Abstract
Partial hepatectomy (PH) can lead to severe complications, including liver failure, due to the low regenerative capacity of the remaining liver, especially after extensive hepatectomy. Liver sinusoidal endothelial cells (LSECs), whose proliferation occurs more slowly and later than hepatocytes after PH, compose the lining of the hepatic sinusoids, which are the smallest blood vessels in the liver. Vascular endothelial growth factor (VEGF), secreted by hepatocytes, promotes LSEC proliferation. Supplementation of exogenous VEGF after hepatectomy also increases the number of LSECs in the remaining liver, thus promoting the reestablishment of the hepatic sinusoids and accelerating liver regeneration. At present, some shortcomings exist in the methods of supplementing exogenous VEGF, such as a low drug concentration in the liver and the reaching of other organs. More-over, VEGF should be administered multiple times and in large doses because of its short half-life. This review summarized the most recent findings on liver regeneration and new strategies for the localized delivery VEGF in the liver.
Keywords: Liver regeneration, Vascular endothelial growth factor, Nano materials, Liver resection
Core Tip: Low regenerative ability of the liver remaining after partial hepatectomy may lead to serious complications including liver failure. The proliferation of liver sinusoidal endothelial cells (LSECs) is slower and occurs later compared to that of hepatocytes, leading to a delayed recovery of the hepatic sinusoids and impaired liver regeneration. Hepatocytes secrete vascular endothelial growth factor (VEGF) that promotes the proliferation of LSECs, and supplementation of exogenous VEGF after hepatectomy also increases the number of LSECs in the remaining liver, thus promoting the reestablishment of the hepatic sinusoids and accelerating liver regeneration. This review summarized the most recent findings on liver regeneration and new strategies for the localized delivery in the liver.
INTRODUCTION
Approximately 300 million people in China are suffering from liver diseases[1]. Liver cancer originates from hepatitis B in 80% of patients, and the incidence and mortality of liver cancer account for more than 50% of worldwide, making liver cancer the second leading cause of death in China[2]. Surgery remains the primary treatment option for liver cancer, but only 15% of patients can undergo this procedure[3]. Insufficient residual liver volume is the primary reason why about 30% of patients are ineligible for surgery[4], thus, becoming the main factor limiting the safe implementation of major liver resection. Therefore, there is an urgent need to improve the regenerative capacity of the liver and promote liver function after partial hepatectomy (PH).
SCHEMATIC DESCRIPTION OF LIVER REGENERATION AFTER PH
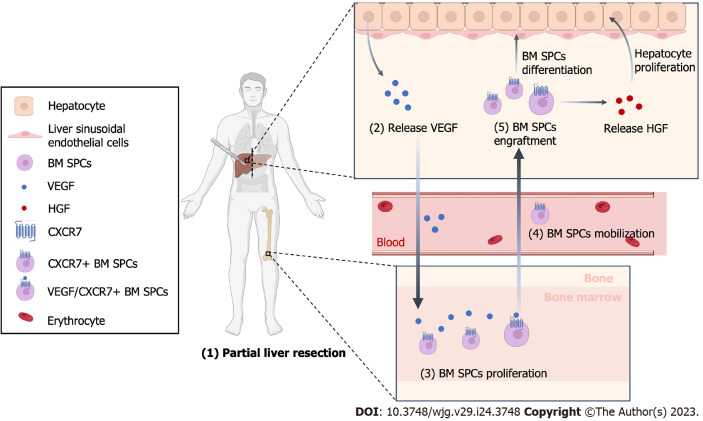
The proliferation time of each cell type in the liver after PH is different[5]. Hepatocytes begin to proliferate within a few hours after PH and reach a peak at 24 h. The proliferation of liver sinusoidal endothelial cells (LSECs) is slower and later compared to that of hepatocytes. Indeed, they start to proliferate at 36 h after PH and reach the peak at 96 h[6]. In the first 72 h after PH, the hepatic plate becomes wider, the proportion of LSECs decreased due to the rapid proliferation of hepatocytes, and the density of the liver sinusoid is temporarily reduced. This leads to hypoxia in some areas of the liver, inducing the secretion of vascular endothelial growth factor (VEGF) by the hepatocyte. VEGF is a vital regulator inducing the proliferation, mobilization, engraftment and differentiation of bone marrow progenitor cells of LSECs (BM SPCs), which is the main of renew LSECs[7]. BM SPCs have a high expression of hepatocyte growth factor (HGF) that promotes hepatocyte proliferation. The different proliferation time of the different cell types in the liver leads to their interaction to complete liver regeneration[7-9].
The regeneration of the liver after PH is a complex process that involves various types of cells and signaling pathways. Hepatocytes highly expressing VEGF are mainly located around the portal vein, and LSECs have the highest proliferation ability also around the portal vein after PH. Inhibition of VEGF significantly impairs the proliferation of hepatocytes and LSECs, while exogenous VEGF significantly promotes their proliferation, suggesting that VEGF promotes the proliferation of hepatocytes by acting on LSECs[10]. Moreover, LSECs regulate the expression of stromal cell-derived factor 1 (SDF-1) after PH, which leads to the recruitment and mobilization of chemokine receptor 7+ (CXCR7+) BM SPCs into the circulation. Furthermore, CXCR7+ BM SPCs are recruited to the liver and eventually differentiated into mature LSECs, being involved in the reconstruction of the hepatic sinusoids and the promotion of liver regeneration[11]. In vitro and in vivo studies demonstrated that the expression of CXCR7 receptor and downstream paracrine factor HGF in the microenvironment is regulated by a unique transcriptional regulator called inhibitor of DNA binding 1 (Id1)[12]. The regeneration and repair response in the liver when injured is regulated by the activation of the intrahepatic CXCR7-Id1-HGF/Wnt2 pathway[13]. Therefore, our hypothesis is that VEGF changes the guiding microenvironment through the SDF-1/CXCR7 pathway, activates Id-1 in LSECs, and up-regulates the expression of HGF and Wnt2 to reprogram the microenvironment during liver regeneration (Figure 1).
Figure 1.
Schematic Illustration of liver after partial hepatectomy. After partial hepatectomy, hepatocytes begin to proliferate and release vascular endothelial growth factor, which promotes the proliferation of bone marrow-derived progenitor cells (BM SPCs) and reconstruction of hepatic sinusoids. BM SPCs secrete hepatocyte growth factor, which provides positive feedback and promotes hepatocytes proliferation. This figure was created with BioRender (BioRender.com). VEGF: Vascular endothelial growth factor; HGF: Hepatocyte growth factor; BM SPCs: Bone marrow-derived progenitor cells.
IMMUNITY AND LIVER REGENERATION
Liver regeneration is a complex process that involves multiple factors closely related to the immune response, which plays a critical role in tissue regeneration[14,15]. Following liver resection or injury, hypoxia occurs in certain liver areas, leading to hepatocyte apoptosis and release of apoptotic extracellular vesicles (aEVs). These aEVs are mainly cleared and phagocytosed by immune cells, while the main factor performing phagocytosis is still controversial. Some studies suggest that these aEVs are phagocytosed by Kupffer cells (KCs). KCs, the resident macrophages in the liver, are necessary for the emergence of liver progenitor-like cells (LPLCs), which are a source of newly regenerated hepatocytes under injury, as demonstrated by a mouse model of macrophage specific knockout[16]. KCs secrete inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, which are crucial early signals to start regeneration[17]. While other studies demonstrated that hepatocytes are reborn from pre-existing hepatocytes[18], aEVs in the liver are mainly cleared and phagocytosed by circulating neutrophils rather than by phagocytes such as KCs[8,19]. Massive cell apoptosis after PH inhibits the phagocytic activity of immune cells resident in the liver[14]. At this time, circulating neutrophils are recruited and responsible for the phagocytosis and clearance of aEVs. Neutrophils phagocytose aEVs secreted by apoptotic hepatic cells and then, they released multiple growth factors including HGF and VEGF to regulate the proliferation of hepatic cells. Liver regeneration decreases under the specific condition of neutrophil exhaustion, and liver function recovery is also delayed[8]. Liver regeneration and immunity are closely related, and further studies should focus more on them, including the role of efferocytosis and metabolism.
RELATIONSHIP BETWEEN VEGF AND LIVER REGENERATION
VEGF belongs to a class of peptides with heparin-binding activity connected by a disulfide bond through a cysteine residue[20]. It is a potent mitogen for endothelial cells by directly acting on their specific surface receptors to exert its biological effects. The asynchronous proliferation of hepatocytes and LSECs delays the regeneration of new liver sinusoids, causing an insufficient liver regeneration in the early postoperative period (within 72 h)[21,22]. Therefore, this early period after PH is the most important period in which VEGF promotes liver regeneration. Endothelial cells under the action of VEGF migrate, proliferate and form tubular protrusions, eventually forming a network structure of arteries and veins, also increasing vascular permeability[23].
Exogenous VEGF promotes LSEC proliferation, but only in a dose-dependent manner, meaning that only above a certain dose of VEGF the proliferation of LSECs is promoted[24]. In vitro studies demonstrated that the addition of VEGF to the culture medium of primary cultured LSECs promotes cell proliferation, while the group without VEGF showed a significantly less number of LSECs at 48 h than that at 16 h, and no significant difference was observed before and after the addition of 10 ng/mL VEGF. However, the number of LSECs at 48 h after the addition of 100 ng/mL VEGF is significantly higher than that at 16 h. Scanning electron microscopy showed that the window structure of LSECs gradually decreases with the increasing of time in the group without VEGF. LSECs in the liver are typically characterized by the presence of pores (fenestrae). These pores in the group with 10 ng/mL VEGF treatment are basically stable, and the number and size of this pores are significantly increased compared with those in the previous two groups. This indicated that VEGF promotes the proliferation of LSECs in a dose-dependent manner, with only a certain dose of VEGF promoting cell proliferation and exerting its function[24].
INTRAVENOUS INJECTION OF EXOGENOUS VEGF
Intravenous (IV) injection of exogenous VEGF is an proven method to induce the regeneration of the liver[25]. Indeed, it promotes the postoperative regeneration of the remaining liver after the detection of the PCNA index of hepatocytes in mice. The results showed that exogenous VEGF intervention significantly stimulates the proliferation of the remaining hepatocytes and promotes postoperative liver regeneration[26]. Another study also used tail vein injection of exogenous VEGF and in vivo microscopy to demonstrate that VEGF is involved in the regeneration after the removal of two-thirds of the liver[27]. The results showed that exogenous VEGF increases the hepatic vascular density, vascular diameter, and liver weight ratio as well as it enhances the proliferation of hepatocyte. Nevertheless, abnormal liver function and inhibition of hepatocyte proliferation are observed when endogenous VEGF is antagonized. However, the treatment with exogenous VEGF is not effective on liver regeneration when 90% of the liver is resected, as demonstrated by a rat resection model, suggesting that proliferation is also related to the degree of liver resection[28]. Although the administration of drugs through IV injection allows a certain degree of liver regeneration, the effective concentration of VEGF in the liver is insufficient due to its short half-life, uptake by other organs and tissues, and abundant hepatic blood flow[29]. Therefore, other efficient and safe liver-targeted drug delivery systems should be designed to overcome these shortcomings.
VEGF RELEASE BY NANOFIBERS
Electrospun nanofibers
The IV delivery of exogenous VEGF increases the content of VEGF only transiently, and long-term high concentrations in the liver cannot be achieved due to the abundant blood flow in the liver. Moreover, the short half-life of VEGF in vivo makes it difficult to achieve a constant effective concentration with a single injection, necessitating repeated injections that increase the complexity of treatment. Therefore, a controllable release nano-matrix membrane loaded with exogenous VEGF was designed and prepared to overcome the shortcomings, which was implanted into the residual liver wound after surgery to realize a targeted delivery of drugs by a local controlled release of VEGF.
The electrospinning technology is a universal and effective method for preparing nanoscale polymer fibers, and its principle is as follows: A polymer solution is connected with a high-voltage power supply through a wire, a receiving plate is grounded, and static electricity exists between the polymer solution and the receiving plate. The continuous increase of the voltage, the polymer solution gradually overcomes the surface tension and is ejected through a nozzle when the electrostatic action is greater than the cohesive force of the polymer flow, and the polymer is split into a fine polymer flow before reaching the collecting plate. The nanoscale polymer fibers in the form of a nonwoven fabric are thus obtained on the receiving sheet[30]. Nano-sized polymer fibers become a hot research topic in polymer material science in recent years due to their rapid preparation and their application in medical field is increasing and becoming mature.
Our research group previously developed a novel electrospun nanofiber loaded with VEGF, and thin layers of collagen were used as support to wrap the nanofiber between two layers of collagen to make a membrane-like structure. Collagen improves the stiffness of the fiber and confers a hemostatic function, which is beneficial to the fixation of the material at the implantation site. After the surgery, the fiber is directly covered on the liver wound surface, and the exogenous VEGF in the nanofiber in the local liver is continuously and efficiently controlled to form a microenvironment with effective VEGF concentration. This approach allows the proliferation of LSECs early after surgery, with an early promotion of liver regeneration[31]. The biodegradable biocompatible polymer and collagen used to make the nanofiber is approved by FDA of the United States and has a long-term traceable biological safety. The results showed that this new method of controlled release of VEGF through nanofibers significantly promotes the proliferation of the remaining hepatocytes at 48 and 72 h after surgery. The expression of endogenous VEGF in the liver is also higher than that in the control group without nanofibers. The above results confirmed that biodegradable nanofibers represent a convenient and effective method for the release of VEGF specifically in the postoperative residual liver to effectively promote liver regeneration[31].
Hydrogel nanofiber technology
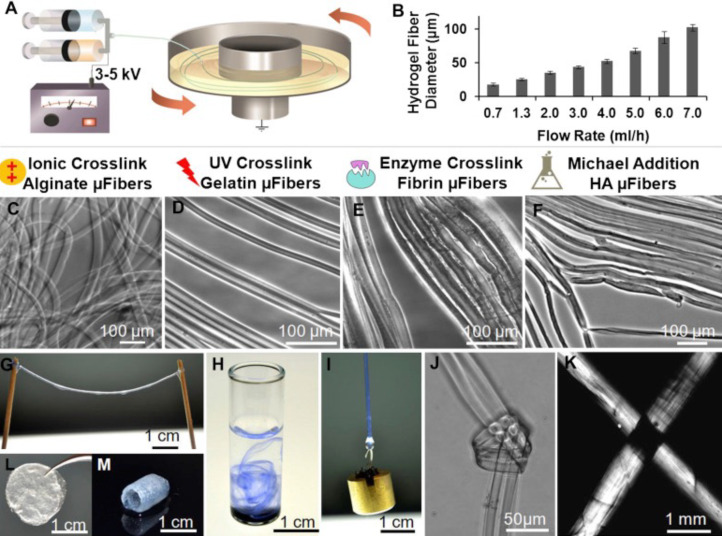
Electrospun nanofibers have some limitations in loading growth factors due to the high shear rate (105 to 106 s-1) of the polymer solution and the use of organic solvents. Moreover, the electrospinning of water-soluble polymers is difficult. These limitations make difficult the maintenance of an efficient activity of growth factors during the manufacturing process. Therefore, our research group prepared polymer composite hydrogel fibers by stretching the water-soluble polymer solution under the combined action of an electric field force and mechanical force[32]. The diameter and composition of the hydrogel fiber is realized by adjusting the components, concentration and stretching conditions of the polymer aqueous solution[33]. An important feature of this method is that the molecular chains in the fiber bundles are oriented using an electric field and a unidirectional mechanical tension. natural or synthetic polymer aqueous solutions were originally used to spin various hydrogel fibers, including alginate, collagen, fibrin, and hyaluronic acid. More importantly, this method without the use of organic solvents allows the formation of multilayered (core-shell) fibers that encapsulate growth factors in hydrogel fibers with a small impact on their bioactivity (Figure 2)[32]. In addition, the release rate of growth factors is controlled by adjusting the fiber composition and the degree of crosslinking, as well as the properties of the composite. The treatment with VEGF loaded with hydrogels accelerates the recovery of liver function after surgery in a rat model of 70% PH, and the peak value of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was 12 h earlier than that in the control group without hydrogels. Hepatocyte proliferation was also increased[34].
Figure 2.
Preparation of hydrogel nanofiber. A: Illustration of the electrostretching setup; B: Effect of alginate solution feeding rate on the diameter of hydrogel microfibres; C-F: Various crosslinking mechanisms have been employed to crosslink alginate, gelatin, fibrin and hyaluronic acid hydrogel microfibers; G: Hydrogel microfibres of any desired length can be prepared; H: When dispersed in water, alginate hydrogel fibres formed a loose network of hydrogel fibres; I: A 10-g metal pillar was lifted with an alginate hydrogel microfibre bundle; J and K: A micro-knot was made with two alginate hydrogel microfibres; L and M: Beyond microfibre bundles, these hydrogel microfibres can also be fabricated into other forms like fibrous films (L) and self-supporting hydrogel tubes (M). Reproduced with permission[32]. Citation: Zhang S, Liu X, Barreto-Ortiz SF, Yu Y, Ginn BP, DeSantis NA, Hutton DL, Grayson WL, Cui FZ, Korgel BA, Gerecht S, Mao HQ. Creating polymer hydrogel microfibres with internal alignment via electrical and mechanical stretching. Biomaterials 2014; 35: 3243-3251. Copyright© The Authors 2013, Published by Elsevier Ltd.
Retrograde intrabiliary infusion of VEGF nanoparticles
All parenchymal cells in the remaining liver after PH develop a proliferative potential, not just an exophytic growth from the wound site. Although the strategy of releasing VEGF from nanofiber results in the local release of growth factors onto the wound surface, the concentration of VEGF inside the liver decreases when the VEGF penetrate into the liver from the liver wound surface, so that the stimulation of VEGF on the cells in the liver is not uniform. Therefore, all cells in the residual liver should be stimulated by VEFG to effectively accelerate the regeneration of the residual liver and the recovery of liver function. Hence, it is necessary to design a new strategy to obtain a wide distribution of growth factors in the liver, allow their efficient uptake by intrahepatic cells, and realize high activity and low clearance rate in the liver.
The ultrastructural surface area of the hepatic biliary system is enormous, and the biliary system provides a surface in direct contact with almost all hepatocytes[35]. In addition, there are no KCs in the intrahepatic biliary system, thus, the non-specific phagocytosis of exogenous molecules by KCs is avoided and the immune inflammatory response of the body is reduced. The large volume of the intrahepatic bile duct makes the intrahepatic biliary system more distensible and able to tolerate the retrograde injection of a certain volume of fluid. Therefore, retrograde intrabiliary infusion (RII) has become a new way to perform a targeted delivery of drugs into the liver[36-38].
Administration of the VEGF gene has some advantages compared with the administration of the VEGF protein: Gene administration induces the transient expression of growth factor in hepatocytes, which is the ideal manner to realize an administration in the full residual liver. Although the conventional transgenic viral vectors effectively transfer the target gene into the parenchymal cells of liver, the side effects and immune responses induced by viruses limit their clinical application. Non-viral gene vectors have the advantages of diverse design, simple synthesis, storage stability, flexibility of the transgene length, low toxicity, and low immune response. In addition, the transfection of a non-viral gene vector is suitable for the transient expression of growth factors in liver regeneration.
At present, researchers have successfully prepared a variety of biodegradable nanoparticle systems for gene delivery[39-41]. Chitosan is a natural polysaccharide with good biodegradability, biocompatibility, anti-inflammatory effects, and its polycation structure confers many excellent properties in drug and gene delivery. Furthermore, the cationic structure of chitosan allows higher entrapment efficiency and loading of drugs, especially negatively charged genes. Finally, positively charged chitosan nanoparticles enhance the cellular uptake thanks to their slightly negatively charged cell membrane surface[42-44]. Ultra-pure, narrow molecular weight fully characterized chitosan is now available, and therefore, it is almost reaching the approval as a vector material than other gene vectors.
Previous studies demonstrated that chitosan-DNA nanoparticles (150-300 nm) achieve high transfection efficiency in vivo in rat hepatocytes after RII. The expression of transgenic proteins began at 12 h, peaks at 3 d after injection, and gradually declines after 14 d. Liver function analysis and histopathological examination show that the approach of using RII of nanoparticles does not produce significant toxic reactions and damage to the liver and bile ducts[45-47]. The expression of transgenic proteins in the kidney, lung, spleen and heart is almost negligible compared with their expression in the liver, which proves that this method results in a local high-efficiency and specific expression of the introduced gene in the liver. Therefore, RII is the best approach for gene delivery in the liver.
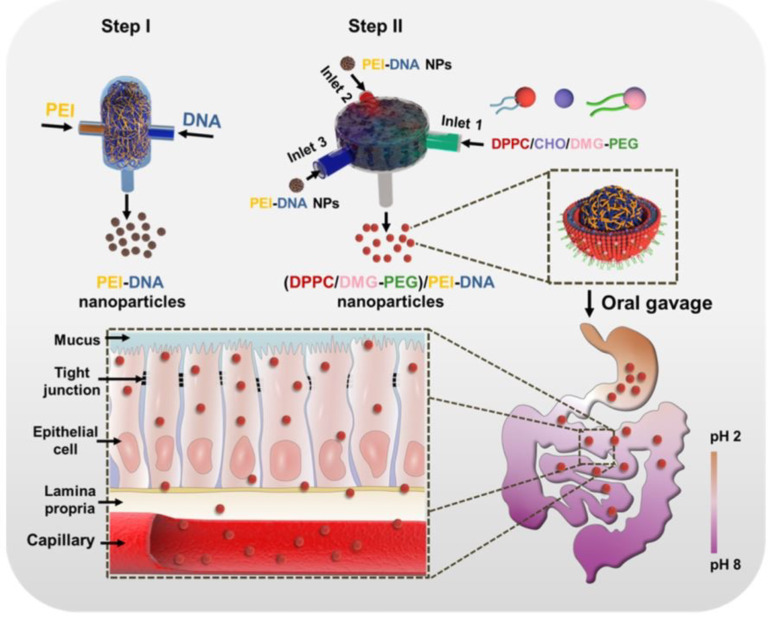
Polycation-DNA nanoparticles are prepared by routine method, which consists of a mechanical mix polycations and plasmid DNA, but this assembly process is subjected to a variety of interference by kinetic factors during the preparation (about tens of milliseconds), easily leading to low homogeneity and unstable properties of nanoparticles. Therefore, our co-investigators developed a rapid nanocomposite method called flash nano-complexation to solve this technical problem, protecting plasmid DNA and working at very high flow rates inside a confined impact jet reactor. The polycationic solution reduces the mixing time of the two substances to approximately 10 milliseconds, avoiding significant folding denaturation of the plasmid DNA[48]. Uniform size, homogeneous properties and controllable oral plasmid DNA nanoparticles are prepared by this innovative method, and successfully used in the treatment of diabetes (Figure 3)[49]. Our group prepared VEGF-chitosan-DNA nanoparticles for RII in rats subjected to 70% hepatectomy and acute liver injury induced by acetaminophen. The preliminary results show that these nanoparticles promote the recovery of liver function and liver regeneration after PH.
Figure 3.
Illustration of PEI-DNA nanoparticle preparation via flash nanocomplexation and the proposed trafficking steps along the GI tract. Reproduced with permission[49]. Citation: Nie T, He Z, Zhou Y, Zhu J, Chen K, Liu L, Leong KW, Mao HQ, Chen Y. Surface Coating Approach to Overcome Mucosal Entrapment of DNA Nanoparticles for Oral Gene Delivery of Glucagon-like Peptide 1. ACS Appl Mater Interfaces 2019; 11: 29593-29603. Copyright© The Authors 2019. Published by American Chemical Society.
LIMITATION AND FUTURE OUTLOOK
A precise spatio-temporal regulation of VEGF level is necessary for the proper functioning of the vascular system. VEGF plays a pivotal role in angiogenesis during embryonic development, tissue regeneration, and wound healing. However, VEGF is also a key mediator of angiogenesis in tumors. It can upregulate the expression of VEGF under various conditions, such as oncogene expression, multiple growth factors, and hypoxia, thereby promoting angiogenesis, which is essential for the development and growth of tumor[50]. In most solid tumors, VEGF is overexpressed. The abnormal expression of VEGF leads to the formation of a new vascular system in and around the tumor. These tumor vessels, formed under the action of VEGF, are structurally and functionally abnormal, thus promoting tumor growth. Given the central role of VEGF in tumor angiogenesis, it represents a rational target for anti-tumor therapy[51]. Therefore, by downregulating the VEGF signal in the tumor, the vascular system returns to a "normal" state. This "normalized" vascular system increases tumor oxygenation and improves drug permeability, leading to a better therapeutic response[52]. Therefore, in the future, we need to more accurately evaluate the VEGF of the recipient tissue and balance between promoting regeneration and inhibiting tumor.
CONCLUSION
VEGF is an important growth factor promoting angiogenesis and tissue regeneration, but it also promotes tumor growth depending on its expression. This contradiction reflects the complex role of VEGF in regulating cell growth and apoptosis[53]. Therefore, more studies are needed to better control VEGF expression to avoid the promotion of tumor growth and to promote tissue regeneration[54].
In the future, the administration of exogenous VEGF might become a potential treatment to promote liver regeneration after liver injury. However, the current intervention methods to administer exogenous VEGF are still limited, and the efficiency needs to be improved. Locally controlled-release VEGF by nanofibers and retrograde injection of VEGF-DNA-chitosan nanoparticles through bile ducts can promote residual liver regeneration, providing an alternative drug delivery mode in clinical practice.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. Mao HQ (Johns Hopkins University), Dr. He ZY (Ocean University of China) and Dr. Nie TQ (Sun Yat-sen University) for the valuable supports.
Footnotes
Conflict-of-interest statement: We declare that we have no conflict of interest to this work.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 3, 2023
First decision: May 4, 2023
Article in press: June 2, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fereydouni N, Iran; Huan C, United States; Kordzaia D, Georgia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
Contributor Information
Yun Jin, Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang Province, China.
Ying-Hao Guo, Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang Province, China.
Jia-Cheng Li, Department of General Surgery, Yuhuan Second People’s Hospital, Taizhou 317600, Zhejiang Province, China.
Qi Li, Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang Province, China.
Dan Ye, Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang Province, China.
Xiao-Xiao Zhang, Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang Province, China.
Jiang-Tao Li, Department of Hepatopancreatobiliary Surgery, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310009, Zhejiang Province, China. zrljt@zju.edu.cn.
References
- 1.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prev. 2018;27:205–212. doi: 10.1097/CEJ.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrero JA, Ahn J, Rajender Reddy K Americal College of Gastroenterology. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109:1328–47; quiz 1348. doi: 10.1038/ajg.2014.213. [DOI] [PubMed] [Google Scholar]
- 4.Chan A, Kow A, Hibi T, Di Benedetto F, Serrablo A. Liver resection in Cirrhotic liver: Are there any limits? Int J Surg. 2020;82S:109–114. doi: 10.1016/j.ijsu.2020.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 6.Gadd VL, Aleksieva N, Forbes SJ. Epithelial Plasticity during Liver Injury and Regeneration. Cell Stem Cell. 2020;27:557–573. doi: 10.1016/j.stem.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 7.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123:1861–1866. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandel V, Schimek V, Göber S, Hammond T, Brunnthaler L, Schrottmaier WC, Mussbacher M, Sachet M, Liang YY, Reipert S, Ortmayr G, Pereyra D, Santol J, Rainer M, Walterskirchen N, Ramos C, Gerakopoulos V, Rainer C, Spittler A, Weiss T, Kain R, Messner B, Gruenberger T, Assinger A, Oehler R, Starlinger P. Hepatectomy-induced apoptotic extracellular vesicles stimulate neutrophils to secrete regenerative growth factors. J Hepatol. 2022;77:1619–1630. doi: 10.1016/j.jhep.2022.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49:121–130. doi: 10.1177/002215540104900112. [DOI] [PubMed] [Google Scholar]
- 11.DeLeve LD, Wang X, Wang L. VEGF-sdf1 recruitment of CXCR7+ bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2016;310:G739–G746. doi: 10.1152/ajpgi.00056.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, Shido K, Rabbany SY, Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shido K, Chavez D, Cao Z, Ko J, Rafii S, Ding BS. Platelets prime hematopoietic and vascular niche to drive angiocrine-mediated liver regeneration. Signal Transduct Target Ther. 2017;2:16044–16044. doi: 10.1038/sigtrans.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di-Iacovo N, Pieroni S, Piobbico D, Castelli M, Scopetti D, Ferracchiato S, Della-Fazia MA, Servillo G. Liver Regeneration and Immunity: A Tale to Tell. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24021176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Moshe S, Veg T, Manco R, Dan S, Papinutti D, Lifshitz A, Kolodziejczyk AA, Bahar Halpern K, Elinav E, Itzkovitz S. The spatiotemporal program of zonal liver regeneration following acute injury. Cell Stem Cell. 2022;29:973–989.e10. doi: 10.1016/j.stem.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Cui L, Lin P, Liu Z, Bao S, Ma X, Nan H, Zhu W, Cen J, Mao Y, Jiang L, Nie Y, Ginhoux F, Li Y, Li H, Hui L. Kupffer-cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers. Cell Stem Cell. 2023;30:283–299.e9. doi: 10.1016/j.stem.2023.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Álvarez-Varela A, Wu P, Jin Y, Zhu J, Li B, Grompe M, Wang B, Nusse R. Inflammatory Cytokine TNFα Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell. 2018;175:1607–1619.e15. doi: 10.1016/j.cell.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu W, Zhu H, Zhang M, Pikiolek M, Ercan C, Li J, Huang X, Han X, Zhang Z, Lv Z, Li Y, Liu K, He L, Liu X, Heim MH, Terracciano LM, Tchorz JS, Zhou B. Bipotent transitional liver progenitor cells contribute to liver regeneration. Nat Genet. 2023;55:651–664. doi: 10.1038/s41588-023-01335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 20.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, Terhardt D, Vogel MJ, Cao L, Korn C, Bartels S, Thomas M, Augustin HG. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 22.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 24.Funyu J, Mochida S, Inao M, Matsui A, Fujiwara K. VEGF can act as vascular permeability factor in the hepatic sinusoids through upregulation of porosity of endothelial cells. Biochem Biophys Res Commun. 2001;280:481–485. doi: 10.1006/bbrc.2000.4148. [DOI] [PubMed] [Google Scholar]
- 25.Rizvi F, Everton E, Smith AR, Liu H, Osota E, Beattie M, Tam Y, Pardi N, Weissman D, Gouon-Evans V. Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat Commun. 2021;12:613. doi: 10.1038/s41467-021-20903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999;30:911–915. doi: 10.1016/s0168-8278(99)80147-0. [DOI] [PubMed] [Google Scholar]
- 27.Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grünewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A, Broelsch CE, Schlaak JF. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291–299. doi: 10.1016/j.jss.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Bockhorn M, Schöllmann S, Opitz B, Sotiropoulos GC, Sheu SY, Niehaus E, Trippler M, Frilling A, Broelsch CE, Schlaak JF. Vascular endothelial growth factor does not improve liver regeneration and survival after 90% subtotal liver resection. Hepatol Res. 2007;37:353–359. doi: 10.1111/j.1872-034X.2007.00047.x. [DOI] [PubMed] [Google Scholar]
- 29.Wei S, Li Z, Shi Q, Luan X, Yuan X, Li Y, Guo C, Wu X, Shi C, Di G. Collagenbinding vascular endothelial growth factor (CBDVEGF) promotes liver regeneration in murine partial hepatectomy. Mol Med Rep. 2022;26 doi: 10.3892/mmr.2022.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin L, Wang T, Zhu ML, Leach MK, Naim YI, Corey JM, Feng ZQ, Jiang Q. Electrospun fibers and tissue engineering. J Biomed Nanotechnol. 2012;8:1–9. doi: 10.1166/jbn.2012.1360. [DOI] [PubMed] [Google Scholar]
- 31.Yu YQ, Jiang XS, Gao S, Ma R, Jin Y, Jin X, Peng SY, Mao HQ, Li JT. Local delivery of vascular endothelial growth factor via nanofiber matrix improves liver regeneration after extensive hepatectomy in rats. J Biomed Nanotechnol. 2014;10:3407–3415. doi: 10.1166/jbn.2014.1872. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Liu X, Barreto-Ortiz SF, Yu Y, Ginn BP, DeSantis NA, Hutton DL, Grayson WL, Cui FZ, Korgel BA, Gerecht S, Mao HQ. Creating polymer hydrogel microfibres with internal alignment via electrical and mechanical stretching. Biomaterials. 2014;35:3243–3251. doi: 10.1016/j.biomaterials.2013.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Zhao Y, Ouyang X, Yang Y, Chen Y, Luo Q, Zhang Y, Zhu D, Yu X, Li L. Biomimetic hybrid hydrogel for hemostasis, adhesion prevention and promoting regeneration after partial liver resection. Bioact Mater. 2022;11:41–51. doi: 10.1016/j.bioactmat.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y, Zhang XX, Yang JJ, Qian CM, Zhou JL, Yu YQ, Zhou DE, Wang L, Peng SY, Mao HQ. A novel strategy for locally deliver VEGF by nanofiber mesh for improving liver regeneration. HPB. 2016;18:e290–e291. [Google Scholar]
- 35.Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147–R1151. doi: 10.1016/j.cub.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai H, Jiang X, Leong KW, Mao HQ. Transient depletion of kupffer cells leads to enhanced transgene expression in rat liver following retrograde intrabiliary infusion of plasmid DNA and DNA nanoparticles. Hum Gene Ther. 2011;22:873–878. doi: 10.1089/hum.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narmada BC, Kang Y, Venkatraman L, Peng Q, Sakban RB, Nugraha B, Jiang X, Bunte RM, So PT, Tucker-Kellogg L, Mao HQ, Yu H. Hepatic stellate cell-targeted delivery of hepatocyte growth factor transgene via bile duct infusion enhances its expression at fibrotic foci to regress dimethylnitrosamine-induced liver fibrosis. Hum Gene Ther. 2013;24:508–519. doi: 10.1089/hum.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai C, Wang M, Zhao L, Xu C, Huang J, Fan Z. Liver gene transfection by retrograde intrabiliary infusion facilitated by temporary biliary obstruction. J Gene Med. 2020;22:e3144. doi: 10.1002/jgm.3144. [DOI] [PubMed] [Google Scholar]
- 39.Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70:399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 40.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 41.Mao HQ, Leong KW. Design of polyphosphoester-DNA nanoparticles for non-viral gene delivery. Adv Genet. 2005;53:275–306. [PubMed] [Google Scholar]
- 42.Cao Y, Tan YF, Wong YS, Liew MWJ, Venkatraman S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar Drugs. 2019;17 doi: 10.3390/md17060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang E, Xing R, Liu S, Qin Y, Li K, Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr Polym. 2019;222:115004. doi: 10.1016/j.carbpol.2019.115004. [DOI] [PubMed] [Google Scholar]
- 44.Ashrafizadeh M, Ahmadi Z, Mohamadi N, Zarrabi A, Abasi S, Dehghannoudeh G, Tamaddondoust RN, Khanbabaei H, Mohammadinejad R, Thakur VK. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer theranostics. Int J Biol Macromol. 2020;145:282–300. doi: 10.1016/j.ijbiomac.2019.12.145. [DOI] [PubMed] [Google Scholar]
- 45.Dai H, Jiang X, Tan GC, Chen Y, Torbenson M, Leong KW, Mao HQ. Chitosan-DNA nanoparticles delivered by intrabiliary infusion enhance liver-targeted gene delivery. Int J Nanomedicine. 2006;1:507–522. doi: 10.2147/nano.2006.1.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Ren Y, Williford JM, Li Z, Mao HQ. Liver-targeted gene delivery through retrograde intrabiliary infusion. Methods Mol Biol. 2013;948:275–284. doi: 10.1007/978-1-62703-140-0_19. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Dai H, Leong KW, Goh SH, Mao HQ, Yang YY. Chitosan-g-PEG/DNA complexes deliver gene to the rat liver via intrabiliary and intraportal infusions. J Gene Med. 2006;8:477–487. doi: 10.1002/jgm.868. [DOI] [PubMed] [Google Scholar]
- 48.Liu HW, Hu Y, Ren Y, Nam H, Santos JL, Ng S, Gong L, Brummet M, Carrington CA, Ullman CG, Pomper MG, Minn I, Mao HQ. Scalable Purification of Plasmid DNA Nanoparticles by Tangential Flow Filtration for Systemic Delivery. ACS Appl Mater Interfaces. 2021;13:30326–30336. doi: 10.1021/acsami.1c05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nie T, He Z, Zhou Y, Zhu J, Chen K, Liu L, Leong KW, Mao HQ, Chen Y. Surface Coating Approach to Overcome Mucosal Entrapment of DNA Nanoparticles for Oral Gene Delivery of Glucagon-like Peptide 1. ACS Appl Mater Interfaces. 2019;11:29593–29603. doi: 10.1021/acsami.9b10294. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y, Cao Y. The impact of VEGF on cancer metastasis and systemic disease. Semin Cancer Biol. 2022;86:251–261. doi: 10.1016/j.semcancer.2022.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 53.Eelen G, Treps L, Li X, Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ Res. 2020;127:310–329. doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad A, Nawaz MI. Molecular mechanism of VEGF and its role in pathological angiogenesis. J Cell Biochem. 2022;123:1938–1965. doi: 10.1002/jcb.30344. [DOI] [PubMed] [Google Scholar]