Abstract
Cancer has recently surpassed heart disease as the leading cause of deaths worldwide for the age group 45–65 and has been the primary focus for biomedical researchers. Presently, the drugs involved in the first-line cancer therapy are raising concerns due to high toxicity and lack of selectivity to cancer cells. There has been a significant increase in research with innovative nano formulations to entrap the therapeutic payload to enhance efficacy and eliminate or minimize toxic effects. Lipid-based carriers stand out due to their unique structural properties and biocompatible nature. The two main leaders of lipid-based drug carriers: long known liposomes and comparatively new exosomes have been well-researched. The similarity between the two lipid-based carriers is the vesicular structure with the core’s capability to carry the payload. While liposomes utilize chemically derived and altered phospholipid components, the exosomes are naturally occurring vesicles with inherent lipids, proteins, and nucleic acids. More recently, researchers have focused on developing hybrid exosomes by fusing liposomes and exosomes. Combining these two types of vesicles may offer some advantages such as high drug loading, targeted cellular uptake, biocompatibility, controlled release, stability in harsh conditions and low immunogenicity.
Keywords: Lipid-Based Vesicles, Cancer Therapeutics, Liposomes, Exosomes, Hybrid Exosomes, Fusion
1. Introduction
Cancer remains one of the most challenging pathological conditions and has been the primary focus for biomedical researchers. For the past several years, drug delivery systems have been aiding to tackle cancer strategies. Even though current anti-cancer drugs and chemotherapies cause serious side effects on healthy cells, they remain the conventional approach to treating many cancers [1]. Although nano formulations such as micelles, protein-based nanoparticles, inorganic and metallic nanoparticles with side effects have been widely used, drug resistance and solubility issues create hurdles for clinical advancement [2]. Thus, employing appropriate drug delivery vehicles and their functionalization would help improve pharmacokinetic parameters such as better distribution, prolonged blood circulation time, controlled release, increased intracellular concentration, superior solubility, enhanced stability, and targeted delivery of the chemotherapeutic agents [3, 4]. It should be noted that many of the FDA-approved commercially successful cancer drug delivery products started their journey in academic research labs. Thus, there is an urgent need to formulate such hypotheses of drug delivery vehicles and test their proof-of-concept to achieve viable therapeutic benefits [5].
There is an increased interest in repurposing potential therapeutic drugs by focusing on different drug delivery methods to provide controlled and targeted delivery [6]. The essential aspects of a suitable drug delivery vehicle are composition, size, and surface properties. Most recent chemotherapeutics use materials such as metal nanoparticles (NPs) due to their ability to overcome issues such as biochemical barrier crossing and photodegradation, tunability of size and shape, and ease of functionalization [7]. Yet, due to the lack of biodegradability, limited knowledge about the fate after surface modifications, and inadequate information about the pharmacokinetic parameters, especially metabolism and elimination, pose a risk regarding patient safety [8]. On the other hand, drug delivery vehicles made up of naturally occurring compounds offer advantages such as high biocompatibility, enhanced stability, and limited immunogenicity. Yet, the scalability issues remain debatable depending upon the availability of the natural source and the infrastructure needed for the isolation [9, 10]. As each material has its unique properties and its synthesis affects the loading and stability of the drug, these may be used as a combination therapy. The characteristics, including the size of the drug delivery vehicle, dictate crucial aspects such as circulation time, protein absorption, biodistribution, extravasation, immunogenicity, internalization, intracellular trafficking, payload delivery, and degradation [11]. The size of the delivery vehicle also dictates the internalization and uptake via the endocytic pathway [12].
The drug delivery vehicles face altered physical and biological barriers (such as shear forces, protein adsorption, and rapid clearance) under pathological conditions, which are difficult to overcome to achieve effective biodistribution and therapeutic effects of the pharmaceutical ingredient [13, 14]. Owing to the specific and non-specific cellular interactions, the drug delivery vehicles are surface functionalized to achieve maximal therapeutic effects. As per the existing knowledge, nano-drug vehicles can cross biochemical barriers [15]. This enhanced permeability and retention (EPR) process is improved by active targeting by attaching different targeting ligands on the surface of the particles [16]. It is well established that the drug delivery systems (DDS) in the nanometric range demonstrate enhanced tumor penetration, lowered systemic toxicity and longer circulation time and, thus, a more significant EPR effect [15].
Out of all the delivery vehicles, lipid-based carriers stand out due to their unique structural properties, tailorable drug release (pH sensitive, temperature sensitive etc.), biodegradable and biocompatible nature, ability to load both lipophilic and hydrophilic drugs, and low-risk profile [17]. Liposomes, the first known artificial vesicles to be identified as lipid-based drug delivery carriers, are usually made up of synthetic lipids and have the ability to carry the payload [18]. Though there are attempts to make these vehicles biocompatible by formulating liposomes using natural phospholipids from soybeans, rape (canola) seed, wheat germ, sunflower, and flax seed, peanuts and animal material, like egg yolk, milk, or krill; yet they face stability issues and thus may result in premature release of the cargo [18, 19]. Exosomes, the relatively new lipid-based vehicles share a similar lipidic structure to that of liposomes and possess surface ligands to facilitate trafficking in the body [20]. Integrating the two lipid-based carriers offers an opportunity to incorporate the advantages of both the carriers and eliminate the limitations to give rise to a promising platform to develop hybrid lipid-based nanocarriers. Unlike most liposomal formulations, hybrid exosomes can be delivered intravenously owing to their ability to escape the mononuclear phagocytic system or the reticuloendothelial system [21]. Currently, studies have shown the potential of hybrid exosomes to be used in various diseases, including cancer [21], fibrotic diseases [22], age-related bone loss [23], nerve damage [24], and genetic diseases [25]. To better understand the current scenario and future perspectives, this review highlights the conventional and emerging lipid-based technologies for cancer therapeutics with commercial potential. The three vital lipid-based carriers widely researched in cancer therapeutics are liposomes, exosomes, and hybrid exosomes. Liposomes are phospholipid bilayer enclosed spherical core-shell structures capable of loading pharmaceutical ingredients inside the core [26]. Since the discovery of liposomes in the 1960s [27, 28], liposomes have come a long way with broad spectra of application. Yet, it was only in the mid and late 1970s that liposomes were thought to be of use in cancer therapeutics [29–32], with slow growth in the research for almost three decades. To this point, research with exosomes started stating its applicability in the cancer field. Exosomes, with their structural similarity to liposomes with an additional payload of protein and nucleic acid along with the surface ligands entered the lipid carriers classification with superior characteristics. It was in the late 1990s that the exosomal technology started taking up pace mainly due to its potential to eradicate murine tumors [33].
This review details the parameters which define the success of cancer DDS and different approaches currently being used to tackle cancer.
2. Drug Loading Strategies
Different strategies for drug loading are classified into pre-loading, co-loading, and post-loading [34]. Pre-loading strategy is about utilizing drug nanoparticles to form a core-shell structure by means of coating. Co-loading uses drug conjugations with carrier molecules which form drug-loaded nanocarriers upon self-assembly. Post-loading strategy comprises of mixing of porous nanocarrier formulations with drug solution to achieve drug loading by adsorption, electrostatic interactions, entrapment, hydrophobic forces, etc. [34]. Considering the pre-loading strategies for exosomes or extracellular vesicles (EVs), it involves EV-producing cells either endogenously overexpressing a particular set of genes or proteins or genetically modified using plasmid or viral vectors to introduce the desired genetic material ultimately to be expressed in EVs [25–28]. Gkionis et al. utilized the co-loading strategy by combining umbelliprenin and lipid mixture in the organic phase, followed by thin lipid film hydration or microfluidic set-up and finally, the post-loading approach achieved doxorubicin (DOX) loading. The resultant liposomes exhibited cytotoxicity in human breast cancer MCF-7, MDA-MB 231, and BT474 cell lines in vitro with delayed release of drug [35].
The post-loading strategy includes electroporation [36], transfection with temperature modulation (heat shock) [37] and pH gradient [35]. This strategy is limited by the possibility of interaction of transfection agents and the genetic material to be loaded which creates discrepancies in the loading efficiency [38]. Also, precipitation and aggregation issues in the buffer have been reported with the electroporation method [39]. Zhao et al. utilized porous CaCO3 nanospheres templates for preloading the anticancer drug DOX within hollow mesoporous silica (HMS@DOX). Interestingly, these formulations were pH sensitive and released the payload in response to acidic pH in vitro. This aspect was proposed for specific delivery to lysosomes, endosomes, and cancerous tissues (pathological sites). Apart from this, the preliminary data regarding the cytotoxicity and internalization studies revealed the therapeutic superiority of HMS@DOX compared to free DOX [40]. Majumder et al. utilized the opposite charges of cationic lipid, DOTAP, and anionic siRNA as a post-loading strategy for anticancer therapy against various non-small cell lung cancer A549, H-1975, PC-9, and PC-9GR cells [41].
3. Targeting Strategies
As conventional DDS is limited for its pharmacodynamic, pharmacokinetic, and pharmacotherapeutic properties, there exists scope for developing targeted DDS to enhance the therapeutic effect at lower doses and reduce the non-specific toxicity associated with the otherwise higher doses [42]. The major advantages of targeted DDS include controlled biodistribution, increased specificity, and improved patient compliance [43].
Conventionally, the tumor targeting approaches are classified into active and passive, yet there cannot be a clear separation between these two approaches. It is true because the efficacy of active tumor targeting relies significantly on the passive accumulation of the nanocarriers in the tumor environment. Thus, the longer circulation property imparted by the passive tumor targeting approach and the active targeting approach’s selectivity and specificity dictates the nano-drug formulations’ therapeutic efficacy [44].
3.1. Passive Targeting:
Passive tumor targeting, also called as EPR effect, was discovered by Matsumura and Maeda [45]. Pathophysiological conditions lead to enhanced vascular permeability due to abnormalities (such as highly proliferating endothelial cells, pericyte remodeling and aberrant basement membrane) in blood vessels in the tumor microenvironment. Due to the size of nano-lipidic carriers being in the order of a few nanometers, these can extravasate and aggregate in the interstitial space. Moreover, these lipidic carriers get retained at the tumor site due to impaired/non-functional lymphatic drainage in tumor tissues [46]. Passive targeting is limited by the degree of tumor vascularization and angiogenesis [44]. Also, it has been long hypothesized that the osmotic pressure in the form of tumor interstitial fluid pressure is very high [47]. This pressure makes drug uptake and uniform distribution at the tumor site difficult [48]. This makes passive targeting less selective and effective.
3.2. Active Targeting:
Active tumor targeting facilitates the selective uptake of nanocarriers functionalized with targeting ligands that bind to the receptors overexpressed in tumors [46]. Functionalization with targeting ligands yields target specificity even at subcellular sites, reducing the unwanted systemic toxicity to healthy cells due to the payload [49]. Such specificity of ligand-receptor interactions leads to internalization by triggering receptor-mediated endocytosis and bypasses P-glycoprotein (PgP)-mediated drug efflux to suppress multidrug resistance [50, 51]. Various targeting ligands such as antibodies, antibody fragments, aptamers, peptides, whole proteins (e.g., transferrin) and different receptor ligands, e.g., folic acid (FA) have been employed for active tumor targeting [52–55].
4. Lipid-Based Nanocarriers
The need for lipid-based drug vehicles can be realized by understanding the fact that almost 40% of the market-approved drugs are poorly water-soluble [56]. Thus, lipid-based carriers come into the picture with advantages such as presenting the compound in a solubilized state, reducing dissolution rate, limiting absorption, improving bioavailability, and circumventing hepatic first-pass metabolism by promoting lymphatic transport [57]. Lipid modification of drugs yields improved pharmacokinetic profile, reabsorption across the tubular epithelium back into circulation, and increased binding to plasma proteins (thus prolonged circulation and reduced renal clearance) [58–60]. Additionally, lipid modification increases absorption across the gastrointestinal epithelium and blood-brain barrier [61]. There has been increasing interest in delivering highly lipophilic, poorly water-soluble cancer drugs which otherwise show exceptional in vitro potency and biological selectivity but remain limited due to their poor dissolution and solubilization when administered orally. This is where lipid-based drug delivery candidates have an advantage of enhanced absorption of drugs [62]. Lipid-based nanocarriers are a broad spectrum (Figure 1) with liposomes and exosomes being the most researched classes.
Figure 1.
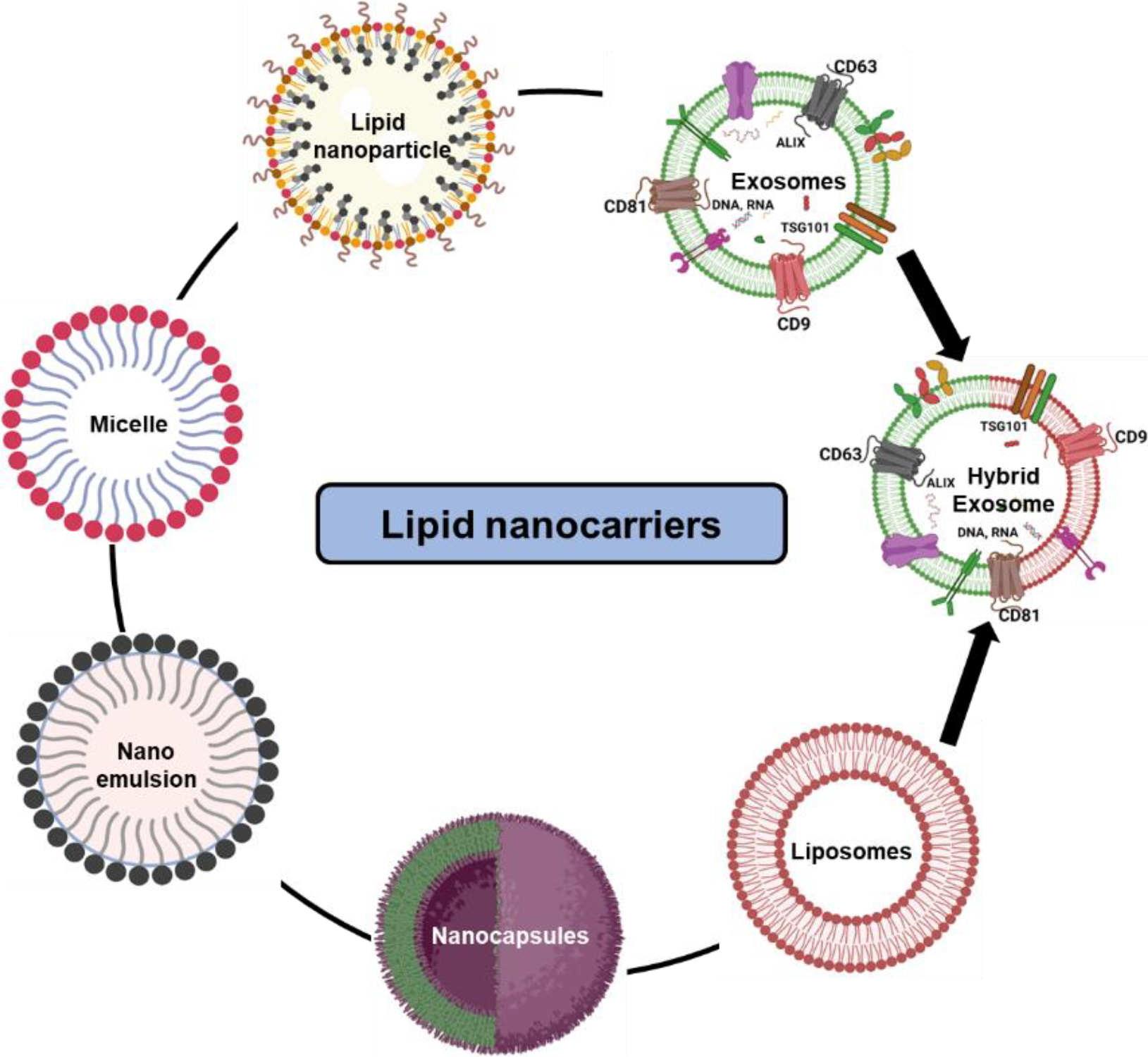
Classification of lipid-based nanocarriers based on their structure and composition. Liposomes, exosomes, and hybrid exosomes exhibit core shell structure enclosed in a lipid bilayer, while microemulsions, nanoemulsions, nanocapsules, micelles and lipid nanoparticles comprise a mono layer of phospholipids to entrap the drug of interest in its lumen.
5. Liposomal Delivery for Cancer Therapeutics
From visualizing the phospholipid bilayer in the mid-60s to being used as lipid-based drug delivery vehicles, liposomes have come a long way in the past 60 years. Liposomes are spherical, hollow, and closed phospholipids (composed of one hydrophilic polar head and two non-polar hydrophobic chains) bilayer structures with a hydrophilic core to entrap the drug of interest [63]. The two key components of liposomes are a) suitable phospholipid, either naturally occurring or chemically synthesized lipid containing a phosphorous polar head and glycerol backbone; and b) sterols (cholesterol) for fluidity and stability of the liposomes [64]. Cholesterol is the linker sterol used as a strategic component as it controls stoutness [65], permeability, and fluidity [66], increases organization of phospholipid packing for alignment of an aliphatic tail region [67, 68], reduces bilayer exchange with surrounding molecules [69] and enhances the microviscosity of the liposomal bilayer [70]. In the current clinical scenario, multiple liposomal anti-cancer formulations (Table 1) are available in the market to treat various cancers.
Table 1.
List of the approved anti-cancer liposomal drugs for use in humans by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
| Product | Active Ingredient | Target treatment/s | Ref |
|---|---|---|---|
|
| |||
| DaunoXome® | Daunorubicin citrate | Kaposi’s sarcoma in HIVpositive patients and Acute myeloid leukemia | [71] |
| Lipodox® | Doxorubicin | Breast and ovarian, multiple myeloma | [72] |
| Doxil®/Caelyx® | Doxorubicin hydrochloride | Breast and ovarian, multiple myeloma and Kaposi’s sarcoma | |
| Promitil® | Mitomycin-C | Solid Tumors and multi-drug resistant cancers | [73] |
| Myocet | Doxorubicin hydrochloride | Breast neoplasms | [74] |
| Onivyde | Irinotecan | Pancreatic neoplasms | [75] |
| Vyxeos | Cytarabine + daunorubicin Hydrochloride | Acute myeloid leukemia | [76] |
| Zolsketil | Doxorubicin hydrochloride | Breast and ovarian, multiple myeloma and Kaposi’s sarcoma | [77] |
| DepoCyt | Cytarabine | Neoplastic meningitis | [78] |
| Marqibo | Vincristine sulfate | Hematologic malignancies and solid tumors | [79] |
| Mepact | Mifamurtide | Non-metastatic osteosarcoma | [80] |
Several factors such as the composition and charge of lipids, number, length and site of fatty acid chains, head polarity of the lipids, degree of saturation/unsaturation, and phospholipid: cholesterol ratio defines the stability, release profile, and pharmacokinetic parameters of the resultant liposomal formulations [65, 81, 82]. Based on the structure, liposomes are classified as:
One phospholipid bilayer: giant unilamellar (>1 μm), large unilamellar (200 nm – 1 μm) and small unilamellar (20–200 nm).
More than one phospholipid bilayer (500 nm – 5 μm): multilamellar (multiple concentric bilayers) and multivesicular (one huge bilayer accommodating randomly placed multiple bilayers) [83–86]
Based on the polar head charge and the lipids utilized, the resultant liposomes can be zwitterionic (phosphatidylcholine), positively charged, negatively charged (phosphatidyl-glycerol), or uncharged [64]. The liposomes can be distinguished further based on their composition and/or mechanism of action, which are discussed below.
5.1. Conventional liposomes:
These are neutral liposomes comprising phosphatidylcholine as the major head group and cholesterol as the linker lipid to form the bilayer structure [87]. Myocet (Liposomal Doxorubicin) is one of the few first-generation liposomal formulations utilized to combat metastatic breast cancer but was limited for use due to its poor stability [88]. Thus, researchers are developing new ideas to modify the liposomal composition to overcome the drawbacks of poor stability and lower half-life. Lee et al. recently engineered conventional liposomes utilizing phosphatidylcholine to encapsulate Indocyanine Green for potential use in cancer phototherapy [89]. As the traditional liposomes lack stability and controlled release, these liposomes are being modified to enhance the pharmacokinetic parameters [90].
5.2. Cationic liposomes:
These comprise positively charged lipids such as DODMA, DOTMA, DOTAP, DOSPA, DOGS, DLinDMA, and DC-Chol, along with helper lipids such as DOPE, DSPC, DOPC, and cholesterol [91, 92]. Cationic liposomes have been regarded as suitable carriers for cancer therapeutics due to their superiority in terms of biodegradability, biocompatibility, and payload encapsulation efficiency [93, 94]. Yan et al. demonstrated that DOTAP-based liposomal cancer vaccine at high doses generates reactive oxygen species (ROS) and causes apoptosis to induce anti-tumor activity [95]. DOTAP/E7-lipopeptide therapeutic vaccines have been developed by Chen et al. (2008) against positive human papillomavirus (HPV) tumors, which showed enhanced CD8+ T-specific lymphocyte response [96, 97]. Interestingly, cargo-free liposomes made up of DOTMA and DOTAP showed therapeutic properties by reducing cell viability due to lipid peroxidation and increased cellular ROS production in HepG2 cells [98].
5.3. Long-circulating liposomes:
Also known as stealth liposomes are sterically stabilized via surface polymerization, usually using polyethylene glycol (PEG) to improve pharmacokinetics and delay renal clearance [99]. PEG-surface functionalization also aids in passive targeting via the EPR effect, decreases the volume of distribution, and reduces therapy-associated cardiotoxicity [100]. Different anticancer therapeutics such as DOX [101, 102], camptothecin [103], oxaliplatin [104], parthenolide [105], and all-trans retinoic acid [106] have been encapsulated into stealth liposomes to prolong the circulation time. Caelyx/Doxil (Schering-Plough, Madison NJ, USA), used for treating AIDS-related Kaposi’s sarcoma, resistant ovarian and metastatic breast cancer, is the only stealth liposomal formulation in the market [107]. In an exciting study, the half-life of the liposomal drug increased 5-fold after PEGylation, and the drug uptake by the organs with a mononuclear phagocytic system was lower than that of the conventional liposomes. The PEGylated formulation was less stable in the hydrated state but relatively more stable in human serum at 37°C for up to 5 to 6 days of incubation [108].
5.4. Ligand-targeted liposomes:
An important tumor-targeting approach is to consider the increased angiogenesis capacity at the tumor microenvironment as compared to the normal cellular environment [109]. To achieve selectivity and specificity, liposomes loaded with targeting ligands such as antibodies (Immunoliposomes) [110], small molecules such as kinase inhibitors, receptor tyrosine kinase inhibitors, non-receptor tyrosine kinase inhibitors, serine/theonine kinase inhibitors, epigenetic inhibitors, BCL-2 inhibitors, hedgehog pathway inhibitors, proteasome inhibitors, PARP inhibitors[111]; and targeting peptides [112] have shown improved tumor targeting and cellular uptake [113–115]. Jain et al. used this approach to target the angiogenesis and make the tumors devoid of nutrients by utilizing VEGF antibody- functionalized liposomes conjugated by carbodiimide chemistry. The resultant formulations showed enhanced uptake and a significant reduction in tumor burden (35%) [116]. The conventional 2-step method of loading targeting ligands involves coupling targeting ligands to pre-formed drug delivery vehicles. To overcome the batch-to-batch variations in ligand coupling yield, Stefanick et al. employed a synthetic strategy to form a lipid conjugate of the targeting ligand, a short cyclic-peptide, which would further become a building block of the HER2-targeted liposomal formulations [117].
5.5. Bubble liposomes:
These are drug delivery vehicles composed of a lipid shell encapsulating poorly water-soluble gas. This liposomal structure disrupts upon external ultrasound actuation, releasing the payload. The conventional method to produce bubble liposomes involves the introduction of inert gas bubbles for coarse dispersion of non-hydrated solid phospholipids to promote hydration [118]. Bubble liposomes are being successfully used to load siRNA [119], pDNA [120], azo initiator [121], laminin-derived peptide AG73 [122], IL-12 gene [123] and many more anti-cancer therapeutics. These have been developed as gene delivery nanocarriers [119, 124, 125].
5.6. Temperature and pH-sensitive liposomes:
The tumor microenvironment demonstrates lower extracellular pH due to an increased metabolism rate than normal healthy cells. Thus, targeting the acidic tumor microenvironment appears to be an attractive approach for current tumor-targeted therapy [126]. Researchers have formulated pH-sensitive liposomes containing DOX [127, 128], ovalbumin [129], paclitaxel (PAC) [130], shRNA and docetaxel [131], afatinib [132], and 5-fluorouracil [133]. This approach has been shown to improve intracytoplasmic delivery and cytotoxic activity and overcome multi-drug resistance compared to free anti-cancer drugs [134]. Jin et al. utilized pH-sensitive peptide derivatives in combination with polymeric lipid, DSPE-PEG200, to load PAC as a therapy for ovarian cancer. The pH-sensitive DDS showed significantly different drug release kinetics at pH 6.4 compared to pH 7. At physiological pH, DDS showed 20% drug release as compared to that at pH 6.4 and a burst release was observed which was attributed to the protonated histidine in the micellar structure which acted as a pH-response on-and-off switch [135].
To achieve a maximal therapeutic effect, liposomes are formulated by combining different lipid components to impart desired outcomes. Jiménez-López and coworkers recently formulated pegylated cationic liposomes in two sizes: large multilamellar vesicles and small unilamellar vesicles (MLV: >180 nm and SUV: <100 nm) to enhance the pharmacological properties of PAC. Surprisingly, unlike the SUVs and free PAC, the MLVs did not induce any peripheral neuropathy in mice. Their biodistribution studies inferred that the MLVs could not pass through the endothelial fenestrations of the dorsal root ganglia, thereby bypassing the side effects of peripheral neuropathy. On the other hand, the SUVs showed increased drug bioavailability in all tissues compared to MLVs by surpassing the RES [136].
5.7. Major issues in the liposomal delivery system:
Despite the extraordinary advantages, a few disadvantages of liposomes as drug delivery vehicles were identified by Daraee et al. 2016 [87]. The need for sophisticated instrumentation, high production cost, short half-life and the possibility of immune toxicity highlights the importance of identifying an alternative for liposomes to deliver drugs [87]. Moreover, immune response and issues of stability, scalability, reliability and reproducibility along with chemical factors such as instability of phospholipid components or denaturation of the encapsulated drug in the manufacturing process is what halts the translation of liposomal research of over 50 years to clinical practice [137, 138].
6. Exosomal Formulations for Cancer Therapeutics
Secreted by different cells, exosomes are naturally occurring EVs in the nanometric range (30–150 nm) containing a complex payload of proteins, lipids, and nucleic acid (mRNA, miRNA, and DNA) [139]. Since the first few reports about the suitability of exosomes for drug delivery in the late 2000s, exosomes have come a long way. Over the years, exosomes have been derived from mesenchymal stem cells (MSCs) [140, 141], hematopoietic stem cells [142], neural stem cells [143], dental pulp stem cells [144], saliva [145], urine [146], milk [147], B lymphoma cells [148], dendritic cells [149], T lymphocytes [150], macrophages [151] etc. For many years, the main source of exosomal isolation has been spent media from cell culture or bodily fluids including milk to collect the exosomes (Figure 2).
Figure 2.
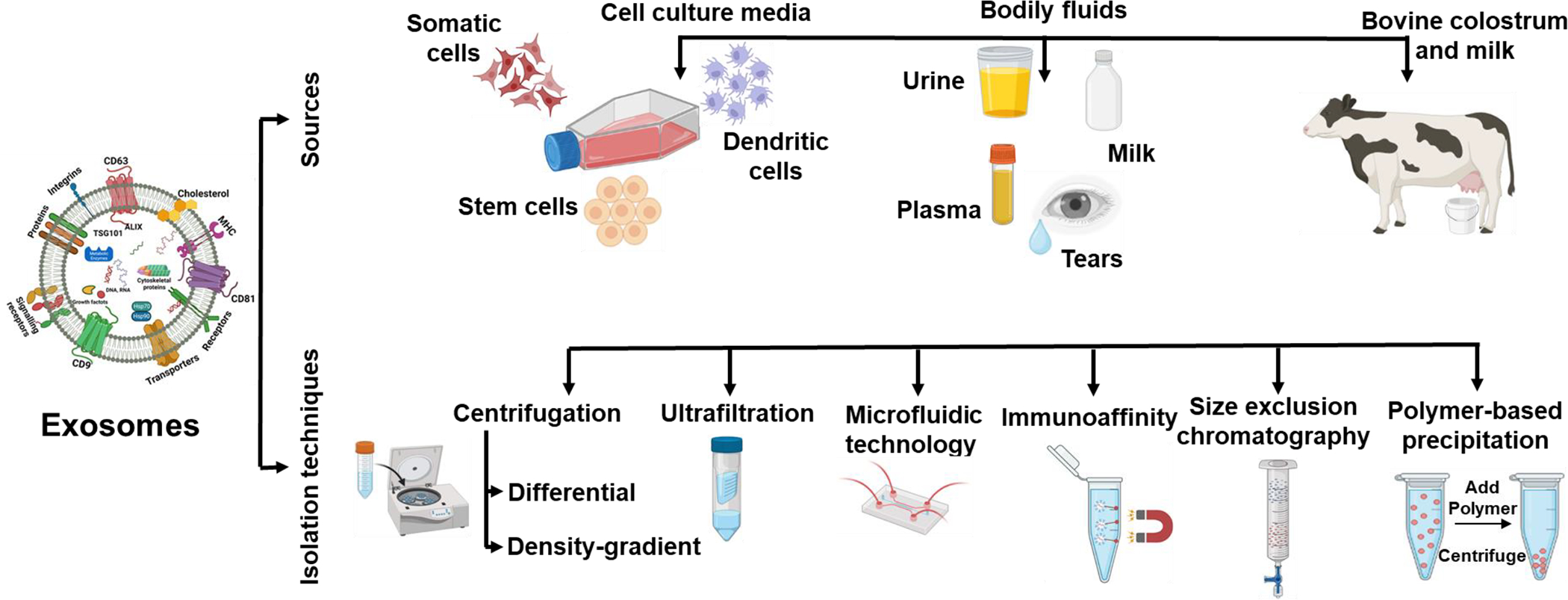
Diversity of exosomes sources and method of exosomes isolation as reported in literature.
6.1. Exosome isolation:
The most widely used method for exosome isolation is a series of centrifugation, including ultracentrifugation, to collect exosomes. A limitation of this process is that it requires large starting volumes and can thus be an expensive investment if the starting material is limited and the yield is low [152]. Another isolation technique similar to ultracentrifugation is density gradient ultracentrifugation which utilizes a nontoxic sucrose or iodixanol media to get high-purity exosomes [153]. Different methods used for the isolation of exosomes have been demonstrated in Figure 2. For further purification of exosomes, techniques such as ultrafiltration, size-exclusion chromatography and immuno-affinity chromatography are used. Ultrafiltration uses porous membranes to isolate exosomes beyond a particular size and is a very convenient method as filters with different cutoff molecular weights in various sizes are available [147]. Size-exclusion chromatography utilizes porous beads and filters or column chromatography to segregate exosomes via sequential elution based on size [154]. Based on the surface of exosomes expressing unique biomarkers such as CD63, CD81, CD82, CD9, and Alix, among others, exosomes are separated using immuno-affinity chromatography. Antibodies against these markers are attached to magnetic beads, plates, chromatography matrices, and microfluidic devices, which aid in immobilizing and capturing the exosomes [155]. Immuno-affinity chromatography has been shown to produce pure and uniform exosomes [156].
6.2. Exosomes as drug delivery vehicle:
Exosomes have been successfully exploited as delivery vectors for small molecules and nucleic acids in cancer treatment [157]. The transmembrane anchoring proteins on the exosome exosomal surface enhance endocytosis and payload transport into cells [158]. At first, Zitvogel and colleagues [159] highlighted the potential of exosomes for cancer therapies in 1988. They utilized the dendritic cells (DCs) to induce primary and secondary immune responses in vivo. They showed that by administering a single dose of acid-eluted tumor peptide-pulsed DC-derived exosomes, the tumor growth either delayed or was completely eradicated and can thus be implemented in cancer immunotherapy [159].
Exosomes have been used to develop novel therapeutic strategies to transport a wide range of hydrophilic and lipophilic drugs for cancer treatment. Exosomes aid to improve drug stability, absorption, circulation time, and accumulation at the target site, which increases drug potency in many circumstances [160]. Sun et al. showed that exosomes from EL-4 murine lymphoma cells improved curcumin stability and bioavailability, improving therapeutic response [161]. In another study, a chemotherapeutic drug, PAC loading on exosomes derived from macrophages displayed 50 times higher cytotoxicity against drug-resistant MDCKMDR1 (PGP+) cells. When compared to free PAC treatment, intranasal delivery of exosomal-PAC formulation resulted in an effective reduction of tumor burden [162].
Exosomes produced from immune cells have been shown to carry characteristics of immune cells in targeting tumor cells. Li et al. isolated exosomes from macrophages and packed with DOX-loaded polymeric nanoparticles on the core. The exosome surface was functionalized with a peptide that targets the triple negative breast cancer (TNBC) which have an overexpression of mesenchymal-epithelial transition factor (c-Met). When tested in a mouse model, these exosomal formulations targeted TNBC tumors reducing tumor burden [151]. In another study, exosomes from bone marrow MSCs were employed to deliver DOX to HER2-positive breast tumors and showed significant tumor inhibition compared to free DOX [163]. Similarly, in vitro potency of the exosomes derived from HEK293 cells was compared with the free drug, DOX and its liposomal formulation. Exosomal-DOX had superior absorption and efficacy, rapid cellular uptake, and redistribution of DOX from endosomes to the cytoplasm and nucleus than the free drug and its liposomal form [164]. The MSC-derived exosomes have also been shown to improve DOX absorption and have potent anti-tumor action against the osteosarcoma MG63 cell line [165].
For instance, most cancer over express folate receptors such as folate receptor-α (FR-α) and reduced folate carriers (RFC) within a broad range of cancerous cells, including lung cancer cell lines A549 and H1299 and A549 tumor xenografts from mice (Figure 3A) [52–55] and are thus a classic and well-recognized biomarker for tumors used in different diagnostic and therapeutic tools [166]. Utilizing this approach, our group determined the tissue distribution of exosomes and FA-functionalized exosomes using highly fluorescent Alexa Fluor™-750 (AF750) tagging. AF750 was covalently attached to the exosomal surface using stable amide chemistry; the unbound dye was removed (>99%) by ultrafiltration. To determine biodistribution, A549 lung tumor xenograft-bearing nude mice were treated i.v. with AF750-labeled Exo and FA-Exo. Animals were euthanized after 4 h. Ex vivo tissue imaging (Figure 3B) showed the highest accumulation of exosomes and FA-exosomes in the kidney and the tumor, followed by the liver, lung, spleen, and brain. However, the tumor accumulation of FA-Exo was twice higher (p <0.05) than nonfunctionalized exosomes (Figure 3C). These data indicate that FA-Exo can deliver a higher payload to the tumor site due to the higher expression of folate receptors. This aspect was utilized by attaching FA ligand to target cancer cells in various studies. FA-Exo exhibited significantly enhanced inhibition of tumor growth with withaferin A against A549 lung tumor xenograft in nude mice as compared to non-functionalized exosomes [167, 168]. Also, it has been shown that FA-Exo can achieve high therapeutic efficiency due to their ability to avoid endosome trapping. FA-functionalized exosomes containing siKRAS also showed higher gene knockdown and therapeutic efficacy against A549 lung tumors [169].
Figure 3.
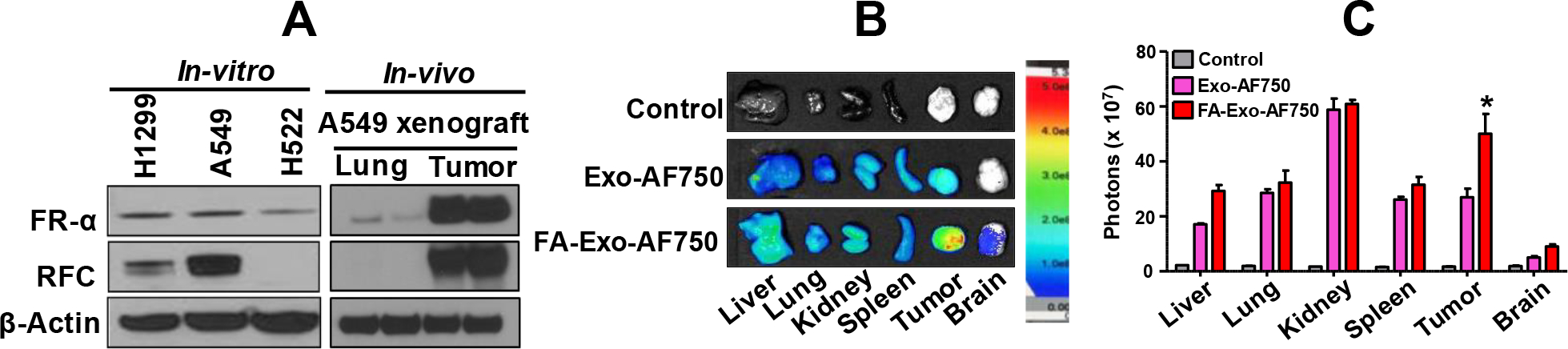
Tissue distribution of exosomes (Exo) and folic acid (FA)-functionalized exosomes (FA-Exo). Folate receptor levels in NSCLC cells in vitro and lung and lung tumor tissues in nude mice, as analyzed by western blot (A). A549-tumor-bearing mice were treated with AF750-labeled Exo and FA-Exo intravenously. Different tissues and tumors were imaged ex vivo after 4 h of the treatment (B). Data for the fluorescence signals represent mean ± SD (n=3). The figure is partially adopted and redrawn from Munagala et al., 2021 [168].
In the last few years, it has been shown that exosomes could be utilized to deliver drugs to the brain. Exosomes have been reported to cross the blood-brain barrier (BBB) to deliver a payload to treat brain disorders, including cancers [170]. Exosomes produced from Raw 264.7 cells and loaded with superparamagnetic iron oxide nanoparticles (SPIONs) and curcumin, with the surface functionalized with a neuropilin-1-targeted peptide (RGERPPR, RGE), can cross the BBB and effectively treat glioma. This technique has also been reported to be beneficial for glioma diagnosis [171].
6.3. Milk exosomes for drug delivery:
The role of exosomes in cell-to-cell communication suggests the ability of exosomes to delivery functional information [172]. In the tumor microenvironment, mesenchymal stem cells (MSCs) act as paracrine mediators to regulate tumor development [173, 174]. Thus, exosomes derived from MSCs have garnered a lot of attention as these exosomes can elucidate both suppressing effects on tumor development and supporting tumor progression [175, 176]. Our laboratory pioneered milk exosomes-drug delivery technology, which eliminates the challenges related to tumorigenicity, starting with abundant, accessible and affordable source. Milk exosomes are biocompatible, non-immunogenic, and could be modified to guide to a targeted site to treat diseases such as cancer [177].
Milk exosomes have been utilized to load various small and large molecules into/onto the exosomes. The hypothesis for loading small molecules is due to the surface lipid-protein nature of the exosomes [147]. We have effectively loaded and delivered compounds such as withaferin A (WFA), celastrol (CEL), curcumin (CUR), anthocyanidins (Anthos), docetaxel (DOC), and PAC to inhibit the growth of different cancers [122, 132–135]. The ability of milk exosomes to improve drug bioavailability, anti-proliferative activity, and potency, as well as reduce dose-related toxicity, is well documented [178]. Milk- and colostrum-derived exosomes also aid in the treatment of drug resistance in cancer cells and display antiproliferative action in exosomal formulations [179]. Milk/colostrum exosomes can be steered to the tumor location for payload delivery by surface functionalization with tumor targeting ligands such as FA [147]. According to biodistribution studies, FA-functionalized exosomes accumulate higher in tumors than nonfunctionalized exosomes due to higher expression of folate receptors [168]. Surface-functionalized exosomes enhance anti-tumor response in animal models compared to non-functionalized exosomal drug formulations [147].
Zhang and colleagues developed a pH/light-sensitive drug system based on milk-exosomes for treating oral squamous cell carcinoma (OSCC) therapy. This drug system demonstrated to rupture the acidic tumor microenvironment and release singlet oxygen to kill cancer cells [180]. Bovine milk exosomes also have a proliferative effect on RAW 264.7 macrophages and a protective effect against cisplatin-induced cytotoxicity [181]. Gao and team isolated exosomes from yak milk and found that these exosomes could inhibit PI3K/AKT/C3 pathway activation and alleviate lipopolysaccharide-induced intestinal inflammation [182]. Bovine milk exosomes have also been used to load miRNA in in-vivo studies and deliver it to the tumor site via the oral route [183–185].
Exosomes have recently been widely exploited for transporting nucleic acids (siRNA, plasmid DNA, and mRNA) to treat various disorders, including cancer. At first, Valadi et al. 2007 identified exosomes’ role in transferring genetic material. After harvesting exosomes from MC/9, BMMC and HMC-1 cells and identifying different mRNAs and miRNAs, they proved that exosomes could shuttle in and out of the cells. This discovery about the capability of exosomes to be utilized for gene delivery and other therapeutics opened doors for researchers to exploit exosomes for cancer treatment technologies [186]. A recent review by Zhang et al. explores recent improvements in exosome-mediated nucleic acid delivery for cancer treatment [187]. In our laboratory, we formulated a gene delivery technique that employs colostrum exosomes and polyethyleneimine (PEI) (so named EPM matrix) to transport a wide range of therapeutic genes to treat many disorders [168, 188]. We were successful in delivering siRNA to knockdown KRAS and introduce p53 plasmid DNA (pDNA) into cancer cells using this technique. KRAS knockdown inhibited lung tumor growth (>70%) in mice having subcutaneous and orthotropic lung tumors [168].
7. Hybrid Exosomes for Cancer Therapeutics
Combining liposomes and exosomes to form hybrid exosomes provides a platform to enrich the exosomal lipid bilayer with exogenous lipids while preserving their intrinsic contents and biophysical and biochemical properties [189]. Thus, the benefits of liposomes, such as easy formulation, longer shelf life, and circulation, along with exosomes’ biocompatibility, natural trafficking, and surface ligands, may bring the best of both in one drug carrier formulation (Figure 4). This hybrid exosome approach offers opportunities for tailoring hybrid exosomes according to the therapeutic need and has garnered much attention lately.
Figure 4.
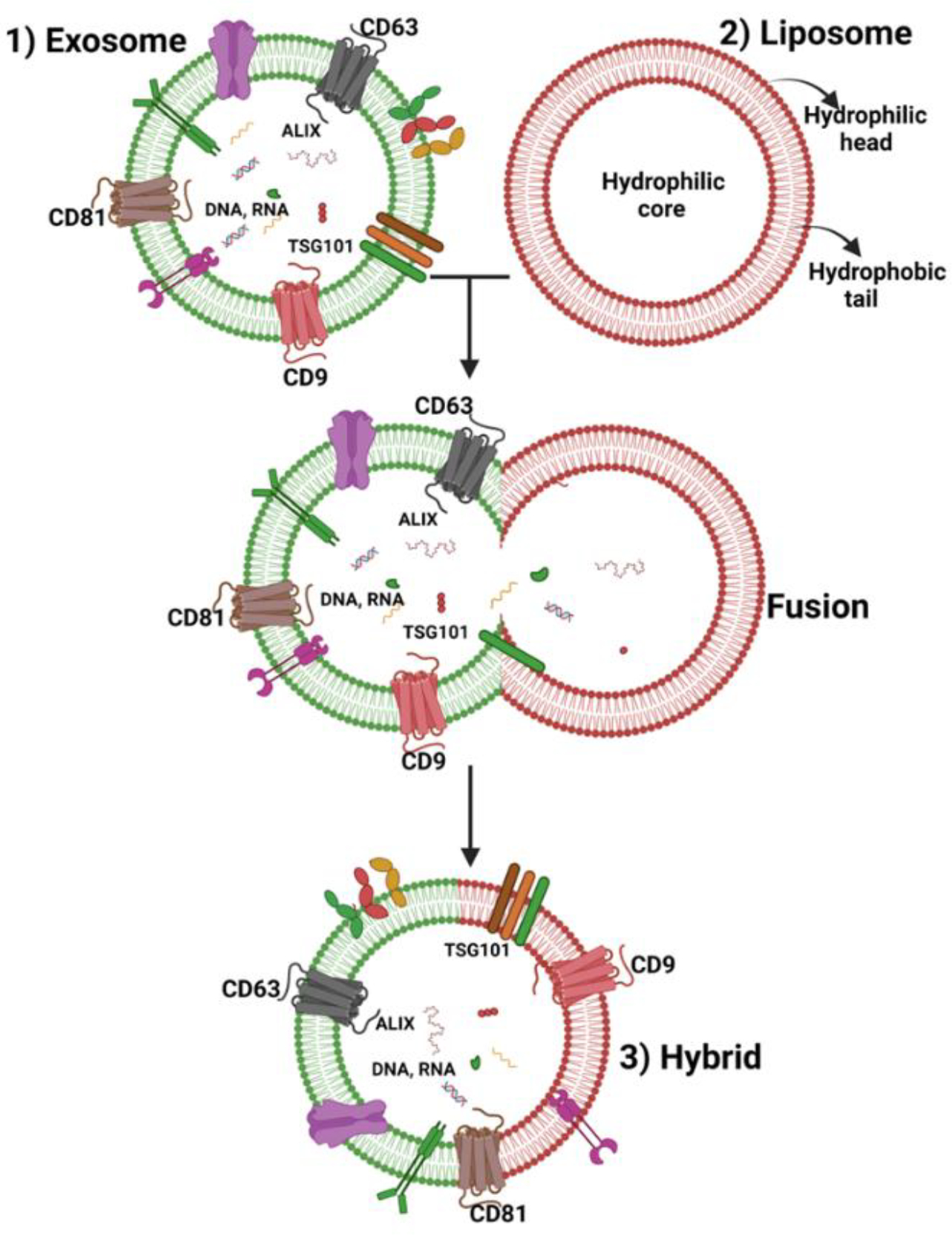
Schematic diagram showing the fusion of exosomal and liposomal bilayer to form hybrid exosomes.
Researchers have employed different methods to fuse liposomes and exosomes. Fusion with lipids can occur either by direct addition of exosomes for the hydration of the lipid thin film [190] or via the addition of exosomes to the preformed liposomes followed by freeze-thaw cycles [191], simple mixing and incubation [25] or sonication followed by extrusion [192]. Sato and coworkers reported the first attempt at fusing exosomal and liposomal bilayers in 2016 [191] by using the freeze-thaw method. They used exosomes from Raw 264.7, CMS7-wt, and CMS7-HE cells and liposomes prepared by thin-film hydration comprising DOPC, DOTAP, NBD-DMPE, and rho-DMPE. The mechanism of fusion in this relies on a well-established fact that freeze-thaw cycles disrupt the plasma membranes and breakdown the large vesicles to form small vesicles [193–195]. Exosomes and liposomes were fused by mixing and freeze-thawing several times. The cellular uptake depended on the lipid composition or the properties of the exosomes. Incorporating anionic and neutral lipids into the exosomes had the same cellular uptake as the exosomes, while cationic lipid DOTAP showed reduced cellular uptake. The addition of PEGylated exogenous lipids showed about 2-fold higher uptake than the non-PEG-lipid incorporation [191]. The degree of fusion can be measured by fluorescence resonance energy transfer (FRET) analysis [196, 197]. Different methods of formation of hybrid exosomes by fusion of lipid thin film with exosomes has been shown in the flowchart (Figure 5).
Figure 5.
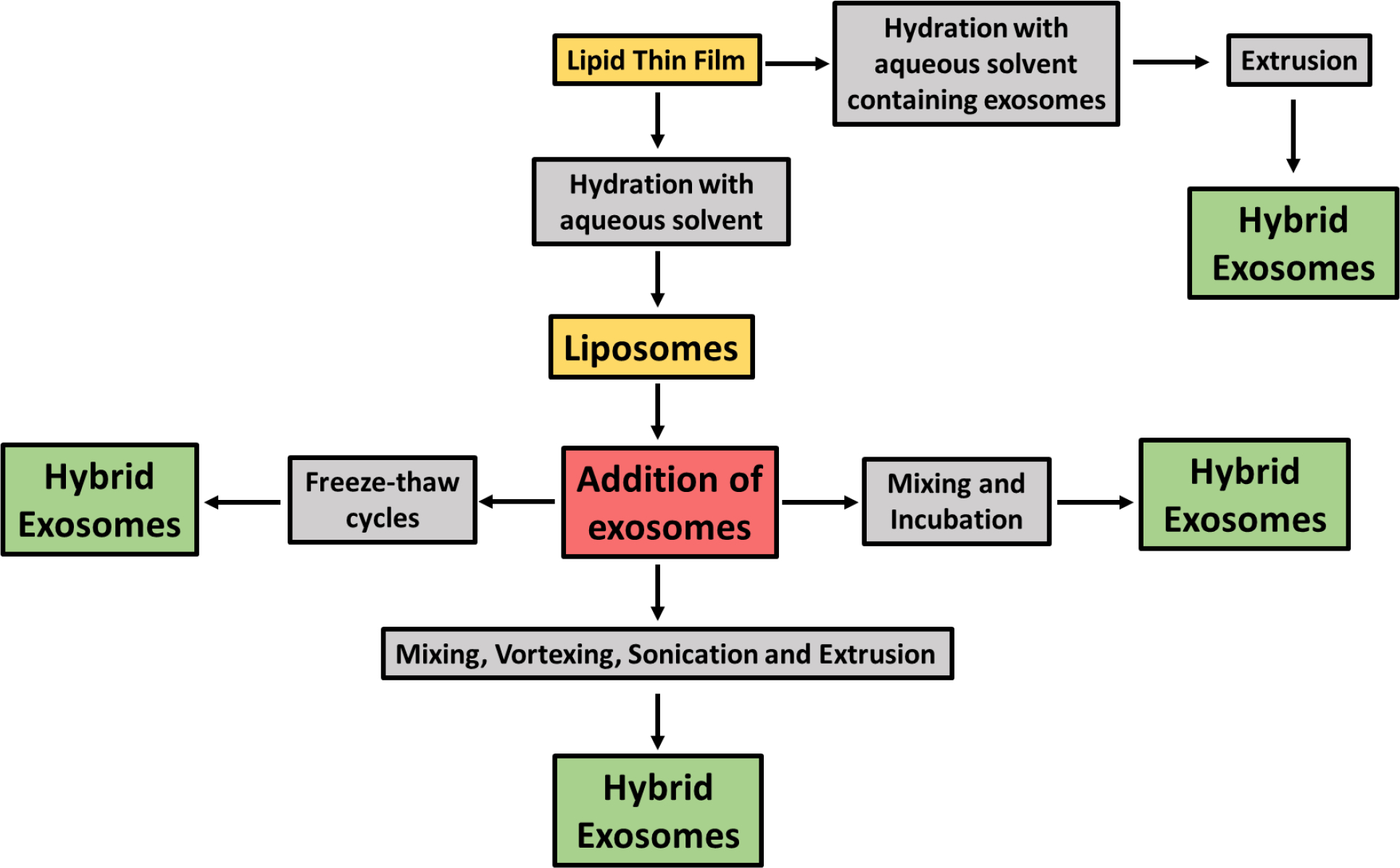
Flowchart of different methods of formation of hybrid exosomes by fusion of lipid thin film with exosomes. Fusion with lipids can occur either by direct addition of exosomes for the hydration of the lipid thin film [190] or via the addition of exosomes to the preformed liposomes followed by freeze-thaw cycles [191], simple mixing and incubation [25] or sonication followed by extrusion [192].
In 2018, Piffoux and coworkers presented the proof-of-concept of the fusion of EVs triggered by PEG. In their study, the optimum EV/liposome ratio of 9/1 led to the highest fusion efficiency. These hybrid lipid carriers can be loaded with hydrophilic and hydrophobic drugs, their interaction with macrophages can be tuned by modifying the PEG concentration and their delivery to cancer microenvironments is enhanced as compared to commercial therapeutic liposomal formulations [189]. Relying on the same principle, Mukherjee et al. utilized different percentages of PEG1000 to fuse exosomes derived from breast cancer MCF-7 cells with cationic liposomes. Hybrid exosomes with 15% PEG1000 offered the best transfection efficiency to the parental MCF-7 cells compared to other hybrid exosomes and exosomes and liposomes alone [198]. Hybrid exosomes can also be prepared by simply incubating exosomes derived from HEK293FT cells, Lipofectamine 2000, and plasmids. Lipofectamine 2000 by itself cannot transfect MSCs. Thus, the researchers tested the transfection ability of the resultant hybrid exosomes compared to exosomes and liposomes alone. While the exosomes and Lipofectamine failed to efficiently transfect plasmids into MSCs, the hybrid exosomes substantially increased mRNA expression. Thus, this is a simplistic yet promising approach for the targeted delivery of clustered regularly interspaced short palindromic repeats system to MSCs [25].
To compensate for the poor yield and loss of functional properties during exosomal isolation, Rayamajhi and coworkers prepared a refined biomimetic structure using immuno-exosomes and synthetic liposomes [190]. The small EVs from mouse macrophage, J774A.1 cells, and the liposomes composed of L-α-phosphatidylcholine and cholesterol were used. The exosomes were used to rehydrate the film to yield hybrid exosomes and DOX was loaded as a model drug using membrane extrusion. These formulations exhibited burst release in basic and acidic environments for the first 8 h and enhanced release kinetics were observed in an acidic environment for the next 48 h. It was concluded that pH dependence and differential targeting and cytotoxicity for cancer cells of hybrid exosome formulations make it suitable for application for cancer therapeutics [190]. However, the hybrid exosomes tend to aggregate at high drug content, therefore, drug load needs to be considered while optimizing the formulations.
Another method to prepare the hybrid exosomes is by sonication following extrusion using a different pore-size membrane. In such an attempt, Jhan and coworkers used exosomes from 3T3 and A549 cells and liposomes and mixed in the different ratios keeping the liposomal concentration constant. The mixture was vortexed, sonicated and serially extruded through different pore sizes to yield the resultant engineered extracellular vesicles (eEVs), and siRNA was then loaded into these vesicles using electroporation [192]. The hybrid exosomes delivery of siRNA using anti-GFP siRNA showed significant knockdown as analyzed by fluorescence microscopy and flow cytometry. However, high toxicity was reported due to the charge of cationic lipids. The electroporation method led to the aggregation of eEVs and siRNA. Aggregation could be minimized by optimizing the use of Opti-MEM + EDTA, trehalose, and hypotonic electroporation buffer for different lipids [192].
Hyperthermic intraperitoneal chemotherapy (HIPEC) has been the standard of care for metastatic peritoneal carcinoma (mPC) but failed to penetrate large tumors. CD47-expressed exosomes from genetically engineered fibroblasts were fused with thermosensitive liposomes by freeze-thaw method to prepare genetically engineered exosomes‐thermosensitive liposomes hybrid Nanoparticles (gETL NPs). The authors found that gETL NPs accumulated in tumors upon intravenous administration, and the release was accelerated under the hypothermia condition of HIPEC. Significant inhibition of tumor progression and enhanced antitumor effects were observed in cell line‐derived xenografts and patient‐derived tumor xenografts when HIPEC was combined with gETL NPs. This study provides new opportunities for treating mPC and similar diseases [21]. However, the major challenge for hybrid exosomes is to determine whether exosomes are fusing with liposomes. Scott and colleagues used fluorescence resonance energy transfer (FRET) analysis to this point. They showed that the EVs isolated from human umbilical vein endothelial cells (HUVEC), and liposomes composed of phosphatidylcholine and phosphatidyl-ethanolamine resulted in hybrid lipid carriers [197]. The fusion of the two vesicles was monitored using FRET analysis which has been previously reported for monitoring liposomal fusion [197]. The fusion was also confirmed by flow cytometry analysis, cryo-TEM analysis and measuring FRET after quenching the NBD fluorescence in the outer monolayer of liposomes. To understand the intracellular trafficking of such hybrid exosomes, Chen and coworkers used exosome/metal nanohybrid equipped with surface-enhanced Raman scattering. Their main findings suggest that these hybrids were internalized by clathrin-mediated endocytosis and then transported to lysosomes for degradation [196]. This approach can be adopted to get a better understanding about the internalization mechanism of the hybrid exosomes.
Other paradigms of hybrid exosome applications: Cheng et al. fused gene-engineered exosomes with thermosensitive liposomes as a hybrid tool for combined photothermal therapy and cancer immunotherapy. The exosomes were genetically engineered to overexpress CD47, thus exhibiting longer blood circulation due to improved escape from the mononuclear phagocyte system (MPS). There in vivo studies showed preferential accumulation of these hybrids at the tumor sites, making them suitable for loading photothermal agents for phototherapies [199]. Evers et al. used EVs derived from SKOV3 cells and siRNA to hydrate the DPPC lipid thin film, followed by extrusion steps to produce hybrid exosomes. They observed that the cellular uptake was dependent on the ratio of exosomes to liposomes and that the uptake was lower for the hybrids than the liposomes alone. For the siRNA delivery to SKOV3 cells, there was no dose dependence of siRNA observed which was attributed to better biocompatibility of the hybrids as compared to liposomes. These hybrids showed the ability to activate endothelial signaling and cell migration which has a pronounced effect on stimulating wound closure and inducing phosphorylation of Akt [200]. Apart from exosomes and liposomes, hybrid exosomes have also been formulated by fusion with block copolymers [201], membrane proteins [202, 203], protease: neutrophil elastase [204], nanoparticles [205, 206] etc.
8. Challenges and future directions
Lipid-based drug delivery vehicles have garnered much attention in recent years due to their superiority in size, biocompatibility, selectivity, ease of loading, and modification. The recent cancer nanomedicine developments based on lipid drug formulations have been challenging and exciting, with the leader being liposomes, emerging exosomes, and most recently, hybrid exosomes. Despite the numerous advantages of liposomes, immunotoxicity becomes a limitation in the clinical translation of liposomes for cancer therapeutics. This is currently being overcome by exosomes, which are structurally like liposomes and contain an endogenous payload. The recent attempt to isolate such EVs from milk and colostrum has led to a revolution, and numerous reports use bovine milk as a source to isolate EVs/exosomes. Milk EVs offer additional advantages such as enhanced drug efficacy, reduced or no immunotoxicity, lower side effects, and reversal of multi-drug resistance. Aiming toward perfection, scientists recently have developed the concept of hybrid exosomes, which fuse the advantages of liposomes and exosomes into a hybrid structure. Hybrid exosomes provide the properties of exosomes and allow to load different types of molecules, such as peptides, siRNA, hydrophilic or hydrophobic drugs etc. Literature has evidenced hybrid exosomes administration both systemically and locally. Compared to liposomes, intravenously injected hybrid exosomes stay in circulation for a longer time and are not easily cleared by the MPS or RES, thus resulting in improved drug delivery.
To extend the efficacy in terms of local administration, the hybrid exosomes can be embedded in matrices such as natural or synthetic hydrogels; which needs further investigation [207]. Though techniques for the characterization of hybrid exosomes are similar to the conventional methods used for the characterization of exosomes and liposomes, methods for the estimation of the efficiency of fusion are scarce and thus require in-depth research. Moreover, the biophysical mechanism responsible for the fusion and stability of the fused lipid structure in equilibrium is not yet completely understood and requires further experimental developments [208]. Additionally, the effect of entropic parameters, lipidic interactions, additives and physical parameters on the stability and efficacy of the fused hybrid exosomes would be an interesting future direction in the advancing the field of hybrid exosomes [209]. Ultimately, in-depth experimental studies of hybrid exosomes in vitro and in animal models would provide a better understanding of their complex biological system. In sum, the clinical translation of hybrid exosomes is a long way to go due to numerous challenges such as large-scale production, optimized purification protocols, development of characterization techniques, optimization of liposome: exosome ratio and cargo loading methods, quality control, and storage stability concerns. Although there are only a handful of articles experimenting with the hybrid exosomes, this concept offers enormous potential and opportunities for the emergence of a “cancer-overcoming era”.
Highlights.
Lipid-based drug delivery systems are favored due to superior biocompatibility.
Liposomes and exosomes are the main lipid-based drug delivery systems.
Liposomes fused with exosomes produce hybrid exosomes with enhanced therapeutic benefits.
Hybrid exosomes offer in-core and on-the-surface loading approaches for cancer drugs.
Acknowledgments:
This work was supported by USPHS grants CA-118114, CA-125152, R41-CA-189517, R44-CA-221487, Helmsley Trust Funds, 3P Biotechnologies, Inc. and Agnes Brown Duggan Endowment (to R.C.G.) and Kentucky Lung Cancer Research Program (to F.A.). DNM is supported by the IPIBS fellowship, University of Louisville. We would like to acknowledge key contributors in the development of the bovine milk/colostrum exosome delivery platform for small molecules and biologics discussed in this review, including Drs. Radha Munagala, Ashish Agrawal, and Margaret Wallen, and Mr. Jeyaprakash Jeyabalan. We would like to thank Christina Davidson, Writing Consultant at the Writing Center, University of Louisville, for proofreading the manuscript for grammatical errors and editing.
Abbreviations:
- DODMA
1,2-Dioleyloxy-3-dimethylaminopropane
- DOTMA
Dioleoyl-3-trimethylammonium propane
- DOTAP
Dioleoyl-3-trimethylammonium propane
- DOSPA
2,3-Dioleyloxy-N-[2-(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium
- OGS
Dioctadecylamidoglycylspermine
- DLinDMA
1,2-Dilinoleyloxy-3-dimethylaminopropane
- DLinMC3DMA
Dilinoleylmethyl-4-dimethylaminobutyrate
- DC-chol
3β-[N-(Nʹ,Nʹ-Dimethylaminoethane)-carbamoyl]cholesterol hydrochloride
- DOPE
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
- DSPC
1,2-Distearoyl-sn-glycero-3-phosphorylcholine
- DOPC
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- NPs
Nanoparticles
- EPR
Enhanced permeability and retention
- MPS
mononuclear phagocyte system
- DDS
Drug delivery systems
- DCs
Dendritic cells
- MScs
Mesenchymal Stem cells
- EVs
Extracellular vesicles
- FA
Folic acid
- Exo
Exosomes
- FA-Exo
FA-functionalized exosomes
- PEI
polyethyleneimine
- EPM
Exomes with PEI matrix
- ROS
Reactive oxygen species
- HPV
Human papillomavirus
- PEG
Polyethylene glycol
- TNBC
Triple negative breast cancer
- BBB
Blood-brain barrier
- SPIONS
Superparamagnetic iron oxide nanoparticles
- MSCs
Mesenchymal stem cells
- WFA
Withaferin
- CEL
Celastrol
- CUR
Curcumin
- Anthos
Anthocyanidins
- DOC
Docetaxel
- PAC
Paclitaxel
- DOX
Doxorubicin
Footnotes
DECLARATION OF INTERESTS DOCUMENTS
Dr. Ramesh C. Gupta holds positions both at the University of Louisville and 3P Biotechnologies, Inc. The authors have filed an international patent application (PCT) based on part of the results reported in this paper.
All other authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Lin A, Giuliano CJ, Palladino A, John KM, Abramowicz C, Yuan ML, Sausville EL, Lukow DA, Liu L, Chait AR, Galluzzo ZC, Tucker C, Sheltzer JM, Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials, Science Translational Medicine, 11 (2019) eaaw8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date, Pharm Res, 33 (2016) 2373–2387. [DOI] [PubMed] [Google Scholar]
- [3].Kim MW, Kwon SH, Choi JH, Lee A, A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy, Int J Mol Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Medina-Alarcón KP, Voltan AR, Fonseca-Santos B, Moro IJ, De Oliveira Souza F, Chorilli M, Soares CP, Dos Santos AG, Mendes-Giannini MJS, Fusco-Almeida AM, Highlights in nanocarriers for the treatment against cervical cancer, Materials Science and Engineering: C, 80 (2017) 748–759. [DOI] [PubMed] [Google Scholar]
- [5].Anselmo AC, Mitragotri S, An overview of clinical and commercial impact of drug delivery systems, J Control Release, 190 (2014) 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK, Drug delivery systems: An updated review, Int J Pharm Investig, 2 (2012) 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nakamura Y, Mochida A, Choyke PL, Kobayashi H, Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer?, Bioconjug Chem, 27 (2016) 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Klębowski B, Depciuch J, Parlińska-Wojtan M, Baran J, Applications of Noble Metal-Based Nanoparticles in Medicine, Int J Mol Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Meng W, He C, Hao Y, Wang L, Li L, Zhu G, Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source, Drug Deliv, 27 (2020) 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butreddy A, Kommineni N, Dudhipala N, Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives, Nanomaterials (Basel), 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mitragotri S, Lahann J, Physical approaches to biomaterial design, Nat Mater, 8 (2009) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rejman J, Oberle V, Zuhorn IS, Hoekstra D, Size-dependent internalization of particles via the pathways of clathrinand caveolae-mediated endocytosis, Biochem. J, 377 (2004) 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Godoy-Gallardo M, Ek PK, Jansman MM, Wohl BM, Hosta-Rigau L, Interaction between drug delivery vehicles and cells under the effect of shear stress, Biomicrofluidics, 9 (2015) 052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, Engineering precision nanoparticles for drug delivery, Nat Rev Drug Discov, 20 (2021) 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nakamura Y, Mochida A, Choyke PL, Kobayashi H, Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer?, Bioconjugate Chemistry, 27 (2016) 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nature Reviews Materials, 1 (2016) 16014. [Google Scholar]
- [17].Shrestha H, Bala R, Arora S, Lipid-Based Drug Delivery Systems, Journal of Pharmaceutics, 2014 (2014) 801820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van der Koog L, Gandek TB, Nagelkerke A, Liposomes and Extracellular Vesicles as Drug Delivery Systems: A Comparison of Composition, Pharmacokinetics, and Functionalization, Advanced Healthcare Materials, 11 (2022) 2100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].van Hoogevest P, Wendel A, The use of natural and synthetic phospholipids as pharmaceutical excipients, European Journal of Lipid Science and Technology, 116 (2014) 1088–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S, Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function, Genomics, Proteomics & Bioinformatics, 13 (2015) 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, Zhou J, Liu B, Liu J, Thermosensitive Exosome-Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer, Adv Sci (Weinh), 7 (2020) 2000515–2000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ji K, Fan M, Huang D, Sun L, Li B, Xu R, Zhang J, Shao X, Chen Y, Clodronate-nintedanib-loaded exosome-liposome hybridization enhances the liver fibrosis therapy by inhibiting Kupffer cell activity, Biomater Sci, 10 (2022) 702–713. [DOI] [PubMed] [Google Scholar]
- [23].Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J, Wang X, Jing Y, Chen X, Su J, Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss, Bioact Mater, 6 (2021) 2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singh A, Raghav A, Shiekh PA, Kumar A, Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation, Bioact Mater, 6 (2021) 2231–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin Y, Wu J, Gu W, Huang Y, Tong Z, Huang L, Tan J, Exosome–Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs, Advanced Science, 5 (2018) 1700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Trucillo P, Campardelli R, Reverchon E, Liposomes: From Bangham to Supercritical Fluids, Processes, 8 (2020) 1022. [Google Scholar]
- [27].Bangham AD, Horne RW, Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope, Journal of Molecular Biology, 8 (1964) 660–IN610. [DOI] [PubMed] [Google Scholar]
- [28].Bangham AD, Standish MM, Watkins JC, Diffusion of univalent ions across the lamellae of swollen phospholipids, Journal of Molecular Biology, 13 (1965) 238–IN227. [DOI] [PubMed] [Google Scholar]
- [29].Kaye SB, Richardson VJ, Potential of liposomes as drug-carriers in cancer chemotherapy: a review, Cancer Chemother Pharmacol, 3 (1979) 81–85. [DOI] [PubMed] [Google Scholar]
- [30].Gregoriadis G, Wills EJ, Swain CP, Tavill AS, Drug-carrier potential of liposomes in cancer chemotherapy, Lancet, 1 (1974) 1313–1316. [DOI] [PubMed] [Google Scholar]
- [31].Fidler IJ, Therapy of spontaneous metastases by intravenous injection of liposomes containing lymphokines, Science, 208 (1980) 1469–1471. [DOI] [PubMed] [Google Scholar]
- [32].Trouet A, Baurain R, Deprez-De Campeneere D, Layton D, Masquelier M, DNA, liposomes, and proteins as carriers for antitumoral drugs, Recent Results Cancer Res, 75 (1980) 229–235. [DOI] [PubMed] [Google Scholar]
- [33].Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S, Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes, Nature Medicine, 4 (1998) 594–600. [DOI] [PubMed] [Google Scholar]
- [34].Liu Y, Yang G, Jin S, Xu L, Zhao CX, Development of High-Drug-Loading Nanoparticles, Chempluschem, 85 (2020) 2143–2157. [DOI] [PubMed] [Google Scholar]
- [35].Gkionis L, Campbell RA, Aojula H, Harris LK, Tirella A, Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity, International Journal of Pharmaceutics, 590 (2020) 119926. [DOI] [PubMed] [Google Scholar]
- [36].Momen-Heravi F, Bala S, Bukong T, Szabo G, Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages, Nanomedicine, 10 (2014) 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y, Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo, Am J Physiol Lung Cell Mol Physiol, 312 (2017) L110–l121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rocco GD, Baldari S, Toietta G, Exosomes and other extracellular vesicles-mediated microRNA delivery for cancer therapy, Translational Cancer Research, (2017) S1321–S1330. [Google Scholar]
- [39].Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, Schiffelers RM, Raemdonck K, Vader P, Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles, J Control Release, 172 (2013) 229–238. [DOI] [PubMed] [Google Scholar]
- [40].Zhao Y, Lin L-N, Lu Y, Chen S-F, Dong L, Yu S-H, Templating Synthesis of Preloaded Doxorubicin in Hollow Mesoporous Silica Nanospheres for Biomedical Applications, Advanced Materials, 22 (2010) 5255–5259. [DOI] [PubMed] [Google Scholar]
- [41].Majumder J, Minko T, Multifunctional Lipid-Based Nanoparticles for Codelivery of Anticancer Drugs and siRNA for Treatment of Non-Small Cell Lung Cancer with Different Level of Resistance and EGFR Mutations, Pharmaceutics, 13 (2021) 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rani K, Paliwal S, A review on targeted drug delivery: Its entire focus on advanced therapeutics and diagnostics, Sch. J. App. Med. Sci, 2 (2014) 328–331. [Google Scholar]
- [43].Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z, Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review, Biomaterials, 85 (2016) 152–167. [DOI] [PubMed] [Google Scholar]
- [44].Bae YH, Drug targeting and tumor heterogeneity, J Control Release, 133 (2009) 2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Matsumura Y, Maeda H, A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs, Cancer Res, 46 (1986) 6387–6392. [PubMed] [Google Scholar]
- [46].Bazak R, Houri M, El Achy S, Kamel S, Refaat T, Cancer active targeting by nanoparticles: a comprehensive review of literature, J Cancer Res Clin Oncol, 141 (2015) 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jain RK, Transport of molecules in the tumor interstitium: a review, Cancer Res, 47 (1987) 3039–3051. [PubMed] [Google Scholar]
- [48].Heldin CH, Rubin K, Pietras K, Ostman A, High interstitial fluid pressure - an obstacle in cancer therapy, Nat Rev Cancer, 4 (2004) 806–813. [DOI] [PubMed] [Google Scholar]
- [49].De Jong WH, Borm PJ, Drug delivery and nanoparticles:applications and hazards, Int J Nanomedicine, 3 (2008) 133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu B, Tai HC, Xue W, Lee LJ, Lee RJ, Receptor-targeted nanocarriers for therapeutic delivery to cancer, Mol Membr Biol, 27 (2010) 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang AZ, Gu F, Zhang L, Chan JM, Radovic-Moreno A, Shaikh MR, Farokhzad OC, Biofunctionalized targeted nanoparticles for therapeutic applications, Expert Opin Biol Ther, 8 (2008) 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, Njardarson JT, Brentjens R, Scheinberg DA, Tumor Targeting with Antibody-Functionalized, Radiolabeled Carbon Nanotubes, Journal of Nuclear Medicine, 48 (2007) 1180–1189. [DOI] [PubMed] [Google Scholar]
- [53].Colcher D, Bird R, Roselli M, Hardman KD, Johnson S, Pope S, Dodd SW, Pantoliano MW, Milenic DE, Schlom J, In Vivo Tumor Targeting of a Recombinant Single-Chain Antigen-Binding Protein, JNCI: Journal of the National Cancer Institute, 82 (1990) 1191–1197. [DOI] [PubMed] [Google Scholar]
- [54].Li Q, Zhao D, Shao X, Lin S, Xie X, Liu M, Ma W, Shi S, Lin Y, Aptamer-Modified Tetrahedral DNA Nanostructure for Tumor-Targeted Drug Delivery, ACS Applied Materials & Interfaces, 9 (2017) 36695–36701. [DOI] [PubMed] [Google Scholar]
- [55].Zwicke GL, Mansoori GA, Jeffery CJ, Utilizing the folate receptor for active targeting of cancer nanotherapeutics, Nano Rev, 3 (2012) 10.3402/nano.v3403i3400.18496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tran P, Pyo YC, Kim DH, Lee SE, Kim JK, Park JS, Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs, Pharmaceutics, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Holm R, Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides, European Journal of Pharmaceutical Sciences, 20 (2003) 91–97. [DOI] [PubMed] [Google Scholar]
- [58].Hackett MJ, Zaro JL, Shen WC, Guley PC, Cho MJ, Fatty acids as therapeutic auxiliaries for oral and parenteral formulations, Adv Drug Deliv Rev, 65 (2013) 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].van der Vusse GJ, Albumin as fatty acid transporter, Drug Metab Pharmacokinet, 24 (2009) 300–307. [DOI] [PubMed] [Google Scholar]
- [60].Smith DA, Brown K, Neale MG, Chromone-2-Carboxylic Acids: Roles of Acidity and Lipophilicity in Drug Disposition, Drug Metabolism Reviews, 16 (1985) 365–388. [DOI] [PubMed] [Google Scholar]
- [61].Zaro JL, Lipid-based drug carriers for prodrugs to enhance drug delivery, AAPS J, 17 (2015) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Porter CJ, Trevaskis NL, Charman WN, Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs, Nat Rev Drug Discov, 6 (2007) 231–248. [DOI] [PubMed] [Google Scholar]
- [63].Barba AA, Bochicchio S, Dalmoro A, Lamberti G, Lipid Delivery Systems for Nucleic-Acid-Based-Drugs: From Production to Clinical Applications, Pharmaceutics, 11 (2019) 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chowdhury DF, Pharmaceutical Nanosystems: Manufacture, Characterization, and Safety, Pharmaceutical Manufacturing Handbook2008, pp. 1289–1325. [Google Scholar]
- [65].Briuglia M-L, Rotella C, McFarlane A, Lamprou DA, Influence of cholesterol on liposome stability and on in vitro drug release, Drug Delivery and Translational Research, 5 (2015) 231–242. [DOI] [PubMed] [Google Scholar]
- [66].Kaddah S, Khreich N, Kaddah F, Charcosset C, Greige-Gerges H, Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule, Food and Chemical Toxicology, 113 (2018) 40–48. [DOI] [PubMed] [Google Scholar]
- [67].Demel RA, De Kruyff B, The function of sterols in membranes, Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes, 457 (1976) 109–132. [DOI] [PubMed] [Google Scholar]
- [68].Liu W, Wei F, Ye A, Tian M, Han J, Kinetic stability and membrane structure of liposomes during in vitro infant intestinal digestion: Effect of cholesterol and lactoferrin, Food Chemistry, 230 (2017) 6–13. [DOI] [PubMed] [Google Scholar]
- [69].Garg T, Goyal AK, Liposomes: Targeted and Controlled Delivery System, Drug Delivery Letters, 4 (2014) 62–71. [Google Scholar]
- [70].Cogan U, Shinitzky M, Weber G, Nishida T, Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes, Biochemistry, 12 (1973) 521–528. [DOI] [PubMed] [Google Scholar]
- [71].Forssen EA, The design and development of DaunoXome® for solid tumor targeting in vivo, Advanced Drug Delivery Reviews, 24 (1997) 133–150. [Google Scholar]
- [72].Burade V, Bhowmick S, Maiti K, Zalawadia R, Ruan H, Thennati R, Lipodox® (generic doxorubicin hydrochloride liposome injection): in vivo efficacy and bioequivalence versus Caelyx® (doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models, BMC Cancer, 17 (2017) 405–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gabizon A, Shmeeda H, Tahover E, Kornev G, Patil Y, Amitay Y, Ohana P, Sapir E, Zalipsky S, Development of Promitil®, a lipidic prodrug of mitomycin c in PEGylated liposomes: From bench to bedside, Advanced Drug Delivery Reviews, 154–155 (2020) 13–26. [DOI] [PubMed] [Google Scholar]
- [74].Batist G, Barton J, Chaikin P, Swenson C, Welles L, Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy, Expert Opinion on Pharmacotherapy, 3 (2002) 1739–1751. [DOI] [PubMed] [Google Scholar]
- [75].Passero FC, Grapsa D, Syrigos KN, Saif MW, The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy, Expert Review of Anticancer Therapy, 16 (2016) 697–703. [DOI] [PubMed] [Google Scholar]
- [76].Tzogani K, Penttilä K, Lapveteläinen T, Hemmings R, Koenig J, Freire J, Márcia S, Cole S, Coppola P, Flores B, Barbachano Y, Roige SD, Pignatti F, EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX‐351) for the Treatment of Adults with Newly Diagnosed, Therapy‐Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia‐Related Changes, The Oncologist, 25 (2020) e1414–e1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zagalo DM, Simões S, Sousa J, Regulatory Science Approach in Pharmaceutical Development of Follow-on Versions of Non-Biological Complex Drug Products, Journal of Pharmaceutical Sciences, 111 (2022) 2687–2713. [DOI] [PubMed] [Google Scholar]
- [78].Phuphanich S, Maria B, Braeckman R, Chamberlain M, A pharmacokinetic study of intra-CSF administered encapsulated cytarabine (DepoCyt®) for the treatment of neoplastic meningitis in patients with leukemia, lymphoma,or solid tumors as part of a phase III study, Journal of Neuro-Oncology, 81 (2007) 201–208. [DOI] [PubMed] [Google Scholar]
- [79].Silverman JA, Deitcher SR, Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine, Cancer Chemotherapy and Pharmacology, 71 (2013) 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Venkatakrishnan K, Liu Y, Noe D, Mertz J, Bargfrede M, Marbury T, Farbakhsh K, Oliva C, Milton A, Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment, British Journal of Clinical Pharmacology, 77 (2014) 998–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Crommelin DJA, Influence of Lipid Composition and Ionic Strength on the Physical Stability of Liposomes, Journal of Pharmaceutical Sciences, 73 (1984) 1559–1563. [DOI] [PubMed] [Google Scholar]
- [82].Ceh B, Lasic DD, A Rigorous Theory of Remote Loading of Drugs into Liposomes: Transmembrane Potential and Induced pH-Gradient Loading and Leakage of Liposomes, J Colloid Interface Sci, 185 (1997) 9–18. [DOI] [PubMed] [Google Scholar]
- [83].Sawant RR, Torchilin VP, Liposomes as ‘smart’ pharmaceutical nanocarriers, Soft Matter, 6 (2010) 4026–4044. [Google Scholar]
- [84].Bochicchio S, Dalmoro A, Barba AA, Grassi G, Lamberti G, Liposomes as siRNA delivery vectors, Curr Drug Metab, 15 (2014) 882–892. [DOI] [PubMed] [Google Scholar]
- [85].Lasic DD, Liposomes, Am. Sci, 80 (1992) 20–31. [Google Scholar]
- [86].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nat Rev Drug Discov, 4 (2005) 145–160. [DOI] [PubMed] [Google Scholar]
- [87].Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A, Application of liposomes in medicine and drug delivery, Artif Cells Nanomed Biotechnol, 44 (2016) 381–391. [DOI] [PubMed] [Google Scholar]
- [88].Swenson CE, Perkins WR, Roberts P, Janoff AS, Liposome technology and the development of Myocet™ (liposomal doxorubicin citrate), The Breast, 10 (2001) 1–7.14965549 [Google Scholar]
- [89].Lee E-H, Lee M-K, Lim S-J, Enhanced Stability of Indocyanine Green by Encapsulation in Zein-Phosphatidylcholine Hybrid Nanoparticles for Use in the Phototherapy of Cancer, Pharmaceutics, 13 (2021) 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tsermentseli SK, Kontogiannopoulos KN, Papageorgiou VP, Assimopoulou AN, Comparative Study of PEGylated and Conventional Liposomes as Carriers for Shikonin, Fluids, 3 (2018) 36. [Google Scholar]
- [91].Simões S, Filipe A, Faneca H, Mano M, Penacho N, Düzgünes N, Pedroso de Lima M, Cationic liposomes for gene delivery, Expert Opinion on Drug Delivery, 2 (2005) 237–254. [DOI] [PubMed] [Google Scholar]
- [92].Farhood H, Gao X, Son K, Yang YY, Lazo JS, Huang L, Barsoum J, Bottega R, Epand RM, Cationic liposomes for direct gene transfer in therapy of cancer and other diseases, Ann N Y Acad Sci, 716 (1994) 23–34; discussion 34–25. [DOI] [PubMed] [Google Scholar]
- [93].Shinkai M, Yanase M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T, Intracellular Hyperthermia for Cancer Using Magnetite Cationic Liposomes: In vitro Study, Japanese Journal of Cancer Research, 87 (1996) 1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liu C, Zhang L, Zhu W, Guo R, Sun H, Chen X, Deng N, Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy, Molecular Therapy - Methods & Clinical Development, 18 (2020) 751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yan W, Chen W, Huang L, Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine, Journal of Controlled Release, 130 (2008) 22–28. [DOI] [PubMed] [Google Scholar]
- [96].Chen W, Huang L, Induction of cytotoxic T-lymphocytes and antitumor activity by a liposomal lipopeptide vaccine, Molecular pharmaceutics, 5 (2008) 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chen W, Yan W, Huang L, A simple but effective cancer vaccine consisting of an antigen and a cationic lipid, Cancer Immunology, Immunotherapy, 57 (2008) 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yun C-H, Bae C-S, Ahn T, Cargo-free nanoparticles containing cationic lipids induce reactive oxygen species and cell death in HepG2 cells, Biological and Pharmaceutical Bulletin, 39 (2016) 1338–1346. [DOI] [PubMed] [Google Scholar]
- [99].Nag OK, Awasthi V, Surface engineering of liposomes for stealth behavior, Pharmaceutics, 5 (2013) 542–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].ElBayoumi TA, Torchilin VP, Tumor-Targeted Nanomedicines: Enhanced Antitumor Efficacy In vivo of Doxorubicin-Loaded, Long-Circulating Liposomes Modified with Cancer-Specific Monoclonal Antibody, Clinical Cancer Research, 15 (2009) 1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Banerjee R, Tyagi P, Li S, Huang L, Anisamide-targeted stealth liposomes: A potent carrier for targeting doxorubicin to human prostate cancer cells, International Journal of Cancer, 112 (2004) 693–700. [DOI] [PubMed] [Google Scholar]
- [102].Symon Z, Peyser A, Tzemach D, Lyass O, Sucher E, Shezen E, Gabizon A, Selective delivery of doxorubicin to patients with breast carcinoma metastases by stealth liposomes, Cancer, 86 (1999) 72–78. [PubMed] [Google Scholar]
- [103].Sivadasan D, Sultan MH, Madkhali OA, Alsabei SH, Alessa AA, Stealth Liposomes (PEGylated) Containing an Anticancer Drug Camptothecin: In Vitro Characterization and In Vivo Pharmacokinetic and Tissue Distribution Study, Molecules, 27 (2022) 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yang C, Liu HZ, Fu ZX, Lu WD, Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma, BMC Biotechnology, 11 (2011) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu Y, Lu W-L, Guo J, Du J, Li T, Wu J-W, Wang G-L, Wang J-C, Zhang X, Zhang Q, A potential target associated with both cancer and cancer stem cells: A combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes, Journal of Controlled Release, 129 (2008) 18–25. [DOI] [PubMed] [Google Scholar]
- [106].Li R-J, Ying X, Zhang Y, Ju R-J, Wang X-X, Yao H-J, Men Y, Tian W, Yu Y, Zhang L, Huang R-J, Lu W-L, All-trans retinoic acid stealth liposomes prevent the relapse of breast cancer arising from the cancer stem cells, Journal of Controlled Release, 149 (2011) 281–291. [DOI] [PubMed] [Google Scholar]
- [107].Cattel L, Ceruti M, Dosio F, From Conventional to Stealth Liposomes a new Frontier in Cancer Chemotherapy, Tumori Journal, 89 (2003) 237–249. [DOI] [PubMed] [Google Scholar]
- [108].Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L, Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes, Journal of Controlled Release, 63 (2000) 19–30. [DOI] [PubMed] [Google Scholar]
- [109].Schiffelers RM, Molema G, ten Hagen TL, Janssen AP, Schraa AJ, Kok RJ, Koning GA, Storm G, Ligand-targeted liposomes directed against pathological vasculature, J Liposome Res, 12 (2002) 129–135. [DOI] [PubMed] [Google Scholar]
- [110].Narayanaswamy R, Torchilin VP, Targeted Delivery of Combination Therapeutics Using Monoclonal Antibody 2C5-Modified Immunoliposomes for Cancer Therapy, Pharmaceutical Research, 38 (2021) 429–450. [DOI] [PubMed] [Google Scholar]
- [111].Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, Yang W, Tian C, Miao Z, Wang T, Yang S, Small molecules in targeted cancer therapy: advances, challenges, and future perspectives, Signal Transduction and Targeted Therapy, 6 (2021) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gray BP, McGuire MJ, Brown KC, A Liposomal Drug Platform Overrides Peptide Ligand Targeting to a Cancer Biomarker, Irrespective of Ligand Affinity or Density, PLOS ONE, 8 (2013) e72938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Marty C, Meylan C, Schott H, Ballmer-Hofer K, Schwendener RA, Enhanced heparan sulfate proteoglycan-mediated uptake of cell-penetrating peptide-modified liposomes, Cellular and Molecular Life Sciences CMLS, 61 (2004) 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lee T-Y, Lin C-T, Kuo S-Y, Chang D-K, Wu H-C, Peptide-Mediated Targeting to Tumor Blood Vessels of Lung Cancer for Drug Delivery, Cancer Research, 67 (2007) 10958–10965. [DOI] [PubMed] [Google Scholar]
- [115].Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC, Development of ligand-targeted liposomes for cancer therapy, Expert Opinion on Therapeutic Targets, 8 (2004) 335–353. [DOI] [PubMed] [Google Scholar]
- [116].Jain S, Deore SV, Ghadi R, Chaudhari D, Kuche K, Katiyar SS, Tumor microenvironment responsive VEGF-antibody functionalized pH sensitive liposomes of docetaxel for augmented breast cancer therapy, Materials Science and Engineering: C, 121 (2021) 111832. [DOI] [PubMed] [Google Scholar]
- [117].Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B, A Systematic Analysis of Peptide Linker Length and Liposomal Polyethylene Glycol Coating on Cellular Uptake of Peptide-Targeted Liposomes, ACS Nano, 7 (2013) 2935–2947. [DOI] [PubMed] [Google Scholar]
- [118].Talsma H, Van Steenbergen MJ, Borchert JCH, Crommelin DJA, A Novel Technique for the One-Step Preparation of Liposomes and Nonionic Surfactant Vesicles without the Use of Organic Solvents. Liposome Formation in a Continuous Gas Stream: The ‘Bubble’ Method, Journal of Pharmaceutical Sciences, 83 (1994) 276–280. [DOI] [PubMed] [Google Scholar]
- [119].Alamoudi K, Martins P, Croissant JG, Patil S, Omar H, Khashab NM, Thermoresponsive pegylated bubble liposome nanovectors for efficient siRNA delivery via endosomal escape, Nanomedicine, 12 (2017) 1421–1433. [DOI] [PubMed] [Google Scholar]
- [120].Endo-Takahashi Y, Negishi Y, Nakamura A, Suzuki D, Ukai S, Sugimoto K, Moriyasu F, Takagi N, Suzuki R, Maruyama K, Aramaki Y, pDNA-loaded Bubble liposomes as potential ultrasound imaging and gene delivery agents, Biomaterials, 34 (2013) 2807–2813. [DOI] [PubMed] [Google Scholar]
- [121].Lin X, Qiu Y, Song L, Chen S, Chen X, Huang G, Song J, Chen X, Yang H, Ultrasound activation of liposomes for enhanced ultrasound imaging and synergistic gas and sonodynamic cancer therapy, Nanoscale Horizons, 4 (2019) 747–756. [Google Scholar]
- [122].Negishi Y, Omata D, Iijima H, Takabayashi Y, Suzuki K, Endo Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y, Enhanced Laminin-Derived Peptide AG73-Mediated Liposomal Gene Transfer by Bubble Liposomes and Ultrasound, Molecular Pharmaceutics, 7 (2010) 217–226. [DOI] [PubMed] [Google Scholar]
- [123].Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, Hirata K, Taira Y, Utoguchi N, Negishi Y, Nakagawa S, Maruyama K, Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure, Journal of Controlled Release, 142 (2010) 245–250. [DOI] [PubMed] [Google Scholar]
- [124].Negishi Y, Ishii Y, Shiono H, Akiyama S, Sekine S, Kojima T, Mayama S, Kikuchi T, Hamano N, Endo-Takahashi Y, Suzuki R, Maruyama K, Aramaki Y, Bubble Liposomes and Ultrasound Exposure Improve Localized Morpholino Oligomer Delivery into the Skeletal Muscles of Dystrophic mdx Mice, Molecular Pharmaceutics, 11 (2014) 1053–1061. [DOI] [PubMed] [Google Scholar]
- [125].Oda Y, Suzuki R, Mori T, Takahashi H, Natsugari H, Omata D, Unga J, Uruga H, Sugii M, Kawakami S, Higuchi Y, Yamashita F, Hashida M, Maruyama K, Development of fluorous lipid-based nanobubbles for efficiently containing perfluoropropane, International Journal of Pharmaceutics, 487 (2015) 64–71. [DOI] [PubMed] [Google Scholar]
- [126].Danhier F, Feron O, Préat V, To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery, Journal of Controlled Release, 148 (2010) 135–146. [DOI] [PubMed] [Google Scholar]
- [127].Paliwal SR, Paliwal R, Pal HC, Saxena AK, Sharma PR, Gupta PN, Agrawal GP, Vyas SP, Estrogen-Anchored pH-Sensitive Liposomes as Nanomodule Designed for Site-Specific Delivery of Doxorubicin in Breast Cancer Therapy, Molecular Pharmaceutics, 9 (2012) 176–186. [DOI] [PubMed] [Google Scholar]
- [128].de Oliveira Silva J, Fernandes RS, Ramos Oda CM, Ferreira TH, Machado Botelho AF, Martins Melo M, de Miranda MC, Assis Gomes D, Dantas Cassali G, Townsend DM, Rubello D, Oliveira MC, de Barros ALB, Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model, Biomedicine & Pharmacotherapy, 118 (2019) 109323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yuba E, Tajima N, Yoshizaki Y, Harada A, Hayashi H, Kono K, Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy, Biomaterials, 35 (2014) 3091–3101. [DOI] [PubMed] [Google Scholar]
- [130].Chen M, Song F, Liu Y, Tian J, Liu C, Li R, Zhang Q, A dual pH-sensitive liposomal system with charge-reversal and NO generation for overcoming multidrug resistance in cancer, Nanoscale, 11 (2019) 3814–3826. [DOI] [PubMed] [Google Scholar]
- [131].Swami R, Kumar Y, Chaudhari D, Katiyar SS, Kuche K, Katare PB, Banerjee SK, Jain S, pH sensitive liposomes assisted specific and improved breast cancer therapy using co-delivery of SIRT1 shRNA and Docetaxel, Materials Science and Engineering: C, 120 (2021) 111664. [DOI] [PubMed] [Google Scholar]
- [132].Almurshedi AS, Radwan M, Omar S, Alaiya AA, Badran MM, Elsaghire H, Saleem IY, Hutcheon GA, A novel pH-sensitive liposome to trigger delivery of afatinib to cancer cells: Impact on lung cancer therapy, Journal of Molecular Liquids, 259 (2018) 154–166. [Google Scholar]
- [133].Udofot O, Affram K, B.e. Israel E. Agyare, Cytotoxicity of 5-fluorouracil-loaded pH-sensitive liposomal nanoparticles in colorectal cancer cell lines, Integr Cancer Sci Ther, 2 (2015) 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Ferreira D.d.S, Lopes S.C.d.A, Franco MS, Oliveira MC, pH-sensitive liposomes for drug delivery in cancer treatment, Therapeutic Delivery, 4 (2013) 1099–1123. [DOI] [PubMed] [Google Scholar]
- [135].Jin X, Zhou J, Zhang Z, Lv H, Doxorubicin combined with betulinic acid or lonidamine in RGD ligand-targeted pH-sensitive micellar system for ovarian cancer treatment, International Journal of Pharmaceutics, 571 (2019) 118751. [DOI] [PubMed] [Google Scholar]
- [136].Jiménez-López J, Bravo-Caparrós I, Cabeza L, Nieto FR, Ortiz R, Perazzoli G, Fernández-Segura E, Cañizares FJ, Baeyens JM, Melguizo C, Prados J, Paclitaxel antitumor effect improvement in lung cancer and prevention of the painful neuropathy using large pegylated cationic liposomes, Biomedicine & Pharmacotherapy, 133 (2021) 111059. [DOI] [PubMed] [Google Scholar]
- [137].Narang A, Chang R-K, Hussain MA, Pharmaceutical Development and Regulatory Considerations for Nanoparticles and Nanoparticulate Drug Delivery Systems, Journal of Pharmaceutical Sciences, 102 (2013) 3867–3882. [DOI] [PubMed] [Google Scholar]
- [138].Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S, Advances and Challenges of Liposome Assisted Drug Delivery, Front Pharmacol, 6 (2015) 286–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Silva M, Melo SA, Non-coding RNAs in Exosomes: New Players in Cancer Biology, Curr Genomics, 16 (2015) 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yin K, Wang S, Zhao RC, Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm, Biomarker Research, 7 (2019) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Banerjee A, Bizzaro D, Burra P, Di Liddo R, Pathak S, Arcidiacono D, Cappon A, Bo P, Conconi MT, Crescenzi M, Pinna CMA, Parnigotto PP, Alison MR, Sturniolo GC, D’Incà R, Russo FP, Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice, Stem Cell Research & Therapy, 6 (2015) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Butler JT, Abdelhamed S, Kurre P, Extracellular vesicles in the hematopoietic microenvironment, Haematologica, 103 (2018) 382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rong Y, Liu W, Wang J, Fan J, Luo Y, Li L, Kong F, Chen J, Tang P, Cai W, Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy, Cell Death & Disease, 10 (2019) 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Zhou H, Li X, Yin Y, He X-T, An Y, Tian B-M, Hong Y-L, Wu L-A, Chen F-M, The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth, Stem Cell Research & Therapy, 11 (2020) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Michael A, Bajracharya S, Yuen P, Zhou H, Star R, Illei G, Alevizos I, Exosomes from human saliva as a source of microRNA biomarkers, Oral Diseases, 16 (2010) 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Pisitkun T, Shen R-F, Knepper MA, Identification and proteomic profiling of exosomes in human urine, Proceedings of the National Academy of Sciences, 101 (2004) 13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Munagala R, Aqil F, Jeyabalan J, Gupta RC, Bovine milk-derived exosomes for drug delivery, Cancer Letters, 371 (2016) 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Gutzeit C, Nagy N, Gentile M, Lyberg K, Gumz J, Vallhov H, Puga I, Klein E, Gabrielsson S, Cerutti A, Scheynius A, Exosomes derived from Burkitt’s lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells, J Immunol, 192 (2014) 5852–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wei G, Jie Y, Haibo L, Chaoneng W, Dong H, Jianbing Z, Junjie G, Leilei M, Hongtao S, Yunzeng Z, Junbo G, Dendritic cells derived exosomes migration to spleen and induction of inflammation are regulated by CCR7, Scientific Reports, 7 (2017) 42996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Azoulay-Alfaguter I, Mor A, Isolation and Characterization of T Lymphocyte-Exosomes Using Mass Spectrometry, Methods Mol Biol, 2184 (2020) 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Li S, Wu Y, Ding F, Yang J, Li J, Gao X, Zhang C, Feng J, Engineering macrophage-derived exosomes for targeted chemotherapy of triple-negative breast cancer, Nanoscale, 12 (2020) 10854–10862. [DOI] [PubMed] [Google Scholar]
- [152].Lobb R, Becker M, Wen S, Wong C, Wiegmans A, Leimgruber A, Möller A, Optimized exosome isolation protocol for cell culture supernatant and human plasma, Journal of extracellular vesicles, 4 (2015) 27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cvjetkovic A, Lötvall J, Lässer C, The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles, Journal of Extracellular Vesicles, 3 (2014) 23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gámez-Valero A, Monguió-Tortajada M, Carreras-Planella L, Franquesa M.l, Beyer K, Borràs FE, Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents, Scientific Reports, 6 (2016) 33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP, Isolation of Extracellular Vesicles: General Methodologies and Latest Trends, BioMed Research International, 2018 (2018) 8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, Whiteside TL, Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma, J Extracell Vesicles, 7 (2018) 1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ha D, Yang N, Nadithe V, Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges, Acta Pharm Sin B, 6 (2016) 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R, Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer, Nature, 546 (2017) 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S, Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes, Nat Med, 4 (1998) 594–600. [DOI] [PubMed] [Google Scholar]
- [160].Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM, Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins, J Control Release, 205 (2015) 35–44. [DOI] [PubMed] [Google Scholar]
- [161].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG, A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes, Molecular Therapy, 18 (2010) 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine, 12 (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Gomari H, Forouzandeh Moghadam M, Soleimani M, Ghavami M, Khodashenas S, Targeted delivery of doxorubicin to HER2 positive tumor models, Int J Nanomedicine, 14 (2019) 5679–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Schindler C, Collinson A, Matthews C, Pointon A, Jenkinson L, Minter RR, Vaughan TJ, Tigue NJ, Exosomal delivery of doxorubicin enables rapid cell entry and enhanced in vitro potency, PLoS One, 14 (2019) e0214545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C, Liu Z, Zhong G, Lin J, A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro, Int J Nanomedicine, 14 (2019) 8603–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Fernández M, Javaid F, Chudasama V, Advances in targeting the folate receptor in the treatment/imaging of cancers, Chem Sci, 9 (2018) 790–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Munagala R, Aqil F, Jeyabalan J, Gupta RC, Bovine milk-derived exosomes for drug delivery, Cancer Lett, 371 (2016) 48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Munagala R, Aqil F, Jeyabalan J, Kandimalla R, Wallen M, Tyagi N, Wilcher S, Yan J, Schultz DJ, Spencer W, Gupta RC, Exosome-mediated delivery of RNA and DNA for gene therapy, Cancer Lett, 505 (2021) 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Zheng Z, Li Z, Xu C, Guo B, Guo P, Folate-displaying exosome mediated cytosolic delivery of siRNA avoiding endosome trapping, Journal of Controlled Release, 311–312 (2019) 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Kandimalla R, Saeed M, Tyagi N, Gupta RC, Aqil F, Exosome-based approaches in the management of Alzheimer’s disease, Neurosci Biobehav Rev, 144 (2023) 104974. [DOI] [PubMed] [Google Scholar]
- [171].Jia G, Han Y, An Y, Ding Y, He C, Wang X, Tang Q, NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo, Biomaterials, 178 (2018) 302–316. [DOI] [PubMed] [Google Scholar]
- [172].Raposo G, Stoorvogel W, Extracellular vesicles: Exosomes, microvesicles, and friends, Journal of Cell Biology, 200 (2013) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ, Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery, Leukemia, 20 (2006) 847–856. [DOI] [PubMed] [Google Scholar]
- [174].Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G, Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche, Cancer Research, 71 (2011) 5346–5356. [DOI] [PubMed] [Google Scholar]
- [175].Vakhshiteh F, Atyabi F, Ostad SN, Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy, Int J Nanomedicine, 14 (2019) 2847–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W, Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo, Cancer Letters, 315 (2012) 28–37. [DOI] [PubMed] [Google Scholar]
- [177].Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Kyakulaga A-H, Wilcher SA, Gupta RC, Milk exosomes - Natural nanoparticles for siRNA delivery, Cancer Letters, 449 (2019) 186–195. [DOI] [PubMed] [Google Scholar]
- [178].Kandimalla R, Aqil F, Tyagi N, Gupta R, Milk exosomes: A biogenic nanocarrier for small molecules and macromolecules to combat cancer, American Journal of Reproductive Immunology, 85 (2021) e13349. [DOI] [PubMed] [Google Scholar]
- [179].Kandimalla R, Aqil F, Alhakeem SS, Jeyabalan J, Tyagi N, Agrawal A, Yan J, Spencer W, Bondada S, Gupta RC, Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Zhang Q, Xiao Q, Yin H, Xia C, Pu Y, He Z, Hu Q, Wang J, Wang Y, Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma, RSC Advances, 10 (2020) 28314–28323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Matic S, D’Souza DH, Wu T, Pangloli P, Dia VP, Bovine Milk Exosomes Affect Proliferation and Protect Macrophages against Cisplatin-Induced Cytotoxicity, Immunological Investigations, 49 (2020) 711–725. [DOI] [PubMed] [Google Scholar]
- [182].Gao HN, Hu H, Wen PC, Lian S, Xie XL, Song HL, Yang ZN, Ren FZ, Yak milk–derived exosomes alleviate lipopolysaccharide-induced intestinal inflammation by inhibiting PI3K/AKT/C3 pathway activation, Journal of Dairy Science, 104 (2021) 8411–8424. [DOI] [PubMed] [Google Scholar]
- [183].del Pozo-Acebo L, López de las Hazas M-C, Tomé-Carneiro J, Gil-Cabrerizo P, San-Cristobal R, Busto R, García-Ruiz A, Dávalos A, Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy, International Journal of Molecular Sciences, 22 (2021) 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Manca S, Upadhyaya B, Mutai E, Desaulniers AT, Cederberg RA, White BR, Zempleni J, Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns, Scientific Reports, 8 (2018) 11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Warren MR, Zhang C, Vedadghavami A, Bokvist K, Dhal PK, Bajpayee AG, Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA, Biomaterials Science, 9 (2021) 4260–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nature Cell Biology, 9 (2007) 654–659. [DOI] [PubMed] [Google Scholar]
- [187].Zhang Y, Liu Q, Zhang X, Huang H, Tang S, Chai Y, Xu Z, Li M, Chen X, Liu J, Yang C, Recent advances in exosome-mediated nucleic acid delivery for cancer therapy, J Nanobiotechnology, 20 (2022) 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wallen M, Aqil F, Kandimalla R, Jeyabalan J, Auwardt S, Tyagi N, Schultz DJ, Spencer W, Gupta RC, A model system for antiviral siRNA therapeutics using exosome-based delivery, Mol Ther Nucleic Acids, 29 (2022) 691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Piffoux M, Silva AKA, Wilhelm C, Gazeau F, Tareste D, Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems, ACS Nano, 12 (2018) 6830–6842. [DOI] [PubMed] [Google Scholar]
- [190].Rayamajhi S, Nguyen TDT, Marasini R, Aryal S, Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery, Acta Biomaterialia, 94 (2019) 482–494. [DOI] [PubMed] [Google Scholar]
- [191].Sato YT, Umezaki K, Sawada S, Mukai S.-a, Sasaki Y, Harada N, Shiku H, Akiyoshi K, Engineering hybrid exosomes by membrane fusion with liposomes, Scientific Reports, 6 (2016) 21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Jhan Y-Y, Prasca-Chamorro D, Palou Zuniga G, Moore DM, Arun Kumar S, Gaharwar AK, Bishop CJ, Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery, International Journal of Pharmaceutics, 573 (2020) 118802. [DOI] [PubMed] [Google Scholar]
- [193].MacDonald RC, Jones FD, Qui R, Fragmentation into small vesicles of dioleoylphosphatidylcholine bilayers during freezing and thawing, Biochimica et Biophysica Acta (BBA) - Biomembranes, 1191 (1994) 362–370. [DOI] [PubMed] [Google Scholar]
- [194].Talsma H, Van Steenbergen MJ, Crommelin DJA, The cryopreservation of liposomes: 3. Almost complete retention of a water-soluble marker in small liposomes in a cryoprotectant containing dispersion after a freezing/thawing cycle, International Journal of Pharmaceutics, 77 (1991) 119–126. [Google Scholar]
- [195].Costa AP, Xu X, Burgess DJ, Freeze-Anneal-Thaw Cycling of Unilamellar Liposomes: Effect on Encapsulation Efficiency, Pharmaceutical Research, 31 (2014) 97–103. [DOI] [PubMed] [Google Scholar]
- [196].Chen H, Luo C, Yang M, Li J, Ma P, Zhang X, Intracellular uptake of and sensing with SERS-active hybrid exosomes: insight into a role of metal nanoparticles, Nanomedicine (Lond), 15 (2020) 913–926. [DOI] [PubMed] [Google Scholar]
- [197].Scott BL, Van Komen JS, Liu S, Weber T, Melia TJ, McNew JA, Liposome Fusion Assay to Monitor Intracellular Membrane Fusion Machines, Methods in Enzymology, Academic Press; 2003, pp. 274–300. [DOI] [PubMed] [Google Scholar]
- [198].Mukherjee D, Paul D, Sarker S, Hasan MN, Ghosh R, Prasad SE, Vemula PK, Das R, Adhikary A, Pal SK, Rakshit T, Polyethylene Glycol-Mediated Fusion of Extracellular Vesicles with Cationic Liposomes for the Design of Hybrid Delivery Systems, ACS Appl Bio Mater, 4 (2021) 8259–8266. [DOI] [PubMed] [Google Scholar]
- [199].Cheng L, Zhang X, Tang J, Lv Q, Liu J, Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy, Biomaterials, 275 (2021) 120964. [DOI] [PubMed] [Google Scholar]
- [200].Evers MJW, van de Wakker SI, de Groot EM, de Jong OG, Gitz-François JJJ, Seinen CS, Sluijter JPG, Schiffelers RM, Vader P, Functional siRNA Delivery by Extracellular Vesicle–Liposome Hybrid Nanoparticles, Advanced Healthcare Materials, 11 (2022) 2101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Lathwal S, Yerneni SS, Boye S, Muza UL, Takahashi S, Sugimoto N, Lederer A, Das SR, Campbell PG, Matyjaszewski K, Engineering exosome polymer hybrids by atom transfer radical polymerization, Proceedings of the National Academy of Sciences, 118 (2021) e2020241118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Molinaro R, Martinez JO, Zinger A, De Vita A, Storci G, Arrighetti N, De Rosa E, Hartman KA, Basu N, Taghipour N, Corbo C, Tasciotti E, Leukocyte-mimicking nanovesicles for effective doxorubicin delivery to treat breast cancer and melanoma, Biomaterials Science, 8 (2020) 333–341. [DOI] [PubMed] [Google Scholar]
- [203].Zhang KL, Wang YJ, Sun J, Zhou J, Xing C, Huang G, Li J, Yang H, Artificial chimeric exosomes for anti-phagocytosis and targeted cancer therapy, Chem Sci, 10 (2019) 1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Li Y-J, Wu J-Y, Hu X-B, Ding T, Tang T, Xiang D-X, Biomimetic Liposome with Surface-Bound Elastase for Enhanced Tumor Penetration and Chemo-Immumotherapy, Advanced Healthcare Materials, 10 (2021) 2100794. [DOI] [PubMed] [Google Scholar]
- [205].Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X, Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy, Nature Communications, 10 (2019) 3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Lee J, Lee J-H, Chakraborty K, Hwang J, Lee Y-K, Exosome-based drug delivery systems and their therapeutic applications, RSC Advances, 12 (2022) 18475–18492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Elkhoury K, Koçak P, Kang A, Arab-Tehrany E, Ellis Ward J, Shin SR, Engineering Smart Targeting Nanovesicles and Their Combination with Hydrogels for Controlled Drug Delivery, Pharmaceutics, 12 (2020) 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Le Meins JF, Schatz C, Lecommandoux S, Sandre O, Hybrid polymer/lipid vesicles: state of the art and future perspectives, Materials Today, 16 (2013) 397–402. [Google Scholar]
- [209].Gordon VD, Beales PA, Zhao Z, Blake C, MacKintosh FC, Olmsted PD, Cates M, Egelhaaf S, Poon WC, Lipid organization and the morphology of solid-like domains in phase-separating binary lipid membranes., Journal of Physics: Condensed Matter, 18 (2006). [DOI] [PubMed] [Google Scholar]


