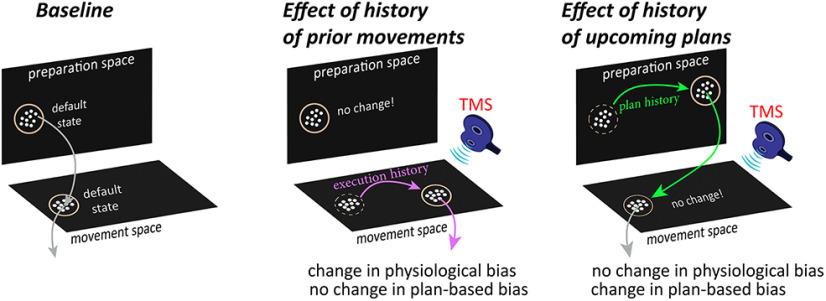
Abstract
Human motor behavior involves planning and execution of actions, some more frequently. Manipulating probability distribution of a movement through intensive direction-specific repetition causes physiological bias toward that direction, which can be cortically evoked by transcranial magnetic stimulation (TMS). However, because evoked movement has not been used to distinguish movement execution and plan histories to date, it is unclear whether the bias is because of frequently executed movements or recent planning of movement. Here, in a cohort of 40 participants (22 female), we separately manipulate the recent history of movement plans and execution and probe the resulting effects on physiological biases using TMS and on the default plan for goal-directed actions using a timed-response task. Baseline physiological biases shared similar low-level kinematic properties (direction) to a default plan for upcoming movement. However, manipulation of recent execution history via repetitions toward a specific direction significantly affected physiological biases, but not plan-based goal-directed movement. To further determine whether physiological biases reflect ongoing motor planning, we biased plan history by increasing the likelihood of a specific target location and found a significant effect on the default plan for goal-directed movements. However, TMS-evoked movement during preparation did not become biased toward the most frequent plan. This suggests that physiological biases may either provide a readout of the default state of primary motor cortex population activity in the movement-related space, but not ongoing neural activation in the planning-related space, or that practice induces sensitization of neurons involved in the practiced movement, calling into question the relevance of cortically evoked physiological biases to voluntary movements.
SIGNIFICANCE STATEMENT Human motor performance depends not only on ability to make movements relevant to the environment/body's current state, but also on recent action history. One emerging approach to study recent movement history effects on the brain is via physiological biases in cortically-evoked involuntary movements. However, because prior movement execution and plan histories were indistinguishable to date, to what extent physiological biases are due to pure execution-dependent history, or to prior planning of the most probable action, remains unclear. Here, we show that physiological biases are profoundly affected by recent movement execution history, but not ongoing movement planning. Evoked movement, therefore, provides a readout of the default state within the movement space, but not of ongoing activation related to voluntary movement planning.
Keywords: evoked movement, history of movement, motor planning, primary motor cortex, TMS
Introduction
Human motor performance depends not only on the ability of the sensorimotor system to plan and execute movements that are relevant to the current state of the environment and/or the body, but also relevant to the recent history of these actions. One emerging approach to understanding the effects of this recent history on the human brain is through the study of directional biases in involuntary movements evoked by transcranial magnetic stimulation (TMS) applied over the primary motor cortex (M1; Classen et al., 1998; Bütefisch et al., 2000). TMS provides a unique and powerful measure compared with other techniques, for example, because of the causal activation of effector-specific motor representation that can be precisely evoked and is not easily observed otherwise. For instance, TMS over the M1 generally evokes thumb movements in a consistent direction, but when participants voluntarily move their thumbs repeatedly over minutes in an opposite direction, subsequent TMS pulses now evoke thumb movements in the recently practiced direction (Classen et al., 1998; Bütefisch et al., 2000). These physiological, practice-dependent, TMS-evoked directional biases are robust and reliable and last for tens of minutes before returning to the baseline direction (Classen et al., 1998; Bütefisch et al., 2000).
What is the mechanism, at the behavioral and neural level, that drives these profound history-dependent physiological biases in the motor area? One possibility is that these are execution-dependent biases that provide a measurable readout of the physiological changes induced by motor practice (Bütefisch et al., 2000) or learning (Diedrichsen et al., 2010) paradigms. Thus, they might reflect a change in potentiation of synapses that are repeatedly activated in the very recent past (Classen et al., 1998; Ziemann et al., 2004; Selvanayagam et al., 2016), or changes in the tuning function of neuron population activity in the direction of a repeated action, such that when a stimulation pulse is applied over the neural population within M1, a new default state is elicited (De Valois et al., 1982; Chapman and Bonhoeffer, 1998; Scott et al., 2001).
Activity in M1, however, is not concerned only with generating motor commands but is also involved in the processing of higher-order signals for motor planning such as action selection (Gold and Shadlen, 2001; Romo et al., 2004; Cisek, 2007; Cisek and Kalaska, 2010). Using noninvasive TMS over M1 as a readout of the functional state of the motor cortex in humans during the planning period (i.e., time before an overt action that is unconfounded by descending motor commands), Klein-Flügge and Bestmann (2012) elegantly showed that the motor system is dynamically shaped by our prior expectations about forthcoming movements. Such prior expectations can be formed by variables that are relevant for action selection, such as the expected reward that can be obtained following an action or the uncertainty about the required action given the instruction cue (Bestmann et al., 2008b; Bestmann, 2012). Thus, an alternative explanation is that the evoked physiological biases might also reflect biases toward prior plans of upcoming movements. When hundreds of single-direction movements are repeatedly planned and executed in the recent past, it is possible that neural activity associated with the practiced movements becomes biased, not only toward the most frequently executed movement, but also toward the most probable movement to come next (Marinovic et al., 2017). Although not directly tested in TMS paradigms, this hypothesis was recently supported in goal-directed behavioral experiments by showing that behavioral biases primarily originate from changes associated with prior planning of movement, and to a much lesser extent, from changes associated with prior executed movement (Marinovic et al., 2017; Tsay et al., 2022). That is, participants seem to generate a default plan associated with a practiced or recent movement that can then be modified when the context requires a different action (Marinovic et al., 2017). Similar plan-based directional biases have been reported following repeated passive movement (Diedrichsen et al., 2010; Javidialsaadi et al., 2021).
Because prior history of movement executions and plans are indistinguishable and largely overlapped in previous work involving evoked movement, it remains unclear to what extent the physiological biases evoked by stimulation over the motor cortex are because of the effect of pure execution-dependent prior history and/or prior planning-dependent history of the most probable action and follow the same plan-based mechanism observed in previous behavioral work. Here, we systematically dissociate these possibilities using novel experimental manipulation of recent execution history and plan history and probe movement execution and plan biases, resulting from the manipulation, using a TMS paradigm and a behavioral timed-response task, respectively. We show that physiological biases reflect a process that is profoundly affected by the recent history of executed movement, with no evidence for a process sensitive to ongoing planning of future actions. We suggest that repetition-induced physiological changes provide a readout of the default state within the movement-related neural space and have little effect on ongoing neural activation related to planning of subsequent voluntary movements. Our findings also raise the possibility that evoked physiological biases and the well-documented behavioral biases reflect dissociable effects likely originating from different neural mechanisms. Finally, we discuss the potential influence of higher-level perceptual decision-making (e.g., processing sensory information or strategic decision) and motor-planning-related processing (e.g., action-selection) on the physiological and plan-based biases we observed.
Materials and Methods
Participants
A total of 40 young participants (22 females; age 24.31 ± 3.44 years; mean ± SD) were recruited to participate in three experiments. All participants were healthy and right-handed. Participants provided written consent to participate in the study, which was approved by the institutional review board and ethics committee of the Technion- Israel Institute of Technology. Participants were given monetary compensation for their participation (100 shekels, ∼$30).
Apparatus
The experimental protocol involved goal-directed and TMS blocks in different sequences for the three experiments (see below, Procedures; Figs. 1C, 2A, 3A) to dissociably manipulate the history of movements and plans while probing physiological and/or plan-based biases before, during, and/or after. In the TMS neurophysiological sessions, participants were seated in a chair and were firmly connected to a frame that kept the head steady and the stimulating coil in a constant position with respect to the head. Each participant's dominant forearm was immobilized using a customized armrest, with the four long fingers supported, and the thumb entirely unconstrained and free to move. Single TMS pulses that evoke involuntary thumb movements were delivered over the motor cortex. The point of TMS application is considered optimal if thumb movements are evoked in a consistent direction with stimulus intensities slightly above the movement threshold (Classen et al., 1998; Bütefisch et al., 2000). In goal-directed behavioral motor task sessions, participants were asked to make fast, voluntary center-out ballistic movements with the same thumb toward 5 mm diameter targets presented at an equal distance of 4 cm from the starting point (Fig. 1A,B). Three TMS behavioral experiments were undertaken to address the study aims.
Figure 1.
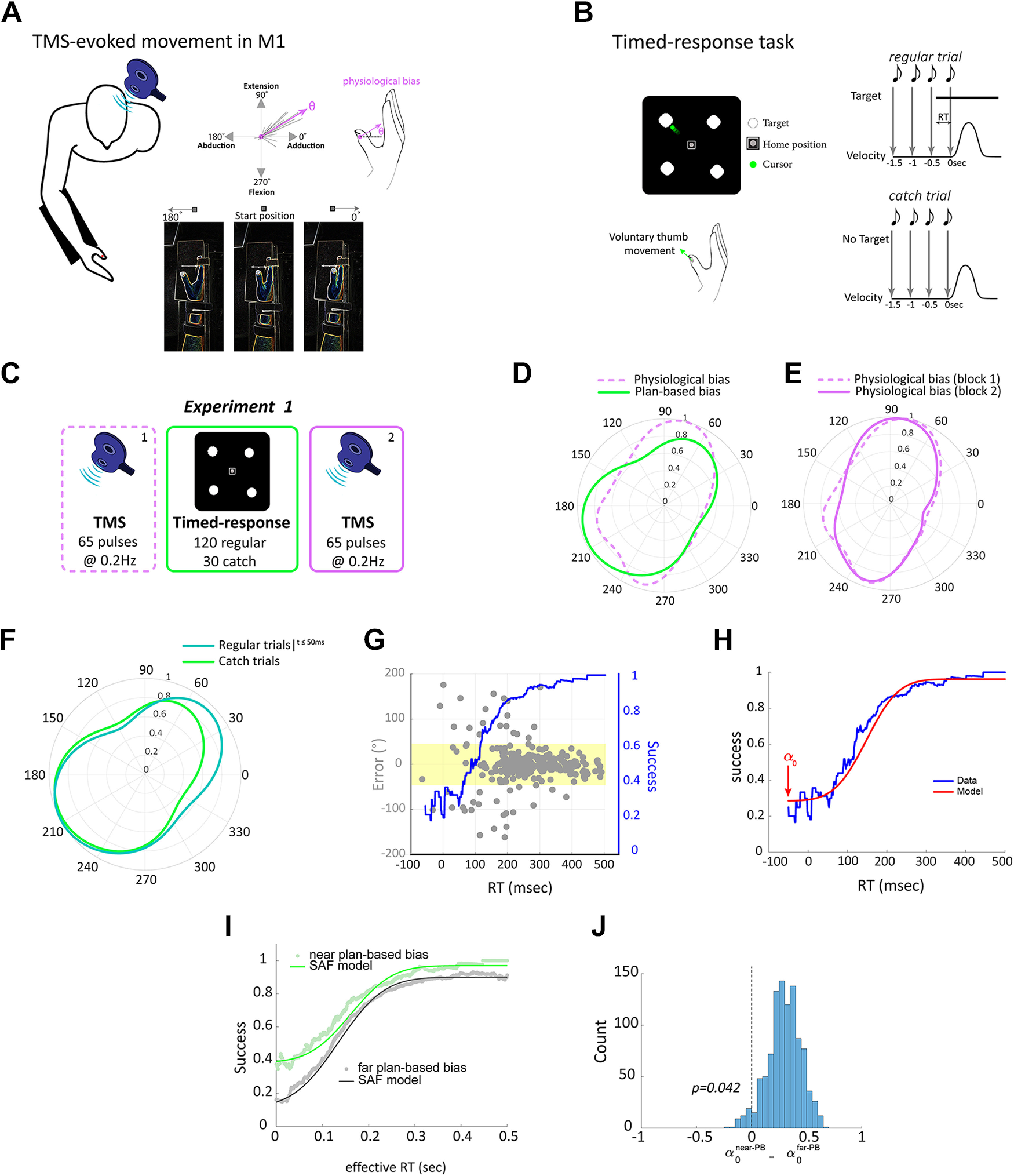
Experimental setup, protocol, and results of experiment 1. A, Schematic overview of the TMS protocol. We delivered TMS over the motor cortex to elicit thumb movements and measured physiological bias as the evoked movement with maximum probability. Top right, Dashed lines represent multiple evoked thumb movements. Bottom, The forearm and the index to the pinky fingers were immobilized and strapped to the armrest using a Velcro strap; only the thumb was free to move. The angles indicated the direction within a two-dimensional movement space (shown on the screen) and not anatomic range of motion. B, Timed-response task. Participants controlled the cursor presented on a monitor by voluntarily moving their thumb. In regular trials, participants initiated their movement synchronously with the onset of the fourth tone toward a target (top). In catch trials, no target ever appeared, but participants were still required to plan and move to any direction with the onset of the fourth tone (bottom). RT indicates effective reaction time. C, Protocol of experiment 1. Participants performed a block of TMS (65 pulses), followed by a block of goal-directed timed-response tasks (150 trials), and last, a block of TMS (65 pulses). The second TMS block was conducted to make sure that the relatively short block of voluntary movements (150 trials) did not lead to significant physiological biases. D, von Mises probability distribution of physiological biases (i.e., TMS-evoked movements; magenta) and plan-based biases (green). E, Distributions of physiological biases in TMS blocks 1 and 2, indicating that short practice of voluntary movement was not sufficient to induce significant physiological changes. Data in D, E are from all participants pooled together. F, The distribution of movement directions in the timed-response trials occurring any time before the target appeared and up to 50 ms immediately after the target was displayed (dark green) were comparable to the directions in the catch trails (light green). G, Example of data during the timed-response condition pooled across participants in experiment 1 (target 2). Gray points indicate direction error (degree) as a function of RT for individual trials; blue line shows the moving average of the probability that a movement is successful for a given RT, which corresponds to the speed-accuracy trade-off. Yellow area indicates the range of where a given movement was considered successful. H, Illustration of maximum likelihood model fit (red line) to empirical speed-accuracy trade-off data (blue line). Arrow indicates the estimated parameters that defined the participant's lower limit accuracy. I, Speed-accuracy trade-off for movements near the plan-based direction revealed that this movement involved the default plan. This was reflected by the increased accuracy at short RTs for targets near the plan-based bias but not for the other targets. J, Fitting the speed-accuracy function revealed significant difference of the parameter (which reflects participant's lower limit accuracy) between near plan-based bias (near-PBB) target and the other targets (far-PBB).
Figure 2.
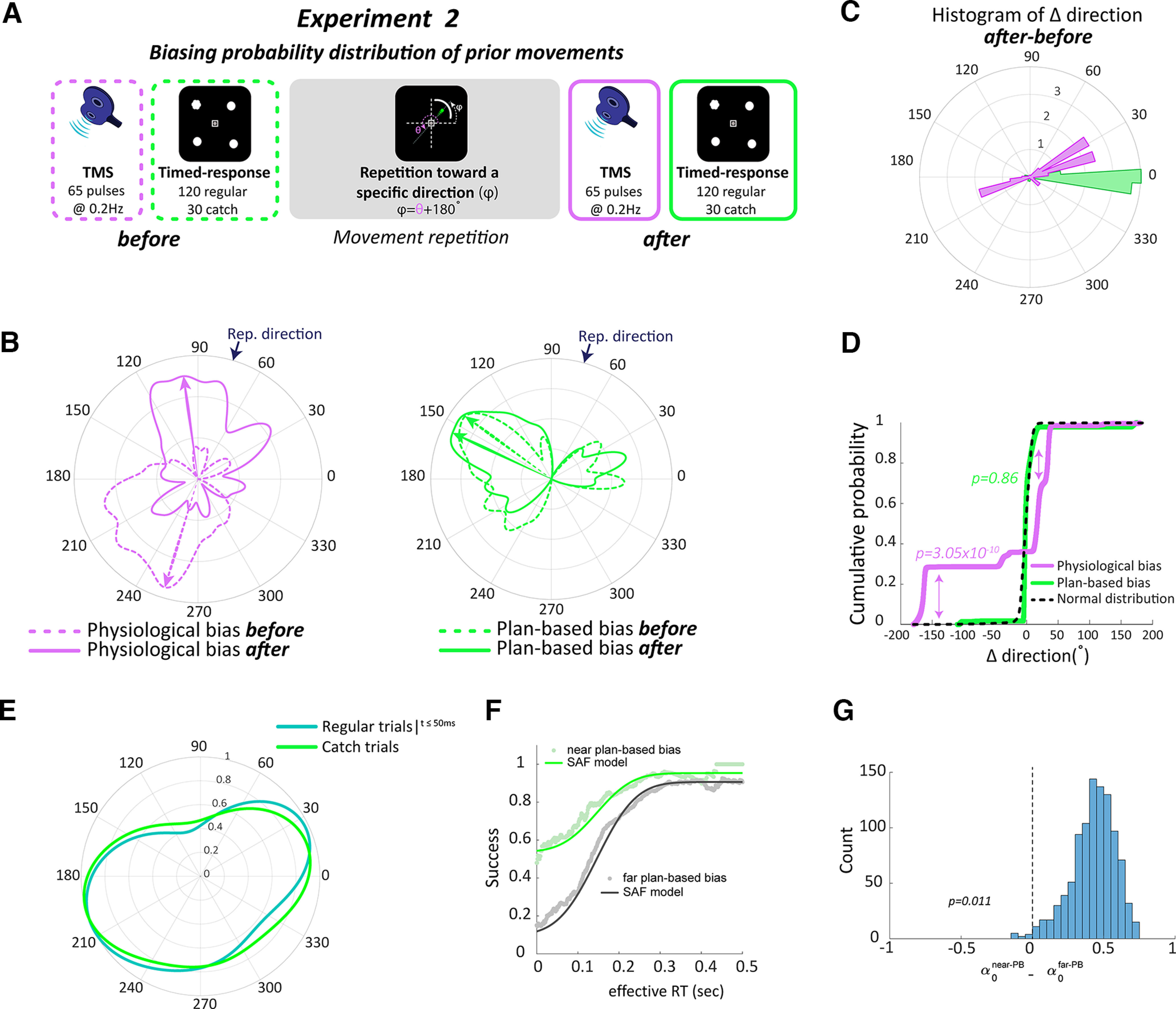
Movement history modulated physiological biases, not plan-based bias of voluntary movements. A, Protocol of experiment 2. B, von Mises probability distribution of physiological biases (left) and plan-based biases (right) before (dashed outlines) and after (solid outlines) changing probability distribution of prior movements through repetition toward a novel direction. Data from an individual participant. C, Histogram of change (after-before) in direction of physiological biases (magenta) and plan-based biases (green). D, Cumulative distribution function of differences in directions for physiological biases, plan-based biases, and null hypothesis of normal distribution (black dashed line). E, The distribution of movement directions in the timed-response trials any time before the target appeared and up to 50 ms immediately after the target was displayed (dark green) were comparable to the plan-based directions in the catch trials (light green). F, Speed-accuracy trade-off for movements near the plan-based direction revealed that this movement involved the default plan. This was reflected by the increased accuracy at short RTs for targets near the plan-based biases, but not for the other targets. G, Fitting the speed-accuracy function revealed significant difference of the parameter (which reflects participant's lower limit accuracy) between near plan-based bias (near-PBB) target and the other targets (far-PBB).
Figure 3.
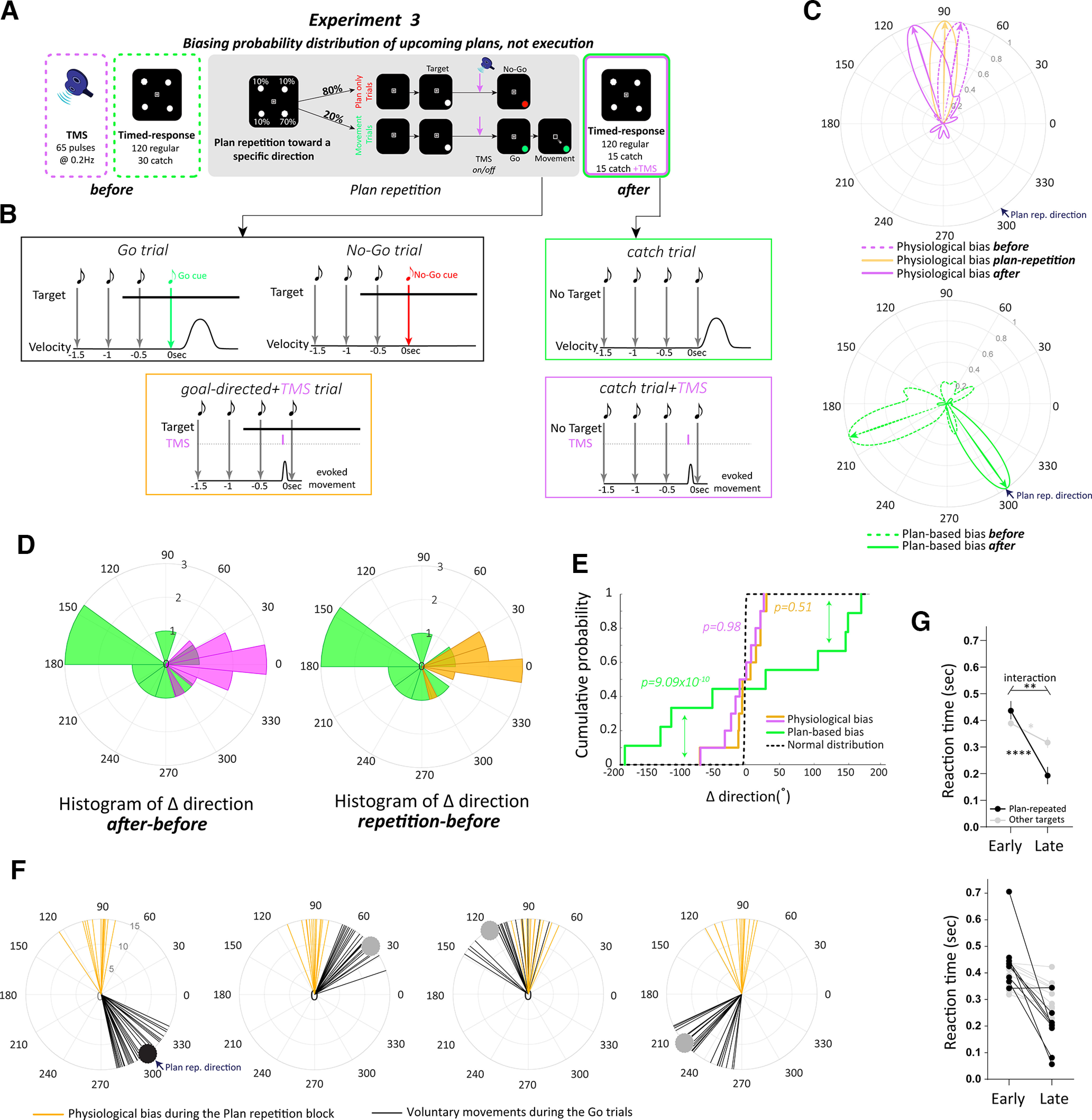
Movement-related physiological changes were distinct from ongoing planning-related processing. A, Protocol of experiment 3. B, Trial schedule of each trial type. During Go/No-Go trials, the target appeared 750 ms before the cue (Target turned green in Go trials, whereas in No-Go trials the target turned red.). In some trials, after presenting the target, a single TMS pulse was delivered 150 ms before the Go/No-Go cue. This allowed sufficient time to prepare the desired movement. During the postplan repetition block, we implemented the timed-response task that included some catch trials where target never appeared. In addition, in some of these catch trials, a single TMS pulse was delivered 150 ms before the fourth tone. C, von Mises probability distribution of physiological biases (top) before (dashed magenta), during (solid orange) and after (solid magenta) the plan repetition block. von Mises probability distribution of plan-based biases (bottom) before (dashed green) and after (solid green) the plan repetition block. Data from an individual participant. D, Histogram of change in direction of physiological biases (plan repetition-before, orange; after-before, magenta), and plan-based biases (green). E, Cumulative distribution function of differences in directions for physiological biases, plan-based biases, and null hypothesis of normal distribution. F, Thumb movements (black lines) during the Go trials and TMS-evoked thumb movement during ongoing planning of the desired movement (orange lines). Each circle represents the distribution of movement for each of the four targets during the plan repetition block. Data suggest robust and invariant physiological biases regardless of the plan. G, Reaction time (seconds) for movements in the Go trials toward the most frequent target (black) and the other targets (gray). sec, Seconds. Early and late represent the first and last 10 trials of the repetition block, respectively. Average across participants (top) and individual data are shown (bottom). Data show mean ± SE, *p < 0.05, **p < 0.01, ****p < 0.0001.
In general, we ensured that the position of the hand and the wrist relative to the table was consistent during all experiments and between all participants. The forearm was immobilized using a customized armrest that was fixed to the table. Additionally, the four fingers (index to pinky) were also immobilized and strapped to the armrest using a Velcro strap. Thus, only the thumb was free to move (Fig. 1A). Also, the starting position of the thumb was defined as the rest position. Using the rest position in which the muscles were always relaxed ensured general kinematic/kinetic consistency within subjects. Further, there was sufficient time between TMS trials for the subject to return to the rest position. Moreover, the positioning of the forearm and hand ensured biomechanical conditions that allowed the thumb to be able to move in any direction within the 360° movement space. Importantly, the angles in our study indicated the direction within a two-dimensional movement space (shown on the screen) and not anatomic range of motion. For example, 180° of thumb movement indicates the opposite direction to the baseline direction but not an actual anatomic range of motion of a full 180°.
TMS neurophysiological paradigm and apparatus
TMS sessions were performed using a PowerMag stimulator (Mag & More) with the participant at complete rest, defined as the absence of visible background electromyography (EMG using Delsys) activity exceeding the noise level of 25 μV (only one single trial of all study trials in experiments 1–3 pretraining and post-training sessions; equal to ∼0%) and confirmed by measuring surface EMG activity from abductor pollicis brevis (APB) and flexor pollicis brevis muscles (agonist and antagonist muscles, respectively) of the right hand, both known to be activated during thumb movements (Classen et al., 1998; Bütefisch et al., 2000; Celnik et al., 2006). A 70 mm figure-of-eight magnetic coil was placed tangential to the scalp with the handle pointing backward and laterally at a 45° angle away from the midline, approximately perpendicular to the central sulcus. To ensure accurate and consistent positioning of the TMS coil throughout the experimental sessions, a frameless 3D neuronavigation system (Mag & More) was used. The optimal scalp position (i.e., hot spot) for activation of the APB was identified using a stimulus intensity sufficient to evoke small thumb movements. In this optimal spot, the resting motor threshold, a measure of neuronal excitability, was determined as the minimum TMS intensity that evoked motor-evoked potentials (MEPs) of ≥ 50 μV in 5 of 10 trials at rest (Rossini et al., 1994; Pascual-Leone et al., 1995). The intensity of TMS for eliciting isolated thumb movements in a consistent direction was set at ∼120–140% of the resting motor threshold. This determination was done on an individual basis for each participant so that the delivered TMS pulses evoked consistent isolated thumb movements (i.e., minimal movement of the other fingers). The direction and amplitude of TMS-evoked thumb movements were recorded using six motion-capture cameras (Prime 13, OptiTrack) to record the trajectory of a spherical reflective marker placed on the thumbnail at a sampling rate of 240 Hz. The position of the reflective marker was mapped onto a screen, in which the x-axis and y-axis represented thumb adduction/abduction and flexion/extension, respectively. The starting position was the rest position for consistency, and before each timed response block, the cursor on the screen was calibrated within the OptiTrack software to appear at the center position of the screen, ensuring that flexion/extension and abduction/adduction movement direction, or any combination of them, would be accurately mapped onto the screen.
Goal-directed behavioral paradigm and apparatus
The participant's dominant forearm was restrained in a molded armrest in a semipronated position so that the four fingers were in a slightly extended position, and the thumb was entirely unconstrained. The use of the armrest allowed consistent positioning of the arm and hand across the different sessions of the experiments. The same motion-capture camera setup as in the TMS paradigm was used to record thumb trajectory. The participant was able to control a screen cursor by moving her/his thumb (Fig. 1B). Participants performed three types of movement trials (each in a separate block), timed-response, movement repetition, and plan repetition trials. In timed-response trials (experiments 1–3), after entering the central start position, participants heard a sequence of four tones, spaced 500 ms apart. One of four potential targets appeared 50–300 ms before the fourth tone, and participants were instructed to move to the target as quickly as possible synchronously with the onset of the fourth tone (Favilla et al., 1989; Ghez et al., 1997; Haith et al., 2016). Manipulation of the timing of the target appearance allowed us to effectively impose a relative reaction time (RT) on each trial and thus control available response preparation time. We characterized the dynamics of response preparation for each target by assessing the accuracy of participants' movements as a function of the imposed RT. The predictable sequence of auditory tones serves to minimize the ambiguity about the time of target presentation, which is known to increase RTs (Frith and Done, 1986; Haith et al., 2016). Movement initiation time was determined online as the time at which the tangential velocity first exceeded 5% of maximum velocity. If participants failed to initiate their movement within ± 150 ms of this time, an on-screen message, Too Early or Too Late, was shown accordingly. The timed-response trials included a subset of catch trials in which no target ever appeared, and participants were required to move with the onset of the fourth tone to any direction of their choice. These catch trials enabled us to assess participants' default preparation in the absence of a specific presented target location (see below, Discussion for interpretation of catch trials). With respect to these trials, participants were instructed to move as quickly as possible to any direction when they heard the fourth tone if no target appeared. In the timed-response trials in general, participants were not given any specific instructions or information about potential targets (e.g., time of target presentation, locations, etc.). The intertrial interval in the timed-response task was set at 2 s. In movement repetition trials (experiment 2), participants were instructed to repeat the same movement toward the center of a semicircular arc target. The center of the arc was 180° from the baseline TMS-evoked direction. Participants were instructed to make quick and accurate movements following presentation of the arc. The plan repetition trials were similar to the timed-response trials but implemented a Go/No-Go cue as well as an altered target probability distribution (i.e., 70, 10, 10, 10%) and consisted of 750 trials. The frequent target in experiment 3 was selected using the following steps: (1) The maximum probability of the TMS-induced movement distribution in the baseline block was calculated (Angle 1), (2) the maximum probability of the planning bias distribution from the time-response block (before) was calculated (Angle 2), (3) the mean angle of the two calculated angles (Angles 1 and 2) was calculated, and (4) the direction of the frequent target was chosen to be as far as possible from the planning bias found in the first time-response block (Angle 2) and to not be close to the direction of the most probable TMS-induced movement (Angle 1). Therefore, to avoid any scenario where there is an overlap between the direction of the frequent target and TMS-induced movement direction, the frequent target direction in the repetition block was set as the direction that is 180° shifted from the mean angle of Angle 1 and Angle 2 (180° minus mean angle). Additionally, a single TMS pulse was delivered during some of these trials, 150 ms before the Go/No-Go cue (see below, experiment 3).
Procedure
Experiment 1
The experiment was designed to determine whether baseline TMS-evoked physiological biases share similar low-level movement kinematics with plan-based biases of voluntary movement estimated using the timed-response task. Participants (n = 15, age 24.3 ± 2.3 years) performed three consecutive blocks (TMS, timed-response, TMS; Fig. 1C). In the first block, participants underwent a TMS paradigm. During this block, the direction of the thumb movement was established by delivering a series of 65 TMS pulses at 0.2 Hz to the optimal scalp position that evoked isolated involuntary thumb movements. The second block included a goal-directed task. Participants were asked to perform 150 timed-response thumb movements, either toward a target (120 trials), selected randomly from four targets [ + (0°, 90°, 180°, and 270°)] positioned 4 cm from the origin or toward the direction of their choice in the absence of a presented target (30 trials). Targets were presented randomly 30 times each. Finally, participants again underwent a TMS block identical to the first TMS block. The second TMS session was conducted to verify that a short practice of timed-response goal-directed tasks is insufficient to elicit physiological biases (Classen et al., 1998; Flöel et al., 2005) so that baseline measurements would be distinct and not confounded by other biases. Time between blocks was ∼3–5 s.
Experiment 2
In this experiment, we systematically manipulated the probability of the recent history of executed movements by asking participants to repeatedly make thumb movements toward a novel direction located in the opposite direction of each participant's baseline TMS bias (). We estimated the physiological biases using TMS and plan-based movement biases using the catch trials of the timed-response block, before and after participants voluntarily repeated a movement toward the center of a semicircular arc target set at 180° from . Specifically, participants (n = 15, age 25.2 ± 4.3 years) were asked to perform three consecutive sessions, baseline, movement repetition, and postmovement repetition (Fig. 2A). The baseline session included one TMS block and one goal-directed behavioral block, as in experiment 1. In the movement repetition session, participants were instructed to repeat the same thumb movement for 40 min toward the repeated direction, opposite to that evoked by the baseline TMS session (). In the postmovement repetition session, participants underwent another TMS block and another goal-directed block.
Experiment 3
To further test the relationship, or lack thereof, between physiological biases and ongoing activity related to motor planning, we systematically dissociated the prior history of movement execution from the prior history of movement plans. To do so, we biased the probability of the upcoming movement plans by making potential target locations (identical to those in the timed-response task) not equally probable and, instead, increased the likelihood of one of the four target locations while implementing a Go/No-Go goal-directed paradigm. Participants (n = 10, age 22.7 ± 2.9 years) were asked to perform three consecutive sessions: baseline, plan repetition, and postplan repetition (Fig. 3A). The baseline session included one TMS block and one goal-directed behavioral block, as in experiments 1 and 2. The plan repetition session included a goal-directed behavioral block with unequal target location probabilities. For each participant, the frequent target was set in the direction with the lowest probability of the fitted von Mises distribution for the movement direction in catch trials during the baseline timed-response block. Unlike the baseline block in which the four targets were uniformly distributed, the frequent target was presented in 70% of trials (525 trials), whereas the other three were each presented in 10% of trials (75 trials per target), with a total of 750 trials (see above, Goal-directed behavioral paradigm and apparatus for a description of the method for selecting the frequent target location). A Go/No-Go paradigm was implemented such that in 80% of trials, participants were led to prepare a movement but were cued thereafter not to execute it (No-Go cue), and in 20% of trials, participants were cued to execute the planned movement (Go cue). The target appeared 750 ms before the Go/No-Go cue, which was indicated by the gray target either turning green or red, respectively, simultaneously with the fourth tone. Within the plan repetition block, TMS was implemented, starting after the participant completed 500 trials of plan repetition, to probe change, or a lack thereof, in physiological bias because of plan history manipulation, and was delivered at random in 15 trials for each target, 150 ms before the Go/No-Go cue. A postplan repetition block was performed following the plan repetition block, which consisted of a modified timed-response block, integrating TMS to test physiological biases within the same context as probing of any changes in the default plan resulting from plan repetition. Thus, the postplan repetition block consisted of an identical timed-response task as the previous ones but with a single pulse of TMS delivered in 15 of the 30 catch trials, 150 ms before the fourth tone.
Data analysis
Physiologic TMS and behavioral data were analyzed using custom routines in MATLAB to compute physiological biases, plan-based biases, repeated direction, reaction time, accuracy, and speed-accuracy trade-off function.
Physiologic biases (i.e., TMS evoked)
The direction and amplitude of TMS-evoked thumb movements were determined using six motion-capture cameras that recorded the trajectory of a spherical reflective marker placed on the thumb at a sampling rate of 240 Hz. The position of the reflective marker was mapped onto a screen in which the x-axis and y-axis represented thumb adduction/abduction and flexion/extension, respectively. Given the x and y position of the marker, the velocity of the involuntary thumb movement was calculated. The thumb direction was defined as the direction at movement onset. Movement onset was specified, off-line, as the time point when the velocity of the cursor exceeded 5% of peak velocity. Then, distribution of the thumb direction was estimated using the von Mises distribution (Gatto and Jammalamadaka, 2007), and the direction with the highest probability of the fitted von Mises distribution was considered as the physiological bias.
Plan-based biases (i.e., default plan)
During catch trials, no target ever appeared, and participants were required to move with the onset of the fourth tone to any direction. These catch trials enabled us to assess participants' default plan in the absence of a specific presented target location. The distribution of thumb direction in these catch trials was estimated using the von Mises distribution, and the direction with the highest probability of the fitted von Mises distribution in catch trials was considered as the plan-based bias. After defining the plan-based direction, we defined the target near the plan-based bias (near-PBB) in the regular timed-response trials as the closest target with minimal distance (≤45°) from the direction of the plan-based bias. All other targets were defined as the far-PBB targets.
RT
During the timed-response trials, RT (or effective RT) was calculated as the time interval between target presentation and movement onset. During the Go trials of the plan repetition block, RT was calculated as the time interval between Go cue and movement onset.
Speed-accuracy trade-off function
For each target, the probability of initiating an accurate movement (the success rate) in the timed-response trials at any given RT was estimated based on the proportion of accurately initiated movements within a 125 ms window around that RT. A movement was considered accurate (i.e., successful) if the initial direction of the movement was within ±45° of the target direction. Otherwise, the movement was considered an error. This yielded an estimate of the speed-accuracy trade-off function (Fig. 1G). To quantitatively characterize this trade-off, we followed the likelihood model presented by previous studies (Haith et al., 2016; Mawase et al., 2018) in which a single preparation event is assumed to occur at a stochastic time , with movements initiated before directed randomly, and movements initiated after directed accurately toward the target. These assumptions lead to the speed-accuracy trade-off following a cumulative Gaussian shape (Fig. 1H). Because we fit this model to the different targets, participants may have expressed preferred default movements at very low RTs and would thus have been either above or below chance. We allowed for this possibility through a parameter that defined the participant's lower limit accuracy. We also allowed for the fact that participants were not 100% accurate even at long RTs through a parameter that defined the participant's asymptotic accuracy.
We estimated these parameters (, , , ) based on maximum likelihood. The probability of the movement in trial being accurate () given that it was initiated at time was then given by the following:
where is the cumulative normal distribution function. The log likelihood function for each trial was therefore defined as follows:
The total log likelihood of the parameters was therefore given by the following:
To prevent overfitting and to improve the generalization of our model, we used a regularization technique, introducing a penalty on the variance (i.e., ) of as follows:
where is the regularization term, which we fixed at , and corresponds to a slope prior, which we set at . Parameter estimates were obtained separately for targets near the plan-based bias direction and for targets far from the plan-based bias direction based on the pooled data from all participants in the timed-response condition. We used the pooled data because the data for individual participants was too sparse to obtain reliable fits. As we were interested in comparing the accuracy at low RTs near the plan-based bias direction versus the other far plan-based bias targets, we collapsed the data of all directions toward targets far from the plan-based bias direction. We calculated confidence intervals for parameter estimates through a bootstrap analysis, using 1000 unique combinations drawn with replacements from the subject pool. The 95% confidence intervals were calculated as the 2.5th and 97.5th percentile values from the distribution for each coefficient obtained across the 1000 fits. To visualize the distribution of reach directions in the catch trials in experiments 1–3, we used a kernel density estimation procedure using von Mises kernels with a kernel width of 30°.
Statistical analysis
The statistical analysis was performed using the Circular Statistics Toolbox (Directional Statistics) in MATLAB software (MathWorks) and Prism software (GraphPad). A two-sample Kuiper's test (Kuiper, 1960) was used to determine the similarity, or dissimilarity, between the distribution of physiological biases and plan-based biases at baseline. Kuiper's test is invariant under cyclic transformations of the independent variable. This invariance under cyclic transformations makes Kuiper's test valuable when testing for differences between circular probability distributions. In the Circular Statistics Toolbox, this test can be performed using the circ_kuipertest() function. To test the effect of manipulating statistics of the history of prior movement (experiment 2) or history of upcoming plans (experiment 3), we ran Kuiper's test on the change (i.e., post-training/pretraining) of physiological biases and plan-based biases and compared the distribution of the deltas with the normal distribution with We also used Kuiper's test to determine whether the direction of movements occurring before and immediately after the target was displayed were comparable to the plan-based directions in the catch trials during the timed-response task. Of note is that the p value of Kuiper's test is taken from tabulated values determined previously (Kuiper, 1960). In this table, any value of p > 0.1 is set to . To determine the accurate value in all cases when p > 0.1 (not significant), we used the equivalent one-sample t test for circular data and tested whether the difference of angles was significantly different from normal distribution with Finally, to assess the accuracy at low RTs of the speed-accuracy trade-off in experiments 1–3, we ran a bootstrap analysis on the parameters that defined the participant's lower limit accuracy, with 1000 resamples (see above, Data analysis). In all comparisons, the significance level was set at 0.05.
Results
Experiment 1: baseline physiological biases shared similar low-level movement kinematic properties with plan-based biases
We started with the question of whether the physiological biases evoked by TMS and the plan-based biases at baseline are similar or dissimilar. We predicted that if physiological biases reflect a distinct process from plan-based biases, then the distribution of TMS-evoked movements should show little overlap with the distribution of participants' baseline default plans. If movement distributions, however, show a large overlap at baseline, then at least two explanations might be possible. First, physiological biases may be sensitive not only to the history of the executed movement, but also to planning, supporting the hypothesis that these processes might reflect similar underlying mechanisms. Alternatively, both processes may be distinct, yet converge to a similar state of the neural activity, presumably reflecting a default movement affected by prior hand use, that can be evoked by the TMS stimulation.
We analyzed the direction of the thumb, our experimental end effector, during a protocol that included a goal-directed behavioral session preceded and followed by a TMS session. In general, in the TMS neurophysiological sessions, single pulses were delivered over the thumb area of the motor cortex that evoked involuntary and isolated thumb movements (Fig. 1A). In the goal-directed behavioral session, participants were asked to make fast, voluntary center-out ballistic movements with the same thumb toward different targets presented at an equal distance from the starting point. Thumb-to-cursor mapping was done using the following angle-to-thumb direction definitions: 0°, adduction; 90°, extension; 180°, abduction; and 270°, flexion. For example, to reach a target at 135°, participants needed to move the thumb in the extension-abduction direction (Fig. 1B, Table 1).
Table 1.
Distribution of thumb directions for the physiological bias and plan-based bias in Experiment 1
| TMS bias |
Plan bias | ||
|---|---|---|---|
| Subject | Block 1 | Block 2 | |
| Subj 1001 | 92 | 209.5 | 266 |
| Subj 1002 | 252 | 251.5 | 21 |
| Subj 1003 | 75 | 141.5 | 177.5 |
| Subj 1004 | 50 | 211.5 | 92 |
| Subj 1005 | 108.5 | 97.5 | 56 |
| Subj 1006 | 190 | 245.5 | 274 |
| Subj 1007 | 185 | 134 | 261 |
| Subj 1008 | 73 | 73.5 | 212.5 |
| Subj 1009 | 192 | 102.5 | 59 |
| Subj 1010 | 98.5 | 98 | 246.5 |
| Subj 1011 | 251 | 252 | 259 |
| Subj 1012 | 59 | 68 | 177.5 |
| Subj 1013 | 140 | 87 | 67.5 |
| Subj 1014 | 186 | 212.5 | 195.5 |
| Subj 1015 | 73.5 | 96.5 | 207.5 |
| MaxLikVonMises (°)a | 82.5 | 91 | 85 |
| Variance (°) | 48.53 | 50.85 | 45.13 |
Distribution of the directions (in degrees) of the physiological bias and plan-based bias in each phase for all participants in experiments 1.
aWe reported (here and in all other tables) the direction with the maximum likelihood of the fitted von Mises function, instead of the circular mean, to adequately demonstrate a representative metric of this type of bimodal distribution. Subj, Subject.
In experiment 1, participants underwent a single TMS block in which 65 pulses were delivered to estimate the physiological biases at baseline, and two goal-directed blocks (before and immediately after the TMS block) in which participants were asked to perform timed-response thumb movements toward a target, selected from four possible targets, each presented randomly 30 times (120 thumb movements in total). In each timed-response trial, participants heard a sequence of four tones, spaced 500 ms apart. The selected target appeared 50–300 ms before the fourth tone, and participants were instructed to move to the target as quickly as possible in synchrony with the onset of the fourth tone (Favilla et al., 1989; Ghez et al., 1997; Haith et al., 2016). The predictable sequence of auditory tones serves to minimize the ambiguity about the time of target presentation, which is known to increase RTs (Frith and Done, 1986; Haith et al., 2016). We also included a subset of catch trials (30 trials) in which no target ever appeared, and participants were required to move with the onset of the fourth tone to any direction (Fig. 1C; see above, Methods and Materials). These catch trials enabled us to assess participants' default plan for upcoming movement in the absence of a target presented in a specific location (see below, Discussion for possible cognitive-related interpretations of what was measured in catch trials). In total, participants performed 150 voluntary thumb movements in the timed-response block.
Distribution of thumb direction (during both evoked and voluntary conditions) was estimated using the von Mises distribution (Gatto and Jammalamadaka, 2007). Two measures of biases were then defined, physiological biases, defined as the direction with the highest probability of the fitted von Mises distribution on the TMS-evoked movements, and plan-based biases, defined as the direction with the highest probability of the fitted von Mises distribution on the plan-based movements during the catch trials of the timed-response block. Our data indicate that physiological biases and plan-based biases at baseline were highly similar, as both distributions showed a large overlap (Kuiper's test, ; Fig. 1D), suggesting that the representation of thumb movement on the motor cortex, as revealed by TMS, shares similar low-level kinematic properties (e.g., direction) with participants' default plan for impending movement execution during the timed-response trials. The distribution of the default plan directions in catch trials, as well as that of TMS-evoked movement, was not uniform but was biased toward particular directions, dominated by the far right (30–60°) and near left (190–270°; Fig. 1D). These movement directions are consistent with previous work (Haith et al., 2016; Mawase et al., 2018) and were likely preferred because they are the least effortful (Shadmehr et al., 2016) and/or influenced by thumb use in daily motor functions.
Consistent with previous work (Classen et al., 1998; Flöel et al., 2005), we confirmed that short practice (∼8.5 min) of voluntary goal-directed movements during the timed-response block, together with the fact that participants made movements equally to different directions, was insufficient to induce physiological biases and thus would not confound subsequent measurements. This was revealed by the similar distribution (no significant difference, ) of physiological biases before (TMS block 1) and after (TMS block 2) the goal-directed block (Fig. 1E). In addition, movements that were inadvertently initiated before, or immediately after the target cue (i.e., too early for the participant to have actually processed the target and planned accordingly; ≤50 ms after target presentation), in the timed-response trials (hereafter termed inadvertent movements, meaning movements that were not resulting from or achieved through deliberate planning) were analyzed and compared with catch trials to test whether they were essentially equivalent. To test this, we analyzed the direction of all inadvertent movements (9.33 ± 5.73% of trials) occurring any time before the target appeared and up to 50 ms immediately after the target was displayed. We found that movement direction in these trials was comparable to that in catch trials (no significant differences between distributions, ; Fig. 1F). Supporting evidence that the inadvertent-movement directions indeed reflected a default plan (i.e., default preparation of upcoming movement in the absence of a presented target) is the fact that the plan for movements to targets that appeared near the default directions should have been readily available, and when the target cue comes in, the participant should accurately reach the target with a shorter RT. We thus expected increased accuracy and shorter RTs for a target near the plan-based biases, defined as the closest target with a minimal distance (≤45°) from the plan-based bias, but not for the other targets. Indeed, fitting the speed-accuracy trade-off function (Fig. 1G,H) revealed significantly () increased accuracy (as reflected by the parameter that defined the participant's lower limit accuracy) at short latencies on targets near the plan-based direction (mean of 0.368 with 95% CI of [0.037, 0.581]), but not near the other targets (mean of 0.0728 with 95% CI of [0.01, 0.169]; Fig. 1J).
Experiment 2: history of prior movements modulated physiological biases, not plan-based biases
Experiment 1 indicated similarity between physiological biases and plan-based biases at baseline. Next, we asked whether manipulating the prior history of executed movement would similarly affect movements evoked by TMS (i.e., physiological biases) and plan-based biases. In experiment 2, we systematically manipulated the recent history of executed movements by asking participants to repeatedly make thumb movements toward a new direction located in the opposite direction of each participant's baseline physiological bias. We estimated the physiological bias using TMS, and the plan-based movement bias via the catch trials of the timed-response block, before and after participants (n = 15) voluntarily repeated, for 40 min (503.14 ± 57.85 movement executions), a movement toward the center () of a semicircular arc target set at 180° from θbase (Fig. 2A; see above, Procedures).
We found a significant effect (Kuiper's test, ) of prior history on physiological biases, as seen by the prominent changes in the evoked movement toward the most recently practiced direction (Fig. 2B–D, Table 1). In contrast, when we probed plan-based bias during the catch trials in the timed-response block, we found no evidence for changes in default plan distribution (, effect size d = 0.100; Fig. 2B–D). These data suggest that physiological biases are largely affected by execution history. This result may be taken as evidence that repeated movements lead to a change in cortical network representing preferred thumb movements, potentially reflecting a new state in the neural space within the motor cortex to which the neural activity converged into following the changes of recent movement history. In contrast, physiological biases were not dependent on plans of upcoming movements.
Control analysis confirmed that movements that were initiated before, or immediately after, the target cue (i.e., inadvertent movements) were essentially equivalent to default directions in catch trials and were processed accordingly, as in experiment 1. The distribution of movement directions of all inadvertent movements in the timed-response trials, occurring any time before the target appeared and up to 50 ms immediately after the target was displayed (10.28 ± 9.29% of trials), was comparable to that of the default movements in the catch trials (no significant differences between distributions, ; Fig. 2E). As expected, and as in experiment 1, fitting the speed-accuracy trade-off function revealed significant () increased accuracy (as reflected by the parameter that defined the participant's lower limit accuracy) at short latencies on targets near the plan-based direction (mean of 0.531 with 95% CI of [0.429 0.694]), but not near the other targets (mean of 0.068 with 95% CI of [0.01 0.3718]; Fig. 2F,G; Table 2).
Table 2.
Distribution of thumb directions for the physiological bias and plan-based bias in Experiment 2
| TMS bias |
Plan bias |
|||
|---|---|---|---|---|
| Subject | Before | After | Before | After |
| Subj 2001 | 253 | 90 | 162 | 150.5 |
| Subj 2002 | 251.5 | 271.5 | 336.5 | 202.5 |
| Subj 2003 | 82.5 | 92.5 | 184.5 | 196.5 |
| Subj 2004 | 286.5 | 306.5 | 30 | 85 |
| Subj 2005 | 90.5 | 60 | 9 | 180 |
| Subj 2006 | 248 | 67.5 | 341 | 352.5 |
| Subj 2007 | 234 | 120.5 | 136.5 | 138 |
| Subj 2008 | 122.5 | 158 | 184.5 | 76 |
| Subj 2009 | 155 | 271 | 11 | 7.5 |
| Subj 2010 | 305 | 89.5 | 205 | 129 |
| Subj 2011 | 207 | 56.5 | 33.5 | 46.5 |
| Subj 2012 | 299.5 | 79.5 | 287.5 | 95.5 |
| Subj 2013 | 66 | 254.5 | 262.5 | 247.5 |
| Subj 2014 | 117 | 89.5 | 40 | 59.5 |
| Subj 2015 | 19.5 | 84 | 286.5 | 348.5 |
| MaxLikVonMises (°) | 257.5 | 87 | 80.5 | 81 |
| Variance (°) | 42.03 | 46.11 | 33.12 | 36.98 |
Distribution of the directions (in degrees) of the physiological bias and plan-based bias in each phase for all participants in experiments 2. Subj, Subject.
Experiment 3: movement-related physiological changes were distinct from ongoing planning-related processing
The results of experiment 2 emphasized that although physiological biases were affected by recent practice, the training had little effect on subsequent movement plans. During catch trials (no target appeared) or regular trials with low RTs (i.e., trials in which the target appeared, but participants respond very early before processing the true location of the target), participants prepared a default plan similar to that at baseline, despite the large post-training biases in movement evoked by TMS. Yet, because repetitive movements in experiment 2 involved both planning and execution to repeatedly reach the same target, it is impossible to determine whether the observed physiological biases were affected by the repetition of planning, execution, or both. In addition, from the results of experiment 2, we cannot determine whether TMS-evoked movements reflect, to any extent, ongoing planning. In fact, probing of the physiological biases by TMS was done after the timed-response task, and thus outside the context of the timed-response task, when the participants were not planning to make any voluntary movements. In experiment 3, we addressed these points by systematically dissociating the effect of prior planning of upcoming movements from prior history of executed movements using a modified Go/No-Go paradigm with interleaved TMS pulses to probe physiological biases during the movement preparation period. We controlled for the repetition frequency as in experiment 2, but instead of executing hundreds of movements toward a specific direction, here, in most trials (80% of trials) participants repeatedly prepared a specific plan without executing the movements.
If physiological biases reflect a readout of ongoing activity that is related to planning of subsequent goal-directed movement, in experiment 3 we would expect a substantial and dynamically increasing bias in the direction of the evoked movement during the preparation period toward the upcoming movement that participants are preparing for. Alternatively, we predicted that if physiological biases do not reflect a readout of ongoing planning-related processing ahead of upcoming movement and have little relevance with respect to goal-directed voluntary behavior, changing the distribution of potential targets should affect only the plan-based biases but not the TMS-evoked physiological biases.
To test this, we biased the probability of upcoming movement plans by making potential target locations in the timed-response task not equally probable and, instead, increased the likelihood of targets presented at specific locations in the workspace (Fig. 3A). Unlike the baseline block in which presentation of the four targets was uniformly distributed, here, the most frequent target was presented in 70% of trials (525 trials), and the other three targets were each presented in 10% of trials (75 trials each target), with a total of 750 trials. To lead participants to repeatedly plan a movement without actually executing it, we used a modified delayed reaching Go/No-Go paradigm. Here, in 80% of trials participants were led to prepare a movement but did not execute it (No-Go condition), and in 20% of trials, participants executed their planned movement (Go condition; see above, Materials and Methods). Importantly, to test the relationship between physiological biases and ongoing planning with respect to goal-directed voluntary behavior, single TMS pulses were delivered in random trials after the target was presented, but 150 ms before the Go/No-Go cue, allowing for sufficient time to prepare the cued movement before delivery of TMS (Fig. 3B). The reason behind choosing this proportion of No-Go/Go trials (80/20%) was to keep a consistent number of repetition trials across experiment 2 (movement repetition) and experiment 3 (planning repetition, without execution, to the frequent target) without contaminating experiment 3 with change in movement execution history that would affect physiological bias. The movement repetition block in experiment 2 resulted in 503.14 ± 57.85 movement executions. Thus, in experiment 3 we aimed to have a similar amount of No-Go trials for plan repetition (420 No-Go trials).
Our data revealed that repetition of a plan for a specific movement had no significant effect on physiological biases. When participants repeatably planned a movement toward a specific direction, the TMS-evoked movement direction did not change but instead revealed robust biases toward the baseline direction regardless of the ongoing plan (Fig. 3C, top). The cumulative distribution function of difference, comparing the distribution of TMS-evoked movement during the plan repetition block with the baseline block, showed a nonsignificant difference (Kuiper's test, ) with the null hypothesis of normal distribution (μ = 0, σ = 10°; Fig. 3D,E). However, in contrast, changing the likelihood of potential targets had a significant effect on the planning of upcoming movements (Fig. 3C, bottom). The cumulative distribution function of difference in plan-based directions during the catch trials showed a significant shift (Kuiper's test, , effect size d = 2.052) from the normal distribution, toward the most frequently planned target (Fig. 3D,E, Table 1).
To further confirm that TMS-evoked physiological biases were indeed distinct from any activity related to ongoing planning, we applied single TMS pulses in some catch trials at 150 ms before the fourth tone in the final timed-response block after plan repetition (Fig. 3A,B). This test allowed direct investigation of the relationship between physiological biases and the newly altered default plan for voluntary movement. Our data revealed consistent results; despite profound biases in the default plan toward the most frequent plan, TMS-evoked movement showed robust biases toward the baseline direction, indifferent to the new plan-based biases (Fig. 3C). The cumulative distribution function of difference, comparing the distribution of TMS-evoked movement after the plan repetition block with the baseline block, showed a nonsignificant difference (Kuiper's test, ) with the null hypothesis of normal distribution (μ = 0, σ = 10°; Fig. 3D,E). Figure 3F shows individual data of evoked movements (orange lines) during the plan repetition block when the participant was preparing, and just before making a goal-directed movement toward the different targets. These elicited movements were largely different from the actual movements (black lines) that the same participant made during the Go trials (each circle represents the distribution of movement for each of the four targets during the plan repetition block).
The No-Go trials allowed us to dissociate planning from execution of a movement, and biasing a specific target allowed us to manipulate the probability distribution of upcoming plans toward that target and then test the effect of this manipulation on physiological biases. Yet, one might be concerned that having many No-Go trials, in which participants did not make any actual movement, might discourage participants from truly planning any movement. If this was true, we would expect increased or unchanged reaction time in the later trials of the plan repetition block. Instead, if participants repeatedly planned the desired movement, we would expect a selective reduction in reaction time for the frequent target. Our data revealed a significant time effect (Early vs Late; ) and a significant interaction between the direction (repeated plan vs other) and time (Early vs Late; Fig. 3G). Post hoc analysis revealed that late in the plan repetition block the average RT across all participants in the direction of the repeated plan was significantly lower than early in the repetition block () and was significantly lower () than the average RT in the nonfrequent target directions. The average RT in the other targets decreased (). Our data rule out the possibility that participants did not truly intend to execute the correct plan during the No-Go trials. Altogether, our data clearly show that physiological biases evoked from the human motor cortex do not reflect a readout of ongoing planning-related processing of goal-directed voluntary movement (Table 3).
Table 3.
Distribution of thumb directions for the physiological bias and plan-based bias in Experiment 3
| TMS bias |
Plan bias |
||||
|---|---|---|---|---|---|
| Subject | Before | After | Before | After 1 | After 2 |
| Subj 3001 | 83 | 67 | 97.5 | 180 | 180 |
| Subj 3002 | 49 | 50.5 | 43.5 | 189 | 159 |
| Subj 3003 | 322.5 | 326 | 328.5 | 183.5 | 34.5 |
| Subj 3004 | 155 | 135 | 148 | 250 | 35.5 |
| Subj 3005 | 271 | 300 | 296 | 113 | 286.5 |
| Subj 3006 | 69 | 52.5 | 50.5 | 195 | 242 |
| Subj 3007 | 209.5 | 211.5 | 180 | 101.5 | 339.5 |
| Subj 3008 | 151.5 | 213 | 214 | 198 | 6 |
| Subj 3009 | 89 | 88 | 109.5 | 255 | 358 |
| Subj 3010 | 80 | 88.5 | 109.5 | 199 | 305.5 |
| MaxLikVonMises (°) | 79 | 76 | 80.5 | 88.5 | 81 |
| Variance (°) | 41.61 | 40.88 | 47.64 | 49.76 | 39.77 |
Distribution of the directions (in degrees) of the physiological bias and plan-based bias in each phase for all participants in experiments 1–3.
We reported the direction with the maximum likelihood of the fitted von Mises function, instead of the circular mean, to adequately demonstrate a representative metric of this type of bimodal distribution. Subj, Subject.
Discussion
In a set of neurophysiological and behavioral paradigms, we systematically uncoupled prior history of movement executions and planning for upcoming movement and showed that physiological biases evoked by TMS over the human motor cortex are because of the effect of execution-dependent prior history and not the history of the most probable plan of action, suggesting that movement repetition induced changes in the state of the neural activity within the motor cortex that can then be read out by noninvasive transcranial stimulation. Our approach was to manipulate the statistics of movement history and the statistics of plans for upcoming movements independently and then to test the effect of each manipulation on physiological biases. Although at baseline physiological biases shared similar low-level movement kinematics (i.e., direction) with plan-based biases, these processes diverged later when the statistics of either movement history or plan history were altered.
Use-dependent plasticity typically refers to the cortical reorganization of the effector (e.g., thumb) representation in the human motor cortex following practice (Kleim et al., 1998; Bontempi et al., 1999; Plautz et al., 2000). Subsequent to a short period of training, consisting of simple, voluntary, and repetitive thumb movements in a specific direction, noninvasive stimulation exhibits the reorganization of the cortical representation of the thumb by eliciting movement that encodes the low-level kinematic details of the newly practiced movement (i.e., bias toward the practiced direction; Kleim et al., 1998; Bontempi et al., 1999; Plautz et al., 2000). These findings suggest that repetition of simple movements leads to rapid establishment of a transient history-dependent change in the cortical motor network representing preferred thumb movements (Classen et al., 1998). Long-term practice over years seems to have a similar use-dependent effect. TMS over the motor cortex in expert musicians (pianists and violinists) is more likely to elicit the same hand movements that occur while musicians actively play their instrument, compared with nonmusician controls or musicians who play other instruments (Gentner et al., 2010).
Multiple other factors might also affect physiological biases, such as reinforcement and motor learning (Mawase et al., 2017). Observations from previous reports show that repetition of successful movements in the face of perturbations elicits stronger physiological biases than movement repetition alone (Huang et al., 2011; Krakauer and Mazzoni, 2011; Haith and Krakauer, 2013). Additionally, evidence from human neuropharmacology studies showed that administration of levodopa, which increases the presynaptic availability of endogenous dopamine, increased the strength of the biases evoked by TMS toward the repeated direction compared with a control group that only repeated the movement. This enhancement in physiological bias was correlated with the dopamine released in the striatum (Flöel et al., 2005; Floel et al., 2008). These results may suggest a hidden process, likely driven by success-related reinforcement signals that might modulate physiological biases. In addition, we have previously shown in a series of TMS experiments that the physiological bias effect can be augmented if reinforcement is coupled with successful goal achievement during skill learning (Mawase et al., 2017).
However, what has not been clear is whether these physiological biases might also reflect changes in planning-based processing that is related to an upcoming action. When hundreds of simple movements are repeatedly planned and executed in the recent past, it is possible that neural activity associated with the practiced movements becomes biased, not only toward the most frequently executed movement, but also toward the most frequent plan of upcoming movement (Marinovic et al., 2017). Although not directly tested in TMS paradigms, this hypothesis was previously supported in goal-directed behavioral experiments by showing that a larger component of the behavioral bias primarily originates from changes associated with motor planning, with a second minor component that is mostly dependent on execution history (Marinovic et al., 2017; Tsay et al., 2022). Our findings revealed that physiological biases in motor cortex are insensitive to ongoing neural activity associated with the movement that participants are most likely planning to make next (i.e., the most frequent plan used in recent history), likely ruling out the functionality of evoked physiological biases with respect to voluntary goal-directed motor behavior (Bestmann and Krakauer, 2015).
Although manipulation of plan history by changing the distribution of the locations of presented targets to include a more frequent target, thus biasing plans toward the more frequent target, significantly affected the default plan for voluntary movement, it did not significantly affect physiological bias. Our result is in line with an earlier neurophysiological study that recorded neural activity from M1 and the dorsal premotor cortex (PMd) while monkeys reached to randomly placed targets with different probability distributions of possible upcoming targets and found that PMd activity, not M1, represents probability distributions of plan-based upcoming reaches, suggesting that such distributions are incorporated by the planning areas of the premotor cortex, outside M1, when coordinating goal-directed voluntary movement (Glaser et al., 2018).
Using a modified version of a delayed reaching task with No-Go trials allowed us to dissociate planning from execution of a movement. Repeating No-Go trials, thereafter, allowed us to bias the probability distribution of upcoming plans toward a specific plan. Nevertheless, having many No-Go trials, in which participants did not make any actual movement, might discourage participants from truly planning to execute the desired movement. This concern might influence the amount of planning and thus weaken the conclusion that TMS-evoked movement truly does not reflect the most probable upcoming plan. If this was true, we would expect increased, or unchanged, reaction time in the Go trials later in the planning repetition block. Instead, if participants repeatedly planned the desired movement, we would expect a selective reduction in reaction time for the frequent target. The significant time, as well as target × time interaction effect, supports the idea that participants repeatedly planned the desired action.
Our findings show that the dependency of physiological biases on execution history, and the insensitivity of these biases to prior planning, is analogous to some degree with the execution-dependent component of the behavioral biases found in goal-directed voluntary tasks (Verstynen and Sabes, 2011; Marinovic et al., 2017; Tsay et al., 2022). Although the consistency between the two observations indicates that physiological and behavioral/movement biases might reflect similar underlying execution-dependent mechanisms, there are several factors that weaken this interpretation and even question the relevance of physiological biases to normal behavior.
First, the strength of physiological biases is 10-fold greater than that of behavioral biases. On average, the magnitude of physiological biases in thumb direction is within the range of 150–180° from baseline distribution of thumb direction (Classen et al., 1998; Bütefisch et al., 2000), whereas behavioral biases exhibited in arm reaching, at most, have a magnitude of 10° (Diedrichsen et al., 2010). Interestingly, Kantak et al. (2013) assessed plasticity of TMS-evoked arm movements after training with a repeated reaching movement in the direction 180° opposite from the baseline-evoked direction, similar to our study and previous thumb studies. Following practice, 6 subjects had a complete reversal of evoked direction, 11 subjects had evoked direction that was somewhere between the original and practiced movements, and 5 subjects showed no consistent change. We speculate that the results were less consistent in the arm compared with the thumb because of the difficulties in using a much larger endpoint effector and the increased representation of the thumb in the human motor cortex. However, the results of Kantak et al.'s (2013) study show very nicely that practice induces execution-based biases not only in the thumb, but also in the arm, and that these biases can be large in many subjects, just as with the thumb. Further, although not reported as a main result in our study, a subanalysis of our data revealed that the execution biases in volitional thumb movements following execution repetition (in experiment 2) are in the range of 5–10°, similar to previous work in hand/arm movement.
Second, the timescale of physiological biases seems to be longer than behavioral biases. It was shown that TMS-evoked biases in thumb movement could last up to ∼40 min following 30 min of repetitions in the biasing direction. On the other hand, behavioral biases last at most for several trials (n = 20–50 trials) before drifting back to baseline level.
Third, in contrast to physiological biases, behavioral biases appear to be task sensitive. During passive tasks, when a robot moves the hand to a target, behavioral biases were also observed (Diedrichsen et al., 2010). However, when the thumb was passively and briskly moved in a direction opposite to the baseline TMS-evoked movement direction for 30 min, subsequent TMS did not reveal any physiological biases toward the repeated direction (Kaelin-Lane et al., 2005). On the basis of these reports and previous observations (Verstynen and Sabes, 2011), we speculate that physiological and behavioral biases might coexist and share similar characteristics in some task setups, but they probably differ in the underlying mechanism. Future work is needed to directly examine the mechanism or mechanisms that underlie each process.
At the neural level, our data can be reconciled with the recent theory of a neural dynamics system. This approach posits that neural dynamics of population activity can be parameterized by a state equation that has different initial conditions and evolves over time. The evolved neural trajectory can span multiple spaces, including separate yet orthogonal spaces for planning and movement execution (Elsayed et al., 2016). The strength of the dynamical systems approach is that it provides a simplified mechanistic basis for understanding the link between time-varying activity of neural populations and planning and execution motor behavior (Afshar et al., 2011; Kaufman et al., 2014; Elsayed et al., 2016). Within this dynamic system, it is believed that movement preparation involves setting the state of the motor cortex to a particular, movement-specific state (Churchland et al., 2006b; Ames et al., 2014). Consequently, this preparation is thought to set the initial state of a dynamical system that generates patterns of activity required for movement (Churchland et al., 2006a; Afshar et al., 2011; Michaels et al., 2015). Our results showing that modulation of execution history significantly affected physiological biases might therefore be a consequence of altering the default state in the movement space in M1 for the repeated direction, with little effect on ongoing activity of the preparatory space (Fig. 4). Stimulation of the motor cortex may thus reflect a readout of the cortical state within the movement space where movements located close to the default state may be more likely to be elicited by TMS. Altering the frequency of potential targets shifted the distribution of plan-based biases toward the frequent target, with no effect on physiological biases. This can be explained by changes in the default preparatory state of cortical activity that converged into a new default state in the preparatory space. For instance, when no target ever appeared, or in regular trials with low RTs (i.e., ≤50 ms), requiring participants to move is likely to trigger a readout of the preparatory state of the motor system at that moment.
Figure 4.
Proposed dynamic of neural activity in the motor cortex underlies physiological and plan-based biases. Recent execution history alters the default state in the movement-related space toward the repeated direction, with little effect on ongoing activity of the preparatory space. Stimulation of M1 may thus reflect a readout of the cortical activity where movements located close to the default state may be more likely to be elicited by TMS. On the other hand, manipulating plan-based expectation changed the default preparatory state.
Alternative to the neural dynamics hypothesis, it seems reasonable that the directional biases of evoked movement reflect instead changes in sensitivity of selective neurons stimulated by the TMS. This hypothesis, and building on the idea that planning effects are more at the level of premotor cortex (e.g., PMd), whereas TMS is more at the level of M1, suggests that TMS-induced movements reflect the tuning of the population, not individual neurons. It is plausible therefore that a shift in TMS-elicited movement after extended practice in a particular direction does not change the tuning of the targeted neurons. Rather, the extended practice increases the strength of the neurons that are engaged by those movements to the extent that they have a higher likelihood of being activated by the TMS pulse in the post-training TMS phase. This implies that a shift in the tuning of the population can occur without any change in the tuning function at the individual neuron level. In accordance with this view, the mapping from plan to movement is unchanged (and thus any bias related to volitional movement is at the plan level), but there is a shift in TMS-elicited movement because the neurons engaged for the practiced direction are sensitized (i.e., dominate the TMS-elicited movement). Future electrophysiological work is needed to reveal which hypothesis is more likely to support changes in evoked movement following extended practice.
The absence of representation of recent motor plans in the TMS-evoked movements does not necessarily mean that M1 cannot reflect functional changes associated with motor planning. In fact, a rich set of studies has now established the general finding that M1 corticospinal excitability is modulated during action preparation in an effector-specific manner (Coxon et al., 2006; Van Elswijk et al., 2007, 2008; Mars et al., 2008; Bestmann et al., 2008b; Duque and Ivry, 2009; Duque et al., 2010). Specifically, it has been shown that the corticospinal excitability (CSE), as measured by MEPs of specific muscles following TMS over M1, allows for differentiating between various intrinsic muscles and thus provides sufficient resolution to distinguish the physiological underpinnings of action preparation and selection for different finger movements (Bestmann et al., 2008b). However, there may not be a straightforward relationship between MEPs and motor output changes (as for example, evoked movement). MEPs can therefore indicate that something is changing physiologically during motor preparation, but the relationship of these measures to the changing behavior remains to be determined.
Our results showing that TMS-evoked movements reflect the history of recently executed movements, but not the planning of upcoming actions, should not be interpreted as evidence that physiological changes in these stimulated motor regions are not involved in the processing or computation of preparation related activity. Changes in CSE, as measured by the amplitude of MEPs of targeted muscles, have also been used to probe the functional state of the motor system. For example, Bestmann et al. (2008a) showed that uncertainty and surprise modulated CSE during preparation for action, in that an increase in CSE was reported during expected (i.e., without surprise) upcoming actions. In addition, Klein-Flügge and Bestmann (2012) showed that change in MEP amplitude was selectivity modulated during the preparation period based on the value of the selected action and specific to the effector muscle required to make that action. Other factors might also affect MEP amplitude during the planning period, such as the amount of upcoming forces needed to be exerted in reach-to-grasp tasks (Parikh et al., 2014), proactive inhibition when participants anticipated the need to stop or change movements (Rawji et al., 2022), value and effort requirements of upcoming actions (Vassena et al., 2015), and the temporal constraints associated with anticipation and urgency on inhibitory processes recruited during response preparation (Lebon et al., 2016). Altogether, these results support the notion that influences on action representations as measured by MEPs are not just stimulus driven but also could reflect dynamic adjustments of motor representations occurring during cognitive planning-related decision processes.
How then could it be that a single TMS pulse evokes movement that is not related to an upcoming movement plan, whereas MEPs in previous work were correlated with planning-related decision variables? We can think of at least three possible explanations of this puzzling finding. First, MEPs elicited by stimulation may also contain contributions from transcortical signals arising from the direct stimulation of neurons in a nearby area (e.g., dorsal premotor areas and/or supplementary motor area) or from the stimulation of axons projecting from frontal, parietal, and subcortical regions to motor cortex. This predicts that MEPs are subject to influences from high-order cognitive planning-related variables such as uncertainty or surprise and subjective expected values, which are probably computed previously but affect the excitability of the motor cortex and action representations (Cisek and Pastor-Bernier, 2014; Bestmann and Duque, 2016). Despite TMS being delivered over the primary motor cortex, single-pulse MEPs likely reflect a summary of contributions of corticospinal, intracortical (e.g., premotor M1), and transcortical elements, and thus single TMS pulses are unlikely to selectively activate any of these elements, but instead target all three to varying degrees (Bestmann and Duque, 2016; Bestmann and Krakauer, 2015). In contrast, evoked movement reflects local changes in movement-related variables within the primary motor cortex, and TMS pulses therefore activate only the sum activity of these movement-related neurons in M1.
Second, it is possible that the dynamical modulation of these physiological signatures varies differently across learning and practice. For example, at the beginning of learning, MEP amplitude increases in linear fashion with increases in grip force required to maintain a constant motor output. However, subsequently, although the newly acquired motor behavior can be retained, MEPs return to baseline levels (Muellbacher et al., 2001). In contrast, following prolonged learning of an isometric force task (Mawase et al., 2017), or repetition of isotonic thumb movement (Classen et al., 1998), evoked movement showed gradual evolvement and robust modulation, an effect that was retained for several minutes after the practice ended.
Third, it is possible that the MEPs and evoked movements have a different sensitivity to the anticipation or urgency during response preparation. In this view, MEPs and evoked movement might show different recruitment timescales in relation to the preparatory cue, and/or to the operative signal (e.g., urgency to respond), and/or to the onset of the voluntary movement itself. For example, in very short latencies between the preparatory cue and the imperative signal (200 ms and less), TMS right before the imperative cue showed nonselective MEP changes. However, in longer delays between the preparatory and imperative cues, TMS pulses right before the imperative cue showed increased inhibition selectively to the mover muscle (Lebon et al., 2016). The fact that in our third experiment, evoked movements were not biased toward the planned target despite the TMS pulse being given 150 ms before the imperative cue in a long-delay reaching task (target appeared ∼1500 ms before the imperative cue) further supports the idea that physiological biases of evoked movement might mechanistically differ from those that underlie MEPs. Nevertheless, our experiment was not directly designed to probe evoked movement at different time points during the preparatory period, and therefore it is hard to determine whether the evoked movement might show (or not) dynamic modulation during the preparation period; further investigation, beyond the scope of the current study, is needed.
What exactly is probed in our catch trials when no visual target ever appeared? We believe that when requiring the participant to move on an imperative cue (i.e., the fourth audible tone) without the target being presented, the current state of the motor plan (i.e., default plan) becomes accessible and can be revealed via behavioral experimentation (e.g., catch trials). Before we discuss the potential cognitive-motor processes that might occur in catch trials, let us first consider a regular trial with a sufficient RT when the target appeared at some point before the fourth imperative tone. During the reaction time of these trials, a series of processes occurred that enabled the brain to perceive the surrounding environment, identify a particular target (or object) of interest, determine the required action in response to that target, and send a motor command to implement the desired action. These processes involve higher-level perceptual decision-making (e.g., processing sensory information to identify the location of the target), conception of the motor goal to be achieved, and movement-related processing (e.g., action selection for the thumb to achieve the goal) that may occur in parallel (Cisek, 2005; Cisek and Kalaska, 2010; Wong et al., 2015). In catch trials, the motor goal is not well defined as the visual target never appeared, thus limiting the perceptual decision-making process for identifying target location. It is possible, however, that participants made a strategic guess about which target would appear or a strategic decision to move in the most comfortable or easiest direction. Nevertheless, our observation that the distribution of movement directions with very low RTs in the timed-response trials when targets did appear is very similar to that of movement in catch trials suggests that the motor plan was successfully probed in these trials. Factors such as comfort and ease (e. g., minimization of energy expenditure, minimization of opposition from mechanical constraints such as tendons and ligaments, etc.) may constitute part of the default plan for choosing how an action will be generated to achieve a goal, as may be the case in low RT trials and catch trials. At this stage, we cannot resolve the default plan into its constituent components or completely disentangle the influence of higher-level cognitive planning from movement plan per se, and further work, beyond the scope of the present investigation will be required to fully understand the relative contribution of each component of the cognitive-motor processes.
Together, the present results may imply that physiological change of the effector representation in the motor system incorporates an abstract representation of the kinematic structure of the executed behavior of recent prior movement. These physiological markers of change, however, were not indicative of ongoing activation that is related to planning upcoming actions.
Footnotes
This work was supported by Israel Science Foundation Grant 1634/19 (F.M.) and United States–Israel Binational Science Foundation Grant 2021323 (F.M.). We like to thank Adrian Haith and Mona Khoury-Mireb for comments and discussions.
The authors declare no competing financial interests.
References
- Afshar A, Santhanam G, Yu BM, Ryu SI, Sahani M, Shenoy KV (2011) Single-trial neural correlates of arm movement preparation. Neuron 71:555–564. 10.1016/j.neuron.2011.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames KC, Ryu SI, Shenoy KV (2014) Neural dynamics of reaching following incorrect or absent motor preparation. Neuron 81:438–451. 10.1016/j.neuron.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S (2012) Functional modulation of primary motor cortex during action selection. In: Cortical connectivity (Chen R, Rothwell J, eds), pp 183–205. Berlin: Springer. [Google Scholar]
- Bestmann S, Duque J (2016) Transcranial magnetic stimulation: decomposing the processes underlying action preparation. Neuroscientist 22:392–405. 10.1177/1073858415592594 [DOI] [PubMed] [Google Scholar]
- Bestmann S, Krakauer JW (2015) The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res 233:679–689. 10.1007/s00221-014-4183-7 [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R (1999) Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400:671–675. 10.1038/23270 [DOI] [PubMed] [Google Scholar]
- Bestmann S, Harrison LM, Blankenburg F, Mars RB, Haggard P, Friston KJ, Rothwell JC (2008a) Influence of uncertainty and surprise on human corticospinal excitability during preparation for action. Curr Biol 18:775–780. 10.1016/j.cub.2008.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Ruff CC, Driver J, Blankenburg F (2008b) Concurrent TMS and functional magnetic resonance imaging: methods and current advances. Oxford handbook of transcranial stimulation (Epstein CM, Wassermann EM, Ziemann U, eds), Oxford Library of Psychology. Online edn, Oxford Academic, 21 Nov. 2012. Accessed 1 June 2023. [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG (2000) Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A 97:3661–3665. 10.1073/pnas.97.7.3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG (2006) Encoding a motor memory in the older adult by action observation. Neuroimage 29:677–684. 10.1016/j.neuroimage.2005.07.039 [DOI] [PubMed] [Google Scholar]
- Chapman B, Bonhoeffer T (1998) Overrepresentation of horizontal and vertical orientation preferences in developing ferret area 17. Proc Natl Acad Sci U S A 95:2609–2614. 10.1073/pnas.95.5.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Santhanam G, Shenoy KV (2006a) Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol 96:3130–3146. 10.1152/jn.00307.2006 [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV (2006b) A central source of movement variability. Neuron 52:1085–1096. 10.1016/j.neuron.2006.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P (2005) Neural representations of motor plans, desired trajectories, and controlled objects. Cogn Process 6:15–24. 10.1007/s10339-004-0046-7 [DOI] [Google Scholar]
- Cisek P (2007) Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362:1585–1599. 10.1098/rstb.2007.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF (2010) Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33:269–298. 10.1146/annurev.neuro.051508.135409 [DOI] [PubMed] [Google Scholar]
- Cisek P, Pastor-Bernier A (2014) On the challenges and mechanisms of embodied decisions. Phil Trans R Soc B 369:20130479. 10.1098/rstb.2013.0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123. 10.1152/jn.1998.79.2.1117 [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD (2006) Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 95:3371–3383. 10.1152/jn.01334.2005 [DOI] [PubMed] [Google Scholar]
- De Valois RL, Yund EW, Hepler N (1982) The orientation and direction selectivity of cells in macaque visual cortex. Vision Res 22:531–544. 10.1016/0042-6989(82)90112-2 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N (2010) Use-dependent and error-based learning of motor behaviors. J Neurosci 30:5159–5166. 10.1523/JNEUROSCI.5406-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Ivry RB (2009) Role of corticospinal suppression during motor preparation. Cereb Cortex 19:2013–2024. 10.1093/cercor/bhn230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Lew D, Mazzocchio R, Olivier E, Ivry RB (2010) Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci 30:3793–3802. 10.1523/JNEUROSCI.5722-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed GF, Lara AH, Kaufman MT, Churchland MM, Cunningham JP (2016) Reorganization between preparatory and movement population responses in motor cortex. Nat Commun 7:13239. 10.1038/ncomms13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favilla M, Hening W, Ghez C (1989) Trajectory control in targeted force impulses VI. Independent specification of response amplitude and direction. Exp Brain Res 75:280–294. 10.1007/BF00247934 [DOI] [PubMed] [Google Scholar]
- Flöel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG (2005) Dopaminergic influences on formation of a motor memory. Ann Neurol 58:121–130. 10.1002/ana.20536 [DOI] [PubMed] [Google Scholar]
- Floel A, Garraux G, Xu B, Breitenstein C, Knecht S, Herscovitch P, Cohen LG (2008) Levodopa increases memory encoding and dopamine release in the striatum in the elderly. Neurobiol Aging 29:267–279. 10.1016/j.neurobiolaging.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Done DJ (1986) Routes to action in reaction time tasks. Psychol Res 48:169–177. 10.1007/BF00309165 [DOI] [PubMed] [Google Scholar]
- Gatto R, Jammalamadaka SR (2007) The generalized von Mises distribution. Stat Methodol 4:341–353. 10.1016/j.stamet.2006.11.003 [DOI] [Google Scholar]
- Gentner R, Gorges S, Weise D, Aufm Kampe K, Buttmann M, Classen J (2010) Encoding of motor skill in the corticomuscular system of musicians. Curr Biol 20:1869–1874. 10.1016/j.cub.2010.09.045 [DOI] [PubMed] [Google Scholar]
- Ghez C, Favilla M, Ghilardi MF, Gordon J, Bermejo R, Pullman S (1997) Discrete and continuous planning of hand movements and isometric force trajectories. Exp Brain Res 115:217–233. 10.1007/pl00005692 [DOI] [PubMed] [Google Scholar]
- Glaser JI, Perich MG, Ramkumar P, Miller LE, Kording KP (2018) Population coding of conditional probability distributions in dorsal premotor cortex. Nat Commun 9:1788. 10.1038/s41467-018-04062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN (2001) Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci 5:10–16. 10.1016/s1364-6613(00)01567-9 [DOI] [PubMed] [Google Scholar]
- Haith AM, Krakauer JW (2013) Model-based and model-free mechanisms of human motor learning. Adv Exp Med Biol 782:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Pakpoor J, Krakauer JW (2016) Independence of movement preparation and movement initiation. J Neurosci 36:3007–3015. 10.1523/JNEUROSCI.3245-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW (2011) Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70:787–801. 10.1016/j.neuron.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javidialsaadi M, Albert ST, Wang J (2021) A conversion from slow to fast memory in response to passive motion. bioRxiv. 434594 10.1101/2021.03.09.434594. [DOI] [Google Scholar]
- Kaelin-Lane A, Sawaki L, Cohen LG (2005) Role of voluntary drive in encoding an elementary motor memory. J Neurophysiol 93:1099–1103. 10.1152/jn.00143.2004 [DOI] [PubMed] [Google Scholar]
- Kantak SS, Jones-Lush LM, Narayanan P, Judkins TN, Wittenberg GF (2013) Rapid plasticity of motor corticospinal system with robotic reach training. Neuroscience 247:55–64. 10.1016/j.neuroscience.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV (2014) Cortical activity in the null space: permitting preparation without movement. Nat Neurosci 17:440–448. 10.1038/nn.3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ (1998) Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol 80:3321–3325. 10.1152/jn.1998.80.6.3321 [DOI] [PubMed] [Google Scholar]
- Klein-Flügge MC, Bestmann S (2012) Time-dependent changes in human corticospinal excitability reveal value-based competition for action during decision processing. J Neurosci 32:8373–8382. 10.1523/JNEUROSCI.0270-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P (2011) Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21:636–644. 10.1016/j.conb.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Kuiper NH (1960) Tests concerning random points on a circle. Indagationes Mathematicae (Proceedings) 63:38–47. 10.1016/S1385-7258(60)50006-0 [DOI] [Google Scholar]
- Lebon F, Greenhouse I, Labruna L, Vanderschelden B, Papaxanthis C, Ivry RB (2016) Influence of delay period duration on inhibitory processes for response preparation. Cereb Cortex 26:2461–2470. 10.1093/cercor/bhv069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinovic W, Poh E, De Rugy A, Carroll TJ (2017) Action history influences subsequent movement via two distinct processes. Elife 6:e26713. 10.7554/eLife.26713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Hulstijn W, Toni I (2008) Selection, preparation, and monitoring: current approaches to studying the neural control of action. Cortex 44:479–481. 10.1016/j.cortex.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Mawase F, Lopez D, Celnik PA, Haith AM (2018) Movement repetition facilitates response preparation. Cell Rep 24:801–808. 10.1016/j.celrep.2018.06.097 [DOI] [PubMed] [Google Scholar]
- Mawase F, Uehara S, Bastian AJ, Celnik P (2017) Motor learning enhances use-dependent plasticity. J Neurosci 37:2673–2685. 10.1523/JNEUROSCI.3303-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels JA, Dann B, Intveld RW, Scherberger H (2015) Predicting reaction time from the neural state space of the premotor and parietal grasping network. J Neurosci 35:11415–11432. 10.1523/JNEUROSCI.1714-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M (2001) Role of the human motor cortex in rapid motor learning. Exp Brain Res 136:431–438. 10.1007/s002210000614 [DOI] [PubMed] [Google Scholar]
- Parikh P, Davare M, McGurrin P, Santello M (2014) Corticospinal excitability underlying digit force planning for grasping in humans. J Neurophysiol 111:2560–2569. 10.1152/jn.00815.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74:1037–1045. 10.1152/jn.1995.74.3.1037 [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ (2000) Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem 74:27–55. 10.1006/nlme.1999.3934 [DOI] [PubMed] [Google Scholar]
- Rawji V, Modi S, Rocchi L, Jahanshahi M, Rothwell JC (2022) Proactive inhibition is marked by differences in the pattern of motor cortex activity during movement preparation and execution. J Neurophysiol 127:819–828. 10.1152/jn.00359.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Hernández A, Zainos A (2004) Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron 41:165–173. 10.1016/s0896-6273(03)00817-1 [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C (1994) Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92. 10.1016/0013-4694(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Scott SH, Gribble PL, Graham KM, Cabel DW (2001) Dissociation between hand motion and population vectors from neural activity in motor cortex. Nature 413:161–165. 10.1038/35093102 [DOI] [PubMed] [Google Scholar]
- Selvanayagam VS, Riek S, De Rugy A, Carroll TJ (2016) Strength training biases goal-directed aiming. Med Sci Sports Exerc 48:1835–1846. 10.1249/MSS.0000000000000956 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Huang HJ, Ahmed AA (2016) A representation of effort in decision-making and motor control. Curr Biol 26:1929–1934. 10.1016/j.cub.2016.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay JS, Kim HE, Saxena A, Parvin DE, Verstynen T, Ivry RB (2022) Dissociable use-dependent processes for volitional goal-directed reaching. Proc Biol Sci 289:20220415. 10.1098/rspb.2022.0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elswijk G, Kleine BU, Overeem S, Stegeman DF (2007) Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci 19:121–131. 10.1162/jocn.2007.19.1.121 [DOI] [PubMed] [Google Scholar]
- Van Elswijk G, Schot WD, Stegeman DF, Overeem S (2008) Changes in corticospinal excitability and the direction of evoked movements during motor preparation: a TMS study. BMC Neurosci 9:51. 10.1186/1471-2202-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena E, Cobbaert S, Andres M, Fias W, Verguts T (2015) Unsigned value prediction-error modulates the motor system in absence of choice. Neuroimage 122:73–79. 10.1016/j.neuroimage.2015.07.081 [DOI] [PubMed] [Google Scholar]
- Verstynen T, Sabes PN (2011) How each movement changes the next: an experimental and theoretical study of fast adaptive priors in reaching. J Neurosci 31:10050–10059. 10.1523/JNEUROSCI.6525-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AL, Haith AM, Krakauer JW (2015) Motor planning. Neuroscientist 21:385–398. 10.1177/1073858414541484 [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ili TV, Pauli C, Meintzschel F, Ruge D (2004) Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci 24:1666–1672. 10.1523/JNEUROSCI.5016-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]