Summary
Mucosa-associated invariant T (MAIT) cells are evolutionarily conserved, innate-like T lymphocytes with enormous immunomodulatory potentials. Due to their strategic localization, their invariant T cell receptor (iTCR) specificity for major histocompatibility complex-related protein 1 (MR1) ligands of commensal and pathogenic bacterial origin, and their sensitivity to infection-elicited cytokines, MAIT cells are best known for their antimicrobial characteristics. However, they are thought to also play important parts in the contexts of cancer, autoimmunity, vaccine-induced immunity, and tissue repair. While cognate MR1 ligands and cytokine cues govern MAIT cell maturation, polarization, and peripheral activation, other signal transduction pathways, including those mediated by costimulatory interactions, regulate MAIT cell responses. Activated MAIT cells exhibit cytolytic activities and secrete potent inflammatory cytokines of their own, thus transregulating the biological behaviors of several other cell types, including dendritic cells, macrophages, natural killer cells, conventional T cells, and B cells, with significant implications in health and disease. Therefore, an in-depth understanding of how costimulatory pathways control MAIT cell responses may introduce new targets for optimized MR1/MAIT cell-based interventions. Herein, we compare and contrast MAIT cells and mainstream T cells for their expression of classic costimulatory molecules belonging to the immunoglobulin superfamily and the tumor necrosis factor (TNF)/TNF receptor superfamily, based not only on the available literature but also on our transcriptomic analyses. We discuss how these molecules participate in MAIT cells’ development and activities. Finally, we introduce several pressing questions vis-à-vis MAIT cell costimulation and offer new directions for future research in this area.
Keywords: MAIT cells, costimulation, CD28, ICOS, CD40 ligand, OX40, 4-1BB, CD27
The CD40L-CD40 costimulatory axis modulates mucosa-associated invariant T (MAIT) cells’ functions and mediates their bidirectional crosstalk with other important immunocytes. Activated MAIT cells rely on this pathway to promote dendritic cell maturation, resulting in type-I cytokine production as well as follicular helper T cell expansion in lymph nodes. In addition, CD40L signaling in MAIT cells enhances B cell activation and antibody responses.
Graphical Abstract
Graphical Abstract.
MAIT cells: a brief introduction
Definition and homing characteristics
Mucosa-associated invariant T (MAIT) cells are innate-like T lymphocytes with a unique gene rearrangement pattern in their invariant T cell receptor (iTCR) α chain, typically TRAV1-2-TRAJ33/12/20 (Vα7.2-Jα33/12/20) in humans and Trav1-Traj33 (Vα19-Jα33) in mice [1–3]. The TCR Vβ repertoires of human and mouse MAIT cells are also limited in diversity, with the predominant usage of Vβ2/13 and Vβ6/8, respectively [3, 4].
Unlike conventional T (Tconv) cells that see antigenic peptides presented by the highly polymorphic major histocompatibility complex (MHC) molecules, MAIT cells are restricted by the monomorphic MHC-related protein 1 (MR1) [5]. MR1 can display vitamin B metabolites synthesized by commensal and pathogenic bacteria and fungi, typified by 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU) [6–8], among other potential ligands. MR1 appeared early in mammals, co-evolved with TRAV1, and remained conserved ever since [9]. The cross-reactivity between mouse and human MAIT cells points to the similarity of MR1 ligands recognized by these cells [10].
MAIT cells are scarce in standard laboratory mouse strains [11] in which they primarily show a CD4−CD8− phenotype [12]. Therefore, in vivo studies on MAIT cells have often relied on experimental pre-enrichment [13], or on genetically altered strains with an adequately large MAIT cell compartment, such as Vα19i TCR transgenic [14] and C57BL/6 (B6)-MAITCAST mice [11]. In contrast, human MAIT cells, which are predominantly CD4−CD8+, are abundant in the peripheral blood (PB), in the liver, and in barrier tissues, including in the skin and in gastrointestinal, respiratory, and urogenital tracts, where they help maintain homeostasis and combat pathogens at their entry sites [15, 16]. Human MAIT cells express high levels of CD161, which has enabled their identification as CD3+Vα7.2+CD161+ cells in many investigations. The advent of MR1 tetramer reagents has allowed for the accurate detection of mouse and human MAIT cells and their comparative analyses [12, 17].
Intrathymic and extrathymic development
MAIT cell maturation in the thymus occurs in three stages. In mice, CD24+CD44−, CD24−CD44− and CD24−CD44+ cells define stages 1, 2, and 3, respectively [18]. In humans, the CD161−CD27− stage is followed by the appearance of CD161−CD27+ cells before CD161+CD27−/+ cells emerge [18]. While early thymic MAIT cell precursors are CD4+CD8+, their differentiation into more mature cells is accompanied by a gradual loss of CD4 expression. In both mice and humans, the transition from stage 2 to stage 3 is dictated by the transcription factor promyelocytic leukemia zinc finger (PLZF) and interleukin (IL)-18 [18, 19].
Preserved iTCR signaling events and intermediates, including ZAP-70 (zeta chain-associated protein kinase 70) and diacylglycerol, are essential for mouse MAIT cell development [19, 20]. In addition, mice lacking miR-181a/b-1, a microRNA pair serving as a rheostat in TCR signal strength regulation, exhibit early MAIT cell maturation arrest [18, 21]. The potential roles of microRNAs during human MAIT cell education are currently unclear. Normal mouse MAIT cell development appears to depend on SAP (signaling lymphocytic activation molecule-associated protein) [19, 22], RIPK3 (receptor-interacting protein kinase 3), [23] and CXCR6 [19], among other signaling entities.
Unlike Tconv cells whose positive selection is executed by cortical thymic epithelial cells displaying self-peptide:MHC complexes, MAIT cells are positively selected by MR1-expressing CD4+CD8+ thymocytes [24]. Another major difference between Tconv and MAIT cells is the location in which functional commitments are made. Tconv cells leave the thymus as mature but naïve cells before they encounter cognate antigens (Ags) in secondary lymphoid tissues, proliferate, and differentiate into various effector cell subsets. By contrast, mouse MAIT cells develop in the thymus as transcriptionally and functionally distinct subpopulations, primarily as MAIT1 and MAIT17 lineages that express T-bet (T-box expressed in T cells) and RORγt (retinoic acid receptor-related orphan receptor γt), respectively [18–20, 22]. These transcription factors are not mutually exclusive in the case of human MAIT cells. In their stage 3, thymic human MAIT cells are RORγt+T-bet+ and capable of producing interferon (IFN)-γ and tumor necrosis factor (TNF)-α [18], likely among other cytokines.
The thymic differentiation of mouse MAIT1 cells and their accumulation in the thymus and spleen depend on IL-2 and IL-15, whereas signaling through inducible costimulator (ICOS) promotes MAIT17 cell development [20]. Furthermore, the presence of commensal microbes and stronger TCR signals, as judged by Nur77 expression, seems to favor the MAIT17 program in the thymus [25].
Mouse MAIT cells complete their maturation in the periphery after birth and in the presence of B lymphocytes and commensal microbes [26, 27]. In humans, MAIT cell development occurs primarily in utero before commensal microbial communities are established [28]. During the second trimester, functional human MAIT cells are detectable within the fetal small intestine, liver, and lungs [28]. It is possible that microbial MR1 ligands present in maternal circulation and mucosal tissues might cross the placenta to access the foetal thymus and mediate positive MAIT cell selection [28]. This is not far-fetched since 5-OP-RU has been detected in the mouse thymus shortly after its cutaneous application [25].
Activation pathways and consequences
MAIT cell activation occurs through iTCR-dependent and -independent mechanisms. Exposure to microbes that metabolize riboflavin or to 5-OP-RU, a riboflavin derivative of bacterial and fungal origin, primes mouse and human MAIT cells in an MR1/iTCR-dependent manner [6–8]. A photodegradation product of folic acid called 6-formylpterin [29], certain drugs and drug-like compounds, such as diclofenac and methotrexate [30], and the dietary molecules vanillin and ethylvanillin [31], can also serve as MR1 ligands with various functional outcomes as far as MAIT cell responses are concerned.
Unlike mature Tconv cells that are either CD4+ or CD8+ and that rely on their respective co-receptor for stable TCR–MHC interactions, the vast majority of mature MAIT cells in mice are double-negative but still responsive to MR1 ligands or iTCR cross-linking. Therefore, co-receptor expression is not required for MR1/iTCR-driven MAIT cell activation in mice. By comparison, CD8 expression on human MAIT cells enhances MR1 binding and cytokine production [32].
In addition to or in the absence of iTCR signaling, MAIT cells can be activated by cytokines released after infection or Toll-like receptor (TLR) engagement. This pathway is particularly critical in antiviral host defence since viruses lack the riboflavin biosynthesis machinery [33]. IL-7, IL-12, IL-15, IL-18, IL-33, type I IFNs (T1-IFNs), and TNF-like protein 1A (TL1A) are among cytokines to which MAIT cells react in the face of infection, sterile inflammation, tissue damage and other microenvironmental changes [34–38].
Once activated, MAIT cells swiftly release potent immunomodulatory mediators, including IFN-γ, TNF-α, IL-4, IL-5, IL-13, IL-17, and IL-22, in various combinations. This is likely influenced by the strength and/or duration of cognate, cytokine and costimulatory signals MAIT cells receive, their functional pre-programming, their tissue imprinting, and their epigenetic regulation [39], among other factors. Owed to their remarkable cytokine production capacity, and also through contact-dependent mechanisms, activated MAIT cells modulate the physiological or pathological behaviors of many cell types, including myeloid and plasmacytoid dendritic cells (DCs) [40], monocytes and macrophages [40–43], natural killer (NK) cells [40, 44–46], type-2 innate lymphoid cells (ILC2) [47], NKT cells [44], Tconv cells, [44, 48, 49] and B cells [50, 51].
MAIT cells express NK group 2, member D (NKG2D), a C-type lectin-like receptor that enables iTCR-independent cytotoxicity against infected, stressed, damaged, or transformed cells [52, 53]. They also harbor a powerful arsenal of cytolytic effector molecules, including perforin, granzymes, and granulysin, which can be upregulated and mobilized toward MR1+ target cells, attack extracellular bacteria, and help overcome resistance to carbapenems, a class of last-resort antibiotics [53, 54].
Steady-state and activated MAIT cells demonstrate transcriptomic signatures suggesting potential functions in tissue repair and remodeling mechanisms [26, 38, 55–57]. For instance, following skin incision, dermal mouse MAIT cells secrete amphiregulin to promote wound healing [57].
For all of the above reasons, one could envisage protective, pathogenic, or sometimes seemingly paradoxical roles for MAIT cells in a wide variety of conditions, including infections and superinfections [58, 59], autoimmunity [44, 60–62], malignancy, [63–65] and fibrotic processes [46, 66]. Therefore, understanding signaling cascades that control MAIT cell responses, including those mediated by costimulatory molecules, is key to successful MR1/MAIT cell-based interventions.
MAIT cell costimulation: basic principles
MAIT cell activation can be tuned by several molecules not falling under the “classic” costimulatory families of receptors. For instance, signaling through IFN-α/β receptor and several TLRs may potentiate, augment, or regulate MAIT cell responses to 5-OP-RU [40, 49, 67–69]. This mode of action is often indirect and reliant on accessory cells, such as Ag-presenting cells (APCs), in co-culture systems or in complex in vivo settings. Mouse MAIT cell responses to certain bacterial infections also depend on concurrent stimulation through IL-12 and IL-23 receptor signaling [70, 71]. As another example, the iTCR-driven TNF-α production capacity of MAIT cells was boosted in the presence of α-ketoglutarate, a cofactor for the epigenetic regulator histone lysine demethylase 6B (KDM6B), in human peripheral blood mononuclear cell (PBMC) cultures [39]. In this review, we only cover classic costimulatory molecules belonging to the immunoglobulin, TNF, and TNF receptor (TNFR) superfamilies.
Naive Tconv cell priming requires at least two signals. Signal 1 is generated when cognate peptide:MHC complexes are detected by TCRs. This signal, which accounts for the specificity of T cell-mediated immunity, is necessary but insufficient for optimal naïve Tconv cell activation, which also depends on costimulatory interactions that supply signal 2. In fact, TCR triggering in the absence of costimulation may lead to T cell anergy or death [72]. Cytokines present in the T cell priming milieu can provide a third signal, which is responsible for or contributes to proliferation, differentiation and functional polarization of the ensuing effector cells and memory cell precursors [73].
Costimulatory molecules work through several mechanisms to enable naïve Tconv cell activation. For instance, CD28 engagement makes signaling-rich lipid rafts cluster [74] and triggers actin cytoskeletal remodeling within the immunological synapses formed between Tconv cells and APCs [75]. Moreover, CD28 costimulation upregulates the anti-apoptotic protein Bcl-xL to promote T cell survival [76], enhances IL-2 gene transcription [77] and messenger RNA (mRNA) stability [78] to enable T cell growth, and increases glycolytic flux to meet the suddenly raised demand for energy [79]. Effector and memory Tconv cells have much less stringent costimulatory requirements compared with their naïve counterparts.
MAIT cells are effector memory-like T lymphocytes, as judged by their CD45RO+CD45RA−CD127hiCD95hiCD62Llo phenotype in humans [80], and mount rapid and robust responses to antigenic stimulation. However, within an “innateness gradient,” MAIT cells stand closer to Tconv cells when transcriptionally compared with iNKT and γδ T cells [81]. Accordingly, MAIT cells contain lower levels of pre-formed mRNA encoding cytokines and cytolytic molecules while appearing to exhibit the highest proliferative capacity among innate-like T cells.
Importantly, in response to TCR cross-linking, MAIT cells launch weaker and more transient effector functions than do CD8+ conventional memory T cells [82]. On the contrary, MAIT cells respond more rigorously to inflammatory cytokines. A higher activation threshold for iTCRs may serve as a checkpoint to prevent unnecessary MAIT cell activation in mucosal layers where commensal bacterial metabolites are plentiful [82]. However, MAIT cells stay poised and prepared to react to infectious agents and inflammatory stimuli by virtue of the cytokine receptors they express.
We previously compared the costimulatory needs of iNKT and Tconv cells [83]. In this review, we focus on MAIT cells, which are more frequent than iNKT cells in humans. We highlight differences in the expression of classic costimulatory molecules (Table 1) and discuss the significance of these molecules in MAIT cell responses.
Table 1.
Expression of classic costimulatory molecules by conventional T and MAIT cells
| Family | Costimulatory molecule | Cell type | Expression during MAIT cell development |
Ligand(s) | Ligand expression | Reference(s) | |
|---|---|---|---|---|---|---|---|
| MAIT cells | Tconv cells | ||||||
| IgSF | CD28 (Tp44) | + | + |
Stage 1: +++*
Stage 2: ++ Stage 3: + |
CD80 (B7-1) |
APCs Activated Tconv cells |
[19, 71, 84–86] |
| CD86 (B7-2) |
APCs | ||||||
| ICOS (CD278, AILIM, CVID1) | + | ↑ | Stage 3:+++* MAIT17 | ICOSL (LICOS, B7h, B7RP-1, B7-H2, GL50, GL50-B, CD275) | B cells DCs Macrophages Non-lymphoid cells |
[20, 51, 70, 71, 87, 88] | |
| TNFSF | CD40L (CD154, TNFSF5, gp39, TRAP, TBAM) | ↑* | ↑ | ND | CD40 (TNFRSF5, Bp50, CDW50, p50) | B cells DCs Macrophages Monocytes Platelets Non-hematopoietic cells |
[36, 40, 55, 89–91] |
| TNFRSF | OX40 (CD134, TNFRSF4) | ↑ * | ↑ | ND | OX40L (CD252, TNFSF4) | B cells DCs Macrophages Tconv cells Endothelial cells |
[92–97] |
| 4-1BB (CD137, TNFRSF9, ILA) | ↑ | ↑ | ND | 4-1BBL (CD137L, TNFSF9) | B cells DCs Macrophages Monocytes Tconv cells |
[40, 55, 70, 98, 99] | |
| CD27 (TNFRSF7, Tp55, S152) | + | + |
Stage 1: -*
Stage 2: + Stage 3: + |
CD70 (CD27L, TNFSF7) | Thymic epithelial cells Activated DCs Activated B cells Activated Tconv cells |
[21, 27, 40, 70, 100] | |
APC: antigen-presenting cell(s); DC(s): dendritic cell(s); IgSF: immunoglobulin superfamily; Tconv: conventional T [cell(s)]; TNFRSF: tumor necrosis factor receptor superfamily; TNFSF: tumor necrosis factor superfamily.
+: constitutive expression; ↑: inducible expression; ND: not determined.
*Bold and italics fonts/arrows refer to mouse and human data, respectively. Results applicable to both species are shown using regular fonts.
Costimulatory molecules of the immunoglobulin superfamily
The CD28-CD80/CD86 system
In the mouse thymus, CD28 expression is highest among CD4+CD8+ thymocytes before its gradual decline as MAIT cell development progresses from stage 1 to stage 3 [19]. Using CD319 and CD138 as surrogate markers for stage-3 MAIT1 and MAIT17 cells, respectively, Koay et al. [19] reported a link between higher CD28 expression and the MAIT1 functional program.
Pulmonary mouse MAIT cells constitutively express CD28 at levels comparable to those found on Tconv cells [70]. To assess the in vivo significance of CD28 costimulation in MAIT cell functions, or lack thereof, Wang et al. compared wild-type (WT) and Cd80/Cd86 double-knockout B6 mice following intravenous administration of a live vaccine strain of Francisella tularensis, which elicits a protective MAIT1-skewed response [71]. In this model, defective CD28 signaling reduced non-MAIT αβ T cell, but not MAIT cell, numbers in the lungs. However, pulmonary MAIT1 cell proportions were slightly increased, rather than decreased, in infected Cd80/Cd86−/− animals. These investigators also demonstrated that CD28 signaling was dispensable for pulmonary MAIT cell expansion after intranasal inoculation of Salmonella typhimurium or Legionella longbeachae, which induces a dominant MAIT17 response [70, 71]. MAIT cell responses and their functional bias in these models depend partially, if not largely, on IL-12 and/or IL-23 [70, 71]. Therefore, it would be interesting to explore the contribution of CD28 costimulation to iTCR-driven antibacterial responses when cytokine receptor signaling is experimentally ablated.
In rhesus macaques, PB MAIT cells show a memory phenotype with elevated levels of CD69, CD95, IL-18 receptor and CCR6, along with a larger CD28+ fraction, compared with non-MAIT T cells [84, 85]. In fact, CD28 expression helps distinguish between central memory (CD28+CD95+) and effector memory (CD28−CD95+) MAIT cell subsets in both rhesus macaques and cynomolgus macaques [84, 85]. Moreover, at the peak of simian immunodeficiency virus viremia in the latter species, CD28+ memory MAIT cells transiently increased their expression levels of Ki-67, CD69, CD39, T-bet, and RORγt, whereas their CD28− counterparts exhibited Ki-67 upregulation only among the tested parameters [84, 85]. Whether CD28 signaling directly alters the activation threshold of nonhuman primate MAIT cells is not known.
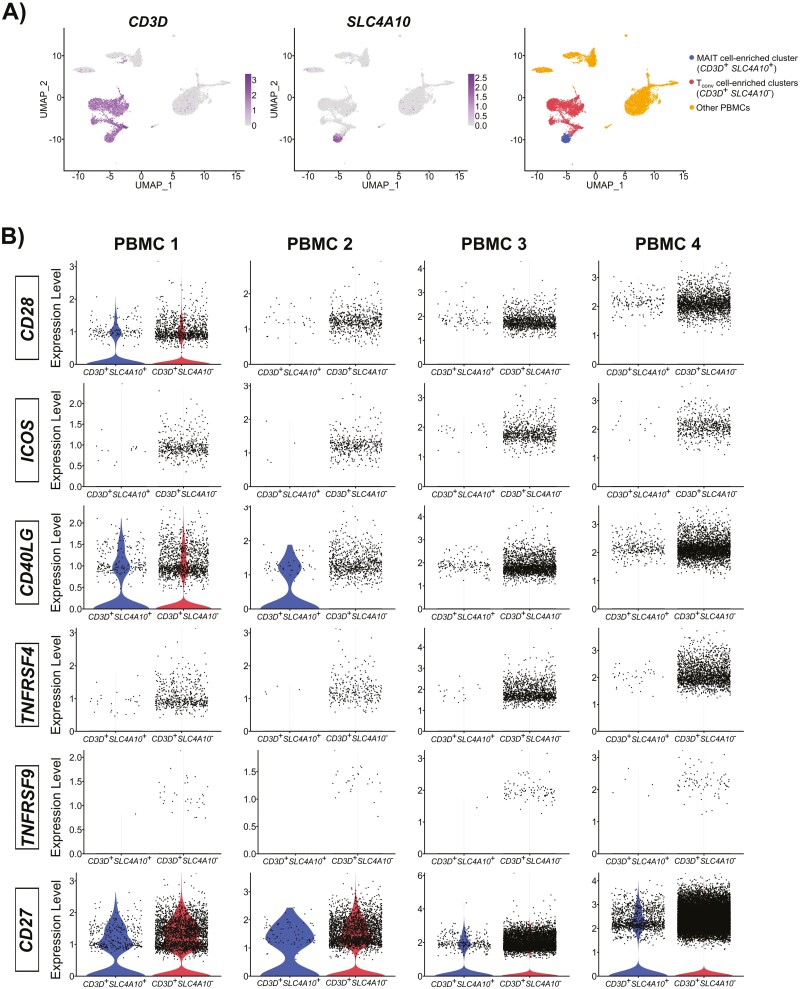
In humans, PB MAIT cells express CD28 at their steady state [40, 80]. Using publicly available PBMC [101–104] and CD45+ non-parenchymal hepatic [105] single-cell RNA-sequencing (scRNA-seq) datasets, we found a modest but still detectable presence of CD28 in PB (Fig. 1A and B and Supplementary Fig. 1) and hepatic (Fig. 2A and B) human MAIT cell-enriched populations. Similar levels of CD28 transcripts were also detectable in non-MAIT Tconv cells in both compartments (Figs. 1B and 2B). For these analyses, MAIT cell-enriched clusters were identified by CD3D and SLC4A10 expression [64], and non-MAIT Tconv cell clusters were defined by CD3D expression only (Figs. 1A and 2A).
Figure 1.
Transcriptomic expression of classic costimulatory molecules by MAIT cell-enriched populations identified in human peripheral blood mononuclear cell datasets. Four pre-processed single-cell RNA sequencing datasets [101–104] were accessed on February 22, 2023, from 10x Genomics (https://www.10xgenomics.com/resources/datasets). These include 10k PBMCs from a healthy donor (v3 chemistry), 8k PBMCs from a healthy donor, 33k PBMCs from a healthy donor, and fresh 68k PBMCs (donor A), which are referred to as PBMC 1, PBMC 2, PBMC 3, and PBMC 4, respectively, in this figure. Datasets were analyzed with default parameters using the standard Seurat (4.3.0) workflow [106]. Briefly, matrices were filtered to remove doublets and non-viable cells, transformed by the LogNormalize function, and adjusted with a scale factor of 10 000. The top 2000 variable features were selected and the ScaleData function was applied. Principal component analysis (PCA) was performed, and the top 25 principal components were selected for graph-based clustering and visualization with Uniform Manifold Approximation and Projection (UMAP) plots. Resolution was set to enable MAIT cell-enriched cluster selection for downstream analyses (0.3, 0.7, 0.5, and 1.1 for PBMC 1, PBMC 2, PBMC 3, and PBMC 4, respectively). (A) Representative UMAP plots from the PBMC 1 dataset. UMAP plots of CD3D and SLC4A10 depict T cells and the MAIT cell-enriched population, respectively. The latter population was defined by concomitant CD3D and SLC4A10 expression while CD3D expression in the absence of SLC4A10 was used to identify non-MAIT T cells. Clusters containing other PBMCs expressing neither CD3D nor SLC4A10 are also illustrated. (B) Violin plots for CD28, ICOS, CD40LG, TNFRSF4 (OX40), TNFRSF9 (4-1BB), and CD27 expression in MAIT and non-MAIT T cells were generated.
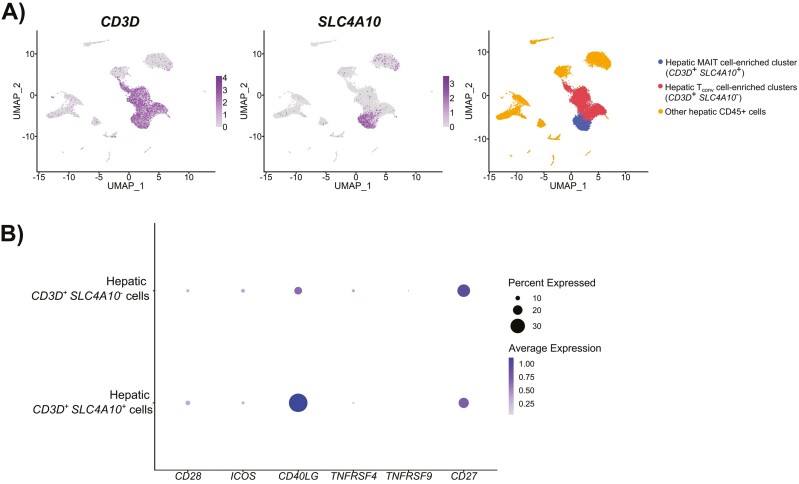
Figure 2.
Transcriptomic expression of classic costimulatory molecules by hepatic MAIT cells. A pre-processed single-cell RNA sequencing dataset [105] generated using CD45+ cells from five healthy liver samples was obtained from the gene expression omnibus (GEO) public repository (accession number GSE48452). Samples used to create the above dataset were from four males and one female (57.4 ± 7.9 years of age). Standard Seurat (4.3.0) workflow [106] was used for dataset analysis with default parameters. Briefly, the count matrix was filtered to remove doublets and non-viable cells, transformed by the LogNormalize function, and adjusted with a scale factor of 10 000. The top 2000 variable features were selected, and the ScaleData function was then applied. Principal component analysis (PCA) was performed, and the top 25 principal components were selected for graph-based clustering and visualization with uniform manifold approximation and projection (UMAP) plots. Resolution was set to 0.5 to enable MAIT cell-enriched cluster selection for downstream analyses. (A) UMAP plots of CD3D and SLC4A10 illustrate T cells and the MAIT cell-enriched population, respectively. The latter cluster was defined by concomitant CD3D and SLC4A10 expression while CD3D expression in the absence of SLC4A10 was used to identify non-MAIT T cells. Clusters containing other CD45+ hepatic mononuclear cells that expressed neither CD3D nor SLC4A10 are also depicted. (B) A DotPlot for CD28, ICOS, CD40LG, TNFRSF4, TNFRSF9, and CD27 expression in MAIT and non-MAIT T cells was generated.
Due to the existence of redundant systems within the costimulatory machinery of human T cells, CD28 appears dispensable for protective immunity against many, if not most, infections. However, intact CD28 signaling controls human papillomavirus (HPV) infection in keratinocytes. This notion is supported by a recent article reporting three patients with inherited CD28 deficiency and cutaneous papillomatosis, including a male with an HPV-2-driven “tree man” phenotype and two relatives with severe, recurrent HPV-4-induced warts [107]. Of note, PB MAIT cells were the only innate-like T lymphocytes with low frequencies in these patients compared with healthy controls. This is especially intriguing in light of another case report describing a person with tattoo-associated persistent HPV+ warts who was found to carry a homozygous point mutation in MR1 resulting in MAIT cell deficiency [108]. Whether and how CD28 signaling in MAIT cells contributes to anti-HPV immunity warrants mechanistic investigations.
Several studies have reported reduced CD28 expression by human MAIT cells in the contexts of viral infections and cancer, accompanied by increases in co-inhibitory molecules and exhaustion markers. For instance, PB MAIT cells from patients with chronic hepatitis delta virus infection exhibited low CD28 and high programmed death-1 (PD-1) levels compared with healthy donor MAIT cells [109]. Similarly, in patients with hepatocellular carcinoma, intratumoral MAIT cells expressed lower CD28 but higher PD-1, cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), and T cell immunoglobulin and mucin-3 (TIM-3) levels than did matched healthy liver MAIT cells [110]. In both scenarios, MAIT cells were hyporesponsive to iTCR stimulation with fixed Escherichia coli (E. coli) [109, 110].
Using cell-free biochemical assays and PD-1-transduced Jurkat T cells, Hui et al. [111] demonstrated that PD-1-recruited Src homology region 2 domain-containing protein tyrosine phosphatase 2 (SHP-2) dephosphorylates CD28, thus terminating its signaling cascade. Using mouse models of chronic viral infection and cancer, Kamphorst et al. [112] found CD28 costimulation to be necessary for CD8+ T cell proliferation following PD-1 blockade. In addition, anti-PD-1 therapies in lung cancer patients expanded a population of CD8+ T cells that were predominantly CD28+ [112].
It is tempting to suggest that PD-1 upregulation in MAIT cells may interfere with their CD28 signaling . To test this hypothesis, however, the expression kinetics of these molecules and their relationship need to be explored first. Nonetheless, in in vitro culture systems, CD28 triggering augments iTCR-based responses. For instance, an anti-CD28 monoclonal antibody (mAb) raised the frequency of CD69+IFN-γ+ cells among purified Vα7.2+ T lymphocytes that were co-cultured with monocytes in the presence of fixed E. coli [113]. In a separate study, biotin beads coated with anti-CD3 and anti-CD28, which were used as artificial APCs, were superior to those coated with anti-CD3 alone in inducing IFN-γ and TNF-α production by CD8+Vα7.2+ T cells [114]. This response was further boosted when CD161 was additionally ligated.
The ICOS-ICOSL system
ICOS-ICOS ligand (ICOSL) interactions play a pivotal role in MAIT cell development, functional fate choice determination, and immunomodulatory responses, at least in mice. Tao et al. [20] found no ICOS expression by mouse TCRβ−CD4+CD8+ thymocytes and low ICOS levels in TCRβ+ thymocytes. However, ICOS was readily detectable in developing MAIT cells with the lowest and highest levels present in thymic stages 1 and 3, respectively.
Approximately 70% of thymic MAIT cells are lost in Icos−/− mice, which can be attributed to a numerical drop in stage-3 cells [20]. In addition, ICOS deficiency selectively but incompletely impedes MAIT17 cell development/maintenance, which reduces the number of splenic, peripheral lymph node, hepatic and pulmonary MAIT cells as a result.
TCR-, ICOS- and cytokine receptor-coupled signaling pathways are known to activate the mammalian target of rapamycin (mTOR) in Tconv cells [115–118]. Using mice with a selective deficiency of Rictor in their CD4+ cells and bone marrow chimeras, Tao et al. found intrinsic mTOR complex 2 (mTORC2) signaling to be strictly required for stage-3 MAIT cell maturation/homeostasis and for ICOS+RORγt+ MAIT17 cell development [20].
Given the more pronounced impact of mTORC2 deficiency on MAIT17 cell development, compared with ICOS deficiency, it is likely that mTOR integrates cytokine cues with other signals generated by TCR and/or ICOS stimulation, to control functional MAIT cell programs. Accordingly, Tao et al. proposed a model in which IL-1β works with IL-23 and ICOS to signal through mTORC2 and promote MAIT17 cell polarization. On the other hand, IL-2 and IL-15 involve mTORC1 signaling to enable MAIT1 cell differentiation [20].
It is noteworthy that using ICOS-sufficient mice, Tao et al. found CD122+ICOSlo and CD122−ICOS+ phenotypes to be prevalent among T-bet+ and RORγt+ MAIT cells in the thymus and peripheral tissues, which were potent IFN-γ and IL-17A producers, respectively, upon ex vivo stimulation with a combination of phorbol myristate acetate (PMA) and ionomycin [20]. Therefore, surface CD122 expression is indicative of the MAIT1 lineage while ICOS can serve as a surrogate marker for MAIT17 cells. In a separate study, transcriptomic and cytofluorimetric analyses of mouse MAIT cells also strongly correlated ICOS expression with RORγt, while the expression of the chemokine receptor CXCR3 was associated with T-bet [119].
Wang et al. [70] demonstrated a much higher basal expression level of ICOS in naïve B6 pulmonary MAIT cells than in non-MAIT T cells [70]. This was in contrast with 4-1BB that was absent in MAIT cells, and unlike CD27, CD28 (as noted before) and CD40 ligand whose expression levels were comparable between MAIT and non-MAIT T cells. The authors found lower MAIT17 cell numbers in the lungs of Icos−/− mice following infection with Salmonella typhimurium or Legionella longbeachae [70]. However, this is not surprising since ICOS is necessary for the thymic development of MAIT17 cells before antibacterial responses are initiated in the periphery.
Jensen et al. [51] reported negligible expression levels of ICOS by unstimulated pediatric PB MAIT cells, a finding that was corroborated in our bioinformatic analyses of adult human PB (Fig. 1A and B and Supplementary Fig. S1) and hepatic (Fig. 2B) CD3D+SLC4A10+ cells. However, a large fraction of “T follicular helper (Tfh)-like MAIT (MAITfh) cells”, which were discovered in the tonsils of children who had undergone tonsillectomy, expressed ICOS. These MAITfh cells were CXCR5+ MAIT cells that co-expressed the Tfh lineage-defining transcription factor BCL6, preferentially homed to germinal centers, and produced IL-21 in response to PMA and ionomycin. Together, these features point to a potential role for MAIT cells in providing help to B lymphocytes in mucosal lymphoid tissues. Importantly, MAIT cell transfer into TCRα−/− mice, which are devoid of conventional Tfh cells, gave rise to MAITfh-like cells that supported B cell differentiation into plasmablasts/plasma cells or memory B cells and restored Vibrio cholerae-specific IgA production after an intranasal challenge with this pathogen. Future investigations will need to address the significance of ICOS in MAITfh cell activities.
Costimulatory molecules of the TNFR/TNF superfamily
The CD40L-CD40 system
The interaction between CD40 ligand (CD40L, CD154) and CD40 results in bidirectional signaling in T cells and their engagement partners, such as DCs and B cells, with clearly important outcomes in adaptive immunity.
In the mouse lung, a substantial proportion of MAIT cells are CD40L+ [70]. In humans, unlike unstimulated PB MAIT cells, a fraction of hepatic MAIT cells display CD40L on their surface [55, 89]. We similarly detected CD40LG transcripts in hepatic CD3D+SLC4A10+ cells at levels that far exceeded those found in CD3D+SLC4A10− cells (Fig. 2B). In addition, varying levels of CD40LG were found in PB MAIT cell-enriched populations from healthy donors (Fig. 1B). Steady-state CD40LG expression is reportedly enriched among human MAIT cells isolated from the rectal mucosae [82]. However, the expression of CD40L by intestinal MAIT cells at the protein level, or lack thereof, and its potential significance remain to be defined.
As with Tconv cells, CD40L expression is inducible upon MAIT cell activation [36, 55, 89, 90]. Lamichhane et al. [55] found CD40L upregulation at both mRNA and protein levels in PB human MAIT cells following E. coli stimulation. This was phenocopied in cultures containing recombinant IL-12 and IL-18, albeit to a lesser extent. Jeffery et al. similarly detected CD40L on human PB MAIT cells in a co-culture system in which T cells were exposed to E. coli-primed biliary epithelial cells [89]. This response was partially dependent on MR1, IL-12 and IL-18. Finally, Lepore et al. [4] showed that stimulation with E. coli-infected THP-1 macrophages prompts human PB MAIT cell clones to secrete soluble CD40L. The in vivo relevance of this finding remains unclear.
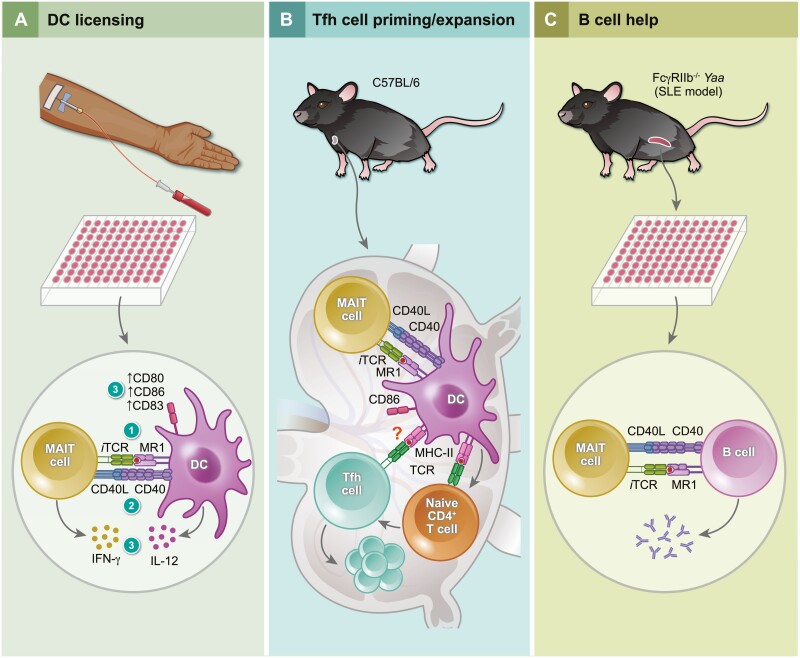
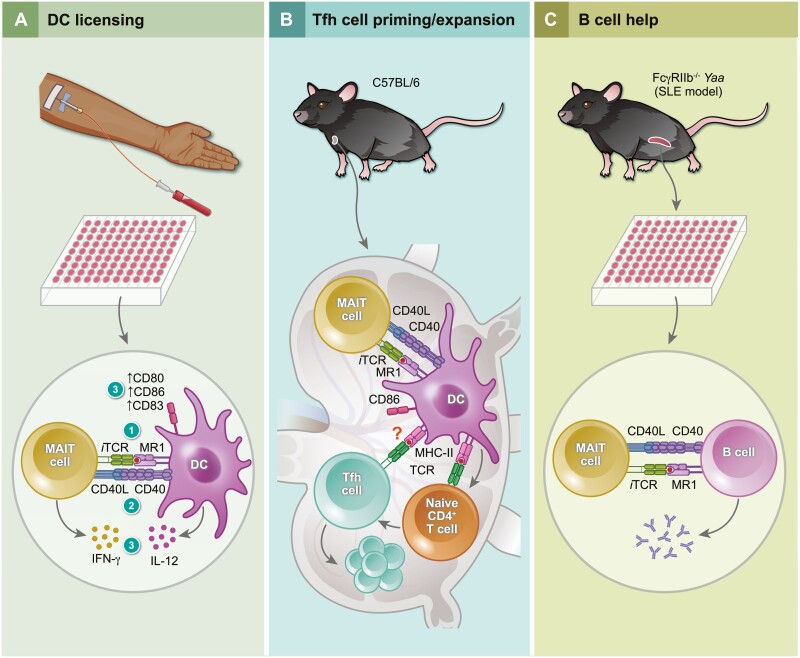
To study MAIT cells’ crosstalk with APCs, Salio et al. [40] co-incubated monocyte-derived DCs and MAIT cells in the presence of 5-amino-6-D-ribitylaminouracil (5-A-RU) and methylglyoxal (MG), which together form 5-OP-RU. This resulted in reciprocal activation of DCs and MAIT cells as evidenced by IL-12p40 and IFN-γ production, respectively. Importantly, IFN-γ production was prevented by MR1 blockade and also significantly diminished by CD40L blockade (Fig. 3A). This study yielded two additional findings of interest. First, iTCR stimulation of MAIT cells enhanced DC maturation, as judged by the expression of several maturation markers, including B7-1 and B7-2 that bind CD28 to costimulate T cells. Second, CD40L upregulation was evident exclusively among CD161bright IFN-γ-secreting cells that also expressed 4-1BB, another prominent costimulatory molecule expressed by T cells. Interestingly, an MR1-blocking mAb completely abrogated CD40L upregulation but only marginally reduced 4-1BB levels [40]. It is reasonable to assume differences between CD40L and 4-1BB expression patterns in terms of activation thresholds and/or expression kinetics and based also on MAIT cell stimulation means and modes. CD40L upregulation occurs quickly after exposure to 5-A-RU or fixed E. coli and reaches its peak within 6 h. By comparison, a combination of IL-12 and IL-18 induces CD40L gradually [55].
Figure 3.
CD40L-CD40 interactions mediate MAIT cells’ crosstalk with dendritic cells and B cells. (A) In vitro riboflavin-derived antigen presentation by MR1 (step 1) licenses dendritic cells (DCs) to activate MAIT cells, which in turn upregulate their CD40L expression to engage CD40 on DCs (step 2). This results in DC maturation, as evidenced by their CD80, CD86 and CD83 upregulation (step 3). At or around the same time, IL-12 and IFN-γ are released by DCs and MAIT cells, respectively (step 3), to promote a type-I response [40]. (B) Intranasal administration of 5-amino-6-D-ribitylaminouracil (5-A-RU)/methylglyoxal to wild-type C57BL/6 mice activates MAIT cells, which in turn promotes conventional DC maturation in mediastinal lymph nodes [120]. This leads to antigen-specific CD4+ T follicular helper (Tfh) cell expansion in the lymph node directly or indirectly [120]. (C) Activated splenic MAIT cells from systemic lupus erythematosus (SLE)-prone FcγRIIb−/−Yaa mice increase autoantibody production upon in vitro interaction with lipopolysaccharide (LPS)-activated splenic B cells in a CD40L-CD40-dependent manner [121].
A multi-omics approach by Schubert et al. [90] identified CD40L as a key driver of transcription in IL-12/18-, but not in anti-CD3/CD28-stimulated human PB MAIT cells. Nonetheless, the strongest MAIT cell responses, either helpful or harmful to the host, are likely to be elicited when both pathways are engaged. In addition, MAIT cell subsets residing in different tissues may behave differently when signaled through iTCRs and/or cytokine receptors.
Certain cytokines other than IL-18 may also induce CD40L expression in the absence of iTCR stimulation. For example, IL-33, an alarmin implicated in inflammatory responses, homeostasis, and tissue repair [122], synergizes with IL-12 to make CD40LG transcripts detectable in human PB MAIT cells [36]. Whether IL-33 alone, or in conjunction with iTCR and/or other cytokine signals, promotes or improves the healing capacity of MAIT cells [26, 38, 55–57] and whether the CD40L-CD40 costimulatory axis plays a role in this context are important questions to address.
The significance of CD40L-CD40 interactions after MAIT cell priming has been documented in mice. Pankhurst et al. [120] recently reported that CD40L blockade prior to and after intranasal 5-A-RU/MG administration interferes with migratory DC maturation, as judged by CD86 expression in mediastinal lymph nodes. In addition, repeated co-administration of chicken ovalbumin, a model protein Ag, with 5-A-RU/MG increased total and Ag-specific Tfh cell frequencies among activated CD4+ Tconv cells. These responses could be efficiently ablated by a CD40L-blocking mAb [120]. Therefore, the CD40L-CD40 axis mediates MAIT cell activities in licensing DCs and in optimizing downstream Tfh cell priming (Fig. 3A and 3B). These activities are apart from MAITfh cell roles in humoral immunity [50, 51] whose dependence on CD40L–CD40 interactions remains to be established.
MAIT cells’ ability to directly help B cells via CD40L-CD40 signaling has been demonstrated using FcγRIIb−/−Yaa mice, which serve as a model for systemic lupus erythematosus (SLE) [121] (Fig. 3C). In these animals, and also in patients with class III or class IV lupus nephritis, activated MAIT cells were detectable in the kidney. Moreover, MR1 deficiency ameliorated the course of lupus in FcγRIIb−/−Yaa mice, suggesting an important role for MAIT cells in this model. Finally, lipopolysaccharide (LPS)-stimulated splenic B cells from these animals produced larger quantities of total IgG, total anti-double-stranded DNA (dsDNA) autoantibodies, and anti-dsDNA IgG when MAIT cells were present in cultures. Importantly, these responses were reversed by a CD40L-blocking mAb, but not through ICOS blockade [121]. It will be interesting to adoptively transfer CD40L-sufficient and -deficient MAIT cells into Mr1−/−FcγRIIb−/−Yaa mice to ask whether CD40L signaling in MAIT cells contributes to the SLE-like phenotype in vivo.
The CD40L-CD40 pathway is known to participate in the pathogenesis of SLE [123], and several clinical trials have tested the efficacy of CD40L antagonists, such as toralizumab and dapirolizumab pegol, in SLE patients [124, 125]. It will be important to determine where human CD40L+ MAIT cells might fit in such clinical pictures.
The OX40-OX40 ligand (OX40L) system
OX40 serves as a “second-wave” costimulatory molecule whose expression is induced by TCR triggering and then sustained by CD28-B7 interactions to support Tconv cell survival, differentiation and memory, especially in the CD4+ compartment [83, 126]. OX40 signaling promotes Bcl-2 and Bcl-xL expression, which bestows upon Tconv cells a survival advantage [126]. Conversely, OX40 is considered a death receptor in mouse iNKT cells since its engagement activates caspase 1 and induces pyroptotic cell demise [127].
Our bioinformatic analyses of PBMC samples from four healthy donors (Fig. 1B) and hepatic CD45+ cells (Fig. 2B) have demonstrated very low, if any, TNFRSF4 levels in unfractionated CD3D+SLC4A10+ cells, which consist primarily of CD8+CD4− MAIT cells. Somewhat consistent with our transcriptomic analyses, two independent studies have reported the cell surface expression of OX40 by circulating MAIT cells in some, but certainly not all, healthy individuals [92, 93].
Vorkas et al. [92] demonstrated selective OX40 expression by CD4+CD8− MAIT cells in a few healthy donor PBMC samples, but not in all samples tested. Interestingly, healthy household contacts of patients with active tuberculosis (TB) from the same community had upregulated levels of OX40 on CD4+CD8− MAIT cells, which are known to respond to Mycobacterium tuberculosis (Mtb) [128]. This is reminiscent of the OX40 expression pattern in Tconv cell subsets after TCR stimulation.
Zhang et al. [93] reported elevated OX40 levels on PB MAIT cells from type 2 diabetic (T2D) patients compared with healthy controls. Furthermore, OX40+ MAIT cells showed higher CD69 and Fas levels on their surface and increased IFN-γ and IL-17A production capacities when compared with OX40− cells. Finally, cleaved caspase-3 became detectable within sorted T2D MAIT cells shortly after they were exposed to recombinant OX40L and an anti-CD3 mAb. This stimulation mode also resulted in activation-induced MAIT cell death. Therefore, as with iNKT cells [127], OX40 signaling can promote MAIT cell death at least in certain conditions. It will be interesting to examine other T cell subsets, along with MAIT cells, for their OX40 expression and survival versus apoptotic potentials not only in T2D but also in other clinical scenarios that have been linked to OX40. These include rheumatoid arthritis, autoimmune colitis, autoimmune encephalomyelitis, asthma, and tissue fibrosis [129].
OX40-OX40L interactions have also been implicated in proinflammatory MAIT cell responses. In patients with Helicobacter pylori (H. pylori), gastric mucosal MAIT cells reportedly express high levels of surface OX40, with the OX40+ population co-expressing more Ki-67, CD69 and CD25 [94]. This was accompanied by increased intracellular IL-9, a suspected culprit of H. pylori-induced gastritis [130], in MAIT cells [94]. In addition, OX40L expression was increased in gastric mucosal DCs at levels that could be positively correlated with IL-9+ MAIT cell proportions. Other positive correlations were found between OX40+ MAIT cell frequencies in gastric biopsies and both IL-9+ MAIT cell percentages and IL-9 serum levels in patients. Finally, IL-9 was readily detectable in MAIT:DC co-culture supernatants after stimulation with recombinant OX40L and/or anti-CD3. By the same token, MAIT cell proliferation in response to H. pylori-primed DCs was partially inhibited by OX40- and/or OX40L-blocking mAbs [94].
How OX40-OX40L interactions modulate MAIT cell functions and those of their engagement partners is ill-defined at this point. Certain viral infections enhance the expression of OX40 by iNKT cells, which engages OX40L on plasmacytoid DCs to promote T1-IFN production [83, 131]. MAIT cells too respond to a wide variety of viruses and to T1-IFNs [34, 37]. However, the contribution of the OX40-OX40L signaling cascade to antiviral MAIT cell responses remains an open question.
The 4-1BB-4-1BB ligand (4-1BBL) system
The 4-1BB-coupled signaling cascade appears dispensable in the early phase of Tconv cell activation but plays a crucial role subsequently when several other costimulatory molecules are less available. Although 4-1BB can be expressed at comparable levels by CD4+ and CD8+ Tconv cells, it preferentially drives or amplifies CD8+ T cell proliferation and cytotoxicity [132].
4-1BB/TNFRSF9 is not constitutively expressed by mouse pulmonary MAIT cells [70] or by human PB and hepatic CD3D+SLC4A10+ cells (Figs. 1B and 2B and Supplementary Fig. S1). However, in vitro iTCR stimulation with 5-A-RU/MG or fixed E. coli induces rapid and robust expression of 4-1BB at both mRNA and protein levels in human MAIT cells [40, 55, 98]. Interestingly, while MR1 blockade in 6-h PBMC cultures with E. coli completely abolished 4-1BB upregulation, it was only modestly effective in 24-h cultures [55], suggesting an important role for other signals, such as inflammatory cytokines, in maintaining 4-1BB expression at later time points. Indeed, a combination of exogenous IL-12 and IL-18 can induce 4-1BB expression by MAIT cells [55, 98]. However, this response is much weaker than that generated through E. coli stimulation.
The strong linkage between iTCR signaling and 4-1BB expression is further supported by functional RNA sequencing using pseudotime analysis that revealed select genes associated with iTCR and cytokine stimulation trajectories [133]. Accordingly, while TNFRSF9 (encoding 4-1BB), CCL3 and CCL4 were of great importance for the iTCR trajectory, IFNG and IL26 were specifically linked to the IL-12/IL-18 stimulation pathway. This approach provides dynamic information not easily acquired by other methods.
Lamichhane et al. sought to determine how viral infection alters MAIT cell responses to 5-A-RU/MG [67]. Interestingly, while adding the influenza A virus (IAV) strain A/Puerto Rico/8/34 or T1-IFNs to human PBMC stimulation cultures enhanced the expression of several activation, effector, or costimulatory molecules by MAIT cells, 4-1BB levels were slightly lowered. Therefore, 4-1BB may need to be tightly regulated during viral infections through a T1-IFN-dependent mechanism(s).
4-1BB also appears to participate in antibacterial MAIT cell responses. Upon in vitro stimulation with Mtb antigens, 4-1BB was upregulated on PB MAIT cells and more strongly on MAIT cells from the tuberculous pleural effusions of TB patients in an IL-2-dependent fashion [134]. In addition, 4-1BB+ MAIT cells expressed significantly more CD25, Ki-67, IFN-γ, IL-17A and IL-17F compared with 4-1BB− MAIT cells [134]. These findings partially simulate Tconv cell responses to Mtb in TB patients in whom 4-1BB expression was associated with IFN-γ production [135].
The CD27-CD70 system
The CD27 is constitutively expressed on naïve CD4+ and CD8+ Tconv cells and upregulated upon T cell activation [136]. After CD27–CD70 interactions, the extracellular domain of CD27 can be cleaved off to form a soluble, active form found in bodily fluids.
Similarly to Tconv cells, the majority of MAIT cells express CD27 at steady state [27, 40, 70], a finding that was reproducible in our transcriptomic analyses of PB and hepatic CD3D+SLC4A10+ cells (Figs. 1B and 2B and Supplementary Fig. S1). Whether MAIT cells release soluble CD27 upon activation is unknown to our knowledge.
Several studies have focused on pathological conditions in which a lack of CD27 on MAIT cells is encountered. For instance, circulating CD27− MAIT cell frequencies are reportedly increased in newly diagnosed, untreated multiple myeloma [137], human immunodeficiency virus (HIV) infection [138], juvenile type 1 diabetes (T1D) [139, 140], and T2D with obesity [141]. Interestingly, CD27− PB MAIT cell frequencies were associated with disease progression in T2D since they were positively correlated with hemoglobin A1c levels and also negatively correlated with the homeostatic model assessment of β cell function (HOMA-β) index [141].
In healthy donors and T2D patients, CD27− MAIT cells were found to produce more IL-17A in response to PMA and ionomycin when compared to CD27+ cells [139, 141]. Consistent with these observations, a higher RORγt:T-bet ratio was evident in CD27− MAIT cells from obese/overweight but otherwise healthy adults and also in obese T2D patients [141]. Of note, CD27–CD70 interactions reportedly promote TH1 differentiation and inhibit IL-17 production by CD4+ Tconv cells [142–144], and a lack of CD27 expression by CD4+ Tconv and γδ T cells confers upon them a T helper 17 (TH17)-like phenotype [142, 145]. Therefore, a role for CD27 signaling in MAIT17 cell differentiation is conceivable.
Examination of the MAIT cell compartment in T2D patients has also revealed a gene signature related to antibacterial responses in circulating CD27+, but not CD27−, MAIT cells [141]. This was accompanied by the overt presence of Bacteroides ovatus, a common commensal of the human colon, in circulation, which drove the accumulation of IL-17-producing CD27− MAIT cells in the PB [141]. Ex vivo, T2D PBMC stimulation with heat-killed B. ovatus resulted in IL-17A and TNF-α by MAIT cells, which was much more pronounced in the CD27− subset [141]. Since microbiota dysbiosis can lead to IL-17 production with pathological consequences, a role for IL-17-secreting CD27− MAIT cells was speculated in T2D exacerbations [141].
Future directions
Despite recent progress in our understanding of costimulatory pathways that operate in MAIT cells, many questions remain unanswered.
Costimulatory signals that are exchanged between MAIT cells and APCs or target cells to promote or stabilize the corresponding immunological synapses need to be deciphered. Such signals may work bidirectionally to alter the biological behaviors of both parties involved. Costimulatory molecules that regulate MAIT cell priming and effector functions are not always simultaneously available. Therefore, their spatiotemporal expression should be assessed in MAIT cells residing in different microenvironments.
The strength of costimulatory and co-inhibitory signals and their balanced (or imbalanced) expression are likely to determine MAIT cell stimulation outcomes. The classical example is CTLA-4, which is upregulated on Tconv cells and outcompetes CD28 for access to B7 molecules [146]. This is due to the much higher avidity of CTLA-4, compared to CD28, for binding to B7, and apart from CTLA-4’s inherent negative signaling properties. We have reported the selective upregulation of lymphocyte-activation gene 3 (LAG-3) and T cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) on MAIT cells in the contexts of toxic shock [147] and chronic psychological stress [148, 149], respectively, with functional repercussions for antimicrobial immunity. Whether costimulation can be experimentally or therapeutically enforced to help overcome MAIT cell dysfunctions, exhaustion, or even undesired skewedness will be an attractive area of research.
Mouse models will continue to shed mechanistic light on T cell costimulation pathways, especially in clarifying which interactions work in T cell-autonomous and/or -extrinsic manners to shape a given phenotype. MAIT cells, which appear to be the most similar innate-like T lymphocytes to Tconv cells [81], are no exception. Importantly, mouse and human MAIT cells are more phenotypically and functionally similar than previously assumed [12]. However, although MAIT cells in these species recognize and respond to similar MR1 ligands, they show drastic differences in terms of tissue abundance, homing properties, and co-receptor usage. Therefore, caution needs to be exercised in interpreting the translatability of findings from mouse models.
iNKT cell subsets defined by their co-receptor expression and tissue localization exhibit functional heterogeneity [150–154] and somewhat distinct costimulatory requirements [83, 155]. While CD4−CD8− cells are the predominant MAIT cell subset in mice, CD4+ and CD8+ MAIT cells are also found in certain locations, for instance in lymph nodes [12]. Whether these subsets fulfil different or complementary functions that may potentially rely on select costimulatory signals remains to be clarified.
In response to PMA and ionomycin, which work together to bypass iTCR engagement, CD4+ human MAIT cells were much more potent producers of IL-2 than all other MAIT cell subsets [156]. This stimulation mode also generated more IL-17A-producing MAIT cells within the human CD4−CD8− subpopulation than in CD8+ cells [157]. Most human PB MAIT cells express CD8αα homodimer or CD8αβ heterodimer as their co-receptors, which enhance MR1 binding and cytokine responses [32]. In addition to the functional MAIT1, MAIT17, and MAIT1/17 subsets that are commonly studied in humans, CD4+ and CD8+ MAIT2-like cells with a delayed capacity to produce IL-13 can be generated in long-term cultures [158] although their significance and signaling requirements need to be validated in vivo. Finally, there exist γδ T cells with MR1 reactivity [159, 160], whose degree of innateness and costimulatory requirements warrant future investigation.
Although typical MAIT cells express TRAV1-2 in their TCRα chain, MR1-restricted T cell repertoires additionally harbor TRAV1-2− cells [161]. Within tissue-resident TRAV1-2+ cell populations, TRAJ33 is enriched in the spleen and liver, and TRAJ12 and TRAJ29/TRAJ36 are frequently used in the liver and jejunum, respectively [162]. This suggests distinct TRAV1-2+ TCR specificities for cognate Ags depending on the metabolomic features of various tissues. It will be interesting to learn whether TRAV and TRAJ gene usage in MAIT and other MR1-restricted T cells influences not only the range of Ags these cells may recognize but also the costimulatory signals they may require for their activation, antimicrobial functions, and potentially for their tissue repair activities.
The tremendous antimicrobial, cytolytic and immunomodulatory activities of MAIT cells may be harnessed in vaccine design or future immunotherapies. The monomorphic nature of MR1 [5] means its ligands may be used to target MAIT cells in genetically diverse populations [163]. Using mouse models of prime-boost immunization against IAVs and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we have recently demonstrated that repeated 5-OP-RU administration is possible without inducing MAIT cell anergy [49]. This is in stark contrast with the prototypic CD1d ligand α-galactosylceramide, which induces long-term iNKT cell anergy even after a single injection [164].
MR1 ligands and/or certain cytokines may be employed to expand MAIT cells in in vivo settings [49, 165] and in in vitro protocols generating many “donor-unrestricted” MAIT cells [166]. Future “off-the-shelf” cell therapies of this kind may restore, reinforce, or rejuvenate MAIT cell compartments when they are depleted [167, 168], aged [169], or functionally impaired [147–149, 170]. Using “costimulation-optimized” strategies and protocols will likely yield better outcomes or products.
The cytolytic potentials of MAIT cells can be exploited against cancer, for instance by engineering MAIT cells that express chimeric Ag receptors (CAR-MAIT cells) with a 4-1BB costimulatory domain [47]. Another potential advantage of MAIT cells in cancer immunotherapy is their resistance to certain chemotherapeutic agents by virtue of their unusually high expression of multi-drug resistance protein 1 [80, 171]. This should enable combined interventions with costimulation-optimized, drug-resistant MAIT cells with chemotherapy, when applicable.
We anticipate new and important knowledge arising from future studies in which costimulatory interactions will be interrogated, augmented when MAIT cells are protective, or interfered with when MAIT cells participate in pathological conditions.
Supplementary Material
Acknowledgements
We thank members of the Haeryfar Laboratory for helpful discussions. We apologize to investigators whose contributions to the area of T cell costimulation could not be cited due to space constraints.
Glossary
Abbreviations
- Ag(s)
antigen(s)
- APC(s)
Ag-presenting cell(s)
- 5-A-RU
5-amino-6-D-ribitylaminouracil
- B6
C57BL/6 [mouse strain]
- 4-1BBL
4-1BB ligand
- CAR
chimeric Ag receptor
- CD
cluster of differentiation
- CD40L
CD40 ligand
- CTLA-4
cytotoxic T-lymphocyte-associated antigen-4
- DC(s)
dendritic cell(s)
- dsDNA
double-stranded deoxyribonucleic acid
- E. coli
Escherichia coli
- HIV
human immunodeficiency virus
- HOMA-β
homeostatic model assessment of β cell function [index]
- HPV
human papillomavirus
- H. pylori
Helicobacter pylori
- IAV(s)
influenza A virus(es)
- ICOS
inducible costimulator
- ICOSL
ICOS ligand
- IFN
interferon
- IL
interleukin
- ILC2
type-2 innate lymphoid cell(s)
- iNKT
invariant natural killer T [cell(s)]
- iTCR
invariant T cell receptor
- KDM6B
histone lysine demethylase 6B
- LAG-3
lymphocyte-activation gene 3
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MAIT
mucosa-associated invariant T [cell(s)]
- MAITfh
T follicular helper-like MAIT [cell(s)]
- MG
methylglyoxal
- MHC
major histocompatibility complex
- MR1
MHC-related protein 1
- mRNA
messenger ribonucleic acid
- Mtb
Mycobacterium tuberculosis
- mTOR
mammalian target of rapamycin
- mTORC
mTOR complex
- NK
natural killer [cell]
- NKG2D
NK group 2, member D
- 5-OP-RU
5-(2-oxopropylideneamino)-6-D-ribitylaminouracil
- OX40L
OX40 ligand
- PB
peripheral blood
- PBMC(s)
peripheral blood mononuclear cell(s)
- PD-1
programmed death-1
- PLZF
promyelocytic leukaemia zinc finger [transcription factor]
- PMA
phorbol myristate acetate
- RIPK3
receptor-interacting protein kinase 3
- RORγt
retinoic acid receptor-related orphan receptor γt
- SAP
signaling lymphocytic activation molecule-associated protein
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- scRNA-seq
single-cell RNA-sequencing
- SHP-2
Src homology region 2 domain-containing protein tyrosine phosphatase 2
- SLE
systemic lupus erythematosus
- T-bet
T-box expressed in T cells
- TB
tuberculosis
- Tconv
conventional T [cell(s)]
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- Tfh
T follicular helper [cell(s)]
- TIGIT
T cell immunoreceptor with immunoglobulin and ITIM domains
- T1-IFN(s)
type 1 IFN(s)
- TIM-3
T cell immunoglobulin and mucin-3
- TL1A: TLR(s)
tumour necrosis factor-like protein 1A
- TLR:
Toll-like receptor(s)
- TNFR
TNF receptor
- ZAP-70
zeta chain-associated protein kinase 70.
Contributor Information
Nicole I Wang, Department of Microbiology and Immunology, Western University, London, Ontario, Canada.
Marina Ninkov, Department of Microbiology and Immunology, Western University, London, Ontario, Canada.
S M Mansour Haeryfar, Department of Microbiology and Immunology, Western University, London, Ontario, Canada; Division of Clinical Immunology and Allergy, Department of Medicine, Western University, London, Ontario, Canada; Division of General Surgery, Department of Surgery, Western University, London, Ontario, Canada; Lawson Health Research Institute, London, Ontario, Canada.
Ethical Approval
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was funded by the Canadian Institutes of Health Research through a Project Grant (PJT 174984) to SMMH. NIW is a recipient of a Frederick Banting and Charles Best Canada Graduate Scholarship-Master’s (CGS-M) from the Canadian Institutes of Health Research.
Conflict of Interest: None declared.
Data Availability
Publicly available peripheral blood mononuclear cell datasets were obtained from 10x Genomics (https://www.10xgenomics.com/resources/datasets), and transcriptomic data from healthy liver samples were generated using Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) under the accession code GSE48452.
Author Contributions
N.I.W. investigation, visualisation, bioinformatic analyses, writing (initial draft). M.N. Investigation, visualisation, writing (initial draft). S.M.M.H. conceptualization, funding acquisition, supervision, investigation, visualization, editing, writing (final manuscript).
Permission to Reproduce
This manuscript does not contain data that would require permission to reproduce.
Clinical Trial Registration
Not Applicable.
References
- 1. Lantz O, Bendelac A.. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8− T cells in mice and humans. J Exp Med 1994, 180, 1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Porcelli S, Yockey CE, Brenner MB, Balk SP.. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8− alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 1993, 178, 1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 1999, 189, 1907–21. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun 2014, 5, 3866. [DOI] [PubMed] [Google Scholar]
- 5. Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003, 422, 164–9. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 6. Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012, 491, 717–23. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 7. Corbett AJ, Eckle SBG, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014, 509, 361–5. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 8. Soudais C, Samassa F, Sarkis M, Le Bourhis L, Bessoles S, Blanot D, et al. In vitro and in vivo analysis of the gram-negative bacteria-derived riboflavin precursor derivatives activating mouse MAIT cells. J Immunol 2015, 194, 4641–9. doi: 10.4049/jimmunol.1403224. [DOI] [PubMed] [Google Scholar]
- 9. Boudinot P, Mondot S, Jouneau L, Teyton L, Lefranc M-P, Lantz O.. Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals. Proc Natl Acad Sci USA 2016, 113, E2983–92. doi: 10.1073/pnas.1600674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lantz O, Legoux F.. MAIT cells: an historical and evolutionary perspective. Immunol Cell Biol 2018, 96, 564–72. doi: 10.1111/imcb.1034. [DOI] [PubMed] [Google Scholar]
- 11. Cui Y, Franciszkiewicz K, Mburu YK, Mondot S, Le Bourhis L, Premel V, et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J Clin Invest 2015, 125, 4171–85. doi: 10.1172/JCI82424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rahimpour A, Koay HF, Enders A, Clanchy R, Eckle SBG, Meehan B, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med 2015, 212, 1095–108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Wilgenburg B, et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat Commun 2018, 9, 4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawachi I, Maldonado J, Strader C, Gilfillan S.. MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol 2006, 176, 1618–27. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 15. Provine NM, Klenerman P.. MAIT cells in health and disease. Annu Rev Immunol 2020, 38, 203–28. doi: 10.1146/annurev-immunol-080719-015428. [DOI] [PubMed] [Google Scholar]
- 16. Nel I, Bertrand L, Toubal A, Lehuen A.. MAIT cells, guardians of skin and mucosa?. Mucosal Immunol 2021, 14, 803–14. doi: 10.1038/s41385-021-00391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013, 210, 2305–20. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koay H-F, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol 2016, 17, 1300–11. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 19. Koay H-F, Su S, Amann-Zalcenstein D, Daley SR, Comerford I, Miosge L, et al. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci Immunol 2019, 4, eaay6039. doi: 10.1126/sciimmunol.aay6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tao H, et al. Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals. Nat Commun 2021, 12, 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winter SJ, Kunze-Schumacher H, Imelmann E, Grewers Z, Osthues T, Krueger A.. MicroRNA miR-181a/b-1 controls MAIT cell development. Immunol Cell Biol 2019, 97, 190–202. doi: 10.1111/imcb.12211. [DOI] [PubMed] [Google Scholar]
- 22. Legoux F, Gilet J, Procopio E, Echasserieau K, Bernardeau K, Lantz O.. Molecular mechanisms of lineage decisions in metabolite-specific T cells. Nat Immunol 2019, 20, 1244–55. doi: 10.1038/s41590-019-0465-3. [DOI] [PubMed] [Google Scholar]
- 23. Patton T, Zhao Z, Lim XY, Eddy E, Wang H, Nelson AG, et al. RIPK3 controls MAIT cell accumulation during development but not during infection. Cell Death Dis 2023, 14, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, et al. Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol 2013, 191, 6002–9. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 25. Legoux F, Bellet D, Daviaud C, El Morr Y, Darbois A, Niort K, et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 2019, 366, 494–9. doi: 10.1126/science.aaw2719. [DOI] [PubMed] [Google Scholar]
- 26. Constantinides MG, Link VM, Tamoutounour S, Wong AC, Perez-Chaparro PJ, Han S-J, et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 2019, 366, eaax6624. doi: 10.1126/science.aax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009, 7, e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leeansyah E, Loh L, Nixon DF, Sandberg JK.. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun 2014, 5, 3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mak JYW, Liu L, Fairlie DP.. Chemical modulators of mucosal associated invariant T cells. Acc Chem Res 2021, 54, 3462–75. doi: 10.1021/acs.accounts.1c00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keller AN, Eckle SBG, Xu W, Liu L, Hughes VA, Mak JYW, et al. Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat Immunol 2017, 18, 402–11. doi: 10.1038/ni.3679. [DOI] [PubMed] [Google Scholar]
- 31. Wang CJH, et al. Quantitative affinity measurement of small molecule ligand binding to major histocompatibility complex class-I-related protein 1 MR1. J Biol Chem 2022, 298, 102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Souter MNT, Awad W, Li S, Pediongco TJ, Meehan BS, Meehan LJ, et al. CD8 coreceptor engagement of MR1 enhances antigen responsiveness by human MAIT and other MR1-reactive T cells. J Exp Med 2022, 219, e20210828. doi: 10.1084/jem.20210828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 2010, 11, 701–8. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 34. van Wilgenburg B, et al. MAIT cells are activated during human viral infections. Nat Commun 2016, 7, 11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ussher JE, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 2014, 44, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azzout M, et al. IL-33 enhances IFNγ and TNFα production by human MAIT cells: a new Pro-Th1 effect of IL-33. Int J Mol Sci 2021, 22, 10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haeryfar SMM. Preface: MAIT cells in viral diseases. Crit Rev Immunol 2021, 41, v–x. doi: 10.1615/CritRevImmunol.2022044131. [DOI] [PubMed] [Google Scholar]
- 38. Leng T, Akther HD, Hackstein C-P, Powell K, King T, Friedrich M, et al.; Oxford IBD Investigators. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep 2019, 28, 3077–3091.e5. doi: 10.1016/j.celrep.2019.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howson LJ, Li J, von Borstel A, Barugahare A, Mak JYW, Fairlie DP, et al. Mucosal-associated invariant T cell effector function is an intrinsic cell property that can be augmented by the metabolic cofactor α-ketoglutarate. J Immunol 2021, 206, 1425–35. doi: 10.4049/jimmunol.2001048. [DOI] [PubMed] [Google Scholar]
- 40. Salio M, Gasser O, Gonzalez-Lopez C, Martens A, Veerapen N, Gileadi U, et al. Activation of human mucosal-associated invariant T cells induces CD40L-dependent maturation of monocyte-derived and primary dendritic cells. J Immunol 2017, 199, 2631–8. doi: 10.4049/jimmunol.1700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meierovics AI, Cowley SC.. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med 2016, 213, 2793–809. doi: 10.1084/jem.20160637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toubal A, Kiaf B, Beaudoin L, Cagninacci L, Rhimi M, Fruchet B, et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun 2020, 11, 3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jakob J, Kröger A, Klawonn F, Bruder D, Jänsch L.. Translatome analyses by bio-orthogonal non-canonical amino acid labeling reveal that MR1-activated MAIT cells induce an M1 phenotype and antiviral programming in antigen-presenting monocytes. Front Immunol 2023, 14, 1091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T.. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol 2011, 23, 529–35. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- 45. Petley EV, Koay H-F, Henderson MA, Sek K, Todd KL, Keam SP, et al. MAIT cells regulate NK cell-mediated tumor immunity. Nat Commun 2021, 12, 4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang X, Peng Y, Liu L, Wang Y, Li M, Li W, et al. MAIT cells ameliorate liver fibrosis by enhancing the cytotoxicity of NK cells in cholestatic murine models. Liver Int 2022, 42, 2743–58. doi: 10.1111/liv.15445. [DOI] [PubMed] [Google Scholar]
- 47. Shimizu Y, Horigane-Konakai Y, Ishii Y, Sugimoto C, Wakao H.. Mucosal-associated invariant T cells repress group 2 innate lymphoid cells in Alternaria alternata-induced model of allergic airway inflammation. Front Immunol 2022, 13, 1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Provine NM, Amini A, Garner LC, Spencer AJ, Dold C, Hutchings C, et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science 2021, 371, 521–6. doi: 10.1126/science.aax8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rashu, R., et al. Targeting the MR1-MAIT cell axis improves vaccine efficacy and affords protection against viral pathogens. Preprint at 10.1101/2023.02.20.529311 (2023). [DOI] [PMC free article] [PubMed]
- 50. Bennett MS, Trivedi S, Iyer AS, Hale JS, Leung DT.. Human mucosal-associated invariant T (MAIT) cells possess capacity for B cell help. J Leukoc Biol 2017, 102, 1261–9. doi: 10.1189/jlb.4A0317-116R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jensen O, Trivedi S, Meier JD, Fairfax KC, Hale JS, Leung DT.. A subset of follicular helper-like MAIT cells can provide B cell help and support antibody production in the mucosa. Sci Immunol 2022, 7, eabe8931. doi: 10.1126/sciimmunol.abe8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rha MS, Han JW, Kim JH, Koh J-Y, Park HJ, Kim SI, et al. Human liver CD8+ MAIT cells exert TCR/MR1-independent innate-like cytotoxicity in response to IL-15. J Hepatol 2020, 73, 640–50. doi: 10.1016/j.jhep.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 53. Rudak PT, Choi J, Haeryfar SMM.. MAIT cell-mediated cytotoxicity: roles in host defense and therapeutic potentials in infectious diseases and cancer. J Leukoc Biol 2018, 104, 473–86. doi: 10.1002/JLB.4RI0118-023R. [DOI] [PubMed] [Google Scholar]
- 54. Boulouis C, Sia WR, Gulam MY, Teo JQM, Png YT, Phan TK, et al. Human MAIT cell cytolytic effector proteins synergize to overcome carbapenem resistance in Escherichia coli. PLoS Biol 2020, 18, e3000644. doi: 10.1371/journal.pbio.3000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lamichhane R, Schneider M, de la Harpe SM, Harrop TWR, Hannaway RF, Dearden PK, et al. TCR- or cytokine-activated CD8+ mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep 2019, 28, 3061–3076.e5. doi: 10.1016/j.celrep.2019.08.054. [DOI] [PubMed] [Google Scholar]
- 56. Hinks TSC, Marchi E, Jabeen M, Olshansky M, Kurioka A, Pediongco TJ, et al. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep 2019, 28, 3249–3262.e5. doi: 10.1016/j.celrep.2019.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. du Halgouet A, Darbois A, Alkobtawi M, Mestdagh M, Alphonse A, Premel V, et al. Role of MR1-driven signals and amphiregulin on the recruitment and repair function of MAIT cells during skin wound healing. Immunity 2023, 56, 78–92.e6. doi: 10.1016/j.immuni.2022.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Salou M, Franciszkiewicz K, Lantz O.. MAIT cells in infectious diseases. Curr Opin Immunol 2017, 48, 7–14. doi: 10.1016/j.coi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 59. Haeryfar SMM. MAIT cells in COVID-19: heroes, villains, or both? Crit Rev Immunol 2020, 40, 173–84. doi: 10.1615/CritRevImmunol.2020034943. [DOI] [PubMed] [Google Scholar]
- 60. Gargano F, Guerrera G, Piras E, Serafini B, Di Paola M, Rizzetto L, et al. Proinflammatory mucosal-associated invariant CD8+ T cells react to gut flora yeasts and infiltrate multiple sclerosis brain. Front Immunol 2022, 13, 890298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salou M, et al. Neuropathologic, phenotypic and functional analyses of mucosal associated invariant T cells in multiple sclerosis. Clin Immunol 2016, 166–167, 1–11. [DOI] [PubMed] [Google Scholar]
- 62. Chiba A, Tamura N, Yoshikiyo K, Murayama G, Kitagaichi M, Yamaji K, et al. Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res Ther 2017, 19, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haeryfar SMM, Shaler CR, Rudak PT.. Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe? Cancer Immunol Immunother 2018, 67, 1885–96. doi: 10.1007/s00262-018-2132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yao T, Shooshtari P, Haeryfar SMM.. Leveraging public single-cell and bulk transcriptomic datasets to delineate MAIT cell roles and phenotypic characteristics in human malignancies. Front Immunol 2020, 11, 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan J, Allen S, McDonald E, Das I, Mak JYW, Liu L, et al. MAIT cells promote tumor initiation, growth, and metastases via tumor MR1. Cancer Discov 2020, 10, 124–41. doi: 10.1158/2159-8290.CD-19-0569. [DOI] [PubMed] [Google Scholar]
- 66. Hegde P, Weiss E, Paradis V, Wan J, Mabire M, Sukriti S, et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat Commun 2018, 9, 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lamichhane R, Galvin H, Hannaway RF, de la Harpe SM, Munro F, Tyndall JDA, et al. Type I interferons are important co-stimulatory signals during T cell receptor mediated human MAIT cell activation. Eur J Immunol 2020, 50, 178–91. doi: 10.1002/eji.201948279. [DOI] [PubMed] [Google Scholar]
- 68. Chen Z, Wang H, D'Souza C, Sun S, Kostenko L, Eckle SBG, et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol 2017, 10, 58–68. doi: 10.1038/mi.2016.39. [DOI] [PubMed] [Google Scholar]
- 69. Liu J, Brutkiewicz RR.. The toll-like receptor 9 signalling pathway regulates MR1-mediated bacterial antigen presentation in B cells. Immunology 2017, 152, 232–42. doi: 10.1111/imm.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang H, Kjer-Nielsen L, Shi M, D'Souza C, Pediongco TJ, Cao H, et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci Immunol 2019, 4, eaaw0402. doi: 10.1126/sciimmunol.aaw0402. [DOI] [PubMed] [Google Scholar]
- 71. Wang H, Nelson AG, Wang B, Zhao Z, Lim XY, Shi M, et al. The balance of interleukin-12 and interleukin-23 determines the bias of MAIT1 versus MAIT17 responses during bacterial infection. Immunol Cell Biol 2022, 100, 547–61. doi: 10.1111/imcb.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science 1990, 248, 1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 73. Harty JT, Badovinac VP.. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 2008, 8, 107–19. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 74. Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A.. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 1999, 283, 680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 75. Siokis A, Robert PA, Demetriou P, Dustin ML, Meyer-Hermann M.. F-Actin-driven CD28-CD80 localization in the immune synapse. Cell Rep 2018, 24, 1151–62. doi: 10.1016/j.celrep.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 76. Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 1995, 3, 87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 77. Fraser JD, Irving BA, Crabtree GR, Weiss A.. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 1991, 251, 313–6. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 78. Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB.. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 1989, 244, 339–43. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 79. Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002, 16, 769–77. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 80. Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011, 117, 1250–9. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 81. Gutierrez-Arcelus M, Teslovich N, Mola AR, Polidoro RB, Nathan A, Kim H, et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun 2019, 10, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Slichter CK, McDavid A, Miller HW, Finak G, Seymour BJ, McNevin JP, et al. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight 2016, 1, e86292. doi: 10.1172/jci.insight.86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van den Heuvel MJ, Garg N, Van Kaer L, Haeryfar SMM.. NKT cell costimulation: experimental progress and therapeutic promise. Trends Mol Med 2011, 17, 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Murugesan A, et al. Functional MAIT cells are associated with reduced Simian-human immunodeficiency virus infection. Front Immunol 2019, 10, 3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ellis AL, Balgeman AJ, Larson EC, Rodgers MA, Ameel C, Baranowski T, et al. MAIT cells are functionally impaired in a Mauritian cynomolgus macaque model of SIV and Mtb co-infection. PLoS Pathog 2020, 16, e1008585. doi: 10.1371/journal.ppat.1008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gross JA, St John T, Allison JP.. The murine homologue of the T lymphocyte antigen CD28. Molecular cloning and cell surface expression. J Immunol 1990, 144, 3201–10. [PubMed] [Google Scholar]
- 87. Skartsis N, Muller YD, Ferreira LMR.. Regulatory T cell homeostasis: requisite signals and implications for clinical development of biologics. Clin Immunol 2023, 246, 109201. [DOI] [PubMed] [Google Scholar]
- 88. Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999, 397, 263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 89. Jeffery HC, van Wilgenburg B, Kurioka A, Parekh K, Stirling K, Roberts S, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol 2016, 64, 1118–27. doi: 10.1016/j.jhep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schubert K, Karkossa I, Schor J, Engelmann B, Steinheuer LM, Bruns T, et al. A multi-omics analysis of mucosal-associated-invariant T cells reveals key drivers of distinct modes of activation. Front Immunol 2021, 12, 616967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D.. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol 1992, 22, 2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 92. Vorkas CK, Krishna C, Li K, Aubé J, Fitzgerald DW, Mazutis L, et al. Single-cell transcriptional profiling reveals signatures of helper, effector, and regulatory MAIT cells during homeostasis and activation. J Immunol 2022, 208, 1042–56. doi: 10.4049/jimmunol.2100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang M, Ming S, Gong S, Liang S, Luo Y, Liang Z, et al. Activation-induced cell death of mucosal-associated invariant T cells is amplified by OX40 in type 2 diabetic patients. J Immunol 2019, 203, 2614–20. doi: 10.4049/jimmunol.1900367. [DOI] [PubMed] [Google Scholar]
- 94. Ming S, Zhang M, Liang Z, Li C, He J, Chen P, et al. OX40L/OX40 signal promotes IL-9 production by mucosal MAIT cells during Helicobacter pylori infection. Front Immunol 2021, 12, 626017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kober J, Leitner J, Klauser C, Woitek R, Majdic O, Stöckl J, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol 2008, 38, 2678–88. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sun G, Jin H, Zhang C, Meng H, Zhao X, Wei D, et al. OX40 regulates both innate and adaptive immunity and promotes nonalcoholic steatohepatitis. Cell Rep 2018, 25, 3786–3799.e4. doi: 10.1016/j.celrep.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 97. Jiang J, Liu C, Liu M, Shen Y, Hu X, Wang Q, et al. OX40 signaling is involved in the autoactivation of CD4+CD28− T cells and contributes to the pathogenesis of autoimmune arthritis. Arthritis Res Ther 2017, 19, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lamichhane R, Munro F, Harrop TWR, de la Harpe SM, Dearden PK, Vernall AJ, et al. Human liver-derived MAIT cells differ from blood MAIT cells in their metabolism and response to TCR-independent activation. Eur J Immunol 2021, 51, 879–92. doi: 10.1002/eji.202048830. [DOI] [PubMed] [Google Scholar]
- 99. Kwon BS, Weissman SM.. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA 1989, 86, 1963–7. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Lier RA, Borst J, Vroom TM, Klein H, Van Mourik P, Zeijlemaker WP, et al. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol 1987, 139, 1589–96. [PubMed] [Google Scholar]
- 101. [Dataset] 10x Genomics. 10k PBMCs from a Healthy Donor (v3 chemistry), Single Cell Gene Expression Dataset by Cell Ranger 3.0.0. (2018).
- 102. [Dataset] 10x Genomics. Fresh 68k PBMCs (Donor A), Single Cell Gene Expression Dataset by Cell Ranger 1.1.0. (2016).
- 103. [Dataset] 10x Genomics. 33k PBMCs from a Healthy Donor, Single Cell Gene Expression Dataset by Cell Ranger 1.1.0. (2016).
- 104. [Dataset] 10x Genomics. 8k PBMCs from a Healthy Donor, Single Cell Gene Expression Dataset by Cell Ranger 2.1.0. (2017).
- 105. [Dataset] Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, Henderson BEP, Luu NT, et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 2019, 575, 512–8. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Béziat V, Rapaport F, Hu J, Titeux M, Bonnet des Claustres M, Bourgey M, et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell 2021, 184, 3812–3828.e30. doi: 10.1016/j.cell.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Howson LJ, Awad W, von Borstel A, Lim HJ, McWilliam HEG, Sandoval-Romero ML, et al. Absence of mucosal-associated invariant T cells in a person with a homozygous point mutation in MR1. Sci Immunol 2020, 5, eabc9492. doi: 10.1126/sciimmunol.abc9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dias J, Hengst J, Parrot T, Leeansyah E, Lunemann S, Malone DFG, et al. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of MAIT cells. J Hepatol 2019, 71, 301–12. doi: 10.1016/j.jhep.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Duan M, Goswami S, Shi J-Y, Wu L-J, Wang X-Y, Ma J-Q, et al. Activated and exhausted MAIT cells foster disease progression and indicate poor outcome in hepatocellular carcinoma. Clin Cancer Res 2019, 25, 3304–16. doi: 10.1158/1078-0432.CCR-18-3040. [DOI] [PubMed] [Google Scholar]
- 111. Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017, 355, 1428–33. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–7. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dias J, Sobkowiak MJ, Sandberg JK, Leeansyah E.. Human MAIT-cell responses to Escherichia coli: activation, cytokine production, proliferation, and cytotoxicity. J Leukoc Biol 2016, 100, 233–40. doi: 10.1189/jlb.4TA0815-391RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Rep 2014, 9, 1075–88. doi: 10.1016/j.celrep.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gorentla BK, Wan C-K, Zhong X.. -P. Negative regulation of mTOR activation by diacylglycerol kinases. Blood 2011, 117, 4022–31. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hamilton KS, Phong B, Corey C, Cheng J, Gorentla B, Zhong X, et al. T cell receptor-dependent activation of mTOR signaling in T cells is mediated by Carma1 and MALT1, but not Bcl10. Sci Signal 2014, 7, ra55. doi: 10.1126/scisignal.2005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity 2016, 45, 540–54. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chang J, Burkett PR, Borges CM, Kuchroo VK, Turka LA, Chang C-H.. MyD88 is essential to sustain mTOR activation necessary to promote T helper 17 cell proliferation by linking IL-1 and IL-23 signaling. Proc Natl Acad Sci USA 2013, 110, 2270–5. doi: 10.1073/pnas.1206048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Chandra, S., et al. Transcriptomes and metabolism define mouse and human MAIT cell heterogeneity. 2021.12.20.473182 Preprint at doi: 10.1101/2021.12.20.473182 (2021). [DOI]
- 120. Pankhurst TE, et al. MAIT cells activate dendritic cells to promote TFH cell differentiation and induce humoral immunity. Cell Rep 2023, 42, 112310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Murayama G, Chiba A, Suzuki H, Nomura A, Mizuno T, Kuga T, et al. A critical role for mucosal-associated invariant T cells as regulators and therapeutic targets in systemic lupus erythematosus. Front Immunol 2019, 10, 2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Cayrol C, Girard J.. -P. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev 2018, 281, 154–68. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 123. Desai-Mehta A, Lu L, Ramsey-Goldman R, Datta SK.. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest 1996, 97, 2063–73. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kalunian KC, Davis JC, Merrill JT, Totoritis MC, Wofsy D; IDEC-131 Lupus Study Group. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002, 46, 3251–8. doi: 10.1002/art.10681. [DOI] [PubMed] [Google Scholar]
- 125. Chamberlain C, Colman PJ, Ranger AM, Burkly LC, Johnston GI, Otoul C, et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis 2017, 76, 1837–44. doi: 10.1136/annrheumdis-2017-211388. [DOI] [PubMed] [Google Scholar]
- 126. Rogers PR, Song J, Gramaglia I, Killeen N, Croft M.. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity 2001, 15, 445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 127. Lan P, Fan Y, Zhao Y, Lou X, Monsour HP, Zhang X, et al. TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury. J Clin Invest 2017, 127, 2222–34. doi: 10.1172/JCI91075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vorkas CK, Wipperman MF, Li K, Bean J, Bhattarai SK, Adamow M, et al. Mucosal-associated invariant and γδ T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight 2018, 3, e121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Croft M, So T, Duan W, Soroosh P.. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009, 229, 173–91. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Shamsdin SA, Alborzi A, Ghaderi A, Lankrani KB, Pouladfar GR.. Significance of TC9 and TH9 in Helicobacter pylori-induced gastritis. Helicobacter 2020, 25, e12672. doi: 10.1111/hel.12672. [DOI] [PubMed] [Google Scholar]
- 131. Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron A-S, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity 2009, 30, 289–99. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 132. Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 1997, 186, 47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Garner, L. C., Amini, A., FitzPatrick, M. E. B., Provine, N. M. & Klenerman, P.. Human MAIT cells show clonal diversity but transcriptional and functional homogeneity. 2022.02.26.482031 Preprint at 10.1101/2022.02.26.482031 (2022). [DOI]
- 134. Jiang J, Cao Z, Shan W, Liu H, Cheng X.. 4-1BB expression on MAIT cells is associated with enhanced IFN-γ production and depends on IL-2. Cell Immunol 2018, 328, 58–69. doi: 10.1016/j.cellimm.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 135. Fernández Do Porto DA, Jurado JO, Pasquinelli V, Alvarez IB, Aspera RH, Musella RM, et al. CD137 differentially regulates innate and adaptive immunity against Mycobacterium tuberculosis. Immunol Cell Biol 2012, 90, 449–56. doi: 10.1038/icb.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jacobs J, Deschoolmeester V, Zwaenepoel K, Rolfo C, Silence K, Rottey S, et al. CD70: An emerging target in cancer immunotherapy. Pharmacol Ther 2015, 155, 1–10. doi: 10.1016/j.pharmthera.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 137. Gherardin NA, Loh L, Admojo L, Davenport AJ, Richardson K, Rogers A, et al. Enumeration, functional responses and cytotoxic capacity of MAIT cells in newly diagnosed and relapsed multiple myeloma. Sci Rep 2018, 8, 4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Leeansyah E, Ganesh A, Quigley MF, Sönnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013, 121, 1124–35. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Harms RZ, Lorenzo KM, Corley KP, Cabrera MS, Sarvetnick NE.. Altered CD161 bright CD8+ mucosal associated invariant T (MAIT)-like cell dynamics and increased differentiation states among juvenile type 1 diabetics. PLoS One 2015, 10, e0117335. doi: 10.1371/journal.pone.0117335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gazali AM, Schroderus A-M, Näntö-Salonen K, Rintamäki R, Pihlajamäki J, Knip M, et al. Mucosal-associated invariant T cell alterations during the development of human type 1 diabetes. Diabetologia 2020, 63, 2396–409. doi: 10.1007/s00125-020-05257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li Y, Yang Y, Wang J, Cai P, Li M, Tang X, et al. Bacteroides ovatus-mediated CD27- MAIT cell activation is associated with obesity-related T2D progression. Cell Mol Immunol 2022, 19, 791–804. doi: 10.1038/s41423-022-00871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vogel I, Acolty V, Keler T, Goriely S, Leo O, Moser M.. Agonistic anti-CD27 antibody ameliorates EAE by suppressing IL-17 production. Eur J Immunol 2022, 52, 1620–9. doi: 10.1002/eji.202149698. [DOI] [PubMed] [Google Scholar]
- 143. Xiao Y, Peperzak V, Keller AM, Borst J.. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol 2008, 181, 1071–82. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 144. Coquet JM, Middendorp S, van der Horst G, Kind J, Veraar EAM, Xiao Y, et al. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity 2013, 38, 53–65. doi: 10.1016/j.immuni.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 145. Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 2009, 10, 427–36. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kim G-R, Choi J.. -M. Current understanding of cytotoxic T lymphocyte antigen-4 (CTLA-4) signaling in T-cell biology and disease therapy. Mol Cells 2022, 45, 513–21. doi: 10.14348/molcells.2022.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Shaler CR, Choi J, Rudak PT, Memarnejadian A, Szabo PA, Tun-Abraham ME, et al. MAIT cells launch a rapid, robust and distinct hyperinflammatory response to bacterial superantigens and quickly acquire an anergic phenotype that impedes their cognate antimicrobial function: Defining a novel mechanism of superantigen-induced immunopathology and immunosuppression. PLoS Biol 2017, 15, e2001930. doi: 10.1371/journal.pbio.2001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rudak PT, Gangireddy R, Choi J, Burhan AM, Summers KL, Jackson DN, et al. Stress-elicited glucocorticoid receptor signaling upregulates TIGIT in innate-like invariant T lymphocytes. Brain Behav Immun 2019, 80, 793–804. doi: 10.1016/j.bbi.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 149. Rudak PT, et al. Chronic stress physically spares but functionally impairs innate-like invariant T cells. Cell Rep 2021, 35, 108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 2005, 202, 1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci USA 2008, 105, 11287–92. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lee PT, Benlagha K, Teyton L, Bendelac A.. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med 2002, 195, 637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, et al. Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol 2002, 168, 3140–4. doi: 10.4049/jimmunol.168.7.3140. [DOI] [PubMed] [Google Scholar]
- 154. Gumperz JE, Miyake S, Yamamura T, Brenner MB.. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med 2002, 195, 625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, et al. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J Immunol 2008, 180, 5448–56. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gherardin NA, Souter MN, Koay H-F, Mangas KM, Seemann T, Stinear TP, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol 2018, 96, 507–25. doi: 10.1111/imcb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dias J, Boulouis C, Gorin J-B, van den Biggelaar RHGA, Lal KG, Gibbs A, et al. The CD4-CD8- MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc Natl Acad Sci USA 2018, 115, E11513–22. doi: 10.1073/pnas.1812273115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kelly J, Minoda Y, Meredith T, Cameron G, Philipp M-S, Pellicci DG, et al. Chronically stimulated human MAIT cells are unexpectedly potent IL-13 producers. Immunol Cell Biol 2019, 97, 689–99. doi: 10.1111/imcb.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Le Nours J, Gherardin NA, Ramarathinam SH, Awad W, Wiede F, Gully BS, et al. A class of γδ T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science 2019, 366, 1522–7. doi: 10.1126/science.aav3900. [DOI] [PubMed] [Google Scholar]
- 160. Rice MT, von Borstel A, Chevour P, Awad W, Howson LJ, Littler DR, et al. Recognition of the antigen-presenting molecule MR1 by a Vδ3+ γδ T cell receptor. Proc Natl Acad Sci USA 2021, 118, e2110288118. doi: 10.1073/pnas.2110288118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Meermeier EW, Laugel BF, Sewell AK, Corbett AJ, Rossjohn J, McCluskey J, et al. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat Commun 2016, 7, 12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Domínguez Conde C, Xu C, Jarvis LB, Rainbow DB, Wells SB, Gomes T, et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 2022, 376, eabl5197. doi: 10.1126/science.abl5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Guo T, Chamoto K, Hirano N.. Adoptive T cell therapy targeting CD1 and MR1. Front Immunol 2015, 6, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang C-R, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest 2005, 115, 2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sortino O, Richards E, Dias J, Leeansyah E, Sandberg JK, Sereti I.. IL-7 treatment supports CD8+ mucosa-associated invariant T-cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS 2018, 32, 825–8. doi: 10.1097/QAD.0000000000001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Parrot T, Healy K, Boulouis C, Sobkowiak MJ, Leeansyah E, Aleman S, et al. Expansion of donor-unrestricted MAIT cells with enhanced cytolytic function suitable for TCR redirection. JCI Insight 2021, 6, e140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Rudak PT, Yao T, Richardson CD, Haeryfar SMM.. Measles virus infects and programs MAIT cells for apoptosis. J Infect Dis 2021, 223, 667–72. doi: 10.1093/infdis/jiaa407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Haeryfar SMM. On invariant T cells and measles: a theory of ‘innate immune amnesia’. PLoS Pathog 2020, 16, e1009071. doi: 10.1371/journal.ppat.1009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Jalali S, Harpur CM, Piers AT, Auladell M, Perriman L, Li S, et al. A high-dimensional cytometry atlas of peripheral blood over the human life span. Immunol Cell Biol 2022, 100, 805–21. doi: 10.1111/imcb.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Choi J, Schmerk CL, Mele TS, Rudak PT, Wardell CM, Deng G, et al. Longitudinal analysis of mucosa-associated invariant T cells in sepsis reveals their early numerical decline with prognostic implications and a progressive loss of antimicrobial functions. Immunol Cell Biol 2023, 101, 249–61. doi: 10.1111/imcb.12619. [DOI] [PubMed] [Google Scholar]
- 171. Fergusson JR, Ussher JE, Kurioka A, Klenerman P, Walker LJ.. High MDR-1 expression by MAIT cells confers resistance to cytotoxic but not immunosuppressive MDR-1 substrates. Clin Exp Immunol 2018, 194, 180–91. doi: 10.1111/cei.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available peripheral blood mononuclear cell datasets were obtained from 10x Genomics (https://www.10xgenomics.com/resources/datasets), and transcriptomic data from healthy liver samples were generated using Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo) under the accession code GSE48452.