Abstract
Background:
Lung cancer has been the leading cause of cancer-related deaths worldwide for many years. This study aimed to investigate the global patterns and trends of lung cancer.
Methods:
Lung cancer incidence and mortality were derived from the GLOBOCAN 2020 database. Continuous data from Cancer Incidence in Five Continents Time Trends were used to analyze the temporal trends from 2000 to 2012 using Joinpoint regression, and average annual percent changes were calculated. The association between the Human Development Index and lung cancer incidence and mortality was assessed by linear regression.
Results:
An estimated 2.2 million new lung cancer cases and 1.8 million lung cancer-related deaths occurred in 2020. The age-standardized incidence rate (ASIR) ranged from 36.8 per 100,000 in Demark to 5.9 per 100,000 in Mexico. The age-standardized mortality rate (ASMR) varied from 32.8 per 100,000 in Poland to 4.9 per 100,000 in Mexico. Both ASIR and ASMR were approximately twice higher in men than in women. The ASIR of lung cancer showed a downward trend in the United States of America (USA) between 2000 and 2012, and was more prominent in men. The age-specific incidence rates of lung cancer for ages of 50 to 59 years showed an upward trend in China for both men and women.
Conclusions:
The burden of lung cancer is still unsatisfactory, especially in developing countries like China. Considering the effectiveness of tobacco control and screening in developed countries, such as the USA, there is a need to strengthen health education, accelerate the establishment of tobacco control policies and regulations, and improve early cancer screening awareness to reduce the future burden of lung cancer.
Keywords: Lung neoplasms, Incidence, Mortality, Early detection of cancer, China, United States
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths globally. According to GLOBOCAN 2020, an estimated 2.2 million new lung cancer cases (11.4%) and almost 1.8 million lung cancer deaths (18.0%) occurred in 2020.[1] The burden of lung cancer varies among different countries mainly because of divergent risk factors, such as the prevalence of smoking, environmental pollution, and even dietary habits.[2] In the United States of America (USA), the incidence of lung cancer has gradually declined since 1990,[3] due to the effective tobacco control policies and regulations. However, some developing countries, including China, exhibit a lower incidence but higher mortality from lung cancer. The reason for such patterns includes incomplete early cancer screening, poor medical conditions, and challenges with tobacco control policies and regulations.[4]
Therefore, this study aimed to describe and compare country-specific morbidity and mortality of lung cancer rates in 2020, and analyze the incidence trend in major countries.
Methods
Data sources
The incidence and mortality of lung cancer were derived from the GLOBOCAN 2020 database (https://gco.iarc.fr/).[5] It is a web-based interactive platform that provides the International Agency for Research on Cancer (IARC) global cancer statistics for 36 cancer types in 185 countries or territories, supplying information for cancer control and research.
To analyze the temporal trends of lung cancer incidence, we extracted cancer incidence data from the Cancer in Five Continents Time Trends (CI5plus) database,[6] which contains high-quality population-based cancer registries, including continuous incidence data from 108 countries or territories or subnational population-based cancer registries.
The Human Development Index (HDI) is a comprehensive index based on the three basic variables of “education level, life expectancy and quality of life” according to the following formula[7,8]: HDI = (IEducation × IHealth × IIncome)1/3. Based on the principle of the Human Development Statistic of the United Nations Development Programme,[9] countries were categorized into four levels according to HDI values (very high HDI, ≥0.800; high HDI, 0.700–0.799; medium HDI, 0.550–0.699; and low HDI, <0.550).
In this study, the International Classification of Diseases 10th revision data code C33–34 was derived from the overall cancer database to analyze the lung cancer incidence and mortality rates.
Selected countries
The 20 countries with the highest ASIRs of lung cancer defined by the United Nations Population Division were selected to assess lung cancer incidence and mortality in 2020. A representative sample of five countries (China, South Korea, the USA, the United Kingdom, and Australia) on four continents were selected and they had complete and continuous data for analyzing the incidence trends from 2000 to 2012.
Statistical analysis
The ASIR and ASMR were calculated from Segi's world standard population.[10] Lung cancer incidence and mortality in 2020 were grouped by country, sex, and age. We used Joinpoint regression (version 4.3.1.0, National Cancer Institute, USA) to analyze temporal trends in lung cancer incidence and mortality for five continents and calculated the average annual percent change (AAPC). The Z test was used to examine whether the average annual percent change has shown upward or downward trends and was statistically different from zero. The association of lung cancer ASIR and ASMR with HDI was detected by linear regression. P values <0.05 were considered statistically significant.
Results
Lung cancer incidence and mortality rates in 2020
An estimated 2.2 million new lung cancer cases and 1.8 million lung cancer-related deaths occurred in 2020, accounting for approximately 11.4% of the total cancer cases and 18.0% of the total cancer deaths worldwide. The crude incidence and mortality of lung cancer were 28.3 and 23.0 per 100,000, respectively. The ASIR and ASMR were 22.4 and 18.0 per 100,000, respectively [Tables 1 and 2].
Table 1.
The incidence of lung cancer among the world's major countries/HDI categories in 2020.
| Total | Male | Female | |||||||
| Country/HDI category | Cases | Crude rate (per 100,000) | ASIR (per 100,000) | Cases | Crude rate (per 100,000) | ASIR (per 100,000) | Cases | Crude rate (per 100,000) | ASIR (per 100,000) |
| Total | 2,206,771 | 28.3 | 22.4 | 1,435,943 | 36.5 | 31.5 | 770,828 | 19.9 | 14.6 |
| Asia | 1,315,136 | 28.3 | 22.9 | 891,898 | 37.6 | 32.7 | 423,238 | 18.7 | 14.0 |
| China | 815,563 | 56.3 | 34.8 | 539,181 | 72.7 | 47.8 | 276,382 | 39.2 | 22.8 |
| Japan | 138,532 | 109.5 | 32.1 | 94,487 | 153.0 | 47.0 | 44,045 | 68.1 | 19.5 |
| South Korea | 28,651 | 55.9 | 25.5 | 19,797 | 77.1 | 39.1 | 8854 | 34.6 | 14.8 |
| India | 72,510 | 5.3 | 5.4 | 51,675 | 7.2 | 7.8 | 20,835 | 3.1 | 3.1 |
| Iran | 10,465 | 12.5 | 12.6 | 7184 | 16.9 | 16.9 | 3281 | 7.9 | 8.1 |
| Europe | 477,534 | 63.8 | 29.4 | 315,054 | 87.1 | 43.6 | 162,480 | 42.0 | 18.1 |
| United Kingdom | 51,983 | 76.6 | 32.3 | 26,943 | 80.3 | 35.2 | 25,040 | 72.9 | 29.9 |
| Germany | 64,804 | 77.3 | 31.9 | 38,436 | 92.8 | 39.2 | 26,368 | 62.2 | 25.7 |
| Italy | 41,953 | 69.4 | 25.3 | 28,369 | 96.4 | 36.0 | 13,584 | 43.8 | 16.4 |
| France | 48,299 | 74.0 | 34.9 | 31,941 | 101.1 | 49.1 | 16,358 | 48.6 | 22.7 |
| Poland | 29,509 | 78.0 | 36.2 | 18,277 | 99.7 | 51.5 | 11,232 | 57.6 | 24.6 |
| Denmark | 5047 | 87.1 | 36.8 | 2399 | 83.3 | 36.9 | 2648 | 90.9 | 36.8 |
| Africa | 45,988 | 3.4 | 6.2 | 33,282 | 5.0 | 9.8 | 12,706 | 1.9 | 3.2 |
| South Africa | 8950 | 15.1 | 18.3 | 6144 | 21.0 | 29.6 | 2806 | 9.3 | 10.0 |
| Egypt | 6538 | 6.4 | 8.0 | 4851 | 9.4 | 12.8 | 1687 | 3.3 | 3.8 |
| Oceania | 16,975 | 39.8 | 24.0 | 9277 | 43.4 | 27.4 | 7698 | 36.1 | 21.0 |
| Australia | 13,162 | 51.6 | 25.3 | 7208 | 56.8 | 28.7 | 5954 | 46.5 | 22.3 |
| New Zealand | 2425 | 50.3 | 24.9 | 1164 | 49.1 | 24.7 | 1261 | 51.4 | 25.0 |
| America | 351,138 | 34.3 | 21.9 | 186,432 | 37.0 | 25.5 | 164,706 | 31.8 | 19.0 |
| USA | 227,875 | 68.8 | 33.1 | 116,335 | 71.0 | 36.3 | 111,540 | 66.7 | 30.4 |
| Mexico | 7588 | 5.9 | 5.3 | 4503 | 7.1 | 7.0 | 3085 | 4.7 | 4.0 |
| Argentina | 12,110 | 26.8 | 19.2 | 7738 | 35.1 | 28.1 | 4372 | 18.9 | 12.3 |
| Brazil | 40,409 | 19.0 | 14.3 | 23,162 | 22.2 | 18.4 | 17,247 | 16.0 | 11.1 |
| Canada | 25,574 | 67.8 | 28.9 | 12,706 | 67.8 | 30.4 | 12,868 | 67.7 | 27.7 |
| Cuba | 6689 | 59.1 | 28.5 | 4077 | 72.5 | 36.9 | 2612 | 45.8 | 21.2 |
| HDI | |||||||||
| High HDI | 1,047,707 | 36.0 | 26.2 | 697,411 | 47.5 | 37.1 | 350,296 | 24.3 | 16.5 |
| Very high HDI | 975,665 | 62.4 | 29.9 | 611,867 | 79.1 | 41.1 | 363,798 | 46.0 | 20.6 |
| Medium HDI | 165,943 | 7.1 | 8.0 | 116,316 | 9.8 | 11.5 | 49,627 | 4.4 | 4.6 |
| Low HDI | 16,418 | 1.7 | 3.5 | 9713 | 2.0 | 4.5 | 6705 | 1.4 | 2.6 |
ASIR was calculated by using world Segi's standard population. ASIR: Age-standardized incidence rate; HDI: Human Development Index; USA: The United States of America.
Table 2.
The mortality of lung cancer among the world's major countries/HDI categories in 2020.
| Total | Male | Female | |||||||
| Country/HDI category | Deaths | Crude rate (per 100,000) | ASMR (per 100,000) | Deaths | Crude rate (per 100,000) | ASMR (per 100,000) | Deaths | Crude rate (per 100,000) | ASMR (per 100,000) |
| World | 1,796,144 | 23.0 | 18.0 | 1,188,679 | 30.2 | 25.9 | 607,465 | 15.7 | 11.2 |
| Asia | 1,112,517 | 24.0 | 19.3 | 757,218 | 31.9 | 27.8 | 355,299 | 15.7 | 11.6 |
| China | 714,699 | 49.4 | 30.2 | 471,546 | 63.6 | 41.8 | 243,153 | 34.5 | 19.7 |
| Japan | 82,369 | 65.1 | 14.7 | 57,334 | 92.8 | 24.2 | 25,035 | 38.7 | 7.1 |
| South Korea | 20,505 | 40.0 | 16.5 | 15,147 | 59.0 | 28.9 | 5358 | 20.9 | 7.4 |
| India | 66,279 | 4.8 | 4.9 | 47,701 | 6.7 | 7.2 | 18,578 | 2.8 | 2.8 |
| Iran | 9071 | 10.8 | 10.9 | 6229 | 14.7 | 14.6 | 2842 | 6.8 | 7.0 |
| Europe | 384,176 | 51.3 | 22.6 | 260,019 | 71.9 | 34.9 | 124,157 | 32.1 | 12.9 |
| United Kingdom | 36,518 | 53.8 | 20.8 | 19,109 | 57.0 | 23.1 | 17,409 | 50.7 | 18.9 |
| Germany | 50,282 | 60.0 | 23.1 | 31,664 | 76.5 | 30.6 | 18,618 | 43.9 | 16.8 |
| Italy | 33,602 | 55.6 | 18.0 | 22,792 | 77.4 | 26.3 | 10,810 | 34.8 | 11.4 |
| France | 37,095 | 56.8 | 25.4 | 25,214 | 79.8 | 37.2 | 11,881 | 35.3 | 15.4 |
| Poland | 27,444 | 72.5 | 32.8 | 17,461 | 95.2 | 48.4 | 9983 | 51.2 | 21.2 |
| Denmark | 3927 | 67.8 | 27.0 | 2032 | 70.6 | 29.3 | 1895 | 65.1 | 25.2 |
| Africa | 41,171 | 3.1 | 5.6 | 29,760 | 4.4 | 8.9 | 11,411 | 1.7 | 2.9 |
| South Africa | 7730 | 13.0 | 15.8 | 5280 | 18.1 | 25.4 | 2450 | 8.1 | 8.7 |
| Egypt | 5817 | 5.7 | 7.2 | 4319 | 8.4 | 11.5 | 1498 | 3.0 | 3.4 |
| Oceania | 12,012 | 28.1 | 16.1 | 6902 | 32.3 | 19.5 | 5110 | 24.0 | 13.2 |
| Australia | 8867 | 34.8 | 15.8 | 5139 | 40.5 | 19.1 | 3728 | 29.1 | 12.9 |
| New Zealand | 1924 | 39.9 | 18.4 | 965 | 40.7 | 19.1 | 959 | 39.1 | 18.0 |
| America | 246,268 | 24.1 | 14.9 | 134,780 | 26.7 | 18.0 | 111,488 | 21.5 | 12.3 |
| USA | 138,225 | 41.8 | 18.9 | 73,009 | 44.6 | 21.9 | 65,216 | 39.0 | 16.4 |
| Mexico | 7100 | 5.5 | 4.9 | 4304 | 6.8 | 6.6 | 2796 | 4.2 | 3.6 |
| Argentina | 10,729 | 23.7 | 16.8 | 6881 | 31.2 | 24.8 | 3848 | 16.6 | 10.7 |
| Brazil | 35,160 | 16.5 | 12.3 | 19,743 | 18.9 | 15.5 | 15,417 | 14.3 | 9.8 |
| Canada | 21,362 | 56.6 | 22.5 | 10,907 | 58.2 | 24.4 | 10,455 | 55.0 | 21.2 |
| Cuba | 6173 | 54.5 | 25.9 | 3876 | 68.9 | 34.7 | 2297 | 40.3 | 18.3 |
| HDI | |||||||||
| High HDI | 918,661 | 31.6 | 22.8 | 610,626 | 41.6 | 32.5 | 308,035 | 21.4 | 14.3 |
| Very high HDI | 711,630 | 45.5 | 20.6 | 462,513 | 59.8 | 30.0 | 249,117 | 31.5 | 12.9 |
| Medium HDI | 149,887 | 6.4 | 7.2 | 106,011 | 8.9 | 10.6 | 43,876 | 3.9 | 4.1 |
| Low HDI | 15,108 | 1.5 | 3.2 | 8987 | 1.8 | 4.2 | 6121 | 1.2 | 2.4 |
ASMR was calculated by using world Segi's standard population. ASMR: Age-standardized mortality rate; HDI: Human Development Index; USA: The United States of America.
Regarding the continents, more than half of the lung cancer cases and 61.9% of mortality occurred in Asia in 2020, with ASIR and ASMR of 22.9 and 32.7 per 100,000, respectively. Asia was followed by Europe, which had 21.6% of cases and 21.3% of deaths, and the highest ASIR (29.4 per 100,000) and ASMR (22.6 per 100,000). Africa had the lowest ASIR (6.2 per 100,000) and ASMR (5.6 per 100,000).
For countries, China had the largest number of lung cancer cases and mortality (37.0% and 39.8%, respectively) in 2020, followed by America (10.3% of cases and 7.7% of mortality) and Japan (6.3% of cases and 4.6% of mortality). Both the ASIR and ASMR among countries varied over six folds. The highest ASIR ranged from 36.8 per 100,000 in Denmark to 5.3 per 100,000 in Mexico, while the highest ASMR ranged from 32.8 per 100,000 in Poland to 4.9 per 100,000 in Mexico. The ASIR and ASMR in Demark, Poland, France, China, and the USA were much higher than the global ASIR (22.4 vs. 18.0 per 100,000), whereas the ASIR and ASMR in Iran, Egypt, India, and Mexico were lower than the global ASIR [Tables 1 and 2].
The highest ASIR for both men vs. women occurred in Poland (51.5 vs. 24.6 per 100,000), followed by France (49.1 vs. 30.4 per 100,000) and China (47.8 vs. 29.9 per 100,000), while the lowest ASIR was observed in Mexico (7.0 vs. 4.0 per 100,000) [Table 1]. The highest ASMR in men and women occurred in Poland (48.4 per 100, 000) and Demark (25.2 per 100,000), respectively. The lowest ASMR in men and women was observed in Mexico (6.6 per 100,000) and India (2.8 per 100,000), respectively [Table 2].
Association of HDI with ASIR and ASMR of lung cancer
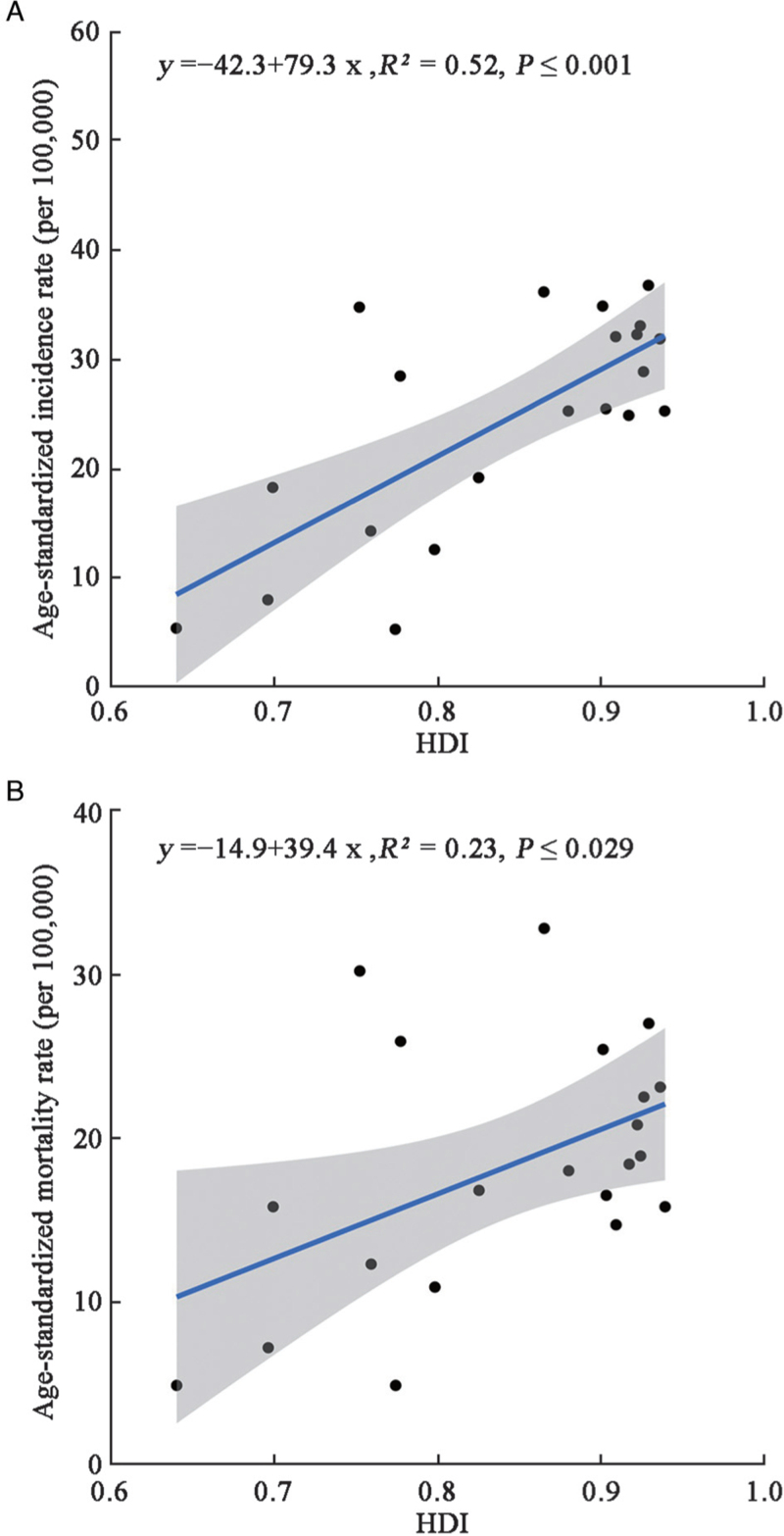
Over 90% of lung cancer cases and 77.1% of lung cancer-related deaths occurred in countries with very high and high HDI. Less than 10% of lung cancer cases and deaths occurred in countries with medium and low HDI. ASIR and ASMR were over three times higher in countries with very high/high HDI than in countries with medium/low HDI [Tables 1 and 2]. The association of ASIR and ASMR with HDI was statistically significant [Figure 1].
Figure 1.
Association between HDI and age-standardized incidence (A) and mortality (B) rates of lung cancer. HDI: Human Development Index.
Temporal trends of lung cancer incidence
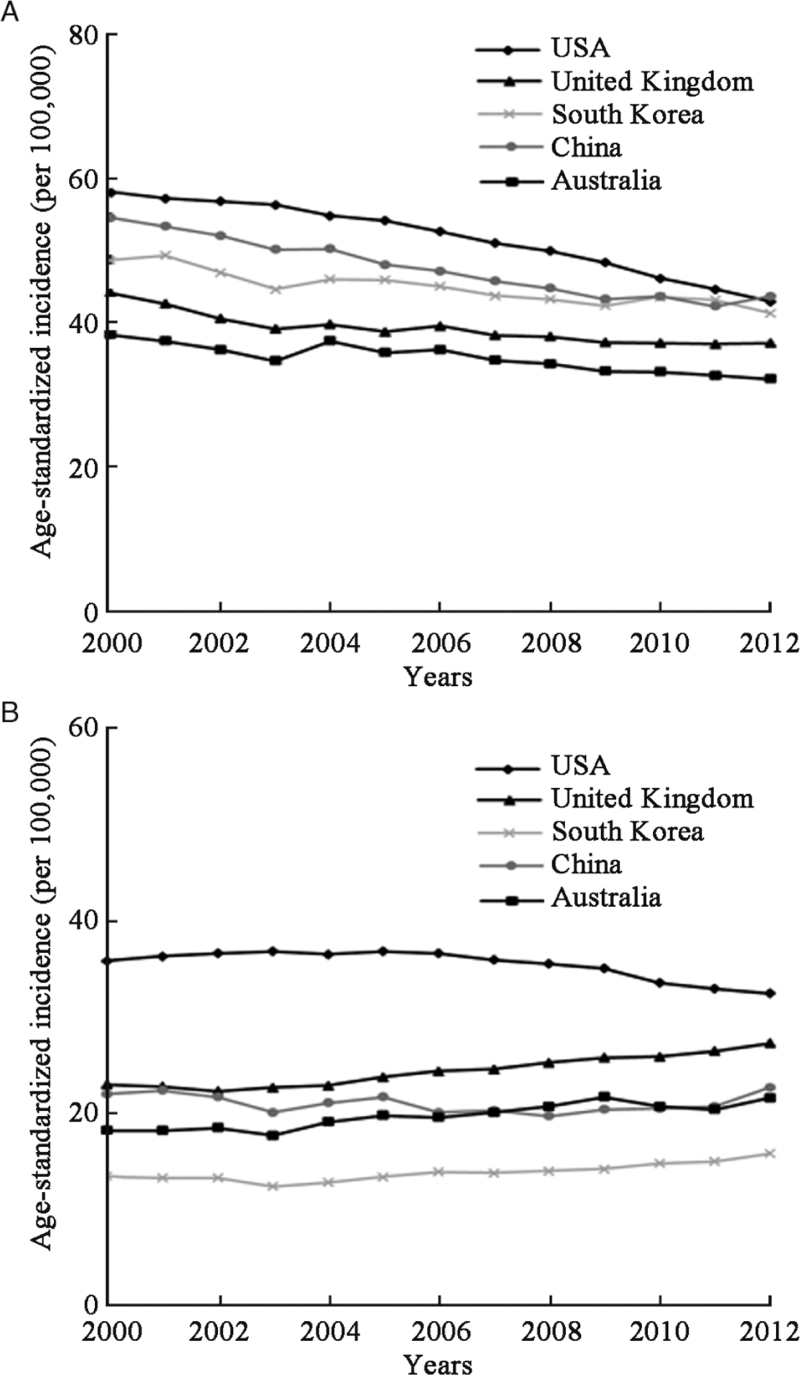
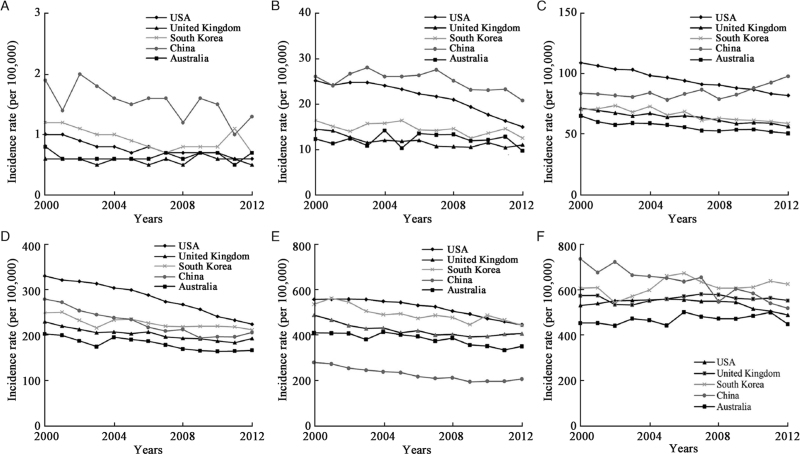
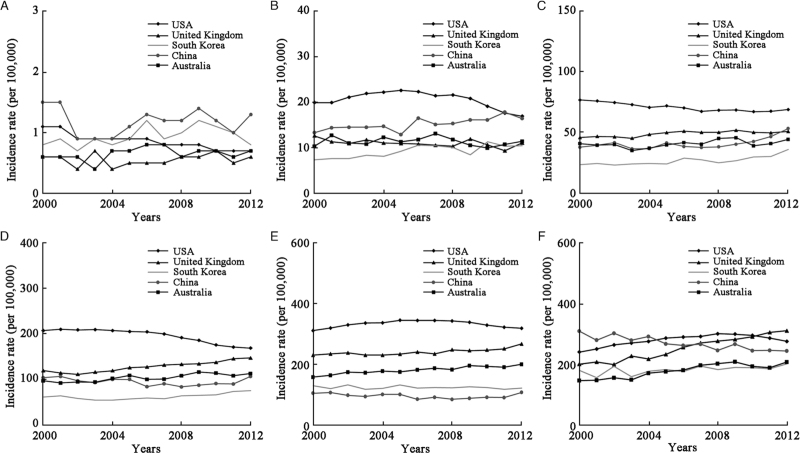
The ASIR of lung cancer in men showed a consistent downward trend in South Korea (AAPC = −1.2%, P < 0.001), Australia (AAPC = −1.3%, P < 0.001), the United Kingdom (AAPC = −1.5%, P < 0.001), China (AAPC = −2.0%, P < 0.001), and the USA (AAPC = −2.5%, P < 0.001) [Table 3 and Figure 2]. For age-specific rates, the greatest decrease occurred in men younger than 40 years in South Korea (AAPC = −3.4%, P = 0.008) and China (AAPC = −3.2%, P = 0.011), and the greatest downward trends were observed in the USA (AAPC = −4.1%, P < 0.001) and the United Kingdom (AAPC = −2.7%, P = 0.003) in age group 40–49 years [Table 3 and Figure 3]. In women, the ASIR of lung cancer showed upward trends in Australia (AAPC = 1.6%, P < 0.001), the United Kingdom (AAPC = 1.4%, P < 0.001), and South Korea (AAPC = 1.1%, P < 0.001), and downward trends in the USA (AAPC = −0.9%, P < 0.001). The largest increases in women were observed in the United Kingdom at age of ≥80 years (AAPC = 4.1%, P < 0.001) and in South Korea at age of 40 to 49 years (AAPC = 3.2%, P < 0.001); the greatest decrease in trends were noted in the USA (AAPC = −3.6%, P < 0.001) [Table 3 and Figure 4]. Detailed information on the trend analyses is provided in [Supplementary Tables 1 and 2].
Table 3.
Trends for age-standardized lung cancer incidence rates by countries, 2000–2012 (%).
| China | South Korea | USA | Australia | United Kingdom | ||||||
| Age groups (years) | AAPC (95% CI) | P value | AAPC (95% CI) | P value | AAPC (95% CI) | P value | AAPC (95% CI) | P value | AAPC (95% CI) | P value |
| Males | ||||||||||
| Total | −2.0∗ (−2.4, −1.5) | <0.001 | −1.2∗ (−1.6, −0.9) | <0.001 | −2.5∗ (−2.8, −2.2) | <0.001 | −1.3∗ (−1.7, −0.9) | <0.001 | −1.5∗ (−1.9, −1.1) | <0.001 |
| <40 | −3.2∗ (−5.5, −0.9) | 0.011 | −3.4∗ (−5.6, −1.1) | 0.008 | −3.9∗ (−4.9, −3.0) | <0.001 | −0.3 (−2.3, 1.8) | 0.780 | −0.2 (−2.0, 1.5) | 0.765 |
| 40–49 | −1.6∗ (−3.1, −0.2) | 0.029 | −1.6∗ (−2.7, −0.5) | 0.008 | −4.1∗ (−4.8, −3.4) | <0.001 | −0.3 (−2.1, 1.6) | 0.760 | −2.7∗ (−4.5, −0.9) | 0.003 |
| 50–59 | 1.5∗ (0.2, 2.8) | 0.026 | −1.8∗ (−2.3, −1.2) | <0.001 | −2.4∗ (−2.5, −2.2) | <0.001 | −1.7∗ (−2.2, −1.3) | <0.001 | −1.8∗ (−2.1, −1.5) | <0.001 |
| 60–69 | −2.6∗ (−3.4, −1.9) | <0.001 | −1.1∗ (−1.7, −0.6) | 0.001 | −3.2∗ (−3.4, −2.9) | <0.001 | −1.7∗ (−2.4, −1.1) | <0.001 | −1.6∗ (−1.9, −1.2) | <0.001 |
| 70–79 | −2.9∗ (−3.4, −2.3) | <0.001 | −1.6∗ (−2.2, −1.0) | <0.001 | −1.9∗ (−2.1, −1.6) | <0.001 | −1.6∗ (−2.2, −1.0) | <0.001 | −1.5∗ (−2.5, −0.6) | 0.002 |
| ≥80 | −2.6∗ (−3.3, −1.9) | <0.001 | 0.6 (−0.3, 1.5) | 0.175 | −0.8∗ (−1.2, −0.3) | <0.001 | 0.5 (−0.1, 1.2) | 0.117 | −0.4 (−1.4, 0.6) | 0.466 |
| Females | ||||||||||
| Total | −0.1 (−1.3, 1.1) | 0.883 | 1.1∗ (0.5, 1.8) | <0.001 | −0.9∗ (−1.2, −0.7) | <0.001 | 1.6∗ (1.1, 2.1) | <0.001 | 1.4∗ (1.0, 1.8) | <0.001 |
| <40 | −1.9 (−8.3, 4.9) | 0.569 | 2.0 (−0.4, 4.6) | 0.100 | −3.6∗ (−4.4, −2.8) | <0.001 | 1.7 (−1.1, 4.6) | 0.219 | 0.9 (−2.1, 4.0) | 0.516 |
| 40–49 | 1.8∗ (0.9, 2.8) | 0.002 | 3.2∗ (1.8, 4.7) | <0.001 | −1.4∗ (−2.5, −0.3) | 0.010 | −0.4 (−1.8, 1.0) | 0.564 | −1.1∗ (−2.1, −0.1) | 0.036 |
| 50–59 | 2.6∗ (0.6, 4.6) | 0.010 | 2.8∗ (1.7, 4.1) | <0.001 | −1.0∗ (−1.4, −0.6) | <0.001 | 1.0 (−0.1, 2.1) | 0.080 | 1.0∗ (0.2, 1.7) | 0.015 |
| 60–69 | −0.2 (−2.4, 2.0) | 0.844 | 1.3∗ (0.1, 2.6) | 0.036 | −1.9∗ (−2.2, −1.6) | <0.001 | 1.7∗ (1.0, 2.4) | <0.001 | 1.7∗ (0.6, 2.9) | 0.004 |
| 70–79 | −1.2∗ (−2.0, −0.5) | 0.004 | −0.3 (−1.0, 0.3) | 0.247 | 0.1 (−0.6, 0.8) | 0.769 | 1.7∗ (1.3, 2.1) | <0.001 | 0.9∗ (0.5, 1.3) | <0.001 |
| ≥80 | −1.9∗ (−2.4, −1.3) | <0.001 | 1.2∗ (0.2, 2.2) | 0.027 | 1.2∗ (0.7, 1.7) | <0.001 | 3.2∗ (2.2, 4.1) | <0.001 | 4.1∗ (3.5, 4.7) | <0.001 |
Average annual percent change during 2000 to 2012 is significantly different from zero (P < 0.05). ASIR was calculated by using world Segi's standard population.
AAPC: Average annual percent change; ASIR: Age-standardized incidence rate; CI: Confidence interval; USA: The United States of America.
Figure 2.
Trends for age-standardized incidence of lung cancer in (A) males and (B) females between 2000 and 2012.
Figure 3.
Trends for age-specific incidence of male lung cancer between 2000 and 2012. (A) 0–39 years; (B) 40–49 years; (C) 50–59 years; (D) 60–69 years; (E) 70–79 years; (F) ≥80 years.
Figure 4.
Trends for age-specific incidence of female lung cancer between 2000 and 2012. (A) 0–39 years; (B) 40–49 years; (C) 50–59 years; (D) 60–69 years; (E) 70–79 years; (F) ≥80 years.
Discussion
In this study, we aimed to analyze the trends of incidence and mortality rates of lung cancer from 2000 to 2012. Our findings revealed that the burden of lung cancer is still unsatisfactory, especially in developing countries like China. The overwhelming cause of the high incidence of lung cancer is smoking,[11] both active and passive.[12] Smoking was prevalent in western developed countries such as European countries, North America, Australia, Japan, and South Korea, earlier in their history, and reached its peak in the middle of the last century. As such, these countries had a heavier burden of lung cancer. Subsequently, the prevalence of smoking among men decreased with the introduction of tobacco control policies in these countries, and the incidence of lung cancer in men gradually declined,[13] which is consistent with the trend observed in this study.
However, the burden of lung cancer in women in these developed countries (United Kingdom, South Korea, and Australia) have reached a plateau but gradually experienced an upward trend, except for the USA. This indicates that smoking among women is still prevalent in developed countries. The heavy burden of lung cancer in China is partly due to the large population. China has the largest number of smokers in the world and consumes about 40% of the world's tobacco every year. Moreover, exposure to secondhand smoke is also notable. Approximately 70% of Chinese people are exposed to secondhand smoking every year, which leads to approximately 60,000 lung cancer deaths. China signed the WHO Framework Convention on Tobacco Control in 2003, and the Chinese government has issued a series of management regulations (regulations on the control of smoking in public places) regarding tobacco control and has vigorously highlighted the dangers of smoking in public media. This has resulted in a significant decline in the prevalence of male lung cancer in recent years. Although these regulations have resulted in positive effects, the efforts to control tobacco are not sufficient, regarding raising tobacco taxes or prices.[14,15]
Another important high-risk factor for lung cancer is ambient air pollution. A number of studies in Europe and America found that the concentration of particulate matter (PM) in the environment, especially PM ≤2.5 μm (PM2.5), is closely related to the risk of lung cancer.[16–18] Therefore, outdoor air pollution was listed as a human carcinogen by the IARC in 2013.[19] Ambient air pollution is also one of the reasons for the high incidence of lung cancer in developing countries such as China, India, and Egypt. With the rapid development of Chinese industrialization and transportation, especially with the preponderant use of coal and petroleum, a large number of harmful substances have been discharged into the atmosphere.[20] The main causes of urban air pollution include the unreasonable energy consumption structure and low utilization rate. In 2012, China first proposed to monitor PM2.5 in key areas such as the Beijing–Tianjin–Hebei region, municipalities directly under the central government, and provincial capital cities. In 2015, it was proposed that all prefecture-level cities conduct the monitoring of PM2.5. The Chinese government has been committed to promoting energy conservation and emission reduction, and has formulated corresponding measures. In 2020, China announced the national goal of reaching a carbon peak by 2030 and becoming carbon neutral by 2060.
Another factor contributing to the high incidence of lung cancer is indoor air pollution, including soot from household burning, heating, or cooking. This might explain why the incidence of lung cancer among non-smoking East Asian women, including China and South Korea, remains high, especially between the ages of 40 and 59 years.[21,22]
Lung cancer is a malignant tumor with a notably poor prognosis. Often, it is initially asymptomatic and typically discovered at advanced stages. Thus, people are paying more attention to the early screening of lung cancer. A number of studies in Europe and America have shown that low-dose computed tomography (CT) for high-risk individuals can help in the early detection of cancer.[23,24] The National Comprehensive Cancer Network, European Society of Radiology/European Respiratory Society, and the National Cancer Center of China have successively issued guidelines for lung cancer screening, and recommend risk assessments for people with smoking history, radon exposure, cancer history, family history of lung cancer in first-degree relatives, and disease history (chronic obstructive pulmonary disease or pulmonary fibrosis). Lung cancer screening experts recommend low-dose CT examinations for high-risk groups. Early diagnosis and treatment can reduce the death rate of lung cancer.[25–29] This explains why the incidence of lung cancer is higher in developed countries than in developing countries, but the mortality rate is lower.
Studies showed that the 5-year survival rate of lung cancer in China was 18.6% in 2000, but increased to only 19.8% in 2010.[30] In comparison, the 5-year survival rate for lung cancer in 2010 was 32.9% in Japan, 21.2% in the USA, and 25.1% in South Korea.[31] The improvement in the survival rate of lung cancer is not only related to cancer screening but also to the medical services available in a country and the pathological subtype of lung cancer. Lung cancer has traditionally been divided into two categories, non-small cell lung cancer (NSCLC) and small cell lung cancer, with the former accounting for about 85% of cases. Lung adenocarcinoma is the most common pathological subtype.[32] Studies have shown that approximately 10% to 15% of NSCLC patients in Europe and 30% to 35% in Asia have epidermal growth factor receptor (EGFR) gene mutation.[33,34] Approximately 60% of Asian, non-smoking female adenocarcinoma patients harbor the EGFR gene mutation.[35,36] A series of studies showed that patients with stage IV NSCLC had EGFR-sensitive mutations and recorded significantly improved progression-free survival and overall survival using epidermal growth factor receptor-tyrosine kinase inhibitor compared with patients who received standard chemotherapy.[37–39] Squamous cell lung cancer is insensitive to chemotherapy and radiotherapy, and only responds to surgery. Small cell lung cancer has the highest degree of malignancy and the worst prognosis, with frequent recurrence and distant metastasis. These two pathological subtypes offer fewer options for the previously described systemic treatments. However, recently, lung cancer had entered the era of immunotherapy, and specific populations are likely to benefit from immunotherapy regardless of the subtype of lung cancer.[40–42] In all, despite the advancements in treatment methods, medical environment, or medical insurance systems, such advancements may be beyond the reach of many developing countries or poor areas.
This study had some limitations. First, the data of this study came from public databases, which lack detailed information such as pathological subtypes, disease stages, and treatment processes. If these data can be combined with trend analysis, they should provide more value for public health and clinical practice. Second, data in the CI5plus database were only available up to 2012. Thus, we only analyzed the time trends between 2000 and 2012.
In conclusion, the burden of lung cancer is still notable globally, and it varies across different countries. The incidence of lung cancer in men showed decreasing trends among the five selected countries, while the incidence showed slightly upward trends in women in South Korea, Australia, and the United Kingdom. Effective tobacco control, strengthening of health education, improving awareness regarding early cancer screening, and the optimization of medical conditions, especially for women, will help to curb the notable lung cancer burden.
Funding
This work was supported by a grant from CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-012).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, Zhang Z, Gao T, Zhang Y, Li L. Global burden and trends of lung cancer incidence and mortality. Chin Med J 2023;136:1583–1590. doi: 10.1097/CM9.0000000000002529
Supplemental digital content is available for this article.
References
- 1.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Chin Med J 2021; 7:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Miller KD, Ma J, Siegel R, Fedewa SA, Islami F, et al. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med 2018; 378:1999–2009. doi: 10.1056/NEJMoa1715907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-Year trends in smoking-related mortality in the United States. N Engl J Med 2013; 368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss PE, Strasser-Weippl K, Lee-Bychkovsky BL, Fan L, Li J, Chavarri-Guerra Y, et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol 2014; 15:489–538. doi: 10.1016/S1470-2045(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 5.Global cancer observatory: Cancer today. Lyon: International Agency for Research on Cancer, 2020. Available from: https://gco.iarc.fr/today. [Assessed March 5, 2022]. [Google Scholar]
- 6.Ferlay J, Colombet M, Bray F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9. 2018; Lyon: IARC, Available from: http://ci5.iarc.fr. [Assessed March 5, 2022]. [Google Scholar]
- 7.United Nations Development Programme. Human Development Report 2018: Human Development for Everyone. New York, NY: United Nations Development Programme; 2018. [Google Scholar]
- 8.Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun 2021; 41:1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations Development Programme Human Development Reports. Available from: http://hdr.undp.org/en. [Accessed March 6, 2022]. [Google Scholar]
- 10.Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer 1967; 2:269–279. doi: 10.1002/ijc.2910020310. [DOI] [PubMed] [Google Scholar]
- 11.Fukumoto K, Ito H, Matsuo K, Tanaka H, Yokoi K, Tajima K, et al. Cigarette smoke inhalation and risk of lung cancer: a case-control study in a large Japanese population. Eur J Cancer Prev 2015; 24:195–200. doi: 10.1097/CEJ.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 12.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum 2004; 83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 13.Jemal A, Thun MJ, Ries LAG, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu L, Cohen JE, Hoe C, Yang T, Wu D. Male smoking reduction behaviour in response to China's 2015 cigarette tax increase. Tob Control 2020; 29:405–411. doi: 10.1136/tobaccocontrol-2019-055053. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Ma C, Xi B. Tobacco control in China: still a long way to go. Lancet 2016; 387:1375–1376. doi: 10.1016/S0140-6736(16)30080-0. [DOI] [PubMed] [Google Scholar]
- 16.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol 2013; 14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 17.Hystad P, Demers PA, Johnson KC, Carpiano RM, Brauer M. Long-term residential exposure to air pollution and lung cancer risk. Epidemiology 2013; 24:762–772. doi: 10.1097/EDE.0b013e3182949ae7. [DOI] [PubMed] [Google Scholar]
- 18.Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long-term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med 2013; 187:1226–1233. doi: 10.1164/rccm.201210-1758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loomis D, Grosse Y, Lauby-Secretan B, Ghissassi FE, Bouvard V, Benbrahim-Tallaa L, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol 2013; 14:1262–1263. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- 20.Luo PF, Lin P, Zhou JY. Progress on epidemiological studies of the relationship between lung cancer and ambient air pollution (in Chinese). China Cancer 2017; 26:792–797. doi:10.11735/j.issn.1004-0242.2017.10.A009. [Google Scholar]
- 21.Kim C, Gao YT, Xiang YB, Barone-Adesi F, Zhang Y, Hosgood HD, et al. Home kitchen ventilation, cooking fuels, and lung cancer risk in a prospective cohort of never smoking women in Shanghai, China. Int J Cancer 2015; 136:632–638. doi: 10.1002/ijc.29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner MC, Andersen ZJ, Baccarelli A, Diver WR, Gapstur SM, Pope CA, 3rd, et al. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin 2020; 70:460–479. doi: 10.3322/caac.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Iersel CA, de Koning HJ, Draisma G, Mali WP, Scholten ET, Nackaerts K, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007; 120:868–874. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]
- 24.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, et al. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med 2015; 191:1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 25.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low dose computed tomographic screening. N Engl J Med 2011; 365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol 2019; 14:1732–1742. doi: 10.1016/j.jtho.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019; 30:1162–1169. doi: 10.1093/annonc/mdz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 29.De Koning H, Van Der Aalst C, Ten Haaf K, Oudkerk M. PL02.05 Effects of volume CT lung cancer screening: mortality results of the NELSON randomised controlled population based trial. J Thorac Oncol 2018; 13:S185.doi: 10.1016/j.jtho.2018.08.012. [Google Scholar]
- 30.Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018; 6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 31.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, et al. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. JAMA Oncol 2019; 5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6:244–285. doi: 10.1513/pats.201107-042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida K, Yatabe Y, Park JY, Shimizu J, Horio Y, Matsuo K, et al. Prospective validation for prediction of gefitinib sensitivity by epidermal growth factor receptor gene mutation in patients with nonsmall cell lung cancer. J Thorac Oncol 2007; 2:22–28. [PubMed] [Google Scholar]
- 35.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004; 64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 36.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba I, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 37.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012; 30:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 38.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 39.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 40.Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 2019; 37:2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol 2021; 39:723–733. doi: 10.1200/JCO.20.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 2021; 39:619–630. doi: 10.1200/JCO.20.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.