Abstract
Description
The recent pandemic of SARS-CoV-2, which causes novel coronavirus disease 2019 (Covid-19), has had devastating impact on a global and national scale. In order to overcome this outbreak it is imperative we find treatments that are safe and effective. To date, no definitive treatment is available that can curtail the spread of this viral syndrome. Convalescent plasma (CP) is one such option that has repeatedly served as an important tool in treatment of various bacterial and viral infections, especially in the setting of no specific antimicrobial or vaccination against an infectious disease. Herein, we review the history of CP, prior usage of CP in various infections and pandemics to date, mechanism of action of the same and conclude with a brief overview of the experience gained so far with use of CP in COVID-19.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, passive immunization, COVID-19 serotherapy, intravenous immunoglobulins, plasma, emerging communicable diseases
Introduction
Convalescent plasma (CP) as a means of therapy against infectious diseases dates back to the late 19th century. Over the course of the past 150 years incremental developments were made on how immunity and protection can be conferred using this mode of therapy. However, advances in antimicrobial and vaccine technologies in the past 50 years that helped eradicate or control several infectious diseases have pushed the CP methodology into the background. The current pandemic of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus-2 (SARS CoV-2), has reignited interest in passive immunity as a therapeutic option. It has been driven largely due to the absence of successful antimicrobials or effective vaccines to counter the infection. As of this publication, there are approximately 10 million cases detected worldwide with approximately 500,000 deaths attributed to the same.1
In this article, we review the history of usage of CP in various infections and pandemics to date, mechanism of action of CP and conclude with a brief overview of the experience gained so far with use of CP in COVID-19.
History of Convalescent Plasma
The concept of using serum therapy, as it was originally known, dates back to as early as late 19th century. In the 1870s, Maurice Raynaud (the same physiologist who first described Raynaud’s disease) described a concept akin to cell mediated immunity while studying vaccinia virus when he concluded that the virus inside the lymph nodes was able to elicit an “elaborated lymph,” which conferred systemic immunity.2, 3
Concurrently in the 1880s, Auguste Chauveau, a French veterinarian, proposed a concept of humoral immunity, wherein microorganisms produced some unknown substance within their host’s blood that are harmful to themselves.2, 4 While his experiments with Bacillus anthracis were deemed a failure, the concept nevertheless led to additional work by Charles Richet and Jules Hericourt. Working with Staphylococcus pyosepticus, they noted dogs were naturally resistant to this bacterium, whereas rabbits were not. They hypothesized that immunity could be transmitted from dogs to rabbits by transfer of blood. In their studies in 1888, they were able to demonstrate protection in rabbits that were transfused with immune blood from healthy dogs. The two sentinel observations in their study on rabbits challenged with S. pyosepticus were: blood transfusion conferred immunity against the bacterium in rabbits, and immunity was stronger if donor blood came from dogs that were accidentally inoculated by the bacterium a few months prior.2, 5, 6 Herein, Richet and Hericourt had discovered a new immunization method against infectious diseases based on transfer of humoral immunity from an immune animal to a nonimmune animal.
With the initial framework laid down by the Frenchmen, in 1890, the German physiologist Emil von Behring and his Japanese student Shibasaburo Kitasato demonstrated that transfer of blood from a rabbit immune to tetanus toxin could confer immunity to the disease in nonimmune rabbits.7, 8 Specifically, they revolutionized the immunization concept using toxins instead of whole/live microbes and proved the clinical success of serotherapy.7 They are credited with the discovery of the immunoglobulin purification technique and its application as a potential therapeutic option in human disease. For this discovery, in 1901, Behring and Kitasato were awarded the first Nobel Prize for Medicine.
By the turn of the century, serum therapy was available for various infectious diseases including diphtheria, tetanus, botulism and scarlet fever (Table 1). Treatment with immune serum was performed successfully and saved the lives of many individuals, specifically children with diphtheria and soldiers with tetanus in World War I.9 As early as 1907, serum from individuals recovering from rubeola (measles) was used to prevent infection in nonimmune individuals.9 Human serum was effective for prophylaxis in measles, which at that time had a mortality rate of 6–7% in some populations.10
Table 1.
Infectious diseases treated with convalescent human serum.
| Bacteria | Disease |
|---|---|
| Bacillus anthracis | Anthrax |
| Bordetella pertussis | Whooping cough |
| Clostridium botulinum | Botulism |
| Clostridium tetani | Tetanus |
| Corynebacterium diphtheria | Diphtheria |
| Group A streptococccus | Erysipelas, Scarlet fever |
| Neisseria meningitidis | Meningitis |
| Streptococcus pneumonia | Pneumonia |
| Viruses | Disease |
| Rubeola | Measles |
| Mumps virus | Mumps |
| Varicella-zoster virus | Chickenpox, Shingles |
| Hepatitis B virus | Hepatitis B |
| HIV-1 | AIDS |
| Influenza A (H1N1) | 1918 Pandemic Influenza |
| Influenza A (H5N1) | Influenza A |
| Respiratory syncytial virus | RSV infection |
| Ebola virus | Ebola |
| SARS-CoV | SARS |
| MERS-CoV | MERS |
| SARS-CoV-2 | COVID-19 |
On a relevant note, the Spanish influenza of 1918 was the first pandemic where the effectiveness of convalescent blood products was clinically documented. A retrospective meta-analysis of eight studies from 1918–1925 involving 1703 patients was performed. An overall case-fatality rate of 16% was found among patients treated with convalescent human blood compared to 37% among those who did not. Given these findings, the authors concluded that patients with Spanish influenza pneumonia who received influenza-convalescent human blood products may have experienced a clinically important reduction in the risk for death.11
Serum therapy was widely applied in pneumococcal disease and was found to be most effective if it was initiated within three days of onset of pneumococcal pneumonia.12, 13 Mortality of type 1 pneumonia could be reduced to 5% by administration of serum within the first 24 hours of onset of symptoms. By the early 1940s, serum therapy for pneumococcal pneumonia was standard practice and commercial, type-specific sera were available for many of the pneumococcal types.13
Serum therapy also had significant clinical and mortality benefits in meningococcal meningitis. In 1905–1906, a major epidemic broke out in New York City and along with a high mortality rate of 70–80% provided a major impetus for the development of serum therapy for Neisseria meningitis. Based on previous experimental animal models, humans were treated with intrathecal and/or intravenous injection of horse anti-meningococcal serum. Retrospective analysis of data from several studies showed statistically significant reduction in the rate of mortality for serum-treated patient when compared to untreated patients. Due to these compelling results, anti-meningococcal serum therapy became standard therapy and was recommended well into 1940s.13
It was not until the second half of the twentieth century when antibody-based therapies, along with convalescent plasma preparations, were developed for various viral syndromes, including rabies, hepatitis A, hepatitis B, varicella-zoster virus and respiratory syncytial virus (RSV).
Convalescent plasma therapy (CPT), as defined now, is collected via plasmapheresis from patient survivors who have developed humoral immunity in the form of disease specific antibodies, which can be transferred to other patients to help them treat the same infection. While antibiotics have largely replaced CPT in bacterial infections, it is still a viable therapy in viral infections in which no vaccine or other treatment has been proven to be effective. Serum therapy, while effective, was associated in up to 10–50% patients with serum sickness, secondary to antigen-antibody complex reaction, characterized by rash, arthralgia and proteinuria. Improved antibody purification methods did reduce toxicity, but the introduction of sulphonamides in the late 1930s led to decreased use of serum therapy. Antimicrobial therapy was less toxic, easier to administer, showed consistent efficiency between lots, and was overall more effective in eradicating bacterial infection.10 Serum therapy use, in contrast, was more time consuming to prepare based on bacterial strains, with significant lot-to-lot and dosing variations, which led to decline and eventual abandonment in serum therapy use by the 1950s.
More recently, CPT has been successfully used in the postexposure setting viral outbreaks such as mumps, polio, measles, rabies, influenza, Middle East Respiratory Syndrome (MERS) and Ebola, with positive changes in clinical outcomes in some instances.14–17 Ebola virus (EBOV) pandemic ravaged mainly Western Africa from December 2013 to June 2016. In the absence of a vaccine, initial management of Ebola virus patients was essentially supportive care, with fluid and electrolyte replacement, and management of secondary complications. In 2014, the World Health Organization proposed using convalescent blood products for Ebola victims. Several patients recovering from the Ebola virus received CPT, even as they were intubated and receiving dialysis for multi-organ failure.18 This recommendation was based on a study during an Ebola outbreak in Democratic Republic of Congo in 1995. In this study, eight patients were transfused with EBOV convalescent whole blood and seven recipients survived accounting for a 12.5% case fatality rate compared with the overall case fatality rate of 80% for the epidemic. However, it must be noted that the authors could not conclude whether CPT by itself or better supportive care primarily accounted for the survival benefit.19
How Does Convalescent Plasma Work?
Convalescent blood products as a therapeutic agent are believed to neutralize the pathogen, while also activating a specific immune response leading to the eventual eradication of the pathogen from the infected host. Several forms of blood products have been used to deliver this form of acquired passive immunity, including convalescent whole blood, convalescent plasma or convalescent serum, pooled immunoglobulins (Ig) for intravenous or intramuscular injections, high titer Ig fractions and concentrated polyclonal or monoclonal antibodies.
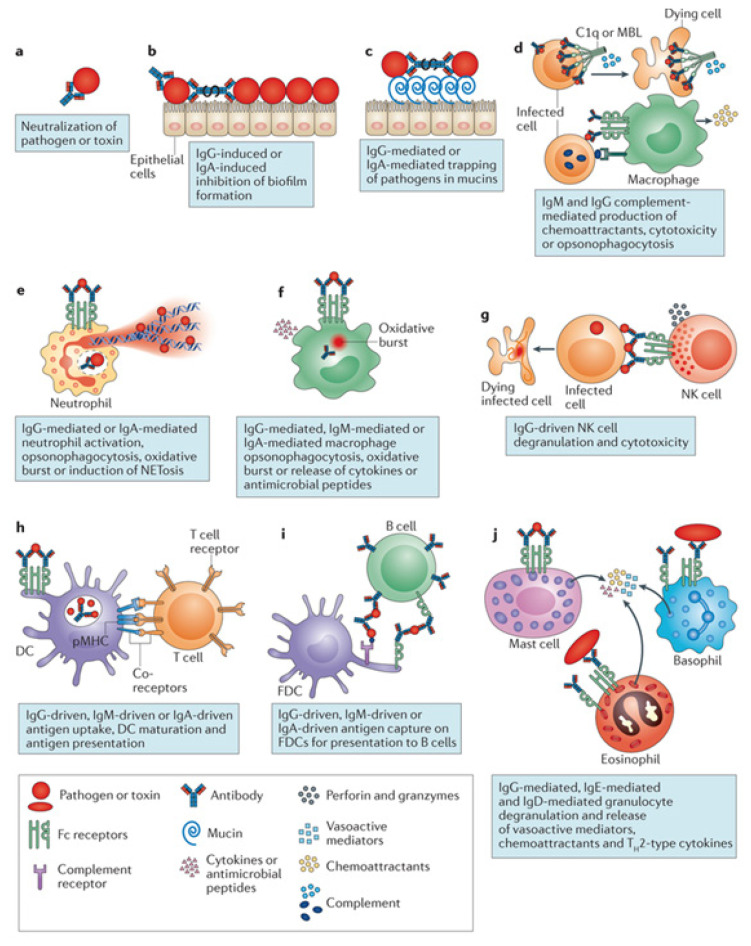
The critical component in all convalescent blood products is the antibody or Ig molecule, especially those specific to the pathogen of interest. These mediators of humoral immunity effect their actions through a variety of mechanisms that involve both the innate and adaptive immune systems in the host they are delivered into. While the degree of an individual mechanism in effecting pathogen clearance might be difficult to assess, the cumulative action is believed to contribute to the eventual protective response.20 (Figure 1) The mechanisms include the following:
Figure 1.
Antibody mechanisms of action in eliminating a pathogen. Reprinted by permission from Springer Nature: Springer Nature, Nature Reviews Immunology (Beyond binding: antibody effector functions in infectious diseases. Lenette L. Lu, Suscovich TJ, Fortune SM, Alter G.), Copyright (2018).
Neutralization of pathogen entry and replication,
Neutralization of toxins,
Neutralization of virulence factors,
Complement activation and evolution of adaptive immune response,
Antibody-dependent cellular cytotoxicity and
Antibody-dependent cellular phagocytosis.
To be clear, there is also the possibility of worsening of disease to be considered when exploiting CP as a therapeutic mode. This phenomenon is seen particularly in viral infections like Dengue and Zika, where multiple serotypes of the pathogen exists. Specifically, protective antibodies against one serotype of Dengue, but cross reactive with other serotypes are known to stabilize the second serotype and thereby facilitate entry within permissive cells, which result in disease.21–23 In the instance of Zika, both plasma from recovering patients and virus specific monoclonal antibodies have been shown to enhance infectivity in cell culture models.24 For this reason, and the general risk of other de novo viral transmission from plasma, the use of CP does need rigorous vetting before implementation as a safe therapy in the general population.
What Is the Role of Convalescent Plasma in COVID-19?
In the case of COVID-19, history suggests as the pool of COVID-19 survivors increase, CPT has the potential to become a viable treatment option.
A small study of 5 critically ill patients with confirmed COVID-19 and acute respiratory distress syndrome (ARDS) was conducted at the Shenzhen Third People’s Hospital in Shenzhen, China, from January 20, 2020 to March 25, 2020, and has served as a proof-of-concept of the benefit of convalescent plasma infusion in this population. Shen et al., found that the administration of CP-containing neutralizing antibodies (SARS-CoV-2-specific anti-IgG binding titer > 1:1000) led to improvements in clinical status, defined as decreased viral load, recovery from COVID-19 and discharge from hospital.25
Similarly, a prospective study of convalescent plasma in 10 patients with severe COVID-19 infection in three participating Chinese hospitals in Wuhan, also showed improved clinical symptoms along with increased oxygen saturations within just 3 days of CPT.26, 27 In addition to the primary treatment endpoint of safety, this study also showed improvement of additional parameters, including improvements in lymphocyte counts, C-reactive protein and radiographic images when compared with pre-transfusion values or images. Of the 10 patients, viral load was undetectable in seven patients after CPT.
Rajendran et al.28 recently published a comprehensive review on the efficacy and safety of CPT in patients with COVID-19. Their review included five independent studies, one conducted in South Korea and four in China, comprising a total of 27 patients. They concluded that, in addition to treatment with other antiviral and antimicrobial drugs, CPT proved to be an effective therapeutic option with promising evidence on safety, improvement of clinical symptoms and reduction in mortality.
As exciting and encouraging as these results are, attention must be drawn to the complexities of using antibodies as a treatment for highly-pathogenic viruses such as the SARS-CoV-2 virus. For example, a 2019 study in Rhesus monkeys showed that monkeys immunized with vaccines (containing SARS-CoV spike proteins) and confirmed to have developed high titers of neutralizing anti-spike antibodies before inoculation with SARS-CoV, experienced a more severe lung injury when compared with their non-immunized controls, despite having lower viral loads.29 A recent retrospective study in China found that when CP was infused immediately after the first detection of viral shedding, all six patients tested negative for SARS-CoV-2 RNA just 3 days after infusion; however, five patients eventually died, suggesting that CPT can halt SARS-CoV-2 shedding but does not improve mortality in critically-ill, end-stage COVID-19 patients.30 A possible explanation for these findings is that in the presence of antibodies, the disease burden is shifted to other immune cells such as macrophages, as has been demonstrated in vitro.31 Dysregulated innate immune responses, typical of severe acute lung injuries, may ensue with the involvement of macrophages.
Based on these small pilot studies, CPT for COVID-19 treatment has gained much attention, especially with no known effective treatment to date. It is important to understand how to best utilize convalescent plasma and in what setting. So far, the emphasis has been on patients with severe disease who have run out of treatment options. However, previous experiences in disease outbreaks mentioned earlier, show that CPT works best when used as prophylaxis or earlier in the disease process, as treatment. This important point was demonstrated in 2002–2004 SARS outbreak, where patients who received CPT within two weeks experience significantly better clinical outcomes when compared to those who received it after two weeks.32 The optimal dose and time of administration, as well as the clinical benefits of CPT in COVID-19, need to be better characterized and further investigated in the context of the above variables in better controlled studies.
As of June 2020, the United States Food and Drug Administration (FDA) has published on its website guidelines on three separate pathways for the use of CPT. Briefly, the following pathways are currently available for administering CPT or studying its utility: (1) Clinical Trials—investigators can submit requests, via email, to the FDA under the traditional investigational new drug (IND) regulatory pathway; (2) Expanded Access—this pathway includes an IND application to include the use of CPT in COVID-19 patients, not eligible or unable to participate in RCTs, and who have immediately life-threatening COVID-19; (3) Single Patient Emergency IND—allows the licensed physician to request a single patient emergency IND for their patients deem to have immediately life-threatening COVID-19, and are unable, for various reasons, to participate in RCTs. Full details about these pathways and how to apply for participation are found on the FDA website.33 Following these provisions, CPT is underway; mass calls for recovered COVID-19 patients to donate plasma is ongoing. For example, the COVID-19 expanded access program already has more than 2000 plasma collection sites, more than 5000 participating licensed physicians and more than 7000 infusions done already.34
According to FDA’s latest guidelines, potential donors must have had a documented SARS-CoV-2 infection, be symptom-free for at least 14 days and meet standard blood donor eligibility requirements. However, a negative result for COVID-19 by a diagnostic test is no longer necessary to qualify as a donor. While testing donor plasma for minimum neutralizing antibody titer (1:160, meaning 1-in-160 dilution of a given unit of plasma has activity against the virus) is recommended, this is not being done at testing facilities due to the lack of widely available, high-throughput, enzyme-linked immunosorbent assay (ELISA) based SARS-CoV-2 tests.33 Typically, plasma donations are only permitted every 28 days; however, due to high demand some collection sites are permitting eligible donors to donate every 7 days for a period of 28 days.
Conclusion
Although supported by a few small studies, and limited numbers of patients, CPT is appearing as a promising therapeutic modality to counter COVID-19. Questions remain on how or whether CPT influences the spectrum of the COVID-19 disease severity. Answers on a number of relevant variables, such as ideal timing of use (prophylactic versus early pre-symptomatic phase versus mildly symptomatic phase versus severe terminal phase), appropriate dose/number of infusions, standardization of donor antibody titers, will help address these questions. In addition, data about induced innate immune response by dysregulation of the immune system by CPT, which could potentially lead to increased toxicity and mortality need to be addressed to ensure safety of this methodology.
Nevertheless, in light of its long history, combined with absence of an effective vaccine or antiviral, CPT remains a worthy candidate as a therapeutic option to address COVID-19.
Funding Statement
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity.
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
The authors are employees of North Florida Regional Medical Center, a hospital affiliated with the journal’s publisher.
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time [published correction appears in Lancet Infect Dis. 2020 Jun 12] Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lahaie YM, Watier H. Contribution of physiologists to the identification of the humoral component of immunity in the 19th century. MAbs. 2017;9(5):774–780. doi: 10.1080/19420862.2017.1325051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raynaud M. Etude experimentale sur le role du sang dans la transmission de l’immunite vaccinale. C R Hebd Seances Acad Sci. 1877;84:453–6. [Google Scholar]
- 4. Chauveau A. Theorie de la contagion mediate ou miasmatique. Des voies par lesquelles s’opere l’infection des sujets sains exposes a la contagion. C R Hebd Seances Acad Sci. 1868;67:898–903. [Google Scholar]
- 5. Hericourt J, Richet C. Sur un microbe pyogene et septique (Staphylococcus pyosepticus) et sur la vaccination contre ses effets. C R Hebd Seances Acad Sci. 1888;107:690–2. [Google Scholar]
- 6. Hericourt J, Richet C. De la transfusion peritoneale, et de l’immunite qu’elle confere. C R Hebd Seances Acad Sci. 1888;107:748–50. [Google Scholar]
- 7. Good RA, Lorenz E. Historic aspects of intravenous immunoglobulin therapy. Cancer. 1991;68(6 Suppl):1415–1421. doi: 10.1002/1097-0142(19910915)68:6+<1415::aid-cncr2820681402>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8. von Behring E, Kitasato S. Uber das zustandekommen der diptherie-immunitat und der tetanus-immunitat bei thieren. Deutsche Medizinische Wochenschrift. 1890;16:1113–1114. doi: 10.1055/s-0029-1207589. [DOI] [PubMed] [Google Scholar]
- 9. Cenci F. Alcune esperienze d sieroimmunizzaziuone e sieroterapie nel norbillo. Rivista di Clinica e Pediatrica. 1907;5:1017–1025. [Google Scholar]
- 10. Casadevall A, Scharff MD. Return to the Past: The Case for Antibody-Based Therapies in Infectious Diseases. Clinical Infectious Diseases. 1995 July;21(1):150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006 Oct 17;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 12. Finland, Maxwell, Brown, John Specific treatment of pneumococcus type I pneumonia: Including the Use of Horse and Rabbit Antipneumococcus Serums and Sulphanilamide. Am J Med Sci. 1939;197(2):151–167. doi: 10.1097/00000441-193902000-00003. [DOI] [Google Scholar]
- 13. Casadevall A, Scharff MD. Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother. 1994;38(8):1695–702. doi: 10.1128/AAC.38.8.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450–1. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 15. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2(9):695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 16. Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–56. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahr F, Ansumana R, Massaquoi TA, Idriss BR, Sesay FR, Lamin JM, et al. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J Infect. 2017;74(3):302–9. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Winkler AM, Koepsell SA. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr Opin Hematol. 2015;22(6):521–526. doi: 10.1097/MOH.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 19. Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl 1):S18–S23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 20. Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawkes RA. Enhancement of the infectivity of arboviruses by specific antisera produced in domestic fowls. Aust J Exp Biol Med Sci. 1964;42:465–482. doi: 10.1038/icb.1964.44. [DOI] [PubMed] [Google Scholar]
- 22. Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A. 2007;104(22):9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beltramello M, Williams KL, Simmons CP, et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe. 2010;8(3):271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dejnirattisai W, Supasa P, Wongwiwat W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17(9):1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen C, Wang Z, Zhao F, et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma [published online ahead of print, 2020 Mar 27] JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: Interim guidance. [Accessed 9 May 2020]. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected .
- 27. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: Systematic review [published online ahead of print, 2020 May 1] J Med Virol. 2020 doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu L, Wei Q, Lin Q, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158. doi: 10.1172/jci.insight.123158. Published 2019 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeng QL, Yu ZJ, Gou JJ, et al. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in Patients With Coronavirus Disease 2019. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perlman S, Dandekar AA. Immunopathogenesis of coronavirus infections: implications for SARS. Nat Rev Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Food and Drug Administration. Recommendations for Investigational COVID-19 Convalescent Plasma. [Accessed 9 May 2020]. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma .
- 34.COVID-19 expanded access program. Convalescent Plasma COVID-19 (Coronavirus) Treatment. [Accessed 9 May 2020]. https://www.uscovidplasma.org/