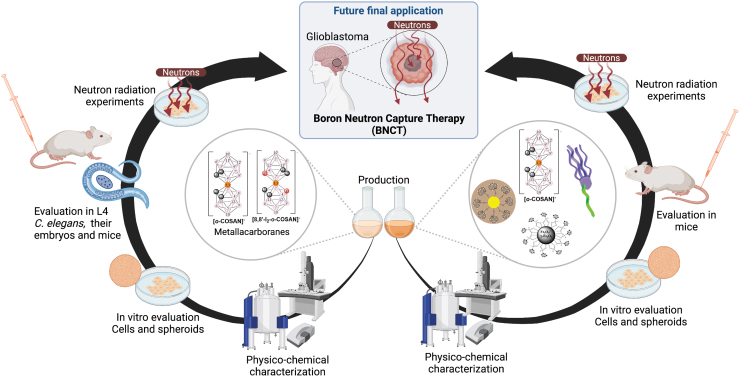
Abstract
This proceeding article compiles current research on the development of boron delivery drugs for boron neutron capture therapy that was presented and discussed at the National Cancer Institute (NCI) Workshop on Neutron Capture Therapy that took place on April 20–22, 2022. The most used boron sources are icosahedral boron clusters attached to peptides, proteins (such as albumin), porphyrin derivatives, dendrimers, polymers, and nanoparticles, or encapsulated into liposomes. These boron clusters and/or carriers can be labeled with contrast agents allowing for the use of imaging techniques, such as PET, SPECT, and fluorescence, that enable quantification of tumor-localized boron and their use as theranostic agents.
Keywords: albumin, BNCT, boron cluster, liposome, peptide, porphyrin
Introduction
Boron Neutron Capture therapy (BNCT) is a binary therapy treatment form of radiotherapy based on the ability of 10B nuclei to capture low-energy neutrons and subsequent fission of the resulting excited nuclei to produce high-linear energy transfer α-particles and recoiling lithium-7 nuclei as shown in equation (1):
| (1) |
The biologically abundant nuclei 12C (0.0034 barn), 1H (0.33 barn), and 14N (1.8 barn) show negligible interference with the 10B (n,α)7Li neutron capture reaction due to their much smaller nuclear cross sections in comparison with 10B (3,838 barns). Since the high-linear energy transfer particles have <10 μm path length in tissue, the BNCT effect is localized to the 10B-containing cells.
Two boron delivery agents have been used in BNCT clinical trials for malignant brain tumors, melanomas, and squamous cell carcinomas: sodium mercaptoundecahydro-closo-dodecaborate (Na210B12H11SH), designated BSH, and (L)-4-dihydroxy-borylphenylalanine, known as l-BPA, often delivered as a water-soluble fructose or sorbitol complex. BSH was the first successful boron delivery agent used for the treatment of malignant brain tumor patients in BNCT. BSH can access a brain tumor when the blood–brain barrier (BBB) is disrupted. BPA, on the contrary, was first used in patients with melanoma. Initially, racemic forms were used, but L-BPA was found to be more potent than D-BPA and is now widely used in BNCT. Further, L-BPA has been found to accumulate in a wide variety of tumors, including malignant brain tumors and head and neck cancers as well as melanoma.
Thus, BSH is not currently used in the clinical practice of BNCT.1–4 Since 2020, the company Stella Pharma is allowed to market Steboronine® (generic name: Borofalan), which is 10B-enriched (99%) l-BPA as its d-sorbitol complex. Also recently, the technology for generation of neutrons using accelerators has been developed in various countries, making the development of novel, selective boron carriers an important and much needed task. Over the last decades, new boron delivery agents emerged for application in BNCT of various cancers. These agents possess several advantages over BSH and l-BPA, including the delivery of higher amounts of boron selectively to tumor cells.
Requirements of a Boron Agent for BNCT
The success of BNCT is highly dependent on the selective accumulation ability of 10B in the tumor cells and its intracellular biodistribution. Ideally, the boron agents used in BNCT should be able to maintain the 10B concentration in tumor at a level of ∼30 μg 10B/g tumor where an antitumor effect can be expected during neutron irradiation, and be safe with low systemic toxicity. In addition, the tumor tissue concentration/normal tissue concentration (T/N) and the tumor tissue concentration/blood concentration (T/B) ratios should be high (>3:1), while at the same time being rapidly expelled from normal tissue and blood after neutron irradiation. Furthermore, boron agents must comply with the International Council for Harmonization of Pharmaceutical Regulations (ICH) guidelines for “neoplastic agents”.
In March 2020, an accelerator-based BNCT for head and neck cancers with Steboronine® was approved by the Pharmaceuticals and Medical Devices Agency in Japan, making BNCT a much more accessible treatment. Although l-BPA is known to actively accumulate in cancer cells via the L-type amino acid transporter 1 (LAT-1), which is overexpressed in many cancer cells, there are still many patients for whom l-BPA is not applicable, which makes the need for new boron agents even more urgent. In addition, because of the rapid clearance of l-BPA, a high-dose infusion (500 mg/kg body weight) is often performed to maintain 10B concentration in blood.
The development of new boron carriers for BNCT has focused on small molecules of high boron content and boron compounds conjugated with biomolecules. Unlike boron-based pharmaceuticals, boron carriers for BNCT should be highly tumor selective and essentially nontoxic. Thus, compounds with a very large boron content5–9 and tumor selectivity have been developed, using specific shuttle systems that accumulate the BNCT agent within tumor cells by internalization processes.5,6,10–12 These include boron compounds conjugated with biomolecules such as peptides, growth factors, antibodies (mAbs), carrier proteins, and/or to porphyrin derivatives, which enable the use of photodynamic therapy (PDT) as an adjuvant to BNCT, and tumor detection via optical microscopy.
Boron Clusters as Scaffolds for BNCT
Aromatic compounds that play important roles in biochemistry find numerous applications ranging from drug delivery to nanotechnology or biological markers. The group of C. Viñas reached an important achievement in demonstrating experimentally and theoretically that neutral and anionic carboranes, as well as anionic metallabis(dicarbollides), display 3D global aromaticity.13 Based on the relationship between stability-aromaticity, they opened new applications of boron clusters as key components in the field of new materials for health care.14,15 One of the most promising boron delivery systems is icosahedral boron clusters, such as carborane, metallacarborane, and their derivatives, due to their high boron content and low toxicity in biological systems.
The group of C. Viñas has developed several strategies to prepare high boron containing nanomaterials for multimodal therapies, including in BNCT, by using icosahedral boron clusters that consist of their attachment onto nanocarriers, such as dendrimers,16 polymers,17 nanoparticles (gold,18,19 magnetic,20,21 or quantum dots22), leading to payloads with a high boron density. Parallel to their use as BNCT plus chemotherapy and/or phototherapy and/or hyperthermal therapy agents simultaneously, boron clusters are excellent scaffolds for diagnostic and therapeutic labeling, opening the door to a wide range of biomedical applications.
Regarding carboranes in biomedicine, research has been focused on the development of new multifunctional hybrid (carboranyl + anilinoquinazolines)23–26 and nanoparticles as nanocarriers and/or, as anticancer drugs that, exhibiting desirable in vitro antitumor activities against F98, HT29, A172, and hCMEC/D3 cancer cell lines, offer the possibility of dual-action (chemotherapy+BNCT23–26 and thermotherapy+BNCT21), which may result in significant clinical benefits for cancer treatment, particularly for glioblastomas.
With respect to the icosahedral metallacarboranes, the anionic metallabis(dicarbollides), [3,3-M(1,2-C2B9H11)2]-, (M = Co, Fe) are the most studied.27 The 3D aromatic Na[3,3-Co(1,2-C2B9H11)2] forms hydrogen and dihydrogen bonds that participate in its self-assembling, water solubility, and aggregates' formation.28 Metallabis(dicarbollides) have attracted much attention in biology because they are inert to biochemical reactions. The Na[3,3-Co(1,2-C2B9H11)2] complex possesses the ability to readily cross cellular membranes,29–31 not being cytotoxic, but cytostatic, and cells recover following its removal.31
Having performed experiments in a round-bottom flask on a chemical scale, the C. Viñas group showed that [3,3-Co(1,2-C2B9H11)2]- and some of its halogenated derivatives interact with biomolecules (amino acids,32 proteins,33,34 ds-DNA35,36 and glucose37). They observed these interactions in vitro experiments by changing the round-bottom flask to a cell and the solutions to the cell physiological components. The chemical scale studies were done individually, whereas the cell study incorporates the effect of all the interacting biomolecules. The group of C. Viñas went a step further by using SR-FTIRM to understand and detect that this anion modifies biomolecules (proteins, DNA, and lipids) and concentrates in the cells' nuclei after their cellular uptake.38 Recently, Na[3,3–57Fe(1,2-C2B9H11)2] demonstrated multitherapies' activity.39
Furthermore, carboranes and Na[3,3-Co(1,2-C2B9H11)2], which can be labeled with contrast agents such as 124I and 125I for in vivo markers by PET and SPECT nuclear imaging techniques make these clusters to be very good scaffolds as theranostic agents,40,41 accumulating selectively in the tumor tissue for diagnosis and multimodal therapies, speeding up action and diminishing secondary effects. Figure 1 summarizes the research of this section.
FIG. 1.
Carboranes and metallabis (dicarbollides) on the road to anticancer therapies: From synthesis, characterization, cellular uptake, and in vitro/in vivo biological evaluations to neutron irradiation to defeat cancer.
Carborane-Peptide Conjugates as Selective Agents for BNCT
Recently, peptides have captured much attention as therapeutic compounds.42,43 Cell-penetrating peptides play an important role in BNCT research for shuttling high amounts of boron into cancer cells. Michiue et al showed high uptake of a compound bearing three arginine (R, Arg) moieties and BSH (BSH-3R) in the tumor, investigated with DOTA-64Cu fused to BSH-3R and studied by PET, which is promising for clinical use.44 This approach was enhanced to eight BSH molecules and 11 arginine molecules in a dendritic lysine structure.3 Peptide derivatives with boron moieties such as boronated starburst dendrimers or BSH, applicable for BNCT, have been described.45–47 Furthermore, carboranes have been integrated into the peptide sequences of well-known therapeutic peptides for the selective delivery of a large amount of boron to tumor cells.
Betzel et al focused on agonists for the somatostatin (SST) receptor, which is overexpressed in many neuroendocrine tumors. Their developed carborane-conjugated Tyr3-octreotate derivatives showed high internalization rates binding affinities to the SST receptor subtype 2 in the nm range depending on the spacer length between the carborane and the cyclic peptide.48 Another attractive target is integrin ανβ3, which is overexpressed in various proliferating endothelial and tumor cells. In vitro cell adhesion assays of cyclic Arg-Gly-Asp (RGD) peptides conjugated with BSH or ortho-carborane demonstrated the high binding affinity of the conjugates to integrin ανβ3.49 Furthermore, biodistribution studies showed a comparable tumor uptake but a significantly longer retention in tumors compared with BSH.49,50 These few selected examples already showcase the suitability of carborane–peptide conjugates as potential boron carriers for BNCT.
Other peptides, which were suggested as shuttle systems for the targeted delivery of pharmaceuticals into tumor cells are SST, epidermal growth factor, neurotensin, substance P, gastrin-releasing peptide, insulin-like growth factor, alpha-melanocyte stimulating hormone, cholecystokinin, vasoactive intestinal peptide, bombesin, and neuropeptide Y (NPY).43,51
The groups of Hey-Hawkins and Beck-Sickinger focus on combining tumor-selective small peptides as highly selective G protein-coupled receptor agonists with meta-carborane derivatives for targeted delivery (Fig. 2).1,2,52–54 They have devised efficient syntheses for novel boron compounds, which provide a combined tumor-targeting system: carborane-containing amino acids and carboxylic acids for incorporation in suitable peptides.53–57 The first tumor-selective peptide-carborane conjugates prepared comprised closo-carborane-modified NPY analogs58,59 or metallacarborane derivatives60; another approach was the incorporation of meta-carboranes in novel ghrelin receptor agonists.52 However, a very high carborane loading (more than two carborane clusters attached to a peptide, including 36 amino acids) resulted in loss of solubility or aggregation in aqueous media, resulting in decreased potency and higher EC50 values.1,2
FIG. 2.
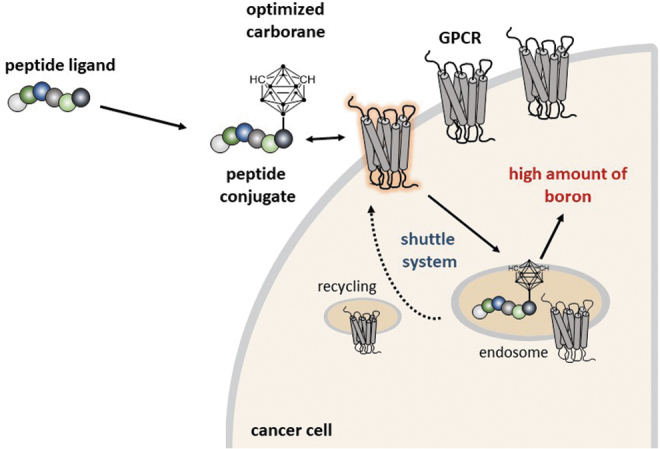
Tumor-selective peptides as highly selective GPCR agonists with meta-carborane derivatives facilitate targeted delivery of high amounts of boron to specific tumor cells. GPCR, G protein-coupled receptor.
Therefore, carborane derivatives bearing water-soluble groups, namely carbohydrate moieties, specifically galactosyl groups, were used to compensate the hydrophobic character of the carborane clusters (up to eight modified carboranes attached to the same peptide comprising 36 amino acids).1 Furthermore, the change from d-galactosyl to l-galactosyl groups increased the selectivity of these derivatives due to a lower unspecific uptake of bioconjugates into liver tissue.2
In summary, the combination of tumor-targeting peptides and carboranes covalently linked to the peptide represents a very efficient shuttle system to transport large amounts of boron into respective target cells and can be considered as a promising approach in tumor-selective boron shuttle system for BNCT.61
Boron-Albumin Conjugates for BNCT
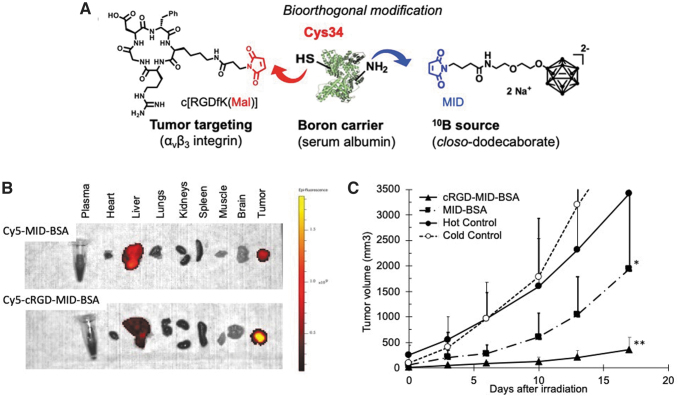
To improve the efficacy of boron transport to tumors, Nakamura's group focused on serum albumin as a boron biocarrier. Serum albumin is the major component of plasma proteins and accounts for ∼55% of the human plasma proteins. Albumin is known to accumulate in malignant tumor and inflamed tissues due to its enhanced permeability and retention (EPR) effect. The Nakamura group chose closo-dodecaborate (B12H12) as a boron source due to its exceptional physical stability, high water solubility, and high boron content. They designed and synthesized a maleimide-functionalized closo-dodecaborate (MID; Fig. 3A) suitable for conjugation with serum albumin at Cys34, which has the only free SH group among 35 Cys residues.62 Interestingly, MID was found to bind not only to Cys34 but also to lysine residues in bovine serum albumin (BSA) under physiological conditions.63
FIG. 3.
Design of cRGD-MID-albumin conjugates (A), their ex vivo imaging (B), and BNCT effect in U87MGxenograft model (C). BNCT, boron neutron capture therapy.
MID-BSA conjugates accumulated in colon 26 cells in a concentration-dependent manner. MID-BSA conjugates efficiently accumulated in mouse tumors, in contrast to boronated liposomes that were highly distributed in other organs, such as liver, kidney, and spleen, 12 h after administration.64,65 Administration of 7.5 mg [10B]/kg of MID-BSA conjugates showed significant tumor growth inhibition in tumor-bearing mice irradiated with thermal neutrons.
Further, Nakamura's group focused on the cyclic RGD (cRGD) peptide, which is known to bind to the integrins that are overexpressed in many cancer cells and designed cRGD-MID-BSA.66 The bioorthogonal modification method of stepwise binding of c[RGDfK(Mal)] peptide and MID to BSA allowed the formation of cRGD-MID-BSA conjugates (Fig. 3A). Selective accumulation of cRGD-MID-BSA was observed against U87MG cells overexpressing integrin αvβ3. In vivo fluorescence live imaging of near infrared dye (Cy5)-conjugated cRGD-MID-BSA and MID-BSA revealed that cRGD-MID-BSA accumulates more selectively than MID-BSA (Fig. 3B). In vivo BNCT studies revealed that the cRGD peptide ligand combination promoted the accumulation of MID-BSA into tumor cells in U87MG human glioblastoma xenograft models.
After neutron irradiation, significant tumor growth suppression was observed at a cRGD-MID-BSA dose of 7.5 mg [10B]/kg (Fig. 3C). In summary, albumin was found to act as a carrier for boron to tumor, and cRGD conjugation of boronated albumin was effective in accumulating in U87MG human glioblastoma cells in vivo.
Boronated Porphyrin Derivatives for BNCT
The preferential accumulation of porphyrin-based compounds within certain tumors versus nearby normal tissues, and their current use in PDT,67–69 led to their investigation as boron delivery vehicles for BNCT. These properties along with their low dark toxicity, high chemical stability, and fluorescence properties led to their development as BNCT agents, as well as BNCT/PDT dual sensitizers. Several boronated porphyrin derivatives and their metal complexes were reported in the 1990s, including BOPP and VCDP.70–72
These porphyrin derivatives were evaluated in multiple in vitro and in vivo studies that revealed high tumor uptake, favorable localizing properties and retention ability in tumor bearing mice, with high T/B and T/N boron concentration ratios (up to 20:1). It was also reported that changing the delivery of boronated porphyrins from i.v. injection to convection-enhanced delivery (CED)73–75 dramatically increased the boron concentration in tumor as well as the T/B and T/N boron ratios, and that the combined administration of BOPP and L-BPA increased the tumor uptake compared with BOPP or l-BPA alone.73
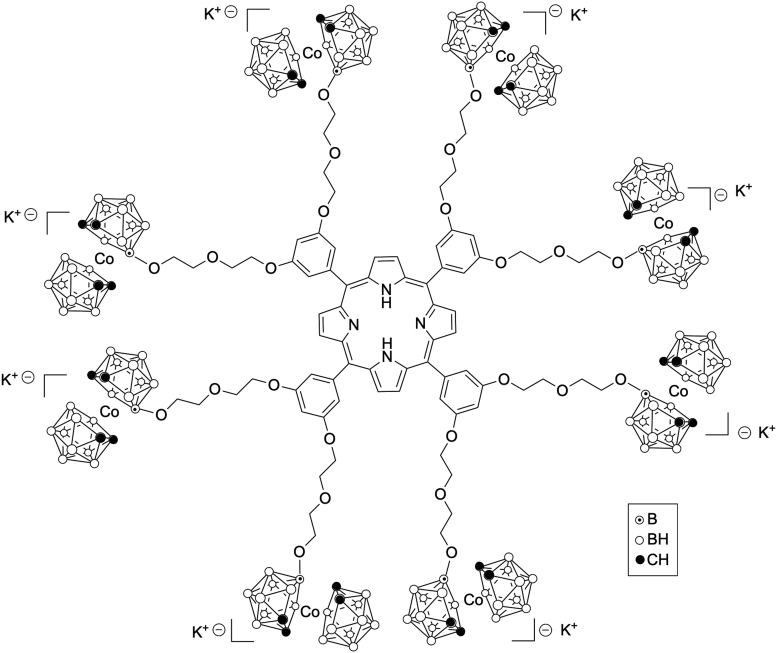
More recently, porphyrin conjugated to boron clusters, including carboranes, nido-carboranes, and cobaltabis(dicarbollides), via hydrolytically stable carbon-carbon links were reported by the Vicente group.76,77 Up to 16 boron clusters were introduced onto a porphyrin macrocycle, resulting in compounds of 35%–45% boron by weight (see example in Fig. 4), with the potential to deliver a high amount of boron to tumor cells.78–80 Despite the bulkiness of the boron clusters at the periphery of porphyrin macrocycles, some of these compounds were shown to interact with DNA and thereby produce in vitro DNA damage following light activation, making them highly promising as BNCT/PDT dual sensitizers.81,82
FIG. 4.
Molecular structure of a porphyrin-bearing multiple cobaltabis (dicarbollide) groups.
With the goal to further increase the tumor uptake of boronated porphyrins, the Vicente group investigated the conjugation of a boronated porphyrin to the cell-penetrating peptide from the human immunodeficiency virus I transcriptional activator HIV-1 Tat (48–60) with the sequence GRKKRRQRRRPPQ. This sequence was found to significantly enhance the uptake of the boronated porphyrin into T98G tumor cells.83 Similar observations were found upon the conjugation of polyamines, due to upregulation of the polyamine transport system in tumor cells.84 However, all boronated porphyrins investigated were found to have low BBB permeability using hCMEC/D3 brain endothelial cells, in part due to their high molecular weights and high degree of hydrophobicity, which could jeopardize their use for glioblastoma treatment unless delivered via CED.
On the contrary, boron dipyrromethenes, known as BODIPYs, have lower molecular weight, higher solubility, and lower toxicity compared with porphyrin derivatives, while displaying high fluorescence quantum yields (ϕf ∼ 0.50). The Vicente group recently reported a series of BODIPYs bearing one or two boron clusters and investigated their tumor cell uptake, toxicity, and BBB permeability.85,86 These studies showed that boronated BODIPYs exhibited low toxicity, high cellular uptake, and moderate BBB permeability, in part, due to their low molecular weight (<400 Da) and favorable hydrophobic properties (log P ∼ 1.50).
In summary, porphyrin derivatives are an important class of pharmacological agents for application in a variety of cancer treatments. The ability of boronated porphyrin derivatives for generation of singlet oxygen upon light activation makes them suitable for tumor treatment by PDT as adjuvant therapy to BNCT. In addition, their fluorescence properties and unique ability for metal complexation and functionalization allows the detection of tumor-localized boron before irradiation, via optical imaging, SPECT, and/or PET. On the contrary, boronated BODIPYs are emerging as very promising BNCT agents due to their low molecular weights, low toxicity, high tumor cellular uptake, high fluorescence, and moderate BBB permeability.
Boron-Rich Liposomes as Nanoscale Delivery Agents for BNCT
Another approach to boron agent development leverages nanotechnology, as explored by Jalisatgi's group. Through this protocol, nanoparticles are targeted to cancerous tissue, with each particle carrying many boron atoms. Nanoparticles of optimal composition are widely observed to accumulate selectively in tumor tissue due to the EPR effect. Furthermore, the surface of each nanoparticle may be modified to enhance stability, plasma circulation time, and tissue specificity. As demonstrated in Jalisatgi's laboratories, liposomal nanoparticles of varying compositions are promising boron agents for BNCT.6,87–90 These agents selectively deliver large quantities of boron to tumor tissues.
Liposomes are spherical nanoparticles composed of a lipid bilayer shell encapsulating an aqueous core. The liposome formulations investigated in the laboratories are small unilamellar vesicles, or SUVs and range in size between 100 to 130 nm in diameter. As liposomes possess both hydrophobic and hydrophilic environments (lipid bilayer and aqueous core), two modes of boron incorporation are available. Hydrophilic, water-soluble species may be encapsulated in the aqueous vesicle interior, and hydrophobic (lipophilic or amphiphilic) species may be embedded within the lipid bilayer.
The liposome project was initiated at UCLA by Professor M. Frederick Hawthorne in the 1990s and continued at the University of Missouri International Institute of Nano and Molecular Medicine (MU-I2NM2). This work has shown that small unilamellar liposomes (MAC-TAC liposomes) are very promising boron delivery agents since they are targeted to the cancer cell interior and or endothelial cells in the tumor vascular supply. This results in high selectivity for tumor as opposed to blood and normal tissue. This mechanism provides a long therapeutic time window. These MAC-TAC liposomes selectively accumulate in tumor tissue over a period of 30 to 48 h giving sufficient time for neutron irradiation procedures.6,89 These exquisite tumor-targeting materials are nontoxic to mice, hamsters, dogs, and cats. Hopefully this will also be true in the case of humans.
Nearly all therapeutic applications of liposomes rely on the stability of the liposome bilayer. This bilayer protects the encapsulated compounds from physiological degradation, thereby providing boron agents with a prolonged circulation lifetime. Liposomes constructed from pure, saturated phospholipids are particularly notable for their high stability and long survival half-lives in human plasma. Because of the similarity of liposomes to biological membranes and their construction from natural body constituents, they exhibit extremely low toxicity and may be safely administered without serious side effects.
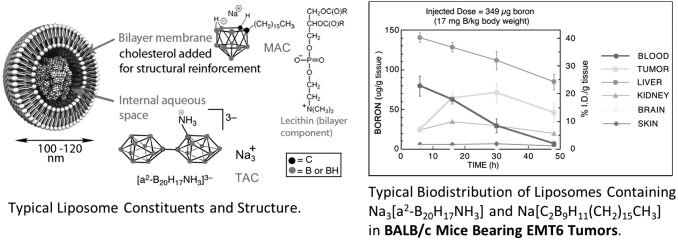
Extensive research was conducted at MU-I2NM2 optimizing liposome formulation in conjunction with animal biodistribution studies. This work made use of liposomes, which contain a water-soluble [B20H17NH2R]−2 polyhedral borane anion derivative and/or an amphiphilic nido-carborane anion species, [nido-7-CH3(CH2)15–7,8-C2B9H11]-, embedded in the bilayer. These liposomes contain 3–5 weight percent boron and are able to deliver therapeutic doses of 30 ppm, or greater, selectively to tumor tissues. Figure 5 is a graph representing a typical biodistribution experiment in BALB/c mice bearing subcutaneous EMT6 mammary adenocarcinoma.
FIG. 5.
Murine biodistribution of boron in BALB/c mice bearing subcutaneous EMT6 tumors, incorporating Na3[a2-B20H17NH3] and Na[C2B9H11(CH2)15CH3] anion species in liposomes, injected dose 349 μg B (17 mg B/kg body weight).
Liposomes are prepared by the probe ultrasonication of a dried lipid film composed of equimolar quantities of distearoylphosphatidylcholine and cholesterol (CH) in the presence of a hydrating solution (buffer or aqueous borane salt solution) incorporating K[nido-7-CH3(CH2)15–7,8-C2B9H11] in the lipid bilayer, and encapsulating Na3[1-(2’-B10H9)-2-NH3B10H8]. The liposome suspension is purified by gel filtration on Sephadex and sterilized by microfiltration through a 0.2 μm membrane. The boron content of the liposome suspension is determined preinjection by ICP-AES, and the liposomal size distribution determined by dynamic light scattering.
Microwave-assisted digestion followed by inductively coupled plasma-optical emission spectroscopy was utilized to determine the biodistribution of boron in various tissues. Single- and double-injection protocols were explored to optimize boron content in the tumor 48 to 72 h subsequent to the initial injection. Significant tumor response for a single BNCT treatment was demonstrated by growth curves versus a control group. Vastly diminished tumor growth was witnessed at 14 d in mice.6
Toxicity issues were not apparent in these experiments and therapeutic boron concentrations were retained in tumor for many hours. In these experiments, tumor boron concentration surpasses that of blood between 10 and 20 h postinjection, with the maximum measured tumor boron concentration after 30 h. This produces a significant concentration of boron in the tumor (up to 60 ppm at 48 h) with very favorable T/B and T/N ratios (> 5.5). Results similar to these were obtained using Fisher rats with subcutaneous RG2 tumors. Hamsters bearing chemically induced tumors were treated in Argentina in collaboration with DOE/Argentina Atomic Energy Commission using MAC-TAC liposomes and the nuclear facilities available there.
As in the case of mice, hamsters tolerated the therapy very well with no loss due to toxicity or radiation effects in both the mouse and hamster cases. Many remissions were observed. All in all, approximately one hundred hamsters were treated in this manner.91 Furthermore, to establish the viability of liposomes as a drug delivery system in a large animal, a preparation of blank (boron-free) liposomes was administered to a canine subject at a dose comparable to those proposed. During this experiment, the dog appeared normal and healthy. Likewise, the blood chemistry analyses (16 factors measured) indicated no significant impact.
The University of Missouri Veterinary School initiated a series of distribution studies using dogs with head-and-neck carcinoma treated with MAC-TAC liposomes. A small number of cases involving tumor-bearing dogs and MAC-TAC liposomes exhibited clinical symptoms resembling CARPA syndrome (complement activation-related pseudoallergy). These dogs developed a marked fever 4 h after liposome infusion followed by profound neutropenia. After 12 h, the temperature of these animals had normalized and blood work demonstrated a rebound leukocytosis.
In vitro biodistribution studies of these liposomes in the EMT6 murine mammary carcinoma cell line show the localization of TAC in cytoplasm specifically in lysosomes and localization of MAC in the cell membrane. These biodistribution studies were done by incubating MAC/TAC liposomes with EMT6 cells for 24 h. After 24 h, the cells were washed to remove any excess of liposomes present in the culture media. The cells were then lysed and cell component separated by filtration. Cell membrane components and the cytoplasm components were separately analyzed for boron by ICP-OES and mass spectrometry methods. Mass spectrometry analysis showed that TAC remains in the cytoplasm and MAC in cell membrane.
Targeted NanoBoron for BNCT
Polymalic acid (PMLA) is naturally derived, biodegradable, nontoxic, and nonimmunogenic nanoplatform and is used as the backbone carrier for the newly developed nanodrugs. PMLA contains pendant carboxylic groups for the covalent attachment of numerous functional units and has been extensively used as a carrier for chemotherapeutic agents, antibodies, oligonucleotides, imaging agents (optical and MRI) that cross the BBB, and demonstrated high potential for clinical application. Patil's research strategy utilizes active targeting by a synthetic peptide Angiopep-2 (Ap2). This peptide has been successfully used for BBB transport by several laboratories to shuttle a variety of nanodrugs based on different carriers, including PMLA-LLL across the BBB.92 In the authors' earlier studies, their carrier has been successfully used not only to efficiently cross BBB but also avidly bound to glioma while demonstrating essentially no binding to normal brain tissues.93,94
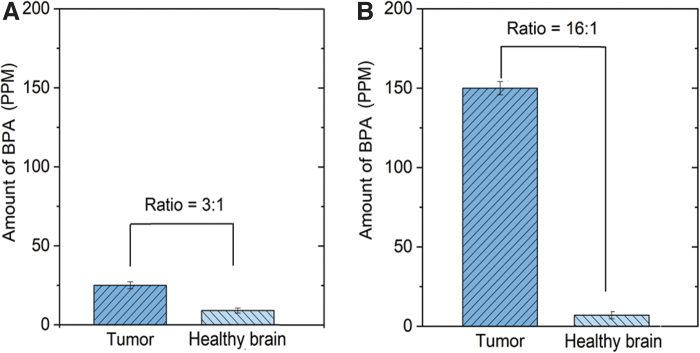
While better selectivity is highly desirable to reduce toxicity to healthy brain tissue, a higher 10B concentration in tumor could be highly useful for superior BNCT response. Clinical studies using a high concentration of L-BPA (excess of 250 mg/kg) have shown that the concentration of L-BPA in tumor versus surrounding healthy brain hovers around 3:1 T/N (Fig. 6A), while the concentration in the blood remains very high. This may cause potential toxicity to the patient receiving BNCT treatment. In addition, high background concentration of BPA in the blood or healthy tissues also limits the dosage of neutrons for higher efficiency of capture reaction. In Patil's animal studies (U87MG xenografts) using a significantly smaller dosage (50 mg/kg BPA dose), they have improved the selectivity from 3:1 to over 16:1 ratio using NanoBoron (Fig. 6B).
FIG. 6.
(A) Traditional BNCT drug has a 3:1 ratio of BPA in tumor (dark blue) versus normal brain (blue) at L-BPA dose of 250 mg/kg (patients data); (B) proposed nanodrug has a 16:1 ratio of BPA in tumor versus healthy brain with L-BPA at a dose of 50 mg/kg (animal study).
Moreover, NanoBoron's long drug retention in tumor by virtue of active targeting along with the ability of high tumor selectivity and accumulation allows lager time window for neutron irradiation, while minimizing the potential toxicity due to significantly lower background BPA concentration in the blood and healthy organs. The authors have used similar nanodrug showing sustained ratio of tumor to healthy brain selectivity over extended periods of time.95
Based on the above data, Patil's group hypothesizes that their novel, PMLA-based proposed nanodrug (NanoBoron) will achieve enhanced and localized intracellular delivery of 10B rich BPA selectively to glioma cells. They expect higher DNA damage and prolonged survival in the treated cohort with the NanoBoron followed by irradiation with thermal neutrons, compared to the saline or BPA-injected cohorts. They also expect to observe high molecular changes in the tumor microenvironment in the nanodrug-treated group in correlation with the survival outcome. This method of drug delivery will in turn make BNCT a practical and highly successful treatment strategy for GBM treatment.
Carborane-Bearing Matrix Metalloproteinase Inhibitors as Ligands for BNCT
Matrix metalloproteinases (MMPs) are calcium- and zinc-dependent endopeptidases, which function to degrade the extracellular matrix. Under normal physiological conditions, the activity of MMP enzymes are well regulated, however, elevated levels of MMP-2, MMP-9, and MMP-13 have been identified and observed in cancer and arthritis patients.96 To combat these maladies, the design and synthesis of MMP inhibitors have been widely explored with the emergence of potent and selective α-sulfone- and sulfonamide-based MMP inhibitors: SC-78080 (SD-2590)97 (MMP-2 = <0.1 nM, MMP-9 = 0.2 nM, MMP-13 = 0.1 nM), SC-7627695 (MMP-2 = 0.2 nM, MMP-9 = 1.5 nM, MMP-13 = 0.1 nM), SC-7796495 (MMP-2 = <0.1 nM, MMP-9 = 0.1 nM, MMP-13 = 0.1 nM), and CGS-27023A98 (MMP-2 = 11 nM, MMP-9 = 8 nM, MMP-13 = 6 nM). The goal of the Becker research group is to synthesize carborane-containing hydroxamates-based MMP inhibitors for use in BNCT.
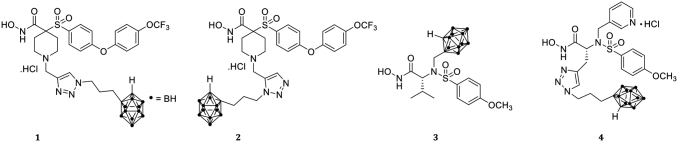
The first series of carborane-containing α-sulfonate hydroxamate-based MMP inhibitors was previously described by the Becker research group.99 The analogs were synthesized via thermal Huisgen 1,3-dipolar cycloaddition or copper-mediated azide-alkyne cycloaddition (CuAAC), or ruthenium-catalyzed azide alkyne cycloaddition (RuAAC) to afford 1,4-triazole 1 and 1,5-triazole 2 compounds containing ortho-closo-carboranes (Fig. 7). Selectivity of 1,4- and 1,5-triazole carborane-containing α-sulfone agents toward MMP-2 and MMP-9 was evaluated through MMP inhibition. Both 1,4-triazole and 1,5-triazoles exhibited low nM potency at MMP-2 (37 and 9.8 nM) and at MMP-9 (46 μM and 13 nM). These compounds show a robust BNCT effect in in vitro experiments with the D37 (dose used to inhibit 63% colony formation) values of 0.27 Gy and 0.32 for the analogs 1 and 2, respectively; 0.82 Gy for BPA, and 1.55 Gy for boron-free control.
FIG. 7.
MMP inhibitor BNCT agents: 1,4-triazole α-sulfone 1, 1,5-triazole α-sulfone 2, enriched 10B analog 3, and 1,4-triazole sulfonamide 4.
The Becker research group's current work focuses on the synthesis of a second series of carborane-containing sulfonamide-based BNCT agents based on the broad-spectrum MMP inhibitor CGS-27023A. Through molecular docking experiments, two areas were identified for incorporation of the carborane, isosteric replacement of pyridine in the S2’ subsite of the enzyme and replacement of the isopropyl group, which extend into the solvent and allow for incorporation of larger moieties. Two novel BNCT agents have been synthesized utilizing incorporation of a carborane via reaction of 10B-enriched complex B10H12(CH3CN)2 with an alkyne resulting in BNCT agent 3, or via a CuAAC-catalyzed reaction yielding BNCT agent 1,4-triazole isomer 4 (Fig. 7).
Both BNCT agents exhibit low nM potency toward MMP-2, MMP-9 and MMP-13. The enriched 10B analog 3 and 1,4-triazole isomer 4 exhibited the following values: MMP-2 (5.4 and 5.2 nM), MMP-9 (125 and 5.1 nM), and MMP-13 (64 and 3.1 nM). Similar to the first series of carborane-containing α-sulfone BNCT agents, the second series of BNCT agents are currently being explored in in vitro and in vivo. In summary, novel BNCT agents have been synthesized with excellent potency toward MMP enzymes that are overexpressed in cancer and show promise in in vitro testing on SCCVII cell line for BNCT in the first series of carborane-containing BNCT analogs.
Biologically Oriented Boron Chemicals for BNCT
The Das research group develops biology oriented boron chemicals as potential probes and pharmacological agents for different diseases that target mitochondrial metabolic and oxidative pathways and also targeting different proteins, as shown in Figure 8.100–103 Their main focus is glioblastomas and neurodegenerative diseases.104 The systematic approach of the Das group to design and synthesize boron-based compounds that target different oncological pathways and proteins opens a new avenue to provide new pipeline compounds to the BNCT community for boron delivery.
FIG. 8.
Development of boron-based potential probes and pharmacological agents in the Das group.
Conclusions and Outlook
BNCT is a radiotherapeutic modality for cancer treatment which has been investigated for decades since the beginning of its conceptional establishment. In addition to a high-quality and stable neutron beam, two challenging tasks of drugs of high selectivity and high loading amount in tumor tissues need to be completed simultaneously for a successful BNCT treatment. Nevertheless, clinically used BPA and BSH cannot fulfill all criteria of the treatment. Therefore, great efforts are being made on the development of the next generation of boron drugs, and various boron entities, including small molecules and macro-/nanocomposites for the definitive success of BNCT. However, all work is still in preclinical stage, and none of the new boron drugs are yet approved, let alone related investigational new drugs, which need to be evaluated and approved by the FDA.
Similar to BPA production, new drugs also need to be produced in the Good Manufacturing Practices facility for clinical usage. In this regard, a feasible, sustainable, and commercially available boron source is becoming much more important. Furthermore, it is highly desired to develop boron drugs for theranostic application because in vivo determination of boron distribution is crucial in BNCT treatment. In this article, the authors discussed the latest research results of new boron drugs for BNCT. Some critical issues must still be addressed, which include the need for more efficient and reliable boron delivery agents, the creation of novel techniques to quantitatively investigate the boron content in tumors after administration, and the improvement of boron delivery agents. Innovative methods developed herein and in the near future may open up new venues for more widespread application of new boron-containing drugs for BNCT treatment.
From the works collected in the previous pages, it can be appreciated that chemists undertook the challenge of discovering new and more effective boron delivery agents for BNCT by conjugating boron clusters to various carriers, including peptides, peptidomimetics, proteins (such as albumin), porphyrin derivatives, and liposomes. In some cases, the boron agents have the ability to bind to DNA and localize in close proximity to cell nucleus, which can enhance their biological efficacy. Preclinical studies suggest that alternative administration methods of boron agents, such as CED, could be used for the delivery of large amounts of boron to tumors, and that the combination of different boron carriers can lead to higher BNCT efficacy, compared with the use of a single boron agent.
Furthermore, to facilitate treatment planning and maximize the tumor killing effect with minimal damage to normal tissues, the neutron irradiation treatment should be applied at the highest T/N and T/B boron concentration ratios. Therefore, promising BNCT agents containing easily detectable moieties, such as a fluorescence label, or a PET, SPECT, or MRI agent, play a prominent role in tracking and quantifying tissue-localized boron, treatment planning, and outcome.
Authors' Contributions
Conceptualization, A.G.B.-S., D.P.B., O.C., B.D., S.F., E.H.-H., N.H., S.S.J., H.N., R.P., M.G.H.V., and C.V.; writing—original draft preparation, A.G.B.-S., D.P.B., O.C., B.D., S.F., E.H.-H., N.H., S.S.J., H.N., R.P., M.G.H.V., and C.V.; writing—review and editing, A.G.B.-S., D.P.B., O.C., B.D., S.F., E.H.-H., N.H., S.S.J., H.N., R.P., M.G.H.V., and C.V.; project administration, N.H. and M.G.H.V. All authors have read and agreed to the published version of the article.
Disclosure Statement
No competing financial interests exist.
Funding Information
C.V. thanks the Spanish Ministerio de Economía y Competitividad (PID2019-106832RB-100) and the Generalitat de Catalunya (2017SGR1720). H.N. received a Grant-in-Aid for Scientific Research (B) (No. 21H02066) from MEXT, Japan. M.G.H.V. thanks the National Institutes of Health grant number T34 GM136452. R.P. thanks the National Institutes of Health, grant number R21 CA259911 and the Department of Neurosurgery, Cedars Sinai Medical Center, Los Angeles.
References
- 1. Hu K, Yang Z, Zhang L, et al. Boron agents for neutron capture therapy. Coord Chem Rev 2020;405:213139. [Google Scholar]
- 2. Dymova MA, Taskaev SY, Richter VA, et al. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun 2020;40:406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lamba M, Goswami A, Bandyopadhyay A.. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem Commun 2021;57:827–839. [DOI] [PubMed] [Google Scholar]
- 4. Malouff TD, Seneviratne DS, Ebner DK, et al. Noron neutron capture therapy: A review of clinical applications. Front Oncol 2021;11:601820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Worm DJ. Hoppenz P, Els-Heindl S, et al. Selective neuropeptide Y conjugates with maximized carborane loading as promising boron delivery agents for boron neutron capture therapy. J Med Chem 2020;63:2358–2371. [DOI] [PubMed] [Google Scholar]
- 6. Hoppenz P, Els-Heindl S, Kellert M, et al. A selective carborane-functionalized gastrin-releasing peptide receptor agonist as boron delivery agent for boron neutron capture therapy. J Org Chem 2020;85:1446–1457. [DOI] [PubMed] [Google Scholar]
- 7. Michiue H, Sakurai Y, Kondo N, et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014;35:3396–3405. [DOI] [PubMed] [Google Scholar]
- 8. Lerouge F, Viñas C, Teixidor F, et al. High boron content carboranylfunctionalized aryl ether derivatives displaying photoluminescent properties. Dalton Trans 2007;92:1898–1903; doi: 10.1039/B618771D [DOI] [PubMed] [Google Scholar]
- 9. Feng B, Tomizawa K, Michiue H, et al. Delivery of sodium borocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials 2009;30:1746–1755. [DOI] [PubMed] [Google Scholar]
- 10. Kueffer PJ, Maitz CA, Khan AA, et al. Boron Neutron Capture Therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc Natl Acad Sci USA 2013;110:6512–6517; doi: 10.1073/pnas.1303437110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubey R, Kushal S, Mollard A, et al. Tumor targeting, trifunctional dendritic wedge. Bioconjugate Chem 2015;26:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciofani G, Raffa V, Menciassi A, et al. Folate functionalized boron nitride nanotubes and their selective uptake by glioblastoma multiforme cells: Implications for their use as boron carriers in clinical boron neutron capture therapy. Nanoscale Res Lett 2008;4:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poater J, Viñas C, Bennour I, et al. Too persistent to give up: Aromaticity in boron clusters survives radical structural changes. J Am Chem Soc 2020;42:9396–9407; doi: 10.1021/jacs.0c02228 [DOI] [PubMed] [Google Scholar]
- 14. Viñas C. The Uniqueness of boron as a novel challenging element for drugs in pharmacology, medicine and for smart biomaterials. Future Med Chem 2013;5:617–619; doi: 10.4155/fmc.13.41 [DOI] [PubMed] [Google Scholar]
- 15. Viñas C, Núñez R, Bennour I, et al. Periphery decorated and core initiated neutral and polyanionic borane large molecules: Forthcoming and promising properties for medicinal applications. Curr Med Chem 2019;26:4935–5076; doi: 10.2174/0929867326666190603123838 [DOI] [PubMed] [Google Scholar]
- 16. Viñas C, Teixidor F, Nuñez R.. Boron clusters-based metallodendrimers. Inorg Chim Acta 2014;409:12–25; doi: 10.1016/j.ica.2013.05.038 [DOI] [Google Scholar]
- 17. Nuñez R, Romero I, Teixidor F, et al. Icosahedral boron clusters: A perfect tool for the enhancement of polymer features. Chem Soc Rev 2016;45:5147–5173; doi: 10.1039/C6CS00159A [DOI] [PubMed] [Google Scholar]
- 18. Cioran AM, Musteti AD, Teixidor F, et al. Mercaptocarborane-capped gold nanoparticles: Electron pools and ion traps with switchable hydrophilicity. J Am Chem Soc 2012;134:212–221; doi: 10.1021/ja203367h [DOI] [PubMed] [Google Scholar]
- 19. Grzelczak MP, Danks SP, Klipp RC, et al. Ion transport across biological membranes by carborane-capped gold nanoparticles. ACS Nano 2017;11:12492–12499; doi: 10.1021/acsnano.7b06600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oleshkevich E, Teixidor F, Rosell A, et al. Merging icosahedral boron clusters and magnetic nanoparticles: Aiming toward multifunctional nanohybrid materials. Inorg Chem 2018;57:462–470; doi: 10.1021/acs.inorgchem.7b02691 [DOI] [PubMed] [Google Scholar]
- 21. Oleshkevich E, Morancho A, Saha A, et al. Combining magnetic nanoparticles and icosahedral boron clusters in biocompatible inorganic nanohybrids for cancer therapy. Nanomedicine 2019;20:101986–101996; doi: 10.1016/j.nano.2019.03.008 [DOI] [PubMed] [Google Scholar]
- 22. Saha A, Oleshkevich E, Vinas C, et al. Biomimetic inspired core–canopy quantum dots: Ions trapped in voids induce kinetic fluorescence switching. Adv Mater 2017;29:1704238–1704245; doi: 10.1002/adma.201704238 [DOI] [PubMed] [Google Scholar]
- 23. Couto M, Alamón C, García MF, et al. Closo-carboranyl- and metallacarboranyl[1,2,3]triazolyl-decorated Lapatinib-scaffold for cancer therapy combining tyrosine kinase inhibition and boron neutron capture therapy. Cells 2020;9:1408–1426; doi: 10.3390/cells9061408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Couto M, Alamón C, Nievas S, et al. Bimodal therapeutic agent against glioblastoma, one the most lethal cancer. Chem Eur J 2020;26:14335–14340; doi: 10.1002/chem.202002963 [DOI] [PubMed] [Google Scholar]
- 25. Alamón C, Dávila B, García MF, et al. Sunitinib containing carborane pharmacophore with ability to inhibit tyrosine kinases receptors, FLT3, KIT, and PDGFR-β, exhibits powerful in vivo anti-glioblastoma activity. Cancers 2020;12:3423–3444; doi: 10.3390/cancers12113423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teixeira RG, Marques F, Robaloc MP, et al. Ruthenium carboranyl complexes with 2,2’-bipyridine derivatives for potential bimodal therapy application. RSC Adv 2020;10:16266–16276; doi: 10.1039/D0RA01522A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grimes R.N. Carboranes. Elsevier: New York, NY; 2016. [Google Scholar]
- 28. Bauduin P, Prevost S, Farràs P, et al. A theta-shaped amphiphilic cobaltabisdicarbollide anion: Transition from monolayer vesicles to micelles. Angew Chem Int Ed 2011;50:5298–5300; doi: 10.1002/anie.201100410 [DOI] [PubMed] [Google Scholar]
- 29. Verdiá C, Alcaraz A, Aguilella VM, et al. Amphiphilic COSAN and I2-COSAN crossing synthetic lipid membranes: Planar bilayers and liposomes. Chem Commun 2014;50:6700–6703; doi: 10.1039/c4cc01283f [DOI] [PubMed] [Google Scholar]
- 30. Tarrés M, Canetta E, Viñas C, et al. , Imaging in living cells using νB–H Raman spectroscopy: Monitoring COSAN uptake. Chem. Commun 2014;50:3370–3372; doi: 10.1039/c3cc49658a [DOI] [PubMed] [Google Scholar]
- 31. Tarrés M, Canetta E, Paul E, et al. Biological interaction of living cells with COSAN-based synthetic vesicles. Sci Rep 2015;5:7804–7812; doi: 10.1038/srep07804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stoica A, Viñas C, Teixidor F.. Cobaltabisdicarbollide anion receptor for enantiomer-selective membraneelectrodes. Chem Commun 2009;4988–4990; doi: 10.1039/B910645F [DOI] [PubMed] [Google Scholar]
- 33. Fuentes I, Pujols J, Viñas C, et al. Dual binding mode of metallacarborane produces a robust shield on proteins. Chem Eur J 2019;25:12820–12829; doi: 10.1002/chem.201902796 [DOI] [PubMed] [Google Scholar]
- 34. Goszczynski TM, Fink K, Kowalski K, et al. Interactions of boron clusters and their derivatives with serum albumin. Sci Rep 2017;7:9800–9812; doi: 10.1038/s41598-017-10314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia-Mendiola T, Bayon-Pizarro V, Zaulet A, et al. Metallacarboranes as tunable redox potential electrochemical indicators for screening of gene mutation. Chem Sci 2016;7:5786–5797; doi: 10.1039/c6sc01567k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fuentes I, García-Mendiola T, Sato S, et al. Metallacarboranes on the road to anticancer therapies: Cellular uptake, DNA interaction, and biological evaluation of Cobaltabisdicarbollide [COSAN]. Chem Eur J 2018;24:17239–17254; doi: 10.1002/chem.201803178 [DOI] [PubMed] [Google Scholar]
- 37. Merhi T, Jonchere A, Girard L, et al. Highlights on the binding of cobalta-bis-(dicarbollide) with glucose units. Chem Eur J 2020;26:13935–13947; doi: 10.1002/chem.202002123. [DOI] [PubMed] [Google Scholar]
- 38. Nuez-Martínez M, Pedrosa L, Martinez-Rovira I, et al. Synchrotron-based fourier-transform infrared micro-spectroscopy (SR-FTIRM) fingerprint of the small anionic molecule Cobaltabis(dicarbollide) uptake in glioma stem cells Int. J Mol Sci 2021;22:9937–9962; doi: 10.3390/ijms22189937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buades AB, Pereira LCJ, Vieira BJC, et al. The Mössbauer effect using 57Fe-ferrabisdicarbollide ([o-57FESAN]−): A glance into the potential of a low-dose approach for glioblastoma radiotherapy. Inorg Chem Front 2022;9:1490–1503; doi: 10.1039/d1qi01513c [DOI] [Google Scholar]
- 40. Winberg KJ, Barberà G, Eriksson L, et al. High yield [125I]iodide-labeling of iodinated carboranes by palladium catalyzed isotopic exchange. J Organomet Chem 2003;680:188–192; doi: 10.1016/S0022-328X(03)00344-9 [DOI] [Google Scholar]
- 41. Gona KB, Zaulet A, Gómez-Vallejo V, et al. COSAN as a molecular imaging platform: Synthesis and ‘‘in vivo’’ imaging. Chem Commun 2014;50:11415–11417; doi: 10.1039/c4cc05058d [DOI] [PubMed] [Google Scholar]
- 42. Ahrens VM, Bellmann-Sickert K, Beck-Sickinge AG.. Peptides and peptide conjugates: Therapeutics on the upward path. Future Med Chem 2012;4:1567–1586. [DOI] [PubMed] [Google Scholar]
- 43. Worm DJ, Els-Heindl S, Beck-Sickinger AG.. Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Peptide Sci 2020;112:e24171. [Google Scholar]
- 44. Iguchi Y, Michiue H, Kitamatsu M, et al. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015;56:10–17. [DOI] [PubMed] [Google Scholar]
- 45. Capala J, Barth RF, Bendayan M, et al. Boronated epidermal growth factor as a potential targeting agent for boron neutron capture therapy of brain tumors. Bioconjugate Chem 1996;7:7–15. [DOI] [PubMed] [Google Scholar]
- 46. Barth RF, Yang W, Adams DM, et al. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer Res 2002;62:3159–3166. [PubMed] [Google Scholar]
- 47. Mier W, Gabel D, Haberkorn U, et al. Conjugation of the closo-borane mercaptoundecahydrododecaborate (BSH) to a tumour selective peptide. Z Anorg Allg Chem 2004;630:1258–1262. [Google Scholar]
- 48. Betzel T, Hess T, Waser B, et al. closo-Borane conjugated regulatory peptides retain high biological affinity: Synthesis of closo-borane conjugated Tyr3-octreotate derivatives for BNCT. Bioconjug Chem 2008;19(9):1796–1802. [DOI] [PubMed] [Google Scholar]
- 49. Kimura S, Masunaga SI, Harada T, et al. Synthesis and evaluation of cyclic RGD-boron cluster conjugates to develop tumor-selective boron carriers for boron neutron capture therapy. Bioorg Med Chem 2011;19:1721–1728. [DOI] [PubMed] [Google Scholar]
- 50. Masunaga SI, Kimura S, Harada T, et al. Evaluating the usefulness of a novel 10B-carrier conjugated with cyclic RGD peptide in boron neutron capture therapy. World J Oncol 2012;3:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khan IU, Beck-Sickinger AG.. Targeted tumor diagnosis and therapy with peptide hormones as radiopharmaceuticals. Anticancer Agents Med Chem 2008;8:186–199. [DOI] [PubMed] [Google Scholar]
- 52. Worm DJ, Els-Heindl S, Kellert M, et al. A stable meta-carborane enables the generation of boron-rich peptide agonists targeting the ghrelin receptor. J Pept Sci 2018;24:e3119. [DOI] [PubMed] [Google Scholar]
- 53. Kellert M, Worm DJ, Hoppenz P, et al. Modular triazine-based carborane-containing carboxylic acids—synthesis and characterisation of potential boron neutron capture therapy agents made of readily accessible building blocks. Dalton Trans 2019;48:10834–10844. [DOI] [PubMed] [Google Scholar]
- 54. Kellert M, Hoppenz P, Lönnecke P, et al. Tuning a modular system-synthesis and characterisation of a boron-rich s-triazine-based carboxylic acid and amine bearing a galactopyranosyl moiety. Dalton Trans 2020;49:57–69. [DOI] [PubMed] [Google Scholar]
- 55. Boehnke S, Saretz S, Kellert M, et al. Facile synthesis of the versatile trifunctionalized building block 1,2-bis(hydroxymethyl)-9-mercapto-1,2-dicarba-closo-dodecaborane(12). Biochem Biophys J Neutron Ther Cancer Treat 2013;1:22–27. [Google Scholar]
- 56. Kellert M, Lönnecke P, Riedl B, et al. Enlargement of a modular system-synthesis and characterization of an s-triazine-based carboxylic acid ester bearing a galactopyranosyl moiety and an enormous boron load. Molecules 2019;24:3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kellert M, Friedrichs JS, Ullrich NA, et al. Modular synthetic approach to carboranyl‒biomolecules conjugates. Molecules 2021;26:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahrens VM, Frank R, Stadlbauer S, et al. Incorporation of ortho-carbaboranyl-Nɛ-modified L-lysine into neuropeptide Y receptor Y1- and Y2-selective analogues. J Med Chem 2011;54:2368–2377. [DOI] [PubMed] [Google Scholar]
- 59. Ahrens VM, Frank R, Boehnke S, et al. Receptor-mediated uptake of boron-rich neuropeptide Y analogues for boron neutron capture therapy. Chem Med Chem 2015;10:164–172. [DOI] [PubMed] [Google Scholar]
- 60. Frank R, Ahrens VM, Boehnke S, et al. Charge-compensated metallacarborane building blocks for conjugation with peptides. Chem Bio Chem 2016;17:308–317. [DOI] [PubMed] [Google Scholar]
- 61. Hoppenz P, Els-Heindl S, Beck-Sickinger AG.. Peptide-drug conjugates and their targets in advanced cancer therapies. Front Chem 2020;8:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kikuchi S, Kanoh D, Sato S, et al. Maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumor for neutron capture therapy. J Control Release 2016;237:160–167. [DOI] [PubMed] [Google Scholar]
- 63. Ishii S, Sato S, Asami H, et al. Design of S-S bond containing maleimide-conjugated closo-dodecaborate (SSMID): Identification of unique modification sites on albumin and investigation of intracellular uptake. Org Biomol Chem 2019;17:5496–5499. [DOI] [PubMed] [Google Scholar]
- 64. Koganei H, Ueno M, Tachikawa S, et al. Development of high boron content liposomes and their promising antitumor effect for neutron capture therapy of cancers. Bioconjugate Chem 2013;24:124–132. [DOI] [PubMed] [Google Scholar]
- 65. Tachikawa S, Miyoshi T, Koganei H, et al. Spermidinium closo-dodecaborate-encapsulating liposomes as efficient boron delivery vehicles for neutron capture therapy. Chem Comm 2014;50:12325–12328. [DOI] [PubMed] [Google Scholar]
- 66. Kawai K, Nishimura K, Okada S, et al. Cyclic RGD-functionalized closo-dodecaborate albumin conjugates as integrin targeting boron carriers for neutron capture therapy. Mol Pharm 2020;17(10):3740–3747. [DOI] [PubMed] [Google Scholar]
- 67. MacDonald IJ, Dougherty, TJ.. Basic principles of photodynamic therapy. J Porphyrins Phthalocyanines 2001;5(2):105–129. [Google Scholar]
- 68. Brown, SB, Brown EA, Walker, I.. The present and future role of photodynamic therapy in cancer. Lancet Oncol 2004;5:497–508. [DOI] [PubMed] [Google Scholar]
- 69. Hamblin MR, Mroz, P.. Advances in Photodynamic Therapy: Basic, Translational, and Clinical. Artech House; Boston, 2008. [Google Scholar]
- 70. Barth RF, Coderre JA, Vicente MGH, et al. Boron neutron capture therapy of cancer: Current status and future prospects. Clin Cancer Res 2005;11:3987–4002. [DOI] [PubMed] [Google Scholar]
- 71. Barth RF, Vicente MGH, Harling OK, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Rad Oncol 2012;7:146–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vicente MGH. Porphyrin-based sensitizers in the detection and treatment of cancer: Recent progress. Curr Med Chem Anti Cancer Agents 2001;1:175–194. [DOI] [PubMed] [Google Scholar]
- 73. Ozawa T, Afzal J, Lamborn KR, et al. Toxicity, biodistribution, and convection-enhanced delivery of the boronated porphyrin Bopp in the 9l intracerebral rat glioma model. Int J Radiat Oncol Biol Phys 2005;63(1):247–252. [DOI] [PubMed] [Google Scholar]
- 74. Ozawa T, Santos RA, Lamborn KR, et al. In vivo evaluation of the boronated porphyrin TABP-1 in U-87MG intracerebral human glioblastoma xenografts. Mol Pharm 2004;1:368–374. [DOI] [PubMed] [Google Scholar]
- 75. Kawabata, S, Yang W, Barth RF, et al. Convection enhanced delivery of carboranylporphyrins for neutron capture therapy of brain tumors. J NeuroOncol 2011;103(2):175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bhupathiraju NVSDK, Vicente MGH.. Synthesis of Carborane-Containing Porphyrin Derivatives for the Boron Neutron Capture Therapy (BNCT) of Tumors. In: Topics in Heterocyclic Chemistry, Applications of Porphyrinoids, Roberto Paolesse, (ed.), Chapter 2, Berlin, Heidelberg: Springer-Verlag; 2014; pp. 31–52. [Google Scholar]
- 77. Xuan S, Vicente MGH.. Recent development of boron delivery agents for boron neutron capture therapy. In: Medicinal Chemistry of Boron Compounds, C. Vinas and E. Hey-Hawkins (eds.). Wiley Publishers: New Jersey; 2019; pp. 298–342. [Google Scholar]
- 78. Gottumukkala V, Luguya R, Fronczek FR, et al. Synthesis and cellular studies of an octa-anionic 5,10,15,20-tetra[3,5-(nido-carboranylmethyl)phenyl]porphyrin (H2OCP) for application in BNCT. Biorg Med Chem 2005;13(13):1633–1640. [DOI] [PubMed] [Google Scholar]
- 79. Hao E, Sibrian-Vazquez M, Serem W, et al. Synthesis, aggregation and cellular investigations of porphyrin–cobaltacarborane conjugates. Chem Eur J 2007;13(32):9035–9042. [DOI] [PubMed] [Google Scholar]
- 80. Bhupathiraju NVSDK, Gottumukkala V, Hao E, et al. Synthesis and toxicity of cobaltabisdicarbollide-containing porphyrins of high boron content. J Porphyrins Phthalocyanines 2012;15(9–10):973–983. [Google Scholar]
- 81. Luguya R, Fronczek FR, Smith, KM, et al. Synthesis of novel carboranylchlorins with dual application in boron neutron capture therapy (BNCT) and photodynamic therapy (PDT). Appl Rad Isotopes 2004;61(5):1117–1123. [DOI] [PubMed] [Google Scholar]
- 82. Hiramatsu R, Kawabata S, Tanaka H, et al. Tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC): Application for photodynamic therapy and boron neutron capture therapy. J Pharmac Sci 2015;104:962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sibrian-Vazquez M, Hao E, Jensen TJ, et al. Enhanced cellular uptake with a cobaltacarborane-porphyrin-HIV-1 Tat 48–60 conjugate. Bioconjugate Chem 2006;17:928–934. [DOI] [PubMed] [Google Scholar]
- 84. Bhupathiraju NVSDK, Hu X, Zhou Z, et al. Synthesis and in vitro evaluation of BBB permeability, tumor cell uptake, and cytotoxicity of a series of carboranylporphyrin conjugates. J Med Chem 2014;57(15):6718–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gibbs JH, Wang H, Bhupathiraju NVSDK, et al. Synthesis and properties of a series of carboranyl-BODIPYs. J Organomet Chem 2015;798Part 1:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Xuan S, Zhao N, Zhou Z, Fronczek FR, et al. Synthesis and in vitro studies of a series of carborane-containing boron dipyrromethenes (BODIPYs). J Med Chem 2016;59(5):2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shelly K, Feakes DA, Hawthorne MF, et al. Model studies directed toward the boron neutron capture therapy of cancer: Boron delivery to murine tumors with liposomes. Proc Natl Acad Sci USA 1992;89(19):9039–9043; doi: 10.1073/pnas.89.19.9039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Feakes DA, Shelly K, Knobler CB, et al. Na3[B20H17NH3]: Synthesis and liposomal delivery to murine tumors. Proc Natl Acad Sci USA 1994;91(8):3029–3033; doi: 10.1073/pnas.91.8.3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Feakes DA, Shelly K, Hawthorne MF.. Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc Natl Acad Sci USA 1995;92(5):1367–1370; doi: 10.1073/pnas.92.5.1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maitz CA, Khan AA, Kueffer PJ, et al. Validation and comparison of the therapeutic efficacy of boron neutron capture therapy mediated by boron-rich lposomes in multiple murine tumor models. Transl Oncol 2017;10(4):686–692; doi: 10.1016/j.tranon.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Heber EM, Hawthorne MF, Kueffer PJ, et al. Therapeutic efficacy of boron neutron capture therapy mediated by boron-rich liposomes for oral cancer in the hamster cheek pouch model. Proc Natl Acad Sci USA 2014;111(45):16077–16081; doi: 10.1073/pnas.1410865111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Israel LL, Braubach O, Galstyan A, et al. A combination of tri-Leucine and Angiopep-2 drives a polyanionic polymalic acid nanodrug platform across the blood-brain barrier. ACS Nano 2019;13(2):1253–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Patil R, Ljubimov AV., Gangalum PR, et al. MRI virtual biopsy and treatment of brain metastatic tumors with targeted nanobioconjugates: Nanoclinic in the brain. ACS Nano 2015;9:5594–5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Patil R, Sun T, Rashid MH, et al. Multifunctional nano polymers for blood-brain barrier delivery and inhibition of glioblastoma growth through EGFR/EGFRvIII, c-Myc and anti-PD-1. Nanomaterials 2021;11:2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Patil R, Galstyan A, Sun T, et al. Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials 2019;206:146–159; doi: 10.1016/j.biomaterials.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Becker DP, Villamil CI, Barta TE, et al. Synthesis and structure-activity relationships of beta- and alpha-piperidine sulfone hydroxamic acid matrix metalloproteinase inhibitors with oral antitumor efficacy. J Med Chem 2005;48(21):6713–6730. [DOI] [PubMed] [Google Scholar]
- 97. Becker DP, Barta TE, Bedell LJ, et al. Orally active MMP-1 sparing α-tetrahydropyranyl and α-piperidinyl sulfone matrix metalloproteinase (MMP) inhibitors with efficacy in cancer, arthritis, and cardiovascular disease. J Med Chem 2010;53(18):6653–6680. [DOI] [PubMed] [Google Scholar]
- 98. MacPherson LJ, Bayburt EK, Capparelli MP, et al. Discovery of CGS 27023A, a non-peptidic, potent, and orally active stromelysin inhibitor that blocks cartilage degradation in rabbits. J Med Chem 1997;40(16):2525–2532. [DOI] [PubMed] [Google Scholar]
- 99. Jr MRL, Flieger S, Colorina A, Wozny J, Hosmane NS, Becker DP.. Carborane-Containing matrix metalloprotease (MMP) ligands as candidates for boron neutron capture therapy (BNCT). Chem Med Chem 2020;15(20):1897–1908. [DOI] [PubMed] [Google Scholar]
- 100. Zhong Y, Wu Y, Liu R, et al. Novel retinoic acid receptor alpha agonists for treatment of kidney disease. PLoS One 2011;6:e27945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Anguiano J, Garner TP, Mahalingam M, et al. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol 2013;9:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Das BC, Nandwana NK, Das S, et al. Boron chemicals in drug discovery and development: Synthesis and medicinal perspective. Molecules 2022;27(9):2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Das BC, Adil Shareef M, Das S, et al. Boron-containing heterocycles as promising pharmacological agents. Bioorg Med Chem 2022;63:116748. [DOI] [PubMed] [Google Scholar]
- 104. Di K, Lomeli N, Bota DA, et al. Magmas inhibition as a potential treatment strategy in malignant glioma. J Neurooncol 2019;141(2):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]