) of surgically resected lung adenocarcinomas from 150 patients (73 female and 77 male) who did not receive neoadjuvant therapy. This cohort was obtained from the Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT) study, developed in 2006 at M.D. Anderson Cancer Center [30]. Expression values were log (base 2) transformed. Correlations between genes, separately in male and female LUAD, were statistically evaluated using Pearson’s correlation coefficients and summarized plotted as correlation matrices. All analysis was performed in the R statistical language and environment (R-project.org; version 3.5.1).
Statistics:
Statistical significance was tested using GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA). For comparison between groups, Student’s t-test and one-way Anova test were used. A p value < 0.05 on two-tailed testing was considered significant.
Results
Estrogen does not enhance the growth of established tumors nor proliferation of NSCLC cells.
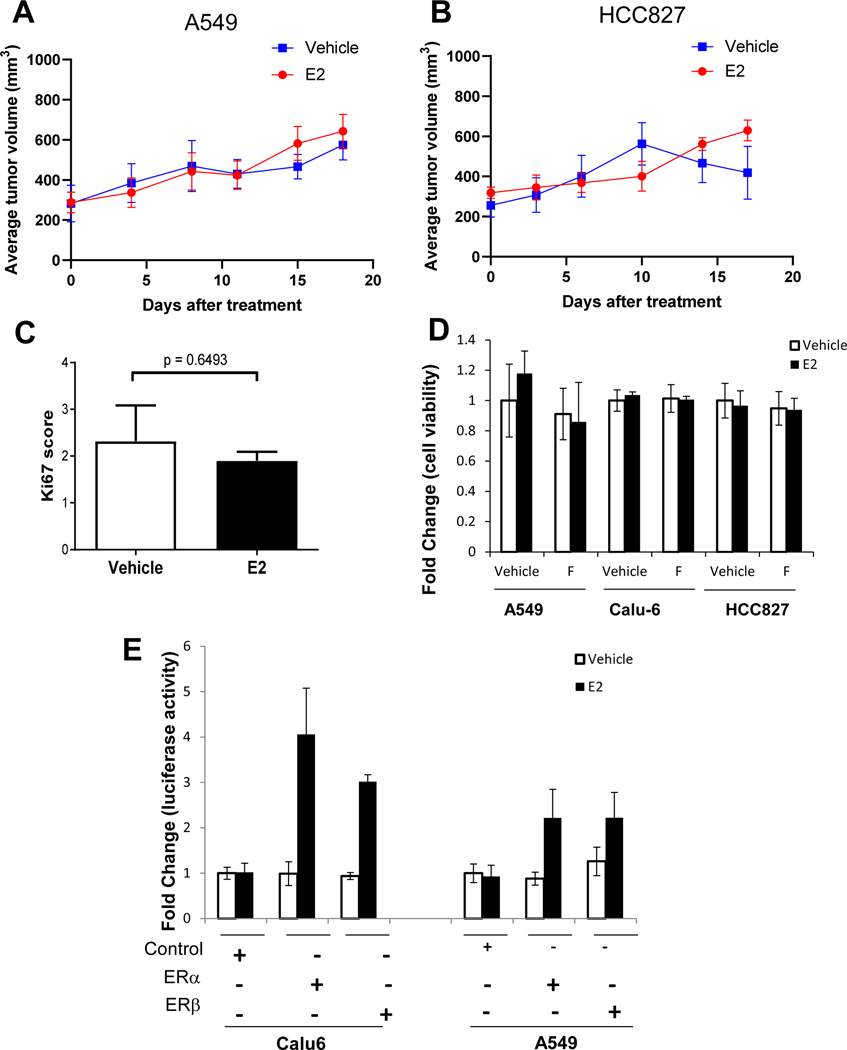
To evaluate the effect of estrogen on the growth of NSCLC xenografts, A549 and HCC827 NSCLC cells were subcutaneously injected into the flank of nude ovariectomized mice, and animals were treated with or without estrogen (Estradiol 17-β) delivered via continuous release (see Methods for details). In both tumor models, estrogen did not significantly impact on the growth of established tumors starting at 200–400 mm3 (Fig. 1A, B). Tumor sections from HCC827 xenografts were stained for Ki-67 to measure proliferation, and we observed no significant difference in Ki-67 staining between vehicle and estrogen-treated tumors (Fig. 1C). To assess the effects of estrogen on tumor cell proliferation rate in vitro, we treated Calu-6, A549, and HCC827 NSCLC cells, all of which express estrogen receptor β (ERβ) [9], with or without estrogen or estrogen in combination with fulvestrant, an ER antagonist for 4 days. Estrogen did not enhance the in vitro proliferation of any cell line tested (Fig. 1D). Likewise, fulvestrant treatment did not inhibit tumor cell proliferation. We next investigated whether estrogen signaling pathways were intact in NSCLC tumor cells. A549 and Calu-6 cells were transfected with expression vectors for ERα or ERβ, and a luciferase reporter assay driven by an estrogen response element was performed. Estrogen stimulation increased luciferase activity in cells ectopically expressing ERα or ERβ (Fig. 1E). Taken together, these findings demonstrate that while estrogen signaling is functionally intact in NSCLC cells, it does not direcly impact tumor cell proliferation or the growth of established tumors.
Figure 1. Estrogen is not sufficient to initiate a growth advantage in NSCLC xenograft tumor models or cell lines.
Average tumor growth rate of tumors starting at (200–400 mm3) in mice xenografts of A549 (A) and HCC827 (B), in presence or absence of estrogen. N =20 mice per group and experiment was repeated twice. The log-rank (Mantel-Cox) test was used to compare the statistical differences among the groups. Mice were sacrificed when the tumor reached 1000mm3. NSCLC xenograft tumors were sectioned and stained by immunohistochemistry for Ki-67 to measure tumor proliferation between vehicle and estrogen-treated NSCLC tumors (C). Cells were serum starved for 24 hours. 10−9M of estrogen (E2) and 10−8 M of pure anti-estrogen fulvestrant (F) was added. MTT assay was performed after 96 hours of incubation (D). Results are presented as a fold change of vehicle-treated proliferation. Calu6 and A549 were serum starved and transfected with ERα or ERβ cDNA. Luciferase activity was measured, and results are expressed as fold change of control (E). Results are presented as mean of biological triplicates ± SEM and experiment was repeated three times.
Estrogen induces resistance to bevacizumab, anti-VEGF, treatment in NSCLC xenografts.
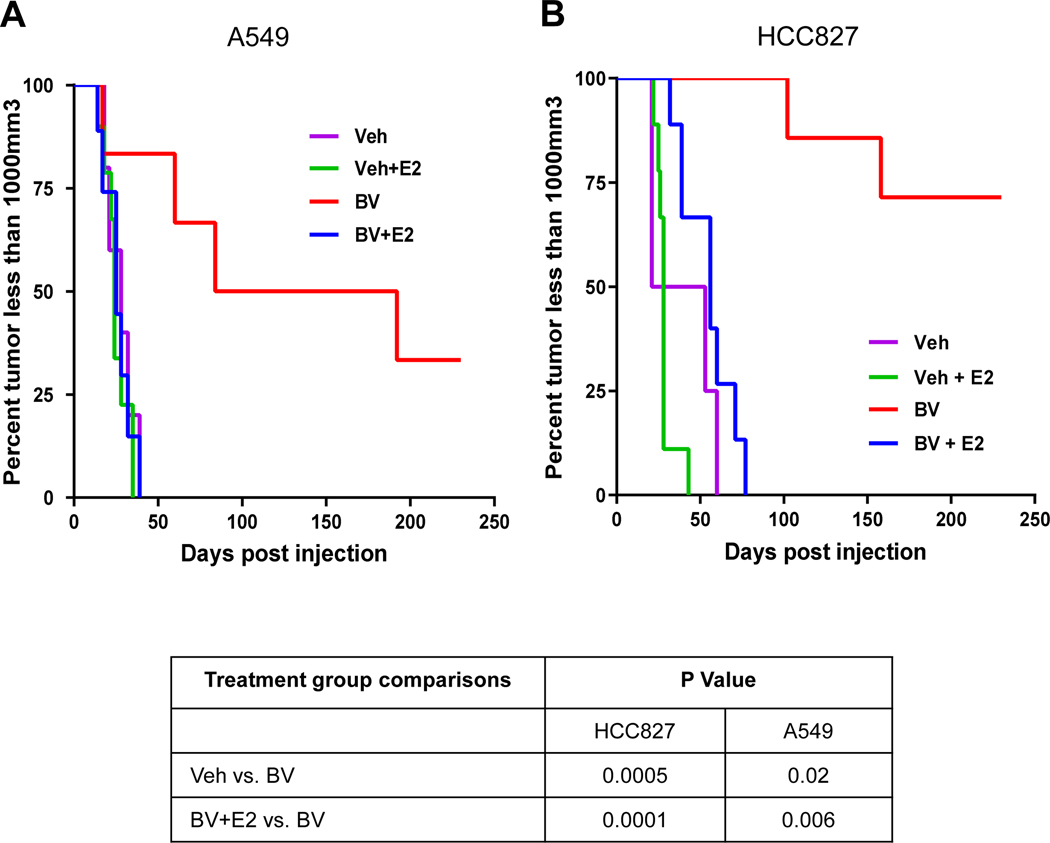
We next investigated whether estrogen promotes resistance of NSCLC tumors to anti-angiogenic therapy. HCC827 and A549 cells were injected subcutaneously into female ovariectomized nude mice with or without estrogen treatment. Once tumors reached a volume of ~250 mm3 animals were randomized to receive vehicle or bevacizumab (10 mg/kg). Animals were euthanized once tumors reached a volume of 1000 mm3. In both HCC827 and A549 xenograft models, estrogen did not accelerate the rate at which tumors reached 1000 mm3 compared to vehicle-treated mice. As expected, in non-estrogen treated mice tumor growth was significantly inhibited by bevacizumab (p = 0.0005, Fig. 2A; p = 0.02, Fig. 2B). However, in both tumor models the efficacy of bevacizumab was diminished when animals were treated with estrogen. In A549 tumor-bearing mice, bevacizumab treatment resulted in a median survival of over 138 days. However, when estrogen was applied the median survival was reduced to 25 days (p = 0.006, Fig. 2A). Similiarly in HCC827 tumor-bearing mice, bevacizumab treatment resulted in a median survival of over 200 days. Whereas in animals receiving estrogen, the median survival with bevacizumab treatment was only 56 days (p = 0.0001, Fig. 2B). These findings demonstrate that estrogen promotes resistance to anti-angiogenic therapy.
Figure 2. Estrogen induces resistance to bevacizumab, anti-VEGF, treatment in NSCLC xenografts.
Nude ovariectomized mice were primed with slow releasing estrogen (80pg/ml) tube. Mice were treated with bevacizumab (BV) 10mg/kg in the presence or absence of estrogen. Mice were sacrificed when tumor size reaches 1000mm3. Kaplan-Meier plot shows survival distribution of mice treated with BV in (A) A549 (B) HCC827 xenografts. The log-rank (Mantel-Cox) test was used to compare the statistical differences among the groups. N =10 mice per group and experiment was performed twice.
Estrogen increases pericyte vessel coverage and pro-angiogenic growth factor secretion in NSCLC xenografts.
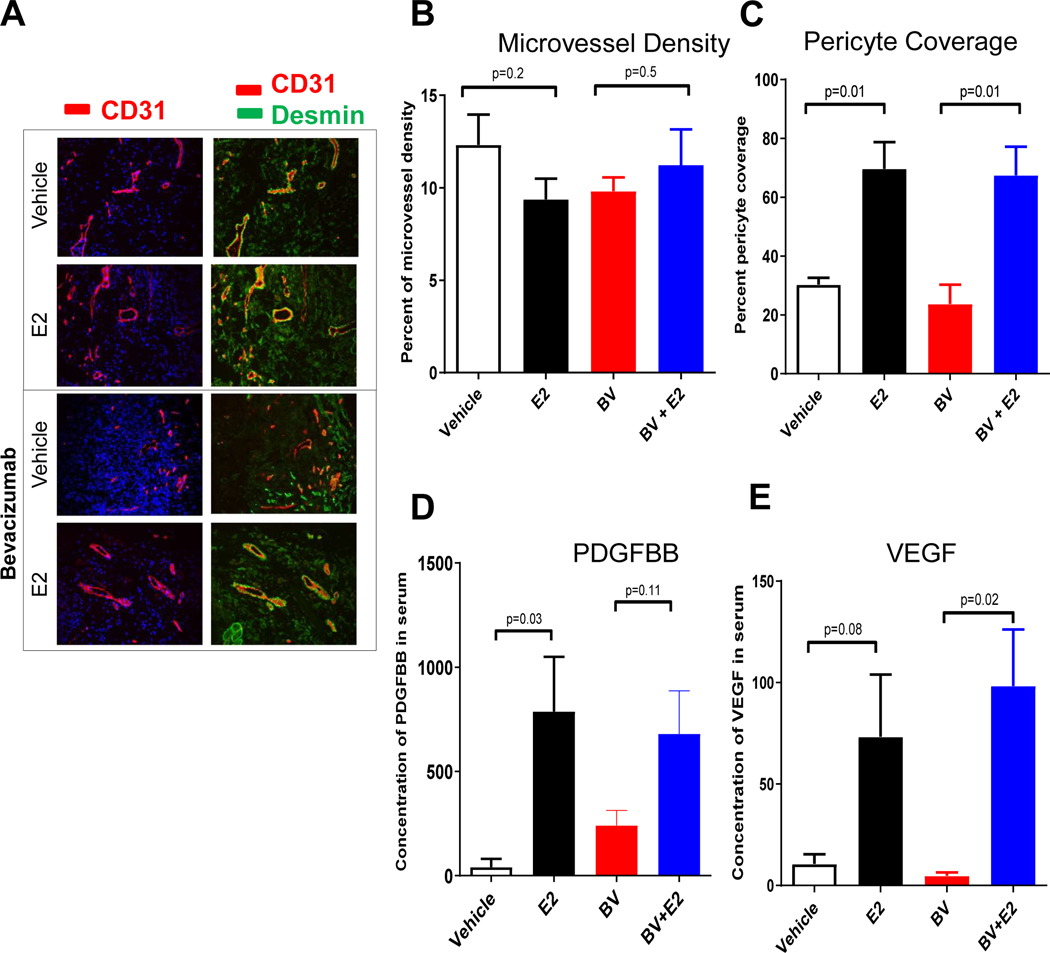
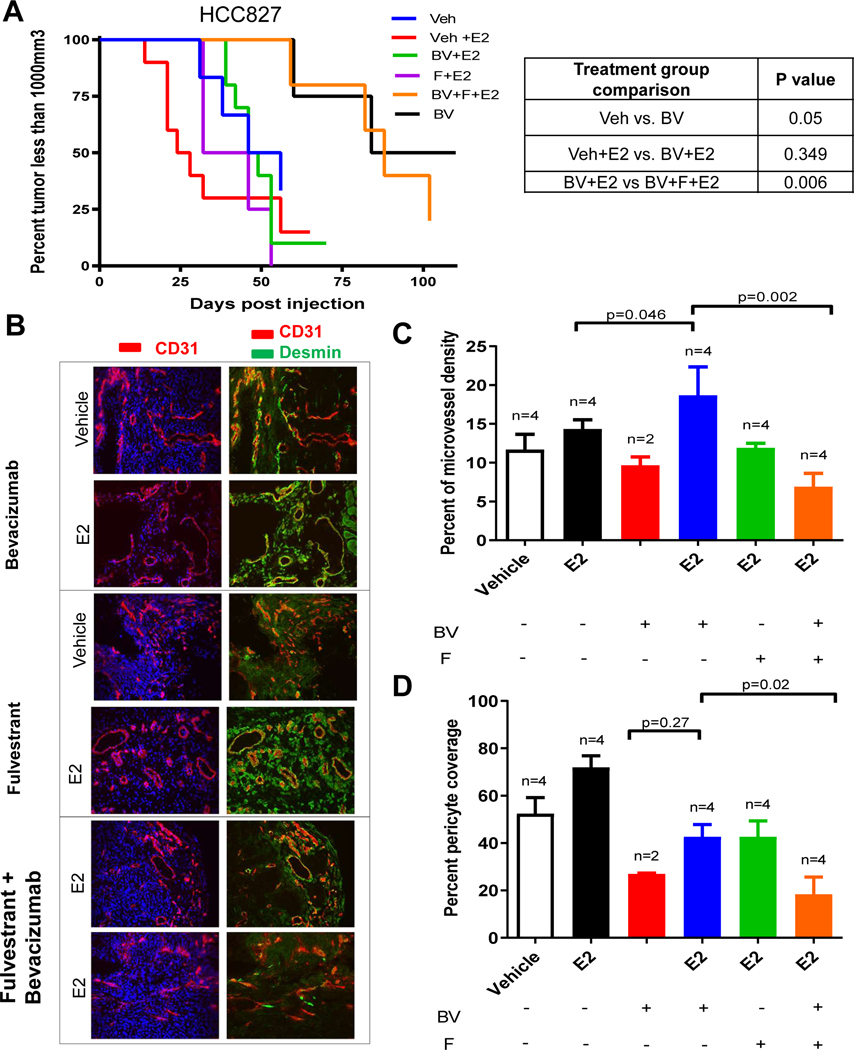
We next evaluated whether estrogen directly impacted tumor vascularization by assessing microvessel density (MVD), a measure of total vascular endothelial staining, as well as pericyte coverage, a measure of vascular maturity, in HCC827 xenografts. Tumors treated with vehicle, estrogen, bevacizimab, or the combination of estrogen and bevacizumab were immunostained with antibodies directed against CD31 and desmin and pericyte coverage was calculated as the percentage of CD31+ vessels that co-localize with desmin positivity (Fig. 3A). Estrogen treatment did not enhance the MVD (Fig. 3B). However, estrogen-treated animals did demonstrate a marked 2.3 fold increase in pericyte-covered vessels (p = 0.01, Fig. 3C). Increased pericyte coverage has enhanced tumor endothelial survival and VEGF inhibitor resistance by our group and others in NSCLC and other tumor types [31–33]. Estrogen had similar effects on tumor vascularization in the A549 model as measured by MVD and pericyte coverage (Fig. S1).
Figure 3. Estrogen increases pericyte vessel coverage and pro-angiogenic growth factor secretion in NSCLC xenografts.
Representative of immunofluorescence staining (20X) of CD31 (red), desmin (green) and nuclei (blue) using immunofluorescence microscopy. A minimum of 4–5 microphotograph (20X) for each sample (n=4/group) were collected (A-B). Quantification of microvessel density (C) and pericyte coverage (D) at tumor progression (100mm3) was performed. Vessels were considered pericyte covered if more than 50% of CD31+ vessel co-localized with desmin. Data are graphed as mean ± SEM. Increased circulating angiogenic factors, PDGFBB and VEGF were measured during tumor growth in presence of estrogen. Mouse PDGFBB (E) and VEGF (F) serum levels were quantified using the Bio-rad multiplex bead assay. For each sample (n=4/group), blood from tumor bearing mouse was drawn by cardiac puncture. The results were plotted as average of the duplicate and graphed as mean ± SEM. Mice were treated with bevacizumab (BV) 10mg/kg in presence or absence of estrogen.
PDGF-BB is a key mediator of pericyte coverage and vascular stability and maturity [34, 35]. We next evaluated circulating levels of mouse PDGF-BB in tumor-bearing animals treated with or without estrogen. Circulating mouse PDGF-BB levels were greater than 10-fold higher in estrogen-treated mice compared to vehicle-treated animals (p = 0.03, Fig. 3D). In addition, circulating levels of mouse VEGF were 7-fold higher in estrogen-treated mice as compared to the vehicle-treated animals (p=0.08, Fig. 3E). These findings suggest that estrogen-induced changes in angiogenic factors may have accelerated the growth of mature tumor vasculature.
Estrogen increases the expression of myeloid recruitment factors CXCL1 and G-CSF and tumor infiltration of CD11b+ myeloid cells.
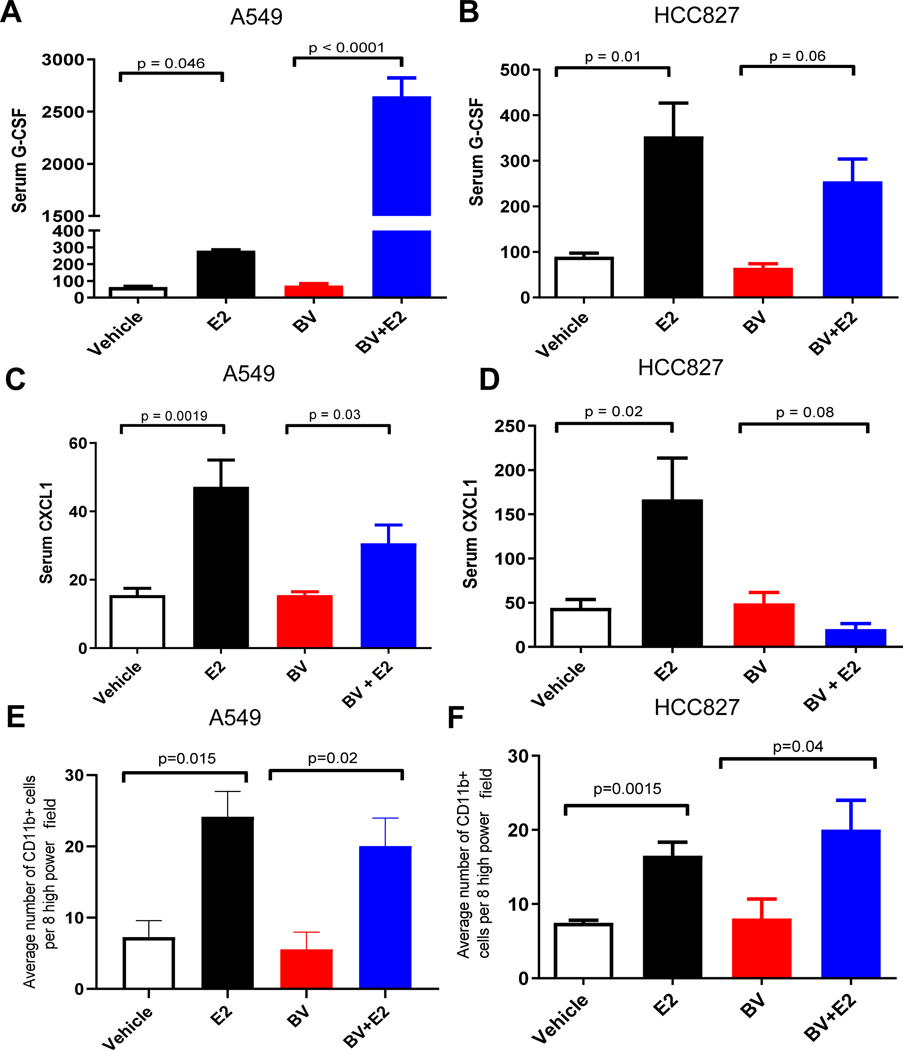
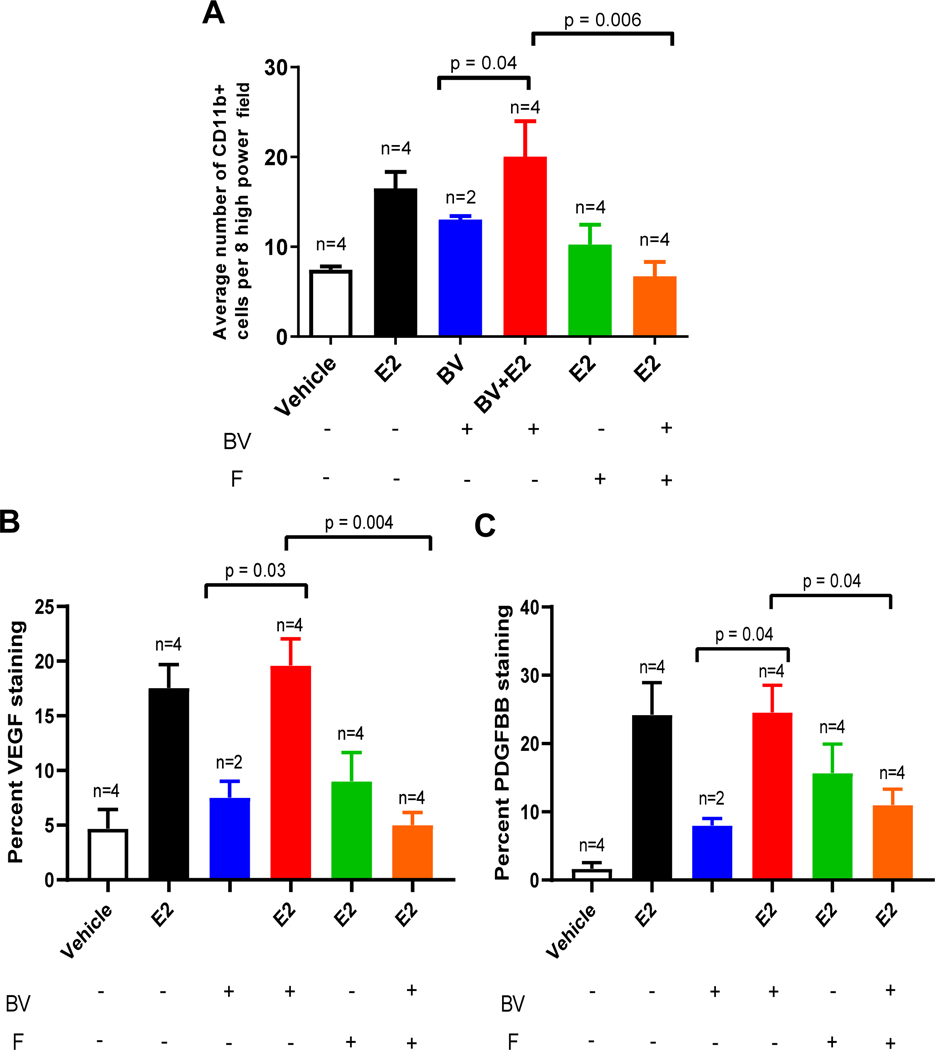
We next sought to identify other growth factors and cytokines that may be influenced by estrogen in our established NSCLC xenografts treated with bevacizumab. Established HCC827 and A549 tumor bearing mice were treated with estrogen, bevacizumab, or the combination, and the changes in serum circulating factors were evaluated by multiplex assay. In the A549 tumor-bearing animals, estrogen treatment resulted in a 3.5-fold increase in serum levels of mouse G-CSF compared to vehicle-treated animals (p = 0.046, Fig. 4A). The combination treatment of bevacizumab and estrogen resulted in an 8-fold increase in serum mouse G-CSF levels, as compared to estrogen alone (p < 0.0001 , Fig. 4A). A significant increase in serum mouse GCSF was also observed in HCC827 tumor-bearing animals treated with estrogen (p = 0.01, Fig. 4B), while G-CSF levels in animals treated with the combination treatment of bevacizumab and estrogen was not significantly modulated compared to animals receiving estrogen alone (p = 0.06, Fig. 4B). Therefore, while E2 lead to an increase in serum G-CSF in both models, the E2/bevacizumab combination had a differential impact in the two models. The mechanism(s) underlying this differential impact are not currently known.
Figure 4. Estrogen increases the recruitment of myeloid cells through the secretion of CXCL1 and G-CSF.
Circulating pro-angiogenic factors, G-CSF from (A) A549 and (B) HCC827 & CXCL1 levels were quantified from (C) A549 and (D) HCC827 mice xenografts in the presence of estrogen. The samples were quantified in duplicate by using the Bio-rad multiplex bead assay. The results were plotted as average of the duplicate and graphed as mean ± SEM. Quantification of CD11b+ staining of A549 (E) and HCC827 (F) xenografts with and without estrogen treatment. A minimum of eight high power field (400X) was counted for CD11b+ cells from the periphery to the center of the tumor from each sample. The results were plotted as average of eight high power field (400X) and graphed as mean ± SEM.
Our multiplex analysis of circulating growth factors also showed that serum human CXCL1 was significantly increased. In mice bearing established A549 or HCC827 xenografts, estrogen treatment induced a significant rise in serum levels of human CXCL1 compared to vehicle-treated animals (p = 0.0019, Fig. 4C; p = 0.02, Fig. 4D). Circulating levels of human CXCL1 were considerably lower in animals treated with bevacizumab in combination with estrogen as compared to animals receiving estrogen alone (p = 0.03, Fig. 4C; p = 0.08, Fig. 4D) suggesting that VEGF may directly or indirectly impact levels of this cytokine. Collectively, our results indicate that estrogen induces production of human CXCL1 and mouse G-CSF.
CXCL1 and G-CSF regulate mobilization of hematopoietic stem cell progenitors and mature myeloid cells into the circulation [36, 37]. Therefore, we examined the effect of estrogen on the infiltration of myeloid cells into the tumor microenvironment by immunostaining tumors with antibodies directed against CD11b, a pan-myeloid cell marker. In both A549 and HCC827 xenograft models, estrogen induced a significant rise in the number of tumor-infiltrating myeloid cells (p = 0.015, Fig. 4E; p = 0.0015, Fig. 4F). Given the established role of CXCL1 and G-CSF in the recruitment of myeloid cells, it is feasible that the estrogen-induced increase in G-CSF levels may mediate the increased infiltration of CD11b+ cells in the tumor observed in both the estrogen and estrogen and bevacizumab combination treatment groups (Fig. S2A and S2B). Interestingly, the recruitment of mature myeloid cells had been demonstrated previously in various tumors including gastric, colon, and bronchioloalveolar carcinoma and is correlated with increased tumor vascular density and poor prognosis [38, 39].
Estrogen promotes increased myeloid infiltration and resistance to bevacizumab which can be reversed with estrogen receptor blockade.
We next assesed whether inhibition of estrogen signaling could enhance the efficacy of bevacizumab. HCC827 tumor cells were injected into ovarectomized female mice receiving vehicle or estrogen combined with the estrogen receptor selective degrader, fulvestrant, or becizumab, once tumors reached a volume of ~250 mm3. Consistent with our earlier observations, bevacizumab delayed tumor growth in mice not treated with estrogen (p = 0.05), but in the presence of estrogen bevacizumab did not reduce tumor growth (p = 0.349, Fig. 5A). The addition of fulvestrant reversed this effect, significantly enhancing the efficacy of bevacizumab in the presence of estrogen (p = 0.006, Fig. 5A). Immunohistochemical analysis of tumors revealed that in estrogen-treated mice receiving bevacizumab, fulvestrant significantly decreased the MVD (p = 0.002) and pericyte coverage (p = 0.02) compared to estrogen-treated mice receiving bevacizumab alone (Fig. 5C and 5D). Additionally, fulvestrant reduced infiltration of CD11b+ myeloid cells (p = 0.006) compared to estrogen-treated mice receiving bevacizumab alone (Fig. 6A). Additionally, a similar relationship was observed in estrogen-treated mice receiving bevacizumab in which fulvestrant significantly decreased protein expression of VEGF and PDGFBB in the tumor as measured by immunohistochemistry staining (Fig. 6B & 6C, Fig. S3).
Figure 5. Estrogen receptor blockade sensitizes NSCLC tumors to anti-VEGF therapy.
Nude ovariectomized mice were primed with slow releasing estrogen (80pg/ml) tube. HCC827 xenograft mice were treated with bevacizumab (BV) 10mg/kg twice in a week, and fulvestrant (F) 200mg/kg, once in a week in presence of estrogen. Kaplan-Meier plot showing survival distribution of HCC827 xenograft treated with BV, F, or estrogen (A). The log-rank (Mantel-Cox) test was used to compare the statistical differences among the groups. Representative images of immunofluoroscence staining (200X) of CD31 (red), desmin (green) and nuclei (blue) using immunofluorescence microscopy were collected (B). A minimum of 4–5 microphotograph (200X) for each sample (n=4/group) were collected. BV alone group had two tumor bearing mice. Quantification of microvessel density (C), pericyte coverage (D), and CD11b+ myeloid cells (E) by staining was measured.
Figure 6 –
Estrogen receptor blockade mediates increased myeloid infiltration in NSCLC xenograft associated with increased in VEGF and PDGFFB.
We next evaluated whether our preclinical findings were representative of the biology associated with estrogen signalling in human lung cancer patients. Using the microarray gene expression data from the MD Anderson cohort of primary lung adenocarcinoma (LUAD) patients prior to neoadjuvant therapy from Profiling of Resistance patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT), we observed significant (p=0.001) positive correlation between ER-α (ESR1) and ER-β (ESR2) expression and expression of CD11b (ITGAM) (Fig. S4A&D) and CD31 (PECAM) (Fig. S4B&E). Moreover, ER-α expression also correlated with expression of CXCL1 (Fig. 4C). Additionally, when stratifying this gene expression analysis by sex (n=77 male patients and n=73 female patients), we surprisingly observed a positive correlation for both males and females. There may be multiple factors which contribute to this positive correlation. First, expression of ESR1 and ESR2 has been observed in both male and female lung cancer tumor cell lines as well as non-malignant cells [22], and estrogen receptor signaling may enhance the expression of paracrine factors promoting myeloid cell recruitment as observed here, and effect that would be expected to be more pronounced in females given the higher levels of circulating estrogen. Additionally, myeloid cells can express estrogen receptor [40] and thus the positive correlation could be indicative of the overall increased infiltration of myeloid cells in these tumors. The relative contribution of paracrine signaling vs direct estrogen receptor signaling in myeloid cells could not be directly addressed here and merits further investigation.
Discussion
Anti-angiogenic therapy has been investigated in multiple malignancies including lung cancer. While patients with advanced NSCLC have experienced a clinical benefit to bevacizumab in combination with chemotherapy in the ECOG4599 study, a sex disparity was observed with females responding more poorly than males [17]. Thus it is critical to determine if the presence of sex hormones and even use of hormone replacement therapy may be associated with differential response to anti-angiogenic therapy. In our study, we assessed the impact of estrogen on the growth of NSCLC tumors and response to anti-angiogenic therapy. The presence of estrogen enhanced pericyte coverage of the tumor vascular network, increased the secretion of angiogenic factors, and the promoted the recruitment of myeloid cells to the tumor. We found that estrogen promoted resistance to VEGF blockade and that targeting estrogen receptor signaling could blunt this effect, suggesting that the use of estrogen receptor antagonist may improve the clinical response of female NSCLC patients receiving VEGF inhibitors.
Expression of ERα and ERβ have been confirmed across multiple tumor cell types including lung cancer cells [41, 42], signifying that sex hormones from hormone replacement therapy may have a direct effect on cancer cell growth. While estrogen did not directly enhance tumor cell proliferation in our in vitro studies, others have also indicated that estrogen receptors signaling could modulate the proliferation of some NSCLC cell lines [43], albeit at estrogen concentrations more than 10-fold higher than those used here, which we selected to more closely represent physiologic levels. [26, 27]. Several studies in NSCLC in vivo models provided insight on the involvement of estrogen in tumorigenesis. Estrogen has been implicated as a driver of tumor growth in lung cancer [44], and others have shown that treatment of NSCLC xenograft with aromatase inhibitors, which reduce estrogen production, significantly decreased tumor growth [21]. Consistent with these earlier reports, our results suggest that estrogen promotes the growth of NSCLC tumors due to alterations to the tumor’s secretome. In support of this, we determined that estrogen induced a rise in circulating levels of mouse PDGF-BB and VEGF, two factors known to play key role in modulating the tumor microenvironment.
The role of VEGF in regulating physiological and pathological angiogenesis has been well characterized [38], and anti-VEGF therapies are approved for multiple malignancies including NSCLC [45]. Pericytes are a critical component of angiogenesis and envelop endothelial cells to regulate vessel stability [46] and promote endothelial survival. Pericyte recruitment is primarily regulated by PDGF-BB [47], enhanced pericyte coverage occurs when there is a high ratio of PDGF-BB to VEGF [48]. Our in vivo observations are supported by other studies showing that estrogen receptor signaling induces VEGF expression by NSCLC cell lines in vitro [49]. In agreement with this, our models displayed an estrogen-induced increase in the PDGF:VEGF ratio, which was associated with enhanced pericyte coverage of the tumor vasculature. Immature blood vessels which lack pericyte coverage are particularly sensitive to anti-VEGF therapies [31].
In addition to the increase in circulating PDGF-BB and VEGF, we also observed elevated mouse G-CSF and human CXCL1 serum concentrations. Reports indicate that tumor refractoriness to anti-angiogenic therapy is mediated in part by G-CSF through the recruitment of myeloid monocytic cells in the tumor microenvironment [36]. Other studies have also illustrated the positive correlation between increased aromatase activity and macrophages infiltration [50]. CXCL1/MIP-2 (chemokine ligand-1/macrophage inhibitory protein-2) leads to recruitment of neutrophils, which in turn induces angiogenesis [51]. In our study, estrogen-mediated increases in G-CSF and CXCL1 correlated with increased recruitment of myeloid cells to the tumor. Bone marrow derived myeloid cells have been shown to contribute to tumor angiogenesis in murine tumor models [52] and human tumors [53] and create an immunosuppressive tumor microenvironment. Others have reported similar findings that 17-β estradiol enhanced the mobilization of bone marrow derived endothelial progenitor cells, which resulted in increased tumor vascularization [54]. The potential role of estrogen in mobilizing bone-marrow derived endothelial and/or myeloid cells merits further investigation. It is worth noting that in the recent Impower-150 study, females appeared to receive less PFS benefit from the addition of atezolizumab to bevacizumab/carboplatin/paclitaxel (BCP) [60], and enhanced mobilization of myeloid cells is one plausible contributor to this difference.
Prior preclinical studies demonstrated synergistic antitumor activity for the combination of fulvestrant with the EGFR tyrosine kinase inhibitor (TKI) gefitinib in human NSCLC xenografts model, suggesting that estrogen signaling may also impact the response to other targeted therapies[11, 55]. Moreover, the interaction between estrogen and the EGFR pathway [56] may contribute to the effects observed in our experiments utilizing HCC827 xenografts, as this cell line harbors an EGFR activating mutation. Additionally, our results build upon previous findings from which show that vandetinib, which blocks VEGFR and EGFR signalling, also has higher efficacy when combined with fulvestrant [57].
In our animal models, dual targeting of estrogen receptors and VEGF significantly reduced pericyte coverage and tumor growth, indicating that targeting the estrogen pathway may enhance the efficacy of anti-angiogenic agents. The roles for estrogen in NSCLC progression proposed here may, at least in part, explain why less benefit was observed in females treated with bevacizumab compared with males in the ECOG 4599 trial. It is worth noting, however, that patients in this study were treated with chemotherapy in combination with bevacizumab, and we cannot rule out the possibility that interactions with chemotherapy also contributed to the the observed sex-specific differences. Nevertheless, both preclinical and clinical data suggest that the addition of anti-estrogens to anti-angiogenic treatment merits further investigation as a potential therapeutic strategy to enhance the efficacy of VEGF inhibitors in NSCLC patients and possibly other cancers.
Supplementary Material
Supplemental Figure 1. Estrogen increases pericyte vessel coverage in A549 NSCLC xenograft.
Representative images of immunofluoroscence staining (200X) of CD31 (red), desmin (green) and nuclei (blue) using immunofluorescence microscopy were collected from A549 xenografts treated with and without estrogen (A) bevacizumab, and the combination of estrogen and bevacizumab treatment (B). Quantification of microvessel density (C) and pericyte coverage (D) by staining was measured.
Supplemental Figure 2. Estrogen is involved in the recruitment of CD11b+ monocytes.
Representative of immunohistochemical images (50X) of H&E and CD11b+ staining from vehicle and estrogen treated A549 xenografts (A). Representative of immunohistochemical images (200X) of CD11b+ staining in A549 and HCC827 xenografts with and without estrogen, bevacizumab, and the combination of estrogen and bevacizumab treatment (B).
Supplemental Figure 3. E2 enhances the production of VEGF and PDGFBB in NSCLC xenografts.
Representative of immunohistochemical images (50X) of VEGF and PDGFBB staining from vehicle (A&E), estrogen (B&F), becavizumab (C&G), and the combination of estrogen and bevacizumab (D&H) treated HCC827 xenografts.
Supplemental Figure 4. Estrogen receptor is positively correlated with gene expression of CD11b, CD31, and CXCL1 in PROSPECT LUAD analysis.
Statistical correlation shows a positive association between estrogen receptor α (ESR1) and gene expression of ITGAM (CD11b) (A&D), PECAM (CD31) (B&E), and CXCL1 (C&F) determined by microarray analysis of surgically resected lung adenocarcinomas (150 patients who did not receive neoadjuvant therapy) from the MD Anderson Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT) study. Additionally these analyses were performed separately between female (G) and male (H) patients in the cohort.
Acknowledgements
This research was supported by the Joan’s Legacy award and the Flight Attendant’s Medical Research Institute. J.V. Heymach is a Damon Runyon-Lilly Clinical Investigator supported in part by Damon Runyon Cancer Research Foundation grant CI 24-04. This work was also supported by the University of Texas Southwestern Medical Center, M. D. Anderson Cancer Center Lung SPORE NIH grant P50 CA070907, the David Bruton, Jr. Endowment, the Rexanna Foundation for Fighting Lung Cancer, NIH P30 CA016672-44 , 1R01 CA190628, Lung Cancer Moon Shot Program, the Hallman Fund, the Richardson fund, the Kopelman Foundation and the Margot Johnson Cancer Research Fund, and the Cancer Center Support Grant P30 CA016672-44.
Footnotes
Disclosures
T.C. reports speaker’s fees from Society for Immunotherapy of Cancer (SITC) and Bristol-Myers Squibb, consulting fees from MedImmune/AstraZeneca and Bristol-Myers Squibb, and advisory role fees from EMD Serono and Bristol-Myers Squibb and clinical research funding to MD Anderson Cancer Center from Boehringer Ingelheim, MedImmune/AstraZeneca, EMD Serono, and Bristol-Myers Squibb. H.T.T. reports research funding from Bayer-AS, Bristol-Myers Squibb, Ziopharm, Guardant Health. X.L. receives consulting/advisory fees from EMD Serono (Merck KGaA), AstraZeneca, Spectrum Pharmaceutics, Eli Lilly, Boehringer Ingelheim, and Daiichi Sanko, and Research Funding from Eli Lilly, and Boehringer Ingelheim. I.W. reports outside the submitted work grants and personal fees from Genentech/Roche, Bayer, Bristol-Myers Squibb, Astra Zeneca/Medimmune, Pfizer, HTG Molecular, Asuragen, Guardant Health, and Merck; personal fees from GlaxoSmithKline, Oncocyte, and MSD; and grants from Oncoplex, DepArray, Adaptive, Adaptimmune, EMD Serono, Takeda, Amgen, Karus, Johnson & Johnson, Iovance, 4D, Novartis, Akoya. J.D.M. reports receiving licensing fees for tumor cell lines from the NIH and University of Texas Southwestern Medical Center. M.B.N. and J.V.H. have filed a patent for the use of poziotinib for treating EGFR and HER2 mutant cancers and licensed the technology to Spectrum Pharmaceuticals and receive royalties and licensing fees. J.V.H. receives grant or research support from AstraZeneca, Boehringer Ingelheim, Spectrum Pharmaceuticals, and GlaxoSmithKline and has served on advisory committees for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Catalyst, EMD Serono, Foundation Medicine, Hengrui Therapeutics, Genentech, GSK, Guardant Health, Eli Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, Seattle Genetics, Spectrum, Takeda. Other authors do not report any relevant conflict of interest.
References
- 1.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2020. CA Cancer J Clin, 2020. 70(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Cronin KA, et al. , Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer, 2018. 124(13): p. 2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Early Lung Cancer Action Program, I., et al. , Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA, 2006. 296(2): p. 180–4. [DOI] [PubMed] [Google Scholar]
- 4.Wakelee HA, et al. , Lung cancer incidence in never smokers. J Clin Oncol, 2007. 25(5): p. 472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risch HA, et al. , Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol, 1993. 138(5): p. 281–93. [DOI] [PubMed] [Google Scholar]
- 6.Chlebowski RT, et al. , Lung cancer among postmenopausal women treated with estrogen alone in the women’s health initiative randomized trial. J Natl Cancer Inst, 2010. 102(18): p. 1413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clague J, et al. , Menopausal hormone therapy and lung cancer-specific mortality following diagnosis: the California Teachers Study. PLoS One, 2014. 9(7): p. e103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganti AK, et al. , Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol, 2006. 24(1): p. 59–63. [DOI] [PubMed] [Google Scholar]
- 9.Garon EB, et al. , Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small-cell lung cancer. J Thorac Oncol, 2013. 8(3): p. 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, et al. , Randomized phase II study of fulvestrant and erlotinib compared with erlotinib alone in patients with advanced or metastatic non-small cell lung cancer. Lung Cancer, 2018. 123: p. 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stabile LP, et al. , Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res, 2005. 65(4): p. 1459–70. [DOI] [PubMed] [Google Scholar]
- 12.Blumenschein G and Heymach JV, Angiogenesis inhibitors for lung cancer: clinical developments and future directions. J Thorac Oncol, 2006. 1(7): p. 744–8. [DOI] [PubMed] [Google Scholar]
- 13.Dudek AZ, et al. , Phase II study of biweekly carboplatin, gemcitabine, and bevacizumab as first-line treatment in patients with stage IIIB/IV NSCLC. Am J Clin Oncol, 2014. 37(2): p. 140–3. [DOI] [PubMed] [Google Scholar]
- 14.Zappa F, et al. , Bevacizumab and erlotinib (BE) first-line therapy in advanced non-squamous non-small-cell lung cancer (NSCLC) (stage IIIB/IV) followed by platinum-based chemotherapy (CT) at disease progression: a multicenter phase II trial (SAKK 19/05). Lung Cancer, 2012. 78(3): p. 239–44. [DOI] [PubMed] [Google Scholar]
- 15.Ellis LM and Hicklin DJ, Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res, 2008. 14(20): p. 6371–5. [DOI] [PubMed] [Google Scholar]
- 16.Jayson GC, et al. , Antiangiogenic therapy in oncology: current status and future directions. Lancet, 2016. 388(10043): p. 518–29. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR, et al. , Sex differences in outcome with bevacizumab therapy: analysis of patients with advanced-stage non-small cell lung cancer treated with or without bevacizumab in combination with paclitaxel and carboplatin in the Eastern Cooperative Oncology Group Trial 4599. J Thorac Oncol, 2011. 6(1): p. 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldini EH and Strauss GM, Women and lung cancer: waiting to exhale. Chest, 1997. 112(4 Suppl): p. 229S–234S. [DOI] [PubMed] [Google Scholar]
- 19.Mah V, et al. , Aromatase expression predicts survival in women with early-stage non small cell lung cancer. Cancer Res, 2007. 67(21): p. 10484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trotter A, et al. , Combined application of 17beta-estradiol and progesterone enhance vascular endothelial growth factor and surfactant protein expression in cultured embryonic lung cells of mice. Int J Pediatr, 2009. 2009: p. 170491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg OK, et al. , Aromatase inhibitors in human lung cancer therapy. Cancer Res, 2005. 65(24): p. 11287–91. [DOI] [PubMed] [Google Scholar]
- 22.Stabile LP, et al. , Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res, 2002. 62(7): p. 2141–50. [PubMed] [Google Scholar]
- 23.Morales DE, et al. , Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation, 1995. 91(3): p. 755–63. [DOI] [PubMed] [Google Scholar]
- 24.Xu L, et al. , Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1alpha in non-small cell lung cancer cells. Oncogene, 2010. 29(18): p. 2616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuqua SA, Blum-Salingaros M, and McGuire WL, Induction of the estrogen-regulated “24K” protein by heat shock. Cancer Res, 1989. 49(15): p. 4126–9. [PubMed] [Google Scholar]
- 26.Cousins SW, et al. , Female gender, estrogen loss, and Sub-RPE deposit formation in aged mice. Invest Ophthalmol Vis Sci, 2003. 44(3): p. 1221–9. [DOI] [PubMed] [Google Scholar]
- 27.Xie Y, et al. , Gender Differences in Low-Molecular-Mass-Induced Acute Lung Inflammation in Mice. Int J Mol Sci, 2021. 22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masamura S, et al. , Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab, 1995. 80(10): p. 2918–25. [DOI] [PubMed] [Google Scholar]
- 29.Weidner N, et al. , Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med, 1991. 324(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 30.Cardnell RJ, et al. , An Integrated Molecular Analysis of Lung Adenocarcinomas Identifies Potential Therapeutic Targets among TTF1-Negative Tumors, Including DNA Repair Proteins and Nrf2. Clin Cancer Res, 2015. 21(15): p. 3480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergers G, et al. , Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest, 2003. 111(9): p. 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cascone T, et al. , Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest, 2011. 121(4): p. 1313–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco M, et al. , Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood, 2011. 118(10): p. 2906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin LE, Hemo I, and Keshet E, A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development, 1998. 125(9): p. 1591–8. [DOI] [PubMed] [Google Scholar]
- 35.Pietras K, et al. , Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med, 2008. 5(1): p. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shojaei F, et al. , G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A, 2009. 106(16): p. 6742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D, et al. , CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res, 2017. 77(13): p. 3655–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara N, Gerber HP, and LeCouter J, The biology of VEGF and its receptors. Nat Med, 2003. 9(6): p. 669–76. [DOI] [PubMed] [Google Scholar]
- 39.Tazzyman S, Niaz H, and Murdoch C, Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol, 2013. 23(3): p. 149–58. [DOI] [PubMed] [Google Scholar]
- 40.Smida T, Bruno TC, and Stabile LP, Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications. Front Oncol, 2020. 10: p. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawai H, et al. , Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res, 2005. 11(14): p. 5084–9. [DOI] [PubMed] [Google Scholar]
- 42.Nose N, et al. , Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol, 2009. 27(3): p. 411–7. [DOI] [PubMed] [Google Scholar]
- 43.Gao X, et al. , Estrogen receptors promote NSCLC progression by modulating the membrane receptor signaling network: a systems biology perspective. J Transl Med, 2019. 17(1): p. 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Lara V, Hernandez-Martinez JM, and Arrieta O, Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis, 2018. 10(1): p. 482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ascha MS, et al. , Bevacizumab for the treatment of non-small cell lung cancer patients with synchronous brain metastases. Sci Rep, 2019. 9(1): p. 17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergers G and Song S, The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol, 2005. 7(4): p. 452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindblom P, et al. , Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev, 2003. 17(15): p. 1835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenberg JI, et al. , A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature, 2008. 456(7223): p. 809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarzynka MJ, et al. , Estradiol and nicotine exposure enhances A549 bronchioloalveolar carcinoma xenograft growth in mice through the stimulation of angiogenesis. Int J Oncol, 2006. 28(2): p. 337–44. [PMC free article] [PubMed] [Google Scholar]
- 50.Stabile LP, et al. , Preclinical Evidence for Combined Use of Aromatase Inhibitors and NSAIDs as Preventive Agents of Tobacco-Induced Lung Cancer. J Thorac Oncol, 2018. 13(3): p. 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieschke GJ, et al. , Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood, 1994. 84(6): p. 1737–46. [PubMed] [Google Scholar]
- 52.Yang L, et al. , Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell, 2004. 6(4): p. 409–21. [DOI] [PubMed] [Google Scholar]
- 53.Schmid MC and Varner JA, Myeloid cell trafficking and tumor angiogenesis. Cancer Lett, 2007. 250(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suriano R, et al. , 17Beta-estradiol mobilizes bone marrow-derived endothelial progenitor cells to tumors. Cancer Res, 2008. 68(15): p. 6038–42. [DOI] [PubMed] [Google Scholar]
- 55.Marquez-Garban DC, et al. , Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids, 2007. 72(2): p. 135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raso MG, et al. , Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res, 2009. 15(17): p. 5359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegfried JM, et al. , Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol, 2012. 7(3): p. 485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson MB, et al. , Altered Regulation of HIF-1alpha in Naive- and Drug-Resistant EGFR-Mutant NSCLC: Implications for a Vascular Endothelial Growth Factor-Dependent Phenotype. J Thorac Oncol, 2021. 16(3): p. 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandler A, et al. , Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med, 2006. 355(24): p. 2542–50. [DOI] [PubMed] [Google Scholar]
- 60.Socinski MA, et al. , Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med, 2018. 378(24): p. 2288–2301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Estrogen increases pericyte vessel coverage in A549 NSCLC xenograft.
Representative images of immunofluoroscence staining (200X) of CD31 (red), desmin (green) and nuclei (blue) using immunofluorescence microscopy were collected from A549 xenografts treated with and without estrogen (A) bevacizumab, and the combination of estrogen and bevacizumab treatment (B). Quantification of microvessel density (C) and pericyte coverage (D) by staining was measured.
Supplemental Figure 2. Estrogen is involved in the recruitment of CD11b+ monocytes.
Representative of immunohistochemical images (50X) of H&E and CD11b+ staining from vehicle and estrogen treated A549 xenografts (A). Representative of immunohistochemical images (200X) of CD11b+ staining in A549 and HCC827 xenografts with and without estrogen, bevacizumab, and the combination of estrogen and bevacizumab treatment (B).
Supplemental Figure 3. E2 enhances the production of VEGF and PDGFBB in NSCLC xenografts.
Representative of immunohistochemical images (50X) of VEGF and PDGFBB staining from vehicle (A&E), estrogen (B&F), becavizumab (C&G), and the combination of estrogen and bevacizumab (D&H) treated HCC827 xenografts.
Supplemental Figure 4. Estrogen receptor is positively correlated with gene expression of CD11b, CD31, and CXCL1 in PROSPECT LUAD analysis.
Statistical correlation shows a positive association between estrogen receptor α (ESR1) and gene expression of ITGAM (CD11b) (A&D), PECAM (CD31) (B&E), and CXCL1 (C&F) determined by microarray analysis of surgically resected lung adenocarcinomas (150 patients who did not receive neoadjuvant therapy) from the MD Anderson Profiling of Resistance Patterns and Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax (PROSPECT) study. Additionally these analyses were performed separately between female (G) and male (H) patients in the cohort.