Abstract
Most of the studies using the colorectal tissue explants challenge model have been conducted after one single dose and before reaching a steady state. We consider that longer exposure as in 28-day postexposure prophylaxis (PEP) course and in an at-risk setting, such as after a sexual risk exposure to HIV could give us valuable information about these drugs. In a substudy we assessed pharmacokinetics, changes on immune system and ex-vivo rectal mucosal susceptibility to HIV-1 infection after taking maraviroc (MVC), raltegravir (RAL), and ritonavir-boosted lopinavir (LPV/r) PEP-based regimens in 30 men who have sex with men. Participants received 28 days of twice-daily MVC (n = 11), RAL (n = 10) or LPV/r (n = 9) all with tenofovir/emtricitabine (TDF/FTC) backbone. Blood, rectal fluid, and rectal tissue samples were collected at days 7, 28, and 90 after starting PEP. The samples obtained at day 90 were considered baseline. All studied antiretrovirals were quantifiable at 7 and 28 days in all tissues. Activation markers were increased in CD4 mucosal mononuclear cells (MMCs) after 28 days of MVC: CD38 + 68.5 versus 85.1, p = .008 and CD38+DR +16.1 versus 26.7, p = .008. Exposure to MVC at both endpoints (7 and 28 days) was associated with significant suppression of HIV-1BAL (p = .005 and p = .028), but we did not observe this effect with RAL or LPV/r. Merging together changes in MMC in all arms, we found a positive correlation in the CD8 T cell lineage between the infectivity at day 7 and activation (CD38+ r = 0.43, p = .025, DR + r = 0.547, p = .003 and 38+DR+ r = 0.526, p = .05), senescence (CD57+CD28− r = 0.479, p = .012), naive cells (RA+CCR7+ r = 0.484, p = .01), and CCR5 expression (r = 0.593, p = .001). We conclude that MVC in combination with TDF/FTC was associated with viral suppression in rectal explants and that overall ex-vivo HIV infectivity correlated with activation and senescence in CD8 MMCs.
Keywords: HIV, postexposure prophylaxis, maraviroc, rectal explants, prevention
Introduction
Currently, we have different biomedical and behavioral strategies that have proven to prevent new HIV infections; however, there are key populations, such as men who have sex with men (MSM), that have 25 times more risk to have an HIV infection, hence we need focused efforts to find the most efficient preventive interventions.1,2
For more than 30 years antiretroviral (ARV) drugs have been used as postexposure prophylaxis (PEP) with an overall low efficacy evidence but widely accepted and prescribed.3 More recently, as pre-exposure prophylaxis (PrEP) with very encouraging results when good adherence is achieved.4–6 PEP and PrEP rationale was initially based in studies conducted in nonhuman primates who were exposed to different ARVs within mucosal challenges and prevention of HIV infection was fairly achieved.7,8
Maraviroc (MVC) is an entry inhibitor that prevents HIV entrance into the host cell by blocking the CCR5 co-receptor,9 and rapidly achieves high concentrations in rectal tissue (RT).10 There is some mixed evidence of MVC efficacy for preventing HIV infection, but overall single-dose studies have failed to demonstrate MVC efficacy after ex-vivo rectal explants challenges11,12 and a large PrEP study, including an explant challenge assay, showed that exposure to one dose of MVC given alone for a 24-week period poorly suppresses ex-vivo HIV infection.13
Raltegravir (RAL) is an integrase inhibitor that blocks the HIV integration into the newly infected cell, this step occurs more than 6 h after infection, which could extend the coital dosing window.14 RAL has been associated with ex-vivo protection15 and nowadays, it is the most recommended third drug for PEP regimens.16,17
The protease inhibitor ritonavir-boosted lopinavir (LPV/r) was considered the standard of care as third drug in all PEP guidelines and still is in some middle- to low-income countries.18 To date, there are no pharmacokinetics (PK) or infectivity studies of this ARV in gastrointestinal tissue.
There are only a few studies assessing different ARVs in rectal mucosa after reaching a steady state.13,19–21 We considered that long exposure to treatment as in 28-day PEP course and in an at-risk setting, as after a sexual risk exposure to HIV, could give us valuable information about these ARVs. We assessed PK, the immunological impact and the effect against rectal mucosa ex-vivo HIV infection of MVC, RAL, and LPV/r in MSM on a three-drug PEP regimen.
Materials and Methods
Ethics
This study was conducted in accordance with Good Clinical Practices procedures and all applicable regulatory requirements. The study protocol was approved by the Ethics Committee for Clinical Research (CEIC) of Hospital Clinic of Barcelona.
Study design, participants, and clinical safety
This is a substudy of two PEP randomized clinical trials22,23 conducted in an HIV clinic in Barcelona. The MARAVIPEP and the RALPEP studies were prospective open randomized clinical trials that evaluated PEP noncompletion at 28 days of MVC or RAL in comparison with LPV/r all in combination with tenofovir/emtricitabine (TDF/FTC) backbone. The following doses were administered: MVC 300 mg bis in die, twice a day (BID), RAL 400 mg BID, LPV/r 400/100 mg BID, and TDF/FTC 245/200 mg quaque die, once a day (QD) for 28 days. Substudy participation was proposed at the first follow-up visit (≤60 h after starting PEP) in the HIV clinic and if acceptance, an informed consent was signed.
Subjects eligible to participate were at-risk24 HIV-uninfected MSM. PEP protocol in our center does not considers testing for HIV before starting the treatment, so we check this and other sexually transmitted infections (STI) during the first follow-up visit. After inclusion, three follow-up visits were scheduled: at day 7 and at day 28 after starting PEP and at day 90 after risk exposure. Since PEP is a treatment that must be started as soon as a risk has been assessed and within 72 h, we could not obtain before-drug baseline samples and the day 90 samples were considered baseline.
Demographics and risk behavior were assessed. Laboratory monitoring, including HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis, was performed following Spanish recommendations for PEP follow-up.24
We did not ask for any changes in sexual practices, even though counseling on HIV and other STI prevention was provided. Adherence to PEP was also reinforced and monitored with the medication event monitoring system (MEMS™ AARDEX Group Ltd., Switzerland). An anal cytology was performed to evaluate intraepithelial lesions.
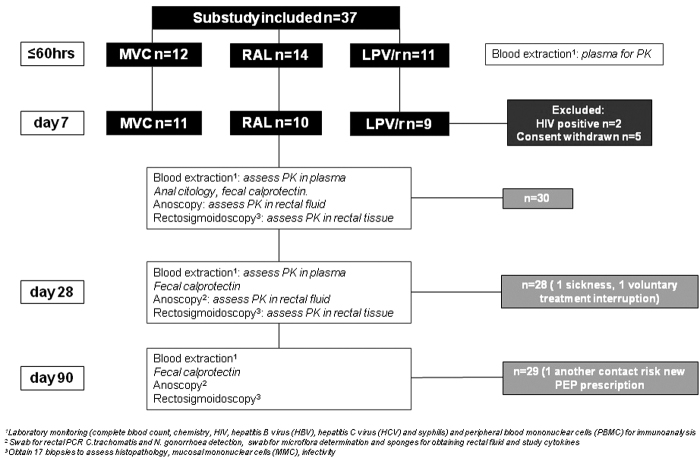
As part of the colorectal tissue evaluation we assessed subepithelial mucosal markers as previously described25,26: (1) participants applied a preparatory enema (Enema Casen® Casen Recordati S. L.) 1 h before procedures and obtained a stool sample to perform a rapid fecal calprotectin test (CalDetect® test distributed by Preventis GmbH); (2) using a lubricated plastic anoscope, 2 swabs were inserted through it and placed in contact with the rectal wall, turned through 360° and removed to determine Chlamydia trachomatis and Neisseria gonorrheae (GC) infection and the microflora (swabs were delivered to the laboratory in ≤30 min). This assessment was conducted to determine mucosal baseline characteristics, any result suggestive of tissue disruption in an asymptomatic individual was considered to have a low impact, hence included in all the evaluations. Flowchart describing different assessments is shown in Figure 1.
FIG. 1.
Flowchart describing the participants' distribution and the different assessments performed during the study. LPV/r, ritonavir-boosted lopinavir; MVC, maraviroc; PK, pharmacokinetics; RAL, raltegravir.
Sample collection
Blood collection
Peripheral blood mononuclear cells (PBMCs) were collected at substudy inclusion (≤60 h after started PEP), days 7, 28, and 90 for immunoassays. Whole blood was obtained using K2 EDTA collection tubes and processed as previously described10 at inclusion, days 7 and 28, and stored at −80°C until PK analysis.
At the same time points serum was obtained to evaluate soluble markers of inflammation [high-sensitive C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α)], and D-dimer. The serum was initially frozen at −80°C. hs-CRP was determined by an immune turbidimetric method (CardioPhase, Siemens Healthcare Diagnostics). A result over 0.5 mg/dL was considered positive. IL-6 and TNF-α were determined by enzime-linked immunosorbent assay (Diasource Immunoassays, Louvain-la-Neuve, Belgium). A result over 5 and 10 pg/mL, respectively, were considered positive. D-dimer was measured with a turbidimetric method (Innovance, Siemens Diagnostics, Marburg, Germany) in a BCS-automated coagulation system (Siemens Diagnostics). Normal cutoff is 500 ng/mL.
Rectal fluid collection
We performed an anoscopy at days 7, 28, and 90 to collect rectal fluid (RF) using four cellulose sponges (Medicalmix BVI WECK-CEL 0008685). Each sponge was processed as previously described,26 weighted before, and after application and stored at −80° until PK or cytokine analysis.
RT collection
Rectal biopsies were obtained by flexible sigmoidoscopy27 at days 7, 28, and 90 each with collection of 17 samples acquired at 20–30 cm from the anal verge, and distributed as follows: (1) histology: one sample fixed on formalin for histological scoring of inflammation,28 these assessments were performed by a single pathologist; (2) PK analysis: five samples snap frozen at first in liquid nitrogen and stored at −80°C until analysis; (3) five samples to obtain mucosal mononuclear cells (MMCs) using enzymatic digestion29; and (4) the remaining six samples were placed in fetal bovine serum plus dimethylsulfoxide (10%) and frozen at −80°C for further explants organization.30 Cryovials were then transferred to the vapor phase of a liquid nitrogen freezer within 1 week.
In brief, biopsies to obtain MMC were digested with a solution of collagenase type II (250 μg/mL), 30 min at 37°C, shaking every 5–10 min to aid tissue dissociation. After that, remaining tissue was passed through a 70 μm filter by a 10 cc syringe and 16G needle to a clean tube containing R15 medium. Complete digestion of biopsies was obtained by repeating at least two rounds of collagenase digestion procedure. Cell yields was 2.5 × 106 ± 1.5 × 106 MMC with a cell viability, measured by Blue Trypan exclusion, >90%.
PK assessment
MVC, RAL y LPV/r in blood plasma, RF, and RT10,31–33 were quantified using a validated high-performance liquid chromatography with tandem mass spectrometric detection methods were performed by the UNC CFAR Clinical Pharmacology and Analytical Chemistry Core.
Flow cytometry and cytokines quantification
All analyses were done in freshly isolated PBMC or MMC, hence we did not use viability dye. PBMC were isolated by density gradient centrifugation using Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO). MMC were obtained using enzymatic digestion.27,29 In both cases, subpopulations of CD4+ and CD8+ T cells were determined using a comprehensive approach of simultaneous measurement34 by a FACSCalibur (Becton Dickinson, San Jose, CA) four-color flow cytometry of different immunological parameters: activation (using CD38 and human leukocyte antigen-DR [HLA-DR] markers), senescence (using CD28 and CD57 markers), co-receptor expression (CCR5, CXCR4), and T cell differentiation stage (using CD45RA and CCR7).
The following monoclonal antibodies were used: CD8-peridinin chlorophyll protein (PerCP), CD4-allophycocyanin (APC), CD28-phycoerythrin (PE), CD57-fluoroisothiocyanate (FITC), CD38-PE, HLA DR-FITC, CD45RA-FITC, CCR7-PE, CCR5-FITC, and CXCR4-PE (all from Becton Dickinson, Mountain View, CA, except CCR7-PE from Milteny Biotec B.V. Leiden, NL). Mouse immunoglobulin isotypes conjugated with PerCP, PE, FITC, or APC were always used as negative controls for nonspecific binding. Thresholds for positive gated populations were defined using the corresponding isotype controls, unstained samples, and/or fluorescence minus one control. Data were analyzed using FlowJo v.7.6.5 software (BD Life Sciences). Gating strategy is represented in Supplementary Figure S1.
The concentrations of the different cytokines: GM-CSF, TNF-α, IL-1β, IL-4, IL-6, MIP-1β, Eotaxin, RANTES, MIG, IL-12, IL-8, IL-17, MIP-1α, IL-10, IL-1RA, INF-γ, IL-13, MCP-1, IL-7, IL-15, INF-α, IL-2R, IP-10, IL-5, and IL2 were analyzed in the RF supernatant with a Luminex™ assay, according to the standard protocol (Thermo Fisher Scientific™, Waltham, MA).
Ex-vivo infection
Owing to our laboratory lack of experience in ex-vivo infection and taking advantage of our colleagues in Instituto de Salud Carlos III (ISCIII) expertise, samples had to be cryopreserved and shipped to Madrid. Explants viability was assessed as previously described by Hughes et al,30 and as they have shown, we expected cryopreserved explants to have as much activity as fresh explants. Infection of endoscopic biopsies was performed with an R5 tropic HIVBal strain (10 ng/well or 104 tissue culture infectious dose 50/mL) in 96-well U-bottom sterile plates with two explants per well and in triplicates for 2 h. Afterward, colorectal explants were extensively washed in phosphate buffered saline and transferred to Dulbecco's modified eagle medium pre-wet espongostan rafts in 24-well microplates.
Colorectal explants were maintained for up to 14 days in complete medium at 37°C and 5% CO2. On days 3, 7, 10, and 14 supernatants were harvested and cultures re-fed with fresh complete medium. Supernatants were frozen at −20°C and quantification of p24 was performed with an Elecsys HIV p24 Ag Test system (Roche). Results are represented as cumulative p24 quantity versus time (days) and reported as area under the curve (AUC) as a model capable of distinguishing between classes. XY analysis and AUC were calculated using GraphPad Prism software. We also analyzed the data dividing the cumulative HIV-1 p24 by CD4+ T cell count in each explant tissue to normalize the infection susceptibility.
Statistics
Characteristics of the study population and the different immunological parameters, translocation, and inflammatory markers were recorded as median [interquartile range (IQR)], and comparisons between groups were made using the Kruskal–Wallis test. Pairwise comparisons were made using a Friedman test and Dunn's post-test for pairwise comparisons. Corrections for multiple comparisons were performed using the Bonferroni method. Correlations between quantitative parameters were explored using the Spearman's test. All statistical analyses were performed using the SPSS software version 20 (SPSS Inc., Chicago, IL).
Results
Subject characteristics, disposition, and safety
A total of 37 MSM were screened and 30 enrolled, distribution of participants is exposed in Figure 1. Median age (IQR) was 35 (22–54), 21 (70%) were Caucasian, 8 (27%) Latin-Americans and 1 participant was Arabic. Ten (33%) participants had a previous STI, 8 (30%) referred ≥10 sexual partners in the past 3 months before entering the study, and 13 (48%) countless sexual partners during lifetime. Medication was well tolerated and there were no significant laboratory abnormalities. In all included participants, HIV, HBV, HCV, and syphilis tests remained negative during the 6-month follow-up.
Two subjects had an abnormal cytology and were referred to a specialist for further evaluation. One subject in the RAL arm had a positive test for rectal GC at day 90 and although he was completely asymptomatic, we treated the infection with antibiotics as recommended.35 No high-grade inflammation in calprotectin test was found and all microflora determinations were saprophytic. As for the rectal histological evaluation, one subject had a persistent spirochetosis and four participants had epithelial erosion or ulceration at different time points, all five individuals were asymptomatic. All participants had ≥85% adherence measured by MEMS.
Pharmacokinetics
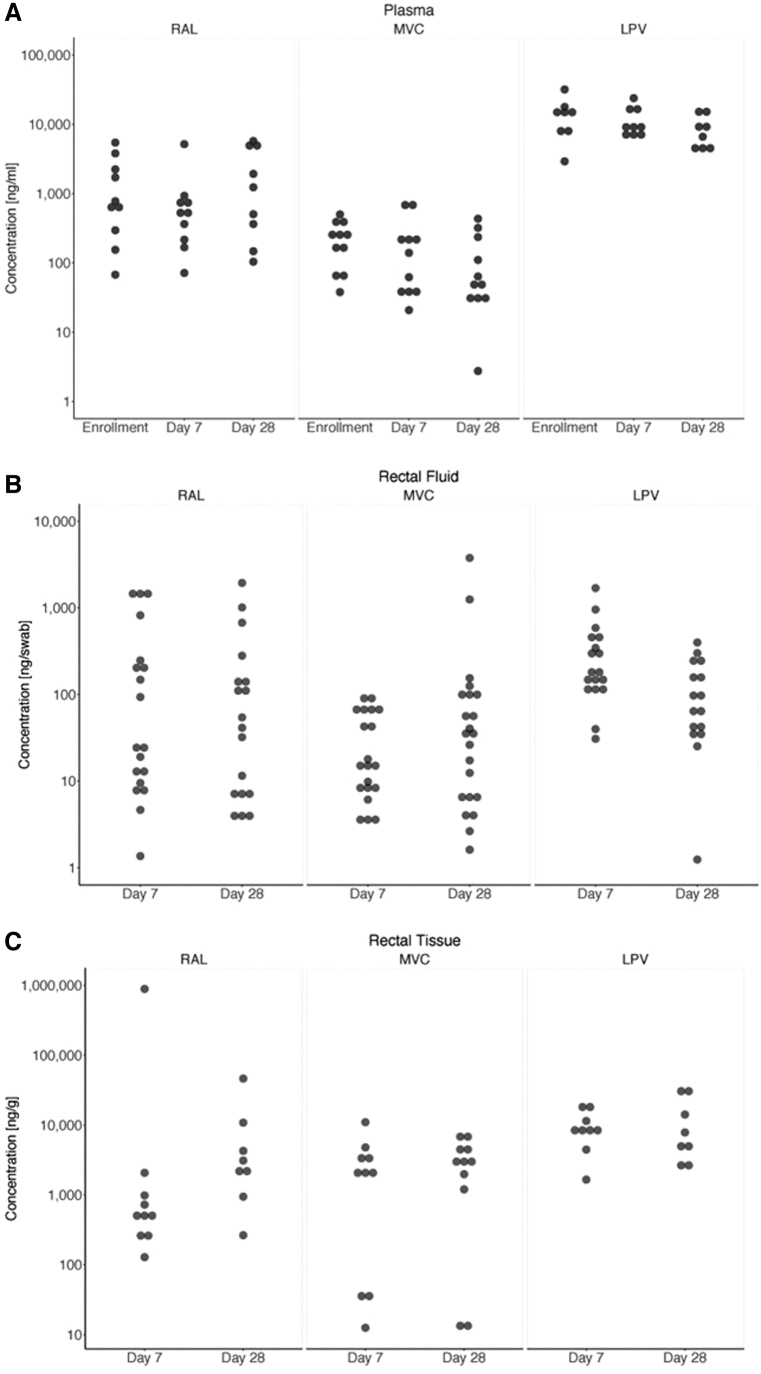
PK blood samples were obtained at substudy inclusion and, as RT samples, at days 7 and 28. The participants started PEP at different hours during the 24-day and due to center logistics the samples could only be obtained during the morning course, hence a wide variety of different drug concentrations were obtained. Plasma ARV median (IQR) concentrations for all study drugs according to PK time points were as follows: MVC 139 ng/mL (221.7), RAL 660 ng/mL (1,820.2), and LPV/r 8,668/542 ng/mL (8,670/816). The RF median (IQR) concentrations were MVC 30 ng/swab (65), RAL 41 ng/swab (224), and LPV/r 151/32 ng/swab (249/47).
Concentrations median (IQR) in RT were MVC 962 ng/g (3,855), RAL 2,714 ng/g (2,979), and LPV 8,151/3,000 ng/g (10,979/2,061) (Fig. 2A–C). We found no significant differences between days 7 and 28 in any of the studied tissues. The ratio of RT versus plasma for all three studied drugs was MVC 16.8, RAL 4.8, and LPV 0.7. We performed an analysis to correlate PK results in different tissues and different time points. We found that RF PK correlates with RT PK at day 7 (r = 0.5, p = .005) and at day 28 (r = 0.45, p = .002), and also with Plasma PK (day 7 r = 0.5, p = .004; day 28 r = 0.44, p = .02). We found a correlation between RT and plasma in the LPV arm (r = 0.51, p = .04).
FIG. 2.
(A–C) PK boxplots of each antiretroviral according to time since last dose, study time points (enrolment, days 7 and 28) and all three studied tissues: plasma, rectal fluid, and rectal tissue. Data collection about time since last dose was incomplete, which explains the large variability seen in the results.
Immunological and inflammation changes
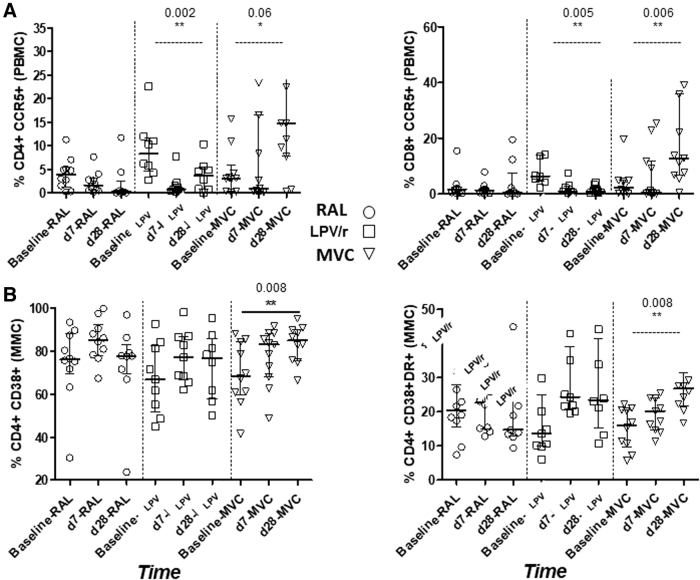
In patients receiving LPV/r there was significant variability in CCR5 expression by PBMC CD4 T cells (p = .002) and a trend in MVC group (p = .06) also relevant in CD8 T cells for LPV/r (p = .005) and MVC (p = .006) over time. Regarding MMC, there was significant variability over time in participants receiving MVC in CD38 and CD38DR expression by CD8 T cells (p = .008) (Fig. 3). Post-test comparisons revealed significant differences between baseline and day 28; for PBMC in LPV/r arm CD4+CCR5 (p = .12) and CD8+CCR5 (p = .01); for MMC in MVC arm CD4 CD38 (p = .10) and CD4 CD38R (p = .005). There were no significant changes in RF cytokines. Inflammation markers such as hs-CRP, IL-6, and TNF-α were assessed in plasma and we found no differences neither between time points nor arms. In the MVC arm, d-dimer levels decreased between baseline and day 7 (339 vs. 220, p = .037). All variations over time regarding immune system homeostasis are shown in Table 1.
FIG. 3.
Significant immunological effects in (A) PBMC and (B) MMC of the three studied antiretrovirals according to different time points. The interquartile range and median of the indicated subsets are shown. Pairwise comparisons were made using a Friedman test and Dunn's post-test for two pairwise comparisons. MMC, mucosal mononuclear cell; PBMC, peripheral blood mononuclear cell.
Table 1.
Immune System Homeostasis: Variations Over Time of Peripheral Blood and Mucosal Mononuclear Cells Phenotype Outcome, and of Inflammation Markers and Rectal Cytokines
| PBMC | LPV/r | MVC | RAL | p |
|---|---|---|---|---|
| CD4% | ||||
| 90 days (baseline) | 32.4 (20.6–38.2) | 40.2 (29.0–43.1) | 37.5 (27.6–42.3) | NS |
| 7 days | 33.5 (27.6–38.7) | 40.7 (30.6–43.6) | 32 (27.8–45.1) | NS |
| 28 days | 26.6 (19.8–44.1) | 42.3 (28.5–46.3) | 43.6 (32.5–46.5) | NS |
| Overall p | NS | NS | .015 | |
| CD4+CD28+ % | ||||
| 90 days (baseline) | 92.1 (83.2–95.8) | 94.2 (86.6–96.1) | 94.5 (92.2–97.9) | NS |
| 7 days | 91.3 (81.1–97.7) | 94.7 (86.3–98.1) | 98.4 (89.3–99.7) | NS |
| 28 days | 94.9 (90.5–97.1) | 94.8 (88.8–98.8) | 98 (93.8–99.6) | NS |
| Overall p | NS | NS | .038 | |
| CD4+RA+CCR7+ % | ||||
| 90 days (baseline) | 36.8 (26.8–62.4) | 42.8 (32.7–84.4) | 28.7 (26.8–46.2) | NS |
| 7 days | 31.9 (19.9–44.7) | 40.8 (30.3–70) | 41.5 (26.2–50.7) | NS |
| 28 days | 31 (26.2–49.9) | 41.4 (30.4–70.8) | 29.5 (25.2–47.2) | NS |
| Overall p | NS | NS | .037 | |
| CD4+CCR5+ % | ||||
| 90 days (baseline) | 10.3 (5.0–17.2) | 3.0 (0.3–5.8) | 2.9 (0.2–4.9) | .015 |
| 7 days | 0.7 (0.3–1.8) | 0.9 (0.4–16.4) | 1.6 (0.5–3.1) | NS |
| 28 days | 3.7 (0.7–5.4) | 14.7 (7.7–30.04) | 0.3 (0.04–2.4) | .002 |
| Overall p | .002 | .06 | NS | |
| CD8+CCR5+ % | ||||
| 90 days (baseline) | 6.5 (4.7–24.6) | 2.3 (0.2–4.8) | 1.6 (0.4–2.4) | .005 |
| 7 days | 0.9 (0.6–3.4) | 0.6 (0.1–11.7) | 1.3 (0.4–2.1) | NS |
| 28 days | 1.0 (0.4–2.8) | 12.8 (6.8–35.8) | 0.4 (0.00–1.7) | .001 |
| Overall p | .005 | .006 | NS | |
| MMC | LPV/r | MVC | RAL | p |
|---|---|---|---|---|
| CD4/CD8 ratio | ||||
| 90 days (baseline) |
1.0 (0.5–1.7) |
1.1 (0.5–1.9) |
2.5 (1.1–3.5) |
.017
|
| 7 days |
1.86 (0.76–2.61) |
1.57 (0.67–2.68) |
3.33 (1.29–4.19) |
NS |
| 28 days |
1.35 (0.63–2.09) |
1.39 (1.14–2.15) |
1.47 (0.83–2.44) |
NS |
| Overall p |
NS |
NS |
NS |
|
| CD4+CD38+ % |
|
|
|
|
| 90 days (baseline) |
66.8 (51.9–82.8) |
68.5 (59.6–84.7) |
76.3 (69.7–88.1) |
NS |
| 7 days |
77.2 (66.9–86.9) |
83.2 (68.2–88.5) |
85.4 (78.0–92.4) |
NS |
| 28 days |
76.8 (57.9–86.0) |
85.1 (75.8–90.1) |
77.8 (69.3–83.0) |
NS |
| Overall p |
NS |
.008
|
NS |
|
| CD4+CD38+DR+ % | ||||
| 90 days (baseline) |
13.5 (9.9–25.0) |
16.1 (9.7–20.5) |
20.3 (15.4–27.8) |
NS |
| 7 days |
24.3 (20.9–39.0) |
20.2 (14.6–24.0) |
22.6 (14.8–26.9) |
NS |
| 28 days |
23.4 (15.1–41.5) |
26.7 (21.8–31.2) |
14.7 (12.9–24.6) |
NS |
| Overall p |
NS |
.008
|
NS |
|
| CD4+CD28+ % | ||||
| 90 days (baseline) |
98.7 (97.3–99.3) |
99.2 (97.9–99.8) |
99.5 (99.2–99.6) |
NS |
| 7 days |
99.9 (99.5–100.0) |
99.2 (97.6–99.7) |
99.9 (98.8–100.0) |
NS |
| 28 days |
99.9 (98.5–100.0) |
99.9 (98.7–100.0) |
98.9 (97.1–99.8) |
NS |
| Overall p |
.02
|
NS |
NS |
|
| CD4+RA+CCR7+ % | ||||
| 90 days (baseline) |
9.8 (5.5–27.2) |
15.9 (8.1–38.1) |
13.1 (8.8–20.3) |
NS |
| 7 days |
30.5 (8.8–70.9) |
32.3 (14.8–37.5) |
27.8 (20.3–53.8) |
NS |
| 28 days |
14.4 (8.4–40.9) |
38.4 (24.9–58.6) |
15.4 (3.5–21.9) |
.001
|
| Overall p |
.02
|
NS |
NS |
|
| CD4+RA−CCR7− % | ||||
| 90 days (baseline) |
28.2 (7.6–74.4) |
18.1 (1.6–41.6) |
11.0 (6.0–40.1) |
NS |
| 7 days |
9.7 (0.3–26.9) |
7.1 (3.1–14.6) |
11.6 (3.2–21.9) |
NS |
| 28 days |
15.5 (6.5–42.0) |
3.4 (2.8–10.5) |
17.8 (9.2–33.1) |
.03
|
| Overall p |
NS |
NS |
NS |
|
| CD8+CD28+ % | ||||
| 90 days (baseline) |
49.4 (35.2–68.5) |
55.3 (46.1–62) |
62.2 (43.7–72.4) |
NS |
| 7 days |
66.1 (56.4–95.7) |
59.3 (50.5–77.5) |
69.4 (49.8–74.5) |
NS |
| 28 days |
51.8 (39.6–88.7) |
67.3 (48.4–80.2) |
53.5 (39.1–80.3) |
NS |
| Overall p |
.008
|
.021
|
NS |
|
| CD8+CD28−CD57+ % | ||||
| 90 days (baseline) |
3.3 (2.5–3.9) |
1.7 (0.7–5.1) |
1.5 (0.7–4.0) |
NS |
| 7 days |
0.0 (0.0–1.9) |
1.8 (0.7–3.7) |
1.4 (0.0–4.3) |
NS |
| 28 days |
1.8 (0.7–2.9) |
1.6 (0.7–3.1) |
3.4 (1.6–6.8) |
NS |
| Overall p |
.01
|
NS |
NS |
|
| CD8+RA+CCR7+ | ||||
| 90 days (baseline) |
9.8 (5.5–27.2) |
15.9 (8.1–38.1) |
13.1 (8.8–20.3) |
NS |
| 7 days |
30.5 (8.8–70.9) |
32.3 (14.8–37.5) |
27.8 (20.3–53.8) |
NS |
| 28 days |
14.4 (8.4–40.9) |
38.4 (24.9–58.6) |
15.4 (3.5–21.9) |
.01
|
| Overall p |
NS |
NS |
.013
|
|
| CD8+RA−CCR7− % | ||||
| 90 days (baseline) |
28.2 (7.6–74.4) |
18.1 (1.6–41.6) |
11.0 (6.0–40.1) |
NS |
| 7 days |
9.7 (0.3–26.9) |
7.1 (3.1–14.6) |
11.6 (3.2–21.9) |
NS |
| 28 days |
15.5 (6.6–42.1) |
3.4 (2.8–10.5) |
17.8 (9.2–33.1) |
.04
|
| Overall p |
NS |
NS |
NS |
|
| CD8+RA+CCR7− % | ||||
| 90 days (baseline) |
4.5 (2.1–7.8) |
1.9 (0.2–6.1) |
1.6 (0.0–2.5) |
NS |
| 7 days |
1.4 (0.9–3.4) |
1.9 (0.5–4.9) |
0.7 (0.0–3.1) |
NS |
| 28 days |
1.7 (0.1–3.3) |
0.9 (0.0–2.9) |
0.8 (0.0–1.8) |
NS |
| Overall p |
.012
|
NS |
NS |
|
| CD8+CCR5+ % | ||||
| 90 days (baseline) |
16.1 (6.3–27.3) |
7.8 (5.0–9.9) |
6.7 (1.8–14.6) |
NS |
| 7 days |
19.6 (2.7–46.6) |
6.6 (2.9–17.6) |
10.1 (1.4–33.6) |
NS |
| 28 days |
12.4 (8.0–19.9) |
17.8 (7.3–33.6) |
5.6 (0.3–10.2) |
.038
|
| Overall p | NS | NS | NS | |
| Inflammation markers | LPV/r | MVC | RAL | p |
|---|---|---|---|---|
| D-DIMER ng/mL | ||||
| 90 days (baseline) |
159 (101–297.5) |
333 (221.5–429.5) |
218 (90–326.5) |
NS |
| 7 days |
194 (124.5–274) |
236 (145–294) |
305 (177.5–364.7) |
NS |
| 28 days |
251 (136–299) |
228 (94.5–396) |
232 (90–290) |
NS |
| Overall p | NS | .037 | NS | |
| Rectal cytokines | LPV/r | MVC | RAL | p |
|---|---|---|---|---|
| IL-6 pg/mL | ||||
| 90 days (baseline) |
77.6 (46.2–375) |
96.2 (47.2–369.2) |
64.5 (18.9–161.6) |
NS |
| 28 days |
103.7 (61.1–212.2) |
71.6 (34.1–94.5) |
16.3 (11.2–26) |
.006
|
| Overall p |
NS |
NS |
NS |
|
| TNF-α pg/mL | ||||
| 90 days (baseline) |
8.8 (0.7–11.6) |
1.7 (0.6–5.7) |
4.3 (1.6–16.3) |
NS |
| 28 days |
8.2 (3.4–10.6) |
1.3 (0.7–3.8) |
1.8 (0.8–6.1) |
.003
|
| Overall p | NS | NS | NS | |
Significant p values in bold.
IL-6, interleukin-6; LPV/r, ritonavir-boosted lopinavir; MMC, mucosal mononuclear cell; MVC, maraviroc; NS, not significant; PBMC, peripheral blood mononuclear cell; RAL, raltegravir; TNF-α, tumor necrosis factor-alpha.
Ex-vivo infection of RT
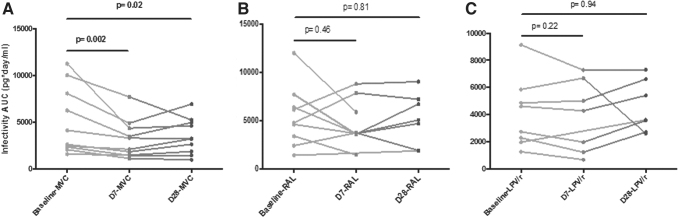
Overall, there were no differences in infectivity neither between time points nor arms. When we analyzed the differences within each arm, we found that AUC infectivity was significantly lower at day 7 (869 pg/mL) and at day 28 (948 pg/mL) compared with baseline (1,402 pg/mL) in the MVC arm (p = .005 and p = .028, respectively). No significant differences were observed between days 7 and 28 in MVC arm. There were no differences within LPV/r or RAL arms (Fig. 4).
FIG. 4.
(A–C) HIV infectivity AUC in rectal tissue according to study drug between time points (baseline D90—no treatment, D7—treatment initiation, D28—end of treatment). AUC, area under the curve.
We also analyzed the cumulative HIV-1 p-24 by CD4+ T cell count in each explant tissue (Supplementary Fig. S2). In this analysis, exposure to MVC and RAL at 7 days showed a trend to suppress HIV-1BAL (p = .07), but this effect was not observed at 28 days or with LPV/r.
Correlations between PK, infectivity, and immunological changes
When we analyzed the associations between PK and other parameters, we found a statistically significant inverse correlation between plasma drug concentrations and the changes in infectivity [merging together the three arms (day 7 r = −0.398, p = .049) (day 28 r = −0.434, p = .03)]. No other significant correlations were found between RF or RT drug concentrations and infectivity or between PK in plasma, RF or RT, and PBMC and MMC changes, cytokines or inflammation markers.
We did not find any significant correlations between infectivity and PBMC changes or inflammation markers in plasma. Merging together the changes in MMC in all three arms, we found a positive correlation in the CD8 T cell lineage between the infectivity at day 7 and activation (CD38+ r = 0.43, p = .025, DR + r = 0.547, p = .003 and 38+DR+ r = 0.526, p = 5), senescence (CD57+ r = 0.479, p = .012), naive cells (RA+CCR7+ r = 0.484, p = .01), and CCR5 expression (r = 0.593, p = .001). A negative correlation was found between infectivity at day 7 and CD8 central memory T cells (RA-CCR7+ r = −0.435, p = .023). When the cohort was separated by arms, we also observed significant correlations in the CD8 T cell lineage in the MVC arm.
There was a positive correlation at day 7 between infectivity and activation (CD38+ r = 0.64, p = .03, DR + r = 0.835, p = .001 and CD38 + 38DR+ r = 0.868, p = .001), senescence (CD28–57+ r = 0.714, p = .014), memory T cells (RA+CCR7+ r = 0.756, p = .007), and CCR5 expression (r = 0.820, p = .002). Also, a negative correlation between infectivity and naive T cells at same time point (RA-CCR7+ r = −0.647, p = .03). Regarding association between infectivity and cytokine concentrations in RF, at day 28 there was a significant positive correlation between infectivity and IP10 (r = 0.457, p = .043) and MIP1β (r = 0.475, p = .019) as well as at day 90 between infectivity and MIG (r = 055, p = .012).
Discussion
Several studies have been conducted evaluating anti-HIV ex-vivo efficacy of different ARVs but to our knowledge very few in an at-risk setting, as after a sexual risk exposure to HIV, and after reaching drug steady state, as in a PEP 28-day treatment.
We studied the PK, the immunological impact, and the effect on HIV-1 ex-vivo infectivity of MVC, an ARV that achieves high concentrations in rectal mucosa and has a relevant mechanism of action, of RAL, nowadays standard of care in PEP regimens and of LPV/r still the standard of care for PEP in resource-limited settings. In this study we have two main findings: (1) participants receiving twice-daily MVC for PEP showed a reduction of viral replication in ex-vivo RT explants; however, this association was not observed with normalized p24 values obtained using the CD4 T cell count; and (2) the ex-vivo infectivity correlated with activation and senescence in CD8 MMC and cytokine concentrations in RF.
Prior studies have shown a lack of efficacy of MVC preventing an HIV infection.11,12,36 These studies were trying to prove MVC preventive efficacy in what could be interpreted as an on-demand modality. In our study MVC was taken twice a day, which could result in tissue drug accumulation and maintained systemic drug concentrations10 that may possibly have a relation with protecting against rectal HIV ex-vivo infection. Other recent study found significant suppression of HIV ex-vivo infectivity after giving MVC 24 weeks as PrEP, but when it was compared with a combined treatment as TDF–disoproxil–fumarate/FTC this effect was considered poor.13 Another study using a PK-pharmacodynamic modeling to predict the tissue concentration profile of MVC alone or in combination with FTC or TDF showed that this combination prolonged the duration of the protection.37
This effect could explain our results with this drug, but we also must consider that, we prescribed a higher dose of MVC (300 mg QD vs. 300 mg BID) and for a longer period of time as compared with most of the studies assessing ex-vivo HIV infectivity, without any significant impact in safety and tolerance. Previous studies in animal models and in HIV-infected individuals have shown an increased T cell CCR5+ expression in PBMC when taking MVC. It has been hypothesized38 that these changes could have implications for preventive efficacy because it might increase the risk of HIV infection, but in our study did not seem to have this negative effect. In fact, according to our previous findings in HIV-exposed uninfected individuals, the immune activation of CD4+ T cells by itself is not sufficient to favor HIV infection.39,40
Unlike MVC, the other studied ARV did not protect against rectal HIV ex-vivo infection. As for RAL, one of the possible reasons for this lack of protection could be the suboptimal drug concentrations we found in RT. Herrera et al showed that RAL can have high levels in RT, but this did not correlate with higher ex-vivo protection.15 Having this into consideration, we could attribute our results due to inadequate drug compliance, although our adherence monitoring showed differently. Further investigation on this drug should be noted.
As for LPV/r, we found no differences on inflammation and activation markers in blood, as previously described,41,42 but there was a significant increase of the senescence and inflammation markers in RT, which could have contributed to HIV replication.43 To the best of our knowledge there are no other studies describing these results.
Finally, we observed that ex-vivo infectivity was correlated with CD8 T cells activation and inflammation in the rectal compartment. We could speculate that those viruses with higher potential to induce inflammation/immune activation in rectal compartments, have more potential to infect their target cells. In any case, all these data should be confirmed. In addition, having more naive cells after treatment could be due to either a reversion of more differentiated cells or to a new production of cells, although to have a more accurate answer to the meaning of this increase in cells with a naive phenotype would probably need not only a phenotypic analysis of cells but also a functional assay, and this could not be done.
Our study has several limitations. Owing to the nature of enrolment, no true baseline samples were available and considering that participants had a potential HIV risk exposure, immunology could have been affected and impact the results, as could be the differences found in several immune markers at baseline between arms. We have performed the ex-vivo HIV infection in cryopreserved RT and there have been some concerns that a frozen sample does not reproduce reliable infectivity data.44 In an attempt to diminish this possible effect an AUC statistical analysis was performed.
A recent study have found noninferiority for HIV-infection assays between fresh and cryopreserved colorectal tissues,30 and there is a suggestion that MVC is lost from tissue during incubation as compared with immediate frozen samples,20 which supports this approach. Participants started PEP at different timings, even at midnights, and all the tests were performed only at mornings, so we obtained a wide variety of concentrations in all different tissues. Also, there are no previous studies available in RF to compare our results and at the same time the amount of mucosal lining fluid on the sponges differed among the participants. Considering all this heterogeneity, it is difficult to draw any strong conclusion, so we need to be cautious. Further studies are required to confirm our findings.
In summary, MVC used twice daily and in a steady state reduces viral replication in ex-vivo RT explants. In contrast, neither RAL nor LPV/r had any impact in HIV ex-vivo RT infection and this absence of effect could be related with poor distribution at this level, but we cannot rule out the possibility that the explants challenge assay may not be suitable for characterizing these ARV profile in RT. The data that we present could be helpful to achieve progress in understanding and predicting which ARV has better characteristics for mucosal tissue penetration, which is necessary to rapid advance in the prevention arena.
Supplementary Material
Acknowledgments
This study would not have been possible without the great dedication of all the participants. We would like to thank Dr. Peter Anton and his team at MICL for their invaluable guidance and teachings before starting this project. We would like to give a special thanks to Prof. Sheena McCormack for her generous help in developing this project. We would like to acknowledge the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410.
Authors' Contributions
L.L., J.M.G., M.P., and F.G. conceived and designed the study. L.L. and F.G. conducted the clinical trial. C.R.M., J.L.L., and C.R. obtained and processed all the different samples. A.D.M.K. conducted the PK analysis. L.M.B., M.B., and J.A. conducted the ex-vivo infections. A.C.G., N.C., and M.P. conducted the immunogenicity analyses. L.L., M.P., and F.G. undertook the statistical analysis. L.L., A.C.G., L.M.B., M.P., and F.G. drafted the article. All authors contributed to the article and approved the submitted version.
Author Disclosure Statement
J.M.G. has received honoraria for speaking and advisory boards and his institution research grants from ViiV, MSD, Janssen, and Gilead. Since May 1st 2018, J.M.G. is a full-time employee of ViiV Healthcare. The rest of the authors do not have a commercial or other association that might pose a conflict of interest.
Funding
This study was partially supported by grants: SAF2015-66193-R, Instituto Carlos III (FIS PI15/00641, ISCIII-FIS PI16CIII/00034) and the Spanish AIDS Research Network RD16/0025/001 that is included in the Spanish I+D+I Plan and is cofinanced by ISCIII-Subdirección General de Evaluación and European Funding for Regional Development (FEDER).
Supplementary Material
References
- 1. UNAIDS Data 2021. Licence: CC BY-NC-SA 3.0 IGO. Joint United Nations Programme on HIV/AIDS: Geneva; 2021. [Google Scholar]
- 2. Sullivan PS, Satcher Johnson A, Pembleton ES, et al. Epidemiology of HIV in the USA: Epidemic burden, inequities, contexts, and responses. Lancet 2021;397(10279):1095–1106; doi: 10.1016/S0140-6736(21)00395-0 [DOI] [PubMed] [Google Scholar]
- 3. Ford N, Shubber Z, Calmy A, et al. Choice of antiretroviral drugs for postexposure prophylaxis for adults and adolescents: A systematic review. Clin Infect Dis 2015;60(Suppl. 3):S170–S176; doi: 10.1093/cid/civ092 [DOI] [PubMed] [Google Scholar]
- 4. Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363(27):2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015;373:2237–2246; doi: 10.1056/NEJMoa1506273 [DOI] [PubMed] [Google Scholar]
- 6. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. García-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med 2008;5(2):0291–0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Irvine C, Egan KJ, Shubber Z, et al. Efficacy of HIV postexposure prophylaxis: Systematic review and meta-analysis of nonhuman primate studies. Clin Infect Dis 2015;60(Suppl. 3):S165–S169; doi: 10.1093/cid/civ069 [DOI] [PubMed] [Google Scholar]
- 9. Lederman MM, Penn-Nicholson A, Cho M, et al. Biology of CCR5 and its role in HIV infection and treatment. J Am Med Assoc 2006;296(7):815–826; doi: 10.1001/jama.296.7.815 [DOI] [PubMed] [Google Scholar]
- 10. Brown KC, Patterson KB, Malone SA, et al. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis 2011;203(10):1484–1490; doi: 10.1093/infdis/jir059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coll J, Moltó J, Boix J, et al. Oral single-dose maraviroc does not prevent ex vivo HIV infection of rectal mucosa in healthy HIV-1 negative human volunteers in tissue explants. AIDS 2015;29(16):2149–2154. [DOI] [PubMed] [Google Scholar]
- 12. Fox J, Tiraboschi JM, Herrera C, et al. Brief Report: Pharmacokinetic/pharmacodynamic investigation of single-dose oral maraviroc in the context of HIV-1 pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2016;73(3):252–257. [DOI] [PubMed] [Google Scholar]
- 13. McGowan I, Wilkin T, Landovitz RJ, et al. The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing PrEP regimens in men who have sex with men. AIDS 2019;33(2):237–246; doi: 10.1097/QAD.0000000000002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massud I, Martin A, Dinh C, et al. Pharmacokinetic profile of raltegravir, elvitegravir and dolutegravir in plasma and mucosal secretions in rhesus macaques. J Antimicrob Chemother 2015;70(5):1473–1481; doi: 10.1093/jac/dku556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrera C, Lwanga J, Lee M, et al. Pharmacokinetic/pharmacodynamic investigation of raltegravir with or without lamivudine in the context of HIV-1 pre-exposure prophylaxis (PrEP). J Antimicrob Chemother 2021;76(8):2129–2136; doi: 10.1093/jac/dkab136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European AIDS Clinical Society (EACS). European AIDS Clinical Society (EACS) Guidelines 11.0. 2021. [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC). Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection Drug Use, or Other Nonoccupational Exposure to HIV—United States, 2016. 2016. [DOI] [PubMed] [Google Scholar]
- 18. Kaplan JE, Dominguez K, Jobarteh K, et al. Postexposure prophylaxis against human immunodeficiency virus (HIV): New guidelines from the WHO: A perspective. Clin Infect Dis 2015;60(Suppl. 3):S196–S199; doi: 10.1093/cid/civ087 [DOI] [PubMed] [Google Scholar]
- 19. Cranston RD, Dezzutti CS, Siegel A, et al. A multiple dose phase 1 assessment of rilpivirine long acting in a model of preexposure prophylaxis against HIV. AIDS Res Hum Retrovir 2019;35(9):794–804; doi: 10.1089/aid.2018.0265 [DOI] [PubMed] [Google Scholar]
- 20. Cranston RD, Lama JR, Richardson BA, et al. MTN-017: A rectal phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin Infect Dis 2017;64(5):614–620; doi: 10.1093/cid/ciw832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGowan IM, Chawki S, Hendrix CW, et al. A randomized, open-label, crossover phase 1 safety and pharmacokinetic study of oral maraviroc and maraviroc 1% gel (the CHARM-03 Study). AIDS Res Hum Retrovir 2022;38(4):269–278; doi: 10.1089/aid.2021.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leal L, León A, Torres B, et al. A randomized clinical trial comparing ritonavir-boosted lopinavir versus maraviroc each with tenofovir plus emtricitabine for post-exposure prophylaxis for HIV infection. J Antimicrob Chemother 2016;71(7):1982–1986; doi: 10.1093/jac/dkw048 [DOI] [PubMed] [Google Scholar]
- 23. Leal L, León A, Torres B, et al. A randomized clinical trial comparing ritonavir-boosted lopinavir versus raltegravir each with tenofovir plus emtricitabine for post-exposure prophylaxis for HIV infection. J Antimicrob Chemother 2016;71(7):1987–1993; doi: 10.1093/jac/dkw049 [DOI] [PubMed] [Google Scholar]
- 24. Gesida. Documento de Consenso sobre Profilaxis postexposición ocupacional y no ocupacional en relación con el VIH, VHB y VHC en adultos y niños. 2015. Available from: www.gesida-seimc.org/guias_clinicas.php [Last accessed: June 10, 2015]. [DOI] [PubMed]
- 25. Fletcher PS, Elliott J, Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006;20(9):1237–1245; doi: 10.1097/01.aids.0000232230.96134.80 [DOI] [PubMed] [Google Scholar]
- 26. McGowan I, Elliott J, Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056). J Acquir Immune Defic Syndr (1999) 2007;46(4):417–425; doi: 10.1097/QAI.0b013e318156ef16 [DOI] [PubMed] [Google Scholar]
- 27. Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 2000;14(March):1761–1765; doi: 10.1097/00002030-200008180-00011 [DOI] [PubMed] [Google Scholar]
- 28. Geboes K. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47(3):404–409; doi: 10.1136/gut.47.3.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shacklett BL, Yang O, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods 2003;279(1–2):17–31; doi: 10.1016/S0022-1759(03)00255-2 [DOI] [PubMed] [Google Scholar]
- 30. Hughes SM, Ferre AL, Yandura SE, et al. Cryopreservation of human mucosal tissues. PLoS One 2018;13(7):e0200653; doi: 10.1371/journal.pone.0200653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson CG, Cohen MS, Kashuba ADM. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr 2013;63(6, Suppl. 2):S240–S247; doi: 10.1097/QAI.0b013e3182986ff8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patterson KB, Prince HA, Stevens T, et al. Differential penetration of raltegravir throughout gastrointestinal tissue. AIDS 2013;27(9):1413–1419; doi: 10.1097/QAD.0b013e32835f2b49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trezza CR, Kashuba ADM. Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: Implications for HIV prevention. Clin Pharmacokinet 2014;53(7):611–624; doi: 10.1007/s40262-014-0148-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guardo AC, Álvarez-Fernández C, Arberas H, et al. Use of RT-defective HIV virions: New tool to evaluate specific response in chronic asymptomatic HIV-infected individuals. PLoS One 2013;8(3):e58927; doi: 10.1371/journal.pone.0058927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mensa J, Gatell JM, García-Sánchez JE, et al. Guía de Terapeútica Antimicrobiana. Editorial Antares; 2013. [Google Scholar]
- 36. Massud I, Aung W, Martin A, et al. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol 2013;87(16):8952–8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Srinivas N, Cottrell M, Maffuid K, et al. Translational approach to predicting the efficacy of maraviroc-based regimens as HIV preexposure prophylaxis. Antimicrob Agents Chemother 2020;64(2):e01729-19; doi: 10.1128/AAC.01729-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Restrepo C, Rallón NI, del Romero J, et al. Low-level exposure to HIV induces virus-specific T cell responses and immune activation in exposed HIV-seronegative individuals. J Immunol 2010;185(2):982–989; doi: 10.4049/jimmunol.1000221 [DOI] [PubMed] [Google Scholar]
- 39. Suy A, Castro P, Nomdedeu M, et al. Immunological profile of heterosexual highly HIV-exposed uninfected individuals: Predominant role of CD4 and CD8 T-cell activation. J Infect Dis 2007;196(8):1191–1201; doi: 10.1086/521193 [DOI] [PubMed] [Google Scholar]
- 40. García F, Plana M, Mestre G, et al. Metabolic and immunological effects of antiretroviral agents in healthy individuals receiving post-exposure prophylaxis. Antivir Ther 2002;7(3):195–197. [PubMed] [Google Scholar]
- 41. Kelesidis T, Moser C, Stein JH, et al. Changes in markers of t-cell senescence and exhaustion with atazanavir-, raltegravir-, and darunavir-based initial antiviral therapy: Actg 5260s. J Infect Dis 2016;214(5):748–752; doi: 10.1093/infdis/jiw253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kelesidis T, Tran TT, Stein JH, et al. Changes in inflammation and immune activation in atazanavir-, raltegravir-, darunavir- based initial antiviral therapy: ACTG 5260s. Clin Infect Dis 2015;61(4):651–660; doi: 10.1093/cid/civ327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seoane R, Vidal S, Bouzaher YH, et al. The interaction of viruses with the cellular senescence response. Biology (Basel) 2020;9(12):455; doi: 10.3390/biology9120455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McGowan I, Tanner K, Elliott J, et al. Nonreproducibility of “snap-frozen” rectal biopsies for later use in ex vivo explant infectibility studies. AIDS Res Hum Retroviruses 2012;28(11):1509–1512; doi: 10.1089/aid.2012.0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.