Abstract
Objective
The purpose of this study was to examine whether there is a causal link between rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) and autism spectrum disorder (ASD)
Methods
We used inverse variance weighted (IVW), weighted median, and MR-Egger regression methods to perform two-sample Mendelian randomization (MR) study using publicly available summary statistics datasets In addition, we employed genome-wide association studies (GWASs) for RA and SLE as exposure and an ASD GWAS as an outcome
Results
Thirty-three and 28 single-nucleotide polymorphisms from RA and SLE GWASs were selected as instrumental variables for ASD The IVW method revealed no evidence supporting a causal association between RA and SLE and risk for ASD (beta=−0077, standard error [SE]=0041, p=0062; beta=0014, SE=0021, p=0493) The weighted median approach yielded no evidence of any causal association between RA and SLE and risk for ASD (beta=−0071, SE=0058, p=0223; beta=0045, SE=0030, p=0130) MR-Egger analysis demonstrated no causal association between RA and SLE and risk for ASD (beta=−0062, SE=0079, p=0434; beta=0048, SE=0043, p=0273) The MR results calculated using IVW, the median weighted and the MR-Egger regression approaches were consistent
Conclusion
The findings of the MR analysis did not support a causal relationship between RA or SLE and the risk of ASD
Keywords: Rheumatoid arthritis, Systemic lupus erythematosus, Autism, Mendelian randomization analysis
INTRODUCTION
Autism spectrum disorder (ASD) refers to a group of early-onset neuropsychiatric diseases characterized by a combination of communication and social interaction deficits, as well as the development of stereotyped and repetitive behaviors and interests [1]. Although the causation of ASD remains unknown, genetic factors are thought to have a role in the pathogenesis of ASD [1].
Rheumatoid arthritis (RA) is a systemic autoimmune disease that causes chronic synovial joint inflammation, disability, and a worse quality of life. Systemic lupus erythematosus (SLE) is a prototypical autoimmune disorder marked by significant inflammation and organ damage due to a disruption in immunological regulation. The pathogenesis of ASD is thought to be influenced by parental autoimmunity and inflammation [2-4]. Observational studies have shown a possible link between ASD and rheumatic autoimmune disorders, including RA and SLE [2,5]. In addition, a meta-anlaysis of observational studies have shown that maternal RA or SLE are linked to a higher risk of ASD in kids, suggesting that genetic factors associated with RA or SLE may have a role in the development of ASD in RA or SLE patients [6].
However, observational studies are prone to biases, including reverse causality and residual confounding, which limits our knowledge of the impact of RA and SLE on ASD [7]. Mendelian randomization (MR) is a method that evaluates whether an observational relationship between a risk factor and an outcome is compatible with a causal impact by using genetic variations as instrumental variables (IVs) [8,9]. Therefore, we tried to determine whether RA and SLE are causally linked to ASD using MR analysis.
MATERIALS AND METHODS
Data sources and selection of genetic variants
The National Human Genome Research Institute–European Bioinformatics Institute Genome-Wide Association Study catalog (https://www.ebi.ac.uk/gwas), a comprehensive library of reported associations from published genome-wide association studies (GWASs), was searched using RA and SLE as exposures and ASD as an outcome. A GWAS meta-analysis of 19,234 RA cases and 61,565 controls from 22 studies of European and Asian ancestry was utilized as an exposure for RA (14,361 RA cases and 43,923 controls from 18 studies of Europeans, and 4,873 RA cases and 17,642 controls from 4 studies of Asians) [10]. As exposure for SLE, summary data from an SLE GWAS, comprising 7,219 SLE and 15,991 control individuals of European ancestry, were utilized [11]. As a result, a publicly available summary statistics dataset from a GWAS meta-analysis of 14,525 ASD patients and 14,890 European ancestry controls was used [12]. Based on a p-value threshold of 5.00×10−8, genetic variations linked with RA or SLE were utilized as IVs to enhance inference (genome-wide significance). The summary statistics (beta coefficient and standard error [SE]) for single-nucleotide polymorphisms (SNPs) linked with RA and SLE as IVs from RA and SLE GWASs were collected, and the summary data of SNPs from ASD GWAS was utilized as an outcome.
MR statistical analysis
The independent association of SNPs with RA or SLE was first evaluated, followed by an investigation of the association between SNP and the risk for ASD. Using MR analysis, these results were then pooled to assess the uncompounded causal relationship between RA or SLE and ASD. A two-sample MR was performed, which is a method for estimating the causal effect of an exposure (i.e., RA and SLE) on outcomes (i.e., ASD) using summary statistics from different GWASs [13], using the summary of the SNPs from RA or SLE and ASD GWASs as IVs. The inverse variance-weighted (IVW) method employs a meta-analytic approach to combine the Wald ratio estimates of the causal effect obtained from different SNPs. It provides a consistent assessment of the causal impact of the exposure (RA and SLE) on the outcome (ASD), when each genetic variant meets the IV assumptions [14]. The weighted median and MR-Egger regression techniques were used to investigate and correct for pleiotropy (the relationship of genetic variations with more than one variable). The existence of imbalanced pleiotropy is accounted for in MR-Egger regression analysis tests by adding a parameter for this bias via the inclusion of summary data estimates of the causal effects of many individual variations, making them robust to invalid instruments [15]. Even if 50% of the information contributing to the study originates from genetic variations that are invalid IVs, the weighted median estimator gives a consistent estimate of the causal impact [16]. The weighted median estimator has the benefit of maintaining more accuracy in estimations than the MR-Egger analysis [16]. At p-value<0.05, the findings were deemed statistically significant. The MR Base platform (app version: 1.2.2 3a435d [January 31, 2019], R version 3.5.1; R Foundation for Statistical Computing, Vienna, Austria) was used for all MR analyses [17].
Tests for heterogeneity and sensitivity
Cochran's Q-statistics and funnel plots were used to evaluate heterogeneity between SNPs [18]. A "leave-one- out" analysis was also carried out to rule out the possibility that the causal relationship was caused by a single SNP.
RESULTS
Studies included in the meta-analysis
1) Instrumental variables for MR
Thirty-three and 28 independent SNPs from RA and SLE GWASs were selected as IVs (Supplementary Tables 1 and 2). These SNPs were linked to RA or SLE with a genome-wide significance (Supplementary Tables 1 and 2). R s12126142 in RA and rs6679677 in SLE were the only SNPs with nominally significant associations with ASD (Supplementary Tables 1 and 2).
2) MR results
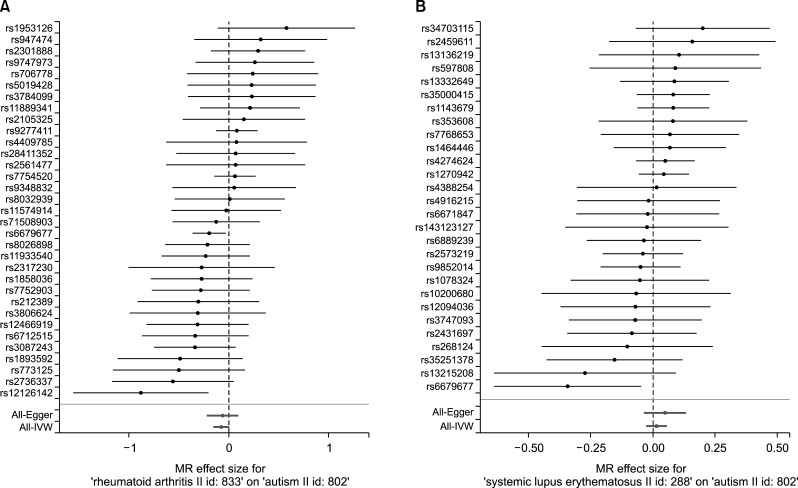
There was no support for an association between RA and SLE using the IVW technique, which showed no relationship (beta=−0.077, SE=0.041, p=0.062; beta=0.014, SE=0.021, p=0.493) (Table 1, Figures 1 and 2). We were unable to find evidence of a causal relationship between RA, SLE and the risk for ASD (beta=−0.071, SE=0.058, p=0.223; beta=0.045, SE=0.030, p=0.130) (Table 1, Figure 2). MR-Egger regression showed that the intercept was −0.003 with a p-value of 0.825 in RA. The intercept was −0.014 with a p-value of 0.375 in SLE. The MR-Egger analysis found no causal connection between RA and SLE and the risk for ASD, which had a beta coefficient of −0.062, with an SE of 0.079, and a p-value of 0.434 in RA; as well, the same analysis had a beta coefficient of 0.048, with an SE of 0.043, and a p-value of 0.273 in SLE (Table 1, Figures 1 and 2).
Table 1.
Mendelian randomization estimates from three methods analyzing the causal effect of RA and SLE on the risk of ASD
| Method | Number of SNPs | Beta coefficient | Standard error | Association p-value | Cochran Q statistic | Heterogeneity p-value |
|---|---|---|---|---|---|---|
| RA | ||||||
| Inverse variance weighted | 33 | −0.077 | 0.041 | 0.062 | 37.25 | 0.240 |
| MR-Egger | 33 | −0.062 | 0.079 | 0.434 | 37.19 | 0.205 |
| Weighted median | 33 | −0.071 | 0.058 | 0.223 | NA | NA |
| SLE | ||||||
| Inverse variance weighted | 28 | 0.014 | 0.021 | 0.493 | 19.34 | 0.857 |
| MR-Egger | 28 | 0.048 | 0.043 | 0.273 | 18.53 | 0.856 |
| Weighted median | 28 | 0.045 | 0.030 | 0.130 | NA | NA |
RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, ASD: autism spectrum disorder, MR: Mendelian randomization, SNP: single nucleotide polymorphism, NA: not available.
Figure 1.
Forest plot of the causal effects of rheumatoid arthritis- (A) and systemic lupus erythematosus- (B) associated single-nucleotide polymorphisms on the risk for autism spectrum disorder (ASD). IVW: inverse variance weighted, MR: Mendelian randomization.
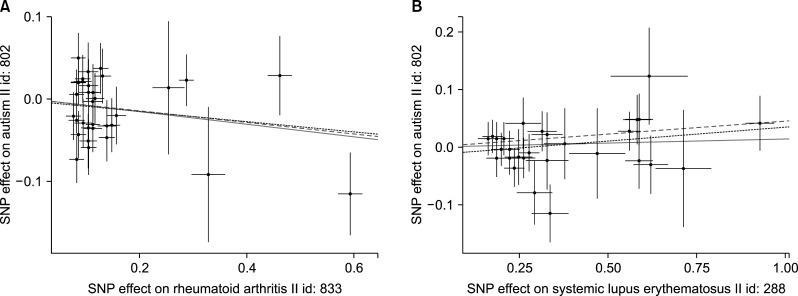
Figure 2.
Scatterplots of genetic associations with rheumatoid arthritis (A) and systemic lupus erythematosus (B) versus genetic associations with autism spectrum disorder. The slopes of each line represent causal associations for each method. The blue, green, and dark lines represent the inverse variance-weighted, weighted median, and mendelian randomization‐Egger estimates, respectively. SNP: single-nucleotide polymorphism.
Heterogeneity and sensitivity tests
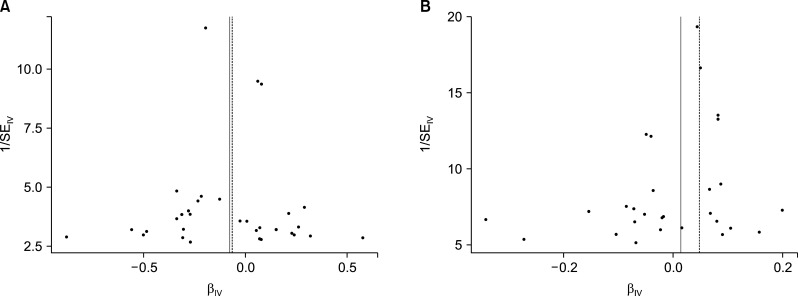
The Cochran's Q test and the funnel test detected no indication of heterogeneity in the IV estimates of the individual variations (Figure 3). The “leave-one-out” method revealed that, except for the 7 SNPs in SLE, no SNP was driving the IVW point estimate.
Figure 3.
Funnel plot assessing heterogeneity.
DISCUSSION
Autoimmunity in RA and SLE may be linked to poor placental development and placenta-mediated pregnancy problems, worsening birth [19]. Maternal autoimmunity may impair fetal brain development through immunological mediators, such as cytokines, T cells, and autoantibodies that reach the fetal bloodstream [19]. Maternal immune activation and fetal exposure to maternal cytokines may result in neurological and behavioral problems [20]. Maternal autoantibodies were shown to disrupt prenatal brain development and induce behavioral problems in offspring in an animal model [21]. Both maternal and paternal RA or SLE have been linked to an elevated risk of ASD in offspring, suggesting that parental genetic variables may play a role in the etiology of ASD in children with RA or SLE parents. In addition, a unique human leukocyte antigen locus has been shown to be shared by ASD and rheumatic diseases such as RA and SLE [22].
Even though maternal autoantibodies were shown to disrupt prenatal brain development and induce behavioral problems in offspring in an animal model and both maternal and paternal RA or SLE have been linked to an elevated risk of ASD in offspring, it has been unclear whether these associations are causal considering confounding factors or biases inherent in observational studies, or a limited number of studies with small sample sizes. We used different MR estimate techniques (IVW, weighted median, and MR-Egger regression). The MR estimates obtained using IVW, MR-Egger, and weighted median analysis were consistent, and did not show any causal relationships between RA, SLE, and ASD. Thus, previously observed correlations between RA or SLE and ASD may be due to confounding variables inherent in observational studies.
The current research had several limitations. First, the small number of SNPs were used as IVs, which may have reduced the ability to find relationships [23]. Therefore, the statistical power to identify a connection between genetically predicted SNPs in relation to RA or SLE and ASD may be low. Second, we used population of European ancestry with ASD for this research. Ethnicity may influence causality; thus, more MR research, including other races, is required. Nonetheless, the current MR analysis is valuable. While RA and SLE have been recognized as possible risk factors for ASD, an MR study has never been conducted. This is the first MR research to look at the possible involvement of RA and SLE in the development of ASD.
CONCLUSION
In conclusion, this MR analysis did not indicate causal correlations between RA or SLE and ASD risk. The epidemiological evidence suggesting a link between RA or SLE and ASD risk does not seem to be causative. Therefore, well-designed epidemiological and MR investigations are required to determine the causality.
SUPPLEMENTARY DATA
Supplementary data can be found with this article online at https://doi.org/10.4078/jrd.2022.29.1.46.
Footnotes
CONFLICT OF INTEREST
YH Lee has been an editorial board member since May 2017, but has no role in the decision to publish this article. No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 2.Rom AL, Wu CS, Olsen J, Jawaheer D, Hetland ML, Mørch LS. Parental rheumatoid arthritis and autism spectrum disorders in offspring: a Danish nationwide cohort study. J Am Acad Child Adolesc Psychiatry. 2018;57:28–32.e1. doi: 10.1016/j.jaac.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Keil A, Daniels JL, Forssen U, Hultman C, Cnattingius S, Söderberg KC, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010;21:805–8. doi: 10.1097/EDE.0b013e3181f26e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wojcik S, Bernatsky S, Platt RW, Pineau CA, Clarke AE, Fombonne É, et al. Risk of autism spectrum disorders in children born to mothers with rheumatoid arthritis: a systematic literature review. Arthritis Care Res (Hoboken) 2017;69:1926–31. doi: 10.1002/acr.23235. [DOI] [PubMed] [Google Scholar]
- 5.Tsai PH, Yu KH, Chou IJ, Luo SF, Tseng WY, Huang LH, et al. Risk of autism spectrum disorder in children born to mothers with systemic lupus erythematosus and rheumatoid arthritis in Taiwan. Joint Bone Spine. 2018;85:599–603. doi: 10.1016/j.jbspin.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Z, Tang S, Deng X, Wang Y. Maternal systemic lupus erythematosus, rheumatoid arthritis, and risk for autism spectrum disorders in offspring: a meta-analysis. J Autism Dev Disord. 2020;50:2852–9. doi: 10.1007/s10803-020-04400-y. [DOI] [PubMed] [Google Scholar]
- 7.Hill HA, Kleinbaum DG. Bias in observational studies. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. Chichester, John Wiley & Sons; 2000. [Google Scholar]
- 8.Burgess S, Daniel RM, Butterworth AS, Thompson SG. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol. 2015;44:484–95. doi: 10.1093/ije/dyu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YH, Song GG. Causal association between rheumatoid arthritis with the increased risk of type 2 diabetes: a Mendelian randomization analysis. J Rheum Dis. 2019;6:131–6. doi: 10.4078/jrd.2019.26.2.131. [DOI] [Google Scholar]
- 10.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–81. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47:1457–64. doi: 10.1038/ng.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross-Disorder Group of the Psychiatric Genomics Consortium, author. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45:1717–26. doi: 10.1093/ije/dyx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. 2013;178:1177–84. doi: 10.1093/aje/kwt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14. doi: 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemani G, Zheng J, Wade KH, Laurin C, Elsworth B, Burgess S, et al. MR-Base: a platform for systematic causal inference across the phenome using billions of genetic associations. BioRxiv. 078972 [Preprint] 2018. [cited 2018 May 30]. https://doi.org/10.1101/78972.
- 18.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SW, Zhong XS, Jiang LN, Zheng XY, Xiong YQ, Ma SJ, et al. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. Behav Brain Res. 2016;296:61–9. doi: 10.1016/j.bbr.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gata-Garcia A, Diamond B. Maternal antibody and ASD: clinical data and animal models. Front Immunol. 2019;10:1129. doi: 10.3389/fimmu.2019.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson WG, Buyske S, Mars AE, Sreenath M, Stenroos ES, Williams TA, et al. HLA-DR4 as a risk allele for autism acting in mothers of probands possibly during pregnancy. Arch Pediatr Adolesc Med. 2009;163:542–6. doi: 10.1001/archpediatrics.2009.74. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow DI, Kuchenbaecker KB, Shah S, Sofat R, Holmes MV, White J, et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol. 2016;45:1600–16. doi: 10.1093/ije/dyw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.