PURPOSE
To assess the efficacy and safety of darolutamide maintenance after successful taxane chemotherapy in patients with metastatic castration-resistant prostate cancer (mCRPC).
PATIENTS AND METHODS
Swiss Group for Clinical Cancer Research (SAKK) 08/16 is a randomized phase II study. Patients with mCRPC who received prior androgen-receptor pathway inhibitors (ARPIs) and subsequently had nonprogressive disease on a taxane were randomly assigned to darolutamide 600 mg twice a day or placebo twice a day. The primary end point was radiographic progression-free survival (rPFS) at 12 weeks. Secondary end points were rPFS, event-free survival, overall survival (OS), prostate-specific antigen (PSA) 50% response rate, and adverse events.
RESULTS
Overall, 92 patients were recruited by 26 centers. Prior taxane was docetaxel in 93% and cabazitaxel in 7%. Prior ARPI was abiraterone in 60%, enzalutamide in 31%, and both in 9%. rPFS at 12 weeks was significantly improved with darolutamide (64.7% v 52.2%; P = .127). Median rPFS on darolutamide was 5.5 versus 4.5 months on placebo (hazard ratio [HR], 0.54 [95% CI, 0.32 to 0.91]; P = .017), and median event-free survival was 5.4 versus 2.9 months (HR, 0.46 [95% CI, 0.29 to 0.73]; P = .001). PSA 50% response rate was improved (22% v 4%; P = .014). Median OS for darolutamide was 24 versus 21.3 months for placebo (HR, 0.62 [95% CI, 0.3 to 1.26]; P = .181). Treatment-related adverse events were similar in both arms.
CONCLUSION
SAKK 08/16 met its primary end point, showing that switch maintenance with darolutamide after prior taxane chemotherapy and at least one ARPI resulted in a statistically significant but clinically modest rPFS prolongation with good tolerability. The median OS with darolutamide maintenance appears promising. Should these findings be confirmed in a larger trial, maintenance treatment could be a novel strategy in managing patients with mCRPC, especially those who responded well to prior ARPI.
INTRODUCTION
Several new agents have recently been introduced for treating patients with metastatic castration-resistant prostate cancer (mCRPC).1 The optimal sequence is still unclear. Yet, for most patients, therapy with docetaxel and one of the novel androgen-receptor pathway inhibitors (ARPIs) is recommended.2,3 Both docetaxel and ARPIs (abiraterone and enzalutamide) were shown to be associated with improved overall survival (OS) as first-line therapy in patients with mCRPC who had previously received androgen-deprivation therapy (ADT) alone for metastatic hormone-sensitive prostate cancer (mHSPC).4-7 Abiraterone and enzalutamide improved OS in patients with progressive mCRPC after docetaxel, whereas no studies prospectively evaluated docetaxel in patients with mCRPC previously treated with ARPI.8,9 In daily clinical practice, most chemotherapy-fit patients who receive ARPI as first-line treatment for mHSPC or mCRPC subsequently receive docetaxel. Cabazitaxel was shown to improve OS in patients with mCRPC progressing either on or after docetaxel, and in patients with mCRPC previously treated with docetaxel and ARPIs.10,11 Recently, two randomized phase III trials demonstrated that olaparib and 177Lu-PSMA-617 improve OS in patients with progressive mCRPC after at least one ARPI.12,13 In patients with mCRPC responding to taxane, no immediate treatment is administered, with patients simply followed up. By contrast, for other cancer types, switch maintenance after response to chemotherapy has become the standard of care.14-17 The first results of mCRPC maintenance treatment have been reported. Indeed, the Swiss Group for Clinical Cancer Research (SAKK) reported a randomized study that switches maintenance with orteronel in patients with mCRPC after docetaxel chemotherapy was beneficial and feasible with a significantly improved event-free survival (EFS).18 In a phase II trial, tasquinimod maintenance resulted in a statistically significant improved radiographic progression-free survival (rPFS) compared with placebo, yet with relevant toxicity.19
CONTEXT
Key Objective
Is darolutamide maintenance a new possible therapeutic strategy in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with an androgen-receptor pathway inhibitor (ARPI) who subsequently have nonprogressive disease after taxane treatment?
Knowledge Generated
In the phase II Swiss Group for Clinical Cancer Research 08/16 trial, darolutamide maintenance therapy improved radiographic progression-free survival at 12 weeks, radiographic progression-free survival, event-free survival, and 50% prostate-specific antigen response rate compared with placebo in patients with mCRPC previously treated with an ARPI and nonprogressing after subsequent taxane treatment without relevantly increasing toxicity. Subgroup analyses of this study revealed that darolutamide maintenance appears especially beneficial in patients who had a radiologic response to their latest ARPI.
Relevance (M.A. Carducci)
-
The addition of darolutamide poststable disease on docetaxel for mCRPC has the potential to extend the time to radiographic progression. This approach will need further evaluation in larger studies before incorporating into clinical practice, yet provides a hint that additional androgen-receptor targeting agents may have a role in this clinical setting.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD.
Darolutamide, which is an ARPI with a distinctly different structure than enzalutamide and apalutamide, was shown to exert fewer side effects, potentially due to decreased blood-brain barrier penetration.20,21 Darolutamide demonstrated a significant OS benefit in patients with non-mCRPC and, recently, in the mHSPC setting, with a maintained quality of life.22-24 Darolutamide displays a favorable safety profile with only few drug-drug interactions.25 This agent is therefore an ideal candidate for maintenance treatment. Despite the cross-resistance between different ARPIs, some data suggest that chemotherapy might reinduce sensitivity to ARPI.26,27
The trial SAKK 08/16 investigated maintenance therapy with darolutamide in patients with mCRPC previously treated with an ARPI, who subsequently had nonprogressive disease after chemotherapy with a taxane.
PATIENTS AND METHODS
Study Design and Conduct
SAKK 08/16 is a multicenter, international, investigator-initiated, randomized, double-blind, and placebo-controlled phase II trial (ClinicalTrials.gov identifier: NCT02933801). The study Protocol (online only) was approved by local independent review boards, with the study conducted according to the Declaration of Helsinki principles and Good Clinical Practice Guidelines. Written informed consent was obtained from all patients.
Patients and Treatments
Eligible patients had confirmed mCRPC and a WHO performance status of 0-2. Patients must have received enzalutamide and/or abiraterone for at least 8 weeks before taxane treatment and must not have progressed to taxane chemotherapy. The minimal cumulative dose was ≥300 mg/m2 or total ≥600 mg for docetaxel; ≥80 mg/m2 or total ≥160 mg for cabazitaxel. Nonprogressive disease was defined as no prostate-specific antigen (PSA) progression and no progression on imaging since taxane initiation according to the Prostate Cancer Working Group 3 (PCWG3) criteria.28 All patients continued ADT.
Patients were centrally randomized via the electronic data capture system secuTrial to either darolutamide (600 mg twice daily) and best supportive care or placebo and best supportive care in a 1:1 ratio using the minimization method with 80% allocation probability. Patients were stratified by country, WHO performance status, visceral metastases, previous ARPI use (abiraterone v enzalutamide v both), and planned start of trial treatment after the last taxane dose.
Patients, investigators, site staff, monitors, data managers, and a designated statistician were blinded to treatment allocation. A scratch-off card was provided when emergency unblinding was necessary.
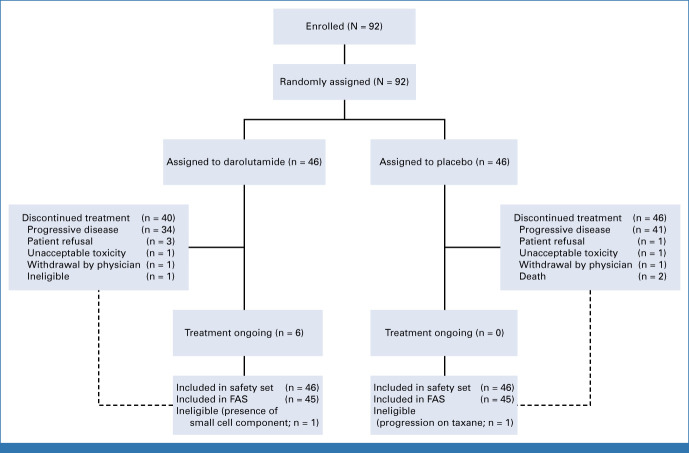
The trial treatment start had to be within 2-8 weeks after last chemotherapy dosing. Treatment was continued until occurrence of unacceptable adverse events (AEs), disease progression, or initiation of a nonprotocol systemic anticancer treatment. The CONSORT diagram is shown in Figure 1.
FIG 1.
CONSORT diagram. FAS, full analysis set.
End Points
The primary end point was rPFS at 12 weeks after treatment initiation (rPFS12). rPFS was defined as the time from the start of treatment to radiographic progression or death from any cause. Radiographic disease progression was defined according to PCWG3 and RECIST 1.1. Patients who did not experience an event were censored at the date of the last available assessment before the initiation of a different treatment, if any. Secondary end points were rPFS, time to PSA progression (defined according to PCWG3), time to symptomatic/clinical progression, EFS (defined as one of the following: death from any cause; radiographic progression and symptomatic/clinical progression; radiographic progression and PSA progression; or symptomatic/clinical progression and PSA progression), OS, PSA response, duration of PSA response, and AEs. Patient-reported fatigue was assessed using the Brief Fatigue Inventory (BFI), a nine-item instrument used to assess the severity of fatigue and its interference with daily living.29
Assessments
Efficacy assessments included computer tomography thorax-abdomen-pelvis with contrast agent and bone scan performed at screening and subsequently every 12 weeks. PSA levels were measured at screening, on day 1 of every cycle (cycle of 28 days), and 30 days after last dose or immediately before initiating a new antineoplastic therapy, whichever occurred first. BFI was completed at baseline, on day 1 of every cycle, and 30 days after last dose or immediately before initiating a new antineoplastic therapy. AEs were graded by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Statistical Analysis
The sample size was calculated based on the primary end point rPFS12. Overall, 88 patients (44 in each arm) had to be randomly assigned to detect an rPFS12 improvement from 50% in the placebo arm to 70% in the experimental arm, with a 15% one-sided type I error and 80% power.30 To account for ineligible patients, the sample size was increased by 5% to 92 patients. All efficacy analyses were based on the full analysis set, including all patients who received at least one darolutamide/placebo dose, yet excluding those with major eligibility violations. Safety analyses were performed on the safety set, which included all patients who received at least one darolutamide/placebo dose.
The rPFS12 was estimated for each treatment arm using Kaplan-Meier methodology assessed at 13 weeks to enable a 1-week delay for the 12-week assessment, along with a 95% CI calculated on the basis of the log hazard. For the primary analysis, a one-sided 85% CI for the difference in rPFS12 between the two treatment arms was estimated using the normal approximation method, with standard errors computed using the Greenwood method along with a corresponding one-sided P value. For all time-to-event end points, the medians and CIs were estimated using Kaplan-Meier methodology and compared between treatment arms using log-rank tests. Hazard ratios (HRs) were estimated using Cox models. Sensitivity analyses were performed to account for nonproportional hazards because of the slight crossing of the curves for rPFS. Sensitivity analyses were carried out to account for competing risks. Subgroup analyses were conducted for predefined variables. Response rates were compared between treatment arms using Fisher's exact test. For the BFI global score, as well as the single items, repeated mixed models were applied to analyze the effects over time by treatment arm.
All analyses were performed using SAS (Version 9.4; SAS Institute, Cary, NC) and R 4.0.3 (The R Foundation).31
RESULTS
From April 20, 2017, to November 19, 2020, 92 patients were randomly assigned by 23 centers from four countries (France, Italy, Spain, and Switzerland). Two patients were excluded due to major eligibility violations; the full analysis set thus consisted of 90 patients, with 45 in each treatment arm (Fig 1). Median follow-up at the time of this analysis was 18 months (95% CI, 14 to 22).
Baseline characteristics were generally well balanced, with some differences to be pointed out. The placebo arm comprised a slightly higher proportion of patients who responded to the latest ARPI, as well as a higher proportion of patients with complete or partial remission to the latest taxane, and a higher number of patients having received cabazitaxel.
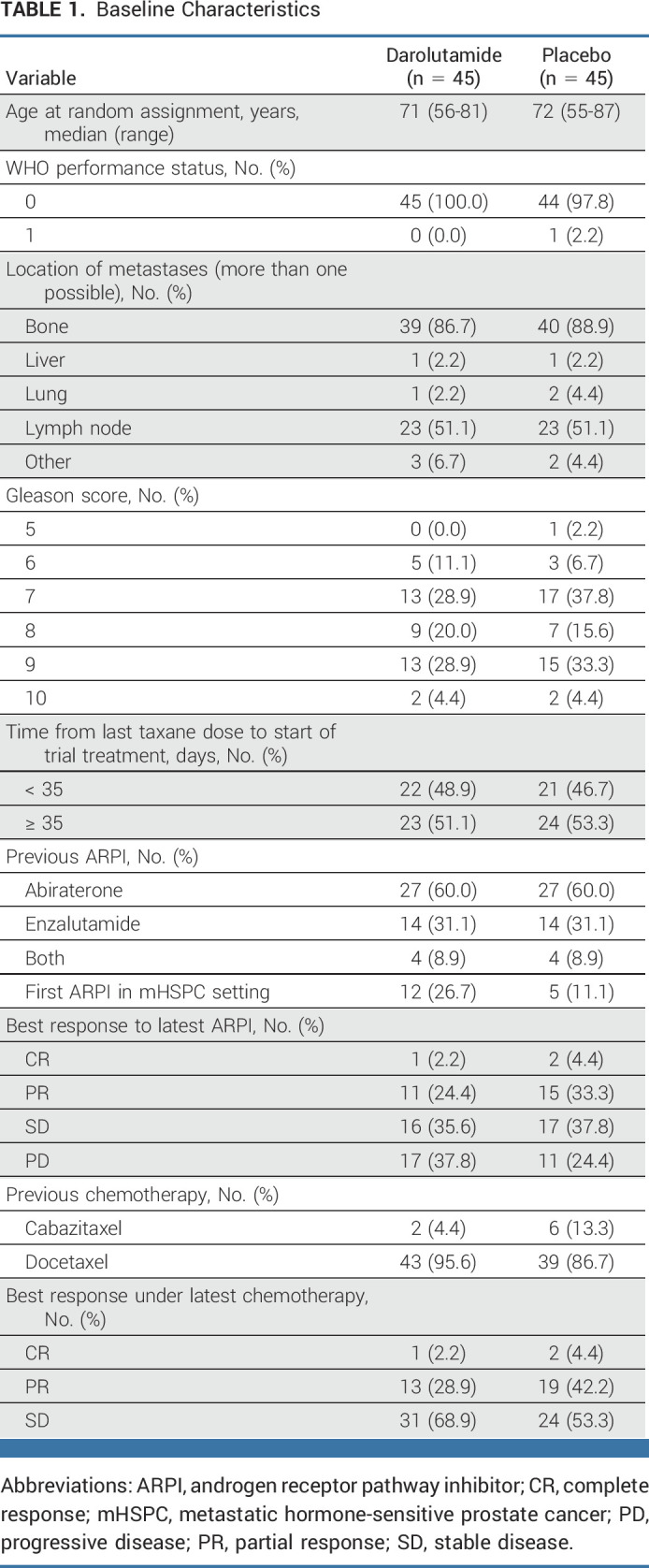
Patient characteristics are summarized in Table 1. Median treatment duration was 5 (95% CI, 0.1 to 46.3) months in the darolutamide group and 3.3 (95% CI, 0.4 to 19.6) months in the placebo group.
TABLE 1.
Baseline Characteristics
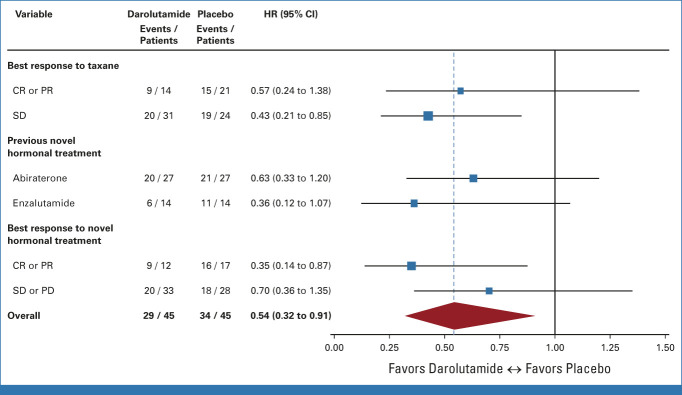
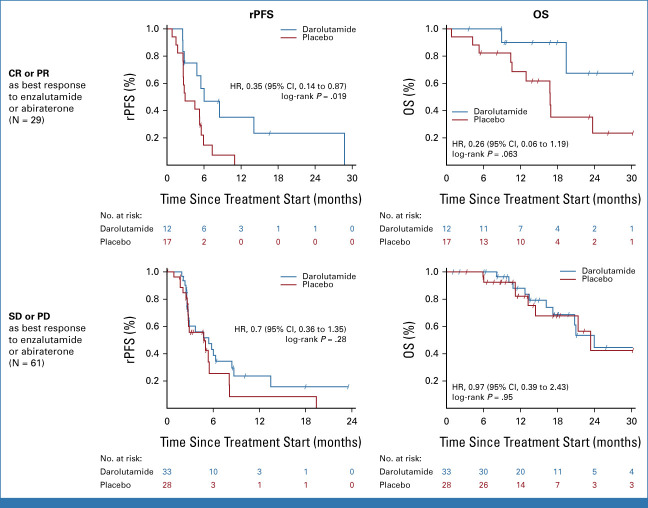
The primary rPFS12 end point was improved with darolutamide at 64.7% (95% CI, 47.6 to 77.5) versus placebo at 52.2% (95% CI, 36.1 to 66.1), which was statistically significant at the .15 significance level (difference 12.5%; one-sided 85% CI [lower bound], 1.1%; one-sided P = .127). Overall, rPFS was 5.5 months on darolutamide compared with 4.5 months on placebo (HR, 0.54 [95% CI, 0.32 to 0.91]; P = .017; Fig 2A). There were eight patients with events recorded before the first scheduled scan, with six of them from the placebo arm. These premature scans were performed because of rising PSA or clinical progression. Sensitivity analyses to account for nonproportional hazards and competing risks supported the primary analysis results (data not shown). EFS was 5.4 months on darolutamide versus 2.9 months on placebo (HR, 0.46 [95% CI, 0.29 to 0.73]; P < .001, Fig 2B). OS was longer in the darolutamide arm with 24.0 months compared with 21.3 months in the placebo arm (HR, 0.62 [95% CI, 0.3 to 1.26]; P = .181; Fig 2C). Time to PSA progression was 2.7 months for darolutamide versus 1.9 months for placebo (HR, 0.48 [95% CI, 0.30 to 0.77]; P = .001). PSA 50% response rate was superior with darolutamide versus placebo (22% v 4%; P = .014; Appendix Fig A1, online only). Median duration of PSA 50% response was 7.7 months with darolutamide compared with 2.8 months with placebo. Time to symptomatic/clinical progression was 8.7 months for darolutamide versus 5.7 months for placebo (HR, 0.67 [95% CI, 0.37 to 1.23]; P = .197). A subgroup analysis by response to the latest ARPI was performed. In the subgroup with a complete or partial radiologic response to latest ARPI (n = 29), darolutamide achieved a highly significant rPFS prolongation (HR, 0.35 [95% CI, 0.14 to 0.87]; P = .019) and OS (HR, 0.26 [95% CI, 0.06 to 1.19]; P = .063), while in the subgroup with stable or progressive disease as best response to latest ARPI (n = 61), no significant between-arm difference was observed (HR, 0.7 [95% CI, 0.36 to 1.35]; P = .28; HR, 0.97 [95% CI, 0.39 to 2.43]; P = .95; Appendix Fig A2, online only). No significant difference in rPFS or OS according to response to prior taxane or prior use of different ARPIs (enzalutamide or abiraterone) was found (Fig 3).
FIG 2.
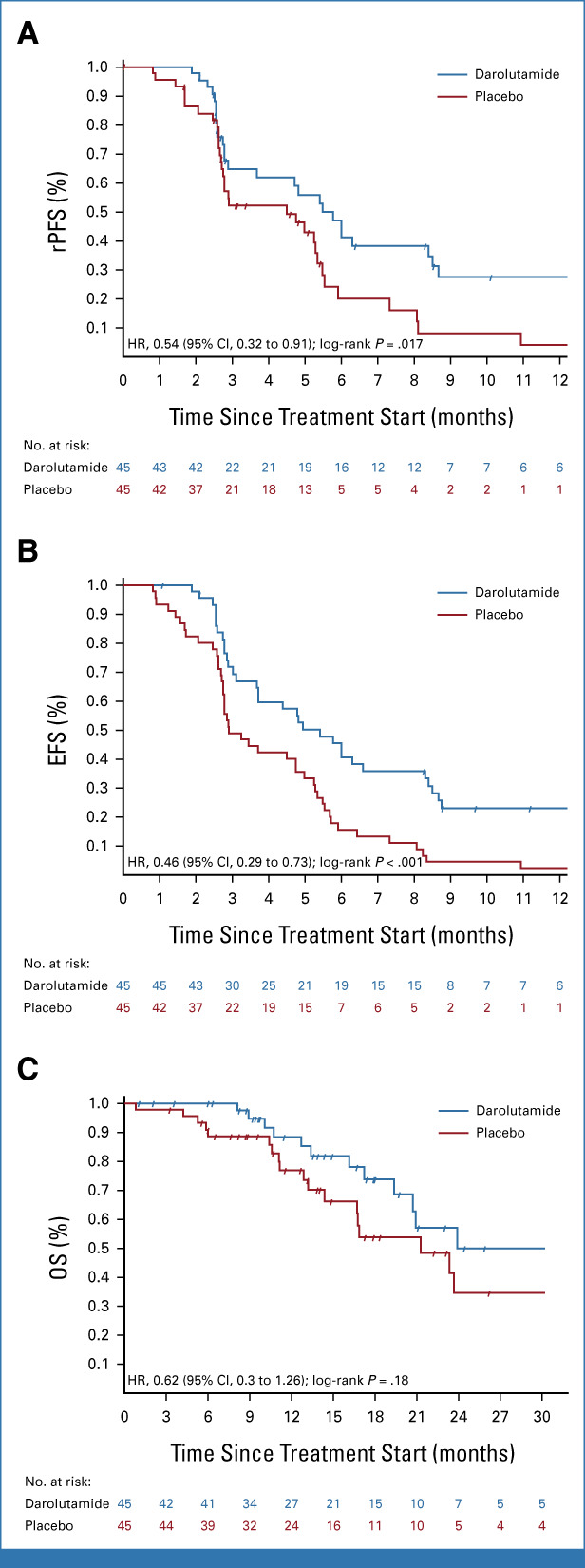
Kaplan-Meier plot for (A) rPFS, (B) EFS, and (C) OS. EFS, event-free survival; HR, hazard ratio; OS, overall survival; rPFS, radiographic progression-free survival.
FIG 3.
Forest plot for prespecified subgroup analyses. CR, complete response; HR, hazard ratio; PD, progressive disease; PR, partial response; SD, stable disease.
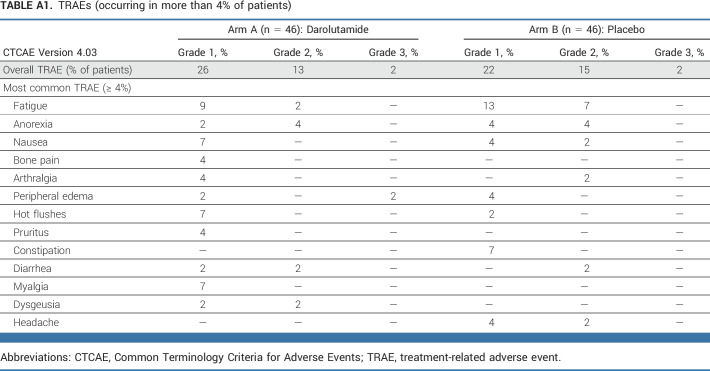
Darolutamide did not result in increased treatment-related AEs (TRAEs) compared with placebo (Appendix Table A1, online only). Overall, 26% and 22% of patients developed a grade 1 TRAE, with 13% and 15% of patients developing a grade 2 TRAE in the darolutamide and placebo groups, respectively. Only 2% of patients from both groups experienced a grade 3 TRAE. No grade 4 or 5 TRAEs occurred in the darolutamide group, yet three non–treatment-related deaths were recorded in the placebo group (one due to sepsis, one due to intracranial hemorrhage, and one due to disease progression). Fatigue was the most common TRAE in both arms, yet numerically less frequent in the darolutamide arm (11% v 20%, Appendix Table A1). For patient-reported fatigue, no significant between-treatment arm differences were found concerning their severity or interference with daily living (Appendix Fig A3, online only).
DISCUSSION
To our knowledge, SAKK 08/16 is the first trial to demonstrate the efficacy and safety of darolutamide in the mCRPC setting. In this study, darolutamide improved rPFS12, rPFS, EFS, and 50% PSA response rate compared with placebo without increasing AEs in patients with mCRPC who had at least stable disease under taxane chemotherapy and who had previously received an ARPI. Since 2004, several treatments have been shown to improve OS in mCRPC, but in this crowded therapeutic scenario, the optimal sequence of these therapies has not yet been established.1 Although ARPIs are usually continued until progression or unacceptable toxicity, taxane chemotherapies are only performed up to a maximum number of cycles, after which patients without progression are simply being followed up, continuing ADT alone. In the TAX-327 and TROPIC trials, the study design comprised up to 10 cycles followed by a follow-up phase.4,10 Usually, the time to progression after ending taxane treatment was only a few months.18 For this reason, a nontoxic maintenance treatment prolonging the achieved disease control would likely be useful so as to prevent progression-related symptomatic events while maintaining patients' quality of life.
The current study findings add to the results of our previous trial, SAKK 08/11, in which orteronel significantly improved EFS in patients with mCRPC with nonprogressive disease on docetaxel.18 This prior trial had an early close, owing to drug development discontinuation. Our SAKK 08/16 trial seems to confirm the validity of ARPI maintenance in patients with mCRPC after chemotherapy. In this setting, an additional darolutamide benefit consisted in its low toxicity, with fatigue less commonly reported in the darolutamide arm. These results confirm the drug's excellent tolerability, as shown in previous randomized phase III trials.21-24 This contrasts with another maintenance trial using tasquinimod, which also revealed a rPFS benefit, yet along with a much less favorable safety profile.19
The rPFS benefit in the darolutamide arm was statistically significant; however, the magnitude for the overall study population was marginal. Furthermore, the benefit was unlikely to be clinically relevant, only consisting of a 1-month improvement in median rPFS versus placebo. The OS was numerically longer under darolutamide, yet without statistical significance. Moreover, the significant financial impact of darolutamide maintenance on health care systems must still be considered when evaluating this strategy's risk/benefit ratio. It thus appears crucial to identify those patients who would most likely benefit from darolutamide maintenance therapy. Our subgroup analyses revealed that darolutamide appears to be especially beneficial in patients with a radiologic response to their latest ARPI. By contrast, there was no difference with respect to the best response to taxane treatment. Interestingly, the outcome did not seem to be dependent on the type of ARPI (CYP17 inhibitor or androgen-receptor antagonist) administered previously. This suggests that a previous response might be a predictive marker of ongoing androgen-receptor pathway dependency and, hence, benefit from further ARPI treatment.
Cross-resistance among different ARPIs is well recognized, and current guidelines recommend avoiding ARPI sequencing in patients with mCRPC.2,26 In our study, all patients were ARPI-treated before undergoing taxane chemotherapy and, hence, before being randomly assigned to either darolutamide or placebo. Published data have suggested that chemotherapy may reinduce sensitivity to ARPI.27 This could account for this study's positive results using darolutamide as a second ARPI in patients with mCRPC. Furthermore, evidence of cross-resistance among ARPIs is derived from studies conducted in the mCRPC setting, while recent evidence in apalutamide-treated patients in nmCRPC or mHSPC settings suggest that the activity and efficacy of sequential ARPI use may not be negligible.32,33 Future studies evaluating the use of a second ARPI in mCRPC are thus required, especially in light of the increased use of ARPIs in the mHSPC setting. Indeed, this may result in a biological disease at progression that differs from that seen when the ARPI is administered in the mCRPC setting.
Therefore, darolutamide maintenance may represent a new therapeutic strategy in patients with mCRPC with at least stable disease under taxane, especially in those with partial or complete radiologic response to the latest androgen-receptor pathway, as suggested by our subgroup analysis results. However, as our sample size was rather small, these results should be regarded rather as hypothesis-generating. They must be further confirmed in a larger trial conducted in patients selected with respect to their latest response to ARPI treatment.
In our study, only very few patients exhibited visceral metastases that are known to be associated with poor prognosis.34 Hence, we currently do not yet know the impact of maintenance darolutamide in this specific population.
In addition to clinical features, molecular characterization could be useful in identifying patients who would most likely benefit from maintenance darolutamide therapy, but the molecular status of our study patients is unknown. One potential predictive molecular biomarker is the androgen-receptor splice variant 7, which is likely associated with resistance to enzalutamide and abiraterone.35 Preclinical studies revealed a link between SPOP point mutations and sensitivity to androgen-receptor inhibition, suggesting that ARPIs might improve the outcome of patients exhibiting such mutations.36 Future studies are required to identify those patients who would most likely benefit from ARPIs and, thus, potentially from darolutamide maintenance therapy. Of note, our study was unable to clarify whether chemotherapy can restore the sensitivity of prostate cancer cells to ARPI therapy.
Finally, these study results could be of interest for evaluating other maintenance therapies for selected patients with prostate cancer, such as the efficacy of poly adenosine diphosphate-ribose polymerase inhibitors as maintenance therapy in patients with alterations in DNA repair genes or immunotherapy in patients with high microsatellite instability.
In conclusion, darolutamide maintenance therapy compared with placebo improved clinical outcomes in patients with mCRPC who were previously treated with an ARPI and nonprogressing after subsequent taxane treatment, yet without increasing toxicity. The most marked response was observed in those patients who had responded to prior ARPI therapy. This may represent a new treatment strategy for selected patients.
ACKNOWLEDGMENT
The authors thank Dr Gabrielle Cremer for editorial assistance with the manuscript.
APPENDIX
TABLE A1.
TRAEs (occurring in more than 4% of patients)
FIG A1.
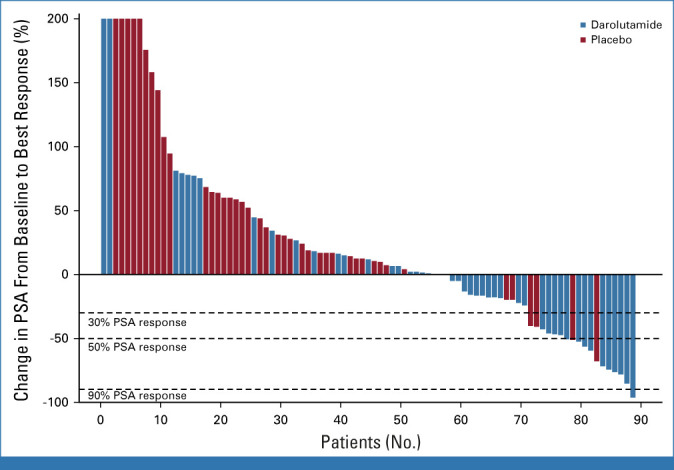
PSA response rate. PSA, prostate-specific antigen.
FIG A2.
Kaplan-Meier plot for subgroup analyses of the best response to the latest androgen receptor pathway inhibitor. CR, complete response; HR, hazard ratio; OS, overall survival; PD, progressive disease; PR, partial response; rPFS, radiographic progression-free survival; SD, stable disease.
FIG A3.
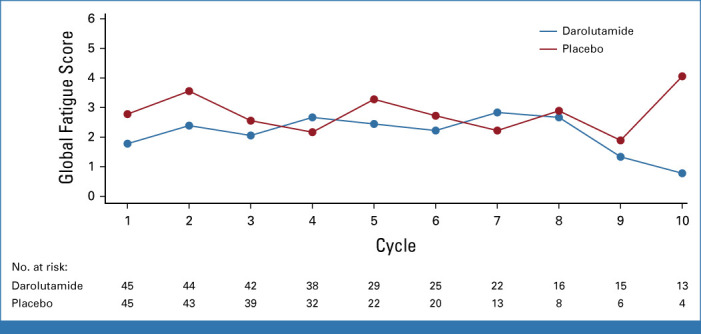
Line plot of medians for the global fatigue score by treatment arm.
Silke Gillessen
Consulting or Advisory Role: Bayer (Inst), MSD Oncology (Inst), Amgen (Inst), Pfizer (Inst), BMS (Inst), Telix Pharma (Inst), AAA HealthCare (Inst), Orion (Inst), Novartis (Inst), Modra Pharmaceuticals (Inst), Myriad Genetics (Inst), AstraZeneca (Inst), TOLREMO (Inst), Silvio Grasso Consulting (Inst), WebMD/Medscape (Inst), ESMO, Swiss Academy of Multidisciplinary oncology (SAMO), Swiss Group for Clinical Cancer Research (SAKK), German-speaking European School of Oncology (DESO), Radiotelevisione Svizzera Italiana (RSI)
Patents, Royalties, Other Intellectual Property: Method for biomarker (WO 3752009138392 A1)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: ProteoMediX
Giuseppe Procopio
Consulting or Advisory Role: Bayer, Bristol Myers Squibb (BMS), Janssen, Novartis, Pfizer, Ipsen, Merck Sharp & Dohme, AstraZeneca, Eisai, MSD Oncology
Research Funding: Astellas Pharma, Ipsen, Janssen Oncology
Orazio Caffo
Honoraria: Astellas Pharma, Janssen Oncology, Bayer, AstraZeneca, Pfizer, Ipsen
Consulting or Advisory Role: Astellas Medivation, Janssen, MSD Oncology, Advanced Accelerator Applications, Bayer
Speakers' Bureau: Astellas Pharma
David Lorente
Consulting or Advisory Role: AstraZeneca Spain, Janssen Oncology, Sanofi, Bayer, Astellas Pharma
Speakers' Bureau: AstraZeneca, Janssen Oncology, Astellas Pharma, Bayer, Ipsen, MSD Oncology, BMSi
Travel, Accommodations, Expenses: Janssen Oncology, Astellas Pharma, Pfizer, Sanofi, Bayer
Guilhem Roubaud
Honoraria: Astellas Pharma (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst)
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen-Cilag (Inst), AstraZeneca (Inst), Ipsen (Inst), AAA/Endocyte/Novartis (Inst), Merck/Pfizer (Inst), Bayer (Inst)
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag, Bayer
Aurelius Omlin
Consulting or Advisory Role: Astellas Pharma (Inst), Bayer (Inst), Sanofi (Inst), Roche (Inst), Janssen (Inst), MSD (Inst), Molecular Partners (Inst), Pfizer (Inst), AstraZeneca (Inst)
Speakers' Bureau: Bayer (Inst), Astellas Pharma (Inst), Janssen-Cilag (Inst)Tripple AAA, AstraZeneca (Inst)
Research Funding: Teva (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Sanofi, Bayer, Astellas Pharma, Janssen
Consuelo ButtIgliero
Consulting or Advisory Role: Janssen-Cilag, Merck, Astellas Pharma, AstraZeneca
Speakers' Bureau: Ipsen
Aránzazu González-del-Alba
Honoraria: Sanofi/Aventis, Roche, Pfizer, Astellas Pharma, Janssen-Cilag, Novartis, Ipsen
Consulting or Advisory Role: Astellas Pharma, Sanofi, Bayer, Janssen Oncology, Pfizer, Bristol Myers Squibb, Eisai, EUSA Pharma, Roche, Novartis, Ipsen, AstraZeneca, MSD Oncology, Eisai, Advanced Accelerator Applications
Speakers' Bureau: Ipsen, Roche, Pfizer, Janssen-Cilag, Advanced Accelerator Applications, Astellas Pharma
Travel, Accommodations, Expenses: Astellas Pharma, Pfizer, Janssen Oncology, Sanofi, Bristol Myers Squibb, MSD Oncology, Roche, Ipsen
Fabio Turco
Travel, Accommodations, Expenses: Bayer
Richard Cathomas
Honoraria: Janssen-Cilag, Astellas Pharma
Consulting or Advisory Role: Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Pfizer, Roche (Inst), MSD Oncology, Janssen-Cilag, Bayer (Inst), Sanofi (Inst), Ipsen (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as a late-breaking abstract at the ESMO Annual Meeting, Paris, France, September 18, 2021 (LBA 26).
SUPPORT
Supported by and investigational study drug provided by Bayer HealthCare Pharmaceuticals Inc.
CLINICAL TRIAL INFORMATION
NCT02933801 (SAKK 08/16)
DATA SHARING STATEMENT
Proposals for data access should be submitted to the corresponding author for consideration. Access to deidentified participant data can be granted if the proposal is approved by SAKK; use of the data is intended only for the approved proposal.
AUTHOR CONTRIBUTIONS
Conception and design: Silke Gillessen, Stefanie Hayoz, Karin Ribi, Richard Cathomas
Administrative support: Eloïse Kremer, Soazig Nenan
Provision of study materials or patients: Silke Gillessen, Giuseppe Procopio, Stefanie Hayoz, Michael Schwitter, Orazio Caffo, David Lorente, Augusto Pedrazzini, Guilhem Roubaud, Aurelius Omlin, Consuelo Buttigliero, Juan Ignacio Delgado Mingorance, Aránzazu González-del-Alba, Maria Teresa Delgado, Franco Nole, Fabio Turco, Ricardo Pereira Mestre, Karin Ribi, Richard Cathomas
Collection and assembly of data: Silke Gillessen, Giuseppe Procopio, Eloïse Kremer, Michael Schwitter, Orazio Caffo, David Lorente, Augusto Pedrazzini, Guilhem Roubaud, Soazig Nenan, Aurelius Omlin, Consuelo Buttigliero, Juan Ignacio Delgado Mingorance, Aránzazu González-del-Alba, Maria Teresa Delgado, Franco Nole, Ricardo Pereira Mestre, Richard Cathomas
Data analysis and interpretation: Silke Gillessen, Stefanie Hayoz, Fabio Turco, Karin Ribi, Richard Cathomas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Darolutamide Maintenance in Patients With Metastatic Castration-Resistant Prostate Cancer With Nonprogressive Disease After Taxane Treatment (SAKK 08/16)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Silke Gillessen
Consulting or Advisory Role: Bayer (Inst), MSD Oncology (Inst), Amgen (Inst), Pfizer (Inst), BMS (Inst), Telix Pharma (Inst), AAA HealthCare (Inst), Orion (Inst), Novartis (Inst), Modra Pharmaceuticals (Inst), Myriad Genetics (Inst), AstraZeneca (Inst), TOLREMO (Inst), Silvio Grasso Consulting (Inst), WebMD/Medscape (Inst), ESMO, Swiss Academy of Multidisciplinary oncology (SAMO), Swiss Group for Clinical Cancer Research (SAKK), German-speaking European School of Oncology (DESO), Radiotelevisione Svizzera Italiana (RSI)
Patents, Royalties, Other Intellectual Property: Method for biomarker (WO 3752009138392 A1)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: ProteoMediX
Giuseppe Procopio
Consulting or Advisory Role: Bayer, Bristol Myers Squibb (BMS), Janssen, Novartis, Pfizer, Ipsen, Merck Sharp & Dohme, AstraZeneca, Eisai, MSD Oncology
Research Funding: Astellas Pharma, Ipsen, Janssen Oncology
Orazio Caffo
Honoraria: Astellas Pharma, Janssen Oncology, Bayer, AstraZeneca, Pfizer, Ipsen
Consulting or Advisory Role: Astellas Medivation, Janssen, MSD Oncology, Advanced Accelerator Applications, Bayer
Speakers' Bureau: Astellas Pharma
David Lorente
Consulting or Advisory Role: AstraZeneca Spain, Janssen Oncology, Sanofi, Bayer, Astellas Pharma
Speakers' Bureau: AstraZeneca, Janssen Oncology, Astellas Pharma, Bayer, Ipsen, MSD Oncology, BMSi
Travel, Accommodations, Expenses: Janssen Oncology, Astellas Pharma, Pfizer, Sanofi, Bayer
Guilhem Roubaud
Honoraria: Astellas Pharma (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst)
Consulting or Advisory Role: Astellas Pharma (Inst), Janssen-Cilag (Inst), AstraZeneca (Inst), Ipsen (Inst), AAA/Endocyte/Novartis (Inst), Merck/Pfizer (Inst), Bayer (Inst)
Research Funding: Bayer (Inst)
Travel, Accommodations, Expenses: Astellas Pharma, Janssen-Cilag, Bayer
Aurelius Omlin
Consulting or Advisory Role: Astellas Pharma (Inst), Bayer (Inst), Sanofi (Inst), Roche (Inst), Janssen (Inst), MSD (Inst), Molecular Partners (Inst), Pfizer (Inst), AstraZeneca (Inst)
Speakers' Bureau: Bayer (Inst), Astellas Pharma (Inst), Janssen-Cilag (Inst)Tripple AAA, AstraZeneca (Inst)
Research Funding: Teva (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Sanofi, Bayer, Astellas Pharma, Janssen
Consuelo ButtIgliero
Consulting or Advisory Role: Janssen-Cilag, Merck, Astellas Pharma, AstraZeneca
Speakers' Bureau: Ipsen
Aránzazu González-del-Alba
Honoraria: Sanofi/Aventis, Roche, Pfizer, Astellas Pharma, Janssen-Cilag, Novartis, Ipsen
Consulting or Advisory Role: Astellas Pharma, Sanofi, Bayer, Janssen Oncology, Pfizer, Bristol Myers Squibb, Eisai, EUSA Pharma, Roche, Novartis, Ipsen, AstraZeneca, MSD Oncology, Eisai, Advanced Accelerator Applications
Speakers' Bureau: Ipsen, Roche, Pfizer, Janssen-Cilag, Advanced Accelerator Applications, Astellas Pharma
Travel, Accommodations, Expenses: Astellas Pharma, Pfizer, Janssen Oncology, Sanofi, Bristol Myers Squibb, MSD Oncology, Roche, Ipsen
Fabio Turco
Travel, Accommodations, Expenses: Bayer
Richard Cathomas
Honoraria: Janssen-Cilag, Astellas Pharma
Consulting or Advisory Role: Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Pfizer, Roche (Inst), MSD Oncology, Janssen-Cilag, Bayer (Inst), Sanofi (Inst), Ipsen (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Leung DKW, Chiu PKF, Ng CF, et al. : Novel strategies for treating castration-resistant prostate cancer. Biomedicines 9:339, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mottet N, van den Bergh RC, Briers E, et al. : EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol 79:243-262, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Schaeffer E, Srinivas S, Antonarakis ES, et al. : NCCN guidelines insights: Prostate cancer, version 1.2021. J Natl Compr Cancer Netw 19:134-143, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. : Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502-1512, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MH, et al. : Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513-1520, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, de Bono JS, et al. : Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer TM, Armstrong AJ, Rathkopf DE, et al. : Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424-433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bono JS, Logothetis CJ, Molina A, et al. : Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995-2005, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, et al. : Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367:1187-1197, 2012 [DOI] [PubMed] [Google Scholar]
- 10.de Bono JS, Oudard S, Ozguroglu M, et al. : Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 376:1147-1154, 2010 [DOI] [PubMed] [Google Scholar]
- 11.de Wit R, de Bono J, Sternberg CN, et al. : Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 381:2506-2518, 2019 [DOI] [PubMed] [Google Scholar]
- 12.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Sartor O, de Bono J, Chi KN, et al. : Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 385:1091-1103, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonia SJ, Villegas A, Daniel D, et al. : Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919-1929, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Golan T, Hammel P, Reni M, et al. : Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381:317-327, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powles T, Park SH, Voog E, et al. : Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383:1218-1230, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Cathomas R, Crabb SJ, Mark M, et al. : Orteronel switch maintenance therapy in metastatic castration resistant prostate cancer after first-line docetaxel: A multicenter, randomized, double-blind, placebo-controlled trial (SAKK 08/11). Prostate 76:1519-1527, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Fizazi K, Ulys A, Sengeløv L, et al. : A randomized, double-blind, placebo-controlled phase II study of maintenance therapy with tasquinimod in patients with metastatic castration-resistant prostate cancer responsive to or stabilized during first-line docetaxel chemotherapy. Ann Oncol 28:2741-2746, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moilanen AM, Riikonen R, Oksala R, et al. : Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep 5:12007, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halabi S, Jiang S, Terasawa E, et al. : Indirect comparison of darolutamide versus apalutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer. J Urol 206:298-307, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Fizazi K, Shore N, Tammela TL, et al. : Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med 383:1040-1049, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Smith MR, Shore N, Tammela TL, et al. : Darolutamide and health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: An analysis of the phase III ARAMIS trial. Eur J Cancer 154:138-146, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Smith MR, Hussain M, Saad F, et al. : Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med 386:1132-1142, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shore N, Zurth C, Fricke R, et al. : Evaluation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: Results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol 14:527-539, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalaf DJ, Annala M, Taavitsainen S, et al. : Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: A multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol 20:1730-1739, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa M, Lu C, Chen Y, et al. : Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol 26:1859-1865, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scher HI, Morris MJ, Stadler WM, et al. : Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol 34:1402-1418, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza TR, Wang XS, Cleeland CS, et al. : The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer 85:1186-1196, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Ratain MJ, Sargent DJ: Optimising the design of phase II oncology trials: The importance of randomisation. Eur J Cancer 45:275-280, 2009 [DOI] [PubMed] [Google Scholar]
- 31.R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2022. https://www.R-project.org
- 32.Agarwal N, Chowdhury S, Bjartell A, et al. : Time to second progression (PFS2) in patients (pts) from TITAN with metastatic castration-sensitive prostate cancer (mCSPC) by first subsequent therapy (hormonal vs. taxane). J Clin Oncol 38, 2020. (suppl 6; abstr 82) [Google Scholar]
- 33.Small EJ, Saad F, Chowdhury S, et al. : Updated analysis of progression-free survival with first subsequent therapy (PFS2) and safety in the SPARTAN study of apalutamide (APA) in patients (pts) with high-risk nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol 37, 2020. (suppl 7; abstr 144) [Google Scholar]
- 34.Shou J, Zhang Q, Wang S, et al. : The prognosis of different distant metastases pattern in prostate cancer: A population based retrospective study. Prostate 78:491-497, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Antonarakis ES, Lu C, Wang H, et al. : AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371:1028-1038, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernasocchi T, Theurillat JPP: SPOP-mutant prostate cancer: Translating fundamental biology into patient care. Cancer Lett 529:11-18, 2022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Proposals for data access should be submitted to the corresponding author for consideration. Access to deidentified participant data can be granted if the proposal is approved by SAKK; use of the data is intended only for the approved proposal.