Summary
Innate lymphoid cells (ILCs) are a family of lymphocytes with essential roles in tissue homeostasis and immunity. Along with other tissue-resident immune populations, distinct subsets of ILCs have important roles in either promoting or inhibiting immune tolerance in a variety of contexts, including cancer and autoimmunity. In solid organ and hematopoietic stem cell transplantation, both donor and recipient-derived ILCs could contribute to immune tolerance or rejection, yet understanding of protective or pathogenic functions are only beginning to emerge. In addition to roles in directing or regulating immune responses, ILCs interface with parenchymal cells to support tissue homeostasis and even regeneration. Whether specific ILCs are tissue-protective or enhance ischemia reperfusion injury or fibrosis is of particular interest to the field of transplantation, beyond any roles in limiting or promoting allograft rejection or graft-versus host disease. Within this review, we discuss the current understanding of ILCs functions in promoting immune tolerance and tissue repair at homeostasis and in the context of transplantation and highlight where targeting or harnessing ILCs could have applications in novel transplant therapies.
Keywords: innate lymphoid cells, natural killer cells, immune tolerance, immune regulation, transplantation, graft-versus-host disease, hematopoietic stem cell transplant, rejection, ischemia reperfusion injury
In this review, Mak and Reid et al. explore protective versus pathogenic functions of innate lymphoid cell (ILCs) family members in transplantation. They further highlight where harnessing or targeting ILCs could be advantageous for transplantation, and discuss challenges limiting clinical translation
Graphical Abstract
Graphical Abstract.
Introduction
Innate lymphoid cells (ILCs) are a family of innate lymphocytes with important roles in tissue homeostasis. Through their rapid production of cytokines and capacity to interface with both immune and non-immune cells, ILCs modulate and direct immune responses involved in host defense, tissue repair, and immune tolerance [1–5]. Differentiated ILCs and ILC precursors in circulation can infiltrate into tissues and develop tissue-adapted functions, a process termed “tissue-poiesis.” [6, 7] Once ILCs take up residency within an organ, they can serve as tissue sentinels, modulating local immunity [8–11], and participating in a broad range of biological processes such as neuronal regulation, epithelial remodeling, adipogenesis, and circadian rhythms [12–15]. While early parabiotic mouse studies of ILCs supported tissue ILCs are “resident” and do not traffic between organs [16], recent studies have observed some ILCs can migrate between the intestines, lung, and liver [17–19], supporting that specific signals promote trafficking of a subset of ILCs to elsewhere in the body. However, features of ILCs that traffic to other locations and signals which direct this are only beginning to be elucidated.
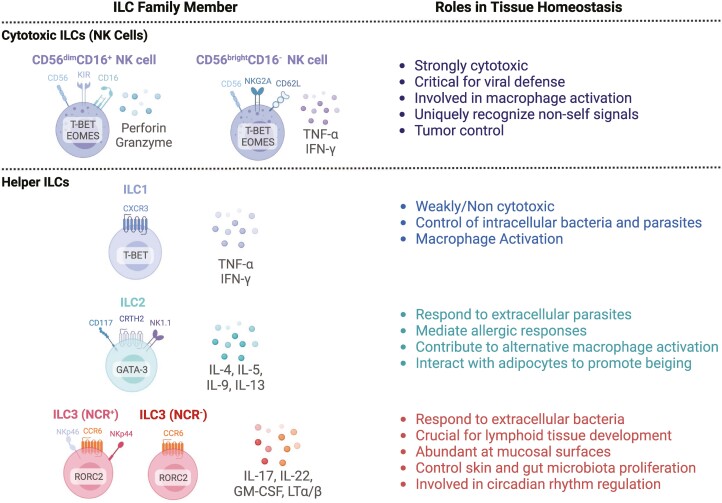
The ILC family includes both cytotoxic and non-cytotoxic or “helper” subsets(Figure 1). Natural killer(NK) cells have important roles in eliminating virally infected cells and tumor cells through their ability to discriminate between self and non-self. This is accomplished primarily via recognition of major histocompatibility complex (MHC) molecules; cells that lack self-MHC molecules due to viral or tumor cell downregulation, or cells of allogeneic origin, are eliminated by NK cells via cytotoxic mechanisms mediated by granzymes and perforin. NK cells also produce pro-inflammatory cytokines interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), which contribute to additional pro-inflammatory processes. In humans, NK cells are further classified as CD56dimCD16+NK cells, which exhibit potent cytotoxic potential, and CD56brightCD16−NK cells which produce high levels of NK cell-associated cytokines [20–23]. Helper ILCs are divided into groups based on cytokine and transcription factor expression. Group 1 ILCs (ILC1s) produce IFN-γ [24] and protect against viral infections, although ILC1s are generally described as lacking cytotoxic potential and do not express molecules such as killer cell immunoglobulin-like receptors (KIRs) important for distinguishing self and non-self, or the transcription factor Eomesodermin (EOMES). Group 2 ILCs (ILC2s) produce interleukin (IL)-4, IL-5, IL-13, and IL-9 to contribute to parasite and helminth immunity, and also maintain tissue homeostasis by interfacing with parenchymal cells to promote epithelial repair in tissues such as the intestine, lung, and skin(Fig. 2) [25–27]. Group 3 ILCs (ILC3s) produce IL-22, IL-17, and granulocyte-macrophage-colony-stimulating-factor(GM-CSF), and respond to extracellular bacteria. Similar to ILC2s, ILC3s are important for tissue homeostasis, possessing roles in skin repair and control of intestinal inflammation [28–32](Fig. 2).
Figure 1.
The innate lymphoid cell family. ILCs are divided by cytotoxic (top) and non-cytotoxic (bottom) subsets. Cytotoxic ILCs include natural killer (NK) cells, which are subdivided into two major subsets based on expression of CD56 and CD16. Helper ILCs include ILC1s, ILC2s, and ILC3s, with ILC3s being further divided based on those that express the natural cytotoxicity receptor (NCR). Human ILC defining surface markers, cytokines, and transcription factors indicated, along with their established roles in host defense and tissue homeostasis. Created with Biorender.com.
Figure 2.
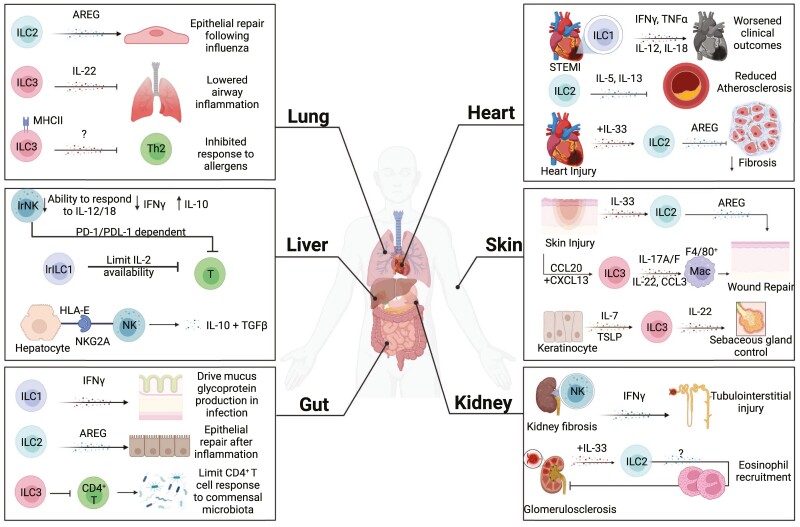
ILC functions in promoting or inhibiting tissue tolerance. ILCs can exhibit organ-adapted functions and can differ in having protective or pathogenic functions, depending on the tissue. (Lung): Within the lung, ILC2s are required to maintain airway epithelial integrity following influenza infection through production of amphiregulin (AREG). Lung ILC3s expressing MHC class II can also limit Th2 cell responses to allergen and ILC3-derived IL-22 reduces inflammation following allergen challenge. (Liver): Liver-resident NK cells (lrNK) are characterized by lower responsiveness to IL-12 and IL-18, reduced production of IFN-g, and the ability to produce IL-10. They can also inhibit T-cell responses via a PD-1/PDL-1 axis. ILC1s within the liver can also promote immune tolerance by limiting IL-2 availability, thereby inhibiting CD8+ T-cell responses. Circulating NK cells were also shown to produce IL-10 and TGF-b following NKG2A interactions with HLA-E on hepatocytes. (Gut): ILC1s are essential for restoring normal mucus glycoprotein production following Salmonella infection. In a murine model of intestinal damage, ILC2s limited inflammation and promoted repair in an amphiregulin (AREG)-dependent mechanism. ILC3s maintain immune homeostasis by limiting intestinal CD4+ T-cell responses to commensal bacteria. (Heart): In acute ST-segment elevation myocardial infarction (STEMI), ILC1s were increased in circulation, and their presence correlated with adverse clinical outcomes that was attributed to ILC1 production of IL-12, IL-18, TNF-a, and IFN-g. In a murine high fat diet model, ILC2s protected from atherosclerosis through IL-5 and IL-13. IL-33 expanded ILC2s were also capable of reducing cardiac fibrosis via amphiregulin (AREG) effects. (Skin): ILC2s are recruited to the site of skin injury via IL-33 and promote repair through amphiregulin (AREG). ILC3s can be recruited to the site of injury via CCL20 and CXCL13, produce IL-17, IL-22, and CCL3 to directly support wound repair and recruitment of reparative macrophages. ILC3s also adopt tissue residency in the skin by IL-7 and TSLP released by keratinocytes, and can then negatively regulate sebaceous gland to control the skin microbiome. (Kidney): NK cells are enriched in fibrotic kidneys and produce the majority of IFN-g, contributing to inflammation and tubulointerstitial injury. In a mouse model of glomerulosclerosis, IL-33 expanded ILC2s and recruited eosinophils to protect from glomerulosclerosis. Created with Biorender.com.
While ILCs have important roles in tissue homeostasis [1], aberrant ILC responses can promote tissue injury [33–39]. For example, ILC2s can recruit eosinophils that exacerbate lung and muscle injury [33–35], and through IL-22 and IL-17A, ILC3s can contribute to airway inflammation [36–38]. Tissue signals have key roles in directing these protective or pathogenic functions of ILCs. For example, IL-33 signaling is critical in facilitating ILC2 activation and production of amphiregulin to promote airway epithelia repair in mice [25], while IL-25 signaling can promote IL-5 production from mouse ILC2s, resulting in eosinophilia that contributes to lung inflammation [40, 41]. Thus ILC-tissue interactions are key for maintaining tissue homeostasis.
In addition to canonical ILCs, recent studies have identified populations of ILCs with immunosuppressive capabilities in contexts such as cancer, allergy, and autoimmunity [42–47]. These regulatory ILCs include IL-10 producing ILC2s, NK-like regulatory ILCs, and ID3+ regulatory ILCs [48]. Understanding, however, of their ontogeny and contexts in which they arise is not well characterized, however, most data currently supports they are canonical ILCs that acquire immunoregulatory functions in response to microenvironment triggers.
Despite wide-spread interest in ILCs, their role in solid organ transplantation and other allogeneic contexts is only beginning to be elucidated. Key questions include whether they contribute to tolerance or rejection, and how donor and infiltrating recipient-derived ILCs interface with other immune and non-immune cells to coordinate responses following transplant. Herein we discuss the known functions of ILCs in tissues and examine the current understanding of ILCs protective or pathogenic functions in allogeneic contexts. We also explore potential therapeutic applications of ILCs for transplantation and identify areas of research needed to evaluate this potential.
Challenges limiting success of transplantation
Transplantation is widely used to treat end-stage organ disease. During transplantation, the organ can suffer ischemia reperfusion injury(IRI). This involves hypoxic damage that occurs during organ retrieval, which can lead to cell death and release of pro-inflammatory cytokines [49]. Additional damage occurs upon restoration of oxygen to the organ, leading to the generation of reactive oxygen species(ROS) that promote expression of cellular adhesion molecules by endothelial cells. This combined with initial hypoxic damage leads to immune infiltration and inflammation [50]. Several immune populations participate in IRI; neutrophils exacerbate damage through the further production of ROS and pro-inflammatory cytokines TNF-α, IL-6, and IL-8 [51]. Damaged and necrotic cells release large amounts of danger-associated molecular patterns (DAMPs) and activate the complement cascades, both of which activate macrophages leading to tissue damage. T cells are also involved in IRI-induced pathology, as T-cell deficient mice experience reduced IRI [52, 53], with CD4+ T helper (Th) 1 cells having harmful functions and CD4+ Th2 cells having protective roles in IRI [54]. NK cells have also been linked to promoting IRI damage, while subsets of ILCs are suggested to have protective functions. Therefore, complex cellular circuits, involving both resident immune cells and infiltrating immune cells from the recipient can contribute to IRI.
Rejection of transplanted organs remains a major challenge for the success of transplantation. Alloantigens can be recognized through direct or indirect pathways; the direct pathway involves recognizing alloantigens presented on donor antigen presenting cells (APCs), while indirect recognition involves the presentation of alloantigens by recipient’s APCs. Both pathways can activate downstream immune mechanisms that lead to graft rejection [55]. Cellular rejection is highly dependent on T-cell responses, which can be directed against non-self MHC molecules or against alloantigens presented by APCs. In addition to cellular rejection, antibody-mediated rejection (ABMR)—or humoral rejection—is mediated by antibody responses against antigens found on the graft, particularly endothelial cells. ABMR leads to vascular damage through complement activation and subsequent macrophage attack, as well as NK cell killing through antibody-dependent cell-mediated cytotoxicity (ADCC). To prevent rejection, optimized human leukocyte antigen (HLA) matching and immunosuppressive regimens aim to limit harmful immune responses. However, specialized populations of immunosuppressive immune cells, such as regulatory T cells (Tregs) also serve to inhibit pathogenic immune cells. Regulatory T cells can suppress T cells, B cells, and APCs, through a variety of mechanisms including secretion of anti-inflammatory cytokines (i. e. IL-10 and TGF-β1), depletion of extracellular ATP through CD39, and sequestering of IL-2 necessary for effector T-cell proliferation [56]. The ability of Tregs to promote tolerance of allogeneic transplants has led to widespread interest in harnessing Tregs as a tolerogenic cell therapy [57–59].
In addition to solid organ transplant, allogeneic hematopoietic stem cell transplant (HSCT) is another common allogeneic therapy. HSCT is used to treat a wide range of immunological disorders and hematological malignancies. The success of HSCT is driven by the ability of donor-derived T cells and NK cells to attack leukemic cells, termed the graft-versus-leukemia effect (GVL). Despite the potential curative effect of alloHSCT, the success of this therapy is limited by the high proportion of individuals that develop graft-versus-host disease(GVHD), where donor T and NK cells attack host tissue [60]. The acute form of GVHD (aGVHD) generally affects the skin, gastrointestinal tract, and liver, while chronic GVHD (cGVHD) affects multiple organs [61]. Despite improvements in donor matching, cell sources, and immunosuppression, ~40% of patients receiving matched-related donor transplants develop aGVHD, with higher rates in other HSCT transplant types [62]. While inflammatory immune interactions between APCs and T cells drive aGVHD initiation [63], important tissue-protective and immunoregulatory roles have been reported for regulatory T cells, myeloid-derived suppressor cells (MDSCs) and ILCs.
Functions of ILCs in allogeneic hematopoietic stem cell transplantation
Several studies have linked NK cells to protection from GVHD, while also still maintaining the GVL response. Early findings demonstrated that a high infused dose of NK cells, rapid reconstitution of NK cells, or a high NK cell:CD8+ T-cell ratio all correlated with protection from GVHD development without impairing GVL responses [64–68]. In haplo-mismatched HSCT, recipients receiving KIR ligand incompatible grafts were completely protected from both leukemia relapse and aGVHD [69]. Mouse models have provided insights into the molecules underlying NK cells protective functions following HSCT. Using a MHC mismatched mouse model, pre-infusion with alloreactive donor NK cells completely prevented GVHD development and resulted in a marked reduction in CD11c+ APCs in the bone marrow, spleen, and gut (Fig. 3A) [69]. This ablation of APCs appears to require natural cytotoxicity receptor NKp46, as NKp46-deficient mice had more severe GVHD, as did mice given NK1.1 depleted grafts [70]. Supporting in vivo studies, NK cells from mice and HSCT recipients could limit T-cell IFN-γ production and directly lyse activated T cells in vitro in an NKG2D-dependent mechanism [71, 72] (Fig. 3A). Additional studies have indicated that reconstitution of specifically CD56bright NK cells reduced the incidence of GVHD [73–75]. Decreased proportions of CD56bright NK cells were observed in HSCT recipients with aGVHD compared to those without [73]. Additionally, when patients were divided into groups based on the number of circulating CD56bright NK cells, patients in the “high” group had an overall survival 2-years post-HSCT of 92.9% compared to only 66.7% and 42.9% in the “middle” and “low” groups, respectively [73].
Figure 3.
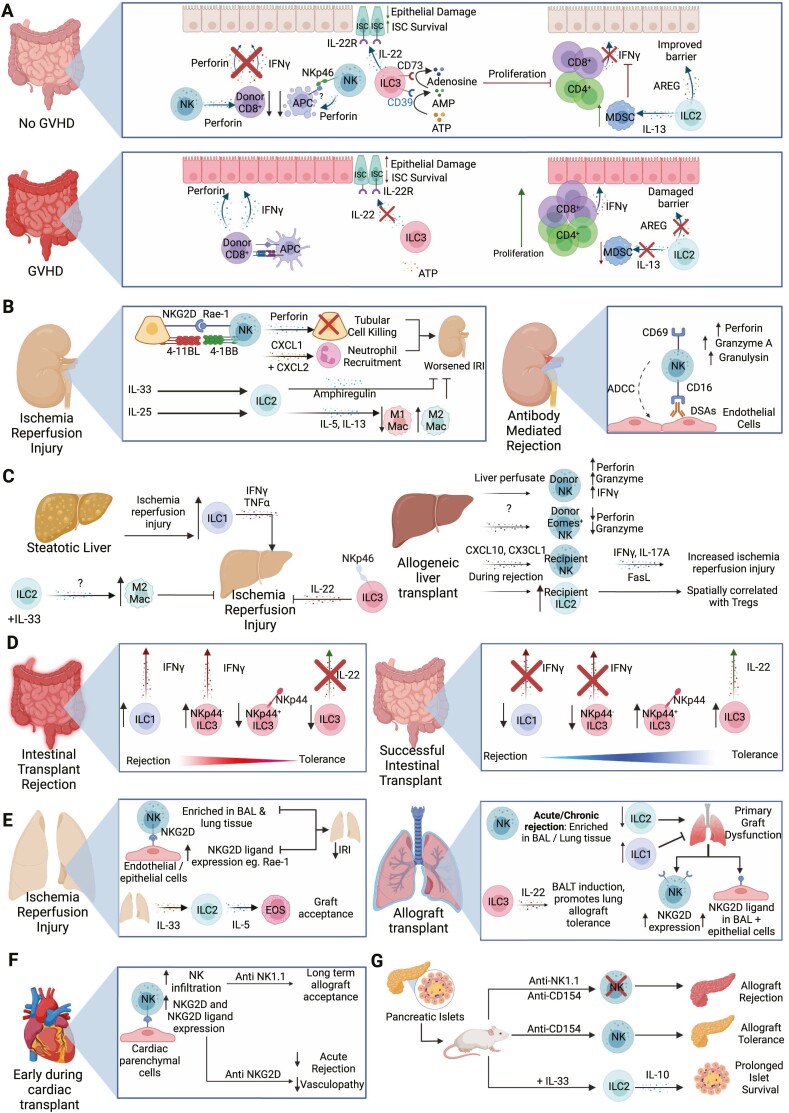
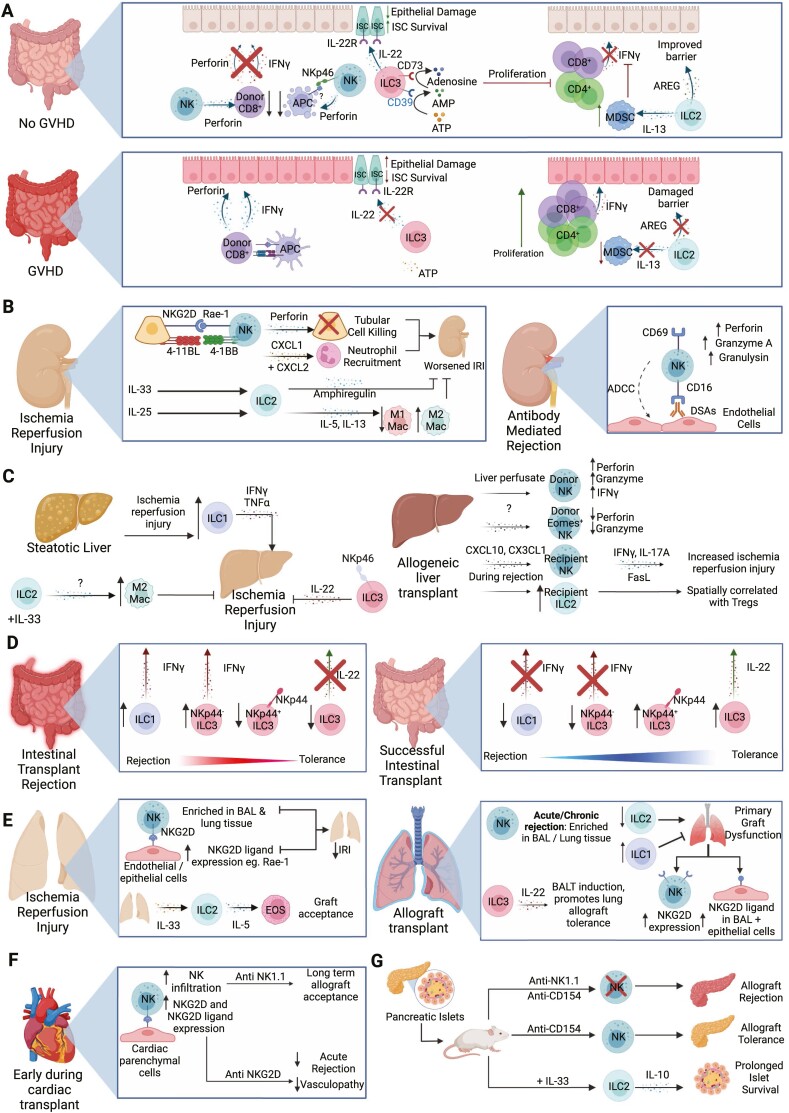
Overview of ILC functions in transplantation. (A) ILCs can help protect from development of GVHD in numerous ways. NK cells inhibit APC in the intestines, reducing activation of CD8+ T cells. NK cells also target alloreactive CD8+ T cells directly, preventing damage to intestinal epithelial cells. ILC3s produce IL-22 in the intestines which protects IL-22R+ intestinal stem cells from destruction. Additionally intestinal ILC3s express ecto-enzymes CD39 and CD73, which degrade inflammatory extracellular ATP into adenosine to suppress immune responses. In vitro expanded mouse ILC2s have dual roles; both improving intestinal barrier function through production of amphiregulin (AREG) and expanding local MDSCs via IL-13, which in turn limit T-cell accumulation and suppress IFN-γ responses. (B) During renal ischemia reperfusion injury (IRI), interactions between tubular epithelial cells (TECS) and NK cells exacerbate IRI. This effect is mediated by perforin and is induced via NKG2D/Rae-1 interactions. The interaction of 4-1BB on NK cells and 4-1BBL on TECs results in the production of CXCL1 and CXCL2 leading to neutrophil recruitment that worsens IRI. In contrast, transferring ILC2s or administrating IL-33 to promote ILC2 development attenuated IRI, an effect dependent on ILC2-derived amphiregulin. When administered IL-25, mouse ILC2s also promoted a Th2 response that induced alternatively activated macrophages which were tissue-protective in IRI. During antibody mediated rejection, NK cells can interact with donor specific antibodies to damage endothelial cells through antibody dependent cell-mediated cytotoxicity (ADCC), and express increased levels of perforin, granzyme A, and granulysin. (C) Using a mouse model of steatotic livers, ILC1s increase with IRI and have increased IFN-γ and TNF-α production that promote tissue injury. In a standard IRI model, administration of IL-33 increases ILC2s, leading to an enrichment of M2 macrophages that decrease damage associated with IRI. NKp46+ ILC3s can reduce hepatic IRI via IL-22. In liver transplant, liver perfusate NK cells exhibit higher expression of perforin, granzymes and IFNγ that may enable them to protect the graft by inhibiting allogeneic immune responses. Conversely, long lasting resident NK cells with high EOMES expression and lower cytotoxic potential are also observed, which may promote long term allograft acceptance as the presence of NK cells following liver transplant is associated with immune tolerance following withdrawal of immunosuppressants. However mouse models of liver transplant suggest increased infiltration of recipient NK cells induced by CXCL10 and CX3CL1 promotes liver IRI through elevated IFN-γ, IL-17A and FasL. ILC2s are increased during rejection and found in proximity to CD4+ regulatory T cells (Tregs), suggesting they are either recruiting or supporting Tregs. (D) In acute cellular rejection, accumulation of IFN-γ producing ILC1s and NKp44- ILC3s were observed, with a reciprocal decrease in IL-22 producing and NKp44+ ILC3s. In contrast, increased IL-22+ NKp44+ ILC3s is associated with graft tolerance. (E) NK cells are enriched within the broncho-alveolar lavage (BAL) and lung tissue during IRI. NKG2D expression by NK cells interacts with Rae-1 on endothelial and epithelial cells during IRI, and blocking the NKG2D receptor or depleting NK cells reduces IRI-induced pathology. IL-33 expression following IRI increases ILC2s within the graft. These ILC2s recruit eosinophils through IL-5 production and can promote allograft tolerance in mice. NK cells increases in the BAL or lung tissue during acute or chronic rejection respectively. The production of IL-22 by ILC3s led to the formation of bronchus associated lymphoid tissue (BALT) and promoted allograft tolerance. In primary graft dysfunction, NKG2D and NKG2D ligand Rae-1 expression is increased on NK cells and epithelial cells, respectively. The development of primary graft dysfunction is correlated with decreased ILC2s and increased ILC1 prior to transplant. (F) Early on in cardiac transplant, NK cells infiltrate the allograft, with increased NKG2D expression by NK cells and NKG2DL by cardiac parenchymal cells. Depletion of NK cells or blocking NKG2D in CD28-deficient mice led to increased cardiac graft survival and attenuated acute allograft rejection and allograft vasculopathy. (G) In a mouse model of islet transplant, treatment with IL-33 expands IL-10 producing ILC2s that can limit allograft rejection and adoptive transfer of IL-10-producing ILC2s blocked islet rejection. NK cells may also have protective functions, as anti-NK1.1 treatment resulted in rejection of transplanted islets in mice receiving anti-CD154 treatment. Created with Biorender.com.
Helper ILC reconstitution is important for establishment of tissue-specific adaptive immunity post-HSCT, whereas ILCs may serve redundant roles in systemic immunity, in line with mouse studies that observe a key role of ILC2 in regulating local but not systemic CD4 T-cell responses [8–11]. However, increased circulating donor-derived ILC2s and ILC3s in HSCT recipients corresponded with decreased aGVHD incidence [76–78]. Munneke et al. longitudinally sampled HSCT recipients during conditioning therapy and post-transplant, and reported circulating helper ILCs were diminished after chemotherapy, and ILC1s, ILC2s, and natural cytotoxicity receptor (NCR)− ILC3s did not recover 12 weeks post-transplant [76]. Phenotyping revealed ILCs in HSCT recipients protected from aGVHD had elevated expression of CD69 and CD69high ILCs had increased expression of gut homing marker α4β7, which correlated with reduced intestinal GVHD [76]. Separately, fewer circulating NKp44+, CD25+ or CCR6+ ILC precursors(ILCPs) were associated with cGVHD development, while fewer ILC2s and ILCPs with aGVHD [77]. Intriguingly, a high proportion of ILCs in granulocyte-colony-stimulating-factor (G-CSF) mobilized grafts correlated with better ILC recovery post-transplant and lower aGVHD incidence, indicating ILCs in HSC grafts may help restore the ILC compartment [78].
In murine studies, ILC3s were shown to protect gut tissues from GVHD pathology. Hanash et al. demonstrated IL-22 produced by recipient-derived intestinal ILC3s protected from development of GVHD in an MHC-mismatch model [79]. IL-22−/− mice had increased epithelial damage, more severe GVHD and lost IL-22R+ intestinal stem cells, supporting that ILC3-derived IL-22 protects intestinal stem cells and decreases intestinal damage(Fig. 3A) [79]. Separately, intestinal CD39+CD73+ILC3s were decreased in GVHD patients compared to non-inflamed tissue from individuals with IBD, and serum adenosine was lower in GVHD patients relative to healthy controls [80]. To support a direct role for ILC3 conversion of ATP into immunosuppressive adenosine underlying these patient observations, human CD39+CD73+ILC3s from healthy donor tonsils suppressed T-cell proliferation in vitro in an adenosine-dependent manner(Fig. 3A) [80].
Emerging evidence supports potential therapeutic applications of ILCs in HSCT. Infusion of in vitro expanded donor or third-party murine ILC2s improved mouse survival, and reduced symptoms of GVHD [81, 82]. Infused ILC2s trafficked to the gastrointestinal tract, where a reduction in donor IFN-γ+ CD4+ and CD8+ T cells was observed, as well as an increase in donor myeloid derived suppressor cells (MDSCs) [82]. When MDSCs were ablated using anti-GR-1 or IL-13−/− ILC2s were transferred that were unable to recruit MDSCs, the benefits of ILC2 infusion were ameliorated [82]. Interestingly, ILC2-derived amphiregulin improved intestinal barrier function, supporting ILC2s may act on both immune and non-immune cells to protect following HSCT(Fig. 3A). These intriguing findings should be further investigated in human studies, as well as contrasted with other ILCs to delineate unique contributions ILC subsets to protection following HSCT.
ILCs in kidney transplant
End stage kidney disease affects over 840 million individuals globally [83, 84], with kidney transplantation being the optimal treatment. IRI and ABMR remain major challenges in kidney transplantation efficacy. While ILCs have essential functions in CMV infection and tissue homeostasis [85–90], several studies have reported circulating recipient NK cells infiltrate the kidney and exacerbate renal IRI [91–94]. For example, depletion of NK cells using anti-asialo GM1 or anti-NK1.1 antibodies ameliorated IRI in mouse models [91, 92](Fig. 3B) and expression of 4-1BB on murine NK cells and 4-1BBL on tubular epithelial cells (TECs) was reported to promote TEC CXCL1 and CXCL2 production, resulting in neutrophil recruitment that exacerbated IRI [93]. In vitro, NKG2D-Rae-1 interactions direct NK cells to lyse renal TECs in a perforin-dependent manner. As TECs upregulated Rae-1 in vivo following IRI, this provides a possible mechanism by which NK cells contribute to IRI [91] (Fig.3B).
NK cells have also been linked to ABMR [95–97]. In mouse studies, donor-specific antibodies (DSAs) triggered activated NK cells to mediate acute rejection [98]. This was attributed to alloreactive NK cell activation from lack of self-recognition, resulting in NK cells interacting with DSAs to target endothelial cells via ADCC [99]. NK cells isolated from ABMR patients upregulated CD69 expression, and had elevated expression of perforin, granzyme A, and granulysin [100] (Fig. 3B). Of note, mismatched KIRs and KIR ligands are associated with a 25% reduction in 10-year graft survival [101] and NK cells may be resistant to conventional immunosuppression such as cyclosporin A, methylprednisolone, hydrocortisone, and azathioprine [102], highlighting that targeting NK cells in kidney transplant may be important to improve clinical outcomes.
In contrast to NK cells, recent studies indicate potential protective roles for ILC2s in kidney transplantation. Utilizing a mouse renal IRI model, Cao et al. reported that transferring ILC2s prior to or following IRI minimized tubular injury and serum creatinine, an effect dependent on amphiregulin and similar to that observed with IL-33 administration [103](Fig. 3B). Separate studies showed that IL-25 alone or in combination with IL-2 and IL-33 co-administration improved IRI outcomes via ILC2-induced M2 macrophage activity(Fig. 3B), which was abrogated with depletion of ILC2s via anti-CD90 antibodies [104, 105]. In contrast, Cameron et al. reported no differences in IRI severity when multiple approaches to deplete or limit ILC2s were used [106]. The authors suggested this may be due to other immune populations being able to compensate for the loss of ILC2s. Although differences in the mouse models may underlie these conflicting findings, a key question is whether functions of ILC2s and other helper ILCs in allogeneic contexts remain unchanged. Studies are therefore needed to evaluate whether helper ILC populations contribute to or protect from allograft rejection or allogeneic IRI.
ILCs in liver transplant
The liver possesses a complex array of liver-resident immune cells that exhibit unique functions. This includes populations of cytotoxic and non-cytotoxic ILCs that are involved in promoting homeostasis and immune tolerance [107–109]. Liver-resident NK cells comprise 30–50% of hepatic lymphocytes [110, 111] and in humans, include conventional NK cells and CD56bright NK cells that express CCR5, CXCR6, CD69, and EOMES. Recent studies have further identified CD7, KLRD1(CD94), and NCR1(NKp46) as additional defining features of liver-resident NK cells [112, 113], which are localized to hepatic sinusoids [114, 115].
Liver-resident NK cells are well-described for their ability to promote immune tolerance, and can directly inhibit hepatic T cells [116]. In a murine lymphocytic choriomeningitis virus model, transfer of liver-resident NK cells decreased hepatic T-cell activity and impaired viral clearance, in contrast to conventional NK cells that promoted T-cell activity and viral clearance [116]. This inhibition of T-cell function was dependent on PD-1 and PD-L1 on T cells and liver-resident NK cells, respectively(Fig. 2) [116]. Liver NK cells are also characterized by decreased inflammatory potential in both mice and humans, possessing reduced ability to produce IFN-γ and respond to IL-12 or IL-18 stimulation, while also being a source of IL-10 [117, 118]. Interestingly in vitro studies by Jinushi et al. demonstrated that circulating NK cells can be induced to produce immunosuppressive cytokines IL-10 and TGF-β when co-cultured with hepatocytes via NKG2A receptor engagement with HLA-E on hepatocytes [119] (Fig. 2).
Depending on the context, liver resident NK cells were reported to promote or inhibit liver regeneration. In studies using immunodeficient mice receiving partial hepatectomy, optimal liver regeneration required NK cells, and blocking NKG2D reduced hepatocyte proliferation [120]. In contrast to these findings, depleting NK cells in C57BL/6J mice using anti-asialo- GM1-antibody enhanced liver regeneration [121]. These differing findings could relate to the degree of NK cell activation, as following liver injury, NK cells in Tigit−-/− mice had increased activation and IFN-γ production which impaired liver regeneration [122]. As new data emerge on the distinct subsets of liver NK cells [113, 123, 124], it will be important to delineate the role of individual NK cell populations in liver regeneration, as these differing reports may be due to distinct NK cell subpopulations being activated depending on the model.
Liver resident NK cell functions have also been explored in the context of transplantation. Analysis of human liver perfusate NK cells before transplant reported that CD56bright NK cells have high perforin and granzyme expression compared to circulating NK cells, express CD69 and IFN-γ, and exhibit enhanced cytotoxic potential [125]. In vitro studies where liver-resident CD56bright NK cells could kill allogeneic CD8+ T cells [126], supports liver NK cells may protect the graft during the acute phase post-transplant by inhibiting allogeneic adaptive immune responses. Notably, a lower percentage of NK cells in the liver perfusate prior to transplant is observed in recipients with acute cellular rejection(ACR) compared to those that did not [127].
While increased proportions of circulating NK cells following liver transplant are correlated with improved operational tolerance following immunosuppression withdrawal [128], these effects may also extend to liver-resident NK cells. For example, donor NK cells can persist within the liver allograft for at least 13 years following transplant and exhibit decreased levels of perforin and granzymes [129] and hypofunctionality [130] (Fig. 3C). Furthermore, introducing donor-derived liver NK cells into rats prolonged allograft survival and reduced histological damage, collectively supporting donor-derived NK cells may possess a role in limiting host versus graft response and promoting long-term allograft acceptance [131]. The same is not necessarily true for recipient-derived infiltrating NK cells. Using a model of rat orthotopic liver transplant, Obara et al. demonstrated early following transplant the liver allograft upregulates CXCL10 and CX3CL1 to recruit recipient circulating recipient NK cells with increased IFN-γ compared to donor NK cells [132] (Fig. 3C). Depletion of NK cells via an anti-asialo GM1 antibody prolonged allograft survival, indicating that infiltrating NK cells acquire a pro-inflammatory signature that contributes to rejection [132]. Infiltrating recipient NK cells were separately shown to promote liver IRI through IFN-γ, IL-17A, and FasL, which was reversed when depleting NK cells using anti-NK1.1 or anti-asialo GM1 antibodies [132–134] (Fig. 3C). Taken together, these murine studies support recipient NK cells are detrimental, whereas donor-derived liver NK cells are protective, highlighting a key difference between NK cells from the donor or recipient.
ILC1s represent the most abundant helper ILC population in the human liver [109], and localize to the perivascular spaces near DCs where they produce IFN-γ downstream of DC-derived IL-12 in viral infection [135, 136]. Similar to NK cells, ILC1s can also control T-cell activity. For example, in a murine hepatitis B virus model, ILC1s limited HBV-specific CD8+ T cell proliferation by controlling IL-2 availability [137] (Figure 2). Beyond regulating T-cell responses, adoptive transfer of ILC1s into immunodeficient mice protected from CCL4-mediated liver injury in a mechanism whereby ILC1-derived IFN-γ increased Bcl-xL expression by hepatocytes [138]. In contrast to these findings, ILC1s worsened IRI-induced tissue damage in a high-fat diet model of steatotic liver disease, where induced IRI resulted in elevated ILC1 numbers that had increased IFN-γ and TNF-α production [139] (Fig. 3C), and IRI studies using immunodeficient and ILC1 knockout mice resulted in decreased IRI-induced pathology [139]. Further studies are needed to clarify ILC1s functions in IRI, particularly in humans, as well as examine if these functions are maintained in allogeneic contexts.
While allogeneic studies on ILC1s are lacking, hepatic ILC2 was reported to significantly increase with rejection in a model of orthotopic liver transplant. ILC2s present within the allograft were of recipient origin and were spatially found in close proximity with liver Tregs, suggesting ILC2s may be attempting to inhibit allograft rejection or respond to damage [140]. In a murine IRI model, IL-33 treatment activated ILC2s that in turn increased M2 macrophage liver accumulation, leading to decreased hepatic damage [141] (Fig. 3C), suggesting possible protective roles ILC2s in liver transplant. However, ILC2s have also been linked to liver fibrosis. Chronic hepatocyte stress upregulated IL-33 that activated mouse ILC2s which in turn produced IL-13 that drove fibrosis [142], indicating that ILC2s protective effects may be context specific.
Current studies support the protective functions of ILC3s in the liver. ILC3s signature cytokine, IL-22, was shown across multiple studies to promote liver regeneration [109, 143, 144]. Using a mouse model of hepatic IRI, mice deficient in NKp46+ IL-22+ cells that resemble ILC3s experienced severe IRI, a phenotype that was reversed with adoptive transfer of these NKp46+ cells [144] (Fig. 3). Furthermore, in a murine hepatitis model, RORγt-deficient mice that lack IL-22+ ILC3s experienced more severe hepatitis [145]. Although the role of ILC3s in allogeneic contexts has yet to be determined, these studies collectively support ILC3s functions in promoting liver regeneration which may have important implications for transplantation.
ILCs in intestinal transplant
Intestinal failure is a life-threatening condition primarily caused by intestinal resections performed to treat inflammatory bowel disease, cancer, or intestinal dysmotility [146]. Intestinal transplants have been increasingly used due to severe catheter-related infections and risk of patients developing metabolic complications [146]. While the success of intestinal transplants has improved, only 50% of grafts remain functional at 5-years [147], due to a wide variety of complications including sepsis, ACR, ABMR, posttransplant lymphoproliferative disorder, IRI, and GVHD [148].
Many complications stem from lymphoid cells present within transplanted tissue, as well as the complex immune network in the gut-associated lymphoid tissue [149]. This includes DCs which stimulate anti-graft lymphocytes, B cells that produce DSAs causing ABMR, and cytotoxic T and NK cells driving ACR. Shortly after transplant, a substantial increase in recipient ILCs in the epithelium and lamina propria is observed in recipients compared to healthy controls [150, 151], which is maintained up to a decade after transplant, gradually skewing towards recipient-derived ILCs [151, 152]. Allograft-resident ILCs are primarily NK cells, ILC1s, and ILC3s, with elevated IFN-γ+ and IL-22+ CD3− intraepithelial lymphocytes, and an increased cytotoxic profile compared to healthy individuals [150]. Despite the cytotoxic profile, none of the grafts in this study failed and patients did not experience infections, suggesting this profile is consistent with successful transplants [150]. Indeed, other studies noted elevated helper ILCs in patients with healthy grafts and those with rejection [151, 152]. Interestingly, Gomez-Massa et al. biopsied a single patient weekly to evaluate ILC replacement kinetics and observed that once the patient progressed to ACR, there was an expansion of ILC1s and a decrease of ILC3s [151]. This could indicate that while an elevation of ILC1s and ILC3s compared to native intestine is beneficial for graft success, a shift in ILC1-ILC3 balance or changes in ILC subpopulations may lead to rejection (Fig. 3D). Separately, patients who developed ACR were reported to have similar proportions of ILC1s, ILC2s, and total ILC3s compared to patients without rejection and healthy controls, however, a decrease in specifically NCR+ILC3s and a reciprocal increase in NCR−ILC3s was observed [153]. Further supporting ILC3s’ importance for intestinal transplant tolerance, when Kang et al. compared ILCs between stable and rejecting allografts, a stark decrease in NCR+ILC3s and an increase in ILC1s occurred in rejecting allografts [154]. Collectively, these data show that a healthy ILC compartment is important for prevention of graft rejection, with NCR+ILC3s being associated with protection.
ILCs in lung transplant
Within the lungs, all ILC subsets are present and have important functions in host defense; NK cells make up 10% of total lymphocytes and are predominantly CD56dimCD16+. Lung NK cells display hyporesponsiveness to stimulation and express more KIRs than circulating NK cells [155]. Other ILCs are also present, with ILC2s and ILC3s comprising the majority of non-cytotoxic ILCs in the lung [156].
During lung transplants, NK cells have been linked to lung allograft failure. NK cell numbers were significantly elevated in bronchoalveolar lavage (BAL) or lung biopsy samples collected from recipients with acute or chronic lung allograft rejection, respectively [157, 158]. In a separate study, an increase of NK cells in areas of lung IRI was noted, which could also be detected in the BAL [159] (Fig. 3E). These human findings were supported by mouse studies, where NK cell-deficient mice had reduced IRI-induced pathology and adoptive transfer of NK cells worsened IRI [159]. NKG2D receptor ligands are present on lung endothelial and epithelial cells following IRI, and blocking the NKG2D receptor similarly reduced IRI, supporting a role for NKG2D in controlling NK cell responses to the lung graft [159] (Fig. 3E). In a murine tracheal transplant model, depletion of NK cells or inhibition of NKG2D attenuated bronchiolitis obliterans in recipients [160], a finding supported by studies of patients with primary graft dysfunction, where NKG2D ligands were enriched in airway epithelial cells and NKG2D was upregulated on NK cells [161, 162]. However, NK cells have also been shown to promote lung transplant tolerance. In a murine model of lung transplant, recipient NK cells expanded with IL-15/IL-15-Ra complex killed allogeneic APCs and protected from rejection, [163] and in humans, HLA-mismatched NK cells correlated with improved outcomes of lung transplantation [164]. These contrasting reports may reflect differences in donor and recipient NK cells, specific NK cell populations, as well as unique donor–recipient interactions. As NK cells are a highly heterogeneous population, additional studies mapping specific subsets of NK cells and their source are needed.
Although helper ILCs functions in lung infection and inflammation have been described (Fig. 2), there are comparatively fewer studies assessing ILCs role in lung allograft tolerance [25, 165, 166]. Monticelli et al. reported the development of primary graft dysfunction is associated with a marked reduction in ILC2s following reperfusion, and patients that did not develop primary graft dysfunction had higher ILC1 frequencies prior to allograft reperfusion [167] (Fig. 3E). In a murine lung transplant model, ILC2s facilitated lung allograft acceptance through eosinophil recruitment via IL-5 production [168] (Fig. 3E). This is intriguing, as ILC2-mediated eosinophilia has been associated with lung inflammation in asthma and other lung pathologies [169–173], yet eosinophilia in models of murine lung transplant has been shown to promote immune tolerance, suggesting that the effects of ILC2 mediated eosinophilia may be context dependent [174]. While not studied in allogeneic context, Seehus et al. report IL-10-producing ILC2s can serve as “tissue sentinels” able to rapidly produce IL-10 to limit immune responses [175]. Whether IL-10-producing ILC2s are protective in lung transplantation is not known, and further investigation is needed to determine if specific ILC2 cytokines support transplant tolerance, or if distinct ILC2 populations are protective.
In the human lung, ~60 % of helper ILCs are ILC3s, with NCR− ILC3s being the predominant ILC population [156]. While studies of ILC3s in human lung transplantation are lacking, murine transplant models support that ILC3s within lung allografts are crucial producers of IL-22 that promotes allograft tolerance [176] (Fig. 3E). Interestingly, lung ILC3s may also directly regulate T-cell responses within the lung. Mouse ILC3s that express MHC class II inhibited CD4+ T-cell responses to allergen, thereby limiting airway inflammation [166] (Fig. 2). Whether ILC3s could regulate allogeneic T-cell responses within the lung remains to be examined, but the possibility of lung ILC3s exhibiting antigen-presenting capabilities warrants investigation into whether this may occur in the transplant setting.
ILCs in cardiac transplant
Studies of ILCs in the human heart are rare, due in part to the complexity and scarcity of cardiac transplant samples. However, cardiac ILC functions were explored at the steady state in mouse models [177–179] (Fig. 2), where NK cells represent 3% of immune cells, ILC2s 1.7%, and ILC1s represent 0.2%, with no ILC3s being detected [180]. Parabiosis and adoptive transfer experiments suggest the proportion of heart ILCs remains stable during homeostatic and inflammatory conditions, implying that circulating ILC progenitors do not infiltrate and alter ILC proportions [180, 181]. Studies in humans corroborate these findings and indicate that a large proportion of non-cytotoxic ILCs are committed cardiac ILC2 precursors. Following ischemic cardiomyopathy or myocarditis, this ILC2 precursor population decreases, and levels of differentiated ILC2s increase, suggesting a mechanism whereby ILC2 precursors are locally activated and differentiate in response to tissue injury [181].
NK cells are the most well-characterized ILC population within cardiac transplantation and are reported to contribute to allograft rejection. Early following transplant, murine NK cells comprise the predominant population of infiltrating lymphocytes, and NKG2D ligands are upregulated in cardiac tissue [182, 183] (Fig. 3F). Early studies involving CD28-deficient mice show that while these mice reject cardiac allografts in a CD8-T-cell mediated mechanism, depletion of NK cells using an anti-NK1.1 antibody prolongs graft survival and established long-term allograft acceptance [184] (Fig. 3F). Blocking the NKG2D receptor was also able to abrogate acute allograft rejection [185], an observation that was replicated in the context of antibody-dependent rejection [186] (Fig. 3F). In addition, separate studies demonstrated NK cells contribute to cardiac allograft vasculopathy through CD16-dependent activation and IFN-γ responses [187, 188]. While human studies are needed, there is consensus from mouse studies to date that NK cells are harmful in cardiac transplants.
ILCs in pancreas and islet transplant
Beta-islet cell (β-cell) replacement therapy is used to treat patients with type 1 diabetes who cannot be stably treated with external insulin [189]. Replacement therapy involves either a whole pancreas transplant requiring major surgery, or the less invasive islet transplantation which can be done laparoscopically [190]. While these are life-changing therapies, both approaches do not resolve underlying immunopathology and recipients require life-long immunosuppression to prevent graft loss [191].
Beilke et al. reported a mouse NK1.1+ILC population-induced islet allograft tolerance following anti-CD154 antibody treatment [192]. Perforin−/− mice were unable to induce tolerance, suggesting this NK1.1+ILC was an NK cell, and supporting NK cell-mediated tolerance involved a cytotoxic mechanism (Fig. 3G). Similarly, induction of islet tolerance using anti-CD45RB and anti-Tim-1 antibodies required both regulatory B cells and NK1.1+ cells for allograft tolerance, and tolerance could be restored with wild-type NK cell infusion [193].
In models of pancreatic stress, ILC2s may have protective capabilities or improve islet function. In mice treated with streptozotocin to induce β-cell destruction, increased IL33 expression was observed in pancreatic mesenchymal cells which activated ILC2s [194]. ILC2s in turn produced IL-13 and GM-CSF, inducing myeloid cells to secrete retinoic acid that increased β-cell insulin secretion [194]. In a murine islet transplant model, IL-33 injection protected from allograft rejection via expansion of IL-10 secreting ILC2s, and adoptive transfer of mouse ILC2s inhibited allograft rejection [195] (Fig. 3G). While promising, additional studies contrasting ILC2s with other ILCs are needed, as are human studies to understand if adoptive transfer of ILCs could limit rejection of transplanted islets.
Harnessing or targeting ILCs to promote transplant tolerance
ILC family members have important roles in tissue homeostasis and immune tolerance. To date, therapeutics that specifically target or harness ILCs have not been used clinically, however, a growing body of evidence supports targeting or harnessing ILCs populations may have applications for transplantation. This includes the possibility of harnessing ILC2 or ILC3s dual reparative and tolerance-promoting functions. Several potential methods of manipulating ILCs have been suggested, including using cytokine and cytokine receptor agonists, targeting receptors expressed by specific subsets using monoclonal antibodies, and even using ILCs in adoptive cell therapies.
Studies of cytokine-based approaches include those where IL-33 injection results in a short-term expansion of ILC2s that protect from islet graft rejection and acute colitis [195, 196]. Expansion of IL-10-producing ILC2s was enhanced with IL-33 co-administration with IL-2/anti-IL-2 receptor antibody complex and prevented rejection in an islet transplant model or decreased renal IRI [43, 195]. Similarly, IL-1β, IL-23, and retinoic acid promoted ILC3 differentiation from ILC1s, which could potentially correct aberrant ILC1 to ILC3 ratios [151, 197]. Similar approaches have been used to expand or activate NK cells, CD8+ T cells, and Th2 cells in a variety of contexts [163, 198], although identifying cytokines that selectively expand protective ILC populations without acting on other immune populations remains challenging.
Adoptive cell transfer of expanded ILCs with immunoregulatory potential has been explored in multiple transplant contexts. Huang et al. transferred in vitro expanded IL-10-producing murine ILC2s into mice shortly before and after islet transplant, which could migrate to the allograft and limit rejection [195]. Similar results were seen in murine GVHD, where infused ILC2s localized in the intestines and indirectly suppressed GVHD through IL-13-mediated MDSC expansion [82]. Additionally, adoptive transfer of mouse ILC2s limited renal IRI in an amphiregulin-dependent manner, supporting ILC2’s dual reparative role could be harnessed for transplantation [103]. Whether ILC3s might have similar beneficial effects in adoptive cell therapies for transplant has not been explored to date, but has been examined in autologous contexts. For example, in a model of experimental type 2 diabetes during Mycobacterium tuberculosis infection, adoptive transfer of IL-22+ILC3s or LTi cells improved mouse survival [199], and adoptive transfer of NKp46+IL-22 producing ILCs minimized liver IRI [144].
Challenges and limitations of ILC research and clinical applications
Limiting translation of adoptive ILC-based therapies to humans is the relatively small numbers of ILCs in peripheral blood, which is typically the source of immune cells for cell therapy. Helper ILCs generally comprise 0.1% of circulating lymphocytes and 0.5% of lymphocytes in cord blood [8]. While several groups have developed protocols for the generation of ILCs from CD34+ cord blood or ILC precursor populations, these expansions result in a mixed ILC population [7, 200]. Separate studies have expanded human ILC3s (referred to as NK22 cells in the study) and ILC2s in vitro. While achieving robust fold expansions, these methods have not been able to obtain sufficient numbers for clinical translation [201, 202].
While NK cells have been widely associated with protection from GVHD following HSCT, and protective in mouse models of islet transplant, they are associated with IRI-induced pathology and rejection in kidney, liver, and cardiac transplant. These varying results across transplant types might be explained by organ-adapted functions of ILCs, and suggest protective or harmful ILC populations could differ between tissues. Human studies examining differences in ILC functions within organ allografts of those with and without rejection are needed to define local ILC responses, not just tracking the presence or absence of ILC family members. Further complicating this, is the diversity of circulating and resident ILC populations between individuals. Even within circulating CD56dim and CD56bright NK cells, there are large variations in phenotypes [203], with overlapping markers of other ILC family members [20], thereby complicating interpretations of early correlative studies of clinical outcomes with NK cell phenotypes. This in turn creates challenges for development of therapeutics targeting NK cells, as both protective and harmful subpopulations of NK cells may be present. New approaches such as single-cell RNA sequencing (scRNAseq)-based technologies are being applied across organs to assess responses that underly rejection and those which occur in healthy individuals. This will allow for better delineation of phenotypic and functional heterogeneity of NK cells and all ILCs in transplantation, which will be important to resolve NK cell populations that drive harmful responses or improve graft outcomes.
Importantly, current immunosuppression has been mostly studied in the context of inhibiting B- and T-cell responses, with some evidence suggesting NK cells and helper ILCs may be resistant to current immunosuppression [102]. Alemtuzumab, an anti-CD52 humanized monoclonal antibody, and polyclonal rabbit anti-thymocyte globulin have been used to treat patients following kidney and pancreas transplantation, resulting in a marked loss of circulating NK cells that exhibited decreased cytotoxicity and increased apoptosis [204]. However, both therapies are unable to target CD52− NK cells found within liver and also deplete T and B cells [205]. A more specific approach that targeted NK cells and CD8+ T cells using anti-NKG2D and anti-CTLA improved allograft survival in a mouse model of islet transplant [206], and similar improvements were seen by blocking NKG2D in in vitro models of airway epithelial cytotoxicity [161]. This emphasizes the possibility of using targeting antibodies to prevent NK cell-mediated IRI and organ rejection. As new data emerges on ILC metabolism, microbiome-derived metabolites effects on ILC functions, chemokines that control ILC trafficking, impact of MHC expression by helper ILCs in allogeneic contexts, and molecules that activate or inhibit ILCs, novel approaches may emerge that more specifically promote ILCs reparative functions, blocking harmful NK cell responses or promoting tolerogenic ILC expansion or activation.
Tissue tolerance is mediated by complex interplay between the various immune cells including DCs [207, 208], mast cells [207], macrophages [209], eosinophils [174, 210], B cells, [211] and ILCs. For example, IFN-γ can induce iNOS+ eosinophils that are protective in lung transplantation and infiltrate early after transplant [210]. ILC2s are similarly protective in lung transplantation, yet they are constrained by IFN-γ [212]. Intriguingly, ILC subsets have been shown to interface with many of these cells and promote regulatory functions [5, 213]. Moving forward, it will be important to disentangle the network of cells and pathways in each organ that leads to protection from adverse outcomes following organ transplantation and explore how ILC family members contribute to these circuits across different tissues. Of particular interest is the role of ILC-Treg crosstalk in promoting tolerance. Currently, the interplay between these immune populations is not well understood in the context of transplantation, but evidence from autologous settings indicates ILCs could work collaboratively with Tregs to promote tolerance. For example, in mice, IL-9 from ILC2s was crucial for activation of Tregs and resolution of inflammation in an arthritis model [214]. In resting or helminth-infected adipose tissue, IL-33 stimulated ILC2s induced Treg activation and accumulation through ICOSL-ICOS interactions [215]. Similarly, ILC3s in the intestine are an important source of IL-2, which supports intestinal Treg maintenance [216] and GM-CSF, which helps maintain Treg homeostasis through DCs and macrophages [217]. It will therefore be important to examine ILC-Treg crosstalk and consequences for tissue tolerance.
A major limitation of our current understanding of the role of ILCs in organ transplantation and HSCT is that most studies have been correlational. While scRNAseq studies investigating human transplantation are shedding new light into the cells and pathways that drive tolerance, these only capture a single point in time, missing important temporal observations of early immune responses that drive organ rejection or promote tolerance. Rodent models of organ transplantation have been essential in bridging the gap between correlational patient data and mechanisms underlying organ transplant and tolerance. Unfortunately, these models do not accurately reflect the diverse human population, and thus have limitations for translation of insights gained from these models [218]. A further complication for the field of ILCs is the differences between mouse and human ILC subsets including markers to identify them, specific functions, and responses to environmental triggers [219]. To gain a better understanding of the functional role of human ILCs in transplantation, newer humanized mouse models, as well as ex vivo approaches such as precision cut-tissue slice models, organoids, organ-on-a-chip, and 3D cultures should be harnessed to better define human ILC interactions with allogeneic tissues and other immune populations that contribute to protective or pathogenic immune responses to organs. This, combined with powerful new single-cell and spatial technologies, will provide valuable insights into mechanisms by which human ILCs coordinate transplant immune responses, which may one day lead to novel therapeutic approaches for transplantation.
Acknowledgements
Figures were created with BioRender.com.
Glossary
Abbreviations
- ACR
acute cellular rejection
- APCs
antigen presenting cell
- ABMR
antibody mediated rejection
- ADCC
antibody-dependent cell-mediated cytotoxicity
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- EOMES
eomesodermin
- G-CSF
granulocyte colony stimulating factor
- GM-CSF
granulocyte macrophage colony stimulating factor
- GVHD
graft versus host disease
- GVL
graft versus leukemia
- HLA
human leukocyte antigen
- HSCT
hematopoietic stem cell transplant
- IFN-γ
interferon-gamma
- IL
interleukin
- ILC
innate lymphoid cell
- ILC1
group 1 innate lymphoid cell
- ILC2
group 2 innate lymphoid cell
- ILC2
group 3 innate lymphoid cell
- ILCP
innate lymphoid cell precursor
- IRI
ischemia reperfusion injury
- KIR
killer cell immunoglobulin-like receptor
- LTi
lymphoid tissue inducer
- MDSC
myeloid derived suppressor cell
- MHC
major histocompatibility complex
- NK cell
natural killer cell
- NCR
natural cytotoxicity receptor
- ROS
reactive oxygen species
- scRNAseq
single cell RNA sequencing
- TEC
tubular epithelial cells
- TNF-α
tumour necrosis factor alpha
- Tregs
CD4 regulatory T cell.
Contributor Information
Martin L Mak, Department of Immunology, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada; Toronto General Hospital Research Institute, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Kyle T Reid, Department of Immunology, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada; Toronto General Hospital Research Institute, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Sarah Q Crome, Department of Immunology, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada; Toronto General Hospital Research Institute, Ajmera Transplant Centre, University Health Network, Toronto, Canada.
Ethical Approval
Not applicable.
Conflict of Interests
The authors declare that they have no competing financial interests. K.T.R and S.Q.C have filed a provisional patent for human ILC expansion methods (Provisional Patent Application#: 63/353,823).
Funding
M.L.M is supported by the Frederick Banting and Charles Best Canada Graduate Scholarships. K.T.R is supported by a MITACs Accelerate Award. S.Q.C holds a Tier 2 Canada Research Chair in Tissue-Specific Immune Tolerance and is supported by funding from the Canadian Institutes for Health Research (169084 and 168960), the Medicine by Design program (Canada First Research Excellence Fund), and the Canada Foundation for Innovation (38308).
Author Contributions
M.L.M and K.T.R. both contributed equally to the work. All authors wrote and edited the manuscript.
Permission to Reproduce
This article does not contain content that requires permission to reproduce. Figures were created with Biorender.com and appropriate license were obtained.
Data availability
Not applicable.
Clinical Trial Registration
Not applicable.
References
- 1. Murphy JM, Ngai L, Mortha A, Crome SQ.. Tissue-dependent adaptations and functions of innate lymphoid cells. Front Immunol 2022, 13, 836999. doi: 10.3389/fimmu.2022.836999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castellanos JG, Longman RS.. The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest 2019, 129, 2640–50. doi: 10.1172/JCI124617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Björkström NK, Kekäläinen E, Mjösberg J.. Tissue-specific effector functions of innate lymphoid cells. Immunology 2013, 139, 416–27. doi: 10.1111/imm.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Symowski C, Voehringer D.. Interactions between innate lymphoid cells and cells of the innate and adaptive immune system. Front Immunol 2017, 8, 1422. doi: 10.3389/fimmu.2017.01422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonnenberg GF, Hepworth MR.. Functional interactions between innate lymphoid cells and adaptive immunity. Nat Rev Immunol 2019, 19, 599–613. doi: 10.1038/s41577-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim AI, Di Santo JP.. ILC-poiesis: Ensuring tissue ILC differentiation at the right place and time. Eur J Immunol 2019, 49, 11–8. doi: 10.1002/eji.201747294. [DOI] [PubMed] [Google Scholar]
- 7. Lim AI, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, et al. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell 2017, 168, 1086–1100.e10. doi: 10.1016/j.cell.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 8. Vély F, Barlogis V, Vallentin B, Neven B, Piperoglou C, Ebbo M, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol 2016, 17, 1291–9. doi: 10.1038/ni.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goncalves P, Doisne J-M, Eri T, Charbit B, Bondet V, Posseme C, et al.; Milieu Intérieur Consortium. Defects in mucosal immunity and nasopharyngeal dysbiosis in HSC-transplanted SCID patients with IL2RG/JAK3 deficiency. Blood 2022, 139, 2585–600. doi: 10.1182/blood.2021014654. [DOI] [PubMed] [Google Scholar]
- 10. Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014, 40, 425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold MJ, Antignano F, Halim TYF, Hirota JA, Blanchet M-R, Zaph C, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol 2014, 133, 1142–8. doi: 10.1016/j.jaci.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 12. Klose CS, Artis D.. Neuronal regulation of innate lymphoid cells. Curr Opin Immunol 2019, 56, 94–9. doi: 10.1016/j.coi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 13. von Moltke J, Ji M, Liang H-E, Locksley RM.. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–5. doi: 10.1038/nature16161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote being of white adipose tissue and limit obesity. Nature 2015, 519, 242–6. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Godinho-Silva C, Domingues RG, Rendas M, Raposo B, Ribeiro H, da Silva JA, et al. Light-entrained and brain-tuned circadian circuits regulate ILC3s and gut homeostasis. Nature 2019, 574, 254–8. doi: 10.1038/s41586-019-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY.. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350, 981–5. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pu Q, Lin P, Gao P, Wang Z, Guo K, Qin S, et al. Gut microbiota regulate gut-lung axis inflammatory responses by mediating ILC2 compartmental migration. J Immunol 2021, 207, 257–67. doi: 10.4049/jimmunol.2001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathä L, Romera-Hernández M, Steer CA, Yin YH, Orangi M, Shim H, et al. Migration of lung resident group 2 innate lymphoid cells link allergic lung inflammation and liver immunity. Front Immunol 2021, 12, 679509. doi: 10.3389/fimmu.2021.679509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willinger T. Metabolic control of innate lymphoid cell migration. Front Immunol 2019, 10, 2010. doi: 10.3389/fimmu.2019.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell 2018, 174, 1054–66. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 21. Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 2013, 13, 145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 22. Fauriat C, Long EO, Ljunggren H-G, Bryceson YT.. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001, 97, 3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 24. Klose CSN, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 2013, 494, 261–5. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 25. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 2011, 12, 1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Monticelli LA, Osborne LC, Noti M, Tran SV, Zaiss DMW, Artis D.. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin-EGFR interactions. Proc Natl Acad Sci U S A 2015, 112, 10762–7. doi: 10.1073/pnas.1509070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, et al. IL-33-dependent group 2 innate lymphoid cells promote cutaneous wound healing. J Invest Dermatol 2016, 136, 487–96. doi: 10.1038/JID.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Z, Hodgkinson T, Gothard EJ, Boroumand S, Lamb R, Cummins I, et al. Epidermal Notch1 recruits RORγ(+) group 3 innate lymphoid cells to orchestrate normal skin repair. Nat Commun 2016, 7, 11394. doi: 10.1038/ncomms11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009, 457, 722–5. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satoh-Takayama N, Vosshenrich CAJ, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008, 29, 958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 31. Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 2013, 498, 113–7. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo J-H, Shih H-Y, et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 2019, 176, 982–997.e16. doi: 10.1016/j.cell.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang K, Jin Y, Lai D, Wang J, Wang Y, Wu X, et al. RAGE-induced ILC2 expansion in acute lung injury due to haemorrhagic shock. Thorax 2020, 75, 209–19. doi: 10.1136/thoraxjnl-2019-213613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kastenschmidt JM, Coulis G, Farahat PK, Pham P, Rios R, Cristal TT, et al. A stromal progenitor and ILC2 niche promotes muscle eosinophilia and fibrosis-associated gene expression. Cell Rep 2021, 35, 108997. doi: 10.1016/j.celrep.2021.108997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer AR, Engevik AC, Madorsky T, Belmont E, Stier MT, Norlander AE, et al. Group 2 innate lymphoid cells coordinate damage response in the stomach. Gastroenterology 2020, 159, 2077–2091.e8. doi: 10.1053/j.gastro.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eken A, Singh AK, Treuting PM, Oukka M.. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol 2014, 7, 143–54. doi: 10.1038/mi.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010, 464, 1371–5. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim HY, Lee HJ, Chang Y-J, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med 2014, 20, 54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shikhagaie MM, Germar K, Bal SM, Ros XR, Spits H.. Innate lymphoid cells in autoimmunity: emerging regulators in rheumatic diseases. Nat Rev Rheumatol 2017, 13, 164–73. doi: 10.1038/nrrheum.2016.218. [DOI] [PubMed] [Google Scholar]
- 40. Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, et al. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential “inflammatory” type 2 innate lymphoid cells. Nat Immunol 2015, 16, 161–9. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 2002, 169, 443–53. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 42. Crome SQ, Nguyen LT, Lopez-Verges S, Yang SYC, Martin B, Yam JY, et al. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat Med 2017, 23, 368–75. doi: 10.1038/nm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cao Q, Wang R, Wang Y, Niu Z, Chen T, Wang C, et al. Regulatory innate lymphoid cells suppress innate immunity and reduce renal ischemia/reperfusion injury. Kidney Int 2020, 97, 130–42. doi: 10.1016/j.kint.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 44. Golebski K, Layhadi JA, Sahiner U, Steveling-Klein EH, Lenormand MM, Li RCY, et al. Induction of IL-10-producing type 2 innate lymphoid cells by allergen immunotherapy is associated with clinical response. Immunity 2021, 54, 291–307.e7. doi: 10.1016/j.immuni.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 45. Bando JK, Gilfillan S, di Luccia B, et al. ILC2s are the predominant source of intestinal ILC-derived IL-10. J Exp Med 2020, 217, 1–9. doi: 10.1084/jem.20191520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bielekova B, Catalfamo M, Reichert-Scrivner S, Packer A, Cerna M, Waldmann TA, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A 2006, 103, 5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, et al. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell 2017, 171, 201–216.e18. doi: 10.1016/j.cell.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 48. Jegatheeswaran S, Mathews JA, Crome SQ.. Searching for the elusive regulatory innate lymphoid cell. J Immunol 2021, 207, 1949–57. doi: 10.4049/jimmunol.2100661. [DOI] [PubMed] [Google Scholar]
- 49. Cowled P, Fitridge R.. Pathophysiology of reperfusion injury. Mech Vasc Dis: Textbook Vasc Special 2011, 17, 415–40. [Google Scholar]
- 50. Kezić A, Stajic N, Thaiss F.. Innate immune response in kidney ischemia/reperfusion injury: potential target for therapy. J Immunol Res 2017, 2017, 6305439. doi: 10.1155/2017/6305439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA.. Neutrophils—a key component of ischemia-reperfusion injury. Shock 2013, 40, 463–70. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 52. Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O'Donnell MP, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 2001, 108, 1283–90. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 2000, 279, F525–31. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 54. Yokota N, Burne-Taney M, Racusen L, Rabb H.. Contrasting roles for STAT4 and STAT6 signal transduction pathways in murine renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 2003, 285, F319–25. doi: 10.1152/ajprenal.00432.2002. [DOI] [PubMed] [Google Scholar]
- 55. Moreau A, Varey E, Anegon I, et al. Effector mechanisms of rejection. Cold Spring Harb Perspect Med 2013, 3, 1–33. doi: 10.1101/cshperspect.a015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vignali DAA, Collison LW, Workman CJ.. How regulatory T cells work. Nat Rev Immunol 2008, 8, 523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant 2008, 8, 1639–51. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 58. Hu M, Wang C, Zhang GY, Saito M, Wang YM, Fernandez MA, et al. Infiltrating Foxp3(+) regulatory T cells from spontaneously tolerant kidney allografts demonstrate donor-specific tolerance. Am J Transplant 2013, 13, 2819–30. doi: 10.1111/ajt.12445. [DOI] [PubMed] [Google Scholar]
- 59. Pilat N, Wiletel M, Weijler AM, Steiner R, Mahr B, Warren J, et al. Treg-mediated prolonged survival of skin allografts without immunosuppression. Proc Natl Acad Sci U S A 2019, 116, 13508–16. doi: 10.1073/pnas.1903165116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garnett C, Apperley JF, Pavlů J.. Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol 2013, 4, 366–78. doi: 10.1177/2040620713489842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015, 21, 389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jamil MO, Mineishi S.. State-of-the-art acute and chronic GVHD treatment. Int J Hematol 2015, 101, 452–66. doi: 10.1007/s12185-015-1785-1. [DOI] [PubMed] [Google Scholar]
- 63. Hill GR, Betts BC, Tkachev V, Kean LS, Blazar BR.. Current concepts and advances in graft-versus-host disease immunology. Annu Rev Immunol 2021, 39, 19–49. doi: 10.1146/annurev-immunol-102119-073227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Soiffer RJ, Gonin R, Murray C, Robertson MJ, Cochran K, Chartier S, et al. Prediction of graft-versus-host disease by phenotypic analysis of early immune reconstitution after CD6-depleted allogeneic bone marrow transplantation. Blood 1993, 82, 2216–23. [PubMed] [Google Scholar]
- 65. Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong ASM, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia 2007, 21, 2145–52. doi: 10.1038/sj.leu.2404892. [DOI] [PubMed] [Google Scholar]
- 66. Yamasaki S, Henzan H, Ohno Y, Yamanaka T, Iino T, Itou Y, et al.; Fukuoka Blood and Marrow Transplantation Group. Influence of transplanted dose of CD56+ cells on development of graft-versus-host disease in patients receiving G-CSF-mobilized peripheral blood progenitor cells from HLA-identical sibling donors. Bone Marrow Transplant 2003, 32, 505–10. doi: 10.1038/sj.bmt.1704165. [DOI] [PubMed] [Google Scholar]
- 67. Minculescu L, Marquart HV, Friis LS, Petersen SL, Schiødt I, Ryder LP, et al. Early natural killer cell reconstitution predicts overall survival in T cell-replete allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2016, 22, 2187–93. doi: 10.1016/j.bbmt.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 68. Tanaka M, Kobayashi S, Numata A, Tachibana T, Takasaki H, Maruta A, et al. The impact of the dose of natural killer cells in the graft on severe acute graft-versus-host disease after unrelated bone marrow transplantation. Leuk Res 2012, 36, 699–703. doi: 10.1016/j.leukres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 69. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 70. Ghadially H, Ohana M, Elboim M, Gazit R, Gur C, Nagler A, et al. NK cell receptor NKp46 regulates graft-versus-host disease. Cell Rep 2014, 7, 1809–14. doi: 10.1016/j.celrep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS.. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010, 115, 4293–301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sheng L, Mu Q, Wu X, Yang S, Zhu H, Wang J, et al. Cytotoxicity of donor natural killer cells to allo-reactive T cells are related with acute graft-vs.-host-disease following allogeneic stem cell transplantation. Front Immunol 2020, 11, 1534. doi: 10.3389/fimmu.2020.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chang Y-J, Zhao X-Y, Huang X-J.. Effects of the NK cell recovery on outcomes of unmanipulated haploidentical blood and marrow transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplant 2008, 14, 323–34. doi: 10.1016/j.bbmt.2007.12.497. [DOI] [PubMed] [Google Scholar]
- 74. Ullrich E, Salzmann-Manrique E, Bakhtiar S, Bremm M, Gerstner S, Herrmann E, et al. Relation between acute GVHD and NK cell subset reconstitution following allogeneic stem cell transplantation. Front Immunol 2016, 7, 595. doi: 10.3389/fimmu.2016.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huenecke S, Cappel C, Esser R, Pfirrmann V, Salzmann-Manrique E, Betz S, et al. Development of three different NK cell subpopulations during immune reconstitution after pediatric allogeneic hematopoietic stem cell transplantation: prognostic markers in GvHD and viral infections. Front Immunol 2017, 8, 109. doi: 10.3389/fimmu.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Munneke JM, Björklund AT, Mjösberg JM, Garming-Legert K, Bernink JH, Blom B, et al. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 2014, 124, 812–21. doi: 10.1182/blood-2013-11-536888. [DOI] [PubMed] [Google Scholar]
- 77. Piperoglou C, Larid G, Vallentin B, Balligand L, Crinier A, Banzet N, et al.; CRYOSTEM Consortium. Innate lymphoid cell recovery and occurrence of GvHD after hematopoietic stem cell transplantation. J Leukoc Biol 2022, 111, 161–72. doi: 10.1002/JLB.5A1019-522RR. [DOI] [PubMed] [Google Scholar]
- 78. Kroeze A, van Hoeven V, Verheij MW, Turksma AW, Weterings N, van Gassen S, et al. Presence of innate lymphoid cells in allogeneic hematopoietic grafts correlates with reduced graft-versus-host disease. Cytotherapy 2022, 24, 302–10. doi: 10.1016/j.jcyt.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 79. Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 2012, 37, 339–50. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hazenberg MD, Haverkate NJE, van Lier YF, Spits H, Krabbendam L, Bemelman WA, et al. Human ectoenzyme-expressing ILC3: immunosuppressive innate cells that are depleted in graft-versus-host disease. Blood Adv 2019, 3, 3650–60. doi: 10.1182/bloodadvances.2019000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bruce DW, Kolupaev O, Laurie SJ, Bommiasamy H, Stefanski H, Blazar BR, et al. Third-party type 2 innate lymphoid cells prevent and treat GI tract GvHD. Blood Adv 2021, 5, 4578–89. doi: 10.1182/bloodadvances.2020001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bruce DW, Stefanski HE, Vincent BG, Dant TA, Reisdorf S, Bommiasamy H, et al. Type 2 innate lymphoid cells treat and prevent acute gastrointestinal graft-versus-host disease. J Clin Invest 2017, 127, 1813–25. doi: 10.1172/JCI91816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Canadian Institute for Health Information. Organ replacement in Canada: CORR annual statistics. 2019. [Google Scholar]
- 84. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011) 2022, 12, 7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ishiyama K, Arakawa-Hoyt J, Aguilar OA, et al. Mass cytometry reveals single-cell kinetics of cytotoxic lymphocyte evolution in CMV-infected renal transplant patients. Proc Natl Acad Sci U S A 2022, 119(8), e2116588119. doi: 10.1073/pnas.2116588119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hadaya K, de Rham C, Bandelier C, Bandelier C, Ferrari-Lacraz S, Jendly S, et al. Natural killer cell receptor repertoire and their ligands, and the risk of CMV infection after kidney transplantation. Am J Transplant 2008, 8, 2674–83. doi: 10.1111/j.1600-6143.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 87. Pickering H, Sen S, Arakawa-Hoyt J, Ishiyama K, Sun Y, Parmar R, et al. NK and CD8+ T cell phenotypes predict onset and control of CMV viremia after kidney transplant. JCI Insight 2021, 6(21), e153175. doi: 10.1172/jci.insight.153175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Law BMP, Wilkinson R, Wang X, Kildey K, Lindner M, Rist MJ, et al. Interferon-γ production by tubulointerstitial human CD56 bright natural killer cells contributes to renal fibrosis and chronic kidney disease progression. Kidney Int 2017, 92, 79–88. doi: 10.1016/j.kint.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 89. Riedel J-H, Becker M, Kopp K, Düster M, Brix SR, Meyer-Schwesinger C, et al. IL-33-Mediated expansion of type 2 innate lymphoid cells protects from progressive glomerulosclerosis. J Am Soc Nephrol 2017, 28, 2068–80. doi: 10.1681/ASN.2016080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pan Z-Y, Liu H-Q, Zhuang Y-P, et al. Reduced type 3 innate lymphoid cells related to worsening kidney function in renal dysfunction. Exp Biol Med (Maywood) 2023, 248, 242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang Z-X, Wang S, Huang X, Min W-P, Sun H, Liu W, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol 2008, 181, 7489–98. doi: 10.4049/jimmunol.181.11.7489. [DOI] [PubMed] [Google Scholar]
- 92. Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, et al. Tissue-resident NK cells mediate ischemic kidney injury and are not depleted by anti-Asialo-GM1 antibody. J Immunol 2015, 195, 4973–85. doi: 10.4049/jimmunol.1500651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kim HJ, Lee JS, Kim JD, Cha HJ, Kim A, Lee SK, et al. Reverse signaling through the costimulatory ligand CD137L in epithelial cells is essential for natural killer cell-mediated acute tissue inflammation. Proc Natl Acad Sci U S A 2012, 109, E13–22. doi: 10.1073/pnas.1112256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kim HJ, Lee JS, Kim A, Koo S, Cha HJ, Han J-A, et al. TLR2 signaling in tubular epithelial cells regulates NK cell recruitment in kidney ischemia-reperfusion injury. J Immunol 2013, 191, 2657–64. doi: 10.4049/jimmunol.1300358. [DOI] [PubMed] [Google Scholar]
- 95. Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 2012, 12, 388–99. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 96. El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant 2009, 9, 527–35. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 97. Naesens M, Kuypers DRJ, de Vusser K, Evenepoel P, Claes K, Bammens B, et al. The histology of kidney transplant failure: a long-term follow-up study. Transplantation 2014, 98, 427–35. doi: 10.1097/TP.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 98. Yagisawa T, Tanaka T, Miyairi S, Tanabe K, Dvorina N, Yokoyama WM, et al. In the absence of natural killer cell activation donor-specific antibody mediates chronic, but not acute, kidney allograft rejection. Kidney Int 2019, 95, 350–62. doi: 10.1016/j.kint.2018.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Koenig A, Mezaache S, Callemeyn J, Barba T, Mathias V, Sicard A, et al. Missing self-induced activation of NK cells combines with non-complement-fixing donor-specific antibodies to accelerate kidney transplant loss in chronic antibody-mediated rejection. J Am Soc Nephrol 2021, 32, 479–94. doi: 10.1681/ASN.2020040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kildey K, Francis RS, Hultin S, Harfield M, Giuliani K, Law BMP, et al. Specialized roles of human natural killer cell subsets in kidney transplant rejection. Front Immunol 2019, 10, 1877. doi: 10.3389/fimmu.2019.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Bergen J, Thompson A, Haasnoot GW, Roodnat JI, de Fijter JW, Claas FHJ, et al. KIR-ligand mismatches are associated with reduced long-term graft survival in HLA-compatible kidney transplantation. Am J Transplant 2011, 11, 1959–64. doi: 10.1111/j.1600-6143.2011.03621.x. [DOI] [PubMed] [Google Scholar]
- 102. Vampa ML, Norman PJ, Burnapp L, Vaughan RW, Sacks SH, Wong W.. Natural killer-cell activity after human renal transplantation in relation to killer immunoglobulin-like receptors and human leukocyte antigen mismatch. Transplantation 2003, 76, 1220–8. doi: 10.1097/01.TP.0000083896.91215.C7. [DOI] [PubMed] [Google Scholar]
- 103. Cao Q, Wang Y, Niu Z, Wang C, Wang R, Zhang Z, et al. Potentiating tissue-resident type 2 innate lymphoid cells by IL-33 to prevent renal ischemia-reperfusion injury. J Am Soc Nephrol 2018, 29, 961–76. doi: 10.1681/ASN.2017070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Huang Q, Niu Z, Tan J, Yang J, Liu Y, Ma H, et al. IL-25 elicits innate lymphoid cells and multipotent progenitor type 2 cells that reduce renal ischemic/reperfusion injury. J Am Soc Nephrol 2015, 26, 2199–211. doi: 10.1681/ASN.2014050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stremska ME, Jose S, Sabapathy V, Huang L, Bajwa A, Kinsey GR, et al. IL233, a Novel IL-2 and IL-33 hybrid cytokine, ameliorates renal injury. J Am Soc Nephrol 2017, 28, 2681–93. doi: 10.1681/ASN.2016121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cameron GJM, Cautivo KM, Loering S, Jiang SH, Deshpande AV, Foster PS, et al. Group 2 innate lymphoid cells are redundant in experimental renal ischemia-reperfusion injury. Front Immunol 2019, 10, 826. doi: 10.3389/fimmu.2019.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dong Z, Wei H, Sun R, Tian Z.. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol 2007, 4, 241–52. [PubMed] [Google Scholar]
- 108. Peng H, Wisse E, Tian Z.. Liver natural killer cells: subsets and roles in liver immunity. Cell Mol Immunol 2016, 13, 328–36. doi: 10.1038/cmi.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim CH, Hashimoto-Hill S, Kim M.. Migration and tissue tropism of innate lymphoid cells. Trends Immunol 2016, 37, 68–79. doi: 10.1016/j.it.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol 1999, 163, 2314–21. [PubMed] [Google Scholar]
- 111. Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol 1998, 28, 84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 112. MacParland SA, Liu JC, Ma X-Z, Innes BT, Bartczak AM, Gage BK, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun 2018, 9, 4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhao J, Zhang S, Liu Y, He X, Qu M, Xu G, et al. Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov 2020, 6, 22. doi: 10.1038/s41421-020-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Marquardt N, Béziat V, Nyström S, Hengst J, Ivarsson MA, Kekäläinen E, et al. Cutting edge: identification and characterization of human intrahepatic CD49a+ NK cells. J Immunol 2015, 194, 2467–71. doi: 10.4049/jimmunol.1402756. [DOI] [PubMed] [Google Scholar]
- 115. Hudspeth K, Donadon M, Cimino M, Pontarini E, Tentorio P, Preti M, et al. Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways. J Autoimmun 2016, 66, 40–50. doi: 10.1016/j.jaut.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhou J, Peng H, Li K, Qu K, Wang B, Wu Y, et al. Liver-resident NK cells control antiviral activity of hepatic T cells via the PD-1-PD-L1 axis. Immunity 2019, 50, 403–417.e4. doi: 10.1016/j.immuni.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 117. Harmon C, Robinson MW, Fahey R, Whelan S, Houlihan DD, Geoghegan J, et al. Tissue-resident Eomes(hi) T-bet(lo) CD56(bright) NK cells with reduced proinflammatory potential are enriched in the adult human liver. Eur J Immunol 2016, 46, 2111–20. doi: 10.1002/eji.201646559. [DOI] [PubMed] [Google Scholar]
- 118. Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS.. Intrahepatic IL-10 maintains NKG2A+Ly49- liver NK cells in a functionally hyporesponsive state. J Immunol 2010, 184, 2693–701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol 2004, 173, 6072–81. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 120. Graubardt N, Fahrner R, Trochsler M, Keogh A, Breu K, Furer C, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology 2013, 57, 1969–79. doi: 10.1002/hep.26008. [DOI] [PubMed] [Google Scholar]
- 121. Sun R, Gao B.. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma). Gastroenterology 2004, 127, 1525–39. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 122. Bi J, Zheng X, Chen Y, Wei H, Sun R, Tian Z.. TIGIT safeguards liver regeneration through regulating natural killer cell-hepatocyte crosstalk. Hepatology 2014, 60, 1389–98. doi: 10.1002/hep.27245. [DOI] [PubMed] [Google Scholar]
- 123. Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T, et al. CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Sci Rep 2016, 6, 26157. doi: 10.1038/srep26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Aw Yeang HX, Piersma SJ, Lin Y, Yang L, Malkova ON, Miner C, et al. Cutting edge: human CD49e-NK cells are tissue resident in the liver. J Immunol 2017, 198, 1417–22. doi: 10.4049/jimmunol.1601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Moroso V, Metselaar HJ, Mancham S, Tilanus HW, Eissens D, van der Meer A, et al. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl 2010, 16, 895–908. doi: 10.1002/lt.22080. [DOI] [PubMed] [Google Scholar]
- 126. Jameson G, Harmon C, Santiago RM, Houlihan DD, Gallagher TK, Lynch L, et al. Human hepatic CD56bright NK cells display a tissue-resident transcriptional profile and enhanced ability to kill allogenic CD8+ T cells. Front Immunol 2022, 13, 921212. doi: 10.3389/fimmu.2022.921212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pagano D, Badami E, Conaldi PG, Seidita A, Tuzzolino F, Barbàra M, et al. Liver perfusate natural killer cells from deceased brain donors and association with acute cellular rejection after liver transplantation: a time-to-rejection analysis. Transplantation 2019, 103, 371–80. doi: 10.1097/TP.0000000000002322. [DOI] [PubMed] [Google Scholar]
- 128. García de la Garza R, Sarobe P, Merino J, Lasarte JJ, D'Avola D, Belsue V, et al. Immune monitoring of immunosuppression withdrawal of liver transplant recipients. Transpl Immunol 2015, 33, 110–6. doi: 10.1016/j.trim.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 129. Cuff AO, Robertson FP, Stegmann KA, Pallett LJ, Maini MK, Davidson BR, et al. Eomeshi NK cells in human liver are long-lived and do not recirculate but can be replenished from the circulation. J Immunol 2016, 197, 4283–91. doi: 10.4049/jimmunol.1601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jamil KM, Hydes TJ, Cheent KS, Cassidy SA, Traherne JA, Jayaraman J, et al. STAT4-associated natural killer cell tolerance following liver transplantation. Gut 2017, 66, 352–61. doi: 10.1136/gutjnl-2015-309395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yu J-D, Long T-Z, Li G-L, Lv L-H, Lin H-M, Huang Y-H, et al. Donor liver natural killer cells alleviate liver allograft acute rejection in rats. Hepatobiliary Pancreat Dis Int 2011, 10, 386–92. doi: 10.1016/s1499-3872(11)60065-9. [DOI] [PubMed] [Google Scholar]
- 132. Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, et al. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am J Transplant 2005, 5, 2094–103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Feng M, Li G, Qian X, Fan Y, Huang X, Zhang F, et al. IL-17A-producing NK cells were implicated in liver injury induced by ischemia and reperfusion. Int Immunopharmacol 2012, 13, 135–40. doi: 10.1016/j.intimp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 134. Nakajima H, Mizuta N, Fujiwara I, Sakaguchi K, Ogata H, Magae J, et al. Blockade of the Fas/Fas ligand interaction suppresses hepatocyte apoptosis in ischemia-reperfusion rat liver. Apoptosis 2008, 13, 1013–21. doi: 10.1007/s10495-008-0234-5. [DOI] [PubMed] [Google Scholar]
- 135. Weizman O-E, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, et al. ILC1 confer early host protection at initial sites of viral infection. Cell 2017, 171, 795–808.e12. doi: 10.1016/j.cell.2017.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Krueger PD, Narayanan S, Surette FA, et al. Murine liver-resident group 1 innate lymphoid cells regulate optimal priming of anti-viral CD8+ T cells. J Leukoc Biol 2017, 101, 329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Fumagalli V, Venzin V, di Lucia P, Moalli F, Ficht X, Ambrosi G, et al. Group 1 ILCs regulate T cell-mediated liver immunopathology by controlling local IL-2 availability. Sci Immunol 2022, 7, eabi6112. doi: 10.1126/sciimmunol.abi6112. [DOI] [PubMed] [Google Scholar]
- 138. Nabekura T, Riggan L, Hildreth AD, O'Sullivan TE, Shibuya A.. Type 1 innate lymphoid cells protect mice from acute liver injury via interferon-γ secretion for upregulating Bcl-xL expression in hepatocytes. Immunity 2020, 52, 96–108.e9. doi: 10.1016/j.immuni.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kang J, Liggett JR, Patil D, Ranjit S, Loh K, Duttargi A, et al. Type 1 innate lymphoid cells are proinflammatory effector cells in ischemia-reperfusion injury of steatotic livers. Front Immunol 2022, 13, 899525. doi: 10.3389/fimmu.2022.899525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sun J, Zhou G-P, Li S-P, Chen X-J, Zhang J-M, Jiang Y-Z, et al. Potential correlation of allograft infiltrating group 2 innate lymphoid cells with acute rejection after liver transplantation. Front Immunol 2022, 13, 953240. doi: 10.3389/fimmu.2022.953240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang H-M, Chen X-J, Li S-P, Zhang J-M, Sun J, Zhou L-X, et al. ILC2s expanded by exogenous IL-33 regulate CD45+CD11b+F4/80high macrophage polarization to alleviate hepatic ischemia-reperfusion injury. Front Immunol 2022, 13, 869365. doi: 10.3389/fimmu.2022.869365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 2013, 39, 357–71. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kudira R, Malinka T, Kohler A, Dosch M, de Agüero MG, Melin N, et al. P2X1-regulated IL-22 secretion by innate lymphoid cells is required for efficient liver regeneration. Hepatology 2016, 63, 2004–17. doi: 10.1002/hep.28492. [DOI] [PubMed] [Google Scholar]
- 144. Eggenhofer E, Sabet-Rashedi M, Lantow M, Renner P, Rovira J, Koehl GE, et al. RORγt(+) IL-22-producing NKp46(+) cells protect from hepatic ischemia reperfusion injury in mice. J Hepatol 2016, 64, 128–34. doi: 10.1016/j.jhep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 145. Matsumoto A, Kanai T, Mikami Y, Chu P-S, Nakamoto N, Ebinuma H, et al. IL-22-producing RORγt-dependent innate lymphoid cells play a novel protective role in murine acute hepatitis. PLoS One 2013, 8, e62853. doi: 10.1371/journal.pone.0062853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Allan P, Lal S.. Intestinal failure: a review. F1000Res 2018, 7, 85. doi: 10.12688/f1000research.12493.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, et al.; Intestinal Transplant Association. Intestinal transplant registry report: global activity and trends. Am J Transplant 2015, 15, 210–9. doi: 10.1111/ajt.12979. [DOI] [PubMed] [Google Scholar]
- 148. Loo L, Vrakas G, Reddy S, Allan P.. Intestinal transplantation: a review. Curr Opin Gastroenterol 2017, 33, 203–11. doi: 10.1097/MOG.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 149. Berger M, Zeevi A, Farmer DG, Abu-Elmagd KM.. Immunologic challenges in small bowel transplantation. Am J Transplant 2012, 12, S2–8. doi: 10.1111/j.1600-6143.2012.04332.x. [DOI] [PubMed] [Google Scholar]
- 150. Talayero P, Mancebo E, Calvo-Pulido J, Rodríguez-Muñoz S, Bernardo I, Laguna-Goya R, et al. Innate lymphoid cells groups 1 and 3 in the epithelial compartment of functional human intestinal allografts. Am J Transplant 2016, 16, 72–82. doi: 10.1111/ajt.13435. [DOI] [PubMed] [Google Scholar]
- 151. Gómez-Massa E, Lasa-Lázaro M, Gil-Etayo FJ, Ulloa-Márquez E, Justo I, Loinaz C, et al. Donor helper innate lymphoid cells are replaced earlier than lineage positive cells and persist long-term in human intestinal grafts—a descriptive study. Transpl Int 2020, 33, 1016–29. doi: 10.1111/tri.13609. [DOI] [PubMed] [Google Scholar]
- 152. Weiner J, Zuber J, Shonts B, Yang S, Fu J, Martinez M, et al. Long-term persistence of innate lymphoid cells in the gut after intestinal transplantation. Transplantation 2017, 101, 2449–54. doi: 10.1097/TP.0000000000001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pucci Molineris M, González Polo V, Rumbo C, Fuxman C, Lowestein C, Nachman F, et al. Acute cellular rejection in small-bowel transplantation impairs NCR+ innate lymphoid cell subpopulation 3/interleukin 22 axis. Transpl Immunol 2020, 60, 101288. doi: 10.1016/j.trim.2020.101288. [DOI] [PubMed] [Google Scholar]
- 154. Kang J, Loh K, Belyayev L, Cha P, Sadat M, Khan K, et al. Type 3 innate lymphoid cells are associated with a successful intestinal transplant. Am J Transplant 2021, 21, 787–97. doi: 10.1111/ajt.16163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Marquardt N, Kekäläinen E, Chen P, Kvedaraite E, Wilson JN, Ivarsson MA, et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69-CD56dim cells. J Allergy Clin Immunol 2017, 139, 1321–1330.e4. doi: 10.1016/j.jaci.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 156. de Grove KC, Provoost S, Verhamme FM, Bracke KR, Joos GF, Maes T, et al. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS One 2016, 11, e0145961. doi: 10.1371/journal.pone.0145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Greenland JR, Jewell NP, Gottschall M, Trivedi NN, Kukreja J, Hays SR, et al. Bronchoalveolar lavage cell immunophenotyping facilitates diagnosis of lung allograft rejection. Am J Transplant 2014, 14, 831–40. doi: 10.1111/ajt.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Fildes JE, Yonan N, Tunstall K, Walker AH, Griffiths-Davies L, Bishop P, et al. Natural killer cells in peripheral blood and lung tissue are associated with chronic rejection after lung transplantation. J Heart Lung Transplant 2008, 27, 203–7. doi: 10.1016/j.healun.2007.11.571. [DOI] [PubMed] [Google Scholar]
- 159. Calabrese DR, Aminian E, Mallavia B, Liu F, Cleary SJ, Aguilar OA, et al. Natural killer cells activated through NKG2D mediate lung ischemia-reperfusion injury. J Clin Invest 2021, 131(3), e137047. doi: 10.1172/JCI137047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kawakami T, Ito K, Matsuda Y, Noda M, Sakurada A, Hoshikawa Y, et al. Cytotoxicity of natural killer cells activated through NKG2D contributes to the development of bronchiolitis obliterans in a murine heterotopic tracheal transplant model. Am J Transplant 2017, 17, 2338–49. doi: 10.1111/ajt.14257. [DOI] [PubMed] [Google Scholar]
- 161. Calabrese DR, Tsao T, Magnen M, Valet C, Gao Y, Mallavia B, et al. NKG2D receptor activation drives primary graft dysfunction severity and poor lung transplantation outcomes. JCI Insight 2022, 7(24), e164603. doi: 10.1172/jci.insight.164603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Aminian E, Mallavia B, Liu F, Cleary S, Wang P, Singer JP, et al. Natural killer cells recognize pulmonary epithelial stress molecules during primary graft dysfunction. J Heart Lung Transplant 2021, 40, S147. doi: 10.1016/j.healun.2021.01.449. [DOI] [Google Scholar]
- 163. Jungraithmayr W, Codarri L, Bouchaud G, Krieg C, Boyman O, Gyülvészi G, et al. Cytokine complex-expanded natural killer cells improve allogeneic lung transplant function via depletion of donor dendritic cells. Am J Respir Crit Care Med 2013, 187, 1349–59. doi: 10.1164/rccm.201209-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Greenland JR, Sun H, Calabrese D, Chong T, Singer JP, Kukreja J, et al. HLA mismatching favoring host-versus-graft NK cell activity via KIR3DL1 is associated with improved outcomes following lung transplantation. Am J Transplant 2017, 17, 2192–9. doi: 10.1111/ajt.14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Taube C, Tertilt C, Gyülveszi G, Dehzad N, Kreymborg K, Schneeweiss K, et al. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One 2011, 6, e21799. doi: 10.1371/journal.pone.0021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Teng F, Tachó-Piñot R, Sung B, Farber DL, Worgall S, Hammad H, et al. ILC3s control airway inflammation by limiting T cell responses to allergens and microbes. Cell Rep 2021, 37, 110051. doi: 10.1016/j.celrep.2021.110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Monticelli LA, Diamond JM, Saenz SA, Tait Wojno ED, Porteous MK, Cantu E, et al. Lung innate lymphoid cell composition is altered in primary graft dysfunction. Am J Respir Crit Care Med 2020, 201, 63–72. doi: 10.1164/rccm.201906-1113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Guo Y, Mei Z, Li D, Banerjee A, Khalil MA, Burke A, et al. Ischemia reperfusion injury facilitates lung allograft acceptance through IL-33-mediated activation of donor-derived IL-5 producing group 2 innate lymphoid cells. Am J Transplant 2022, 22, 1963–75. doi: 10.1111/ajt.17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kwon B-I, Hong S, Shin K, Choi E-H, Hwang J-J, Lee S-H.. Innate type 2 immunity is associated with eosinophilic pleural effusion in primary spontaneous pneumothorax. Am J Respir Crit Care Med 2013, 188, 577–85. doi: 10.1164/rccm.201302-0295OC. [DOI] [PubMed] [Google Scholar]
- 170. Chen R, Smith SG, Salter B, El-Gammal A, Oliveria JP, Obminski C, et al. Allergen-induced increases in sputum levels of group 2 innate lymphoid cells in subjects with asthma. Am J Respir Crit Care Med 2017, 196, 700–12. doi: 10.1164/rccm.201612-2427OC. [DOI] [PubMed] [Google Scholar]
- 171. Bartemes KR, Kephart GM, Fox SJ, Kita H.. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol 2014, 134, 671–678.e4. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H.. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol 2012, 188, 1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 2013, 188, 432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Onyema OO, Guo Y, Mahgoub B, Wang Q, Manafi A, Mei Z, et al. Eosinophils downregulate lung alloimmunity by decreasing TCR signal transduction. JCI Insight 2019, 4(11), e128241. doi: 10.1172/jci.insight.128241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Seehus CR, Kadavallore A, de la Torre B, Yeckes AR, Wang Y, Tang J, et al. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun 2017, 8, 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tanaka S, Gauthier JM, Fuchs A, Li W, Tong AY, Harrison MS, et al. IL-22 is required for the induction of bronchus-associated lymphoid tissue in tolerant lung allografts. Am J Transplant 2020, 20, 1251–61. doi: 10.1111/ajt.15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Newland SA, Mohanta S, Clément M, Taleb S, Walker JA, Nus M, et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat Commun 2017, 8, 15781. doi: 10.1038/ncomms15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chen W-Y, Wu Y-H, Tsai T-H, Li R-F, Lai AC, Li L-C, et al. Group 2 innate lymphoid cells contribute to IL-33-mediated alleviation of cardiac fibrosis. Theranostics 2021, 11, 2594–611. doi: 10.7150/thno.51648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Li J, Wu J, Zhang M, Zheng Y.. Dynamic changes of innate lymphoid cells in acute ST-segment elevation myocardial infarction and its association with clinical outcomes. Sci Rep 2020, 10, 5099. doi: 10.1038/s41598-020-61903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Deng Y, Wu S, Yang Y, Meng M, Chen X, Chen S, et al. Unique phenotypes of heart resident type 2 innate lymphoid cells. Front Immunol 2020, 11, 802. doi: 10.3389/fimmu.2020.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Bracamonte-Baran W, Chen G, Hou X, Talor MV, Choi HS, Davogustto G, et al. Non-cytotoxic cardiac innate lymphoid cells are a resident and quiescent type 2-commited population. Front Immunol 2019, 10, 634. doi: 10.3389/fimmu.2019.00634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Dresske B, Zhu X, Herwartz C, Brötzmann K, Fändrich F.. The time pattern of organ infiltration and distribution of natural killer cells and macrophages in the course of acute graft rejection after allogeneic heart transplantation in the rat. Transplant Proc 1997, 29, 1715–6. doi: 10.1016/s0041-1345(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 183. Feng L, Ke N, Ye Z, Guo Y, Li S, Li Q, et al. Expression of NKG2D and its ligand in mouse heart allografts may have a role in acute rejection. Transplant Proc 2009, 41, 4332–9. doi: 10.1016/j.transproceed.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 184. Maier S, Tertilt C, Chambron N, Gerauer K, Hüser N, Heidecke CD, et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28-/- mice. Nat Med 2001, 7, 557–62. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 185. Kim J, Chang CK, Hayden T, Liu F-C, Benjamin J, Hamerman JA, et al. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol 2007, 179, 6416–20. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- 186. Lin CM, Gill RG, Mehrad B.. The natural killer cell activating receptor, NKG2D, is critical to antibody-dependent chronic rejection in heart transplantation. Am J Transplant 2021, 21, 3550–60. doi: 10.1111/ajt.16690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Paul P, Picard C, Sampol E, Lyonnet L, Di Cristofaro J, Paul-Delvaux L, et al. Genetic and functional profiling of CD16-dependent natural killer activation identifies patients at higher risk of cardiac allograft vasculopathy. Circulation 2018, 137, 1049–59. doi: 10.1161/CIRCULATIONAHA.117.030435. [DOI] [PubMed] [Google Scholar]
- 188. Uehara S, Chase CM, Kitchens WH, Rose HS, Colvin RB, Russell PS, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol 2005, 175, 3424–30. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 189. Shapiro AMJ, Pokrywczynska M, Ricordi C.. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017, 13, 268–77. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- 190. Schuetz C, Anazawa T, Cross SE, Labriola L, Meier RPH, Redfield RR, et al.; IPITA YIC Young Investigator Committee. β cell replacement therapy: the next 10 years. Transplantation 2018, 102, 215–29. doi: 10.1097/TP.0000000000001937. [DOI] [PubMed] [Google Scholar]
- 191. Budd MA, Monajemi M, Colpitts SJ, Crome SQ, Verchere CB, Levings MK.. Interactions between islets and regulatory immune cells in health and type 1 diabetes. Diabetologia 2021, 64, 2378–88. doi: 10.1007/s00125-021-05565-6. [DOI] [PubMed] [Google Scholar]
- 192. Beilke JN, Kuhl NR, van Kaer L, Gill RG.. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med 2005, 11, 1059–65. doi: 10.1038/nm1296. [DOI] [PubMed] [Google Scholar]
- 193. Schuetz C, Lee KM, Scott R, Kojima L, Washburn L, Liu L, et al. Regulatory B cell-dependent islet transplant tolerance is also natural killer cell dependent. Am J Transplant 2017, 17, 1656–62. doi: 10.1111/ajt.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Dalmas E, Lehmann FM, Dror E, Wueest S, Thienel C, Borsigova M, et al. Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity 2017, 47, 928–942.e7. doi: 10.1016/j.immuni.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 195. Huang Q, Ma X, Wang Y, Niu Z, Wang R, Yang F, et al. IL-10 producing type 2 innate lymphoid cells prolong islet allograft survival. EMBO Mol Med 2020, 12, e12305. doi: 10.15252/emmm.202012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ngo Thi Phuong N, Palmieri V, Adamczyk A, Klopfleisch R, Langhorst J, Hansen W, et al. IL-33 drives expansion of type 2 innate lymphoid cells and regulatory T cells and protects mice from severe, acute colitis. Front Immunol 2021, 12, 669787. doi: 10.3389/fimmu.2021.669787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 2015, 43, 146–60. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 198. Mansurov A, Lauterbach A, Budina E, Alpar AT, Hubbell JA, Ishihara J.. Immunoengineering approaches for cytokine therapy. Am J Physiol Cell Physiol 2021, 321, C369–83. doi: 10.1152/ajpcell.00515.2020. [DOI] [PubMed] [Google Scholar]
- 199. Tripathi D, Radhakrishnan RK, Sivangala Thandi R, Paidipally P, Devalraju KP, Neela VSK, et al. IL-22 produced by type 3 innate lymphoid cells (ILC3s) reduces the mortality of type 2 diabetes mellitus (T2DM) mice infected with Mycobacterium tuberculosis. PLoS Pathog 2019, 15, e1008140. doi: 10.1371/journal.ppat.1008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Tang Q, Ahn Y-O, Southern P, Blazar BR, Miller JS, Verneris MR.. Development of IL-22-producing NK lineage cells from umbilical cord blood hematopoietic stem cells in the absence of secondary lymphoid tissue. Blood 2011, 117, 4052–5. doi: 10.1182/blood-2010-09-303081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cella M, Otero K, Colonna M.. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A 2010, 107, 10961–6. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Falquet M, Ercolano G, Jandus P, Jandus C, Trabanelli S.. Healthy and patient Type 2 innate lymphoid cells are differently affected by in vitro culture conditions. J Asthma Allergy 2021, 14, 773–83. doi: 10.2147/JAA.S304126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013, 5, 208ra–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Stauch D, Dernier A, Sarmiento Marchese E, Kunert K, Volk H-D, Pratschke J, et al. Targeting of natural killer cells by rabbit antithymocyte globulin and campath-1H: similar effects independent of specificity. PLoS One 2009, 4, e4709. doi: 10.1371/journal.pone.0004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Hotta R, Ohira M, Matsuura T, Muraoka I, Tryphonopoulos P, Fan J, et al. CD52-Negative NK cells are abundant in the liver and less susceptible to alemtuzumab treatment. PLoS One 2016, 11, e0161618. doi: 10.1371/journal.pone.0161618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Pawlick R, Gala-Lopez B, Pepper AR, McCall M, Ziff O, Shapiro AMJ.. The combination of anti-NKG2D and CTLA-4 Ig therapy prolongs islet allograft survival in a murine model. Am J Transplant 2014, 14, 2367–74. doi: 10.1111/ajt.12838. [DOI] [PubMed] [Google Scholar]
- 207. Min W-P, Zhou D, Ichim TE, Strejan GH, Xia X, Yang J, et al. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol 2003, 170, 1304–12. doi: 10.4049/jimmunol.170.3.1304. [DOI] [PubMed] [Google Scholar]
- 208. Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW.. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol 2007, 178, 7018–31. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 209. Ordikhani F, Pothula V, Sanchez-Tarjuelo R, Jordan S, Ochando J.. Macrophages in organ transplantation. Front Immunol 2020, 11, 582939. doi: 10.3389/fimmu.2020.582939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Onyema OO, Guo Y, Wang Q, Stoler MH, Lau C, Li K, et al. Eosinophils promote inducible NOS-mediated lung allograft acceptance. JCI Insight 2017, 2(24), e96455. doi: 10.1172/jci.insight.96455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Schmitz R, Fitch ZW, Schroder PM, Choi AY, Jackson AM, Knechtle SJ, et al. B cells in transplant tolerance and rejection: friends or foes? Transpl Int 2020, 33, 30–40. doi: 10.1111/tri.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cautivo KM, Matatia PR, Lizama CO, Mroz NM, Dahlgren MW, Yu X, et al. Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation. Immunity 2022, 55, 254–271.e7. doi: 10.1016/j.immuni.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Klose CSN, Artis D.. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res 2020, 30, 475–91. doi: 10.1038/s41422-020-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 2017, 23, 938–44. doi: 10.1038/nm.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Molofsky AB, Van Gool F, Liang H-E, Van Dyken SJ, Nussbaum JC, Lee J, et al. Interleukin-33 and interferon-γ counter-regulate group 2 innate lymphoid cell activation during immune perturbation. Immunity 2015, 43, 161–74. doi: 10.1016/j.immuni.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Zhou L, Chu C, Teng F, Bessman NJ, Goc J, Santosa EK, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature 2019, 568, 405–9. doi: 10.1038/s41586-019-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343, 1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Chong AS, Alegre M-L, Miller ML, Fairchild RL.. Lessons and limits of mouse models. Cold Spring Harb Perspect Med 2013, 3, a015495. doi: 10.1101/cshperspect.a015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Spits H. The road from mouse to human ILCs: a perspective of understanding the roles of ILCs in disease. Adv Exp Med Biol 2022, 1365, 161–6. doi: 10.1007/978-981-16-8387-9_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.