Abstract
The neurobiological effects of mind–body exercise on brain activation, functional neural connections and structural changes in the brain remain elusive. This systematic review and coordinate-based meta-analysis investigated the changes in resting-state and task-based brain activation, as well as structural brain changes before and after mind–body exercise compared to waitlist or active controls based on published structural or functional magnetic resonance imaging randomized controlled trials or cross-sectional studies. Electronic database search and manual search in relevant publications yielded 34 empirical studies with low-to-moderate risk of bias (assessed by Cochrane risk-of-bias tool for randomized trials or Joanna Briggs Institute’s critical appraisal checklist for analytical cross-sectional studies) that fulfilled the inclusion criteria, with 26 studies included in the narrative synthesis and 8 studies included in the meta-analysis. Coordinate-based meta-analysis showed that, while mind–body exercise enhanced the activation of the left anterior cingulate cortex within the default mode network (DMN), it induced more deactivation in the left supramarginal gyrus within the ventral attention network (uncorrected ps < 0.05). Meta-regression with duration of mind–body practice as a factor showed that, the activation of right inferior parietal gyrus within the DMN showed a positive association with increasing years of practice (voxel-corrected p < 0.005). Although mind–body exercise is shown to selectively modulate brain functional networks supporting attentional control and self-awareness, the overall certainty of evidence is limited by small number of studies. Further investigations are needed to understand the effects of both short-term and long-term mind–body exercise on structural changes in the brain.
PROSPERO registration number: CRD42021248984.
Subject terms: Neuroscience, Psychology, Health care
Introduction
Mind and body practices are complementary health approaches that focus on facilitating brain-behavior connections, which aim to promote health and well-being by enhancing the ability of the mind to regulate our physical bodies for optimal daily functioning1,2. Among many mind and body practices, mind–body exercise (i.e., Tai Chi, qigong and yoga) is a specific type of practice that incorporates meditation into the execution of movement routines to improve body balance and the flexibility and strength of the musculoskeletal structures3. Unlike conventional physical exercise that specifically emphasizes the awareness of bodily movements4, mind–body exercise emphasizes the coordination between breathing, bodily sensation awareness, and bodily movement execution5,6. According to a National Health Interview Survey conducted in the United States7, mind–body exercise is listed as one of the most common complementary health approaches among adults. Due to its popularity, researchers have continuously studied the beneficial effects of mind–body exercise on human health. Numerous meta-analytic reviews show that mind–body practices are effective in promoting motor, cognitive and affective functioning of both healthy and clinical populations. For instance, Tai Chi has been shown to be effective at improving motor control in patients with stroke8 and Parkinson’s disease9, promoting psychological well-being in older adults10 and enhancing cognitive functions in both healthy11,12 and clinical13,14 elderly populations. Similar positive clinical effects have been observed when qigong15–18 or yoga19–21 is performed.
Given the promising clinical effects of mind–body exercise, the neurobiological mechanisms associated with the observed behavioral changes have been rigorously studied in recent years22–24. Specifically, functional magnetic resonance imaging (fMRI) has been widely adopted to investigate how mind–body exercise changes brain activation patterns, connections between different regions25 and brain structures, and some studies have shown that mind–body exercise induces brain changes in the frontoparietal regions. For example, Eyre, Acevedo26 showed that a 12-week yoga training program significantly reduced depressive symptoms and improved visuospatial memory in older adults with mild cognitive impairment compared to conventional cognitive training, with enhanced visuospatial memory performance significantly correlated with a reduction in the functional connectivity between the superior and medial parietal cortices that implies greater neural efficiency26 for visual attention and working memory27. Greater activation in the left ventrolateral prefrontal cortex (PFC), a brain region known for inhibitory control28, was found in long-term yoga practitioners compared to age-matched healthy individuals without regular yoga practice when these individuals were presented negative emotional stimuli29. Tao, Chen30 showed that 12-week Tai Chi and qigong practices in older adults increased regional spontaneous neuronal activity in the dorsolateral PFC and the medial PFC, respectively, which was associated with enhanced memory performance. Moreover, significant increases in both whole-brain white matter31 and hippocampal gray matter32 are evident after long-term mind–body practice. However, statistically negative results have also been reported; some studies reported no statistically significant exercise-induced changes in brain activation or functional connectivity patterns were found between the yoga33/Tai Chi34 practice group and the control group.
Notably, the experimental protocols and fMRI outcome measures vary substantially across the existing empirical studies, which might contribute to inconsistent results. For instance, the duration of mind–body practice in different study protocols is highly heterogeneous. Some studies have investigated the neurobiological effects of long-term mind–body practice33,35, but other researchers have examined how several weeks of mind–body practice potentially modify neural activities26,34,36. Moreover, the nature of the control group also varies. In addition, these empirical studies adopted different methods to analyze fMRI data. Some studies investigated the changes in spontaneous neuronal fluctuations before and after mind–body exercises37–39, whereas others investigated the changes in neural connectivity in different regions of interest, including the default mode network (DMN)40 and parietal regions26. Collectively, these differences might complicate interpretations of the overall results, hence limiting our understanding of the neurobiological effects of mind–body practice. Systematic reviews of previously published studies and neuroimaging meta-analyses might help us address the limitations described above. Specifically, using seed-based d mapping with permutation of subject images (SDM-PSI)41, the between-study experimental design heterogeneity discussed above could be controlled by covariate analyses and further explored with meta-regression. In addition, registering consistently coactivated brain regions identified through the meta-analysis on a standardized brain atlas may be possible42, which might help us identify which functional brain networks are consistently modulated by mind–body practice. Finally, the overall study power for the identification of consistently coactivated brain regions by mind–body practice may be enhanced by performing a meta-analysis. Given the aforementioned research gaps, this study aimed to investigate the neurobiological changes (i.e., brain activation, neural connections and structural changes in the brain) associated with mind–body exercise by qualitatively and quantitatively examining currently available fMRI data.
Methods
Literature search
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines43 were used to guide the implementation of this review (PROSPERO registration number: CRD42021248984; see Table S1 for the PRISMA checklist and access the review protocol via https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=248984). All authors first decided on the keywords and electronic databases to search, followed by the first title/abstract/keyword search of the electronic databases Scopus, Embase, ScienceDirect and PubMed conducted on April 2022 using the keywords (“mindful*” OR “mind–body” OR “yoga” OR “tai chi” OR “qigong”) AND (“magnetic resonance imaging” OR “functional magnetic resonance imaging” OR “MRI” OR “fMRI”) to identify relevant papers. Full search strategies were reported in Table S2. An updated search using the same keywords and search engines was performed in June 2023 to ensure the most up-to-date articles were included in the analysis. References from a previously published review44 were also screened. No limitations on the dates of publication were set.
Study inclusion for the systematic review and meta-analysis
The systematic review included published randomized controlled trials (RCTs) and cross-sectional studies investigating the effects of mind–body exercise compared to regular physical exercise (active control)/waitlist control groups. After removing duplicated records, titles and abstracts of the records were screened. Records that were (1) not empirical studies (e.g., review articles, book chapters, conference proceedings and editorials), (2) nonhuman studies and (3) not involving mind–body practice as an intervention were excluded. During the full-text screening, studies that (1) investigated the brain activations during meditative state only, (2) did not report standard stereotactic coordinate space results (either Talairach or MNI), and (3) did not compare the effects of mind–body exercise with a physical exercise/waitlist control group were excluded. Only studies that fulfilled all inclusion criteria were included in the systematic review. For meta-analysis, only fMRI studies comparing whole-brain seed-based resting-state functional connectivity (rsFC)45 between mind–body exercise and active/waitlist control groups were included. The whole screening process was conducted independently by the co-first, second and third authors and recorded separately on each Excel spreadsheet. The first author decided to include or exclude papers when discrepancies appeared. Corresponding authors of the included papers were contacted if there was missing or unclear information.
Data extraction and recoding
Data were independently extracted from the included papers and recorded by the second and third authors. The extracted data were recorded in an Excel spreadsheet independently by the co-first author, second and third authors and were cross-checked by the first author. Demographic characteristics (i.e., participants’ diagnoses, mean age, ratio of sex), experimental details (i.e., types of mind–body exercise, average duration of practice in years, and control group), and outcome measures (i.e., the MRI paradigm and analysis methods, main outcomes in the active group) were extracted. In particular for studies included in the meta-analysis, as we aimed to pool the peaks with significant group differences between mind–body exercise group and control groups reported in previous studies46,47, seed center coordinates and peak coordinates of between-group differences were extracted from each study.
Data recoding was performed to facilitate covariate analyses in the coordinate-base meta-analysis. First, to recode the seed center coordinates of individual studies in a standardized manner such that covariate analyses were possible, the seed center coordinates were registered within the seven resting-state brain networks reported by studies that used large-scale data from human cerebral48,49, cerebellar50, and striatal51 parcellation studies. The seven functional networks included the default mode network (DMN; coordinates other task-positive networks), somatomotor network (SMN; for motor control and execution), frontoparietal network (FPN; coordinates goal-directed behavior), dorsal attention network (DAN; for top-down attention control), ventral attention network (VAN; detects salient stimuli), limbic network (LIM; for emotional processing), and visual network (VIS; processes incoming visual information). Second, regarding the control group conditions, given some studies utilized control groups with participants performing regular physical exercise (e.g. treadmill walking/usual physical exercise regime) while other studies adopted waitlist control groups (e.g. not doing mind–body exercise while also not engaging in any kinds of physical exercise regime), studies with physical exercise as a control condition were recoded as having an “active control group”, while studies with waitlist control groups were recoded as having a "waitlist control group”.
Quality of reporting appraisal and risk of bias within the studies
The revised Cochrane risk-of-bias tool for randomized trials (RoB 2)52 and Joanna Briggs Institute’s (JBI) critical appraisal checklist for analytical cross-sectional studies53 were used to evaluate the methodological quality of the included RCTs and cross-sectional studies, respectively. Bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported result were assessed using RoB 2. Three risk of bias categories (low, high or some concerns) were possible for each domain. The overall rating of the five domains yielded the “overall risk-of-bias judgment” for each of the included studies. Using the JBI critical appraisal checklist, the included cross-sectional studies were qualified, as suggested by Ma, Wang54. By counting the number of “yes” responses from the critical appraisal items among the total items of the study, the quality of each study was then assessed. Criteria with “not applicable” responses were excluded, while “unclear” responses were coded as “no” and did not fulfill the quality criteria. “Yes” scores for 0–33% of the JBI items were assigned a “low” overall quality rating, while 34–66% “yes” scores from the JBI questions were assigned a “medium” rating, and 67% or more “yes” scores for the JBI items were assigned a “high” rating.
Data analysis
Narrative synthesis was performed for studies included in the systematic review. Effect sizes (Cohen’s d) and 95% confidence intervals (CI) were calculated using the formula reported in Lipsey and Wilson55 for all included experiments based on the significance threshold adopted by individual papers. the SDM-PSI software version 6.2141was used to pool the brain imaging data for the meta-analysis. The recommended data preprocessing pipeline was used41. Briefly, preprocessing of extracted fMRI coordinate data was performed with anisotropy = 1, isotropic full width at half maximum (FWHM) set to 20 mm, and a voxel size of 2 mm on a gray matter mask. The between-group analysis was performed with rsFC seed network as a covariate to investigate the effects of mind–body exercise on rsFC. Meta-regression analyses with mean age of participants (years) mean years of mind–body practice as independent variable and peak coordinates of between-group differences (mind–body exercise vs. control group) as dependent variable was performed to explore how duration of mind–body practice modulates the changes in brain activation. Regarding the significance threshold, we first identified significant clusters with the threshold p = 0.05 (uncorrected), Z > 1 and a cluster size (k) larger than 10 voxels, followed by performing voxel-wise error corrections using 1000 permutations to obtain the null distribution of cluster sizes that pass the threshold of p < 0.005, and that distribution was used to set a minimum cluster size56. Each of the local peaks within the significant clusters was classified based on their location within the seven functional networks to understand how mind–body exercise modulate the functional brain networks (i.e., visual, somatomotor, dorsal attention, ventral attention, frontoparietal, limbic and default mode networks). The heterogeneity between studies was indicated by I2 test with I2 = 25%, 50% and 75% corresponds to low, medium and high heterogeneity respectively57, which was calculated for statistically significant peak coordinates yielded from the between-group or meta-regression analyses. Harbord Egger bias tests58 were used to assess “small study effects”. Certainty assessment was conducted using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach59.
Results
Study selection
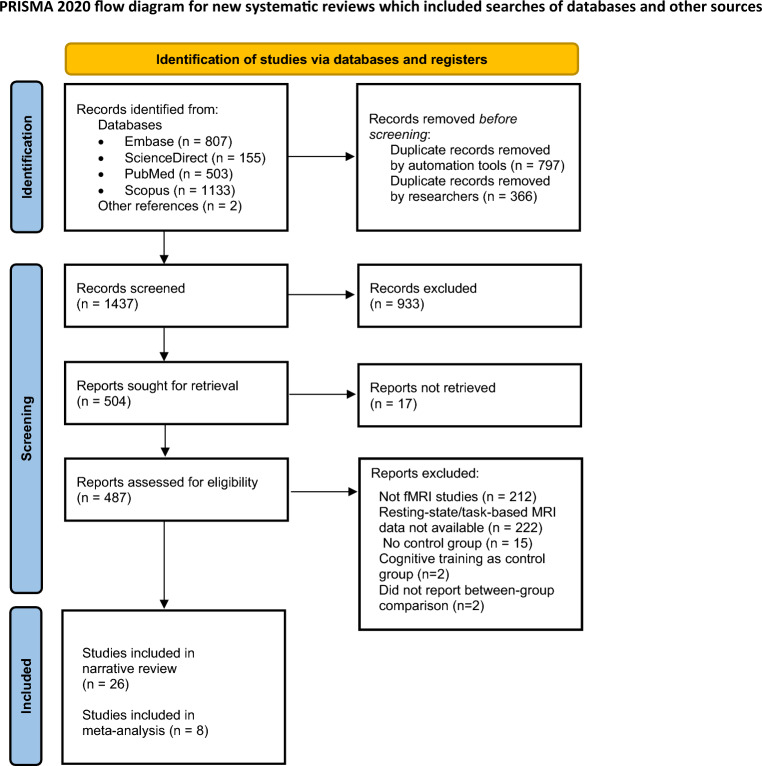
A total of 2600 records were retrieved from multiple electronic databases and references. After the removal of duplicate records, 1437 abstracts were screened. After the exclusion of nonempirical non-fMRI studies and studies that did not involve mind–body interventions, the full text of 487 studies was screened for inclusion in the systematic review. Based on the inclusion and exclusion criteria, 34 fMRI studies (with 42 comparisons) were included, with 26 studies included in the narrative synthesis and 8 studies (with 13 comparisons) included in the meta-analysis. The flow of the article selection process is outlined in Fig. 1.
Figure 1.
Flowchart of the article screening process.
Risk of bias within studies
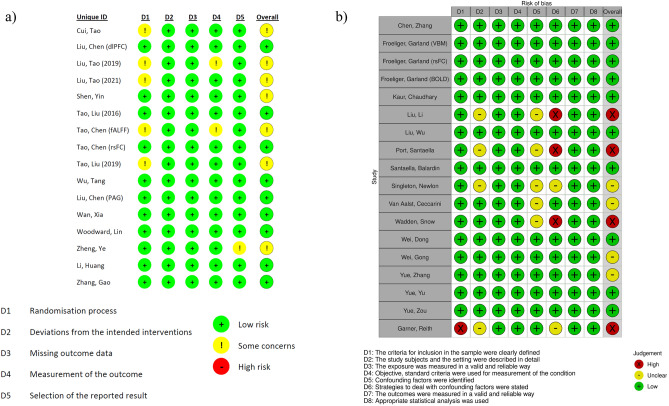
The summary of the authors’ judgment of the risk of bias of the included RCTs and cross-sectional studies is shown in Fig. 2. For the included RCTs (n = 16; Fig. 2a), nine studies had a low overall risk of bias, while the remaining seven studies warranted some concerns. All studies showed a low risk of bias in deviations from the intended interventions and missing outcome data. One study showed unclear bias in selective reporting of results. Unclear bias in outcome measurement was noted in two studies, and an unclear randomization process was noted in five studies. For the included cross-sectional studies (n = 18; Fig. 2b), four studies were rated as having an unclear overall risk of bias, and four studies were rated as having a high overall risk of bias. All studies showed a low risk of bias in defining participants’ inclusion criteria (D1), the use of valid, reliable and objective measurement tools (D3 and D4), outcome measurement (D7) and the choice of statistical analysis (D8). Four of 18 studies showed an unclear risk of bias in the participants’ demographic characteristics (D2). Five of 18 studies showed unclear risk of bias in the identification of confounding factors (D5). Four studies did not report strategies to control for the stated confounding factors (D6).
Figure 2.
Risk of bias summaries: review authors’ judgements about each risk of bias item for each included (a) RCT and (b) cross-sectional study.
Study characteristics
Table 1 lists the demographic characteristics, experimental design, neuroimaging outcome measures and results of the 35 studies included in this review. Detailed narrative and quantitative syntheses are presented in the subsequent paragraphs.
Table 1.
Effects of mind–body exercise on different neuroimaging outcomes (34 studies, 42 experiments).
| Reference (year) | Participants’ details | Experimental protocol | Outcome measures | Statistical thresholdb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population | N | Mean Age (years) | Sex (M:F) | Mind–body practice | Average duration of practice (years) | Control group | MRI paradigm | MRI analysis | Between-group resultsa (rsFC seed; if applicable) | Effect size in d [95%CI] | ||
| Experiments included in the narrative synthesis (26 studies, 29 comparisons) | ||||||||||||
| Chen15 | Healthy | 22 | 52.4 | 15:7 | TCC | 14 | Passive | Resting | rsFC (ROI-ROI) | ↓ R MFG–L MFG (DMN) | 0.643 [0.00470, 1.28] | Family-wise-error corrected p < 0.05 |
| 18 | 54.8 | 10:8 | ||||||||||
| Cui61 | Healthy | 12 | 21.8 | 2:10 | TCC | 0.167 | Active | Resting | Graph theory network analysis |
Nodal clustering coefficient ↑ L thalamus ↑ L olfactory cortex Nodal efficiency ↑ Bilateral posterior cingulate ↑ R cuneus Nodal local efficiency ↑ L thalamus ↑ R olfactory cortex |
0.85 [0.01, 1.68] | Cluster-corrected, p < 0.05 |
| 12 | 21.8 | 2:10 | ||||||||||
| Froeliger73 | Healthy | 7 | 36.4 | 6:1 | Yoga | 9.3 | Regular physical activity | Resting | VBM | ↑ GM volume in frontal, limbic, temporal, occipital, and cerebellar regions | 1.16 [0.03, 2.30] | Cluster-corrected, p < 0.05 |
| 7 | 35.5 | 6:1 | ||||||||||
| Froeliger35 | Resting | Network-based rsFC |
DAN within-network ↑ R anterior IPS–FEF ↑ R anterior IPS–L middle temporal cortex ↑ R posterior IPS–L middle temporal cortex ↑ R middle temporal cortex–L FEF |
|||||||||
| Froeliger29 |
(1) Emotional picture viewing task (2) Stroop task with distractor of negative emotion |
BOLD activation (ROI) |
↓ R dlPFC (negative–neutral) during emotion viewing ↑ L vlPFC (negative–neutral) during Stroop task |
|||||||||
| Garner75 | Healthy | 39 | 22.7 | 5:34 | Yoga | 0.2 | Strengthening/stretching exercise | Resting | VBM |
VBM GM change ↓ L insula ↓ R IFG ↓ L IPL, AG |
− 0.86 [− 1.44, − 0.28] | Cluster-corrected p < 0.05 |
| 32 | 22.9 | 1:31 | ||||||||||
| Kaur71 | Healthy | 13 | 27.2 | 6:7 | Yoga | 3 | Active lifestyle |
N-back task Stroop |
BOLD activation and FC (ROI) |
Activation changes 1-back ↑ STG ↑ L inferior semi lunar lobule ↑ Precentral gyrus ↑ Insula ↑ Parahippocampal gyrus 2-back ↑ MTG ↑ Left cerebellar tonsil Color stroop ↑ Inferior occipital gyrus ↑ Precuneus ↑ Parahippocampal gyrus ↑ Inferior semi lunar lobule ↑ Cerebellar tonsil ↑ STG Task-based FC changes 1-back ↑ L lPFC–R cerebellum ↑ R lPFC–R Insula ↓ L IPS–R PCG/R Insula/L cerebellum 2-back ↑ R FEF–L SFG ↑ L PFC–R cerebellum ↑ R IPS–Right R Cuneus cortex Color stroop ↑ FPN |
0.81 [0.01, 61] | Family-wise-error corrected p < 0.05 |
| 13 | 28.6 | 6:7 | ||||||||||
| Li64 | Parkinson’s disease | 32 | 62.7 | 17:15 | TCC | 1 | Walking | Resting | Resting-state network connectivity | ↑ Visual network associated with better functional balance performance | 0.60 [0.0035, 1.20] | Voxel-wise corrected p < 0.05 |
| 17 | n.r. | n.r. | ||||||||||
| Liu100 | Osteoarthritis | 28 | n.r. | 6:22 | TCC | 0.25 | Cycling | Resting | Seed to whole-brain rsFC |
PAG as seed ↑ L PAG–R MTG ↑ R PAG– R AG ↑ R PAG–L MTG ↓ L PAG–R MFG ↓ L PAG–L PCG ↓ R PAG–R mPFC ↓ R PAG–R NAc ↓ R PAG–bil. ACC |
0.54 [0.0028, 1.08] | Cluster-corrected p < 0.05 |
| 27 | 4:23 | |||||||||||
| 29 | n.r. | 5:24 | BDJ | 0.25 | Cycling | Resting | Seed to whole-brain rsFC |
PAG as seed ↑ R PAG–PAG–R AG ↓ R PAG–R mPFC |
0.54 [0.0027, 1.07] | Cluster-corrected p < 0.05 | ||
| 27 | 4:23 | |||||||||||
| Liu72 | Healthy | 27 | 65.1 | 10:17 | TCC | 10.0 | Regular physical activity | Sequential decision task | BOLD activation and FC (ROI) | ↑Fronto-striatal connectivity | 0.55 [0.00290, 1.10] | Family-wise correction, p < 0.05 |
| 26 | 64.2 | 10:16 | ||||||||||
| Liu62 | MCI | 20 | 66.2 | 5:15 | BDJ | 0.5 | Walking | Resting | Seed to whole-brain rsFC |
LC as seed ↑ L LC–R dlPFC ↑ R LC–R ACC ↓ L LC–Bilateral cerebellum exterior ↓ R LC–R inferior occipital cortex ↓ R LC–Bilateral precentral cortex ↓ R LC–Bilateral postcentral cortex ↓ R LC–L middle occipital cortex ↓ R LC–R cerebellum VTA as seed ↑ L VTA–Bilateral ACC ↑ R VTA–R TPJ |
0.99 [0.304, 1.67] | Voxel-wise Uncorrected, p < 0.005 |
| 17 | 64.3 | 7:10 | ||||||||||
| Port97 | Healthy | 8 | 66.4 | 3:5 3:5 | TCC | 17.4 | Regular physical activity |
(1) n-back task (2) Stroop task |
BOLD activation (whole-brain) |
n-back ↓ R frontal pole ↓ SFG Stroop ↓ R intralcalcarine cortex ↓ Lateral occipital cortex ↓ Occipital pole |
1.07 [0.024, 2.12] | Cluster-corrected p < 0.05 |
| 8 | 66.4 | |||||||||||
| Shen37 | Healthy | 12 | 21.8 | 2:10 | TCC | 0.16 | Brisk walking | Resting | fALFF |
↑ Lateral medial SFG ↑ R FFG ↓ R dorsal SFG ↓ R PCL |
0.85 [0.0114, 1.68] | Family-wise-error corrected p < 0.05 |
| 12 | 21.8 | 2:10 | ||||||||||
| Tao63 | Healthy | 21 | 62.4 | 8:13 | TCC | 0.25 | Regular physical activity | Resting | Seed to whole-brain rsFC | ↑ Bilateral hippocampus–Bilateral mPFC | 0.597 [0.00370, 1.19] | Cluster-corrected p < 0.05 |
| 25 | 59.8 | 6:19 | ||||||||||
| Tao63 | 16 | 62.2 | 6:10 | BDJ | 0.25 | ↑ Bilateral hippocampus–Bilateral mPFC | 0.648 [0.00460, 1.29] | Cluster-corrected p < 0.05 | ||||
| 25 | 59.8 | 6:19 | ||||||||||
| Tao30 | Healthy | 21 | 62.4 | 8:13 | TCC | 0.25 | Regular physical activity | Resting | fALFF | ↑ dlPFC (slow-5 and low-frequency) | 0.597 [0.00370, 1.19] | Cluster-corrected p < 0.05 |
| 25 | 59.8 | 6:19 | ||||||||||
| Tao30 | 15 | 62.3 | 6:9 | BDJ | 0.25 | ↑ Bilateral mPFC (slow-5 and low-frequency) |
0.661 95% C.I. [0.00490, 1.32] |
Cluster-corrected p < 0.005 | ||||
| 25 | 59.8 | 6:19 | ||||||||||
| Tao38 | MCI | 20 | 66.2 | 5:15 | BDJ | 0.25 | Walking | Resting | VBM, fALFF, seed to whole-brain rsFC |
VBM GM volume changes ↑ R hippocampus fALFF changes ↑ mPFC (slow-4) ↑ dlPFC (slow-4) ↑ Bilateral ACC (slow-5) ↓ Bilateral lingual gyrus (slow-4) ↓ L hippocampus (slow-4) ↓ R STG rsFC changes ↑ R hippocampus–L mPFC ↑ R hippocampus–R angular gyrus |
0.988 [0.304, 1.67] | Cluster-corrected p < 0.05 |
| 17 | 64.3 | 7:10 | ||||||||||
| Wadden70 | Healthy | 19 | 35.9 | 3:16 | Yoga | 0.5 | Regular physical activity |
Emotional/non-emotional video viewing Happiness sadness anger |
BOLD activation (whole-brain) |
↑ L SPL ↑R anterior supramarginal gyrus ↑ Postcentral gyrus |
1.12 [0.189, 1.67] | Voxel-wise uncorrected, p < 0.005 |
| 12 | 32.6 | 6:6 | ||||||||||
| Wan98 | Subclinical (cognitive decline) | 26 | 67.4 | 9:17 | BDJ | 0.5 | Regular physical activity | Resting | GM (ROI) |
GM change ↑ R CA1 ↑ R presubiculum ↑ L parasubiculum |
0.57 [0.00320, 1.135] | Cluster-corrected, p < 0.05 |
| 24 | 64.7 | 12:12 | ||||||||||
| Wei65 | Healthy | 18 | 52.4 | 7:11 | TCC | 14.6 | Sedentary lifestyle | Resting | ReHo |
↓ L ACC ↓R SFC of dlPFC ↑ R postcentral gyrus |
0.643 [0.00470, 1.28] | Cluster-corrected p < 0.05 |
| 22 | 54.8 | 8:14 | ||||||||||
| Wei39 | Healthy | 18 | 52.4 | 7:11 | TCC | 14.6 | Sedentary lifestyle | Resting | fALFF |
↓ DMN ↓ Bilateral FPN ↓ Anterior cingulate-FPN network |
0.643 [0.00470, 1.28] | Cluster-corrected p < 0.05 |
| 22 | 54.8 | 8:14 | ||||||||||
| Woodward76 | Early psychosis | 21 | 21.5 | 0:21 | Yoga | 0.25 | Aerobic exercise | Resting | GM (ROI) | Aerobic exercise > yoga: bilateral fusiform GM volume | 0.65 [0.00490, 1.30] | p < 0.05 |
| 18 | 22.3 | 0:18 | ||||||||||
| Wu8 | Healthy | 16 | 64.9 | 3:13 | TCC | 0.25 | Regular physical activity | Task-switching paradigm | BOLD activation (whole-brain, ROI) |
↑ L SFG ↑ R MFG |
1.09 [0.337, 1.85] | Family-wise-error corrected p < 0.005 |
| 15 | 64.9 | 0:15 | ||||||||||
| Yue32 | Healthy | 20 | 62.9 | 0:20 | TCC | 16.6 | Walking | Resting | VBM, ReHo |
VBM GM volume changes ↑ R ITG, L hippocampus and L cerebellum ReHo changes: ↑L hippocampus ↑ Fusiform gyrus |
1.10 [0.448, 1.75] | voxel-wise uncorrected, p < 0.001 |
| 22 | 62.4 | 0:22 | ||||||||||
| Yue31 | Healthy | 20 | 62.9 | 0:20 | TCC | 6 | Walking | Resting | Graph theory network analysis |
White matter network ↑ normalized clustering coefficient ↑ characteristic path length |
0.624 [0.00430, 1.24] | (Correction not mentioned), p < 0.05 |
| 22 | 62.4 | 0:22 | ||||||||||
| Zhang99 | Subclinical (mood/anxiety symptoms) | 9 | 24.2 | 2:7 | TCC | 0.17 | Regular physical activity | Resting | fALFF |
fALFF changes ↑ R MFG (orbital part) ↑ R MTG (temporal pole) ↑ R MOG |
1.00 [0.0194, 1.98] | Cluster-corrected p < 0.05 |
| 9 | 22.5 | 3:6 | ||||||||||
| Zheng 74 | MCI | 23 | 65.8 | 6:17 | BDJ | 0.5 | Walking | Resting | VBM |
VBM GM volume changes ↑ R PCG ↑ R MOG |
0.78 [0.193, 1.394] | Voxel-wise corrected, p < 0.01 |
| 23 | 64.9 | 11:12 | ||||||||||
| Experiments included in the meta-analysis (8 studies, 13 comparisons) | ||||||||||||
| Liu34 | Healthy | 28 | 58.6 | n.r. | TCC | 0.25 | Waitlist control | Resting | Seed to whole-brain rsFC | Bilateral dlPFC (MNI coordinates: ± 36,27,29; FPN) | 0.559 [0.00300, 1.11] | Cluster-corrected p < 0.05 |
| 24 | 56.9 | |||||||||||
| Liu34 | 29 | 59.7 | BDJ | Bilateral dlPFC (MNI coordinates: ± 36,27,29; FPN) | 0.554 [0.00300, 1.11] | Cluster-corrected p < 0.05 | ||||||
| 24 | 56.9 | |||||||||||
| Liu34 | 28 | 58.6 | TCC | Walking | Bilateral dlPFC (MNI coordinates: ± 36,27,29; FPN) | 0.559 [0.00300, 1.11] | Cluster-corrected p < 0.05 | |||||
| 27 | 61.3 | |||||||||||
| Liu34 | 29 | 59.7 | BDJ | Bilateral dlPFC (MNI coordinates: ± 36,27,29; FPN) | 0.554 [0.00300, 1.11] | Cluster-corrected p < 0.05 | ||||||
| 27 | 61.3 | |||||||||||
| Liu40 | 21 | 62.4 | 8:13 | TCC | Waitlist control |
PCC (MNI coordinates: -2, -36, 37; DMN) mPFC (MNI coordinates: 1, 54, 21; DMN) |
0.597 [0.00370, 1.19] | Cluster-corrected p < 0.05 | ||||
| 25 | 60.1 | 6:19 | ||||||||||
| Liu40 | 16 | 62.2 | 6:10 | BDJ | 0.65 [0.00460, 1.29] | Cluster-corrected p < 0.05 | ||||||
| 25 | 60.1 | 6:19 | ||||||||||
| Liu66 | 16 | 65.19 | 8:18 | TCC | 10.4 | Regular physical activity | dlPFC (MNI coordinates: 36,27,29; FPN) |
0.95 [0.292, 1.61] |
Voxel-wise uncorrected, p < 0.005 | |||
| 25 | 63.92 | 9:16 | ||||||||||
| Tao36 | 21 | 62.38 | 8:13 | TCC | 0.25 |
Bilateral dlPFC (MNI coordinates: ± 36.27.29; FPN) Bilateral dlPFC (MNI coordinates: ± 36.27.29; FPN) |
0.597 [0.00370, 1.19] | Cluster-corrected p < 0.05 | ||||
| 25 | 59.76 | 6:19 | ||||||||||
| Tao36 | 16 | 62.33 | 6:9 | BDJ | 0.648 [0.00460, 1.29] | Cluster-corrected p < 0.05 | ||||||
| 25 | 59.76 | 6:19 | ||||||||||
| Santaella67 | 20 | 68.2 | 0:20 | Yoga | 15.1 |
PCC (MNI coordinates: 1, − 61, 38; DMN) mPFC (MNI coordinates: 1, 55, − 3; DMN) |
0.640 [0.00470, 1.28] | Family-wise-error corrected p < 0.05 | ||||
| 20 | 66.5 | 0:20 | ||||||||||
| Singleton68 | 16 | 49.4 | 5:11 | Yoga | 18.3 | PCC (MNI coordinates: − 9.6, − 51.3, 27.5; DMN) | 0.956 [0.235, 1.68] | Cluster-corrected p < 0.01 | ||||
| 17 | 52.5 | 7:10 | ||||||||||
| van Aalst33 | 10 | 36.8 | 2:8 | Yoga | 4.8 | Stationary cycling | WHOLE-brain rsFC |
DMN (MNI coordinates: not specific) DMN (MNI coordinates: not specific) |
1.75 [0.723, 2.79] | Voxel-wise uncorrected, p < 0.001 | ||
| 10 | 34.6 | 2:8 | ||||||||||
| Yue69 | 20 | 62.9 | 0:20 | TCC | 6 | Walking | 0.624 [0.00430, 1.24] | Family-wise-error corrected p < 0.05 | ||||
| 22 | 63.3 | 0:22 | ||||||||||
N number of participants, TCC Tai Chi Chung, BDJ Baduanjin, VBM voxel-based morphometry, GM grey matter, VMHC voxel-mirrored homotopic connectivity, ReHo regional homogeneity, fALFF fractional amplitude of low-frequency fluctuations, BOLD blood-oxygen-level-dependent, rsFC resting-state functional connectivity, ROI regions of interest, DTI diffusion tensor imaging, DMN default mode network, DAN dorsal attention network, FPN frontoparietal network, SN salience network, MFG middle frontal gyrus, MFC medial frontal cortex, PFC prefrontal cortex, aPFC anterior prefrontal cortex, lPFC lateral prefrontal cortex, mPFC medial prefrontal cortex, dmPFC dorsal medial prefrontal cortex, dlPFC dorsal lateral prefrontal cortex, vlPFC ventral lateral prefrontal cortex, SFG superior frontal gyrus, ACC anterior cingulate cortex, PCC posterior cingulate cortex, IPS intraparietal sulcus, SPL superior parietal lobule, FEF frontal eye field, STG superior temporal gyrus, MTG middle temporal gyrus, ITG inferior temporal gyrus, LC locus coeruleus, VTA ventral tegmental area, FFG fusiform gyrus, PCL paracentral lobule, PCG precentral gyrus, AG angular gyrus, PAG periaqueductal gray, NAc nucleus accumbens, n.r. not reported.
aStatistically significant results only; contrast of interest: mind-body practice group > control group, unless otherwise specified.
Can mind–body exercise modulate resting-state brain activities?
Twenty-four studies showed that both long-term and short-term mind–body practice induced changes in resting-state brain activities (Table 1). Most of the studies reported that mind–body exercise is capable of modulating the synchronization between different brain regions. Among studies that reported changes in brain synchronization, 13 experiments (reported in 8 studies) were included in the meta-analysis based on the inclusion criteria. In addition, some studies have shown that mind–body exercise modulates spontaneous neuronal fluctuations.
Nine studies reporting changes in rsFC, as indexed by the statistical correlations of BOLD signals between selected brain regions, were included in the narrative synthesis only. These studies showed that mind–body exercise is capable of reducing rsFC of the cortical brain regions within the DMN60, visual network61, as well as between bilateral periaqueductal gray (PAG) and the right medial prefrontal cortex63, while increasing rsFC between the DMN and hippocampus38,62, between PAG and parietotemporal regions63, and increasing rsFC within the DAN35. The remaining three studies showed significant changes in local brain region synchronization, as indexed by changes in regional homogeneity32,64 and nodal efficiency65.
Thirteen experiments reported in eight studies were included in the SDM-PSI coordinate-based meta-analysis33,34,36,40,66–69. With seed location and control condition as covariates, increased activation was observed in the DMN with a peak at the left anterior cingulate gyri and decreased activation was observed in the VAN with a peak at the left supramarginal gyrus in the mind–body exercise group when compared to the control group (uncorrected p < 0.05; Table 2). These peaks did not survive voxel-corrected significance tests. Meta-regression between brain activation and years of mind–body practice showed that, the activation difference between treatment and control group of right inferior parietal gyri (BA40) increases with increasing years of mind–body practice (voxel-corrected p = 0.003; Fig. 3). Meta-regression of mean age of participants did not reveal significant correlation with brain activation patterns at the whole brain level (p > 0.05). Regarding heterogeneity and small study effects, the left anterior cingulate gyri (I2 < 0.01%; Harbord Egger bias tests p-value = 0.93), left supramarginal gyrus (I2 = 1.79%; Harbord Egger bias tests p-value = 0.94) and right inferior parietal gyri (I2 < 0.01%; Harbord Egger bias tests p-value = 0.99) peaks showed low heterogeneity and nonsignificant small study effects.
Table 2.
Effects of mind–body exercise on the functional connectivity of resting-state networks.
| Brain regions with peak activation | Cluster breakdown | Resting-state network | |||||
|---|---|---|---|---|---|---|---|
| Anatomical region | L/R | Total number of voxels | MNI coordinates | SDM-Z | p | Anatomical regions (Brodmann area) | |
| Mind–body exercise > physical activity | |||||||
| Anterior cingulate/paracingulate gyri | L | 11 | −4, 42, −6 | 1.70 | 0.045 | Anterior cingulate/paracingulate gyri (BA10) | DMN |
| Mind–body exercise < physical activity | |||||||
| Supramarginal gyrus | L | 109 | −58, −42, 26 | −1.85 | 0.033 |
Supramarginal gyrus (BA48) Superior temporal gyrus (BA42) |
VAN |
Analysis conducted with (1) seed location and (2) control group condition as covariates.
Significance threshold: uncorrected p < 0.05.
Figure 3.
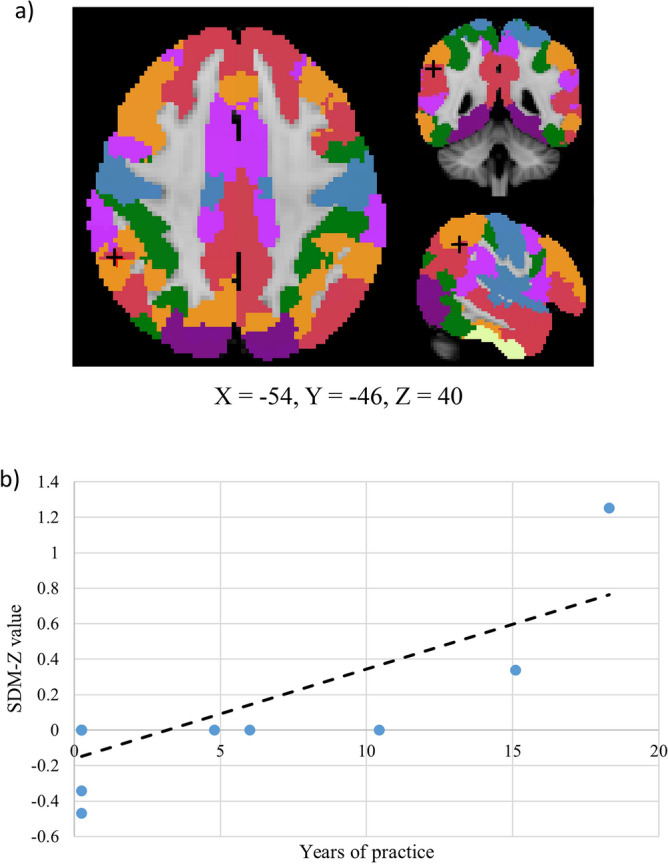
(a) The activation of right inferior parietal gyri (BA40, peak voxel indicated by ‘+’ symbol), which lies within the default mode network (red), increases with years of mind–body practice (voxel-corrected p < 0.005). (b) A scattered plot showing the positive relationship between the activation of right inferior parietal gyri and years of mind–body practice.
Regarding spontaneous neuronal fluctuations that are indicated by the fractional amplitude of low-frequency fluctuations (fALFF), five studies reported significant changes in fALFF after mind–body exercise. Three studies reported changes in fALFF in multiple regions in the frontal, temporal and hippocampal regions after several weeks of mind–body exercise30,37,38,99, and one study reported network-level changes in fALFF after more than 14 years of Tai Chi practice39.
Can mind–body exercise modulate brain activities associated with task performance?
Seven studies reported mind–body exercise-associated changes in brain activation (indicated by changes in BOLD signals) and functional connectivity in practitioners when they performed cognitive and affective tasks (Table 1). Regarding changes in BOLD signals, both short-term and long-term yoga practice induce changes in brain changes in the frontoparietal areas when participants perform various affective tasks. For instance, long-term yoga practice was found to induce a decrease in the activity of the right dlPFC during emotional picture viewing and an increase in activity of the left vlPFC during the emotional Stroop task29, while short-term practitioners were shown to have increased activation in the postcentral gyrus, left superior parietal lobule and right anterior supramarginal gyrus during emotional video viewing tasks70. Changes in cortical and subcortical activation patterns were also evident when long-term yoga practitioners engaged in the color Stroop task and tasks with low (i.e., 1-back) and high (i.e., 2-back) working memory loads71. Some evidence has revealed that short-term Tai Chi practice induces changes in frontal activations during task switching8. Regarding changes in task-based FC, long-term yoga practice strengthens FC within the frontoparietal network during the color Stroop task. During working memory tasks, FC is strengthened between the PFC and various brain regions, including the parietal brain areas, cerebellum and insula, while it is reduced within parietal brain areas, cerebellum and insula71. Some evidence revealed that long-term Tai Chi practice induced stronger frontostriatal FC during a decision-making task embedded with emotional components72.
Can mind–body exercise induce structural changes in the brain?
Eight studies showed that mind–body exercise induced structural changes in the brain, with 7 studies indexed these structural changes in terms of gray matter volume. Multiple studies showed that short-term (i.e., 3 months) Qigong (Baduanjin) practice promotes an increase in gray matter volume the right hemisphere, namely the right precentral and middle occipital gyri74, as well as in the right hippocampus38,98, while for long-term Tai Chi and Yoga practitioners32,73, the gray matter volume is increased in multiple cortical and subcortical areas, including frontal, temporal and occipital lobes, as well as limbic, parahippocampal areas and cerebellum. In contrast, short-term yoga practice is found to induce grey matter reduction in frontoparietal75, as well as temporal regions76. One study showed that long-term Tai Chi practice promotes the overall integrity and efficiency of the white matter network, as evidenced by an increase in the normalized clustering coefficient and the characteristic path length31.
Certainty assessment
A summary of findings and certainty assessment was reported in Table 3. Overall speaking, the certainty of the evidence reported above was low, mainly due to small number of studies, indirectness and inconsistencies.
Table 3.
Summary of findings and certainty assessment.
| Outcome | No. of studies | Effect (SDM-Z) | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication bias | Certainty of the evidence |
|---|---|---|---|---|---|---|---|---|---|
| Resting-state brain activity (seed to whole-brain rsFC) | 8 | 1.70 (anterior cingulate); −1.85 (left supramar-ginal gyrus) | Cross-sectional studies; RCTs | No serious risk of bias | No serious inconsistencies | Serious (healthy controls only) | No serious imprecision | No serious publication bias |
⊕ ⊕ ⊝ ⊝ Low (due to small number of studies and indirectness) |
| Resting-state brain activity (seed-based rsFC, other synchronization parameters) | 9 | Not pooled | Cross-sectional studies; RCTs | No serious risk of bias | Some inconsistencies | No serious indirectness | No serious imprecision | – |
⊕ ⊕ ⊝ ⊝ Low (due to small number of studies and inconsistencies) |
| Resting-state brain activity (fALFF) | 5 | Not pooled | Cross-sectional studies; RCTs | No serious risk of bias | Some inconsistencies | No serious indirectness | No serious imprecision | – |
⊕ ⊕ ⊝ ⊝ Low (due to small number of studies and inconsistencies) |
| Task-based brain activity | 7 | Not pooled | Cross-sectional studies; RCTs | Some risk of bias | Some inconsistencies | No serious indirectness | No serious imprecision | – |
⊕ ⊕ ⊝ ⊝ Low (due to small number of studies and inconsistencies) |
| Structural change (grey and white matter volume) | 8 | Not pooled | Cross-sectional studies; RCTs | No serious risk of bias | Some inconsistencies | No serious indirectness | No serious imprecision | – |
⊕ ⊕ ⊝ ⊝ Low (due to small number of studies and inconsistencies) |
rsFC resting-state functional connectivity, fALFF fractional amplitude of low-frequency fluctuations.
Discussion
By systematically synthesizing the current fMRI literature, this review aimed to examine the effects of mind–body exercise on brain activation, functional neural connections and structural changes in the brain. After performing a systematic literature search, 34 empirical studies were included for either narrative or meta-analytic review. The following results were obtained from the qualitative and quantitative analyses of fMRI studies: (1) mind–body exercise modulates the rsFC of the DMN, VAN and DAN; (2) mind–body exercise-induced changes in activation patterns in the frontal regions, as well as the changes in frontoparietal functional connections; (3) changes in spontaneous neuronal fluctuations at rest in frontal, temporal and hippocampal regions are evident in short-term mind–body practice practitioners; and 4) short-term Qigong and long-term Tai Chi/Yoga practice induces changes in the gray matter volume in various cortical and subcortical brain regions. The clinical implications of these results are discussed in the subsequent paragraphs.
Mind–body exercise modulates functional connections in the brain at rest, as evidenced by changes in correlations of BOLD signals between brain regions, regional homogeneity and nodal efficiency. Specifically, the functional connections in brain regions within the DMN were found to be enhanced when compared to control conditions, and importantly, the increase in activation of a brain region within DMN (i.e. right inferior parietal gyri) is shown to be significantly associated with years of mind–body practice. The DMN has long been suggested to be responsible for self-awareness77 and the coordination of task-positive networks78. Previous studies have shown that the functional connections between the DMN and task-positive functional networks increase only when an individual is preparing to perform cognitive tasks79. The capability of mind–body exercise to enhance rsFC within the DMN may imply that mind–body exercise might promote the readiness to perform goal-directed tasks of an individual. It is interesting to note that the SDM-Z value of the meta-regression is negative at the Y-axis, and one might be tempted to associate this with the possible attenuation effect on the well-documented age-related decline in the right inferior parietal gyri80–82. We recommend cautious interpretation of results given the number of studies are limited for the meta-regression. Further studies are needed to explore how mind–body exercises maintain the function of the parietal network and to examine the associations between behavioral improvements and mind–body exercise-induced changes in DMN.
The systematic review and meta-analysis revealed that mind–body exercise modulates the functional connections of dorsal (DAN) and ventral (VAN) attention networks at rest. The DAN and VAN are the two brain functional networks that are established to be responsible for attention and cognitive control83,84. While the DAN, a network that exerts top-town control, is responsible for regulating the processing of external stimuli in facilitation of the successful engagement of goal-directed behavior, the VAN is a “bottom-up” network that is responsible for the detection of behaviorally relevant stimuli85. These two networks interact with each other during attention shifting; specifically, the VAN interrupts the activation of DAN to allow an individual to reorient his attention to a new external stimulus84. As revealed by our results, the upregulation of DAN and downregulation of VAN at rest may imply that mind–body exercise reduces the reactivity towards external stimuli while also enhancing the ability to focus on goal-directed behaviors. Indeed, this postulation is consistent with previous findings showing that mindfulness-based interventions reduce reactivity to temptations (e.g. food cues)86 and enhance attentional control87 in practitioners.
Apart from resting-state neural connections, mind–body exercise also modulates brain activation patterns and functional connectivity when individuals perform cognitive/affective tasks. Notably, mind–body exercise specifically modulates task-related brain activation patterns in the frontal cortex and the functional connections between frontal and other brain regions. The effect of abnormal frontal lobe activation patterns on behaviors has been extensively studied in individuals with multiple neurodevelopmental, neuropsychiatric and neurodegenerative diagnoses, such as autism88, depression89, and dementia90. As revealed in the currently available literature, mind–body exercise appears to alter brain activation patterns specifically in the prefrontal brain regions, which signifies its applicability as a treatment for patients with frontal abnormalities. Indeed, previous clinical studies have provided positive empirical evidence supporting its applications2,91. Notably, the studies that investigated the effects of mind–body exercise on brain activity during active task performance are heterogeneous in terms of study designs. More studies involving similar cognitive/affective tasks are needed to support meta-analytic synthesis, which will help us further understand how mind–body exercise modulates task-based brain activities and functional connections.
In addition, spontaneous neuronal fluctuation at rest, as indexed by fALFF changes, is modulated by short-term mind–body exercise. Because fALFF has been shown to be associated with cognitive control abilities92,93, changes in fALFF in multiple brain regions may imply a network-based modulation of neuronal activities, although further investigations are needed to dissect the relationship between regional-specific fALFF changes and behavioral enhancement. Moreover, some evidence shows that short-term Qigong and long-term mind–body exercise may increase gray matter volume in various brain regions, including the frontal, temporal and occipital lobes, as well as limbic and parahippocampal areas and cerebellum. These brain regions are involved in various cognitive processes, which have been shown to shrink with increasing age94,95, and the reduction in volume is associated with age-related functional decline96. Long-term mind–body practice reverses age-related neural degeneration, implying that these exercises may play a role in delaying aging in the general population. The finding that short-term Qigong promotes grey matter volume changes in both frontal and hippocampal regions is encouraging; future studies should investigate the effects of long-term Qigong practice on grey matter increment. Future cross-sectional studies might consider studying the associations between years of training and changes in gray matter volume to determine the length of time participants must engage in mind–body exercise practice to yield observable changes in brain structure, and longitudinal studies may help address whether the observed changes in gray matter volume are long lasting.
Limitations
To the best of our knowledge, this was the first study that comprehensively investigated the effects of mind–body exercise on brain activation, neural connectivity and brain structures by means of narrative synthesis and coordinate-based meta-analysis, yet several limitations were noted. Although we attempted to retrieve all possible published fMRI studies that investigated the effects of mind–body exercise on brain activities and narratively synthesized studies that did not report whole-brain rsFC between-group difference, only 13 experiments were included for coordinate-based meta-analysis, which limited the power of our analysis, resulting in small effect sizes observed in the meta-analysis. Regarding the study characteristics, although we attempted to control for the between-study heterogeneity by conducting covariate analyses in the meta-analysis, we were aware of the unaddressed heterogeneity across studies in both narrative synthesis and meta-analysis, for example the heterogeneity induced by the inclusion of both RCTs and cross-sectional studies, participants of different age and biological sexes. We encourage future neuroimaging studies examining the effects of mind–body exercise at the whole-brain level, such that larger-scale meta-analytic reviews that controls for the between-study heterogeneity could be performed, which would deepen our understanding about how mind–body exercise can promote functional changes in different regions of the brain. In addition, researchers may also consider investigating the specific effects of mind–body exercise on DMN, VAN, DAN and their behavioral correlates. Last but not least, it is critical to study the differential effects of meditation-only, conventional-exercise-only, and mind–body exercise on the brain in future studies.
Conclusion
This review examined the neurobiological effects of mind–body exercise on brain activation, functional neural connections and structural changes in the brain. A systematic literature search yielded 34 relevant empirical studies that were included in the review, and data from 13 fMRI experiments were included in the meta-analysis. The results show that mind–body exercise modulates the rsFC of task-negative and attentional control networks, while also changes frontal activation patterns and frontoparietal functional connections during various cognitive tasks. Additionally, preliminary data show that short-term mind–body practice alters spontaneous neuronal fluctuations at rest in frontal, temporal and hippocampal regions, and short-term Qigong practice is further shown to induce both cortical and subcortical grey matter increment. We recommend that future studies include both neuropsychological and neurophysiological/neuroimaging techniques to further understand the neural mechanisms underpinning mind–body exercise.
Supplementary Information
Author contributions
Y.H. and H.T. were responsible for the conception of the study, funding acquisition, data interpretation and manuscript revision. M.C. was responsible for the study design, data acquisition, analysis and interpretation, manuscript writing and revision. C.C. and M.L. were responsible for the data processing, figure/table preparation and manuscript writing. C.C. was also responsible for manuscript revision. D.A. was responsible for the study design and protocol registration.
Funding
This study was supported by the Bright Future Charitable Foundation (Grant number: P0030920) awarded to H.W.H.T.
Data availability
Data extracted from included studies would be available upon reasonable request made to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yvonne M. Y. Han and Melody M. Y. Chan.
These authors jointly supervised this work: Yvonne M. Y. Han and Hector W. H. Tsang.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-37309-4.
References
- 1.NCCIH. Framework for Developing and Testing Mind and Body Interventions. (National Center for Complementary and Integrative Health Bethesda, 2017).
- 2.Chan AS, et al. A Chinese mind-body exercise improves self-control of children with autism: A randomized controlled trial. PLoS ONE. 2013;8(7):e68184. doi: 10.1371/journal.pone.0068184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, et al. The effects of mind-body exercise on cognitive performance in elderly: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2018;15(12):2791. doi: 10.3390/ijerph15122791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro F, Oliveira J. Effect of physical exercise and age on knee joint position sense. Arch. Gerontol. Geriatr. 2010;51(1):64–67. doi: 10.1016/j.archger.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Hartley L. Wisdom of the Body Moving: An Introduction to Body-Mind Centering. North Atlantic Books; 1995. [Google Scholar]
- 6.Mehling WE, et al. Body awareness: A phenomenological inquiry into the common ground of mind-body therapies. Philos. Ethics Hum. Med. 2011;6:6. doi: 10.1186/1747-5341-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke TC, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health Stat. Rep. 2015;79:1. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MT, et al. Task-switching performance improvements after Tai Chi Chuan training are associated with greater prefrontal activation in older adults. Front. Aging Neurosci. 2018;10:280. doi: 10.3389/fnagi.2018.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Q, et al. Effects of Tai Chi on balance and fall prevention in Parkinson’s disease: A randomized controlled trial. Clin. Rehabil. 2014;28(8):748–753. doi: 10.1177/0269215514521044. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, et al. The effects of tai chi on depression, anxiety, and psychological well-being: A systematic review and meta-analysis. Int. J. Behav. Med. 2014;21(4):605–617. doi: 10.1007/s12529-013-9351-9. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, et al. The effects of Tai Chi exercise on cognitive function in older adults: A meta-analysis. J. Sport Health Sci. 2013;2(4):193–203. doi: 10.1016/j.jshs.2013.09.001. [DOI] [Google Scholar]
- 12.Wayne PM, et al. Effect of Tai Chi on cognitive performance in older adults: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2014;62(1):25–39. doi: 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu, R. et al. Effect of Tai Chi on cognitive function among older adults with cognitive impairment: A systematic review and meta-analysis. Evid.-Based Complem. Altern. Med. v2021, 6679153; 10.1155/2021/6679153 (2021). [DOI] [PMC free article] [PubMed]
- 14.Lin, R. et al. Effects of Tai Chi on patients with mild cognitive impairment: A systematic review and meta-analysis of randomized controlled trials. BioMed Res. Int. 2021, 5530149; 10.1155/2021/5530149 (2021). [DOI] [PMC free article] [PubMed] [Retracted]
- 15.Chen S, et al. The effect of Qigong-based therapy on patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2020;34(12):1436–1448. doi: 10.1177/0269215520946695. [DOI] [PubMed] [Google Scholar]
- 16.Zou, L. et al. A systematic review and meta-analysis of Baduanjin Qigong for health benefits: Randomized controlled trials. Evid.-Based Complem. Altern. Med. v2017, 4548706; 10.1155/2017/4548706 (2017). [DOI] [PMC free article] [PubMed]
- 17.Guo L, Kong Z, Zhang Y. Qigong-based therapy for treating adults with major depressive disorder: A meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health. 2019;16(5):826. doi: 10.3390/ijerph16050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, et al. Effects of tai chi and Qigong on cognition in neurological disorders: A systematic review and meta-analysis. Geriatr. Nurs. 2022;46:166–177. doi: 10.1016/j.gerinurse.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Youkhana S, et al. Yoga-based exercise improves balance and mobility in people aged 60 and over: A systematic review and meta-analysis. Age Ageing. 2016;45(1):21–29. doi: 10.1093/ageing/afv175. [DOI] [PubMed] [Google Scholar]
- 20.Cramer H, et al. Yoga for posttraumatic stress disorder—A systematic review and meta-analysis. BMC Psychiatry. 2018;18(1):1–9. doi: 10.1186/s12888-018-1650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gothe NP, McAuley E. Yoga and cognition: A meta-analysis of chronic and acute effects. Psychosom. Med. 2015;77(7):784–797. doi: 10.1097/PSY.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 22.Chan AS, et al. A Chinese Chan-based mind–body intervention for patients with depression. J. Affect. Disord. 2012;142(1–3):283–289. doi: 10.1016/j.jad.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Han YM, et al. A mind-body lifestyle intervention enhances emotional control in patients with major depressive disorder: A randomized, controlled study. Cogn. Affect. Behav. Neurosci. 2020;20(5):1056–1069. doi: 10.3758/s13415-020-00819-z. [DOI] [PubMed] [Google Scholar]
- 24.Sezer, I., Pizzagalli, D.A. & Sacchet, M.D. Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis. Neurosci. Biobehav. Rev. 104583 (2022). [DOI] [PMC free article] [PubMed]
- 25.Young KS, et al. The impact of mindfulness-based interventions on brain activity: A systematic review of functional magnetic resonance imaging studies. Neurosci. Biobehav. Rev. 2018;84:424–433. doi: 10.1016/j.neubiorev.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Eyre HA, et al. Changes in neural connectivity and memory following a yoga intervention for older adults: A pilot study. J. Alzheimers Dis. 2016;52(2):673–684. doi: 10.3233/JAD-150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koenigs M, et al. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 2009;29(47):14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neurosci. 2008;9:102. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froeliger, B. et al. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: A pilot study. Front. Integr. Neurosci. (2012). [DOI] [PMC free article] [PubMed]
- 30.Tao J, et al. Tai Chi Chuan and Baduanjin mind-body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: A resting-state fMRI study. Front. Hum. Neurosci. 2017;11:1–10. doi: 10.3389/fnhum.2017.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue C, et al. Tai chi training evokes significant changes in brain white matter network in older women. Healthcare (Switzerland) 2020;8:1. doi: 10.3390/healthcare8010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue C, et al. Regular Tai Chi practice is associated with improved memory as well as structural and functional alterations of the hippocampus in the elderly. Front. Aging Neurosci. 2020;12:586770. doi: 10.3389/fnagi.2020.586770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Aalst J, et al. Long-term Ashtanga yoga practice decreases medial temporal and brainstem glucose metabolism in relation to years of experience. EJNMMI Res. 2020;10:1. doi: 10.1186/s13550-020-00636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, et al. Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: A multiple mode MRI study. Brain Behav. Immun. 2019;82:253–263. doi: 10.1016/j.bbi.2019.08.193. [DOI] [PubMed] [Google Scholar]
- 35.Froeliger B, et al. Meditation-state functional connectivity (msFC): Strengthening of the dorsal attention network and beyond. Evid.-Based Complem. Altern. Med. 2012;2012:680407. doi: 10.1155/2012/680407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao J, et al. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 2017;7:41581. doi: 10.1038/srep41581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen QQ, et al. The potential advantages of Tai Chi Chuan in promoting inhibitory control and spontaneous neural activity in young adults. Front. Behav. Neurosci. 2021;15:747733. doi: 10.3389/fnbeh.2021.747733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao J, et al. Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. NeuroImage Clin. 2019;23:101834. doi: 10.1016/j.nicl.2019.101834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei GX, et al. Mind–body practice changes fractional amplitude of low frequency fluctuations in intrinsic control networks. Front. Psychol. 2017;8:1049. doi: 10.3389/fpsyg.2017.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, et al. Different modulation effects of Tai Chi Chuan and Baduanjin on resting-state functional connectivity of the default mode network in older adults. Soc. Cognit. Affect. Neurosci. 2019;14(2):217–224. doi: 10.1093/scan/nsz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albajes-Eizagirre A, et al. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage. 2019;186:174–184. doi: 10.1016/j.neuroimage.2018.10.077. [DOI] [PubMed] [Google Scholar]
- 42.Chan MM, Han YM. The functional brain networks activated by music listening: A neuroimaging meta-analysis and implications for treatment. Neuropsychology. 2022;36(1):4. doi: 10.1037/neu0000777. [DOI] [PubMed] [Google Scholar]
- 43.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, et al. Effects of mind–body exercise on brain structure and function: A systematic review on MRI studies. Brain Sci. 2021;11(2):1–19. doi: 10.3390/brainsci11020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gürsel DA, et al. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: A meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2018;87:151–160. doi: 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser RH, et al. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatr. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: Current and future directions. Soc. Cognit. Affect. Neurosci. 2007;2(2):150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer A, et al. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 2018;28(9):3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeo, B.T. et al. The Organization of the Human Cerebral Cortex Estimated by Intrinsic Functional Connectivity.J. Neurophysiol. (2011). [DOI] [PMC free article] [PubMed]
- 50.Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterne JA, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 53.Munn Z, et al. The development of software to support multiple systematic review types: The Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI) JBI Evid. Implement. 2019;17(1):36–43. doi: 10.1097/XEB.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 54.Ma L-L, et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020;7(1):1–11. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lipsey, M.W. & Wilson, D.B. Practical meta-analysis. in Applied Social Research Methods Series. Vol. 49. (Sage Publications, 2001).
- 56.Mirman D, et al. Corrections for multiple comparisons in voxel-based lesion-symptom mapping. Neuropsychologia. 2018;115:112–123. doi: 10.1016/j.neuropsychologia.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 58.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006;25(20):3443–3457. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 59.Mustafa RA, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. J. Clin. Epidemiol. 2013;66(7):736–742. doi: 10.1016/j.jclinepi.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Chen L-Z, et al. Brain functional specialization is enhanced among Tai Chi Chuan practitioners. Arch. Phys. Med. Rehabil. 2020;101(7):1176–1182. doi: 10.1016/j.apmr.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Li G, et al. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 2022;11(1):6. doi: 10.1186/s40035-022-00280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tao J, et al. Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front. Aging Neurosci. 2016;8:25. doi: 10.3389/fnagi.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, et al. Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: a randomised multimodal magnetic resonance imaging study. Br. J. Anaesth. 2019;123(4):506–518. doi: 10.1016/j.bja.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 64.Wei GX, et al. Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front. Aging Neurosci. 2014;6:74. doi: 10.3389/fnagi.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui L, et al. Tai Chi Chuan alters brain functional network plasticity and promotes cognitive flexibility. Front. Psychol. 2021;12:665419. doi: 10.3389/fpsyg.2021.665419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z, et al. Functional connectivity within the executive control network mediates the effects of long-term Tai Chi exercise on elders’ emotion regulation. Front. Aging Neurosci. 2018;10:315. doi: 10.3389/fnagi.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santaella DF, et al. Greater anteroposterior default mode network functional connectivity in long-term elderly yoga practitioners. Front. Aging Neurosci. 2019;10:158. doi: 10.3389/fnagi.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singleton O, et al. Brain structure and functional connectivity correlate with psychosocial development in contemplative practitioners and controls. Brain Sci. 2021;11(6):728. doi: 10.3390/brainsci11060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue C, et al. Differential effects of Tai Chi Chuan (motor-cognitive training) and walking on brain networks: A resting-state FMRI study in Chinese women aged 60. Healthcare (Switzerland) 2020;8(1):67. doi: 10.3390/healthcare8010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wadden KP, et al. Yoga practitioners uniquely activate the superior parietal lobule and supramarginal gyrus during emotion regulation. Front. Integr. Neurosci. 2018;12:60. doi: 10.3389/fnint.2018.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaur H, et al. Comparing cognition, coping skills and vedic personality of individuals practicing yoga, physical exercise or sedentary lifestyle: A cross-sectional fMRI study. Integr. Med. Res. 2022;11(1):100750. doi: 10.1016/j.imr.2021.100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z, et al. Reduced feelings of regret and enhanced fronto-striatal connectivity in elders with long-term Tai Chi experience. Soc. Cognit. Affect. Neurosci. 2020;15(8):861–873. doi: 10.1093/scan/nsaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Froeliger, B., Garland, E.L., & McClernon, F.J. Yoga meditation practitioners exhibit greater gray matter volume and fewer reported cognitive failures: Results of a preliminary voxel-based morphometric analysis. Evid.-Based Complem. Altern. Med. 2012, 821307 (2012). [DOI] [PMC free article] [PubMed]
- 74.Zheng G, et al. Traditional Chinese mind-body exercise Baduanjin modulate gray matter and cognitive function in older adults with mild cognitive impairment: A brain imaging study. Brain Plast. 2021;7(2):131–142. doi: 10.3233/BPL-210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garner M, et al. 10-week Hatha Yoga increases right hippocampal density compared to active and passive control groups: a controlled structural cMRI study. J. Neuroimaging Psychiatry Neurol. 2019;4(1):1–11. doi: 10.17756/jnpn.2019-027. [DOI] [Google Scholar]
- 76.Woodward ML, et al. Medial temporal lobe cortical changes in response to exercise interventions in people with early psychosis: A randomized controlled trial. Schizophr. Res. 2020;223:87–95. doi: 10.1016/j.schres.2020.05.043. [DOI] [PubMed] [Google Scholar]
- 77.Raichle ME. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- 78.Fox MD, et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koshino H, et al. Coactivation of the default mode network regions and working memory network regions during task preparation. Sci. Rep. 2014;4(1):1–8. doi: 10.1038/srep05954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacDonald SW, et al. Increased response-time variability is associated with reduced inferior parietal activation during episodic recognition in aging. J. Cogn. Neurosci. 2008;20(5):779–786. doi: 10.1162/jocn.2008.20502. [DOI] [PubMed] [Google Scholar]
- 81.Thambisetty M, et al. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52(4):1215–1223. doi: 10.1016/j.neuroimage.2010.04.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maillet D, Rajah MN. Age-related differences in brain activity in the subsequent memory paradigm: A meta-analysis. Neurosci. Biobehav. Rev. 2014;45:246–257. doi: 10.1016/j.neubiorev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Dosenbach NU, et al. A dual-networks architecture of top–down control. Trends Cogn. Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 85.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Keesman M, et al. Mindfulness reduces reactivity to food cues: Underlying mechanisms and applications in daily life. Curr. Addict. Rep. 2017;4(2):151–157. doi: 10.1007/s40429-017-0134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson ND, et al. Mindfulness-based stress reduction and attentional control. Clin. Psychol. Psychother. Int. J. Theory Pract. 2007;14(6):449–463. doi: 10.1002/cpp.544. [DOI] [Google Scholar]
- 88.Philip RC, et al. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci. Biobehav. Rev. 2012;36(2):901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Diener C, et al. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61(3):677–685. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 90.Sadigh-Eteghad S, et al. Different patterns of brain activation in normal aging and Alzheimer's disease from cognitional sight: Meta analysis using activation likelihood estimation. J. Neurol. Sci. 2014;343(1–2):159–166. doi: 10.1016/j.jns.2014.05.066. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, et al. Mind–body therapies for older adults with dementia: a systematic review and meta-analysis. Eur. Geriatr. Med. 2022;2022:1–11. doi: 10.1007/s41999-022-00639-z. [DOI] [PubMed] [Google Scholar]
- 92.Mennes M, et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54(4):2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng Y, et al. The relevance of fractional amplitude of low-frequency fluctuation to interference effect. Behav. Brain Res. 2016;296:401–407. doi: 10.1016/j.bbr.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 94.Smith C, et al. Age and gender effects on human brain anatomy: A voxel-based morphometric study in healthy elderly. Neurobiol. Aging. 2007;28(7):1075–1087. doi: 10.1016/j.neurobiolaging.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 95.Smith C, et al. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68(16):1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 96.Ferreira D, et al. Cognitive decline is mediated by gray matter changes during middle age. Neurobiol. Aging. 2014;35(5):1086–1094. doi: 10.1016/j.neurobiolaging.2013.10.095. [DOI] [PubMed] [Google Scholar]
- 97.Port AP, et al. Cognition and brain function in elderly Tai Chi practitioners: A case–control study. Explore (NY) 2018;14(5):352–356. doi: 10.1016/j.explore.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Wan M, et al. Baduanjin exercise modulates the hippocampal subregion structure in community-dwelling older adults with cognitive frailty. Front. Aging Neurosci. 2022;14:956273. doi: 10.3389/fnagi.2022.956273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang J, et al. The effect of Bafa Wubu of Tai Chi on college students’ anxiety and depression: A randomized, controlled pilot study. Front. Physiol. 2023;14:78. doi: 10.3389/fphys.2023.1036010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J, et al. Mind–body exercise modulates locus coeruleus and ventral tegmental area functional connectivity in individuals with mild cognitive impairment. Front. Aging Neurosci. 2021;13:646807. doi: 10.3389/fnagi.2021.646807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data extracted from included studies would be available upon reasonable request made to the corresponding author.