Abstract
There has been substantial research on adult COVID-19 and how to treat it. But how do severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections afflict children? The COVID-19 pandemic has yielded many surprises, not least that children generally develop less severe disease than older adults, which is unusual for a respiratory disease. However, some children can develop serious complications from COVID-19, such as multisystem inflammatory syndrome in children (MIS-C) and Long Covid, even after mild or asymptomatic COVID-19. Why this occurs in some and not others is an important question.
Moreover, when children do contract COVID-19, understanding their role in transmission, especially in schools and at home, is crucial to ensuring effective mitigation measures. Therefore, in addition to nonpharmaceutical interventions, such as improved ventilation, there is a strong case to vaccinate children so as to reduce possible long-term effects from infection and to decrease transmission. But questions remain about whether vaccination might skew immune responses to variants in the long term. As the experts discuss below, more is being learned about these important issues, but much more research is needed to understand the long-term effects of COVID-19 in children.
–Gemma K. Alderton
Why is COVID-19 generally milder in children?
Why infants and young children have milder clinical courses with COVID-19, accounting for ~0.1% of deaths, but are more vulnerable to established pathogens has been a key question since the onset of the pandemic. This may be attributed to differences in susceptibility. All segments of the population were naive to SARS-CoV-2, whereas older children and adults have protective immunity to established viruses because of prior exposures and vaccines. Although the underrepresentation of children in severe COVID-19 cases was initially hypothesized to reflect decreased susceptibility, no age-associated difference in household transmission, expression of angiotensin-converting enzyme 2 (ACE2, the receptor for viral cell entry), antibody titers against other coronaviruses, or SARS-CoV-2 viral loads in nasopharyngeal swabs have been detected (1-4). These findings, coupled with immunological studies, suggest that the more favorable outcomes likely reflect rapid engagement of the pediatric mucosal immune system.
Studies of immune responses in hospitalized children and adults during the first wave of the COVID-19 pandemic found that pediatric patients had less robust memory T cell responses and lower neutralizing and Fcγ-receptor activating antibody responses than adults (2, 5). Coupled with data showing an age-dependent decrease in serum levels of the cytokines interferon-γ and interleukin-17, these findings suggested that children may mount a more vigorous innate (pathogen nonspecific) response that promotes viral clearance and precludes the robust adaptive immune response to SARS-CoV-2 that contributes to severe disease in adults (2). This hypothesis was strengthened by direct study of mucosal responses at the time of COVID-19 diagnosis: RNA sequencing data and measurement of cytokines demonstrated a brisk innate response in the nasal mucosa of children versus adults (3). These findings may be a consequence of trained immunity resulting from more frequent respiratory infections in children, leading to higher baseline innate activity in pediatric relative to adult nasal mucosae (6, 7).
Another hypothesis is that the naïve character of the pediatric adaptive immune system contributes to milder outcomes. Analysis of peripheral blood immune cells in pediatric and adult COVID-19 patients showed an increased frequency of naïve T cells, depletion of natural killer (NK) cells, and a lower frequency of cytotoxic T cells in children compared with adults (6). Likewise, the T cell receptor repertoire from young children who seroconverted after COVID-19 showed reduced clonal expansion compared with adults (8). The skewing away from cytotoxic activity may avert an over-exuberant adaptive immune response that contributes to hyperinflammation, which is characteristic of severe disease.
Clinical experiences with newer SARS-CoV-2 variants, which are potentially more transmissible, suggest that the innate response continues to play an important role, although there is concern that future variants with greater capacity to evade innate immunity may emerge. Thus, vaccinations, which are also approved for young children, are critical as SARS-CoV-2 transitions from a pandemic to an endemic virus. Notably, vaccines also protect against MIS-C, a rare but potentially severe post-COVID-19 pediatric sequela. The findings underscore that differences in outcomes with new and old infections depend on the host, their prior infectious history and immune status, and the pathogen(s) involved. Studies across the age spectrum are needed to identify these contributing factors and guide public health policies.
Why do some children develop MIS-C?
An obese but otherwise healthy 9-year-old boy develops fevers, rashes, vomiting, neutrophilia, lymphopenia, elevated inflammatory markers, and coagulopathy. He had asymptomatic COVID-19 4 weeks before displaying these symptoms. Owing to myocarditis and hypotension, he receives immunosuppressive dosing of intravenous immunoglobulin (IVIG) and glucocorticoids, enabling recovery in 1 week. This case is representative of MIS-C (9, 10), defined by fever, inflammation, and involvement of at least two organ systems and requiring hospitalization. Coronary artery aneurysms occur in over 8% of patients (11). Unlike acute COVID-19, MIS-C is a postinfectious syndrome occurring within 3 to 6 weeks of mild or asymptomatic SARS-CoV-2 infection (9). Although severe COVID-19 occurs most frequently in the elderly, healthy children between 6 and 12 years of age most commonly develop MIS-C. In 2020, MIS-C occurred in ~3 in 10,000 US persons under 21 years, with a mortality rate of ~0.8%. Obesity is present in ~30% of children with MIS-C (12), but its role and what triggers MIS-C remain unknown. The incidence and severity of MIS-C are lower after infections with the Omicron variant, compared to the Alpha or Delta variants. This has been attributed to differences in inflammatory responses associated with each variant and enhanced host immunity after COVID-19 vaccinations or SARS-CoV-2 reinfections (13, 14).
Patients with MIS-C have activated neutrophils, monocytes, T cells, B cells, NK cells, and dendritic cells (15, 16). Increased circulating chemokines and cytokines facilitate homing of these activated immune cells to inflamed organs, most commonly the gastrointestinal and cardiovascular systems. Autoantibodies occur in some patients with MIS-C, but it is unknown if they drive autoimmunity or are byproducts of a hyperinflamed state. The SARS-CoV-2 spike protein has structural similarities to superantigens that can nonspecifically activate T cells (12). The expansion of T cells with specific T cell receptor Vb repertoires, a characteristic of superantigen activation, has been observed in some patients with MIS-C (17).
Although effective treatments for MIS-C are available, the causes of this postinfectious syndrome remain unclear. It is challenging to obtain biospecimens from critically ill children before treatment, and there are no animal models of this disease. The hypothesis of persistent, low-level SARS-CoV-2 replication driving MIS-C is based on one study finding circulating SARS-CoV-2 spike protein in a subset of patients with MIS-C (12), although this was not replicated (18). Similarly, extremely rare cases of MIS-C occurring after COVID-19 vaccination prompted concerns that vaccine-derived spike protein could be a trigger (19). However, studies have shown that COVID-19 vaccination reduces the risk of MIS-C (20), possibly by reducing the risk of SARS-CoV-2 infection, and the overall incidence of MIS-C is decreasing. Drivers of hyperinflammation, including genetic variants impairing restraint of immune activation, have been identified as additional risk factors (12). Future studies need to resolve this paradox of a hyperinflammatory disease affecting previously healthy children with antecedent asymptomatic or mild SARS-CoV-2 infections. The identification of early biomarkers of hyperinflammation may address the unpredictable onset of MIS-C, a feature shared by the development of Long Covid, another incompletely understood syndrome that occurs after SARS-CoV-2 infection.
Long Covid in children and young people
Although most children recover fully from COVID-19, some will have persistent symptoms. Global prevalence estimates of Long Covid in children vary from 1:4 to 1:100 depending on cohort, methodology, and definition (21). The Office of National Statistics (ONS) estimates that ~120,000 children in the UK are suffering the effects of Long Covid, with 26,000 having symptoms for >1 year (22). A quarter of children experienced persistent symptoms months after hospitalization with acute COVID-19 infection, with almost 1 in 10 experiencing multisystem involvement (23). In total, over 200 different symptoms have been reported, affecting every organ system. Emerging evidence from case studies, patient-led charities, and pediatric services set up to treat Long Covid highlight that children are also affected by Long Covid after initial mild or asymptomatic illness (24). However, there are challenges in interpreting the existing literature, particularly with regards to defining “control groups” in a population that has had high levels of COVID-19 infection.
Owing to the heterogeneity of symptoms, diagnosing Long Covid in children is challenging, especially in the absence of established biomarkers of disease. The first research definition of Long Covid in children and young people was published recently (25). This acknowledges that the lack of complete recovery after acute infection with SARS-CoV-2 for >4 weeks is concerning, but symptoms persistent at 12 weeks are required to meet the definition of Long Covid. An international consensus for a pediatric Long Covid definition is anticipated in 2023; this will be an important step in the illness being recognized and managed consistently.
Children with Long Covid can be divided into those unable to return to normal life because of symptoms such as (but not limited to) pain, fatigue, post-exertional symptom exacerbation, headaches, and cognitive difficulties, and those who have additional postinfectious complications, such as pediatric multisystem inflammatory syndrome (PIMS), acute neurological disease, myocarditis, and other potential acute or subacute illnesses (21). Although the evidence for underlying biomedical causes of the long-term impact of SARS-CoV-2 infection in adults is growing quickly, there is a paucity of similar studies in children, limiting our understanding of the disease in young people. Potential mechanisms underlying Long Covid include viral persistence in tissues, disorders of coagulation, immune dysfunction, and autoantibody production (21). At the population health level, there is a suggestion that the incidence of type 1 diabetes is increased in children with Long Covid, although the cause of this is unclear and requires further investigation (26). There are currently no longitudinal studies that report outcomes for recovery in children.
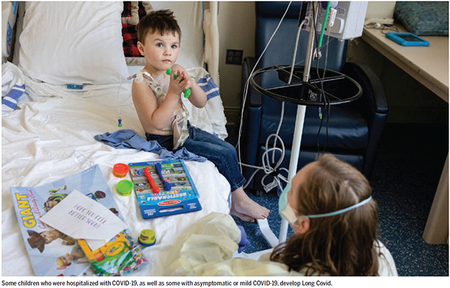
A substantial global biomedical research effort, alongside cooperation with patients and families, is required to understand the long-term impact of Long Covid in children and to develop effective services and therapies. Limiting severity of disease through vaccination and taking preventive steps to mitigate against airborne virus transmission, such as improving indoor ventilation in schools, remain important tools to prevent the burden of Long Covid increasing in children. This is especially important for low- and middle-income countries (LMICs) where additional factors such as poor sanitation, overcrowding, and malnutrition pose further risks to child health (27).
What is the role of children in transmission of SARS-CoV-2?
There is now unequivocal evidence that children play an important role in the transmission of SARS-CoV-2, particularly within school and household settings. The role that a person or group plays in transmission is dictated by a combination of exposure (how likely they are to get exposed to infection), susceptibility (how likely they are to get infected if exposed), and transmissibility (how likely they are to transmit to others if infected). Although the different contributions of these are difficult to disentangle (28, 29), the important role of children in SARS-CoV-2 transmission is likely due to higher levels of exposure in schools. Early studies probably underestimated susceptibility in children (28-30) by not considering both lower relative exposure in children during periods of lockdown and school closures, and that children are less likely to be symptomatic than adults (31, 32), and so are less likely to be identified as cases.
School exposure is high for two main reasons. Schools involve hundreds of children mixing daily in crowded indoor spaces that are often poorly ventilated, facilitating transmission. Additionally, infection in children is easily missed because they are more likely to have asymptomatic, relatively mild, or atypical symptoms compared to adults (31, 32); and they can be harder to test if COVID-19 is suspected. Studies have also shown that SARS-CoV-2 infection may go undetected among children, who can be “silent” asymptomatic spreaders in school outbreaks, which then spread into the community (33). Contemporaneous surveillance data from the UK ONS (based on random COVID-19 testing of households in England) (32) showed that symptom-based testing vastly underestimated actual case incidence and prevalence in children.
The clearest findings about the role of children in community transmission come from studies that showed substantial reductions and increases in pandemic growth when schools were closed and opened, respectively (34). Random survey data from the ONS (32) indicate repeated increases in, and spread from, school-age children into parental age groups, with increases in infection rates in children predating increases in other age groups after school opening. Very large studies of adults living with children in the US (35), UK (36), and Denmark (37) have shown a higher risk of infection among households with children. More recently, genomic studies have also confirmed superspreading events within schools that then spread infections back into the community (33). Fortunately, studies also show that robust multilayered mitigation measures within schools can greatly reduce school outbreaks and are associated with lower community prevalence (35).

To reduce the role that children play in transmission of SARS-CoV-2, and to limit the impact of COVID-19 on children’s health and that of their families, it is important to reduce exposure and transmission through safer school environments (improving air quality through investing in better ventilation and air cleaning), using N95/FFP2 masks during high or increasing community transmission, vaccination, accessible case ascertainment (e.g., saliva testing), and public information on how to make homes safer environments. Key areas of future research include understanding reinfections in children (How often do they occur? What are the risks of Long Covid or severe disease?); variant-specific effects on immune escape, reinfection, and transmission; and developing vaccines or boosters that are safe for children but provide longer protection against infection, particularly for under-12s; quantifying the long-term benefits of reduction in airborne disease; and better air quality, through investment in cleaner air in schools.
The importance of vaccinating children against COVID-19
The rationale for vaccinating children is clear: The recent waves of COVID-19 caused by the Delta and Omicron variants have taught us that infections with SARS-CoV-2 are not as benign for children as previously thought. In the US, more than 1200 children have lost their lives due to COVID-19 since the pandemic began (38), a number equivalent to or higher than the 300 children who die annually from all vaccine-preventable illnesses (39). Globally, the United Nations Children’s Fund (UNICEF) reports that ~17,000 children and adolescents have lost their lives to COVID-19, with ~50% of those being under the age of 10 years (40). However, this number is based on 4.4 million overall global COVID-19 deaths from 91 countries, which is generally considered an underestimate. The new World Health Organization determination of 14.9 million excess global deaths (41), together with a UNICEF finding that 0.4% of COVID-19 deaths occur among children and adolescents (40), could suggest that as many 60,000 children and adolescents have died so far. This number would place pediatric COVID-19 deaths on par with those caused by some other vaccine-preventable diseases such as pediatric meningitis and other illnesses caused by the bacterium Haemophilus influenzae type b (Hib) and almost as high as measles-related deaths (42).
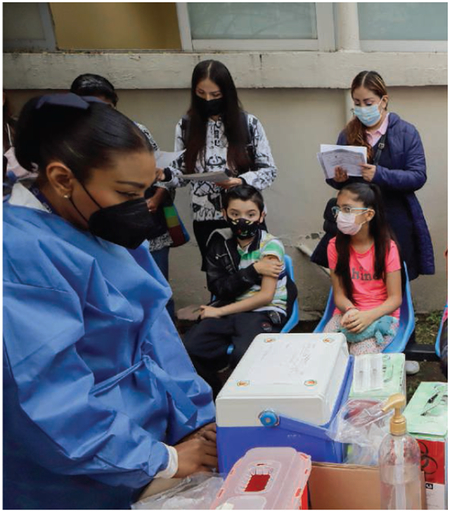
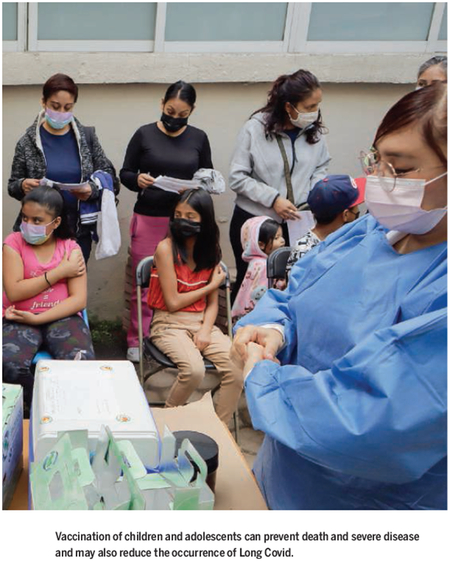
COVID-19 vaccines are now approved or increasingly available for immunizing children over the age of 6 months. Beyond their potential to reduce deaths from COVID-19 are the actual measured benefits of vaccinating children. In the US Delta variant wave in the summer–fall of 2021, most hospitalizations occurred among unvaccinated adolescents—only 8% of hospitalized adolescents were fully vaccinated—although this number rose to just over 20% when Omicron infections first accelerated in the final month of 2021 (43). Another study found that two doses of the Pfizer-BioNTech mRNA COVID-19 vaccine were over 90% effective at protecting against MIS-C (44). Vaccinated children are also less susceptible to COVID-19 infection overall (45), although the current vaccines are less effective in children against symptomatic infection with the Omicron variant (46). Still missing are data on vaccinating children to prevent Long Covid or its neurological complications. However, based on evidence from adults, some pediatric societies currently advocate for pediatric COVID-19 vaccinations on this basis (47). Also lacking are studies on vaccine access, equity, and effectiveness for children with intellectual and developmental disabilities. Additionally, extensive pharmacovigilance studies in the US and Europe have so far confirmed the safety of pediatric COVID-19 immunizations, but such studies need to be extended to LMICs.
Despite the benefits of pediatric COVID-19 vaccinations and the enthusiastic support from most pediatric societies, parental acceptance for vaccinating their children remains low. For instance, in many US Southern states, only 14 to 15% of children aged 5 to 11 have received a COVID-19 vaccination. Adding to these concerns is rising antivaccine activism that targets all pediatric vaccinations, including those for COVID-19. Therefore, there is urgency to accelerate educational efforts for parents about the seriousness of COVID-19 infections in children and the positive health impact of immunizations. In parallel, expanded studies on vaccine effectiveness are needed for pediatric COVID-19 vaccinations. This is true for both the recently authorized mRNA vaccines and newer and next-generation platform technologies (including universal coronavirus vaccines). These vaccines might eventually be incorporated into routine childhood immunization programs to avoid future coronavirus infection vulnerabilities among new birth cohorts. Pediatric COVID-19 immunizations must be prioritized by policymakers and practitioners committed to global child health.
Original antigenic sin and childhood immune responses against SARS-CoV-2
Children’s first exposures to SARS-CoV-2, whether through infection or vaccination, might shape the specificity of their immune responses to SARS-CoV-2 variants for the rest of their lives. Previous studies on influenza virus infections may inform how childhood SARS-CoV-2 infections affect long-lived immunity. Thomas Francis Jr. coined the phrase “original antigenic sin” in 1960 to describe the observation that antibody responses elicited by childhood influenza virus infections can be recalled later in life upon exposure to antigenically distinct influenza viruses (48). This recall can lead to different patterns of antibody specificity among individuals exposed to the same influenza virus strain. But it remains uncertain whether de novo immune responses to new antigens are diminished by these previous exposures and recalled immune memory. The specificity of antibody responses to influenza virus arises from targeting different epitopes. Often, these responses target epitopes that are conserved between contemporary and childhood viral strains. This bias can be beneficial when antibodies are neutralizing, but potentially detrimental when these epitopes change in the virus, leading to a loss of antibody recognition and greater risk of infection (49). Evidence suggests that variation in risk of medically attended influenza virus infection and vaccine effectiveness between influenza seasons and age groups arises partly from these idiosyncratic interactions with immune memory (50-54).
Is original antigenic sin relevant to COVID-19 vaccination and SARS-CoV-2 infections of children? Although the answer is currently unknown, it is likely that the specificity of antibody responses to a SARS-CoV-2 variant is influenced by exposure history. For example, Omicron infection elicits a broader neutralizing antibody response in vaccinated adults compared to those who are unvaccinated, leading to higher neutralizing antibody titers to Omicron and Delta strains in vaccinated individuals (55). The specificity and magnitude of antibodies elicited by SARS-CoV-2 infection appear to be influenced by vaccination status (56), but it is difficult to interpret differences in immune data between vaccinated and unvaccinated individuals because unvaccinated individuals typically encounter more antigen during their infections. Furthermore, it is unknown how different exposures affect immune memory over the long term in children and adults.
To understand the role of antigenic sin in SARS-CoV-2 immunity, it is important to continue to investigate the magnitude, specificity, and functionality of B and T cell responses elicited by sequential SARS-CoV-2 exposures in animal models and humans. It will also be important to directly compare different types of SARS-CoV-2 vaccines in people with different exposure histories. For example, mRNA vaccines might promote better de novo responses than some other vaccine platforms in individuals with prior SARS-CoV-2 immunity because these vaccines elicit very long-lived germinal centers (where B cells evolve to become high-affinity plasma cells that produce specific antibodies) (57) that prolong the availability of antigens to both naïve and memory B cells. Longitudinal studies are important to elucidate the short- and long-term effects of different SARS-CoV-2 exposures on immune memory, infection risk, and vaccine effectiveness in children.
ACKNOWLEDGMENTS
B.C.H. and K.C.H. are funded by NIH NIAID R01 AI134367. C.A.P. was supported by the Medical Scientist Training Program of the Albert Einstein College of Medicine (T32GM007288) and the institutional training grant in HIV/AIDS pathogenesis (T32AI007501). J.C. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases to study MIS-C. C.P. and B.K. are members of Independent SAGE (www.independentsage.org/). P.H. is a coinventor of a COVID-19 recombinant protein vaccine technology owned by Baylor College of Medicine (BCM) that was recently licensed by BCM nonexclusively and with no patent restrictions to several companies committed to advance vaccines for LMICs. S.C. and S.E.H. are funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. 75N93021C00015.
REFERENCES AND NOTES
- 1.McLean HQ et al. , Pediatrics 149, e2021054178 (2022).35194642 [Google Scholar]
- 2.Pierce CA et al. , Sci. Transl. Med 12, eabd5487 (2020).32958614 [Google Scholar]
- 3.Pierce CA et al. , JCI Insight 6, e148694 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madera S et al. , Sci. Rep 11, 3044 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP et al. , Nat. Immunol 22, 25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida M et al. , Nature 602, 321 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loske J et al. , Nat. Biotechnol 40, 319 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Rowntree LC et al. , Immunity 55, 1299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldstein LR et al. , JAMA 325, 1074 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein LR et al. , N. Engl. J. Med 383, 334 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsaied T et al. , Circulation 143, 78 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Chou J, Thomas PG, Randolph AG, Nat. Immunol 23, 177 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm M et al. , JAMA Pediatr. 176, 821 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy N et al. , JAMA 327, 2452 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vella LA et al. , Sci. Immunol 6, eabf7570 (2021).33653907 [Google Scholar]
- 16.Consiglio CR et al. , Cell 183, 968 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sancho-Shimizu V et al. , J. Exp. Med 218, e20210446 (2021).33904890 [Google Scholar]
- 18.Sigal GB et al. , Clin. Infect. Dis 10.1093/cid/ciac160 (2022). [DOI] [Google Scholar]
- 19.Salzman MB, Huang C-W, O’Brien CM, Castillo RD, Emerg. Infect. Dis 27, 1944 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zambrano LD et al. , MMWR Morb. Mortal. Wkly. Rep 71, 52 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buonsenso D et al. , Future Microbiol. 17, 551 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Office for National Statistics (ONS), Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK (ONS, London, 2022). [Google Scholar]
- 23.Osmanov IM et al. , Eur. Respir. J 59, 2101341 (2022).34210789 [Google Scholar]
- 24.Buonsenso D et al. , Future Microbiol. 17, 577 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephenson T et al. , Arch. Dis. Child 107, 674 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Kamrath C et al. , Diabetes Care 45, 1762 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Zar HJ, Dawa J, Fischer GB, Castro-Rodriguez JA, Paediatr. Respir. Rev 35, 70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyde Z, Med. J. Aust 214, 190 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyde Z, Clin. Infect. Dis 74, 152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi Q et al. , Lancet Infect. Dis 20, 911 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poletti P et al. , JAMA Netw. Open 4, e211085 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hippich M et al. , Med 2, 149 (2021).33163984 [Google Scholar]
- 33.Meuris C et al. , , JAMA Netw. Open 4, e2128757 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haug N et al. , Nat. Hum. Behav 4, 1303 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Lessler J et al. , Science 372, 1092 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forbes H et al. , BMJ 372, n628 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Husby A, Corn G, Krause TG, MedRxiv 02.28.21250921 (2021). [Google Scholar]
- 38.National Center for Health Statistics, Provisional COVID-19 Deaths: Focus on Ages 0-18 Years; https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Focus-on-Ages-0-18-Yea/nr4s-juj3, accessed 2 July 2022.
- 39.Office of Disease Prevention and Health Promotion, Immunization and Infectious Diseases; https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases, accessed 2 July 2022.
- 40.UNICEF, COVID-19 confirmed cases and deaths, age- and sex-disaggregated data. March 2022; https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dash-board/, accessed 2 July 2022.
- 41.World Health Organization, 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021, 5 May 2022; https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021, accessed 2 July 2022.
- 42.Frenkel LD, Allergy Asthma Proc. 42, 378 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks KJ et al. , MMWR Morb. Mortal. Wkly. Rep 71, 271 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zambrano LD et al. , MMWR Morb. Mortal. Wkly. Rep 71, 52 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yigit M et al. , Pediatr. Infect. Dis. J 10.1097/INF.0000000000003625 (2022). [DOI] [Google Scholar]
- 46.Cohen-Stavi CJ et al. , N. Engl. J. Med 387, 227 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stein M et al. , Vaccines 10, 81 (2022).35062742 [Google Scholar]
- 48.Francis T, Proc. Am. Philos. Soc 104, 572 (1960). [Google Scholar]
- 49.Cobey S, Hensley SE, Curr. Opin. Virol 22, 105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flannery B et al. , J. Infect. Dis 218, 189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gostic KM et al. , PLOS Pathog. 15, e1008109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arevalo P, McLean HQ, Belongia EA, Cobey S, eLife 9, e50060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skowronski DM et al. , J. Infect. Dis 216, 1487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO, Science 354, 722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan K et al. , Nature 607, 356 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynolds CJ et al. , Science, eabq1841 (2022).35699621 [Google Scholar]
- 57.Pardi N et al. , J. Exp. Med 215, 1571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


