Abstract
In recent years, significant progress has been made in transdermal drug delivery systems, but there is still a search for enhancers that can improve the absorption of active substances through the stratum corneum. Although permeation enhancers have been described in the scientific literature, the use of naturally occurring substances in this role is still of particular interest, because they can offer a high level of safety of use, with a low risk of skin irritation, and high efficiency. In addition, these ingredients are biodegradable, easily available, and widely accepted by consumers due to the growing trust in natural compounds. This article provides information on the role of naturally derived compounds in transdermal drug delivery systems that help them penetrate the skin. The work focuses on the components found in the stratum corneum such as sterols, ceramides, oleic acid, and urea. Penetration enhancers found in nature, mainly in plants, such as terpenes, polysaccharides, and fatty acids have also been described. The mechanism of action of permeation enhancers in the stratum corneum is discussed, and information on the methods of assessing their penetration efficiency is provided. Our review mainly covers original papers from 2017 to 2022, supplemented with review papers, and then older publications used to supplement or verify the data. The use of natural penetration enhancers has been shown to increase the transport of active ingredients through the stratum corneum and can compete with synthetic counterparts.
Keywords: penetration enhancers, raw materials, skin barrier, terpenes, fatty acids, polysaccharides
1. Introduction
Transdermal drug delivery (TDD) systems are one of the most widely researched pharmaceutical products.1 TDD is a convenient alternative to intravenous, intramuscular, and oral routes of administration. The transdermal route avoids the first-pass effect in the liver making this method suitable for drugs that have low bioavailability when administered through the oral route or exhibit adverse effects due to biotransformation.2 This method also has the advantage of being painless, noninvasive, and easy to apply and having controlled release (modified release) which can prolong the therapeutic effect.3 Nevertheless, the skin acts as an external barrier to prevent exogenous compounds from entering the body, including drugs.4 This presents a significant challenge for TDD researchers in the development of methods to penetrate the top layer of the epidermis: the stratum corneum (SC).5 The nonpolar, hydrophobic nature of the SC and the physiological property of being permeated by only those particles whose atomic weight does not exceed 500 Da substances makes polar, hydrophilic particles cross this barrier rarely or not at all.6 An intact SC is crucial in maintaining legitimate skin function. Its dysfunctions are seen during dermatological diseases, such as atopic dermatitis or ichthyosis.5 Transdermal drug delivery systems should be applied to healthy skin, so methods are being sought that will temporarily interact with the components of the epidermis, temporarily changing its permeability to therapeutic substances, at the same time not changing the activity of the active substance, not causing its degradation, and not causing skin irritation and permanent skin damage.7
The key to producing an effective transdermal system is the right choice of the type of formulation. The degree of drug diffusion through the skin is influenced by the type of TDD formulation, i.e., ointment, cream, gel, or transdermal patch.8,9 These formulations include different types of gels (hydrogels, organogels, bigels, emulgels, and nano gels), emulsions, (microemulsions, nanoemulsions, and multiple emulsions), and liquid crystals, which are intermediate between the solid and liquid form.10−12 Furthermore, the development of nanotechnology has enabled the incorporation of APIs into carriers such as liposomes, niosomes, nanostructured lipid carriers, solid lipid nanoparticles, polymer nanoparticles, micelles, dendrimers, carbon nanotubes, etc.13,14
Moreover, active and passive methods are used to facilitate TDD. Active methods involve the use of external energy to increase the penetration of active pharmaceutical ingredients (APIs) through the skin by using electrical energy (iontophoresis, electroporation), ultrasound (sonophoresis), radiofrequency electromagnetic waves (radiofrequency), and laser energy.15−20 These methods include mechanical techniques such as micropuncture to create pores in the SC for APIs.21−24 Passive methods involve the interactions between the drug, vehicle, and SC layer. They aim to modify the API physicochemical properties, e.g., by changing its solubility or ionization and/or leading to an increase in skin permeability. Given this, eutectic systems, prodrugs, ion-pair technique, and supersaturated systems are used. Developments in pharmaceutical and chemical sciences have now made it possible to use chemical compounds that, by interacting with SC components, improved the permeability of drugs.25,26
The eutectic system is a mixture of two or more substances formed by entropy changes associated with both hydrogen bonds and van der Waals forces. These interactions result in a mutual decrease in the melting point of the mixture with respect to each of its individual components.11,27,28 The pro-drug technique is based on the modification of a substance’s active molecules by attaching a lipophilic group, such as an ester group, to create a compound that is more easily partitioned between the SC and the pharmaceutical formulation causing a change in the partition coefficient (LogP).29−32 The so-called ion-pair technique which involves neutralization of the electrical charge of the active substance by through formation its salt is also used.33−35 Supersaturated systems allow for faster transdermal penetration of the drug, as they have increased thermodynamic activity.36
Among chemical compounds that increase the permeability of the skin to drugs are those otherwise known as chemical penetration enhancers (CPEs) or sorption promoters: terpenes, terpenoids, sulfoxides, laurocapram (Azone), pyrrolidones, fatty acids, fatty alcohols, alcohols containing glycols, urea, and surfactants.25,26 In 2021, 649 compounds classified as CPEs were collected in a database which included the following groups of chemicals: alcohols and polyols, lactams and their analogues (azepane, azone, caprolactam, morpholine, piperazine, piperidine, piperidone, pyrrolidine, pyrrolidone, and succinimide), esters and ethers, fatty acids, terpenes and steroids, and miscellaneous additives such as amino acids, aliphatic compounds, aromatic compounds, and inorganic compounds.37 However, some of the listed groups of compounds, primarily morpholine and morpholine derivatives and among them Azone,38 surfactants,39 aromatic compounds,40 and many others,38 carry the risk of causing skin irritation, permanent disorganization of the skin barrier, and toxic effects on skin cells. These effects may not be acceptable in the application of transdermal drug delivery systems to the skin. High potential for safe use and a low risk of skin irritation is presented by transdermal formulations based on substances of natural origin. In addition, these ingredients are biodegradable, readily available, and widely accepted by consumers due to growing reliance on natural occurring compounds.41,42 These advantages make them commonly used in cosmetic and pharmaceutical formulations. They are increasingly used as substrates for the application of active ingredients43,44 and as well as compounds that facilitate the penetration of other substances, including APIs.45,46
The most popular permeation enhancers that occur in SC are sterols, ceramides, fatty acids (oleic acid), and urea. The second group of natural enhancers that occur in nature, mainly in plants, are fatty acids and terpenes. The main sources of fatty acids are plant oils, while terpenes and terpenoids are the main components of essential oils.47−49
Plant oils possess skin barrier restoration and regenerative features, as well as antioxidant, and anti-inflammatory properties.50 They are valuable ingredients in pharmaceutical and cosmetic products, where they act as both active substances43,44 and compounds that facilitate the penetration of other substances, including medications.45,46 They are considered nontoxic and safe for topical use. Some divergence has been observed in the effects of plant oils on the skin. While sunflower seed oil contributes to improving the hydrolipidic layer of the skin, olive oil negatively affects the integrity of SC components.51 It has been suggested that these properties depend on the ratio of oleic to linoleic acid in the oil composition, as only the former contributes to an increase in SC permeability, facilitating the penetration of the therapeutic substance through the skin.44,52,53 Not without significance is the content of the unsaponifiable fraction in oils, which include compounds such as triterpene alcohols, squalene, phytosterols, flavonoids, and phospholipids, which may also potentially affect the barrier properties of the epidermis.44
Essential oils contain medicinal properties such as antiseptic, antiparasitic, antiviral, antifungal, and antibacterial activities.54 However, essential oils carry a risk of skin irritation if used undiluted or in too high of a concentration. In topical preparations, essential oils are used in concentrations between 0.5–5% and sometimes up to 10%, depending on the specific oil.55
It should be noticed that by using them in pharmaceutical and cosmetic products for external application as a base and/or active substance their disorganizing effect on the skin is overlooked. Consequently, a given preparation may have unforeseen and undesired effects on the skin, such as increased transepidermal water loss and skin inflammation. When used in daily skin care, these compounds can have the above-mentioned adverse effects, but on the other hand, they can be a component of the transdermal therapeutic systems, using their disorganizing effect on the protective barrier to facilitate the penetration of active substances. Therefore, it was deemed necessary to report the role of these natural compounds used in systems that facilitate the penetration of APIs through the skin.
This article focuses on the role of individual substances in enhancing the penetration of APIs. The use of natural substances as penetration enhancers of active substances in TDD systems is in line with the trend of using naturally occurring raw materials in pharmaceutical and cosmetic formulations.41,42 Their popularity is due to their easy availability. In addition, these compounds are considered environmentally safe and biodegradable.
The mechanism of action of natural penetration enhancers is important in determining the safety of the different ingredients. This information is particularly important for pharmaceutical and cosmetic manufacturers, as it helps in the selection of ingredients derived from natural raw materials to be used in transdermal systems. This paper provides information on which components should be avoided when a restorative effect on the epidermal barrier is needed. This is important in the pathogenesis of many dermatological defects.
Our literature review demonstrates the relationship between natural substances used in TDD systems and their effects on the SC in terms of functional changes, as well as highlighting the analytical methods used to assess these changes. The electronic databases such as Scopus, PubMed, and Medline formed the basis of the information search.
The databases were searched from March 15, 2022, to July 1, 2022, with papers from 2017–2022 considered first, followed by older publications to supplement or verify the data. Information was supplemented in the period from March 1 to March 15, 2023. Only publications in English were included. Our review consisted of mainly original papers that were supplemented with review papers. The search method for scientific articles consisted of entering keywords ranging from general ones such as TDD systems, chemical penetration enhancers, natural chemical penetration enhancers, SC, skin barrier, and biological barriers, to more specific ones such as ceramides, sterols (especially cholesterol and cholesterol sulfate), fatty acids, terpenes, urea, vegetable oils, essential oils, and polysaccharides, linking them together with AND and OR logical connectors. These substances were chosen because they naturally occur in the SC or are commonly present as active ingredients in plants. The safety of their use is well-defined, but information on their use and benefits is not obvious. The present article gives information on the concentrations of the applied promoters, the APIs that can be used with natural enhancers, and the effects that can be obtained by using the APIs together with the absorption promoters (Table 1).
Table 1. Efficacy of Applying Natural Permeation Enhancers with the Active Substances Used in TDDs.
| no. | API | isolated permeation enhancer or oil from a natural orign | substances naturally present in the skin | permeation enhancer in drug formulations | research type | research model | research technique | concentration of the enhancer | efficiency | refs |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | theophylline | CER NS (C6) | in vitro | model lipid membranes | Franz diffusion cells | a | (56) | |||
| indomethacin | ||||||||||
| 2 | ethyl-p-aminobenzoate | CER NS (C16) | in vitro | model lipid membranes | PermeGear in-line diffusion cells | a | (57) | |||
| 3 | interferon alfa-2B | oleic acid | in vitro | full thickness human breast skin | in-line Bronaugh flow-through diffusion cells | 10% | c | (58) | ||
| 4 | urea | oleic acid | in vitro | model lipid membranes | Franz-type diffusion cells | 10% | b | (59) | ||
| 5 | caffeine | |||||||||
| 6 | diclofenac sodium | |||||||||
| 7 | insulin | oleic acid | in vitro | rat skin | Franz-type diffusion cells | 4.2 μL | b | (60) | ||
| 8 | insulin | linolenic acid | c | |||||||
| 9 | caffeine | urea | in vitro | porcine skin | Franz-type diffusion cells | 10% | a | (61) | ||
| 10 | haloperidol | limonene | in vitro | epidermal membranes from abdomal human skin | Franz-type diffusion cells | 5% | a | (62) | ||
| 11 | ligustrazine hydrochloride | menthol | in vitro | porcine skin | Franz-type diffusion cells | 3% | a | (63) | ||
| 12 | indometacin | camphor | in vitro | rat skin | TK-20B diffusion apparatus | 3% | a | (64) | ||
| 13 | lidocaine | |||||||||
| 14 | aspirin | |||||||||
| 15 | antipyrine | |||||||||
| 16 | Tegafur | |||||||||
| 17 | 5-fluorouracil | |||||||||
| 18 | osthole | borneol | in vitro | rat skin | Franz-type diffusion cells | 1.2% | a | (65) | ||
| 19 | theophyline | 6-(dimetyloamino)heksanian cytronellyl | in vitro | epidermal membranes from abdomal human skin | Franz-type diffusion cells | 30 mM concentration | a | (66) | ||
| 20 | hydrokortyzon | |||||||||
| 21 | naproxen sodium | Lavandula angustifoli oil | in vitro | rat skin | Franz-type diffusion cells | 0.5% | a | (67) | ||
| 22 | ibuprofen | Radix angelicae sinensis oil | in vitro | rat skin | Franz-type diffusion cells | 3% | a | (68) | ||
| Rhizoma chuanxiong oil | ||||||||||
| Rhizoma cyperi oil | ||||||||||
| Cinnamomum cassia oil | ||||||||||
| Flos caryophylli oil | ||||||||||
| 23 | 5-fluorouracil | Sinapis alba oil | in vitro | rat skin | Franz-type diffusion cells | 5% | a | (69) | ||
| 24 | Paeonol | |||||||||
| 25 | Osthol | |||||||||
| 26 | ibuprofen | Epilobium angustifolium oil | in vitro | porcine skin | Franz-type diffusion cells | a | (70) | |||
| 27 | lidocaine | |||||||||
| 28 | caffeine | |||||||||
| 29 | donepezil hydrochloride | Ledum palustre oil | in vitro | rat skin | horizontal diffusion cells | 10% | a | (71) | ||
| 30 | dihydroquercetin | soybean oil | in vitro | abdomal human skin | Bronaugh-type flow-through diffusion cells | 0.5% | a | (72) | ||
| olive oil | ||||||||||
| 31 | flurbiprofen | olive oil | in vitro | abdomal human skin | Franz-type diffusion cells | 20% | a | (73) | ||
| 32 | diltiazem hydrochloride | Mesua ferrea oil | ex vivo | porcine skin | Keshary–Chien glass | 15% | a | (74) | ||
| 33 | trans-resveratrol | Punica granatum oil | in vitro | porcine skin | Franz-type diffusion cells | 2.5–10% | a | (75) | ||
| 34 | caffeine | Hibiscus rosa-sinensis L. leaves mucilage | in vitro | rat skin | vertical diffusion cells | 2% | a | (76) | ||
| 35 | minoxidil | nanoemulsion with oleic acid or eucalyptol | in vitro | epidermal membranes from abdomal human skin | Franz-type diffusion cells | 14.63–15.93% | a | (77) | ||
| 36 | caffeine | nanoemulsion with oleic acid or eucalyptol | in vitro | epidermal membranes from abdomal human skin | Franz-type diffusion cells | 14.63–15.93% | a | (78) | ||
| 37 | naproxen | |||||||||
| 38 | caffeic acid from propolis (Apis trigona) | nanoemulgel with oleic acid | in vitro | rat skin | Franz-type diffusion cells | 1.25–2.5% | a | (79) | ||
| 39 | 2,3,5,4′-tetrahydroxystilbene 2-O-β-d-glucoside | vesicles with oleic acid | in vitro | porcine skin | TP-6 Franz diffusion cell | 32 μL | a | (80) | ||
| 40 | recombinant human growth hormone | complex urea/hydroxy propyl-beta cyclodextrin | in vitro | rat skin | Franz-type diffusion cells | 12.1% | a | (81) | ||
| 41 | methotrexate | nanoemulsion containing chaulmoogra oil | in vitro | rabbits skin | Franz-type diffusion cells | 84.54 μg/mL | a | (82) | ||
| 42 | diltiazem hydrochloride | Ficus reticulata L. fruit mucilage as a matrix in transdermal patches | in vitro | rat skin | Keshary–Chien diffusion cell | 5–25% | a | (83) | ||
| 43 | diclofenac sodium | Ficus carica L. fruit mucilage as a matrix in transdermal patches | in vitro | rat skin | Keshary–Chien diffusion cell | 4–20% | a | (84) | ||
| 44 | diltiazem hydrochloride | complex Colocasia esculenta (taro) corms mucilage/hydroxypropylmethylcellulose as a matrix in transdermal patches | in vitro | dialysis cellulose membrane | Franz-type diffusion cells | 0.5–2% | a | (85) | ||
| 45 | isoliquiritigenin | hydrogels containing complex hyaluronic acid/hydroxyethyl cellulose | in vitro | rat skin | Franz-type diffusion cells | a | (86) | |||
| 46 | luteolin | hydrogels containing complex hyaluronic acid/poly(N-isopropylacrylamide) | in vitro | micropig dorsal skin | Franz-type diffusion cells | a | (87) | |||
| 47 | bovine serum albumin | hydrogels with hyaluronic acid | in vitro | pig skin | Franz-type diffusion cells | 5% | a | (88) | ||
| 48 | ketoprofen | liposomes with hyaluronic acid | ex vivo | pig skin | Franz-type diffusion cells | 1% | a | (89) | ||
| 49 | rhodamine B | ethosomes with hyaluronic acid | in vitro | rat skin | Franz-type diffusion cells | a | (64) | |||
| 50 | curcumin | ethosomes with hyaluronic acid | in vitro | mouse skin | vertical diffusion cells | a | (90) | |||
| 51 | tacrolimus | polymeric nanoparticles with hyaluronic acid | ex vivo | mouce skin | Franz-type diffusion cells | 0.1–0.5% | a | (91) | ||
| 52 | betamethasone valerate | polymeric nanoparticles with hyaluronic acid | ex vivo | rat skin | Franz-type diffusion cells | 1–6 mg/mL | a | (92) |
Improved drug penetration.
There was no significant change in the drug’s permeation.
Reduced drug penetration.
2. Characterization of the Epidermal Barrier in Terms of APIs Delivered by the Transdermal Route
Studies evaluating the permeation efficacy of substances applied to the skin surface for potential use in TDDs most often refer to the SC region. Research aimed at understanding the mechanism of action of permeation enhancers at a molecular level is particularly important. For this purpose, a brief description of the structure of the SC is necessary.
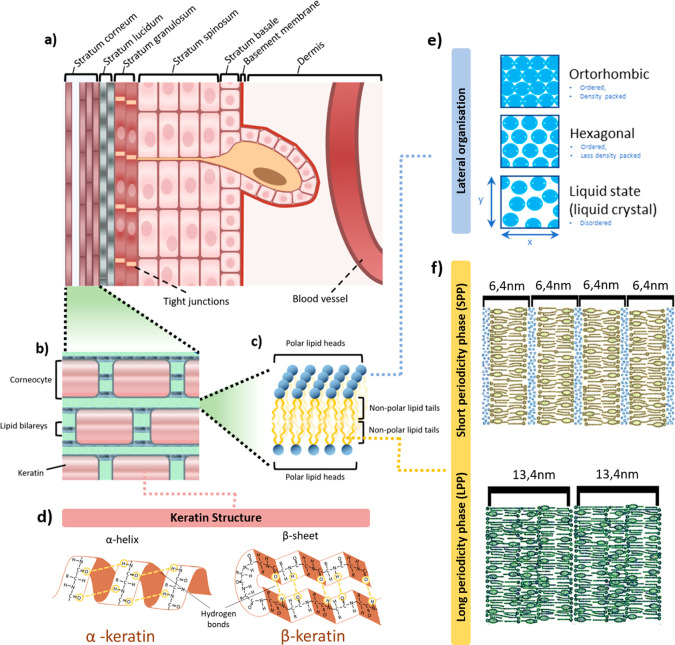
The SC is 10–20 μm thick and consists of 15–20 layers of flattened, densely packed keratin-filled corneocytes separated by a lamellar intercellular lipid system (Figure 1a,b). The remainder of the epidermis is 50–120 μm thick of stratified squamous epithelium in which no blood vessels are present. It consists of a lamina propria, granular layer, squamous layer, and basement membrane separating the epidermis from the dermis.93
Figure 1.
Skin structure. (a) Structure of the epidermis. (b) Schematic diagram of the structure of the SC. (c) Schematic diagram of the arrangement of lamellar lipids. (d) Schematic structure of keratin. (e) Three packing forms of the barrier lipids: orthorhombic, hexagonal, or liquid state (liquid crystal) arrangement. (f) Repeated phases of lamellar lipids: LPP and SPP with a water layer (highlighted in blue) between the polar lipid heads.
Small-angle X-ray scattering data obtained by Bouwsta et al. indicates that the SC lamellar lipid system can be divided into two recurring, characteristic phases, namely, a 6.4 nm short periodicity phase (SPP) and a 13.4 nm long periodicity phase (LPP)94 (Figure 1f). In the SPP, the lipid hydrocarbon chains can form three packing forms with the cross-section of the hydrocarbon alkyl chains indicating an orthorhombic, hexagonal, or liquid state (liquid crystal) arrangement (Figure 1e). The hexagonal and liquid crystal forms are characterized by greater mobility of the hydrocarbon chains as they are loosely packed and therefore have greater mobility and permeability compared to lipid chains in a orthorhombic arrangement.95,96 The thickness of the SPP depends on the amount of water contained in the SC, as with increasing hydration, up to 60% w/w, the length of the SPP increases linearly indicating the formation of a new aqueous phase between the SC lamellar lipid layers (directly affecting the increase in SPP phase length).94 Phase separation in the lamellar lipid region can lead to a rearrangement of the hydrocarbon lipid chains into micelles.
The study by Ogawa et al.97 cited previous findings98 and confirmed that a hydrated SC results in increased permeability to hydrophilic APIs. A study by Yamamoto et al.99 discussed the important role of the inhibition of transepidermal water loss through occlusion in the penetration of ketoprofen. Physiologically, the water content of the epidermis decreases from the viable epidermal layers of the stratum spinosum and stratum granulosum (about 70% by weight) to the SC. On average, the water content in the SC is 25%, but authors have reported different results which may have been influenced by measurement conditions as well as the individual nature of the epidermal barrier.100
Protein components account for 60–85% of the weight of SC. They are formed by keratin fibers, among which acidic type I keratin and neutral to basic type II keratin can be identified. Acidic keratins have a greater number of negatively charged amino acid side chains, such as aspartic or glutamic acid. Basic proteins have a greater number of positively charged side chains such as lysine, arginine, or histidine.101 It is known that under the influence of increasing humidity, the secondary structure of keratin changes from an α- to a β-helix conformation (Figure 1d). Keratin in α-helix conformation has a coiled-coil structure in which the side chains do not interact with water molecules.102 In the β-helix conformation, the side chains are exposed to the main chain of the protein so that water molecules have access to the peptide bonds and bind to them through hydrogen bonds.102,103
A study by Jokura et al. provided information that water molecules not bound to corneocyte proteins are part of the natural moisturizer factor (NMF).104 NMF further consists of amino acids, urea, lactic acid salts, pyroglutamic acid (PCA) and its salts, sugars, sodium, magnesium, potassium, calcium, chlorine, and phosphate ions.105,106 NMF form ionic bonds to keratin fibers which alter the elasticity of keratin by reducing intermolecular forces.104
The lipid mixture of the SC, organized in a double layer, provides protective properties to the epidermis and at the same time forms a barrier to API diffusion. It consists of ceramides (40–50% by weight), cholesterol (20–30%), cholesterol sulfate (2–5%), and free fatty acids (7–13%).107 Ceramides are particularly important in providing a barrier function to the epidermis. Ceramides in the SC are constructed from a sphingoid base, which can be sphingosine, dihydrosphingosine, or phytosphingosine and 6-hydroxysphingosine linked by an amide bond to the acyl residue of a fatty acid which can be nonsubstituted, α-hydroxylated, or ω-hydroxylated and contain an ω-linoleoyl group.108−111 Recently, 1-O-acylceramides have also been identified in the human SC, where their esterified fatty acid is attached to the headgroup of sphingosine in position 1. This component is described as one of the key components of the SC because its deficiency leads to impaired protective properties and increased water loss.112 It is generally accepted that ceramides are composed of a small polar head and two simple saturated hydrophobic aliphatic chains. The polar part (polar fragment, the head) consists of 2–4 hydroxyl groups and an amide group. This structure ensures the formation of a strong hydrogen bonding network that maintains the stability of the lamellar structure and the strength and barrier properties of SC.113,114
The free fatty acids in human SC generally consist of saturated (chain lengths ranging from C14:0 to C34:0) and unsaturated (C16:1 to C18:1, C30:1 to C36:1, C18:2) fatty acids.115 The most abundant group of fatty acids present in the SC (>50% of all SC fatty acids) are saturated, linear chain lipids with 16 to 30 carbon atoms with the most common being lignoceric acid (C24:0) and hexacosanoic acid (C26:0).115−117
The SC is not only a physical barrier, as its protective properties are enhanced by chemical and immunological factors. Scientific literature indicates that the presence of the skin microbiome, which forms the natural physiological flora and is responsible for both chemical and immunological factors.118 The skin’s physiological flora consists of microorganisms such as Corynebacterium species, Cutibacterium acnes, coagulase-negative Staphylococcus species including Staphylococcus epidermidis, and Malassezia spp. The important role of the skin microbiome in creating the skin’s protective barrier is supported by the fact that it promotes the differentiation and integrity of the epithelium. Additionally, some skin bacteria secrete sphingomyelinases, which are responsible for the production of ceramides in the SC.119 Furthermore, microorganisms that colonize the skin produce lipase enzymes that break down sebum triglycerides into free fatty acids. These free fatty acids strengthen the acidic nature of the skin, which limits the colonization of pathogenic microorganisms on the skin.120 The physiological pH of the skin surface is acidic, falling within the pH range of 4.1 to 5.8. However, as one moves closer to the living layers of the epidermis, the pH increases to neutral levels of pH 7 to 7.4. In a low pH environment, fatty acids exist in a nonionized form, which causes minimal repulsion of lipid head groups and promotes the formation of lamellar structures, ultimately affecting the integrity of the protective barrier. Increased pH of the skin surface is associated with impaired function of the protective barrier of the SC.121
To summarize, the SC forms a hydrophobic, nonpolar protective barrier, which is determined by the homeostatic composition and quantity of the individual components of the SC. Fluctuations in the quantity of SC components as well as increased hydration of this layer significantly alter its properties. Regarding the transdermal administration of APIs, the information above is a valuable clue for researchers, as it encourages the search for permeation-enhancing agents that can temporarily affect the chemical composition and hydration of the SC. By doing so, the permeability of the SC to APIs can be increased, facilitating their transdermal delivery.
3. Natural Components of the SC Are Compounds That Regulate the Permeability of APIs through the Skin
Substances applied externally that penetrate the structure of the SC interact with its components. Substances containing compounds that physiologically occur in the SC, such as ceramides, cholesterol and its sulfate, and fatty acids affect the proportions of their counterparts resulting in supplementation of their deficiencies.122−128 Conversely, they may disrupt the natural quantitative balance of lipid components to induce an increase in skin permeability. It has been found that changes in the composition and chain length of fatty acids present in their free forms as well as bound to SC ceramides change their melting point and membrane permeability.129 The potential to modulate the barrier properties of the SC appears to be helpful in assisting the transport of APIs across the skin.130
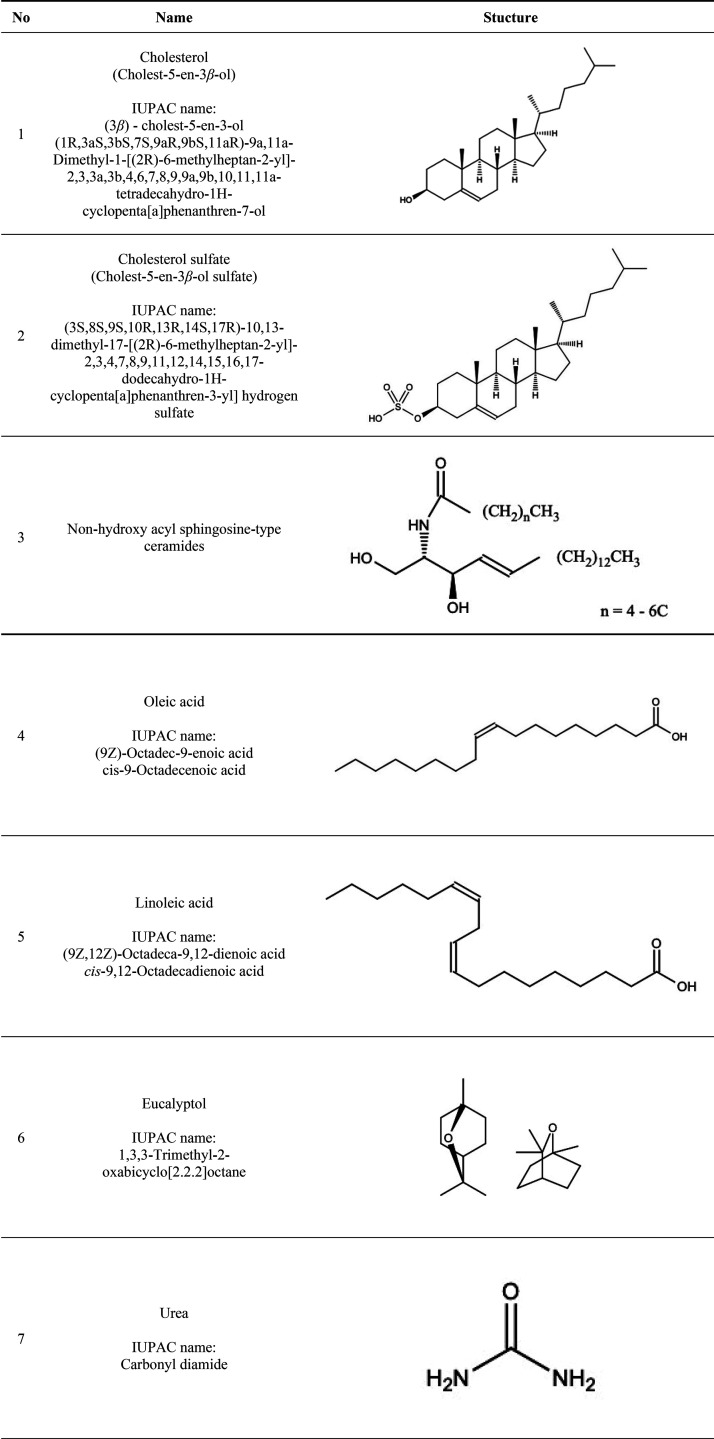
3.1. Sterols
In the SC, the ratio of cholesterol (Table 2, item 1) to cholesterol sulfate (Table 2, item 2) is important in maintaining the SC barrier, as it is indicated that with increasing amounts of cholesterol sulfate, an increased fluidity of the lipid fraction is observed leading to a greater permeability of the SC,131,132 while at the same time, the chains of fatty acid residues or chains of acyl groups and ceramides in the SC remain rigid.131,132 Cholesterol sulfate, by weakening the barrier functions, may serve as a substance that facilitates API permeation.132 The explanation for the observed effects was based on an analysis showing that the polar, acidic, sulfate group of cholesterol sulfate exhibits a stronger hydrogen bonding capacity compared to the rest of the nonpolar, hydrophobic components of the lipid matrix. Cholesterol sulfate groups, as a result of repulsive electrostatic forces and the solvation effect of charged atoms, increase the hydration region between lipids creating a pathway for hydrophilic APIs.131,132 Furthermore, cholesterol sulfate is a highly amphiphilic molecule with the ability to penetrate cell membranes by diffusion.133
Table 2. Structural Patterns of Natural SC Components Regulating Skin Permeability of APIs (Entries 1–5 and 7) and Natural Compounds with the Permeation-Enhancing Potential of APIs (Entries 6 and 8–16).
3.2. Ceramides
Current scientific research does not directly describe topically applied ceramides as penetration enhancers for APIs, but their role in modulating the barrier properties of the epidermis has been confirmed. Studies have evaluated the influence of the fatty chain and the size of the polar head of ceramides on the permeability of externally applied substances, which suggests the potential for ceramides as future penetration enhancers for APIs.113,134−136 In healthy human SC, ceramides contain fatty acid residue chains of more than 22 carbon atoms (Table 2, item 3).108,137,138 Shorter ceramide chains are observed in dermatoses that damage the skin barrier, such as atopic dermatitis, lamellar ichthyosis, Netherton syndrome, psoriasis, and autosomal recessive congenital ichthyosis.113,134−136 The effect of ceramide acyl chain length on SC barrier integrity has been confirmed in studies,139−141 in which it was observed that replacing the acyl chain in nonhydroxy acyl sphingosine-type ceramides (CER NS) of C24 length with a shorter one (C4–6) resulted in a significantly increased SC lipid permeability. An in vitro permeability study by Školova et al.56 using Franz diffusion cells of 5% theophylline (180 g/mol, LogP = 0) and 2% indomethacin (358 g/mol, LogP = 4.3) showed that replacement of CER NS (C24) with CER NS (C6) resulted in an increase of approximately 3.5 times the permeation of theophylline and nearly 6.5 times that of indomethacin. The paper by Uche et al.57 evaluated the effect of substituting a CER NS with a 16-carbon chain at the expense of CER NS (C24) on lipid membrane permeability of ethyl-p-aminobenzoate (E-PABA). An in vitro study using PermeGear in-line diffusion cells showed that the amount of API permeated through the lipid membrane increased with CER NS (C16) concentrations to 3.5 times more API at 50% CER NS (C16) concentrations and 6 times more API at 75% CER NS (C16) concentrations compared to long-chain ceramide membranes. The increased permeability of the model lipid membrane consisting of ceramides, fatty acids, cholesterol, and cholesterol sulfate was accompanied by an increased distance between the LPP and a change in the spatial arrangement of the lipids, as some of the lipid chains formed a hexagonal phase at the expense of the rhomboid phase as confirmed by SAXS (small-angle X-ray scattering). The increased space between the SC lipid layers and decreased packing density of the lipid chains explain the easier penetration of APIs. Table 1 (items 1 and 2) presents the permeation effectiveness of APIs used with ceramides in TTDs.
3.3. Fatty Acids
3.3.1. Oleic Acid
The most frequently used fatty acid in TDD systems is the unsaturated oleic acid (OA), cis-octadecenoic acid (Table 2, item 4). An ex vivo study on rat skin using Raman spectroscopy provided information on the disorganizing effect of OA on SC lipid conformations.53 The double bond in the configuration cis of the OA structure, disrupts the organization of the alkyl chains of the lamellar lipids leading to separation and increased fluidization of the lipid bilayers.142,143In vitro study using in-line Bronaugh flow-through diffusion cells showed that the flow of the protein interferon alpha-2B drug (19 kDa) through the SC was not enhanced in the presence of a solution containing 10% OA in propylene glycol. However, a separate liquid phase in the SPP region of the lamellar lipids was simultaneously observed in an ex vivo study on human skin using small- and wide-angle X-ray scattering analysis, which formed a potential pathway predisposing to the penetration of API molecules.58 Research into the mechanism of action of OA dates back to the 1990s, when Naik’s research team used attenuated total reflectance infrared spectroscopy. It was shown that a 5% solution of OA in ethanol applied to the human skin in vivo creates a separate liquid phase leading to the separation of the lamellar lipid bilayers and causes fluidization. This finding indicated that an observed effect of changing the conformation of epidermal lipids predisposes it to the permeation of small molecules.144 Similar conclusions were presented by Jiang et al. following an in vivo study on rat skin in which 10% OA in propylene glycol was applied.145
An in vitro permeation study using Franz-type diffusion cells with a lipid membrane mimicking the quantitative and qualitative composition of human SC in the presence of 10% OA showed that, of the three model drugs used, 10% urea (60.06 g/mol, LogP = −2.11), 2% caffeine (194.19 g/mol, LogP = −0.07), and 0.1% diclofenac sodium (318.14 g/mol, LogP = 4.28), urea was the most hydrophilic substance and had the fastest diffusion rate. However, there were no differences in lipid membrane permeability in the presence and absence of OA, indicating the natural penetrating properties of urea.59 Therefore, it can be inferred that OA does not provide significant API permeation-enhancing properties, despite its disorganizing effect on lipid fractions. The in vitro permeation effect on Franz diffusion cells using a rat skin-insulin model was also assessed with three different fatty acids: OA (C18:1, LogP: 7.64), linoleic acid (C18:2, LogP = 7.05), and linolenic acid (C18:3, LogP = 6.46). OA was shown to have the best permeation enhancement profile of these three fatty acids, but not enough to be considered an insulin permeation facilitator. Linolenic acid (Table 2, item 5) reduced the permeation of insulin through the skin,60 which correlates with reports by other authors on the lipid-barrier-rebuilding properties of this fatty acid.51,146 The paper explains that this phenomenon is due to the lower oil/water partition coefficient, higher structural rigidity related to the number of double bonds, and higher surface tension of linolenic acid compared to acids with fewer double bonds.60
Currently, OA has also started to be used as a component of carrier formulations, e.g., in micro- or nanoemulsions, whose dermal absorption-enhancing properties for APIs are used in various medical areas.147−151
The work of Abd et al.77 evaluated an in vitro Franz diffusion cell model mimicking human skin to determine the permeation efficiency of 2% minoxidil (LogP = 1.24) incorporated into an oil-in-water (o/w) nanoemulsion with OA or eucalyptol (Table 2, item 6) entering the oil phase. Both nanoemulsion formulations (the one with OA and the one with terpene) were shown to increase the amount of API penetration through the SC and its solubility compared to the API alone, with a greater efficacy observed in the formulation containing eucalyptol. Nanoemulsions with OA and eucalyptol were also found to be effective in increasing the permeation of 3% caffeine and 2% naproxen.78
A nanoemulgel with OA was used to deliver caffeic acid from propolis (Apis trigona). An in vitro permeation study on Franz diffusion cells through rat skin showed that the highest API retention was obtained using OA at a concentration of 2.5%, compared to 5% and 1.25%.79 Lai et al.80 published results showing a 4-fold more effective (compared to a control trial without carriers) delivery of 2,3,5,4′-tetrahydroxystilbene 2-O-β-d-glucoside (extracted from Polygonum multiflorum) using OA-containing vesicles loaded into a complex gel. The permeation-enhancing properties of OA were modified by the use of carrier and gel forms in TDD systems. Table 1 (items 3–8 and 35–39) presents the permeation effectiveness of APIs used with oleic acid in TTDs.
3.4. Urea
Urea, also known as carbamide, is an acyclic, polar compound containing a carbonyl group attached to two amine groups (Table 2, item 7). It is naturally included in human NMF (7% of NMF composition).152,153 It is a compound that exhibits hygroscopic and keratolytic properties in a concentration-dependent manner. In formulations for external application, a 2–10% concentration shows a moisturizing effect, a 10–20% concentration shows a moisturizing, keratolytic, and API-permeation-enhancing effect, and a 30–50% concentration no longer shows a moisturizing effect.153,154
An in vitro human epidermal barrier permeation study on Franz diffusion cells under infinite dosing conditions showed that urea had a higher permeation rate than more hydrophilic substances like glycerol and mannitol but less than the more lipophilic estradiol. In comparison, under finite dosing conditions, the amount of urea permeated was the highest among substances used in the study, which according to the authors was related to the difference in the skin hydration status between the two experiments, i.e., fully hydrated skin in the infinite dosing technique and partially hydrated skin in the finite dosing technique, the type of solvent, and the interactions between it and the substance and membrane used for the study.155 The differences between in vitro testing under infinite dosing and finite dosing conditions were described by Franz,156 which indicated that greater reliability of in vitro test results is obtained with the finite dosing technique. Intarakumhaeng et al. found that nonvolatile substances with a molecular weight up to 60 g/mol, as in the case of urea, show a relatively high percentage of a penetrating dose.155
The mechanism of action responsible for the potential permeation enhancement of SC proposed by Mueller et al. is based on its ability to bind water and import it into the corneocytes. A large amount of bonded water can lead to an increase in cell volume of up to 50% and an increase in osmotic pressure.157 Water can also accumulate between corneocytes in the SC, which can affect the barrier function of the skin. In the lipid bilayer region, water can also disrupt the local electrostatic interactions and lead to the formation of pores in the lipid membrane. This can occur due to the reorientation of hydrophilic lipid headgroups toward the center of the bilayer and the formation of inverted micelles.158,159
Additionally, varying the water content of the SC can potentially alter the degree of ionization of fatty acids, which are commonly assumed to exist in a nonionized form.159 NMF results published by Pham et al. confirmed that the final segments of the keratin structure, on which glycine and serine residues are located, are fluidized in the presence of urea.160 Urea is identified in the literature as a chemical “denaturant” that alters the structure of proteins indirectly by affecting the hydrogen bond network of bound water at the boundary of the protein.161−163 Another concept concerning the denaturing effect of urea has also been proposed, stating that urea directly interacts with the protein by breaking intramolecular hydrogen bonds.164
In an in vitro study, a gel with 10% urea increased transdermal caffeine delivery by almost 50%, at a level similar to the permeation-enhancing effect of the surfactant sodium laureth sulfate. Furthermore, the gel formulation appeared to be more effective in promoting caffeine absorption than the use of an emulsion.61 Shams et al.81 used urea at a concentration of 2 M (12.1%) together with the cyclodextrin derivative hydroxy propyl-β cyclodextrin (HP-β-CD) to deliver recombinant human growth hormone (rhGH) to the deeper layers of the skin. The use of two permeation enhancers showing a synergistic effect had a positive effect on the delivery of the API, with the greatest amount of substance permeated after 120 min. The synergistic effect was due to the protective effect of HP-β-CD on the structure of rhGH in the presence of urea, such that without HP-β-CD the activity of the hormone decreased. Higher concentrations (4–8 M) of urea resulted in significantly lower levels of rhGH in the skin, which, according to the authors, could be due to the denaturing effect of urea. Table 1 (item 9) presents the permeation effectiveness of APIs used with urea in TTDs.
4. Natural Compounds with the Penetration-Enhancing Potential of the API
4.1. Terpenes
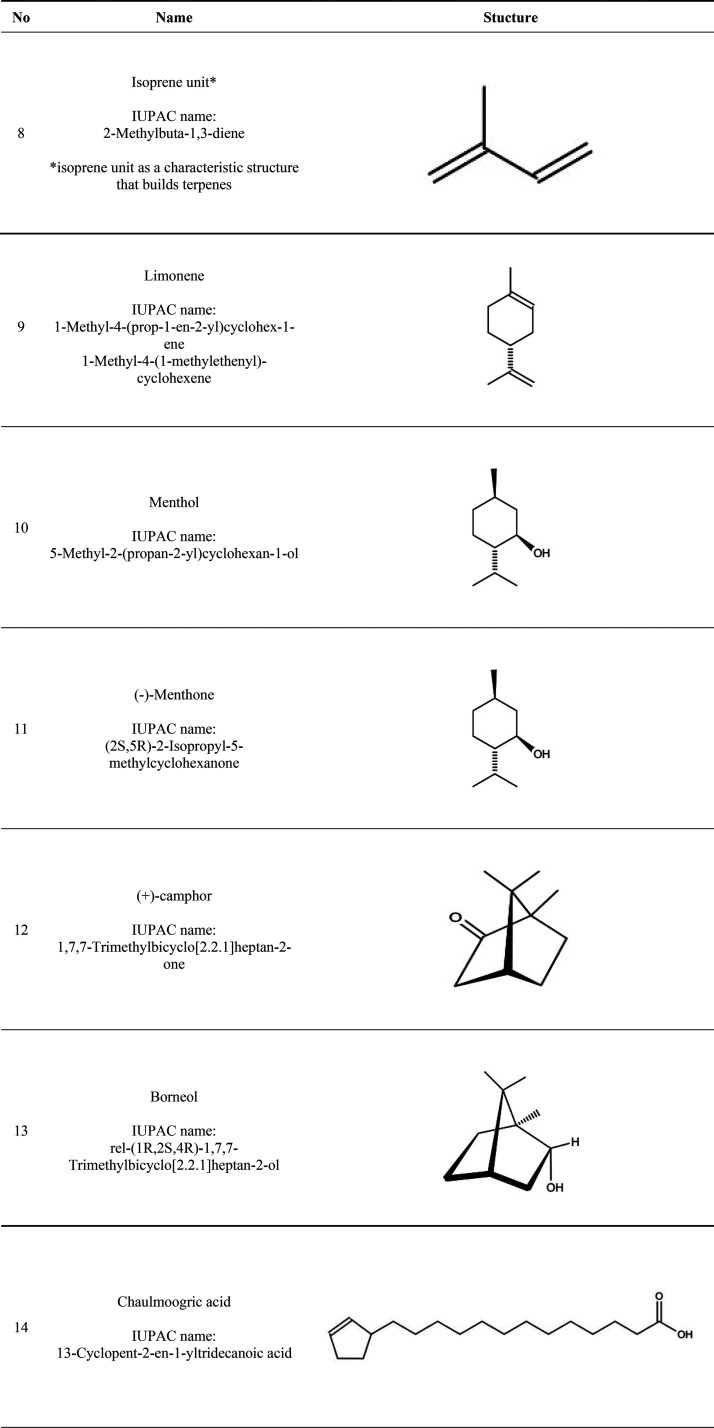
Terpenes represent a group of compounds with the general formula, (C5H8)n, consisting of two or more five-carbon isoprene units (IUs) (Table 2, item 8). They are classified according to the number of isoprene units in the carbon skeleton: monoterpenes (2 IUs), sesquiterpenes (3 IUs), diterpenes (4 IUs), sesterterpenes (5 IUs), triterpenes (6 IUs), tetraterpenes (8 IUs), and polyterpenes (>8 IUs). The structure of terpenes can be formed either by carbon chains or carbon rings. These arranged carbon rings can be further classified as monocyclic (one carbon ring) and successively bicyclic, tricyclic, etc. Acyclic compounds do not contain a carbon ring.165 Isoprene is one of the most abundant volatile hydrocarbons produced by living organisms in the world including bacteria.165,166
Terpenes have a high LogP which is indicative of their lipophilic properties determining their solubility in SC lipids. However, the presence of polar and nonpolar groups in the terpene molecule makes them exhibit the potential to promote the permeation of both hydrophilic and lipophilic APIs.167,168 The different degrees of lipophilicity of terpenes (e.g., LogP = 2.13 for camphor and LogP = 5.32 for nerolidol), structure (linear or ring), and presence of additional functional groups give them different permeation enhancement efficiencies.169
In section 3.3.1, we described eucalyptol because its use was related to the OA study.
An in vitro study showed a higher amount of permeation of the API haloperidol (375.9 Da, LogP = 3.36) across the human epidermal membrane in the presence of limonene (Table 2, item 9) (LogP = 4.45) than in the presence of the oxygen-containing terpenes linalool (LogP = 3.28) and 1,4-cineole (cineole LogP = 2.31 1,8-cineole LogP = 2.82). The work does not indicate exactly which terpene was used, which was explained by the facilitation of API solubility in the skin by limonene.62 FTIR (Fourier-transform infrared spectroscopy) results confirmed that limonene interacts with the alkyl chain region of SC lipids causing an increase in fluidization, as it relaxes the lamellar organization of the lipids.170
Zhu et al.171 evaluated seven oxygen-containing terpenes (1,8-cineole, citral, geraniol, linalool, menthol, terpinen-4-ol, and α-terpineol) at a concentration of 5%; both cyclic and linear forms were evaluated using the method of measuring skin electrical resistance (SER), the changes of which reflect the degree of skin integrity after application of compounds considered to be API permeation enhancers.172,173 Cyclic terpenes (1,8-cineole, terpinen-4-ol, menthol, and α-terpineol) were shown to reduce SER values and impair the skin’s barrier function to a greater extent than linear terpenes (linalool, geraniol, and citral). Molecular computer simulations indicated that the SER values for cyclic terpenes were associated with the formation of stronger hydrogen bonds with the polar head of SC ceramides171 creating a pathway for hydrophilic API penetration (LogP > 3), as well as a pathway for water to escape through the epidermis.171,174
In an in vitro study conducted in Franz diffusion cells, differences in the enhancement of ligustrazine hydrochloride permeation using the monocyclic monoterpenes menthol (Table 2, item 10) and menthone (Table 2, item 11) were assessed. Menthol contains a hydroxyl group attached to its ring and was found to be more effective than menthone with a carbonyl group. The hydroxyl group more easily forms hydrogen bonds with the amide group of ceramides and, as confirmed by FTIR analysis, menthol exhibits stronger epidermal lipid-extracting activity.63 In the study of Huang et al.,175 the simulation of skin permeability using molecular dynamics confirmed the interaction of menthol with ceramide 2 by forming hydrogen bonds in a single-component bilayer model, which facilitated the penetration of quercetin. The study showed that quercetin tended to localize in the area of the polar heads of the lipid bilayer, which created barriers for its deeper penetration through the hydrophobic region of the lipids. By hydrogen bonding with menthol, quercetin reduced its chances of interacting with ceramide. Meanwhile, menthol inserted itself into the lipid bilayer, breaking the hydrogen bonds between ceramides and facilitating the diffusion of quercetin. However, the effect of the interaction of menthol with quercetin on the SC should be tested on a more complicated bilayer model that reflects the natural composition of the SC lipid mixture.
An in vitro rat skin transdermal permeation study using a TK-20B diffusion apparatus showed that (+)-camphor (Table 2, item 12) (monoterpene, LogP = 2.13) at a concentration of 3% increased the permeation of APIs with different lipophilicities: indomethacin (LogP = 3. 80), lidocaine (LogP = 2.56), aspirin (LogP = 1.23), antipyrine (LogP = 0.23), tegafur (LogP = −0.48), and 5-fluorouracil (LogP = −0.95). At the same time, camphor was found to increase the permeation efficiency of the APIs linearly with decreasing LogP values, as the highest amount of API permeation through the skin was observed for hydrophilic APIs (LogP of approximately 0). Camphor was found to increase the partitioning of the API in the SC, i.e., to increase the release of the API from the carrier into the SC.176 This is the first step in drug delivery through the skin, resulting in a concentration gradient as molecules diffuse into deeper layers of the epidermis and dermis.177 Camphor also extracts some of the lipids and disrupts the molecular organization of SC lipids, as confirmed by FTIR analysis.64 Borneol (Table 2, item 13), a monoterpene with similar lipophilicity (LogP = 2.71) and structural similarity to camphor, was reported by Dai et al.65 Franz diffusion tests showed that at a concentration of 0.54%, borneol inhibited the penetration of the lipophilic API osthole (LogP = 3.8), while increasing API penetration was observed at the higher borneol concentration of 1.02%. TEM (transmission electron microscope) imaging provided information on the disorganizing effect of 0.54% borneol on lipids in rat skin, while a concentration of 1.02% resulted in the complete destruction of the lamellar lipid arrangement. Using CGMD (coarse-grained molecular dynamics), it was observed that borneol at concentrations above 10–15% disorganized the arrangement of lipid alkyl chains and caused the extraction of some lipids and formed aqueous spaces and inverted micelles, while concentrations up to 10% localized to the space of lipid alkyl chains without affecting their structure. The disorganizing and extracting effects on SC borneol lipids at concentrations of 1%, 3%, and 5% were confirmed using ATR-FTIR (attenuated total reflectance–Fourier-transform infrared spectroscopy) by Yi et al.178 Thus, the disorganizing effect on the lamellar lipid structures is not sufficient for the penetration of lipophilic compounds when using borneol at concentrations below 1%.
The study by Kopečná et al.66 describes a novel class of penetration enhancers, which are a combination of an amino acid derivative with various mono- and sesquiterpene alcohols (namely, 6-(dimethylamino)hexanoic acid with citronellol, geraniol, nerol, farnesol, linalool, menthol, borneol, and carveol esters). The researchers tested the effectiveness of these enhancers in delivering two different APIs, theophylline (MW = 180 g/mol, LogP = −0.02) and hydrocortisone (MW = 362 g/mol; LogP = 1.61), through human skin in vitro. Among all the terpene alcohols used, citronellyl 6-(dimethylamino)hexanoate was found to be the most effective at a concentration of 30 mM. Importantly, these enhancers did not show any cellular toxicity in vitro, and their mechanism of action was found to be based on the fluidization of epidermal lipids, as confirmed by FTIR analysis on the isolated human epidermis.
Terpenes are the main components of essential oils. Natural essential oils are extracted from the herb, leaves, flowers, and fruit. The methods used to extract essential oils from the plants include supercritical fluid extraction, subcritical liquid extraction, solvent-free microwave extraction, hydrodistillation, steam-distillation, hydrodiffusion, and solvent extraction. Essential oils include nitrogen- and sulfur-containing compounds (isocyanates, e.g., allyl isothiocyanate), aromatic compounds (benzene derivatives, e.g., eugenol), and others, including long-chain unbranched compounds.179,180 In an in vitro study on rat skin, the permeation of naproxen sodium from a gel matrix in the presence of an absorption promoter in the form of 0.5% essential oil of Lavandula angustifolia. The use of this oil formulation allowed the API to be delivered to the skin at a higher concentration (222.19 ± 24.87 μg/cm2) compared to naproxen gel alone (107.65 ± 6.38 μg/cm2). The main constituents of lavender oil, as confirmed by gas chromatography–mass spectrometry (GC-MS) analysis included 1,8-cineole (22.3%), linalool (11.2%), camphor (7.9%), β-pinene (5.8%), α-terpineol (4.9%), α-pinene (4.6%), terpinen-4-ol (4.2%), and borneol (4.0%).67 An in vitro rat skin permeation study showed that essential oils at a concentration of 3% extracted from Radix Angelicae sinensis [Angelica sinensis (Oliv.) Diels (Umbelliferae)], Rhizoma chuanxiong [Ligusticum chuanxiong Hort. (Umbelliferae)], Rhizoma cyperi [Cyperus rotundus L. (Sedge)], Cinnamomum cassia Presl. (Lauraceae), and Flos caryophylli [Eugenia caryophyllata Thunb. (Myrtaceae)] are effective when used alone in facilitating the penetration of ibuprofen. The main constituents of these oils included ligustilide (79.32%), ligustilide (41.00%), (E)-cinnamaldehyde (83.30%), and eugenol (80.22%), respectively.68 In contrast, the essential oil of Sinapis alba L. when used at concentrations of 0.5%, 2%, and 5% (with the highest efficacy at 5%) showed promising permeation enhancement properties for the following APIs with different lipophilicities: 5-fluorouracil (LogP = −0.95), paeonol (LogP = 2.054), and osthol (LogP = 3.85). The main constituents of the oil were 3-butenenitrile (16.62%), allyl isothiocyanate (57.02%), and isothiocyanato cyclopropane (17.46%).69
The compounds that make up the majority of essential oil compositions do not necessarily interact with the SC constituents and are responsible for the permeation-enhancing effects of APIs, as confirmed by Nowak et al.70 The essential oil from Epilobium angustifolium L. consists mainly of cosanes (23.70%), 5-methyldocosane (14.95%), caryophyllenes (9.22%), and oxygen derivatives of caryophyllenes (11.30%). In an in vitro study on Franz cells, the oil promoted the penetration of ibuprofen, lidocaine, and caffeine through pig skin. Analysis of the composition of the pig skin and acceptor fluid after 24 h by GC-MS analysis revealed the presence of α-terpineol, (S)-carvone, thymol, anethole, secalciferol, and trimethylpentadecan-2-one in the skin, which accounted for 1.94–4.54% of the oil composition, while no compounds were found in the acceptor fluid.70 The results suggest that the compounds present in the skin are responsible for the beneficial effects of API penetration.
Due to the difficulty in identifying which components are responsible for the penetration-enhancing effect, it may be considered fair to compare the effect of a śś of essential oil with individual compounds isolated from the oil.169 A question that is worth considering is whether a mixture of these compounds shows a synergistic effect resulting in improved API absorption through the skin. Oil extracted from Ledum palustre L. var. angustum N. Busch was used in an in vitro horizontal diffusion cells study to evaluate its efficacy in facilitating the permeation of donepezil hydrochloride. The compound facilitating the penetration of the API was cuminaldehyde, which accounted for 5.72% of the oil composition. Cuminaldehyde, used alone in the same study, was found to be 2 times more effective than the oil alone. Terpenes included in the oil were sabinene (33.40%), 4-terpineol (20.33%), and p-cymene (18.31%), but when used alone, they showed little or no efficacy.71
Table 1 (items 10–29) presents the permeation effectiveness of APIs used with terpens and essentials oils in TTDs.
4.2. Fatty Acids
Plant fats such as tri-, mono-, and diglycerides, free fatty acids, phosphatides, sterols, and fatty alcohols occur in nature in plant tissue.181 They are most abundant in seeds, pulp, stone fruit, tubers, and sprouts. The main source of plant lipids are oilseed-producing plants such as sunflower, soybean, and rapeseed, oilseed-producing fruits such as olive, coconut, and palm, oilseed tubers such as peanuts, or oilseed germ such as maize. Methods for extracting the lipid compounds are based on chemical extraction, supercritical fluid extraction, steam-distillation, mechanical extraction, and most commonly mechanical pressing by which vegetable oil is extracted.181,182 Volatile essential oils and fatty vegetable oils differ in the content of the compounds predominantly present in the same plant source such as terpenes as well as other bioactive compounds, such as flavonoids.182 Plant oils have been found to influence the penetration of active substances through the skin, and fatty acids are mainly responsible for this effect.72
Results published by Cizinauskas et al.72 showed that of all the oils used (0.5% w/w) olive oil, soybean, coconut, avocado, sea-buckthorn pulp, and raspberry seed oils contained the same fatty acids in different proportions: C16:0 (palmitic), C18:0 (stearic), C18:1 (oleic), C18:2 (linoleic), and C16:1 (palmitoleic). Of these, only soybean oil and olive oil increased dihydroquercetin penetration (LogP < 3) in vitro using Bronaugh-type flow-through diffusion cells. The API in the presence of soybean oil was localized in the epidermis and dermis, while with olive oil the API penetrated deeper by localizing only in the dermis, as confirmed by TOF-SIMS (time-of-flight secondary ion mass spectrometry) analysis in vivo using human skin. The unsaturated fatty acids from the oils used in the study penetrated into the deeper layers of the epidermis and dermis, but at the same time, no correlation was observed in the study between the concentration of individual fatty acids and the effect of increased API penetration through the skin. According to the authors, this may be due to the presence of other components in the oil, not detected by the GC-MS analytical method used (after derivatization into methyl esters), and the synergistic effect of a mixture of penetration enhancers. Using flurbiprofen, which is more lipophilic than dihydroquercetin (LogP = 4.16), olive oil proved to be the best penetration enhancer compared to avocado oil, coconut oil, and oils of animal origin: emu and crocodile. This effect correlated with the highest amount of oleic acid (OA) in the oil formulation (76%). The second highest amount of OA was avocado oil (68%). However, it was coconut oil that showed greater penetration enhancement effects despite containing a high concentration (52%) of saturated, short-chain lauric acid (C12:0).73 An ex vivo study by Singh et al. using porcine skin and a Keshary–Chien glass diffusion target showed that seed kernel oil of Mesua ferrea Linn. at a concentration of 15% significantly increased the penetrating amount of diltiazem hydrochloride. FTIR spectra showed changes in the peak positions of the methylene groups of SC lipids and the amide groups in SC keratin indicating disordered lipid organization, lipid fluidization, and an altered conformation of keratin fibers in mesua oil-treated skin samples. Scanning electron microscope (SEM) imaging of the epidermis confirmed the disruption of protein structure in the SC.74 The mechanism of action was not correlated with the composition of the oil; however, other researchers have provided information on the content of OA, stearic acid, linoleic acid, palmitic acid, myristic acid, and arachidic acid,183 as well as coumarins, terpenoids, phenolics, and flavonoids in the oil.184
Another in vitro Franz diffusion cell study showed that oil extracted from Punica granatum seeds at concentrations of 2.5%, 5.0%, and 10% increased the amount of trans-resveratrol penetrating pig skin by 1.25, 2.25, and 3.14 times, respectively, compared to a control sample without oil. Analysis of the oil composition by GC-MS showed a composition of punicic acid (C18:3, 73.93%) with OA (C18:1), eicosenoic acid (C20:1), and linoleic acid (C18:2) which together accounted for 13.46% of the oil composition.75 A study82 demonstrated the penetration-enhancing potential of chaulmoogra oil extracted from the seeds of a tree from the genus Hydnocarpus and family Flacourtiaceae.(185) A nanoemulsion containing chaulmoogra oil at approximately 84 μg/mL, Tween surfactant at approximately 90 μg/mL, and cosurfactant at approximately 2 μg/mL was developed for the transdermal delivery of methotrexate in the dermatological treatment of psoriasis. Drug retention studies showed that higher amounts of the API were retained in the epidermis and dermis, the layers that are mainly affected by psoriasis. Fluorescent microscopy analysis of skin cells also confirmed the presence of the drug in the deeper layers of the skin. In an in vivo clinical evaluation of the efficacy of this psoriasis treatment using the Psoriatic Area Severity Index (PASI) score, the nanoemulsion reduced the PASI by approximately 95% after 28 days. Chaulmoogra oil alone reduced the PASI by an average of 46%.82 Chaulmoogra oil is characterized by the presence of predominantly cyclopentenyl fatty acids: chaulmoogric acid (C18:1) (Table 2, item 14), hydnocarpic acid (C16:1), and gorlic acid (C18:2) (Table 2, item 15) containing a five-carbon ring with one unsaturated bond attached to the carbon chain.186 These fatty acids exhibit unusual biological activity against acid-resistant bacteria, and the oil itself was once used as a API to treat leprosy in humans.187,188 Cyclopentenyl acids from chaulmoogra oil have been shown to incorporate into triacylglycerols and cell membrane phospholipids to further disrupt membrane processes leading to the inhibition of Mycobacterium vaccae proliferation.185,189
Table 1 (items 30 to 33) presents the permeation effectiveness of APIs used with oils in TTDs.
4.3. Polysaccharides
Polysaccharides are naturally occurring polymers that are found in plants (e.g., starch, cellulose, and pectin), marine sources (e.g., agarose, alginate, chitosan, and carrageenan), microorganisms (e.g., dextran and pullulan), and animals (e.g., hyaluronic acid (HA), chondroitin sulfate, and heparin). Some of them have been described in the literature as compounds showing the potential to modify the skin barrier which supports their use in TDD, mainly for hydrophilic active ingredients.190,191 They have beneficial properties that make them widely used in the pharmaceutical industry. They exhibit susceptibility to chemical modification, sensitivity to environmental changes, and ability to swell in aqueous environments, and they are nontoxic, readily available, and biodegradable. These natural polymers via chemical or physical cross-linking form hydrogels, which are used in TDD. Most commonly, polysaccharides are physically cross-linked by means of electrostatic interactions, hydrophobic interactions, and ionic cross-linking supported by multivalent ions, van der Waals forces, or host–guest complexes.192
Mucilage is a complex heteropolysaccharide, which in contact with water becomes a viscous gel with a slimy appearance. It is extracted from plant parts such as fruits, pods, seeds, flowers, and leaves. The mucilage structure consists of hydrophilic groups namely −OH, −CONH–, −CONH2, and −SO3H entities with the ability to form noncovalent bonds with biological tissue.193 Saidin et al. extracted mucilage from fresh leaves of Hibiscus rosa-sinensis L. When applied to rat skin at concentrations of 1%, 1.5%, and 2%, it resulted in extraction and fluidization of corneocyte lipids and proteins and a conformational change in keratin SC proteins, as confirmed by ATR-FTIR analysis. Moreover, spectra analysis of the peak characteristic of the OH and NH groups showed the formation of hydrogen bonds between the gel components and SC ceramides, indicating a mechanism of action similar to terpenes (formation of new polar pathways for API diffusion). An in vitro permeation study using vertical diffusion cells showed an enhanced diffusion of caffeine in the presence of Hibiscus leaf mucilage with the best effect occurring at a concentration of 2%. The SEM image of the morphology of skin treated with Hibiscus gel showed an increase in skin smoothness after the application of the 2% gel compared to skin treated with a caffeine solution alone. The smooth surface of the SC permeation area had a reduced diffusional resistance to API transport.76 The natural saccharide gel also exhibited controlled drug-release properties. Mucilage extracted from the fruit of Ficus reticulata L. acted as a matrix in transdermal patches with properties that delayed the release of the API diltiazem hydrochloride into the SC.83 Mucilage extracted from Ficus carica L. also acted as a matrix in transdermal patches delaying the release of diclofenac sodium.84 Mucilage from Colocasia esculenta (taro) corms was combined with hydroxypropyl methylcellulose, which together served as a matrix in transdermal patches. An in vitro study demonstrated that these patches provided a safe, nonirritating control system for the release of diltiazem hydrochloride through the skin. Drug release slowed over time as the concentration of mucilage in the formulation increased.85
Hyaluronic acid (HA) is a linear glycosaminoglycan consisting of N-acetyl-d-glucosamine and d-glucuronic acid (Table 2, item 16) that is naturally found in the extracellular matrix of human connective tissue.194 For pharmaceutical and cosmetic purposes, it is extracted by microbial fermentation of microbial sources such as Streptococcus zooepidemicus and Corynebacterium glutamicum.(195) HA is used in a variety of drug delivery systems including nanoemulsion hydrogels, microemulsions, nanostructured carriers, and microneedles.196,197
Hydrogels form a three-dimensional network with a porous morphology. Due to the swelling and water-attracting properties of natural polymers, the hydrogel pores form a reservoir for water molecules or drug solutions. Drug release occurs under the influence of environmental changes such as pH, temperature, the presence of enzymes, and reactive oxygen species.198,199 HA is frequently used in pH-sensitive transdermal systems, such as hydrogels, as the presence of a COOH carboxyl group that dissociates at a pH equal to 6.7 creates a negatively charged COO– group that contributes to the release of the API into the skin through electrostatic interactions.87 The potential of HA-based hydrogels has been used in TTD systems for polar phenolic compounds. A study by Kong et al. indicated that hydrogels using HA (MW = 800 kDa) and another natural plant polymer, hydroxyethyl cellulose, facilitated the in vitro permeation through rat skin of the phenolic compound isoliquiritigenin extracted from Glycyrrhiza uralensis.(86) In a study by Kim, a hydrogel-based on HA (MW = 0.48 MDa) and poly(N-isopropylacrylamide) was found to be effective in transporting luteolin into the epidermis and dermis, as confirmed by UV–vis spectrophotometric analysis of in vitro drug-penetrated skin samples.87 The transport of proteins through the skin is hindered mainly due to their hydrophilicity and high molecular weights.200 The potential to enhance the permeation of the protein drug bovine serum albumin (66 kDa) hydrogel with 5% HA with different molecular weights of 5 kDa, 100 kDa and 1 MDa were evaluated.88 Using fluorescence resonance energy transfer (FRET)-FLIM, it was observed that low-molecular-weight HA (5 kDa) cotransported with the protein drug into viable epidermal layers, i.e., stratum basale and stratum spinosum, which was not observed with the higher HA molecular weights of 100 kDa and 1 MDa.88 Furthermore, the relationship between HA molecular weight with the ability to interact with SC proteins was confirmed by FTIR. Low-molecular-weight HA dramatically increased skin hydration and caused a conformational change in keratin structure from an α-helix to a β-sheet, which affects the organization of the lipid bilayer in the SC and the permeability to API. This effect was not observed for 100 kDa HA, which only increased the hydration of the SC.88 The SC penetration of HA is limited by its molecular weight, which can range from 5.000 to 5.000 000 Da. Raman microimaging of human skin sections provided information that only low-molecular-weight HA (20–300 kDa) was able to cross the SC barrier. In contrast, the SC barrier is impermeable to high-molecular-weight HA of 1.000–1.400 kDa.201 A new promising solution to improve the penetration of HA through the skin was proposed by Yan et al. The solution involves combining HA (in this study, HA with a molecular weight of 50 kDa) with a binding peptide called HaPP and a peptide called Pep-1. Together, these peptides promote the penetration of HA into the dermis.202 These penetrating peptides linked to HA are covered by patent application No. CN107226846B. In the discussed study by Yan et al., it has been suggested that the discussed solution opens the possibility of potentially combining HA with APIs.
Kawar et al. published information on a new type of liposome with a gel core formed from HA (hyaluosomes). Hyaluosomes were three times more effective in delivering ketoprofen through porcine skin in vitro compared to conventional liposomes (60 μg/h versus 20 μg/h).89 The beneficial effects of HA in enhancing API permeation are also exploited in the modification of other liposomal carriers and ethosomes to form phospholipid-ethanol complexes with HA.203 Fluorescence microscopic imaging of rat skin showed that ethosomes containing sodium forms of HA (MW = 150 kDa) facilitated the penetration of more rhodamine B into the dermis compared to classical ethosomes.176 Research indicates that HA can be included in topical skin drug delivery systems for the treatment of psoriasis and atopic dermatitis. Its mucoadhesive properties enable controlled drug release over time and absorption rate.204 The use of etosomes, which are based on propylene glycol coated with a gel made of HA with MW = 240 kDa, resulted in the delivery of 1% curcumin locally to the dermis and transdermally after 8 h in vitro on mouse skin at levels 1.4 and 1.6 times higher, respectively, compared to ethosomes without HA. Additionally, the delivery was 3.3 and 3.1 times higher compared to the curcumin solution alone.90
In the treatment of atopic dermatitis, polymeric nanoparticles coated with HA with a MW of 100 kDa have been shown to be effective in the ex vivo delivery of tacrolimus to rat skin, according to Zhuo et al.91 Other polymer nanoparticles coated with HA of the same molecular weight have also been used to deliver betamethasone valerate to ex vivo rat skin.92
Table 1 (items 34–52) presents the permeation effectiveness of APIs used with HA in TTDs.
5. Conclusion
The use of natural substances possessing properties that facilitate API permeation through the SC is undoubtedly the direction in which modern pharmaceuticals are heading.
The disorganizing effect on lamellar lipid fractions is considered the most crucial factor in achieving improved permeation. Urea is one such compound that can cause this effect. Terpenes are widely recognized as the most versatile permeation enhancers due to their molecular structure, which contains both polar and nonpolar groups. This structure enables terpenes to promote the permeation of both hydrophilic and lipophilic APIs. Additionally, HA possesses mucoadhesive properties that allow for controlled drug release over time and absorption rate.
API penetrations appear to be most effective when utilizing a compound formulation containing a mixture of permeation enhancers that complement each other, as they can interact with both the lipids and proteins of the SC, thereby creating transport pathways for both hydrophobic and hydrophilic APIs. This method also avoids the application of high concentrations of a single permeation enhancer, which could induce overly potent changes in the epidermis and cause skin irritation. Furthermore, this approach offers the possibility of effective use of both polar and nonpolar APIs.
We believe that proposed look on so diverse group of substances that contribute to transdermal delivery system offers new perspective for further study leading to discovery of new, natural permeation enhancers.
Acknowledgments
The authors would like to thank Karol Jasinski, M.D., for his assistance in preparing the figures for this manuscript.
Glossary
Abbreviations
- TDDs
transdermal delivery systems
- SC
stratum corneum
- CPEs
chemical permeation enhancers
- API
active pharmaceutical ingredient
- SPP
short periodicity phase
- LPP
long periodicity phase
- NMF
natural moisturizer factor
- CER NS
nonhydroxy acyl sphingosine-type ceramide
- E-PABA
ethyl-p-aminobenzoate
- OA
oleic acid
- HP-β-CD
hydroxy propyl-beta cyclodextrin
- rhGH
recombinant human growth hormone
- IU
isoprene unit
- SER
skin electrical resistance
- TEM
transmission electron microscope
- CGMD
coarse-grained molecular dynamics
- GC-MS
gas chromatography–mass spectrometry
- TOF-SIMS
time-of-flight secondary ion mass spectrometry
- SEM
scanning electron microscope
- PASI
psoriatic area severity index
- HA
hyaluronic acid
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Conceptualization, writing (review and editing), and visualization, R.B.; methodology and writing (original draft preparation), N.S. and R.B.; literature review N.S. and P.B.; analysis, N.S., P.B., and R.B.; supervision, W.O.S. and J.L.
The authors did not receive support from any organization for the submitted work.
The authors declare no competing financial interest.
References
- Ramadon D.; McCrudden M. T. C.; Courtenay A. J.; Donnelly R. F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12 (4), 758–791. 10.1007/s13346-021-00909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani A.; McCrudden M. T.; Donnelly R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7 (4), 438–470. 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prausnitz M. R.; Langer R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26 (11), 1261–1268. 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Tryon T. A.; Grice E. A. Microbiota and Maintenance of Skin Barrier Function. Science (1979) 2022, 376 (6596), 940–945. 10.1126/science.abo0693. [DOI] [PubMed] [Google Scholar]
- Vitorino C.; Sousa J.; Pais A. Overcoming the Skin Permeation Barrier: Challenges and Opportunities. Curr. Pharm. Des 2015, 21 (20), 2698–2712. 10.2174/1381612821666150428124053. [DOI] [PubMed] [Google Scholar]
- Cal K. Across Skin Barrier: Known Methods, New Performances.. Front. Drug Design Discov. 2012, 4, 162–188. 10.2174/978160805202810904010162. [DOI] [Google Scholar]
- Finnin B. C.; Morgan T. M. Transdermal Penetration Enhancers: Applications, Limitations, and Potential. J. Pharm. Sci. 1999, 88 (10), 955–958. 10.1021/js990154g. [DOI] [PubMed] [Google Scholar]
- Jaipakdee N.; Jarukamjorn K.; Putalun W.; Limpongsa E. Permeation, Stability and Acute Dermal Irritation of Miroestrol and Deoxymiroestrol from Pueraria Candollei Var. Mirifica Crude Extract Loaded Transdermal Gels. Pharm. Dev Technol. 2021, 26 (9), 967–977. 10.1080/10837450.2021.1967982. [DOI] [PubMed] [Google Scholar]
- al Hanbali O. A.; Khan H. M. S.; Sarfraz M.; Arafat M.; Ijaz S.; Hameed A. Transdermal Patches: Design and Current Approaches to Painless Drug Delivery. Acta Pharmaceutica 2019, 69 (2), 197–215. 10.2478/acph-2019-0016. [DOI] [PubMed] [Google Scholar]
- Nnamani P. O.; Ugwu A. A.; Nnadi O. H.; Kenechukwu F. C.; Ofokansi K. C.; Attama A. A.; Lehr C.-M. Formulation and Evaluation of Transdermal Nanogel for Delivery of Artemether. Drug Deliv Transl Res. 2021, 11 (4), 1655–1674. 10.1007/s13346-021-00951-4. [DOI] [PubMed] [Google Scholar]
- Kim D.; Jang S.; Kim I. W. Eutectic Formation of Naproxen with Some Dicarboxylic Acids. Pharmaceutics 2021, 13 (12), 2081. 10.3390/pharmaceutics13122081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X.; Wang X.; Tian C.; Liu L.; Xia M.; Jiang J.; Gui S. Dual Drug-Loaded Cubic Liquid Crystal Gels for Transdermal Delivery: Inner Structure and Percutaneous Mechanism Evaluations. Drug Dev. Ind. Pharm. 2019, 45 (12), 1879–1888. 10.1080/03639045.2019.1672716. [DOI] [PubMed] [Google Scholar]
- Yu Y. Q.; Yang X.; Wu X. F.; Fan Y. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. 10.3389/fbioe.2021.646554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiei M.; Kashanian S.; Samavati S. S.; Jamasb S.; McInnes S. J. P. Nanomaterial and Advanced Technologies in Transdermal Drug Delivery. J. Drug Target 2020, 28 (4), 356–367. 10.1080/1061186X.2019.1693579. [DOI] [PubMed] [Google Scholar]
- Lee H.; Song C.; Baik S.; Kim D.; Hyeon T.; Kim D.-H. Device-Assisted Transdermal Drug Delivery. Adv. Drug Deliv Rev. 2018, 127, 35–45. 10.1016/j.addr.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Seah B. C.-Q.; Teo B. M. Recent Advances in Ultrasound-Based Transdermal Drug Delivery. Int. J. Nanomedicine 2018, 13, 7749–7763. 10.2147/IJN.S174759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegre-Sánchez A.; Jiménez-Gómez N.; Boixeda P. Vehiculización de Fármacos Asistida Por Láser. Actas Dermosifiliogr 2018, 109 (10), 858–867. 10.1016/j.ad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Nieboer M. J.; Meesters A. A.; Almasian M.; Georgiou G.; de Rie M. A.; Verdaasdonk R. M.; Wolkerstorfer A. Enhanced Topical Cutaneous Delivery of Indocyanine Green after Various Pretreatment Regimens: Comparison of Fractional CO2 Laser, Fractional Er:YAG Laser, Microneedling, and Radiofrequency. Lasers Med. Sci. 2020, 35 (6), 1357–1365. 10.1007/s10103-020-02950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. v.; Mehrabi J. N.; Zachary C. B.; Geronemus R. G. Evaluation of Device-Based Cutaneous Channels Using Optical Coherence Tomography: Impact for Topical Drug Delivery. Dermatologic Surgery 2022, 48 (1), 120–125. 10.1097/DSS.0000000000003275. [DOI] [PubMed] [Google Scholar]
- Wenande E.; Erlendsson A. M.; Haedersdal M. Opportunities for Laser-Assisted Drug Delivery in the Treatment of Cutaneous Disorders. Semin Cutan Med. Surg 2017, 36 (4), 192–201. 10.12788/j.sder.2017.046. [DOI] [PubMed] [Google Scholar]
- Damiri F.; Kommineni N.; Ebhodaghe S. O.; Bulusu R.; Jyothi V. G. S. S.; Sayed A. A.; Awaji A. A.; Germoush M. O.; Al-Malky H. S.; Nasrullah M. Z.; Rahman M. H.; Abdel-Daim M. M.; Berrada M. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, Vol. 15, Page 190 2022, 15 (2), 190. 10.3390/ph15020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don T.-M.; Chen M.; Lee I.-C.; Huang Y.-C. Preparation and Characterization of Fast Dissolving Ulvan Microneedles for Transdermal Drug Delivery System. Int. J. Biol. Macromol. 2022, 207, 90–99. 10.1016/j.ijbiomac.2022.02.127. [DOI] [PubMed] [Google Scholar]
- Ramalheiro A.; Paris J. L.; Silva B. F. B.; Pires L. R. Rapidly Dissolving Microneedles for the Delivery of Cubosome-like Liquid Crystalline Nanoparticles with Sustained Release of Rapamycin. Int. J. Pharm. 2020, 591, 119942. 10.1016/j.ijpharm.2020.119942. [DOI] [PubMed] [Google Scholar]
- Dabholkar N.; Gorantla S.; Waghule T.; Rapalli V. K.; Kothuru A.; Goel S.; Singhvi G. Biodegradable Microneedles Fabricated with Carbohydrates and Proteins: Revolutionary Approach for Transdermal Drug Delivery. Int. J. Biol. Macromol. 2021, 170, 602–621. 10.1016/j.ijbiomac.2020.12.177. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Quan P.; Liu X.; Wang M.; Fang L. Novel Chemical Permeation Enhancers for Transdermal Drug Delivery. Asian J. Pharm. Sci. 2014, 9 (2), 51–64. 10.1016/j.ajps.2014.01.001. [DOI] [Google Scholar]
- Kováčik A.; Kopečná M.; Vávrová K. Permeation Enhancers in Transdermal Drug Delivery: Benefits and Limitations. Expert Opin Drug Deliv 2020, 17 (2), 145–155. 10.1080/17425247.2020.1713087. [DOI] [PubMed] [Google Scholar]
- Al-Akayleh F.; Adwan S.; Khanfar M.; Idkaidek N.; Al-Remawi M. Correction to: A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2021, 22 (1), 13. 10.1208/s12249-020-01903-w. [DOI] [PubMed] [Google Scholar]
- van den Bruinhorst A.; Kollau L. J. B. M.; Vis M.; Hendrix M. M. R. M.; Meuldijk J.; Tuinier R.; Esteves A. C. C. From a Eutectic Mixture to a Deep Eutectic System via Anion Selection: Glutaric Acid + Tetraethylammonium Halides. J. Chem. Phys. 2021, 155 (1), 014502 10.1063/5.0050533. [DOI] [PubMed] [Google Scholar]
- Ossowicz-Rupniewska P.; Klebeko J.; Świątek E.; Bilska K.; Nowak A.; Duchnik W.; Kucharski Ł.; Struk Ł.; Wenelska K.; Klimowicz A.; Janus E. Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. Int. J. Mol. Sci. 2022, 23 (8), 4158. 10.3390/ijms23084158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther R.; Rautio J.; Zelikin A. N. Prodrugs in Medicinal Chemistry and Enzyme Prodrug Therapies. Adv. Drug Deliv Rev. 2017, 118, 65–77. 10.1016/j.addr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Chen K.-J.; Plaunt A. J.; Leifer F. G.; Kang J. Y.; Cipolla D. Recent Advances in Prodrug-Based Nanoparticle Therapeutics. Eur. J. Pharm. Biopharm. 2021, 165, 219–243. 10.1016/j.ejpb.2021.04.025. [DOI] [PubMed] [Google Scholar]
- Chaulagain B.; Jain A.; Tiwari A.; Verma A.; Jain S. K. Passive Delivery of Protein Drugs through Transdermal Route. Artif Cells Nanomed Biotechnol 2018, 46 (sup1), 472–487. 10.1080/21691401.2018.1430695. [DOI] [PubMed] [Google Scholar]
- Wang H.; Tian Q.; Quan P.; Liu C.; Fang L. Probing the Role of Ion-Pair Strategy in Controlling Dexmedetomidine Penetrate Through Drug-in-Adhesive Patch: Mechanistic Insights Based on Release and Percutaneous Absorption Process. AAPS PharmSciTech 2020, 21 (1), 4. 10.1208/s12249-019-1539-0. [DOI] [PubMed] [Google Scholar]
- Uchino T.; Miyazaki Y.; Ishikawa A.; Kagawa Y. Development of a Novel Simple Gel Formulation Containing an Ion-Pair Complex of Diclofenac and Phenylephrine. Skin Pharmacol Physiol 2019, 32 (6), 318–327. 10.1159/000501734. [DOI] [PubMed] [Google Scholar]
- Torky A. S.; Freag M. S.; Nasra M. M. A.; Abdallah O. Y. Novel Skin Penetrating Berberine Oleate Complex Capitalizing on Hydrophobic Ion Pairing Approach. Int. J. Pharm. 2018, 549 (1–2), 76–86. 10.1016/j.ijpharm.2018.07.051. [DOI] [PubMed] [Google Scholar]
- Hirakawa Y.; Ueda H.; Miyano T.; Kamiya N.; Goto M. New Insight into Transdermal Drug Delivery with Supersaturated Formulation Based on Co-Amorphous System. Int. J. Pharm. 2019, 569, 118582. 10.1016/j.ijpharm.2019.118582. [DOI] [PubMed] [Google Scholar]
- Vasyuchenko E. P.; Orekhov P. S.; Armeev G. A.; Bozdaganyan M. E. CPE-DB: An Open Database of Chemical Penetration Enhancers. Pharmaceutics 2021, 13 (1), 66. 10.3390/pharmaceutics13010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořáková K.; Štěpánek P.; Kroupová J.; Zbytovská J. N-Alkylmorpholines: Potent Dermal and Transdermal Skin Permeation Enhancers. Pharmaceutics 2022, 14 (1), 64. 10.3390/pharmaceutics14010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares R. S. N.; Tao T. P.; Maschmeyer I.; Maria-Engler S. S.; Schäfer-Korting M.; Winter A.; Zoschke C.; Lauster R.; Marx U.; Gaspar L. R. Toxicity of Topically Applied Drugs beyond Skin Irritation: Static Skin Model vs. Two Organs-on-a-Chip. Int. J. Pharm. 2020, 589, 119788. 10.1016/j.ijpharm.2020.119788. [DOI] [PubMed] [Google Scholar]
- Vitale C. M.; Gutovitz S.. Aromatic Toxicity. In StatPearls [Internet]; StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK532257/?report=reader#_NBK532257 (accessed 2023–01–12). [PubMed]
- Marchev A. S.; Georgiev M. I. Plant In Vitro Systems as a Sustainable Source of Active Ingredients for Cosmeceutical Application. Molecules 2020, 25 (9), 2006. 10.3390/molecules25092006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur D.; Kaur G.; Puri A.; Nanda R. Therapeutic Potential of Essential Oil-Based Microemulsions: Reviewing State-of-the-Art. Curr. Drug Deliv 2021, 18 (9), 1218–1233. 10.2174/1567201818666210217161240. [DOI] [PubMed] [Google Scholar]
- Sharmeen J. B.; Mahomoodally F. M.; Zengin G.; Maggi F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26 (3), 666. 10.3390/molecules26030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poljšak N.; Kreft S.; Kočevar Glavač N. Vegetable Butters and Oils in Skin Wound Healing: Scientific Evidence for New Opportunities in Dermatology. Phytotherapy Research 2020, 34 (2), 254–269. 10.1002/ptr.6524. [DOI] [PubMed] [Google Scholar]
- Eid R. K.; Essa E. A.; el Maghraby G. M. Essential Oils in Niosomes for Enhanced Transdermal Delivery of Felodipine. Pharm. Dev Technol. 2019, 24 (2), 157–165. 10.1080/10837450.2018.1441302. [DOI] [PubMed] [Google Scholar]
- Kumar L.; Verma S.; Kumar S.; Prasad D. N.; Jain A. K. Fatty Acid Vesicles Acting as Expanding Horizon for Transdermal Delivery. Artif Cells Nanomed Biotechnol 2017, 45 (2), 251–260. 10.3109/21691401.2016.1146729. [DOI] [PubMed] [Google Scholar]
- Fox L. T.; Gerber M.; Plessis J. du; Hamman J. H. Transdermal Drug Delivery Enhancement by Compounds of Natural Origin. Molecules 2011, 16 (12), 10507–10540. 10.3390/molecules161210507. [DOI] [Google Scholar]
- Sarkic A.; Stappen I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5 (1), 11. 10.3390/cosmetics5010011. [DOI] [Google Scholar]
- Saporito F.; Sandri G.; Bonferoni M. C.; Rossi S.; Boselli C.; Icaro Cornaglia A.; Mannucci B.; Grisoli P.; Vigani B.; Ferrari F. Essential Oil-Loaded Lipid Nanoparticles for Wound Healing. Int. J. Nanomedicine 2018, 13, 175–186. 10.2147/IJN.S152529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-K.; Zhong L.; Santiago J. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19 (1), 70. 10.3390/ijms19010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn A. R.; Clark A. K.; Sivamani R. K.; Shi V. Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin Dermatol 2018, 19 (1), 103–117. 10.1007/s40257-017-0301-1. [DOI] [PubMed] [Google Scholar]
- Yu B.; Langer R.; Blankschtein D.; Kim K. H.; So P. T. C. Visualization of Oleic Acid-Induced Transdermal Diffusion Pathways Using Two-Photon Fluorescence Microscopy. Journal of Investigative Dermatology 2003, 120 (3), 448–455. 10.1046/j.1523-1747.2003.12061.x. [DOI] [PubMed] [Google Scholar]
- Atef E.; Altuwaijri N. Using Raman Spectroscopy in Studying the Effect of Propylene Glycol, Oleic Acid, and Their Combination on the Rat Skin. AAPS PharmSciTech 2018, 19 (1), 114–122. 10.1208/s12249-017-0800-7. [DOI] [PubMed] [Google Scholar]
- Koyama S.; Heinbockel T. The Effects of Essential Oils and Terpenes in Relation to Their Routes of Intake and Application. Int. J. Mol. Sci. 2020, 21 (5), 1558. 10.3390/ijms21051558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelan U. S.; de Oliveira A. C.; Cacoci É. S. P.; Martins T. E. A.; Giacon V. M.; Velasco M. V. R.; Lima C. R. R. de C. Potential Use of Essential Oils in Cosmetic and Dermatological Hair Products: A Review. J. Cosmet Dermatol 2022, 21 (4), 1407–1418. 10.1111/jocd.14286. [DOI] [PubMed] [Google Scholar]
- Školová B.; Janůšová B.; Zbytovská J.; Gooris G.; Bouwstra J.; Slepička P.; Berka P.; Roh J.; Palát K.; Hrabálek A.; Vávrová K. Ceramides in the Skin Lipid Membranes: Length Matters. Langmuir 2013, 29 (50), 15624–15633. 10.1021/la4037474. [DOI] [PubMed] [Google Scholar]
- Uche L. E.; Gooris G. S.; Bouwstra J. A.; Beddoes C. M. Increased Levels of Short-Chain Ceramides Modify the Lipid Organization and Reduce the Lipid Barrier of Skin Model Membranes. Langmuir 2021, 37 (31), 9478–9489. 10.1021/acs.langmuir.1c01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam S. H.; Saliaj E.; Wettig S. D.; Dong C.; Ivanova M. v.; Huzil J. T.; Foldvari M. Effect of Chemical Permeation Enhancers on Stratum Corneum Barrier Lipid Organizational Structure and Interferon Alpha Permeability. Mol. Pharmaceutics 2013, 10 (6), 2248–2260. 10.1021/mp300441c. [DOI] [PubMed] [Google Scholar]
- Ochalek M.; Podhaisky H.; Ruettinger H. H.; Neubert R. H. H.; Wohlrab J. SC Lipid Model Membranes Designed for Studying Impact of Ceramide Species on Drug Diffusion and Permeation, Part III: Influence of Penetration Enhancer on Diffusion and Permeation of Model Drugs. Int. J. Pharm. 2012, 436 (1–2), 206–213. 10.1016/j.ijpharm.2012.06.044. [DOI] [PubMed] [Google Scholar]
- Harjoh N.; Wong T. W.; Caramella C. Transdermal Insulin Delivery with Microwave and Fatty Acids as Permeation Enhancers. Int. J. Pharm. 2020, 584, 119416 10.1016/j.ijpharm.2020.119416. [DOI] [PubMed] [Google Scholar]
- Pavlačková J.; Egner P.; Polašková J.; Hojerová J.; Pind’áková L.; Mokrejš P.; Varad’ová V. Transdermal Absorption of Active Substances from Cosmetic Vehicles. J. Cosmet. Dermatol. 2019, 18 (5), 1410–1415. 10.1111/jocd.12873. [DOI] [PubMed] [Google Scholar]
- Lim P. F. C.; Liu X. Y.; Kang L.; Ho P. C. L.; Chan Y. W.; Chan S. Y. Limonene GP1/PG Organogel as a Vehicle in Transdermal Delivery of Haloperidol. Int. J. Pharm. 2006, 311 (1–2), 157–164. 10.1016/j.ijpharm.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Wang J.; Dong C.; Song Z.; Zhang W.; He X.; Zhang R.; Guo C.; Zhang C.; Li F.; Wang C.; Yuan C. Monocyclic Monoterpenes as Penetration Enhancers of Ligustrazine Hydrochloride for Dermal Delivery. Pharm. Dev Technol. 2017, 22 (4), 571–577. 10.1080/10837450.2016.1189936. [DOI] [PubMed] [Google Scholar]
- Xie J.; Ji Y.; Xue W.; Ma D.; Hu Y. Hyaluronic Acid-Containing Ethosomes as a Potential Carrier for Transdermal Drug Delivery. Colloids Surf. B Biointerfaces 2018, 172, 323–329. 10.1016/j.colsurfb.2018.08.061. [DOI] [PubMed] [Google Scholar]
- Dai X.; Yin Q.; Wan G.; Wang R.; Shi X.; Qiao Y. Effects of Concentrations on the Transdermal Permeation Enhancing Mechanisms of Borneol: A Coarse-Grained Molecular Dynamics Simulation on Mixed-Bilayer Membranes. International Journal of Molecular Sciences 2016, Vol. 17, Page 1349 2016, 17 (8), 1349. 10.3390/ijms17081349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopečná M.; Macháček M.; Nováčková A.; Paraskevopoulos G.; Roh J.; Vávrová K. Esters of Terpene Alcohols as Highly Potent, Reversible, and Low Toxic Skin Penetration Enhancers. Scientific Reports 2019 9:1 2019, 9 (1), 1–12. 10.1038/s41598-019-51226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iman M.; Sohrab Rostamkalaei S.; Ataee R.. Percutaneous Absorption Enhancer Properties of Lavandula Angustifolia Essential Oil on Percutaneous Absorption of Naproxen Sodium from Topical Gel. Research Square, April 19, 2021, rs.3.rs-344039, ver. 1. DOI: 10.21203/RS.3.RS-344039/V1.
- Jiang Q.; Wu Y.; Zhang H.; Liu P.; Yao J.; Yao P.; Chen J.; Duan J. Development of Essential Oils as Skin Permeation Enhancers: Penetration Enhancement Effect and Mechanism of Action. Pharm. Biol. 2017, 55 (1), 1592–1600. 10.1080/13880209.2017.1312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan S.; Wang Z.; Xiang S.; Chen H.; Shen Q.; Liu L.; Wu W.; Cao S.; Wang Z.; Yang Z.; Weng L.; Zhu H.; Liu Q. Mechanisms of White Mustard Seed (Sinapis Alba L.) Volatile Oils as Transdermal Penetration Enhancers. Fitoterapia 2019, 138, 104195 10.1016/j.fitote.2019.104195. [DOI] [PubMed] [Google Scholar]
- Nowak A.; Duchnik W.; Makuch E.; Kucharski Ł.; Ossowicz-Rupniewska P.; Cybulska K.; Sulikowski T.; Moritz M.; Klimowicz A. Epilobium Angustifolium L. Essential Oil—Biological Activity and Enhancement of the Skin Penetration of Drugs—In Vitro Study. Molecules 2021, 26 (23), 7188. 10.3390/molecules26237188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan L.; Liu C.; Li Q.; Wan X.; Guo J.; Quan P.; Fang L. Investigation of the Enhancement Effect of the Natural Transdermal Permeation Enhancers from Ledum Palustre L. Var. Angustum N. Busch: Mechanistic Insight Based on Interaction among Drug, Enhancers and Skin. European Journal of Pharmaceutical Sciences 2018, 124, 105–113. 10.1016/j.ejps.2018.08.025. [DOI] [PubMed] [Google Scholar]
- Čižinauskas V.; Elie N.; Brunelle A.; Briedis V. Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. Molecules 2017, 22 (9), 1536. 10.3390/molecules22091536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen J. M.; Cowley A.; du Preez J.; Gerber M.; du Plessis J. Penetration Enhancing Effects of Selected Natural Oils Utilized in Topical Dosage Forms. Drug Dev. Ind. Pharm. 2015, 41 (12), 2045–2054. 10.3109/03639045.2015.1047847. [DOI] [PubMed] [Google Scholar]
- Singha L. R.; Das M. K. Effect of Mesua Ferrea Linn. Seed Kernel Oil on Percutaneous Absorption of Diltiazem Hydrochloride through Pig Ear Epidermis: A Mechanistic Study. J. Drug Deliv Sci. Technol. 2021, 64, 102628. 10.1016/j.jddst.2021.102628. [DOI] [Google Scholar]
- Liu W.; Zhao Q.; Lv L.; Yan S.; Song Q.; Chen T.; Yao Q. Pomegranate Seed Oil Enhances the Percutaneous Absorption of trans-Resveratrol. J. Oleo Sci. 2018, 67 (4), 479–487. 10.5650/jos.ess17144. [DOI] [PubMed] [Google Scholar]
- Saidin N. M.; Anuar N. K.; Tin Wui W.; Meor Mohd Affandi M. M. R.; Wan Engah W. R. Skin Barrier Modulation by Hibiscus Rosa-Sinensis L. Mucilage for Transdermal Drug Delivery. Polym. Bull. 2022, 79 (5), 3099–3115. 10.1007/s00289-021-03658-1. [DOI] [Google Scholar]
- Abd E.; Benson H. A. E.; Roberts M. S.; Grice J. E. Minoxidil Skin Delivery from Nanoemulsion Formulations Containing Eucalyptol or Oleic Acid: Enhanced Diffusivity and Follicular Targeting. Pharmaceutics 2018, Vol. 10, Page 19 2018, 10 (1), 19. 10.3390/pharmaceutics10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd E.; Namjoshi S.; Mohammed Y. H.; Roberts M. S.; Grice J. E. Synergistic Skin Penetration Enhancer and Nanoemulsion Formulations Promote the Human Epidermal Permeation of Caffeine and Naproxen. J. Pharm. Sci. 2016, 105 (1), 212–220. 10.1002/jps.24699. [DOI] [PubMed] [Google Scholar]
- Viqhi A. V.; Manggau M. A.; Sartini S.; Wahyudin E.; Rahman L.; Yulianti R.; Permana A. D.; Awal S. A. Development of Propolis (Apis Trigona)-Loaded Nanoemulgel for Improved Skin Penetration of Caffeic Acid: The Effect of Variation of Oleic Acid Concentration. Open Access Maced J. Med. Sci. 2020, 9 (B), 1264–1278. 10.3889/oamjms.2021.6672. [DOI] [Google Scholar]
- Lai W.-F.; Tang R.; Wong W.-T. Ionically Crosslinked Complex Gels Loaded with Oleic Acid-Containing Vesicles for Transdermal Drug Delivery. Pharmaceutics 2020, 12 (8), 725. 10.3390/pharmaceutics12080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams L.; Shahraky M. K.; Mirtaleb M. S. Transdermal Co-Delivery of Urea and Recombinant Human Growth Hormone. Iran J. Biotechnol. 2021, 19 (4), e2891. 10.30498/IJB.2021.252676.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajitha P.; Shammika P.; Aiswarya S.; Gopikrishnan A.; Jayakumar R.; Sabitha M. Chaulmoogra Oil Based Methotrexate Loaded Topical Nanoemulsion for the Treatment of Psoriasis. J. Drug Deliv Sci. Technol. 2019, 49, 463–476. 10.1016/j.jddst.2018.12.020. [DOI] [Google Scholar]
- Ahad H.; Kumar C.; Venkatnath L.; Gangadhar P. Characterization and Permeation Studies of Diltiazem Hydrochloride-Ficus Reticuleta Fruit Mucilage Transdermal Patches. Int. J. Pharm. Sci. Rev. Res. 2010, 1 (2), 32–37. [Google Scholar]
- Ahad H.; Kumar C.; Kumar B.; AmarnathReddy B.; Shekar A.; Sagar V.; Gangadhar Permeation Studies of Diclofenac Sodium From Ficus Carica Fruit Mucilage Matrices for Transdermal Delivery. Int. J. Pharmtech Res. 2010, 2 (2), 1013–1017. [Google Scholar]
- Sarkar G.; Saha N. R.; Roy I.; Bhattacharyya A.; Bose M.; Mishra R.; Rana D.; Bhattacharjee D.; Chattopadhyay D. Taro Corms Mucilage/HPMC Based Transdermal Patch: An Efficient Device for Delivery of Diltiazem Hydrochloride. Int. J. Biol. Macromol. 2014, 66, 158–165. 10.1016/j.ijbiomac.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Kong B. J.; Kim A.; Park S. N. Properties and in Vitro Drug Release of Hyaluronic Acid-Hydroxyethyl Cellulose Hydrogels for Transdermal Delivery of Isoliquiritigenin. Carbohydr. Polym. 2016, 147, 473–481. 10.1016/j.carbpol.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Kim A. R.; Lee S. L.; Park S. N. Properties and in Vitro Drug Release of PH- and Temperature-Sensitive Double Cross-Linked Interpenetrating Polymer Network Hydrogels Based on Hyaluronic Acid/Poly (N-Isopropylacrylamide) for Transdermal Delivery of Luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. 10.1016/j.ijbiomac.2018.06.061. [DOI] [PubMed] [Google Scholar]
- Witting M.; Boreham A.; Brodwolf R.; Vávrová K.; Alexiev U.; Friess W.; Hedtrich S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharmaceutics 2015, 12 (5), 1391–1401. 10.1021/mp500676e. [DOI] [PubMed] [Google Scholar]
- Kawar D.; Abdelkader H. Hyaluronic Acid Gel-Core Liposomes (Hyaluosomes) Enhance Skin Permeation of Ketoprofen. Pharm. Dev Technol. 2019, 24 (8), 947–953. 10.1080/10837450.2019.1572761. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xia Q.; Li Y.; He Z.; Li Z.; Guo T.; Wu Z.; Feng N. CD44 Assists the Topical Anti-Psoriatic Efficacy of Curcumin-Loaded Hyaluronan-Modified Ethosomes: A New Strategy for Clustering Drug in Inflammatory Skin. Theranostics 2019, 9 (1), 48–64. 10.7150/thno.29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo F.; Abourehab M. A. S.; Hussain Z. Hyaluronic Acid Decorated Tacrolimus-Loaded Nanoparticles: Efficient Approach to Maximize Dermal Targeting and Anti-Dermatitis Efficacy. Carbohydr. Polym. 2018, 197, 478–489. 10.1016/j.carbpol.2018.06.023. [DOI] [PubMed] [Google Scholar]
- Pandey M.; Choudhury H.; Gunasegaran T. A. P.; Nathan S. S.; Md S.; Gorain B.; Tripathy M.; Hussain Z. Hyaluronic Acid-Modified Betamethasone Encapsulated Polymeric Nanoparticles: Fabrication, Characterisation, in Vitro Release Kinetics, and Dermal Targeting. Drug Deliv. Transl. Res. 2019, 9 (2), 520–533. 10.1007/s13346-018-0480-1. [DOI] [PubMed] [Google Scholar]
- Baroni A.; Buommino E.; de Gregorio V.; Ruocco E.; Ruocco V.; Wolf R. Structure and Function of the Epidermis Related to Barrier Properties. Clin Dermatol 2012, 30 (3), 257–262. 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Bouwstra J. A.; Gooris G. S.; van der Spek J. A.; Bras W. Structural Investigations of Human Stratum Corneum by Small-Angle X-Ray Scattering. Journal of Investigative Dermatology 1991, 97 (6), 1005–1012. 10.1111/1523-1747.ep12492217. [DOI] [PubMed] [Google Scholar]
- Groen D.; Berthaud F.; Bouwstra J. A.; Chapuis C.; Gooris G. S.; Boncheva M. In Vitro Model Systems for Studying the Impact of Organic Chemicals on the Skin Barrier Lipids. Biochimica et Biophysica Acta (BBA) - Biomembranes 2014, 1838 (1), 310–318. 10.1016/j.bbamem.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Bouwstra J. A.; Gooris G. S.; Dubbelaar F. E. R.; Ponec M. Phase Behavior of Lipid Mixtures Based on Human Ceramides: Coexistence of Crystalline and Liquid Phases. J. Lipid Res. 2001, 42 (11), 1759–1770. 10.1016/S0022-2275(20)31502-9. [DOI] [PubMed] [Google Scholar]
- Ogawa-Fuse C.; Morisaki N.; Shima K.; Hotta M.; Sugata K.; Ichihashi T.; Oguri M.; Yoshida O.; Fujimura T. Impact of Water Exposure on Skin Barrier Permeability and Ultrastructure. Contact Dermatitis 2019, 80 (4), 228–233. 10.1111/cod.13174. [DOI] [PubMed] [Google Scholar]
- Zimmerer R. E.; Lawson K. D.; Calvert C. J. The Effects of Wearing Diapers on Skin. Pediatr Dermatol 1986, 3 (2), 95–101. 10.1111/j.1525-1470.1986.tb00497.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Yamauchi R.; Ohno S.; Asai K.; Fukami T.; Koide T. Evaluation of the Water Content and Skin Permeability of Active Pharmaceutical Ingredients in Ketoprofen Poultice Formulations Removed from Their Airtight Containers and Left at Room Temperature. Biol. Pharm. Bull. 2019, 42 (12), 2102–2108. 10.1248/bpb.b19-00758. [DOI] [PubMed] [Google Scholar]
- Hatta I.; Nakazawa H.; Ohta N.; Uchino T.; Yanase K. Stratum Corneum Function: A Structural Study with Dynamic Synchrotron X-Ray Diffraction Experiments. J. Oleo Sci. 2021, 70 (9), 1181–1199. 10.5650/jos.ess21159. [DOI] [PubMed] [Google Scholar]
- Wickett R. R.; Visscher M. O. Structure and Function of the Epidermal Barrier. Am. J. Infect Control 2006, 34 (10), S98–S110. 10.1016/j.ajic.2006.05.295. [DOI] [Google Scholar]
- Choe C.; Schleusener J.; Choe S.; Ri J.; Lademann J.; Darvin M. E. Stratum Corneum Occlusion Induces Water Transformation towards Lower Bonding State: A Molecular Level in Vivo Study by Confocal Raman Microspectroscopy. Int. J. Cosmet Sci. 2020, 42 (5), 482–493. 10.1111/ics.12653. [DOI] [PubMed] [Google Scholar]
- Schleusener J.; Salazar A.; von Hagen J.; Lademann J.; Darvin M. E. Retaining Skin Barrier Function Properties of the Stratum Corneum with Components of the Natural Moisturizing Factor—A Randomized, Placebo-Controlled Double-Blind In Vivo Study. Molecules 2021, 26 (6), 1649. 10.3390/molecules26061649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokura Y.; Ishikawa S.; Tokuda H.; Imokawa G. Molecular Analysis of Elastic Properties of the Stratum Corneum by Solid-State 13C-Nuclear Magnetic Resonance Spectroscopy. Journal of Investigative Dermatology 1995, 104 (5), 806–812. 10.1111/1523-1747.ep12607005. [DOI] [PubMed] [Google Scholar]
- Darvin M. E.; Schleusener J.; Lademann J.; Choe C.-S. Current Views on Noninvasive in Vivo Determination of Physiological Parameters of the Stratum Corneum Using Confocal Raman Microspectroscopy. Skin Pharmacol Physiol 2022, 35 (3), 125–136. 10.1159/000521416. [DOI] [PubMed] [Google Scholar]
- Rawlings A. v.; Harding C. R. Moisturization and Skin Barrier Function. Dermatol Ther 2004, 17 (s1), 43–48. 10.1111/j.1396-0296.2004.04S1005.x. [DOI] [PubMed] [Google Scholar]
- Knox S.; O’Boyle N. M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055. 10.1016/j.chemphyslip.2021.105055. [DOI] [PubMed] [Google Scholar]
- Masukawa Y.; Narita H.; Sato H.; Naoe A.; Kondo N.; Sugai Y.; Oba T.; Homma R.; Ishikawa J.; Takagi Y.; Kitahara T. Comprehensive Quantification of Ceramide Species in Human Stratum Corneum. J. Lipid Res. 2009, 50 (8), 1708–1719. 10.1194/jlr.D800055-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováčik A.; Pullmannová P.; Opálka L.; Šilarová M.; Maixner J.; Vávrová K. Effects of (R)- and (S)-α-Hydroxylation of Acyl Chains in Sphingosine, Dihydrosphingosine, and Phytosphingosine Ceramides on Phase Behavior and Permeability of Skin Lipid Models. Int. J. Mol. Sci. 2021, 22 (14), 7468. 10.3390/ijms22147468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessner D.; Ruettinger A.; Kiselev M. A.; Wartewig S.; Neubert R. H. H. Properties of Ceramides and Their Impact on the Stratum Corneum Structure. Skin Pharmacol Physiol 2008, 21 (2), 58–74. 10.1159/000112956. [DOI] [PubMed] [Google Scholar]
- Wertz P. W. Lipids and the Permeability and Antimicrobial Barriers of the Skin. J. Lipids 2018, 2018, 1–7. 10.1155/2018/5954034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabionet M.; Bernard P.; Pichery M.; Marsching C.; Bayerle A.; Dworski S.; Kamani M. A.; Chitraju C.; Gluchowski N. L.; Gabriel K. R.; Asadi A.; Ebel P.; Hoekstra M.; Dumas S.; Ntambi J. M.; Jacobsson A.; Willecke K.; Medin J. A.; Jonca N.; Sandhoff R. Epidermal 1-O-Acylceramides Appear with the Establishment of the Water Permeability Barrier in Mice and Are Produced by Maturating Keratinocytes. Lipids 2022, 57 (3), 183–195. 10.1002/lipd.12342. [DOI] [PubMed] [Google Scholar]
- Uchida Y.; Park K. Ceramides in Skin Health and Disease: An Update. Am. J. Clin Dermatol 2021, 22 (6), 853–866. 10.1007/s40257-021-00619-2. [DOI] [PubMed] [Google Scholar]
- Li Q.; Fang H.; Dang E.; Wang G. The Role of Ceramides in Skin Homeostasis and Inflammatory Skin Diseases. J. Dermatol Sci. 2020, 97 (1), 2–8. 10.1016/j.jdermsci.2019.12.002. [DOI] [PubMed] [Google Scholar]
- van Smeden J.; Boiten W. A.; Hankemeier T.; Rissmann R.; Bouwstra J. A.; Vreeken R. J. Combined LC/MS-Platform for Analysis of All Major Stratum Corneum Lipids, and the Profiling of Skin Substitutes. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2014, 1841 (1), 70–79. 10.1016/j.bbalip.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Wertz P. W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22 (10), 5229. 10.3390/ijms22105229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr N. J.; Khan M. H.; Edney M. K.; Trindade G. F.; Kern S.; Pirkl A.; Kleine-Boymann M.; Elms C.; O’Mahony M. M.; Bell M.; Alexander M. R.; Scurr D. J. Elucidating the Molecular Landscape of the Stratum Corneum. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (12), e2114380119 10.1073/pnas.2114380119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney M. H.; Kalan L. R. Living in Your Skin: Microbes, Molecules, and Mechanisms. Infect. Immun. 2021, 89 (4), 00695-20. 10.1128/IAI.00695-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Tryon T. A.; Grice E. A. Microbiota and Maintenance of Skin Barrier Function. Science (1979) 2022, 376 (6596), 940–945. 10.1126/science.abo0693. [DOI] [PubMed] [Google Scholar]
- Harris-Tryon T. A.; Grice E. A. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376 (6596), 940–945. 10.1126/science.abo0693. [DOI] [PubMed] [Google Scholar]
- Proksch E. PH in Nature, Humans and Skin. J. Dermatol 2018, 45 (9), 1044–1052. 10.1111/1346-8138.14489. [DOI] [PubMed] [Google Scholar]
- Čuříková-Kindlová B. A.; Vovesná A.; Nováčková A.; Zbytovská J. In Vitro Modeling of Skin Barrier Disruption and Its Recovery by Ceramide-Based Formulations. AAPS PharmSciTech 2022, 23 (1), 21. 10.1208/s12249-021-02154-z. [DOI] [PubMed] [Google Scholar]
- Vovesná A.; Zhigunov A.; Balouch M.; Zbytovská J. Ceramide Liposomes for Skin Barrier Recovery: A Novel Formulation Based on Natural Skin Lipids. Int. J. Pharm. 2021, 596, 120264 10.1016/j.ijpharm.2021.120264. [DOI] [PubMed] [Google Scholar]
- Draelos Z.; Baalbaki N.; Cook S.; Raab S.; Colón G. The Effect of a Ceramide-Containing Product on Stratum Corneum Lipid Levels in Dry Legs. Journal of Drugs in Dermatology 2020, 19 (4), 372–376. 10.36849/JDD.2020.4796. [DOI] [PubMed] [Google Scholar]
- Huth S.; Schmitt L.; Marquardt Y.; Heise R.; Lüscher B.; Amann P. M.; Baron J. M. Effects of a Ceramide-Containing Water-in-Oil Ointment on Skin Barrier Function and Allergen Penetration in an IL-31 Treated 3D Model of the Disrupted Skin Barrier. Exp Dermatol 2018, 27 (9), 1009–1014. 10.1111/exd.13697. [DOI] [PubMed] [Google Scholar]
- Matsuoka M.; Okoshi K.; Ito S.; Kume T.; Seki T.; Nishizaka T.; Okada J.; Nagasawa A.; Iijima M.; Abe M.; Nemoto O. Efficacy of Heparinoid Cream Containing Pseudo-Ceramide for Remission of Atopic Dermatitis. Clin Cosmet Investig Dermatol 2021, 14, 1839–1847. 10.2147/CCID.S337930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. M.; Lee B.-M. Safety and Risk Assessment of Ceramide 3 in Cosmetic Products. Food Chem. Toxicol. 2015, 84, 8–17. 10.1016/j.fct.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Tamura E.; Ishikawa J.; Yasuda Y.; Yamamoto T. The Efficacy of Synthetic Pseudo-ceramide for Dry and Rough Lips. Int. J. Cosmet Sci. 2021, 43 (2), 158–164. 10.1111/ics.12677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwara A.; Wertheim-Tysarowska K.; Mika A. Alterations of Ultra Long-Chain Fatty Acids in Hereditary Skin Diseases—Review Article. Front Med. (Lausanne) 2021, 8, 730855 10.3389/fmed.2021.730855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwstra J. Structure of the Skin Barrier and Its Modulation by Vesicular Formulations. Prog. Lipid Res. 2003, 42 (1), 1–36. 10.1016/S0163-7827(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Elias P. M.; Williams M. L.; Choi E. H.; Feingold K. R. Role of Cholesterol Sulfate in Epidermal Structure and Function: Lessons from X-Linked Ichthyosis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2014, 1841 (3), 353–361. 10.1016/j.bbalip.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandrei F.; Engberg O.; Opálka L.; Jančálková P.; Pullmannová P.; Steinhart M.; Kováčik A.; Vávrová K.; Huster D. Cholesterol Sulfate Fluidizes the Sterol Fraction of the Stratum Corneum Lipid Phase and Increases Its Permeability. J. Lipid Res. 2022, 63 (3), 100177. 10.1016/j.jlr.2022.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponec M.; Williams M. L. Cholesterol Sulfate Uptake and Outflux in Cultured Human Keratinocytes. Arch Dermatol Res. 1986, 279 (1), 32–36. 10.1007/BF00404355. [DOI] [PubMed] [Google Scholar]
- Fujii M. The Pathogenic and Therapeutic Implications of Ceramide Abnormalities in Atopic Dermatitis. Cells 2021, 10 (9), 2386. 10.3390/cells10092386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imokawa G.; Terrinoni A. Cutting Edge of the Pathogenesis of Atopic Dermatitis: Sphingomyelin Deacylase, the Enzyme Involved in Its Ceramide Deficiency, Plays a Pivotal Role. International Journal of Molecular Sciences 2021, Vol. 22, Page 1613 2021, 22 (4), 1613. 10.3390/ijms22041613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M.; Sassa T.; Kyono Y.; Uemura H.; Kugo M.; Hayashi H.; Imai Y.; Yamanishi K.; Kihara A. Comprehensive Stratum Corneum Ceramide Profiling Reveals Reduced Acylceramides in Ichthyosis Patient with CERS3Mutations. J. Dermatol 2021, 48 (4), 447–456. 10.1111/1346-8138.15725. [DOI] [PubMed] [Google Scholar]
- Jia Z. X.; Zhang J. L.; Shen C. P.; Ma L. Profile and Quantification of Human Stratum Corneum Ceramides by Normal-Phase Liquid Chromatography Coupled with Dynamic Multiple Reaction Monitoring of Mass Spectrometry: Development of Targeted Lipidomic Method and Application to Human Stratum Corneum of Different Age Groups. Anal. Bioanal. Chem. 2016, 408 (24), 6623–6636. 10.1007/s00216-016-9775-6. [DOI] [PubMed] [Google Scholar]
- Masukawa Y.; Narita H.; Shimizu E.; Kondo N.; Sugai Y.; Oba T.; Homma R.; Ishikawa J.; Takagi Y.; Kitahara T.; Takema Y.; Kita K. Characterization of Overall Ceramide Species in Human Stratum Corneum. J. Lipid Res. 2008, 49 (7), 1466–1476. 10.1194/jlr.M800014-JLR200. [DOI] [PubMed] [Google Scholar]
- Janůšová B.; Zbytovská J.; Lorenc P.; Vavrysová H.; Palát K.; Hrabálek A.; Vávrová K. Effect of Ceramide Acyl Chain Length on Skin Permeability and Thermotropic Phase Behavior of Model Stratum Corneum Lipid Membranes. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2011, 1811 (3), 129–137. 10.1016/j.bbalip.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Novotný J.; Janůšová B.; Novotný M.; Hrabálek A.; Vávrová K. Short-Chain Ceramides Decrease Skin Barrier Properties. Skin Pharmacol Physiol 2009, 22 (1), 22–30. 10.1159/000183923. [DOI] [PubMed] [Google Scholar]
- Mojumdar E. H.; Kariman Z.; van Kerckhove L.; Gooris G. S.; Bouwstra J. A. The Role of Ceramide Chain Length Distribution on the Barrier Properties of the Skin Lipid Membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes 2014, 1838 (10), 2473–2483. 10.1016/j.bbamem.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Haque T.; Talukder M. M. U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8 (2), 169–179. 10.15171/apb.2018.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.; Wei T.; Goldberg H.; Wang W.; Cullion K.; Kohane D. S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29 (37), 1606596. 10.1002/adma.201606596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A.; Pechtold L. A. R. M.; Potts R. O.; Guy R. H. Mechanism of Oleic Acid-Induced Skin Penetration Enhancement in Vivo in Humans. J. Controlled Release 1995, 37 (3), 299–306. 10.1016/0168-3659(95)00088-7. [DOI] [Google Scholar]
- Jiang S. J.; Zhou X. J. Examination of the Mechanism of Oleic Acid-Induced Percutaneous Penetration Enhancement: An Ultrastructural Study. Biol. Pharm. Bull. 2003, 26 (1), 66–68. 10.1248/bpb.26.66. [DOI] [PubMed] [Google Scholar]
- Simard M.; Tremblay A.; Morin S.; Martin C.; Julien P.; Fradette J.; Flamand N.; Pouliot R. α-Linolenic Acid and Linoleic Acid Modulate the Lipidome and the Skin Barrier of a Tissue-Engineered Skin Model. Acta Biomater 2022, 140, 261–274. 10.1016/j.actbio.2021.11.021. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Cao Y.; Meng X.; Li C.; Wang H.; Zhang S. Enhancement of Transdermal Delivery of Artemisinin Using Microemulsion Vehicle Based on Ionic Liquid and Lidocaine Ibuprofen. Colloids Surf. B Biointerfaces 2020, 189, 110886. 10.1016/j.colsurfb.2020.110886. [DOI] [PubMed] [Google Scholar]
- Islam Md. R.; Uddin S.; Chowdhury Md. R.; Wakabayashi R.; Moniruzzaman M.; Goto M. Insulin Transdermal Delivery System for Diabetes Treatment Using a Biocompatible Ionic Liquid-Based Microemulsion. ACS Appl. Mater. Interfaces 2021, 13 (36), 42461–42472. 10.1021/acsami.1c11533. [DOI] [PubMed] [Google Scholar]
- Park C.; Zuo J.; Somayaji V.; Lee B.-J.; Löbenberg R. Development of a Novel Cannabinoid-Loaded Microemulsion towards an Improved Stability and Transdermal Delivery. Int. J. Pharm. 2021, 604, 120766 10.1016/j.ijpharm.2021.120766. [DOI] [PubMed] [Google Scholar]
- Assaf S.; Maaroof K.; Altaani B.; Ghareeb M.; Abu Alhayyal A. Jojoba Oil-Based Microemulsion for Transdermal Drug Delivery. Res. Pharm. Sci. 2021, 16 (4), 326. 10.4103/1735-5362.319572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P.; Pol A.; Kalaria D.; Date A. A.; Kalia Y.; Patravale V. Microemulsion-Based Gel for the Transdermal Delivery of Rasagiline Mesylate: In Vitro and in Vivo Assessment for Parkinson’s Therapy. Eur. J. Pharm. Biopharm. 2021, 165, 66–74. 10.1016/j.ejpb.2021.04.026. [DOI] [PubMed] [Google Scholar]
- Gunnarsson M.; Mojumdar E. H.; Topgaard D.; Sparr E. Extraction of Natural Moisturizing Factor from the Stratum Corneum and Its Implication on Skin Molecular Mobility. J. Colloid Interface Sci. 2021, 604, 480–491. 10.1016/j.jcis.2021.07.012. [DOI] [PubMed] [Google Scholar]
- Celleno L. Topical Urea in Skincare: A Review. Dermatol Ther 2018, 31 (6), e12690 10.1111/dth.12690. [DOI] [PubMed] [Google Scholar]
- Piquero-Casals J.; Morgado-Carrasco D.; Granger C.; Trullàs C.; Jesús-Silva A.; Krutmann J. Urea in Dermatology: A Review of Its Emollient, Moisturizing, Keratolytic, Skin Barrier Enhancing and Antimicrobial Properties. Dermatol Ther (Heidelb) 2021, 11 (6), 1905–1915. 10.1007/s13555-021-00611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intarakumhaeng R.; Alsheddi L.; Wanasathop A.; Shi Z.; Li S. K. Skin Permeation of Urea Under Finite Dose Condition. J. Pharm. Sci. 2019, 108 (2), 987–995. 10.1016/j.xphs.2018.10.026. [DOI] [PubMed] [Google Scholar]
- Franz T. J. The Finite Dose Technique as a Valid in Vitro Model for the Study of Percutaneous Absorption in Man. Curr. Probl Dermatol. Basel, Karger 1979, 7, 58–68. 10.1159/000401276. [DOI] [PubMed] [Google Scholar]
- Mueller J.; Oliveira J. S. L.; Barker R.; Trapp M.; Schroeter A.; Brezesinski G.; Neubert R. H. H. The Effect of Urea and Taurine as Hydrophilic Penetration Enhancers on Stratum Corneum Lipid Models. Biochimica et Biophysica Acta (BBA) - Biomembranes 2016, 1858 (9), 2006–2018. 10.1016/j.bbamem.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Oliveira M. C.; Yusupov M.; Bogaerts A.; Cordeiro R. M. Molecular Dynamics Simulations of Mechanical Stress on Oxidized Membranes. Biophys Chem. 2019, 254, 106266. 10.1016/j.bpc.2019.106266. [DOI] [PubMed] [Google Scholar]
- Åberg C.; Wennerström H.; Sparr E. Transport Processes in Responding Lipid Membranes: A Possible Mechanism for the PH Gradient in the Stratum Corneum. Langmuir 2008, 24 (15), 8061–8070. 10.1021/la800543r. [DOI] [PubMed] [Google Scholar]
- Pham Q. D.; Björklund S.; Engblom J.; Topgaard D.; Sparr E. Chemical Penetration Enhancers in Stratum Corneum — Relation between Molecular Effects and Barrier Function. J. Controlled Release 2016, 232, 175–187. 10.1016/j.jconrel.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Hédoux A.; Krenzlin S.; Paccou L.; Guinet Y.; Flament M.-P.; Siepmann J. Influence of Urea and Guanidine Hydrochloride on Lysozyme Stability and Thermal Denaturation; a Correlation between Activity, Protein Dynamics and Conformational Changes. Phys. Chem. Chem. Phys. 2010, 12 (40), 13189. 10.1039/c0cp00602e. [DOI] [PubMed] [Google Scholar]
- Ikkai F.; Naito S. Dynamic Light Scattering and Circular Dichroism Studies on Heat-Induced Gelation of Hard-Keratin Protein Aqueous Solutions. Biomacromolecules 2002, 3 (3), 482–487. 10.1021/bm010160i. [DOI] [PubMed] [Google Scholar]
- Panuszko A.; Bruździak P.; Zielkiewicz J.; Wyrzykowski D.; Stangret J. Effects of Urea and Trimethylamine- N-Oxide on the Properties of Water and the Secondary Structure of Hen Egg White Lysozyme. J. Phys. Chem. B 2009, 113 (44), 14797–14809. 10.1021/jp904001m. [DOI] [PubMed] [Google Scholar]
- Muthuselvi L.; Miller R.; Dhathathreyan A. How Does Urea Really Denature Myoglobin?. Chem. Phys. Lett. 2008, 465 (1–3), 126–130. 10.1016/j.cplett.2008.09.037. [DOI] [Google Scholar]
- Hillier S. G.; Lathe R. Terpenes, Hormones and Life: Isoprene Rule Revisited. Journal of Endocrinology 2019, 242 (2), R9–R22. 10.1530/JOE-19-0084. [DOI] [PubMed] [Google Scholar]
- McGenity T. J.; Crombie A. T.; Murrell J. C. Microbial Cycling of Isoprene, the Most Abundantly Produced Biological Volatile Organic Compound on Earth. ISME J. 2018, 12 (4), 931–941. 10.1038/s41396-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtunik-Kulesza K. A.; Kasprzak K.; Oniszczuk T.; Oniszczuk A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers 2019, 16 (12), e1900434 10.1002/cbdv.201900434. [DOI] [PubMed] [Google Scholar]
- Yang D.; Liu C.; Quan P.; Fang L. A Systematic Approach to Determination of Permeation Enhancer Action Efficacy and Sites: Molecular Mechanism Investigated by Quantitative Structure–activity Relationship. J. Controlled Release 2020, 322, 1–12. 10.1016/j.jconrel.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Chen J.; Jiang Q.-D.; Chai Y.-P.; Zhang H.; Peng P.; Yang X.-X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21 (12), 1709. 10.3390/molecules21121709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard E. C.; Tfayli A.; Laugel C.; Baillet-Guffroy A. Molecular Interactions of Penetration Enhancers within Ceramides Organization: A FTIR Approach. European Journal of Pharmaceutical Sciences 2009, 36 (2–3), 192–199. 10.1016/j.ejps.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Li Y.; Xu F.; Gu W.; Yan G.; Dong J.; Chen J. Skin Electrical Resistance Measurement of Oxygen-Containing Terpenes as Penetration Enhancers: Role of Stratum Corneum Lipids. Molecules 2019, Vol. 24, Page 523 2019, 24 (3), 523. 10.3390/molecules24030523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. J.; Heylings J. R.; McCarthy T. J.; Correa C. M. Development of an in Vitro Model for Studying the Penetration of Chemicals through Compromised Skin. Toxicology in Vitro 2015, 29 (1), 176–181. 10.1016/j.tiv.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Guth K.; Schäfer-Korting M.; Fabian E.; Landsiedel R.; van Ravenzwaay B. Suitability of Skin Integrity Tests for Dermal Absorption Studies in Vitro. Toxicology in Vitro 2015, 29 (1), 113–123. 10.1016/j.tiv.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Machado M.; Salgado T. M.; Hadgraft J.; Lane M. E. The Relationship between Transepidermal Water Loss and Skin Permeability. Int. J. Pharm. 2010, 384 (1–2), 73–77. 10.1016/j.ijpharm.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Huang C.; Wang H.; Tang L.; Meng F. Penetration Enhancement of Menthol on Quercetin through Skin: Insights from Atomistic Simulation. J. Mol. Model. 2019, 25 (8), 235. 10.1007/s00894-019-4135-z. [DOI] [PubMed] [Google Scholar]
- Xie F.; Chai J. K.; Hu Q.; Yu Y. H.; Ma L.; Liu L. Y.; Zhang X. L.; Li B. L.; Zhang D. H. Transdermal Permeation of Drugs with Differing Lipophilicity: Effect of Penetration Enhancer Camphor. Int. J. Pharm. 2016, 507 (1–2), 90–101. 10.1016/j.ijpharm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Surber C.; Wilhelm K. P.; Hori M.; Maibach H. I.; Guy R. H. Optimization of Topical Therapy: Partitioning of Drugs into Stratum Corneum. Pharm. Res. 1990, 7 (12), 1320–1324. 10.1023/A:1015958526423. [DOI] [PubMed] [Google Scholar]
- Yi Q.-F.; Yan J.; Tang S.-Y.; Huang H.; Kang L.-Y. Effect of Borneol on the Transdermal Permeation of Drugs with Differing Lipophilicity and Molecular Organization of Stratum Corneum Lipids. Drug Dev. Ind. Pharm. 2016, 42 (7), 1086–1093. 10.3109/03639045.2015.1107095. [DOI] [PubMed] [Google Scholar]
- Aziz Z. A. A.; Ahmad A.; Setapar S. H. M.; Karakucuk A.; Azim M. M.; Lokhat D.; Rafatullah M.; Ganash M.; Kamal M. A.; Ashraf G. M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential - A Review. Curr. Drug Metab 2018, 19 (13), 1100–1110. 10.2174/1389200219666180723144850. [DOI] [PubMed] [Google Scholar]
- Dehsheikh A. B.; Sourestani M. M.; Dehsheikh P. B.; Mottaghipisheh J.; Vitalini S.; Iriti M. Monoterpenes: Essential Oil Components with Valuable Features. Mini-Reviews in Medicinal Chemistry 2020, 20 (11), 958–974. 10.2174/1389557520666200122144703. [DOI] [PubMed] [Google Scholar]
- Gunstone F. D.Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; Blackwell Publishing Ltd., 2021. [Google Scholar]
- Amra K.; Momin M.; Desai N.; Khan F. Therapeutic Benefits of Natural Oils along with Permeation Enhancing Activity. Int. J. Dermatol 2022, 61 (4), 484–507. 10.1111/ijd.15733. [DOI] [PubMed] [Google Scholar]
- Kshirsagar P. R.; Patil S. M.. Phytochemistry and Pharmacology of Mesua ferrea L. In Bioactive Compounds in Underutilized Fruits and Nuts; Murthy H. N., Bapat V. A., Eds.; Springer Cham, 2020; pp 223–256. [Google Scholar]
- Chahar M. K.; Sanjaya Kumar D. S.; Lokesh T.; Manohara K. P. In-Vivo Antioxidant and Immunomodulatory Activity of Mesuol Isolated from Mesua Ferrea L. Seed Oil. Int. Immunopharmacol 2012, 13 (4), 386–391. 10.1016/j.intimp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Koranappallil B.; Rameshkumar Abdulkhader Hisham L. P. G.. Recent Progress in Medicinal Plants: Chemistry and Therapeutic Potential of Chaulmoogra Oil; Studium Press, 2011; Vol. 33. [Google Scholar]
- Sengupta A.; Gupta J. K.; Dutta J.; Ghosh A. The Component Fatty Acids of Chaulmoogra Oil. J. Sci. Food Agric 1973, 24 (6), 669–674. 10.1002/jsfa.2740240606. [DOI] [PubMed] [Google Scholar]
- Norton S. A. Useful Plants of Dermatology. I. Hydnocarpus and Chaulmoogra. J. Am. Acad. Dermatol 1994, 31 (4), 683–686. 10.1016/S0190-9622(08)81744-6. [DOI] [PubMed] [Google Scholar]
- Kar H. K.; Gupta R. Treatment of Leprosy. Clin Dermatol 2015, 33 (1), 55–65. 10.1016/j.clindermatol.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Sahoo M. R.; Dhanabal S. P.; Jadhav A. N.; Reddy V.; Muguli G.; Babu U. V.; Rangesh P. Hydnocarpus: An Ethnopharmacological, Phytochemical and Pharmacological Review. J. Ethnopharmacol 2014, 154 (1), 17–25. 10.1016/j.jep.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Li J.; Xiang H.; Zhang Q.; Miao X. Polysaccharide-Based Transdermal Drug Delivery. Pharmaceuticals 2022, 15 (5), 602. 10.3390/ph15050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes de Moraes F.; Trauthman S. C.; Zimmer F.; Pacheco P. P.; Pont Morisso F. D.; Ziulkoski A. L.; Κanis L. A.; Zepon K. M. A Polysaccharide-Based Hydrogel as a Green Platform for Enhancing Transdermal Delivery. Sustain. Chem. Pharm. 2022, 25, 100604 10.1016/j.scp.2022.100604. [DOI] [Google Scholar]
- Rial-Hermida M. I.; Rey-Rico A.; Blanco-Fernandez B.; Carballo-Pedrares N.; Byrne E. M.; Mano J. F. Recent Progress on Polysaccharide-Based Hydrogels for Controlled Delivery of Therapeutic Biomolecules. ACS Biomater Sci. Eng. 2021, 7 (9), 4102–4127. 10.1021/acsbiomaterials.0c01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.; Tyagi S.; Gupta S. K.; Kulkarni G. T.; Bhatnagar A.; Kumar N. Development and Gamma-Scintigraphy Study of Hibiscus Rosasinensis Polysaccharide-Based Microspheres for Nasal Drug Delivery. Drug Dev. Ind. Pharm. 2016, 42 (11), 1763–1771. 10.3109/03639045.2016.1173050. [DOI] [PubMed] [Google Scholar]
- Sudha P. N.; Rose M. H. Beneficial Effects of Hyaluronic Acid. Adv. Food Nutr. Res. 2014, 72, 137–176. 10.1016/B978-0-12-800269-8.00009-9. [DOI] [PubMed] [Google Scholar]
- Karami M.; Shahraky M. K.; Ranjbar M.; Tabandeh F.; Morshedi D.; Aminzade S. Preparation, Purification, and Characterization of Low-Molecular-Weight Hyaluronic Acid. Biotechnol. Lett. 2021, 43 (1), 133–142. 10.1007/s10529-020-03035-4. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Tang X.; Jia Y.; Ho C.-T.; Huang Q. Applications and Delivery Mechanisms of Hyaluronic Acid Used for Topical/Transdermal Delivery – A Review. Int. J. Pharm. 2020, 578, 119127. 10.1016/j.ijpharm.2020.119127. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Wei D.; Xu Y.; Zhu Q. Hyaluronic Acid in Ocular Drug Delivery. Carbohydr. Polym. 2021, 264, 118006. 10.1016/j.carbpol.2021.118006. [DOI] [PubMed] [Google Scholar]
- Buwalda S. J.; Boere K. W. M.; Dijkstra P. J.; Feijen J.; Vermonden T.; Hennink W. E. Hydrogels in a Historical Perspective: From Simple Networks to Smart Materials. J. Controlled Release 2014, 190, 254–273. 10.1016/j.jconrel.2014.03.052. [DOI] [PubMed] [Google Scholar]
- Cao H.; Duan L.; Zhang Y.; Cao J.; Zhang K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct Target Ther 2021, 6 (1), 426. 10.1038/s41392-021-00830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A.; Ukai H.; Morishita M.; Katsumi H. Approaches to Improve Intestinal and Transmucosal Absorption of Peptide and Protein Drugs. Pharmacol Ther 2020, 211, 107537. 10.1016/j.pharmthera.2020.107537. [DOI] [PubMed] [Google Scholar]
- Essendoubi M.; Gobinet C.; Reynaud R.; Angiboust J. F.; Manfait M.; Piot O. Human Skin Penetration of Hyaluronic Acid of Different Molecular Weights as Probed by Raman Spectroscopy. Skin Research and Technology 2016, 22 (1), 55–62. 10.1111/srt.12228. [DOI] [PubMed] [Google Scholar]
- Yan L. H.; Zhang Y. J.; Hu H. J.; Zhang C.; Wang Y.; Xu X. T.; Zhang T. C.; Su R.; Luo X. G. Enhanced Transdermal Absorption of Hyaluronic Acid via Fusion with Pep-1 and a Hyaluronic Acid Binding Peptide. Macromol. Biosci 2023, 23 (3), 2200173. 10.1002/mabi.202200173. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zhang Z.; Xin Y.; Zhou R.; Jiang K.; Sun X.; He D.; Song J.; Zhang Y. Synergistic Transdermal Delivery of Nanoethosomes Embedded in Hyaluronic Acid Nanogels for Enhancing Photodynamic Therapy. Nanoscale 2020, 12 (28), 15435–15442. 10.1039/D0NR03494K. [DOI] [PubMed] [Google Scholar]
- How K. N.; Yap W. H.; Lim C. L. H.; Goh B. H.; Lai Z. W. Hyaluronic Acid-Mediated Drug Delivery System Targeting for Inflammatory Skin Diseases: A Mini Review. Front. Pharmacol. 2020, 11, 1105. 10.3389/fphar.2020.01105. [DOI] [PMC free article] [PubMed] [Google Scholar]