Abstract
This review aimed to map current evidence on the association between dietary factors and colorectal cancer (CRC) risk in Asia. This review was conducted based on Arksey and O’Malley methodological framework. Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) flow diagram was used to record the review process. For the purpose of searching for articles, three electronic databases namely PubMed, EBSCOHost and ScienceDirect were employed. The inclusion criteria for articles selection were articles with association analysis between diet and CRC risk among Asians, had adults as participants, articles were written in English, open-accessed and published between years 2009 and 2021. Thus, 35 out of 369 screened articles were eventually included in this review which covered 28 case-control studies, six prospective cohort studies and one randomised clinical trial. Foods such as meats, alcohol and westernised diet have been shown to be associated with increase of CRC risk while fruits, vegetables and traditional meals decreased the risk of CRC. Only a few interventional and dietary patterns studies were identified. Specific single foods and nutrients and dietary patterns have been found to increase the risk but also protected the Asian population against CRC. The findings of this review will guide health professionals, researchers and policy makers to conduct a suitable study design and topic for future research.
Keywords: colorectal neoplasms, diet, dietary exposure, Asia
Introduction
Colorectal cancer (CRC) is currently a major public health concern worldwide due to the increasing trend of new cases from 1.36 million in 2015 (GLOBOCAN 2015) to 1.8 million in 2018 (GLOBOCAN 2018) (1, 2). CRC is the second leading cause of cancer-related mortality and the third most common cancer globally (1, 3). Despite having a lower overall frequency of CRC than Western countries, Asia has the highest number of prevalent instances, according to the International Agency for Research on Cancer’s analysis of the WHO database (4). The 5-year prevalence rate was reportedly higher in China, Japan, Korea, Malaysia, Singapore and Turkey than that of other Asian countries (≥ 46.5/100,000) while India, Indonesia, Vietnam and Iran had relatively low prevalence rate (5). The reason for these differences between Asian countries is not entirely clear. However, the large population size in China might be a contributing factor to its highest number of prevalent cases, new cases and deaths (4).
The root causes of CRC can be due to non-modifiable and environmental risk factors (4). Non-modifiable factors include genetic factors, age, gender, ethnicity, body height and family history of CRC while environmental risk factors consisted of smoking, alcohol drinking, overweight and obesity, westernised diet, physical inactivity, chronic diseases and microbiota (4). A genetic predisposition was estimated to cause 25% of CRC while 5% had reported to be inherited factors that contributed to its growth. Approximately 70% of CRC cases were sporadic cases and they were affected by environmental factors (6, 7). A systematic review and meta-analysis by Magalhães et al. (8) reported that a westernised diet characterised by a high intake of red and processed meat had increased the colon cancer risk. However, some foods might be protective factors against CRC risk such as whole grains, dairy products and foods containing dietary fibre based on a recent report from the continuous update project (CUP) (9).
Studies from various diet-related to CRC were conducted widely around the world (10–15). However, the literature mapping and research gaps especially in Asia are limited. Therefore, this review proposed to map the current evidence on the linkage between diet and CRC risk in Asia.
Methods
This review was conducted following the methodological framework developed by Arksey and O’Malley (16) which included identifying the research question, identifying relevant studies, study selection, charting data and summarising the results. This review will be following the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (17). A protocol for the review does not exist and ethics approval was not required as the review relied solely on publicly available information.
Identifying the Research Question
The review or research question was determined prior to identifying relevant studies. Population, Intervention, Comparison, Outcomes, Study type (PICOS) framework or model was applied to develop a review question and search strategy (18, 19). Three PICOS elements developed were: Asia (populations), diet (intervention or exposure) and CRC (outcomes) while another two elements (comparison and study type) were not applicable. The proposed review question was ‘What is the association between diet and CRC risk among Asians?’ Three key terms identified through the review question were diet, CRC and Asia. This is because the dietary habits of each country and region were different (20), Asia had the highest number of prevalent cases as compared to Western countries (4) and investigating the Asians habits may help to identify foods, nutrients and dietary patterns that causes CRC among the population.
Identifying Relevant Studies
This review used three electronic databases namely PubMed, EBSCOHost and ScienceDirect for article searching. A simple search using the key terms: diet and CRC was performed in the electronic databases to determine the keywords (synonyms). Keywords were also obtained from Medical Subject Headings (MeSH) database. The key terms and keywords (Supplementary 1) obtained were then used in PubMed, EBSCOHost and ScienceDirect databases to search for the articles. The Boolean operator of AND was used to combine key terms and keywords (1 AND 2 AND 3) during the articles search. The number of studies available and selected based on the key terms and keywords search is shown in Supplementary 1.
Study Selection
The article selections were conducted in two stages. For the first stage, two researchers were responsible to screen the titles and abstracts of the articles independently based on the inclusion and exclusion criteria and search terms. The inclusion criteria for the article’s search included articles that reported the association between diets and CRC risk among Asians, adults (aged 18 years old and above), all types of study designs except review articles, systematic reviews, narrative reviews, opinion papers, letters and conference proceedings with abstract only, the articles have to be written in English, open-accessed and published in between 1 January 2009 and 7 October 2021. Any non-human, animal studies and studies involving benign colorectal diseases were excluded. The PRISMA-ScR diagram was used as a guide to record the review process (17). After the selected titles and abstracts were screened and checked to ensure their relevance to the review question, researchers then retrieved the full text articles of selected abstracts and excluded unrelated abstracts.
In the second stage, the full articles were checked and reviewed by two researchers independently to ensure it meets the review objective. Furthermore, articles that do not reflect the association between dietary intake and CRC risk in Asia and articles that do not meet the review objective were excluded. Then, relevant articles were assessed to answer the review question. Discrepancies between researchers were discussed and a consensus was sought at the end. Throughout the process, EndNote® software was used to manage the results from the search.
Charting the Data
A standardised data charting form (Table 1) was developed and reviewed by the study team for relevance and appropriateness. The form’s extraction fields captured relevant information on study characteristics, including author(s), year of publication, aims of the study, study design, type of scale or instrument, sample size, participant’s characteristics and results that were relevant to the objectives of the review. The charting form was pilot tested by researchers with a random sample of five sources of evidence to ensure all relevant data were captured. Eligible sources of evidence were charted using Microsoft Excel. The goal of this scoping review was to provide an overview of the existing literature regardless of quality and a formal appraisal of the methodological quality of sources of evidence that were included in the review was not performed.
Table 1.
Characteristics of the studies included in the review
| Author, year | Aim | Study design/Scale | Sample size | Participant characteristics | Results |
|---|---|---|---|---|---|
| Abu Mweis et al. (28), 2015 | To examine the association between food groups (including grains, fruits, vegetables, milk, and meat and legumes) and CRC risk in Jordan | Case-control study/FFQ known as DHQ I | 167 cases and 240 controls | CRC patients and hospital-derived controls, Jordan, percentage age groups < 50 yr. (44.8%), > 50 yr. (55.2%) for cases and < 50 yr. (53.2%), > 50 yr. (46.8%) for controls | There was a direct association between the risk of CRC and the frequency of consumption of chicken (OR = 2.52; 95% CI: 1.33, 4.77) Increased consumption of white bread was associated with increased CRC risk (OR = 3.13; 95% CI: 1.18, 9.25; Ptrend = 0.042) Increased consumption of whole bread was associated with decreased CRC risk (OR = 0.32; 95% CI: 0.12, 0.84; Ptrend = 0.042) |
| Arafa et al. (22), 2011 | To evaluate the dietary pattern, sociodemographic and other lifestyle risk factors of CRC patients as compared to control subjects in Jordan | Case-control study/SQFFQ | 220 cases and 220 controls | CRC patients and hospital-derived control, Jordan, mean age for cases and controls (56.3 ± 12.3 yr. for males and 53.7 ± 6.8 yr. for females), males (53.6 %), females (46.4%) | The frequency of consumption of fruits (P < 0.001) and vegetables (P < 0.001) was lower among CRC cases while the frequency of consumption of red meat and saturated fat (P < 0.001) was higher and positively associated with CRC risk |
| Cho et al. (46), 2018 | To examine whether a specific dietary pattern reflecting inflammation was associated with a risk of CRC | Age and sex matched case-control study/SQFFQ-106 items | 695 cases and 1,846 controls | CRC patients and hospital-derived controls, Korea, mean age 56.4 yr. (cases) and 56.1 yr. (controls) | High CRP-dietary pattern score was associated with increased risk of CRC (OR = 9.98; 95% CI: 6.81, 14.62, for highest vs. lowest quartile; Ptrend < 0.001) The association of CRP-dietary pattern score was slightly stronger with rectal cancer (OR = 11.69; 95% CI: 6.81, 20.05 for highest vs. lowest quartile; Ptrend < 0.001) compared to colon cancer (OR = 8.87; 95% CI: 5.55, 14.18 for highest vs. lowest quartile; Ptrend < 0.001) The stronger association between CRP-dietary pattern score with rectal cancer than colon cancer was observed in women but not in men |
| Cho et al. (47), 2016 | To examine the association between the DII and the risk of CRC through a case-control study conducted in Korea | Age-gender matched case-control study/SQFFQ-106 items | 923 cases and 1,846 controls | CRC patients and hospital-derived controls, Korea, mean age 56.6 yr. (cases) and 56.1 yr. (controls) | A higher DII score was associated with an increased incidence of CRC (OR = 2.16; 95% CI: 1.71, 2.73 for highest vs. lowest tertile; Ptrend < 0.001) A slightly weaker association was observed with proximal colon cancer (OR = 1.68; 95% CI: 1.08, 2.61 for highest vs. lowest tertile; Ptrend = 0.02) A stronger association was observed among women (OR = 2.50; 95% CI: 1.64, 3.82 for highest vs. lowest tertile; P trend < 0.001) compared to men (OR = 1.72; 95% CI: 1.30, 2.28 for highest vs. lowest tertile; P trend < 0.001) Stronger associations were observed among subjects who were ≥ 50 yr. (OR = 2.61; 95% CI: 1.98, 3.43 for highest vs. lowest tertile; P for interaction = 0.004), engaged in physical activity (OR = 3.42; 95% CI: 2.37, 4.95 for highest vs. lowest tertile; P for interaction < 0.001) and did not smoke (OR = 2.58; 95% CI: 1.81, 3.68 for highest vs. lowest tertile; P for interaction = 0.03) |
| DellaValle et al. (58), 2014 | To investigate the association between dietary nitrate and nitrite intake and risk of CRC in the Shanghai Women’s Health Study | Prospective cohort study/FFQ-77 items | 73,188 | Women, Shanghai, aged 40 yr.–70 yr. | Overall, nitrate intake was not associated with CRC risk (HR = 1.08; 95% CI: 0.73, 1.59; Ptrend = 0.39) Among women with vitamin C intake below the median (83.9 mg/day) and hence higher potential exposure to NOCs, risk of CRC increased with increasing quintiles of nitrate intake (highest vs. lowest quintile HR = 2.45; 95% CI: 1.15, 5.18; Ptrend = 0.02) No association among women with higher vitamin C intake (HR = 0.93; 95% CI: 0.44, 1.96; Ptrend = 0.69) No association between nitrite intake and risk of CRC overall (HR = 1.05; 95% CI: 0.77, 1.42; Ptrend = 0.78) or by low (HR = 1.10; 95% CI: 0.68, 1.79; Ptrend = 0.74) or high (HR = 0.90; 95% CI: 0.55, 1.49; Ptrend = 0.85) intake of vitamin C |
| Ganesh et al. (23), 2009 | To determine the various factors associated with CRC such as tobacco, alcohol drinking and dietary items | Case-control study/Food items questionnaire | 203 cases and 1,628 controls | CRC patients and hospital-derived controls, Mumbai, percentage age groups < 35 yr. (11.3%), 35 yr.–44 yr. (27.1%), 45 yr.–54 yr. (26.6%), 55 yr.–64 yr. (24.1%), 65+ yr. (10.8%) for cases and < 35 yr. (13.4%), 35 yr.–44 yr. (26.3%), 45 yr.–54 yr. (27.7%), 55 yr.–64 yr. (21.0%), 65+ yr. (11.5%) for controls | No significant excess risk for chewers (OR = 0.9; CI: 0.7, 1.4), (OR = 0.5; CI: 0.2, 1.1), (OR = 0.8; CI: 0.6, 1.2) among men, women and both sexes respectively compared to those without the habits No significant excess risk for alcohol drinkers (OR = 1.2; CI: 0.7, 2.1), (OR = 1.2; CI: 0.7, 2.2) among men and both sexes, respectively compared to those without the habits Cabbage-eaters among men (OR = 0.6; CI: 0.3, 0.9) and both sexes (OR = 0.5; CI: 0.3, 0.8) had 40% and 50% reduction in risk, respectively, compared to those who did not eat cabbage. No significant observed among women (OR = 0.5; CI: 0.2, 1.1) Sprout eaters among women (OR = 0.7; CI: 0.4, 1.2) and both sexes (OR = 0.5; CI: 0.4, 2.4) had 30% and 50% reduction in risk, respectively. No significant observed among men (OR = 0.5; CI: 0.3, 0.7) Men who consume dry-fish had an increase of 1.6-fold risk compared to those who did not eat dry-fish (OR = 1.6; CI: 1.0, 2.6) Fresh-fish eaters among both sexes (OR = 0.6; CI: 0.4, 0.9) and women (OR = 0.3; CI: 0.1, 0.5) had 40% and 70% reduction in risk compared to those who did not eat fresh-fish Meat-eaters among women (OR = 2.4; CI: 1.2, 4.7) had a 2.4-fold excess risk than non-meat-eaters. Meat-eaters among men also had excess risk (OR = 1.0; CI: 0.6, 1.7) than non-meat-eaters Dark-green-leafy-vegetables consumed among men (OR = 1.1; CI: 0.6, 2.0), women (OR = 1.3; CI: 0.6, 3.2) and both sexes (OR = 1.2; CI: 0.7, 1.9) did not have protective effect against CRC Root vegetables consumed among men (OR = 1.0; CI: 0.5, 2.0), women (OR = 1.9; CI: 0.7, 5.7) and both sexes (OR = 1.3; CI: 0.7, 2.2) did not show any protective effect against CRC Other vegetables eaten among men (OR = 1.8; CI: 0.5, 6.8), women (OR = 0.3; CI: 0.1, 1.1) and both sexes (OR = 1.0; CI: 0.4, 2.4) did not have protective effect against CRC |
| Huang et al. (34), 2018 | To evaluate the association between dietary intake of total carbohydrate, non-fibre carbohydrate, total fibre, starch, GI and GL and CRC risk | Age-gender matched case–control study/FFQ-81 items | 1,944 cases and 2,027 controls | CRC patients, hospital-derived controls and community-derived controls, Guangzhou, mean age 56.4 yr. (cases) and 56.2 yr. (controls) | Total fibre was related to a 53% reduction in CRC risk (aORquartile 4 v. 1 0.47; 95% CI: 0.39, 0.58; Ptrend < 0.01) Dietary GI was positively associated with CRC risk (aOR quartile 4 v. 1 3.10; 95% CI: 2.51, 3.85; Ptrend < 0.01) |
| Khan et al. (32), 2015 | To examine associations of dietary practices, addictive behaviour and bowel habits in developing CRC among patients in a low-resource setup | Age-gender matched case-control study/Structured questionnaire | 74 cases and 148 controls | CRC patients, healthy individuals, Karachi, average age of cases 41.47 ± 15.48 yr | Individuals who consumed high fats diet had 98% higher chances to endure CRC compared to those who did not consume those diets (OR = 1.98; 95% CI: 1.13, 3.49; P = 0.017) |
| Kim et al. (35), 2012 | To investigate the association between folate and alcohol intake, methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and CRC risk in Koreans | Case-control study/FFQ-dietary intake, structured questionnaire-alcohol | 787 cases and 656 controls | CRC patients, hospital-derived controls, Seoul, age of cases between 30 yr. and 79 yr. | High folate intake was associated with reduced CRC risk (OR = 0.64; 95% CI: 0.49, 0.84; Ptrend = 0.002 for high compared with low intake) High alcohol consumption was associated with increased risk of CRC (OR = 1.76; 95% CI: 1.26, 2.46; Ptrend = 0.001 for high compared with low intake) |
| Mafiana et al. (59), 2018 |
|
Case-control study/FFQ-dietary intake, structured questionnaire-alcohol | 109 cases and 170 controls | CRC patients, hospital-derived controls, Muscat, mean age 53.7 yr. (cases) and 57.4 yr. (control) | Alcohol consumption was not associated with CRC (OR = 0.91; 95% CI: 0.32, 2.58; P = 0.86) |
| Nayak et al. (24), 2009 | To identify the dietary predispositions of the indigenous population of Malabar region of the state of Kerala, India | Age and sex matched case-control study/FFQ | 108 cases and 324 controls | Adenocarcinoma of colon patients, hospital-derived controls, Kerala, mean age of the cases 55.6 ± 0.98 yr | A strong association was found between CRC and tapioca (OR = 2.7; P = 0.001), beef (OR = 4.25; P < 0.001) and pungent spices (OR = 9.62; P = 0.018) Fruits and vegetables showed a strong negative association (OR = 0.15; P = 0.002) Fish consumption on a daily basis showed a 25% reduction in risk Heavy consumption of sugar (OR = 2.80) showed significant high risk |
| Park et al. (44), 2016 | To identify major dietary patterns among Koreans and to evaluate the associations of these patterns with CRC risk by gender, taking into account different anatomical subsites | Case-control study/SQFFQ-106 items | 923 cases and 1,846 controls | CRC patients, hospital-derived controls, Korea, mean age of cases 56.6 ± 9.7 yr | Traditional and prudent patterns were inversely associated with CRC risk (OR = 0.35; 95% CI: 0.27, 0.46 for the highest intake tertile of pattern score vs. the lowest; Ptrend < 0.001) and (OR = 0.37; 95% CI: 0.28, 0.48 for the highest intake tertile of pattern score vs. the lowest; Ptrend < 0.001), respectively Westernised pattern showed a positive association especially among women (OR = 2.13; 95% CI: 1.35, 3.34 for the highest vs. lowest tertile; Ptrend < 0.01) A decrease in CRC risk among those with the highest intake of the prudent pattern was observed in all anatomical subsites in both men (OR = 0.36; 95% CI: 0.19, 0.68; Ptrend < 0.01 for proximal colon; OR = 0.21; 95% CI: 0.12, 0.36; Ptrend < 0.001 for distal colon; OR = 0.28; 95% CI: 0.18, 0.44; Ptrend < 0.001 for rectum) and women (OR = 0.28; 95% CI: 0.11, 0.71; Ptrend < 0.01; OR = 0.27; 95% CI: 0.13, 0.54; Ptrend < 0.001; OR = 0.45; 95% CI: 0.25, 0.83; Ptrend = 0.01), respectively |
| Poomphakwaen et al. (25), 2014 | To investigate the interaction between the presence of a polymorphism of the XRCC1 gene and known risk factors for colorectal cancer in Thailand | Age and sex matched case-control study/Semi quantitative food and beverage intake frequency questionnaire | 230 cases and 230 controls | CRC patients, hospital-derived controls, Khon Kaen Province or neighbouring provinces, percentage age groups of cases < 45 yr. (23.9%), 45 yr.–55 yr. (27.8%), 56 yr.–65 yr. (31.3%), > 65 yr. (17%) | High frequency of pork consumption was significantly associated with an increased risk of CRC (OR = 1.49; 95% CI: 1.00, 2.21; P = 0.047) Fish (OR = 0.94; P = 0.862), fruit (OR = 0.91; P = 0.669), vegetables (OR = 0.94; P = 0.753), beef (OR = 1.20; P = 0.37), poultry (OR = 1.45; P = 0.06) and others were not associated with CRC |
| Rafiee et al. (50), 2019 | To examine the relationship between E-DII and the risk of CRC and CAP in a case-control study in Iran | Age and sex matched case-control study/Semi-quantitative FFQ-148 items | 134 CRC, 130 CAP, 240 controls | CRC patients, CAP patients, hospital-derived controls, Tehran, mean age 59 (49.25–64) yr. (CRC), 58 (51–64) yr. (CAP) | A significant positive association was observed between E-DII and CRC risk (aORcontinuous = 1.71; 95% CI: 1.41, 2.07) Subjects in the third (highest) tertile was five times more likely to have CRC compared to subjects in the first (lowest) tertile (ORtertile 3 vs. 1 = 5.08; 95% CI: 2.70, 9.56; Ptrend < 0.0001) |
| Safari et al. (45), 2013 | To identify dietary patterns and its association with the risk of CRC in Tehran, Iran | Age and sex matched case-control study/SQFFQ-125 items | 71 cases and 142 controls | CRC patients and hospital-derived controls, Tehran, mean age 59.9 yr. (men) and 55.7 yr. (women) | Healthy dietary pattern was significantly associated with a decreased risk of CRC (OR = 0.227; 95% CI: 0.108, 0.478; P < 0.001) while an increased risk of CRC was observed with the Western dietary pattern (OR = 2.616; 95% CI: 1.361, 5.030; P = 0.004) |
| Shivappa et al. (48), 2017 | To investigate the association between DII scores and CRC in a case-control study in a Jordanian population | Age, sex and occupation matched case-control study/FFQ | 232 cases and 271 controls | CRC patients, hospital-derived controls, Jordan, mean age 53.8 yr. | Subjects with higher DII scores were at increased odds of CRC (ORcontinuous = 1.45; 95% CI: 1.13, 1.85; P = 0.002; and ORtertile 3 vs. tertile 1 = 2.13; 95% CI: 1.23–3.72; Ptrend = 0.007) |
| Shivappa et al. (49), 2018 | To examine the association between DII scores and CRC in a case-control study conducted in Iran | Case-control study/FFQ-125 items | 71 cases and 142 controls | CRC patients, hospital-derived controls, Tehran, mean age of cases 58.2 ± 10.5 yr. | Subjects with higher DII scores had a higher odds of CRC (OR continuous = 2.20; 95% CI: 1.22, 3.96; P = 0.01; and OR tertile 3 vs. tertile 1 = 2.47; 95% CI: 1.10, 5.55; Ptrend = 0.02) |
| Takachi et al. (21), 2011 | To examine associations between the consumption of red and processed meat and the risk of subsite-specific CRC by gender in a large Japanese cohort | Prospective cohort study | 80,658 | Men and women, Japan, aged 45 yr.–74 yr. | Higher consumption of red meat was significantly associated with a higher risk of colon cancer among women (HR = 1.48; 95% CI: 1.01, 2.17; Ptrend = 0.03) Higher consumption of total meat was significantly associated with a higher risk of colon cancer among men (HR = 1.44; 95% CI: 1.06, 1.98; Ptrend = 0.07) Red meat was positively associated with proximal colon cancer in women (HR = 1.57; 95% CI: 0.95, 2.58; Ptrend = 0.08) and distal colon cancer in men (HR = 1.42; 95% CI: 0.92, 2.19; Ptrend = 0.12) No association between processed meat and risk of either colon (HR = 1.19; 95% CI: 0.82, 1.74; Ptrend = 0.64) or rectal (HR = 0.98; 95% CI: 0.53, 1.79; Ptrend = 1.00) cancer in women and colon (HR = 1.27; 95% CI: 0.95, 1.71; Ptrend = 0.10) or rectal (HR = 0.70; 95% CI: 0.45, 1.09; Ptrend = 0.25) cancer in men |
| Tayyem et al. (33), 2015 | To investigate the association between macro- and micronutrient intake and CRC risk using data from a case-control study conducted in Jordan | Age, sex, occupation and marital status matched case-control study/FFQ | 169 cases and 248 control | CRC patients, hospital-derived controls, Jordan, average age for cases 53.8 ± 12.2 yr. | Total energy intake was associated with a higher risk of developing CRC (OR = 2.60; 95% CI: 1.21, 5.56 for the highest vs. lowest quartile; Ptrend = 0.03). Intakes of protein (OR = 3.62; 95% CI: 1.63, 8.05; Ptrend = 0.002), carbohydrates (OR = 1.41; 95% CI: 0.67, 2.99; Ptrend = 0.043) and percentage of energy from fat (OR = 2.10; 95% CI: 0.38,11.70; Ptrend = 0.009) were significantly increased the CRC risk Saturated fat, dietary cholesterol and sodium intake were significantly associated with CRC risk (OR = 5.23; 95% CI: 2.33, 11.76; Ptrend = 0.009; OR = 2.48; 95% CI: 1.18, 5.21; Ptrend = 0.027; and OR = 3.42; 95% CI: 1.59, 7.38, respectively) Vitamin E and caffeine intake were protective factors against CRC risk (OR = 0.002; 95% CI: 0.0003, 0.011; Ptrend= 0.001) and (OR = 0.023; 95% CI: 0.008, 0.067) respectively |
| Wang et al. (60), 2014 | To investigate the associations of sugars and sucrose intake with CRC risk in a community-based case–control study in Japan | Age and sex matched case-control study/Computer-assisted interview-148 items | 816 cases and 815 controls | CRC patients, community-derived controls, Fukuoka and three adjacent areas, mean age 60.9 (8.8) yr. (men) and 60.0 (9.4) yr. (women) | Sugars (OR = 1.15; 95% CI: 0.75, 1.75; Ptrend = 0.31; OR = 1.07; 95% CI: 0.64, 1.78; Ptrend = 0.87) intakes were not related to CRC risk in men and women, respectively Sucrose (OR = 1.09; 95% CI: 0.71, 1.67; Ptrend = 0.50; OR = 1.16; 95% CI: 0.69, 1.96; Ptrend = 0.95) intakes were not related to CRC risk in men and women, respectively In men, sugars intake was associated with CRC risk inversely among never-smokers and positively among male ever-smokers (P for interaction = 0.01). Sugars intake was associated with an increased risk among men with no alcohol consumption but was unrelated to the risk among male alcohol drinkers (P for interaction = 0.02) |
| Xu et al. (36), 2016 | To evaluate the associations between flavonoid intake from different dietary sources and CRC risk in a Chinese population | Age and sex matched case-control study/FFQ-81 items | 1,632 cases and 1,632 controls | CRC patients, hospital-derived controls, healthy community controls, Guangzhou, mean age of cases 56.5 yr. | No significant association between total flavonoids and CRC risk (aOR = 1.06; 95% CI: 0.85, 1.32 for highest vs. lowest quartile, Ptrend = 0.78) Flavanones and flavones intakes from total diet were negatively associated with CRC risk (aOR = 0.28; 95% CI: 0.22, 0.36 for highest vs. lowest quartile; Ptrend < 0.01) |
| Abe et al. (61), 2016 | To assess the association between GI, GL, and CRC using a prospective Japanese population-based cohort | Prospective cohort study/FFQ | 73,501 | Men and women, Japan, aged 40 yr.–69 yr. | Overall, no association was observed between GI and GL with CRC risk |
| Nakamura et al. (40), 2010 | To investigate whether dietary instruction optimising the fat energy ratio suppresses the recurrence of colorectal tumours | Randomised clinical trial/3-day DR | 373 | Patients with two colorectal tumours (adenomas and/or early cancers) and has been removed endoscopically, Osaka, mean age 54.8 ± 6.1 yr. (men) and 56.3 ± 6.3 yr. (women) | In men, the risk of tumours decreased significantly as the intake of PUFA (OR = 0.48; 95% CI: 0.23, 1.02; Ptrend = 0.04) and LA (OR = 0.42; 95% CI: 0.19, 0.89; Ptrend = 0.02) increased In women, the risk of tumours decreased significantly as the intake of ALA (OR = 0.68; 95% CI: 0.18, 2.65; Ptrend = 0.03) increased |
| Peterson et al. (62), 2009 | To investigate whether coffee consumption was associated with decreased risk of CRC and whether cigarette smoking and stage of disease modify the association in the Singapore Chinese Health Study | Prospective cohort study/FFQ-165 items | 61,321 | Men and women, Singapore, mean age 56.5 yr. | No overall association between coffee intake and CRC In analysis by subsite and stage restricted to ever smokers, the coffee–colon cancer association between coffee and colon became significant for advanced disease (HR = 0.56; 95% CI: 0.35, 0.90; Ptrend= 0.01). Coffee may protect against smoking related advanced colon cancer |
| Panprathip et al. (63), 2019 | To investigate the associations between folate status, gene polymorphisms and the risk of CRC in Thais and to identify the interactions of folate-gene polymorphism and their impact on CRC risk | Case-control study/SQFFQ-66 high-folate Thai food items | 105 CRC, 101 CRA and 182 controls | CRC patients, CRA patients, hospital derived controls, Bangkok, percentage age groups < 50 yr. (10.5%), 50 yr.–59 yr. (23.8%), 60 yr.–69 yr. (31.4%), ≥ 70 yr. (34.3%) for CRC and < 50 yr. (4.0%), 50 yr.–59 yr. (25.7%), 60 yr.–69 yr. (26.7%), ≥ 70 yr. (43.6%) for CRA | The lowest quartile group of serum folate and folate intake had higher risk of CRC than the highest quartile group (OR = 11.45; 95% CI = 4.43, 29.59; P < 0.001) and (OR = 10.29; 95% CI = 4.17, 25.41; P < 0.001), respectively The risk of CRC was increased with alcohol consumption when combined with low folate status (P < 0.05) |
| Ting et al. (64), 2018 | To identify factors associated with negative colonoscopy and positive iFOBT results obtained during CRC screening | Case-control study/- | 648 colorectal neoplasia and 559 without colorectal neoplasia | Patients with colorectal neoplasia (positive colonoscopy group), patients without colorectal neoplasia (negative colonoscopy group), Taiwan, mean age of colorectal neoplasia patients 66.9 ± 10.6 yr. | Alcohol was not associated with colorectal neoplasia in positive iFOBT patients (aOR = 2.017; 95% CI: 0.840, 4.838; P = 0.116) |
| Wu et al. (65), 2009 | To investigate the impact of betel quid chewing, cigarette smoking or alcohol consumption on CRC risk in Taiwan | Age and sex matched case-control study/Standardised questionnaire | 258 CRC and 533 controls | CRC patients, hospital-derived controls, southern Taiwan, mean age of cases 63.1 ± 11.7 yr. | Alcohol drinking (aOR = 1.1; 95% CI: 0.7, 1.8; P for interaction = 0.608) and betel quid chewing (aOR = 1.3; 95% CI: 0.7, 2.4; P for interaction = 0.277) were not associated with CRC |
| Abulimiti et al. (51), 2020 | To investigate whether the DII was associated with the risk of CRC in a Chinese population | Age and sex matched case-control study/FFQ-81 items | 2,502 cases and 2,538 controls | CRC patients, hospital and community-derived controls, Guangzhou, mean age of cases 57.0 ± 10.3 yr. | A positive association was found between the E-DII and CRC risk (OR = 1.40; 95% CI: 1.16, 1.68; for highest vs. lowest quartile, Ptrend < 0.01) Significant associations were not observed in women or underweight individuals when stratified based on cancer subsite, sex, BMI and smoking status |
| Bahrami et al. (41), 2020 | To determine the relationship between the INQ and the risk of CRC and CRA | Age and sex matched case-control study/FFQ-148 items | 129 CRC, 130 CRA and 240 controls | CRC and CRA patients, hospital-derived controls, Tehran, mean age of cases 56.6 ± 11.5 yr. | The INQ of calcium, riboflavin, vitamin C, fiber and folate were associated with decreased risk of CRC (ORcalcium = 0.21; 95% CI: 0.08, 0.52; P = 0.001; ORvitB2 = 0.35; 95% CI: 0.18, 0.65; P = 0.001; ORvitC = 0.16; 95% CI: 0.09, 0.28; P < 0.0001; ORfiber = 0.35; 95% CI: 0.21, 0.58; P < 0.0001; ORfolate = 0.33; 95% CI: 0.16, 0.65; P = 0.002, respectively) |
| Dao et al. (27), 2020 | To examine the association between heterocyclic amines 2-amino-1-methyl-6-phenylimidazo pyridine (PhIP) and CRC in Vietnam | Case-control study/SQFFQ-97 items | 512 CRC and 1,096 controls | CRC patients, hospital-derived controls, Hanoi | There was a significant association between PhIP intake and the risk of CRC among all participants (aOR = 4.89; 95% CI: 3.03, 7.89 for high intake vs. non-intake; Ptrend < 0.01), men (aOR = 5.27; 95% CI: 2.83, 9.81 for high intake vs. non-intake; Ptrend < 0.01) and women (aOR = 4.58; 95% CI: 2.10, 10.01 for high intake vs. non-intake; Ptrend < 0.01) The significant positive association was observed for proximal colon (OR = 8.05; 95% CI: 3.86, 16.77; Ptrend < 0.01 for total proximal colon; OR = 6.91; 95% CI: 2.65, 18.07; Ptrend < 0.01 for men; and OR = 12.15; 95% CI: 3.73, 39.57; Ptrend < 0.01 for women) The significant positive association was observed for distal colon (OR = 1.69; 95% CI: 0.49, 5.86; Ptrend < 0.01 for total distal colon; and OR = 2.82; 95% CI: 0.75, 10.52; Ptrend = 0.05 for men) The significant positive association was observed for rectum (OR = 4.24; 95% CI: 2.29, 7.84; Ptrend < 0.01 for total rectum cancer; OR = 3.49, 95% CI: 1.48, 8.23; Ptrend < 0.01 for men; and OR = 5.50; 95% CI: 2.10, 14.39; Ptrend < 0.01 for women) |
| Jafari Nasab et al. (52), 2020 | To investigate the association of HEI-2010 and MSDPS with CRC and CRA risk | Age and sex matched case-control study/FFQ-148 items | 129 CRC, 130 CRA and 240 controls | CRC and CRA patients, hospital-derived controls, Tehran, median age 59 (49.25–64) yr. (CRC), 58 (51–64) yr. (CRA) and 56 (50–61.75) yr. (controls) | HEI-2010 (OR = 0.04; 95% CI: 0.01, 0.12 for highest vs. first tertiles, Ptrend = 0.00) and MSDPS (OR = 0.19; 95% CI: 0.09, 0.38 for highest vs. first tertiles, Ptrend = 0.00) were significantly associated with lower odds of CRC |
| Khankari et al. (37), 2020 | To evaluate the association between soy isoflavones and soy protein and CRC risk using four prospective cohort studies from China and Japan | Prospective cohort studies/FFQ-11 and 138 items | 205,060 | Men and women, China and Japan, mean age 54.8 ± 8.9 yr. | No significant association between soy isoflavones (HRisoflavones = 0.97; 95% CI: 0.86, 1.11; Ptrend = 0.68) and soy protein (HRsoy protein = 0.98; 95% CI: 0.86, 1.12; Ptrend = 0.67) with CRC risk in the pooled analysis No association among ever smokers consuming higher isoflavones (HRisoflavones = 0.83; 95% CI: 0.68, 1.00; Ptrend = 0.69) and soy protein (HRsoy protein = 0.81; 95% CI: 0.39, 1.10) Risk reductions were observed among premenopausal women with a BMI < 23.0 kg/m2 at baseline for higher isoflavone (HRisoflavones = 0.58; 95% CI: 0.34, 0.98; Ptrend = 0.65) |
| Kunnavuttivanich et al. (66), 2020 | To explore the association between dietary patterns and disease recurrence among Thai CRC patients | Case-control study/FFQ-45 items | 74 cases (recurrence), 151 controls (disease-free) | CRC patients (recurrence and disease-free), Thailand, percentage age groups < 65 yr. (56.8%), ≥ 65 yr. (43.2%) for cases and < 65 yr. (46.4%), ≥ 65 yr. (53.6%) for controls | Significantly low recurrence rates among patients consumed high amounts of pickled fish or chili-paste compared to patients who had never eaten those foods (P < 0.01) No significant association between meat/wheat (P = 0.58), fast-food/processed fruit (P = 0.34) or vegetarian (P = 0.85) dietary patterns and CRC recurrence |
| Luo et al. (42), 2021 | To evaluate whether the association of dietary zinc and selenium intake and risk of CRC were modified by SOD1, SOD2, GPX and CAT polymorphisms in a Chinese population | Age and sex matched case-control study/FFQ-81 items | 493 cases and 498 controls | CRC patients, hospital-derived controls and community-derived controls, Guangdong, mean age 57.8 yr. (cases) and 57.7 yr. (controls) | Intake of selenium was inversely associated with CRC risk (OR = 0.42; 95% CI: 0.28, 0.64; for highest vs. lowest quartile, Ptrend < 0.001) Zinc was not associated with CRC risk. (OR = 0.96; 95% CI: 0.63, 1.47; Ptrend = 0.505) |
| Nguyen et al. (53), 2020 | To evaluate the association between CRC incidence and adherence to the CHFP, AHEI-2010 and DASH | Prospective cohort studies/SQFFQ-81 and 77 items | 132,606 | Men and women, Shanghai, median age for CRC 33.7 (6.2) and for non-CRC was 34.0 (6.0) yr. | CHFP score was inversely associated with risk of CRC (HRQ2 vs Q1 = 0.88; 95% CI: 0.77, 1.00; HRQ3 vs Q1 = 0.86; 95% CI: 0.75, 0.98; and HRQ4 vs Q1 = 0.84; 95% CI: 0.73, 0.96) with Ptrend = 0.01 The inverse association appeared stronger for rectal cancer (Ptrend = 0.01); age < 50 yr. (Ptrend < 0.01); BMI < 25 kg/m2 (Ptrend = 0.01) or without any metabolic conditions (Ptrend < 0.01) at baseline, although no multiplicative interactions were noted The modified DASH (Ptrend = 0.23) score and AHEI-2010 (Ptrend = 0.27) showed no association with CRC risk |
Notes: DII = dietary inflammatory index; GI = glycaemic index; GL = glycaemic load; CAP = colorectal adenomatous polyps; 3-day DR = 3-day diet record; SQFFQ = semi-quantitative food frequency questionnaire; CRC = colorectal cancer; CRA = colorectal adenoma; yr. = years old; E-DII = energy-adjusted DII; OR = odd ratio; aOR = adjusted odds ratio; DHQ I = diet history questionnaire I; LA = linoleic acid; PUFA = polyunsaturated fatty acids; ALA = linolenic acid; CI = confidence interval; CRP = C-reactive protein; iFOBT = immunochemical feacal occult blood test; INQ = index of nutritional quality; PhIP = heterocyclic amines 2-amino-1-methyl-6-phenylimidazo pyridine; HEI-2010 = Healthy Eating Index-2010; MSDPS = Mediterranean-Style Dietary Pattern Score; CHFP = Chinese Food Pagoda; AHEI-2010 = Alternative Healthy Eating Index-2010; DASH = Dietary Approaches to Stop Hypertension; vs. = versus; BMI = body mass index
Summarising the Results
A numerical and descriptive summary of the charting results was used to present findings. Besides that, a summary diagram was used to better visualise the linkage between diet and CRC risk in Asia. The limitations of this study were also assembled to allow identification of research gap and produce useful recommendations for future research.
Results
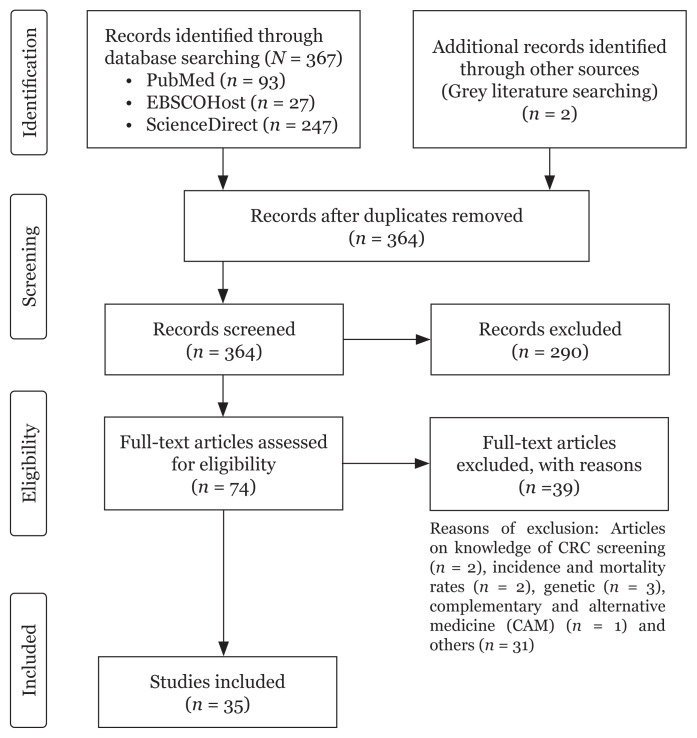
A total of 367 articles were identified from three search engines namely PubMed (n = 93), EBSCOHost (n = 27) and ScienceDirect (n = 247) while another two articles were obtained from other sources (Figure 1). Five duplicate articles were removed and the remaining 364 articles were screened based on the title and abstracts. Out of 364 articles, 290 were excluded because the titles and abstract did not match the research question of the study. The remaining 74 articles were assessed for eligibility based on their full text. A total of 39 articles were not eligible and excluded from the study as the articles did not analyse the association between CRC and diet. The final total articles included in this review were 35 and the outcome was presented in Table 1.
Figure 1.
Process of articles selection based on PRISMA-ScR flow diagram
The included articles were carried out in 10 Asian countries which were China (n = 12), India (n = 2), Iran (n = 7), Japan (n = 6), Jordan (n = 4), Korea (n = 4), Oman (n = 1), Pakistan (n = 1), Taiwan (n = 2), Thailand (n = 4) and Vietnam (n = 2). These studies consisted of three study designs namely case-control (n = 28), prospective cohort (n = 6) and randomised clinical trial (n = 1) study.
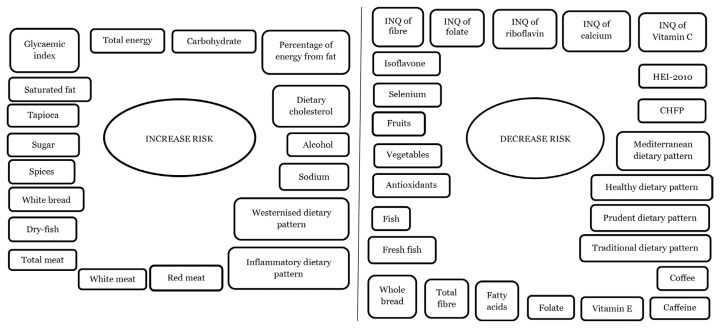
The results showed that various single foods and nutrients and dietary patterns (multiple food groups and multiple foods groups and nutrients) were related to the risk of CRC. A total of 24 primary studies indicated the association of CRC risk with single foods and nutrients while 11 studies, with dietary patterns. Single foods and nutrients and dietary patterns that increased risks were red, white and total meat, dry-fish, white bread, spices, sugar, tapioca, saturated fat, dietary glycemic index (GI), total energy, protein, carbohydrate, percentage of energy from fat, dietary cholesterol, alcohol, sodium, westernised diet and inflammatory factors. On the other hand, single foods and nutrients, and dietary patterns that decreased risks were fruits, vegetables, antioxidants, isoflavone, fish, fresh fish, whole bread, total fibre, fatty acids, index of nutritional quality (INQ) of fibre, folate, riboflavin, calcium, and vitamin C, folate, vitamin E, caffeine, coffee, selenium, traditional, prudent, healthy, Mediterranean, Chinese Food Pagoda (CHFP) and Healthy Eating Index-2010 (HEI-2010) (Figure 2).
Figure 2.
Single foods and nutrients and dietary patterns associated with increased and decreased risk of CRC
Notes: INQ = index of nutritional quality; HEI-2010 = Healthy Eating Index-2010; CHFP = Chinese Food Pagoda
Discussion
The present review aims to systematically identify the existing literature on the relationship between diet and CRC risk in Asia. Based on the included literature (Table 1), total meat (21) and red meat (22, 23) including beef (24) and pork (25) are considered as single foods were associated with increase CRC risk in Asian countries. According to Hur et al. (26) the harmful substance known as heterocyclic amines (HCAs), polycyclic aromatic hydrocarbons (PAHs) and N-nitroso compounds (NOCs) that were typically produced during cooking and processing of red meat might induce cancer. Furthermore, Dao et al. (27) reported that heterocyclic amines 2-amino-1-methyl-6-phenylimidazo pyridine (PhIP) which was produced from cooked beef was associated with an increase in CRC risk. Besides red meat, white meat (23) including chicken (28) was found to contribute to CRC development. In addition, Clostridium perfringens/histolyticum spp. was found abundant in chicken meat than beef or salmon based on in vitro faecal batch culture investigation (29). It has been proclaimed to produce other enzymes involved in genotoxic or carcinogenic metabolites production in the colon such as β-glucosidase, nitroreductase and azoreductase (29).
Ganesh et al. (23) have observed that the consumption of dry-fish increased the risk of CRC among non-vegetarian Indian population. Dry-fish was considered as a risk factor probably due to the methods of preservation which consisted of salting and drying (23). Besides that, the high GI of white bread (grains) might explain why this diet was also associated with CRC risk among Jordanians (28). Moreover, Franceschi et al. (30) stated that refined carbohydrate was responsible for CRC based on their positive relationship with GI and glycaemic load (GL). Although the exact mechanism for cancer genesis with high GI type of dietary pattern is not clear, but insulin resistance or insulin growth factor might be involved (31). Spices such as chillies and pepper, sugar and tapioca were also associated with increased CRC risk (24). Chillies have carcinogenic properties due to their capsaicin compound and might explain the positive association between spices and CRC risk (24). Furthermore, the unprocessed tapioca contains toxins such as linamarin and cyanide derivatives. These toxins could directly act on the bowel mucosa to cause CRC (24).
Besides food items and food groups, macro- and micronutrients were also identified as contributors to the CRC risk in this current review. High fats diet (32), saturated fat (22, 33), dietary GI (34), total energy (33), protein (33), carbohydrate intakes (33), percentage of energy from fat (33), dietary cholesterol (33), alcohol (35) and sodium (33) intake were associated with increased risk of CRC where by, high intake of these nutrients among certain populations could be the reason why it leads to disease development.
However, there are certain food items and groups have been found to be protective against CRC. The consumption of fruits (24) and vegetables (24) including cabbage (23) and sprout (23) may reduce the development of CRC among the Indian population. A study by Xu et al. (36) reported that, antioxidants from fruits and vegetables such as flavan-3-ols, flavanones, flavones and flavonols were associated with decreased CRC risks among Chinese population. According to Khankari et al. (37), higher intake of isoflavone could reduce the CRC risk among premenopausal women with BMI < 23.0 kg/m2 at baseline assessment. Antioxidants trap the reactive oxygen molecules and free radicals at cellular level, thus intervening in a protective mechanism against oxidative damage (33). CRC risk could be reduced by approximately 25% among individuals who consume fish daily like the community in Kerala, India (24). They commonly consumed sea fish that were rich in vitamin D and selenium. According to Du and Fang (38), these nutrients may protect the pathogenesis and development of CRC. Another study conducted also in India showed that, fresh-fish intake had a 40% to 70% reduction in CRC development as compared to individuals who do not consume fresh-fish (23). Moreover, whole bread was classified as having a protective effect against CRC in a Jordanian (28) study and this is because whole grain products could be rich in antioxidants including trace minerals and phenolic compounds (39).
Macronutrients such as total fibre (34) were associated with a 53% reduction of CRC risk while the increased intake of polyunsaturated fatty acids (PUFA) (40) and linoleic acid (LA) (40) might decrease the risk in men and linolenic acid (ALA) (40) in women. For INQ of fibre, it was negatively associated with CRC risk (41). Micronutrients that could prevent the formation of CRC included folate (35, 63), vitamin E (33), caffeine (33), selenium (42) and INQ of folate, riboflavin, calcium and vitamin C (41). Folate influences the availability of methyl groups in the CRC pathway, which enhances deoxyribonucleic acid (DNA) synthesis and repair (35). Besides that, vitamin E and caffeine act as an antioxidant to fight against free radicals and effectively scavenge reactive oxygen molecules and hydroxyl radicals (33) while selenium which also act as an antioxidant protects against oxidative stress (42). On the other hand, Riboflavin (vitamin B2) plays an important role in DNA methylation, stability, synthesis and repair (43). For calcium, it can modulate the CRC-related cell signalling pathways and inhibit oxidative DNA damage while vitamin C, has antioxidative and pro-apoptosis effect, inhibit cell proliferation and reduce inflammation (43).
The CRC risk factors and protective factors were not due only to single foods and nutrients, but it was also influenced by dietary patterns. According to Park et al. (44), westernised dietary patterns characterised by high intake of meats, carbohydrates (fast foods), oil and sugar were associated with enhanced risk of CRC especially among Korean women. A positive association was also observed between the western patterns and CRC in Iran (45). Cho et al. (46) reported that higher consumption of C-reactive protein (CRP)-dietary pattern score (inflammatory diet) derived from reduced rank regression (RRR) was associated with increased CRC risk and a stronger risk was observed in rectal cancer than colon cancer. Inflammatory diet patterns not only could be obtained from RRR but they could also be derived from an index-based method such as dietary inflammatory index (DII). All studies included inflammatory diet patterns using DII (47–51) (n = 5) and showed a positive association with CRC risk. Another six dietary patterns including traditional (44), prudent (44), healthy (45), Mediterranean (52), CHFP (53) and HEI-2010 (52) dietary patterns were found to be protective against CRC.
This review found that findings of diet relationship with CRC between Asia and Western countries had similarities. The similarities could be seen in several studies conducted by Bradbury et al. (54), Sanjoaquin et al. (55), Castelló et al. (56) and Fung et al. (57). Bradbury et al. (54) indicated that red meat and alcohol enhanced the risk while fibre was found protective against CRC among United Kingdom (UK) population. Another study done in the UK population by Sanjoaquin et al. (55) revealed that white bread and alcohol use were associated with increased risk, but regular fruit eating was associated with decreased risk of CRC. According to Castelló et al. (56), high and low adherence to Mediterranean and westernised dietary patterns respectively may decreased CRC risk among the Spain population. According to Fung et al. (57), westernised eating habits were associated with colon cancer in United States (US) women. Even while the majority of foods were comparable, there were others such tapioca and dry fish, that were only available in Asia and had ties to the illness. Thus, investigating the food habits of the Asian population is essential.
There were several strengths and limitations of the current scoping review. Strength-wise, the current and updated evidence provided in this review are useful to develop dietary intervention programmes for the mitigation and prevention of CRC. Limitation-wise, first there was insufficient interventional study designs with the majority were observational studies (28 case-control and six prospective cohort studies) which are known to have bias. Secondly, only 11 studies were conducted on dietary patterns as compared to 24 studies that were on single foods and nutrients. Dietary patterns are preferred because this approach captures the overall diet which also includes variation of foods and combination of nutrients in a studied population. Thirdly, most of the included studies were not stratified based on the disease outcome or anatomical subsites such as proximal colon, distal colon and rectum.
Conclusion
In conclusion, single foods and nutrients and dietary patterns such as meats, alcohol and westernised diet have been found to be associated with the increase risks of CRC while fruits, vegetables and traditional decrease the risks of CRC among Asian population. The limitations or gaps identified from this review will assist and guide health professionals, researchers and policymakers to design suitable study designs and topics related to diet and CRC for future studies.
Supplementary Data
Search strategy using PubMed, EBSCOHost and ScienceDirect
| Search terms | No. of studies available | No. of studies selecteda |
|---|---|---|
| 1. (“Colorectal cancer*” OR colorectal OR “colon cancer” OR colon OR “rectal cancer” OR rectum OR “colorectal neoplasms” OR “colorectal neoplasia” OR “colorectal carcinoma” OR “colorectal adenocarcinoma” OR “lynch syndrome” OR “hereditary nonpolyposis colorectal neoplasms”). | PubMed: 492, 736 EBSCOHost: 7,110 ScienceDirect: 172, 597 | PubMed: 29 EBSCOHost: 3 ScienceDirect: 3 |
| 2. (Diet* OR “dietary pattern*” OR “dietary habits” OR “dietary factors” OR “dietary risk factors” OR “dietary intake” “dietary flavonoids” OR “dietary non-enzymatic antioxidant capacity” OR “diet quality” OR “inflammatory diet*” OR “inflammatory diet* pattern” OR nutri* OR “nutrient patterns” OR “eating patterns” OR food* OR meat OR poultry OR fish OR fiber OR whole-grain OR “whole grains” OR nut OR curcumin OR beverage* OR alcohol OR “green tea” OR “black tea” OR coffee OR milk OR dairy OR macronutrients OR micronutrient* OR calcium OR “vitamin D” OR iron OR “portion size*”). | PubMed: 3, 243, 871 EBSCOHost: 49, 468 ScienceDirect: 1, 337, 220 | PubMed: 27 EBSCOHost: 2 ScienceDirect: 1 |
| 3. (Asia OR Malaysia OR China OR India OR Indonesia OR Pakistan OR Bangladesh OR Japan OR Philippines OR Vietnam OR Turkey OR Iran OR Thailand OR Myanmar OR South Korea OR Iraq OR Afghanistan OR Saudi Arabia OR Uzbekistan OR Yemen OR Nepal OR North Korea OR Sri Lanka OR Kazakhstan OR Syria OR Cambodia OR Jordan OR Azerbaijan OR United Arab Emirates OR Tajikistan OR Israel OR Laos OR Lebanon OR Kyrgyzstan OR Turkmenistan OR Singapore OR Oman OR Palestine OR Kuwait OR Georgia OR Mongolia OR Armenia OR Qatar OR Bahrain OR Timor-Leste OR Cyprus OR Bhutan OR Maldives OR Brunei). | PubMed: 1, 214, 094 EBSCOHost: 22,573 ScienceDirect: 1, 171, 376 | PubMed: 19 EBSCOHost: 2 ScienceDirect:1 |
Notes:
For ScienceDirect only these terms were used: “Colorectal cancer”, diet, “dietary patterns”, “dietary factors” and Asia
Acknowledgements
The authors extend their gratitude to Mrs. Noraida Hassan, Deputy Chief Librarian, Hamdan Tahir Library, Universiti Sains Malaysia and Mrs. Siti Hamizah Abd Rahman, Research Assistant, Universiti Sains Malaysia for guiding the researchers on how to search articles via electronic databases. An appreciation also goes to the Ministry of Higher Education Malaysia for funding the research study and the USM Fellowship scheme for providing financial support to postgraduate student, Ms. Lydiatul Shima Ashari.
Footnotes
Conflict of Interest
None.
Funds
Ministry of Higher Education Malaysia via Long Term Research Grant Scheme (LRGS)-Malaysia Research University Network (MRUN) (203/PPSK/6720021 and LR001-2019) and USM Fellowship scheme.
Author’s Contributions
Conception and design: HJJM, LSA
Analysis and interpretation of the data: LSA, AAAR
Drafting of the article: LSA, AAAR
Critical revision of the article for important intellectual content: SMR, LYY, HJJM
Final approval of the article: SMR, LYY, HJJM
Obtaining of funding: SMR, LYY, HJJM
Collection and assembly of data: LSA, AAAR
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Lee DH, Keum N, Giovannucci EL. Colorectal cancer epidemiology in the nurses’ health study. Am J Public Health. 2016;106(9):1599–1607. doi: 10.2105/AJPH.2016.303320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest Res. 2019;17(3):317–329. doi: 10.5217/ir.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Cancer today: data visualization tools for exploring the global cancer burden in 2018. Lyon: International Association of Cancer Registries; [Retrieved 2018 May 23]. Available at: https://gco.iarc.fr/today/home. [Google Scholar]
- 6.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migliore L, Migheli F, Spisni R, Coppedè F. Genetics, cytogenetics, and epigenetics of colorectal cancer. Biomed Res Int. 2011;2011:1–19. doi: 10.1155/2011/792362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magalhães B, Peleteiro B, Lunet N. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev. 2012;21(1):15–23. doi: 10.1097/CEJ.0b013e3283472241. [DOI] [PubMed] [Google Scholar]
- 9.World Cancer Research Fund International/American Institute for Cancer Research. Continuous update project report: diet, nutrition, physical activity and colorectal cancer. [Retrieved 2017 May 23]. Available at: wcrf.org/colorectal-cancer-2017.
- 10.Rosato V, Tavani A, Negri E, Serraino D, Montella M, Decarli A, et al. Processed meat and colorectal cancer risk: a pooled analysis of three Italian case-control studies. Nutr Cancer. 2017;69(5):732–738. doi: 10.1080/01635581.2017.1310259. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Wang PP, Woodrow J, Zhu Y, Roebothan B, Mclaughlin JR, et al. Dietary patterns and colorectal cancer: results from a Canadian population-based study. Nutr J. 2015;14(8):1–9. doi: 10.1186/1475-2891-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Kinany K, Deoula MMS, Hatime Z, Boudouaya HA, Huybrechts I, El Asri A, et al. Consumption of modern and traditional Moroccan dairy products and colorectal cancer risk: a large case control study. Eur J Nutr. 2019;59(3):953–963. doi: 10.1007/s00394-019-01954-1. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Lee J, Woo HD, Kim DW, Oh JH, Chang HJ, et al. Dietary mercury intake and colorectal cancer risk: a case-control study. Clin Nutr. 2019;39(7):2106–2113. doi: 10.1016/j.clnu.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 14.Zhivotovskiy AS, Kutikhin AG, Azanov AZ, Yuzhalin AE, Magarill YA, Brusina EB. Colorectal cancer risk factors among the population of south-east Siberia: a case-control study. Asian Pac J Cancer Prev. 2012;13(10):5183–5188. doi: 10.7314/apjcp.2012.13.10.5183. [DOI] [PubMed] [Google Scholar]
- 15.Annema N, Heyworth JS, McNaughton SA, Iacopetta B, Fritschi L. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J Am Diet Assoc. 2011;111(10):1479–1490. doi: 10.1016/j.jada.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 17.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 18.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14(1):1–10. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106(4):420–431. doi: 10.5195/jmla.2018.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q, Tong Y, Wu T, Li J, Tong N. Comparison of the hypoglycemic effect of acarbose monotherapy in patients with type 2 diabetes mellitus consuming an Eastern or Western diet: a systematic meta-analysis. Clin Ther. 2013;35(6):880–899. doi: 10.1016/j.clinthera.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Takachi R, Tsubono Y, Baba K, Inoue M, Sasazuki S, Iwasaki M, et al. Red meat intake may increase the risk of colon cancer in Japanese, a population with relatively low red meat consumption. Asia Pac J Clin Nutr. 2011;20(4):603–612. [PubMed] [Google Scholar]
- 22.Arafa MA, Waly MI, Jriesat S, Al Khafajei A, Sallam S. Dietary and lifestyle characteristics of colorectal cancer in Jordan: a case-control study. Asian Pac J Cancer Prev. 2011;12(8):1931–1936. [PubMed] [Google Scholar]
- 23.Ganesh B, Talole SD, Dikshit R. A case-control study on diet and colorectal cancer from Mumbai, India. Cancer Epidemiol. 2009;33(3–4):189–193. doi: 10.1016/j.canep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Nayak SP, Sasi MP, Sreejayan MP, Mandal S. A case-control study of roles of diet in colorectal carcinoma in a South Indian population. Asian Pac J Cancer Prev. 2009;10(4):565–568. [PubMed] [Google Scholar]
- 25.Poomphakwaen K, Promthet S, Suwanrungruang K, Chopjitt P, Songserm N, Wiangnon S. XRCC1 gene polymorphism, diet and risk of colorectal cancer in Thailand. Asian Pac J Cancer Prev. 2014;15(17):7479–7486. doi: 10.7314/apjcp.2014.15.17.7479. [DOI] [PubMed] [Google Scholar]
- 26.Hur SJ, Yoon Y, Jo C, Jeong JY, Lee KT. Effect of dietary red meat on colorectal cancer risk: a review. Compr Rev Food Sci Food Saf. 2019;18(6):1812–1824. doi: 10.1111/1541-4337.12501. [DOI] [PubMed] [Google Scholar]
- 27.Dao HV, Nguyen TTM, Tran HH, Dang LT, Dinh MT, Le NT. A case-control study of meat mutagens and colorectal cancers in Viet Nam. Asian Pac J Cancer Prev. 2020;21(8):2217–2223. doi: 10.31557/apjcp.2020.21.8.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abu Mweis SS, Tayyem RF, Shehadah I, Bawadi HA, Agraib LM, Bani-Hani KE, et al. Food groups and the risk of colorectal cancer: Results from a Jordanian case-control study. Eur J Cancer Prev. 2015;24(4):313–320. doi: 10.1097/cej.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 29.Shen Q, Chen YA, Tuohy KM. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe. 2010;16(6):572–577. doi: 10.1016/j.anaerobe.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Franceschi S, Dal Maso L, Augustin L, Negri E, Parpinel M, Boyle P, et al. Dietary glycemic load and colorectal cancer risk. Ann Oncol. 2001;12(2):173–178. doi: 10.1023/a:1008304128577. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 32.Khan NA, Hussain M, ur Rahman A, Farooqui WA, Rasheed A, Memon AS. Dietary practices, addictive behavior and bowel habits and risk of early onset colorectal cancer: a case control study. Asian Pac J Cancer Prev. 2015;16(17):7967–7973. doi: 10.7314/apjcp.2015.16.17.7967. [DOI] [PubMed] [Google Scholar]
- 33.Tayyem RF, Bawadi HA, Shehadah IN, Abu-Mweis SS, Agraib LM, Bani-Hani KE, et al. Macro- and micronutrients consumption and the risk for colorectal cancer among Jordanians. Nutrients. 2015;7(3):1769–1786. doi: 10.3390/nu7031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Fang YJ, Xu M, Luo H, Zhang NQ, Huang WQ, et al. Carbohydrate, dietary glycaemic index and glycaemic load, and colorectal cancer risk: a case-control study in China. Br J Nutr. 2018;119(8):937–948. doi: 10.1017/s000711451800051x. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Cho YA, Kim DH, Lee BH, Hwang DY, Jeong J, et al. Dietary intake of folate and alcohol, MTHFR C677T polymorphism, and colorectal cancer risk in Korea. Am J Clin Nutr. 2012;95(2):405–412. doi: 10.3945/ajcn.111.020255. [DOI] [PubMed] [Google Scholar]
- 36.Xu M, Chen YM, Huang J, Fang YJ, Huang WQ, Yan B, et al. Flavonoid intake from vegetables and fruits is inversely associated with colorectal cancer risk: a case-control study in China. Br J Nutr. 2016;116(7):1275–1287. doi: 10.1017/s0007114516003196. [DOI] [PubMed] [Google Scholar]
- 37.Khankari NK, Yang JJ, Sawada N, Wen W, Yamaji T, Gao J, et al. Soy intake and colorectal cancer risk: results from a pooled analysis of prospective cohort studies conducted in China and Japan. J Nutr. 2020;150(9):2442–2450. doi: 10.1093/jn/nxaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du W, Fang J-Y. Nutrients impact the pathogenesis and development of colorectal cancer. Gastrointest Tumors. 2015;2(4):203–207. doi: 10.1159/000441212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slavin J. Whole grains and human health. Nutr Res Rev. 2004;17(1):99–110. doi: 10.1079/NRR200374. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura T, Ishikawa H, Takeyama I, Kawano A, Ishiguro S, Otani T, et al. Excessive fat restriction might promote the recurrence of colorectal tumors. Nutr Cancer. 2010;62(2):154–163. doi: 10.1080/01635580903305292. [DOI] [PubMed] [Google Scholar]
- 41.Bahrami A, Rafiee P, Jafari Nasab S, Hekmatdoost A, Sohrab G, Sadeghi A, et al. The relationship between the index of nutritional quality and the risk of colorectal cancer and adenoma: a case-control study. Eur J Cancer Prev. 2020;29(3):222–228. doi: 10.1097/cej.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 42.Luo H, Fang YJ, Zhang X, Feng XL, Zhang NQ, Abulimiti A, et al. Association between dietary zinc and selenium intake, oxidative stress-related gene polymorphism, and colorectal cancer risk in Chinese population: a case-control study. Nutr Cancer. 2021;73(9):1621–1630. doi: 10.1080/01635581.2020.1804950. [DOI] [PubMed] [Google Scholar]
- 43.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1–26. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park Y, Lee J, Oh JH, Shin A, Kim J. Dietary patterns and colorectal cancer risk in a Korean population: a case-control study. Medicine (Baltimore) 2016;95(25):1–8. doi: 10.1097/md.0000000000003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safari A, Shariff ZM, Kandiah M, Rashidkhani B, Fereidooni F. Dietary patterns and risk of colorectal cancer in Tehran province: a case-control study. BMC Public Health. 2013;13:1–9. doi: 10.1186/1471-2458-13-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, et al. Inflammatory dietary pattern, IL-17F genetic variant, and the risk of colorectal cancer. Nutrients. 2018;10(6):1–11. doi: 10.3390/nu10060724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho YA, Lee J, Oh JH, Shin A, Kim J. Dietary inflammatory index and risk of colorectal cancer: a case-control study in Korea. Nutrients. 2016;8(8):1–11. doi: 10.3390/nu8080469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivappa N, Hebert JR, Steck SE, Hofseth LJ, Shehadah I, Bani-Hani KE, et al. Dietary inflammatory index and odds of colorectal cancer in a case-control study from Jordan. Appl Physiol Nutr Metab. 2017;42(7):744–749. doi: 10.1139/apnm-2017-0035. [DOI] [PubMed] [Google Scholar]
- 49.Shivappa N, Hebert JR, Steck SE, Safari A, Sedaghat F, Rashidkhani B. Dietary Inflammatory Index and odds of colorectal cancer in a case-control study from Iran. Asian Pac J Cancer Prev. 2018;19(7):1999–2006. doi: 10.22034/apjcp.2018.19.7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rafiee P, Shivappa N, Hebert JR, Nasab SJ, Bahrami A, Hekmatdoost A, et al. Dietary inflammatory index and odds of colorectal cancer and colorectal adenomatous polyps in a case-control study from Iran. Nutrients. 2019;11(6):1–13. doi: 10.3390/nu11061213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abulimiti A, Zhang X, Shivappa N, Hébert JR, Fang YJ, Huang CY, et al. The dietary inflammatory index is positively associated with colorectal cancer risk in a Chinese case-control study. Nutrients. 2020;12(1):1–15. doi: 10.3390/nu12010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jafari Nasab S, Bahrami A, Rafiee P, Hekmatdoust A, Ghanavati M, Rashidkhani B, et al. Healthy Eating Index-2010 and Mediterranean-Style Dietary Pattern Score and the risk of colorectal cancer and adenoma: a case-control study. Nutr Cancer. 2020;72(8):1326–1335. doi: 10.1080/01635581.2019.1683212. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen S, Li H, Yu D, Gao J, Gao Y, Tran H, et al. Adherence to dietary recommendations and colorectal cancer risk: results from two prospective cohort studies. Int J Epidemiol. 2020;49(1):270–280. doi: 10.1093/ije/dyz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020;49(1):246–258. doi: 10.1093/ije/dyz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanjoaquin M, Appleby P, Thorogood M, Mann J, Key T. Nutrition, lifestyle and colorectal cancer incidence: a prospective investigation of 10998 vegetarians and non-vegetarians in the United Kingdom. Br J Cancer. 2004;90(1):118–121. doi: 10.1038/sj.bjc.6601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castelló A, Amiano P, Fernández de Larrea N, Martín V, Alonso MH, Castaño-Vinyals G, et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr. 2019;58(4):1495–1505. doi: 10.1007/s00394-018-1674-5. [DOI] [PubMed] [Google Scholar]
- 57.Fung T, Hu FB, Fuchs C, Giovannucci E, Hunter DJ, Stampfer MJ, et al. Major dietary patterns and the risk of colorectal cancer in women. Arch Intern Med. 2003;163(3):309–314. doi: 10.1001/archinte.163.3.309. [DOI] [PubMed] [Google Scholar]
- 58.DellaValle CT, Xiao Q, Yang G, Shu XO, Aschebrook-Kilfoy B, Zheng W, et al. Dietary nitrate and nitrite intake and risk of colorectal cancer in the Shanghai Women’s Health Study. Int J Cancer. 2014;134(12):2917–2926. doi: 10.1002/ijc.28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mafiana RN, Al Lawati AS, Waly MI, Al Farsi Y, Al Kindi M, Al Moundhri M. Association between dietary and lifestyle indices and colorectal cancer in Oman: A case-control study. Asian Pac J Cancer Prev. 2018;19(11):3117–3122. doi: 10.31557/apjcp.2018.19.11.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, Uchida K, Ohnaka K, Morita M, Toyomura K, Kono S, et al. Sugars, sucrose and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol. 2014;49(5):581–588. doi: 10.3109/00365521.2013.822091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe S, Inoue M, Sawada N, Ishihara J, Iwasaki M, Yamaji T, et al. Glycemic index and glycemic load and risk of colorectal cancer: a population-based cohort study (JPHC Study) Cancer Causes Control. 2016;27(4):583–593. doi: 10.1007/s10552-016-0733-6. [DOI] [PubMed] [Google Scholar]
- 62.Peterson S, Yuan J, Koh W, Sun C, Wang R, Turesky RJ, et al. Coffee intake and risk of colorectal cancer among Chinese in Singapore: the Singapore Chinese Health Study. Nutr Cancer. 2009;62(1):21–29. doi: 10.1080/01635580903191528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panprathip P, Petmitr S, Tungtrongchitr R, Kaewkungwal J, Kwanbunjan K. Low folate status, and MTHFR 677C > T and MTR 2756A > G polymorphisms associated with colorectal cancer risk in Thais: a case-control study. Nutr Res. 2019;72:80–91. doi: 10.1016/j.nutres.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Ting P-H, Lin X-H, Jiang J-K, Luo J-C, Chen P-H, Wang Y-P, et al. The factors associated with negative colonoscopy in screening subjects with positive immunochemical stool occult blood test outcomes. J Chin Med Assoc. 2018;81(9):759–765. doi: 10.1016/j.jcma.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Wu IC, Lee C-H, Kuo C-H, Kuo F-C, Wu D-C, Ko Y-C, et al. Consumption of cigarettes but not betel quid or alcohol increases colorectal cancer risk. J Formos Med Assoc. 2009;108(2):155–163. doi: 10.1016/S0929-6646(09)60046-2. [DOI] [PubMed] [Google Scholar]
- 66.Kunnavuttivanich V, Pramyothin P, Ithimakin S. Association between dietary patterns and disease recurrence in Thai colorectal cancer patients. Medicine (Baltimore) 2020;99(11):1–7. doi: 10.1097/md.0000000000019522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy using PubMed, EBSCOHost and ScienceDirect
| Search terms | No. of studies available | No. of studies selecteda |
|---|---|---|
| 1. (“Colorectal cancer*” OR colorectal OR “colon cancer” OR colon OR “rectal cancer” OR rectum OR “colorectal neoplasms” OR “colorectal neoplasia” OR “colorectal carcinoma” OR “colorectal adenocarcinoma” OR “lynch syndrome” OR “hereditary nonpolyposis colorectal neoplasms”). | PubMed: 492, 736 EBSCOHost: 7,110 ScienceDirect: 172, 597 | PubMed: 29 EBSCOHost: 3 ScienceDirect: 3 |
| 2. (Diet* OR “dietary pattern*” OR “dietary habits” OR “dietary factors” OR “dietary risk factors” OR “dietary intake” “dietary flavonoids” OR “dietary non-enzymatic antioxidant capacity” OR “diet quality” OR “inflammatory diet*” OR “inflammatory diet* pattern” OR nutri* OR “nutrient patterns” OR “eating patterns” OR food* OR meat OR poultry OR fish OR fiber OR whole-grain OR “whole grains” OR nut OR curcumin OR beverage* OR alcohol OR “green tea” OR “black tea” OR coffee OR milk OR dairy OR macronutrients OR micronutrient* OR calcium OR “vitamin D” OR iron OR “portion size*”). | PubMed: 3, 243, 871 EBSCOHost: 49, 468 ScienceDirect: 1, 337, 220 | PubMed: 27 EBSCOHost: 2 ScienceDirect: 1 |
| 3. (Asia OR Malaysia OR China OR India OR Indonesia OR Pakistan OR Bangladesh OR Japan OR Philippines OR Vietnam OR Turkey OR Iran OR Thailand OR Myanmar OR South Korea OR Iraq OR Afghanistan OR Saudi Arabia OR Uzbekistan OR Yemen OR Nepal OR North Korea OR Sri Lanka OR Kazakhstan OR Syria OR Cambodia OR Jordan OR Azerbaijan OR United Arab Emirates OR Tajikistan OR Israel OR Laos OR Lebanon OR Kyrgyzstan OR Turkmenistan OR Singapore OR Oman OR Palestine OR Kuwait OR Georgia OR Mongolia OR Armenia OR Qatar OR Bahrain OR Timor-Leste OR Cyprus OR Bhutan OR Maldives OR Brunei). | PubMed: 1, 214, 094 EBSCOHost: 22,573 ScienceDirect: 1, 171, 376 | PubMed: 19 EBSCOHost: 2 ScienceDirect:1 |
Notes:
For ScienceDirect only these terms were used: “Colorectal cancer”, diet, “dietary patterns”, “dietary factors” and Asia