Abstract
Background:
Randomized controlled trials (RCTs) provide essential evidence to inform practice, but the many necessary steps result in lengthy times to initiation, which is problematic in the case of rapidly emerging infections such as COVID-19. This study aimed to describe the start-up timelines for the Canadian Treatments for COVID-19 (CATCO) RCT.
Methods:
We surveyed hospitals participating in CATCO and ethics submission sites using a structured data abstraction form. We measured durations from protocol receipt to site activation and to first patient enrolment, as well as durations of administrative processes, including research ethics board (REB) approval, contract execution and lead times between approvals to site activation.
Results:
All 48 hospitals (26 academic, 22 community) and 4 ethics submission sites responded. The median time from protocol receipt to trial initiation was 111 days (interquartile range [IQR] 39–189 d, range 15–412 d). The median time between protocol receipt and REB submission was 41 days (IQR 10–56 d, range 4–195 d), from REB submission to approval, 4.5 days (IQR 1–12 d, range 0–169 d), from REB approval to site activation, 35 days (IQR 22–103 d, range 0–169 d), from protocol receipt to contract submission, 42 days (IQR 20–51 d, range 4–237 d), from contract submission to full contract execution, 24 days (IQR 15–58 d, range 5–164 d) and from contract execution to site activation, 10 days (IQR 6–27 d, range 0–216 d). Processes took longer in community hospitals than in academic hospitals.
Interpretation:
The time required to initiate RCTs in Canada was lengthy and varied among sites. Adoption of template clinical trial agreements, greater harmonization or central coordination of ethics submissions, and long-term funding of platform trials that engage academic and community hospitals are potential solutions to improve trial start-up efficiency.
There is an imperative to initiate clinical trials rapidly during a health emergency. However, during recent infectious disease outbreaks, such as SARS (2003), H1N1 influenza (2009) and Ebola virus disease (2014–2016), investigators were generally unable to initiate randomized clinical trials in time to inform management while the outbreaks were still active.1–3 In contrast, during the COVID-19 pandemic, hundreds of clinical trials have been initiated, both globally and in Canada, many with overlapping interventions. Many trials did not complete planned enrolment, as their start-up was delayed by logistical difficulties, and results were superseded by those from jurisdictions that were able to initiate and recruit more quickly.
In Canada, clinical trial initiation requires several steps, including Health Canada approval in the case of novel or repurposed medications; establishing a supply of study medications; obtaining ethics approval at each site (facilitated in some provinces by provincial or regional research ethics boards [REBs]); establishing legal contracts between the trial sponsor (the institution responsible for trial conduct) and each participating site; training research, pharmacy and clinical staff involved in the study; and obtaining site operational approval to ensure all start-up activities are completed and regulatory standards have been met. The duration of each step depends on site experience, site-specific requirements, the pragmatism of the study design and the availability of multisite harmonization of processes.
Canadian Treatments for COVID-19 (CATCO) is a multicentre randomized controlled trial (RCT) examining therapeutic interventions for patients admitted to hospital with COVID-19 in Canada funded by the Canadian Institutes of Health Research (CIHR) (NCT04330690).4 In this paper, we describe the start-up timelines for CATCO at 48 hospitals across the country during the early phase of the COVID-19 pandemic, and highlight opportunities for improvement in trial initiation and logistical conduct in Canada, for both pandemic and nonpandemic research.
Methods
Setting and design
This was a time–motion study of the start-up of CATCO, a multicentre, adaptive, open-label RCT of therapeutics for the treatment of COVID-19 in patients admitted to hospital.4 For context, CATCO evaluated the efficacy of remdesivir and interferon-β-1a, and optimized support care for patients with COVID-19 admitted to a hospital that was a participating study site.
We adapted the methods of this study from a previous study.5 The present study included data from 48 hospitals in 8 provinces (British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, Nova Scotia, and Newfoundland and Labrador), as well as 4 provincial research ethics boards or coordinated submission systems (in British Columbia, Alberta, Ontario and Quebec) participating in CATCO.
Data collection
Between May and September 2021, sites participating in CATCO were asked to submit the following information electronically via a data abstraction form: date of initial REB submission, date of REB approval, date of contract submission to site legal services, date of full contract execution (defined by receipt of all required signatures), date of site activation (defined by the sponsor as having completed all necessary start-up procedures) and date of first patient enrolment. We used the date that the CATCO protocol was distributed electronically to the sites as a proxy for protocol receipt. We categorized sites as academic or community based on self-designation as fully affiliated (or not) with a university,6 expecting that different research infrastructure may exist between academic and community sites.
Data analysis
Using the survey data, we calculated the following durations: protocol receipt to site activation (i.e., total time required to initiate the trial), protocol receipt to REB submission, protocol receipt to legal contract submission, REB submission to approval, legal contract submission to execution, contract execution to site activation, and REB approval to site activation. Durations were represented as medians with interquartile range (IQR) and full range. All analysis was done in Microsoft Excel version 16.57.
Ethics approval
Research ethics review was not required for this study because it was deemed quality-improvement and not human research.
Results
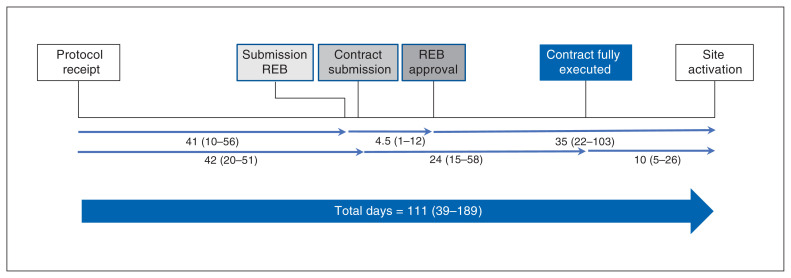
All 48 hospitals (26 academic, 22 community) and 4 ethics submission sites provided data for the study. The complete response rate was 15/26 (58%) for academic hospitals, 13/22 (59%) for community hospitals and 3/4 (75%) for regional ethics submission sites. Figure 1 shows the stepwise and parallel processes required in trial initiation and their respective durations. Some sites did the REB and legal steps in parallel. Many did not enter the submission dates for either the REB or legal steps. Quite a few obtained REB approval on the same day as submission. The median length of time required for each of these steps at the different types of sites is shown in Table 1.
Figure 1:
Schematic of processes leading to clinical trial initiation at site. Values are median number of days and interquartile range. Total days = number of days between protocol sent to sites and first patient enrolled. Note: REB = research ethics board.
Table 1:
Time required for incremental steps in clinical trial initiation across participating sites
| Type of site; parameter | Overall; time, d | Research ethics board; time, d | Legal; time, d | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Protocol sent to site activation | Protocol sent to first patient enrolled | Site activation to first patient enrolled | Protocol sent to REB submission | REB submission to approval | REB approval to site activation | Protocol sent to contract submission | Contract submission to full contract execution | Full contract execution to site activation | |
| All hospitals | |||||||||
|
| |||||||||
| Mean ± SD | 128.0 ± 99.6 | 151.3 ± 93.3 | 48.8 ± 54.5 | 44.8 ± 44.1 | 13.4 ± 29.4 | 65.1 ± 57.6 | 50.7 ± 49.7 | 43.3 ± 40.0 | 31.4 ± 45.1 |
|
| |||||||||
| Median (IQR) | 111 (38.8–189.3) | 169 (53–232) | 32 (4–76) | 41 (10–56) | 4.5 (1–12) | 35 (22–103.5) | 42 (20.5–50.8) | 24.5 (15–57.8) | 10 (5.5–26.5) |
|
| |||||||||
| Range | 15–412 | 14–358 | 1–210 | 4–195 | 0–169 | 0–239 | 4–237 | 5–164 | 0–216 |
|
| |||||||||
| Academic hospitals, n = 26 | |||||||||
|
| |||||||||
| Mean ± SD | 102.7 ± 94.4 | 109.7 ± 96.5 | 33.9 ± 47.8 | 24.5 ± 24.2 | 19.4 ± 39.0 | 52.4 ± 50.1 | 30.6 ± 22.4 | 38.7 ± 42.8 | 15.9 ± 21.0 |
|
| |||||||||
| Median (IQR) | 68.5 (25.3–182.3) | 71 (27.5–218.8) | 5 (2.3–58.5) | 16 (5–38) | 5 (1–16) | 32.5 (13.3–93.5) | 41 (4–43) | 22 (15–40) | 8 (4–21) |
|
| |||||||||
| Range | 15–392 | 14–266 | 1–140 | 4–84 | 0–169 | 0–160 | 4–82 | 5–164 | 0–73 |
|
| |||||||||
| Missing | 0 | 4 | 4 | 6 | 6 | 0 | 9 | 9 | 1 |
|
| |||||||||
| Community hospitals, n = 22 | |||||||||
|
| |||||||||
| Mean ± SD | 158.3 ± 101.5 | 199.4 ± 66.3 | 65.9 ± 59.4 | 70.1 ± 51.9 | 5.8 ± 6.2 | 80.8 ± 64.1 | 73.5 ± 63.5 | 48.5 ± 38.9 | 49.0 ± 58.6 |
|
| |||||||||
| Median (IQR) | 118.5 (91.3–192) | 206 (169–235) | 45 (24–103.5) | 51.5 (43–76.3) | 4 (1.8–8.3) | 60 (30–133) | 50 (42–84) | 49 (21–59.5) | 19.5 (8–87.5) |
|
| |||||||||
| Range | 28–412 | 39–358 | 1–210 | 11–195 | 0–23 | 10–239 | 5–237 | 7–157 | 0–216 |
|
| |||||||||
| Missing | 0 | 3 | 3 | 6 | 6 | 1 | 7 | 7 | 0 |
|
| |||||||||
| Regional ethics submission sites, n = 4 | |||||||||
|
| |||||||||
| Mean ± SD | – | – | – | – | 7 ± 4.9 | – | – | – | – |
|
| |||||||||
| Median (IQR) | – | – | – | – | 7 (4–10) | – | – | – | – |
|
| |||||||||
| Range | – | – | – | – | 1–13 | – | – | – | – |
|
| |||||||||
| Missing | – | – | – | – | 1 | – | – | – | – |
Note: REB = research ethics board.
Overall, the median time to initiate the trial was 111 days (IQR 39–189 d, range 15–412 d). The median time between protocol receipt and REB submission was 41 days (IQR 10–56 d, range 4–195 d), between protocol receipt and contract submission, 42 days (IQR 20–51 d, range 4–237 d), between REB submission and approval, 4.5 days (IQR 1–12 d, range 0–169 d), between contract submission and full contract execution, 24 days (IQR 15–58 d, range 5–164 d), between REB approval and site activation, 35 days (IQR 22–103 d, range 0–169 d) and between contract execution and site activation, 10 days (IQR 6–26 d, range 0–216 d).
When examined by academic versus community status, the median time from protocol dissemination to site activation was 68 days (IQR 25–182 d, range 15–392 d) at academic sites and 118 days (IQR 91–192 d, range 28–412 d) at community sites. Aside from a similar time between REB submission and approval, all incremental steps in study activation took longer at community sites than at academic sites (Appendix 1, available at www.cmajopen.ca/content/11/4/E615/suppl/DC1).
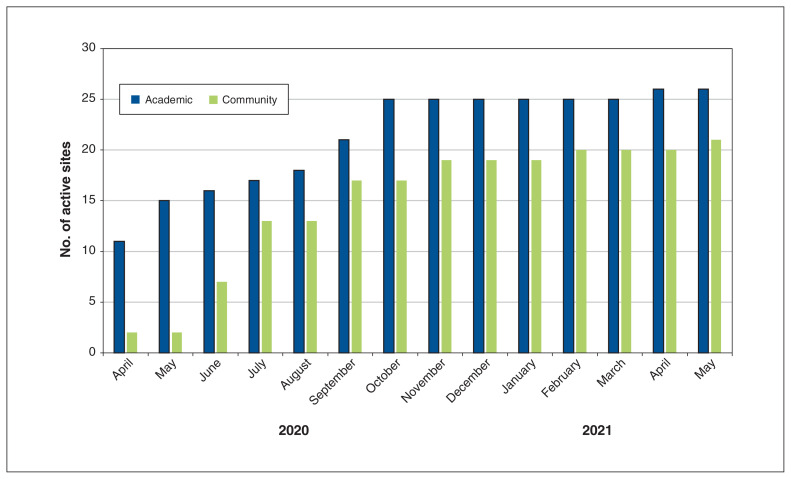
The CATCO protocol was finalized on Mar. 13, 2020, the first site was activated on Apr. 2, 2020, and the first patient was enrolled on Apr. 3, 2020. The cumulative number of activated study sites from April 2020 to May 2021 is illustrated in Figure 2.
Figure 2:
Cumulative number of activated sites for the Canadian Treatments for COVID-19 randomized controlled trial, April 2020 to May 2021.
Interpretation
In this descriptive study of start-up times for a large, multicentre RCT, we found that the median time to initiate study enrolment was 111 days (3.6 mo). The first site was activated on Apr. 2, only 2 weeks after the CATCO protocol was finalized. However, the majority of sites initiated recruitment after the first wave of the pandemic had already passed (Figure 2). This lengthy interval represents a lost opportunity to generate evidence early in the pandemic.
The most time-consuming steps in site initiation were submission and approval of trial contracts. Research ethics board approvals occurred quickly, after a median of 4.5 days. Therefore, improving the timeliness of contract writing and execution is crucial to augment the efficiency of clinical trial start-up in Canada.
Currently in Canada, clinical trial agreements are negotiated between the trial sponsor and each individual site, typically via the site’s research institute or university. Most sponsors have a template trial agreement, but subsequent negotiations proceed in parallel, with legal review required at each site. Although the core elements of such agreements are commonly accepted, there can be substantial back-and-forth negotiations around individual and shared responsibilities, site payments, intellectual property, trial insurance and indemnification. Prior work establishing model contract trial agreements for pharmaceutical-based trials has been helpful,7 but such agreements are not commonly used by Canadian research institutes. Although there were other factors at play that caused delays, such as the inability of some sites to proceed in parallel with the REB and legal submissions, failure to adopt a model clinical trial agreement likely created a major barrier to timely initiation of CATCO. The lack of harmonization resulting in a delay in contract approval and ultimately to delay in trial initiation has also been reported by Crow and colleagues.8 This shortcoming represents an urgent collective responsibility of trialists, legal and paralegal experts, and hospital and research institute administration to improve and refocus the purpose of these legal agreements, and work collectively toward creation of a model clinical trial agreement. This problem might also be addressed by CIHR (which funds the vast majority of investigator-initiated trials in Canada) by encouraging the use and implementation of model clinical trial agreements as a priority to improve the impact of Canada’s investment in health research.
In addition to the above-mentioned barriers, investigator and staff time for initiating the study need to be acknowledged. These research administrative tasks are time-consuming, and, although efficiencies are required, there will inevitably be tasks required to be performed for study initiation at local institutions. Ensuring that there are trained staff to accomplish these tasks in every relevant institution is crucial for efficient start-up and conduct of research.
In contrast, our anecdotal experience during the COVID-19 pandemic, reinforced by the findings in this time–motion study, is that individual REBs and coordinated ethics submission systems were comparatively rapid in reviewing, commenting on and approving pandemic-related proposals. The development of provincial clinical trial ethics organizations has greatly improved research ethics efficiencies over the last 2 decades. These organizations enable trial protocol submission to the REB of one collaborating research institute, which subsequently becomes the REB of record, facilitating efficient review by the REBs of the other participating sites in the province. Having such a system active within each province would further improve efficiency. In addition, mechanisms to harmonize across provincial ethics organizations would streamline ethics approvals for multisite studies. This might include the existing provincial ethics organizations’ populating a national operational research ethics committee (perhaps in distinction to a national REB) to encourage common provincial REB submission platforms and streamline submission activities at individual sites. In parallel, agreement on the necessary core elements of a research ethics submission would also facilitate harmonization.
Importantly, we found substantial variability in total start-up time across sites, from 15 to 412 days. Typically, the sponsoring site has greater lead time and strong participation incentive, which ensure rapid start-up. Not surprisingly, we found that community sites often lacked the infrastructure to examine protocols, assess ethics submissions and respond to contracting requests, which led to longer site initiation times. Closing this gap is important because community sites look after the vast majority of Canada’s critically ill patients.6 Template clinical trial agreements developed in consultation with stakeholders would help to meet these infrastructure challenges, which might encourage more community sites to participate in research.
Although this study examined the timelines of only 1 large clinical trial, many other observational studies and clinical trials were carried out during the first phase of the pandemic. Traditional clinical trials assess a single intervention. For each subsequent intervention, a new clinical trial is designed, and the same set of start-up procedures is repeated. Canadian Treatments for COVID-19 is an adaptive-platform trial, meaning that it compares prioritized interventions using a common, durable clinical trial platform that can remain operational throughout the pandemic, with interventions stopped according to efficacy determination, followed by the addition of new interventions. This strategy avoids the need for repeated start-up procedures, as described in the present study. Durable, year-on-year funding for coordinated, centrally operational adaptive-platform trial infrastructure is a necessary evolution in the conduct of clinical trials and learning health care systems, and might lead to improved efficiency in evaluating interventions as well as reduced per-patient costs.9–11
Improved clinical trial infrastructure is essential to the timely and efficient conduct of clinical trials that generate evidence to inform patient care.12,13 Adoption of harmonized clinical trial agreements, greater interprovincial coordination in research ethics review, streamlining of Health Canada regulations for low-risk clinical trials14 and a transition toward funding durable research networks across health care systems in Canada would address many of the challenges currently faced by clinical trials. A more harmonized clinical trials infrastructure would emulate elements of the United Kingdom’s National Institute for Health and Care Research,13,14 which integrates clinical research with clinical care in the UK. An ideal system would be embedded and funded from within the Canadian health care system and would allow shifting of resources to meet the evolving research needs of the system while alleviating the administrative workload on individual research institutes.
Limitations
The strengths of this study include its focus on a pressing contemporary challenge in clinical research. In addition, we were able to collect detailed timeline information from the largest multicentre COVID-19 clinical trial in the Canadian context and estimate the relative importance of various steps in the research pathway. We engaged a relatively large number of community sites in the study, which better reflected the balance of where most Canadians receive health care. Finally, based on our findings, we offer concrete solutions to the challenges highlighted.
This study also has limitations. The study survey relied on site staff to retrospectively recall time points in the clinical trial initiation process. Accordingly, we encountered missing data at some sites for some milestones. Second, site-to-site variations existed in the process itself, which may have affected timelines. For example, 1 site did not require a legal contract, and some, but not all, sites used a coordinated ethics submission system. These factors may have contributed to over- or underestimation of some of the time intervals. Third, the data describe only 1 trial that took place during the first waves of the COVID-19 pandemic, which imparted some unique aspects to study management, including the rapid funding decision from CIHR, prioritization of CATCO by the site sponsor over other studies and diversion of resources toward CATCO. Although our findings may be generalizable to other large investigator-initiated clinical trials, a broader examination across many trials and studies during the pandemic, and beyond the pandemic, might identify additional important barriers.
Conclusion
Randomized controlled trials provide essential evidence to inform treatments; however, they take time to initiate. Even in a pandemic setting, the time required to initiate CATCO, a large clinical trial, was lengthy and varied considerably from site to site. Adoption of model clinical trial agreements, coordination of research ethics submission reviews, and funding of durable clinical platform trials that engage both academic and community hospitals are all potential solutions to the barriers identified in this study.
Supplementary Material
Footnotes
Competing interests: Jennifer Tsang reports a Physicians’ Services Incorporated Foundation grant to her institution, outside the submitted work. She is cochair of the Canadian Community ICU Research Network (CCIRNet) and vice-chair of the Quest Community Health Centre board of directors. Alexandra Binnie reports a Physicians’ Services Incorporated Foundation grant and a Mohawk Medbuy grant to her institution, outside the submitted work. She is cochair of CCIRNet. Srinivas Murthy reports a grant from the Health Research Foundation, Innovative Medicines Canada, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Koren Teo, Robert Fowler, Neill Adhikari and Srinivas Murthy conceived and designed the work, and acquired and analyzed the data. All authors interpreted the data, drafted the manuscript and revised it critically for important intellectual content, approved the final version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by the Canadian Institutes of Health Research (WST-171496).
Data sharing: Data from this study will not be available.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/4/E615/suppl/DC1.
References
- 1.Muller MP, McGeer A, Straus SE, et al. Clinical trials and novel pathogens: lessons learned from SARS. Emerg Infect Dis. 2004;10:389–94. doi: 10.3201/eid1003.030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson CR, Beever D, Challen K, et al. The UK’s pandemic influenza research portfolio: a model for future research on emerging infections. Lancet Infect Dis. 2019;19:e295–300. doi: 10.1016/S1473-3099(18)30786-2. [DOI] [PubMed] [Google Scholar]
- 3.Acharya M. Ebola viral disease outbreak — 2014: implications and pitfalls. Front Public Health. 2014;2:263. doi: 10.3389/fpubh.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali K, Azher T, Baqi M, et al. Canadian Treatments for COVID-19 (CATCO); Association of Medical Microbiology and Infectious Disease Canada (AMMI) Clinical Research Network and the Canadian Critical Care Trials Group. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194:E242–51. doi: 10.1503/cmaj.211698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rishu AH, Marinoff N, Dumitrascu M, et al. Canadian Critical Care Trials Group. Time required to initiate outbreak and pandemic observation research. J Crit Care. 2017;40:7–10. doi: 10.1016/j.jcrc.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsang JL, Fowler R, Cook DJ, et al. How can we increase participation in pandemic research in Canada? Can J Anaesth. 2022;69:293–97. doi: 10.1007/s12630-021-02119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical trials and research in Canada: an overview. Canadian Clinical Trials Coordinating Centre; [accessed 2022 Jan. 22]. Available: https://www.cctcc.ca. [Google Scholar]
- 8.Crow RA, Hart KA, McDermott MP, et al. A checklist for clinical trials in rare disease: obstacles and anticipatory actions — lessons learned from the FORDMD trial. Trials. 2018;19:29. doi: 10.1186/s13063-018-2645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO) Clinicaltrials.gov.: NCT04330690. 2020. [accessed 2022 Jan. 22]. Available: https://clinicaltrials.gov/ct2/show/NCT04330690.
- 10.Houston BL, Lawler PR, Goligher EC, et al. Anti-Thrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC): study design and methodology for an international, adaptive Bayesian randomized controlled trial. Clin Trials. 2020;17:491–500. doi: 10.1177/1740774520943846. [DOI] [PubMed] [Google Scholar]
- 11.Randomised evaluation of COVID-19 therapy. Recoverytrial.net: ISRCTN50189673. 2020. [accessed 2022 Jan. 22]. Available: https://www.recoverytrial.net/
- 12.Murthy S, Fowler RA, Laupacis A. How Canada can better embed randomized trials into clinical care [editorial] CMAJ. 2020;192:E928–9. doi: 10.1503/cmaj.201764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamontagne F, Rowan KM, Guyatt G. Integrating research into clinical practice: challenges and solutions for Canada. CMAJ. 2021;193:E127–31. doi: 10.1503/cmaj.202397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consultation: Health Canada’s Clinical Trials Regulatory Modernization Initiative. Ottawa: Health Canada; 2021. [accessed 2022 Jan. 22]. Available: https://www.canada.ca/en/health-canada/programs/consultation-clinical-trials-regulatory-modernization-initiative.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.