Abstract
Mitochondria regulate multiple aspects of neuronal development, physiology, plasticity, and pathology through their regulatory roles in bioenergetic, calcium, redox, and cell survival/death signalling. While several reviews have addressed these different aspects, a comprehensive discussion focussing on the relevance of isolated brain mitochondria and their utilities in neuroscience research has been lacking. This is relevant because the employment of isolated mitochondria rather than their in situ functional evaluation, offers definitive evidence of organelle-specificity, negating the interference from extra mitochondrial cellular factors/signals. This mini-review was designed primarily to explore the commonly employed in organello analytical assays for the assessment of mitochondrial physiology and its dysfunction, with a particular focus on neuroscience research. The authors briefly discuss the methodologies for biochemical isolation of mitochondria, their quality assessment, and cryopreservation. Further, the review attempts to accumulate the key biochemical protocols for in organello assessment of a multitude of mitochondrial functions critical for neurophysiology, including assays for bioenergetic activity, calcium and redox homeostasis, and mitochondrial protein translation. The purpose of this review is not to examine each and every method or study related to the functional assessment of isolated brain mitochondria, but rather to assemble the commonly used protocols of in organello mitochondrial research in a single publication. The hope is that this review will provide a suitable platform aiding neuroscientists to choose and apply the required protocols and tools to address their particular mechanistic, diagnostic, or therapeutic question dealing within the confines of the research area of mitochondrial patho-physiology in the neuronal perspective.
Keywords: Intrasynaptic mitochondria, density gradient centrifugation, mitochondrial membrane potential (MMP), electron transport chain (ETC), calcium capacitance, reactive oxygen species (ROS), glutathione (GSH), Trolox equivalent antioxidant capacity (TEAC)
1. INTRODUCTION
Neuronal processes of synaptic transmission, membrane potential maintenance, and Ca2+ exchange/extrusion require high amounts of energy, making the brain among the most prominent sites of energy consumption in the body. Mitochondria, the cellular power houses, hence are critical mediators of neuronal physiology, fulfilling the high-energy demands of neurons. In addition, mitochondria regulate calcium and redox signalling, and cellular death through regulation of the apoptotic and necrotic signals [1-3]. In particular, the case of intrasynaptic mitochondria is very interesting. Owing to the high-energy demands associated with the processes of neuronal signalling and plasticity, synapses, the major sites of interneuronal communication are enriched in mitochondria. Recent studies have indicated that these synapse-localized mitochondria (synaptic mitochondria or SM) are significantly different from the free mitochondrial pool (although both share the same origin) in terms of physical sizes as well as proteome, lipidome, and enzyme profiles [4-6]. Moreover, SM are configured for rapid adaptive responses to match the dynamic changes associated with synaptic signalling and plasticity [7, 8]. Interestingly, SM are more vulnerable to insults such as redox and calcium dyshomeostasis (and swelling) than their free neuronal mitochondrial counterparts [5, 8-10]. For these reasons, recent studies, including from our group, have been primarily directed to the evaluation of synapse-specific mitochondria in pathophysiological brain states, ranging from early-life insults [11, 12] to ageing-induced neurodegeneration [13-15].
Given the peculiar and multifactorial roles of brain mitochondria, it is hardly surprising that dysfunction of mitochondria is implicated as a primary pathogenic mechanism in almost all brain pathologies [16-21]. A comprehensive understanding of the brain's mitochondrial functions both under normal conditions and in pathological states is warrantied for understanding the disease pathology, identification of biomarkers, and for the development of effective neuroprotective and disease-modifying therapies. While appreciable progress has been made in the previous decades, many aspects of mitochondrial functions (and dysfunction) unique to brain mitochondria have still remained obscure. In this regard, biochemically isolated viable mitochondrial preparations retain their compartment properties and provide a suitable platform for in organello (patho)physiological assessment in a mitochondrial-specific manner [22-24]. Indeed, there are clear advantages of using isolated mitochondria for the assessment of their roles in neuronal (patho)physiology. First, these preparations negate the interference from cellular signalling cascades and other factors that regulate mitochondrial function. Second, they allow a high-throughput and in-depth analysis of functions, minimizing time and sample-to-sample variation. Third, preparation of viable and metabolically active mitochondria is possible even from long-term stored (cryopreserved) tissues of human and rodent origin. This indeed constitutes a powerful experimental tool in clinical research involving post-mortem human brain tissue samples in view of the technical challenges associated with their analyses.
Since the late 1940s, when biochemical isolation of liver mitochondria was pioneered by the Palade lab [25], considerable progress has been made in the techniques for functional assessment of intact viable mitochondrial preparations from fresh and frozen brain tissues. This has in turn been instrumental in providing distinct information regarding mitochondrial function and dysfunction in diseased states [22, 26]. While there are several reviews delineating the relevance of mitochondrial biology from the perspective of neuroscience research, a comprehensive review of the methodological procedures and protocols for an in organello assessment of isolated brain mitochondria has been lacking. The purpose of this review is to assemble the commonly used protocols for mitochondrial isolation, their quality assessment, and functional characterization in a single place. While the authors do not claim to discuss all the in organello techniques for mitochondrial assessment, the present review does aim to cover the widely employed and relevant biochemical assays and will provide considerable information to aid neuroscience researchers in choosing and applying the selected protocols, tools, and citations to address a particular research question pertaining to mitochondrial patho-physiology in the neuronal health and disease. Efforts have also been made to emphasize protocols which require only the basic biochemical equipment commonly available in most life sciences laboratories, rather than specialized cost-incurring instruments. This has also allowed us to choose methodologies which are simple, systematic and efficient, and simultaneously time- and cost-efficient.
2. ISOLATION OF MITOCHONDRIAL PREPARATIONS, QUALITY ASSESSMENT AND CRYOPRESERVATION
2.1. Density Gradient Isolation of Brain Mitochondria
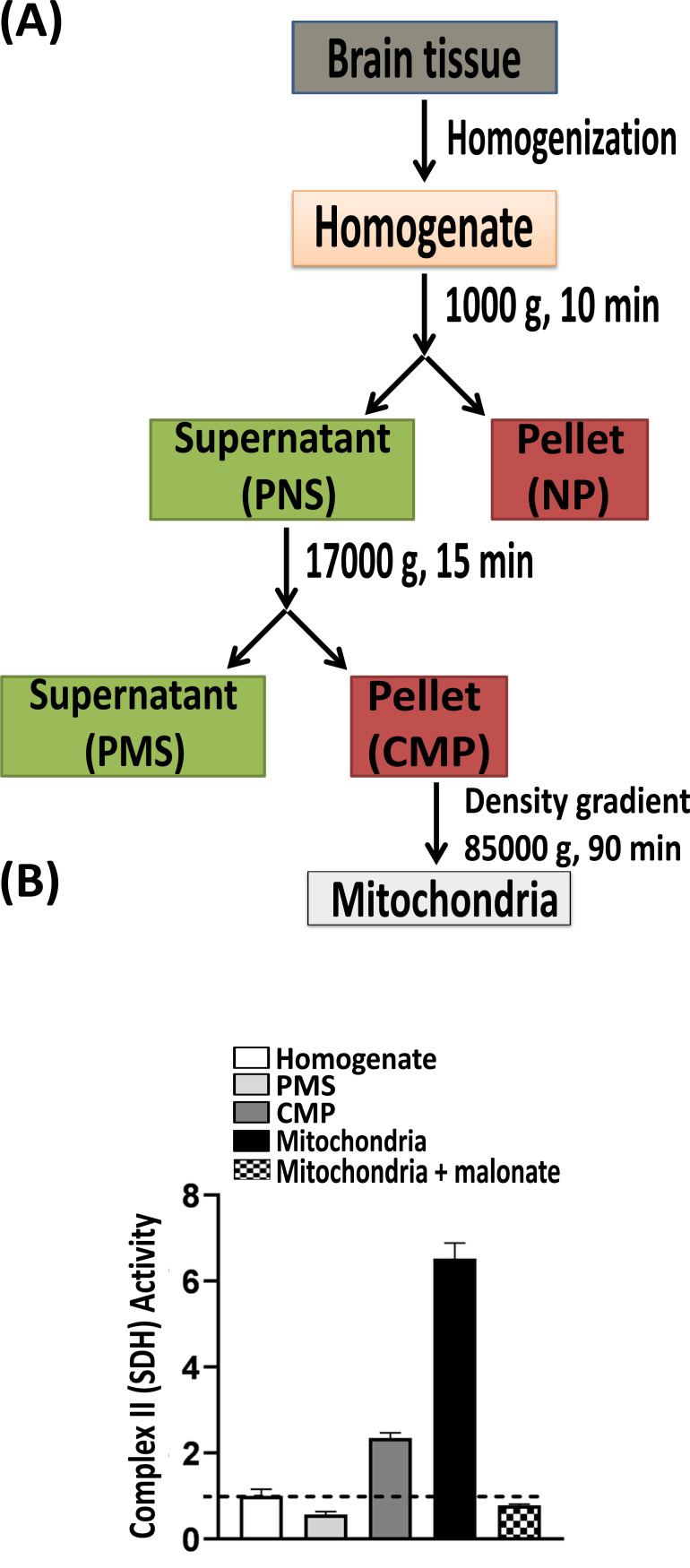
Different protocols exist for the isolation of ex vivo preparations of viable mitochondria from the different starting materials (e.g., different tissue- and cell-type and organism). The basic principle however is similar and relies on a) tissue/cell homogenization and b) sequential centrifugation (Fig. 1). Sequential centrifugation of the homogenate with increasing sedimentation forces enables a rough fractionation of the cellular environment. Thus, centrifugation of the brain homogenate at low speed (ca. 600-800 g) pellets down nuclei and cellular debris, and a second high-speed centrifugation step (ca. 7000-8000 g) generates a mitochondrial pellet. This crude mitochondrial pellet has sometimes been used in organello studies; however, it should be noted that this crude preparation contains appreciable extra-mitochondrial contaminants [24, 27]. Further isolation of high-purity enriched mitochondrial sample requires equilibrium density gradient centrifugation-based protocols which result in the acquirement of mitochondria as a focused layer at the isopycnic point in the gradient [27-29].
Fig. (1).
Biochemical isolation of brain mitochondria. (A) Flowchart reveals the basic protocol for isolation of mitochondria using an equilibrium density gradient sub-cellular fractionation protocol. The first slow speed centrifugation of the mechanically homogenized brain tissue sample results in a post nuclear supernatant (PNS) and a crude nuclear pellet (NP). Further high speed centrifugation of the PNS generates the crude mitochondrial pellet (CMP) and the cytosolic post mitochondrial supernatant (PMS). Pure free (nonsynaptic) mitochondria are separated from the CMP using density gradient centrifugation. (B) Assessment of enrichment (and integrity) of the mitochondrial fraction in comparison to the starting brain homogenate and other intermediate fractions can be performed using a spectrophotometric assay of succinate dehydrogenase (SDH) activity (section 2.2.7.1; [61]). Malonate, a competitive SDH inhibitor was used to establish the specificity of the assay.
Density gradient centrifugation for mitochondrial isolation from rat liver was first proposed in the late 1940s [25] and the first protocols for brain mitochondria isolation were published in the 1960s [30, 31]. Since then, a number of specialized protocols have also been proposed for brain mitochondria isolation, employing different density gradient media; such as sucrose [32], Ficoll [11, 12, 33-35], and Percoll [27, 36-39]. It is relevant to note here that cryopreserved rodent and human tissues can be effectively used as a source of functionally and bioenergetically viable mitochondria [40-44]. Readers are directed to the specific protocols for primary neurons and astrocytes [28, 45, 46], rodent brain tissue [32, 37-39] and spinal cord [33, 36], and human brain tissues obtained post-mortem [42, 47, 48] and post-surgery [49, 50]. Specific protocols have also been designed to isolate viable and bioenergetically well-coupled mitochondria from neonatal rodent brains [51]. Crude mitochondria have also been isolated from small amounts of mouse brain tissue, which is very convenient for differential assessment of region-specific mitochondrial activity and viability [41].
Interestingly, subcellular fractionation of synaptic and non-synaptic brain mitochondria has also been used for comparative studies [12, 52], which has been instrumental in deducing the differences in the two mitochondrial populations in terms of bioenergetics profile, Ca2+, and redox regulatory roles and susceptibility to insults/stress [12, 35, 39, 53, 54]. Similar studies have also been carried out for differential characterization of neuronal and glial mitochondria [34]. It is interesting for the readers to note here that subcellular fractionation protocols also exist for isolation of membrane fraction containing mitochondria-associated membranes (MAM) [55-57], which are thought to be physically associated contact sites between endoplasmic reticulum membrane proteins and outer mitochondrial membrane. However, functional analyses of these preparations are beyond the scope of this review and will not be discussed here.
Lastly, other methods are also available for mitochondrial isolation. For example, immunopurification (and subsequent magnetic extraction) of brain mitochondria has been carried out using antibodies specific for outer mitochondrial membrane proteins, such as translocase of the outer membrane (TOM) complex component TOM22 [50]. Isolation of mitochondria by integrated zone electrophoresis has also been performed [58]; however, it requires specialized skills and instruments such as a free-flow apparatus.
2.2. Quality Control Procedures: Mitochondrial Yield and Integrity
2.2.1. Electron Microscopy (EM)
EM is obviously the best and the most direct method to assess the enrichment and morphological intactness of ex vivo mitochondrial preparations. Free mitochondria are easily identifiable as ribbed structures with a clear double membrane [59, 60]. Time and cost constraints along with the requirement of specialized skills and equipment however limit the use of electron microscopy for the sole purpose of evaluation of mitochondrial purity and intactness.
2.2.2. Mitochondrial Stains
We and others have employed Janus green B, a supravital lipophilic cationic stain for a quick and simple assessment of the enrichment, viability, and integrity of isolated mitochondria. Since the dye can only be taken up by metabolically active mitochondria with an intact membrane potential (see section 3.1), colorimetric [61, 62] or microscopic [63, 64] evaluation of Janus green B retention serves as a good indicator of the intactness of isolated mitochondria.
The enrichment of mitochondria (and determination of their mass) in the ex vivo preparations can also be evaluated using the fluorescent MitoTracker® dyes [65, 66]. Similar to Janus green B, these potential-indicating cationic dyes accumulate electrophoretically into mitochondria. Unlike Janus green B, however, MitoTracker® forms covalent adducts with the thiol groups of the matrix proteins, making their fluorescence independent of mitochondrial potential. Visualisation of mitochondria can be performed using either flow cytometry [67], fluorescence microscopy [68], or spectroscopy [44, 69].
2.2.3. Marker Proteins: Immunoblotting and Activity Assays
Enrichment of mitochondria in the biochemically isolated preparations can be confirmed by immunoblotting (with respect to the starting homogenates) using antibodies against mitochondrial marker proteins [70]. Several candidates can be used for this purpose; such as electron transport chain complexes, TOM20, and voltage-dependent anion-selective channel (VDAC), etc. However, we strongly recommend using activity assays of marker proteins such as succinate dehydrogenase (see section 3.4.2; [11, 12, 61]) or citrate synthase (section 2.2.7.2) for determining the enrichment.
2.2.4. Lactate Dehydrogenase (LDH; EC 1.1.1.27) Activity
LDH is a cytosolic enzyme and its absence is sometimes taken as an indicator of mitochondrial enrichment [46, 67]. Although it is not very often used for this purpose, it is worth mentioning it here because of the utility of the assay in determining the integrity of cells as well as isolated synaptic terminals [71, 72]. Briefly, the assay relies on spectrophotometric measurement of the oxidation of exogenously added NADH in the presence of an exogenous LDH substrate, pyruvate [73]. Similarly, absence of activity of a synaptic marker enzyme, acetylcholinesterase in mitochondrial preparations has sometimes been employed for confirmation of minimal synaptic contamination [46]. This is particularly relevant for the quality assessment of mitochondrial samples isolated from brain tissue samples.
2.2.5. Cytochrome c Release
Similar to the LDH release assay, cytochrome c release assay is often employed to evaluate the intactness of isolated mitochondria [26]. Cytochrome c release from mitochondrial samples can be assessed by immunoblotting [70] or by evaluating the differential (under normal oxidized conditions, and in dithionite-treated reduced conditions) absorption spectra of the extracellular medium (supernatant) [74].
2.2.6. Oxidation of Cytochrome c and Ascorbate
Several other assays have also been routinely used to assess the intactness of the outer mitochondrial membrane (OMM) in isolated mitochondrial preparations. The basic principle of all these methodologies are based upon the impermeability of OMM for certain chemicals (cytochrome c and ascorbate) or localization of certain enzymes in the mitochondrial intermembrane space (e.g. adenylate kinase; section 2.2.7.4). Evaluation of the KCN-sensitive (complex IV-mediated) oxidation of exogenously added cytochrome c (see section 3.4.5) has been employed as a measure of OMM intactness [75, 76]. Similarly, the ascorbate test for the integrity of OMM of mitochondrial preparations evaluates the oxidation of exogenous ascorbate. Monitoring ascorbate oxidation however, requires indirect polarographic evaluation of oxygen consumption using a Clark-type electrode (see section 3.5) in the presence and absence of N, N, N’, N’-tetramethyl-p-phenylenediamine (TMPD). The detailed procedure has been previously published [41, 77].
2.2.7. Enzyme Activity Assays
In organello activity assays of several mitochondrial marker enzymes have been employed to evaluate both enrichment (yield) and integrity of mitochondrial preparations. We discuss below the most-widely used assays.
2.2.7.1. Succinate Dehydrogenase (SDH; E.C. 1.3.5.1) Activity
As previously indicated, succinate dehydrogenase (complex II) is localized in the inner mitochondrial membrane and hence its activity serves as a good indicator of both enrichment and integrity of isolated mitochondria, as demonstrated by us and others [12, 61, 78]. Refer to section 3.4.2 for details of the colorimetric assay. Generally, the integrity of isolated mitochondrial preparations is thought to be good if 90-95% mitochondria are intact [12, 79].
2.2.7.2. Citrate Synthase (CS; EC 2.3.3.1) Activity
The yield of mitochondria (i.e. the efficiency of the isolation protocol) can also be evaluated by measuring the activity of the mitochondrial marker enzyme, citrate synthase (CS) [80, 81]. The assay spectrophotometrically measures the reduction of 5, 5’-dithiobis(2-nitrobenzoic acid) (DTNB) to 5’-thionitrobenzoate (TNB-) anion in the presence of exogenous substrates, acetyl-CoA and oxaloacetate [41, 82]. Since CS is localized in the mitochondrial matrix, its activity in isolated mitochondria before and after membrane disruption by freeze-thaw cycles (2-3 cycles of quick freezing at -85°C and thawing at 4°C on ice) or by extraction with Triton X-100 is regarded as a good measure of the integrity of the inner mitochondrial membrane (IMM). CS activity is often also employed as a normalization control for the various in organello assays (section 7).
2.2.7.3. Fumarase (E.C. 4.2.1.2) Activity
We and others have used activity assessment of another mitochondrial matrix enzyme, fumarase as an indicator of mitochondrial yield and integrity of its inner membrane [12, 77, 83]. The spectrophotometric assay follows the formation of fumarate upon addition of exogenous malate to isolated mitochondria [84]. Whether using CS or fumarase as the marker enzyme, the integrity of mitochondria can be expressed in %, calculated as [1-(γb/γa)]*100; where γb and γa are the enzyme activity rates before and after membrane disruption.
2.2.7.4. Adenylate Kinase (AK; EC 2.7.4.3) and Glutamate Dehydrogenase (GDH; EC 1.4.1.3) Activities
In organello activity assays for adenylate kinase (AK) and glutamate dehydrogenase (GDH) are sometimes also employed to evaluate the intactness of mitochondrial OMM and IMM, respectively as the two enzymes are localized in mitochondrial intermembrane space and matrix, respectively [85].
2.2.8. Calcium Buffering Capacity
Lastly, evaluation of mitochondrial calcium buffering capacity serves as a fast and efficient fluorimetric measure of the integrity of isolated mitochondria since the uptake of Ca2+ occurs through the inner membrane-localized mitochondrial calcium uniporter and is dependent on an intact mitochondrial membrane potential [27]. Refer to section 3.3 for details of the assay.
2.3. Cryopreservation of Isolated Mitochondria
The time taken for mitochondrial isolation and the batch-to-batch variability may hinder high throughput assessment of their functions [86]. To improve assay efficiency and consistency, long-term storage of isolated mitochondria is recommended and can be performed using trehalose [87], dimethyl sulfoxide (DMSO; [88]), glycerol [89], or ethylene glycol [90]. These methods of cryopreservation of isolated mitochondria have been demonstrated to optimally preserve mitochondrial outer membrane integrity and bioenergetic functions. Nevertheless, we would like to emphasize here that wherever possible, in organello assays of mitochondrial functions should be performed freshly isolated samples.
3. MITOCHONDRIAL ACTIVITY AND BIOENERGETIC ASSAYS
3.1. Mitochondrial Membrane Potential (MMP or Δψm)
Mitochondria use oxidizable substrates to generate an electrochemical proton gradient across the inner mitochondrial membrane. The direction of this mitochondrial membrane potential (Δψm) is such that the inside is electronegative, and hence promotes inward transport of cations and forms the basis for ATP generation [65]. As such, Δψm is a robust indicator of mitochondrial integrity, activity and bioenergetic capacity. Indeed, any dysregulation of Δψm (whether low or persistently high) indicates possible functional and structural dysfunction of the mitochondria [2, 91].
Cationic lipophilic fluorescent dyes have been envisioned as excellent tools to measure MMP for a long time [65, 92-94]. For example, we used safranin O, a rhodamine derivative for the assessment of Δψm of isolated mitochondrial preparations at both the basal and energized states (in presence of substrates, malate and glutamate) [11, 12]. Basal Δψm can be simply measured by the amount of safranin O retained in the mitochondrial pellet. On the other hand, fluorescence quenching of safranin O after addition of energy substrates serves as a suitable measure of mitochondrial Δψm generation (i.e. Δψm at energized state) [11, 12, 41]. Moreover, safranin O can also be employed to deduce the absolute values of Δψm in isolated mitochondria with calibration using a K+ gradient [95, 96].
Other cationic fluorescent dyes have also been used for assessment of Δψm in isolated mitochondrial preparations; however 5, 5’, 6, 6’-tetrachloro-1, 1’, 3, 3’-tetraethylbenzi-midazolylcarbocyanine iodide (more commonly known as JC-1) which exhibits a green-to-red emission shift upon mitochondrial accumulation, has become the gold standard for measuring Δψm [67, 97-100]. Noteworthy, JC-1 or safranin O mediated Δψm measurements have been shown to be performed using fluorescence spectroscopy [11, 12, 54, 101], fluorescence microscopy [65], or fluorescence-activated cell sorting (FACS) [102].
3.2. Mitochondrial Permeability Transition (Induced Swelling)
Mitochondrial permeability transition (mPT) or the opening of the mitochondrial permeability transition pore (mPTP) is the sudden increase in the permeability of the inner mitochondrial membrane to solutes up to 1.5 kDa (which are normally impermeable) [103]. mPT is primarily caused by stressors such as calcium overload and oxidative stress and results in disruption of the proton gradient, uncoupling of respiration, cessation of ATP production, and bioenergetic failure. mPT ultimately leads to osmotic swelling and outer membrane rupture and signals cell death [104, 105]. Not surprisingly, vulnerability of mitochondria to Ca2+-induced swelling has been evaluated in several pathological states [36, 106-109]. Interestingly, recent evidences also suggest that transient mPTP opening may also be a regulator of brain development [110].
In organello induction of mPT in isolated mitochondria is done in the presence of high Ca2+ in the extracellular medium. This Ca2+-induced swelling can be monitored by light scattering at 520 nm, with a steep reduction in the absorbance at this wavelength; both under energized (in presence of substrates malate and glutamate) and de-energized states [36, 67, 70].
3.3. Calcium Capacitance (Buffering Capacity)
Calcium buffering capacity or calcium retention capacity of mitochondria is essential for cellular functions, particularly in neuronal systems for protection against excitotoxicity [111]. Importantly, intramitochondrial calcium is significant not only for intracellular buffering but also for metabolic regulation of many key mitochondrial enzymes (such as pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, etc.) [2]. Calcium-sensitive fluorescent probes such as Fura 6F and Calcium Green 5N are excellent tools to monitor calcium uptake and release in isolated mitochondrial samples. Readers are advised to consult the fluorescence spectroscopy-based methodology described in several previous studies [27, 36, 54, 70, 74, 101, 112, 113].
3.4. Activities of the Electron Transport Chain (ETC) Complexes
The ETC and oxidative phosphorylation serves as the major energy source for neurons, particularly during high-energy demands (Fig. 2) [3, 114]. As such, brain is very sensitive to the loss of substrates or other impediments to ETC and oxidative phosphorylation. Not surprisingly, activity assays for the ETC complexes have been performed to evaluate brain mitochondrial bioenergetic profiles in several disease states and pathologies. ETC complex activity assays can be performed using both spectrophotometry-based kinetics assessments [40, 115-117] as well as by polarographic measurements of oxygen consumption (section 3.5) [115, 118-120]. For the purpose of the review, we will only focus on the in organello spectroscopic assays as they can be easily performed in a time- and cost-efficient manner with the requirement of only the basic reagents and experimental setup.
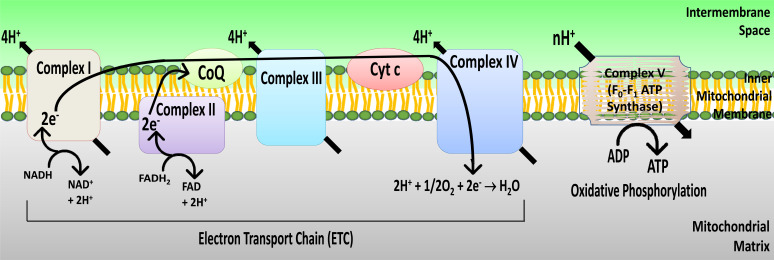
Fig. (2).
Schematic representation of the electron transport chain (ETC) and oxidative phosphorylation. The ETC consists of a series of oxidoreduction reactions in which the electrons are transferred from the substrate NADH to molecular oxygen through a series of electron carriers and involves oxidoreductase complexes (I to IV) localized in the inner mitochondrial membrane. There is a concomitant release of H+ from the mitochondrial matrix into the intermembrane space across the inner mitochondrial membrane, creating a proton gradient which is utilized by the ATP synthase (complex V) for the synthesis of ATP.
While a number of slightly different variants exist for spectrophotometric analyses of ETC complexes, the general approach and principle are the same. We have, for instance, evaluated ETC complex activities in both mitochondrial [12] and synaptosomal [11] preparations. Readers are directed to excellent methods-based articles for an extensive overview of the principle and protocols involved [40, 117]. For this review, we focus only on the spectrophotometric assays that have been used in our studies [11, 12].
3.4.1. Complex I (NADH Dehydrogenase; E.C. 1.6.5.3)
The activity of complex I is measured in terms of the reduction of exogenously added potassium ferri(FeIII)cyanide to ferro(FeII)cyanide at 420 nm in the presence of NADH. Rotenone is used to obtain complex I-specific NADH oxidation.
3.4.2. Complex II (Succinate Dehydrogenase; E.C. 1.3.5.1)
The assay for complex II is carried out in a similar manner as the assay for complex I. The difference is in the substrate used, which in this case is succinate. The reductive conversion of the indicator chromogen, potassium ferricyanide to ferrocyanide is again monitored at 420 nm. Pre-treatment of mitochondria with malonate, a competitive complex II inhibitor can be performed as a negative control.
3.4.3. Complex I-III (NADH: Cytochrome c Oxidoreductase)
Complex I-III activity is assessed as the reduction of exogenous cytochrome c which is monitored at 550 nm. The reaction is initiated by the addition of the substrate NADH and in the presence of KCN (an inhibitor of complex IV which oxidizes cytochrome c). Again, only rotenone-sensitive NADH oxidation (and consequent cytochrome c reduction) is taken as the measure of complex I-III activity.
3.4.4. Complex II-III (Succinate: Cytochrome c Oxidoreductase)
The activity of complex II-III is monitored by following the reduction of cytochrome c (exogenous) upon the addition of substrate, succinate. KCN is again used to inhibit complex IV.
3.4.5. Complex IV (Cytochrome c Oxidase; E.C. 1.9.3.1)
For this assay, cytochrome c is first reduced by dithionite. Dithionite-induced reduction of ferri(FeIII)cytochrome (oxidised) to ferro(FeII)cytochrome (reduced) can be conveniently followed as a clear colour change from blood red to pink. Reduced cytochrome c is then quickly purified on a desalting column (e.g., Sephadex G-25). Mitochondria-mediated oxidation of exogenous cytochrome c is then evaluated by following the decrease in the absorbance at 550 nm. An air oxidation control for ferrocytochrome c is included for each set.
3.4.6. Complex V (F0F1 ATP Synthase; E.C. 7.1.2.2)
Oligomycin-sensitive F0F1 ATP synthase (sometimes called complex V) activity in isolated mitochondria is evaluated by following the hydrolysis of exogenous ATP coupled with the oxidation of exogenous NADH at 340 nm. The detailed procedure is described elsewhere [121-123].
In our studies, we have measured ATP synthesis indirectly utilizing a glucose-hexokinase trap method (excess of hexokinase and glucose-6-phosphate dehydrogenase-coupled enzymes) to ensure a non-rate limiting ADP-regeneration system [11, 12, 41]. Consumption of exogenous inorganic phosphate (Pi) can then be measured employing a phosphomolybdic acid-ascorbic acid-based assay at 820 nm [11, 12, 124] or by following the reduction of NADP+ spectrophotometrically at 340 nm in the extramitochondrial phase [41, 125]. Similarly, Radiolabelled [32]Pi has also been employed to monitor the residual Pi in a liquid scintillator counter [126].
The most commonly used method for in organello mitochondrial ATP synthesis now-a-days is a bioluminescence-based assay that utilizes exogenous luciferase from firefly (Photinus pyralis), an enzyme that oxidizes substrate protein luciferin with the generation of light in an ATP-dependent manner [79, 126]. Since both the enzyme and substrate luciferin are not rate-limiting, bioluminescence measured on a luminometer is directly proportional to ATP concentration. Bioluminescent measurement of ATP levels in isolated mitochondria can also be performed in the presence of different energy substrates [79]. Thus, addition of glutamate and malate have been used to evaluate ATP production with electron flow exclusively through complex I; and succinate and rotenone have been employed to evaluate the contribution of complex II in ATP generation.
3.5. Oxygen Consumption and Mitochondrial Coupling
Oxygen consumption is one of the classical end-points that has been evaluated as a measure of mitochondrial function, viability, and oxidative capacity. Oxygen consumption in isolated mitochondria was pioneered by Britton Chance in the 1950s [127]. Recent advances in respirometry have significantly reduced the amount of mitochondria (and hence, the starting tissue) required [128]. The most commonly used method for monitoring mitochondrial oxygen consumption is based on a Clark-type oxygen electrode which polarographically measures oxygen in the solution. Different respiratory states of isolated mitochondria can be measured; oxygen consumption in the presence of substrates alone (state 2), in the presence of substrates and ADP (state 3), and after ADP depletion (state 4). The ratio of oxygen consumption in state 3/ state 4 is the respiratory control ratio (RCR) which is an indicator of mitochondrial coupling (of ATP synthesis and oxygen consumption), and can be conveniently used to calculate mitochondrial integrity and viability [70, 129]. Readers are directed to previously published protocols that have described the method in detail [70, 79, 130].
A fluorescence-based assay employing a MitoXpress® probe (Luxcel Biosciences) has also become popular for high-throughput measurement of oxygen consumption of cells [131, 132] and isolated mitochondrial preparations [69, 133]. The assay is based upon the principle that fluorescence of the MitoXpress® probe is quenched by O2 through molecular collision, and hence the amount of fluorescence signal is inversely proportional to the amount of extracellular O2 in the sample. A very detailed methodology has been provided elsewhere [134]. Recent studies have also evaluated the rates of oxygen consumption under different energy states using a high throughput Seahorse XF analyser [23, 135]. It should be pointed out here that barring the MitoXpress®-based fluorimetric assay, all other assays require specialized equipment (and accessories).
3.6. Tricarboxylic Acid Cycle (TCA) Enzyme Activities
The TCA or Krebs cycle is the central pathway of metabolism and is involved in the oxidation of acetyl coenzyme A derivatives obtained by the catabolism of carbohydrates, amino acids, and fatty acids. Pyruvate dehydrogenase which generates acetyl coenzyme A is a key link between glycolysis and the TCA cycle. TCA enzymes are localized in the mitochondrial matrix wherein they are organized as a supramolecular complex, called the Krebs metabolon. However, some of the TCA enzymes (e.g., aconitase, fumarase, malate dehydrogenase) have cytosolic counterparts that perform extramitochondrial functions. Of note, TCA enzymes may have additional moonlighting functions such as stabilization of mitochondrial DNA (mtDNA) and mitochondrial mRNA translation, etc. [136].
Activity assays of pyruvate dehydrogenase complex and TCA cycle enzymes can be performed easily in organello in isolated mitochondrial preparations using simple spectrophotometric protocols [137]. We have previously listed the activity assays for fumarase (section 2.2.7.3), CS (section 2.2.7.2), and SDH (section 3.4.2). Readers are directed to detailed activity assay protocols for pyruvate dehydrogenase and other TCA enzymes (aconitase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, succinyl CoA synthase, and malate dehydrogenase) published elsewhere [138].
4. OXIDATIVE DAMAGE AND REDOX HOMEOSTASIS
Mitochondrial ETC and several other oxidases are the major sources of reactive oxygen species (ROS) such as superoxide ion (O2.-), hydrogen peroxide (H2O2), and hydroxyl radical (•OH). Unsurprisingly, there are a multitude of sophisticated endogenous mitochondrial antioxidant mechanisms, ranging from low molecular weight antioxidants like glutathione (GSH) to detoxifying enzymes such as superoxide dismutases (SODs), catalase, and peroxidases, acting as primary lines of defence [139]. Nevertheless, dysregulation of these endogenous antioxidants and oxidative damage to proteins and lipids appears to be a chief feature of several neuropathologies [140, 141]. Hence, assessment of redox signalling, oxidative damage, and antioxidant capacity of ex vivo mitochondrial preparations has been a key focus area of research evaluating the role of mitochondria in brain diseases. This section attempts to briefly summarize some of the in organello biochemical assays used for evaluation of the major indices of mitochondrial redox signalling.
4.1. Reactive Oxygen (ROS) and Nitrogen (RNS) Species Production
4.1.1. DCF Assay
The most widely used fluorogenic probe to measure ROS/RNS production is dichlorodihydrofluorescein diacetate (DCFH-DA). In presence of ROS/RNS, non-fluorescent DCFH-DA is converted to a brightly fluorescent dichlorofluorescein (DCF) [142]. DCF fluorescence can be monitored in isolated mitochondrial samples, both at basal and energized states as described in previous studies, either as a kinetic assay or as an end-point assay [11, 12, 54].
4.1.2. Superoxide Anion (O2.-)/H2O2 Production
ROS production by the ETC in mitochondria occurs predominantly by the premature leak of electrons from complexes I, II, and III, resulting in partial reduction of oxygen to superoxide (O2.-) which is then dismutated to hydrogen peroxide (H2O2) [143]. Mitochondrial production of H2O2 is most commonly evaluated using Amplex Red [95, 142]. In the presence of mitochondrial H2O2 and exogenous horseradish peroxidase, Amplex Red is oxidized to resorufin which can be monitored fluorimetrically [144, 145]. In organello mitochondrial O2.- production can also be measured spectrophotometrically by following the reduction of exogenously added oxidized-cytochrome c (Fe3+-cyt c) [85, 146] or epinephrine [147].
4.1.3. Nitric Oxide (NO•) End Products
Nitrosative species such as NO• are known to be detrimental to mitochondrial functions, particularly the respiratory proteins [148]. In our studies, we have used a spectrophotometric assay for evaluating NO• end products (nitrites and nitrates), employing Griess reagent [11, 12]. A spectrophotometric assay based on the oxidation of exogenous oxyhemoglobin (HbO2) for NO production in mitochondria (activity assay of mitochondrial nitric oxide synthase; mtNOS) has also been employed in literature [149, 150].
4.2. Oxidative Damage to Lipids and Proteins
Simple in organello spectrophotometric assays for assessment of oxidative damage to lipids (peroxidation) and proteins (carbonylation and thiol oxidation) have been carried out in our studies for both isolated mitochondria [12] and synaptosomes [11].
4.3. Mitochondrial Antioxidant Potential
4.3.1. General Antioxidant Capacity
In organello evaluation of the free radical scavenging capacity of mitochondria has been performed by us utilizing 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) or ABTS dye [11, 12, 151]. The radical form of ABTS (denoted as ABTS+•) is generated in the presence of (potassium) persulfate and has absorption maxima at 660, 734, and 820 nm. As reduction of ABTS+• results in its decolourization, mitochondrial scavenging of ABTS+• can be evaluated at 734 nm. The degree of antioxidant capacity of ABTS+• radical scavenging can be quantified in comparison with a standard curve of Trolox (a water-soluble analogue of vitamin E), and expressed in terms of Trolox equivalent antioxidant capacity (TEAC) [152]. Similar assay involving quenching of peroxy radicals from azo initiators, such as 2, 2’-azobis(2-amidinopropane) dihydrochloride or ABAP (Total radical-trapping antioxidant parameter assay or TRAP; [153]) and 2, 2’-azobis(2-methylpropionamidine) dihydrochloride or AAPH (Oxygen radical absorbance capacity or ORAC; [154]) have also been employed for measuring the endogenous antioxidant ability of isolated mitochondria.
Another commonly used method to evaluate mitochondrial antioxidant power is the ferric reducing antioxidant power (FRAP) assay which is based upon the ability to reduce ferric compounds (such as FeIII-tripyridyltriazine) to ferrous counterparts [151, 155].
4.3.2. Glutathione (GSH) Antioxidant Signalling
GSH is the most abundant small molecule thiol present in the brain [156, 157] and is the major mitochondrial antioxidant [158]. Scavenging of oxidative species like H2O2 and other hydroperoxides involves oxidation of GSH to GSSG by the action of glutathione peroxidase (GPx). Recycling of GSSG back to its reduced GSH form is carried out by glutathione reductase (GR). Yet another mechanism of detoxification of oxidizing species is their conjugation with GSH catalysed by the enzyme glutathione S-transferase (GST) [157, 159]. Assessment of the endogenous mitochondrial GSH-mediated antioxidant activity hence requires assessment of the levels of reduced GSH and activities of the GSH-related enzymes; GPx, GR, and GST. The spectroscopy based in organello protocols for each of these assays have been described in detail in our previous publication [11].
4.3.3. Superoxide Dismutase (SOD) Activity
Several variants of the assays for SOD activity have been efficiently employed in the literature. These are based upon detection of superoxides by using dyes such as nitroblue tetrazolium (NBT; [160]), adrenaline [161], or sulphanilamide [162]. One of the most commonly employed assays that deserve a special mention here is the spectrophotometric assay employing a xanthine-xanthine oxidase system to generate superoxide ion and oxidized cytochrome c as the superoxide-trapping detection system. The methodology for this assay is described in detail elsewhere [163].
5. MITOCHONDRIAL PROTEIN TRANSLATION
While over 99% of the mitochondrial proteins are encoded by the nuclear DNA and translated in the cytosolic ribosomes, mtDNA encodes 37 genes, of which 13 code for mitochondrial proteins which are essential components of the ETC, and the rest code for rRNAs and tRNAs needed for their translation [164]. Recent studies are establishing the critical roles of local de novo translation of mitochondrial proteins in maintaining neuronal function, particularly synaptic physiology and plasticity [164, 165]. In this regard, in organello approaches to study protein translation in isolated mitochondria have been successfully used with yeast, plant, and mammalian cells [166]. It should be noted that biochemically isolated mitochondrial preparations are often contaminated, at least to some degree with microsomes and other membrane fractions studded with cytosolic ribosomes. Hence, it is essential to use blockers of cytoplasmic protein synthesis, such as emetine and cycloheximide to study mitochondria-specific protein translation. Correspondingly, our assays of synapse-specific protein translation in isolated synaptic terminals are indispensable without a mitochondrial protein synthesis blocker, such as chloramphenicol because of the presence of intrasynaptic mitochondria and free mitochondrial contaminants [167-169].
Local de novo protein translation in isolated mitochondria has been assayed utilizing [35S]-radiolabelled cysteine and methionine, followed by either gel autoradiography or radioactivity counting [166, 170-173]. In the literature, we only found references for radioactivity-based protocols for in organello mitochondrial protein translation. It will be interesting to follow the development of alternative non-radioactive methods for the evaluation of mitochondrial protein synthesis, such as those based on puromycin [167, 169], an aminoacyl-tRNA analogue that has similar action on cytosolic and mitochondrial protein translation [174]. Intriguingly, puromycin-mediated assessment of mitochondrial translation has been carried out in an adherent cell culture system with the concomitant presence of emetine, a blocker of cytosolic protein synthesis [175].
6. PROTEOMIC CHARACTERIZATION
Proteomic characterization is the most obvious and most widely employed application of isolated mitochondria, both in terms of the global proteomic characterization of mitochondrial proteins and their post-translational modifications. Both gel-based and gel-free comparative mitochondrial proteomics methodologies have been widely-used to evaluate the quantitative and qualitative differences in mitochondrial proteome as a function of subcellular localization (e.g., synaptic versus non-synaptic mitochondria; [9]), developmental stage (e.g., embryonic versus postnatal, or young versus aged [176]), disease conditions (e.g., in Alzheimer’s disease [177]; and multiple sclerosis [178]) as well as treatment with therapeutic agents (e.g., bis(7)-tarcine [179]; and simvastatin [180]). Since excellent recent review articles have delineated both the techniques of mitochondrial proteomics as well as their contribution to our understanding the of pathology of brain diseases [177, 181-190], we will not discuss this topic further.
7. NORMALIZATION OF MITOCHONDRIAL CONTENT
Normalization for in organello assays is essential in order to evaluate the actual inherent mitochondrial properties, independent of the differences in the mitochondrial content of different preparations. There are several ways to do this. The simplest of these are normalizing mitochondrial protein content using the established protein quantitation assays such as Bradford or bicinchoninic acid (BCA) methods. However, this method is not specific for mitochondria (i.e. it does not differentiate between the mitochondrial content and the other minute impurities that may be present in the preparation) and hence may be erroneous. A better normalization control is the activity of CS [79, 191]. In our opinion, normalization at both levels (total protein content and citrate synthase activity) should be performed for functional analyses of mitochondria.
CONCLUSION
While studies have provided some evidences for the utility of ex vivo mitochondrial preparations in the characterization of the physiology and pathophysiology of neuronal mitochondria, it is clear that the use of these preparations has been under-utilized in neurobiology research.
In particular, research on the synaptic mitochondrial pool is lagging behind, in spite of the knowledge that synapses are the major sites of interneuronal communication and substrates for almost all higher-order functions and their dysfunction is a common and primary pathogenic event in brain pathologies across the whole development span, from early-life insults to ageing-related neurodegeneration. Given the critical roles of synaptic mitochondria in neurotransmission, it is surprising that only a few studies have been directed against evaluating their functions specifically. In this regard, biochemically isolated synaptic mitochondria seem to be the most important step forward to evaluate their function (and dysfunction) in relation to synaptic physiology and brain functions.
The purpose of the review is to solely summarize the methodologies and protocols of mitochondrial research, specifically using isolated brain mitochondrial samples (Table 1). However, in doing so, the hope is that it will help neuroscientists to identify and appreciate the utilities of these biochemical fractions and encourage them to employ their expertise in understanding the finer complexities of mitochondrial (and synaptic) pathology in healthy and diseased brain states. We also hope that through this review, researchers will be motivated to identify the gaps and device novel assays to evaluate mitochondrial (patho)physiology. One can only hope for an escalation in the employment of ex vivo mitochondria from cellular and animal models and human subjects to further delineate the contribution of mitochondria to brain function and dysfunction, and to evaluate the efficiency of potential therapeutic agents/strategies in prevention and amelioration of multiple neuropathologies.
Table 1.
Summary of the in organello assays for mitochondrial quality assessment, viability and functions.
| S. No. | Assay Name | Assay Type | Information Provided |
|---|---|---|---|
| Quality Control Procedure | |||
| 1. | Electron microscopy | Transmission and Scanning electron microscope (TEM and SEM) | Mitochondrial enrichment/yield and morphological integrity |
| 2. | Mitochondrial stains | Spectroscopy-, Flow cytometry-, or Fluorescence microscopy-based | Mitochondrial enrichment and integrity |
| 3. | Marker proteins | Immunoblotting | Enrichment |
| 4. | Lactate dehydrogenase activity assay | Spectroscopy-based | Mitochondrial enrichment/yield and integrity |
| 5. | Cytochrome c release | Immunoblotting or spectroscopy-based | Mitochondrial enrichment/yield and integrity |
| 6. | Oxidation of exogenous ascorbate/ cytochrome c |
Spectroscopy-based or polarographic assay | Mitochondrial intactness |
| 7. | Succinate dehydrogenase (Complex II) activity assay | Spectroscopy-based | Mitochondrial enrichment/yield and integrity |
| 8. | Citrate synthase activity assay | Spectroscopy-based | Mitochondrial enrichment/yield and integrity, and as a normalization control |
| 9. | Fumarase activity assay | Spectroscopy-based | Mitochondrial integrity |
| 10. | Adenylate kinase activity assay | Spectroscopy-based | Mitochondrial integrity |
| 11. | Glutamate dehydrogenase activity assay | Spectroscopy-based | Mitochondrial integrity |
| 12. | Calcium buffering capacity | Fluorimetric | Mitochondrial integrity |
| Functional and Bioenergetic Assays | |||
| 1. | Mitochondrial membrane potential (cationic fluorescent dyes) | Fluorimetric, fluorescence spectroscopy- or cell cytometry-based | Mitochondrial integrity, viability and bioenergetic capacity |
| 2. | Ca2+-induced swelling (mitochondrial permeability transition) | Light scattering (spectroscopy)-based | Mitochondrial integrity, viability and bioenergetic capacity |
| 3. | Calcium buffering capacity (Ca sensitive fluorescent dyes) |
Fluorescence spectroscopy-based | Mitochondrial integrity and viability |
| 4. | Electron transport chain complexes activity assays - Complex I - Complex II - Complex I-III - Complex II-III - Complex IV - Complex V |
Spectroscopy-based, polarographic, or bioluminescence-based assay |
Mitochondrial viability and bioenergetic capacity |
| 5. | Oxygen consumption | Polarographic or fluorescence spectroscopy-based | Mitochondrial viability and bioenergetic capacity |
| 6. | Tricarboxylic acid cycle enzyme activity assays | Spectroscopy-based | Mitochondrial viability and bioenergetic capacity |
| Assays for Oxidative Damage and Redox Homeostasis | |||
| 1. | Dichlorofluorescein (DCF) assay | Fluorescence spectroscopy-based | Amounts of oxidative species |
| 2. | Superoxide/peroxide production | Spectroscopy- or fluorescence spectroscopy-based | Production of superoxide anion and hydrogen peroxide |
| 3. | Griess assay | Spectroscopy-based | Amounts of nitric oxide end products |
| 4. | Lipid peroxidation | Spectroscopy-based | Lipid oxidation |
| 5. | Protein carbonylation | Immunoblotting or spectroscopy-based | Protein oxidative damage |
| 6. | Protein thiol oxidation | Spectroscopy-based | Protein oxidative damage |
| 7. | Antioxidant capacity | Spectroscopy-based | Free radical scavenging |
| 8. | Glutathione (GSH) signalling - Reduced GSH levels - Glutathione reductase - Glutathione peroxidase - Glutathione S-transferase |
Spectroscopy- or fluorescence spectroscopy-based |
Antioxidant GSH signalling |
| 9. | Activity assay for superoxide dismutase | Spectroscopy- or fluorescence spectroscopy-based | Superoxide scavenging |
| Protein Translation Assays | |||
| 1. | Radiolabeled cysteine/methionine incorporation assay | Liquid scintillation or gel autoradiograhy | De novo protein translation |
| 2. | Puromycin incorporation assay | Immunoblotting | De novo protein translation |
ACKNOWLEDGEMENTS
The author, SH, and MYA sincerely thank Jazan University for providing access to the Saudi Digital Library for this study.
LIST OF ABBREVIATIONS
- AAPH
2, 2’-azobis(2-methylpropionamidine) dihydrochloride
- ABAP
2,2’-azobis(2-amidinopropane) dihydrochloride
- ABTS
2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- AK
Adenylate Kinase
- BCA
Bicinchoninic Acid
- CMP
Crude Mitochondrial Pellet
- CS
Citrate Synthase
- DCF
Dichlorofluorescein
- DCFH-DA
Dichlorodihydrofluorescein Diacetate
- DMSO
Dimethyl Sulfoxide
- DTNB
5,5’-dithiobis(2-nitrobenzoic acid)
- EM
Electron Microscopy
- ETC
Electron Transport Chain
- FACS
Fluorescence-activated Cell Sorting
- FRAP
Ferric Reducing Antioxidant Power
- GDH
Glutamate Dehydrogenase
- GPx
Glutathione Peroxidase
- GR
Glutathione Reductase
- GSH
Glutathione, Reduced
- GSSG
Glutathione, Oxidized
- GST
Glutathione S-transferase
- HbO2
Oxyhemoglobin
- IMM
Inner Mitochondrial Membrane
- JC-1
5,5’,6,6’,-tetrachloro-1,1’,3,3’-tetraethyl-benzimidazolylcarbocyanine iodide
- LDH
Lactate Dehydrogenase
- MAM
Mitochondria-associated Membranes
- MMP (Δψm)
Mitochondrial Membrane Potential
- mPT
Mitochondrial Permeability Transition
- mPTP
Mitochondrial Permeability Transition Pore
- mtDNA
Mitochondrial DNA
- mtNOS
Mitochondrial Nitric Oxide Synthase
- NBT
Nitroblue Tetrazolium
- NO
Nitric Oxide
- NP
Nuclear Pellet
- OMM
Outer Mitochondrial Membrane
- ORAC
Oxygen Radical Absorbance Capacity
- PMS
Post Mitochondrial Supernatant
- PNS
Post Nuclear Supernatant
- RCR
Respiratory Control Ratio
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SDH
Succinate Dehydrogenase
- SM
Synaptic Mitochondria
- SOD
Superoxide Dismutase
- TCA
Tricarboxylic Acid Cycle
- TEAC
Trolox Equivalent Antioxidant Capacity
- TMPD
N,N,N’,N’-tetramethyl-p-phenylenedia-mine
- TNB-
5’-Thionitrobenzoate Anion
- TOM
Translocase of the Outer Membrane
- TRAP
Total Radical Trapping Antioxidant Parameter Assay
- VDAC
Voltage-dependent Anion-selective Channel
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The authors, FA and SR thank Vellore Institute of Technology, Vellore for providing ‘VIT SEED Grant – RGEMS Fund (Sanction Order No. SG20220054)’ for carrying out this research work.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Khacho M., Harris R., Slack R.S. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat. Rev. Neurosci. 2019;20(1):34–48. doi: 10.1038/s41583-018-0091-3. [DOI] [PubMed] [Google Scholar]
- 2.Belenguer P., Duarte J.M.N., Schuck P.F., Ferreira G.C. Mitochondria and the brain: bioenergetics and beyond. Neurotox. Res. 2019;36(2):219–238. doi: 10.1007/s12640-019-00061-7. [DOI] [PubMed] [Google Scholar]
- 3.Kann O., Kovács R. Mitochondria and neuronal activity. Am. J. Physiol. Cell Physiol. 2007;292(2):C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 4.Völgyi K., Gulyássy P., Háden K., Kis V., Badics K., Kékesi K.A., Simor A., Györffy B., Tóth E.A., Lubec G., Juhász G., Dobolyi A. Synaptic mitochondria: A brain mitochondria cluster with a specific proteome. J. Proteomics. 2015;120:142–157. doi: 10.1016/j.jprot.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Dubinsky J.M. Heterogeneity of nervous system mitochondria: Location, location, location! Exp. Neurol. 2009;218(2):293–307. doi: 10.1016/j.expneurol.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 6.Fedorovich S.V., Waseem T.V., Puchkova L.V. Biogenetic and morphofunctional heterogeneity of mitochondria: The case of synaptic mitochondria. Rev. Neurosci. 2017;28(4):363–373. doi: 10.1515/revneuro-2016-0077. [DOI] [PubMed] [Google Scholar]
- 7.Ly C.V., Verstreken P. Mitochondria at the synapse. Neuroscientist. 2006;12(4):291–299. doi: 10.1177/1073858406287661. [DOI] [PubMed] [Google Scholar]
- 8.Vos M., Lauwers E., Verstreken P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front. Synaptic Neurosci. 2010;2:139. doi: 10.3389/fnsyn.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stauch K.L., Purnell P.R., Fox H.S. Quantitative proteomics of synaptic and nonsynaptic mitochondria: insights for synaptic mitochondrial vulnerability. J. Proteome Res. 2014;13(5):2620–2636. doi: 10.1021/pr500295n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raefsky S.M., Mattson M.P. Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic. Biol. Med. 2017;102:203–216. doi: 10.1016/j.freeradbiomed.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmad F., Haque S., Ravinayagam V., Ahmad A., Kamli M.R., Barreto G.E. Developmental lead (Pb)-induced deficits in redox and bioenergetic status of cerebellar synapses are ameliorated by ascorbate supplementation. Toxicology. 2020;440:152492. doi: 10.1016/j.tox.2020.152492. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad F., Salahuddin M., Alamoudi W., Acharya S. Dysfunction of cortical synapse-specific mitochondria in developing rats exposed to lead and its amelioration by ascorbate supplementation. Neuropsychiatr. Dis. Treat. 2018;14:813–824. doi: 10.2147/NDT.S148248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Guo L., Lu L., Sun H., Shao M., Beck S.J., Li L., Ramachandran J., Du Y., Du H. Synaptosomal mitochondrial dysfunction in 5xFAD mouse model of Alzheimer’s disease. PLoS One. 2016;11(3):e0150441. doi: 10.1371/journal.pone.0150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du H., Guo L., Yan S., Sosunov A.A., McKhann G.M., ShiDu Yan S. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc. Natl. Acad. Sci. USA. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo L., Tian J., Du H. Mitochondrial dysfunction and synaptic transmission failure in Alzheimer’s Disease. J. Alzheimers Dis. 2017;57(4):1071–1086. doi: 10.3233/JAD-160702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm A., Eckert A. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017;143(4):418–431. doi: 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei L., Wallace D.C. Mitochondrial etiology of neuropsychiatric disorders. Biol. Psychiatry. 2018;83(9):722–730. doi: 10.1016/j.biopsych.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeanneteau F., Arango-Lievano M. Linking mitochondria to synapses: New insights for stress-related neuropsychiatric disorders. Neural Plast. 2016;2016:3985063. doi: 10.1155/2016/3985063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabral-Costa J.V., Kowaltowski A.J. Neurological disorders and mitochondria. Mol. Aspects Med. 2020;71:100826. doi: 10.1016/j.mam.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Mattson M.P., Gleichmann M., Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60(5):748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin L.J. Biology of mitochondria in neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2012;107:355–415. doi: 10.1016/B978-0-12-385883-2.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frezza C., Cipolat S., Scorrano L. Organelle isolation: Functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat. Protoc. 2007;2(2):287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 23.Sperling J.A., Sakamuri S.S.V.P., Albuck A.L., Sure V.N., Evans W.R., Peterson N.R., Rutkai I., Mostany R., Satou R., Katakam P.V.G. Measuring respiration in isolated murine brain mitochondria: Implications for mechanistic stroke studies. Neuromolecular Med. 2019;21(4):493–504. doi: 10.1007/s12017-019-08552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartwig S., Feckler C., Lehr S., Wallbrecht K., Wolgast H., Müller-Wieland D., Kotzka J. A critical comparison between two classical and a kit-based method for mitochondria isolation. Proteomics. 2009;9(11):3209–3214. doi: 10.1002/pmic.200800344. [DOI] [PubMed] [Google Scholar]
- 25.Hogeboom G.H., Schneider W.C., Pallade G.E. Cytochemical studies of mammalian tissues; isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. J. Biol. Chem. 1948;172(2):619–635. doi: 10.1016/S0021-9258(19)52749-1. [DOI] [PubMed] [Google Scholar]
- 26.Lampl T., Crum J.A., Davis T.A., Milligan C., Del Gaizo Moore V. Isolation and functional analysis of mitochondria from cultured cells and mouse tissue. J. Vis. Exp. 2015;(97):52076. doi: 10.3791/52076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wettmarshausen J., Perocchi F. Isolation of functional mitochondria from cultured cells and mouse tissues. Methods Mol. Biol. 2017;1567:15–32. doi: 10.1007/978-1-4939-6824-4_2. [DOI] [PubMed] [Google Scholar]
- 28.Kristián T., Hopkins I.B., McKenna M.C., Fiskum G. Isolation of mitochondria with high respiratory control from primary cultures of neurons and astrocytes using nitrogen cavitation. J. Neurosci. Methods. 2006;152(1-2):136–143. doi: 10.1016/j.jneumeth.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham J.M. Purification of a crude mitochondrial fraction by density‐gradient centrifugation. Curr. Protoc. Cell Biol. 1999:4. doi: 10.1002/0471143030.cb0304s04. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa K., Seta K., Takeda H., Ando K., Handa H., Araki C. On the isolation of mitochondria with high respiratory control from rat brain. J. Biochem. 1966;59(5):501–510. doi: 10.1093/oxfordjournals.jbchem.a128334. [DOI] [PubMed] [Google Scholar]
- 31.Stahl W.L., Smith J.C., Napolitano L.M., Basford R.E. Brain mitochondria. I. Isolation of bovine brain mitochondria. J. Cell Biol. 1963;19(2):293–307. doi: 10.1083/jcb.19.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clayton DA, Shadel GS. Purification of mitochondria by sucrose step density gradient centrifugation. Cold Spring Harb Protoc. 2014;2014(10):pdb.prot080028. doi: 10.1101/pdb.prot080028. [DOI] [PubMed] [Google Scholar]
- 33.Sauerbeck A., Pandya J., Singh I., Bittman K., Readnower R., Bing G., Sullivan P. Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. J. Neurosci. Methods. 2011;198(1):36–43. doi: 10.1016/j.jneumeth.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamberger A., Blomstrand C., Lehninger A.L. Comparative studies on mitochondria isolated from neuron-enriched and glia-enriched fractions of rabbit and beef brain. J. Cell Biol. 1970;45(2):221–234. doi: 10.1083/jcb.45.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai J.C.K., Walsh J.M., Dennis S.C., Clark J.B. Synaptic and non-synaptic mitochondria from rat brain: isolation and characterization. J. Neurochem. 1977;28(3):625–631. doi: 10.1111/j.1471-4159.1977.tb10434.x. [DOI] [PubMed] [Google Scholar]
- 36.Morota S., Hansson M.J., Ishii N., Kudo Y., Elmér E., Uchino H. Spinal cord mitochondria display lower calcium retention capacity compared with brain mitochondria without inherent differences in sensitivity to cyclophilin D inhibition. J. Neurochem. 2007;103(5):2066–2076. doi: 10.1111/j.1471-4159.2007.04912.x. [DOI] [PubMed] [Google Scholar]
- 37.Sims N.R. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J. Neurochem. 1990;55(2):698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 38.Sims N.R., Anderson M.F. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat. Protoc. 2008;3(7):1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 39.Kristian T. Isolation of mitochondria from the CNS. Curr. Protoc. Neurosci. 2010 doi: 10.1002/0471142301.ns0722s52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spinazzi M., Casarin A., Pertegato V., Salviati L., Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012;7(6):1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 41.Valenti D., de Bari L., De Filippis B., Ricceri L., Vacca R.A. Preservation of mitochondrial functional integrity in mitochondria isolated from small cryopreserved mouse brain areas. Anal. Biochem. 2014;444:25–31. doi: 10.1016/j.ab.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Barksdale K.A., Perez-Costas E., Gandy J.C., Melendez-Ferro M., Roberts R.C., Bijur G.N. Mitochondrial viability in mouse and human postmortem brain. FASEB J. 2010;24(9):3590–3599. doi: 10.1096/fj.09-152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Roche M., Casal A., Carriquiry M., Radi R., Quijano C., Cassina A. Respiratory analysis of coupled mitochondria in cryopreserved liver biopsies. Redox Biol. 2018;17:207–212. doi: 10.1016/j.redox.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acin-Perez R., Benador I.Y., Petcherski A., Veliova M., Benavides G.A., Lagarrigue S., Caudal A., Vergnes L., Murphy A.N., Karamanlidis G., Tian R., Reue K., Wanagat J., Sacks H., Amati F., Darley-Usmar V.M., Liesa M., Divakaruni A.S., Stiles L., Shirihai O.S. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 2020;39(13):e104073. doi: 10.15252/embj.2019104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clayton DA, Shadel GS. Isolation of mitochondria from tissue culture cells. Cold Spring Harb Protoc. 2014;2014(10):pdb.prot080002.. doi: 10.1101/pdb.prot080002. [DOI] [PubMed] [Google Scholar]
- 46.Almeida A., Medina J.M. A rapid method for the isolation of metabolically active mitochondria from rat neurons and astrocytes in primary culture. Brain Res. Brain Res. Protoc. 1998;2(3):209–214. doi: 10.1016/S1385-299X(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 47.Devi L., Prabhu B.M., Galati D.F., Avadhani N.G., Anandatheerthavarada H.K. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J. Neurosci. 2006;26(35):9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prajapati P., Wang W.X., Nelson P.T., Springer J.E. Methodology for subcellular fractionation and MicroRNA examination of mitochondria, mitochondria associated ER membrane (MAM), ER, and cytosol from human brain. Methods Mol. Biol. 2020;2063:139–154. doi: 10.1007/978-1-0716-0138-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansson M.J., Morota S., Chen L., Matsuyama N., Suzuki Y., Nakajima S., Tanoue T., Omi A., Shibasaki F., Shimazu M., Ikeda Y., Uchino H., Elmér E. Cyclophilin D-sensitive mitochondrial permeability transition in adult human brain and liver mitochondria. J. Neurotrauma. 2011;28(1):143–153. doi: 10.1089/neu.2010.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khattar N.K., Yablonska S., Baranov S.V., Baranova O.V., Kretz E.S., Larkin T.M., Carlisle D.L., Richardson R.M., Friedlander R.M. Isolation of functionally active and highly purified neuronal mitochondria from human cortex. J. Neurosci. Methods. 2016;263:1–6. doi: 10.1016/j.jneumeth.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Leverin A.L., Han W., Zhu C., Johansson B.R., Jacotot E., Ten V.S., Sims N.R., Hagberg H. Isolation of brain mitochondria from neonatal mice. J. Neurochem. 2011;119(6):1253–1261. doi: 10.1111/j.1471-4159.2011.07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pallotti F., Lenaz G. Isolation and subfractionation of mitochondria from animal cells and tissue culture lines. Methods Cell Biol. 2007;80:3–44. doi: 10.1016/S0091-679X(06)80001-4. [DOI] [PubMed] [Google Scholar]
- 53.Hill R.L., Kulbe J.R., Singh I.N., Wang J.A., Hall E.D. Synaptic mitochondria are more susceptible to traumatic brain injury-induced oxidative damage and respiratory dysfunction than non-synaptic mitochondria. Neuroscience. 2018;386:265–283. doi: 10.1016/j.neuroscience.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 54.Brown M.R., Sullivan P.G., Geddes J.W. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J. Biol. Chem. 2006;281(17):11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 55.Annunziata I., Weesner J.A., d’Azzo A. Isolation of mitochondria-associated ER membranes (MAMs), synaptic MAMs, and glycosphingolipid enriched microdomains (GEMs) from brain tissues and neuronal cells. Methods Mol. Biol. 2021;2277:357–370. doi: 10.1007/978-1-0716-1270-5_22. [DOI] [PubMed] [Google Scholar]
- 56.Wieckowski M.R., Giorgi C., Lebiedzinska M., Duszynski J., Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 2009;4(11):1582–1590. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- 57.Schreiner B., Ankarcrona M. Isolation of mitochondria-associated membranes (MAM) from mouse brain tissue. Methods Mol. Biol. 2017;1567:53–68. doi: 10.1007/978-1-4939-6824-4_5. [DOI] [PubMed] [Google Scholar]
- 58.Islinger M., Wildgruber R., Völkl A. Preparative free-flow electrophoresis, a versatile technology complementing gradient centrifugation in the isolation of highly purified cell organelles. Electrophoresis. 2018;39(18):2288–2299. doi: 10.1002/elps.201800187. [DOI] [PubMed] [Google Scholar]
- 59.Hackenbrock C.R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc. Natl. Acad. Sci. USA. 1968;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frey T.G., Mannella C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000;25(7):319–324. doi: 10.1016/S0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad F., Alamoudi W., Haque S., Salahuddin M., Alsamman K. Simple, reliable, and time-efficient colorimetric method for the assessment of mitochondrial function and toxicity. Bosn. J. Basic Med. Sci. 2018;18(4):367–374. doi: 10.17305/bjbms.2018.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghazi-Khansari M., Mohammadi-Bardbori A., Hosseini M-J. Using Janus green B to study paraquat toxicity in rat liver mitochondria: role of ACE inhibitors (thiol and nonthiol ACEi). Ann. N. Y. Acad. Sci. 2006;1090(1):98–107. doi: 10.1196/annals.1378.010. [DOI] [PubMed] [Google Scholar]
- 63.Kundu T., Bhattacharjee B., Hazra S., Ghosh A.K., Bandyopadhyay D., Pramanik A. Synthesis and biological assessment of pyrrolobenzoxazine scaffold as a potent antioxidant. J. Med. Chem. 2019;62(13):6315–6329. doi: 10.1021/acs.jmedchem.9b00717. [DOI] [PubMed] [Google Scholar]
- 64.Wang X., Yan X., Yang Y., Yang W., Zhang Y., Wang J., Ye D., Wu Y., Ma P., Yan B. Dibutyl phthalate-mediated oxidative stress induces splenic injury in mice and the attenuating effects of vitamin E and curcumin. Food Chem. Toxicol. 2020;136:110955. doi: 10.1016/j.fct.2019.110955. [DOI] [PubMed] [Google Scholar]
- 65.Lemasters J.J., Ramshesh V.K. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell Biol. 2007;80:283–295. doi: 10.1016/S0091-679X(06)80014-2. [DOI] [PubMed] [Google Scholar]
- 66.Chazotte B. Labeling mitochondria with MitoTracker dyes. Cold Spring Harb. Protoc. 2011;2011(8):990–2. doi: 10.1101/pdb.prot5648. [DOI] [PubMed] [Google Scholar]
- 67.Lecoeur H., Langonné A., Baux L., Rebouillat D., Rustin P., Prévost M.C., Brenner C., Edelman L., Jacotot E. Real-time flow cytometry analysis of permeability transition in isolated mitochondria. Exp. Cell Res. 2004;294(1):106–117. doi: 10.1016/j.yexcr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 68.Tanji K., Bonilla E. Optical imaging techniques (histochemical, immunohistochemical, and in situ hybridization staining methods) to visualize mitochondria. Methods Cell Biol. 2007;80:135–154. doi: 10.1016/S0091-679X(06)80006-3. [DOI] [PubMed] [Google Scholar]
- 69.Westensee I.N., Brodszkij E., Qian X., Marcelino T.F., Lefkimmiatis K., Städler B. Mitochondria encapsulation in hydrogel‐based artificial cells as ATP producing subunits. Small. 2021;17(24):2007959. doi: 10.1002/smll.202007959. [DOI] [PubMed] [Google Scholar]
- 70.Bhosale G., Duchen M.R. Investigating the mitochondrial permeability transition pore in disease phenotypes and drug screening. Curr. Protocols Pharmacol. 2019;85(1):e59. doi: 10.1002/cpph.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koh J.Y., Choi D.W. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J. Neurosci. Methods. 1987;20(1):83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 72.Xu J., Chen Q., Zen K., Zhang C., Zhang Q. Synaptosomes secrete and uptake functionally active microRNAs via exocytosis and endocytosis pathways. J. Neurochem. 2013;124(1):15–25. doi: 10.1111/jnc.12057. [DOI] [PubMed] [Google Scholar]
- 73.Vassault A. L-Lactate dehydrogenase. UV method with pyruvate and NADH. Methods Enzym Anal. 1983;3:118–126. [Google Scholar]
- 74.Nuñez-Figueredo Y., Pardo-Andreu G.L., Ramírez-Sánchez J., Delgado-Hernández R., Ochoa-Rodríguez E., Verdecia-Reyes Y., Naal Z., Muller A.P., Portela L.V., Souza D.O. Antioxidant effects of JM-20 on rat brain mitochondria and synaptosomes: Mitoprotection against Ca2+-induced mitochondrial impairment. Brain Res. Bull. 2014;109:68–76. doi: 10.1016/j.brainresbull.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Colombini M. Measurement of VDAC permeability in intact mitochondria and in reconstituted systems. Methods Cell Biol. 2007;80:241–260. doi: 10.1016/S0091-679X(06)80012-9. [DOI] [PubMed] [Google Scholar]
- 76.Douce R., Bourguignon J., Brouquisse R., Neuburger M. Isolation of plant mitochondria: General principles and criteria of integrity. Methods Enzymol. 1987;148:403–415. doi: 10.1016/0076-6879(87)48039-7. [DOI] [Google Scholar]
- 77.Vacca R.A., Valenti D., Bobba A., Merafina R.S., Passarella S., Marra E. Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death. Plant Physiol. 2006;141(1):208–219. doi: 10.1104/pp.106.078683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dietrich P., Alli S., Mulligan M.K., Cox R., Ashbrook D.G., Williams R.W., Dragatsis I. Identification of cyclin D1 as a major modulator of 3-nitropropionic acid-induced striatal neurodegeneration. Neurobiol. Dis. 2022;162:105581. doi: 10.1016/j.nbd.2021.105581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lanza I.R., Nair K.S. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–372. doi: 10.1016/S0076-6879(09)05020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rasmussen H.N., Andersen A.J., Rasmussen U.F. Optimization of preparation of mitochondria from 25-100 mg skeletal muscle. Anal. Biochem. 1997;252(1):153–159. doi: 10.1006/abio.1997.2304. [DOI] [PubMed] [Google Scholar]
- 81.Rasmussen H.N., Rasmussen U.F. Small scale preparation of skeletal muscle mitochondria, criteria of integrity, and assays with reference to tissue function. Mol. Cell. Biochem. 1997;174(1/2):55–60. doi: 10.1023/A:1006851705996. [DOI] [PubMed] [Google Scholar]
- 82.Srere P.A. Citrate synthase. Methods Enzymol. 1969;13:3–11. doi: 10.1016/0076-6879(69)13005-0. [DOI] [Google Scholar]
- 83.Valenti D., Vacca R.A., de Pinto M.C., De Gara L., Marra E., Passarella S. In the early phase of programmed cell death in Tobacco Bright Yellow 2 cells the mitochondrial adenine nucleotide translocator, adenylate kinase and nucleoside diphosphate kinase are impaired in a reactive oxygen species-dependent manner. Biochim. Biophys. Acta Bioenerg. 2007;1767(1):66–78. doi: 10.1016/j.bbabio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Bergmeyer H.U., Gawehn K., Grassl M. Enzymatic assay of fumarase. Methods Enzym Anal. 1974;1:543–545. [Google Scholar]
- 85.Atlante A., Calissano P., Bobba A., Azzariti A., Marra E., Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J. Biol. Chem. 2000;275(47):37159–37166. doi: 10.1074/jbc.M002361200. [DOI] [PubMed] [Google Scholar]
- 86.De Loecker P., Fuller B.J., De Loecker W. The effects of cryopreservation on protein synthesis and membrane transport in isolated rat liver mitochondria. Cryobiology. 1991;28(5):445–453. doi: 10.1016/0011-2240(91)90053-Q. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi R., Andreyev A., Murphy A.N., Perkins G.A., Ellisman M.H., Newmeyer D.D. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007;14(3):616–624. doi: 10.1038/sj.cdd.4402035. [DOI] [PubMed] [Google Scholar]
- 88.Nukala V.N., Singh I.N., Davis L.M., Sullivan P.G. Cryopreservation of brain mitochondria: A novel methodology for functional studies. J. Neurosci. Methods. 2006;152(1-2):48–54. doi: 10.1016/j.jneumeth.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 89.Kuznetsov A.V., Kunz W.S., Saks V., Usson Y., Mazat J.P., Letellier T., Gellerich F.N., Margreiter R. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal. Biochem. 2003;319(2):296–303. doi: 10.1016/S0003-2697(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 90.Schieber O., Dietrich A., Maréchal-Drouard L. Cryopreservation of plant mitochondria as a tool for protein import or in organello protein synthesis studies. Plant Physiol. 1994;106(1):159–164. doi: 10.1104/pp.106.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zorova L.D., Popkov V.A., Plotnikov E.Y., Silachev D.N., Pevzner I.B., Jankauskas S.S., Babenko V.A., Zorov S.D., Balakireva A.V., Juhaszova M., Sollott S.J., Zorov D.B., Zorov S.D., Balakireva A.V., Juhaszova M., Sollott S.J., Zorov D.B. Mitochondrial membrane potential. Anal. Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perry S.W., Norman J.P., Barbieri J., Brown E.B., Gelbard H.A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scaduto R.C., Jr, Grotyohann L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 1999;76(1):469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bunting J.R., Phan T.V., Kamali E., Dowben R.M. Fluorescent cationic probes of mitochondria. Metrics and mechanism of interaction. Biophys. J. 1989;56(5):979–993. doi: 10.1016/S0006-3495(89)82743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tahara E.B., Navarete F.D.T., Kowaltowski A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic. Biol. Med. 2009;46(9):1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Åkerman K.E.O., Wikström M.K.F. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- 97.Sivandzade F., Bhalerao A., Cucullo L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc. 2019;9(1):e3128. doi: 10.21769/BioProtoc.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lugli E., Troiano L., Cossarizza A. Polychromatic analysis of mitochondrial membrane potential using JC-1. Curr. Protoc. Cytom. 2007:Chapter 7: Unit7.32. doi: 10.1002/0471142956.cy0732s41. [DOI] [PubMed] [Google Scholar]
- 99.Cossarizza A., Salvioli S. Flow cytometric analysis of mitochondrial membrane potential using JC‐1. Curr. Protoc. Cytom. 2000:Chapter 9:Unit 9.14. doi: 10.1002/0471142956.cy0914s13. [DOI] [PubMed] [Google Scholar]
- 100.Reers M., Smiley S.T., Mottola-Hartshorn C., Chen A., Lin M., Chen L.B. Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- 101.Noterman M.F., Chaubey K., Lin-Rahardja K., Rajadhyaksha A.M., Pieper A.A., Taylor E.B. Dual-process brain mitochondria isolation preserves function and clarifies protein composition. Proc. Natl. Acad. Sci. USA. 2021;118(11):e2019046118. doi: 10.1073/pnas.2019046118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lores-Arnaiz S., Lombardi P., Karadayian A.G., Orgambide F., Cicerchia D., Bustamante J. Brain cortex mitochondrial bioenergetics in synaptosomes and non-synaptic mitochondria during aging. Neurochem. Res. 2016;41(1-2):353–363. doi: 10.1007/s11064-015-1817-5. [DOI] [PubMed] [Google Scholar]
- 103.Hurst S., Hoek J., Sheu S.S. Mitochondrial Ca2+ and regulation of the permeability transition pore. J. Bioenerg. Biomembr. 2017;49(1):27–47. doi: 10.1007/s10863-016-9672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chinopoulos C. Mitochondrial permeability transition pore: Back to the drawing board. Neurochem. Int. 2018;117:49–54. doi: 10.1016/j.neuint.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Halestrap A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 2009;46(6):821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 106.Mnatsakanyan N., Beutner G., Porter G.A., Alavian K.N., Jonas E.A. Physiological roles of the mitochondrial permeability transition pore. J. Bioenerg. Biomembr. 2017;49(1):13–25. doi: 10.1007/s10863-016-9652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choo Y.S., Johnson G.V.W., MacDonald M., Detloff P.J., Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004;13(14):1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 108.Mirandola S.R., Melo D.R., Saito A., Castilho R.F. 3-nitropropionic acid-induced mitochondrial permeability transition: Comparative study of mitochondria from different tissues and brain regions. J. Neurosci. Res. 2010;88(3):630–639. doi: 10.1002/jnr.22239. [DOI] [PubMed] [Google Scholar]
- 109.Brustovetsky N., Brustovetsky T., Purl K.J., Capano M., Crompton M., Dubinsky J.M. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J. Neurosci. 2003;23(12):4858–4867. doi: 10.1523/JNEUROSCI.23-12-04858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pérez M.J., Quintanilla R.A. Development or disease: Duality of the mitochondrial permeability transition pore. Dev. Biol. 2017;426(1):1–7. doi: 10.1016/j.ydbio.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 111.Li V., Brustovetsky T., Brustovetsky N. Role of cyclophilin D-dependent mitochondrial permeability transition in glutamate-induced calcium deregulation and excitotoxic neuronal death. Exp. Neurol. 2009;218(2):171–182. doi: 10.1016/j.expneurol.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amigo I., Menezes-Filho S.L., Luévano-Martínez L.A., Chausse B., Kowaltowski A.J. Caloric restriction increases brain mitochondrial calcium retention capacity and protects against excitotoxicity. Aging Cell. 2017;16(1):73–81. doi: 10.1111/acel.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chalmers S., Nicholls D.G. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J. Biol. Chem. 2003;278(21):19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 114.Hyder F., Rothman D.L., Bennett M.R. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc. Natl. Acad. Sci. USA. 2013;110(9):3549–3554. doi: 10.1073/pnas.1214912110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barrientos A., Fontanesi F., Díaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 2009. Available from: https://onlinelibrary.wiley.com/doi/10.1002/0471142905.hg1903s63. [DOI] [PMC free article] [PubMed]
- 116.Agbas A., Krishnamurthy P., Michaelis M.L., Michaelis E.K. Mitochondrial electron transfer cascade enzyme activity assessment in cultured neurons and select brain regions. Curr. Protoc. Toxicol. 2019;80(1):e73. doi: 10.1002/cptx.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kirby D.M., Thorburn D.R., Turnbull D.M., Taylor R.W. Biochemical assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:93–119. doi: 10.1016/S0091-679X(06)80004-X. [DOI] [PubMed] [Google Scholar]
- 118.Doerrier C., Garcia-Souza L.F., Krumschnabel G., Wohlfarter Y., Mészáros A.T., Gnaiger E. High-resolution fluorespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol. Biol. 2018;1782:31–70. doi: 10.1007/978-1-4939-7831-1_3. [DOI] [PubMed] [Google Scholar]
- 119.Villani G., Attardi G. Polarographic assays of respiratory chain complex activity. Methods Cell Biol. 2007;80:121–133. doi: 10.1016/S0091-679X(06)80005-1. [DOI] [PubMed] [Google Scholar]
- 120.Connolly N.M.C., Theurey P., Adam-Vizi V., Bazan N.G., Bernardi P., Bolaños J.P., Culmsee C., Dawson V.L., Deshmukh M., Duchen M.R., Düssmann H., Fiskum G., Galindo M.F., Hardingham G.E., Hardwick J.M., Jekabsons M.B., Jonas E.A., Jordán J., Lipton S.A., Manfredi G., Mattson M.P., McLaughlin B., Methner A., Murphy A.N., Murphy M.P., Nicholls D.G., Polster B.M., Pozzan T., Rizzuto R., Satrústegui J., Slack R.S., Swanson R.A., Swerdlow R.H., Will Y., Ying Z., Joselin A., Gioran A., Moreira Pinho C., Watters O., Salvucci M., Llorente-Folch I., Park D.S., Bano D., Ankarcrona M., Pizzo P., Prehn J.H.M. Guidelines on experimental methods to assess mitochondrial dysfunction in cellular models of neurodegenerative diseases. Cell Death Differ. 2018;25(3):542–572. doi: 10.1038/s41418-017-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Morava E, Rodenburg RJ, Hol F, De Vries M, Janssen A, Van Den Heuvel L. Clinical and biochemical characteristics in patients with a high mutant load of the mitochondrial T8993G/C mutations. Am J Med Genet. 2006;140 A:863–8. doi: 10.1002/ajmg.a.31194. [DOI] [PubMed] [Google Scholar]
- 122.Haraux F., Lombès A. Kinetic analysis of ATP hydrolysis by complex V in four murine tissues: Towards an assay suitable for clinical diagnosis. PLoS One. 2019;14(8):e0221886. doi: 10.1371/journal.pone.0221886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bosetti F., Brizzi F., Barogi S., Mancuso M., Siciliano G., Tendi E.A., Murri L., Rapoport S.I., Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol. Aging. 2002;23(3):371–376. doi: 10.1016/S0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 124.Katewa S.D., Katyare S.S. A simplified method for inorganic phosphate determination and its application for phosphate analysis in enzyme assays. Anal. Biochem. 2003;323(2):180–187. doi: 10.1016/j.ab.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 125.Valenti D., Tullo A., Caratozzolo M.F., Merafina R.S., Scartezzini P., Marra E., Vacca R.A. Impairment of F1F0-ATPase, adenine nucleotide translocator and adenylate kinase causes mitochondrial energy deficit in human skin fibroblasts with chromosome 21 trisomy. Biochem. J. 2010;431(2):299–310. doi: 10.1042/BJ20100581. [DOI] [PubMed] [Google Scholar]
- 126.Vives-Bauza C., Yang L., Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol. 2007;80:155–171. doi: 10.1016/S0091-679X(06)80007-5. [DOI] [PubMed] [Google Scholar]
- 127.Chance B., Williams G.R. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J. Biol. Chem. 1956;221(1):477–489. doi: 10.1016/S0021-9258(18)65266-4. [DOI] [PubMed] [Google Scholar]
- 128.Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 2001;128(3):277–297. doi: 10.1016/S0034-5687(01)00307-3. [DOI] [PubMed] [Google Scholar]
- 129.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Silva A.M., Oliveira P.J. Evaluation of respiration with clark type electrode in Isolated mitochondria and permeabilized animal cells. Methods Mol. Biol. 2018;1782:7–29. doi: 10.1007/978-1-4939-7831-1_2. [DOI] [PubMed] [Google Scholar]
- 131.Diepart C., Verrax J., Calderon P.B., Feron O., Jordan B.F., Gallez B. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Anal. Biochem. 2010;396(2):250–256. doi: 10.1016/j.ab.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 132.Hynes J., Hill R., Papkovsky D.B. The use of a fluorescence-based oxygen uptake assay in the analysis of cytotoxicity. Toxicol. In Vitro. 2006;20(5):785–792. doi: 10.1016/j.tiv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Hynes J., Marroquin L.D., Ogurtsov V.I., Christiansen K.N., Stevens G.J., Papkovsky D.B., Will Y. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicol. Sci. 2006;92(1):186–200. doi: 10.1093/toxsci/kfj208. [DOI] [PubMed] [Google Scholar]
- 134.Hynes J., Natoli E., Will Y. Fluorescent pH and oxygen probes of the assessment of mitochondrial toxicity in isolated mitochondria and whole cells. 2009:Chapter 2: Unit 2.16. doi: 10.1002/0471140856.tx0216s40. [DOI] [PubMed] [Google Scholar]
- 135.Iuso A., Repp B., Biagosch C., Terrile C., Prokisch H. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using Seahorse XF96 analyzer. Methods Mol. Biol. 2017;1567:217–230. doi: 10.1007/978-1-4939-6824-4_13. [DOI] [PubMed] [Google Scholar]
- 136.Sriram G., Martinez J.A., McCabe E.R.B., Liao J.C., Dipple K.M. Single-gene disorders: What role could moonlighting enzymes play? Am. J. Hum. Genet. 2005;76(6):911–924. doi: 10.1086/430799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Acin-Perez R., Benincá C., Shabane B., Shirihai O.S., Stiles L. Utilization of human samples for assessment of mitochondrial bioenergetics: Gold standards, limitations, and future perspectives. Life (Basel) 2021;11(9):949. doi: 10.3390/life11090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reisch A.S., Elpeleg O. Biochemical assays for mitochondrial activity: Assays of TCA cycle enzymes and PDHc. Methods Cell Biol. 2007;80:199–222. doi: 10.1016/S0091-679X(06)80010-5. [DOI] [PubMed] [Google Scholar]
- 139.Andreyev A.Y., Kushnareva Y.E., Starkov A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc.) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 140.Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018;592(5):692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 141.Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019;2019:1–18. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Armstrong J.S., Whiteman M. Measurement of reactive oxygen species in cells and mitochondria. Methods Cell Biol. 2007;80:355–377. doi: 10.1016/S0091-679X(06)80018-X. [DOI] [PubMed] [Google Scholar]
- 143.Zhao R.Z., Jiang S., Zhang L., Yu Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Starkov A.A. Measurement of mitochondrial ROS production. Methods Mol. Biol. 2010;648:245–255. doi: 10.1007/978-1-60761-756-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhou M., Diwu Z., Panchuk-Voloshina N., Haugland R.P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal. Biochem. 1997;253(2):162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 146.Forman H.J., Fridovich I. Superoxide dismutase: A comparison of rate constants. Arch. Biochem. Biophys. 1973;158(1):396–400. doi: 10.1016/0003-9861(73)90636-X. [DOI] [PubMed] [Google Scholar]
- 147.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247(10):3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 148.Brown G.C. Nitric oxide as a competitive inhibitor of oxygen consumption in the mitochondrial respiratory chain. Acta Physiol. Scand. 2000;168(4):667–674. doi: 10.1046/j.1365-201x.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- 149.Navarro A., Boveris A., Bández M.J., Sánchez-Pino M.J., Gómez C., Muntané G., Ferrer I. Human brain cortex: Mitochondrial oxidative damage and adaptive response in Parkinson disease and in dementia with Lewy bodies. Free Radic. Biol. Med. 2009;46(12):1574–1580. doi: 10.1016/j.freeradbiomed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 150.Boveris A., Arnaiz S.L., Bustamante J., Alvarez S., Valdez L., Boveris A.D., Navarro A. Pharmacological regulation of mitochondrial nitric oxide synthase. Methods Enzymol. 2002;359:328–339. doi: 10.1016/S0076-6879(02)59196-5. [DOI] [PubMed] [Google Scholar]
- 151.Katalinic V., Modun D., Music I., Boban M. Gender differences in antioxidant capacity of rat tissues determined by 2, 2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;140(1):47–52. doi: 10.1016/j.cca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 152.Shea T.B., Rogers E., Ashline D., Ortiz D., Sheu M.S. Quantification of antioxidant activity in brain tissue homogenates using the ‘total equivalent antioxidant capacity’. J. Neurosci. Methods. 2003;125(1-2):55–58. doi: 10.1016/S0165-0270(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 153.Siqueira I.R., Fochesatto C., Andrade A., Santos M., Hagen M., Bello-Klein A., Netto C.A. Total antioxidant capacity is impaired in different structures from aged rat brain. Int. J. Dev. Neurosci. 2005;23(8):663–671. doi: 10.1016/j.ijdevneu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 154.Sofic E., Sapcanin A., Tahirovic I., Gavrankapetanovic I., Jellinger K., Reynolds G.P., Tatschner T., Riederer P. Antioxidant capacity in postmortem brain tissues of Parkinson’s and Alzheimer’s diseases. J. Neural Transm. Suppl. 2006;(71):39–43. doi: 10.1007/978-3-211-33328-0_5. [DOI] [PubMed] [Google Scholar]
- 155.Raukas M., Rebane R., Mahlapuu R., Jefremov V., Zilmer K., Karelson E., Bogdanovic N., Zilmer M. Mitochondrial oxidative stress index, activity of redox-sensitive aconitase and effects of endogenous anti- and pro-oxidants on its activity in control, Alzheimer’s disease and Swedish Familial Alzheimer’s disease brain. Free Radic. Res. 2012;46(12):1490–1495. doi: 10.3109/10715762.2012.728286. [DOI] [PubMed] [Google Scholar]
- 156.Cooper A.J.L., Kristal B.S. Multiple roles of glutathione in the central nervous system. Biol. Chem. 1997;378(8):793–802. [PubMed] [Google Scholar]
- 157.Dringen R., Hirrlinger J. Glutathione pathways in the brain. Biol. Chem. 2003;384(4):505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- 158.Nolfi-Donegan D., Braganza A., Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iskusnykh I.Y., Zakharova A.A., Pathak D. Glutathione in brain disorders and aging. Molecules. 2022;27(1):324. doi: 10.3390/molecules27010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun Y., Oberley L.W., Li Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988;34(3):497–500. doi: 10.1093/clinchem/34.3.497. [DOI] [PubMed] [Google Scholar]
- 161.Bannister J.V., Calabrese L. Assays for superoxide dismutase. Methods Biochem. Anal. 1987;32:pp. 279–312. doi: 10.1002/9780470110539.ch5. Available from: https://onlinelibrary.wiley.com/doi/10.1002/9780470110539.ch5. [DOI] [PubMed] [Google Scholar]
- 162.Elstner E.F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- 163.Vives-Bauza C., Starkov A., Garcia-Arumi E. Measurements of the antioxidant enzyme activities of superoxide dismutase, catalase, and glutathione peroxidase. Methods Cell Biol. 2007;80:379–393. doi: 10.1016/S0091-679X(06)80019-1. [DOI] [PubMed] [Google Scholar]
- 164.Harbauer A.B. Mitochondrial health maintenance in axons. Biochem. Soc. Trans. 2017;45(5):1045–1052. doi: 10.1042/BST20170023. [DOI] [PubMed] [Google Scholar]
- 165.Liang Y. Mitochondrial support and local translation of mitochondrial proteins in synaptic plasticity and function. Histol. Histopathol. 2021;36(10):1007–1019. doi: 10.14670/HH-18-345. [DOI] [PubMed] [Google Scholar]
- 166.Fernández-Silva P., Acín-Pérez R., Fernández-Vizarra E., Pérez-Martos A., Enriquez J.A. In vivo and in organello analyses of mitochondrial translation. Methods Cell Biol. 2007;80:571–588. doi: 10.1016/S0091-679X(06)80028-2. [DOI] [PubMed] [Google Scholar]
- 167.Ahmad F., Salahuddin M., Alsamman K., Herzallah H.K., Al-Otaibi S.T. Neonatal maternal deprivation impairs localized de novo activity-induced protein translation at the synapse in the rat hippocampus. Biosci. Rep. 2018;38(3):BSR20180118. doi: 10.1042/BSR20180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ahmad F., Singh K., Das D., Gowaikar R., Shaw E., Ramachandran A., Rupanagudi K.V., Kommaddi R.P., Bennett D.A., Ravindranath V. Reactive oxygen species-mediated loss of synaptic Akt1 signaling leads to deficient activity-dependent protein translation early in Alzheimer’s disease. Antioxid. Redox Signal. 2017;27(16):1269–1280. doi: 10.1089/ars.2016.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ahmad F., Salahuddin M., Alsamman K., AlMulla A.A., Salama K.F. Developmental lead (Pb)-induced deficits in hippocampal protein translation at the synapses are ameliorated by ascorbate supplementation. Neuropsychiatr. Dis. Treat. 2018;14:3289–3298. doi: 10.2147/NDT.S174083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Côté C., Poirier J., Boulet D. Expression of the mammalian mitochondrial genome. Stability of mitochondrial translation products as a function of membrane potential. J. Biol. Chem. 1989;264(15):8487–8490. [PubMed] [Google Scholar]
- 171.Almajan E.R., Richter R., Paeger L., Martinelli P., Barth E., Decker T., Larsson N.G., Kloppenburg P., Langer T., Rugarli E.I. AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival. J. Clin. Invest. 2012;122(11):4048–4058. doi: 10.1172/JCI64604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Formosa L.E., Hofer A., Tischner C., Wenz T., Ryan M.T. Translation and assembly of radiolabeled mitochondrial DNA-encoded protein subunits from cultured cells and isolated mitochondria. Methods Mol. Biol. 2016;1351:115–129. doi: 10.1007/978-1-4939-3040-1_9. [DOI] [PubMed] [Google Scholar]
- 173.Prem P.N., Kurian G.A. High-fat diet increased oxidative stress and mitochondrial dysfunction induced by renal ischemia-reperfusion injury in rat. Front. Physiol. 2021;12:715693. doi: 10.3389/fphys.2021.715693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Estell C., Stamatidou E., El-Messeiry S., Hamilton A. In situ imaging of mitochondrial translation shows weak correlation with nucleoid DNA intensity and no suppression during mitosis. J. Cell Sci. 2017;130(24):jcs.206714. doi: 10.1242/jcs.206714. [DOI] [PubMed] [Google Scholar]
- 175.Konovalova S., Hilander T., Loayza-Puch F., Rooijers K., Agami R., Tyynismaa H. Exposure to arginine analog canavanine induces aberrant mitochondrial translation products, mitoribosome stalling, and instability of the mitochondrial proteome. Int. J. Biochem. Cell Biol. 2015;65:268–74. doi: 10.1016/j.biocel.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 176.Villeneuve L.M., Stauch K.L., Fox H.S. Proteomic analysis of the mitochondria from embryonic and postnatal rat brains reveals response to developmental changes in energy demands. J. Proteomics. 2014;109:228–239. doi: 10.1016/j.jprot.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Abyadeh M., Gupta V., Chitranshi N., Gupta V., Wu Y., Saks D., Wander Wall R., Fitzhenry M.J., Basavarajappa D., You Y., Salekdeh G.H., Haynes P., Graham S.L., Mirzaei M. Mitochondrial dysfunction in Alzheimer’s disease-a proteomics perspective. Expert Rev. Proteomics. 2021;18(4):295–304. doi: 10.1080/14789450.2021.1918550. [DOI] [PubMed] [Google Scholar]
- 178.Broadwater L., Pandit A., Clements R., Azzam S., Vadnal J., Sulak M., Yong V.W., Freeman E.J., Gregory R.B., McDonough J. Analysis of the mitochondrial proteome in multiple sclerosis cortex. Biochim. Biophys. Acta Mol. Basis Dis. 2011;1812(5):630–641. doi: 10.1016/j.bbadis.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fu H., Li W., Liu Y., Lao Y., Liu W., Chen C., Yu H., Lee N.T.K., Chang D.C., Li P., Pang Y., Tsim K.W.K., Li M., Han Y. Mitochondrial proteomic analysis and characterization of the intracellular mechanisms of bis(7)-tacrine in protecting against glutamate-induced excitotoxicity in primary cultured neurons. J. Proteome Res. 2007;6(7):2435–2446. doi: 10.1021/pr060615g. [DOI] [PubMed] [Google Scholar]
- 180.Pienaar I.S., Schallert T., Hattingh S., Daniels W.M.U. Behavioral and quantitative mitochondrial proteome analyses of the effects of simvastatin: implications for models of neural degeneration. J. Neural Transm. (Vienna) 2009;116(7):791–806. doi: 10.1007/s00702-009-0247-4. [DOI] [PubMed] [Google Scholar]
- 181.Kabiri Y., von Toerne C., Fontes A., Knolle P.A., Zischka H. Isolation and purification of mitochondria from cell culture for proteomic analyses. Methods Mol. Biol. 2021;2261:411–419. doi: 10.1007/978-1-0716-1186-9_25. [DOI] [PubMed] [Google Scholar]
- 182.Pienaar I.S., Dexter D.T., Burkhard P.R. Mitochondrial proteomics as a selective tool for unraveling Parkinson’s disease pathogenesis. Expert Rev. Proteomics. 2010;7(2):205–226. doi: 10.1586/epr.10.8. [DOI] [PubMed] [Google Scholar]
- 183.Ahmad F., Haque S., Chavda V., Ashraf G.M. Recent advances in synaptosomal proteomics in Alzheimer’s Disease. Curr. Protein Pept. Sci. 2021;22(6):479–492. doi: 10.2174/1389203722666210618110233. [DOI] [PubMed] [Google Scholar]
- 184.Butterfield D.A., Palmieri E.M., Castegna A. Clinical implications from proteomic studies in neurodegenerative diseases: Lessons from mitochondrial proteins. Expert Rev. Proteomics. 2016;13(3):259–274. doi: 10.1586/14789450.2016.1149470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen X., Li J., Hou J., Xie Z., Yang F. Mammalian mitochondrial proteomics: Insights into mitochondrial functions and mitochondria-related diseases. Expert Rev. Proteomics. 2010;7(3):333–345. doi: 10.1586/epr.10.22. [DOI] [PubMed] [Google Scholar]
- 186.Butterfield D.A., Boyd-Kimball D. Mitochondrial oxidative and nitrosative stress and Alzheimer disease. Antioxidants. 2020;9(9):818. doi: 10.3390/antiox9090818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jiang Y., Wang X. Comparative mitochondrial proteomics: Perspective in human diseases. J. Hematol. Oncol. 2012;5(1):11. doi: 10.1186/1756-8722-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ingram T., Chakrabarti L. Proteomic profiling of mitochondria: What does it tell us about the ageing brain? Aging (Albany NY) 2016;8(12):3161–3179. doi: 10.18632/aging.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zanfardino P., Doccini S., Santorelli F.M., Petruzzella V. Tackling dysfunction of mitochondrial bioenergetics in the brain. Int. J. Mol. Sci. 2021;22(15):8325. doi: 10.3390/ijms22158325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fountoulakis M. Application of proteomics technologies in the investigation of the brain. Mass Spectrom. Rev. 2004;23(4):231–258. doi: 10.1002/mas.10075. [DOI] [PubMed] [Google Scholar]
- 191.Gu F., Chauhan V., Kaur K., Brown W.T., LaFauci G., Wegiel J., Chauhan A. Alterations in mitochondrial DNA copy number and the activities of electron transport chain complexes and pyruvate dehydrogenase in the frontal cortex from subjects with autism. Transl. Psychiatry. 2013;3(9):e299. doi: 10.1038/tp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]