Abstract
In the present study, we identified the ectoparasite communities of red foxes in three regions of Poland that encompassed two endemic regions for the occurrence of Dermacentor reticulatus, as well as a region that is free of this tick species (‘gap’ area). Our study sites were selected to enable the role of foxes as hosts for juvenile (nest dwelling) and adult (exophilic) D. reticulatus ticks to be determined, and to assess their contribution to the spread of this important vector of Babesia canis. We compared also ectoparasite communities between adult foxes with those of fox cubs. Finally, we carried out a systematic search for subcutaneous ticks determining their prevalence and abundance. In 2016–2018, 366 adult foxes and 25 live-trapped cubs were examined for ectoparasites. Ectoparasites were identified based on morphological features, PCR amplification and sequencing. The total prevalence of ectoparasites was higher in cubs (68%) than in adults (62.8%). In adults, 15 parasite species were recorded, including four tick species, seven flea species, scabies, and one Anopluran species each in the genera Felicola and Lipoptena. In cubs, six ectoparasite species were found, including Ixodes kaiseri, a species not found in adults. Although Ixodes ricinus and D. reticulatus were the dominant tick species on adult foxes, no D. reticulatus ticks were found on cubs. Subcutaneous ticks were common (38%) and abundant in all areas. Molecular analysis of subcutaneous nodules allowed the identification of 17 I. ricinus and five D. reticulatus. In conclusion, red foxes play a minor role as hosts of D. reticulatus.
Key words: Dermacentor reticulatus, ectoparasites community, fleas, Ixodes canisuga, Ixodes hexagonus, Ixodes kaiseri, Ixodes ricinus, subcutaneous ticks
Introduction
The red fox (Vulpes vulpes) has become the most abundant and widespread carnivore and apex predator in numerous countries of Central Europe, including Poland (Panek and Bresiński, 2002; Goszczyński et al., 2008). The increasing population of foxes in Europe and their synurbanization raise questions about the role of this species as a reservoir host of pathogens/parasites of veterinary and medical importance. Foxes contribute to the maintenance of wildlife reservoirs of a range of ectoparasites, fleas, ticks, mites (including scabies) and therefore also to pathogens vectored by these ectoparasitic arthropods (Sréter et al., 2003; Kočišová et al., 2006; Foley et al., 2017). Red foxes are known to be hosts for two important but ecologically distinct ecotypes of ticks. As foxes explore large areas in search of food (territory about 20–30 ha) (Trewhella et al., 1989; Adkins and Stott, 1998) they may become infested with exophilic tick species: Ixodes ricinus, adult Dermacentor reticulatus or Haemaphysalis spp. However, since they inhabit burrows, they are also important hosts for several endophilic nest-dwelling tick species, including Ixodes hexagonus and Ixodes canisuga (Petney et al., 2012; Mihalca et al., 2012; Sándor et al., 2017).
In recent year, studies have focused on the role of foxes as reservoir hosts of canine vector-borne diseases (Duscher et al., 2015). Among the tick-borne pathogens for which foxes may serve as reservoir hosts is Babesia canis, the causative agent of canine babesiosis that manifests mostly as an acute life-threatening disease in domestic dogs that are not treated appropriately with preventive chemotherapy. Foxes may constitute the important source of infection for vectors (ticks) but there is still little known about their role in this context. Therefore, in order to evaluate the role of foxes as reservoir hosts of this tick-borne pathogen, the priority is to determine whether the specific vector of B. canis, the tick D. reticulatus, actually feeds on foxes and thereby becomes infected.
The ornate dog tick is a species that has been spreading recently in many Central European countries. The geographical range of this tick is divided in Europe with separate populations occurring in eastern and western macro regions (Rubel et al., 2016). These macro regions are separated by ‘a gap’ spanning the area between Western and Eastern Poland (Mierzejewska et al., 2016). Thus, in Poland, there are two separate D. reticulatus populations, a western population in Western Poland (Mierzejewska et al., 2016) and an eastern population in Central and Eastern Poland which are separated by an area that has been historically (Szymański, 1986), and is still currently (our monitoring), free of this tick species (gap). In the current study, we sampled foxes from these three regions of Poland in order to verify the role of red foxes as hosts of D. reticulatus.
The spread of ticks to new regions is often accompanied by the emergence of pathogens that were not previously endemic in the region, and in the case of D. reticulatus, canine babesiosis caused by B. canis is an obvious example (Bajer et al., 2014a, 2014b). Dermacentor reticulatus is known to be the main vector of B. canis and B. canis DNA has been identified in adult questing ticks in numerous studies (Mierzejewska et al., 2015; Jongejan et al., 2015; Radzijevskaja et al., 2018). However, how the ticks become infected still remains an unresolved issue. Juvenile D. reticulatus ticks are nest-dwelling and spend their entire life in rodent burrows, and are strictly seasonal: in Poland, D. reticulatus larvae occur on small rodents in June–July, and nymphs in late July and August (Paziewska et al., 2010; Dwużnik et al., 2019). Rodents, on the other hand, are not hosts/reservoirs of B. canis (Pawełczyk et al., 2004), so cannot serve as the source of infection for ticks. Here we propose a hypothesis that a proportion of juvenile D. reticulatus ticks (larvae and nymphs) feed on fox cubs, which spend the first few weeks of life in burrows, in spring and early summer and provide a possible source of infection with B. canis. To verify this hypothesis, we live-trapped fox cubs and inspected them for ectoparasites. During the current study, numerous ticks were found also in the subcutaneous tissues of the autopsied adult foxes. Although this phenomenon was first recorded in 1914 by Nutall (1914), reports of the subcutaneous occurrence of ticks, including in humans (Chang et al., 2006), are still rare (Smith et al., 1986; D'Amico et al., 2017) and the reasons for their presence in these tissues are not totally understood.
In the present study, we determined the ectoparasite communities of red foxes from three regions of Poland, covering two separate areas in which D. reticulatus is known to occur (Mierzejewska et al., 2016) and a region that is free of this tick species. Additionally, we compared the ectoparasite communities of adult individuals hunted in the autumn/winter season with those of fox cubs, live-trapped in spring/early summer. Finally, we carried out a meticulous search of subcutaneous tissues for ticks and determined their prevalence and abundance in these tissue types, and exploited molecular typing to establish the identity of the tick species involved.
Materials and methods
Fox carcasses were obtained during three hunting seasons in the autumns/winters of 2015/2016, 2016/2017 and 2017/2018. A number of carcasses consisted of road-killed animals. These individuals were obtained from various location in the vicinity of, and even from the city centre of Warsaw and were supplied by the city waste management company.
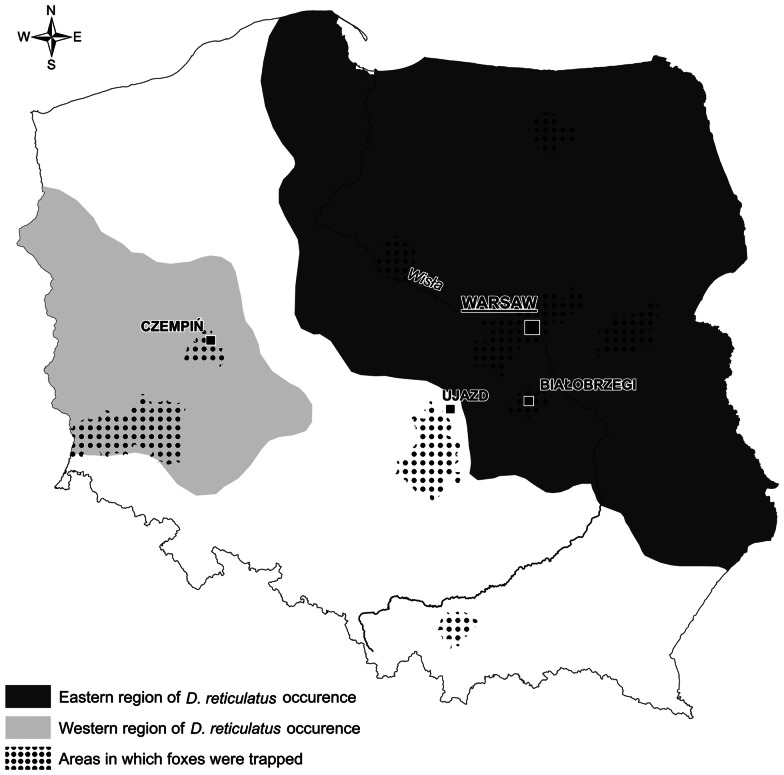
Among 366 examined carcasses, 225 originated from the old D. reticulatus-endemic area (Central and Eastern Poland), 73 originated from the new western D. reticulatus population in Western Poland and 68 carcasses originated from the non-endemic region between western and central/eastern areas (Fig. 1). To facilitate the presentation of the results, we refer to the first region as the eastern region, the second as the western region and the non-endemic region between them as the gap region (Fig. 1). Fox carcasses were frozen separately in plastic bags and transported to the Department of Parasitology for subsequent further investigation.
Fig. 1.
Origin of fox samples by region.
After freezing for a period of 2 weeks at −80°C temperature, fox carcasses were weighed, sexed and measured. Then the carcasses were carefully examined for ectoparasites, with particular attention to the skin around the ears, around the eyes and mouth, neck and groin (ticks), abdomen and comb (fleas). The bags in which the animals were kept were also checked to minimize the loss of ectoparasites. In addition, scrapings were collected from foxes in which skin lesions (alopecia, non-specific damages, inflammation) had been observed, and these were then examined carefully under a light microscope to detect Sarcoptes mites. Finally, the carcasses were carefully skinned to detect parasites in subcutaneous tissues.
Live-trapping of juvenile foxes
The methods used for live-trapping of foxes and collection of ectoparasites from juvenile foxes have been described in Mierzejewska et al. (2020). Ectoparasites were collected from 25 cubs over the course of three breeding seasons. Eight cubs were trapped in the eastern region, close to the town of Białobrzegi (N 51.6460, E 20.9566), 16 cubs were trapped in the western region, near Czempiń(N 52.1427, E 16.7612) and one cub was trapped in the gap region, close to the town Ujazd (N 51.5995, E 19.9212) (Fig. 1). Ectoparasites were fixed in 70% ethanol, but selected specimens of fleas were additionally cleared in 10% NaOH solution. Ectoparasites were identified on the basis of morphological characters, using published keys (Skuratowicz, 1967; Nosek and Sixl, 1972; Wegner, 1972; Złotorzycka, 1972).
Molecular typing of ticks
Genomic DNA from ticks was extracted using the A&A Biotechnology DNA extraction kit (Gdańsk, Poland). PCR amplification and sequencing of the 440 bp long fragment of mitochondrial 16S rDNA (Kulakova et al., 2014) were implemented for the identification of damaged ticks derived from subcutaneous nodules and for confirmation of tick identity based on morphological features. Additionally, 36 Ixodes were genotyped to distinguish between I. ricinus and I. inopinatus following the PCR method of Estrada-Peña et al. (2014).
PCR products were sequenced by a private company (Genomed S.A., Warsaw, Poland). DNA sequence alignments and analyses were conducted using MEGA 7. Consensus sequences were compared with sequences deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) the latter being then used as reference sequences for phylogenetic tree. For the phylogenetic analysis, the Akaike information criterion was used in the jModel Test to identify the most appropriate model of nucleotide substitution. A representative tree for mt 16S rDNA was constructed using MEGA 7, by the Maximum Likelihood method and the General Time Reversible model with γ distribution. The new nucleotide sequences have been deposited in the GenBank database under the accession numbers: MK613135–MK613137; MK613139–MK613140; MK643534–MK643535; MK671574–MK671590 and MK880191–MK880193.
Statistical analysis
For the analysis of prevalence (% infected), we applied maximum likelihood techniques based on log-linear analysis of contingency tables in the IBM SPSS v. 21 software package (IBM Corporation). REGION of fox origin (three levels: eastern, western and gap regions, Fig. 1) and SEX of foxes (males and females), were used as the factors in models with the presence or absence of ectoparasites considered as a binary factor (0, 1) and referred to as INFESTATION. For each level of analysis in turn, beginning with the most complex model, involving all possible main effects and interactions, those combinations that did not contribute significantly to explaining variation in the data were eliminated in a stepwise fashion beginning with the highest level interaction (backward selection procedure). A minimum sufficient model was then obtained, for which the likelihood ratio of χ2 was not significant, indicating that the model was sufficient in explaining the data. The importance of each term in interactions involving INFESTATION in the final model was assessed by the probability that its exclusion would alter the model significantly and these values are given in the text, assessed by a likelihood ratio test between nested models with and without each factor of interest.
General linear models (GLMs in SPSS v.21) were used for the analysis of mean ectoparasite abundance, using models with normal errors, incorporating REGION of fox origin and SEX of foxes as fixed factors. Means are presented with the standard error of the mean (s.e.).
Results
Ectoparasites of adult foxes
The overall prevalence of ectoparasites (all species combined including ticks in subcutaneous tissues) was 62.8%, and was similar in male and female foxes (not significant, NS) (Table 1). Excluding Sarcoptes infestation and ticks found in subcutaneous tissues, 691 ectoparasites of 14 species were collected, including four tick species, seven flea species, one species each of the Anopluran genera, Felicola and Lipoptena (Table 1). Fourteen species were found on male foxes and 11 species on females (Table 1). The mean species richness (MSR) was 0.49 ± 0.05.
Table 1.
Comparison of prevalence and mean abundance of ectoparasites on foxes from regions differing in D. reticulatus status
| Region | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ectoparasites species | East n = 225 | West n = 73 | Gap n = 68 | Total n = 366 | ||||
| Prevalence (%) | Mean abundance ± s.e. | Prevalence (%) | Mean abundance ± s.e. | Prevalence (%) | Mean abundance ± s.e. | Prevalence (%) | Mean abundance ± s.e. | |
| Ticks: | ||||||||
| D. reticulatus | 6.7 | 0.11 ± 0.03 | 12.3 | 0.18 ± 0.06 | 0 | – | 6.6 | 0.09 ± 0.03 |
| I. ricinus | 5.3 | 0.17 ± 0.06 | 8.2 | 0.09 ± 0.12 | 1.5 | 0.01 ± 0.11 | 5.2 | 0.09 ± 0.06 |
| I. canisuga | 5.3 | 0.12 ± 0.07 | 5.5 | 0.11 ± 0.12 | 8.8 | 0.36 ± 0.12 | 6.0 | 0.19 ± 0.06 |
| I. hexagonus | 4.9 | 0.11 ± 0.04 | 0 | – | 2.9 | 0.16 ± 0.07 | 3.6 | 0.09 ± 0.04 |
| Total external ticks | 15.1 | 0.51 ± 0.12 | 20.5 | 0.38 ± 0.21 | 11.8 | 0.53 ± 0.22 | 15.6 | 0.46 ± 0.11 |
| Ticks in subcutaneous tissues* | 32.9 | 1.83 ± 0.42 | 37.0 | 3.95 ± 0.74 | 47.1 | 2.09 ± 0.77 | 36.3 | 2.60 ± 0.39 |
| Total ticks (external and subcutaneous) | 44.0 | 2.30 ± 0.43 | 49.3 | 4.26 ± 0.76 | 51.5 | 2.60 ± 0.80 | 46.4 | 0.48 ± 0.03 |
| Fleas: | ||||||||
| C. canis | 4.0 | 0.06 ± 0.02 | 2.7 | 0.04 ± 0.03 | 0 | – | 3.0 | 0.03 ± 0.02 |
| C. felis | 0.4 | 0.01 ± 0.01 | 0 | – | 0 | – | 0.3 | 0.01 ± 0.01 |
| C. globiceps | 28.4 | 1.91 ± 0.41 | 4.1 | 0.16 ± 0.71 | 10.3 | 0.18 ± 0.74 | 20.2 | 0.73 ± 0.37 |
| C. trichosa | 1.3 | 0.02 ± 0.01 | 0 | – | 0 | – | 0.8 | 0.01 ± 0.01 |
| P. irritans | 1.8 | 0.02 ± 0.01 | 0 | – | 0 | – | 1.1 | 0.01 ± 0.01 |
| A. erinacei | 0 | – | 1.4 | 0.01 ± 0.01 | 0 | – | 0.3 | 0.01 ± 0.01 |
| M. sciurorum | 0.4 | 0.01 ± 0.01 | 0 | – | 0 | – | 0.3 | 0.01 ± 0.01 |
| Total fleas | 29.3 | 2.03 ± 0.41 | 8.2 | 0.21 ± 0.73 | 10.3 | 0.18 ± 0.75 | 21.6 | 0.78 ± 0.38 |
| Other ectoparasites | ||||||||
| Scabies | 11.1 | – | 0 | – | 13.2 | – | 9.3 | – |
| Anoplura | 0.4 | 0.02 ± 0.01 | 0 | – | 0 | – | 0.8 | 0.01 ± 0.01 |
| F. vulpes | 0.4 | 0.01 ± 0.01 | 0 | – | 0 | – | 0.3 | 0.01 ± 0.01 |
| L. cervi | 0.9 | 0.01 ± 0.01 | 1.4 | – | 0 | – | 0.3 | 0.01 ± 0.01 |
| Total external ectoparasites | 40.9 | 2.56 ± 0.43 | 27.4 | 0.60 ± 0.75 | 19.1 | 0.71 ± 0.78 | 41.8 | 1.88 ± 0.34 |
| Total ectoparasites (external and subcutaneous) |
67.1 | 4.36 ± 0.59 | 50.7 | 4.54 ± 1.03 | 61.8 | 2.76 ± 1.06 | 62.8 | 3.88 ± 0.53 |
*(%) out of 366 examined foxes
Output of General Linear Model (GLM) analyses of ectoparasite abundance and ML HILOGLINEAR analyses of ectoparasite prevalence:
REGION × total ectoparasites INFESTATION: χ22 = 13.50, P < 0.001
Main effect of REGION on ectoparasites abundance (excluding subcutaneous ticks and scabies): F2, 365 = 3.83, P = 0.01
Main effect of REGION on the abundance of total fleas: F2, 365 = 3.89, P = 0.02
REGION × D. reticulatus INFESTATION: χ22 = 12.43, P < 0.001
REGION × I. hexagonus INFESTATION: χ21 = 6.41, P = 0.04
REGION × Sarcoptes INFESTATION: χ22 = 16.20, P < 0.001
Main effect of REGION on the abundance of tick in subcutaneous tissues: F2, 349 = 3.00, P = 0.05
MSR in the eastern region (0.72 ± 0.05) was twice as high as that in the western (0.37 ± 0.09) and gap areas (0.37 ± 0.10) (main effect of REGION on MSR: F2, 365 = 8.24, P < 0.001).
There were some significant differences in the prevalence and abundance of total ectoparasites/ectoparasite species between three regions (Table 1), including the expected lack of D. reticulatus ticks in the gap area.
Mean abundance of combined species of ectoparasites (excluding subcutaneous ticks and Sarcoptes) was 1.88 ± 0.34 (Table 1).
Fleas
Fleas constituted a large proportion of the collected ectoparasites (seven flea species of 14 identified ectoparasite species, 508 flea specimens of 691 ectoparasites) with a total prevalence of 21.6% (25.3% in females, 18.6% in males) (Table 1). There were some differences in flea prevalence between males and females depending on the region (REGION × SEX × total flea INFESTATION: χ22 = 9.80, P = 0.01). Generally, the prevalence of fleas was higher in females than in males in each of the regions (eastern region: 31.3 vs 27.8%; western region: 9.4 vs 6.7%); however, the difference was most pronounced in foxes from the gap area, where no males were found infested with fleas in comparison to 22.6% of females. The most common (20.2%) and abundant flea species was Chaetopsylla globiceps (Table 1).
Ctenocephalides canis was found on foxes from the eastern and western regions (3%; Table 1). The anthropophilic flea species, Pulex irritans, was recovered from four foxes (total prevalence 1.1%) (Table 1). Chaetopsylla trichosa was found only on foxes from the eastern region (Table 1). Only single specimens of Ctenocephalides felis, Archaeopsylla erinacei and Monopsylla sciurorum were collected (Table 1).
External ticks
Four tick species were detected in adult foxes: adult D. reticulatus (25 females and 14 males), I. ricinus (32 females and 16 males), I. canisuga (31 females, 22 nymphs and two larvae) and I. hexagonus (18 females, 14 nymphs and one larva). External tick infestation was lower than flea infestation, with a prevalence of 15.6% (Table 1). Total prevalence was also similar in the three regions (NS) (Table 1).
The most common tick species was D. reticulatus with a prevalence of 6.6%, found only on foxes from the eastern and western regions (Table 1).
Ixodes canisuga was the second most common tick species with an overall prevalence of 6.0%. The prevalence of I. canisuga was twice as high on females (9.3%) compared with males (3.4%) (SEX × I. canisuga INFESTATION: χ21 = 5.44, P = 0.02) (Table 1).
Ixodes ricinus was found on 5.2% of the examined foxes (Table 1). Among 36 specimens, primarily identified as I. ricinus, no I. inopinatus ticks were identified by molecular typing.
The prevalence of I. hexagonus was low (3.6%) (Table 1).
Abundance of external ticks
The mean overall abundance of ticks was low (0.46 ± 0.11/fox) and the number of ticks collected from any single individual ranged from one to 14. There was a borderline significant effect of host sex on the abundance of I. hexagonus and I. canisuga (main effect of SEX on the abundance of I. hexagonus: F1,365 = 4.07, P = 0.05; main effect of SEX on the abundance of I. canisuga: F1,365 = 7.63, P = 0.01), and in both cases, mean abundance was higher on females than on males (I. hexagonus: 0.15 ± 0.05 vs 0.02 ± 0.05; I. canisuga: 0.28 ± 0.09 vs 0.09 ± 0.08, respectively).
Only a few specimens of the remaining ectoparasites were detected, too few to merit statistical analysis. The lice Anoplura sp. and Felicola vulpis were found in one fox each from the eastern region and the deer ked Lipoptena cervi was recorded on 0.3% of the foxes (Table 1).
Ticks in subcutaneous tissues
In total, 842 ticks were recovered from subcutaneous tissues of 350 skinned adult foxes (Fig. 2). A single nodule was observed in just 36.3% of foxes; between two and 10 nodules were found in 45.7% and in excess of 10 nodules were recorded in 18.0% of the foxes. The highest number of nodules (n = 72) was found in a fox from the western region. The mean abundance of subcutaneous ticks was 2.6 ± 0.39. Most ticks were found on the lateral thigh and paddle surfaces of fox bodies (Fig. 2).
Fig. 2.
Nodules with ticks and ticks derived from subcutaneous tissues: (A) Nodule in subcutaneous tissues. (B) Tick of unidentified species isolated from subcutaneous tissues. (C) Ixodes ricinus isolated from subcutaneous tissues
Genotyping of ticks in subcutaneous tissues
Identification of tick species, solely based on morphological features, was not possible in the majority of cases due to the high level of nodules degradation (Fig. 2). Ticks from only nine nodules could be reliably identified using morphological keys, all turning out to be I. ricinus females. Genomic DNA was extracted from 64 ticks found in subcutaneous tissues. Successful PCR amplification and sequencing of PCR products were obtained from only 22 of these 64 samples (34.4%). BLAST analysis allowed typing of 17 I. ricinus (dominant species, 77.3%) and five D. reticulatus (22.7%) among these samples. These sequences were further incorporated into phylogenetic analysis.
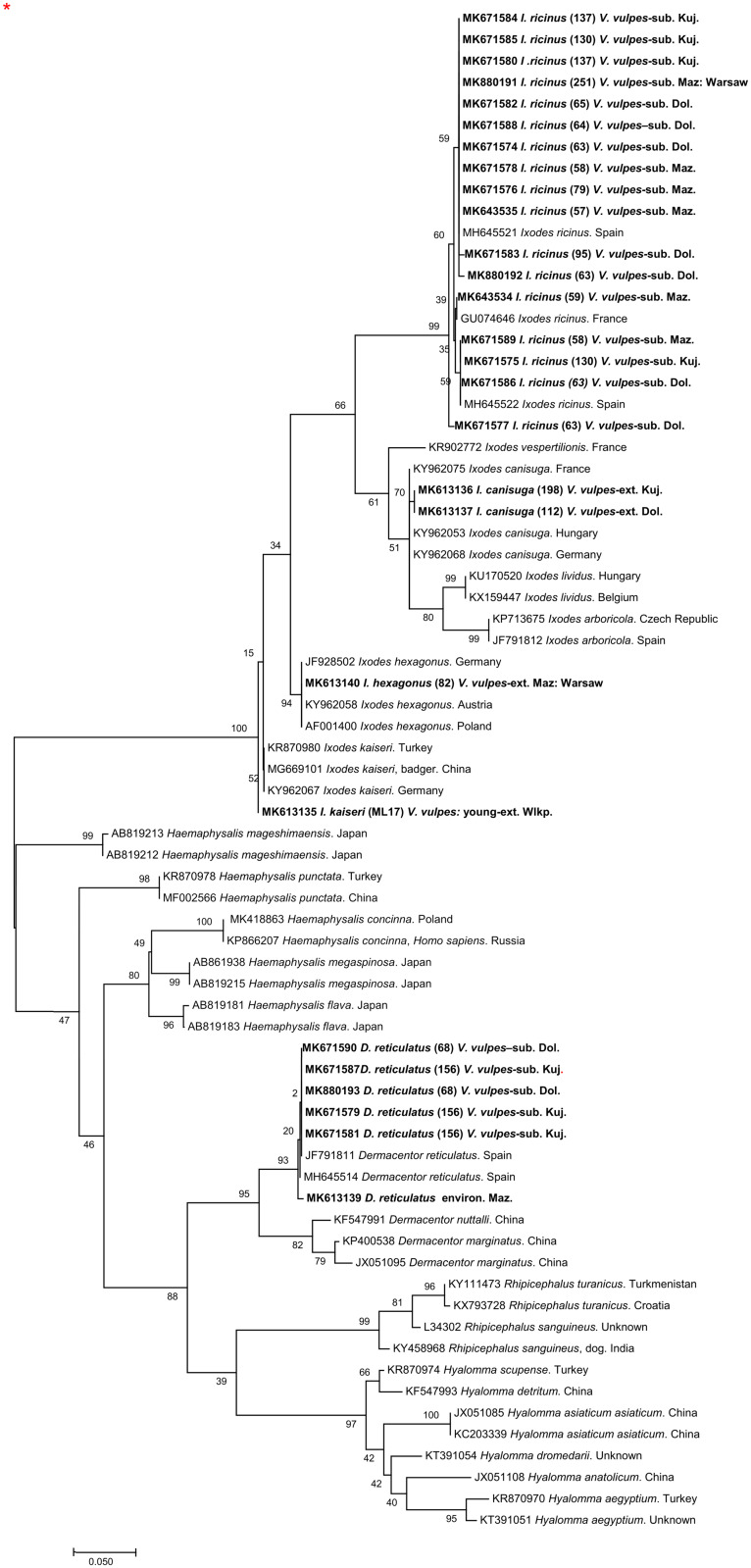
Additional sequencing and further genotyping of four selected PCR products from external ticks (see below) was totally congruent with identification based on the morphological characters of I. hexagonus (one sequence), I. canisuga (two sequences) and I. kaiseri (one sequence) and these sequences have been implemented into the phylogenetic analysis together with one additional sequence from questing D. reticulatus and reference sequences from the GenBank database. A phylogenetic tree incorporating a total of 27 sequences obtained in this study is presented in Fig. 3. The tree topology showed that mt 16S rDNA sequences from ticks in our study cluster on two separate branches, as expected from BLAST analysis, constituting the Ixodes genus clade and Dermacentor genus clade. While the Dermacentor sequences grouped only with the D. reticulatus reference sequences, our Ixodes sequences grouped on four different sub-clades containing I. kaiseri, I. canisuga, I. hexagonus and I. ricinus reference sequences, thus confirming their species identity.
Fig. 3.
Phylogenetic analysis of Ixodidae by Maximum Likelihood method. General Time Reversible model (Nei and Kumar, 2000). The tree with the highest log likelihood (−2217, 0448) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete γ distribution was used to model evolutionary rate differences among sites [5 categories (+G, parameter = 0.2220)]. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 82 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 252 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016).
Ectoparasites of foxes from Warsaw area
Among a subset of 22 foxes originating from Warsaw (12 males and 10 females), 50% were infested with ectoparasites. Mean abundance was very low, 1.00 ± 0.69. Ticks were collected from 18% of these foxes, adult D. reticulatus were found on two foxes, and a single I. hexagonus nymph was observed on one fox. An individual fox was infested with eight I. canisuga females. Flea infestation with only C. globiceps was noted on 14% of foxes, flea numbers ranging from two to seven fleas/fox. Interestingly, 36% of these urban foxes were highly infested with Sarcoptes. Co-occurrence of two or more ectoparasitic species on the same host was rare, only three of these foxes harboured more than one ectoparasite species (Sarcoptes × C. globiceps × D. reticulatus; Sarcoptes × C. globiceps × I. canisuga; C. globiceps × I. hexagonus). Finally, the occurrence of ticks in subcutaneous tissues was also detected in 23% of foxes from the Warsaw area (mean abundance: 1.23 ± 0.65).
Ectoparasites of juvenile live-trapped foxes
Twenty-five cubs were examined for ectoparasite presence, following anaesthesia. Ectoparasites were collected from 68% of cubs. Six ectoparasite species were identified (Table 2). MSR was 1.23 ± 0.20/cub
Table 2.
Prevalence and mean abundance of ectoparasites on fox cubs by region and host sex
| Region | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| East | West | Gap | Total | Total | ||||||||||||||||
| Male n = 5 | Female n = 3 | Total East | Male n = 9 | Female n = 7 | Total West | Male n = 1 | Male n = 15 | Female n = 10 | n = 25 | |||||||||||
| Prev. (%) | Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | Presence (%) | Number of ectoparasites ± s.e. | Prev. (%) |
Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | Prev. (%) | Mean abundance ± s.e. | |
| I. ricinus | 100 | 4.4 ± 1.35 | 66.7 | 2.67 ± 1.74 | 67.7 | 3.53 ± 0.10 | 22.2 | 0.67 ± 1.01 | 57.1 | 2.00 ± 1.14 | 37.5 | 1.33 ± 0.76 | 100 | 4.00 ± 3.02 | 53.3 | 3.02 ± 1.15 | 60.0 | 2.33 ± 1.04 | 56.0 | 2.75 ± 0.81 |
| I. canisuga | 20 | 0.20 ± 0.16 | 0 | – | 12.5 | 0.10 ± 0.13 | 11.1 | 0.11 ± 0.12 | 14.3 | 0.14 ± 0.14 | 12.5 | 0.13 ± 0.09 | 100 | 8.00 ± 0.36 | 20.0 | 2.78 ± 0.14 | 10.0 | 0.07 ± 0.12 | 16.0 | 1.70 ± 0.10 |
| I. hexagonus | 0 | – | 0 | – | 0 | – | 11.1 | 0.56 ± 0.35 | 0 | – | 6.2 | 0.28 ± 0.27 | 0 | – | 6.7 | 0.19 ± 0.40 | 0 | – | 4.0 | 0.11 ± 0.28 |
| I. kaiseri | 0 | – | 0 | – | 0 | – | 0 | – | 14.3 | – | 6.2 | 0.07 ± 0.05 | 0 | – | 0 | – | 10.0 | 0.71 ± 0.71 | 4.0 | 0.03 ± 0.06 |
| Total ticks | 100 | 4.36 ± 1.43 | 66.7 | 2.67 ± 1.85 | 87.5 | 3.63 ± 1.17 | 44.4 | 1.33 ± 1.07 | 71.4 | – | 56.2 | 1.81 ± 0.81 | 100 | 12.00 ± 3.20 | 66.7 | 5.98 ± 1.22 | 70.0 | 2.48 ± 1.11 | 68.0 | 4.58 ± 0.86 |
| C. globiceps | 60 | 0.60 ± 0.11 | 0 | – | 37.5 | 0.3 ± 0.09 | 0 | – | 0 | 0.14 ± 0.08 | 0 | – | 0 | – | 20.0 | 0.20 ± 0.09 | 0 | – | 12.0 | 0.12 ± 0.07 |
| Scabies | 0 | – | 0 | – | 0 | – | 0 | – | 14.3 | – | 6.2 | – | 0 | – | 0 | – | 10.0 | – | 4.0 | – |
| Total ectoparasites | 100 | 5.20 ± 1.47 | 66.7 | 2.67 ± 1.89 | 87.5 | 3.93 ± 1.20 | 44.4 | 1.33 ± 1.09 | 71.4 | 2.87 ± 1.24 | 56.2 | 1.81 ± 0.83 | 100 | 12.00 ± 3.28 | 66.7 | 6.18 ± 1.25 | 70.0 | 2.48 ± 1.13 | 68.0 | 4.68 ± 0.88 |
The most common tick species was I. ricinus, which was found on 56% of cubs (Table 2). This tick species occurred in all regions with high prevalence, as compared to other ticks species (REGION × I. ricinus INFESTATION: χ22 = 7.10, P = 0.03; Table 2). The second most prevalent tick species, occurring in all regions with a similar prevalence, was I. canisuga (Table 2). Ixodes hexagonus was collected from one male cub and a single I. kaiseri female was found on a female cub (Table 2).
Among the identified tick species, I. ricinus was the most abundant (2.75 ± 0.81/cub; Table 2). The mean abundance of I. canisuga (1.70 ± 0.10) was lower than I. ricinus (Table 2).
The only flea species found on cubs was C. globiceps. High Sarcoptes infestation was observed in one female cub from the western region (Table 2).
Comparison of the ectoparasites community of adult and juvenile foxes
The ectoparasite communities of adult and juvenile foxes were sampled at different times of the year for the reasons given earlier, and therefore since it would not be possible to disentangle age (juvenile vs adult) and seasonal (spring/summer vs autumn/winter) effects, neither factor could be included in statistical models. Nevertheless, with that proviso, some comparison between the age classes was worthwhile and proved to be instructive. MSR was twice as high for juvenile foxes compared to adults (1.23 ± 0.20 and 0.49 ± 0.05, respectively) (F1, 390 = 17.68, P < 0.001). The total prevalence of ectoparasites, including ticks in subcutaneous tissues, was higher in cubs (68%) than adults (62.8%) (χ21 = 6.52, P = 0.01; Tables 1 and 2). Among external ticks, I. ricinus was the dominant species (56%) in cubs, followed by I. canisuga (16%) but no D. reticulatus ticks were recovered. In adults, I. ricinus and D. reticulatus were most common, both externally and in subcutaneous sites. Differences in mean ectoparasites abundance between cubs and adults were not significant (NS). The total prevalence of ticks was higher in juvenile foxes compared to adults (χ21 = 31.43, P < 0.001; Tables 1 and 2). Total fleas prevalence was almost twice as high in adults compared with cubs (χ21 = 4.24, P = 0.04; Tables 1 and 2).
Discussion
In the present study, we assessed the ectoparasite communities of red foxes in three areas of Poland, covering the old historic endemic and new regions colonized recently by D. reticulatus, as well as a region where this tick species has never been recorded. Although the prevalence of infestation with D. reticulatus was highest among the tick species recovered from adult foxes, no ticks of this species were found on cubs, and thus, on the basis of our data, we can discount red foxes as a source of transmissible infection with B. canis for D. reticulatus. The ectoparasite communities differed between adult individuals and cubs, although as emphasized earlier these two age groups of foxes were sampled at different times of the year. Additionally, a meticulous search for subcutaneous ticks in adult foxes revealed a high frequency of ticks occurring in subcutaneous tissues, much higher even than the prevalence of ticks on the skin surface of these same animals. Combining morphological and molecular methods of species identification allowed for providing an insight into red foxes’ competence as hosts for various tick species and confirmed that both I. ricinus and D. reticulatus were found in subcutaneous localization.
Our finding of a high infestation of red foxes with ectoparasites (62.8% in adults and 68% in cubs) corresponds well with comparably high prevalence recorded in other European countries (Sréter et al., 2003; Kočišová et al., 2006). The most prevalent external tick species in adults was D. reticulatus. The adult foxes for our study were acquired mostly from driven hunts organized in the hunting season beginning in the autumn and from individual hunts in the winter season, which corresponds well with the seasonal activity of adult D. reticulatus ticks (Kiewra et al., 2016). In Central Poland, adult D. reticulatus are most active in early spring (March–April) and autumn (October–November) (Bajer et al., 2014a, 2014b, 2017). Foxes obtained at these times of the year are therefore more likely to be infested with D. reticulatus than I. ricinus, the latter species showing peak activity in late spring (May–June) and late summer/early autumn (September) (Kowalec et al., 2017). Accordingly, in our study, the tick I. ricinus was most prevalent and abundant on juvenile foxes trapped in the spring season (May–June) when all the developmental stages of this species show highest activity. Since fox cubs tend to stay close to their dens for the first 2–3 months of life (Goszczyński et al., 2008) and do not explore more distant areas, the risk of contracting adult D. reticulatus (a species that is typically encountered as an adult in open habitats) is low. Fox cubs spend most of their time in burrows, similarly to rodents, which are known to be competent hosts for nidicolous juvenile D. reticulatus. (Paziewska et al., 2010; Pfäffle et al., 2015; Dwużnik et al., 2019). Despite this, we did not find any juvenile D. reticulatus on fox cubs. This tick was found in the western and eastern regions of Poland, which are known to lie in the geographic range of the occurrence of D. reticulatus, but was not observed on foxes from the gap area, and this finding is consistent with our current understanding of the distribution of D. reticulatus in Poland (Mierzejewska et al., 2016; Dwużnik et al., 2019). The low frequency of infestation and a lack of D. reticulatus on foxes from the gap area in our data suggest that red foxes play only a minor role in spreading this species. However, considerable variations in the prevalence of D. reticulatus (0.3–27%) on foxes have been reported in other studies (Sréter et al., 2003; Kočišová et al., 2006; Meyer-Kayser et al., 2012), and therefore further investigation is required to consolidate the role of red foxes as hosts of D. reticulatus. The absence of juvenile D. reticulatus on cubs suggests that red foxes do not constitute a source of B. canis infection for tick instars. We found only a low prevalence (less than 4%) and low abundance of the nidicolous tick species, I. hexagonus and I. canisuga, in adult and juvenile foxes. In other studies, I. hexagonus has been reported to constitute a greater part of the tick community of red foxes (Sándor et al., 2017; Checa et al., 2018). Interestingly, in the present study, both species, I. hexagonus and I. canisuga, were found on foxes from the Warsaw area. Harris and Thompson (1978) also found these two tick species on urban foxes in London, so it is likely that these tick species are exchanged between red foxes and dogs. We found significant associations between I. canisuga and I. hexagonus abundance and host sex in adult foxes, with a higher mean abundance on females. Vixens generally spend more time in dens compared to males, especially in the breeding season, so they may be particularly prone to infestation by nidicolous ticks (Harris and Thompson, 1978; Cavallini, 1996). Both I. canisuga and I. hexagonus were also recovered from juvenile foxes, confirming that red foxes are important hosts for nest-dwelling tick species.
Ixodes kaiseri, a species of which we recovered only one female from one fox cub, was among the rarest in this study. There are very few reports of I. kaiseri on foxes or even on other mammals in the literature, although the species has been found in Germany and there are some reports also of its occurrence from other European countries (e.g. Hornok et al., 2017). In Poland, it has been recorded previously only from raccoon dogs (Wodecka et al., 2016).
The phylogenetic tree, illustrated in Fig. 3, summarizes the range of tick species in red foxes. As a consequence of the high level of degradation of DNA material extracted from subcutaneous ticks and because we used a short fragment of a conserved molecular marker (mt 16S rDNA), it was not possible in this study to explore genetic diversity within species of ticks. However, the sequences we obtained and incorporated into the phylogenetic analysis were sufficient in confirming the species status of our isolates.
The flea community was the most diverse ectoparasite group in our study. The most abundant flea species was C. globiceps, which was detected in both adults (also urban) and cubs and is known as a species that occurs commonly in fox populations (Karbowiak et al., 2016; Foley et al., 2017). The prevalence of combined flea species in adults was much higher in the autumn/winter season than in juvenile foxes trapped in May–June, in which only C. globiceps was found, with a very low prevalence. Besides the common C. globiceps infestations in adults, we also found flea species that have been rarely reported from foxes: the squirrel flea M. sciurorum and hedgehog flea A. erinacei (Sréter et al., 2003; Víchová et al., 2018).
Our finding of P. irritans, C. canis and C. felis and Sarcoptes infestation on adult foxes suggests that foxes explore anthroponotic habitats, and as a result, acquire ectoparasites from domestic animals (Domínguez-Peñafiel et al., 2011; Carricondo-Sanchez et al., 2017). Most of the fleas on foxes are picked up during the exploration of their habitats (Buckle and Harris, 1980). The young foxes inspected for ectoparasites in our study were about 2.5–3.5 months old. At this age, cubs spend most of their time in dens or in close proximity to the burrows (Goszczyński et al., 2008), as they do not hunt for themselves. Then, when they start to explore larger areas of their habitat and eventually try to establish their own territory, they become more exposed to flea infestation (Buckle and Harris, 1980). Fleas can accumulate on adults in the autumn/winter seasons during territory penetrations, breeding season or contact with prey.
This current research project is the first to study the ectoparasite community of juvenile foxes in Poland sampled by a live-trapping method. Examination of live-trapped foxes is likely to reduce the risk of some species of ectoparasites escaping from the host (e.g. fleas), and may result in higher burdens of ectoparasites than on foxes that have been shot, indeed as we found. Examining a host under anaesthesia reduces the likelihood of loss of ectoparasites, and consequently, ectoparasite burdens can be relatively high compared with those on animals provided by hunters (Galloway, 2005; Whitaker et al., 2009; Foley et al., 2017).
One of the interesting and largely unexpected findings of the current study was our demonstration that ticks in subcutaneous tissues are very common among foxes, and indeed we found tick-containing subcutaneous nodules in all regions and more often than ticks attached to the body surface. Most of the ticks involved were identified as I. ricinus and only a few were D. reticulatus. To the best of our knowledge, the reasons for and mechanisms of tick penetration under host skin are not known precisely. One of the hypotheses on the origin of such nodules is based on the generation of hyper-inflammation by the host at the place of tick attachment. This may lead to the excessively deep penetration of the tick hypostome and an inability of the tick to release itself from the host after feeding, resulting in the tick ‘sinking’ into the skin (D'Amico et al., 2017). The hypostome of all the developmental stages of I. ricinus is definitely longer than that of D. reticulatus. This may explain why female I. ricinus with their very long hypostomes become ‘stuck’ in host tissues and cannot easily detach, compared with species with shorter hypostomes (Nutall, 1914; Tugwell and Lancaster, 1962; D'Amico et al., 2017). A high level of infestation and a long duration of exposure to I. ricinus bites are likely to result in enhanced immune responses to the presence of the ticks. This could lead to ticks being unable to free themselves from host tissues after completion of engorgement and consequently they become encapsulated and killed by the cutaneous host response. However, the majority of nodules that we found were under intact skin, with no signs of inflammation or scars. We observed skin damage in only one case and this was about a 1 cm wide oval reddish area, free of fur and likely resembling a tick attachment site. At this location, under a small perforation in the skin, we found several ticks surrounded by host tissue. However, for the remaining 842 nodules that we examined, we did not observe any signs of previous tick attachment on the surface of the local skin. As the majority of ticks in nodules were highly degenerated, this suggests that encapsulation had taken place sufficiently earlier to provide the tissue with appropriate time for regeneration. This could explain why skin damage was not observed on the foxes. We were able to identify only nine specimens by morphological characters and we consider this to be evidence in support of the idea that a rapid immune response to tick attachment in foxes results in the death of ticks and their eventual resorption (Tugwell and Lancaster, 1962). An alternative explanation to hyper-inflammation trapping ticks in the skin may be aggregation around bleeding wounds/cuts leading to D. reticulatus accidentally occurring in subcutaneous tissues.
In conclusion, although infestation with D. reticulatus was relatively high in adult foxes, no ticks of this species were found on cubs, thus red foxes are unlikely to play a significant role as the source of infection for D. reticulatus with microorganisms such as B. canis. The ectoparasite communities of adult individuals and cubs were distinct and differed. A meticulous search for subcutaneous ticks in culled adult foxes revealed a high frequency of tick occurrence in subcutaneous tissues, but the mechanism by which ticks penetrate beneath the skin surface remains unknown.
Acknowledgements
We would like to express our sincere gratitude to Professor Grzegorz Karbowiak from W. Stefański Institute of Parasitology of the Polish Academy of Sciences, Twarda 51/55, Warsaw, Poland for his help in identifying the ectoparasitic species recorded in this study. We sincerely thank the employees of Forest Districts: Dobieszyn, Konstantynowo and Spała for their assistance in trapping juvenile foxes. Our special thanks are due to Marek Panek, PhD., Director of PZŁ Research Station in Czempiń. We also thank Bartosz Szarek, of ‘Dzik’ Hunting Club Żyrardów for help and support in acquiring research materials and creating cartographic presentations, and Professor Jerzy M Behnke, University of Nottingham, UK, for the linguistic proofreading of this article.
Financial support
The study was financially supported by the National Science Centre (NCN) Sonata Bis grant no. 2014/14/E/NZ7/00153.
Ethical standards
Not applicable.
Conflict of interest
None.
References
- Adkins CA and Stott P (1998) Home ranges, movements and habitat associations of red foxes Vulpes vulpes in suburban Toronto, Ontario, Canada. Journal of Zoology 244, 335–346. [Google Scholar]
- Bajer A, Mierzejewska EJ, Rodo A, Bednarska M, Kowalec M and Welc-Falęciak R (2014a) The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland: Part 1: a population study on sled dogs during the racing season. Veterinary Parasitology 202, 276–286. http://dxdoiorg/101016/jvetpar201312033. [DOI] [PubMed] [Google Scholar]
- Bajer A, Mierzejewska EJ, Rodo A and Welc-Falęciak R (2014b) The risk of vector-borne infections in sled dogs associated with existing and new endemic areas in Poland: Part 2: occurrence and control of babesiosis in a sled dog kennel during a 13-year-long period. Veterinary Parasitology 202, 234–240. [DOI] [PubMed] [Google Scholar]
- Bajer A, Rodo A, Alsarraf M, Dwuznik D, Behnke JM and Mierzejewska EJ (2017) Abundance of the tick, Dermacentor reticulatus, in an ecosystem of abandoned meadows: experimental intervention and the critical importance of mowing. Veterinary Parasitology 246, 70–75. [DOI] [PubMed] [Google Scholar]
- Buckle A and Harris S (1980) The flea epifauna of a suburban fox (Vulpes vulpes) population. Journal of Zoology 190, 431–439. [Google Scholar]
- Carricondo-Sanchez D, Odden M, Linnell JDC and Odden J (2017) The range of the mange: spatiotemporal patterns of sarcoptic mange in red foxes (Vulpes vulpes) as revealed by camera trapping. PLoS ONE 12, e0176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallini P (1996) Ranging behaviour of red foxes during the mating and breeding seasons. Ethology Ecology & Evolution 8, 57–65 DOI: 101080/0892701419969522935. [Google Scholar]
- Chang SH, Park JH, Kwak JE, Joo M, Kim H, Chi JG, Hong ST and Chai JY (2006) A case of histologically diagnosed tick infestation on the scalp of a Korean child Korean. Journal of Parasitology 44, 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checa R, López-Beceiro AM, Montoya A, Barrera JP, Ortega N, Gálvez R, Marino V, González J, Olmeda AS, Fidalgo LU and Miró G (2018) Babesia microti-like piroplasm (syn. Babesia vulpes) infection in red foxes (Vulpes vulpes) in NW Spain (Galicia) and its relationship with Ixodes hexagonus. Veterinary Parasitology 252, 22–28. [DOI] [PubMed] [Google Scholar]
- D'Amico G, Juránkoá J, Tăbăran FA, Frgelecová L, Forejtek P, Matei IA, Ionică AM, Hodžić A, Modrý D and Mihalca and AD (2017) Occurrence of ticks in the subcutaneous tissue of red Vulpes vulpes in Czech Republic and Romania. Ticks and Tick Borne Diseases 8, 309–312. [DOI] [PubMed] [Google Scholar]
- Domínguez-Peñafiel G, Giménez-Pardo C, Gegúndez MI and Lledó L (2011) Prevalence of ectoparasitic arthropods on wild animals and cattle in the Las Merindades area (Burgos, Spain). Parasite 18, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscher GG, Leschnik M, Fuehrer HP and Joachim A (2015) Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. International Journal for Parasitology. Parasites and wildlife 4, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwużnik D, Mierzejewska EJ, Drabik P, Kloch A, Alsarraf M, Behnke JM and Bajer A (2019) The role of juvenile Dermacentor reticulatus ticks as vectors of microorganisms and the problem of ‘meal contamination’. Experimental and Applied Acarology 78, 181–202. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Nava S and Petney T (2014) Description of all the stages of Ixodes inopinatus sp. (Acari: Ixodidae). Ticks and Tick-Borne Diseases 5, 734. [DOI] [PubMed] [Google Scholar]
- Foley P, Foley J, Sándor AD, Ionica AM, Matei IA, D'Amico G, Gherman CM, Doms C and Mihalca, AD (2017) Diversity of flea (Siphonaptera) parasites on red foxes (Vulpes vulpes) in Romania. Journal of Medical Entomology 54, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Galloway TD (2005) Ectoparasites from native and introduced birds from Christchurch and surrounding areas, New Zealand. Tuhinga 16, 13–20. [Google Scholar]
- Goszczyński J, Misiorowska M and Juszko S (2008) Changes in the density and spatial distribution of red fox dens and cub numbers in central Poland following rabies vaccination. Acta Theriologica 53, 121–127. [Google Scholar]
- Harris S and Thompson GB (1978) Populations of the ticks Ixodes (Pholeoixodes) hexagonus and Ixodes (Pholeoixodes) canisuga infesting suburban foxes, Vulpes vulpes. Journal of Zoology 186, 83–93. [PubMed] [Google Scholar]
- Hornok S, Sándor AD, Beck R, Farkas R, Beati L, Kontschán J, Takács N, Földvári G, Silaghi C, Meyer-Kayser E, Hodžić A, Tomanović S, Abdullah S, Wall R, Estrada-Peña A, Duscher GG and Plantard O (2017) Contributions to the phylogeny of Ixodes (Pholeoixodes) canisuga, I. (Ph) kaiseri, I. (Ph) hexagonus and a simple pictorial key for the identification of their females. Parasites & Vectors 10, 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F, Ringenier M, Putting M, Berger L, Burgers S, Kortekaas R, Lenssen J, Roessel M, Wijnveld M and Madder M (2015) Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasites & Vectors 8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowiak G, Szewczyk T and Werszko J (2016) Ectoparasites of carnivores in north-eastern Poland. Annals of Parasitology 62 (suppl.), 184. [Google Scholar]
- Kiewra D, Czułowska A and Lonc E (2016) Winter activity of Dermacentor reticulatus (Fabricius, 1794) in the newly emerging population of Lower Silesia, south-west Poland. Ticks and Tick-Borne Diseases 7, 1124–1127. [DOI] [PubMed] [Google Scholar]
- Kočišová A, Lazar P and Letko V (2006) Ectoparasitic species from red foxes (Vulpes vulpes) in East Slovakia. Veterinarski Arhiv 76, 599–563. [Google Scholar]
- Kowalec M, Szewczyk T, Welc-Falęciak R, Siński E, Karbowiak G and Bajer A (2017) Ticks and the city – are there any differences between city parks and natural forests in terms of tick abundance and prevalence of spirochaetes? Parasites & Vectors 10, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulakova NV, Khasnatinov MA, Sidorova EA, Adel`shin RV and Belikov SI (2014) Molecular identification and phylogeny of Dermacentor nuttalli (Acari: Ixodidae). Parasitology Research 113, 1787–1793. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G and Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 70 for bigger datasets. Molecular Biology and Evolution 3, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Kayser E, Hoffmann L, Silaghi C, Pfister K, Mahling M and Passos LM (2012) Dynamics of tick infestations in foxes in Thuringia, Germany. Ticks and Tick-Borne Diseases 3, 232–239. [DOI] [PubMed] [Google Scholar]
- Mierzejewska EJ, Pawełczyk A, Radkowski M, Welc-Falęciak R and Bajer A (2015) Pathogens vectored by the tick, Dermacentor reticulatus, in endemic regions and zones of expansion in Poland. Parasites & Vectors 8, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierzejewska EJ, Estrada-Peña A, Alsarraf M, Kowalec M and Bajer A (2016) Mapping of Dermacentor reticulatus expansion in Poland in 2012–2014. Ticks and Tick Borne Diseases 7, 94–106. [DOI] [PubMed] [Google Scholar]
- Mierzejewska EJ, Dwużnik D, Tołkacz K, Bajer A, Panek M and Grzybek M (2020) The efficiency of live-capture traps for the study of red fox (Vulpes vulpes) cubs: A three-year study in Poland. Animals 10, 374. doi: 10.3390/ani10030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalca AD, Dumitrache MO, Sándor AD, Magdaş C, Oltean M, Györke A, Matei IA, Ionică A, D'Amico G, Cozma V and Gherman CM (2012) Tick parasites of rodents in Romania: host preferences, community structure and geographical distribution. Parasites & Vectors 5:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M and Kumar S (2000) Molecular Evolution and Phylogenetics. Oxford, US: Oxford University Press. [Google Scholar]
- Nosek J and Sixl W (1972) Central-European ticks (Ixodoidea) key for determination.
- Nutall GH (1914) Penetration of Ixodes beneath the skin. Parasitology 7, 258–259. [Google Scholar]
- Panek M and Bresiński W (2002) Red fox Vulpes vulpes density and habitat use in a rural area of western Poland in the end of 1990s, compared with the turn of 1970s. Acta Theriologica 4, 433–442. [Google Scholar]
- Pawełczyk A, Bajer A, Behnke JM, Gilbert FS and Siński E (2004) Factors affecting the component community structure of haemoparasites in common voles (Microtus arvalis) from the Mazury Lake District region of Poland. Parasitology Research 92, 270–284. [DOI] [PubMed] [Google Scholar]
- Paziewska A, Zwolińska L, Harris PD, Bajer A and Siński E (2010) Utilisation of rodent species by larvae and nymphs of hard ticks (Ixodidae) in two habitats in NE Poland. Experimental and Applied Acarology 50, 79–91. [DOI] [PubMed] [Google Scholar]
- Petney TN, Pfäffle MP, Jasmin D and Skuballa JD (2012) An annotated checklist of the ticks (Acari: Ixodida) of Germany. Systematic And Applied Acarology UK 17, 115–170. [Google Scholar]
- Pfäffle M, Littwin N and Trevor P (2015) Host preferences of immature Dermacentor reticulatus (Acari: Ixodidae) in a forest habitat in Germany. Ticks and Tick-Borne Diseases 6, 508–515. 101016/jttbdis201504003. [DOI] [PubMed] [Google Scholar]
- Radzijevskaja J, Mardosaitė-Busaitienė D, Aleksandravičienė A and Paulauskas A (2018) Investigation of Babesia spp. in sympatric populations of Dermacentor reticulatus and Ixodes ricinus ticks in Lithuania and Latvia. Ticks and Tick-Borne Diseases 9, 270–274. [DOI] [PubMed] [Google Scholar]
- Rubel F, Brugger K, Pfeffer M, Chitimia-Dobler L, Didyk YM, Leverenz S, Dautel H and Kahl O (2016) Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Tick and Tick Borne Diseases 7, 224–233. [DOI] [PubMed] [Google Scholar]
- Sándor AD, D'Amico G, Gherman CM, Dumitrache MO, Domșa C and Mihalca DA (2017) Mesocarnivores and macroparasites: altitude and land use predict the ticks occurring on red foxes (Vulpes vulpes). Parasites & Vectors 10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuratowicz W (1967) Klucze do Oznaczania Owadów Polski; Część XXIX Pchły – Siphonaptera (Aphaniteptera). Opracowanie Zbiorowe. Warszawa, PL: Państwowe Wydawnictwo Naukowe, (In Polish). [Google Scholar]
- Smith DD, Frenkel JK and Smith EI (1986) Intradermal infestation of a red fox (Vulpes vulpes) by the lone star tick (Amblyomma americanum). Journal of Wildlife Diseases 22, 122–124. [DOI] [PubMed] [Google Scholar]
- Sréter T, Széll Z and Varga I (2003) Ectoparasite infestations of red foxes (Vulpes vulpes) in Hungary. Veterinary Parasitology 115, 349–354. [DOI] [PubMed] [Google Scholar]
- Szymański S (1986) Distribution of the tick Dermacentor reticulatus (Fabricius, 1794) (Ixodidae) in Poland. Acta Parasitologica Polonica 31, 143–154. [Google Scholar]
- Trewhella WJ, Harris A and McAllister FE (1989) Dispersal distance, home range size and population density in the red fox (Vulpes vulpes): a quantitative analysis. Journal of Applied Ecology 25, 423–434. [Google Scholar]
- Tugwell P and Lancaster JL (1962) Results of a tick-host study in northwest Arkansas. Journal of the Kansas Entomological Society 35, 202–211. [Google Scholar]
- Víchová B, Bona M, Miterpáková M, Kraljik J, Čabanová V, Nemčíková G, Hurníková Z and Oravec M (2018) Fleas and ticks of red foxes as vectors of canine bacterial and parasitic pathogens, in Slovakia, Central Europe. Vector-Borne and Zoonotic Diseases 18, 611–619 [DOI] [PubMed] [Google Scholar]
- Wegner Z (1972) Klucze do Oznaczania Owadów Polski; Część XVI Wszy – Anoplura. Opracowanie Zbiorowe. Warszawa, PL: Państwowe Wydawnictwo Naukowe, (In Polish). [Google Scholar]
- Whitaker J, Dick C and Ritzi C (2009) Collecting and preserving ectoparasites for ecological study. In Parsons S and Kuntz TH (eds), Ecological and Behavioral Methods for the Study of Bats. Baltimore, US: Johns Hopkins University Press, 806–882. [Google Scholar]
- Wodecka B, Michalik J, Lane RS, Nowak-Chmura M and Wierzbicka A (2016) Differential associations of Borrelia species with European badgers (Meles meles) and raccoon dogs (Nyctereutes procyonoides) in western Poland. Ticks and Tick-Borne Diseases 7, 1010–1016. [DOI] [PubMed] [Google Scholar]
- Złotorzycka J (1972) Klucze do Oznaczania Owadów Polski; Część XV Wszoły – Mallophga. Opracowanie Zbiorowe. Warszawa, PL: Państwowe Wydawnictwo Naukowe, (In Polish). [Google Scholar]