Letter to the Editor
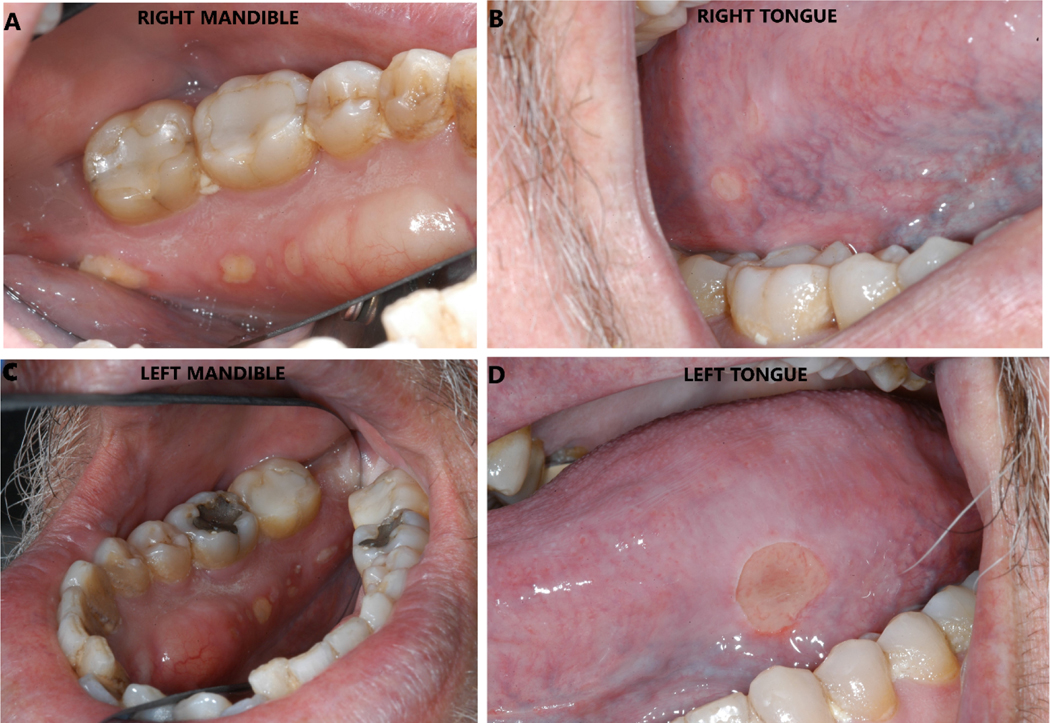
A 59-year-old male patient, diagnosed with Stage IV non-small cell lung cancer (NSCLC) with brain metastasis, status post gamma knife to the left frontal lobe lesion, palliative radiation therapy with a dose of 30Gy in 10 fractions to the left upper lobe and mediastinum and post 5 cycles of carboplatin/pemetrexed/pembrolizumab received at an outside institution, presented to Memorial Sloan Kettering Cancer Center for further management. He was a former heavy smoker and his medical history was also significant for type II diabetes mellitus. Due to the absence of driver mutations and given PDL 1 expression less than 50%, he was recommended a combination of chemotherapy and immunotherapy as the mainstay treatment for his metastatic disease. He received a total of 3 cycles of pemetrexed 500mg/m2 (1000mg) and pembrolizumab 200mg/flat dose every 3 weeks and was evaluated for response to therapy after 3 months. A CT chest-abdomen-pelvis scan revealed new lung nodules with increased thoracic and abdominal nodes suspicious for metastases. An MRI brain scan was also performed for surveillance which showed new ill-defined foci of enhancement within left frontal lobe, right occipital lobe and midline cerebellum and possibly small metastatic foci. Due to the disease progression, he was planned for a second line of chemotherapy with docetaxel and ramucirumab. A total of five cycles of docetaxel 60mg flat dose and ramucirumab were administered 8mg/kg (700mg) by intravenous infusion for 60 minutes every 2 weeks over a 2.5-month period. Seven days following the fifth cycle of docetaxel and ramucirumab, the patient consulted his local dentist for tongue irritation while eating, who noticed bilateral exposed bone area (bEBA) in the mandible and severe dry mouth. His treatment was discontinued at that time and he was then referred to the dental service by his treating oncologist for further evaluation and management. A thorough oral examination confirmed bEBA in the right and left postero-lingual mandibular alveolus measuring 4×5mm (Fig. 1A) and 3×3mm (Fig. 1C) in size respectively, with trailing smaller lesions and primarily located in the region of bilateral mandibular tori. The patient denied any oral trauma to these areas. There was no active purulence or acute infection seen and the bEBA was non tender on palpation. However, symptomatic ulcerative lesions were noticed on the right and left posterolateral borders of the opposing tongue measuring 2×2mm (Fig. 1B) and 8×6mm (Fig. 1D) in size, respectively, secondary to trauma from the rough bEBA. The remaining oral cavity was unremarkable. To alleviate the existing tongue irritation, bEBA was smoothened using a high-speed dental hand piece and a diamond dental bur after which patient felt immediate relief in symptoms. He was prescribed chlorhexidine gluconate 0.12% oral rinse twice daily and was instructed to keep bEBA clean. Nine days later, he presented to our dental service with continued irritation on the right side of the tongue. On examination, the ulcerative lesion on the right posterolateral border of the tongue appeared larger in size than before now measuring 6×7mm in size. The exposed bone on the right postero-lingual mandibular area was further smoothened to patient’s satisfaction. During a follow up with his treating oncologist 10 days later, he confirmed that he was feeling better as the tongue irritation had resolved and was scheduled to return later that month. Unfortunately, the patient succumbed to his disease prior to his next dental evaluation.
Figure 1.
A: A 4×5 mm area of exposed bone with trailing smaller lesions seen on the right postero-lingual mandibular alveolus.
B: A 2×2 mm ulceration seen on the right postero-lateral border of the tongue, secondary to trauma from the sharp exposed bone.
C: A 3×3 mm area of exposed bone with trailing smaller lesions seen on the right postero-lingual mandibular alveolus.
D: A 8×6 mm ulceration seen on the left postero-lateral border of the tongue, secondary to trauma from the sharp exposed bone.
The clinical presentation of bEBA following treatment with ramucirumab, an angiogenesis inhibitor (AI), in this patient was consistent with Stage 1 medication-related osteonecrosis of jaw (MRONJ) [1]. MRONJ is a well-documented complication associated with antiresorptive drugs such as bisphosphonates and denosumab, less common with other therapies [1,2,3,4]. AI are being increasingly used in the management of metastatic cancer patients including ovarian cancer, renal cell cancer, breast cancer, colorectal cancer, NSCLC, and glioblastoma multiforme [5]. The incidence of MRONJ associated with AI alone or in a combination has been reported in the literature [6,7]. The mandible is the most affected site than the maxilla, which could be attributed to poor vascularization, with a higher predilection noted for the posterior lingual mandible [8]. Exposed bone, pain and infection is common in patients with MRONJ [9]. Pimolbutr et al. reported 35 cases of MRONJ associated with AI other than ramucirumab. Most patients presented with bone exposure and subsequently had a complete resolution of MRONJ [7].
The first approval of ramucirumab by the US Food and Drug Administration was for the treatment of patients with metastatic gastric/gastroesophageal junction carcinoma [10]. This medication has now been approved for the treatment of second-line advanced or metastatic non-small cell lung cancer (NSCLC) in combination with the chemotherapy agent, docetaxel [11]. Ramucirumab (Cyramza™, IMC-1121B; Eli Lilly and Company, Indianapolis, IN, USA) is a fully human monoclonal antibody of the IgG1 class that causes blockade of angiogenesis pathways that are critical for tumor growth, proliferation tumor and metastasis of many malignant tumors including NSCLC [12]. It inhibits angiogenesis by binding with high affinity to the extracellular domain of vascular endothelial growth factor receptor −2 (VEGFR-2) inhibiting binding of VEGF receptor ligands VEGF-A, VEGF-C, and VEGF-D [13]. Observed toxicities with ramucirumab include neutropenia, hypertension, diarrhea, fatigue, febrile neutropenia, leukopenia, and hypertension [11,14,15]. Bevacizumab, the first clinically used AI, has also been associated with MRONJ [16,17]. However, ramucirumab provides a different mechanism of action compared to bevacizumab, which binds to VEGF-A and avoids the interaction of this ligand with both VEGFR-1 and VEGFR-2 receptors. Iijima et al. reported a case of dry socket condition and delayed healing (about 150 days) of the extraction socket in the right posterior mandible of the patient receiving ramucirumab for gastric cancer with liver metastasis [18].
To date, our case report is the first to describe spontaneous exposed bone in a patient treated with ramucirumab, without any previous treatment with antiresorptive or any other AI. This reinforces the need for medical and dental providers to be aware of MRONJ as a possible complication in patients undergoing metastatic cancer treatment with ramucirumab.
Funding:
This study was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Ethical approval:
This report was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center.
Informed Consent: An informed consent for clinical research was obtained from the patient.
Conflict of interest: Dr Isabel Preeshagul is consulting and advisory for AstraZeneca, Blueprint Medicines, Curio Science LLC, Dava Oncology, Healio, Lilly Oncology, Pfizer Inc. The authors have no other conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw−−2014 update. J Oral Maxillofac Surg. 2014. Oct;72(10):1938–56. [DOI] [PubMed] [Google Scholar]
- [2].Troeltzsch M, Woodlock T, Kriegelstein S, Steiner T, Messlinger K, Troeltzsch M. Physiology and pharmacology of nonbisphosphonate drugs implicated in osteonecrosis of the jaw. J Can Dent Assoc. 2012;78:c85. [PubMed] [Google Scholar]
- [3].Owosho AA, Scordo M, Yom SK, Randazzo J, Chapman PB, Huryn JM, Estilo CL. Osteonecrosis of the jaw a new complication related to Ipilimumab. Oral Oncol. 2015. Dec;51(12): e100–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vallina C, Ramírez L, Torres J, Casañas E, Hernández G, López-Pintor RM. Osteonecrosis of the jaws produced by sunitinib: a systematic review. Med Oral Patol Oral Cir Bucal. 2019. May 1;24(3): e326–e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].De Falco S. Antiangiogenesis therapy: an update after the first decade. Korean J Intern Med. 2014. Jan;29(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, Elad S, Campisi G, Tsoukalas N, Bektas-Kayhan K, Tan W, Body JJ, Migliorati C, Lalla RV; MASCC Bone Study Group. Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Support Care Cancer. 2019. Feb;27(2):383–394. [DOI] [PubMed] [Google Scholar]
- [7].Pimolbutr K, Porter S, Fedele S. Osteonecrosis of the Jaw Associated with Antiangiogenics in Antiresorptive-Naïve Patient: A Comprehensive Review of the Literature. Biomed Res Int. 2018. Apr 23; 2018:8071579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schiodt M, Vadhan-Raj S, Chambers MC, Nicolatou-Galitis O, Politis C, Coropciuc R, Fedele S, Jandial D, Zhang J et al. (2018) A multicenter case registry study on medication-related osteonecrosis of the jaw in patients with advanced cancer. Support Care Cancer 26:1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, Elad S, Campisi G, Tsoukalas N, Bektas-Kayhan K, Tan W, Body JJ, Migliorati C, Lalla RV; MASCC Bone Study Group. Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review. Support Care Cancer. 2019. Feb;27(2):383–394. [DOI] [PubMed] [Google Scholar]
- [10].Calvetti L, Pilotto S, Carbognin L, et al. The coming of ramucirumab in the landscape of anti-angiogenic drugs: potential clinical and translational perspectives. Expert Opin Biol Ther. 2015;15(9):1359–1370. [DOI] [PubMed] [Google Scholar]
- [11].Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. [DOI] [PubMed] [Google Scholar]
- [12].Cobo M, Gutiérrez V, Villatoro R, Trigo JM, Ramos I, López O, Ruiz M, Godoy A, López I, Arroyo M. Spotlight on ramucirumab in the treatment of nonsmall cell lung cancer: design, development, and clinical activity. Lung Cancer (Auckl). 2017. Jul 12; 8:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Spratlin J, Cohen R, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Camidge DR, Berge EM, Doebele RC, et al. A phase II, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol. 2014;9(10):1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Doebele RC, Spigel D, Tehfe M, et al. Phase 2, randomized, open-label study of ramucirumab in combination with first line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/ metastatic non-small cell lung cancer. Cancer. 2015;121(6):883–892. [DOI] [PubMed] [Google Scholar]
- [16].Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G 3rd, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008. Aug 20;26(24):4037–8. [DOI] [PubMed] [Google Scholar]
- [17].Kudva A, Koshy J, Jacob JG. Oral mucosal pseudotumor - Novelty complication in patient undergoing bevacizumab therapy. Oral Oncol. 2021. Nov; 122:105543. [DOI] [PubMed] [Google Scholar]
- [18].Iijima Y, Yamada M, Hino S, Sano M, Kaneko T, Horie N. Delayed Healing of Tooth Extraction Sockets with Ramucirumab Use. Case Rep Dent. 2020. Sep 30; 2020:8881749. [DOI] [PMC free article] [PubMed] [Google Scholar]