Primary familial brain calcification (PFBC) is a rare neurodegenerative disorder characterized by a combination of neurological, psychiatric, and cognitive decline associated with calcium deposition on brain imaging. Its prevalence is estimated from 4.5/10000 to 3.3/1000 [1]. To date, mutations in five genes have been linked to PFBC [1].
The SLC20A2 gene (MIM * 158378) encodes for the transmembrane type III sodium-dependent phosphate transporter 2 (PiT2), which is largely expressed in the brain, especially in the regions of globus pallidus, thalamus and cerebellum. Heterozygous pathogenic variants in SLC20A2 are predicted to cause a loss of function of the PiT2 protein through haploinsufficiency or dominant negative mechanism, leading to idiopathic basal ganglia calcification-1 (IBGC1) (MIM # 213600), also known as Fahr disease [2]. PiT2 dysfunction results in an accumulation of inorganic phosphate (Pi) in the extracellular matrix, causing insidious and progressive calcium accumulation in the brain parenchyma, typically in the basal ganglia, and vessels [2].
IBGC1 may be asymptomatic or manifest with various neuropsychiatric symptoms, usually with onset in adulthood. Penetrance is incomplete and it has been suggested that the asymptomatic condition may be related to the “resilencing” state of the brain [2]. Around 60–70% of patients develop symptoms (e.g., parkinsonism, extrapyramidal symptoms, dysarthria, and psychiatric symptoms) [1]. Pediatric cases are instead uncommon [2]. The term “Primary Familial Brain Calcification” has recently been proposed to highlight the genetic aetiology of this condition [1]. Indeed, several genes have been associated with an overlapping phenotype of brain calcifications. Among these, SLC20A2 is the most commonly involved in the autosomal dominant PFBC, together with PDGFB (MIM * 190040), PDGFRB (MIM * 173410) and XPR1 (MIM * 605237) [1]. Alternatively, MYORG variants lead to a recessive form of the disorder [1]. Of note, biallelic SLC20A2 variants have been very recently reported in an adult patient developing neuropsychiatric manifestations and in two siblings with congenital Cytomegalovirus (CMV) infection-like phenotype [3, 4].
We report the fourth case of recessive PFBC, associated with severe moyamoya vasculopathy in a child harboring a missense variant in SLC20A2 in homozygous state.
Exome sequencing revealed the homozygous NM_006749.5:c.560 A > G p.(Tyr187Cys) variant in SLC20A2. It is absent in gnomAD, predicted pathogenic by several in silico tools (e.g., CADD score 29, Mutation Taster score 1), and classified as likely pathogenic according to the American College of Medical Genetics and Genomics (ACMG) Guidelines (PM2, PP2, and PP3 criteria). Both parents were confirmed to be carriers of the SLC20A2 variant. Next-generation sequencing (NGS)-based copy number variation (CNV) analysis did not detect any additional CNVs in known or candidate disease genes. No other pathogenic or likely pathogenic neither candidate variants that could be related to the phenotype were identified.
This 5-year-old boy is a single-born to consanguineous healthy parents (first cousins). There was no reported history of neurological diseases or vasculopathies in the two preceding generations. The proband was born at 36 gestational weeks, following a regular pregnancy. Birth parameters were within normal ranges and the neonatal course was uneventful except for mild respiratory distress in the first day of life. At the age of 2 months, focal motor seizures occurred and levetiracetam (20 mg/Kg/day) and carbamazepine (30 mg/Kg/day) led to good seizure control. Brain magnetic resonance imaging (MRI) revealed left temporo-parieto-occipital arterial ischemic stroke due to moyamoya vasculopathy involving both the anterior and posterior circulation with complete occlusion of the basilar artery. Ophthalmological investigations revealed bilateral congenital cataracts. At the age of 14 months, he underwent direct and indirect revascularization surgery in the territory of the middle and anterior cerebral artery, with good clinical and radiological outcome.
The patient was diagnosed with severe psychomotor delay and developed a progressive spastic tetraparesis, more marked on the right side. His communication skills were limited to social smile and vocalizations. Physical examination showed minor facial dysmorphisms (synophrium, small and spaced teeth, thickened tragus and lobes) and acquired microcephaly (−3.5 standard deviations). Height and weight were within normal ranges. Video-electroencephalography showed interhemispheric asymmetry with fast activity on the left and slower rhythms on the right, mixed with bilateral and multifocal epileptic abnormalities.
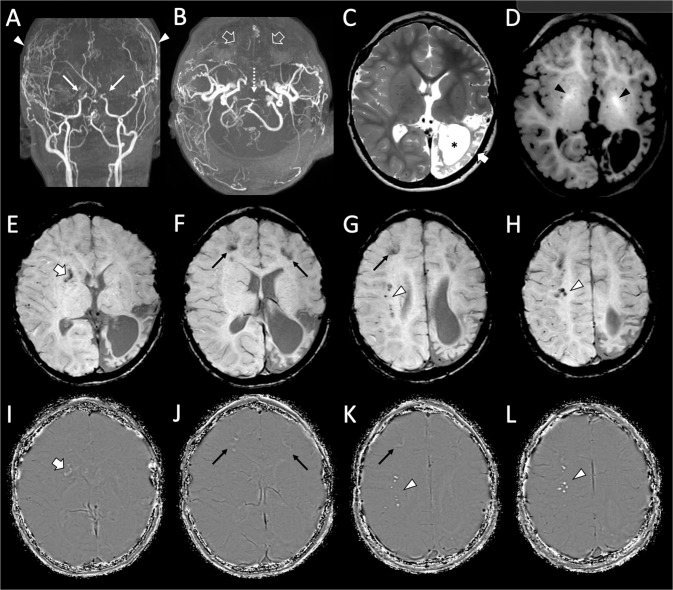
Brain MRI, at the age of 4 years and 9 months, revealed severe moyamoya vasculopathy, left chronic temporo-parieto-occipital arterial ischemic stroke, a small right frontal white matter chronic ischemic lesion, spontaneous T1 hyperintensity of the globi pallidi and several scattered calcifications at the level of the basal ganglia and frontal cortico-subcortical and profound white matter regions (Fig. 1).
Fig. 1. Brain MR angiography and MRI of the patient performed at 4 years and 3 months of age.
A, B Arterial MR angiography images reveal very severe moyamoya vasculopathy with bilateral stenosis of the terminal internal carotid arteries (thin arrows), absent flow in the middle and anterior cerebral arteries (empty arrows) with signs of bilateral direct and indirect surgical revascularizations (arrowheads). Note the severe stenosis of the apical portion of the basilar artery (dotted arrow). C Axial T2-weighted image shows a chronic arterial ischemic stroke in the left temporo-parieto-occipital regions (thick arrow) with marked white matter volume reduction and consequent ventricular enlargement (asterisk). D Axial T1-weighted image depicts spontaneous hyperintensity of the globi pallidi (black arrowheads), in keeping with mineralization of these regions. E–H Axial susceptibility weighted images with (I–L) corresponding phase maps images demonstrate several scattered calcifications in the right basal ganglia (thick arrow), bilateral frontal cortico-subcortical regions (thin arrows), right profound frontal white matter (arrowheads).
Our patient is the fourth subject harboring a homozygous SLC20A2 variant (Table 1). The first case was a woman with basal ganglia calcifications on MRI performed for dizziness at the age of 20 years. Over the years, she also developed psychosis and dysarthria, later evolving into asymmetric parkinsonism. The authors concluded that homozygous status of the variant likely led to a more severe IBGC phenotype [3]. A biallelic SLC20A2 variant with splicing effect was identified more recently in two siblings presenting with developmental delay, failure to thrive, microcephaly, epilepsy, bilateral cataracts, mimicking congenital CMV infection [4].
Table 1.
Clinical spectrum of recessive SLC20A2-related PFBC.
| Arteche-López 2021 [3] | Ceylan 2022 [4] | Ceylan 2022 [4] | Our patient | |
|---|---|---|---|---|
| Gender | F | F | M | M |
| Consanguinity | Yes | Yes | Yes | Yes |
| Carrier parents | Healthy (Brain CT not performed) | Healthy mother, father with migraine (MRI of both parents showed symmetrical signal loss on SWI sequences at bilateral BG) | Healthy mother, father with migraine (MRI of both parents showed symmetrical signal loss on SWI sequences at bilateral BG) | Healthy (Brain CT not performed) |
| Variant | c.211 C > T | c.1794 + 1 G > A | c.1794 + 1 G > A | c.560 A > G |
| Protein | p.(Arg71Cys) | p.Ser570Argfs∗30 | p.Ser570Argfs∗30 | p.(Tyr187Cys) |
| Exon/Intron | Exon 2 | Intron 10 | Intron 10 | Exon 5 |
| Ethnicity | NR | NR | NR | Egyptian |
| Symptoms onset | 20 y (vertigo) | birth | birth | 2 m (focal motor seizures) |
| Age at last follow-up | 55 y | 2 y 5 m | 13 m | 5 y |
| Psychomotor delay | No | Yes | Yes | Yes |
| Language | Dysarthria (>40 y) | NR | NR | Non-verbal communication |
| Epilepsy | No | Yes | Yes | Yes (symptomatic lesional) |
| Motor involvement | Motor clumsiness, balance disturbance, asymmetric Parkinsonism (>40–50 y) | Spasticity in four extremities | Hemiplegic right side | Spastic tetraparesis (right > left) |
| Psychiatric symptoms | Psychosis (Paranoid delirium, improved in aripiprazole) at 28 y | NR | NR | No |
| Neuroimaging findings | Extensive cortical bi-hemispheric calcifications in BG, periventricular WM, posterior cortex and cerebellum | Cerebral atrophy, abnormal signal changes in cerebral WM-thalamus, corpus callosum hypoplasia, possible microcalcifications and subdural effusion | Chronic sequela changes in both cerebral hemispheres, especially in the posterior parts and ventricular dilatation | Severe anterior and posterior moyamoya vasculopathy, T1 hyperintensity of the globi pallidi, and BG, cortico-subcortical, and white matter calcifications |
| Dysmorphism | NR | NR | NR | Minor (synophrium, small and spaced teeth, thickened tragus and lobes) |
| Other features | NR | Bilateral congenital cataracts, microcephaly, growth retardation | Bilateral congenital cataracts, microcephaly, growth retardation, secundum atrial septal defect | Bilateral congenital cataracts, acquired microcephaly |
BG basal ganglia, CT computerized tomography, F female, M male, m months, NR not reported, SWI susceptibility weighted imaging, y years, WM white matter.
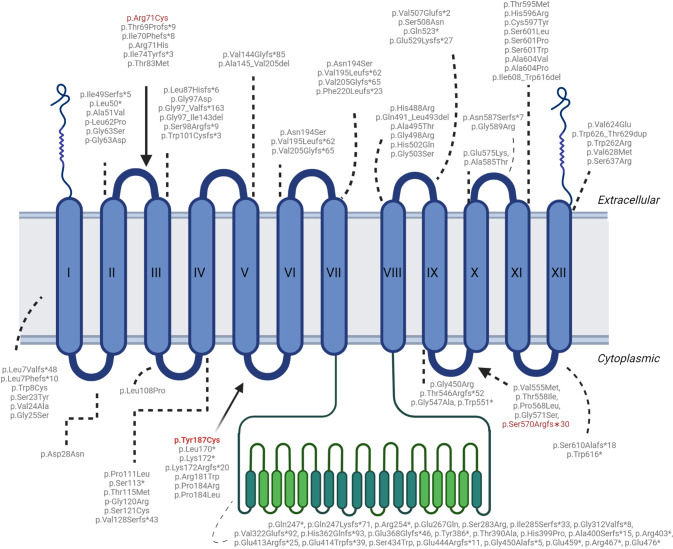
According to a recent review, heterozygous SLC20A2 variants are more often missense [5]. Their localization is shown in Fig. 2. The variant detected in our patient, p.(Tyr187Cys), is located in exon 5 of the gene. It is an extremely rare missense change predicted to impact on protein function according to in silico tools. More in detail, this variant has been previously reported in heterozygous state in two members of the same family with PFBC, with no apparent connection to the family of our proband [6]. Functional studies in Xenopus oocyte model demonstrated that this amino acid change affects the ability to modulate phosphate uptake, but not its efflux [7]. Interestingly, all three biallelic variants do not fall in the transmembrane domains, affecting instead an extracellular and a cytosolic loop.
Fig. 2. Localization of pathogenic SLC20A2 variants in PFBC patients.
Schematic drawing of the SLC20A2 transporter showing the localization of the three biallelic variants (in red) associated with SLC20A2-related PFBC so far, the p.(Arg71Cys) [7], the p.(Tyr187Cys) (our patient) and the p.(Ser570Argfs∗30) [8]. Note that all three biallelic variants do not fall in the transmembrane domains, affecting instead an extracellular and a cytosolic loop.
The phenotype of our patient is suggestive of a severe clinical involvement, likely caused by the presence of this variant in both alleles. However, neuroimaging data of his parents were not available for review, thus limiting the possibility to rule out that similar though milder neuroradiological features were present. Although the carrier parents are healthy at the time of diagnosis, we cannot exclude that they may also develop neurological signs and symptoms during their lifetime.
Cerebrovascular symptoms have already been reported with SLC20A2 haploinsufficiency due to heterozygous loss of function variants. Among them, migraine, transient ischemic attack, and stroke were the most common [1]. To our knowledge, this is the first case associated with moyamoya vasculopathy. This is a severe condition characterized by progressive mono/bilateral stenosis of intracranial internal carotid arteries and their proximal branches, accompanied by formation of collateral arterial circulation. It may occur as isolated (moyamoya disease) or associated with several human disorders (moyamoya syndrome). The etiology and pathogenesis are currently elusive, although the high frequency in certain medical conditions suggest possibly involved mechanisms. Interestingly, very severe moyamoya vasculopathy involving both the anterior and posterior circulation was observed in our case, associated with signs of mineralization of the globi pallidi and scattered calcifications in the basal ganglia, frontal cortico-subcortical regions and centrum semiovale.
The association of brain calcifications with moyamoya vasculopathy is occasional and a direct link with SLC20A2 has not been previously suggested [8]. Noteworthy, the autopsy in a 62-year-old Japanese woman with IBGC caused by a heterozygous missense variant in exon 11 of SLC20A2 revealed progressive calcifications in the tunica media of small arteries, arterioles and capillaries were observed, especially involving basal ganglia, cerebellar white matter and deeper layers of cerebral cortex [9]. In a small number of arteries, these calcifications led to lumen obstruction. Additionally, in a murine model of SLC20A2 haploinsufficiency, elevated phosphate levels in cerebrospinal fluid (CSF) were detected in association with increased susceptibility to arteriolar calcifications [10]. Although the involvement of SLC20A2 in moyamoya vasculopathy remains to be clarified, these findings lead to suggest that its deficiency might be involved in the pathogenesis of the cerebral vasculopathy in our case, also based on the large expression of SLC20A2 in brain vessels.
Our patient was also diagnosed with severe congenital cataracts, supporting the idea that homozygous mutations may result in more complex clinical manifestations. This hypothesis is also endorsed by Ceylan et al. [4]. In their report, the two siblings featured not only bilateral congenital cataracts, but also psychomotor delay with severe motor involvement, early-onset epilepsy and microcephaly. Further reports should confirm these findings and we cannot exclude a gene dosage effect underlying the higher severity of the rare recessive SLC20A2-related PFBC. The contribution of other genetic modulators might also affect the phenotype. This hypothesis has been suggested to explain the variable penetrance of monoallelic variants, whose effect could be partially mitigated by the increased expression of homologous genes and/or a biologically activated protein conformation forced by Heat Shock Proteins [2]. A contribution to the understanding of the pathophysiological role of recessive SLC20A2 variants could be provided by functional assays investigating the impact of these variants on the transporter activity.
Supplementary information
Acknowledgements
We gratefully acknowledge the family for their participation in this study.
Author contributions
MS and VC contributed to the study conception and design. GD’O and MS drafted the paper. FR, PDM, and FZ conducted the genetic studies. MS processed the neuroradiological features. RR, MC, and PS were involved in the clinical care of the patients and in collecting clinical and genetic data. All authors have critically revised the paper and approved the final one as submitted.
Funding
This work was supported by a grant of the Italian Ministry of Health (Ricerca Finalizzata RF-2019-12369247).
Data availability
All data generated or analyzed during this study are included in this published paper. Further inquiries can be directed to the corresponding author.
Competing interests
The authors declare no competing interests.
Ethics approval
The family involved in the study provided informed consent for being part of this study. The study was reviewed and approved by the Ethical Committee of the Gaslini Children’s Hospital (Genoa, Italy).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gianluca D’Onofrio, Marcello Scala.
Contributor Information
Marcello Scala, Email: mscala.md@gmail.com.
Valeria Capra, Email: valeriacapra@gaslini.org.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-023-01349-1.
References
- 1.Donzuso G, Mostile G, Nicoletti A, Zappia M. Basal ganglia calcifications (Fahr’s syndrome): related conditions and clinical features. Neurol Sci. 2019;40:2251–63. doi: 10.1007/s10072-019-03998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira DF, de Lemos RR, de Oliveira JR. Mutations at the SLC20A2 gene and brain resilience in families with idiopathic basal ganglia calcification (“Fahr’s disease”) Front Hum Neurosci. 2013;7:420. doi: 10.3389/fnhum.2013.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arteche-López A, Álvarez-Mora MI, Sánchez Calvin MT, Lezana Rosales JM, Palma Milla C, Gómez, et al. Biallelic variants in genes previously associated with dominant inheritance: CACNA1A, RET and SLC20A2. Eur J Hum Genet. 2021;29:1520–6. doi: 10.1038/s41431-021-00919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceylan AC, Kireker Köylü O, Özyürek H, Özaydin E, Yön Mİ, Kasapkara ÇS. Homozygous SLC20A2 mutations cause congenital CMV infection-like phenotype. Acta Neurol Belg. 2022. 10.1007/s13760-022-02044-6. [DOI] [PubMed]
- 5.Balck A, Schaake S, Kuhnke NS, Domingo A, Madoev H, Margolesky J, et al. Genotype-Phenotype Relations in Primary Familial Brain Calcification: Systematic MDSGene Review. Mov Disord. 2021;36:2468–80. doi: 10.1002/mds.28753. [DOI] [PubMed] [Google Scholar]
- 6.Lemos RR, Ramos EM, Legati A, Nicolas G, Jenkinson EM, Livingston JH, et al. Update and Mutational Analysis of SLC20A2: A Major Cause of Primary Familial Brain Calcification. Hum Mutat. 2015;36:489–95. doi: 10.1002/humu.22778. [DOI] [PubMed] [Google Scholar]
- 7.López-Sánchez U, Tury S, Nicolas G, Wilson MS, Jurici S, Ayrignac X, et al. Interplay between primary familial brain calcification-associated SLC20A2 and XPR1 phosphate transporters requires inositol polyphosphates for control of cellular phosphate homeostasis. J Biol Chem. 2020;295:9366–78. doi: 10.1074/jbc.RA119.011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Troedson C, Wong M, Dalby-Payne J, Wilson M, Dexter M, Rice GI, et al. Systemic lupus erythematosus due to C1q deficiency with progressive encephalopathy, intracranial calcification and acquired moyamoya cerebral vasculopathy. Lupus. 2013;22:639–43. doi: 10.1177/0961203313486950. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Miura T, Aoki K, Saito S, Hondo H, Konno T, et al. Familial idiopathic basal ganglia calcification: Histopathologic features of an autopsied patient with an SLC20A2 mutation. Neuropathology. 2016;36:365–71. doi: 10.1111/neup.12280. [DOI] [PubMed] [Google Scholar]
- 10.Wallingford MC, Chia JJ, Leaf EM, Borgeia S, Chavkin NW, Sawangmake C, et al. SLC20A2 Deficiency in Mice Leads to Elevated Phosphate Levels in Cerbrospinal Fluid and Glymphatic Pathway-Associated Arteriolar Calcification, and Recapitulates Human Idiopathic Basal Ganglia Calcification. Brain Pathol. 2017;27:64–76. doi: 10.1111/bpa.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published paper. Further inquiries can be directed to the corresponding author.