Abstract
Background.
Moradabad district in Uttar Pradesh reported the highest number of paralytic polio cases in India during 2001–2007. We conducted a study in Moradabad in 2007 to assess seroprevalence against poliovirus types 1, 2, and 3 in children 6–12 and 36–59 months of age to guide future strategies to interrupt wild poliovirus transmission in high-risk areas.
Methods.
Children attending 10 health facilities for minor illnesses who met criteria for study inclusion were eligible for enrollment. We recorded vaccination history, weight, and length and tested sera for neutralizing antibodies to poliovirus types 1, 2, and 3.
Results.
Poliovirus type 1, 2, and 3 seroprevalences were 88% (95% confidence interval [CI], 84%–91%), 70% (95% CI, 66%–75%), and 75% (95% CI, 71%–79%), respectively, among 467 in the younger age group (n = 467), compared with 100% (95% CI, 99%–100%), 97% (95% CI, 95%–98%), and 93% (91%–95%), respectively, among 447 children in the older age group (P < .001 for all serotypes).
Conclusions.
This seroprevalence study provided extremely useful information that was used by the program in India to guide immunization policies, such as optimizing the use of different OPV formulations in vaccination campaigns and strengthening routine immunization services. Similar surveys in populations at risk should be performed at regular intervals in countries where the risk of persistence or spread of indigenous or imported wild poliovirus is high.
Keywords: seroprevalence, wild poliovirus, neutralizing antibodies, OPV formulations, immunogenecity, immunization policies, Moradabad
The number of polio-endemic countries dropped from 125 in 1988, the year the World Health Assembly passed a resolution to eradicate polio, to just 4 countries in 2005, one of them India [1, 2]. The number of polio cases globally dropped from an estimated 350 000 in 1988 to 1000–2000 annually during 2004–2010. While India had been using oral poliovirus vaccine (OPV) in routine immunization since 1978, mass polio vaccination campaigns were initiated in the country in 1995 and intensified in 1999. In 1997, the World Health Organization (WHO) Country Office for India established the National Polio Surveillance Project, which deployed medical officers at the district level to support surveillance for acute flaccid paralysis (AFP) and intensification of vaccination campaigns. A polio laboratory network composed of 8 laboratories provided support to the field surveillance for poliovirus detection among AFP cases [3]. India interrupted transmission of wild poliovirus type 2 (WPV2) in 1999 and by 2001 had successfully eliminated endemic circulation of WPV1 and WPV3 in all 35 states and union territories except Uttar Pradesh (UP) and Bihar [4]. High population density and poor sanitation in these 2 states required very high levels of individual and herd immunity to interrupt WPV transmission, compared with other areas of India or other countries [5–8].
Large outbreaks of WPV1 infection were observed in 2002 and 2006. Moradabad district in western Uttar Pradesh reported more polio cases than any other district of India between 2001 and 2007. Genetic sequencing data also showed that the virus lineages circulating in Moradabad led to reinfections and caused outbreaks, not only in neighboring districts of UP, but also in other states of India. Following the relicensing of monovalent polio vaccines, which had better efficacy against the respective vaccine type [9,10], the polio eradication program in India conducted intensive polio immunization campaigns with type 1 monovalent OPV (mOPV1) beginning in 2005 in western UP, including Moradabad, to interrupt transmission of WPV1 in UP, which was responsible for the majority of paralytic polio cases. The bivalent OPV (bOPV) was introduced in India during January 2010 and has since been extensively used. The last case of paralytic polio due to WPV in the country was detected in January 2011. As of March 2014, India has completed 3 years without any WPV detection.
Rapid assessments of the serological response to OPV have been recommended by the WHO to guide polio immunization activities [11]. Such studies have been conducted in several countries including India [12–15], but previous studies conducted in India occurred in 1992, before the use of mass vaccination campaigns and were conducted in states that had become polio-free by 2006 [16]. In order to better understand the underlying reasons for persistence of WPV1 despite repeated mass mOPV1 vaccination campaigns with reported high levels of coverage, the Government of India and partners in the Global Polio Eradication Initiative decided to conduct a rapid assessment of polio immunity levels in Moradabad during 2007.
The study was designed to compare the proportion of children with detectable antibody titers to poliovirus types 1, 2, and 3 in 2 age groups with different risks for paralytic polio. There was a high incidence of polio in India among infants 6–12 months of age, who were, therefore, considered at highest risk for paralytic polio, whereas children 36–59 months of age were considered at lower risk. In addition to age and vaccination, other risk factors for low seroprevalence to poliovirus were also investigated. The study aimed to assess poliovirus seroprevalence among children living in district blocks with a high incidence of polio. The results of this study were expected to guide future strategies and immunization activities to ensure interruption of WPV transmission in western UP.
The study was approved by the Ethical Review Board of the Enterovirus Research Center in Mumbai and by the Government of Uttar Pradesh.
METHODS
Site Selection
Moradabad district comprises 13 blocks and 1 town. Sampling of healthy children in the community was not feasible for this study because of the logistical challenges and safety issues associated with collecting blood samples in households. Enrollment of children brought to health facilities offered a safer and more feasible method. Ten health facilities (3 in the Moradabad urban area and 7 in rural areas) were selected on the basis of their catchment populations, which largely represented blocks with historically high incidence of polio, and the willingness of pediatricians to participate in the study (Figure 1). Seven study sites were located in government healthcare facilities, while 3 were popular private health facilities.
Figure 1.
Distribution of study sites for the study.
Sample Size
It was assumed that at least 70% of children would have antibodies against the 3 poliovirus types. The sample size was calculated to allow for the detection of a difference of ≥8% in the seroprevalence to any poliovirus type between the 2 age groups at different risk for paralytic polio (90% power; α = .05; 2 tailed). A minimum of 400 children were required in each of the 2 age groups (total, 800 children); to account for children who withdrew or had an insufficient level of sera available for analysis, the final sample was 500 children per group.
Eligibility Criteria
Any child aged 6–12 months or 36–59 months who had resided in Moradabad district since the age of 2 months and whose parent or caregiver gave informed consent for participation in the study was eligible for enrollment (inclusion) in the study. The exclusion criteria were age <6 months, >12 to <36 months, or ≥60 months; not a resident in the district since 2 months of age; contraindication for blood specimen collection (eg, bleeding disorder); or lack of consent from the parent or caregiver for blood specimen collection.
Study Procedures
Parents of children eligible for the study were contacted by study staff when they reported at the selected health facilities for medical consultation. Eligible children of the 2 target age groups were recruited consecutively at each clinic or study site until a predetermined sample size was achieved. The total target sample size was distributed across all the study sites in proportion to the patient loads in the outpatient clinics.
The study physician explained the objectives of the study to the parent or caregiver and requested their consent for participation. After obtaining informed consent from their parent or caregiver, the child was allotted a unique study identification number. The physician recorded demographic information and poliovirus vaccination history in a standardized questionnaire. To assist in parent and caregiver recall of doses of OPV received or missed during immunization campaigns, a calendar with dates of campaigns and social and religious festivals was used. Weight and length (or height) were measured using standardized weighing scales and height measuring boards. The presence of stunting (low length for age) and wasting (low weight for length) was evaluated using reference tables from a standard international population [17]. Values for length for age or weight for length ≥2 SDs below the standard mean were considered indicative of moderate stunting or wasting. Values ≥3 SDs below the mean were considered indicative of severe stunting or wasting.
In a subset of 76 enrolled children selected randomly, the number of OPV doses received, according to parent or caregiver recall, was validated with information recorded in tally sheets from polio vaccination campaigns, immunization cards, and records from healthcare workers conducting routine immunization.
Blood Collection
A phlebotomist or trained laboratory technician collected blood samples by venipuncture using aseptic techniques. Two milliliters of blood was collected in a vacutainer tube with a clot separator for poliovirus serological testing, and 1 mL was collected in a separate tube to measure hemoglobin level and blood cell count. Blood samples were sent to the district hospital, where serum was separated by centrifugation on the same day and stored frozen until shipment for testing at the Enterovirus Research Laboratory (Mumbai, India) and the Enterovirus Laboratory at the Centers for Disease Control and Prevention (Atlanta, GA). Hemoglobin levels and blood cell counts were provided to the physicians within 48 hours of blood collection, for appropriate follow-up and treatment.
Poliovirus Neutralizing Antibody Test
The serum samples were tested using a standard microneutralization assay for poliovirus antibodies described elsewhere [18, 19]. Each serum dilution was tested in triplicate. Titers below the starting dilution and those above the ending dilution were assigned values of <6 (log2 = 2.5) and >1448 (log2 = 10.5), respectively. On the assumption that maternal antibody titers decline to undetectable levels by the age of 6 months, seropositivity was defined as the presence of a neutralizing antibody titer of 1:8 (log2 = 3.0) or greater.
Data Analysis
Data from questionnaires were entered into a database and validated for errors, using Microsoft Access 2003 (Microsoft). Analyses were conducted with SAS 9.2 (SAS Institute, Cary, NC) [20].
Seroprevalences and 95% confidence intervals (CIs) were estimated for each serotype, stratified by age group and demographic factors. χ2 analysis and the Cochran-Mantel test for trend were used to compare categorical variables. The Wilcoxon nonparametric test was used to compare age, number of OPV doses, and antibody titer distributions. Two-sided P values are reported.
Multivariate logistic regression was used to calculate adjusted odds ratios. The number of doses of each type of OPV received was entered as a continuous variable, and other risk factors were entered as dichotomous variables. Age was excluded because of the strong correlation with the number of OPV doses received in campaigns (r = 0.86). The final multivariable model was chosen using a stepwise selection procedure that assessed main effects and all 2-way interactions (P > .05).
Enrollment of study subjects and collection of blood samples was conducted between 30 October and 21 November 2007. Antibody testing of the samples was completed by 25 January 2008. After the data were analyzed, the main conclusions were presented in the first week of March 2008 to the Ministry of Health and Family Welfare, Government of India.
RESULTS
Enrollment, Exclusions, and Final Study Group
A total of 527 children 6–12 months of age and 473 children 36–59 months of age were enrolled in the study. The rate of consent for participation in the study was >90% in both age groups. Of these 1000 children, 86 were excluded from the final analysis because of incomplete vaccination information or an insufficient level of sera for testing. The results are presented for 914 participants, including 467 children aged 6–12 months and 447 children aged 36–59 months.
A total of 52 cases of WPV1 infection and 24 cases of WPV3 infection were reported in Moradabad district between January 2006 and October 2007. During the same period, 13 polio vaccination campaigns with mOPV1, 4 with mOPV3, and 1 with trivalent OPV (tOPV) were conducted in the district (Figure 2).
Figure 2.
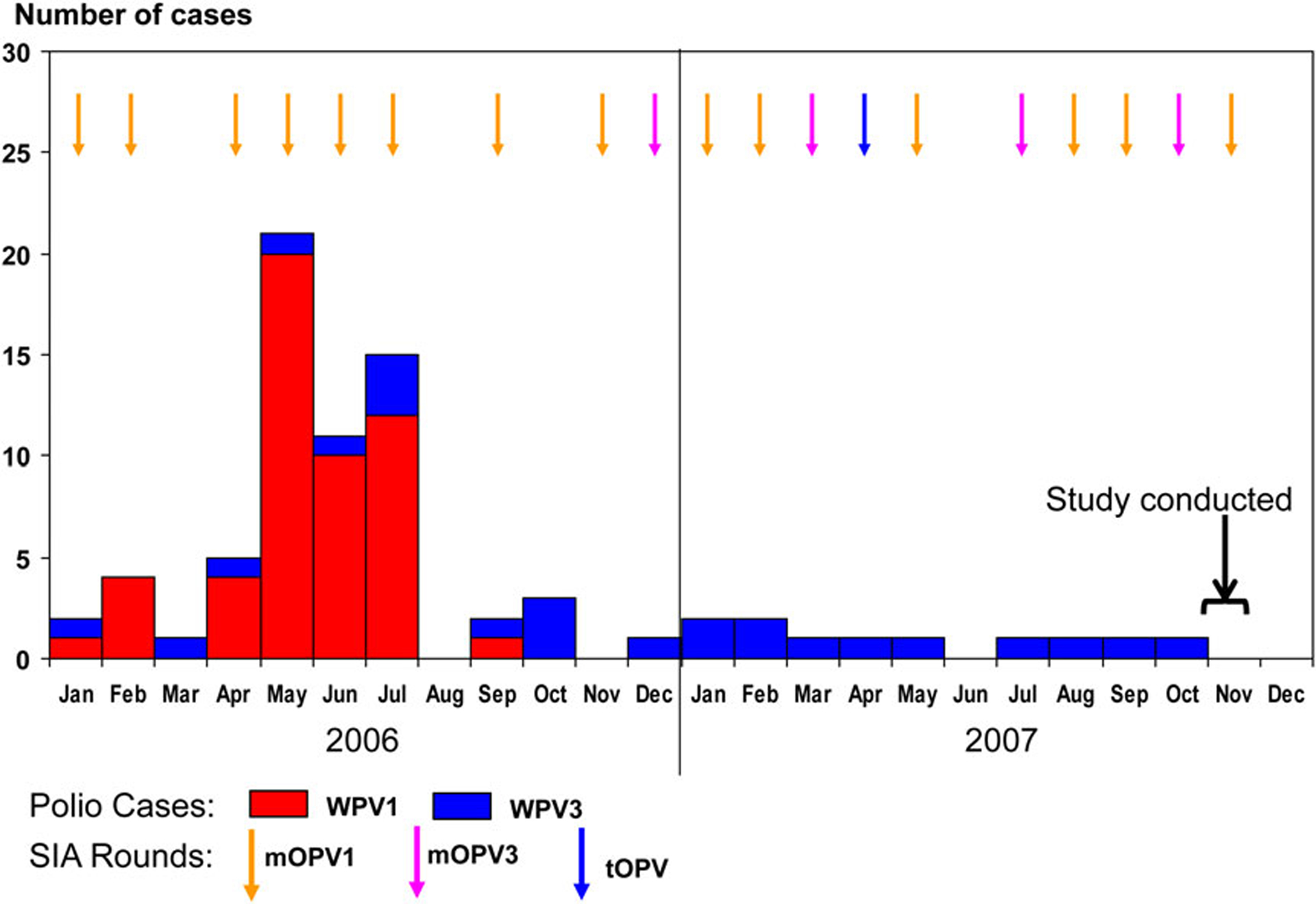
Polio cases and mass polio vaccination campaigns, Moradabad, 2006–2007. Abbreviations: mOPV1, type 1 monovalent oral poliovirus vaccine; mOPV3, type 3 monovalent oral poliovirus vaccine; SIA, supplementary immunization activity; tOPV, trivalent oral poliovirus vaccine; WPV1, wild poliovirus type 1; WPV3, wild poliovirus type 3.
Seroprevalence, by Age Group
Seroprevalences for poliovirus types 1, 2, and 3 were 94% (95% CI, 92%–95%), 83% (95% CI, 81%–86%), and 84% (95% CI, 81%–86%), respectively, for all participants in the study. Among children 6–12 months of age, 88% (95% CI, 84%–91%), 70% (95% CI, 66%–75%), and 75% (95% CI, 71%–79%) were seropositive for types 1, 2, and 3, respectively. Among children 36–59 months of age, 100% (95% CI, 99%–100%), 97% (95% CI, 95%–98%), and 93% (95% CI, 91%–95%) were seropositive for the 3 serotypes, respectively (P < .0001, compared with younger children, for all serotypes; Table 1).
Table 1.
Seroprevalence of Antibody to Poliovirus Types 1, 2, and 3, by Age Group, Moradabad, 2007
| Estimate | Age 6–12 mo (n = 467) | Age 36–49 mo (n = 447) | ||||
|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | Type 1 | Type 2 | Type 3 | |
| Seroprevalence, % (95% CI) | 88a (84–91) | 70a (66–75) | 75a (71–79) | 100 (99–100) | 97 (95–98) | 93 (91–95) |
| Reciprocal median titer | 910 | 181a | 228b | 724 | 362 | 362 |
Abbreviation: CI, confidence interval.
P < .0001, compared with values for children 36–59 months of age.
P < .001, compared with values for children 36–59 months of age.
OPV Doses Received by Study Subjects
The median number of tOPV doses received through routine immunization services was 2 (range, 0–5 doses) for younger children and 1 (range, 0–5 doses) for older children (P = .99, by the Wilcoxon test). During the 12 months before the serosurvey, 11 immunization campaigns had been conducted in Moradabad district, using different vaccines. As a result and based on parent or caregiver recall, children 6–12 months of age had received a median of 3 mOPV1 doses (range, 0–6 mOPV1 doses), 2 tOPV doses (range, 0–6 tOPV doses), and 3 mOPV3 doses (range, 0–4 mOPV3 doses). Children 36 to 59 months of age had received 18 (13–18) mOPV1 doses, 14 (6–24) tOPV doses, and 5 (1–5) mOPV3 doses.
The validation of the number of OPV doses received, according to parent and caregiver recall and other sources, was performed in a subset of 76 children and showed that parent and caregiver recall tended to overestimate the number of doses received through supplementary immunization activities (SIAs), whereas the number of doses received through routine immunization was more accurate. The mean difference in the number of doses received in SIAs between parent and caregiver recall and documented sources was +0.67 (95% CI, .09–1.24) for younger children and +6.58 (95% CI, 5–7.95) for older children (P < .0001, compared with a 0 difference for both groups). The number of tOPV doses received through routine immunization, as reported by parents, and the number recorded in immunization cards or routine immunization records did not differ in any age group.
Seroprevalence, by Type of Vaccine and Doses Received
Because of the inaccuracy of the number of doses received among older children, we looked at seroprevalence by number of vaccine doses and by type of vaccine received only in the younger age group. The percentage of children seropositive for all 3 serotypes increased from 6 months to 12 months of age, almost in parallel with the median number of doses of different vaccine types received through vaccination campaigns (Table 2). Six-month-old children should have received at least 3 doses of tOPV through routine immunization, according to the routine immunization schedule followed in India that administers tOPV at 6, 10, and 14 weeks of age, but only 18 of 57 (32%) had received ≥3 doses.
Table 2.
Seroprevalence of Antibodies to Poliovirus Types 1, 2, and 3, by Age, Among Infants 6–12 Months of Age, Moradabad, 2007
| Age, mo | Subjects, No. | Seroprevalence, %, Subjects | Dose(s), No., Median | ||||
|---|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | mOPV1 | mOPV3 | tOPVa | ||
| 6 | 57 | 79 | 49 | 65 | 3 | 2 | 1 |
| 7 | 64 | 77 | 63 | 73 | 3 | 2 | 2 |
| 8 | 84 | 86 | 76 | 73 | 3 | 2 | 2 |
| 9 | 79 | 90 | 76 | 78 | 3 | 3 | 3 |
| 10 | 76 | 95 | 75 | 71 | 4 | 3 | 3 |
| 11 | 83 | 94 | 73 | 80 | 5 | 3 | 2 |
| 12 | 24 | 96b | 79c | 92d | 5 | 4 | 4 |
Abbreviations: mOPV1, type 1 monovalent oral poliovirus vaccine; mOPV3, type 3 monovalent oral poliovirus vaccine; tOPV, trivalent oral poliovirus vaccine.
Included 0–5 doses received through routine immunization and 0–1 dose received through vaccination campaigns.
P < .0001.
P < .01.
P < .05, by the Cochran test for trend.
Seroprevalence data estimated on the basis of the number of doses of type-specific OPV received is shown in Table 3.
Table 3.
Seroprevalence of Antibodies to Poliovirus Types 1, 2, and 3 Among Infants Aged 6–12 Months, by Number of Doses of Specific Poliovirus Vaccine Received, Moradabad, 2007
| Dose(s), No. | Seroprevalence, % (Proportion) | ||
|---|---|---|---|
| Type 1, mOPV1 Recipientsa | Type 2, tOPV Recipientsb | Type 3, mOPV3 Recipientsa | |
| 0 | 0 (0/3) | 33 (14/43) | 50 (1/2) |
| 1 | 67 (4/6) | 63 (92/146) | 44 (4/9) |
| 2 | 70 (7/10) | 71 (39/55) | 65 (43/66) |
| 3 | 84 (66/79) | 74 (56/76) | 70 (53/76) |
| 4 | 92 (24/26) | 86 (86/100) | 75 (18/24) |
| 5 | 96 (48/50) | 91 (39/43) | … |
| 6 | 100c(4/4) | 75c (3/4) | … |
| Overall | 86 (153/178) | 70 (329/467) | 67 (119/177) |
Abbreviations: mOPV1, type 1 monovalent oral poliovirus vaccine; mOPV3, type 3 monovalent oral poliovirus vaccine; RI, routine immunization; SIA, supplementary immunization activity; tOPV, trivalent oral poliovirus vaccine.
Excluding children who received tOPV through RI and including 135 children (76%) who had received 1 tOPV dose in SIAs.
Including children who received tOPV doses through SIAs and RI.
P < .0001, by the Cochran test for trend.
Children who had received any tOPV dose through routine immunization were excluded from the analysis for seroprevalence by dose of mOPV1 and mOPV3. After 3 mOPV1 doses, 84% of infants were seropositive for type 1 poliovirus, and 96% were seropositive after 5 doses. After 3 mOPV3 doses, 70% of infants were seropositive, and 75% seropositive after 4 doses. The estimates of seroprevalence based on the number of mOPV1 and mOPV3 doses have the limitation that 76% of children received an additional dose of tOPV, but exclusion of these children would have resulted in groups too small to compare (Table 3).
Additional Factors Influencing Poliovirus Seroprevalence
Univariate analysis showed that male sex was associated with higher seropositivity for poliovirus 1 and 3 (P < .001 and P < .05, respectively; Table 4). A lower seroprevalence of antibody to type 2 poliovirus was associated with belonging to a Muslim family (P < .05, vs belonging to a Hindu family) and with a lower education duration of the father or mother (P < .05, by the Cochran test for trend). Stunting and wasting were not associated with poliovirus antibody positivity (Table 4)
Table 4.
Seroprevalence of Antibodies to Each Poliovirus Type, by Demographic and Clinical Risk Factors, Among Children 6–12 Months of Age, Moradabad, 2007
| Factor | Subjects, No. | Type 1, Subjects, % | Type 2, Subjects, % | Type 3, Subjects, % |
|---|---|---|---|---|
| Sex | ||||
| Male | 283 | 92 | 72 | 78 |
| Female | 184 | 82a | 68 | 70b |
| Religion | ||||
| Muslim | 327 | 86 | 65a | 73 |
| Hindu | 140 | 92 | 83 | 80 |
| RI doses | ||||
| <3 | 298 | 86 | 62 | 70 |
| ≥3 | 169 | 91 | 85a | 84a |
| Health facility type | ||||
| Private | 207 | 85 | 67 | 73 |
| Public | 260 | 90 | 73 | 76 |
| Mother’s education duration, y | ||||
| 0 | 308 | 86 | 65 | 73 |
| 1–9 | 67 | 87 | 72 | 72 |
| ≥10 | 91 | 93 | 88a | 84 |
| Father’s education duration, y | ||||
| 0 | 213 | 86 | 61 | 72 |
| 1–9 | 129 | 87 | 68 | 71 |
| ≥10 | 124 | 91 | 90a | 84 b |
| Diarrhea in prior 14 d | ||||
| Yes | 177 | 87 | 68 | 70 |
| No | 290 | 88 | 72 | 78 |
| Stunting (low height for age)c | ||||
| Severe | 38 | 87 | 66 | 61 |
| Moderate | 63 | 86 | 62 | 78 |
| None | 366 | 88 | 72 | 76 |
| Wasting (low weight for age)c | ||||
| Severe | 56 | 86 | 68 | 68 |
| Moderate | 100 | 88 | 72 | 74 |
| None | 311 | 88 | 70 | 77 |
Abbreviation: RI, routine immunization.
P < .001, compared with the reference value, using χ2 analysis or Cochran test for trend (variables with >2 categories).
P < .05, compared with the reference value, using χ2 analysis or the Cochran test for trend (variables with >2 categories).
According to World Health Organization tables for standard weight and height by age, moderate stunting or wasting was defined as 2–2.9 SDs below the mean, and severe stunting or wasting was defined as >2.9 SDs below the mean.
To better understand the differences in seroprevalence with respect to sex and religion, we compared demographic and clinical characteristics after stratifying by these factors. There were no differences in parent’s education duration, religion, routine immunization coverage, diarrhea prevalence, and malnutrition prevalence between male and female children, although there was a significant sex-based difference in the proportion attending private clinics (51% for males and 34% for females; P < .0001). On the other hand, the comparison between children from Muslim and Hindu religious communities showed that illiteracy among parents was more frequent, and a higher proportion of children from Muslim families had severe chronic malnutrition and/or diarrhea ≤14 days before blood specimen collection (Table 5). Furthermore, only 29% of Muslim children had received ≥3 tOPV doses during routine immunization, compared with 71% of Hindu children (P < .001). However, there were no religion-associated differences in doses received through vaccination campaigns.
Table 5.
Comparison of Epidemiological and Clinical Characteristics of Children From Hindu and Muslim Families
| Characteristic | Muslim (n = 647) | Hindu (n = 267) | Pa |
|---|---|---|---|
| Male sex | 59 | 65 | NS |
| Age, mo | 12 | 12 | NS |
| SIA OPV doses, no. | 11 | 10 | NS |
| RI OPV doses, no. | 0 | 3 | <.0001 |
| ≥3 RI doses received | 29 | 71 | <.0001 |
| Mother’s education duration, y | |||
| 0 | 71 | 55 | <.001 |
| 1–9 | 14 | 19 | |
| ≥10 | 15 | 26 | |
| Father’s education duration, y | |||
| 0 | 55 | 29 | <.001 |
| 1–9 | 28 | 22 | |
| ≥10 | 17 | 49 | |
| Attendance at a private clinic | 37 | 28 | .008 |
| Diarrhea in last 14 d | 30 | 21 | .007 |
| Stuntingb | |||
| No | 61 | 70 | <.05 |
| Moderate | 21 | 14 | |
| Severe | 19 | 16 | |
| Wastingb | |||
| No | 64 | 63 | NS |
| Moderate | 22 | 22 | |
| Severe | 13 | 14 | |
Data are % of subjects or median value.
Abbreviations: NS, not significant; OPV, oral poliovirus vaccine; RI, routine immunization; SIA, supplementary immunization activity.
Compared with the reference value, using χ2 analysis or the Cochran test for trend
According to World Health Organization tables for standard weight and height by age, moderate stunting or wasting was defined as 2–2.9 SDs below the mean, and severe stunting or wasting was defined as >2.9 SDs below the mean.
The final logistic regression model for seroprevalence of antibodies to poliovirus types 1, 2, or 3 included number of doses of tOPV, mOPV1, or mOPV3; sex; religion; father’s education duration; and severe stunting. The OR (and 95% CIs) of each additional dose of each vaccine are shown on Table 6. After adjustment for vaccine type and doses, male sex was associated with a higher odds of seropositivity for type 1 poliovirus, father’s education duration of >10 years was associated with an increased odds of seropositivity for type 2 poliovirus, and severe stunting was associated with a decreased odds of seropositivity for type 3 poliovirus (Table 6).
Table 6.
Multivariate Odds Ratios (ORs) for Seroprevalence of Antibodies to Poliovirus Types 1, 2 and 3 Among Children 6–12 Months of Age
| Factor | Type 1 (n = 467) | Type 2 (n = 467) | Type 3 (n = 466) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| tOPV doses | 1.23 (1.01–1.50) | .04 | 1.49 (1.28–1.74) | <.0001 | 1.38 (1.18–1.60) | <.0001 |
| mOPV1 doses | 1.77 (1.34–2.33) | <.0001 | … | … | ||
| mOPV3 doses | … | … | 1.33 (.99–1.79) | .06 | ||
| Male sex | 2.58 (1.43–4.64) | .04 | … | 1.64 (1.06–2.55) | .04 | |
| Father’s education duration >10 y | … | 3.31 (1.75–6.28) | .001 | … | ||
| Severe stunting | … | 0.40 (.19–.83) | .03 | |||
Abbreviations: CI, confidence interval; mOPV1, type 1 monovalent oral poliovirus vaccine; mOPV3, type 3 monovalent oral poliovirus vaccine; tOPV, trivalent oral poliovirus vaccine.
DISCUSSION
According to the 2001 Census of India, the total population of Moradabad district was 3 810 983. The number of children <5 years of age was 513 631. A total of 54% of the population is Hindu, and 46% follow Islam [21]. A district-level household survey conducted during 2007–2008 showed routine immunization coverage of 34% for 3 doses of OPV [22]. The last case of WPV2 infection in Moradabad was reported in 1998 [23].Between 2000 and 2006, 235 virologically confirmed cases of paralytic poliomyelitis were reported in Moradabad district (189 cases due to WPV1, 44 due to WPV3, and 2 due to WPV1 plus WPV3) [24]. Most of the WPV1 cases were reported during the outbreaks of 2002 (n = 75) and 2006 (n = 52), whereas WPV3 transmission occurred at low intensity. About 80% of paralytic poliomyelitis cases were in the 6–24-month age group, and children among Muslim families were disproportionately affected [24].
Monovalent OPVs (mOPV1 and mOPV3) were introduced in the polio program in 2005 because the type 2 vaccine strain had an interfering effect with seroconversion to the other 2 serotypes [9, 25]. The India Expert Advisory Group on polio eradication recommended in 2005 that the interruption of WPV1 transmission should be prioritized by more-frequent use of mOPV1. Serotype 1 has a higher propensity than WPV3 to spread into polio-free areas and cause large outbreaks. As a result, the frequency of campaigns administering mOPV1 increased during 2006 and 2007.
In Moradabad, by the end of 2007 immunity against WPV1 was <80% among infants 6 months of age and increased with age in direct correlation with the number of mOPV1 doses received in immunization campaigns, reaching 96% after receipt of 5 doses. The results of this survey also confirmed the high immunogenicity of mOPV1 observed in clinical trials [9, 25, 26]. Seroprevalence levels observed in infants aged 6–12 months were most likely induced by vaccine because no cases of WPV1 infection had been reported in Moradabad between the birth of the study cohort and collection of their serum samples.
Seroprevalence against type 3 poliovirus was 75% among subjects aged 6–12 months, compared with 88% against type 1 poliovirus. Fewer doses of mOPV3 than mOPV1 were administered during vaccination campaigns in Moradabad during 2006–2007. This is also partially expected because of the known lower immunogenicity of tOPV against type 3 poliovirus, especially in tropical countries [27]. We could not confirm the expected immunogenic advantage of mOPV3, compared with tOPV, from this study. The median prevalence of seroconversion in tropical countries was 70% after 1 mOPV3 dose (range, 52%–80%) [9], but in this study at least 3 doses of mOPV3 appeared to be necessary to reach a seroprevalence of around 70%. Suboptimal immunogenicity among children with severe chronic malnutrition may help to explain the lower seroprevalence to type 3 in settings with a high prevalence of malnutrition. During the study period, there was active transmission of WPV3 in the district, and some study subjects may have been seropositive as a result of natural immunity.
No case of paralytic poliomyelitis due to WPV1 was reported in western Uttar Pradesh between September 2007 and May 2008 [24].In 2008, western Uttar Pradesh was reinfected by WPV1 imported from Bihar, causing a small outbreak in which 8 cases were reported in Moradabad [24].The high population immunity against poliovirus 1 achieved with intensive campaigns using mOPV1 possibly prevented the occurrence of a large-scale outbreak similar to the one in 2002. On the other hand, a large outbreak of WPV3 infection caused 484 and 662 cases of paralytic polio during 2008 and 2009 in India, respectively, of which 10 and 72 cases were reported in Moradabad district [24, 28]. This increase in WPV3 transmission was likely due to the persistent immunity gap for type 3 poliovirus observed in this study.
Our study also confirmed the low routine immunization coverage in Moradabad district. Only 70% of infants were seropositive to type 2 poliovirus. Infants had received a median of 2 tOPV doses through routine immunization, and only 36% had received 3 tOPV doses. Coverage with routine immunization was especially low in children from Muslim families: only 26% of Muslim children 6–12 months of age had received ≥3 tOPV doses, compared with 59% of Hindu children (P < .001). During the lifetime of these children, only 1 vaccination campaign had provided tOPV. This immunity gap against type 2 poliovirus increased the risk of an outbreak of type 2 circulating vaccine-derived poliovirus (cVDPV) infection in Moradabad and other districts in Uttar Pradesh with low levels of coverage for routine immunization. Older children were better protected, with a type 2 seroprevalence of >95%, as they had received a higher number of tOPV doses through vaccination campaigns. As predicted by this study, an outbreak of type 2 cVDPV infection, with 18 paralytic cases, occurred in western Uttar Pradesh during 2009–2010 [29].
These findings were important in guiding the supplementary immunization strategies in India by confirming that more-frequent administration of mOPV1 was necessary to achieve high seroprevalence against type 1 poliovirus by 6 months of age, especially considering that the highest incidence of polio in northern India was among children 6–12 months of age. A supplementary vaccination regimen with more-frequent mOPV1 doses and much fewer doses of tOPV and mOPV3 vaccines was successfully implemented to stop WPV1 transmission. The availability and use of bivalent OPV (types 1 and 3) by 2010 enabled interruption of type 3 poliovirus transmission while maintaining a high seroprevalence of antibody against type 1 poliovirus.
This study has several limitations. By design, it is not possible to generalize the data to be representative of the total population of children in the district. Given that most of the children had received multiple doses of several vaccines, calculating the perdose efficacy in this study was difficult. Estimations of the number of OPV doses received was based on parent and caregiver recall, which has high likelihood of error, especially in the older age group, as demonstrated by the OPV dose validation process performed in a subset of children. Since this was a health facility–based study, individuals who do not have access to healthcare may have been underrepresented. Children attending outpatient clinics may also have an immunity status different from that of healthy children. Finally, parents who did not give consent to participate in the study may also be more likely to refuse vaccination, resulting in an overestimation of seroprevalence.
In conclusion, information of high predictive value was obtained from this seroprevalence survey evaluating population immunity against poliovirus. These data were used by the polio eradication program to guide immunization policies, such as optimizing the use of different OPV formulations in campaigns and urgent strengthening of routine immunization services. Similar surveys that are strategically designed and include populations at risk should be performed at regular intervals in countries where the risk of persistence or spread of indigenous or imported WPV is high.
Acknowledgments.
We thank the surveillance medical officers and field volunteers from the National Polio Surveillance Project, WHO Country Office for India, for their contribution to the study; the medical officers/physicians at the study sites, for the enrollment of study subjects; the data management staff of the National Polio Surveillance Project, WHO Country Office for India, for performing data entry; the laboratory staff at the Enterovirus Research Center, Mumbai, and the Centers for Disease Control and Prevention, for serological testing; and the Government of India (Ministry of Health and Family Welfare) and the Government of Uttar Pradesh, for supporting the field work.
Financial support.
This work was supported by the Ministry of Health and Family Welfare, Government of India, and by the World Health Organization.
Supplement sponsorship.
This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–India, January 2005–June 2006. MMWR Morb Mortal Wkly Rep 2006; 55:772–6. [PubMed] [Google Scholar]
- 2.Dowdle WR, Birmingham ME. The biologic principles of poliovirus eradication. J Infect Dis 1997; 175(suppl 1):S286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–India, 1998. Morb Mortal Wkly Rep 1998; 47:778–81. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–India, January 2004–May 2005. MMWR Morb Mortal Wkly Rep 2005; 54:655–9. [PubMed] [Google Scholar]
- 5.Bottiger M The elimination of polio in the Scandinavian countries. Public Health Rev 1993; 94:27–33. [PubMed] [Google Scholar]
- 6.Diedrich S, Claus H, Schreier E. Immunity status against poliomyelitis in Germany: determination of cut-off values in International Units. BMC Infect Dis 2002; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol 1999; 150:1001–21. [DOI] [PubMed] [Google Scholar]
- 8.Ghendon Y, Robertson SE. Interrupting the transmission of wild polioviruses with vaccines: immunological considerations. Bull World Health Organ 1994; 72:973–83. [PMC free article] [PubMed] [Google Scholar]
- 9.Caceres VM, Sutter RW. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin Infect Dis 2001; 33:531–41. [DOI] [PubMed] [Google Scholar]
- 10.El-Sayed N, Al-Jorf S, Hennessey KA, et al. Survey of poliovirus antibodies during the final stage of polio eradication in Egypt. Vaccine 2007; 25:5062–70. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Rapid assessment of serological response to oral polio vaccine. Wkly Epidemiol Rec 1990; 5:34–5. [PubMed] [Google Scholar]
- 12.Frantzidou F, Diza E, Halkia D, Antoniadis A. A seroprevalence study of poliovirus antibody in the population of northern Greece. Clin Microbio Infect 2005; 11:68–71. [DOI] [PubMed] [Google Scholar]
- 13.Chen RT, Hausinger S, Dajani AS, et al. Seroprevalence of antibody against poliovirus in inner-city preschool children. Implications for vaccination policy in the United States. JAMA 1996; 275:1639–45. [PubMed] [Google Scholar]
- 14.Rumke HC, Oostvogel PM, Van Steenis G, Van Loon AM. Poliomyelitis in The Netherlands: a review of population immunity and exposure between the epidemics in 1978 and 1992. Epidemiol Infect 1995; 115:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoub BD, Blackburn NK, McAnerney JM. Seroprevalence to polio in personnel at a virology institute. J Infect 2001; 43:128–31. [DOI] [PubMed] [Google Scholar]
- 16.Deshpande JM, Kamat JR, Rao VK, et al. Prevalence of antibodies to polioviruses & enteroviruses excreted by healthy children in Bombay. Indian J Med Res 1995; 101:50–4. [PubMed] [Google Scholar]
- 17.World Health Organization. Multicentre Growth Reference Study Group: WHO Child Growth Standards: Length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization, 2006. http://www.who.int/childgrowth/standards/technical_report/en/index.html. Accessed on 17 March 2010. [Google Scholar]
- 18.Expanded Programme on Immunization. Report of a WHO Informal consultation on polio neutralizing antibody assays, Nashville, 5–6 December 1991. Geneva: World Health Organization, 1991. (WHO/EPI/RD/91.3 Rev 1). [Google Scholar]
- 19.World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine. Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and the Gambia. J Infect Dis 1995; 171:1097–106. [DOI] [PubMed] [Google Scholar]
- 20.SAS/STAT user’s guide, version 6.4 4th ed, vol. 1. Cary, NC: SAS Institute, 1989. [Google Scholar]
- 21.Census of India. Basic data sheet. District Moradabad (04), Uttar Prades (09). http:censusindia.ogv.in/Dist_File/datasheet-0904.pdf. Accessed 12 November 2013. [Google Scholar]
- 22.Government of India Ministry of Health and Family Welfare. District household and facility survey (DLHS-3): 2007–08. http://www.rchiips.org. Accessed 12 November 2013. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–South-East Asia, January 2000–June 2001. Morb Mortal Wkly Rep 2001; 50:738–42, 751. [PubMed] [Google Scholar]
- 24.National Polio Surveillance Project. AFP and polio data. http://www.npspindia.org. Accessed 12 November 2013.
- 25.el-Sayed N, el-Gamal Y, Abbassy AA, et al. Monovalent type 1 oral poliovirus vaccine in newborns. N Eng J Med 2008; 359:1655–65. [DOI] [PubMed] [Google Scholar]
- 26.John TJ, Jain H, Ravishankar K, et al. Monovalent type 1 oral poliovirus vaccine among infants in India: report of two randomized double-blind controlled clinical trials. Vaccine 2011; 29:5793–801. [DOI] [PubMed] [Google Scholar]
- 27.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis 1991; 13:926–39. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication–India, January 2007-May 2009. MMWR Morb Mortal Wkly Rep 2009; 58:719–23. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses — worldwide, July 2009–March 2011. MMWR Morb Mortal Wkly Rep 2011; 60:846–50. [PubMed] [Google Scholar]


