Abstract
Neuroendocrine prostate cancer, generally arising late in the disease trajectory, is a heterogenous subtype that infers a worse prognosis and limited treatment options for patients. Characterization of the complex landscape of this disease subtype and scrutiny of the relationship between tumor cells and cells of the surrounding tumor microenvironment have aided in elucidating some of the mechanisms of neuroendocrine disease biology and have uncovered a multitude of signaling pathways involved in disease transdifferentiation under therapeutic selection. In this review, we discuss current efforts to better understand the heterogenous landscape of neuroendocrine prostate cancer and summarize research efforts to define the interplay between tumor cells and the microenvironment, with an emphasis on the immune component. Research efforts have uncovered several potential therapeutic approaches that may improve disease outcomes for patients diagnosed with neuroendocrine prostate cancer, including the potential for combination immunotherapies. However, additional research is required to fully address and exploit the contribution of tumor cell and microenvironment heterogeneity in developing effective treatment strategies.
Keywords: Neuroendocrine prostate cancer, heterogeneity, tumor microenvironment, androgen receptor, phenotype, immune composition
Introduction
In 2022, it is estimated that there will be more than 260,000 new diagnoses of prostate cancer (PC) and 34,000 deaths from this disease in the United States alone (Siegel et al., 2022). PC is the second most common cause of cancer related mortality in men after lung cancer, with cases of regional and distant metastases noted to be rising over the past decade (Siegel et al., 2022). While survival rates for localized disease are promising, the onset of disease spread contributes significant risk to the patient and increases the mortality burden. The majority of de novo PCs are driven by the androgen receptor (AR), a transcription factor activated by androgenic hormones such as testosterone and dihydrotestosterone (DHT). Testosterone, the most common androgen, is metabolized into DHT, which is a more active hormone capable of increased AR binding (Feldman and Feldman, 2001). Androgen deprivation therapy (ADT), an anti-hormone therapy suppressing androgen synthesis, has become the mainstay of treatment for progressive PC, leading to decreased levels of serum testosterone (Yang et al., 2016). The emergence of second-generation AR signaling inhibitors (ARSI) such as enzalutamide and abiraterone acetate have further contributed to the treatment spectrum of hormone driven PC. However, eventually, patients will become resistant to current AR targeting therapies, leading to a disease state termed castration resistant prostate cancer (CRPC) during which PC cells can adapt to low androgen status and continue to proliferate (Montgomery et al., 2008). Metastatic CRPC responds briefly to chemotherapy, and specific subtypes such as those with BRCA2 mutations can be treated with targeted therapies, but overall metastatic CRPC is incurable.
Recent research efforts have focused on understanding the complex landscape of CRPC. In 2017, Bluemn et al., undertook in-depth genomic and phenotypic analyses of Rapid Autopsy CRPC tumors derived from the University of Washington and determined that while the majority of treatment resistant CRPCs retain AR signaling (ARPC), there was an increase in both small cell or neuroendocrine prostate cancer (NEPC) and double negative prostate cancer (DNPC) over a 20 year time span (Bluemn et al., 2017). These findings noted that cells were adapting under therapeutic pressure to no longer rely on AR for growth and were undergoing a process of transdifferentiation to divergent phenotypes with distinct molecular profiles (Bluemn et al., 2017).
The occurrence of de novo NEPC is extremely rare, and more frequently develops later in the disease trajectory, often as a mechanism of treatment resistance. A subset of NEPCs exhibit differentiated small cell carcinoma histology, with other NEPCs distinguished primarily by the expression of neuroendocrine-associated transcription factors and neuroendocrine factors such as chromogranin, as well as subsets with combinations of prostate adenocarcinoma mixed with small cell or neuroendocrine subtypes (Conteduca et al., 2019). Notably, the NEPC phenotype often exhibits the hallmark gene expression patterns and genomic features of small cell carcinoma, such as TP53 and RB1 loss (Beltran et al., 2016), which are associated with poor outcomes for patients. Cell line experiments observed that restriction of REST activity (a potent suppressor of neuroendocrine gene expression) is not sufficient alone in driving the onset of small cell PC disease (Labrecque et al., 2019). In recent years, consensus guidelines have been published to aid in the identification of neuroendocrine tumors, noting that morphology and underlying genomic characteristics can vary based on distinct anatomical sites and how organ specific neoplasms arise (Rindi et al., 2018). General pathology classification guidelines from the North American Neuroendocrine Tumor Society for poorly differentiated neuroendocrine tumors suggest a range of pathology criteria which include immunohistochemical staining for cytokeratin and neuroendocrine markers synaptophysin and chromogranin A, and in-depth clinical guidelines from diagnosis to continuance of care are also provided (Kunz et al., 2013). Multiple studies and review articles have further described the mechanisms of cellular plasticity involved in the proposed NEPC differentiation, with both genomic and epigenomic features at play, highlighting the often heterogenous landscape of this disease subtype.
As noted above, epigenetic reprogramming may have a key role to play in cellular plasticity. Analysis in genetically engineered mouse models described a synergistic relationship between Rb1 and MYCN that promoted the overexpression of N-Myc and the emergence of a neuroendocrine-like phenotype that incurred alterations to chromatin binding (Brady et al., 2021b). Studies have noted that reprogramming of the AR cistrome allows epigenetic regulator EZH2 to drive neuronal gene expression and stem cell factors, assisting lineage plasticity (Davies et al., 2021). In addition to EZH2, FOXA1, a transcription factor involved in AR chromatin binding, can become reprogrammed to enhance neuroendocrine driven proliferation and the expression of neuroendocrine regulated genes (Baca et al., 2021). Improved insight into the complex characteristics of this disease state is key to the development of therapeutic options for patients.
A pan-cancer analysis of neuroendocrine tumors observed transcription factor ONECUT2 to be a regulator of poorly differentiated neuroendocrine tumors that are associated with poor prognosis. Expression of ONECUT2 causes an upregulation of hypoxia related genes with the authors noting the potential for use of therapies that target hypoxic conditions (Guo et al., 2019). Additionally, the transcription factor E2F1 has also been described as a potential driver of neuroendocrine lineage plasticity in partnership with bromodomain chromatin reader BRD4, with cell line experiments observing a decrease in neuroendocrine cell growth after treatment with a BET inhibitor which paved the way for a future clinical trial (Kim et al., 2021). Molecular imaging studies have further emphasized a divergence of NEPC from ARPCs with evidence demonstrating the limited accuracy of PSMA-targeted imaging methods to detect and monitor patients with NEPC. A study examining over 900 tumors determined that FOLH1 (the gene encoding for PSMA) expression was low in some NEPC tumors profiled thus making PSMA-targeted imaging more difficult in this cohort (Bakht et al., 2018). The authors described a potential future candidate, SSTR2, that could improve image-based monitoring for patients with NEPC (Bakht et al., 2018).
While ARPC and NEPC tumors may comprise homogeneous populations of neoplastic cells with uniform genomic and phenotypic features, others are clearly substantially more complex and may be comprised of mixtures of ARPC and NEPC cell types or include ‘amphicrine’ tumor cells that individually express both ARPC and NEPC programs (Labrecque et al., 2019, Brady et al., 2021a). There is also evidence that NEPCs, as with small cell lung cancer, may be driven by distinct differentiation drivers such as those expressing the lineage-distinguishing transcription factors ASCL1 versus NEUROD1. Improved understanding of this complicated heterogenous landscape of NEPC and the contribution of the surrounding microenvironment is essential for improving outcomes for this patient population and deriving targetable treatment strategies that consider intra-tumor diversity manifest by tumor cell heterogeneity and variation in the composition of the tumor microenvironment.
Heterogeneity of neuroendocrine prostate cancer
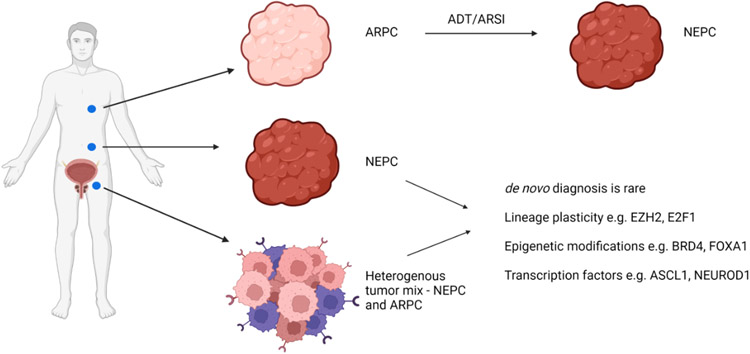
Efforts to develop targeted therapies for patients with NEPC, for whom other treatment options have been exhausted, are hampered by the aforementioned substantial heterogeneity that has emerged across this subtype (Figure 1). Of relevance for understanding the interplay between and within tumor subtypes, the genomic and phenotypic integrity of NEPC and other CRPC subtypes is maintained in patient derived xenograft models (PDX) and organoids allowing for continuous investigation into disease morphology and furthering advancements into treatment strategies (Beshiri et al., 2018). Digital spatial profiling of distinct sites of metastasis in patients with CRPC demonstrated considerable inter-patient heterogeneity with respect to the expression of gene signatures associated with different phenotypes of CRPC (Brady et al., 2021a). Notably, although intra-patient tumor lesions were primarily homogenous – there were exceptions. Intra-patient/inter-tumor heterogeneity was observed more commonly (Brady et al., 2021a), further complicating the treatment spectrum for this population.
Figure 1:
Schematic of intra-patient heterogenous tumor lesions and the emergence of NEPC.
Created with BioRender.com. ADT – androgen deprivation therapy, ARPC – androgen receptor driven prostate cancer, ARSI – androgen receptor signaling inhibitors, NEPC – neuroendocrine prostate cancer.
Whole genome sequencing analysis of NEPC tumors identified a distinct NEPC expression profile, with differential expression of genes such as E2F1 and CDKN2a, and NEPC tumors were less likely to exhibit AR gene amplification (Aggarwal et al., 2019). The same study also observed intra-patient heterogeneity among NEPC tumors, proposing that neuroendocrine differentiation emerges late into disease progression with the onset of distant metastases causing divergent CRPC phenotypes to co-exist (Aggarwal et al., 2019). Conversely, a recent study utilizing single-cell sequencing technology assessed tumor cells isolated from primary prostate adenocarcinoma, and local and distant CRPC cases, and noted that those patients with NEPC histology upon progression had a primary diagnosis of adenocarcinoma (Cheng et al., 2022). The authors observed a rare population of highly plastic NEPC cells present in hormone-naive early PC that strongly correlated with disease progression outcomes and demonstrated how lineage plasticity of these pre-existing cells can contribute to castration resistance in a process distinct from cell divergence under therapeutic pressure (Cheng et al., 2022). These findings suggest that multiple mechanisms for the onset of resistance exist, adding further complexity and requiring additional investigation into the causality of divergence involved in this disease state.
As outlined previously, NEPC demonstrates the hallmarks of small cell lung cancer, often exhibiting similar genomic and transcriptomic expression signatures. Advances in small cell lung cancer have focused on understanding the drivers of malignant disease and their associated gene signatures, advances that may be reproducible in NEPC. Small cell lung cancer, once understood to be almost homogenous in nature due to TP53 and RB1 loss, has been demonstrated to be an aggressive, heterogenous disease, subtypes of which can be delineated by differential expression of transcription factors such as YAP1, POU2F3, ASCL1 or NEUROD1 (Borromeo et al., 2016, Gay et al., 2021, Baine et al., 2020). Intra-patient heterogeneity defined by single cell sequencing of expression profiles of both ASCL1 and NEUROD1 in spatially distinct regions has also been observed (Gay et al., 2021). By interrogating chromatin binding sites in models of small cell lung cancer, it has been determined that ASCL1 and NEUROD1 subtypes can be further defined by differential gene expression patterns; MYCL1, RET, SOX2 and NFIB are driven by ASCL1 and MYC expression is driven by NEUROD1 (Borromeo et al., 2016). Immunohistochemical analysis of small cell lung cancer patient samples noted that ASCL1 and NEUROD1 subtypes were associated with high expression of a neuroendocrine associated marker (e.g., synaptophysin, INSM1) and high expression of DLL3, a member of the NOTCH signaling pathway with targeted therapeutic potential (Baine et al., 2020).
Similar improvements in the understanding of subtype heterogeneity in NEPC have been undertaken. When assessing NEPC tumors that evolved under therapeutic pressure, the chromatin landscape detailed two distinct NEPC subtypes, driven by either ASCL1 or NEUROD1 as observed in small cell lung cancer (Cejas et al., 2021). These subtypes appear homogenous in NEPC cell lines or PDX models, but intra-tumor heterogeneity has been established in patient samples with distinct genomic profiles associated with each subtype (Cejas et al., 2021); events that may further complicate treatment options. Redirection efforts to develop tangible therapeutic strategies have identified potential candidates linked to these NEPC subtypes. ASCL1 strongly correlates with CEACAM5 expression, a cell adhesion molecule present on the surface of NEPC cells, promoting CEACAM5 expression during reprogramming to neuroendocrine disease (DeLucia et al., 2021, Lee et al., 2018). Labetuzumab govitecan, an anti-CEACAM5 antibody drug conjugate, demonstrated efficacy in in vivo models of NEPC with robust responses observed in tumors treated with the drug compared to the controls, leading to the planning for a phase I/II clinical trial to further test the efficacy and safety of this treatment (DeLucia et al., 2021). Moreover, research detailing the promising effects of an additional antibody drug conjugate, SC16LD6.5, in treating NEPC by targeting DLL3 has been reported (Puca et al., 2019). DLL3 expression is strongly associated with neuroendocrine features and RB1 loss in NEPC, and in in vivo models, treatment with SC16LD6.5 elicited a complete response in DLL3 positive tumors compared to DLL3 negative tumors (Puca et al., 2019), demonstrating promise as a potential NEPC therapeutic candidate.
The influence of the microenvironment
While many research studies have focused on the transdifferentiation mechanisms involved in NEPC emergence and progression, and the complex genomic and epigenomic intricacies of this disease, research efforts are simultaneously focused on the interplay between the tumor microenvironment and tumor cells. The tumor microenvironment, a diverse network of blood vessels, immune cells, fibroblasts, and the extracellular matrix, is thought to have a role to play in PC progression and metastatic spread (Mo et al., 2018, Andersen et al., 2016). Notably in NEPC, treatment with hormonal based therapies, such as ADT, may modulate interactions with the tumor microenvironment One cell type in particular, cancer associated fibroblasts, an activated fibroblast subtype known to be involved in cellular plasticity and the secretion of factors involved with tumor development and metastasis (Ping et al., 2021), has been demonstrated to trigger the growth of primary PC tumors and aid in the metastatic spread in xenograft mouse models (Linxweiler et al., 2020). Epigenetic changes within these cancer associated fibroblasts are thought to be linked to NEPC reprogramming. Whole genome methylation analysis of fibroblasts isolated from PC tissue determined epigenetic silencing of RASAL3, a Ras inhibitor (Mishra et al., 2018). This silencing, induced by treatment with ADT, leads to a cascade of events which involves Ras activation, the induction of macropinocytosis, and glutamine synthesis. These effects allow stromal glutamine to mediate neuroendocrine differentiation by providing energy required by prostate epithelial cells, and glutamine was observed at increased levels in patients treated with ADT (Mishra et al., 2018), suggesting a role in the neuroendocrine differentiation process. Further examination of the ability of stromal cells to influence NEPC observed an additional potential mechanism, also thought to be triggered by castration resistance to ADT and ARSI treatment (Enriquez et al., 2021). The onset of castration conditions caused the upregulation of GRP78 causing microRNA (miR29-b) downregulation of SPARC, an extracellular matrix protein, in the surrounding stroma. The downregulation of SPARC prompted the production of IL-6, known to be involved in promoting a neuroendocrine environment. Promisingly, the authors demonstrated the ability of GRP78 to act as a NEPC therapeutic target by treating castrated mouse tumors with a potent inhibitor of GRP78, isoliquiritigenin, observing a decrease in neuroendocrine differentiation (Enriquez et al., 2021).
Heterogeneity also exists within the fibroblast populations present in CRPC, with a subset of fibroblasts expressing CD105, a membrane glycoprotein linked to epithelial mesenchymal transition (Kato et al., 2019). This subset of CD105 expressing cells effected the expression of SFRP1 which can regulate NEPC differentiation, as demonstrated in cell line models that when treated with SFRP1 exhibited increased expression of neuroendocrine related genes (Kato et al., 2019). Moreover, treatment with enzalutamide greatly increased CD105 cell surface expression on fibroblasts and epithelial cells (Kato et al., 2019), again suggesting that ADT incites the tumor microenvironment to encourage a neuroendocrine phenotype.
An essential intermediary of cancer associated fibroblasts is fibroblast activation protein (FAP) a protein that has potential utility as a prognostic marker and is associated with poor clinical outcomes. Knockdown of FAP in ovarian cancer models correlated with a decrease in cancer associated fibroblasts, suggesting its role as a key regulator of this cell type (Lai et al., 2012). By assessing PC tumors available through public database cBioportal, it was established that FAP was strongly correlated with worse overall survival in CRPC, and high expression of FAP was associated with strong neuroendocrine pathway scores and lower AR pathway scores (Vlachostergios et al., 2022) further demonstrating the interactions with the microenvironment. Additional examination of FAP by immunohistochemistry highlighted an increased expression pattern with the advancement of disease from primary to metastatic CRPC (Kesch et al., 2021). Importantly, imaging modalities targeting FAP ([68Ga] Ga-FAPI-04 PET/CT) has demonstrated strong positivity in CRPC and could potentially be used as theranostic strategy in future for patients with NEPC (Kesch et al., 2021).
The diverse interactions observed between stroma and fibroblasts of the tumor microenvironment and PC cells further delineates the multiplicity of cell signaling pathways involved in the evolution of NEPC. Moreover, it is clear that existing anti-androgen therapies aid in promoting a neuroendocrine rich environment suggesting that amending standard therapeutic approaches may be of benefit.
Tumor immune microenvironment of neuroendocrine tumors
An additional component of the tumor microenvironment is the diverse range of immune cells that are present and targeting immunogenicity of a tumor has gained considerable traction in recent years, a movement that could enable improved outcomes for NEPC. Of note, immunotherapies targeting immune checkpoint molecules, such as PD-1, PDL-1 and CTLA4, have been developed and investigated with effective tumor cell killing observed in certain cancer types (e.g. metastatic melanoma) leading to promising outcomes for eligible patients (Robert, 2020). However, the tumor immune microenvironment is diverse and distinct cancer types exhibit both ‘immune hot’ and ‘immune cold’ environments, with many ‘immune cold’ tumors having no durable response to treatment.
A recent review article succinctly described the immune landscape of CRPC, noting how it is generally ‘immune cold’ and how this immune suppressive environment may be attributed to the interplay between cells of the microenvironment and the function of different immune cell populations such as regulatory T cells and myeloid derived suppressor cells (Stultz and Fong, 2021). PC tumors have not exhibited robust responses to immune checkpoint inhibition: for example, for almost a decade research has established that CRPC tumor cells express extremely limited levels of PDL-1 on their surface (Taube et al., 2014). Extensive investigation into the tumor immune microenvironment of various neuroendocrine cancer types is ongoing to better understand these immune related complexities and has established heterogeneous expression profiles of immune cells and a generally ‘immune cold’ tumor microenvironment although some examples of immune rich environments have been documented. For example, in neuroendocrine pancreatic cancer, an aggressive, rare disease of which four distinct molecular subtypes have been described, only one subtype was demonstrated to have elevated immune expression after differential gene expression analysis and spatial profiling (Young et al., 2021). Moreover, analysis of pituitary neuroendocrine tumors found that chemokine signaling from the surrounding microenvironment recruits macrophages, and neutrophils to the tumor which can contribute to aggressive tumor behavior and increased invasive potential (Marques et al., 2019).
Subsets of immune cells thought to closely interact with tumor cells are linked to better disease outcomes and response to immunotherapies, however the interaction between neuroendocrine tumors and the tumor immune microenvironment is not well elucidated. Expression of PD-L1 and PD-1 was determined to be extremely rare in small intestine and pancreatic neuroendocrine tumors, although strong PD-L2 cytoplasmic expression was observed, the therapeutic significant of which is still under investigation (da Silva et al., 2018). T cell infiltrates (CD3+, CD8+, FOXP3+) were present in higher numbers in pancreatic neuroendocrine tumors, compared to small intestinal neuroendocrine tumors (da Silva et al., 2018). An additional study noted that higher numbers of T cells (CD3+ and CD8+) were primarily present outside the tumor interface, however, tumors with higher infiltration of T cells, specifically CD3+ T cells, were demonstrated to be significantly associated with progression free survival in gastroenteropancreatic neuroendocrine tumors (Baretti et al., 2021).
Small cell lung cancer is a prominent example of the ‘immune hot’ versus ‘immune cold’ tumor immune microenvironments observed across neuroendocrine cancer and can be subdivided into neuroendocrine high and neuroendocrine low subtypes. Immunohistochemical analysis performed to examine expression of prominent immune infiltrates, such as CD45, CD3, CD8 and TIM3, determined that the majority of immune cells present were confined to surrounding stromal compartments and not within the tumor mass (Dora et al., 2020). However, by comparing intra-tumoral immune expression between neuroendocrine low and neuroendocrine high subtypes, significantly higher expression was observed in the neuroendocrine low subtype (Dora et al., 2020).
Understanding the mechanisms involved in this interplay between tumor cells and immune cells of the microenvironment is important to improve treatment responses. Assessment of neuroendocrine tumors derived from distinct primary sites (e.g., lung, pancreas, stomach) described negative PDL-1 expression and very low expression of T cells (de Hosson et al., 2020), consistent with previous reports, and stromal cells present adjacent to tumor cells were determined by immunohistochemical staining to be cancer associated fibroblasts with the authors suggesting these cells could have a role to play in the maintenance of an ‘immune cold’ environment (de Hosson et al., 2020).
Additional immune cell subtypes are also thought to have a role to play in neuroendocrine disease, including macrophages. In a heterogenous neuroendocrine patient population (patients with mixed neuroendocrine and non-neuroendocrine neoplasms) it was established that macrophages were more commonly present in neuroendocrine lesions when compared to adenocarcinoma or mucinous carcinoma lesions, with CD68+ and CD163+ macrophages found to be present in higher numbers in intra-tumoral regions (Tsunokake et al., 2022). A recent study in small cell lung cancer observed tumor associated macrophages to be the most abundant cell type of the tumor immune microenvironment, exceeding T-cells in number (Dora et al., 2021). Although immune pathways are thought to be upregulated in neuroendocrine low tumors when compared to neuroendocrine high tumors, the same study observed two rare immune phenotypes - immune rich in a subset of neuroendocrine high tumors and immune low in a subset of neuroendocrine low tumors – that had distinct genomic profiles with potential as therapeutic targets (Dora et al., 2021).
Tumor immune microenvironments of neuroendocrine prostate cancer
As NEPC often reflects the complexities and diversity of other neuroendocrine tumors, understanding the tumor immune microenvironment is likely of great importance, however, the immune composition of NEPC remains poorly elucidated. NEPC is generally associated with visceral metastases, however the role the immune microenvironment of these organs may play in aiding the development of NEPC remains understudied and future research may aid in providing additional insight. In line with other neuroendocrine tumors, PC is thought to give rise to an immune suppressive microenvironment, a finding that has been frequently documented in non-neuroendocrine subtypes. Previous review articles have noted the ability of androgens to modulate the immune response, for example by suppressing anti-inflammatory cells such as dendritic cells and modifying regulatory T cell production (Gamat and McNeel, 2017, Trigunaite et al., 2015). Altering androgen levels with hormonal therapy and anti-androgens can promote an immune suppressive microenvironment. Treatment with ARSI enzalutamide blocked AR expression on myeloid derived suppressor cells, resulting in an increase in the immunosuppressive ability of the myeloid cells which limited adaptive immune response and increased tumor growth (Consiglio et al., 2020). However, promisingly, AR blockade inhibited AR expression on CD8+ T cells, reducing levels of T cell exhaustion and improving response to PD-1 directed immunotherapy (Guan et al., 2022). PC frequently metastasizes to the bone, an immune suppressive environment that is driven in part by myeloid derived suppressor cells that overexpress chemokine CCL20 and is further characterized by the presence of exhausted cytotoxic and helper T cells (Kfoury et al., 2021). In the DNPC subtype, an immune suppressive microenvironment is thought to involve recruitment of tumor associated macrophages and regulatory T cells by chemokine CCL2, itself regulated by polycomb repressor complex 1 (PRC1), that promotes immune suppression within the tumor (Su et al., 2019). In a PTEN-deficient mouse model of PC, knockout of Chd1 cumulated in a reduction of myeloid derived suppressor cells suggesting a role of CHD1 in driving an immune suppressive environment (Zhao et al., 2020).Anti-androgen treatment may contribute in part to the immune suppressive environment often observed in NEPC.
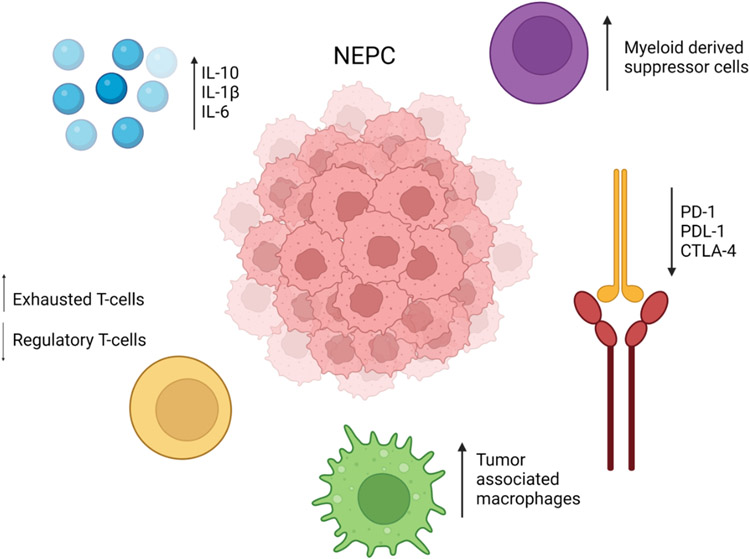
In NEPC, various cytokines and immune mediators have been investigated to address the knowledge gap of the role of the immune microenvironment in driving disease progression, transdifferentiation, and response to immunotherapies (Figure 2). For over 20 years, the role of IL-6 in NEPC differentiation has been investigated, with in vitro models implying a role for IL-6 signaling in promoting NEPC differentiation from AR dependent adenocarcinoma in LNCaP cells (Deeble et al., 2001). In in vitro PC cell line experiments, IL1-β facilitated the onset of skeletal metastasis, and was correlated with the expression of neuroendocrine related markers after treatment with ADT (Liu et al., 2013), implying a potential role in the transdifferentiation process again facilitated by hormone driven treatment. An additional cytokine, IL-10, although generally thought to aid in an anti-tumor response, in PC is linked to the induction of neuroendocrine differentiation in PC cell lines, as well as promoting upregulation of PD-L1 on tumor cells aiding in prolonged tumor survival by promoting an exhausted T cell state (Samiea et al., 2020).
Figure 2:
Interactions from multiple immune mediators contribute to the tumor immune interactions observed in NEPC.
Created with BioRender.com. NEPC – neuroendocrine prostate cancer.
In addition to cytokines, other immune mediators and processes are also thought to drive NEPC. Increased expression of CD46, a complement regulator of the innate immune system, is present on both primary and metastatic PC tumors, including high expression in NEPCs (Su et al., 2018). While the mechanism of action of CD46 in PC is not wholly established, as CD46 is only present in low levels in normal prostate tissue, a CD46 antibody-drug conjugate has been developed with promising killing effects on in vitro NEPC models and in in vivo studies (Su et al., 2018).
The aforementioned lack of response to immune checkpoint blockade therapy for patients with NEPC or CRPC, has raised questions about the infiltration of T-cells into prostate tumors. The transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model of PC develops a NEPC phenotype following castration and consequently allows for the study of both ARPC and NEPC within one model system. Hypoxia is correlated with worse prognosis in PC, and in TRAMP models, areas with hypoxic conditions were found to have decreased levels of infiltrating T cells compared to normal conditions thus promoting resistance to checkpoint blockade therapy (Jayaprakash et al., 2018). By targeting these hypoxic conditions with a hypoxia-activated prodrug, TH-302, and combination immune checkpoint blockade therapy, T-cell activity was restored, myeloid derived suppressor cells were decreased, and no evidence of neuroendocrine tumors was observed after treatment leading the authors to hypothesize that these targeted mechanisms can improve CRPC response to immunotherapies (Jayaprakash et al., 2018).
One of the most extensively studied and abundant immune cell types in NEPC are macrophages. A pro-inflammatory cytokine, macrophage migration inhibitory factor, is increased during neuroendocrine differentiation of LNCaP PC cells and sustained high levels of this factor promote tumor cell progression and is actively involved in maintaining signaling of pro-tumor pathways AKT and ERK1/2 (Tawadros et al., 2013). Mechanistic studies investigating the role of macrophages in neuroendocrine differentiation under therapeutic pressure have defined a prominent role of tumor associated macrophages. In vitro co-culture assays described a synergistic relationship between PC cells and activated macrophages, whereby BMP-6 released from tumor cells triggers the release of IL-6 from macrophages contributing to the onset of a neuroendocrine phenotype (Lee et al., 2011). NEPC induced by treatment with enzalutamide was linked to increased levels of circulating tumor associated macrophages when compared to treatment naïve patient samples, with markedly high protein expression of HMGB1 observed (Wang et al., 2018). HMGB1 recruits tumor associated macrophages which in turn secrete IL-6 driving the neuroendocrine differentiation process. Importantly, this mechanism of action can be targeted by combined HMGB1 knockdown and IL-6 repression, successfully reducing resistance to enzalutamide (Wang et al., 2018).
Immunohistochemical analysis of metastatic CRPC samples has reported the prominent presence of M2 macrophages, which increase across the disease trajectory, a further indicator of the contributors to the immune suppressive microenvironment attributed to CRPC, although it must be noted that the inclusion of neuroendocrine samples in this study was limited (Zarif et al., 2019). As previously discussed, CRPC is generally thought to have low expression of PD-L1 (Brady et al., 2021a), limiting response to immune checkpoint blockade therapies. However, expression of PD-L1 and presence of tumor infiltrating lymphocytes in NEPC is not well documented. Analysis of NEPC tumor samples observed expression of PD-L1 at the mRNA level in 50% of samples tested, with the presence of tumor infiltrating lymphocytes also observed in a portion of NEPC tumors suggesting potential expression in subgroups of NEPC of PD-L1 that requires further investigation (von Hardenberg et al., 2019). Stimulating the immune microenvironment of CRPC, and NEPC specifically, may hold promise for improving treatment outcomes, with several immunotherapies currently under investigation in PC, as reviewed recently, including Bi-Specific T-cell engagers and chimeric antigen receptor T-cell (CAR-T) therapy (Zorko and Ryan, 2021).
Conclusion
It is evident that the molecular landscape of NEPC is complex, heterogenous in nature, and involves crosstalk from both genomic and epigenomic pathways that complicate the disease spectrum. Ongoing research efforts are encouraging with respect to identifying the underlying mechanisms that trigger neuroendocrine differentiation and improving understanding of the role and interplay of cells of the surrounding tumor microenvironment., Therapeutic options for patients with NEPC remain extremely limited, considerably effecting quality of life and patient outcomes. However, the emergence of a number of potential treatment approaches mentioned throughout this review are promising for this patient population, moving away from AR directed therapies and focusing on candidate markers specific for NEPC such as DLL3 and CEACAM5, and the development of antibody drug conjugates. Systematic characterization of ‘druggable’ characteristics differentiating NEPC from ARPC point to several vulnerabilities including the regulation of apoptosis (Corella et al., 2020). Further, theranostic imaging that is not reliant on AR expression, can aid in the identification of NEPC emergence. To further address this treatment gap, ongoing work is focused on better understanding the individual immune landscapes of subtypes of CRPCs, with the goal of defining specific targets that are likely to respond to existing immunotherapies or targeted therapies. As a recent example, research efforts have identified B7H3, a checkpoint molecule, to be highly expressed in CRPC (Brady et al., 2021a), and although expression is less in NEPC when compared to adenocarcinoma (Guo et al., 2022), it has demonstrated targeted therapeutic potential for this patient population. Given the diverse nature of this disease subtype, personalized, combination therapeutic strategies that target the multifaceted tumor burden, in addition to harnessing the advancements made in other neuroendocrine subtypes such as small cell lung cancer, may drive improved outcomes for patients with NEPC.
Acknowledgements
We thank colleagues in the Nelson, Lee, Haffner and Morrissey research groups for helpful advice, comments, and collaboration. We gratefully acknowledge support from the Pacific Northwest Prostate Cancer SPORE CA097186; R01CA234715, 1R01 CA266452, PC170350P1, the Prostate Cancer Foundation, the Institute for Prostate Cancer Research, and a Department of Defense Young Investigator Award (W81XWH-21-1-0028).
Abbreviations
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ARPC
androgen receptor driven prostate cancer
- ARSI
androgen receptor signaling inhibition
- CRPC
castration resistant prostate cancer
- DHT
dihydrotestosterone
- DNPC
double negative prostate cancer
- FAP
fibroblast activation protein
- NEPC
neuroendocrine prostate cancer
- PC
prostate cancer
- PDX
patient derived xenograft
- TRAMP
transgenic adenocarcinoma of the mouse prostate
Footnotes
Declaration of Competing Interests
The authors declare no conflicts related to this work. PSN received consulting fees from Janssen, BMS and Pfizer for work unrelated to this review.
References
- AGGARWAL RR, QUIGLEY DA, HUANG J, ZHANG L, BEER TM, RETTIG MB, REITER RE, GLEAVE ME, THOMAS GV, FOYE A, PLAYDLE D, LLOYD P, CHI KN, EVANS CP, LARA PN, FENG FY, ALUMKAL JJ & SMALL EJ 2019. Whole-Genome and Transcriptional Analysis of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer Demonstrates Intraclass Heterogeneity. Mol Cancer Res, 17, 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN S, RICHARDSEN E, MOI L, DONNEM T, NORDBY Y, NESS N, HOLMAN ME, BREMNES RM & BUSUND LT 2016. Fibroblast miR-210 overexpression is independently associated with clinical failure in Prostate Cancer - a multicenter (in situ hybridization) study. Sci Rep, 6, 36573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACA SC, TAKEDA DY, SEO JH, HWANG J, KU SY, ARAFEH R, ARNOFF T, AGARWAL S, BELL C, O'CONNOR E, QIU X, ALAIWI SA, CORONA RI, FONSECA MAS, GIAMBARTOLOMEI C, CEJAS P, LIM K, HE M, SHEAHAN A, NASSAR A, BERCHUCK JE, BROWN L, NGUYEN HM, COLEMAN IM, KAIPAINEN A, DE SARKAR N, NELSON PS, MORRISSEY C, KORTHAUER K, POMERANTZ MM, ELLIS L, PASANIUC B, LAWRENSON K, KELLY K, ZOUBEIDI A, HAHN WC, BELTRAN H, LONG HW, BROWN M, COREY E & FREEDMAN ML 2021. Reprogramming of the FOXA1 cistrome in treatment-emergent neuroendocrine prostate cancer. Nat Commun, 12, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAINE MK, HSIEH MS, LAI WV, EGGER JV, JUNGBLUTH AA, DANESHBOD Y, BERAS A, SPENCER R, LOPARDO J, BODD F, MONTECALVO J, SAUTER JL, CHANG JC, BUONOCORE DJ, TRAVIS WD, SEN T, POIRIER JT, RUDIN CM & REKHTMAN N 2020. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J Thorac Oncol, 15, 1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKHT MK, DERECICHEI I, LI Y, FERRAIUOLO RM, DUNNING M, OH SW, HUSSEIN A, YOUN H, STRINGER KF, JEONG CW, CHEON GJ, KWAK C, KANG KW, LAMB AD, WANG Y, DONG X & PORTER LA 2018. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr Relat Cancer, 26, 131–146. [DOI] [PubMed] [Google Scholar]
- BARETTI M, ZHU Q, ZAHURAK M, BHAIJEE F, XU H, ENGLE EL, KOTTE A, PAWLIK TM, ANDERS RA & DE JESUS-ACOSTA A 2021. Prognostic Implications of the Immune Tumor Microenvironment in Patients With Pancreatic and Gastrointestinal Neuroendocrine Tumors. Pancreas, 50, 719–726. [DOI] [PubMed] [Google Scholar]
- BELTRAN H, PRANDI D, MOSQUERA JM, BENELLI M, PUCA L, CYRTA J, MAROTZ C, GIANNOPOULOU E, CHAKRAVARTHI BV, VARAMBALLY S, TOMLINS SA, NANUS DM, TAGAWA ST, VAN ALLEN EM, ELEMENTO O, SBONER A, GARRAWAY LA, RUBIN MA & DEMICHELIS F 2016. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med, 22, 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BESHIRI ML, TICE CM, TRAN C, NGUYEN HM, SOWALSKY AG, AGARWAL S, JANSSON KH, YANG Q, MCGOWEN KM, YIN J, ALILIN AN, KARZAI FH, DAHUT WL, COREY E & KELLY K 2018. A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic Screening. Clin Cancer Res, 24, 4332–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUEMN EG, COLEMAN IM, LUCAS JM, COLEMAN RT, HERNANDEZ-LOPEZ S, THARAKAN R, BIANCHI-FRIAS D, DUMPIT RF, KAIPAINEN A, CORELLA AN, YANG YC, NYQUIST MD, MOSTAGHEL E, HSIEH AC, ZHANG X, COREY E, BROWN LG, NGUYEN HM, PIENTA K, ITTMANN M, SCHWEIZER M, TRUE LD, WISE D, RENNIE PS, VESSELLA RL, MORRISSEY C & NELSON PS 2017. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell, 32, 474–489.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORROMEO MD, SAVAGE TK, KOLLIPARA RK, HE M, AUGUSTYN A, OSBORNE JK, GIRARD L, MINNA JD, GAZDAR AF, COBB MH & JOHNSON JE 2016. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep, 16, 1259–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY L, KRINER M, COLEMAN I, MORRISSEY C, ROUDIER M, TRUE LD, GULATI R, PLYMATE SR, ZHOU Z, BIRDITT B, MEREDITH R, GEISS G, HOANG M, BEECHEM J & NELSON PS 2021a. Inter- and intra-tumor heterogeneity of metastatic prostate cancer determined by digital spatial gene expression profiling. Nat Commun, 12, 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY NJ, BAGADION AM, SINGH R, CONTEDUCA V, VAN EMMENIS L, ARCECI E, PAKULA H, CARELLI R, KHANI F, BAKHT M, SIGOUROS M, BAREJA R, SBONER A, ELEMENTO O, TAGAWA S, NANUS DM, LODA M, BELTRAN H, ROBINSON B & RICKMAN DS 2021b. Temporal evolution of cellular heterogeneity during the progression to advanced AR-negative prostate cancer. Nat Commun, 12, 3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEJAS P, XIE Y, FONT-TELLO A, LIM K, SYAMALA S, QIU X, TEWARI AK, SHAH N, NGUYEN HM, PATEL RA, BROWN L, COLEMAN I, HACKENG WM, BROSENS L, DREIJERINK KMA, ELLIS L, ALAIWI SA, SEO JH, BACA S, BELTRAN H, KHANI F, POMERANTZ M, DALL'AGNESE A, CROWDIS J, VAN ALLEN EM, BELLMUNT J, MORRISEY C, NELSON PS, DECAPRIO J, FARAGO A, DYSON N, DRAPKIN B, LIU XS, FREEDMAN M, HAFFNER MC, COREY E, BROWN M & LONG HW 2021. Subtype heterogeneity and epigenetic convergence in neuroendocrine prostate cancer. Nat Commun, 12, 5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG Q, BUTLER W, ZHOU Y, ZHANG H, TANG L, PERKINSON K, CHEN X, JIANG XS, MCCALL SJ, INMAN BA & HUANG J 2022. Pre-existing Castration-resistant Prostate Cancer-like Cells in Primary Prostate Cancer Promote Resistance to Hormonal Therapy. Eur Urol, 81, 446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSIGLIO CR, UDARTSEVA O, RAMSEY KD, BUSH C & GOLLNICK SO 2020. Enzalutamide, an Androgen Receptor Antagonist, Enhances Myeloid Cell-Mediated Immune Suppression and Tumor Progression. Cancer Immunol Res, 8, 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTEDUCA V, OROMENDIA C, ENG KW, BAREJA R, SIGOUROS M, MOLINA A, FALTAS BM, SBONER A, MOSQUERA JM, ELEMENTO O, NANUS DM, TAGAWA ST, BALLMAN KV & BELTRAN H 2019. Clinical features of neuroendocrine prostate cancer. European Journal of Cancer, 121, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORELLA AN, CABILIZA ORDONIO MVA, COLEMAN I, LUCAS JM, KAIPAINEN A, NGUYEN HM, SONDHEIM D, BROWN LG, TRUE LD, LEE JK, MACPHERSON D, NGHIEM P, GULATI R, MORRISSEY C, COREY E & NELSON PS 2020. Identification of Therapeutic Vulnerabilities in Small-cell Neuroendocrine Prostate Cancer. Clin Cancer Res, 26, 1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DA SILVA A, BOWDEN M, ZHANG S, MASUGI Y, THORNER AR, HERBERT ZT, ZHOU CW, BRAIS L, CHAN JA, HODI FS, RODIG S, OGINO S & KULKE MH 2018. Characterization of the Neuroendocrine Tumor Immune Microenvironment. Pancreas, 47, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES A, NOURUZI S, GANGULI D, NAMEKAWA T, THAPER D, LINDER S, KARAOĞLANOĞLU F, OMUR ME, KIM S, KOBELEV M, KUMAR S, SIVAK O, BOSTOCK C, BISHOP J, HOOGSTRAAT M, TALAL A, STELLOO S, VAN DER POEL H, BERGMAN AM, AHMED M, FAZLI L, HUANG H, TILLEY W, GOODRICH D, FENG FY, GLEAVE M, HE HH, HACH F, ZWART W, BELTRAN H, SELTH L & ZOUBEIDI A 2021. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat Cell Biol, 23, 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE HOSSON LD, TAKKENKAMP TJ, KATS-UGURLU G, BOUMA G, BULTHUIS M, DE VRIES EGE, VAN FAASSEN M, KEMA IP & WALENKAMP AME 2020. Neuroendocrine tumours and their microenvironment. Cancer Immunol Immunother, 69, 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEEBLE PD, MURPHY DJ, PARSONS SJ & COX ME 2001. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol, 21, 8471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELUCIA DC, CARDILLO TM, ANG L, LABRECQUE MP, ZHANG A, HOPKINS JE, DE SARKAR N, COLEMAN I, DA COSTA RMG, COREY E, TRUE LD, HAFFNER MC, SCHWEIZER MT, MORRISSEY C, NELSON PS & LEE JK 2021. Regulation of CEACAM5 and Therapeutic Efficacy of an Anti-CEACAM5-SN38 Antibody-drug Conjugate in Neuroendocrine Prostate Cancer. Clin Cancer Res, 27, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA D, RIVARD C, YU H, BUNN P, SUDA K, REN S, LUEKE PICKARD S, LASZLO V, HARKO T, MEGYESFALVI Z, MOLDVAY J, HIRSCH FR, DOME B & LOHINAI Z 2020. Neuroendocrine subtypes of small cell lung cancer differ in terms of immune microenvironment and checkpoint molecule distribution. Mol Oncol, 14, 1947–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA D, RIVARD C, YU H, PICKARD SL, LASZLO V, HARKO T, MEGYESFALVI Z, DINYA E, GERDAN C, SZEGVARI G, HIRSCH FR, DOME B & LOHINAI Z 2021. Characterization of Tumor-Associated Macrophages and the Immune Microenvironment in Limited-Stage Neuroendocrine-High and -Low Small Cell Lung Cancer. Biology (Basel), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENRIQUEZ C, CANCILA V, FERRI R, SULSENTI R, FISCHETTI I, MILANI M, OSTANO P, GREGNANIN I, MELLO-GRAND M, BERRINO E, BREGNI M, RENNE G, TRIPODO C, COLOMBO MP & JACHETTI E 2021. Castration-Induced Downregulation of SPARC in Stromal Cells Drives Neuroendocrine Differentiation of Prostate Cancer. Cancer Res, 81, 4257–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN BJ & FELDMAN D 2001. The development of androgen-independent prostate cancer. Nat Rev Cancer, 1, 34–45. [DOI] [PubMed] [Google Scholar]
- GAMAT M & MCNEEL DG 2017. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer, 24, T297–t310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAY CM, STEWART CA, PARK EM, DIAO L, GROVES SM, HEEKE S, NABET BY, FUJIMOTO J, SOLIS LM, LU W, XI Y, CARDNELL RJ, WANG Q, FABBRI G, CARGILL KR, VOKES NI, RAMKUMAR K, ZHANG B, DELLA CORTE CM, ROBSON P, SWISHER SG, ROTH JA, GLISSON BS, SHAMES DS, WISTUBA II, WANG J, QUARANTA V, MINNA J, HEYMACH JV & BYERS LA 2021. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell, 39, 346–360.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUAN X, POLESSO F, WANG C, SEHRAWAT A, HAWKINS RM, MURRAY SE, THOMAS GV, CARUSO B, THOMPSON RF, WOOD MA, HIPFINGER C, HAMMOND SA, GRAFF JN, XIA Z & MORAN AE 2022. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature, 606, 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO C, FIGUEIREDO I, GUREL B, NEEB A, SEED G, CRESPO M, CARREIRA S, REKOWSKI J, BURONI L, WELTI J, BOGDAN D, GALLAGHER L, SHARP A, FENOR DE LA MAZA MD, RESCIGNO P, WESTABY D, CHANDRAN K, RIISNAES R, FERREIRA A, MIRANDA S, CALÌ B, ALIMONTI A, BRESSAN S, NGUYEN AHT, SHEN MM, HAWLEY JE, OBRADOVIC A, DRAKE CG, BERTAN C, BAKER C, TUNARIU N, YUAN W & DE BONO JS 2022. B7-H3 as a Therapeutic Target in Advanced Prostate Cancer. Eur Urol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUO H, CI X, AHMED M, HUA JT, SOARES F, LIN D, PUCA L, VOSOUGHI A, XUE H, LI E, SU P, CHEN S, NGUYEN T, LIANG Y, ZHANG Y, XU X, XU J, SHEAHAN AV, BA-ALAWI W, ZHANG S, MAHAMUD O, VELLANKI RN, GLEAVE M, BRISTOW RG, HAIBE-KAINS B, POIRIER JT, RUDIN CM, TSAO MS, WOUTERS BG, FAZLI L, FENG FY, ELLIS L, VAN DER KWAST T, BERLIN A, KORITZINSKY M, BOUTROS PC, ZOUBEIDI A, BELTRAN H, WANG Y & HE HH 2019. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat Commun, 10, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAYAPRAKASH P, AI M, LIU A, BUDHANI P, BARTKOWIAK T, SHENG J, AGER C, NICHOLAS C, JAISWAL AR, SUN Y, SHAH K, BALASUBRAMANYAM S, LI N, WANG G, NING J, ZAL A, ZAL T & CURRAN MA 2018. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest, 128, 5137–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO M, PLACENCIO-HICKOK VR, MADHAV A, HALDAR S, TRIPATHI M, BILLET S, MISHRA R, SMITH B, ROHENA-RIVERA K, AGARWAL P, DUONG F, ANGARA B, HICKOK D, LIU Z & BHOWMICK NA 2019. Heterogeneous cancer-associated fibroblast population potentiates neuroendocrine differentiation and castrate resistance in a CD105-dependent manner. Oncogene, 38, 716–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESCH C, YIRGA L, DENDL K, HANDKE A, DARR C, KRAFFT U, RADTKE JP, TSCHIRDEWAHN S, SZARVAS T, FAZLI L, GLEAVE M, GIESEL FL, HABERKORN U & HADASCHIK B 2021. High fibroblast-activation-protein expression in castration-resistant prostate cancer supports the use of FAPI-molecular theranostics. Eur J Nucl Med Mol Imaging, 49, 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KFOURY Y, BARYAWNO N, SEVERE N, MEI S, GUSTAFSSON K, HIRZ T, BROUSE T, SCADDEN EW, IGOLKINA AA, KOKKALIARIS K, CHOI BD, BARKAS N, RANDOLPH MA, SHIN JH, SAYLOR PJ, SCADDEN DT, SYKES DB & KHARCHENKO PV 2021. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell, 39, 1464–1478.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM DH, SUN D, STORCK WK, WELKER LENG K, JENKINS C, COLEMAN DJ, SAMPSON D, GUAN X, KUMARASWAMY A, RODANSKY ES, URRUTIA JA, SCHWARTZMAN JA, ZHANG C, BELTRAN H, LABRECQUE MP, MORRISSEY C, LUCAS JM, COLEMAN IM, NELSON PS, COREY E, HANDELMAN SK, SEXTON JZ, AGGARWAL R, ABIDA W, FENG FY, SMALL EJ, SPRATT DE, BANKHEAD A 3RD, RAO A, GESNER EM, ATTWELL S, LAKHOTIA S, CAMPEAU E, YATES JA, XIA Z & ALUMKAL JJ 2021. BET Bromodomain Inhibition Blocks an AR-Repressed, E2F1-Activated Treatment-Emergent Neuroendocrine Prostate Cancer Lineage Plasticity Program. Clin Cancer Res, 27, 4923–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNZ PL, REIDY-LAGUNES D, ANTHONY LB, BERTINO EM, BRENDTRO K, CHAN JA, CHEN H, JENSEN RT, KIM MK, KLIMSTRA DS, KULKE MH, LIU EH, METZ DC, PHAN AT, SIPPEL RS, STROSBERG JR & YAO JC 2013. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas, 42, 557–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABRECQUE MP, COLEMAN IM, BROWN LG, TRUE LD, KOLLATH L, LAKELY B, NGUYEN HM, YANG YC, DA COSTA RMG, KAIPAINEN A, COLEMAN R, HIGANO CS, YU EY, CHENG HH, MOSTAGHEL EA, MONTGOMERY B, SCHWEIZER MT, HSIEH AC, LIN DW, COREY E, NELSON PS & MORRISSEY C 2019. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J Clin Invest, 129, 4492–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI D, MA L & WANG F 2012. Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol, 41, 541–50. [DOI] [PubMed] [Google Scholar]
- LEE GT, KWON SJ, LEE JH, JEON SS, JANG KT, CHOI HY, LEE HM, KIM WJ, LEE DH & KIM IY 2011. Macrophages induce neuroendocrine differentiation of prostate cancer cells via BMP6-IL6 Loop. Prostate, 71, 1525–37. [DOI] [PubMed] [Google Scholar]
- LEE JK, BANGAYAN NJ, CHAI T, SMITH BA, PARIVA TE, YUN S, VASHISHT A, ZHANG Q, PARK JW, COREY E, HUANG J, GRAEBER TG, WOHLSCHLEGEL J & WITTE ON 2018. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci U S A, 115, E4473–e4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINXWEILER J, HAJILI T, KÖRBEL C, BERCHEM C, ZEUSCHNER P, MÜLLER A, STÖCKLE M, MENGER MD, JUNKER K & SAAR M 2020. Cancer-associated fibroblasts stimulate primary tumor growth and metastatic spread in an orthotopic prostate cancer xenograft model. Sci Rep, 10, 12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Q, RUSSELL MR, SHAHRIARI K, JERNIGAN DL, LIONI MI, GARCIA FU & FATATIS A 2013. Interleukin-1β promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res, 73, 3297–305. [DOI] [PubMed] [Google Scholar]
- MARQUES P, BARRY S, CARLSEN E, COLLIER D, RONALDSON A, AWAD S, DORWARD N, GRIEVE J, MENDOZA N, MUQUIT S, GROSSMAN AB, BALKWILL F & KORBONITS M 2019. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol Commun, 7, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISHRA R, HALDAR S, PLACENCIO V, MADHAV A, ROHENA-RIVERA K, AGARWAL P, DUONG F, ANGARA B, TRIPATHI M, LIU Z, GOTTLIEB RA, WAGNER S, POSADAS EM & BHOWMICK NA 2018. Stromal epigenetic alterations drive metabolic and neuroendocrine prostate cancer reprogramming. J Clin Invest, 128, 4472–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MO F, LIN D, TAKHAR M, RAMNARINE VR, DONG X, BELL RH, VOLIK SV, WANG K, XUE H, WANG Y, HAEGERT A, ANDERSON S, BRAHMBHATT S, ERHO N, WANG X, GOUT PW, MORRIS J, KARNES RJ, DEN RB, KLEIN EA, SCHAEFFER EM, ROSS A, REN S, SAHINALP SC, LI Y, XU X, WANG J, WANG J, GLEAVE ME, DAVICIONI E, SUN Y, WANG Y & COLLINS CC 2018. Stromal Gene Expression is Predictive for Metastatic Primary Prostate Cancer. Eur Urol, 73, 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTGOMERY RB, MOSTAGHEL EA, VESSELLA R, HESS DL, KALHORN TF, HIGANO CS, TRUE LD & NELSON PS 2008. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res, 68, 4447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PING Q, YAN R, CHENG X, WANG W, ZHONG Y, HOU Z, SHI Y, WANG C & LI R 2021. Cancer-associated fibroblasts: overview, progress, challenges, and directions. Cancer Gene Therapy, 28, 984–999. [DOI] [PubMed] [Google Scholar]
- PUCA L, GAVYERT K, SAILER V, CONTEDUCA V, DARDENNE E, SIGOUROS M, ISSE K, KEARNEY M, VOSOUGHI A, FERNANDEZ L, PAN H, MOTANAGH S, HESS J, DONOGHUE AJ, SBONER A, WANG Y, DITTAMORE R, RICKMAN D, NANUS DM, TAGAWA ST, ELEMENTO O, MOSQUERA JM, SAUNDERS L & BELTRAN H 2019. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINDI G, KLIMSTRA DS, ABEDI-ARDEKANI B, ASA SL, BOSMAN FT, BRAMBILLA E, BUSAM KJ, DE KRIJGER RR, DIETEL M, EL-NAGGAR AK, FERNANDEZ-CUESTA L, KLÖPPEL G, MCCLUGGAGE WG, MOCH H, OHGAKI H, RAKHA EA, REED NS, ROUS BA, SASANO H, SCARPA A, SCOAZEC J-Y, TRAVIS WD, TALLINI G, TROUILLAS J, VAN KRIEKEN JH & CREE IA 2018. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern Pathology, 31, 1770–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERT C. 2020. A decade of immune-checkpoint inhibitors in cancer therapy. Nature Communications, 11, 3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMIEA A, YOON JSJ, ONG CJ, ZOUBEIDI A, CHAMBERLAIN TC & MUI AL 2020. Interleukin-10 Induces Expression of Neuroendocrine Markers and PDL1 in Prostate Cancer Cells. Prostate Cancer, 2020, 5305306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL RL, MILLER KD, FUCHS HE & JEMAL A 2022. Cancer statistics, 2022. CA Cancer J Clin, 72, 7–33. [DOI] [PubMed] [Google Scholar]
- STULTZ J & FONG L 2021. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer and Prostatic Diseases, 24, 697–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU W, HAN HH, WANG Y, ZHANG B, ZHOU B, CHENG Y, RUMANDLA A, GURRAPU S, CHAKRABORTY G, SU J, YANG G, LIANG X, WANG G, ROSEN N, SCHER HI, OUERFELLI O & GIANCOTTI FG 2019. The Polycomb Repressor Complex 1 Drives Double-Negative Prostate Cancer Metastasis by Coordinating Stemness and Immune Suppression. Cancer Cell, 36, 139–155.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU Y, LIU Y, BEHRENS CR, BIDLINGMAIER S, LEE NK, AGGARWAL R, SHERBENOU DW, BURLINGAME AL, HANN BC, SIMKO JP, PREMASEKHARAN G, PARIS PL, SHUMAN MA, SEO Y, SMALL EJ & LIU B 2018. Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBE JM, KLEIN A, BRAHMER JR, XU H, PAN X, KIM JH, CHEN L, PARDOLL DM, TOPALIAN SL & ANDERS RA 2014. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res, 20, 5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAWADROS T, ALONSO F, JICHLINSKI P, CLARKE N, CALANDRA T, HAEFLIGER JA & ROGER T 2013. Release of macrophage migration inhibitory factor by neuroendocrine-differentiated LNCaP cells sustains the proliferation and survival of prostate cancer cells. Endocr Relat Cancer, 20, 137–49. [DOI] [PubMed] [Google Scholar]
- TRIGUNAITE A, DIMO J & JØRGENSEN TN 2015. Suppressive effects of androgens on the immune system. Cellular Immunology, 294, 87–94. [DOI] [PubMed] [Google Scholar]
- TSUNOKAKE J, FUJISHIMA F, WATANABE H, SATO I, MIURA K, SAKAMOTO K, SUZUKI H, SAWAI T, ITAKURA Y, HOSHI T, KUNIMITSU A, YAMAUCHI T, AKAISHI R, OZAWA Y, FUKUTOMI T, OKAMOTO H, SATO C, TANIYAMA Y, KAMEI T & SASANO H 2022. Tumor Microenvironment in Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: Interaction between Tumors and Immune Cells, and Potential Effects of Neuroendocrine Differentiation on the Tumor Microenvironment. Cancers (Basel), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VLACHOSTERGIOS PJ, KARATHANASIS A & TZORTZIS V 2022. Expression of Fibroblast Activation Protein Is Enriched in Neuroendocrine Prostate Cancer and Predicts Worse Survival. Genes (Basel), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON HARDENBERG J, HARTMANN S, NITSCHKE K, WORST TS, TING S, REIS H, NUHN P, WEIS CA & ERBEN P 2019. Programmed Death Ligand 1 (PD-L1) Status and Tumor-Infiltrating Lymphocytes in Hot Spots of Primary and Liver Metastases in Prostate Cancer With Neuroendocrine Differentiation. Clin Genitourin Cancer, 17, 145–153.e5. [DOI] [PubMed] [Google Scholar]
- WANG C, PENG G, HUANG H, LIU F, KONG DP, DONG KQ, DAI LH, ZHOU Z, WANG KJ, YANG J, CHENG YQ, GAO X, QU M, WANG HR, ZHU F, TIAN QQ, LIU D, CAO L, CUI XG, XU CL, XU DF & SUN YH 2018. Blocking the Feedback Loop between Neuroendocrine Differentiation and Macrophages Improves the Therapeutic Effects of Enzalutamide (MDV3100) on Prostate Cancer. Clin Cancer Res, 24, 708–723. [DOI] [PubMed] [Google Scholar]
- YANG YC, BANUELOS CA, MAWJI NR, WANG J, KATO M, HAILE S, MCEWAN IJ, PLYMATE S & SADAR MD 2016. Targeting Androgen Receptor Activation Function-1 with EPI to Overcome Resistance Mechanisms in Castration-Resistant Prostate Cancer. Clin Cancer Res, 22, 4466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG K, LAWLOR RT, RAGULAN C, PATIL Y, MAFFICINI A, BERSANI S, ANTONELLO D, MANSFIELD D, CINGARLINI S, LANDONI L, PEA A, LUCHINI C, PIREDDA L, KANNAN N, NYAMUNDANDA G, MORGANSTEIN D, CHAU I, WIEDENMANN B, MILELLA M, MELCHER A, CUNNINGHAM D, STARLING N, SCARPA A & SADANANDAM A 2021. Immune landscape, evolution, hypoxia-mediated viral mimicry pathways and therapeutic potential in molecular subtypes of pancreatic neuroendocrine tumours. Gut, 70, 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZARIF JC, BAENA-DEL VALLE JA, HICKS JL, HEAPHY CM, VIDAL I, LUO J, LOTAN TL, HOOPER JE, ISAACS WB, PIENTA KJ & DE MARZO AM 2019. Mannose Receptor-positive Macrophage Infiltration Correlates with Prostate Cancer Onset and Metastatic Castration-resistant Disease. Eur Urol Oncol, 2, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO D, CAI L, LU X, LIANG X, LI J, CHEN P, ITTMANN M, SHANG X, JIANG S, LI H, MENG C, FLORES I, SONG JH, HORNER JW, LAN Z, WU CJ, LI J, CHANG Q, CHEN KC, WANG G, DENG P, SPRING DJ, WANG YA & DEPINHO RA 2020. Chromatin Regulator CHD1 Remodels the Immunosuppressive Tumor Microenvironment in PTEN-Deficient Prostate Cancer. Cancer Discov, 10, 1374–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZORKO NA & RYAN CJ 2021. Novel immune engagers and cellular therapies for metastatic castration-resistant prostate cancer: do we take a BiTe or ride BiKEs, TriKEs, and CARs? Prostate Cancer Prostatic Dis, 24, 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]