Abstract
Background
Transgender and gender diverse (TGD) individuals experience an incongruence between their assigned birth sex and gender identity. They may have a higher prevalence of health conditions associated with cancer risk than cisgender people.
Aim
To examine the prevalence of several cancer risk factors among TGD individuals compared with cisgender individuals.
Design and setting
A cross-sectional analysis was conducted using data from the UK’s Clinical Practice Research Datalink to identify TGD individuals between 1988–2020, matched to 20 cisgender men and 20 cisgender women on index date (date of diagnosis with gender incongruence), practice, and index age (age at index date). Assigned birth sex was determined from gender-affirming hormone use and procedures, and sex-specific diagnoses documented in the medical record.
Method
The prevalence of each cancer risk factor was calculated and the prevalence ratio by gender identity was estimated using log binomial or Poisson regression models adjusted for age and year at study entry, and obesity where appropriate.
Results
There were 3474 transfeminine (assigned male at birth) individuals, 3591 transmasculine (assigned female at birth) individuals, 131 747 cisgender men, and 131 827 cisgender women. Transmasculine people had the highest prevalence of obesity (27.5%) and ‘ever smoking’ (60.2%). Transfeminine people had the highest prevalence of dyslipidaemia (15.1%), diabetes (5.4%), hepatitis C infection (0.7%), hepatitis B infection (0.4%), and HIV infection (0.8%). These prevalence estimates remained elevated in the TGD populations compared with cisgender persons in the multivariable models.
Conclusion
Multiple cancer risk factors are more prevalent among TGD individuals compared with cisgender individuals. Future research should examine how minority stress contributes to the increased prevalence of cancer risk factors in this population.
Keywords: cancer, health disparities, LGBTQ, morbidity, transgender persons
INTRODUCTION
Transgender and gender diverse (TGD) individuals experience an incongruence between their assigned sex at birth and gender identity. Between 0.1% and 2.0% of the worldwide population identify as TGD.1 Several cancer risk factors, including obesity, alcohol use, exogenous hormone use, smoking, and viral infections are associated with multiple cancer types.2,3 The prevalence of these risk factors among TGD people has not been well characterised.
The minority stress framework4 posits that institutionalised stigma and social norms marginalise TGD individuals resulting in chronic stress.5 For example, 23% of transgender people in the US have stated they avoided seeking necessary medical care in the past year due to discrimination and stigma.6
Individuals reported experiencing harassment from clinicians or refusal of care because of their gender identity. These experiences may lead TGD persons to delay medical care or participate in harmful behaviours that can impact the prevention and treatment of conditions that predispose to cancer. Indeed, studies have shown associations between discrimination against TGD people in health care and increased tobacco use.7,8 Transgender individuals may be more likely to smoke and to have alcohol use disorders (for example, substance misuse and alcohol poisoning) than cisgender individuals.9,10 Additionally, TGD individuals present on average with more comorbidities than cisgender people and may be at a higher risk of most chronic conditions, including obesity and dyslipidaemia.11
The evidence regarding the effects of gender-affirming hormone therapy on long-term health is mixed.12 Gender-affirming hormone therapy can produce physiological and metabolic changes that require monitoring.13–15 Testosterone and oestrogen use has been linked with short-term changes in body mass index (BMI) and lean body mass. Unfavourable changes in lipid composition have been associated with testosterone and oestrogen use,16,17 particularly among transmasculine individuals.18
Much of the literature to date is cross-sectional or limited to TGD individuals on gender-affirming hormone therapy, which overlooks the social and environmental conditions affecting the TGD experience. Furthermore, an understanding of these morbidities in the context of cancer risk is necessary as this population ages. Given these gaps in the literature, this study focused on estimating the prevalence of key cancer risk factors in the TGD community compared with cisgender people.
How this fits in
| Transgender and gender diverse (TGD) individuals experience an incongruence between their assigned sex at birth and gender identity. Little research has been conducted on the prevalence of cancer risk factors among TGD individuals. In this analysis using primary care data it was found that factors such as smoking, alcohol use, obesity, dyslipidaemia, diabetes, and HIV and hepatitis infections are elevated among TGD persons, likely due to the increased stigma and discrimination this population faces. Awareness of the higher prevalence of these risk factors among TGD people can enable GPs to offer more opportunistic patient education and investigation. |
METHOD
A cross-sectional analysis of risk factors for cancer was conducted using the UK’s Clinical Practice Research Datalink (CPRD). CPRD is a longitudinal primary care database that includes patients across participating practices within the UK.19,20 Data was combined from CPRD GOLD (which includes patients from England, Wales, Scotland, and Northern Ireland) and CPRD Aurum (which includes patients from England only).19,20 CPRD is representative of the UK and comparable to the UK census in terms of age, sex, and ethnicity.19,20 There were 7151 TGD individuals diagnosed with gender incongruence (formerly gender identity disorder) from 1988 to 2020 aged ≥18 years using Read and SNOMED codes from GOLD and Aurum, respectively (see Supplementary Tables S1 and S2). The index date was defined as the first occurrence of a gender incongruence diagnosis among TGD individuals. Each TGD person was individually matched to 20 cisgender men and 20 cisgender women from the same medical practice on index age (age at gender incongruence diagnosis, ±1 year) and index year (year of gender incongruence diagnosis, ±1 year). The matched cisgender cohort consisted of 140 983 cisgender men and 141 060 cisgender women. Exclusion criteria were applied to exclude individuals:
with a diagnosis of gender incongruence who were believed to be misclassified cisgender people, such as those taking finasteride for benign prostatic hypertrophy, or individuals taking menopausal hormone therapy after a hysterectomy or mastectomy;
in CPRD Aurum who had been referred to LGBT services only and no other gender incongruence codes with no evidence of gender affirming hormone therapy or surgery (∼1.7% of TGD persons in Aurum);
over the age of 90 years; and
with variations of sex characteristics (formerly disorders of sex development; see Supplementary Tables S3 and S4).
The final analysis population consisted of 6603 TGD adults matched to 263 574 cisgender adults (see Supplementary Figure S1). The cancer risk factors of interest included smoking status (current, former, or never smoker), alcohol use (current, former, or never user), and obesity (BMI ≥30 kg/m2), obtained from the first documentation of the condition closest to the index date. Chronic conditions like HIV infection, hepatitis B infection, hepatitis C infection, dyslipidaemia, and diabetes were based on documentation of diagnosis codes or medications related to the diagnosis closest to the index date.
Statistical analyses
The prevalence of each risk factor by gender identity was estimated using Poisson regression with sandwich estimator for factors with high prevalence (all except for viral infection outcomes) or log binomial regression to yield the prevalence ratio (PR) with a 95% confidence interval (CI).21,22 All models were adjusted for continuous index age and continuous index year. Models were further adjusted for dyslipidaemia and diabetes, and for obesity.
Sex assigned at birth was determined from the medical record based on a combination of gender-affirming hormone therapy and procedures, and sex-specific diagnosis terms (see Supplementary Tables S5–S7). Because the authors were unable to identify the sex assigned at birth for a total of 3725 TGD individuals, multiple imputation was performed for missing values in sex assigned at birth, in addition to missing values for BMI, alcohol use, and smoking status based on height, weight, index age, index year, and all cancer risk factors. Multiple imputation was performed using proc MI in SAS (version 9.4) to create five imputed datasets. PROC SURVEYFREQ and PROC MIANALYZE (SAS, version 9.4) was used to obtain pooled frequencies and proportions. In a sensitivity analysis, frequencies, proportions, and PRs were reported without imputation. The analysis where sex assigned at birth was imputed but individuals with missing smoking use, alcohol use, and/or BMI data were excluded are also reported. All counts less than five are suppressed per CPRD policy to protect privacy. All analyses were carried out in accordance with CPRD guidelines and regulations.
This work is a collaboration between authors who are researchers, community organisers, advocates for, and members of the TGD community. TGD insight was instrumental during the design and execution of this research. This analysis was reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.23
RESULTS
The analysis included 6603 TGD individuals (see Supplementary Table S8) and, following imputation, 3258 TGD persons were categorised as transmasculine and 3345 persons as transfeminine (see Table 1). The matched cisgender cohort consisted of 131 747 cisgender men and 131 827 cisgender women. The mean age at index date for transmasculine people was 30.2 years and the mean age for transfeminine people was 35.6 years.
Table 1.
Characteristics of transgender and gender diverse individuals and cisgender individuals in CPRD 1988–2020 using multiple imputationa
| Characteristic |
n (%)b
|
|||
|---|---|---|---|---|
| Transmasculine people (n = 3258) | Transfeminine people (n = 3345) | Cisgender men (n = 131 747) | Cisgender women (n = 131 827) | |
| Age, mean (SE) | 30.2 (0.22) | 35.6 (0.26) | 32.9 (0.04) | 32.8 (0.04) |
|
| ||||
| Body mass index | ||||
| Underweight/normal | 1600 (49.1) | 1605 (47.9) | 61 543 (46.7) | 67 157 (51.0) |
| Overweight | 773 (23.7) | 1018 (30.4) | 43 435 (33.0) | 33 178 (25.2) |
| Obese | 884 (27.5) | 721 (21.6) | 26 770 (20.3) | 31 492 (23.9) |
|
| ||||
| Smoking Status | ||||
| Never | 1299 (39.9) | 1216 (36.3) | 55 138 (41.9) | 60 650 (46.0) |
| Former | 980 (30.1) | 1001 (30.0) | 33 083 (25.1) | 37 156 (28.2) |
| Current | 980 (30.1) | 1128 (33.7) | 43 527 (33.0) | 34 021 (25.8) |
|
| ||||
| Alcohol use | ||||
| Never | 484 (14.9) | 328 (9.8) | 13967 (10.6) | 17 522 (13.3) |
| Former | 278 (8.5) | 193 (5.8) | 5484 (4.2) | 7556 (5.7) |
| Current | 2496 (76.6) | 2824 (84.4) | 112 296 (85.2) | 106 750 (81.0) |
|
| ||||
| Dyslipidaemia | ||||
| Yes | 280 (8.6) | 507 (15.1) | 14 839 (11.3) | 11 127 (8.4) |
| No | 2978 (91.4) | 2838 (84.8) | 116 908 (88.7) | 120 700 (91.6) |
|
| ||||
| Diabetes | ||||
| Yes | 150 (4.6) | 179 (5.4) | 5732 (4.4) | 5359 (4.1) |
| No | 3108 (95.4) | 3166 (94.6) | 126 015 (95.6) | 126 468 (95.9) |
|
| ||||
| Hepatitis C infection | ||||
| Yes | 13 (0.4) | 22 (0.7) | 459 (0.3) | 258 (0.2) |
| No | 3245 (99.6) | 3323 (99.3) | 131 288 (99.7) | 131 569 (99.8) |
|
| ||||
| Hepatitis B infection | ||||
| Yes | 9 (0.3) | 15 (0.4) | 372 (0.3) | 313 (0.2) |
| No | 3249 (99.7) | 3330 (99.6) | 131 375 (99.7) | 131 514 (99.8) |
|
| ||||
| HIV infection | ||||
| Yes | 18 (0.5) | 28 (0.8) | 317 (0.2) | 173 (0.1) |
| No | 3240 (99.4) | 3317 (99.2) | 131 430 (99.8) | 131 654 (99.9) |
Numbers and percentages may not add up or round to the total due to the imputation procedure.
Unless otherwise stated. CPRD = Clinical Practice Research Datalink.
Transmasculine people had the highest prevalence of obesity (27.5%) but the lowest prevalence of current alcohol use (76.6%). Transfeminine individuals had the highest prevalence of current smoking (33.7%), dyslipidaemia (15.1%), and diabetes (5.4%). HIV infection was higher among transmasculine individuals (0.5%) and transfeminine individuals (0.8%), compared with cisgender men (0.2%) and cisgender women (0.1%) (Table 1).
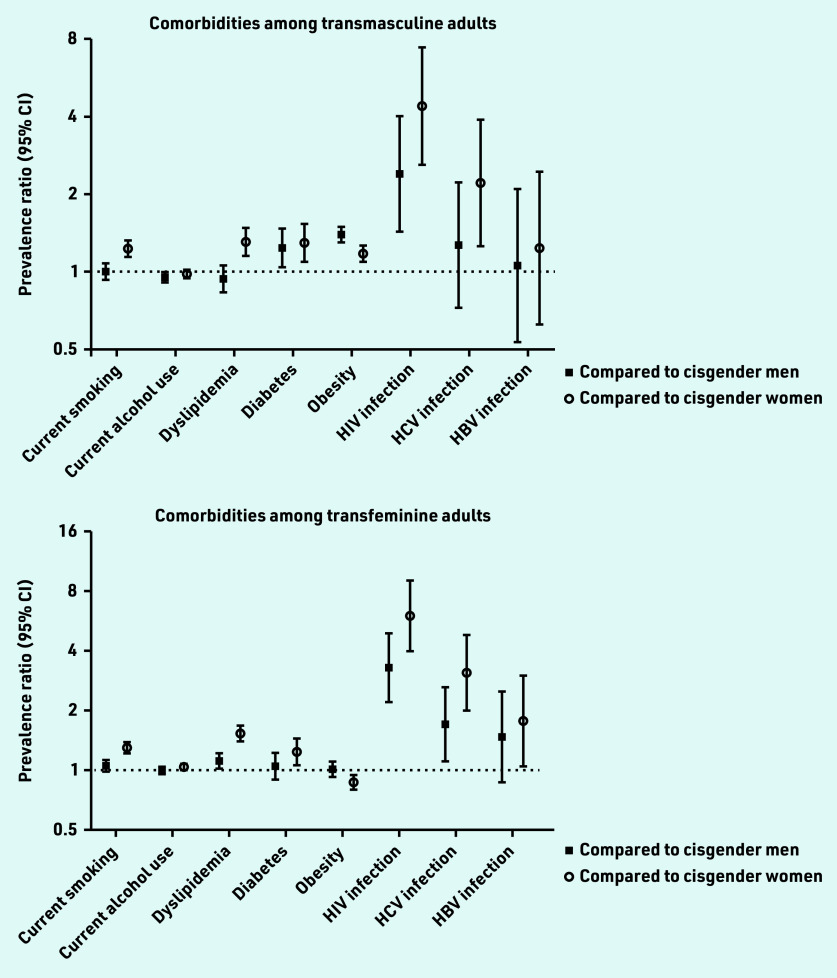
In the multivariable models, obesity was elevated for transmasculine individuals compared with cisgender men (PR 1.39; 95% CI = 1.30 to 1.49) and cisgender women (PR 1.17; 95% CI = 1.09 to 1.26) (see Table 2). Transfeminine adults had a lower prevalence of obesity than cisgender women (PR 0.88; 95% CI = 0.80 to 0.95) but the same prevalence as cisgender men (PR 1.02; 95% CI = 0.93 to 1.11). Transmasculine adults had a higher prevalence of dyslipidaemia compared with cisgender women (PR 1.31; 95% CI = 1.15 to 1.48), but not compared with cisgender men (PR 0.94; 95% CI = 0.83 to 1.06). Transfeminine adults had elevated prevalence of dyslipidaemia compared with cisgender men (PR 1.12; 95% CI = 1.02 to 1.22) and cisgender women (PR 1.53; 95% CI = 1.40 to 1.68). Diabetes prevalence was elevated for transmasculine adults compared with cisgender men (PR 1.24; 95% CI = 1.04 to 1.47) and cisgender women (PR 1.29; 95% CI = 1.09 to 1.53). Also, transfeminine adults showed elevated diabetes prevalence compared with cisgender women (PR 1.24; 95% CI = 1.06 to 1.45), but not compared with cisgender men (PR 1.05; 95% CI = 0.90 to 1.23).
Table 2.
Prevalence ratios of cancer risk factors for transgender and gender diverse individuals compared with cisgender individuals in the UK’s Clinical Practice Research Datalink (with imputation)
| Outcome | Transmasculine people versus cisgender men, PR (95% CI) | Transmasculine people versus cisgender women, PR (95% CI) | Transfeminine people versus cisgender men, PR (95% CI) | Transfeminine people versus cisgender women, PR (95% CI) |
|---|---|---|---|---|
| Obesity (BMI ≥30 kg/m2)a | 1.39 (1.30 to 1.49) | 1.17 (1.09 to 1.26) | 1.02 (0.93 to 1.11) | 0.88 (0.80 to 0.95) |
| Current smokera | 1.00 (0.93 to 1.08) | 1.23 (1.14 to 1.32) | 1.05 (0.99 to 1.13) | 1.30 (1.22 to 1.39) |
| Former smokera | 1.27 (1.18 to 1.35) | 1.21 (1.13 to 1.30) | 1.11 (1.04 to 1.18) | 1.11 (1.04 to 1.19) |
| Current alcohol usea | 0.95 (0.91 to 0.99) | 0.98 (0.94 to 1.02) | 1.00 (0.96 to 1.04) | 1.04 (1.00 to 1.08) |
| Former alcohol usea | 1.33 (1.17 to 1.51) | 1.27 (1.12 to 1.44) | 1.13 (0.97 to 1.32) | 1.15 (0.99 to 1.35) |
| Dyslipidaemiaa | 0.94 (0.83 to 1.06) | 1.31 (1.15 to 1.48) | 1.12 (1.02 to 1.22) | 1.53 (1.40 to 1.68) |
| Diabetesa,b | 1.24 (1.04 to 1.47) | 1.29 (1.09 to 1.53) | 1.05 (0.90 to 1.23) | 1.24 (1.06 to 1.45) |
| HIV infectionc | 2.40 (1.43 to 4.02) | 4.41 (2.60 to 7.45) | 3.29 (2.20 to 4.91) | 6.02 (3.98 to 9.12) |
| Hepatitis C infectionc | 1.27 (0.72 to 2.23) | 2.21 (1.25 to 3.91) | 1.71 (1.11 to 2.63) | 3.10 (2.00 to 4.82) |
| Hepatitis B infectionc | 1.05 (0.53 to 2.08) | 1.23 (0.62 to 2.44) | 1.48 (0.87 to 2.50) | 1.78 (1.05 to 3.01) |
Prevalence ratios were estimated using multiple imputation to impute missing values for sex assigned at birth, BMI, smoking, and alcohol use with log-binomial regression adjusted for age, index year, and index practice.
Models also adjusted for obesity.
Prevalence ratios were estimated using multiple imputation to impute missing values for sex assigned at birth, BMI, smoking, and alcohol use with Poisson regression with a robust variance estimator adjusted for age, index year, and index practice.
BMI = body mass index. PR = prevalence ratio.
Compared with cisgender women, current smoking was elevated for both transmasculine individuals (PR 1.23; 95% CI = 1.14 to 1.32) and transfeminine individuals (PR 1.30; 95% CI = 1.22 to 1.39). Compared with cisgender men, there was no difference in the current smoking prevalence for either transfeminine adults (PR 1.05; 95% CI = 0.99 to 1.13) or transmasculine adults (PR 1.00; 95% CI = 0.93 to 1.08) (Table 2).
For former smoking, transfeminine people showed elevated prevalence compared with cisgender men (PR 1.11; 95% CI = 1.04 to 1.18) and cisgender women (PR 1.11; 95% CI = 1.04 to 1.19). Transmasculine people were also more likely to be former smokers than cisgender men (PR 1.27; 95% CI = 1.18 to 1.35) and cisgender women (PR 1.21; 95% CI = 1.13 to 1.30) (Table 2).
Compared with cisgender men, transmasculine adults were less likely to be current alcohol users (PR 0.95; 95% CI = 0.91 to 0.99), but transfeminine adults were not (PR 1.00; 95% CI = 0.96 to 1.04). Transmasculine individuals (PR 0.98; 95% CI = 0.94 to 1.02) and transfeminine individuals (PR 1.04; 95% CI = 1.00 to 1.08) were just as likely to be current drinkers as cisgender women. Transmasculine individuals were more likely to be former drinkers than cisgender men (PR 1.33; 95% CI = 1.17 to 1.51) and cisgender women (PR 1.27; 95% CI = 1.12 to 1.44). Transfeminine individuals were just as likely to be former drinkers compared with cisgender men (PR 1.13; 95% CI = 0.97 to 1.32) and cisgender women (PR 1.15; 95% CI = 0.99 to 1.35) (Table 2).
Compared with cisgender men, transmasculine people (PR 2.40; 95% CI = 1.43 to 4.02) and transfeminine people (PR 3.29; 95% CI = 2.20 to 4.91) had an elevated prevalence of HIV infection. Compared with cisgender women, there was an increased prevalence of HIV infection among transmasculine people (PR 4.41; 95% CI = 2.60 to 7.45) and transfeminine people (PR 6.02; 95% CI = 3.98 to 9.12) (Table 2).
Among transmasculine people, hepatitis C infection was two times higher compared with cisgender women (PR 2.21; 95% CI = 1.25 to 3.91), but not elevated compared with cisgender men (PR 1.27; 95% CI = 0.72 to 2.23). The prevalence of hepatitis C infection was three times higher for transfeminine people compared with cisgender women (PR 3.10; 95% CI = 2.00 to 4.82), and almost two times higher compared with cisgender men (PR 1.71; 95% CI = 1.11 to 2.63). Hepatitis B prevalence for transmasculine individuals was not elevated compared with cisgender women (PR 1.23: 95% CI = 0.62 to 2.44) or compared with cisgender men (PR 1.05; 95% CI = 0.53 to 2.08). Transfeminine adults had an elevated prevalence of hepatitis B infection compared with cisgender women (PR 1.78; 95% CI = 1.05 to 3.01), but not compared with cisgender men (PR 1.48; 95% CI = 0.87 to 2.50) (Table 2).
In sensitivity analyses, where the prevalence and prevalence ratios were calculated without imputing assigned birth sex (see Supplementary Table S9) and individuals with missing smoking, alcohol, and BMI information were removed (see Supplementary Table S10), the results did not materially differ.
DISCUSSION
Summary
In this large analysis using primary care data, it was found that there is an increased prevalence of cancer risk factors among TGD individuals. Transmasculine individuals showed an elevated prevalence of obesity, smoking, dyslipidaemia, and hepatitis C infection compared with cisgender women and an elevated prevalence of obesity, current alcohol use, diabetes, and HIV infection compared with cisgender men. Transfeminine individuals showed elevated prevalence of smoking, dyslipidaemia, diabetes, and hepatitis B, hepatitis C, and HIV infections compared with cisgender women, but a decreased prevalence of obesity.
Transfeminine people also showed elevated dyslipidaemia, hepatitis C infection, and HIV infection compared with cisgender men. These findings suggest that minority stress due to stigma and discrimination, in addition to factors like hormone use, may increase comorbidity risk.
Figure 1.
Prevalence ratios for common comorbidities among transmasculine and transfeminine adults compared with cisgender men and cisgender women.
Strengths and limitations
A strength of this study is the inclusion of TGD individuals using diagnosis codes for gender incongruence and not exclusive to those receiving gender-affirming care such that the results may be more generalisable to the wider TGD community engaged in health care. However, as TGD people are less likely to engage with the medical system than cisgender people,6–8,24 these results may not be generalisable to all TGD adults in the UK. Diagnosis codes and medications were used to define many of the risk factors of interest, which are likely to be more accurate than self-report.
A limitation of this study is that TGD identity was inferred based on gender incongruence codes and assumed sex assigned at birth through electronic medical record information, as opposed to self-reported gender identity as is the gold standard.25 Relying on diagnosis codes for gender incongruence may miss people who have not disclosed to their providers or who do not seek medical transition.
Furthermore, the prevalence ratios may be inflated if clinicians were more likely to ascertain health history from TGD persons than cisgender persons due to ascertainment bias. The prevalence of some conditions may have been underestimated by using diagnosis codes and medications rather than laboratory values.26 Lastly, the authors were unable to adjust for important confounders, including socioeconomic status, race/ethnicity, income level, physical activity, and immigration status due to the lack of documentation of these factors in CPRD.
In this article, the terms transmasculine (assigned female at birth) and transfeminine (assigned male at birth) have been used to best represent the potential gender diversity captured by the study’s inclusion criteria. Terminology used by clinical and TGD populations can vary, with the most widely accepted terms often being introduced and upheld by the latter.27 This, along with the study's intention to balance inclusivity and specificity, forms the rationale for the choice of terminology. Due to the nature of the CPRD data it is difficult to reliably identify and report on non-binary identities. The authors recognise this limitation and acknowledge that this should be addressed in future research.
Comparison with existing literature
Current literature suggests that transmasculine individuals experience an increase in body mass related to hormone therapy.16,17,28 However, the increased prevalence of obesity among this population compared with cisgender individuals may also involve social and environmental factors such as living in poverty or reduced physical activity.29,30 Changes to lipid profiles shortly after initiating gender-affirming hormone therapy have been documented in transfeminine and transmasculine adults and adolescents.18,31 Specifically unfavourable changes, such as an increase in total cholesterol, triglycerides, and low-density lipoprotein cholesterol levels along with a decrease in high-density lipoprotein cholesterol levels was observed for transmasculine individuals.18 However, these changes may not necessarily equate to poor cardiovascular outcomes.
A systematic review of TSG individuals on gender-affirming hormone therapy, showed very few deaths due to stroke and myocardial infarction, especially among transgender men.32 The present study found higher diabetes prevalence among TGD individuals compared with cisgender individuals; however, studies from Europe and the US have found no increase in diabetes prevalence among TGD populations.33–35
Previous studies have shown higher tobacco use among TGD individuals compared with cisgender individuals.9,11,36 In this study, the cohort includes patients seeking primary health care, many of whom are seeking gender-affirming care that may require cessation of smoking.37 Regarding alcohol use, a decreased prevalence of self-reported alcohol use was observed among Veterans Health Administration TSG patients in the US; however, the same study also found an increase in alcohol use disorder diagnoses relative to cisgender patients.10 Likewise, Hughto et al11 showed that TGD individuals had a higher prevalence of alcohol use disorder diagnoses compared with cisgender people. It is possible that the findings from the present study that former alcohol use is elevated among transmasculine individuals reflects abstinence following an alcohol use disorder diagnosis.
This study also found that the prevalence of HIV infection was two to six times higher among TGD individuals than cisgender individuals. Additionally, hepatitis C infection was two to three times higher among TGD individuals compared with cisgender women. Increased prevalence, incidence, and diagnosis of HIV infection may be due to engaging in condomless sexual intercourse, possibly in the context of survival sex work and/or injection drug use.38–40 The population included in the present study had a low overall prevalence of HIV compared with national estimates of 0.46–4.78 per 1000 TSG persons,41 likely due to the study population being engaged with primary care and potentially more aware of prevention measures. However, these data suggest that the HIV and hepatitis C epidemics for TGD persons, particularly transfeminine individuals, are still ongoing and that targeted interventions are needed to reduce the number of newly acquired infections each year.
Implications for research and practice
Chronic health conditions may be increased among TGD patients for a number of reasons, including, but not limited to, minority stress due to societal discrimination and stigma. Chronic stress from institutionalised stigma and social norms results in TGD individuals’ rejection of healthcare needs as a priority, resulting in worse health outcomes.24,42,43 GPs should be aware of the increased risk of chronic conditions among TGD patients to provide proper prevention and treatment. For example, the increased prevalence of smoking and alcohol use among TGD patients in this study cohort suggests that harm reduction or cessation counselling in primary care settings may significantly benefit TGD patients.
Additionally, GPs should be aware that a significant amount of discrimination occurs in healthcare settings, with more than half of TGD people reporting avoiding going to a doctor when feeling unwell.24 A 2021 survey of almost 700 TSG individuals in the UK found that 70% experienced transphobia in medical settings and 14% reported being refused health care (of any kind) by a GP for being transgender.24 These instances were more common for non-binary and Black people and people of colour.24 Consequently, TGD individuals may delay addressing their healthcare needs in the face of this stigma and stress, resulting in worse health outcomes. GP practices may wish to undertake additional training for all staff to address discrimination.44–46 Awareness of delayed presentations by TGD people may enable GPs to offer more opportunistic patient education or investigation when patients do present.
This analysis of TGD individuals in primary care found an elevated prevalence of at least one risk factor for cancer, including viral infections, such as HIV, as well as diseases of metabolic origin, like obesity, diabetes, and dyslipidaemia. Reasons impacting the presence of these risk factors may include social and environmental determinants of health that remain underaddressed in this population. Further longitudinal research is required to elucidate the factors driving the increase of these morbidities and if these factors result in increases in diseases like cancer in this population.
Acknowledgments
The authors thank Emily Carver, BS, and David Ruggieri, BS, from Information Management Services Inc. (Calverton, MD).
Funding
This study was funded by the Intramural Research Program of the National Cancer Institute.
Ethical approval
This study is based on data from the February 2020 Clinical Practice Research Datalink (CPRD) GOLD and Aurum database releases (obtained under license from the UK Medicines and Healthcare products Regulatory Agency; the data are provided by patients and collected by the NHS as part of their care and support). The interpretation and conclusions of this analysis are those of the authors alone. GPs do not require patient consent when sharing data with CPRD, though patients have the option of opting out, as CPRD data are anonymised. This study was approved by the CPRD Independent Scientific Advisory Committee (proposal #19_177) and exempted from full institutional review board review by the National Institutes of Health Office of Human Subjects Research.
Data
The data that support the findings of this study are available from CPRD, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available with permission of CPRD.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Goodman M, Adams N, Corneil T, et al. Size and distribution of transgender and gender nonconforming populations: a narrative review. Endocrinol Metab Clin North Am. 2019;48(2):303–321. doi: 10.1016/j.ecl.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Lewandowska AM, Rudzki M, Rudzki S, et al. Environmental risk factors for cancer — review paper. Ann Agric Environ Med. 2019;26(1):1–7. doi: 10.26444/aaem/94299. [DOI] [PubMed] [Google Scholar]
- 3.Yıldırım-Kahrıman S. Non-intrinsic cancer risk factors. Exp Oncol. 2021;43(4):290–297. doi: 10.32471/exp-oncology.2312-8852.vol-43-no-4.16804. [DOI] [PubMed] [Google Scholar]
- 4.Meyer IH. Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull. 2003;129(5):674–697. doi: 10.1037/0033-2909.129.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan KKH, Treharne GJ, Ellis SJ, et al. Gender minority stress: a critical review. J Homosex. 2020;67(10):1471–1489. doi: 10.1080/00918369.2019.1591789. [DOI] [PubMed] [Google Scholar]
- 6.Grant JM, Mottet LA, Tanis J, et al. Injustice at every turn: a report of the national transgender discrimination survey. 2011. https://transequality.org/sites/default/files/docs/resources/NTDS_Report.pdf (accessed 7 Jun 2023).
- 7.Ruben MA, Livingston NA, Berke DS, et al. Lesbian, gay, bisexual, and transgender veterans’ experiences of discrimination in health care and their relation to health outcomes: a pilot study examining the moderating role of provider communication. Health equity. 2019;3(1):480–488. doi: 10.1089/heq.2019.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reisner SL, Pardo ST, Gamarel KE, et al. Substance use to cope with stigma in healthcare among US female-to-male trans masculine adults. LGBT Health. 2015;2(4):324–332. doi: 10.1089/lgbt.2015.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchting FO, Emory KT, Scout Kim Y, et al. Transgender use of cigarettes, cigars, and e-cigarettes in a national study. Am J Prev Med. 2017;53(1):e1–e7. doi: 10.1016/j.amepre.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams EC, Frost MC, Rubinsky AD, et al. Patterns of alcohol use among transgender patients receiving care at the veterans health administration: overall and relative to nontransgender patients. J Stud Alcohol Drugs. 2021;82(1):132–141. doi: 10.15288/jsad.2021.82.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughto JMW, Quinn EK, Dunbar MS, et al. Prevalence and co-occurrence of alcohol, nicotine, and other substance use disorder diagnoses among US transgender and cisgender adults. JAMA Netw Open. 2021;4(2):e2036512. doi: 10.1001/jamanetworkopen.2020.36512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.T’Sjoen G, Arcelus J, Gooren L, et al. Endocrinology of transgender medicine. Endocr Rev. 2018;40(1):97–117. doi: 10.1210/er.2018-00011. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SS, Nambiar KZ, O’Callaghan S, Berner AM. Understanding the role of sex hormones in cancer for the transgender community. Trends Cancer. 2022;8(4):273–275. doi: 10.1016/j.trecan.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal SM. Challenges in the care of transgender and gender-diverse youth: an endocrinologist’s view. Nat Rev Endocrinol. 2021;17(10):581–591. doi: 10.1038/s41574-021-00535-9. [DOI] [PubMed] [Google Scholar]
- 16.Wierckx K, Van Caenegem E, Schreiner T, et al. Cross sex hormone therapy in trans persons is safe and effective at short time follow up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11(8):1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 17.Kyinn M, Banks K, Leemaqz SY, et al. Weight gain and obesity rates in transgender and gender-diverse adults before and during hormone therapy. Int J Obes (Lond) 2021;45(12):2562–2569. doi: 10.1038/s41366-021-00935-x. [DOI] [PubMed] [Google Scholar]
- 18.Cocchetti C, Castellini G, Iacuaniello D, et al. Does gender-affirming hormonal treatment affect 30-year cardiovascular risk in transgender persons? A two-year prospective European study (ENIGI) J Sex Med. 2021;18(4):821–829. doi: 10.1016/j.jsxm.2021.01.185. [DOI] [PubMed] [Google Scholar]
- 19.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44(3):827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf A, Dedman D, Campbell J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol. 2019;48(6):1740–1740. doi: 10.1093/ije/dyz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Qian L, Shi J, Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18(1):63. doi: 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TransActual Trans lives survey 2021: enduring the UK’s hostile environment. 2021. https://www.transactual.org.uk/trans-lives-21 (accessed 7 Jun 2023).
- 25.Tordoff DM, Morgan J, Dombrowski JC, et al. Increased ascertainment of transgender and non-binary patients using a 2-step versus 1-step gender identity intake question in an STD clinic setting. Sex Transm Dis. 2019;46(4):254–259. doi: 10.1097/OLQ.0000000000000952. [DOI] [PubMed] [Google Scholar]
- 26.Persson R, Vasilakis-Scaramozza C, Hagberg KW, et al. CPRD Aurum database: Assessment of data quality and completeness of three important comorbidities. Pharmacoepidemiol Drug Saf. 2020;29(11):1456–1464. doi: 10.1002/pds.5135. [DOI] [PubMed] [Google Scholar]
- 27.TransActual Trans health: information for healthcare professionals. https://www.transactual.org.uk/healthcare-professionals (accessed 7 Jun 2023).
- 28.Klaver M, de Blok CJM, Wiepjes CM, et al. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178(2):163–171. doi: 10.1530/EJE-17-0496. [DOI] [PubMed] [Google Scholar]
- 29.VanKim NA, Erickson DJ, Eisenberg ME, et al. Weight-related disparities for transgender college students. Health Behav Policy Rev. 2014;1(2):161–171. doi: 10.14485/HBPR.1.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conron KJ, Scott G, Stowell GS, Landers SJ. Transgender health in Massachusetts: results from a household probability sample of adults. Am J Public Health. 2012;102(1):118–122. doi: 10.2105/AJPH.2011.300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millington K, Finlayson C, Olson-Kennedy J, et al. Association of high-density lipoprotein cholesterol with sex steroid treatment in transgender and gender-diverse youth. JAMA Pediatr. 2021;175(5):520–521. doi: 10.1001/jamapediatrics.2020.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maraka S, Singh Ospina N, Rodriguez-Gutierrez R, et al. Sex steroids and cardiovascular outcomes in transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017;102(11):3914–3923. doi: 10.1210/jc.2017-01643. [DOI] [PubMed] [Google Scholar]
- 33.Meyer IH, Brown TNT, Herman JL, et al. Demographic characteristics and health status of transgender adults in select US regions: behavioral risk factor surveillance system, 2014. Am J Public Health. 2017;107(4):582–589. doi: 10.2105/AJPH.2016.303648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Velzen D, Wiepjes C, Nota N, et al. Incident diabetes risk is not increased in transgender individuals using hormone therapy. J Clin Endocrinol Metab. 2021;107(5):e2000–e2007. doi: 10.1210/clinem/dgab934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nokoff NJ, Scarbro S, Juarez-Colunga E, et al. Health and cardiometabolic disease in transgender adults in the United States: behavioral risk factor surveillance system 2015. J Endocr Soc. 2018;2(4):349–360. doi: 10.1210/js.2017-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawyer AN, Bono RS, Kaplan B, Breland AB. Nicotine/tobacco use disparities among transgender and gender diverse adults: findings from wave 4 PATH data. Drug Alcohol Depend. 2022;232:109268. doi: 10.1016/j.drugalcdep.2022.109268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. 2nd edn. 2016. https://transcare.ucsf.edu/sites/transcare.ucsf.edu/files/Transgender-PGACG-6-17-16.pdf (accessed 7 Jun 2023).
- 38.Crepaz N, Hess KL, Purcell DW, Hall HI. Estimating national rates of HIV infection among MSM, persons who inject drugs, and heterosexuals in the United States. AIDS. 2019;33(4):701–708. doi: 10.1097/QAD.0000000000002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Operario D, Soma T, Underhill K. Sex work and HIV status among transgender women: systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2008;48(1):97–103. doi: 10.1097/QAI.0b013e31816e3971. [DOI] [PubMed] [Google Scholar]
- 40.Baral SD, Poteat T, Strömdahl S, et al. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. [DOI] [PubMed] [Google Scholar]
- 41.Kirwan PD, Hibbert M, Kall M, et al. HIV prevalence and HIV clinical outcomes of transgender and gender-diverse people in England. HIV Med. 2021;22(2):131–139. doi: 10.1111/hiv.12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poteat T, German D, Kerrigan D. Managing uncertainty: a grounded theory of stigma in transgender health care encounters. Soc Sci Med. 2013;84:22–29. doi: 10.1016/j.socscimed.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 43.Hendricks ML, Testa RJ. A conceptual framework for clinical work with transgender and gender nonconforming clients: an adaptation of the Minority Stress Model. Prof Psychol Res Pr. 2012;43(5):460–467. [Google Scholar]
- 44.Royal College of General Practitioners. LGBT health hub. https://elearning.rcgp.org.uk/course/view.php?id=584 (accessed 7 Jun 2023).
- 45.LGBT Foundation Pride in practice. https://lgbt.foundation/how-we-can-help-you/pride-in-practice (accessed 7 Jun 2023).
- 46.Live Through This. Support for professionals. Support for people working with LGBTIQ+ people affected by cancer. https://livethroughthis.co.uk/professional-support (accessed 7 Jun 2023).