Abstract
Aims
High precordial leads (HPL) on the resting electrocardiogram (ECG) are widely used to improve diagnostic detection of type 1 Brugada ECG pattern (Br1ECGp). A parasympathetic activation marks the initial recovery phase of treadmill stress testing (TET), and this can be useful for detecting the typical ECG pattern. Our study aimed to evaluate the role of a new HPL-treadmill exercise testing (TET) protocol in detecting Br1ECGp fluctuation compared to resting HPL-ECG.
Methods and results
74 out of 163 patients of a Brugada syndrome (BrS) Brazilian cohort (GenBra Registry) underwent exercise testing with HPL-TET protocol. Precordial leads were displayed in strategic positions in the right and left parasternal spaces. The step-by-step analysis included ECG classification (as presence or absence of Br1ECGp) in standard vs. HPL leads placement in the following sequences: resting phase, maximal exercise, and the passive recovery phase (including ‘quick lay down’). For heart rate recovery (HRR) measurements and comparisons, a Student’s t-test was applied. McNemar tests compared the detection of Br1ECGp. The significance level was defined as P < 0.05. Fifty-seven patients (57/74; 77%) were male, the mean age was 49.0 ± 14, 78.4% had spontaneous BrS, and the mean Shanghai score was 4.5. The HPL-TET protocol increased Br1ECGp detection by 32.4% against resting HPL-ECG (52.7% vs. 20.3%, P = 0.001) alone.
Conclusion
Stress testing using HPL with the passive recovery phase in the supine position offers an opportunity to unmask the type 1 Br1ECGp, which could increase the diagnostic yield in this population.
Keywords: Brugada syndrome, Type 1 Brugada ECG pattern, Treadmill exercise testing, Diagnosis
Graphical Abstract
Graphical abstract.
What’s new?
The present study highlights the possibility of using stress testing with high precordial leads to detect ECG fluctuation in Brugada syndrome and might open the window for a new diagnostic tool. It is a widely available and low-cost exam.
Stress testing using high precordial leads improved BrS1 pattern detection by 32%.
The incorporation of a provocative manoeuvre for sudden autonomic changes during stress testing is a promising strategy to unmask the Brugada type 1 ECG pattern.
Introduction
Brugada syndrome (BrS) is an inherited arrhythmia diagnosed in the presence of type 1 Brugada Syndrome electrocardiographic pattern (Br1ECGp), defined as a coved-shaped 2 mm ST-segment elevation in at least V1 or V2 on a 12-lead electrocardiogram (ECG), in standard or high precordial leads (HPL).1
The presence of a spontaneous Br1ECGp, usually obtained by a random ECG, has been historically associated with a higher risk of life-threatening arrhythmic events (LAE).2 However, Br1ECGp definition is challenging due to ST-segment fluctuation over time, highlighted by previous studies with 24-h Holter monitoring using HPL3,4 or standard leads.5 The Brugada pattern in inferior or lateral leads has also been linked to higher-risk patients.6,7 Additional strategies to find spontaneous BrS1 ECG pattern might be valuable, whereas, beyond ECG, genetic testing has a low yield for BrS diagnosis.
Body surface mapping taking BrS from leads V1–V3 at a higher intercostal space (ICS) (third or second) has detected the Br1ECGp with a higher sensitivity than that obtained in the standard lead positioning.8,9
As far as we know, the role of treadmill stress testing (TET) using HPL (HPL-TET) for Br1ECGp evaluation has yet to be demonstrated. Our study aimed to evaluate the role of HPL-TET in detecting the Br1ECGp fluctuation.
Methods
GenBra cohort—Brugada syndrome
From 1999 to 2022, 163 consecutive patients with BrS1 ECG pattern were included and followed up prospectively at the Instituto do Coração, Faculdade de Medicina, Universidade de São Paulo, Brazil. Baseline characteristics of 138/163 patients were previously published.10
Consent
All the patients signed an informed consent to the study, which was conducted following the Declaration of Helsinki and had the approval of our Institutional Review Board (Protocol #404214022).
A multicenter national survey on Inherited Channelopathy and Right Ventricular Cardiomyopathy was organized to provide genetic testing for patients from our and other institutions (Genetic of Brazilian Arrhythmias—GenBra registry), A governmental funding of Fundação de Amparo à Pesquisa do Estado de São Paulo- FAPESP, project #2016/15223-3, provided financial support for the genetic testing. All patients signed specific consent forms for genetic testing.
Periodic medical visits obtained baseline characteristics. The clinical variables of interest were age at enrolment at the institution and stress testing protocol, gender, body mass index (BMI), self-declared race, proband status, spontaneous or drug-induced Br1ECGp, clinical presentation at diagnosis, and Shanghai score.11 Patients were considered symptomatic in the presence of aborted sudden cardiac death (SCD), nocturnal agonal respiration, or arrhythmogenic syncope cases.
Eligibility
From GenBra Cohort, subjects were eligible if they had a Shanghai score ≥ 3.5. All patients had to be free of arrhythmic events 6 months before stress testing, with no history of substrate Brugada ablation and had to be able to perform stress testing on a treadmill. All patients underwent routine examinations (echocardiography and laboratory tests) to exclude underlying structural heart disease, myocardial ischaemia, and metabolic or electrolyte abnormalities.
Stress testing ‘proposed protocol’
From 2015 to 2022, 74 out of 163 patients underwent HPL-TET. All these tests were performed on treadmills, and Bruce or Ellestad protocols were chosen according to exercise tolerance.12,13 A symptom-limited or maximal graded treadmill exercise testing was used, using the Karvonen prediction equation (220—age).
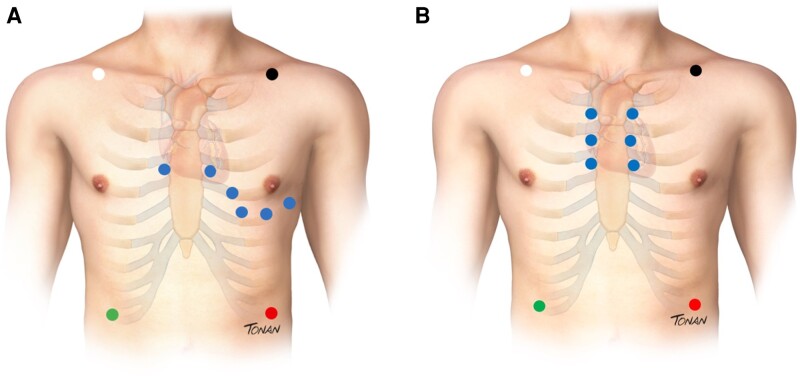
Six precordial leads were displayed in a ‘superior ECG manner’ chosen as V1 and V2 in a standard position (fourth ICS), replacing V3 and V4 in the third ICS, and finally V5 and V6 to the second ICS. (Figure 1).
Figure 1.
(A) Standard precordial leads position using modified (Mason Likar) exercise lead system. (B) HPL positioning.
The ECG tracings were recorded at rest, at the end of each exercise stage, at peak exercise, and every minute during the 6 min of the recovery phase.
L.S. and N.L.P. classified the ECG tracings independently while blinded to the patient’s data to ensure the presence of Br1ECGp defined as an ST-segment elevation ≥ 2 mm in one or more precordial leads. The ‘non-type 1’ (non-BrS type 1) pattern was any change that did not fulfil the abovementioned classification. A third blinded observer solved occasional disagreements by consensus (F.C.C.D.). If BrS type 1 pattern was revealed in any peripheral leads, it was described as an additional finding.
All traces were recorded every minute in each phase. The step-by-step analysis included heart rate (HR) value and ECG classification (as presence/absence of Br1ECGp) in standard vs. HPL in the following phase sequences:
Resting phase in orthostatic position
Resting phase in a supine position
Maximal exercise
Quick lay down—passive recovery
Passive recovery phase in a supine position
We also recorded HR and blood pressure (BP) during exercise testing. Heart rate recovery (HRR) was defined as HR decay in the first minute after exercise cessation. To explore the HRR, we divided the patients into two groups, absent or present, according to the development of Br1ECGp in the recovery phase of HPL-TET.
Frequent supraventricular and ventricular arrhythmias were considered when >7 premature beats per minute or two pairs of non-sustained ventricular tachycardia (NSVT) occurred during the exercise or recovery phase. Symptom-limited exercise ECG involves graded exercise until physical fatigue. For safety reasons, HPL-TET was discontinued in case of achievement of maximum HR predicted for age; exacerbated rise or fall in BP (increase in systolic BP >260 mmHg and diastolic BP >140 mmHg; decrease in systolic BP > 20 mmHg); symptoms of intolerance; frequent premature ventricular complexes (PVC); onset of ventricular tachycardia; new atrial fibrillation or sustained supraventricular tachycardia, new second or third-degree heart block; limiting chest pain (or discomfort), ataxia or cardiac arrest.13,14
Follow-up
The clinical parameters analysed were the age at which the exercise test was performed, family history of sudden cardiac death at age 45 years, results of pharmacological challenge with inhibitor Na-blocking drugs (if performed), the incidence of VT and/or ventricular fibrillation (VF), syncope, and other BrS-related symptoms, electrophysiological study (EPS) (if performed), and whether an implantable cardioverter defibrillator (ICD) was implanted. Follow-up visits were scheduled every six months. Follow-up time was defined from the treadmill exercise testing to the last evaluation or death. Patients were considered to have LAE if they presented sustained VT/VF, aborted SCD, and/or appropriate ICD therapies during the follow-up period.
Statistical analysis
Data were analysed with IBM-SPSS for Windows statistical package (version 22.0). Categorical variables were presented as absolute values and percentages. Continuous variables were analysed as means, standard deviations, and median if they presented normal distribution. Heart rate recovery in the first minute was measured and compared with the Student’s t-test, according to the Br1ECGp presentation.
For the statistical analysis, McNemar tests were used to compare the detection of Br1ECGp at rest (orthostatic and supine position) and during the treadmill test (maximal exercise and recovery phases) in the standard (fourth ICS) vs. the HPL positioning. The significance level was defined as P-values <0.05.
Results
Baseline characteristics
A total of 74 patients were enrolled, and their demographic characteristics are summarized in Table 1. At the time of stress testing, the patients’ mean age was 49.0 ± 14 years, two years more than the mean age at the presentation diagnosis (47 ± 13.4 years). Most patients were men (57; 77%), probands (60; 81%), and self-declared white race (patients, 57; 77%, and probands 60; 81%). Regarding comorbidities, 8 patients (10.8%) had essential hypertension, 15 (20.3%) dyslipidemia, 3 (4.1%) type 2 diabetes mellitus, and 1 (1.9%) with both Long QT syndrome and BrS (‘overlapping’).
Table 1.
Baseline characteristics in patients who underwent treadmill exercise testing
| Variable | Descriptive (n = 74) |
|---|---|
| Demographic profile | |
| Age at TET (years), mean ± SD | 49.0 ± 14 |
| Male sex, n (%) | 57 (77) |
| BMI (Kg/m²), mean ± SD | 26.6 ± 4 |
| Self-declared race, n (%) | |
| White | 57 (77) |
| Brown | 13 (17.6) |
| Yellow | 3 (4.1) |
| Black | 1 (1.4) |
| Comorbidities | |
| Hypertension, n (%) | 8 (10.8) |
| Dyslipidemia, n (%) | 15 (20.3) |
| Diabetes mellitus, n (%) | 3 (4.1) |
| Long QT syndrome, n (%) | 1 (1.4) |
| BrS presentation | |
| Probands, n (%) | 60 (81) |
| Spontaneous type 1 ECG, n (%) | 58 (78.4) |
| Fever-induced type 1 ECG, n (%) | 3 (4.1) |
| Shanghai score, mean ± SD | 4.5 ± 1.1 |
| FH of unexplained SCD < 45 yrs (%) | 19 (25.7) |
| VF induced by EPS, n/n (%) | 12/40 (30) |
| ICD, n/n (%) | 21/74 (28.4) |
| SCN5A mutation, n/n (%) | 13/60 (21.7) |
| Symptoms, n (%) | 11 (14.9) |
| Nocturnal agonal respiration, n (%) | 3 (4.1) |
| Arrhythmogenic syncope, n (%) | 5 (6.7) |
| Aborted sudden death, n (%) | 3 (4.1) |
| Follow-up data | |
| Follow-up (months) | 37.9 ± 17 |
| Follow-up LAEs after TET, n/N (%) | 3/74 (4.1) |
| Death or SCD | 0 |
| Ablation of RVOT | 11/74 (14.9) |
Data are expressed as mean ± standard deviation or n (%).
Abbreviations: BMI, body mass index; EPS, electrophysiological study; FH, family history; ICD, implantable cardioverter defibrillator; LAE, Life-threatening arrhythmic event; RVOT: right ventricular outflow tract; SCD, sudden cardiac death; TET, treadmill exercise testing; VF, ventricular fibrillation; VT, ventricular tachycardia; Yrs, years.
In the baseline evaluation, they were classified as spontaneous (58/74; 78%), drug-induced (13/74; 18%), and fever-induced Br1ECGp (3/74; 4%). The mean Shanghai score was 4.5 ± 1.1, ranging from 3.5 to 8.
At initial presentation, 11/74 (14.9%) patients were symptomatic (3 had nocturnal agonal respiration, 5 had arrhythmogenic syncope, and 3 had aborted SCD), and the remaining 63/74 (85%) were asymptomatic. Programmed electrical stimulation was performed in 40/63 (63.5%) asymptomatic patients, and 12 out of 40 patients (30%) met the criteria for inducibility. ICD was implanted in 21/74 (28.4%) patients of the entire cohort; 2 refused ICD implantation. Genetic testing revealed a pathogenic or likely pathogenic SCN5A variant in 13/60 patients (21.7%).
Treadmill exercise testing results
Bruce protocol was used in 49/74 (66.2%). All subjects were in sinus rhythm, 1 patient had a first-degree atrioventricular block, and 1 had a concomitant right bundle branch block.
On average, 93% of the predicted maximum heart rate (MHR) was achieved: 7/74 (9.5%) failed to achieve 85% of the age-predicted maximum HR (chronotropic incompetence); 37/74 (50%) reached the submaximal HR (>85%), and 30/74 (40.5%) reached more than 95%, which were considered maximal tests (11 patients exceeded 100% MHR).
The mean functional capacity assessed was 10.8 metabolic equivalent (MET). Only 1 (1.4%) woman had appropriate classification (6.7 MET). Most patients’ haemodynamic response was within the normal range; 19/74 (25.7%) were hypertensive. General information is described in Table 2.
Table 2.
Characteristics of treadmill exercise testing
| TET variables | Descriptive (n = 74) |
|---|---|
| Bruce protocol n (%) | 49 (66.2) |
| MET (units), mean ± SD | 10.8 ± 3.3 |
| Age-predicted MHR (%), mean ± SD | 93 ± 9.3 |
| HRR (%), mean ± SD | 41.5 ± 6.4 |
| Normal BP ST, n (%) | 55 (74.3) |
| Arrhythmias, n (%) | |
| Absence | 52 (70) |
| PVC | 14 (19) |
| PVC pair (couplets) | 3 (4.1) |
| NSVT | 2 (2.7) |
| PAC | 6 (8.1) |
| Symptoms, n (%) | 1 (1.4) |
| AVL +, n (%) | 6 (8.1) |
Data are expressed as mean ± standard deviation or n (%).
Abbreviations: HRR, heart rate recovery; MET, metabolic equivalent of task; MHR, maximum heart rate; NSVT, non-sustained ventricular tachycardia; PAC, premature atrial contractions; PVC, premature ventricular complexes; TET, treadmill exercise testing.
Only 5/74 (6.7%) were on antiarrhythmic drugs when HPL-TET was performed: 1/74 (1.4%) used Sotalol 160 mg (frequent PVCs), 3/74 (3.1%) beta-blockers, and 1/74 amiodarone (1.4%).
ECG classification according to HPL-TET phases
1) Resting phase in orthostatic position
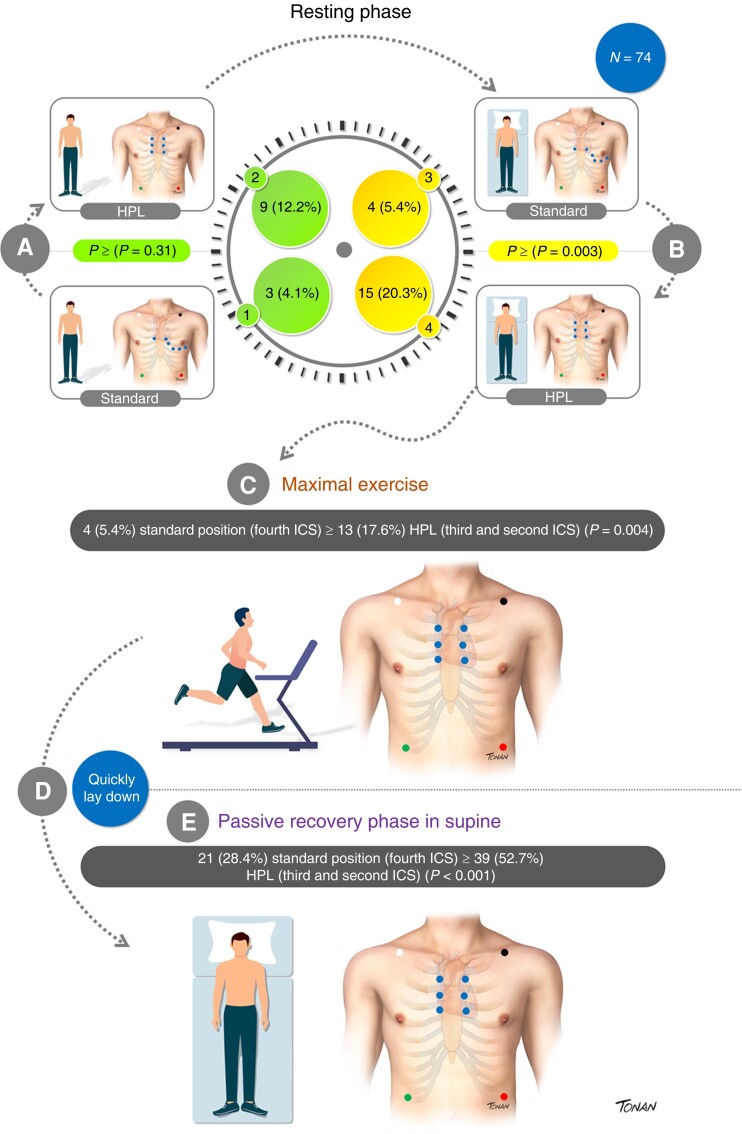
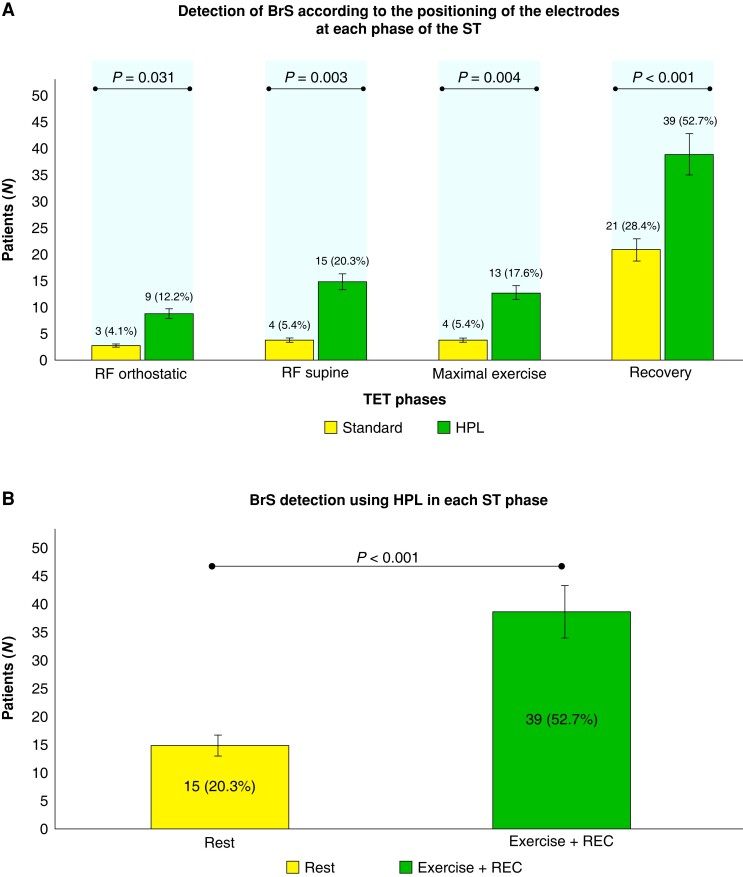
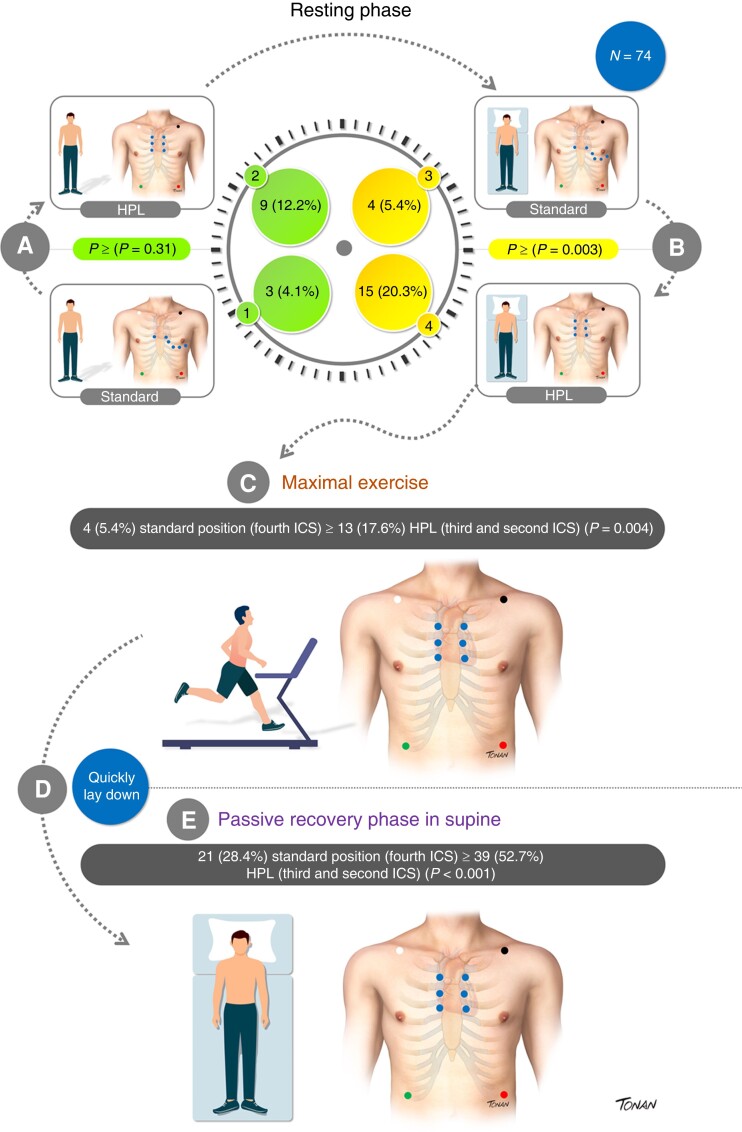
In the orthostatic resting phase (RF) in the standard position, 3/74 (4.1%) had a BrS1 ECG pattern. Its detection increased to 9/74 (12.2%) in HPL (P = 0.031).
2) Resting phase in the supine position
In the standard position, 4/74 (5.4%) had Br1ECGp. Its detection increased to 15/74 (20.3%) in HPL (P = 0.003).
3) Maximal exercise
At this phase, only 4 out of 74 subjects (5.4%) presented with Br1ECGp in the standard position and 13/74 (17.6%) in HPL (P = 0.004).
4) Quick lay down and passive recovery in the supine position
Up to 6 min, Br1ECGp was observed in 21/74 (28.4%) patients in the standard leads and 39/74 (52.7%) in HPL (P < 0.001).
Among the 41 patients who developed Br1ECGp at any time of HPL-TET, Br1ECGp was only observed in 16 (39%) during passive recovery in the supine position. ST-segment elevation in aVL was also observed in 6/74 (8.1%) patients, and all of them had concomitant Br1ECGp in precordial leads.
The detection of Br1ECGp according to TET phases and leads positioning is described in Figure 2; additional detailed information about the HPL-TET protocol is illustrated in Figure 3 (Graphical Abstract). Examples of different HPL-TET response are in supplementary materials (see Supplementary material online, Figures A–C).
Figure 2.
(A) Detection of type 1 Br1ECGp according to the leads positioning and TET phases. (B) Comparison of Br1ECGp detection between ‘resting phase’ (as in any other ECG), and incorporation of treadmill exercise testing (exercise and recovery phase). Br1ECGp, BBrugada ECG pattern; REC, recovery phase.
Figure 3.
Summary of a newly proposed treadmill exercise protocol to unmask type 1 Br1ECGp. (A) Orthostatic resting phase in standard (1) and HPL position (2); (B) supine resting phase in standard (3) and HPL position (4); (C) maximal exercise; and (D) quick lay down and passive recovery in a supine position. Data are expressed as mean or n (%). HPL, high precordial leads; ICS, intercostal space.
Heart rate recovery and type 1 BrS fluctuation
The HRR was greater in patients who developed Br1ECGp in the recovery phase of HPL-TET (43.2 ± 10.7 vs. 38.1 ± 11.2 P = 0.050). (Table 3).
Table 3.
Heart rate recovery and brugada type 1 detection
| Variable | BrS | Total | P | |
|---|---|---|---|---|
| HRR | Absent | Present | ||
| Mean ± SD | 38.1 ± 11.2 | 43.2 ± 10.7 | 40.9 ± 11.2 | 0.05* |
| Median (min.; max.) | 39 (14; 62) | 42 (23; 75) | 41.5 (14; 75) | |
*Student’s t-test.
Mean values, standard deviation, and median of HRR in the first minute in the recovery phase of TET relating to the detection of the type 1 Br1ECGp. Data are expressed as mean ± standard deviation and median.
Abbreviations: BrS, Brugada syndrome; HRR, heart rate recovery; Min, minimum; Max, maximum.
Treadmill exercise testing safety in Brugada syndrome patients
All patients tolerated the HPL-TET protocol. One patient had self-limiting vasovagal symptoms and a drop in BP during the recovery phase while in sinus rhythm. No other symptoms were observed.
Three patients had discontinued HPL-TET (3/74; 4.1%), two of them due to short polymorphic non-sustained VT (four beats), and the other patient due to a 13-second monomorphic non-sustained VT (spontaneous recovery), both during the exercise phase. There were no ICD therapies or sudden cardiac arrest on the treadmill exercise test.
Follow-up
The mean follow-up period was 37.9 (range 7–67) months. Three patients (3/74, 4.1%) had LAE (appropriate ICD therapies) at the end of this study, and 11/74 (14.9%) patients underwent substrate ablation after treadmill protocol, according to clinical judgment. No patients had sudden cardiac death or death from any causes.
Discussion
Population and protocol
The overall characteristics of our middle-aged men population were similar to the usual presentation of BrS in the literature.2 In our registry, there is a high frequency of spontaneous type 1 Br1ECGp when adding the HPL technique (78.2%). In the baseline evaluation, 78% were classified as spontaneous; however, the ‘Brugada burden’ was low (only 20% of BrS patients had Br1ECGp at the beginning of HPL-TET). This new term refers to extensive ECG alteration in peripheral and precordial leads and the persistence of ST-segment elevation in ECG follow-up. The higher the Brugada burden, the greater the probability of arrhythmic events.15 Indeed, in our cohort, the rate of arrhythmic events was low in agreement with this concept. Our rate of inducible VF/VT by EPS (12/40, 30%) was similar to that of the Finger Registry.2
The proportion of SCN5A pathogenic and likely pathogenic variants (13/60; 21.7%) is expected for the recognized low yield of genetic testing in BrS.16
The purpose of choosing an exercise protocol based on the patient’s profile was for an individual to achieve maximal exercise tests and efforts to ensure similar autonomic imbalance among them. Up to 90% of the patients completed the MHR. The age profile and low prevalence of comorbidities could explain the good average performance.12
Increased body temperature induced by exercise could be a risk for life-threatening arrhythmias in patients with BrS and performing HPL-TET could raise some concerns. Our HPL-TET protocol, like in other series,5,17,18 was safe since no patient developed sustained VT or VF.
HPL-TET findings
The primary goal of stress testing in HPL would not be to replace the sodium channel blocker challenge in the diagnosis of Brugada syndrome. Instead, we aimed to evaluate whether stress testing using HPL could provide any additional spontaneous detection of the type 1 ECG pattern. This is an important issue to address, as the use of sodium channel blockers can be associated with significant risks and is not always practical or feasible. Although our patients had established Br1ECGp (medium Shanghai score of 4.5, ranging from 3.5 to 8), the intermittent pattern of Brugada ECG is challenging to diagnose.
Recognition of true Br1ECGp instead of phenocopies is paramount. Twelve-lead-Holter monitoring and provocative tests are the proposed options for elusive ECGs.4 Indeed, the first is scarce in clinical settings, and the second is limited to countries where proper drugs are available. Furthermore, recently an induced Br1ECGp was not defined by itself as Brugada syndrome and misleading diagnoses must be refuted.1
ECG recording in Brugada syndrome patients is routinely done by repositioning precordial leads to HPL to increase the likelihood of the typical pattern detection.1 The increased sensitivity had been stated by several authors,8,17,19–22 and electroanatomic studies ratified its utility. Little is known about the diagnostic yield of HPL-TET. In general, TET is underutilized in clinical settings for channelopathies, as pointed out by Giudicessi and Ackerman23 for SCD survivors, and by Behr24 for family screening.
We found several case reports regarding standard stress testing with ST-segment augmentation, once called ‘Exercise-induced Brugada sign’25,26 during the recovery and the exercise phase of the test in Brugada syndrome patients. Our primary finding was the increase of Br1ECGp detection using HPL-TET instead of only resting HPL-ECG (52.7% vs. 20.3%, P = 0.001), representing a diagnostic increment of 32.4%. The recovery phase was associated with the Br1ECGp detection, reinforced by HPL positioning.
Parasympathetic reactivation is thought to occur at early recovery after treadmill exercise testing, especially in the first minute after exercise cessation.27,28 In line with the pathophysiological role of the parasympathetic nervous system in BrS, some authors realized that dynamic ECG patterns might fluctuate according to autonomic balance, usually more prominent during vagal tone.29 When performing the recovery phase passively in horizontal dorsal decubitus, we aimed to reduce the adrenergic tone of the orthostatic position.
Of note, our study confirmed that the higher the HRR value, the greater the likelihood of Br1ECGp detection under an exacerbated parasympathetic activity. Makimoto and colleagues performed stress testing using standard leads and showed higher ST-segment augmentation in patients with greater HRR as well.5
Cerrato et al. demonstrated that 20% of the drug-induced patients with Br1ECGp were, in fact, spontaneous BrS type 1 patients in 12-lead 24-h Holter monitoring when the protocol HPL-ST was added.30 Segment elevation burden is higher in the afternoon and early evening, also observed in the ‘Brugada clock’ study,4 possibly reinforcing the role of vagal tone after meals.
Exertion leads to sympathetic activation, and parasympathetic inhibition increases the HR, a reliable indicator to evaluate cardiac autonomic function.31 In our study, only some patients developed Br1ECGp in peak exercise (4/74; 5%), although the prevalence of this finding in the literature could not be found. Leong et al. assessed the ventricular conduction stability test in BrS patients immediately after peak exercise and detected greater conduction heterogeneity in SCD survivors compared to non-survivors.32,33
QRS widening was observed by Amin et al. predominantly in patients with SCN5A mutation during treadmill exercise testing.17 Although QRS duration was not systematically measured in our study, among four patients who presented with a Br1ECGp and an enlarged QRS complex, only one had an SCN5A mutation.
Some studies have already reported the potential role of exercise testing in BrS prognosis.5,18,34–36 Amin et al.17 found a higher parasympathetic reactivation during early recovery after exertion in BrS patients with prior VF. Makimoto et al.5 reported 93 patients who underwent standard stress testing. Likewise, they observed a marked augmentation of ST-segment elevation in the early recovery phase. Besides, ST-segment elevation ≥ 0.05 mV in leads V1 to V3 was also observed as a predictor of poor prognosis by Subramanian.18 In our low-risk type 1 Brugada patients from GenBra,10 we could not perform risk association analysis with HPL-TET findings. In contrast, type 1 development was observed with slightly greater HRR in patients who developed Br1ECGp (43.2 vs. 38.1, P = 0.05).
Our study has some limitations. The primary obstacles in identifying independent predictors of increased arrhythmic risk are low event rates, limited follow-up time, and phenotypic variability among different patient populations. The lack of a non-dynamic golden standard tool in diagnosis is a barrier to genuinely recognizing the sensitivity and specificity of Brugada syndrome induced by stress testing as a prognostic tool.
Further studies in the general population are required to explore the prevalence of type 1 Br1ECGp in the healthy population and its relationship with true Brugada syndrome.
Conclusion
The treadmill exercise testing protocol, using HPL and the passive recovery phase in the supine position, is a valuable tool that offers an opportunity to unmask the type 1 Brugada pattern ECG, which could increase the diagnostic yield in this population.
Perspectives of the treadmill exercise stress testing
Keeping the recovery phase under watchful eyes could provide data on ST-segment fluctuation in established BrS patients and raise new diagnosis opportunities.
Consortium: Cesar Gruppi1 (interpretation and conception), Denise Tessariol Hachul (interpretation)2, Gabrielle Darezzo Pessente (acquisition)4, Jose Eduardo Krieger4 (acquisition of data), Jose Grindler3 (interpretation), Leonardo Filipe Benedeti Marinucci1 (acquisition of data), Livia Ozzetti Azzouri1 (acquisition of data), Mariana Lombardi Peres de Carvalho3 (acquisition), Mirella Faccin2 (conception), Pedro Veronese (acquisition)2, Rodrigo Imada1 (acquisition of data), Sávia Christina Pereira Bueno2 (acquisition), Silvio Barbosa1 (interpretation), Tan Chen Wu2 (acquisition)—Instituto do Coracao (InCor), Departments of Ergometry(1), Arrhythmias(2), Electrocardiography Service(3) and Laboratory of Genetics and Molecular Cardiology(4)—Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil.
Supplementary Material
Acknowledgements
Medical illustrations—Rodrigo Ricieri Tonan—ORCID https://orcid.org/0009-0004-2530-8148. contato@mentovery.com.br
Contributor Information
Nemer L Pichara, Departament of Ergometry—Instituto do Coracao (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Luciana Sacilotto, Arrhythmia Unit—Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Av. Dr. Eneas de Carvalho Aguiar, 44 AB, CEP 05403-900 Sao Paulo, Brazil.
Maurício I Scanavacca, Arrhythmia Unit—Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Av. Dr. Eneas de Carvalho Aguiar, 44 AB, CEP 05403-900 Sao Paulo, Brazil.
Acácio Fernandes Cardoso, Electrocardiology Service, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Beatriz Moreira Ayub Ferreira Soares, Departament of Ergometry—Instituto do Coracao (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Paola P PN F Falcochio, Departament of Ergometry—Instituto do Coracao (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Andrea M G Falcão, Departament of Ergometry—Instituto do Coracao (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Natalia Olivetti, Laboratory of Genetics and Molecular Cardiology (LGMC) Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Francisco Carlos da Costa Darrieux, Arrhythmia Unit—Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Av. Dr. Eneas de Carvalho Aguiar, 44 AB, CEP 05403-900 Sao Paulo, Brazil.
William A Chalela, Departament of Ergometry—Instituto do Coracao (Incor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - 05403-900.
Supplementary material
Supplementary material is available at Europace online.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo- FAPESP, a governmental funding provided financial support for the genetic testing—grant #2016/15223-3 (to Prof. F.C.C. Darrieux).
Data availability
Relevant data on the significance of type-1 BrS ECG pattern detection with the treadmill exercise stress test with HPL have been incorporated into the article and its Supplementary material online. Additional data are available on request.
References
- 1. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. . 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 2. Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HLet al. . Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada syndrome registry. Circulation 2010;121:635–43. [DOI] [PubMed] [Google Scholar]
- 3. Zorzi A, Mastella G, Cipriani A, Berton G, Del Monte A, Gusella Bet al. . Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur J Prev Cardiol 2018;25:2003–11. [DOI] [PubMed] [Google Scholar]
- 4. Gray B, Kirby A, Kabunga P, Freedman SB, Yeates L, Kanthan Aet al. . Twelve-lead ambulatory electrocardiographic monitoring in Brugada syndrome: potential diagnostic and prognostic implications. Heart Rhythm 2017;14:866–74. [DOI] [PubMed] [Google Scholar]
- 5. Makimoto H, Nakagawa E, Takaki H, Yamada Y, Okamura H, Noda Tet al. . Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol 2010;56:1576–84. [DOI] [PubMed] [Google Scholar]
- 6. Brugada J, Pappone C, Berruezo A, Vicedomini G, Manguso F, Ciconte Get al. . Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ Arrhythm Electrophysiol 2015;8:1373–81. [DOI] [PubMed] [Google Scholar]
- 7. Honarbakhsh S, Providencia R, Garcia-Hernandez J, Martin CA, Hunter RJ, Lim WYet al. . A primary prevention clinical risk score model for patients with brugada syndrome (BRUGADA-RISK). JACC Clin Electrophysiol 2021;7:210–22. [DOI] [PubMed] [Google Scholar]
- 8. Shimizu W, Matsuo K, Takagi M, Tanabe Y, Aiba T, Taguchi Aet al. . Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol 2000;11:396–404. [DOI] [PubMed] [Google Scholar]
- 9. Batchvarov VN, Govindan M, Camm AJ, Behr ER. Brugada-Like changes in the peripheral leads during diagnostic ajmaline test in patients with suspected Brugada syndrome. Pacing Clin Electrophysiol 2009;32:695–703. [DOI] [PubMed] [Google Scholar]
- 10. Sacilotto L, Scanavacca MI, Olivetti N, Lemes C, Pessente GD, Wulkan Fet al. . Low rate of life-threatening events and limitations in predicting invasive and noninvasive markers of symptoms in a cohort of type 1 Brugada syndrome patients: data and insights from the GenBra registry. J Cardiovasc Electrophysiol 2020;31:2920–8. [DOI] [PubMed] [Google Scholar]
- 11. Kawada S, Morita H, Antzelevitch C, Morimoto Y, Nakagawa K, Watanabe Aet al. . Shanghai Score system for diagnosis of Brugada syndrome. JACC Clin Electrophysiol 2018;4:724–30. [DOI] [PubMed] [Google Scholar]
- 12. Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJet al. . Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007;116:329–43. [DOI] [PubMed] [Google Scholar]
- 13. Meneghelo RS, Araújo CGS, Stein R, Mastrocolla LE, Albuquerque PF, Serra SMet al. . /Sociedade brasileira de cardiologia. III diretrizes da sociedade brasileira de cardiologia sobre teste ergométrico. [III of sociedade brasileira de cardiologia on the exercise test]. Arq Bras Cardiol 2010;95:1–26. [DOI] [PubMed] [Google Scholar]
- 14. Committee Members, Gibbons RJ, Balady GJ, Timothy Bricker J, Chaitman BR, Fletcher GFet al. . ACC/AHA 2002 guideline update for exercise testing: summary article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to update the 1997 exercise testing guidelines). Circulation 2002;106:1883–92. [DOI] [PubMed] [Google Scholar]
- 15. Stazi F, Battisti P. When Brugada syndrome is at risk of sudden death: clinical and anatomical aspects. Eur Heart J Suppl 2022;24:I165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster Jet al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amin AS, de Groot EAA, Ruijter JM, Wilde AAM, Tan HL. Exercise-induced ECG changes in Brugada syndrome. Circ Arrhythm Electrophysiol 2009;2:531–9. [DOI] [PubMed] [Google Scholar]
- 18. Subramanian M, Prabhu MA, Harikrishnan MS, Shekhar SS, Pai PG, Natarajan K. The utility of exercise testing in risk stratification of asymptomatic patients with type 1 Brugada pattern. J Cardiovasc Electrophysiol 2017;28:677–83. [DOI] [PubMed] [Google Scholar]
- 19. Sangwatanaroj S. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J 2001;22:2290–6. [DOI] [PubMed] [Google Scholar]
- 20. Papadakis M, Papatheodorou E, Mellor G, Raju H, Bastiaenen R, Wijeyeratne Yet al. . The diagnostic yield of Brugada syndrome after sudden death with normal autopsy. J Am Coll Cardiol 2018;71:1204–14. [DOI] [PubMed] [Google Scholar]
- 21. Márquez MF, Allende R, Morales JL. Unmasking the Brugada syndrome with high parasternal leads. Europace 2007;9:1216.Epub 2007 Oct 15 [DOI] [PubMed] [Google Scholar]
- 22. Miyamoto K, Yokokawa M, Tanaka K, Nagai T, Okamura H, Noda Tet al. . Diagnostic and prognostic value of a type 1 Brugada electrocardiogram at higher (third or second) V1 to V2 recording in men with Brugada syndrome. Am J Cardiol 2007;99:53–7. [DOI] [PubMed] [Google Scholar]
- 23. Giudicessi JR, Ackerman MJ. Exercise testing oversights underlie missed and delayed diagnosis of catecholaminergic polymorphic ventricular tachycardia in young sudden cardiac arrest survivors. Heart Rhythm 2019;16:1232–9.. Epub 2019 Feb 11. PMID: 30763784. [DOI] [PubMed] [Google Scholar]
- 24. Behr ER, Scrocco C, Wilde AAM, Marijon E, Crotti L, Iliodromitis KEet al. . Investigation on sudden unexpected death in the young (SUDY) in Europe: results of the European Heart Rhythm Association Survey. Europace 2022;24:331–9. [DOI] [PubMed] [Google Scholar]
- 25. Jayasuriya C, Whitman M. Exercise-induced Brugada sign. Europace 2011;13:446–7.. Epub 2010 Sep 29. [DOI] [PubMed] [Google Scholar]
- 26. Grimster A, Segal OR, Behr ER. Type I Brugada electrocardiogram pattern during the recovery phase of exercise testing. Europace 2008;10:897–8. Epub 2008 Apr 28. [DOI] [PubMed] [Google Scholar]
- 27. Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama Het al. . Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994;24:1529–35. [DOI] [PubMed] [Google Scholar]
- 28. Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJet al. . Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol-Heart Circ Physiol 1989;256:H132–41. [DOI] [PubMed] [Google Scholar]
- 29. Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol 1996;27:1061–70. [DOI] [PubMed] [Google Scholar]
- 30. Cerrato N, Giustetto C, Gribaudo E, Richiardi E, Barbonaglia L, Scrocco Cet al. . Prevalence of type 1 Brugada electrocardiographic pattern evaluated by twelve-lead twenty-four-hour Holter monitoring. Am J Cardiol 2015;115:52–6. [DOI] [PubMed] [Google Scholar]
- 31. Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease. J Am Coll Cardiol 2008;51:1725–33. [DOI] [PubMed] [Google Scholar]
- 32. Leong KMW, Ng FS, Shun-Shin MJ, Koa-Wing M, Qureshi N, Whinnett ZIet al. . Non-invasive detection of exercise-induced cardiac conduction abnormalities in sudden cardiac death survivors in the inherited cardiac conditions. Europace 2021;23:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shun-Shin MJ, Leong KMW, Ng FS, Linton NWF, Whinnett ZI, Koa-Wing Met al. . Ventricular conduction stability test: a method to identify and quantify changes in whole heart activation patterns during physiological stress. Europace 2019;21:1422–31. [DOI] [PubMed] [Google Scholar]
- 34. Masrur S, Memon S, Thompson PD. Brugada syndrome, exercise, and exercise testing. Clin Cardiol 2015;38:323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calvo M, Gomis P, Romero D, Le Rolle V, Béhar N, Mabo Pet al. . Heart rate complexity analysis in Brugada syndrome during physical stress testing. Physiol Meas 2017;38:387–96. [DOI] [PubMed] [Google Scholar]
- 36. Calvo M, Romero D, Le Rolle V, Béhar N, Gomis P, Mabo Pet al. . Multivariate classification of brugada syndrome patients based on autonomic response to exercise testing. Tolkacheva EG, editor. PLoS One 2018;13:e0197367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Relevant data on the significance of type-1 BrS ECG pattern detection with the treadmill exercise stress test with HPL have been incorporated into the article and its Supplementary material online. Additional data are available on request.