Abstract
Since their invention in the early 2000s, tyrosine kinase inhibitors (TKIs) have gained prominence as the most effective pathway-directed anti-cancer agents. TKIs have shown significant utility in the treatment of multiple hematological malignancies and solid tumors, including chronic myelogenous leukemia, non-small cell lung cancers, gastrointestinal stromal tumors, and HER2-positive breast cancers. Given their widespread applications, an increasing frequency of TKI-induced adverse effects has been reported. Although TKIs are known to affect multiple organs in the body including the lungs, liver, gastrointestinal tract, kidneys, thyroid, blood, and skin, cardiac involvement accounts for some of the most serious complications. The most frequently reported cardiovascular side effects range from hypertension, atrial fibrillation, reduced cardiac function, and heart failure to sudden death. The potential mechanisms of these side effects are unclear, leading to critical knowledge gaps in the development of effective therapy and treatment guidelines. There are limited data to infer the best clinical approaches for the early detection and therapeutic modulation of TKI-induced side effects, and universal consensus regarding various management guidelines is yet to be reached. In this state-of-the-art review, we examine multiple pre-clinical and clinical studies and curate evidence on the pathophysiology, mechanisms, and clinical management of these adverse reactions. We expect that this review will provide researchers and allied healthcare providers with the most up-to-date information on the pathophysiology, natural history, risk stratification, and management of emerging TKI-induced side effects in cancer patients.
Subject terms: Cardiology, Oncology, Pathogenesis, Gastrointestinal diseases
Introduction
Innovations in the field of cancer therapies have led to the effective management of different cancers, which were previously considered to be incurable. Although these treatments have dramatically changed the natural course of many cancers, they may result in cardiac and extracardiac complications, which can be manifested either during therapy or after the completion of treatment. Both traditional therapies like anthracyclines, alkylating agents, and antimetabolites,1 and newer cancer therapies such as pathway-directed targeted therapies, and immunotherapies2,3 can have cardiovascular (CV) and metabolic sequelae, resulting in heart failure (HF), coronary artery disease (CAD), myocarditis, arrhythmias, and vascular and metabolic disturbances.
In the human genome, ninety tyrosine kinases have been identified, including fifty-six receptor tyrosine kinases and thirty-two cellular tyrosine kinases.4 Tyrosine kinase inhibitors (TKIs) used as targeted therapies are designed to perturb the cellular pathways that regulate malignant cell growth.1 TKI can be generally categorized into small molecules and macromolecules (e.g., monoclonal antibodies, polypeptides, antibody–drug conjugates, and nucleic acids).5,6 TKIs target signaling pathways involving the receptor tyrosine kinase and/or intracellular kinases that regulate cellular proliferation and tumor angiogenesis.2,6,7 The selectivity of binding of various TKIs to their targets influences the potency, mechanism of action, selectivity, and safety profile of these agents.8 Only select TKIs exhibit selectivity to specific protein kinases whereas most of the TKIs inhibit multiple kinases (~10–100) leading to the increased risk of toxicities.9 For instance, prior studies reported a ~ 5% rate of discontinuation of multi-kinase inhibitor (MKIs), imatinib therapy due to TKI-associated adverse effects.10 Toxicity can be attributed to both on-target effects through excessive inhibition of the intended TK function; and/or off-target effects resulting from the simultaneous inhibition of multiple other kinases due to limited selectivity.8,11,12 On-target effects have been implicated in the causation of adverse reactions such as hypertension (HTN), hypothyroidism, skin reactions, and proteinuria.13 However, it is important to identify the close relationship between the effectiveness of therapy and the occurrence of toxic reactions. The side effect profile is frequently used as a monitoring tool to identify the desired outcomes of cancer therapy.14 However, a positive correlation has been identified between the number of kinases inhibited and the extent of their toxic potential.15
Several TKIs have been approved by the US Food and Drug Administration (Table 1). TKI-associated side effects can be mild to life-threatening affecting various organ systems, and in some cases, severe adverse effects require premature discontinuation of life-saving cancer therapies. Cardiotoxicity has been asserted as a notable effect of several agents including TKIs. Although TKIs are often used as therapeutics in oncology, it should also be noted that TKIs utilized in other fields of pharmacotherapy, for instance, TKIs used as antipsychotics, can also induce cardiotoxicity.16 TKI-related cardiovascular dysfunction (TRCD) can have serious consequences ultimately leading to premature treatment cessation or interruption. In this review, we provide a comprehensive summary of commonly reported cardiac and extracardiac side effects of TKIs used as therapy in oncology, with a specific focus on the early detection and clinical management of these side effects. Special emphasis is given to the CV side effects due to the complexity of screening and management of these complications.
Table 1.
Small molecule TKIs approved by US FDA for use in cancer therapy, their indications, and common adverse effects
| Receptor targets | Drugs | Applications | Cardiovascular Adverse Effects | Extra-Cardiac Adverse Effects | |
|---|---|---|---|---|---|
| ALK family (Anaplastic lymphoma kinase) | First-generation: Crizotinib (Type Ia c-MET inhibitor) | ALK+, ROS1 + NSCLC | Arrhythmia, QT Prolongation, Bradycardia | Visual disturbances (flashes, light columns, blurred vision), Neutropenia | |
| Second-generation: | Ceritinib | ALK + NSCLC as first-line treatment or after crizotinib resistance | QT Prolongation Arrhythmia, MI, Bradycardia | GI disorders | |
| Alectinib | ALK + NSCLC | QT Prolongation, Arrhythmias | Anemia | ||
| Brigatinib | ALK+ NSCLC after crizotinib | Arrhythmia, Bradycardia, HTN | ILD | ||
| Ensartinib | ALK+ NSCLC | – | Rash, Elevated transaminase levels (AST/ALT), Pruritus, GI disorders, Edema, Anemia, Increased levels of blood ALP blood creatinine, GGT, Increased bilirubin level, Increased CPK level, and Hyponatremia | ||
| Third-generation: | Lorlatinib | ALK + NSCLC | MI, PR interval prolongation, AV block | Fatigue, Increased ALT, Cough, Anemia, Decreased neutrophil count, Mental disorders, Mood, speech, and sleep disorders | |
| Entrectinib | ROS1 + NSCLC; solid tumors with NTRK fusion proteins | QT Prolongation | Dysgeusia, Dizziness, Weight Gain, Paresthesia, Fatigue, GI Disorders, Peripheral edema, Myalgia, Anemia, Increased Blood Creatinine, and Arthralgia. | ||
| Repotrectinib (Next- Generation ROS1/TRK/ALK Inhibitor | ROS1+ advanced NSCLC | – | Dizziness | ||
| BCR–ABL family [Fusion protein of ABL1 (Abelson murine leukemia viral oncogene homolog 1) and Breakpoint cluster region protein (BCR)-cytoplasmic fusion tyrosine kinase] | First-generation: Imatinib (also targets VEGFR and PDGFR) | Ph+ CML or ALL, CEL, DFSP, HES, GIST, MDS/MDP | HF, LVD |
Nausea, Vomiting, Diarrhea, Edema, Myelosuppression, Immunosuppression, Fatigue, Insomnia, Depression, Dizziness, URTI, influenza, Pyrexia, Cough, Abdominal pain, Myalgia, Arthralgia, Skin rash, Hemorrhage Rare association with appendiceal carcinoma |
|
| Second-generation: | Nilotinib | Ph+ ALL | MI, QT prolongation, HTN | Myelosuppression, Hyperbilirubinemia, Pancreatitis, Fatigue, Headache, Nausea, Vomiting, Diarrhea, Constipation, Skin rash, Pruritis, Edema | |
| Dasatinib [also targets Src (cytoplasmic tyrosine kinase)] | Ph+ CML and ALL | Pleural/Pericardial Effusion, QT prolongation, HF, LVD, MI |
Myelosuppression, Panniculitis, Bleeding, Fatigue, Headache, Dyspnea, Infection, Fluid retention, Abdominal pain, Nausea, Diarrhea, Edema Myalgia, Arthralgia, Skin rash, Hemorrhage |
||
| Bosutinib (also targets Src) | CML resistant or intolerant to prior TKI therapy | QT prolongation, PHTN, Pericardial Effusion | Myelosuppression, Fatigue, Headache, Dyspnea, Cough, Pyrexia, GI disorders, Edema, Increased ALT, Arthralgia, Skin rash | ||
| Third-generation: Ponatinib | Ph+ CML or ALL | HTN, HF, Arrhythmias |
Myelosuppression, Fatigue, Headache, Dyspnea, Pyrexia, Pancreatitis, Pyrexia, Abdominal pain, Nausea, Vomiting, Diarrhea, Constipation, Increased ALT, Myalgia, Arthralgia, Skin rash, Dry skin, Hepatotoxicity, Liver failure, and Death |
||
| B-Raf family (B-rapid accelerating fibrosarcoma/v-raf murine sarcoma viral oncogene homolog B1) | Vemurafenib | Melanoma with BRAFV600E mutation, and ECD | LVD, QT prolongation, AF | Photosensitivity, Skin rash, Increased LFTs, Arthralgia, Nausea, Fatigue, Edema, Cutaneous squamous- cell carcinoma, Pruritus, Palmar–plantar dysesthesia | |
| Dabrafenib | Melanoma and NSCLC with BRAF mutations | LVD | Fever, Neutropenia, Arthralgia, Fatigue, Headache, Peripheral edema | ||
| Encorafenib | BRAF V600E/K mutant melanoma with binimetinib | QT prolongation, LVD | Anemia, Transient Bell’s palsy, Myalgia, Nausea, Palmoplantar erythrodysesthesia, Arthralgia, Alopecia, Hyperkeratosis. | ||
| BTK family (Bruton’s tyrosine kinase) | Ibrutinib | MCL, CLL, WM, GVD, MZL | Arrhythmia, HTN | Hemorrhage, Cytopenias Diarrhea, Increased risk of Infections, Arthralgia, Fatigue, Muscle Spasms or Myalgias, Pyrexia, Skin Rash, Headaches | |
| Acalabrutinib | MCL | Arrhythmia | Hemorrhage, Headache, Diarrhea, Fatigue, Myalgias, Cough, Neutropenia, Nausea, Skin Rash, and Infections. | ||
| Zanubrutinib | Refractory MCL and WM | Arrhythmia | Neutropenia, Increased Risk of Infections | ||
| c-MET family (Hepatocyte Growth Factor (HGF)/Mesenchymal al-Epithelial Transition Factor Receptor) | Capmatinib (Type Ib inhibitor) | NSCLC with METex1 4 (MET exon 14 skipping mutation) | – | ILD/Pneumonitis, Peripheral Edema, Fatigue, GI Disorders, Grade 3 hepatotoxicity, Elevated Creatinine clearance, Photosensitivity | |
| Tepotinib (Type Ib inhibitors) | NSCLC) with METex14 | – | Peripheral edema, Nausea, Diarrhea, Increased blood creatinine, Upper abdominal pain, Hypoalbuminemia, Increased ALT/AST, amylase and lipase levels, Asthenia, Decreased appetite, Pleural effusion, Alopecia, Fatigue, General edema | ||
| EGFR/ERBB family ErbB1 [EGFR (human epidermal growth factor receptor) or HER1 (erythroblastic leukemia viral oncogene B), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4) [EGFR family receptor tyrosine kinase] | First-generation (Competitive and Reversible) | Gefitinib | NSCLC | MI | Skin rash, Diarrhea, Nausea, ILD, Dry Skin, Pruritus, Stomatitis, Anorexia |
| Erlotinib | NSCLC and Pancreatic Cancer | Edema | Skin rash, Diarrhea, Fatigue, Appetite loss, Nausea, ILD, Hematologic, Alopecia, Arthralgia, Neuropathy | ||
| Lapatinib (reversible dual inhibitor- EGFR and HER2) | Breast Cancer | HF, LVD, QTProlongation | Skin rash, Diarrhea | ||
| Second-generation (Covalent and Irreversible) | Vandetanib (also targets Src/VEGFR 2/RET) | MTC | Asymptomatic QT prolongation, Torsades de pointes, Arrhythmia, HF, HTN | Rash, Diarrhea, Proteinuria | |
| Afatinib | NSCLC | HTN | Severe Diarrhea, Rash-Acne, Stomatitis, Paronychia, Dry Skin, Appetite loss | ||
| Dacomitinib | EGFR-mutated NSCLC | – | Diarrhea, Paronychia, Rash-Acne, Stomatitis, Dry Skin, Appetite loss, Weight Loss, Alopecia, Cough, Hemorrhoids, Wound, Back pain, Headache | ||
| Neratinib | HER2+ breast cancer | Low rates of symptomatic decline in LVEF and QT prolongation | Diarrhea, GI disorders, Fatigue, Headache | ||
| Third-generation (Irreversible): Osimertinib | NSCLC | QT prolongation, LVD, HF, Arrhythmia, MI, Pericardial Effusion | Diarrhea, Rash, Dry Skin, Paronychia, Stomatitis, Fatigue | ||
| Mobocertinib (Irreversible) | EGFR exon 20 insertions (EGFRex20i ns) mutation-driven NSCLC | – | GI Disorders, Rash, Dry skin, Stomatitis, Fatigue, Maculopapular Rash, Paronychia, Anemia, Dermatitis acneiform, Increased lipase, Pruritus | ||
| Pyrotinib (Irreversible dual pan-ErbB receptor inhibitor) | HER2- positive | Diarrhea, Hand-foot syndrome, Leukopenia, Neutropenia, GI disorders, Increased ALT, Anemia, Asthenia | |||
| FGFR family (fibroblast growth factor receptor) | Erdafitinib | Urothelial carcinoma | MI | Ocular disorders (central retinopathy), Hyperphosphatemia, Embryo-fetal toxicity, Fatigue, Dysgeusia, Paronychia, Alopecia, HFSR, Xerosis | |
| Flt3 family (Fms-like tyrosine kinase 3) | Gilteritinib | AML with FLT3 mutation5 | QT prolongation | Arthralgia/myalgia, Dizziness, Dyspnea, Edema, Fatigue, Noninfectious diarrhea, Pneumonia, Rash, Transaminitis | |
| Midostaurin | ALL Flt3 mutation+ | HTN, Pericardial Effusion | Nausea, Vomiting, Diarrhea, Fatigue, Headaches | ||
| JAK family (Janus Kinase) | Ruxolitinib | MF and PV | MI, Venous thromboembolism | Myelosuppression, Hematological toxicity particularly thrombocytopenia, anemia - dose-limiting toxicity, ecchymosis, Dizziness, and Fatigue | |
| Fedratinib | Primary or secondary myelofibrosis | HF, Cardiogenic Shock | Anemia, GI symptoms, Increased levels of liver transaminases, serum creatinine, and pancreatic enzymes, Encephalopathy | ||
| MAPKK family/ MEK ½ (Mitogen-activated protein kinase kinase) | Trametinib | Melanoma (2013) and NSCLC (2017) with BRAF mutations | LVD | Rash, Diarrhea, Central Serous Retinopathy, Papulopustular Exanthema, Peripheral edema, | |
| Binimetinib | BRAF V600E/Kmelanoma with encorafenib | QT prolongation, LVD | Papulopustular rash, Central Serous-like Retinopathy, GI Disorders | ||
| Cobimetinib | Melanoma with BRAF V600E/K mutations with vemurafenib | LVD, QT prolongation | Rash, Fatigue, Edema, GI Disorders | ||
| NTRK family (Neurotrophic Tyrosine Receptor Kinase) | Larotrectinib | Solid tumors with NTRK gene fusion proteins | – | Anemia, Increased AST/ALT, Weight increase, Decreased neutrophil count, Fatigue, Cough | |
| PDGFR family (PDGFR α/β, KIT [CD117, stem cell factor receptor, Colonial stimulating factor-1 receptor (CSF1R), the stem cell growth factor receptor (SCGFR), FLK2/FLK3][Receptor tyrosine kinase] & VEGFR family (VEGFR1, VEGFR2, VEGFR3) [Receptor tyrosine kinase] | Sorafenib (also targets CDK, B-RAF, KIT, FLT-3, RET, c-MET) | RCC, DTC, and HCC | HTN, ACS, HF, Arterial thromboembolism, QT prolongation | Skin rash, Hemorrhage, HFSR, Mucositis, Hypothyroidism, Fatigue, Renal toxicities, including proteinuria and acute renal failure, Dyspnea, Diarrhea | |
| Sunitinib (also targets Src) | HES, GIST, MDS/MDP | HTN, MI, HF, Reduced LVEF, LVD, Arterial thrombosis, QT prolongation, Torsades de pointes | Hemorrhage, Hypothyroidism, Adrenal Dysfunction | ||
| Ponatinib (also targets Src and FGFR) | Ph+ CML or ALL | HF, Arrhythmia, HTN, Cardiomyopathy, Vascular occlusion, Arterial, and Venous thrombosis | Rash, Abdominal pain, Nausea, Constipation, Headaches, Dry skin, Fatigue, Fever, Myalgia. Dyspnea, Arthralgia, Increased ALT/ lipase, Pancreatitis and amylase increase, Hematologic adverse effects (thrombocytopenia > neutropenia > anemia), Hepatotoxicity, Liver failure, and Death | ||
| Axitinib (also targets KIT) | RCC |
HTN, HF, Arterial Thrombosis. Hypotension |
Fatigue, Nausea, Diarrhea, Vomiting, Headache, Hemoptysis, Stomatitis, Erythema, Anorexia, Limb Pain, Arthralgia, Myalgia Hand-Foot Syndrome, Dyspnea, Dehydration | ||
| Regorafenib | CRC, GIST | MI, HTN, Arterial thrombosis | HFSR reactions, Diarrhea, Fatigue, Anemia, Thrombocytopenia, Proteinuria | ||
| Pazopanib | RCC, STS | LVD, HTN, HF, Arterial thrombosis, Cardiomyopathy, QT Prolongation, Torsades de pointes | Fatigue, GI disorders, Elevated ALT, Neutropenia, Leukopenia, Lymphocytopenia, Anemia, Asthenia | ||
| Lenvatinib (also targets Src) | DTC | QT Prolongation, LVD, HF, HTN | Hypothyroidism, Diarrhea, Fatigue, Decreased appetite | ||
| Cabozantinib (Type II c-MET inhibitor) (also targets Src and) | Metastatic MTC, advanced RCC, and HCC | HTN, Arterial thrombosis | Palmar–plantar erythrodysesthesia, GI disorders, Fatigue, Stomatitis. | ||
| Praseltinib (also inhibits RET, DDR1, TRKC, FLT3, JAK1–2, TRKA, VEGFR2, PDGFRb, and FGFR1) |
Metastatic RET fusion + NSCLC Advanced or metastatic RET-mutant MTC Advanced or metastatic RET fusion + thyroid cancer |
HTN | Rash, Anemia, Cough, Fatigue, Pyrexia, GI disorders, Edema, Musculoskeletal pain, Decreased WBCs, Hyperphosphatemia, Increased AST/ALT, Pneumonitis, Headache, Peripheral neuropathy, Dizziness, Dysgeusia | ||
| RET family | Alectinib | ALK + NSCLC | QT Prolongation, Bradycardia | GI disorders, Myalgia, Peripheral edema, Elevated liver enzymes, Elevated blood bilirubin, Anemia | |
| Selpercatinib | Metastatic RET fusion-positive NSCLC, advanced or metastatic RET-mutant MTC | HTN, QT prolongation, | Hepatotoxicity, Hemorrhagic events, Hypersensitivity, risk of impaired wound healing, and Embryo-fetal toxicity. | ||
ACS Acute Coronary Syndrome, AF Atrial Fibrillation, ALL Acute lymphocytic leukemia, ALP, Alkaline Phosphatase, ALT Alanine Transaminase, AST Aspartate Transaminase, AV Atrioventricular, CEL Chronic Eosinophilic Leukemia, CLL Chronic lymphocytic leukemia, CML, Chronic myeloid leukemia, CPK Creatine Phosphokinase, CRC Colorectal cancer, DFSP Dermatofibrosarcoma protuberans, DTC Differentiated thyroid cancer, ECD Erdheim-Chester Disease, GGT Gamma Glutamyl Transference, GI Gastrointestinal, GIST Gastrointestinal Stromal Tumor, GVD graft versus host disease, HCC Hepatic Cell Carcinoma, HES Hyper eosinophilic syndrome, HF Heart Failure, HFSR hand–foot skin reaction, HTN Hypertension, ILD Interstitial Lung Disease, LFT Liver function test, LVD Left Ventricular Dysfunction, LVEF Left ventricular ejection fraction, MCL Mantle cell lymphoma, MDP Myeloproliferative Disorders, MDS Myelodysplastic syndromes, MF Myelofibrosis, MI Myocardial Infarction, MTC Medullary thyroid cancer, MZL Marginal zone lymphoma, NSCLC Non-Small Cell Lung Cancer, PDGFR Platelet-Derived Growth Factor Receptor, Ph+ Philadelphia chromosome, PHT Pulmonary hypertension, PV Polycythemia vera, RCC Renal Cell Carcinoma, ROS Reactive Oxygen Species, STS Soft tissue sarcoma, TKI Tyrosine Kinase Inhibitor, URTI Upper Respiratory Tract Infection, VEGFR Vascular Endothelial Growth Factor Receptor, WBC White Blood cell, WM Waldenstrom macroglobulinemia
Cardiovascular adverse effects
Seven cardiotoxic cancer therapy classes (including four classes of targeted therapies) are proposed based on the range of CV toxicities.17 CV complications are reported in both single-targeted and multi-targeted TKIs. The list of commonly used and novel TKIs with common CV adverse effects is listed in Table 2. In the heart, TKIs can adversely affect vascular endothelial cells and cardiac post-mitotic cells, predominantly cardiomyocytes (CMs).18,19 TKIs, specifically those with multikinase activities, affect several signal transduction pathways, of which the most affected ones include cardiotrophin and its receptor gp130, epidermal growth factor receptor (EGFR, ErbB2, or HER2), phosphoinositide 3-kinase (PI3K), AMP-activated protein kinase (AMPK), ubiquitin proteasomal system, and lysosomal autophagy pathways.20 Similarly, vascular endothelial growth factors (VEGF) involved in angiogenesis, microvascular function, and myocardial perfusion are often implicated in the development of TKI-induced CV target-organ damage.21
Table 2.
Novel TKIs used in cancer therapy and their common cardiovascular side effects
| Drug Class and Name | Molecular target | Common cardiovascular complications |
|---|---|---|
| Her2 inhibitors (trastuzumab, pertuzumab, lapatinib, neratinib) | erbB2/HER2 | A decline in LVEF, Congestive heart failure |
| VEGF signaling pathway inhibitors (bevacizumab, ramucirumab, aflibercept) | VEGF-A | Hypertension, Cardiomyopathy |
| TKI with anti-VEGF activities (sunitinib, sorafenib, pazopanib, axitinib, vandetanib, regorafinib, cabozatenib, lenvatinib | VEGFR and other kinases e.g. PDGFR | Hypertension |
| Multi-targeted TKIs (imatinib, dasatinib, ponatinib, nilotinib) | Abl, abl mutant (except T315I), EGFR, PDGFR, SRC, KIT, BRAF, DDR1, DDR2, Ephrin receptor | Vascular events, QTc prolongation, Pulmonary hypertension (with dasatinib) |
| ALK inhibitors (crizotinib, ceritinib) | ALK | QTc prolongation, Bradycardia |
| BTK inhibitors (ibrutinib) | BTK | Atrial fibrillation, Ventricular arrhythmia |
| MEK inhibitors(trametinib) | MEK1/MEK2 | Cardiomyopathy |
ALK Anaplastic lymphoma kinase, BRAF B-rapid accelerating fibrosarcoma, BTK Bruton’s Tyrosine Kinase, EGFR Epidermal Derived Growth Factor Receptor, LVEF Left ventricular ejection fraction, MEK Mitogen-activated protein kinase kinase, PDGFR Platelet-Derived Growth Factor Receptor, TKI Tyrosine kinase inhibitors, VEGFR Vascular Endothelial Growth Factor Receptor
Mechanisms such as reactive oxygen species (ROS), accumulation of drug metabolites disrupting the structure and function of sarcomeres, and mitochondrial biogenesis leading to vacuole formation, contractile element disarray, disruption of mitochondrial transport chains leading to activation of various cell death pathways, including ferroptosis, pyroptosis, and necrosis have been implicated.11,16,22 Specifically, apoptosis can be induced through the activation of extrinsic (cell surface death receptors) and intrinsic [mitochondria and the endoplasmic reticulum (ER)] pathways.23 Necroptosis and ferroptosis are caspase-independent and iron-dependent processes, respectively.24,25 Necroptosis can be induced mainly through the stimulation of various death receptors and Toll-like receptors.23
Major TKI related cardiovascular events
Myocardial dysfunction and heart failure
In various clinical studies, TKIs were reported to be associated with the highest relative risk (RR 5.6) for high-grade cardiotoxicity. A meta-analysis of clinical trials in 10,647 patients with a wide range of malignancies treated with TKIs (axitinib, pazopanib, sorafenib, sunitinib, and vandetanib) showed a combined incidence of asymptomatic left ventricular dysfunction (LVD) of 2.4% with no differences between more specific TKIs and MKIs.26 Among the TKIs, HER2 molecular-targeted therapies such as lapatinib, vascular endothelial growth factor inhibitors (VEGFIs), and BCR-ABL TKIs were associated with a higher risk of HF or LVD.27,28 Similarly, VEGFIs are known to induce chronic progressive microvascular changes which reduce the myocardial capillary network (rarefaction) leading to myocardial hypoperfusion and impaired contractility.29
Traditionally, the term type I and type II cardiotoxicity are used to describe the pattern of cancer therapy-induced cardiotoxicity.30,31 Anthracyclines are the prototype drugs in type I cardiotoxicity and are responsible for acute as well as progressive and cumulative myocellular injury or loss.32–34 In contrast, trastuzumab and TKIs are classic examples of type II cardiotoxicity characterized by late-onset, non-progressive, and reversible features (Table 3).22 However, the growing evidence suggests that this categorization is both incomplete and fundamentally incorrect. A study involving 45,000 breast cancer patients treated with anthracycline, trastuzumab, or the combination revealed that trastuzumab caused more long-term damage than anthracyclines in real-world patients with breast cancer. These findings debunk the notion that trastuzumab-treated patients aren’t susceptible to “sequential stress-induced cardiomyopathy” in the same fashion as anthracycline-treated patients.35 Several other TKIs have been known to cause irreversible cardiac changes.
Table 3.
Types of cardiotoxicity
| Type I Cardiotoxicity | Type II Cardiotoxicity |
|---|---|
| • Early Onset | • Late Onset |
| • Myocardial damage | • Myocardial Dysfunction |
| • Permanent / Irreversible | • Reversible in nature |
| • Dose-dependent effects | • Cumulative Dose-independent effects |
| • Greater association with Cardiac Dysfunction and Clinical HF | • Increased loss of contractility and less myocyte death |
| • Typically, with Anthracyclines (Doxorubicin), Alkylating Agents Taxanes, Topoisomerase Inhibitors, Antimetabolites | • Typically, with Trastuzumab, Bevacizumab, and other Tyrosine Kinase Inhibitors, Immunomodulatory drugs, and Proteasome inhibitors but patients may develop type I cardiotoxicity in the long run |
From the mechanistic perspective, CM death was reported in cardiac-specific VEGF knockout mice, and also in mice treated with VEGFI.36 Similar effects were observed in mice treated with platelet-derived growth factor inhibitors (PDGFI), further highlighting the effects of VEGF in the development of microvascular dysfunction through PDGF inhibition.37 Importantly, VEGFI-induced LVD is predisposed by previous or concurrent cardiotoxic chemotherapies creating a vulnerable myocardium.26 Sunitinib and sorafenib impair the angiogenic response necessary to overcome the effects of HTN-induced pressure overload to the heart, thus resulting in an increased incidence of cardiac dysfunction and HF.2 Sunitinib is also reported to inhibit platelet-derived growth factor receptors (PDGFR) resulting in decreased myocardial pericytes and microvascular density.38 Similar findings were reported in pazopanib-treated patients with some unique case reports of the apical ballooning syndrome and rapidly progressive fulminant HF.39
The neuregulin-HER pathway (NRG-1/HER-4/HER-2 axis) inhibition is responsible for the anti-HER-2 TKI-related HF pathophysiology. Based on the cardioprotective role of neuregulin-1 (NRG-1), clinical trials assessing its safety and efficacy in HF are currently underway.40 Cardiotoxicity with anti-HER2 TKIs was seen at a higher rate in metastatic trials compared to adjuvant trials.28 In a meta-analysis evaluating the cardiac adverse events of anti-HER2 drug lapatinib-treated patients, the overall incidence associated with breast cancer was found to be higher than in other cancers (3% vs 2.7%). This could be explained by the confounding effects of radiotherapy but needs further analysis.41
A meta-analysis of pivotal studies involving osimertinib reported an increased risk of HF (about 19.3%), which developed on an average of 29 days after the initiation of therapy.42 The AURA3 Randomized Phase III Study (Osimertinib vs Platinum-Pemetrexed) and the FLAURA trial (osimertinib vs first-generation EGFR-TKI) in EGFR-Mutated Advanced non-small cell lung cancers (NSCLC) reported the frequency of LVD at 5% and 10%, respectively.43 Paradoxically, a larger population study with a 2-year follow-up in 942 gastrointestinal stromal tumor (GIST) patients postulated that imatinib does not significantly induce cardiac failure. Instead, as an add-on therapy for pulmonary arterial hypertension (PAH), it improves exercise capacity, cardiac hemodynamics, right ventricular (RV) function, and left ventricular (LV) early diastolic relaxation.44
A combination of BRAF and MEK inhibitors is often used to treat metastatic BRAF-mutated melanoma and is associated with cardiotoxicity in 5% to 11% of patients. However, BRAF monotherapy is rarely associated with LVD.45 The COLUMBUS trial conducted in BRAF mutant metastatic melanoma patients investigating a combination of encorafenib plus binimetinib/encorafenib/vemurafenib showed a 6% incidence of all-grade, 6% grades 1–2, and 2% grade 3 LVD.46
Coronary artery disease and myocardial ischemia/infarction
VEGF inhibition is known to increase mitochondrial superoxide generation and decrease nitric oxide (NO) production.47 This results in the acceleration of atherosclerosis in apolipoprotein E (apo E) knockout mice with no discernible effects on plaque vulnerability.26 Experiments in rats treated with sunitinib demonstrated reduced vasorelaxation due to a reduction in endothelial NO release.48 Small molecule TKIs such as ponatinib and nilotinib are associated with increased incidence of myocardial ischemia or infarction.49 Ponatinib is also associated with an increased risk of angina pectoris. In addition to acute coronary syndromes, nilotinib has off-target vascular pro-atherogenic properties causing arterial stenosis and vasospasm.46 Erlotinib and sorafenib exhibited an increased incidence of cerebrovascular accidents and myocardial infarction (MI).49 It is important to note that biologics such as bevacizumab are very target-specific, and their side effects are mostly related to exaggerated pharmacological effects. On the other hand, small-molecule drugs such as sunitinib, sorafenib, and nilotinib are more prone to induce harmful non-target effects.50
QTc prolongation and cardiac arrhythmias
The incidence of QTc prolongation and various arrhythmias with VEGFI is about 0.1%.51 Some reports suggested that the QTc interval is unaffected by VEGF inhibition.26 No correlation was reported between the duration of drug exposure and QT prolongation effect.21 Studies have shown that the effect on QTc interval differs in the same drug depending on indications, duration of therapy, drug combinations, and pathophysiology of the underlying disease.26 Infrequently, high-grade atrioventricular (AV) blocks requiring permanent pacemaker implantation have been observed.52,53 However, due to the smaller number of studies, the true incidence is yet to be defined. Third-generation EGFR TKIs- rociletinib and osimertinib are reported to have a higher incidence of grade 3–4 QTc prolongation.43 Nilotinib has a black-box warning for QTc prolongation and sudden cardiac death (SCD).54 Importantly, it has been reported that the QTc prolongation potential is not directly proportional to the increased risk of ventricular arrhythmias.55 Concurrent administration of other QTc-prolonging drugs (oral chemotherapeutic agents, antibiotics, psychiatric medications) in cancer patients can have additive effects.56
Ibrutinib used in the treatment of chronic lymphocytic leukemia/lymphoma (CLL) is predominantly associated with atrial fibrillation (AF) based on numerous studies.57,58 Primarily, AF has been described as an off-target effect of ibrutinib.59 In the HELIOS study, AF incidence was higher when ibrutinib was combined with other drugs compared to monotherapy. The median time to onset of AF was around 2.8 months. Ventricular arrhythmias including non-sustained ventricular tachycardia, ventricular fibrillation, and SCD were reported with a cumulative incidence of 1991 events per 100,000 person-years.60 An ibrutinib-associated ventricular arrhythmia occurs to a lesser extent compared to AF. No association between ibrutinib and QT prolongation was observed. Paradoxically, there was evidence of QT shortening with ibrutinib use.55
Acalabrutinib, a highly selective Bruton’s tyrosine kinase inhibitor (BTKI) was shown to be associated with an increased incidence of AF in the ELEVATE-TN and ASCEND trials (Table 2). The former study included acalabrutinib and obinutuzumab, which were associated with 3% and 4% incidence of AF respectively.61 Similarly, the ASCEND trial which compared acalabrutinib with the investigator’s drug of choice demonstrated AF in 5% and 3% of patients, respectively.62 The ASPEN study involving a selective TKI, zanubrutinib demonstrated a reduced risk of AF compared to ibrutinib.63 However, while considering the results of these studies, we should also acknowledge the pitfalls of these being open-label with no specific screening for AF. Surprisingly, BTKI is known to be associated with a lower incidence of sudden death. A meta-analysis that included 4 studies (RESONATE, RESONATE-2, HELIOS, and RAY) with 1000 ibrutinib-treated patients reported sudden death only in 10 patients.64
Bradycardia is mostly associated with ALK inhibitors including crizotinib and ceritinib used in the treatment of NSCLC.65 Mechanistically, drug interactions having a significant effect on CYP (Cytochrome P450) 3A4 is reported.55 Clinical trials with pazopanib reported sinus bradycardia in about 19% of patients.26 However, most patients remained asymptomatic and dose reduction was rarely necessary.55
Systemic arterial hypertension
HTN remains a frequently reported adverse event with TKIs, with evidence suggesting possible dose-independent class effects.55,66 A meta-analysis of 77 studies reported a significantly increased risk of HTN, cardiac ischemia, and cardiac dysfunction associated with TKIs.67 HTN is usually detected within the first month of treatment. Across different trials, the incidences of HTN with individual drugs were 42% with pazopanib, 63–68% with ponatinib, 7–43% with sorafenib, 5–24% with sunitinib, 40% with axitinib, and 30–59% with regorafenib.58,68,69 The highest rate of both all-grade and high-grade (grade 3 or 4) HTN was reported in ibrutinib and lenvatinib. Vandetanib, cabozantinib, and vatalanib were associated with all grades in 24–29% and high-grade HTN in 7–22% of patients, respectively.66
Among the small molecule TKIs, VEGFI showed increased association with HTN in about 30–80% of patients.70 In a study with metastatic renal cell carcinoma (RCC) patients treated with sunitinib, an average increase in blood pressure (BP) of 14/11 mmHg was observed through 24-h ambulatory monitoring. A new baseline was noted after the first drug cycle, which never returned to the pre-treatment level even after completion of therapy demonstrating a more long-lasting change in vasculature with VEGF inhibition.55 Sunitinib was associated with reduced plasma renin concentration and activity with unchanged aldosterone levels suggesting mineralocorticoid-receptor activation.71 Alivon et al. noted that sunitinib or sorafenib induces inhibition of vascular signaling pathways increasing arterial stiffness partially independent of the change in BP.72 Patients with pre-existing HTN, high body mass index (BMI), advanced age (≥60 years), underlying metastatic RCCs, and use of more potent agents such as axitinib were reported to be more vulnerable.55,66
A single-arm cohort study by Dickerson et al. estimated that 78.3% of ibrutinib-treated patients developed a new or worsening HTN (17.7% with BP > 160/100 mmHg) over a median time duration of 30 months. The incidence of HTN is lower in more selective agents compared with ibrutinib.73 The cumulative incidence of HTN in ibrutinib was 78% with a median time of onset ranging from 1.8 to 6 months, but it can also develop in a very short time.60 ELEVATE-TN and ASCEND trials showed a higher incidence of HTN with acalabrutinib. Zanubrutinib and ibrutinib were studied in the ASPEN trial, where the incidence of HTN was lower in zanubrutinib compared to the ibrutinib group.57 Other TKIs including erlotinib, osimertinib, ruxolitinib, and a combination of BRAF and MEK inhibitors showed significant association with systemic HTN.43,66
Pulmonary arterial hypertension
PAH has a relatively lower incidence in TKIs and usually appears as a late complication. Studies have shown a proven risk with dasatinib, and a possible risk with ponatinib, bosutinib, lapatinib, and lorlatinib therapies.74 Dasatinib, the second-generation drug used to treat chronic myeloid leukemia (CML) was the first TKI reported to induce severe pre-capillary PAH with an incidence of 0.45% as reported in the French registry.75 It usually occurs 8–40 months after the initiation of therapy.68 Interestingly, PAH was more frequent in women, and the presence of hormonal or immunological factors may increase the risk.74 Mechanistic studies in pre-clinical models demonstrated that dasatinib administered alone was not sufficient for the development of PAH, suggesting the requirement of an additional predisposing factor(s) in the form of pulmonary vascular insult.74 In the rat models of pulmonary fibrosis, PAH was exacerbated by VEGF inhibition. In addition to these direct effects, indirect mechanisms such as the development of pulmonary venous thromboembolism in the setting of a cancer-induced hypercoagulable state could be contributory. Studies also suggested that VEGF inhibition can cause the impairment of RV CMs in response to cardiac stress. RV failure can also be explained by the multi-hit hypothesis through CMs, coronary, pulmonary, and venous vasculature modifications.26
Pericardial diseases
Pleural and pericardial effusion is associated with drugs such as dasatinib, nilotinib, imatinib, ponatinib, and FLT3 inhibitors.58,60 Dasatinib is frequently associated with pleuropericardial effusion.76 FAERS database examining CV toxicities of VEGFI demonstrated a 0.3% incidence of pericardial effusion.77
Thromboembolic diseases
The mechanism of thromboembolism involves the alteration of vascular protective properties of endothelial cells.66 Arterial and venous thrombosis results from the reduction of NO synthesis and endothelial dysfunction.40 Risk factors include specific cancer types, metastasis, central venous catheter, HF, immobility, AF, obesity, previous episodes of thromboembolic diseases, high-dose chemotherapy, hormonal therapies, advanced age, and the female gender.58 The increased incidence of all-grade arterial thromboembolism is predominantly associated with VEGFI including erlotinib, nilotinib, dabrafenib, and trametinib.28,58 These agents exacerbate the pro-coagulant state of cancer thus increasing the risk of arterial and venous thromboembolism. Central nervous system (CNS) hemorrhagic or ischemic events are associated with ibrutinib.78 Bosutinib used in refractory/relapsed CML is associated with increased cardiac adverse events, especially cerebrovascular events. Ponatinib is reported to have an increased incidence of cerebrovascular (6%) and peripheral arterial occlusive events (8%).60
In a meta-analysis including eligible VEGFI patients, an increased risk of MI and arterial thrombotic episodes was observed with therapy but the risk of stroke was not increased.79 The incidence of arterial thrombosis is reported at 1.7% for sorafenib and 1.4% for sunitinib.68 Ponatinib is associated with venous thromboembolism and is reported in up to 5% of patients. Ibrutinib shows pharmacological interactions with anticoagulants owing to its selective metabolism by CYP3A4, leading to increased plasma concentrations.60
To summarize, the main CV complications of cardiotoxic targeted therapies can be classified based on their pathway specificity.17 They include-
VEGFIs (also known as angiogenesis inhibitors: Systemic HTN, LVD and HF, QTc prolongation, and arterial thrombosis including MI.38,83–85
MKIs targeting BCR-ABL (often called BCR-ABL TKIs): Arterial thrombosis leading to MI, stroke and peripheral arterial occlusive disease (ponatinib), venous thromboembolism, systemic HTN, LVD, and HF, accelerated atherosclerosis (ponatinib and nilotinib), QTc prolongation (nilotinib) and PAH (dasatinib).86–93
Compared to other TKIs, the VEGFI have wider use in solid tumors of various types due to their broad antiangiogenic activities.94 Both VEGFR-antibodies (such as Bevacizumab and Ramucirumab) and small molecule TKIs targeting VEGFR (such as Sunitinib, and Sorafenib) have been used in clinical practice. They however could display different efficacy and toxicity profiles.95 While VEGFR-antibodies are very target-specific, and their side effects are mostly related to exaggerated pharmacological effects, small-molecule drugs such as sunitinib, sorafenib, and pazopanib are more prone to induce harmful non-target effects.50 In a meta-analysis comparing the efficacy and toxicity profile of antiangiogenic therapies in gastric cancer, VEGR-antibodies were found to be more effective in controlling cancer and showed a better patient survival rate. In addition, a lesser degree of treatment-related adverse events including HTN, proteinuria, and hand-foot syndrome was reported with VEGFR-antibodies.96 No differences were noted in terms of bleeding. Antibody development, however, is relatively more laborious and more cost prohibitory compared to the development of small molecule TKIs.
Pathophysiology and proposed mechanisms of cardiovascular side effects
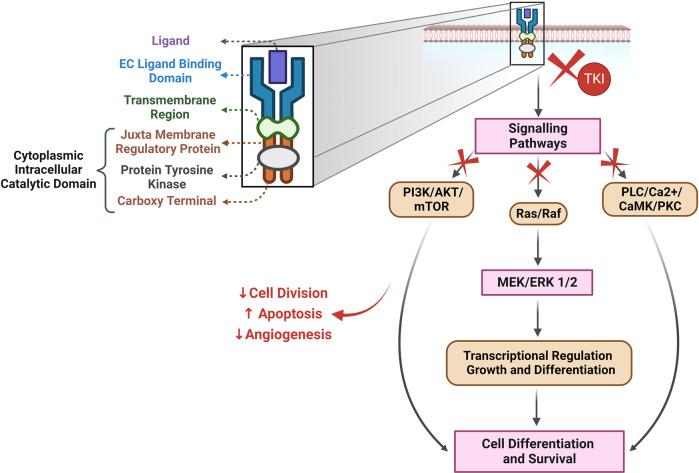
Most of the evidence on the mechanisms of TKI function and their side effects has been derived from preclinical studies. In mouse myocardial tissues, increased expression of various proteins and their transcription factors was detected after TKI therapy. These include NRG1 (paracrine factor), which along with ErbB2 activates the mitogenic pathways crucial for the maintenance of CM health and survival.97 Studies also reported JunB expression after erlotinib therapy, forkhead box transcription factor (FOXO3), and SRY- Box Transcription Factor 6 (Sox6) expression in sunitinib-treated hearts.98 Examining genome‑wide association studies (GWAS) datasets, Li et al. reported single nucleotide polymorphism (SNP) of the various HF-related genes in subjects treated with sunitinib, pazopanib, sorafenib, dasatinib, and nilotinib.99 The role of ROS is also recognized as a mechanism of endothelial and vascular smooth muscle dysfunction resulting from TKI use. ROS accumulation can lead to the production of toxic metabolites leading to unpredictable specificities of targeted tumor antigens which can induce unexpected tumor lysis and cytokine release. This can lead to CM contractile dysfunction, apoptosis, and autophagy (Fig. 1).100,101 The cardiotoxic effects are also exerted through TKI activities in cardiac fibroblasts, and endothelial and vascular smooth muscle cells, with some studies suggesting the involvement of immune cells and cardiac progenitor cells.101 Cardiac pathologies exhibit regulated cell death in the form of apoptosis, necroptosis, mitochondrial-mediated necrosis, pyroptosis, ferroptosis, and autophagic cell death.23 TKIs used in both oncologic as well as other nononcologic conditions can lead to molecular changes in the CMs leading to various pathways related to cell death.102
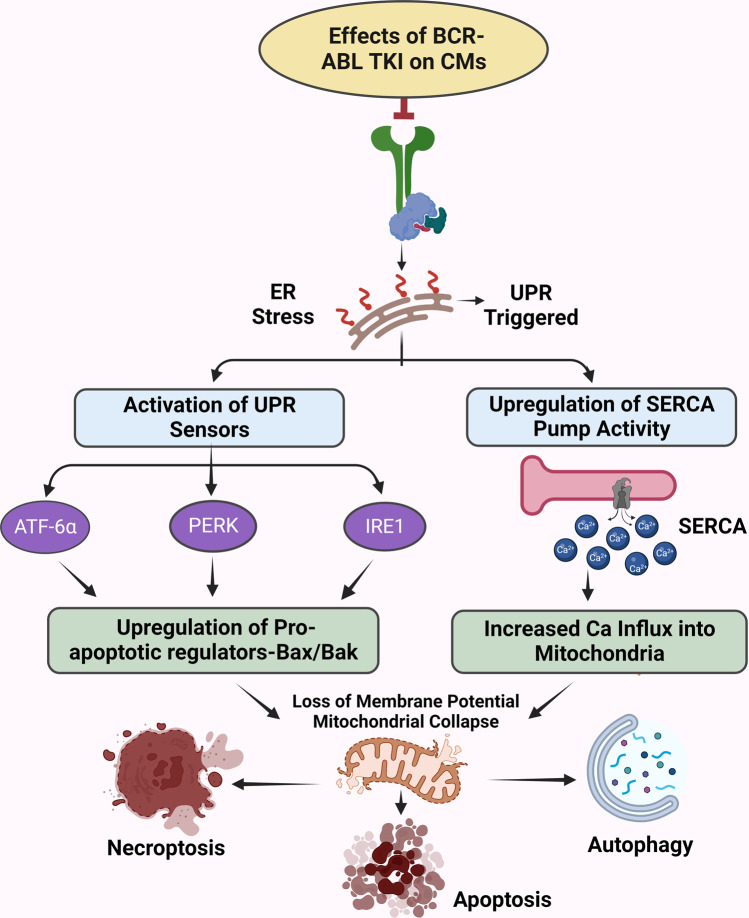
Fig. 1.
Proposed mechanisms of cardiovascular toxicity induced by on-target effects of BCR-ABL TKI. The on-target effect is defined as toxicity arising from the inhibition of the intended tyrosine kinase targets. Inhibition of BCR-ABL TK activity induces prolonged ER stress leading to stimulation of unfolded protein response (UPR) pathways. The UPR pathways constitute 3 major arms, including protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme-1 α (IRE-1), and activating transcription factor 6 α (ATF6-α) transmembrane proteins of ER. When activated, PERK phosphorylates the eIF2α factor, which in turn leads to attenuation of protein synthesis. IRE-1-mediated downstream target activation of JNK signaling promotes both apoptotic and non-apoptotic cell death. ATF6 is a basic leucine zipper transcription factor activated by translocation to the Golgi apparatus. ATF6 and ATF4 lead to the expression of CHOP, a pro-apoptotic transcription factor inducing cell death signaling. By activating pro-apoptotic proteins and inhibiting anti-apoptotic proteins, it directly activates Bax and Bak on the mitochondrial membrane. Altogether CHOP and JNK signaling produce significant mitochondrial dysfunction due to loss of MP, the release of cytochrome c, and ultimately apoptosis and necroptosis. Additionally, there is an abnormal sarco/endoplasmic reticulum Ca+2 ATPase pump (SERCA) activity in response to ER stress specifically due to calcium depletion in ER or activation of the UPR pathway. This results in increased calcium influx into mitochondria, the opening of mitochondrial permeability transition pore with a subsequent decline in ATP concentration, and markedly impaired energy generation. ATF4, Activating transcription factor 4; ATF6, Activating transcription factor 6; BAK, Bcl-2 antagonist/killer; Bax, Bcl-2-associated X protein; BCL-2, B-cell lymphoma 2; BCR-ABL, breakpoint cluster region-Abelson; BCL-2, B-cell lymphoma 2; Bid, BH3 interacting domain death agonist; Bim, Bcl-2-interacting mediator of cell death; Ca2+, Calcium; CHOP, C/EBP homologous protein; c-kit, Receptor tyrosine kinase; eIF2α, eukaryotic Initiation Factor 2α; ER, Endoplasmic Reticulum; IRE-1, Inositol-requiring transmembrane kinase/endoribonuclease; JNK, c-Jun N-terminal kinase; MLKL, Mixed lineage kinase domain-like protein; MP, Membrane Potential; PERK, protein kinase RNA like ER kinase; RIPK1, receptor-interacting serine/threonine kinase; SERCA, Sarcoendoplasmic Reticulum Calcium ATPase; SR, Sarcoplasmic Reticulum; TK, Tyrosine Kinase; TKI, Tyrosine Kinase Inhibitor; UPR, unfolded protein response. The figures were created using scientific image and illustration software, BioRender (BioRender.com)
Effects on cardiomyocyte survival signaling
Dual action of ponatinib induces inhibition of pro-survival pathways (AKT and ERK) and upregulation of pro-apoptotic pathways (Bax, Bcl-xL, and Caspase) was demonstrated by Singh et al. in zebrafish embryos. Importantly, the toxic effects were irreversible even after the drug withdrawal.103 These reactions are a result of both on-target and off-target inhibition of signaling pathways (Fig. 2).104 Sorafenib, an MKI, concurrently inhibits protein kinase ERK through inhibition of Raf-1 (in response to stressors) and disinhibits pro-apoptotic kinases leading to on-target cardiotoxicity. Dual action involving inhibition of pro-survival signaling pathways and activation of cell death signaling pathways eventually results in apoptosis, autophagy, and necrosis; necroptosis, and ferroptosis. Imatinib suppresses the transcription factor GATA4 by downregulating Bcl-2 and Bcl-xL pathways resulting in cellular apoptosis.105
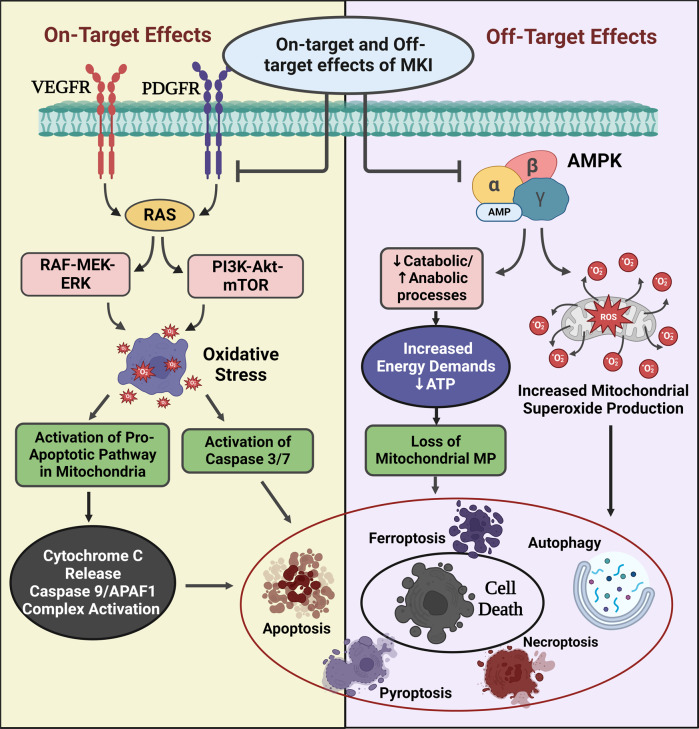
Fig. 2.
Proposed cellular mechanisms of on- and off-target effects of multi-kinase inhibitors. Off-target toxic effects are a result of the inhibition of kinases other than the intended target of the TKIs. Off-target blockage of AMPK by drugs like sunitinib leads to superoxide production in mitochondria resulting in cell death. Additionally, it inhibits catabolic processes such as glycolysis, GLUT 4 expression, and upregulation of anabolic processes such as glycogen synthesis, FA oxidation, and lipolysis which in turn leads to dysregulation of energy homeostasis in CMs along with ATP depletion and loss of membrane potential. It is also known to directly inhibit eIF2, resulting in the impairment of protein synthesis. Its on-target toxicity is mediated through the inhibition of PDGFR and VEGFR, which affects Raf-MEK-ERK and PI3K-Akt-mTOR pathways. This leads to oxidative stress and activation of pro-apoptotic pathways involving the release of Cyt c and activation of caspases and APAF1. Altogether these processes result in increased apoptosis, decreased cell survival and impaired compensatory myocardial response to stress, and ultimately cardiac contractile dysfunction. ATP, Adenosine tri-phosphate; Akt, Ak strain transforming; AMPK, AMP-activated protein kinase; APAF 1, Apoptotic protease activating factor 1; ATP, Adenosine triphosphate; CaMKII, Calcium/Calmodulin Dependent Protein Kinase 2; CM, cardiomyocytes; Cyt C, Cytochrome C; ERK, Extracellular signal-regulated kinase; eIF2, FA, Fatty Acid; GLUT, Glucose transporter; MEK, Mitogen-activated protein kinase kinase; MP, membrane potential; mTOR, mammalian target of rapamycin; P, phosphate; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol-3 kinase; Raf, rapidly accelerated fibrosarcoma; Ras, Rat sarcoma virus; ROS, reactive oxygen species; TKI, tyrosine kinase inhibitors; VEGFR, Vascular Endothelial Growth Factor Receptor. The figures were created using scientific image and illustration software, BioRender (BioRender.com)
Overexpression of VEGF was shown to be associated with increased capillary density and mitochondrial energetics.106 The inhibition of the VEGFR signaling pathway, on contrary, is associated with left ventricular hypertrophy which is a prognostic factor for CV mortality. Animal experiments also demonstrated that VEGF signaling has a significant role in normal cardiac physiology extending beyond angiogenesis.107 In clinical practice, CV events are reported to be associated with all approved anti-VEGF agents. Sunitinib and sorafenib can have a direct toxic effect on CMs, mediated either through cell damage or by inhibiting the tissue repair process.108,109
Effects on mitochondrial and sarcoplasmic reticulum homeostasis
TKIs are responsible for mitochondrial oxidative/nitrosative stress through a disproportionate production of ROS/reactive nitrogen species and increased deactivation of antioxidant enzymes, especially in CMs.40 This has been exhibited in imatinib and dasatinib by a reduction in membrane potential and complex 1 of the electron transport chain resulting in the cleavage of caspase 3.110 Kerkela et al. identified off-target inhibition of AMPK by sunitinib, affecting mitochondrial function important for maintaining CV homeostasis, particularly during increased stress conditions.111,112 Sorafenib lowers S16 phospholamban phosphorylation, leading to the reduced sarcoplasmic reticulum (SR) calcium load and delayed removal through SR Ca2+-ATPase (SERCA). These effects were reported as rapid, concentration-dependent, and reversible resulting in the net negative inotropy.113 Similar mechanisms were also demonstrated with imatinib, dasatinib, and sunitinib therapies2 (Fig. 2).
Effects on cardiomyocyte contraction and excitation
The primary mechanisms of QTc prolongation and cardiac arrhythmias associated with TKIs are postulated to be an off-target blockade of the human Ether-à-go-go-Related Gene (hERG) encoded potassium (K+) channel proteins in CMs which carries the repolarizing rapid delayed rectifier (IKr) current. This results in impaired depolarization and delay in impulse conduction.39 An alternate hypothesis suggests that protein misfolding due to specific drug activity or modification of chaperone interactions with other proteins results in the inhibition of sorting of the K+ channel proteins. This interferes with the chaperones of hERG channels leaving the ER leading to a reduction of flow through the K+ channels.57 Adverse effects are also attributed to the downregulation of PI3K/Akt pathways resulting in the regulation of various channel-forming proteins responsible for cardiac arrhythmias. Their direct inhibition activates late sodium current (INa-L), affects L-type calcium current (ICa-L), and modulates calcium cycling. This favors QT prolongation, abnormal automaticity, and early and late after depolarization. Impairment of VEGFR, PDGFR, and c-Src leads to contractile abnormalities and a fall in the heart rate.60 The arrhythmogenic effects of BTKI, ibrutinib, can be an on-target (BTK-dependent) or off-target (PI3K/AKT pathway or C-terminal SRC kinase-dependent) effect. However, because of its high specificity to the B lymphocyte receptors, off-target effects are relatively lower.55,114 On the other hand, VEGFI toxicity is a multiple-hit phenomenon leading to micro-and macro-vascular dysfunction, further potentiated by increased afterload due to HTN. It is often associated with mitochondrial ATP (adenosine triphosphate) depletion, activation of pro-apoptotic kinases, and profound vasoconstriction in some cases.26
Risk factors associated with TKI-related cardiovascular dysfunction
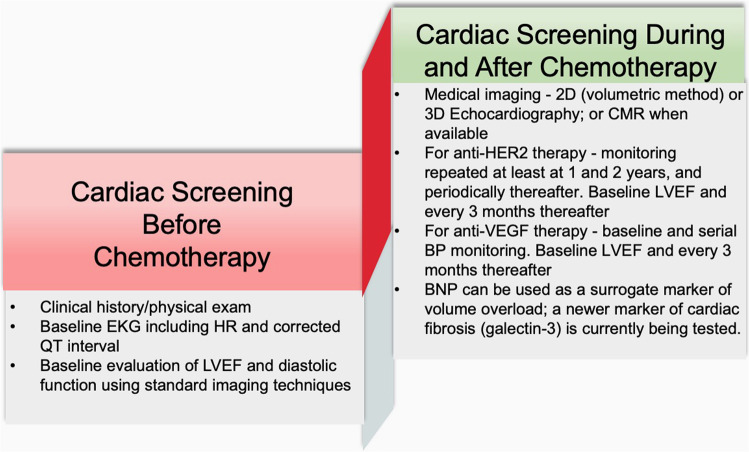
Patient-related risk factors such as CAD, advanced age, HTN, diabetes mellitus (DM), and smoking.22 Other risk factors including atherothrombotic risk can be detected through circulating biomarkers such as high-sensitivity C-reactive protein (hs-CRP), and other markers of inflammation such as interleukin-1 (IL-1), IL-6, and fibrinogen.115 Elevated cardiac biomarkers including high-sensitive troponin T (hsTnT), N-terminal pro-BNP (NT-proBNP), mid-regional pro-atrial natriuretic peptide (MR-proANP), mid-regional pro-adrenomedullin (MR-proADM), and C-Terminal pro-endothelin-1 are also considered as the risk predictors.116,117 Elevation of these biomarkers in cancer patients, before the initiation of cardiotoxic chemotherapy, is a higher risk of all-cause mortality.117 A schematic representation of cardiotoxicity screening before, during, and after cancer therapy is listed in Fig. 3.
Fig. 3.
Schematic representation of cardiotoxicity screening before, during, and after cancer therapy. Specific screening criteria based on the types the therapies are proposed. CMR, cardiac MRI; and HER, human epidermal growth factor receptor
Therapy-related risk factors include high-dose therapy, administration as a bolus or in combination with other drugs, prior anthracycline use, mediastinal radiation with the heart as a target, and concomitant use of specific agents such as anthracyclines, trastuzumab, and cyclophosphamide are linked to increased risk.118 The presence of concomitant HTN before the initiation of therapy is associated with an increased risk of cancer therapy-induced LVD. However, there is no evidence to suggest that the prior HTN treatment reduces risk when compared to other comorbidities such as DM and chronic kidney disease (CKD).66 On the contrary, a large retrospective study showed no correlation between TKI-induced cardiotoxicity and CV risk factors suggesting a possibility of genetic predisposition to TRCD in some cases.119 CV diseases, chemotherapy-induced cardiotoxicity, and cancer are more common in post-menopausal women (associated with metabolic changes, oxidative stress, and subclinical inflammation). Herrmann et al. proposed an objective Cardiotoxicity Risk score (CRS) which estimates the risk by considering both patient and therapy-related risk factors. Considering such risk predictive algorithms, ponatinib, sunitinib, and sorafenib are moderate-risk; whereas imatinib, lapatinib, and dasatinib are low-risk.22
Schema for the clinical management of TRCD
Early detection and prompt therapy for cardiotoxicity provide opportunities to prevent irreversible cardiac damage. Early cardiotoxicity is often asymptomatic or subclinical with no structural damage present and can be reversible. The late-stage cardiomyopathy results from irreversible structural changes and patients are often symptomatic.100 The most recent American and European guidelines (American Society of Clinical Oncology-ASCO-2017 and ASCO-2018), European Society for Medical Oncology (ESMO-2017, ESMO-2020), and European Society of Cardiology (ESC-2016) were analyzed by a cardio-oncology expert panel from the French Working Group of Cardio-Oncology. They have put forth cardio-oncological evaluation protocols for the proper screening and management of patients exposed to cardiotoxic agents.28
Pre-treatment assessment and prevention
The key principles include screening and management of pre-existing CV risk factors (Age > 60 years, CAD or MI, AF, HF, tobacco use, hyperlipidemia, HTN, DM, and obesity).28,55,68 Screening before anticancer therapy including baseline risk assessment is imperative in all patients before the initiation of therapy, focusing on early pre-clinical detection of cardiotoxicity.119 This may significantly reduce the possibility of their occurrence during treatment and helps in identifying patients who would benefit from cardioprotective therapy and adjustment of therapy before irreversible injury occurs.28,119 BP measurement, baseline electrocardiogram (ECG), serum fasting lipid profile, HbA1C, glomerular filtration rate (GFR) calculation, and cardiac biomarkers should be considered.28,55,120
The HF status and its underlying causes (including CAD and valvular heart disease) should be evaluated as part of baseline screening. In the case of CAD, provocative testing is recommended to rule out any residual disease.121 The severity of valvular diseases should be assessed to determine the requirement of correction before the initiation of cancer therapy. In the case of heart failure with reduced ejection fraction (HFrEF) and left bundle branch block (LBBB), cardiac resynchronization therapy (CRT) should be considered.122 The novel CV risk stratification model which was proposed by the Cardio-Oncology Study Group of the Heart Failure Association (HFA) and ESC in collaboration with the International Cardio‐Oncology Society (ICOS) was used to stratify the CV risk. In studies with CML patients, the HFA-ICOS baseline stratification proforma has proven to be more sensitive than the older Systematic Coronary Risk Evaluation (SCORE) charts.123
Cardiac functional assessment
In patients treated with targeted therapies, a sustained low normal left ventricular ejection fraction (LVEF) is considered to be of high risk.45,119 LVEF of 53% was considered a key metric to discern normal function vs. cardiac dysfunction, as proposed by the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI).124 Global longitudinal strain (GLS), a parameter of myocardial deformation, is also used as an early cardiac imaging parameter to assess TRCD. A GLS below 18% or more than 15% drop from the lower limit of normal (LLN) is considered abnormal.27 Recommendations from the ESC, ASE, EACVI, and ESMO state that a reduction of LVEF by more than 10% from baseline to a value below the LLN, or more than 15% relative reduction in GLS from the baseline suggests LVD.125 Tissue Doppler imaging (TDI) is also used to evaluate diastolic dysfunction with indexes such as septal and lateral-mitral annular e’ velocity and average E/e’ ratio used for prognosis.126
The general principles of cardiac imaging related to cardiotoxicity include BP measurement during echocardiography (ECHO), a combination of clinical and biomarkers with imaging, measurement of 2-dimensional (2D) (modified biplane Simpson’s technique) or three-dimensional (3D) LVEF, LV volumes and GLS and cardiovascular magnetic resonance (CMR) in some instances.27,55 Currently, techniques such as 3D ECHO and 3D speckle tracking ECHO are used depending on the prior CV history and planned therapies with single or combined cardiotoxic agents.100 CMR imaging is an emerging advanced cardiac imaging tool considered a gold standard to assess changes in ventricular volumes and EF is suggested in cases of substandard ECHO images if a discrepancy between LV function measurements exists or in patients with pre-existing complex heart diseases or if ischemic myocardial perfusion assessment is simultaneously planned.27,55 It provides better spatial resolution compared to 2D which is highly accurate and reproducible.55 It also offers helpful information regarding the presence of prior MI scar, diffuse fibrosis, and intercellular or interstitial edema during cancer treatment. ASE/EACVI guidelines require CMR if LVEF is close to 53% or the ECHO image is of poor quality.127
Role of biomarkers
Cardiac troponin I and brain natriuretic peptide (BNP) can be used to detect myocardial injury and elevation in LV filling pressure and wall stress, respectively.119,128 While no specific biomarkers are identified with TRCD, a rise in troponins is often reported. A rise in troponin occurs early, within 12 hours in 53% of patients and a rise within ≈3 days of high-dose chemotherapeutic drugs is highly predictive of cardiotoxicity.55,119 Therefore, it is important to have the baseline biomarkers profile followed by sequential measurements at the appropriate intervals. According to ESC and American College of Cardiology (ACC) guidelines, the measurement of B-type NP (BNP) and N-terminal pro-BNP (NT-proBNP)28,68 is an HF criterion and plays an important role in monitoring for chemotherapy-related HF.129 Novel Biomarkers such as C-reactive protein (CRP), IL-6, myeloperoxidase (MPO) for inflammation; Plasminogen activator inhibitor (PAI), tissue-type plasminogen activators (tPA), and soluble intercellular adhesion molecule (ICAM) for endothelial dysfunction, glycogen phosphorylase BB, and NRG-1 for myocardial ischemia; circulating microRNAs, galectin-3 for myocardial remodeling and development of cardiac fibrosis; and sST2 for cardiac remodeling have also been reported.130–132
To conclude, the decision to treat patients with TKI therapy is based on a multitude of factors including the urgency of cancer care, the availability of alternative agents, and the level of risks. HFA of the ESC Cardio-Oncology Study Group in collaboration with the ICOS had developed a baseline CV risk assessment proformas for seven cardiotoxic cancer therapy classes known to cause a range of CV toxicities.28 Separate proformas are used for these seven drug classes including Anthracycline chemotherapy, HER2 targeted therapies, VEGFIs, MKIs for CML targeting BCR-ABL, MKIs for CML targeting BCR-ABL, Proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs), Combination RAF and MEK inhibitor treatment, Androgen deprivation therapies (ADT), Immune checkpoint inhibitors. Completion of the baseline CV risk assessment proformas in all patients scheduled to receive one of the seven oncology drug classes with potential cardiotoxicity is recommended. Low-risk level cancer patients continue with treatment with CV surveillance as appropriate according to local, national, and international guidelines. Medium-risk cancer patients require closer monitoring of CV health during treatment or consideration for referral for a cardio-oncology or cardiology assessment. High and very high-risk level patients are referred for a cardio-oncology or cardiology assessment, ideally in a specialist cardio-oncology service (if available) to optimize the management of their pre-existing CV disease and modifiable CV risk factors and provide a personalized management plan for surveillance during cancer treatment.17
Primary prevention in high-risk patients
Strategies to reduce cardiotoxicity risk include encouraging a healthy lifestyle and identification and management of risk factors.119 Secondary causes of HTN, including untreated obstructive sleep apnea (OSA) should be considered.55 Other considerations include limiting or using lower doses of anti-cancer drugs when feasible. Cardioprotective drugs include dexrazoxane, beta-blockers (BB) (prevent LVEF reduction and decrease the incidence of HF), angiotensin-converting enzyme inhibitor (ACEI) (prevent LVEF deterioration), and combination therapies (shows no reduction in LVEF in 6 months).119 BP of below 140/90 mmHg is advocated for all patients; 130/80 mmHg in case of CKD or DM.32 Chang et al. suggested that cardio-protection should be considered in patients with EF < 50% or a drop of > 10%, GLS > 15% drop, and elevated cardiac biomarkers.49 All patients exposed to cardiotoxins should be treated as American Heart Association (AHA) HF stage A-at risk for HF but without structural heart disease.22 ACEI/angiotensin receptor blockers (ARBs) and BB (newer generation such as carvedilol and nebivolol) combination therapy are recommended in cancer patients with an LVEF of <40% and also in patients with an asymptomatic decline in LVEF ( < 10% decrease from baseline or <53%) by the Canadian Cardiovascular Society.40,128
Baseline BP should always be measured before the initiation of TKI therapy, and every week thereafter for the first 8 weeks and before any infusion or treatment cycle.133 In the presence of cardiac risk factors (pre-existing CV disease, CKD, DM, age ≥ 75, Systolic BP ≥ 130 mmHg), antihypertensive medications should be started before chemotherapy.32,57 The BP ideally should be maintained below 140/90 mmHg but in the case of CKD, it should be <135/85 mmHg.133 Both HTN and ibrutinib are independent risk factors of AF, therefore the ideal BP to initiate antihypertensives is ≥140/90 mmHg.57 Spironolactone, a mineralocorticoid antagonist with the potential to reduce fibrosis in HF can also play a role in cardioprotection.128 Rao et al. recommended a baseline fasting lipid profile and prophylactic statin and aspirin therapy in BCR-ABL TKIs which have an increased risk of atherosclerosis.55
In patients with drug-induced AF, thromboembolic events can be controlled by using LMWH (Low Molecular Weight Heparin) for the short-term, and warfarin and DOACs (Direct Oral Anticoagulants) can be utilized for long-term therapy.120 HF has an increased incidence of venous thromboembolism. DOACs are beneficial in these conditions showing efficacy, and safety compared to warfarin. Since the long-term safety of DOACs is still under investigation, LMWH remains the preferred option for prophylaxis.122 A few cancer types are associated with bleeding themselves and the agent used should be weighed against the risk associated, and the presence of drug-drug interactions carefully evaluated.120
Cardiac surveillance in patients receiving TKIs
There is no standardized protocol currently available regarding surveillance.45 In the case of VEGFI and BCR-ABL TKI-treated patients, an ECHO should be repeated at least every 4 months for the first year. However, in high-risk patients, an early assessment is done 2 to 4 weeks after the initiation of drug therapy. In patients undergoing long-term therapy who remain asymptomatic during the first year, an ECHO can be done every 6–12 months thereafter.27,120 BCR-ABL TKIs are primarily metabolized by CYP3A4 and their levels are increased when administered concomitantly with inhibitors of CYP3A4 such as diltiazem and verapamil.134 Peripheral atherosclerosis is increasingly associated with BCR-ABL TKIs such as nilotinib and ponatinib.135 Ankle Brachial Index (ABI) is highly sensitive and specific and a value of <0.9 is specific for peripheral arterial disease.136 ABI (or duplex ultrasonography) is assessed every 6–12 months as per the European LeukemiaNet recommendations.137
The drugs used for the chemotherapy regimen should be selected based on their known effects on pre-existing CV disease. If a cardiotoxic drug is required, effective management of the pre-existing comorbidities is essential.115 For example, the interrupted HER2 targeted can be resumed, once the LVEF improves.28 However, it is usually avoided when LVEF is less than 40% unless no effective alternative treatment exists.120 It is important to note that the toxicity due to HER2 TKI therapy is often irreversible.114 During therapy, asymptomatic low-risk patients require no follow-up, however medium- to high-risk patients require CV assessment after the last dose, at 3–6 months, and then at 12 months. In patients with new symptoms of LVD, the safety of continuing therapy should be evaluated. During the long-term therapy in asymptomatic metastatic diseases, a 3-month follow-up during the first year is recommended. Thereafter, if the cardiac functional parameters are within the normal range (low risk) less frequent follow-up is recommended.27
For cardiac surveillance, the screening test of choice is a transthoracic echocardiogram (TTE). Other modalities include ventilation-perfusion (VQ) scan to study pulmonary embolism and right heart catheterization (RHC) to establish the presence of PAH (increase in the mean pulmonary artery pressure ≥ 25 mmHg at rest and pulmonary capillary wedge pressure < 15 mmHg).58,115 RV function and estimation of pulmonary pressure using ECHO with spectral Doppler screening are required to establish the pre-treatment baseline, detect pre-existing PAH, and if cardiac symptoms develop, maintain a low threshold for repeat ECHO. EACVI suggests that routine RV-free wall strain measurement is more characteristic of RV longitudinal deformation than septal strain. Currently, the estimation of RV ejection fraction is possible by both CMR and 3D ECHO.138,139 Routine monitoring for right HF and PAH is recommended only in patients with cardiopulmonary symptoms before the initiation of therapy, and every 3 months thereafter as per ESC.115 TKI-induced PAH can be fatal and therefore early screening and prompt management are required.74
The German consensus guideline recommends ECHO after a month of starting BRAF and MEK inhibitors, and thereafter every 3 months during therapy. Treatment can be withdrawn if LVEF is below 40% or when there is an incremental decrease of >10% in the setting of LVEF of 40–49%.45 In ibrutinib-treated patients, in addition to the baseline evaluation, they require further monitoring every 3 months in the first year. This is advantageous since most of the rhythm disorders associated with the drug therapy occur with the peak incidence within the first 30 days. AF, HF, and ventricular arrhythmias are diagnosed within the first 2–3 months. Additionally, drug-induced HTN is established within the first 4–5 months of therapy, and most of the abnormal echocardiographic findings are noted during the first year.140 If patients are symptomatic, Holter monitoring along with 12-lead ECG is recommended.120 Diagnosis of deep vein thrombosis (DVT) is made by compression ultrasound and pulmonary embolism is by spiral CT (computed tomography) angiography (CTA). VQ scan has a low sensitivity and specificity. When allergic to iodinated contrast, magnetic resonance (MR) pulmonary angiography is employed.58 D-dimer levels are associated with poor prognosis in the setting of cancer, independent of venous thromboembolism. They can be used to rule out venous thromboembolism, but not for diagnosis. The proposed optimum cut-off is between 981–1,500 ng/mL but these values are not externally validated.129,141
Management of TKI-induced hypertension and cardiac dysfunction
Regular monitoring for HF during chemotherapy helps early detection of cardiotoxicity either leading to the continuation of cardioprotective measures or permanently discontinuing chemotherapy.122 The presence of HF is not always a plausible reason for the interruption of chemotherapy.120 The benefit versus the potential ill effects of the continuation of therapy should always be considered.55 Cancer drugs can be continued in the absence of a hypertensive emergency or HTN-mediated end-organ damage along with prompt initiation of HTN management and optimization.142 The goal is to maintain BP < 140/90 mmHg in uncomplicated HTN and <140/85 mmHg in patients with DM and renal failure.119 Most of these patients can be managed by dose modifications and normal anti-hypertensive medications (ACEI, ARBs, BBs, amlodipine, and aldosterone antagonists).119,133 Some studies suggested that the presence of HTN serves as a biomarker for treatment efficacy.28,133 Renal findings including proteinuria >1 g/d, hematuria, or acute renal failure will require a nephrology referral. Cancer treatment can be safely resumed once the BP management is within the expected goal.120
ACEI and BB are the first-line treatment in HF or LVD with HTN, especially with VEGFI.68,120 Non-dihydropyridine calcium channel blockers (DHP CCBs) are avoided owing to drug-drug interactions.120 It is suggested that greater efficacy and lower adverse effects are seen with lower-dose antihypertensive combination therapy.55 HTN in the setting of VEGFI can be a sign of its efficacy and should not dictate mandatory discontinuation.143 It should be interrupted and evaluated to see if resumption is appropriate.28 Treatment of HTN has significant beneficial effects on major adverse cardiovascular events (MACE), such as CAD, HF, stroke, end-stage renal failure, and overall mortality.66 HTN can develop from the initiation of therapy until one year after therapy, especially in sunitinib.68 TKI therapy can be continued as long as there is no severe HTN. In case of proteinuria, ACEI, ARBs, or DHP CCBs can be given.133 Non-DHP CCBs are contraindicated with oral angiogenesis inhibitors since these agents inhibit CYP3A4 resulting in increased VEGFI levels.133
Management of arrhythmias
Caution is advised when combining antiarrhythmic agents with cancer therapy owing to drug-drug interactions and QTc prolongation.120 ECG is recommended at baseline, 7 days after initiation, after every treatment cycle, after initiation of new medication, when plasma concentration reaches a steady state, after any dose adjustments, or in the event of electrolyte imbalance.137,144 In patients with QTc prolongation, correctable causes like electrolyte abnormalities (hypokalemia, hypomagnesemia, hypocalcemia) should be identified and corrected (Magnesium > 2.0 mEq/l, Potassium at > 4.0 mmol/l) before starting chemotherapy.55,144 In general, cessation of cancer therapy due to any cardiotoxicity should be considered only if all other alternatives have been exhausted.49 BTKIs, apart from AF, possess an antiplatelet effect leading to increased bleeding risk; therefore, caution should be exercised when combining ibrutinib with antithrombotic agents.145 The majority of cases can be managed without the interruption of therapy. Alternatives include more selective agents (acalabrutinib, zanubrutinib) and venetoclax-based strategies.57
Management of pleural and pericardial effusion
The reduction of risk of pleural and pericardial effusion can be achieved by dose reduction of drugs such as nilotinib, ponatinib, or FLT3 inhibitors or using a single dose of dasatinib in a day.60 When effusion occurs, apart from discontinuing dasatinib, diuretics or a short course of steroids can be helpful for reversal.146 Emergency pericardiocentesis is required in response to a large pericardial effusion (≥2 cm) of hemodynamic significance or less emergent for the diagnosis. Surgery is required to create a pericardial window for recurrent pericarditis.58
Management of pulmonary arterial hypertension
In the case of PAH, permanent discontinuation of dasatinib is necessary.146
Management of thromboembolism
The previous history of arterial thromboembolism is not an absolute contraindication for VEGFI but if an event occurred within the last 6–12 months, caution is warranted. Discontinuation of therapy is only recommended in grade 3 or higher thromboembolic events. Therapy can be restarted once the symptoms resolve.147 Antiplatelet and anticoagulant drugs can be given with VEGFI despite the increased bleeding risk. Other drugs include aspirin (prophylaxis in high-risk patients), LMWH, or unfractionated heparin (UFH). When venous thromboembolism develops on chemotherapy, the therapy should be stopped and anticoagulants started immediately. Thrombolytic therapy can be added if necessary. Cancer therapy is restarted once stabilization and anticoagulation are achieved.58
Post-treatment management
Cancer survivors require long-term care for therapy-induced CV comorbidities. Management of LVD is carried out in line with AHA/ACC HF guidelines and individualized according to the patient’s risk factors.148 In a large prospective cohort study involving non-metastatic breast cancer patients, a strong inverse relationship was reported between exercise intensity and CV conditions such as CAD and HF.128 A routine exercise with a goal of ≥ 9 MET-hour per week showed a reduction in CV morbidities in a prospective study involving 2,973 non-metastatic breast cancer patients. Exercise has been recommended for patients in cardiac rehabilitation with an increased risk of cardiotoxicity by AHA and all survivors of cancer by ESMO.149
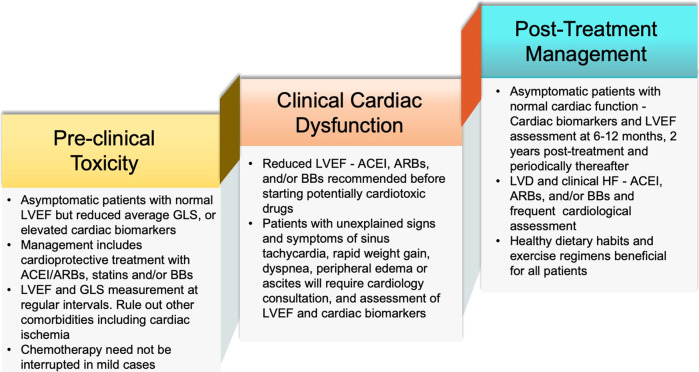
Long-term surveillance is recommended for patients with pre-existing conditions and also to assess the risk for late cardiotoxicity.150,151 In HER2-targeted therapy, assessment should be carried out right after treatment and at a 3-month follow-up. CV evaluation including detailed clinical history and physical examination should be undertaken.152 When cardiotoxicity is suspected, further testing in the form of imaging [ECHO or cardiac magnetic resonance imaging (MRI)] is recommended. If asymptomatic, further testing is unnecessary.128 An outline for the management of cardiotoxicity is depicted in Fig. 4.
Fig. 4.
Management of cardiotoxicity based on the clinical manifestations and the severity of the cardiotoxicity. It is recommended to utilize identical imaging modalities, biomarkers, and other laboratory parameters during different follow-up stages for comparability. Reduction in GLS is considered significant when there is a ≥ 12% relative decrease or ≥ 5% absolute decrease. 2D- 2-dimensional; 3D, 3-dimensional; ACEI, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blockers; BB, Beta Blockers; CMR, Cardiac Magnetic Resonance imaging; GLS, Global Longitudinal Strain; HER, human epidermal growth factor receptor; IHD, Ischemic Heart Disease; PAD, Peripheral Artery Disease; and VEGF, Vascular Endothelial Growth Factor
Gastrointestinal adverse reactions
Gastrointestinal (GI) symptoms are frequently reported in cancer patients treated with TKIs.153,154 Mild diarrhea with a grade of 1–2 CTCAE (Common Terminology Criteria for Adverse Events) and colitis were identified as the most common occurrences.155 Other symptoms include nausea, vomiting, stomatitis, mucositis, dysgeusia, dyspepsia, anorexia, constipation, abdominal discomfort, and weight loss (Table 1).156 These symptoms were considered non-specific due to their common occurrence in most of the chemotherapeutic drugs utilized in the treatment of solid organ, hematologic and endocrine malignancies.155 The majority of these side effects were rapid-onset, mild, and often self-limiting. They persisted for a short duration and were mostly TKI dose-related.157 Importantly, high rates of incidence and severity, but reduced rates of recurrence of GI side-effects were noted in solid organ cancers such as genitourinary and lung compared to hematologic malignancies.158 This explains the rationale behind frequent dose reductions of TKIs in solid organ cancers with better tolerance in hematologic cancers. Other more severe manifestations such as colonic perforation and severe and life-threatening diarrhea and colitis were reported in some cases.159–162
Mechanisms of GI side effects
The potential mechanisms are widely debated, though many theories have been proposed. Diarrhea may be a consequence of EGFR-related inhibition of epithelial growth and limited healing of the GI mucosa lining.163 Other mechanisms including direct toxic effects on mucosal cells (Fig. 5) and increased GI inflammation have also been implicated.164,165 Endoscopic luminal appearance and inflammatory findings on histology are attributed to the direct toxicity but these mechanisms are yet to be confirmed.166
Fig. 5.
Receptor tyrosine kinase and its downstream pathways affected by tyrosine kinase inhibitors: Upon binding with the ligand the tyrosine kinase receptor gets activated resulting in the activation of multiple pathways, which are critical in cell survival, growth, and differentiation. Aberrant activation of these tyrosine kinase pathways is implicated in carcinogenesis. These effects are blunted by tyrosine kinase inhibitors (TKIs), which not only affect tumor growth but also hinder the function of normal cells in various organs, including cardiomyocytes in the heart. EC, extracellular; Akt, Ak strain transforming /protein kinase B; RAF, rapidly accelerate fibrosarcoma; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; Ca2+, Calcium; CAMK, calmodulin-dependent protein kinase; CM, cardiomyocyte; EGFR, epidermal growth factor receptor; ERK, extracellular-signal-regulated kinase; MEK, Mitogen-activated protein kinase kinase; PKC, Protein kinase C. The figures were created using scientific image and illustration software, BioRender (BioRender.com)
BCR-ABL TKIs and BTKIs exhibit GI toxicity during the initial months of therapy, or after any change in the therapeutic algorithm.167,168 Stomatitis is frequently associated with fibroblast growth factor receptor (FGFR) TKIs with the inflammatory lesions reported as well-defined and painful.169 About 12% of patients treated with regorafenib to 58% treated with erdafitinib reported stomatitis which was self-limiting but painful, affecting the quality of life.170 Bosutinib is reportedly associated with diarrhea.171 The ENESTnd and DASISION trials in CML patients reported a higher GI toxicity profile associated with imatinib compared to nilotinib and dasatinib.172 Ponatinib, used after treatment failure with imatinib, was associated with abdominal pain and constipation.173 As part of phase III clinical trial, all-grade diarrhea was increasingly associated with second-generation irreversible EGFR TKIs compared to reversible inhibitors.174 In studies involving VEGFI, sorafenib, regorafenib, lenvatinib, and cabozantinib, rates of nausea and vomiting were higher.175 Furthermore, drug combinations such as BRAF and MEK inhibitors reported more frequent episodes of nausea, vomiting, and diarrhea than alone.176
Clinical management
Metoclopramide, levosulpiride, or ondansetron can be used as antiemetics. However, caution should be exercised in the case of ondansetron given its interactions with TKIs and the additive effect on QTc prolongation.175 Prophylaxis of stomatitis/mucositis involves maintaining good oral hygiene, non-alcoholic mouthwashes, and consumption of non-irritating food to avert stomatitis. Guidelines for fungal, viral, and/or bacterial prophylaxis with topical or systemic antimicrobials. Grade 2/3 stomatitis warrants dose reduction or drug cessation with no interventions needed for grade 1 stomatitis. Diarrhea can be managed with dietary modifications including a low-fat, low-fiber diet. Any underlying infection or other causes of diarrhea should be further examined.175 Fluid status should be monitored and adequate hydration maintained.177 Diarrhea usually resolves with supportive treatment, and immunosuppressive medications or antibiotics are usually not needed.178 Severe or persistent episodes, though uncommon, necessitate medication switches or discontinuation.173 In patients treated with erlotinib and afatinib, dose reductions hasten treatment, but not with gefitinib.100 Severe manifestations, such as GI perforation, warrant immediate drug cessation.154
Hepatotoxicity
TKIs are associated with frequent elevations of hepatic enzymes, mainly aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT) during the initial months of treatment.179 The highest levels of elevated hepatic enzymes were reported in nilotinib, bosutinib, and ponatinib.180 Nilotinib is also known to cause bilirubin elevations. In general, dose reduction is not necessary since modest hyperbilirubinemia does not constitute toxicity (Table 1).181 Imatinib and ponatinib are associated with late-onset hepatotoxicity and rare cases of hepatic failure.182 Severe cases have been reported when imatinib is concurrently taken with acetaminophen.173 Studies with EGFR TKIs, particularly gefitinib, and erlotinib can cause transient elevations of enzyme levels without clinical manifestations. Toxicity is more prominent with specific EGFR mutations in patients of Asian descent.166 Among ALK TKIs, various clinical trials provided evidence of hepatotoxicity with crizotinib and alectinib, with the higher grades seen in ceritinib and brigatinib (Table 1).183 Similarly, ibrutinib and acalabrutinib were more toxic than zanubrutinib, necessitating dose reductions or avoidance.184 The association of pazopanib with polymorphism of the UGT1A1 gene in patients with Gilbert’s syndrome was reported by Xu et. al. The resultant hyperbilirubinemia was reported as a benign manifestation requiring no interventions.185 Additionally, pazopanib is highly protein bound and requires dose adjustment in hypoalbuminemia.186
Most cases of TKI-induced liver dysfunctions are asymptomatic and require no treatment.166 Baseline liver functions need to be evaluated before the initiation of therapy. During treatment, regular monitoring of hepatic enzymes is needed- biweekly in the first 2 months and every 1–2 months thereafter.187 Any major changes (e.g. grade 3 CTCAE) in the levels of the enzymes require dose modifications with reconstitution of regular doses once the values return to grade 1 or baseline levels.166 Severe cases of TKI-induced hepatitis require drug substitution.173 Caution should be exerted in cases of long treatment duration and concurrent use of other hepatotoxic medications.166
Pancreatic Dysfunction
Acute pancreatitis was observed to a lower extent in TKI-treated patients (ranging from 0 to 4.3%). Increased levels of pancreatic enzymes including serum amylase, and/or lipase levels are reported, which are mostly asymptomatic.188 TKIs exert dose-dependent pancreatic toxicity mostly during the initial months of therapy.173 The enzyme levels were highest with sorafenib and lowest with nilotinib.188 However, in the ENESTnd trial, both imatinib and nilotinib were identified to have very high incidence.87
The mechanisms of pancreatic enzyme elevation after sorafenib therapy are unclear. VEGF inhibition results in pancreatic tissue ischemia and acute pancreatitis may ensue.189,190 In the case of nilotinib, its high-affinity inhibition of non-receptor TK c-Abl proteins can affect multiple signaling pathways regulating pancreatic cell death. Mechanisms affecting the calcium release and increased accumulation of fatty acid inside the pancreatic acinar cells have also been proposed. It has been documented that monitoring pancreatic enzymes is important during TKI therapy, especially with sorafenib and ponatinib.191 Immediate drug cessation is required for grade 3–4 enzyme elevations. On resolution, treatment can be restarted albeit at a lower dose. Switching the drug is necessary for recurrent pancreatitis.173
Dermatological adverse reactions
TKIs are known to be associated with a wide range of mild to moderate skin reactions in more than 70% of patients (with severe reactions in 2–16% of patients) localized to the head and trunk.166 Side effects include rash, dry skin, pruritus, and inflammation of nail/periungual tissues as seen specifically in EGFR TKIs and BCR-ABL TKIs.15,100 Common cutaneous reactions to EGFR TKIs are known as the PRIDE syndrome (papulopustular and/ or paronychia, regulatory abnormalities of hair growth, itching, and dryness due to the EGFR TKIs).192 Toxicity is dose-related and for the most part, self-limited.173 The highest incidence of skin lesions is seen in sorafenib and the lowest in sunitinib and vandetanib.193 Perifollicular, hyperkeratotic, and erythematous maculopapular eruptions are the most frequent lesions with nilotinib. Skin rashes were frequently reported with imatinib, bosutinib, ponatinib, and cabozantinib (Table 4).194 General management involves supportive care with emollients and antihistamines with or without steroids. Severe cases require a change of drug dosage or alternative TKIs.173 Treatment regimen with TKIs should be continued in cases of grade 1 and 2 adverse events and discontinued for grade 3 adverse events. When the reactions improve to grade ≤ 1, a reduced dose is again recommended.170
Table 4.
Various Dermatological Lesions Associated with TKIs
| Tyrosine Kinase Inhibitors | Associated dermatological lesions |
|---|---|
| Imatinib |
Maculopapular eruptions along with pigmentary changes, Photosensitization, Superficial edema affecting the periorbital area (epiphora, chemosis, and conjunctivochalasis), Urticarial, Lichenoid, and Psoriasiform eruptions, Exacerbations of the existing Psoriasis, Hypo-/Hyperpigmentation, Neutrophilic eccrine hidradenitis, and neutrophilic panniculitis, Mycosis fungoides like reaction, Follicular mucinosis, Malpighian epithelium, Porphyria Cutanea trade, and Pseudoporphyria, Exanthematous pustulosis, Sweet Syndrome, Panniculitis, SJS Rare cases - Small vessel vasculitis, Erythema nodosum, and GVHD of skin |
| Nilotinib | Perifollicular, Hyperkeratotic, and Erythematous Maculopapular Eruptions, Mild to moderate pruritus, Skin xerosis, Alopecia, Body hair loss, and Bullous Sweet syndrome |
| Bosutinib | Erythema, Maculopapular Eruption, Pruritic Rash, Allergic Dermatitis, Acne, Folliculitis, and Skin Exfoliation |
| Ponatinib |
Erythematous and papular rashes Skin xerosis |
| Dasatinib | Macular, Papular or Exfoliative rash, Pruritus, Localized and generalized erythema, Papular Eruptions, Exfoliative rash, Pruritus, Hyperhidrosis, Alopecia, Xerosis, Acne, Urticaria, Dermatitis, Photosensitivity, Nail Disorders, and Pigmentary Changes, Skin Ulcers, Palmoplantar Erythrodysesthesia Syndrome. Rare Cases - Panniculitis, Acute Febrile Neutrophilic Dermatosis, And Bullous Disorders |
| Sorafenib | Stomatitis, Seborrheic dermatitis-like facial and scalp erythema, Subungual splinter hemorrhages, Periungual erythema, EM and EM-like eruptions, SJS, Eruptive melanocytic nevi and Drug-induced lentigines, Pyoderma gangrenosum, Generalized keratosis pilaris like eruption, Epidermal cysts, Nipple hyperkeratosis and/ or dysesthesia or dyskeratotic plaque with milia, New squamous cell carcinomas, Keratoacanthomas, and Inflammation of Preexisting Actinic Keratosis |
| Sunitinib |
All grade hand-foot skin reactions, Seborrheic dermatitis-like facial and scalp erythema, Skin discoloration, Hair depigmentation, Facial edema, Transient scalp dysesthesia, Yellow skin pigmentation of the face Dry skin, Dermatitis, Stomatitis, Alopecia, Subungual splinter hemorrhages Phototoxicity, Pyoderma gangrenosum, Generalized keratosis pilaris-like eruption, Epidermal cysts, Nipple hyperkeratosis and/ or dysesthesia or Dyskeratotic plaque with milia |
| Erlotinib | Acneiform rash, Xeroderma, Pruritus, Paronychia |
| Cabozantinib | Hand-foot skin reaction, Generalized pigment dilution and/or Hair depigmentation, Xerosis, Scrotal erythema/ulceration, Subungual splinter hemorrhages |
| Vemurafenib | Dose-dependent papulopustular rash, Photosensitivity, Xerosis, Pruritus, Paronychia, Alopecia and hair follicle alterations, Hyperkeratosis, Pyogenic granulomas, Verrucous keratosis, Acantholytic dermatosis, Seborrheic keratosis, Verruca vulgaris, Hypertrophic actinic keratosis, Cutaneous squamous cell carcinoma and/ or Keratoacanthoma |
| Dabrafenib | Dose-dependent papulopustular rash, Xerosis, Pruritus, Paronychia, Alopecia and Hair follicle alterations, Hyperkeratosis, Pyogenic granulomas Verruca vulgaris, Hypertrophic actinic keratosis, Cutaneous squamous cell carcinoma and/ or Keratoacanthoma |
| Trametinib | Acneiform rash, Pruritus, Xerosis. Squamous cell carcinoma |
Rash
Rash is one of the commonest cutaneous manifestations of TKIs. Cutaneous manifestations resulting from EGFR inhibition affect multiple molecular pathways involved in cell growth and differentiation (Fig. 5 and Fig. 2) resulting in growth arrest, decreased migration, abnormal differentiation, and stimulation of inflammatory systems.195 Erlotinib is known to possess off-target inhibition of TK proteins and also explains the difference in the severity of lesions compared to gefitinib.196 Imatinib usually causes an erythematous, macular-papular rash a few days after initiation of treatment mediated through direct pharmacologic toxicity and is usually self-limited.197 Additionally, inhibition of KIT, VEGFR, and PDGFR may play a similar role.193 Use of emollients, and in case of infection, topical antibiotics for 2 weeks are recommended. Topical corticosteroids and/or oral antibiotics can be added to the regimen for a few weeks. Dose reductions are sometimes needed for erlotinib and afatinib.100 Acute generalized exanthematous pustulosis and neutrophilic dermatoses (Sweet syndrome) were also identified in imatinib-treated CML patients.198,199 BTKI therapy exhibited asymptomatic nonpalpable petechial rash, palpable rash, and erythema nodosum. The nonpalpable petechial rash can be explained by underlying ibrutinib-induced platelet dysfunction. They respond well to dose reduction and steroids.168,200,201
Stevens–Johnson syndrome (SJS)
Numerous cases of SJS have been reported within 1 week of imatinib-treated CML patients.202 Imatinib desensitization along with prednisone is recommended for the initial management of severe mucocutaneous eruptions.203 However, newer alternatives like dasatinib and nilotinib have largely avoided this need.197 Similar reports have been published with the use of vandetanib.154
Metabolic dysfunctions
Glucose metabolism
Increasing evidence suggests an association between imatinib, sunitinib, and nilotinib and glucose metabolism. In the adjuvant GIST trial, hyperglycemia was demonstrated in patients on imatinib therapy. Imatinib was associated with the severity of hypoglycemia in GIST patients.204 Studies showed enhancement of B-cell survival by imatinib, potentially contributing to the observed hypoglycemic effects.205 Sunitinib is associated with the incidence of hypoglycemia in 15–19% of patients who present mostly 4 weeks after treatment.206 Regression of pancreatic islets, modulation of IGF-1 (insulin-like growth factor 1) signaling, and decreased glucose uptake are the proposed hypotheses. In diabetic patients on TKIs, assessment of glycemic control is recommended. In a nondiabetic patient on TKIs, periodic monitoring of HbA1c and blood glucose levels are recommended.207
Fluid retention
This is commonly reported after BCR-ABL TKI therapy, especially imatinib and to a lesser extent nilotinib, dasatinib, and bosutinib in CML patients.173 The most common edema locations associated with imatinib are the periorbital space and the lower limbs.208 Increased edema risk is reported in patients 65 years or older, and those with cardiac disease, renal insufficiency, HTN, hypercholesterolemia, and a history of autoimmune diseases.15 Regular monitoring of body weight, heart and lung symptoms, chest imaging, and peripheral tissue tone to detect edema is recommended. Edema is often reversible after treatment with diuretics and steroids. TKI cessation is required in severe cases, at least until the symptoms are controlled.15 Peripheral edema is a common side effect associated with MEK inhibitors. Mild cases involve treatment with compression therapy and head elevation after ruling out other causes such as renal failure and hypoalbuminemia.179
Electrolyte imbalances
BCR-ABL TKIs are associated with electrolyte abnormalities (Table 1).15 Specifically, an increased incidence of early-onset, persistent hypophosphatemia is noted with imatinib, nilotinib, dasatinib, bosutinib, and ponatinib therapy. It is accompanied by increased phosphaturia with low-to-normal calcium levels, and secondary hyperparathyroidism.209,210 Therefore, recommendations for periodic monitoring and correction of electrolyte abnormalities in TKI therapy are of clinical significance.15
Bone and mineral homeostasis
Studies are in progress to identify an association between imatinib and bone mineral density. However significant outcomes have yet to be determined.167 The proposed mechanisms include nonspecific inhibition of TKs expressed by osteoclasts and osteoblasts, including c-KIT and PDGFRA. Slowing of growth velocity and growth retardation was observed in children undergoing imatinib or dasatinib therapy for CML, a mechanism involving disturbances in the growth hormones/IGF-1 axis.167,173 Dasatinib leads to dysregulation of bone remodeling via osteoclast inhibition.211
Endocrine dysfunctions
Thyroid metabolism
Abnormal thyroid metabolism was reported with BCR-ABL TKIs such as imatinib, dasatinib, and nilotinib. Side effects are usually subclinical and transient, with no treatment necessary.173 Hypothyroidism was associated with imatinib, sunitinib, and sorafenib across several studies. Routine thyroid function tests are recommended before and after drug therapy, and after any changes in the TKI regimen. Overt hypothyroidism is treated with levothyroxine but TKI therapy can still be continued.212 Imatinib either worsens pre-existing hypothyroidism or causes de-novo hypothyroidism. Non-deiodination clearance has been described as the underlying mechanism of imatinib-associated hypothyroidism.213 A similar association is seen in studies with sunitinib used during therapy for gastrointestinal stromal cells and metastatic RCC. Sunitinib when given to a patient pre-treated with imatinib, showed higher TSH levels.212 Fatigue associated with sunitinib is possibly related to underlying hypothyroidism, which is an off-target effect of TKIs. Sorafenib is associated with a lower incidence of hypothyroidism when compared to sunitinib due to the difference in the degree of receptor inhibition.214 Serial monitoring of thyroid function tests at baseline and during TKI therapy is recommended. Symptomatic patients are often managed with thyroid hormone replacement with the continuation of cancer treatment.212
Adrenal insufficiency
No overt suppression of the adrenal gland has been identified with sunitinib therapy. It is recommended to monitor adrenal functions in patients exposed to major stressors such as surgery, trauma, or severe infection.215
Hypogonadism
Alterations in testosterone was reported in imatinib and dasatinib-treated patient with concomitant gynecomastia.167 Case reports showed that the development of painful gynecomastia with sunitinib therapy is due to its inhibition of the c-KIT/PDGFRA axis.216
Pulmonary toxicity
Pleural effusion
Pleural effusion is mostly seen with BCR-ABL TKIs with dasatinib having a high likelihood of pleural effusion as the late toxicity.167 This was reported in the DASISION-dasatinib trial which reported a 10% incidence of pleural effusion in the 1st year, and 29% by the end of the study. Importantly, 20% of the patients had to discontinue dasatinib therapy due to toxicity.217 The incidence is higher in bosutinib in comparison to imatinib as per the BELA trial demonstrating discontinuation of bosutinib in 250 patients vs. no changes in imatinib.218 Dasatinib is associated with pulmonary toxicity in the form of unilateral or bilateral pleural effusions, and in some cases, it is associated with lung parenchymal infiltrates.219,220 An efficient strategy to reduce risk includes dose optimization.221 Management depends on the severity, and withdrawal of dasatinib in symptomatic patients until spontaneous resolution is recommended. Later, a lower dose of dasatinib or a switch to another TKI is recommended. Additional therapies may include a short course of steroids and thoracentesis in severe cases.173
Interstitial lung disease (ILD)
ILD is a heterogeneous group of diseases ranging from mild radiographic lung infiltrates to fatal acute respiratory distress syndrome.166 ILD is associated with both EGFR TKIs and ALK TKIs. It has also been reported in ALK-TKIs such as crizotinib, ceritinib, and alectinib. The exact mechanism of ILD precipitated by EGFR TKIs is unknown but it is probably due to a decreased protective function of the EGF receptors localized on type 2 pneumocytes. The risk factors include a previous diagnosis of lung fibrosis, older age, poor performance status, male gender, smoking history, previous or concomitant ILD, comorbid pleural effusion, and coexisting heart disease.65,166 It is also the main cause of death in EGFR TKI therapy. If ILD is suspected, TKIs are discontinued. Once ILD is confirmed, the culprit agents should be stopped regardless of the severity.166
Drug-induced pneumonitis
Pneumonitis is associated with several TKIs, including EGFR inhibitors222 and MEK inhibitors. In the case of pulmonary symptoms, a CT chest is performed. Once the infectious cause is excluded via bronchoalveolar lavage, a high dose of corticosteroids is indicated. Severe cases warrant permanent discontinuation of the culprit drugs with the reintroduction of BRAF inhibitors after remission, which is possible.179 Both capmatinib and tepotinib are associated with about 2–6% of drug-induced pneumonitis as per the GEOMETRY and VISION trials, respectively.223–225 Management involves permanent discontinuation of MET inhibitor along with steroid treatment.226 Switching with another TKI after the resolution of pneumonitis can be considered.227
Neurological dysfunction
Clinical trials enrolling 23 CML patients treated with nilotinib therapy demonstrated an increased incidence of vascular adverse effects in response to an increase in TKI doses and duration of treatment leading to cerebral infarction (CI). CI is considered one of the fatal complications of treatment. The incidence of CI per 1000 person-years was 2.25 with imatinib, 6.47 with nilotinib, and 2.43 with dasatinib. The adjusted incidence rates of CI in the age and gender-matched Japanese general population was 3.342 per 1000 person-years.228 However, a large-scale population-based study in Sweden investigating vascular adverse effects in TKI therapy reported no increase in the CI incidence, though it was relatively higher with nilotinib.86 Another study from Korea demonstrated an increased incidence of stroke in nilotinib in comparison to dasatinib.229 This was further corroborated by the ENESTnd trial studying imatinib and dasatinib during a 5-year follow-up period.87 Studies also reported that males had a higher incidence compared to females.228 Imatinib is known to have a relatively safer vascular profile compared to other TKIs. Nilotinib is associated with an increased incidence of arterial occlusive disease as per a report by Hadzijusufovic et. al.230 In comparison, ponatinib showed a significant episode of serious cerebrovascular events.231. Fedratinib has been associated with cases of encephalopathy and MRI demonstrated findings consistent with Wernicke encephalopathy.232
Pathogenesis
Nilotinib inhibits angiogenesis and endothelial cell proliferation leading to a slow blood flow recovery rate post-ischemia. It also promotes the expression of various pro-atherogenic cytoadhesion molecules.233 Nilotinib demonstrated an imbalance between pro/anti-inflammatory processes, leading to a hypothesis that the pro-inflammatory state activates pro-atherothrombosis via enhanced lipid peroxidation. Genetic pro-atherothrombotic predisposition conferred by LOX-1 may play an important role.234 These changes were absent in imatinib. Ponatinib inhibits neo-angiogenesis of vascular endothelial cells. However, more trials are necessary to study the vascular adverse effects of ponatinib and dasatinib.235
Management
Neurovascular risk scores help with patient selection requiring closer monitoring and detection of early signs. Periodic lipid profiling and blood glucose examinations, BP monitoring, Doppler ultrasound of the selected blood vessels, and intimal medial thickness measurement have been employed in cases to monitor TKI side effects.236 Decisions regarding therapy modifications should be based on the severity of the vascular event(s), the response state of CML, and the availability of alternative treatment options for CML.231 In the case of mild cases, a dose reduction with close monitoring has been recommended. It is evidenced by data that there is a reduction in the risk of arterial thrombotic events by about 40% when the ponatinib dose is reduced by 15 mg.237
Renal toxicity
Although uncommon, renal dysfunction has been associated with TKI therapy, particularly reported with imatinib and bosutinib (Table 1). Imatinib has shown a decline in GFR and new-onset CKD in 22% of patients. Renal toxicity is also reported in <10% of patients taking bosutinib.167 TKIs, specifically VEGFIs, are associated with the development of proteinuria. It has been predominantly seen with sunitinib, sorafenib, and pazopanib therapies. Further, sorafenib and sunitinib manifest as albuminuria, thrombotic microangiopathy, and acute interstitial nephritis.154 Sorafenib requires dose adjustments for creatinine clearance less than 40 mL/min.186 Cediranib, a newer potent VEGF inhibitor, has shown proteinuria in 30% of patients within 2 weeks of therapy. Long-term data are still unavailable.238
The underlying mechanisms are suggested to be due to endothelial damage by TKIs resulting in local thrombosis (Fig. 5). The resultant thrombotic microangiopathy manifests as low-grade proteinuria with no systemic findings. Renal biopsy is performed only in selected patients with high-grade proteinuria.238 Since the risk of proteinuria is associated with the progression of renal disease, measures must be taken to screen these patients using baseline urinalysis and protein-to-creatinine ratio before starting TKIs. A urine protein to creatinine ratio of ≥ 1 or 24-hour urine with ≥ 1 gr/dL/24 hours of protein should prompt intervention.154 In the case of severe and persistent proteinuria when thrombotic microangiopathy is evident through renal biopsy, the benefits vs. risks of continuation or TKI therapy modifications should be considered. This is particularly difficult in conditions where TKIs are the last line of therapy for advanced cancer. Proteinuria is often accompanied by HTN. Thus, ACEIs or ARBs are generally viable treatments. Diuretics, CCBs, or BBs can be used, although benefits are yet to be demonstrated.238
Acute renal failure or electrolyte disorders were reported in combination therapy of BRAF and MEK inhibitors with an increase in serum creatinine.239,240 Pathology includes acute on chronic tubular interstitial damage.241,242 Drug cessation with reintroduction at a lower dose is done in higher-grade kidney injuries. Rehydration may be beneficial in a few patients with pre-renal failure.179
Ocular reactions
Ocular inflammation (uveitis, conjunctivitis)
These side effects are rarely seen with BRAF inhibitors. Mild cases are treated with topical steroids. Discontinuation or dose reduction with severe episodes is recommended.243
Retinal vein occlusion is observed with both BRAF inhibitor monotherapy or a combination of BRAF and MEK inhibitor therapy.243 Since the frequency is similar to patients not on therapy, the causal relationship is still up for debate. It is generally recommended to discontinue therapy with the constitution of ophthalmologic therapy.244
Central serous chorioretinopathy is an adverse effect with fluid accumulation in the retina and is associated with a combination of BRAF and MEK inhibitor therapies.245 It is seen in about 25% of patients treated with erdafitinib, an FGFR inhibitor used to treat metastatic urothelial carcinoma. This is often asymptomatic but can rarely lead to transient visual disturbances. It is usually bilateral, multifocal occurring within hours, days, or weeks of initiation and for the most part, attributed to the MEK inhibitors. The contributory mechanism is not completely understood. It requires frequent monitoring246 and can be diagnosed with optical coherence tomography.245 Usually, no treatment is required. However, discontinuation or reduction of MEK inhibitors with severe manifestations can be considered.179
Opportunistic infections
These are seen in >50% of patients taking BTK TKIs. The patients receiving these TKIs are immunocompromised and are at increased risk for infections. It is most likely to occur in patients with relapsed or refractory type cancers.247 It is hypothesized that irreversible248 inhibition of ITK52249 and impairment of macrophages increase susceptibility to infection.250,251
Pneumonia
This is the most common infective complication and the major contributor to death, in about 2% of patients.248 Pneumonia was reported in about 12% of patients with ibrutinib therapy. Aspergillus fumigatus and Pneumocystis jirovecii (PJP) have been isolated as the culprit organisms.168 Other organisms included Histoplasma, Cryptococcus, and Nocardia.248 In high-risk patients or those with a previous history of infections, PJP prophylaxis should be considered.252 In the case of Aspergillus fumigatus, a high incidence of infection has been reported early in treatment with higher rates in patients receiving steroids.168 There are differing opinions regarding management which dictates cessation of TKIs in acute infection until a definitive diagnosis has been established or during the grade 4 infection.253,254 Nevertheless, therapy can be restarted on improvement as per the healthcare provider. The administration of vaccines against influenza and pneumococcus has been advocated before the initiation of therapy along with the addition of recombinant, adjuvant vaccine against varicella-zoster virus.255 Intravenous immunoglobulin therapy should be considered in patients with recurrent infections or for known hypogammaglobulinemia.168
Invasive Aspergillosis
Combination drug studies in primary CNS lymphoma (PCNSL) reported invasive aspergillosis infections in 39% of patients. Single-agent studies demonstrated aspergillosis in 5–11% of patients.248
Urinary tract infections (UTIs)
These were the most common infections found in fedratinib-treated patients.232
Hematological reactions
Cytopenias
These side effects include anemia, leucopenia, neutropenia, and thrombocytopenia most commonly identified in CML patients being treated with BTK TKIs.256 Cytopenias were often reported at the initiation of therapy.15 Nilotinib or dasatinib are associated with an increased incidence of cytopenias. However, when the second-generation drugs were given as first-line therapy for CML rates of cytopenias were lower for both drugs. Nilotinib is associated with neutropenia in 4–12% and thrombocytopenia in 2–12% of patients. Similarly, dasatinib is associated with neutropenia in 21% and thrombocytopenia in 10–19% of patients.15 In the ENESTnd and DASISION trials, nilotinib and dasatinib were demonstrated to be less hemotoxic than imatinib.168 According to a meta-analysis including 17 studies, dasatinib appeared to be the most hemotoxic drug used in CML compared to nilotinib which was the safest.256 Ruxolitinib has been associated with dose-dependent anemia, high-grade neutropenia, and dose-limiting thrombocytopenia.257 Fedratinib is frequently associated with anemia during treatment.232
Mechanisms of cytopenia
Anemia is due to the inhibition of c-KIT by TKIs.258 Leukopenia and neutropenia can be due to the binding of drugs to several key kinase targets of the immune system, such as Lck, Lyn, Btk, and Src.259 Cytopenia in BCR-ABL TKIs is likely due to decreased levels of healthy residual Philadelphia chromosome-negative bone marrow being unable to reconstitute peripheral blood counts rather than direct toxicity of the drugs towards normal hematopoietic cells.260 Additionally, dasatinib has inhibitory effects against platelets which are rapidly reversible with the interruption of therapy.261,262
Management of cytopenia
Most cases are mild to moderate severity cytopenias which are self-limited. Severe manifestations are carefully observed necessitating monitoring of blood counts weekly in the first month and monthly thereafter for the next 3 months.263 Supportive treatment with changes in dosage or temporary cessations of TKI is undertaken.264 Supportive treatments include recombinant erythropoietin or granulocyte-stimulating factor and thrombopoietin receptor agonists. Additional immunosuppressive therapies such as short-course corticosteroids and anti-CD20 monoclonal antibodies can be considered.173
Bleeding risk
BTK TKIs are most commonly associated with bruising in two-thirds of patients with no increased risk of major bleeding. Major bleeding occurs rarely in about 2–9% of patients.168 In an integrated analysis, ibrutinib was associated with an increased risk of bleeding of 4.4% compared to the test drug with 2.8%. Additionally, both groups experienced increased use of anticoagulants or antiplatelet agents.265 On the other hand, acalabrutinib in the ELEVATE-TN trial reported major bleeding in 2% of patients and minor bleeding in 37% of patients.266 Zanubrutinib is more selective in its BTK inhibition with no effects on platelet function or receptor shedding compared to ibrutinib.267 Dasatinib and ponatinib can cause platelet dysfunction, potentially increasing the risk of bleeding.268 As per a combined report of 4 multi-center studies dasatinib use resulted in grade 3 or 4 bleeding in up to 10% of patients.269 Dasatinib also causes thrombocytopenia by impairing megakaryocyte formation.270 This is of particular importance in the application of anticoagulation and anti-platelet drugs in vascular disease in ponatinib and dasatinib-treated patients.231 Mild mucosal bleeding can occur from inhibition of VEGFR-2 leading to microvascular leaks from endothelial cell damage.154
BTK TKIs can be held for 3 days or 7 days before and after minor and major invasive procedures respectively due to the increased risk of periprocedural bleeding.271 For minor bleeds, temporary cessation of therapy is done until resolution. In severe grade ≥3 bleeding, platelet transfusion irrespective of platelet count is considered to overcome bleeding.272 Concurrent use of drugs with increased bleeding risk is avoided. Alternative CLL therapy agents are considered if the patient requires dual antiplatelet therapy.273
Miscellaneous side effects
Headache
This symptom is most commonly associated with the second-generation BTK TKIs, acalabrutinib. Grade 1 or 2 headaches were mostly reported in 70% of patients during the first few weeks of therapy.266,274 Comparatively, in ibrutinib, this symptom presents at a later stage of treatment. Symptomatic relief can be achieved with extended treatment and the administration of acetaminophen with or without caffeine.168
Secondary malignancies
Case reports published from a review of over 9000 CML patients showed no clear increase in any malignancies, though it has to be mentioned that the patients had a relatively short follow-up.275 Similarly, a single large institutional study with a follow-up of 107 months reported no second cancers.276 On the contrary, a small study of 52 Japanese patients and a Swedish registry study with 868 patients suggested an increased risk of malignancies over the general population.277
TKI and pregnancy
Limited data are available on the safety of TKIs in pregnancy. TKI’s effects on fetal growth and development are not known. Current guidelines suggest avoiding pregnancy while undergoing therapy.278 Some studies suggest that imatinib is associated with fetal malformations such as low birth weight, whereas erlotinib and lapatinib can cause oligohydramnios. Therefore, close follow-up of growth through ultrasonography and amniotic fluid index is recommended in these patients.279 In individuals who desire pregnancy, a 3-month washout period of TKI therapy is recommended before conception.280 In the case of TKI therapy being necessary during pregnancy, interferons may be used safely. However, its toxicity and slow response time should be considered.281 An alternate therapy with hydroxyurea is also safe during pregnancy, given in pulses to avoid high white blood cell counts.282
Drug–drug interactions
Cytochrome P450 metabolism
Sorafenib, regorafenib, lenvatinib, and cabozantinib are metabolized by the liver enzyme CYP3A4, and as such concurrent use of drugs modulating the activity of CYP3A4 can affect tolerability.283 Thus, the administration of a potent CYP3A4 inhibitor, antifungal ketoconazole can cause increased plasma levels. Thus, CYP3A4 inhibitors should be avoided with regorafenib and cabozantinib. Similarly, CYP3A4 inducers have the potential to reduce plasma TKI levels and decrease efficacy.284
P-glycoprotein interactions
P-glycoprotein (P-gp) is inhibited by sorafenib and cabozantinib leading to the accumulation of P-gp substrates. Thus measures should be undertaken to avoid these drugs in patients receiving sorafenib or cabozantinib.285,286 Furthermore, metabolites of regorafenib which are P-gp substrates should therefore be avoided with P-gp inhibitors or inducers.228
Future perspectives
The development of small molecule TKIS for cancer therapies has been growing at a rapid pace. Despite significant progress in cancer care, the utility of TKIs still faces significant challenges in terms of their tissue selectivity, efficacy, adverse events, and drug resistance. In addition to TKIs, there are several new categories of cancer-targeted drug discovery in development. For example, transcript-targeted therapies based on the use of RNA interference (RNAi) and antisense oligonucleotides (ASOs),287 and other RNA-based therapeutics such as Apatorsen, Cenersen, Custirsen288 for use in cancer. Targeting transcription and translation may affect multiple pathways, rather than the targeted one, and there are inevitable side effects arising from the fact that all cells require transcription and translation for their proper function. Similarly, humanized antibodies such as Pembrolizumab (PD-1antibody) or a combination of TKIs and PD-1 blockers are gaining extensive popularity in cancer care.289–291 The antibodies can induce immune-related and other side effects affecting multiple organ systems. Others include antibody-drug conjugate (ADC) drugs such as polatuzumab vedotin-piiq (Polivy), enfortumab vedotin-ejfv (Padcev), that are either already approved by the FDA or are in the development phase.292–294 Each of these new classes of drugs carries a separate set of challenges and can cause complex cardiac and extracardiac complications that would require in-depth knowledge and expertise to manage such complications in cancer patients.
When drug-associated toxicity is identified, it is imperative to understand the precise mechanisms of specific organ toxicity and to identify patients at risk for such events. A personalized approach must be incorporated in toxicity assessment in drug selection.295 Future studies should focus on the utilization of targeted therapies using personalized pharmacogenomics tools. This approach will address the variability of drug responses in individual patients, resulting in the reduction of adverse events.296 The combination of TKIs with nanoscaled transporters is another promising approach, resulting in the prevention of bystander adverse effects. Nanomedicine has many other advantages beyond the reduction of toxic effects to improve patient outcomes.297 The main objective is to facilitate specific drug biodistribution to the target areas in an adequate local concentration with minimal loss of volume or activity in blood. It efficiently reduces the toxicity towards healthy cells and tissues, as well as avoids the development of drug resistance.298
Acknowledgements
S.P. received support from NIH/NHLBI (grant no. R01HL150266). U.C.S. received support from NIH/NHLBI (grant no. R01HL152090). All figures were created from BioRender.com with permission for publication.
Author contributions
The authors confirm contribution to the paper as follows: draft manuscript preparation, S.S., study conception, design and revision, U.C.S. and S.P. All authors have read and approved the article.
Competing interests
The authors declare no competing interests.
References
- 1.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Nair P, Prado M, Perea-Gil I, Karakikes I. Concise review: precision matchmaking: induced pluripotent stem cells meet cardio-oncology. Stem Cells Transl. Med. 2019;8:758–767. doi: 10.1002/sctm.18-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 4.Broekman F, Giovannetti E, Peters GJ. Tyrosine kinase inhibitors: multi-targeted or single-targeted? World J. Clin. Oncol. 2011;2:80. doi: 10.5306/wjco.v2.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkes G. Targeted therapy: attacking cancer with molecular and immunological targeted agents. Asia-Pac. J. Oncol. Nurs. 2018;5:137–155. doi: 10.4103/apjon.apjon_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu F, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021;6:1–35. doi: 10.1038/s41392-021-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong L, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021;6:1–48. doi: 10.1038/s41392-021-00572-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman, M. & Adler, E. Protein Kinase Inhibitors—Selectivity or Toxicity? (IntechOpen, 2021).
- 9.Hantschel O. Unexpected off-targets and paradoxical pathway activation by kinase inhibitors. ACS Chem. Biol. 2015;10:234–245. doi: 10.1021/cb500886n. [DOI] [PubMed] [Google Scholar]
- 10.Lamore SD, Kohnken RA, Peters MF, Kolaja KL. Cardiovascular toxicity induced by kinase inhibitors: mechanisms and preclinical approaches. Chem. Res. Toxicol. 2020;33:125–136. doi: 10.1021/acs.chemrestox.9b00387. [DOI] [PubMed] [Google Scholar]
- 11.CHEN Z, AI D. Cardiotoxicity associated with targeted cancer therapies. Mol. Clin. Oncol. 2016;4:675–681. doi: 10.3892/mco.2016.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miljković F, Bajorath J. Computational analysis of kinase inhibitors identifies promiscuity cliffs across the human kinome. ACS Omega. 2018;3:17295–17308. doi: 10.1021/acsomega.8b02998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah DR, Shah RR, Morganroth J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013;36:413–426. doi: 10.1007/s40264-013-0050-x. [DOI] [PubMed] [Google Scholar]
- 14.Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Relationship between skin rash and outcome in non-small-cell lung cancer patients treated with anti-EGFR tyrosine kinase inhibitors: a literature-based meta-analysis of 24 trials. Lung Cancer. 2012;78:8–15. doi: 10.1016/j.lungcan.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Jabbour E, Deininger M, Hochhaus A. Management of adverse events associated with tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia. 2011;25:201–210. doi: 10.1038/leu.2010.215. [DOI] [PubMed] [Google Scholar]
- 16.Li X-Q, Tang X-R, Li L-L. Antipsychotics cardiotoxicity: What’s known and what’s next. World J. Psychiatry. 2021;11:736–753. doi: 10.5498/wjp.v11.i10.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon AR, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020;22:1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez JA. Vascular endothelial growth factor-tyrosine kinase inhibitors: Novel mechanisms, predictors of hypertension and management strategies. Am. Hear. J. Cardiol. Res. Pr. 2022;17:100144. doi: 10.1016/j.ahjo.2022.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, et al. Adaptation of human iPSC-derived cardiomyocytes to tyrosine kinase inhibitors reduces acute cardiotoxicity via metabolic reprogramming. Cell Syst. 2019;8:412–426.e7. doi: 10.1016/j.cels.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eschenhagen T. Exaggerated cardiotoxicity of sunitinib in stressed 3-dimensional heart muscles. JACC: Basic Transl. Sci. 2018;3:277–279. doi: 10.1016/j.jacbts.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaszewska B, Muzolf M, Grabysa R, Bodnar L. Cardiotoxicity of antiangiogenic drugs: causes and mechanisms. Oncol. Rev. 2021;11:12–18. [Google Scholar]
- 22.Perez IE, Taveras Alam S, Hernandez GA, Sancassani R. Cancer therapy-related cardiac dysfunction: an overview for the clinician. Clin. Med. Insights: Cardiol. 2019;13:1179546819866445. doi: 10.1177/1179546819866445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra PK, et al. Guidelines for evaluating myocardial cell death. Am. J. Physiol. Heart Circ. Physiol. 2019;317:H891. doi: 10.1152/ajpheart.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishihara S, et al. Mechanisms of EGFR-TKI-induced apoptosis and strategies targeting apoptosis in EGFR-mutated non-small cell lung cancer. Genes. 2022;13:2183. doi: 10.3390/genes13122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen AV, et al. The Lck inhibitor, AMG-47a, blocks necroptosis and implicates RIPK1 in signalling downstream of MLKL. Cell Death Dis. 2022;13:291. doi: 10.1038/s41419-022-04740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbin SJH, Petrie MC, Myles RC, Touyz RM, Lang NN. Cardiotoxic effects of angiogenesis inhibitors. Clin. Sci. 2021;135:71–100. doi: 10.1042/CS20200305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Čelutkienė J, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC) Eur. J. Heart Fail. 2020;22:1504–1524. doi: 10.1002/ejhf.1957. [DOI] [PubMed] [Google Scholar]
- 28.Curigliano G, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrmann J, et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133:1272–1289. doi: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J. Clin. Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 31.Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. 2008;31:459–467. doi: 10.2165/00002018-200831060-00002. [DOI] [PubMed] [Google Scholar]
- 32.Thomas SA. Chemotherapy agents that cause cardiotoxicity. U. S. Pharm. 2017;42:HS24–HS33. [Google Scholar]
- 33.Volkova M, Raymond Russell I. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr. Cardiol. Rev. 2011;7:214. doi: 10.2174/157340311799960645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann. Intern. Med. 1978;88:168–175. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- 35.Du XL, Xia R, Burau K, Liu CC. Cardiac risk associated with the receipt of anthracycline and trastuzumab in a large nationwide cohort of older women with breast cancer, 1998-2005. Med. Oncol. 2011;28:S80–S90. doi: 10.1007/s12032-010-9717-7. [DOI] [PubMed] [Google Scholar]
- 36.Touyz RM, Lang NN, Herrmann J, Van Den Meiracker AH, Danser AHJ. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension. 2017;70:220–226. doi: 10.1161/HYPERTENSIONAHA.117.08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aslan G, et al. Evaluation of serum platelet-derived growth factor receptor-ß and brain-derived neurotrophic factor levels in microvascular angina. Anatol. J. Cardiol. 2020;24:397. doi: 10.14744/AnatolJCardiol.2020.44388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis. Oncol. 2018;2:1–11. doi: 10.1038/s41698-018-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinkhas D, Ho T, Smith S. Assessment of pazopanib-related hypertension, cardiac dysfunction and identification of clinical risk factors for their development. Cardio-Oncol. 2017;3:5. doi: 10.1186/s40959-017-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varricchi G, et al. Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front. Physiol. 2018;9:167. doi: 10.3389/fphys.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi HD, Chang MJ. Cardiac toxicities of lapatinib in patients with breast cancer and other HER2-positive cancers: a meta-analysis. Breast Cancer Res. Treat. 2017;166:927–936. doi: 10.1007/s10549-017-4460-9. [DOI] [PubMed] [Google Scholar]
- 42.Anand K, Ensor J, Trachtenberg B, Bernicker EH. Osimertinib-Induced cardiotoxicity: a retrospective review of the FDA adverse events reporting system (FAERS) JACC CardioOncol. 2019;1:172–178. doi: 10.1016/j.jaccao.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Díaz-Serrano A, Gella P, Jiménez E, Zugazagoitia J, Paz-Ares Rodríguez L. Targeting EGFR in lung cancer: current standards and developments. Drugs. 2018;78:893–911. doi: 10.1007/s40265-018-0916-4. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Russo M, Pirozzi F, Tocchetti CG, Ghigo A. Autophagy and cancer therapy cardiotoxicity: From molecular mechanisms to therapeutic opportunities. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118493. doi: 10.1016/j.bbamcr.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Stone JR, Kanneganti R, Abbasi M, Akhtari M. Monitoring for chemotherapy-related cardiotoxicity in the form of left ventricular systolic dysfunction: a review of current recommendations. JCO Oncol. Pract. 2021;17:228–236. doi: 10.1200/OP.20.00924. [DOI] [PubMed] [Google Scholar]
- 46.Jin Y, et al. A comprehensive review of clinical cardiotoxicity incidence of FDA-approved small-molecule kinase inhibitors. Front. Pharmacol. 2020;11:891. doi: 10.3389/fphar.2020.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winnik S, et al. Systemic VEGF inhibition accelerates experimental atherosclerosis and disrupts endothelial homeostasis—implications for cardiovascular safety. Int. J. Cardiol. 2013;168:2453–2461. doi: 10.1016/j.ijcard.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Thijs AMJ, et al. Impaired endothelium-dependent vasodilation does not initiate the development of sunitinib-associated hypertension. J. Hypertens. 2015;33:2075–2082. doi: 10.1097/HJH.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 49.Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 1. J. Am. Coll. Cardiol. 2017;70:2536–2551. doi: 10.1016/j.jacc.2017.09.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sierra JR, Cepero V, Giordano S. Molecular mechanisms of acquired resistance to tyrosine kinase targeted therapy. Mol. Cancer. 2010;9:75. doi: 10.1186/1476-4598-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK. QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br. J. Cancer. 2015;112:296–305. doi: 10.1038/bjc.2014.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buza, V., Rajagopalan, B. & Curtis, A. B. Cancer treatment-induced arrhythmias: focus on chemotherapy and targeted therapies. Circ. Arrhythm. Electrophysiol. 10, e005443 (2017). [DOI] [PubMed]
- 53.Tolcher AW, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother. Pharmacol. 2011;67:751–764. doi: 10.1007/s00280-010-1372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandrasekhar S, Fradley MG. QT interval prolongation associated with cytotoxic and targeted cancer therapeutics. Curr. Treat. Options Oncol. 2019;20:1–17. doi: 10.1007/s11864-019-0657-y. [DOI] [PubMed] [Google Scholar]
- 55.Rao VU, et al. Clinical approach to cardiovascular toxicity of oral antineoplastic agents: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;77:2693–2716. doi: 10.1016/j.jacc.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fradley MG, Moslehi J. QT prolongation and oncology drug development. Card. Electrophysiol. Clin. 2015;7:341–355. doi: 10.1016/j.ccep.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Sestier M, Hillis C, Fraser G, Leong D. Bruton’s tyrosine kinase inhibitors and cardiotoxicity: more than just atrial fibrillation. Curr. Oncol. Rep. 2021;23:113. doi: 10.1007/s11912-021-01102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J. Am. Coll. Cardiol. 2017;70:2552–2565. doi: 10.1016/j.jacc.2017.09.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mulder TA, et al. Ibrutinib has time-dependent on- and off-target effects on plasma biomarkers and immune cells in chronic lymphocytic leukemia. HemaSphere. 2021;5:e564. doi: 10.1097/HS9.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giudice V, Vecchione C, Selleri C. Cardiotoxicity of novel targeted hematological therapies. Life. 2020;10:1–22. doi: 10.3390/life10120344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimopoulos M, et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: A substudy of the phase 3 ASPEN trial. Blood Adv. 2020;4:6009–6018. doi: 10.1182/bloodadvances.2020003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghia P, et al. Ascend: Phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2020;38:2849–2861. doi: 10.1200/JCO.19.03355. [DOI] [PubMed] [Google Scholar]
- 63.Tam CSL, et al. ASPEN: Results of a phase III randomized trial of zanubrutinib versus ibrutinib for patients with Waldenström macroglobulinemia (WM) J. Clin. Oncol. 2020;38:8007–8007. doi: 10.1200/JCO.2020.38.15_suppl.8007. [DOI] [Google Scholar]
- 64.Lampson BL, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–2584. doi: 10.1182/blood-2016-10-742437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Wang W. Safety and efficacy of anaplastic lymphoma kinase tyrosine kinase inhibitors in non‑small cell lung cancer (Review) Oncol. Rep. 2020;45:13–28. doi: 10.3892/or.2020.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung R, et al. Hypertensive cardiotoxicity in cancer treatment—systematic analysis of adjunct, conventional chemotherapy, and novel therapies—epidemiology, incidence, and pathophysiology. J. Clin. Med. 2020;9:1–22. doi: 10.3390/jcm9103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat. Rev. 2017;53:120–127. doi: 10.1016/j.ctrv.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Vaitiekus D, Kastanjeda -Martines E, Lingytė E, Dambrauskas L, Juozaitytė E. Tyrosine kinase inhibitors induced cardiotoxicity. J. Med. Sci. Med. Sci. 2017;1:1–10. [Google Scholar]
- 69.Agarwal M, Thareja N, Benjamin M, Akhondi A, Mitchell GD. Tyrosine kinase inhibitor-induced hypertension. Curr. Oncol. Rep. 2018;20:65. doi: 10.1007/s11912-018-0708-8. [DOI] [PubMed] [Google Scholar]
- 70.Turner, J. R., Karnad, D. R. & Kothari, S. Oncology Drug Therapy: Cardiotoxicity and the Discipline of Cardio-oncology. In: Cardiovascular Safety in Drug Development and Therapeutic Use. 201-221 (Adis, Cham. 2017).
- 71.Kappers MHW, et al. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 72.Alivon M, et al. Large artery stiffness and hypertension after antiangiogenic drugs: Influence on cancer progression. J. Hypertens. 2015;33:1310–1317. doi: 10.1097/HJH.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 73.Dickerson T, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–1928. doi: 10.1182/blood.2019000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satoh T. Tyrosine kinase inhibitor for pulmonary arterial hypertension. Ther. Res. 2013;34:1221–1223. [Google Scholar]
- 75.Montani D, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–2137. doi: 10.1161/CIRCULATIONAHA.111.079921. [DOI] [PubMed] [Google Scholar]
- 76.Mishra AK, Sahu KK, Kaul S, Lal A. Dasatinib induced pleuro-pericardial effusion. Acta Biomed. 2020;91:142–143. doi: 10.23750/abm.v91i1.8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldman A, et al. Cardiovascular toxicities of antiangiogenic tyrosine kinase inhibitors: a retrospective, pharmacovigilance study. Target. Oncol. 2021;16:471–483. doi: 10.1007/s11523-021-00817-2. [DOI] [PubMed] [Google Scholar]
- 78.Bernardeschi P, Pirrotta MT, Del Rosso A, Fontanelli G, Milandri C. Sudden ventricular fibrillation and death during ibrutinib therapy—a case report. Eur. J. Haematol. 2019;103:442–443. doi: 10.1111/ejh.13290. [DOI] [PubMed] [Google Scholar]
- 79.Faruque LI, et al. Systematic review of the risk of adverse outcomes associated with vascular endothelial growth factor inhibitors for the treatment of cancer. PLoS ONE. 2014;9:e101145. doi: 10.1371/journal.pone.0101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slamon D, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 82.Cameron D, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ewer MS, et al. Cardiovascular events among 1090 cancer patients treated with sunitinib, interferon, or placebo: a comprehensive adjudicated database analysis demonstrating clinically meaningful reversibility of cardiac events. Eur. J. Cancer. 2014;50:2162–2170. doi: 10.1016/j.ejca.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 84.Wittes J. Pazopanib versus sunitinib in renal cancer. N. Engl. J. Med. 2013;369:1968–1970. doi: 10.1056/NEJMc1311795. [DOI] [PubMed] [Google Scholar]
- 85.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Hear. Fail. 2013;1:72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Dahlén T, et al. Cardiovascular events associated with use of tyrosine kinase inhibitors in chronic myeloid leukemia. Ann. Intern. Med. 2016;165:161–166. doi: 10.7326/M15-2306. [DOI] [PubMed] [Google Scholar]
- 87.Hochhaus A, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor–associated cardiovascular toxicity in chronic myeloid leukemia. J. Clin. Oncol. 2015;33:4210. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shah NP, et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am. J. Hematol. 2015;90:1060–1064. doi: 10.1002/ajh.24174. [DOI] [PubMed] [Google Scholar]
- 90.Fox LC, et al. The incidence and natural history of dasatinib complications in the treatment of chronic myeloid leukemia. Blood Adv. 2017;1:802–811. doi: 10.1182/bloodadvances.2016003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caocci G, et al. Recurrent arterial occlusive events in patients with chronic myeloid leukemia treated with second- and third-generation tyrosine kinase inhibitors and role of secondary prevention. Int. J. Cardiol. 2019;288:124–127. doi: 10.1016/j.ijcard.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 92.Giles FJ, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27:1310–1315. doi: 10.1038/leu.2013.69. [DOI] [PubMed] [Google Scholar]
- 93.Cortes JE, et al. Ponatinib efficacy and safety in Philadelphia chromosome–positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393–404. doi: 10.1182/blood-2016-09-739086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat. Rev. Cancer. 2012;12:699. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sampat KR, O’Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18:430. doi: 10.1634/theoncologist.2012-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu D, Ma X, Xiao D, Jia Y, Wang Y. Efficacy and safety of targeting VEGFR drugs in treatment for advanced or metastatic gastric cancer: a systemic review and metaanalysis. Oncotarget. 2018;9:8120–8132. doi: 10.18632/oncotarget.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shim JV, et al. Mechanistic systems modeling to improve understanding and prediction of cardiotoxicity caused by targeted cancer therapeutics. Front. Physiol. 2017;8:651. doi: 10.3389/fphys.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stuhlmiller, T. J. et al. Kinome and transcriptome profiling reveal broad and distinct activities of erlotinib, sunitinib, and sorafenib in the mouse heart and suggest cardiotoxicity from combined signal transducer and activator of transcription and epidermal growth factor recepto. J. Am. Heart Assoc. 6, e006635 (2017). [DOI] [PMC free article] [PubMed]
- 99.Li Y, Wang W, Gao R, Xu X, Zhang Y. Genome-wide prioritization reveals novel gene signatures associated with cardiotoxic effects of tyrosine kinase inhibitors. Oncol. Lett. 2021;21:94. doi: 10.3892/ol.2020.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zheng PP, Li J, Kros JM. Breakthroughs in modern cancer therapy and elusive cardiotoxicity: Critical research-practice gaps, challenges, and insights. Med. Res. Rev. 2018;38:325–376. doi: 10.1002/med.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McMullen CJ, Chalmers S, Wood R, Cunningham MR, Currie S. Sunitinib and Imatinib Display Differential Cardiotoxicity in Adult Rat Cardiac Fibroblasts That Involves a Role for Calcium/Calmodulin Dependent Protein Kinase II. Front. Cardiovasc. Med. 2021;7:630480. doi: 10.3389/fcvm.2020.630480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li L, et al. CB1R-stabilized NLRP3 inflammasome drives antipsychotics cardiotoxicity. Signal Transduct. Target. Ther. 2022;7:190. doi: 10.1038/s41392-022-01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh AP, et al. Ponatinib-induced cardiotoxicity: delineating the signalling mechanisms and potential rescue strategies. Cardiovasc. Res. 2019;115:966–977. doi: 10.1093/cvr/cvz006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gozgit JM, et al. Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol. Cancer Ther. 2012;11:690–699. doi: 10.1158/1535-7163.MCT-11-0450. [DOI] [PubMed] [Google Scholar]
- 105.Ma W, Wei S, Zhang B, Li W. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Front. Cell Dev. Biol. 2020;8:434. doi: 10.3389/fcell.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu XH, et al. VEGF attenuates development from cardiac hypertrophy to heart failure after aortic stenosis through mitochondrial mediated apoptosis and cardiomyocyte proliferation. J. Cardiothorac. Surg. 2011;6:1–9. doi: 10.1186/1749-8090-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zentilin L, et al. Cardiomyocyte VEGFR‐1 activation by VEGF‐B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 2010;24:1467–1478. doi: 10.1096/fj.09-143180. [DOI] [PubMed] [Google Scholar]
- 108.Chu TF, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat. Rev. Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 110.Bouitbir J, Panajatovic MV, Frechard T, Roos NJ, Krähenbühl S. Imatinib and dasatinib provoke mitochondrial dysfunction leading to oxidative stress in C2C12 myotubes and human RD cells. Front. Pharmacol. 2020;11:1. doi: 10.3389/fphar.2020.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Truitt R, et al. Increased afterload augments sunitinib-induced cardiotoxicity in an engineered cardiac microtissue model. JACC Basic Transl. Sci. 2018;3:265–276. doi: 10.1016/j.jacbts.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kerkela R, et al. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin. Transl. Sci. 2009;2:15–25. doi: 10.1111/j.1752-8062.2008.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schneider C, et al. The anti-cancer multikinase inhibitor sorafenib impairs cardiac contractility by reducing phospholamban phosphorylation and sarcoplasmic calcium transients. Sci. Rep. 2018;8:5295. doi: 10.1038/s41598-018-23630-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu J, Bian SY. Late-chronic cardiotoxicity and heart failure caused by ibrutinib: a case report and literature review. J. Geriatr. Cardiol. 2021;18:307–311. doi: 10.11909/j.issn.1671-5411.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aghel N, Delgado DH, Lipton JH. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance. Vasc. Health Risk Manag. 2017;13:293. doi: 10.2147/VHRM.S108874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ibrahim NE, Januzzi JL. Established and emerging roles of biomarkers in heart failure. Circ. Res. 2018;123:614–629. doi: 10.1161/CIRCRESAHA.118.312706. [DOI] [PubMed] [Google Scholar]
- 117.Pavo N, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–1880. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 118.Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog. Cardiovasc. Dis. 2010;53:94–104. doi: 10.1016/j.pcad.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 119.Bojan A, Torok-Vistai T, Parvu A. Assessment and management of cardiotoxicity in hematologic malignancies. Dis. Markers. 2021;2021:1–10. doi: 10.1155/2021/6616265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alexandre J, et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the american and european cardio-oncology guidelines. J. Am. Heart Assoc. 2020;9:e018403. doi: 10.1161/JAHA.120.018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zamorano JL, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 122.Ameri P, et al. Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur. J. Heart Fail. 2018;20:879–887. doi: 10.1002/ejhf.1165. [DOI] [PubMed] [Google Scholar]
- 123.Mercurio V, et al. Redox imbalances in ageing and metabolic alterations: Implications in cancer and cardiac diseases. An overview from the working group of cardiotoxicity and cardioprotection of the Italian society of cardiology (SIC) Antioxidants. 2020;9:1–20. doi: 10.3390/antiox9070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van de Bovenkamp AA, et al. Validation of the 2016 ase/eacvi guideline for diastolic dysfunction in patients with unexplained dyspnea and a preserved left ventricular ejection fraction. J. Am. Heart Assoc. 2021;10:21165. doi: 10.1161/JAHA.121.021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oikonomou EK, et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4:1007. doi: 10.1001/jamacardio.2019.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guan J, Zhang M. Cardiotoxicity of anthracycline-free targeted oncological therapies in HER2-positive breast cancer (Review) Oncol. Lett. 2021;21:100. doi: 10.3892/ol.2020.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferreira de Souza T, Quinaglia T, Neilan TG, Coelho-Filho OR. Assessment of cardiotoxicity of cancer chemotherapy: the value of cardiac MRI. Magn. Reson. Imaging Clin. N. Am. 2019;27:533. doi: 10.1016/j.mric.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mehta LS, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J. Thorac. Dis. 2018;10:S4282–S4295. doi: 10.21037/jtd.2018.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bi J, Garg V, Yates AR. Galectin-3 and sST2 as prognosticators for heart failure requiring extracorporeal life support: Jack n’ Jill. Biomolecules. 2021;11:1–10. doi: 10.3390/biom11020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Henri C, Heinonen T, Tardif J-C. The role of biomarkers in decreasing risk of cardiac toxicity after cancer therapy. Biomark. Cancer. 2016;8s2:39–45. doi: 10.4137/BIC.S31798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bargieł W, et al. Recognized and potentially new biomarkers—their role in diagnosis and prognosis of cardiovascular disease. Medicina. 2021;57:701. doi: 10.3390/medicina57070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ancker OV, Wehland M, Bauer J, Infanger M, Grimm D. The adverse effect of hypertension in the treatment of thyroid cancer with multi-kinase inhibitors. Int. J. Mol. Sci. 2017;18:625. doi: 10.3390/ijms18030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pophali PA, Patnaik MM. The role of new tyrosine kinase inhibitors in chronic myeloid leukemia. Cancer J. 2016;22:40. doi: 10.1097/PPO.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haguet H, et al. The risk of arterial thrombosis in patients with chronic myeloid leukemia treated with second and third generation BCR-ABL tyrosine kinase inhibitors may be explained by their impact on endothelial cells: an in-vitro study. Front. Pharmacol. 2020;11:1007. doi: 10.3389/fphar.2020.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khan TH, Farooqui FA, Niazi K. Critical review of the ankle brachial index. Curr. Cardiol. Rev. 2008;4:101. doi: 10.2174/157340308784245810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Steegmann JL, et al. European LeukemiaNet recommendations for the management and avoidance of adverseevents of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Galderisi M, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur. Hear. J. Cardiovasc. Imaging. 2017;18:1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 139.Muraru D, et al. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur. Hear. J. Cardiovasc. Imaging. 2016;17:1279–1289. doi: 10.1093/ehjci/jev309. [DOI] [PubMed] [Google Scholar]
- 140.Salem JE, et al. Cardiovascular toxicities associated with ibrutinib. J. Am. Coll. Cardiol. 2019;74:1667–1678. doi: 10.1016/j.jacc.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 141.Ramspek CL, Jager KJ, Dekker FW, Zoccali C, Van DIepen M. External validation of prognostic models: what, why, how, when and where? Clin. Kidney J. 2021;14:49. doi: 10.1093/ckj/sfaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mouhayar E, Salahudeen A. Hypertension in cancer patients. Tex. Hear. Inst. J. 2011;38:263. [PMC free article] [PubMed] [Google Scholar]
- 143.Markman TM, Markman M. Cardio-oncology: management of cardiovascular toxicity. F1000Research. 2019;7:113. doi: 10.12688/f1000research.12598.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Porta-Sánchez A, et al. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J. Am. Heart Assoc. 2017;6:e007724. doi: 10.1161/JAHA.117.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chai KL, et al. Practical recommendations for the choice of anticoagulants in the management of patients with atrial fibrillation on ibrutinib. Leuk. Lymphoma. 2017;58:2811–2814. doi: 10.1080/10428194.2017.1315115. [DOI] [PubMed] [Google Scholar]
- 146.Lee H, Basso IN, Kim DDH. Target spectrum of the BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia. Int. J. Hemat. 2021;113:632–641. doi: 10.1007/s12185-021-03126-6. [DOI] [PubMed] [Google Scholar]
- 147.Kearon C, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 148.Bozkurt B, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure) Circ. Cardiovasc. Qual. Outcomes. 2021;14:E000102. doi: 10.1161/HCQ.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bikiewicz A, Banach M, von Haehling S, Maciejewski M, Bielecka-Dabrowa A. Adjuvant breast cancer treatments cardiotoxicity and modern methods of detection and prevention of cardiac complications. Esc. Heart Fail. 2021;8:2397–2418. doi: 10.1002/ehf2.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herrmann J, et al. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin. Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie Y, et al. Breast cancer survivorship and cardiovascular disease: emerging approaches in cardio-oncology. Curr. Treat. Options Cardiovasc. Med. 2015;17:60. doi: 10.1007/s11936-015-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Plana JC, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 153.De Leo A, Di Simone E, Spano A, Puliani G, Petrone F. Nursing management and adverse events in thyroid cancer treatments with tyrosine kinase inhibitors. A narrative review. Cancers. 2021;13:5961. doi: 10.3390/cancers13235961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J. Thyroid. Res. 2011;2011:985780. doi: 10.4061/2011/985780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu, C. et al. Clinical characteristics and outcomes of tyrosine kinase inhibitor-related lower GI adverse effects. J. Cancer Res. Clin. Oncol. 1–12 (2022). [DOI] [PMC free article] [PubMed]
- 156.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr. Oncol. 2011;18:126–138. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maroun JA, et al. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr. Oncol. 2007;14:13–20. doi: 10.3747/co.2007.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hartmann J, Haap M, Kopp H-G, Lipp H-P. Tyrosine Kinase Inhibitors—a review on pharmacology, metabolism and side effects. Curr. Drug Metab. 2009;10:470–481. doi: 10.2174/138920009788897975. [DOI] [PubMed] [Google Scholar]
- 160.Flaig TW, et al. Colonic pneumatosis and intestinal perforations with sunitinib treatment for renal cell carcinoma. Invest. N. Drugs. 2009;27:83–87. doi: 10.1007/s10637-008-9146-z. [DOI] [PubMed] [Google Scholar]
- 161.Hur H, et al. Perforation of the colon by invading recurrent gastrointestinal stromal tumors during sunitinib treatment. World J. Gastroenterol. 2008;14:6096–6099. doi: 10.3748/wjg.14.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Walraven M, et al. Antiangiogenic tyrosine kinase inhibition related gastrointestinal perforations: a case report and literature review. Angiogenesis. 2011;14:135–141. doi: 10.1007/s10456-010-9197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Secombe KR, et al. Diarrhea induced by small molecule tyrosine kinase inhibitors compared with chemotherapy: potential role of the microbiome. Integr. Cancer Ther. 2020;19:1534735420928493. doi: 10.1177/1534735420928493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lu JF, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin. Pharmacol. Ther. 2006;80:136–145. doi: 10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 165.Glabbeke MVan, et al. Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: a study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Gr. Eur. J. Cancer. 2006;42:2277–2285. doi: 10.1016/j.ejca.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 166.Płużański, A. & Piórek, A. Side effects of tyrosine kinase inhibitors-management guidelines. Oncol. Clin. Pr. 12, 113–118 (2016).
- 167.Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia. Curr. Hematol. Malig. Rep. 2016;11:71–79. doi: 10.1007/s11899-016-0309-2. [DOI] [PubMed] [Google Scholar]
- 168.Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol. Am. Soc. Hematol. Educ. Program. 2020;20:336–345. doi: 10.1182/hematology.2020000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chae YK, et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget. 2017;8:16052–16074. doi: 10.18632/oncotarget.14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lacouture ME, et al. Dermatologic adverse events associated with selective fibroblast growth factor receptor inhibitors: overview, prevention, and management guidelines. Oncologist. 2021;26:e316–e326. doi: 10.1002/onco.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Keller-von Amsberg G, Schafhausen P. Bosutinib in the management of chronic myelogenous leukemia. Biologics. 2013;7:115–122. doi: 10.2147/BTT.S30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sasaki K, et al. Clinical safety and efficacy of nilotinib or dasatinib in patients with newly diagnosed chronic-phase chronic myelogenous leukemia and pre-existing liver and/or renal dysfunction. Clin. Lymphoma Myeloma Leuk. 2016;16:152–162. doi: 10.1016/j.clml.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rea D. Management of adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukemia. Ann. Hematol. 2015;94:149–158. doi: 10.1007/s00277-015-2318-y. [DOI] [PubMed] [Google Scholar]
- 174.Tao G, Chityala PK. Epidermal growth factor receptor inhibitor-induced diarrhea: Clinical incidence, toxicological mechanism, and management. Toxicol. Res. 2021;10:476–486. doi: 10.1093/toxres/tfab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat. Rev. 2019;77:20–28. doi: 10.1016/j.ctrv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 176.Chen P, Chen F, Zhou B. Therapeutic efficacy and safety of combined BRAF and MEK inhibition in patients with malignant melanoma: a meta-analysis. Onco. Targets Ther. 2017;10:5391–5403. doi: 10.2147/OTT.S147438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang JCH, et al. Diarrhea associated with afatinib: An oral ErbB family blocker. Expert Rev. Anticancer Ther. 2013;13:729–736. doi: 10.1586/era.13.31. [DOI] [PubMed] [Google Scholar]
- 178.Som A, et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J. Clin. Cases. 2019;7:405–418. doi: 10.12998/wjcc.v7.i4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Heinzerling L, et al. Tolerability of BRAF/MEK inhibitor combinations: Adverse event evaluation and management. ESMO Open. 2019;4:491. doi: 10.1136/esmoopen-2019-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang Z, et al. Comparison of hepatotoxicity associated with new BCR-ABL tyrosine kinase inhibitors vs imatinib among patients with chronic myeloid leukemia: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4:2120165. doi: 10.1001/jamanetworkopen.2021.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saglio G, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 182.Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf. 2013;36:491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 183.Omar NE, et al. Postmarketing safety of anaplastic lymphoma kinase (ALK) inhibitors: an analysis of the FDA Adverse Event Reporting System (FAERS) ESMO Open. 2021;6:100315. doi: 10.1016/j.esmoop.2021.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tam CS, Ou YC, Trotman J, Opat S. Clinical pharmacology and PK/PD translation of the second-generation Bruton’s tyrosine kinase inhibitor, zanubrutinib. Expert Rev. Clin. Pharmacol. 2021;14:1329–1344. doi: 10.1080/17512433.2021.1978288. [DOI] [PubMed] [Google Scholar]
- 185.Xu CF, et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br. J. Cancer. 2010;102:1371–1377. doi: 10.1038/sj.bjc.6605653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Keisner SV, Shah SR. Pazopanib: the newest tyrosine kinase inhibitor for the treatment of advanced or metastatic renal cell carcinoma. Drugs. 2011;71:443–454. doi: 10.2165/11588960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 187.Qian J, et al. Tyrosine kinase inhibitor-related hepatotoxicity in patients with advanced lung adenocarcinoma: a real-world retrospective study. Cancer Manag. Res. 2020;12:3293–3299. doi: 10.2147/CMAR.S237968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pezzilli R, Corinaldesi R, Morselli-Labate AM. Tyrosine kinase inhibitors and acute pancreatitis. J. Pancreas. 2010;11:291–293. [PubMed] [Google Scholar]
- 189.Pierce GF, et al. Platelet-derived growth factor and transforming growth factor-β enhance tissue repair activities by unique mechanisms. J. Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ueda T, et al. Vascular endothelial growth factor increases in serum and protects against the organ injuries in severe acute pancreatitis. J. Surg. Res. 2006;134:223–230. doi: 10.1016/j.jss.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 191.Pasquale R, et al. Case report: pleural effusion during tyrosine-kinase inhibitor treatment in chronic myeloid leukemia: Not only a dasatinib-related adverse event. Front. Oncol. 2022;12:1012268. doi: 10.3389/fonc.2022.1012268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J. Am. Acad. Dermatol. 2008;58:545–570. doi: 10.1016/j.jaad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 193.Ye L, Santarpia L, Gagel RF. The evolving field of tyrosine kinase inhibitors in the treatment of endocrine tumors. Endocr. Rev. 2010;31:578–599. doi: 10.1210/er.2009-0031. [DOI] [PubMed] [Google Scholar]
- 194.Zuo RC, et al. Cutaneous adverse effects associated with the tyrosine-kinase inhibitor cabozantinib. JAMA Dermatol. 2015;151:170–177. doi: 10.1001/jamadermatol.2014.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Terada T, Noda S, Inui KI. Management of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitors. Pharmacol. Ther. 2015;152:125–134. doi: 10.1016/j.pharmthera.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 196.Yamamoto N, Honma M, Suzuki H. Off-target serine/threonine kinase 10 inhibition by erlotinib enhances lymphocytic activity leading to severe skin disorders. Mol. Pharmacol. 2011;80:466–475. doi: 10.1124/mol.110.070862. [DOI] [PubMed] [Google Scholar]
- 197.Pretel-Irazabal M, Tuneu-Valls A, Ormaechea-Pérez N. Adverse skin effects of imatinib, a tyrosine kinase inhibitor. Actas Dermosifiliogr. 2014;105:655–662. doi: 10.1016/j.ad.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 198.Dib EG, Ifthikharuddin JJ, Scott GA, Partilo SR. Neutrophilic eccrine hidradenitis induced by imatinib mesylate (Gleevec) therapy. Leuk. Res. 2005;29:233–234. doi: 10.1016/j.leukres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 199.Ayirookuzhi SJ, Ma L, Ramshesh P, Mills G. Imatinib-induced sweet syndrome in a patient with chronic myeloid leukemia. Arch. Dermatol. 2005;141:368–370. doi: 10.1001/archderm.141.3.368. [DOI] [PubMed] [Google Scholar]
- 200.Iberri DJ, et al. Ibrutinib-associated rash: a single-centre experience of clinicopathological features and management. Br. J. Haematol. 2018;180:164–166. doi: 10.1111/bjh.14302. [DOI] [PubMed] [Google Scholar]
- 201.Sibaud V, et al. Dermatological toxicities of Bruton’s tyrosine kinase inhibitors. Am. J. Clin. Dermatol. 2020;21:799–812. doi: 10.1007/s40257-020-00535-x. [DOI] [PubMed] [Google Scholar]
- 202.Hsiao LT, et al. Stevens-Johnson syndrome after treatment with STI571: A case report. Br. J. Haematol. 2002;117:620–622. doi: 10.1046/j.1365-2141.2002.03499.x. [DOI] [PubMed] [Google Scholar]
- 203.Nelson RP, et al. Desensitization to imatinib in patients with leukemia. Ann. Allergy, Asthma Immunol. 2006;97:216–222. doi: 10.1016/S1081-1206(10)60016-6. [DOI] [PubMed] [Google Scholar]
- 204.Hamberg P, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J. Clin. Oncol. 2006;24:e30–e31. doi: 10.1200/JCO.2006.06.5318. [DOI] [PubMed] [Google Scholar]
- 205.Hägerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec®): role of ‐cell NF‐KB activation and anti‐apoptotic preconditioning. FASEB J. 2007;21:618–628. doi: 10.1096/fj.06-6910com. [DOI] [PubMed] [Google Scholar]
- 206.Billemont B, et al. Blood glucose levels in patients with metastatic renal cell carcinoma treated with sunitinib. Br. J. Cancer. 2008;99:1380–1382. doi: 10.1038/sj.bjc.6604709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lodish MB, Stratakis CA. Endocrine side effects of broad-acting kinase inhibitors. Endocr. Relat. Cancer. 2010;17:R233. doi: 10.1677/ERC-10-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deininger MWN, O’Brien SG, Ford JM, Druker BJ. Practical management of patients with chronic myeloid leukemia receiving imatinib. J. Clin. Oncol. 2003;21:1637–1647. doi: 10.1200/JCO.2003.11.143. [DOI] [PubMed] [Google Scholar]
- 209.Berman E, et al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N. Engl. J. Med. 2006;354:2006–2013. doi: 10.1056/NEJMoa051140. [DOI] [PubMed] [Google Scholar]
- 210.O’Sullivan S, et al. Decreased bone turnover despite persistent secondary hyperparathyroidism during prolonged treatment with imatinib. J. Clin. Endocrinol. Metab. 2009;94:1131–1136. doi: 10.1210/jc.2008-2324. [DOI] [PubMed] [Google Scholar]
- 211.Vandyke K, et al. The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J. Bone Miner. Res. 2010;25:1759–1770. doi: 10.1002/jbmr.85. [DOI] [PubMed] [Google Scholar]
- 212.Torino F, Corsello SM, Longo R, Barnabei A, Gasparini G. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat. Rev. Clin. Oncol. 2009;6:219–228. doi: 10.1038/nrclinonc.2009.4. [DOI] [PubMed] [Google Scholar]
- 213.De Groot JWB, Zonnenberg BA, Plukker JTM, Van Der Graaf WTA, Links TP. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin. Pharmacol. Ther. 2005;78:433–438. doi: 10.1016/j.clpt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 214.Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 215.Rock EP, et al. Food and drug administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107–113. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 216.Ballardini P, Margutti G, Aliberti C, Manfredini R. Onset of male gynaecomastia in a patient treated with sunitinib for metastatic renal cell carcinoma. Clin. Drug Investig. 2009;29:487–490. doi: 10.2165/00044011-200929070-00007. [DOI] [PubMed] [Google Scholar]
- 217.Cortes JE, et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-Naïve chronic myeloid leukemia patients trial. J. Clin. Oncol. 2016;34:2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brümmendorf TH, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: Results from the 24-month follow-up of the BELA trial. Br. J. Haematol. 2015;168:69–81. doi: 10.1111/bjh.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Bergeron A, et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: A case series. Am. J. Respir. Crit. Care Med. 2007;176:814–818. doi: 10.1164/rccm.200705-715CR. [DOI] [PubMed] [Google Scholar]
- 220.Goldblatt M, Huggins JT, Doelken P, Gurung P, Sahn SA. Dasatinib-induced pleural effusions: A lymphatic network disorder? Am. J. Med. Sci. 2009;338:414–417. doi: 10.1097/MAJ.0b013e3181ae9227. [DOI] [PubMed] [Google Scholar]
- 221.Rousselot P, et al. Dasatinib dose optimisation based on therapeutic drug monitoring reduces pleural effusion rates in chronic myeloid leukaemia patients. Br. J. Haematol. 2021;194:393–402. doi: 10.1111/bjh.17654. [DOI] [PubMed] [Google Scholar]
- 222.Suh CH, et al. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer. 2018;123:60–69. doi: 10.1016/j.lungcan.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 223.Kanemura H, Takeda M, Shimizu S, Nakagawa K. Interstitial lung disease associated with capmatinib therapy in a patient with non-small cell lung cancer harboring a skipping mutation of MET exon 14. Thorac. Cancer. 2021;12:549–552. doi: 10.1111/1759-7714.13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hashiguchi, M. H. et al. Successful tepotinib challenge after capmatinib-induced interstitial lung disease in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation: case report. JTO Clin. Res. Rep.3, (2022). [DOI] [PMC free article] [PubMed]
- 225.Le X, et al. Tepotinib efficacy and safety in patients with met exon 14 skipping NSCLC: outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin. Cancer Res. 2022;28:1117–1126. doi: 10.1158/1078-0432.CCR-21-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ohmori T, et al. Molecular and clinical features of egfr-tki-associated lung injury. Int. J. Mol. Sci. 2021;22:1–18. doi: 10.3390/ijms22020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tseng LW, Chang JWC, Wu CE. Safety of tepotinib challenge after capmatinib-induced pneumonitis in a patient with non-small cell lung cancer harboring MET exon 14 skipping mutation: a case report. Int. J. Mol. Sci. 2022;23:11809. doi: 10.3390/ijms231911809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fujioka I, et al. Features of vascular adverse events in Japanese patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a retrospective study of the CML Cooperative Study Group database. Ann. Hematol. 2018;97:2081–2088. doi: 10.1007/s00277-018-3412-8. [DOI] [PubMed] [Google Scholar]
- 229.Suh KJ, et al. Analysis of adverse events associated with dasatinib and nilotinib treatments in chronic-phase chronic myeloid leukemia patients outside clinical trials. Int. J. Hematol. 2017;106:229–239. doi: 10.1007/s12185-017-2225-1. [DOI] [PubMed] [Google Scholar]
- 230.Emir H, et al. Nilotinib exerts direct pro-atherogenic and anti-angiogenic effects on vascular endothelial cells: a potential explanation for drug-induced vasculopathy In CML. Blood. 2013;122:257–257. doi: 10.1182/blood.V122.21.257.257. [DOI] [Google Scholar]
- 231.Pasvolsky O, et al. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardio-Oncol. 2015;1:1–10. doi: 10.1186/s40959-015-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pardanani A, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1:643–651. doi: 10.1001/jamaoncol.2015.1590. [DOI] [PubMed] [Google Scholar]
- 233.Hadzijusufovic E, et al. Nilotinib-induced vasculopathy: Identification of vascular endothelial cells as a primary target site. Leukemia. 2017;31:2388–2397. doi: 10.1038/leu.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Aprile L, et al. Atherothrombotic Risk and TKIs treatment in chronic myeloid leukemia patients: a role for genetic predisposition and pro-inflammatory/pro-oxidative status? Blood. 2013;122:1482–1482. doi: 10.1182/blood.V122.21.1482.1482. [DOI] [Google Scholar]
- 235.Gover-Proaktor A, et al. Pathogenesis of ponatinib associated vascular disease in chronic myeloid leukemia: an in vitro study. Blood. 2015;126:2798–2798. doi: 10.1182/blood.V126.23.2798.2798. [DOI] [Google Scholar]
- 236.Rea D, et al. Usefulness of the 2012 European CVD risk assessment model to identify patients at high risk of cardiovascular events during nilotinib therapy in chronic myeloid leukemia. Leukemia. 2015;29:1206–1209. doi: 10.1038/leu.2014.342. [DOI] [PubMed] [Google Scholar]
- 237.Pinilla-Ibarz J, et al. Clinical impact of dose modification on response to ponatinib in patients with chronic phase chronic myeloid leukemia (CP-CML) Blood. 2013;122:4007–4007. doi: 10.1182/blood.V122.21.4007.4007. [DOI] [Google Scholar]
- 238.Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011;80:1271–1277. doi: 10.1038/ki.2011.288. [DOI] [PubMed] [Google Scholar]
- 239.Teuma C, et al. Adjunction of a MEK inhibitor to Vemurafenib in the treatment of metastatic melanoma results in a 60% reduction of acute kidney injury. Cancer Chemother. Pharmacol. 2017;79:1043–1049. doi: 10.1007/s00280-017-3300-2. [DOI] [PubMed] [Google Scholar]
- 240.Launay-Vacher V, et al. Acute renal failure associated with the new BRAF inhibitor vemurafenib: A case series of 8 patients. Cancer. 2014;120:2158–2163. doi: 10.1002/cncr.28709. [DOI] [PubMed] [Google Scholar]
- 241.Wanchoo R, Jhaveri KD, Deray G, Launay-Vacher V. Renal effects of BRAF inhibitors: A systematic review by the Cancer and the Kidney International Network. Clin. Kidney J. 2016;9:245–251. doi: 10.1093/ckj/sfv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jhaveri KD, Sakhiya V, Fishbane S. Nephrotoxicity of the BRAF inhibitors vemurafenib and dabrafenib. JAMA Oncol. 2015;1:1133–1134. doi: 10.1001/jamaoncol.2015.1713. [DOI] [PubMed] [Google Scholar]
- 243.Choe CH, McArthur GA, Caro I, Kempen JH, Amaravadi RK. Ocular toxicity in BRAF mutant cutaneous melanoma patients treated with vemurafenib. Am. J. Ophthalmol. 2014;158:831–837.e2. doi: 10.1016/j.ajo.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 244.Rogers S, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313. doi: 10.1016/j.ophtha.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Francis JH, et al. Clinical and morphologic characteristics of MEK inhibitor–associated retinopathy: differences from central serous chorioretinopathy. Ophthalmology. 2017;124:1788–1798. doi: 10.1016/j.ophtha.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Claiborne RT, Tsan GL. Case report: erdafitinib-induced central serous chorioretinopathy. Optom. Vis. Sci. 2022;99:88–92. doi: 10.1097/OPX.0000000000001833. [DOI] [PubMed] [Google Scholar]
- 247.Mato AR, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the united states: a real-world analysis. Haematologica. 2018;103:874–879. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur. J. Haematol. 2018;100:325–334. doi: 10.1111/ejh.13020. [DOI] [PubMed] [Google Scholar]
- 249.Dubovsky JA, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Borge M, et al. Ibrutinib impairs the phagocytosis of rituximab-coated leukemic cells from chronic lymphocytic leukemia patients by human macrophages. Haematologica. 2015;100:e140–e142. doi: 10.3324/haematol.2014.119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-ĸB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132:1985–1988. doi: 10.1182/blood-2017-12-823393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ryan CE, Cheng MP, Issa NC, Brown JR, Davids MS. Pneumocystis jirovecii pneumonia and institutional prophylaxis practices in CLL patients treated with BTK inhibitors. Blood Adv. 2020;4:1458–1463. doi: 10.1182/bloodadvances.2020001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood. 2019;133:1298–1307. doi: 10.1182/blood-2018-11-846808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Brown JR. How i treat CLL patients with ibrutinib. Blood. 2018;131:379–386. doi: 10.1182/blood-2017-08-764712. [DOI] [PubMed] [Google Scholar]
- 255.Pleyer C, et al. Response to the Shingrix Varicella Zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I) Blood. 2019;134:3053–3053. doi: 10.1182/blood-2019-121675. [DOI] [Google Scholar]
- 256.Fachi MM, et al. Haematological adverse events associated with tyrosine kinase inhibitors in chronic myeloid leukaemia: a network meta-analysis. Br. J. Clin. Pharmacol. 2019;85:2280–2291. doi: 10.1111/bcp.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ostojic A, Vrhovac R, Verstovsek S. Ruxolitinib for the treatment of myelofibrosis: Its clinical potential. Ther. Clin. Risk. Manag. 2012;8:95–103. doi: 10.2147/TCRM.S23277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Galanis A, Levis M. Inhibition of c-Kit by tyrosine kinase inhibitors. Haematologica. 2015;100:e77. doi: 10.3324/haematol.2014.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sillaber C, et al. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur. J. Clin. Invest. 2009;39:1098–1109. doi: 10.1111/j.1365-2362.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 260.Henkes M, van der Kuip H, Aulitzky WE. Therapeutic options for chronic myeloid leukemia: Focus on imatinib (Glivec®, GleevecTM) Ther. Clin. Risk. Manag. 2008;4:163–187. doi: 10.2147/TCRM.S1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Quintás-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261–263. doi: 10.1182/blood-2008-09-180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gratacap MP, et al. The new tyrosine-kinase inhibitor and anticancer drug dasatinib reversibly affects platelet activation in vitro and in vivo. Blood. 2009;114:1884–1892. doi: 10.1182/blood-2009-02-205328. [DOI] [PubMed] [Google Scholar]
- 263.Galinsky I, Buchanan S. Practical management of dasatinib for maximum patient benefit. Clin. J. Oncol. Nurs. 2009;13:329–335. doi: 10.1188/09.CJON.329-335. [DOI] [PubMed] [Google Scholar]
- 264.Quintás-Cardama A, et al. Dynamics and management of cytopenias associated with dasatinib therapy in patients with chronic myeloid leukemia in chronic phase after imatinib failure. Cancer. 2009;115:3935–3943. doi: 10.1002/cncr.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.O’Brien S, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin. Lymphoma Myeloma Leuk. 2018;18:648–657.e15. doi: 10.1016/j.clml.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 266.Sharman JP, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278–1291. doi: 10.1016/S0140-6736(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Dobie G, et al. Ibrutinib, but not zanubrutinib, induces platelet receptor shedding of GPIb-IX-V complex and integrin αIIbβ3 in mice and humans. Blood Adv. 2019;3:4298–4311. doi: 10.1182/bloodadvances.2019000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhang Y, Diamond SL. Src family kinases inhibition by dasatinib blocks initial and subsequent platelet deposition on collagen under flow, but lacks efficacy with thrombin generation. Thromb. Res. 2020;192:141–151. doi: 10.1016/j.thromres.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yim E, Choi YG, Nam YJ, Lee J, Kim JA. Dasatinib induces severe hemorrhagic colitis in a patient with accelerated phase of chronic myelogenous leukemia. Korean J. Intern. Med. 2018;33:446–448. doi: 10.3904/kjim.2015.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mazharian A, Ghevaert C, Zhang L, Massberg S, Watson SP. Dasatinib enhances megakaryocyte differentiation but inhibits platelet formation. Blood. 2011;117:5198–5206. doi: 10.1182/blood-2010-12-326850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brown JR, et al. Incidence of and risk factors for major haemorrhage in patients treated with ibrutinib: An integrated analysis. Br. J. Haematol. 2019;184:558–569. doi: 10.1111/bjh.15690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jones JA, et al. Use of anticoagulants and antiplatelet in patients with chronic lymphocytic leukaemia treated with single‐agent ibrutinib. Br. J. Haematol. 2017;178:286. doi: 10.1111/bjh.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shatzel JJ, et al. Ibrutinib-associated bleeding: pathogenesis, management and risk reduction strategies. J. Thromb. Haemost. 2017;15:835–847. doi: 10.1111/jth.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Byrd JC, et al. Acalabrutinib monotherapy in patients with relapsed/ refractory chronic lymphocytic leukemia: Updated phase 2 results. Blood. 2020;135:1204–1213. doi: 10.1182/blood.2018884940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pilot PR, Sablinska K, Owen S, Hatfield A. Epidemiological analysis of second primary malignancies in more than 9500 patients treated with imatinib. Leukemia. 2006;20:148. doi: 10.1038/sj.leu.2404025. [DOI] [PubMed] [Google Scholar]
- 276.Verma D, et al. Malignancies occurring during therapy with tyrosine kinase inhibitors (TKIs) for chronic myeloid leukemia (CML) and other hematologic malignancies. Blood. 2011;118:4353–4358. doi: 10.1182/blood-2011-06-362889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Togasaki-Yoshimoto E, Shono K, Onoda M, Yokota A. The occurrence of second neoplasms after treatment with tyrosine kinase inhibitors for chronic myeloid leukemia. Leuk. Lymphoma. 2014;55:453–456. doi: 10.3109/10428194.2013.806805. [DOI] [PubMed] [Google Scholar]
- 278.Dou X, Qin Y, Huang X, Jiang Q. Planned pregnancy in female patients with chronic myeloid leukemia receiving tyrosine kinase inhibitor therapy. Oncologist. 2019;24:e1141–e1147. doi: 10.1634/theoncologist.2019-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Robinson AA, Watson WJ, Leslie KK. Targeted treatment using monoclonal antibodies and tyrosine-kinase inhibitors in pregnancy. Lancet Oncol. 2007;8:738–743. doi: 10.1016/S1470-2045(07)70242-5. [DOI] [PubMed] [Google Scholar]
- 280.Rambhatla A, Strug MR, De Paredes JG, Cordoba Munoz MI, Thakur M. Fertility considerations in targeted biologic therapy with tyrosine kinase inhibitors: a review. J. Assist. Reprod. Genet. 2021;38:1897–1908. doi: 10.1007/s10815-021-02181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abruzzese E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine kinase inhibitors and pregnancy. Mediterr. J. Hematol. Infect. Dis. 2014;6:2014028. doi: 10.4084/mjhid.2014.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Granatowicz A, et al. An overview and update of chronic myeloid leukemia for primary care physicians. Korean J. Fam. Med. 2015;36:197–202. doi: 10.4082/kjfm.2015.36.5.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Deng S, Solinas A, Calvisi DF. Cabozantinib for HCC treatment, from clinical back to experimental models. Front. Oncol. 2021;11:756672. doi: 10.3389/fonc.2021.756672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hakkola J, Hukkanen J, Turpeinen M, Pelkonen O. Inhibition and induction of CYP enzymes in humans: an update. Arch. Toxicol. 2020;94:3671–3722. doi: 10.1007/s00204-020-02936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang X, et al. The drug-drug interaction of sorafenib mediated by P-glycoprotein and CYP3A4. Xenobiotica. 2016;46:651–658. doi: 10.3109/00498254.2015.1109160. [DOI] [PubMed] [Google Scholar]
- 286.Xiang QF, et al. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int. 2015;35:1010–1023. doi: 10.1111/liv.12524. [DOI] [PubMed] [Google Scholar]
- 287.Barresi V, Musmeci C, Rinaldi A, Condorelli DF. Transcript-targeted therapy based on RNA interference and antisense oligonucleotides: current applications and novel molecular targets. Int. J. Mol. Sci. 2022;23:8875. doi: 10.3390/ijms23168875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zogg H, Singh R, Ro S. Current advances in RNA therapeutics for human diseases. Int. J. Mol. Sci. 2022;23:2736. doi: 10.3390/ijms23052736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Taylor MH, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J. Clin. Oncol. 2020;38:1154. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chau V, Bilusic M. Pembrolizumab in combination with axitinib as first-line treatment for patients with renal cell carcinoma (RCC): evidence to date. Cancer Manag. Res. 2020;12:7321. doi: 10.2147/CMAR.S216605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rini BI, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 292.Thomas A, Teicher BA, Hassan R. Antibody–drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–e262. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yaghoubi S, et al. Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy. J. Cell. Physiol. 2020;235:31–64. doi: 10.1002/jcp.28967. [DOI] [PubMed] [Google Scholar]
- 294.de la Torre BG, Albericio F. The pharmaceutical industry in 2021. An analysis of FDA drug approvals from the perspective of molecules. Molecules. 2022;27:1075. doi: 10.3390/molecules27031075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]
- 296.Kalash, R. S., Lakshmanan, V. K., Cho, C. S. & Park, I. K. Theranostics. Biomaterials Nanoarchitectonics. 197–215 (William Andrew Publishing, 2016).
- 297.Russo E, Spallarossa A, Tasso B, Villa C, Brullo C. Nanotechnology of tyrosine kinase inhibitors in cancer therapy: A perspective. Int. J. Mol. Sci. 2021;22:6538. doi: 10.3390/ijms22126538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Du W, Elemento O. Cancer systems biology: Embracing complexity to develop better anticancer therapeutic strategies. Oncogene. 2015;34:3215–3225. doi: 10.1038/onc.2014.291. [DOI] [PubMed] [Google Scholar]