Abstract
BACKGROUND
As a novel endogenous anti-angiogenic molecule, vasohibin 1 (VASH1) is not only expressed in tumor stroma, but also in tumor tissue. Moreover, studies have shown that VASH1 may be a prognostic marker in colorectal cancer (CRC). Knockdown of VASH1 enhanced transforming growth factor-β1 (TGF-β1)/Smad3 pathway activity and type I/III collagen production. Our previous findings suggest that ELL-associated factor 2 (EAF2) may play a tumor suppressor and protective role in the development and progression of CRC by regulating signal transducer and activator of transcription 3 (STAT3)/TGF-β1 signaling pathway. However, the functional role and mechanism of VASH1-mediated TGF-β1 related pathway in CRC has not been elucidated.
AIM
To investigate the expression of VASH1 in CRC and its correlation with the expression of EAF2. Furthermore, we studied the functional role and mechanism of VASH1 involved in the regulation and protection of EAF2 in CRC cells in vitro.
METHODS
We collected colorectal adenocarcinoma and corresponding adjacent tissues to investigate the clinical expression of EAF2 protein and VASH1 protein in patients with advanced CRC. Following, we investigated the effect and mechanism of EAF2 and VASH1 on the invasion, migration and angiogenesis of CRC cells in vitro using plasmid transfection.
RESULTS
Our findings indicated that EAF2 was down-regulated and VASH1 was up-regulated in advanced CRC tissue compared to normal colorectal tissue. Kaplan-Meier survival analysis showed that the higher EAF2 Level group and the lower VASH1 Level group had a higher survival rate. Overexpression of EAF2 might inhibit the activity of STAT3/TGF-β1 pathway by up-regulating the expression of VASH1, and then weaken the invasion, migration and angiogenesis of CRC cells.
CONCLUSION
This study suggests that EAF2 and VASH1 may serve as new diagnostic and prognostic markers for CRC, and provide a clinical basis for exploring new biomarkers for CRC. This study complements the mechanism of EAF2 in CRC cells, enriches the role and mechanism of CRC cell-derived VASH1, and provides a new possible subtype of CRC as a therapeutic target of STAT3/TGF-β1 pathway.
Keywords: ELL-associated factor 2, Vasohibin 1, Transforming growth factor-β1, Signal transducer and activator of transcription 3, Colorectal cancer, Angiogenesis
Core Tip: Endothelial cell-derived vasohibin 1 (VASH1) can regulate tumor angiogenesis and lymphangiogenesis, and tumor cell-derived VASH1 also has a potential functional role in inhibiting cancer cell proliferation and invasion. Knockdown of VASH1 enhanced transforming growth factor beta 1 (TGF-β)/Smad3 pathway activity and type I/III collagen production. Our previous findings suggest that ELL-associated factor 2 (EAF2) may play a tumor suppressor and protective role in the development and progression of colorectal cancer (CRC) by regulating signal transducer and activator of transcription 3 (STAT3)/TGF-β1 signaling pathway. In this study, we found that EAF2 was down-regulated and VASH1 was up-regulated in advanced CRC tissue compared to normal colorectal tissue by immunohistochemistry and Western blot. And the expression levels of EAF2 and VASH1 were positively correlated in advanced CRC tissue. Furthermore, in vitro cellular mechanism studies suggest that overexpression of EAF2 may inhibit the activity of STAT3/TGF-β1 pathway by up-regulating the expression of VASH1, thereby attenuating the invasion, migration and angiogenesis of CRC cells. This study provides new ideas and theoretical basis for the development of new diagnostic markers for CRC and the exploration of the mechanism of targeted therapy.
INTRODUCTION
The diagnosis and treatment of colorectal cancer (CRC) have greatly improved in recent years, and its incidence and mortality have steadily decreased by about 1.7% and 3.2% per year respectively[1]. This has benefited from the continuous in-depth research on the pathogenesis of CRC, especially the application of protein molecular markers and targeted therapy. However, the pathogenesis of CRC has not been fully elucidated, and its diagnosis and treatment remain a major challenge in clinical work. Malignant tumor metastasis is one of the main factors leading to poor survival of patients with CRC[2]. Therefore, there is an urgent need to clarify the potential mechanisms of invasion and metastasis of CRC and explore new therapeutic targets.
Current studies have shown that not only the proliferation, invasion, and metastasis of cancer cells themselves contribute to the growth, progression, and distant metastasis of CRC, but tumor microenvironmental factors such as tumor angiogenesis also play a crucial role. As a novel endogenous anti-angiogenic molecule, vasohibin 1 (VASH1) increased ΔY-tubulin level to attenuate endocytosis of vascular endothelial growth factor (VEGF) receptor 2 and fibroblast growth factor (FGF) receptor 1, resulting in suppressing pro-angiogenic factor-induced endothelial cells migration[3]. Several studies have shown that the expression of VASH1 in tumor tissue and stroma can be used as a new molecular marker for tumor prognosis. In addition, cancer cell-derived VASH1 regulates tumor growth and metastasis both in vitro and in vitro[4-6]. And studies have shown that VASH1 may be a marker for the prognosis of CRC[7-9]. It has been suggested that stroma VASH1 Level is negatively correlated with tumor size, clinical stage, and distant metastasis in colon cancer patients[8]. Whereas, other studies have suggested that the overall survival (OS) of patients with high VASH1 expression in CRC tissue are significantly lower than those with low VASH1 expression[9]. To date, the expression of VASH1 in CRC cancer tissue and cancer cells remains controversial, the effect and mechanism of cancer cell-derived VASH1 have not been clarified.
Ingenuity Pathway Analysis showed that transforming growth factor (TGF)-β/SMAD signaling pathway was one of the most common mutated pathways in CRC metastasis[10]. Knockdown of VASH1 enhanced Smad3 phosphorylation and type I/III collagen production in renal fibroblasts stimulated by TGF-β[11]. What’s more, inhibition of VASH1 in islet β cells can activate GF-β1 pathway and promote insulin resistance in gestational diabetes mellitus patients[12]. TGF-β1 is abundantly expressed in the tumor microenvironment of a variety of human tumors, which is an important molecular pathway connecting tumor cells with the surrounding microenvironment and leading to resistance to checkpoint blockade therapy[13]. More notably, blockade of TGF-β1 signaling is a strategic approach to treat CRC tumor metastasis[14]. Nevertheless, whether CRC cell-derived VASH1 regulates TGF-β1 related pathways in tumor development has not been investigated.
Androgen-responsive protein ELL-associated factor 2 (EAF2) has been shown to inhibit the growth of tumor size in prostate cancer models in vivo[15], and inhibit the proliferation and migration of prostate cancer cells in vitro[16]. Recent studies have found that EAF2 acts as a tumor suppressor in a variety of malignant tumors, such as renal cancer cells, human liver cancer cells and breast cancer cells[17,18]. In-depth mechanistic studies showed that EAF2 was involved in the regulation of cancer cell function by attenuating TGF-β1-induced G1 phase cell cycle arrest and cell migration[18]. In addition, silencing of EAF2 expression could modulate the cytotoxic response to statins in HCT-116 cells[19]. Our previous findings suggested that EAF2 may play a tumor suppressor and protective role in the development and progression of CRC by regulating STAT3/TGF-β1 signaling pathway[20]. Therefore, we ventured to speculate whether the mechanism of VASH1 in CRC was related to the EAF2-mediated TGF-β1 pathway.
In this study, we aimed to investigate the expression of VASH1 in CRC and its correlation with the expression of EAF2. To further explore the functional role and mechanism of VASH1-mediated TGF-β1 related pathway in CRC cells. To investigate whether VASH1 is involved in the regulation and protection of EAF2 in CRC cells by regulating STAT3/TGF-β1 signaling pathway. It provides a clinical basis for discovering new clinical diagnostic and prognostic markers of CRC, and a theoretical basis for elucidating its development mechanism and exploring new molecular targeted therapy.
MATERIALS AND METHODS
CRC patients and tissue samples
A total of 120 cases of colorectal adenocarcinoma and corresponding adjacent tissues were collected at the First Hospital of China Medical University from 2012 to 2017. Patients who received preoperative radiotherapy or chemotherapy were excluded. The American Joint Committee on Cancer/Union for International Cancer Control 8th edition TNM staging system was used for tumor staging. The study was approved by the institutional review board of the First Affiliated Hospital of China Medical University (registration number 2021-68-2, informed consent waived).
Cell culture
We cultured NCM460 and CRC cell lines (SW480, RKO, HCT116, HT29, and LoVo) (Chinese Academy of Sciences, Shanghai, China) with PMIS 1640 medium (Gibco, Grand Island, NY, United States) containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, United States) in a humidified incubator at 37 °C with 5% CO2. And we treated CRC cells with 5 ng/mL recombinant human TGF-β1 protein (100-21, PeproTech, United States) for 24 h. Human Umbilical Vein Endothelial Cells (HUVECs, Chinese Academy of Sciences, Shanghai, China) were cultured with DMEM medium (Gibco, Grand Island, NY, United States) containing 10% FBS (Gibco, Grand Island, NY, United States) in a humidified incubator at 37 °C and 5% CO2.
Cell transfection
Gene transfection plasmids designed and synthesized by GeneChem (Shanghai, China) were used to treat CRC cells, including overexpressing human EAF2 plasmid (GenBank No. NM_018456), overexpressing human VASH1 plasmid (GenBank No. NM_014909), silencing human STAT3 plasmids (GenBank registration No. NM_139276) and silencing VASH1 plasmids (GenBank No. NM_014909). Table 1 Lists three gene-silencing plasmids targeting human STAT3 plasmids and three targeting human VASH1 plasmids. Lipofectamine 2000 Kit (Invitrogen, Carlsbad, CA, United States) was used to transfect plasmids into CRC cells for cell transfection.
Table 1.
Gene sequences used for transfection
|
Gene
|
Sequences
|
|
| STAT3-siRNA | siRNA-S1-a | 5’-CCG GGC TGA CCA ACA ATC CCA AGA ACT CGA GTT CTT GGG ATT GTT GGT CAG CTT TTT G-3’ |
| siRNA-S1-b | 5’-AAT TCA AAA AGC TGA CCA ACA ATC CCA AGA ACT CGA GTT CTT GGG ATT GTT GGT CAG C-3’ | |
| siRNA-S2-a | 5’-CCG GGC ACA ATC TAC GAA GAA TCA ACT CGA GTT GAT TCT TCG TAG ATT GTG CTT TTT G-3’ | |
| siRNA-S2-b | 5’- AAT TCA AAA AGC ACA ATC TAC GAA GAA TCA ACT CGA GTT GAT TCT TCG TAG ATT GTG C-3’ | |
| siRNA-S3-a | 5’- CCG GGC TGA AAT CAT CAT GGG CTA TCT CGA GAT AGC CCA TGA TGA TTT CAG CTT TTT G-3’ | |
| siRNA-S3-b | 5’- AAT TCA AAA AGC TGA AAT CAT CAT GGG CTA TCT CGA GAT AGC CCA TGA TGA TTT CAG C-3’ | |
| VASH1-siRNA | siRNA-V1-a | 5’-CCG GCT GCC AAT CAA ATG CCT GGA ACT CGA GTT CCA GGC ATT TGA TTG GCA GTT TTT G -3’ |
| siRNA-V1-b | 5’-AAT TCA AAA ACT GCC AAT CAA ATG CCT GGA ACT CGA GTT CCA GGC ATT TGA TTG GCA G -3’ | |
| siRNA-V2-a | 5’-CCG GGA GCT GCA GTA CAA TCA CAC ACT CGA GTG TGT GAT TGT ACT GCA GCT CTT TTT G-3’ | |
| siRNA-V2-b | 5’-AAT TCA AAA AGA GCT GCA GTA CAA TCA CAC ACT CGA GTG TGT GAT TGT ACT GCA GCT C-3’ | |
| siRNA-V3-a | 5’-CCG GCA CAG GAC ATA GTG GTG CTT TCT CGA GAA AGC ACC ACT ATG TCC TGT GTT TTT G-3’ | |
| siRNA-V3-b | 5’-AAT TCA AAA ACA CAG GAC ATA GTG GTG CTT TCT CGA GAA AGC ACC ACT ATG TCC TGT G -3’ | |
STAT3: Signal transducer and activator of transcription 3; VASH1: Vasohibin 1.
Immunohistochemistry
Tissue blocks were fixed and serially sectioned. Antigen repair was performed with citric acid or repair solution, and endogenous peroxidase was blocked with hydrogen peroxide (3%). Rabbit anti-EAF2 monoclonal antibody (1:200 dilution, ab237753) (Abcam, United Kingdom) and rabbit anti-VASH1 polyclonal antibody (5 μg/mL) (Abcam, United Kingdom) were then incubated overnight at 4 °C. Subsequently, secondary antibodies were incubated at 37 °C for 40 min. The stains were stained with diamine benzidine and counterstained with hematoxylin. Finally, the slices are air-dried, dehydrated and installed.
Slides were read in a double-blind manner by two experienced pathologists. The final staining score is the proportion score multiplied by the intensity score. Five 200 × high-power fields were randomly selected for each section, and the intensity of immunostaining was classified into four categories: 0 (negative immunostaining), 1 (weak immunostaining), 2 (moderate immunostaining), and 3 (strong immunostaining). The percentage of positive cells was also counted: 0 (positive cells < 5%); 1 (5%-25% positive cells), 2 (26%-50% positive cells), 3 (51%-75% positive cells), and 4 (> 75% positive cells).
Western blot assay
Tissues and cells were lysed with RIPA lysates containing PMSF protease inhibitors. Protein was extracted and assayed by BCA kit (Beyotime Institute of Biotechnology, China). Equal amounts of proteins (50 μg per sample) were separated by 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) at 80 V and then transferred to polyvinylidene difluoride (PVDF) membranes at a constant current of 200 mA. PVDF membranes were incubated with primary antibodies overnight at 4 °C. Primary antibodies to EAF2 (ab151692), VASH1 (ab176114) (Abcam, United Kingdom), TGF-β1 (#3709), phospho-STAT3 (Tyr705) (#9145), and STAT3 (#30835) (Cell Signaling, Danvers, MA, United States) were used at a 1:1000 dilution ratio and normalized to a 1:1000 dilution ratio for GAPDH (FL-335) (Santa Cruz, CA, United States). The PVDF membranes were further incubated with secondary antibody (1:5000) for 2 h at 37 °C. The immunoreactive bands were washed with PBS and detected by the Microchemi 4.2 bioimaging system using ECL-Plus chemiluminescence detection HRP reagent (Beyotime Institute of Biotechnology, China). ImageJ software was used to analyze the gray value of the Western blot results. The experiment was repeated at least 3 times under the same experimental conditions (Supplementary Figure 1).
Transwell invasion and migration assay
Matrigel (BD Biosciences, San Diego, CA, United States) was diluted in serum-free medium at 1:8 and spread in an 8-μM transwell chamber (3422, Corning, NY, United States) at 37 °C for 6 h. After the matrix gel for invasion assay was successfully prepared, 4 × 104 cells/well were diluted with 200 μL serum-free medium and inoculated into the upper chamber of 8-μM transwell chamber (3422), and 600 μL medium containing 20% FBS was added to the lower chamber.
In the transwell migration experiment, 2 × 105 cells/well diluted with 200 μL serum-free medium were inoculated into the upper chamber of Matrigel-free transwell chamber (3422) with a diameter of 8 μM, and 600 μL culture medium containing 10% FBS was injected into the lower chamber.
The chambers were collected after incubation for 72 h at 37 °C under constant temperature regulation. The chamber filters were fixed with methanol after removing the cells that were not invaded or migrated on the inner surface of the chamber filters. Cells that had been invaded or migrated on the fixed filter membrane were further stained with 0.1% crystal violet. The filter membrane was then removed from the chamber and fixed. Finally, the invasive and metastatic cells were counted in 5 randomly selected fields under a light microscope (200 ×). Repeat the experiment at least 3 times under the same experimental conditions.
Wound healing assay
Cells were seeded in 6-well plates at 2 × 105 cells/well. When the cell confluence reached 90%, a 200 μL pipette tip was used to scratch the wound. Incubation was continued with medium containing 2% serum after gentle washing. The scratch wounds of five regions were selected and photographed (40 ×) at 0 h, 6 h, 12 h, 24 h, 48 h, and 72 h, respectively. The ImageJ software was used to measure the wound area. Repeat the experiment at least 3 times under the same experimental conditions.
Tube formation assay
Undiluted Matrigel (BD Biosciences) was plated in 96-well plates and incubated at 37 °C for 1 h in preparation for tube formation experiments. RKO cells were treated with TGF-β1 recombinant protein or EAF2 overexpression plasmid or VASH1 overexpression plasmid, and then conditioned medium was collected for later use. HUVECs were suspended with the collected conditioned medium and seeded in Matrigel coated 96-well plates at a density of 1.0 × 104 cells/well, and then incubated at 37 °C in an incubator containing 5% CO2. After 6 h, the best tube formation was observed under inverted microscope (100 ×). Finally, the images were analyzed using the angiogenesis analyzer developed by Image J software to evaluate branch points. The experiment was repeated at least 3 times under the same experimental conditions.
HUVEC transwell migration assay
A total of 1 × 105 HUVECs were diluted with 200 μL serum-free medium and seeded into the upper chamber of Matrigel-free transwell chamber (3422) with a diameter of 8 μM. Conditioned medium was collected from RKO cells treated with TGF-β1 recombinant protein or EAF2 overexpression plasmid or VASH1 overexpression plasmid and placed in the lower chamber.
The chambers were collected after incubation for 6 h at 37 °C under constant temperature regulation. The chamber filters were fixed with methanol after removing the cells that were not invaded or migrated on the inner surface of the chamber filters. Cells that had been invaded or migrated on the fixed filter membrane were further stained with 0.1% crystal violet. The filter membrane was then removed from the chamber and fixed. Finally, the invasive and metastatic cells were counted in 5 randomly selected fields under a light microscope (200 ×). Repeat the experiment at least 3 times under the same experimental conditions.
HUVEC scratch assay
A total of 2 × 105 HUVECs/well was seeded in 6-well plates. When the cells reached about 90% confluence, a 200 μL tip was used for scratch. The cells were then washed three times with PBS and the incubation was continued with 2 mL of conditioned medium collected from RKO cells treated with TGF-β1 recombinant protein or EAF2 overexpression plasmid or VASH1 overexpression plasmid. Five areas (40 ×) were selected and photographed under a microscope at 0 h, 12 h, and 24 h time points. The ImageJ software was used to measure the wound area. The experiment was repeated at least 3 times under the same experimental conditions.
Statistical analysis
SPSS software (Version 26) was used for statistical analysis of the result data. Wilcoxon signed-rank test and Spearman rank correlation analysis were used to evaluate the expression of EAF2 and VASH1 and their correlation. Pearson’s chi-square test was used to evaluate the correlation between proteins expression in tissues and clinicopathological parameters. All data were expressed as mean ± SD. Kaplan-Meier method and Log-rank tests were used for survival analysis. Student’s t test was used for comparison between two groups, and one-way analysis of variance was used for comparison between three or more groups. One-way analysis of variance and Bonferroni test were used for statistical analysis. All P values were two-sided. A value of P < 0.05 was considered statistically significant.
RESULTS
Protein expression of EAF2 and VASH1 in CRC tissue
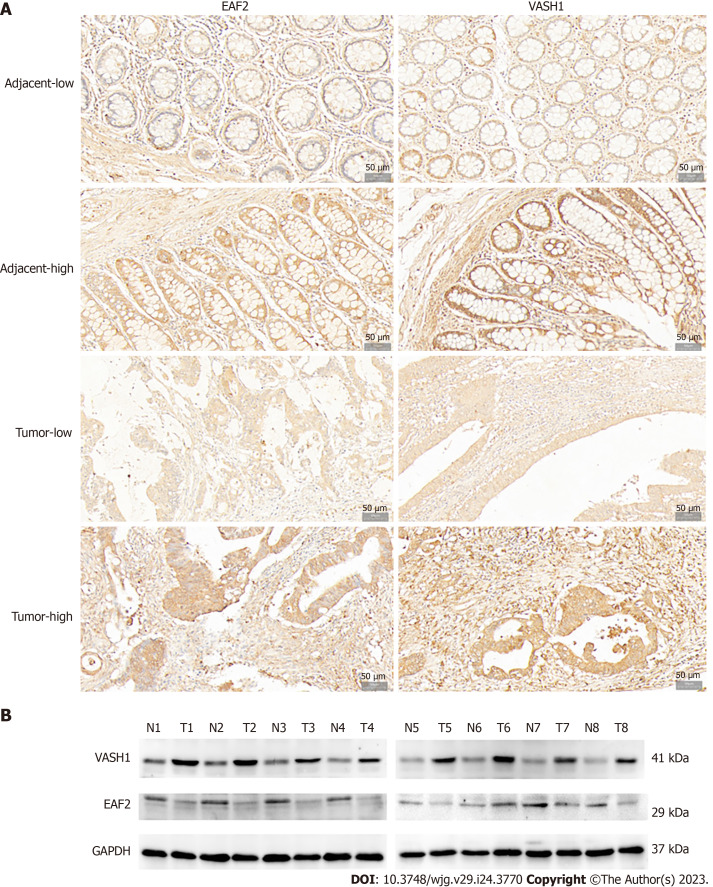
Immunohistochemical analysis was performed on paired adenocarcinoma and paracancerous tissues. The results of semi-quantitative analysis showed that the median score of EAF2 was 4.0 points in adenocarcinoma tissue and 7.2 points in paired paracancer tissue, with a median difference of -2.0 points. Wilcoxon signed-rank test showed that the expression level of EAF2 protein in adenocarcinoma tissue was significantly lower than that in paracancerous tissue (Z = -5.843, P < 0.001). Meanwhile, the median score of VASH1 was 12.0 points in adenocarcinoma tissue and 5.0 points in paired non-cancer tissue, with a median difference of 6.0 points. The results of Wilcoxon signed-rank test showed that the expression of VASH1 protein in adenocarcinoma tissue was significantly higher than that in paracancerous tissue (Z = -8.594, P < 0.001). In addition, Spearman’s rank correlation analysis showed that the expression of EAF2 and VASH1 protein in colorectal adenocarcinoma tissue was significantly positively correlated (r = 0.208, P = 0.022). Besides, localization analysis showed that EAF2 and VASH1 proteins were mainly located in the cytoplasm of colon epithelial cells (Figure 1A).
Figure 1.
Expression of ELL-associated factor 2 and Vasohibin 1 proteins in tissues of patients with advanced colorectal cancer. A: Representative immunohistochemical staining of ELL-associated factor 2 (EAF2) and vasohibin 1 (VASH1) in colorectal adenocarcinoma tissue and the adjacent non-cancerous tissue (magnification 200 ×); B: The protein bands of EAF2 and VASH1 in 8-matched pairs of colorectal adenocarcinoma tissue and adjacent tissue were detected by Western blot assay. EAF2: ELL-associated factor 2; VASH1: Vasohibin 1; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; T: Colorectal adenocarcinoma tissue; N: Adjacent non-tumor tissue.
Not only that, Western blot was used to detect the protein expression of EAF2 and VASH1 in 8 paired fresh colorectal adenocarcinoma tissue and adjacent normal tissue. Compared with adjacent normal tissue, EAF2 protein expression was significantly lower (P = 0.023), while VASH1 protein expression was significantly higher (P < 0.001) in colorectal adenocarcinoma tissues (Figure 1B).
Association of EAF2 and VASH1 expression with clinicopathological parameters and the survival analysis in advanced CRC patients
The semi-quantitative immunohistochemical results of EAF2 and VASH1 in CRC patients were analyzed by ROC curve and the Youden index was determined. The results suggested that the cut-off value delimiting high and low expression of EAF2 (dependent variable is non-cancer) was 6.2 points (P < 0.001), and the cut-off value delimiting high and low expression of VASH1 (dependent variable is cancer, P < 0.01 indicated that it was significant for the diagnosis of tumor) was 8.5 points (P < 0.001). In this study, 100 of 120 CRC tissues were found to have EAF2 Low-expression and 84 were found to have VASH1 high-expression.
After defining the high and low expression of EAF2 and VASH1 proteins in colorectal adenocarcinoma tissues, the relationship between them and major clinicopathological features was further analyzed. We found that EAF2 protein was negatively correlated with angiolymphatic and/or perineural invasion (r = -0.180, P = 0.049), tumor stage (r = -0.194, P = 0.034), distant metastasis (r = -0.244, P = 0.007), and CEA (r = -0.211, P = 0.021), as well as positively correlated with the degree of differentiation (r = 0.225, P = 0.013) (Table 2). VASH1 protein was positively correlated with degree of differentiation (r = 0.186, P = 0.042), angiolymphatic and/or perineural invasion (r = 0.249, P = 0.006), distant metastasis (r = 0.190, P = 0.037), but not with other clinical characteristics (Table 2).
Table 2.
Baseline characteristics of patients with advanced colorectal cancer and the correlation with immunohistochemical expression of ELL-associated factor 2 and vasohibin 1
|
Clinicopathologic characteristics
|
Cases, n (%)
|
EAF2
|
P value
|
VASH1
|
P value
|
||
|
Low (n = 100)
|
High (n = 20)
|
Low (n = 36)
|
High (n = 84)
|
||||
| Age (yr) | 0.569 | 0.248 | |||||
| < 61 (median) | 44.17 | 43 | 10 | 13 | 40 | ||
| ≥ 61 | 55.83 | 57 | 10 | 23 | 44 | ||
| Gender | 0.935 | 0.843 | |||||
| Male | 54.17 | 54 | 11 | 20 | 45 | ||
| Female | 45.83 | 46 | 9 | 16 | 39 | ||
| Tumor site | 0.657 | 0.515 | |||||
| Colon | 29.17 | 30 | 5 | 9 | 26 | ||
| Rectum | 70.83 | 70 | 15 | 27 | 58 | ||
| Size of the tumor, cm | 0.250 | 0.689 | |||||
| ≤ 4 | 41.67 | 44 | 6 | 16 | 34 | ||
| > 4 | 58.33 | 56 | 14 | 20 | 50 | ||
| Histological type | 0.695 | 0.566 | |||||
| Mucinous | 21.67 | 21 | 5 | 9 | 17 | ||
| Non-mucinous | 78.33 | 79 | 15 | 27 | 67 | ||
| Degree of differentiation | 0.013 | 0.042 | |||||
| Well/moderately differentiated | 64.17 | 69 | 8 | 28 | 49 | ||
| Poor/mucinous differentiated | 35.83 | 31 | 12 | 8 | 35 | ||
| Angiolymphatic and/or perineural invasion | 0.049 | 0.006 | |||||
| Absent | 45.00 | 41 | 13 | 23 | 31 | ||
| Present | 55.00 | 59 | 7 | 13 | 53 | ||
| TNM | 0.034 | 0.068 | |||||
| I-II | 48.33 | 44 | 14 | 22 | 36 | ||
| III-IV | 51.67 | 56 | 6 | 14 | 48 | ||
| Tumor invasion depth | 0.219 | 0.084 | |||||
| T2-T3 | 57.50 | 55 | 14 | 25 | 44 | ||
| T4 | 42.50 | 45 | 6 | 11 | 40 | ||
| Lymph node status | 0.073 | 0.095 | |||||
| N0 | 46.67 | 43 | 13 | 21 | 35 | ||
| N1-N2 | 53.33 | 57 | 7 | 15 | 49 | ||
| Distant metastasis | 0.007 | 0.037 | |||||
| Absent | 70.00 | 65 | 19 | 30 | 54 | ||
| Present | 30.00 | 35 | 1 | 6 | 30 | ||
| CEA | 0.021 | 0.874 | |||||
| Normal | 56.67 | 52 | 16 | 20 | 48 | ||
| High | 43.33 | 48 | 4 | 16 | 36 | ||
| CA19-9 | 0.321 | 0.809 | |||||
| Normal | 40.00 | 38 | 10 | 15 | 33 | ||
| High | 60.00 | 62 | 10 | 21 | 51 | ||
| P53 | 0.219 | 0.905 | |||||
| Normal | 42.50 | 40 | 11 | 15 | 36 | ||
| High | 57.50 | 60 | 9 | 21 | 48 | ||
| CDX2 | 0.224 | 0.921 | |||||
| Normal | 20.00 | 18 | 6 | 7 | 17 | ||
| High | 80.00 | 82 | 14 | 29 | 67 | ||
EAF2: ELL protein-associated factor 2; TNM: Tumor-node-metastasis; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; VASH1: Vasohibin 1.
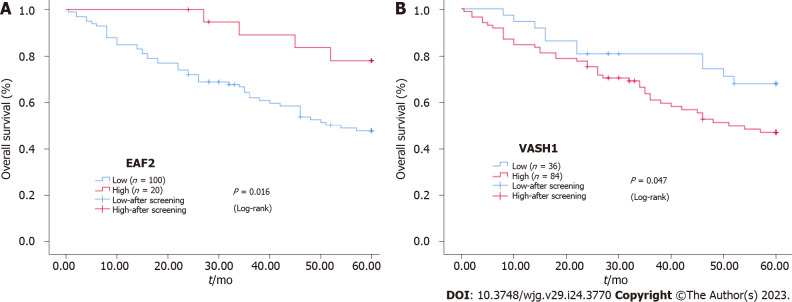
Furthermore, Kaplan-Meier survival analysis and Log-rank test showed that the survival rate of EAF2High group was higher than that of EAF2Low group (P = 0.016), and the survival rate of VASH1Low group was higher than that of VASH1High group (P = 0.047) (Figure 2).
Figure 2.
Kaplan-Meier survival curves. A and B: Kaplan-Meier survival curves for the protein expression of ELL-associated factor 2 and vasohibin 1 in colorectal cancer tissue. EAF2: ELL-associated factor 2; VASH1: Vasohibin 1.
Cox proportional-hazards model was used to analyze the influencing factors of OS in patients with advanced CRC. By univariate analysis, we found angiolymphatic and/or perineural invasion (P < 0.001), tumor stage (P < 0.001), tumor invasion depth (P < 0.001), lymph node status (P < 0.001), distant metastasis (P < 0.001) and EAF2 protein expression level (P = 0.024), p53 protein expression level (P = 0.048) were significantly associated with OS (Table 3). Confounding factors were further adjusted to evaluate the independent prognostic factors of OS in patients with advanced CRC. The clinicopathological features with P < 0.300 in the univariate analysis were included in the multivariate analysis, and the results suggested that, angiolymphatic and/or perineural invasion (P = 0.033), distal metastasis (P < 0.001), CEA protein expression (P = 0.004), p53 protein expression level (P = 0.048) were independent prognostic factors for OS (Table 3).
Table 3.
Univariate and multivariate survival analysis of overall survival for colorectal cancer by Cox regression.
|
Variables
|
Univariate analysis
|
Multivariate analysis
|
||||
|
HR
|
95%CI
|
P value
|
HR
|
95%CI
|
P value
|
|
| Age (yr) (≥ 61/< 61) | 1.703 | 0.969-2.991 | 0.064 | 1.413 | 0.764-2.613 | 0.270 |
| Gender (female/male) | 0.962 | 0.561-1.651 | 0.890 | |||
| Tumor site (colon/rectum) | 1.410 | 0.798-2.492 | 0.237 | 1.211 | 0.638-2.298 | 0.558 |
| Size of the tumor, cm (> 4/≤ 4) | 0.777 | 0.446-1.356 | 0.375 | |||
| Histological type (mucinous/non-mucinous) | 1.218 | 0.612-2.424 | 0.574 | |||
| Degree of differentiation (poor/mucinous, well/moderate) | 0.917 | 0.523-1.608 | 0.761 | |||
| Angiolymphatic and/or perineural invasion (present/absent) | 0.209 | 0.107-0.408 | < 0.001 | 0.255 | 0.072-0.898 | 0.033 |
| Tumor stage (III-IV/I-II) | 0.240 | 0.128-0.450 | < 0.001 | 1.455 | 0.339-6.256 | 0.614 |
| Tumor invasion depth (T4/T2-T3) | 0.353 | 0.202-0.614 | < 0.001 | 0.547 | 0.225-1.331 | 0.184 |
| Lymph node status (N1-N2/N0) | 0.288 | 0.155-0.532 | < 0.001 | 2.150 | 0.511-9.038 | 0.296 |
| Distant metastasis (present/absent) | 0.161 | 0.091-0.284 | < 0.001 | 0.200 | 0.090-0.444 | < 0.001 |
| EAF2 (low/high) | 3.244 | 1.170-8.999 | 0.024 | 2.049 | 0.698-6.014 | 0.191 |
| VASH1 (high/low) | 0.518 | 0.266-1.007 | 0.052 | 0.785 | 0.389-1.584 | 0.499 |
| CEA (high/low) | 1.590 | 0.906-2.790 | 0.106 | 2.506 | 1.336-4.702 | 0.004 |
| CA19-9 (high/low) | 0.817 | 0.466-1.433 | 0.481 | |||
| P53 (high/low) | 0.558 | 0.313-0.994 | 0.048 | 0.545 | 0.299-0.995 | 0.048 |
| CDX2 (high/low) | 0.705 | 0.332-1.495 | 0.362 | |||
HR: Hazard ratio; CI: Confidence interval; EAF2: ELL protein-associated factor 2; CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9.
VASH1 overexpression inhibited STAT3/TGF-β1 pathway activity in RKO
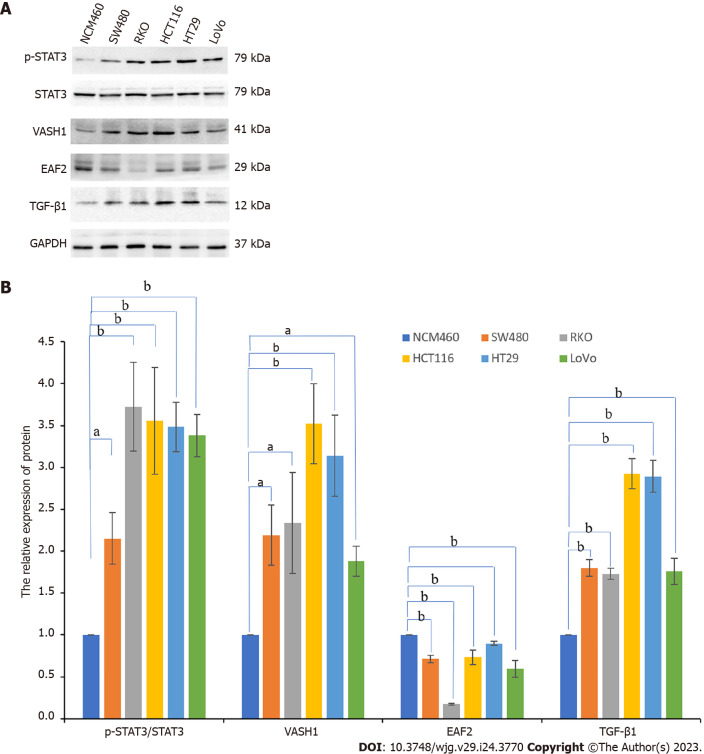
Normal colorectal epithelial cells (NCM460) and a variety of human CRC cell lines (SW480, RKO, HCT116, HT29, and LoVo) were cultured in vitro. Western blot assay showed that, compared with NCM460 cells, VASH1 protein levels were increased to varying degrees in human CRC cell lines (Figure 3), and the expression was upregulated 2.336-fold in RKO cells (P = 0.002).
Figure 3.
Protein expression of pathway-related factors in a variety of human colorectal cancer cells and colorectal epithelial cells. A and B: The protein bands of ELL-associated factor 2, vasohibin 1, phosphorylated-signal transducer and activator of transcription 3, and transforming growth factor-β1 in colorectal cancer cell lines (SW480, RKO, HCT116, HT29, and LoVo) and normal colorectal epithelial cells (NCM460) by Western blot assay and the corresponding data. aP < 0.05, bP < 0.001. EAF2: ELL-associated factor 2; VASH1: Vasohibin 1; p-STAT3: Phosphorylated-signal transducer and activator of transcription 3; TGF-β1: Transforming growth factor-β1.
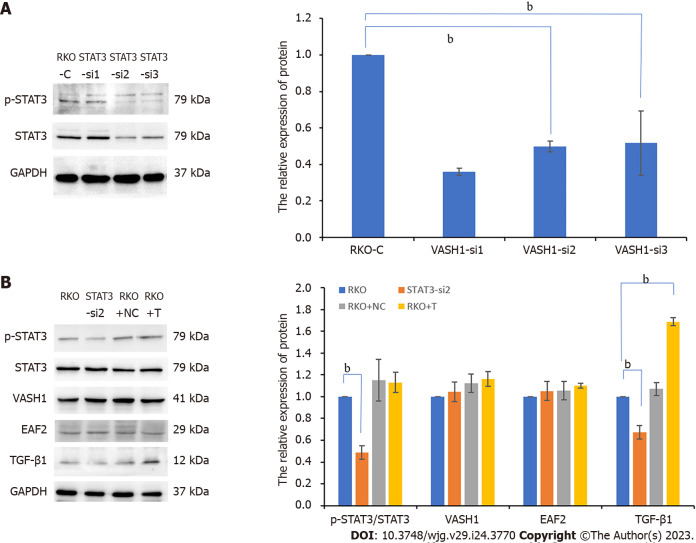
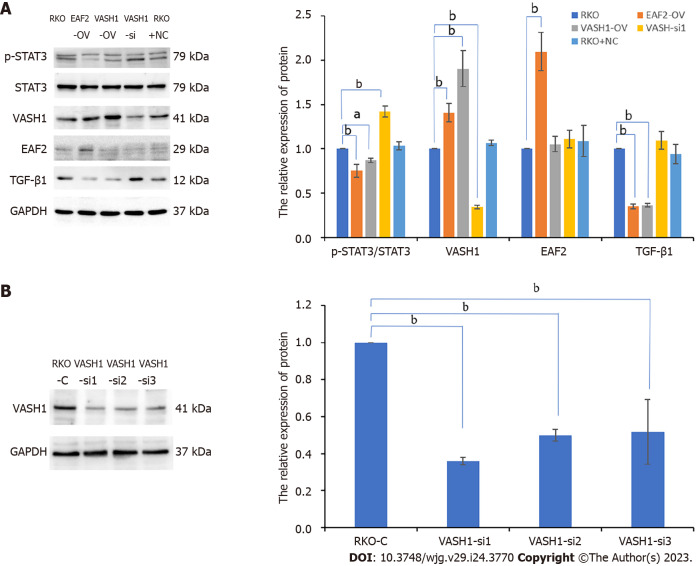
In addition, the expression level of phosphorylated STAT3 (Tyr705) (P < 0.001) and TGF-β1 protein (P < 0.001) in RKO were significantly higher than those in normal colorectal epithelial cells (NCM460). And then we suppressed STAT3 phosphorylation by transfecting RKO cells with silencing plasmid. In this study, RKO cells were transfected with three different STAT3 siRNAs (STAT3-si1, STAT3-si2, and STAT3-si3) targeting the STAT3 gene (Figure 4A). When compared to the control group (RKO-C), transfection with the three different STAT3-siRNAs decreased phosphorylated STAT3 expression. Additionally, STAT3-si2 was the most efficient, with a reduction of 61.8% in phosphorylated STAT3 expression (P < 0.001) (Figure 4A). Further study showed that when STAT3-si2 was transfected to silence STAT3, the expression of TGF-β1 protein in RKO was significantly lower than that in the normal control group (Figure 4B).
Figure 4.
Silencing signal transducer and activator of transcription 3 phosphorylation significantly inhibits the expression of transforming growth factor-β1 protein in RKO cells. A: The protein bands of phospho-signal transducer and activator of transcription 3 (STAT3) in RKO cells transfected with STAT3 siRNA plasmids by Western blot assay and the corresponding data; B: The protein bands of pathway-related factors in RKO cells treated with STAT3 siRNA or exogenous transforming growth factor-β1 recombinant protein by Western blot assay. bP < 0.001. RKO-C: RKO cells were cultured in 1640 medium containing 10% fetal bovine serum (FBS). STAT3-si: RKO cells were transfected with STAT3 siRNA plasmids for 6 h and then cultured in 1640 medium containing 10% FBS for 24 h. RKO + NC: RKO cells were transfected empty vector for 6 h and then cultured in 1640 medium containing 10% FBS for 24 h. RKO + T: RKO cells were cultured for 24 h in 1640 medium containing 10% FBS in which 5 ng/mL TGF-β1 recombinant protein was added. VASH1: Vasohibin 1; STAT3: Signal transducer and activator of transcription 3; EAF2: ELL-associated factor 2; TGF-β1: Transforming growth factor-β1.
We used gene transfection technique to overexpress or silence the VASH1 gene in RKO cells and determined the activity and expression of STAT3/TGF-β1 signaling pathway. The results showed that overexpression of VASH1 (with 1.906-fold higher, P < 0.001, Figure 5B) significantly inhibited STAT3 phosphorylation (Tyr705) as well as TGF-β1 protein expression (Figure 5B). What’s more, we transfected RKO cells with three different VASH1 siRNAs (VASH1-si1, VASH1-si2, and VASH1-si3) targeting the VASH1 gene (Figure 5A). When compared to the control group (RKO-C), transfection with the three different VASH1-siRNAs decreased VASH1 protein expression. Additionally, VASH1-si1 was the most efficient, with a reduction of 64.02% in VASH1 protein expression (P < 0.001) (Figure 5A). As a result, transfection with VASH1 siRNA significantly promoted STAT3 phosphorylation (Figure 5B, P < 0.001). However, when RKO cells were transfected with STAT3 siRNA, the expression level of VASH1 protein was not significantly different from that of RKO control group (Figure 4B). Treatment of RKO cells with exogenous TGF-β1 recombinant protein did not affect the expression of VASH1 protein (Figure 4B).
Figure 5.
Overexpressing vasohibin 1 inhibits signal transducer and activator of transcription 3 phosphorylation (Tyr705) as well as transforming growth factor-β1 protein expression in RKO cells. A: The protein bands of pathway-related factors in RKO cells transfected with ELL-associated factor 2 (EAF2) overexpression plasmids, or vasohibin 1 (VASH1) overexpression plasmids, or VASH1 silencing plasmids by Western blot assay; B: The protein bands of VASH1 in RKO cells transfected with VASH1 siRNA plasmids by Western blot assay and the corresponding data. aP < 0.05, bP < 0.001. VASH1-si: RKO cells transfected with VASH1 siRNA plasmids for 6 h and then cultured in 1640 medium containing 10% fetal bovine serum (FBS) for 24 h. VASH1-OV: The group of RKO cells transfected with VASH1 overexpression plasmids for 6 h and then cultured in 1640 medium containing 10% FBS for 24 h. EAF2-OV: RKO cells transfected with EAF2 overexpression plasmids for 6 h and then cultured in 1640 medium containing 10% FBS for 24 h. VASH1: Vasohibin 1; EAF2: ELL-associated factor 2; TGF-β1: Transforming growth factor-β1.
EAF2 overexpression inhibited STAT3/TGF-β1 pathway activity in RKO
Meanwhile, Western blot results also showed that the expression levels of EAF2 protein in human CRC cell lines (SW480, RKO, HCT116, HT29, and LoVo) were decreased to varying degree compared with NCM460 cells (Figure 3), and the decrease was 82.7% in RKO cells (P < 0.001). Therefore, we transfected RKO cells with EAF2 overexpression plasmid in vitro to investigate the potential function and molecular mechanism of EAF2 in CRC cells.
Transfecting RKO cells with EAF2 overexpression plasmids significantly upregulated the expression of EAF2 protein (with 2.1-fold higher, P < 0.001, Figure 5B). At the same time, we also found that the levels of phosphorylated STAT3 (Tyr705) protein and TGF-β1 protein were significantly decreased in RKO cells transfected with EAF2 overexpression plasmids compared with RKO control group. However, silencing STAT3 gene or exogenous TGF-β1 recombinant protein did not affect EAF2 protein expression in RKO cells (Figure 4B).
EAF2 overexpression or VASH1 overexpression inhibited biological function of RKO through STAT3/TGF-β1 pathway
Subsequently, we preliminarily explored the regulatory effects of EAF2 and VASH1 proteins on the biological function of RKO cells through the STAT3/TGF-β1 pathway in vitro, in an attempt to provide a scientific basis for exploring the role of EAF2 and VASH1 in CRC.
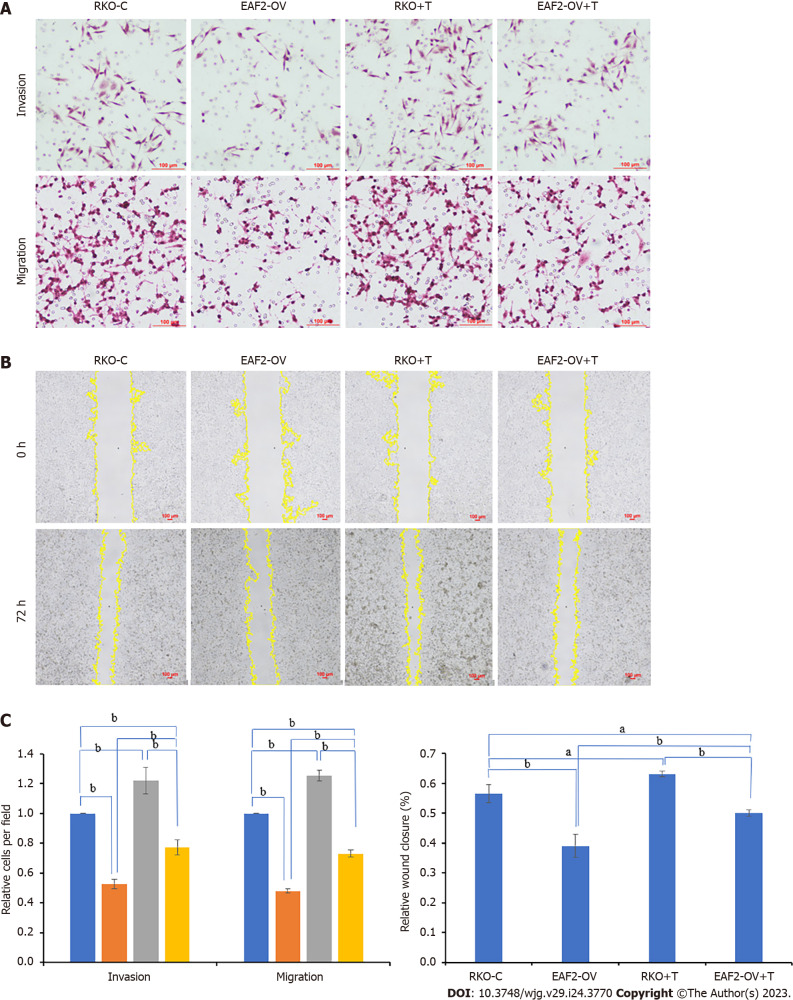
Transwell invasion assay of matrigel envelope showed that the invasion ability of RKO cells was significantly weakened after transfection of EAF2 overexpression plasmids (Figure 6A, P < 0.001). In transwell migration assay, RKO cells transfected with EAF2 overexpressing plasmids showed a significantly reduced migration ability compared with the control group (Figure 6A, P < 0.001). Consistent with this, overexpression of EAF2 also significantly reduced the scratch healing ability of RKO cells (Figure 6B, P < 0.001). Our treatment of RKO cells with exogenous TGF-β1 recombinant protein significantly promoted the transwell invasion, transwell migration, and scratch healing abilities of RKO cells (Figure 6). More importantly, when the RKO cells transfected with EAF2 overexpression plasmids were treated with TGF-β1 recombinant protein for 24 h, it was found that the transwell invasion, transwell migration and scratch healing abilities of the RKO cells inhibited by EAF2 overexpression were reversed by TGF-β1 recombinant protein (Figure 6).
Figure 6.
Overexpressing ELL-associated factor 2 inhibits the invasion and migration of RKO cells by suppressing transforming growth factor-β1 related pathway. A: The invasion and migration abilities were evaluated by the transwelll assay; B: The migration ability was analyzed by wound healing assay after 72 h; C: The results of migration and invasion abilities of RKO cells were measured using ImageJ software. aP < 0.05, bP < 0.001. ELL-associated factor 2 (EAF2)-OV + T: The group of RKO cells were transfected with EAF2 overexpression plasmids for 24 h and then cultured with 5 ng/mL transforming growth factor-β1 recombinant protein for 24 h. EAF2: ELL-associated factor 2.
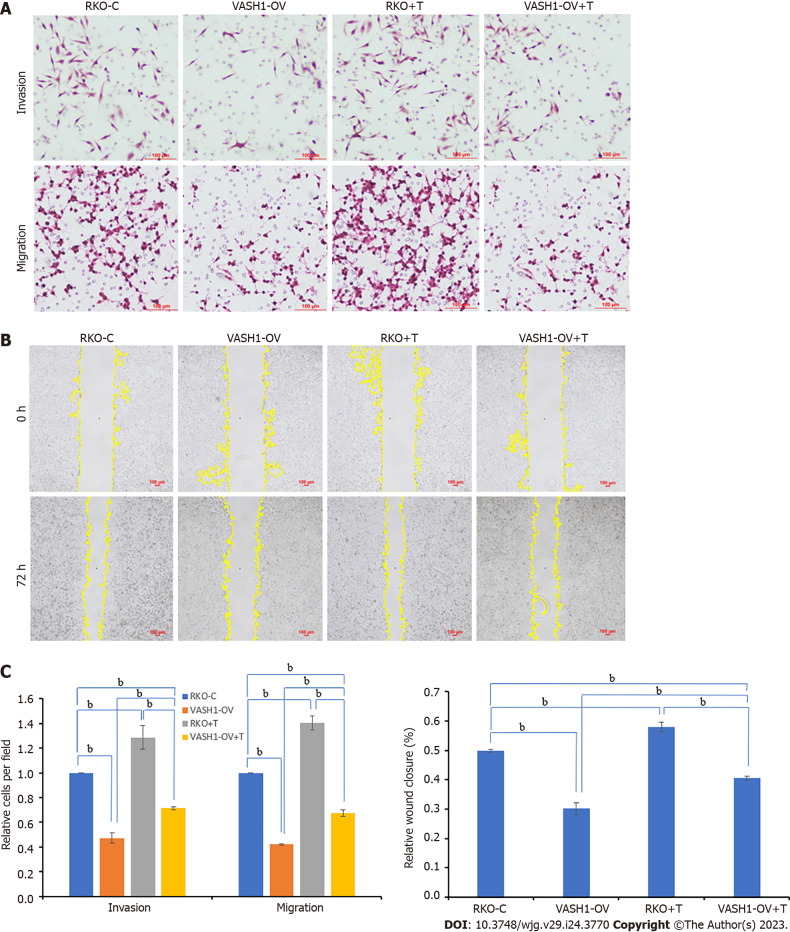
Furthermore, the RKO cells were transfected with VASH1 overexpression plasmids for transwell invasion assay, transwell migration assay and wound healing assay. Combining the results of Matrigel-coated transwell invasion assay, transwell migration assay and wound healing assay, it was found that compared with RKO control group, RKO cells overexpressing VASH1 protein showed lower transwell invasion, transwell migration, and scratch healing abilities (Figure 7, P < 0.001). It was also of concern to us that the transwell invasion, transwell migration, and scratch healing of RKO cells transfected with VASH1 overexpression plasmids could be reversed after 24 h treatment with TGF-β1 recombinant protein (Figure 7, P < 0.001).
Figure 7.
Overexpressing Vasohibin 1 inhibits the invasion and migration of RKO cells by suppressing transforming growth factor-β1 related pathway. A: The invasion and migration abilities were evaluated by the transwelll assay; B: The migration ability was analyzed by wound healing assay after 72 h; C: The results of migration and invasion abilities of RKO cells were measured using ImageJ software. bP < 0.001. vasohibin 1 (VASH1)-OV + T: The group of RKO cells were transfected with VASH1 overexpression plasmids for 24 h and then cultured with 5 ng/mL transforming growth factor-β1 recombinant protein for 24 h. VASH1: Vasohibin 1.
EAF2 overexpression or VASH1 overexpression inhibited CRC angiogenesis through STAT3/TGF-β1 pathway
Conditioned medium from RKO cells treated with TGF-β1 recombinant protein, EAF2-overexpressed plasmids, or VASH1-overexpressed plasmids was collected to treat HUVECs, respectively. Following, we further examined the tube formation and migration ability of HUVECs. In this study, the potential effects and mechanisms of EAF2 and VASH1 on CRC angiogenesis were investigated in vitro.
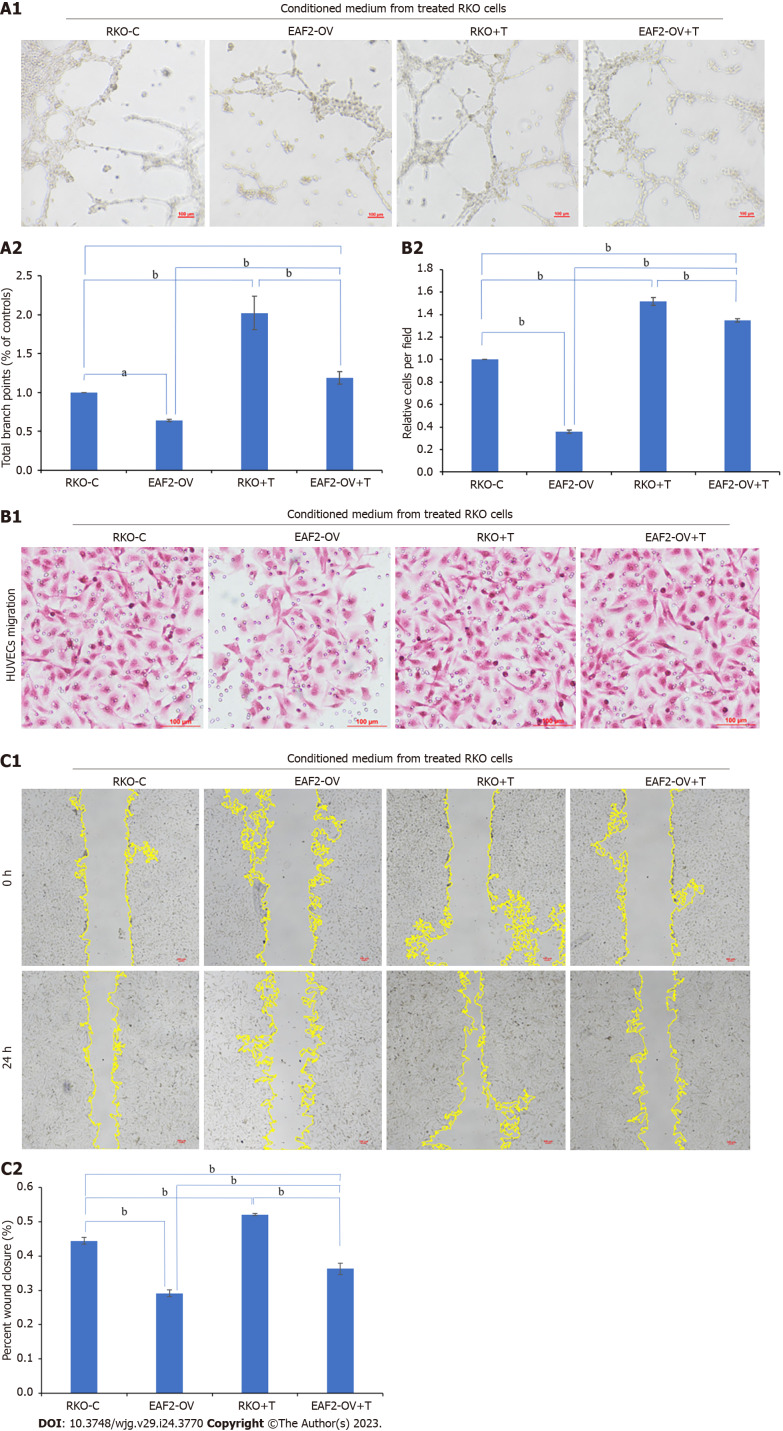
Tube formation assay showed that the tubular ability of HUVECs treated with the conditioned medium from RKO cells transfected with EAF2-overexpressed plasmids (EAF2-OV group) was significantly lower than that in the control group (RKO-C group), in which HUVECs were treated by conditioned medium from RKO cells grown in medium containing 10% serum. From the results of transwell migration assay and scratch assay, we also found that the migration ability of HUVECs in EAF2-OV group was significantly lower than that in RKO-C group (Figure 8, P < 0.001). In addition, the tube formation and invasion ability of HUVECs in the RKO + T group cultured with conditioned medium collected from RKO cells treated with exogenous TGF-β1 recombinant protein were significantly increased compared with those in the RKO-C group (Figure 8, P < 0.001). HUVECs were further cultured with conditioned medium from RKO cells in the EAF2-OV + T group, which were transfected with EAF2-overexpressed plasmids and treated with TGF-β1 recombinant protein for 24 h. The results of tube formation assay, transwell migration assay and scratch assay showed that TGF-β1 recombinant protein could reverse the inhibitory effect of EAF2 overexpression in RKO cells on tube formation and migration of HUVECs (Figure 8, P < 0.001).
Figure 8.
Overexpressing ELL-associated factor 2 inhibits tumor-induced angiogenesis in RKO cells via transforming growth factor-β1 related pathway. A: Conditioned medium from RKO cells transfected with ELL-associated factor 2-overexpressed plasmids or treated with transforming growth factor-β1 recombinant protein was collected to perform tube formation assay; B and C: The migration ability of Human Umbilical Vein Endothelial Cells was analyzed by transwell migration assay (B) and wound healing assay (C). And the results were measured by ImageJ software. aP < 0.05, bP < 0.001. EAF2: ELL-associated factor 2; TGF-β1: Transforming growth factor-β1.
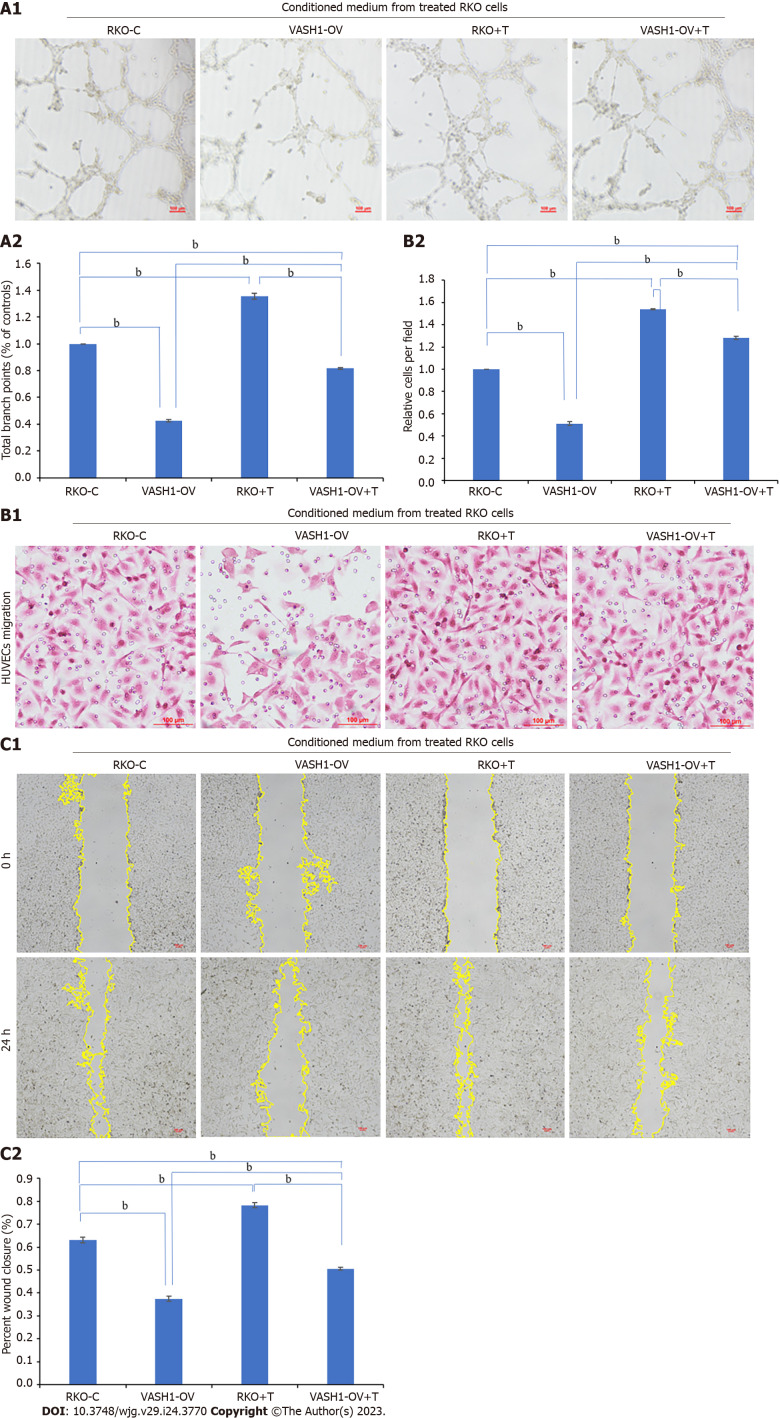
At the same time, we treated HUVECs for 24 h with conditioned medium from RKO cells in the VASH1-OV group, which were transfected with VASH1-overexpressed plasmids, to investigate the role and mechanism of CRC cell-derived VASH1 in CRC angiogenesis. Then, the tube formation assay showed that the tubular ability of HUVECs in the VASH1-OV group was significantly lower than that in the control group (RKO-C group) (Figure 9, P < 0.001). The results of migration experiment and scratch experiment showed that the migration ability of HUVECs in VASH1-OV group was significantly lower than that in RKO-C group (Figure 9, P < 0.001). After treating RKO cells transfected with VASH1 overexpression plasmid with TGF-β1 recombinant protein (VASH1-OV + T group), it was observed that the tube forming ability and migration ability of HUVECs were significantly increased (Figure 9, P < 0.001).
Figure 9.
Overexpressing Vasohibin 1 inhibits tumor-induced angiogenesis in RKO cells via transforming growth factor-β1 related pathway. A: Conditioned medium from RKO cells transfected with VASH1-overexpressed plasmids or treated with transforming growth factor-β1 recombinant protein was collected to perform tube formation assay; B and C: The migration ability of Human Umbilical Vein Endothelial Cells was analyzed by transwell migration assay (B) and wound healing assay (C). And the results were measured by ImageJ software. bP < 0.001. VASH1: Vasohibin 1; EAF2: ELL-associated factor 2.
Overexpression of EAF2 up-regulated VASH1 to inhibit the activity of STAT3/TGF-β1 pathway
The previous results suggest that the expression levels of EAF2 and VASH1 proteins are positively correlated in CRC tissues. Therefore, it is of great significance to investigate whether there is a mutual regulatory effect between EAF2 and VASH1 in CRC cells.
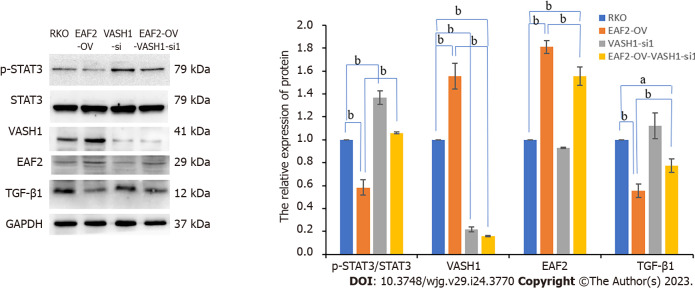
Compared with RKO control group, transfection of the EAF2 overexpression plasmid significantly increased VASH1 protein expression in RKO cells (Figure 5B, P = 0.001). However, transfection of RKO cells with VASH1 overexpression plasmid or VASH1 siRNA did not significantly modulate the expression level of EAF2 protein (Figure 5B).
In the following experiments, we transfected the RKO cells with VASH1 siRNA to further determine whether VASH1 was involved in the activation of STAT3/TGF-β1 crosstalk pathway by overexpression of EAF2. The results showed that silencing VASH1 reversed the STAT3/TGF-β1 signaling pathway inhibited by EAF2 overexpression (Figure 10).
Figure 10.
Silencing Vasohibin 1 reverses the signal transducer and activator of transcription 3/transforming growth factor-β1 pathway inhibited by ELL-associated factor 2 overexpression. aP < 0.05, bP < 0.001. ELL-associated factor 2 (EAF2)-OV- Vasohibin 1 (VASH1-si): The group of RKO cells transfected with EAF2 overexpression plasmids and VASH1 siRNA plasmids. VASH1: Vasohibin 1; EAF2: ELL-associated factor 2.
DISCUSSION
CRC metastasis is a dynamic process that undergoes detachment of the primary cancer, infiltration into adjacent tissues and blood/lymph circulation, and metastasis to distant CRC colony formation. The mechanism is complex and affected by many factors. Angiogenesis is an important link in the process of tumor occurrence, invasion and metastasis, which can meet the oxygen and nutrients needed for the survival and growth of tumor cells[21]. The revolution of targeted agents has brought new hope for the treatment of patients with advanced tumors. Checkpoint inhibitors can significantly inhibit tumors and even may cure them, but currently only 3.8% of metastatic CRC are available[22]. The identification of predictive biomarkers and the exploration of new molecular targets contribute to the improvement of clinical diagnosis and the development of new drugs for advanced CRC.
The androgen response gene EAF2, a member of the EAF family, has tumor suppressive properties in multiple organs, including liver, lung and prostate[15]. EAF2 protein is under-expressed in prostate cancer tissues, and knocking down EAF2 in prostate cancer cell lines can enhance proliferation, migration, and invasion. Prostatic luminal epithelial cells have the potential to develop prostatic intraepithelial neoplasia in the EAF2 knockout mouse model[23]. In addition, the high expression of EAF2 also indicates a better prognosis of skin cutaneous melanoma patients[24]. Recent studies have identified EAF2 inactivation in MSI-H CRC, and not only that, but also intratumor heterogeneity (ITH) of EAF2 frameshift mutations in CRC[25]. Our previous determination of EAF2 expression levels in CRC tissues suggested that EAF2 expression was significantly lower in cancer tissues than in adjacent normal tissues[20]. In addition, the level of EAF2 protein in CRC tissues was negatively correlated with distant metastasis and CEA expression level in patients with advanced CRC[20]. In the present study, we further expanded the clinical sample size and similarly found that EAF2 protein level was significantly lower in CRC tissue than in non-cancer tissue. Further correlation analysis showed that advanced CRC tissue with low EAF2 protein expression had poor angiolymphatic and/or perineural invasion, poor TNM stage, poor distant metastasis, and high CEA protein expression compared with high EAF2. Meanwhile, advanced CRC tissue with high EAF2 protein expression had poor degree of differentiation compared with low EAF2. Therefore, by expanding the sample size, we still found that the expression level of EAF2 protein was lower in advanced CRC tissue. The results of Kaplan-Meier survival analysis were also consistent with previous studies[20], suggesting that the survival rate of the group with EAF2 high expression was higher than that of the group with EAF2 Low expression. These results further suggest that EAF2 protein expression in CRC tissues is closely related to tumor invasion and metastasis. EAF2 protein may be a new diagnostic and prognostic marker for CRC, and its low expression may be associated with poor prognosis of patients with advanced CRC. All these suggest that EAF2 may play a role in the occurrence and development of CRC, especially in the process of invasion and metastasis.
Vasohibin was originally identified and isolated as a novel angiogenesis inhibitor by microarray analysis of transcripts induced by VEGF in endothelial cells[26]. In addition, transfecting Lewis lung carcinoma (LLC) cells with vasohibin cDNA effectively inhibited tumor angiogenesis and tumor growth, indicating that vasohibin can act on endothelial cells from the outside[26]. VASH1, one of vasohibin family, is not only selectively expressed in endothelial cells under the induction of angiogenic growth factors (VEGF and FGF-2), which inhibits the migration, proliferation and tube formation of endothelial cells[26], but also expressed in tumor cells and tumor stroma, participating in the inhibition of tumor angiogenesis and prevention of tumor growth and metastasis[27,28]. Although previous studies have focused more on how endothelial cell-derived VASH1 regulates tumor angiogenesis and lymphangiogenesis[29], preliminary studies have also confirmed the potential functional role of tumor cell-derived VASH1 in inhibiting cancer cell proliferation and invasion[30,31]. It has also been shown that overexpression of VASH1 inhibits tumorigenesis and metastasis in a human colon cancer model[32]. However, the expression and function of VASH1 in CRC tissues and cells remain controversial, and its direct effects on tumor cells and mechanisms remain to be elucidated.
Xiong et al[7] used RT-qPCR and Western blot to determine the RNA and protein expression levels of VASH1, and the results showed that VASH1 expression was significantly down-regulated in CRC tissues compared with adjacent normal tissues. However, through Western blot detection, Yan et al[9] found that the expression level of VASH1 protein in CRC tissue was significantly higher than that in adjacent normal tissues. Immunohistochemical analysis also indicated that 88.64% (117/132) of CRC tissue expressed VASH1 protein. Moreover, the expression of VASH1 protein and mRNA in colon cancer cell lines was significantly higher than that in normal colon mucosa cell lines. In this study, we also used immunohistochemistry and Western blot to detect the expression of VASH1 protein in advanced CRC tissue, and the results showed that the level of VASH1 protein in colorectal adenocarcinoma tissue was significantly higher than that in paracancer tissues. In addition, our results found that advanced CRC tissue with high VASH1 protein expression had poor degree of differentiation, poor angiolymphatic and/or perineural invasion, poor distant metastasis compared with those with low VASH1. Kaplan-Meier survival analysis showed that the survival rate of patients with low VASH1 expression was higher than that of patients with high VASH1 expression. It is consistent with the findings of Yan et al[9], who showed that high VASH1 protein expression is an independent risk factor for worse OS and PFS. It is of great significance to further explore the function and mechanism of VASH1 protein in the occurrence and development of CRC, especially in the process of invasion and metastasis.
Univariate analysis showed that vascular lymphatic and/or neural invasion, tumor stage, depth of tumor invasion, lymph node status, distant metastasis, EAF2 protein expression level and p53 protein expression level were prognostic factors for the survival of patients with advanced CRC. However, multivariate analysis did not show that EAF2 or VASH1 expression levels were an independent prognostic factor for survival in patients with advanced CRC. More cases and more comprehensive clinical studies are needed to clarify the clinical significance of EAF2 and VASH1 proteins in CRC. In addition, this study is the first to investigate the correlation between the expression of the tumor suppressor EAF2 and the anti-angiogenic factor VASH1 in CRC tissues. The expressions of EAF2 and VASH1 proteins in advanced CRC tissue are positively correlated. Immunohistochemical localization showed that EAF2 and VASH1 proteins were mainly expressed in the cytoplasm of CRC epithelial cells. Therefore, we also ventured to speculate whether EAF2 and VASH1 interact in the development of CRC.
And then, we conducted in vitro cell studies to investigate the role and potential mechanism of CRC cell-derived EAF2 and VASH1 protein. Western blot assay showed that EAF2 protein expression was down-regulated and VASH1 protein expression was up-regulated in RKO cells compared with normal colon epithelial cells in vitro. In addition, RKO cells overexpressing EAF2 protein expressed higher levels of VASH1 protein. These results suggest that EAF2 overexpression can up-regulate the expression of VASH1 protein in RKO cells. Further investigation of the potential mechanism of EAF2 and VASH1 in CRC cells may provide a research direction for exploring the role of EAF2 and VASH1 in CRC.
In parallel, our results also found that phospho-STAT3 (Tyr705) and TGF-β1 protein levels were significantly increased in RKO cells. TGF-β signaling participates in and promotes the development of metastatic CRC through multiple mechanisms[33,34]. TGF-β1 expression is also significantly increased in CRC tissue and in distant metastases of CRC[35]. Targeting TGF-β signaling is emerging as a new therapy for CRC metastasis[36]. Not only that, increased TGF-β expression specifically promotes endothelial cell angiogenesis induced by the human CRC cell line HT-29 by upregulating VEGF secretion[37]. STAT3 is also one of the main factors closely related to the occurrence and development of CRC, and is involved in tumor angiogenesis and lymphangiogenesis to promote the early occurrence of advanced CRC. Immunohistochemical evaluation of pSTAT3 in a study containing 724 CRC patients showed that pSTAT3 expression was significantly associated with high mortality and increased peritumoral lymphoid reaction[38]. What’s more, TGF-β1 promotes CRC invasion by inducing EMT via Src/Annexin A2 (ANXA2)/STAT3 pathway[39]. We also found in vitro that silencing STAT3 phosphorylation significantly downregulated TGF-β1 protein expression in RKO cells. Therefore, exploring the regulatory proteins of STAT3/TGF-β1 pathway is helpful to find new therapeutic targets for CRC.
EAF2 has been shown to be a repressor of STAT3 signaling pathway in prostate cancer[40]. Down-regulation of EAF2 protein in human prostate cancer specimens correlated with the immunostaining of phosphorylated STAT3 (Tyr705), and knockdown of EAF2 gene induced STAT3 phosphorylation (Tyr705) in prostate cancer both in vivo and in vitro. Besides, EAF2 attenuates TGF-β1-induced G1 phase cell cycle arrest and cell migration in a variety of tumor cells[18]. Encouragingly, when we overexpressed EAF2 protein, the activity of STAT3/TGF-β1 pathway was inhibited in RKO cells. Furthermore, in vitro functional studies in CRC cells showed that overexpression of EAF2 inhibited not only the invasion and migration of RKO cells, but also the angiogenesis induced by RKO cells. However, the inhibitory effect of EAF2 overexpression on RKO cell function was reversed by recombinant TGF-β1 protein.
In this study we also found that overexpressing VASH1 protein in RKO cells significantly inhibited the activity of the STAT3/TGF-β1 pathway. More meaningfully, the silencing of VAH1 gene with variable activity in the STAT3/TGF-β1 pathway was reversed by overexpression of EAF2. It is suggested that CRC-derived VASH1 protein is involved in the EAF2-mediated STAT3/TGF-β1 pathway in CRC cells. Overexpression of VASH1 protein in colon cancer cells not only inhibited cancer cell growth and colony formation in vitro, but also inhibited tumor growth in vivo[8]. This is consistent with the results of our study, which revealed that overexpressing VASH1 protein inhibited RKO cell invasion, migration and angiogenesis. What’s more, this effect was reversed by TGF-β1 recombinant protein. Similarly, VASH1 siRNA significantly increased the mobility of gastric cancer cell and endothelium cells vessel formation in Matrigel[41]. Hence, CRC cell-derived VASH1 may inhibit the invasion, migration and angiogenesis ability of RKO cells by inhibiting STAT3/TGF-β1 pathway. In addition, overexpression of EAF2 may inhibit the activity of STAT3/TGF-β1 pathway by up-regulating the expression of VASH1.
However, further studies are needed to clarify whether there is a direct effect of EAF2 on regulating VASH1 protein in CRC cells and the mechanism by which EAF2 and VASH1 regulate STAT3/TGF-β1 pathway. In addition, more objective and scientific experimental methods should be combined in vivo and in vitro to further comprehensively discuss and evaluate the functional effects of EAF2 and VASH1 on CRC, so as to make up for the limitations and one-sidedness of this study which only conducted in vitro invasion, migration and angiogenesis studies.
Moreover, in-depth study on the mechanism and methods of overexpressing EAF2 and VASH1 may provide new targets and ideas for the treatment of CRC. Deletion of EAF2 and Von Hippel-Lindau (VHL) produced a synergistic effect on angiogenesis in male fractional liver and prostate, accompanied by an increase in the expression of VHL target proteins hypoxia-inducible factor 1α and VEGF[42]. Further study on the effect and mechanism of EAF2-mediated VASH1 on endothelial cells in tumor microenvironment may help to elucidate the pathogenesis of CRC. Inhibition of cytotoxic T lymphocyte protein 4 can reactivate immune cells, especially T cells, in patients with CRC, thereby enhancing the ability of immune cells to fight tumors[43]. Regulatory T cells (Tregs) in tumor microenvironment can mediate mechanisms such as immune escape, and may also participate in the regulation of ovarian cancer angiogenesis through VEGFA and VASH1[44]. The synergistic effect of VASH1-related pathway anti-angiogenesis therapy and immunotherapy may become a new idea for CRC treatment.
CONCLUSION
In conclusion, our study found that EAF2 was down-regulated and VASH1 was up-regulated in advanced CRC tissue. Kaplan-Meier survival analysis showed that the high EAF2 Level group and the low VASH1 Level group had a higher survival rate. This study suggests that EAF2 and VASH1 may serve as new diagnostic and prognostic markers for CRC. What’s more, overexpression of EAF2 protein or VASH1 protein in CRC cells inhibited STAT3/TGF-β1 pathway, and further inhibited invasion, migration and angiogenesis of CRC cells. Overexpression of EAF2 may provide tumor inhibition and protection against CRC by up-regulating VASH1 expression and inhibiting the activity of downstream STAT3/TGF-β1 pathway (Figure 11). This study provides new ideas and theoretical basis for the development of new diagnostic markers for CRC and the exploration of the mechanism of targeted therapy.
Figure 11.
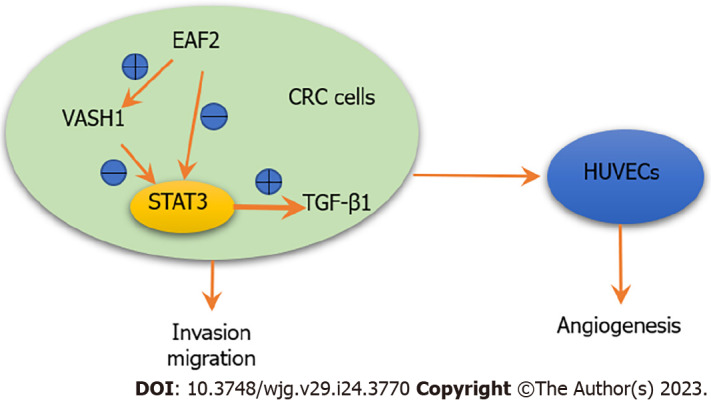
Schematic depiction of ELL-associated factor 2 and vasohibin 1 regulating the signal transducer and activator of transcription 3/ transforming growth factor-β1 signaling pathway in this study. Overexpression of ELL-associated factor 2 may inhibit the activity of signal transducer and activator of transcription 3/transforming growth factor-β1 pathway by up-regulating the expression of vasohibin 1, thereby inhibiting cell invasion, migration and angiogenesis. EAF2: ELL-associated factor 2; TGF-β1: Transforming growth factor-β1; STAT3: Signal transducer and activator of transcription 3; CRC: Colorectal cancer; HUVECs: Human umbilical vein endothelial cells; VASH1: Vasohibin 1.
ARTICLE HIGHLIGHTS
Research background
More and more studies have suggested that the anti-angiogenic factor vasohibin 1 (VASH1) is not only expressed in the stroma of tumor cells, but also expressed in tumor cells. It also plays a certain role in the regulation of tumor growth, invasion and tumor microenvironment. Recent studies have found that ELL-associated factor 2 (EAF2) can mediate signal transducer and activator of transcription 3 (STAT3)/transforming growth factor-β1 (TGF-β1) pathway to act on colorectal cancer (CRC). Knockdown of VASH1 enhanced TGF-β1/Smad3 pathway activity and type I/III collagen production.
Research perspectives
Further study on the effect and mechanism of EAF2-mediated VASH1 on endothelial cells in tumor microenvironment may help to elucidate the pathogenesis of CRC. The synergistic effect of VASH1-related pathway anti-angiogenesis therapy and immunotherapy may become a new idea for CRC treatment.
Research conclusions
This study suggests that EAF2 and VASH1 may serve as new diagnostic and prognostic markers for CRC. It complements the mechanism of EAF2 in CRC cells, enriches the role and mechanism of CRC cell-derived VASH1, and provides a new possible subtype of CRC as a therapeutic target of STAT3/TGF-β1 pathway.
Research results
Our findings indicated that EAF2 was down-regulated and VASH1 was up-regulated in advanced CRC tissue compared to normal colorectal tissue. Overexpression of EAF2 might inhibit the activity of STAT3/TGF-β1 pathway by up-regulating the expression of VASH1, thereby attenuating the invasion, migration and angiogenesis of CRC cells.
Research methods
We collected colorectal adenocarcinoma and corresponding adjacent tissues in patients with advanced CRC. Following, we investigated the effect and mechanism of EAF2 and VASH1 on the invasion, migration and angiogenesis of CRC cells in vitro using plasmid transfection.
Research objectives
To investigate the expression of VASH1 in CRC and its correlation with the expression of EAF2. Furthermore, we explored the functional role and mechanism of EAF2 and VASH1-mediated TGF-β1 related pathway in CRC cells.
Research motivation
Detection of EAF2 and VASH1 protein expression in CRC tissues may help to explore new diagnostic and prognostic markers for CRC. The study of the potential mechanism of EAF2 and VASH1 can provide new ideas for the treatment of CRC.
Footnotes
Institutional review board statement: The study was reviewed and approved by the institutional review board of the First Affiliated Hospital of China Medical University (approval No. 2021-68-2).
Informed consent statement: The informed consent was waived from the patients.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 18, 2023
First decision: March 26, 2023
Article in press: May 12, 2023
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Ekine-Afolabi B, United Kingdom; Solimando AG, Italy S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
Contributor Information
Ming-Liang Feng, Department of Endoscopy, The First Hospital Affiliated to China Medical University, Shenyang 110001, Liaoning Province, China.
Ming-Jun Sun, Department of Endoscopy, The First Hospital Affiliated to China Medical University, Shenyang 110001, Liaoning Province, China.
Bo-Yang Xu, Department of Endoscopy, The First Hospital Affiliated to China Medical University, Shenyang 110001, Liaoning Province, China.
Meng-Yuan Liu, Department of Endoscopy, The First Hospital Affiliated to China Medical University, Shenyang 110001, Liaoning Province, China.
Hui-Jing Zhang, Department of Endoscopy, The First Hospital Affiliated to China Medical University, Shenyang 110001, Liaoning Province, China.
Can Wu, Department of Endoscopy, The First Hospital Affiliated to China Medical University, Shenyang 110001, Liaoning Province, China. wucanydyy@163.com.
Data sharing statement
Data can be acquired from the corresponding author.
References
- 1.Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Wu Y, Tian T. TGF-β Signaling in Metastatic Colorectal Cancer (mCRC): From Underlying Mechanism to Potential Applications in Clinical Development. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232214436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi M, Wakabayashi I, Suzuki Y, Fujiwara K, Nakayama M, Watabe T, Sato Y. Tubulin carboxypeptidase activity of vasohibin-1 inhibits angiogenesis by interfering with endocytosis and trafficking of pro-angiogenic factor receptors. Angiogenesis. 2021;24:159–176. doi: 10.1007/s10456-020-09754-6. [DOI] [PubMed] [Google Scholar]
- 4.Tamaki K, Moriya T, Sato Y, Ishida T, Maruo Y, Yoshinaga K, Ohuchi N, Sasano H. Vasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci. 2009;100:88–94. doi: 10.1111/j.1349-7006.2008.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao G, Yang Y, Tang Y, Han R, Sun Y. Reduced expression of vasohibin-1 is associated with clinicopathological features in renal cell carcinoma. Med Oncol. 2012;29:3325–3334. doi: 10.1007/s12032-012-0313-x. [DOI] [PubMed] [Google Scholar]
- 6.Kosaka T, Miyazaki Y, Miyajima A, Mikami S, Hayashi Y, Tanaka N, Nagata H, Kikuchi E, Nakagawa K, Okada Y, Sato Y, Oya M. The prognostic significance of vasohibin-1 expression in patients with prostate cancer. Br J Cancer. 2013;108:2123–2129. doi: 10.1038/bjc.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong HL, Zhong XH, Guo XH, Liao HJ, Yuan X. circASS1 overexpression inhibits the proliferation, invasion and migration of colorectal cancer cells by regulating the miR-1269a/VASH1 axis. Exp Ther Med. 2021;22:1155. doi: 10.3892/etm.2021.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Han B, Zhang Q, Dou J, Wang F, Lin W, Sun Y, Peng G. Vasohibin-1 suppresses colon cancer. Oncotarget. 2015;6:7880–7898. doi: 10.18632/oncotarget.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y, Shen Z, Ye Y, Jiang K, Zhang H, Shen C, Mustonen H, Puolakkainen P, Wang S. A novel molecular marker of prognosis in colorectal cancer: Vasohibin-1. Med Oncol. 2014;31:816. doi: 10.1007/s12032-013-0816-0. [DOI] [PubMed] [Google Scholar]
- 10.Fang W, Radovich M, Zheng Y, Fu CY, Zhao P, Mao C, Zheng S. 'Druggable' alterations detected by Ion Torrent in metastatic colorectal cancer patients. Oncol Lett. 2014;7:1761–1766. doi: 10.3892/ol.2014.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watatani H, Maeshima Y, Hinamoto N, Yamasaki H, Ujike H, Tanabe K, Sugiyama H, Otsuka F, Sato Y, Makino H. Vasohibin-1 deficiency enhances renal fibrosis and inflammation after unilateral ureteral obstruction. Physiol Rep. 2014;2 doi: 10.14814/phy2.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang XW, Qin QX. miR-335-5p induces insulin resistance and pancreatic islet β-cell secretion in gestational diabetes mellitus mice through VASH1-mediated TGF-β signaling pathway. J Cell Physiol. 2019;234:6654–6666. doi: 10.1002/jcp.27406. [DOI] [PubMed] [Google Scholar]
- 13.Martin CJ, Datta A, Littlefield C, Kalra A, Chapron C, Wawersik S, Dagbay KB, Brueckner CT, Nikiforov A, Danehy FT Jr, Streich FC Jr, Boston C, Simpson A, Jackson JW, Lin S, Danek N, Faucette RR, Raman P, Capili AD, Buckler A, Carven GJ, Schürpf T. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay8456. [DOI] [PubMed] [Google Scholar]
- 14.Villalba M, Evans SR, Vidal-Vanaclocha F, Calvo A. Role of TGF-β in metastatic colon cancer: it is finally time for targeted therapy. Cell Tissue Res. 2017;370:29–39. doi: 10.1007/s00441-017-2633-9. [DOI] [PubMed] [Google Scholar]
- 15.Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, Cai X, Hahn J, Liu J, Pins M, Doglio L, Dhir R, Gingrich J, Wang Z. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene. 2008;27:1536–1544. doi: 10.1038/sj.onc.1210786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, Keener AL, Jing Y, Cai L, Ai J, Zhang J, Fisher AL, Fu G, Wang Z. FOXA1 modulates EAF2 regulation of AR transcriptional activity, cell proliferation, and migration in prostate cancer cells. Prostate. 2015;75:976–987. doi: 10.1002/pros.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zang Y, Dong Y, Yang D, Xue B, Li F, Gu P, Zhao H, Wang S, Zhou S, Ying R, Wang Z, Shan Y. Expression and prognostic significance of ELL-associated factor 2 in human prostate cancer. Int Urol Nephrol. 2016;48:695–700. doi: 10.1007/s11255-015-1210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Chen Z, Ouyang G, Song T, Liang H, Liu W, Xiao W. ELL Protein-associated Factor 2 (EAF2) Inhibits Transforming Growth Factor β Signaling through a Direct Interaction with Smad3. J Biol Chem. 2015;290:25933–25945. doi: 10.1074/jbc.M115.663542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savas S, Azorsa DO, Jarjanazi H, Ibrahim-Zada I, Gonzales IM, Arora S, Henderson MC, Choi YH, Briollais L, Ozcelik H, Tuzmen S. NCI60 cancer cell line panel data and RNAi analysis help identify EAF2 as a modulator of simvastatin and lovastatin response in HCT-116 cells. PLoS One. 2011;6:e18306. doi: 10.1371/journal.pone.0018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng ML, Wu C, Zhang HJ, Zhou H, Jiao TW, Liu MY, Sun MJ. Overexpression of ELL-associated factor 2 suppresses invasion, migration, and angiogenesis in colorectal cancer. World J Gastrointest Oncol. 2022;14:1949–1967. doi: 10.4251/wjgo.v14.i10.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, Shi T, Chen W. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis. 2020;11:55. doi: 10.1038/s41419-020-2252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akagi K, Oki E, Taniguchi H, Nakatani K, Aoki D, Kuwata T, Yoshino T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021;112:1105–1113. doi: 10.1111/cas.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascal LE, Rigatti LH, Ai J, Zhang A, Zhou J, Nelson JB, Wang Z. EAF2 loss induces prostatic intraepithelial neoplasia from luminal epithelial cells in mice. Am J Clin Exp Urol. 2020;8:18–27. [PMC free article] [PubMed] [Google Scholar]
- 24.Han W, Shen GL. Systematic expression analysis of EAF family reveals the importance of EAF2 in melanoma. Int Immunopharmacol. 2020;88:106958. doi: 10.1016/j.intimp.2020.106958. [DOI] [PubMed] [Google Scholar]
- 25.Jo YS, Kim SS, Kim MS, Yoo NJ, Lee SH. Candidate Tumor Suppressor Gene EAF2 is Mutated in Colorectal and Gastric Cancers. Pathol Oncol Res. 2019;25:823–824. doi: 10.1007/s12253-018-0461-1. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda H, Sato Y. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, Hojo K, Matsumoto M, Yamauchi C, Ohta H, Sonoda H, Sato Y. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol. 2010;176:1950–1958. doi: 10.2353/ajpath.2010.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito S, Miyashita H, Suzuki Y, Kobayashi M, Satomi S, Sato Y. Enhanced cancer metastasis in mice deficient in vasohibin-1 gene. PLoS One. 2013;8:e73931. doi: 10.1371/journal.pone.0073931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyashita H, Watanabe T, Hayashi H, Suzuki Y, Nakamura T, Ito S, Ono M, Hoshikawa Y, Okada Y, Kondo T, Sato Y. Angiogenesis inhibitor vasohibin-1 enhances stress resistance of endothelial cells via induction of SOD2 and SIRT1. PLoS One. 2012;7:e46459. doi: 10.1371/journal.pone.0046459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120:2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 32.Kern J, Bauer M, Rychli K, Wojta J, Ritsch A, Gastl G, Gunsilius E, Untergasser G. Alternative splicing of vasohibin-1 generates an inhibitor of endothelial cell proliferation, migration, and capillary tube formation. Arterioscler Thromb Vasc Biol. 2008;28:478–484. doi: 10.1161/ATVBAHA.107.160432. [DOI] [PubMed] [Google Scholar]
- 33.Haque S, Morris JC. Transforming growth factor-β: A therapeutic target for cancer. Hum Vaccin Immunother. 2017;13:1741–1750. doi: 10.1080/21645515.2017.1327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colak S, Ten Dijke P. Targeting TGF-β Signaling in Cancer. Trends Cancer. 2017;3:56–71. doi: 10.1016/j.trecan.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Stanilova S, Stanilov N, Julianov A, Manolova I, Miteva L. Transforming growth factor-β1 gene promoter -509C/T polymorphism in association with expression affects colorectal cancer development and depends on gender. PLoS One. 2018;13:e0201775. doi: 10.1371/journal.pone.0201775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2018;50:121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Hu S, Tan J, Zhang X, Yuan W, Wang Q, Xu L, Liu J, Liu Z, Jia Y, Huang X. Pregnancy-specific glycoprotein 9 (PSG9), a driver for colorectal cancer, enhances angiogenesis via activation of SMAD4. Oncotarget. 2016;7:61562–61574. doi: 10.18632/oncotarget.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 39.Rocha MR, Barcellos-de-Souza P, Sousa-Squiavinato ACM, Fernandes PV, de Oliveira IM, Boroni M, Morgado-Diaz JA. Annexin A2 overexpression associates with colorectal cancer invasiveness and TGF-ß induced epithelial mesenchymal transition via Src/ANXA2/STAT3. Sci Rep. 2018;8:11285. doi: 10.1038/s41598-018-29703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pascal LE, Wang Y, Zhong M, Wang D, Chakka AB, Yang Z, Li F, Song Q, Rigatti LH, Chaparala S, Chandran U, Parwani AV, Wang Z. EAF2 and p53 Co-Regulate STAT3 Activation in Prostate Cancer. Neoplasia. 2018;20:351–363. doi: 10.1016/j.neo.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Z, Yan Y, Ye C, Wang B, Jiang K, Ye Y, Mustonen H, Puolakkainen P, Wang S. The effect of Vasohibin-1 expression and tumor-associated macrophages on the angiogenesis in vitro and in vivo. Tumour Biol. 2016;37:7267–7276. doi: 10.1007/s13277-015-4595-4. [DOI] [PubMed] [Google Scholar]
- 42.Pascal LE, Ai J, Rigatti LH, Lipton AK, Xiao W, Gnarra JR, Wang Z. EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate. Angiogenesis. 2011;14:331–343. doi: 10.1007/s10456-011-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derakhshani A, Hashemzadeh S, Asadzadeh Z, Shadbad MA, Rasibonab F, Safarpour H, Jafarlou V, Solimando AG, Racanelli V, Singh PK, Najafi S, Javadrashid D, Brunetti O, Silvestris N, Baradaran B. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers (Basel) 2021;13 doi: 10.3390/cancers13102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao S, Hou Y, Rong Q, Han B, Liu P. Tregs are involved in VEGFA/ VASH1-related angiogenesis pathway in ovarian cancer. Transl Oncol. 2023;32:101665. doi: 10.1016/j.tranon.2023.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be acquired from the corresponding author.