Abstract
Introduction
The efficacy of abatacept is enhanced in anti-citrullinated protein antibody (ACPA) and rheumatoid factor (RF)-positive versus -negative patients with rheumatoid arthritis (RA). Four early RA abatacept trials were analyzed to understand the differential impact of abatacept among patients with SeroPositive Early and Active RA (SPEAR) compared to non-SPEAR patients.
Methods
Pooled patient-level data from AGREE, AMPLE, AVERT, and AVERT-2 were analyzed. Patients were classified as SPEAR if they were ACPA +, RF +, disease duration < 1 year, and Disease Activity Score-28 (DAS28) C-reactive protein (CRP) ≥ 3.2 at baseline; non-SPEAR otherwise. Outcomes included: American College of Rheumatology (ACR) 20/50/70 at week 24; mean change from baseline to week 24 for DAS28 (CRP), Simple Disease Activity Index (SDAI), ACR core components; DAS28 (CRP) and SDAI remission. Adjusted regression analyses among abatacept-treated patients compared SPEAR and non-SPEAR patients, and in full trial population estimating how the efficacy of abatacept versus comparators [adalimumab + methotrexate, methotrexate] was modified by SPEAR status.
Results
The study included 1400 SPEAR and 673 non-SPEAR patients; most were female (79.35%), white (77.38%), and with a mean age 49.26 (SD 12.86) years old. Around half with non-SPEAR were RF + and three-quarters ACPA +. Stronger improvements from baseline to week 24 were observed in almost all outcomes for abatacept-treated SPEAR versus non-SPEAR patients or versus SPEAR patients treated with comparators. Larger improvements were observed for SPEAR patients among the abatacept-treated population, and more strongly improved efficacy among SPEAR patients for abatacept than comparators.
Conclusions
This analysis, including large patient numbers of early-RA abatacept trials, confirmed beneficial treatment effects of abatacept in patients with SPEAR versus non-SPEAR.
Keywords: Abatacept, Anti-citrullinated protein antibody, Cross-trial analysis, Rheumatoid arthritis, Rheumatoid factor, Seropositive
Key Summary Points
| Why carry out this study? |
| While anti-citrullinated protein antibodies and rheumatoid factor biomarkers have demonstrated value in diagnosing rheumatoid arthritis (RA) and prognosticating more severe disease, evidence has indicated that these biomarkers may also interact with therapeutic outcomes in RA. |
| This study analyzed patient-level data from four early RA trials of abatacept and compared clinical outcomes between patients with SeroPositive Early and Active RA (SPEAR) and patients without these characteristics (non-SPEAR) to understand the differential treatment impact of abatacept on various efficacy endpoints. |
| We hypothesized that abatacept is associated with stronger improvements across multiple efficacy endpoints among SPEAR patients compared to non-SPEAR patients. |
| What was learned from the study? |
| This pooled analysis of four abatacept trials demonstrated a differential treatment effect of abatacept on change from baseline to week 24 across various efficacy outcomes among patients with SPEAR versus non-SPEAR, which was not seen with comparators. |
| The results of this study confirm prior literature demonstrating the predictive value of anti-citrullinated protein antibodies and rheumatoid factor seropositivity in abatacept treatment and highlight the potential utility of serostatus to guide treatment selection. |
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by inflammation of the synovial membrane, leading to erosion of cartilage and bone and eventual destruction of joints if left untreated [1]. Active RA causes joint pain, stiffness, and fatigue, and substantially reduces physical functioning and quality of life [2, 3]. In the United States (US), the prevalence of RA is estimated to range from 0.5 to 1% [4, 5]; women and the elderly are particularly affected [6].
The current “treat-to-target” approach to RA management involves frequent disease monitoring and adapting therapy as needed to achieve the predefined target of low disease activity or remission [7]. Therapeutic options include conventional disease-modifying antirheumatic drugs (DMARDs, e.g., methotrexate), biological DMARDs (e.g., adalimumab, abatacept [ABA]), and targeted synthetic DMARDs (e.g., JAK inhibitors) to treat moderately-to-highly active disease (e.g., Disease Activity Score 28 for RA with C-reactive protein [DAS28 (CRP)] ≥ 3.2)[7].
While biomarkers such as anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF) have demonstrated prognostic value in RA and can indicate more severe disease [8–10], evidence suggests that these biomarkers may also interact with therapeutic outcomes in RA [11, 12]. In an exploratory biomarker-driven phase IV clinical trial (Early AMPLE), ABA was associated with more pronounced clinical responses and remission rates compared to adalimumab among the shared epitope-positive patients, while no significant differences were observed among shared epitope-negative patients [13]. Notably, this population with early RA (mean disease duration of 5.5 months) generally demonstrated a greater clinical response than the population in the original AMPLE study (mean disease duration of 1.7–1.9 years), highlighting the importance of early treatment [13]. Moreover, in the AVERT and AMPLE clinical trials, treatment with ABA was found to be associated with improved clinical outcomes in the enriched populations (e.g., anti-cyclic citrullinated peptide [anti-CCP] 2 immunoglobulin M [IgM]-positive) compared to the non-enriched populations [14, 15]. In addition, in a US registry-based study, treatment with ABA was associated with greater Clinical Disease Activity Index (CDAI) responses among ACPA-positive patients than ACPA-negative patients [16]. While individual data sources evaluated the impact of ABA on different clinical outcomes in seropositive and seronegative patients, heterogeneity exists in the comparisons of efficacy outcomes between the populations across studies. Additionally, the potential for predictive value in a patient population with particularly poor outcomes further underlines the importance of assessing the role of biomarkers in the treatment of seropositive RA. Finally, combining data from multiple trials increases power compared to analyses of individual trial data sets.
This study therefore conducted a post hoc analysis of large patient-level clinical trial data from four early RA trials of ABA and compared clinical outcomes between patients with SeroPositive Early and Active RA (SPEAR) and patients without these characteristics (non-SPEAR) to understand the differential treatment impact of ABA on various efficacy endpoints.
Methods
Study Design and Population
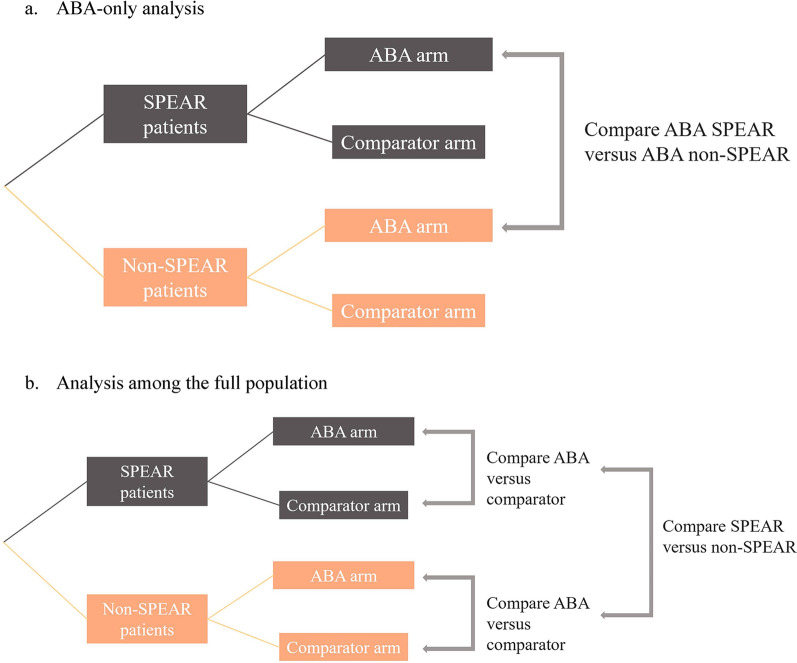
A cross-trial analysis using individual patient data from historical ABA trials was conducted to assess differences in efficacy outcomes between SPEAR and non-SPEAR populations among ABA-treated arms, and between ABA and comparator arms relative to SPEAR and non-SPEAR status (Fig. 1). Individual patient data were pooled from four early-RA ABA trials (AGREE [17]: NCT00122382, 2005–2009; AMPLE [18]: NCT00929864, 2009–2012; AVERT [19]: NCT01142726, 2010–2014; AVERT-2: NCT02504268, 2015–2020), which included patients who were biologic-naïve and had moderate-to-severe, active, early RA (≤ 5 years). A fifth early-RA trial of ABA (Early AMPLE: NCT02557100) included only patients categorized as SPEAR; as the primary purpose of this analysis was to identify differences between SPEAR and non-SPEAR patients, Early AMPLE was excluded from the analysis.
Fig. 1.
Analysis schema for ABA-treated population and full population. ABA abatacept, SPEAR SeroPositive Early and Active RA
Patients who satisfied all the following criteria at baseline were defined as SPEAR:
were ACPA-positive (i.e., anti-CCP level higher than 1 time the upper limit of normal [ULN]);
were RF-positive;
had a disease duration < 1 year (based on greater clinical response in early RA observed in Early AMPLE [13]); and
had a DAS28 (CRP) ≥ 3.2 (since ABA is indicated for moderately-to-severely active RA [20])
Those with missing data on any of the four criteria and otherwise fulfilled all other criteria were considered to have missing SPEAR status and thus excluded from analyses; in contrast, patients who failed to meet one of the criteria were considered non-SPEAR regardless of any missing other criteria.
Patients were grouped according to treatment status in the analyses (treated with ABA [monotherapy or with methotrexate] or with a comparator [adalimumab plus methotrexate or methotrexate alone]).
The trials analyzed in this study (AGREE, AMPLE, AVERT, and AVERT-2) had IRB approval, patient consent, and were conducted in accordance with the Helsinki Declaration. Patient IDs from AGREE, AMPLE, AVERT, and AVERT-2 were encrypted and not identified in this study. Confidentiality of patient records was maintained throughout the conduct of the study, and only aggregate data were reported. Therefore, IRB approval was not required for this specific study.
Outcome Measures
The timepoint of interest was week 24 for all outcomes. Primary outcomes measured included DAS28 (CRP) mean change from baseline to week 24, DAS28 (CRP) remission defined as DAS28 [CRP] score < 2.6 at week 24, American College of Rheumatology 20/50/70 criteria (ACR20/50/70) rate at week 24, and mean changes from baseline to week 24 in the individual ACR core components, which included patient-reported outcomes (Health Assessment Questionnaire Disability Index (HAQ-DI) score, Patient Global Assessment, Pain visual analog scale), laboratory and clinical values (CRP level (mg/dl), tender joint count [TJC] out of 68 joints, and swollen joint count [SJC] out of 66 joints), and Physician Global Assessment. Secondary outcomes measured included Simple Disease Activity Index (SDAI) mean change from baseline to week 24, and SDAI remission defined as SDAI score ≤ 3.3 at week 24.
Statistical Analysis
For each outcome, two separate linear (for continuous outcomes) or logistic regressions (for binary outcomes) were specified. Regression analyses were conducted separately among ABA-treated patients comparing SPEAR and non-SPEAR patients and among the full population estimating how the efficacy of ABA versus comparators is modified by SPEAR status.
The regression models were set up to estimate the mean change from baseline at week 24 for continuous outcomes; for categorical outcomes, the regression models estimated the log odds ratios of the outcomes at week 24. Regressions models among the ABA-only population included covariates such as trial fixed effect, SPEAR status, baseline measures of the outcome (for all outcomes except for the ACR outcomes, for which no baseline outcomes were included), baseline demographics (age, sex, race [non-White, White], and region [North America, Europe, other]), and baseline ACR core measures. For the full population, in addition to the covariates from the ABA-only regression, treatment type and an interaction between treatment type and SPEAR status were included. In the ABA-only population, the contrast of interest was the SPEAR status indicator, reflecting the difference in outcomes between patients with SPEAR status versus those without. In the full population, the contrast of interest was the interaction between SPEAR status and treatment, reflecting the difference in clinical outcomes between SPEAR and non-SPEAR patients among ABA-treated patients compared to the same difference among comparator treatments. Sensitivity analyses that defined SPEAR status using only ACPA and RF seropositivity were conducted to assess the robustness of the results to a different definition of SPEAR status. Two additional analyses were conducted to assess the relationship of the individual SPEAR criteria with SPEAR status:
the analyses of DAS28 (CRP) mean change from baseline were replicated using one of the four individual criteria replacing SPEAR status, and
a logistic regression was conducted with SPEAR status as outcome and the four SPEAR criteria as separate covariates.
Missing data imputation for outcomes at week 24 was conducted using last observation carried forward for continuous outcomes and non-responder imputation for binary outcomes. Otherwise, the analyses were restricted to those patients without any missing data in the baseline variables.
Results
Baseline Characteristics
A total of 2073 patients (n = 1400 SPEAR, n = 673 non-SPEAR) were included in the analysis; 23.73% were from AGREE, 24.51% from AMPLE, 16.35% from AVERT, and 35.41% from AVERT-2 (Table 1). Most patients were female (79.35%), White (77.38%), and had a mean ± standard deviation (SD) age of 49.26 ± 12.86 years. Average age and proportion of women were lower among SPEAR patients than non-SPEAR patients (both p < 0.01). More SPEAR patients reported being Asian and fewer SPEAR patients reported being Black, other, or White race.
Table 1.
Baseline characteristics, overall and by SPEAR status and treatment type
| All patients | SPEAR patients | Non-SPEAR patients | SPEAR versus non-SPEAR | ||||
|---|---|---|---|---|---|---|---|
| ABA | Comparator | ABA versus comparator | ABA | Comparator | |||
| N = 2073 | n = 819 | n = 581 | p value | n = 341 | n = 332 | p value | |
| Trial, n (%) | |||||||
| AGREE | 492 (23.73) | 177 (21.61) | 155 (26.68) | < 0.001 | 68 (19.94) | 92 (27.71) | < 0.001 |
| AMPLE | 508 (24.51) | 62 (7.57) | 69 (11.88) | 189 (55.43) | 188 (56.63) | ||
| AVERT | 339 (16.35) | 165 (20.15) | 83 (14.29) | 60 (17.60) | 31 (9.34) | ||
| AVERT-2 | 734 (35.41) | 415 (50.67) | 274 (47.16) | 24 (7.04) | 21 (6.33) | ||
| Demographics | |||||||
| Age (years) | 49.26 ± 12.86 | 48.49 ± 12.53 | 48.95 ± 13.47 | 0.51 | 49.87 ± 12.57 | 51.08 ± 12.74 | < 0.01 |
| Female, n (%) | 1,645 (79.35) | 630 (76.92) | 453 (77.97) | 0.69 | 282 (82.70) | 280 (84.34) | < 0.01 |
| Race, n (%) | 0.53 | < 0.001 | |||||
| Asian | 206 (9.94) | 106 (12.94) | 71 (12.22) | 17 (4.99) | 12 (3.61) | ||
| Black | 118 (5.69) | 38 (4.64) | 34 (5.85) | 27 (7.92) | 19 (5.72) | ||
| Other | 145 (6.99) | 55 (6.72) | 31 (5.34) | 28 (8.21) | 31 (9.34) | ||
| White | 1604 (77.38) | 620 (75.70) | 445 (76.59) | 269 (78.89) | 270 (81.33) | ||
| Disease characteristics | |||||||
| Duration of disease (months) | 8.53 ± 12.55 | 2.50 ± 2.90 | 2.62 ± 3.00 | 0.48 | 21.94 ± 15.68 | 19.99 ± 15.13 | < 0.001 |
| Duration of disease < 1 year | 1577 (76.07) | 819 (100.00) | 581 (100.00) | – | 86 (25.22) | 91(27.41) | 0.58 |
| RF + , n (%) | 1931 (93.29) | 819 (100.00) | 581 (100.00) | – | 260 (76.47) | 271 (82.12) | < 0.001 |
| ACPA + , n (%) | 1886 (91.07) | 819 (100.00) | 581 (100.00) | – | 250 (73.75) | 236 (71.08) | < 0.001 |
| DAS28 (CRP) Score | 5.71 ± 1.13 | 5.69 ± 1.09 | 5.75 ± 1.08 | 0.29 | 5.68 ± 1.22 | 5.67 ± 1.21 | 0.46 |
| DAS28 (CRP) ≥ 3.2 | 2045 (98.84) | 819 (100.00) | 581 (100.00) | – | 329 (97.34) | 316 (95.47) | 0.27 |
| CRP value (mg/dl) | 2.16 ± 3.10 | 2.21 ± 2.83 | 2.39 ± 3.64 | 0.29 | 1.84 ± 2.44 | 1.96 ± 3.29 | < 0.01 |
| TJC out of 68 joints | 25.11 ± 14.88 | 23.83 ± 14.32 | 24.80 ± 14.01 | 0.21 | 27.20 ± 16.41 | 26.67 ± 15.71 | < 0.001 |
| SJC out of 66 joints | 16.78 ± 10.78 | 15.90 ± 10.37 | 16.59 ± 10.19 | 0.21 | 18.04 ± 12.18 | 17.98 ± 11.03 | < 0.001 |
| HAQ-DI Score | 1.56 ± 0.69 | 1.57 ± 0.68 | 1.58 ± 0.70 | 0.95 | 1.51 ± 0.69 | 1.55 ± 0.68 | 0.17 |
| Patient Global Assessment | 62.95 ± 22.68 | 64.26 ± 22.23 | 62.48 ± 23.28 | 0.15 | 61.95 ± 22.58 | 61.55 ± 22.73 | 0.10 |
| Physician Global Assessment | 63.05 ± 19.23 | 63.72 ± 19.08 | 64.28 ± 19.42 | 0.59 | 61.21 ± 18.27 | 61.12 ± 20.05 | < 0.01 |
| Pain visual analog scale | 65.11 ± 22.06 | 65.16 ± 22.29 | 64.82 ± 21.92 | 0.78 | 64.65 ± 21.96 | 65.96 ± 21.90 | 0.79 |
Data are mean ± SD unless otherwise stated and presented for non-missing data
ABA abatacept, ACPA anti-citrullinated protein antibodies, CI confidence interval, CRP C-reactive protein, DAS28 (CRP) Disease Activity Score 28 for RA with C-reactive protein, HAQ-DI Health Assessment Questionnaire Disability Index, RF rheumatoid factor, SD standard deviation, SJC swollen joint count, SPEAR SeroPositive Early and Active RA, TJC tender joint count
The average ± SD disease duration was 8.53 ± 12.55 months across all patients at baseline, and most patients were RF-positive (93.29%) and ACPA-positive (91.07%; Table 1). The average DAS28 (CRP) score was 5.71 at baseline. As expected, non-SPEAR patients had longer disease duration and a lower percentage were RF-positive or ACPA-positive than SPEAR patients (all p < 0.01). However, baseline DAS28 (CRP) scores were similar between SPEAR and non-SPEAR patients (p = 0.46). SPEAR patients had lower baseline TJC and SJC than non-SPEAR patients, but worse Physician Global Assessment at baseline (all p < 0.01).
Primary Outcomes—DAS28 (CRP) and ACR 20/70/90
Larger improvements from baseline to week 24 were observed for all DAS28 (CRP) and ACR outcomes for ABA-treated SPEAR patients relative to SPEAR patients treated with comparators or relative to non-SPEAR patients treated with ABA (Table 2).
Table 2.
Descriptive outcome summary at week 24, overall and stratified by SPEAR status and treatment type
| Outcome | Total | SPEAR patients | Non-SPEAR patients | ABA: SPEAR versus non-SPEAR | Difference in differences [A] − [B] | ||||
|---|---|---|---|---|---|---|---|---|---|
| ABA | Comparator | ABA versus comparator [A] | ABA | Comparator | ABA versus comparator [B] | ||||
| N = 2004 | N = 796 | n = 558 | n = 329 | n = 321 | |||||
| DAS28 (CRP) change from baseline | − 2.36 ± 1.40 | − 2.67 ± 1.34 | − 2.13 ± 1.35 | − 0.54, p < 0.001* | − 2.27 ± 1.41 | − 2.10 ± 1.49 | − 0.17, p = 0.14 | − 0.40, p < 0.001* | − 0.37, p < 0.01* |
| DAS28 (CRP) remission | 587 (29.29) | 301 (37.81) | 138 (24.73) | 13.08, p < 0.001* | 83 (25.23) | 65 (20.25) | 4.98, p = 0.16 | 12.58, p < 0.001* | 8.10, p = 0.14 |
| ACR20 | 1425 (71.11) | 614 (77.14) | 366 (65.59) | 11.55, p < 0.001* | 228 (69.30) | 217 (67.60) | 1.70, p = 0.70 | 7.84, p < 0.01* | 9.85, p < 0.05* |
| ACR50 | 955 (47.65) | 448 (56.28) | 235 (42.11) | 14.17, p < 0.001* | 147 (44.68) | 125 (38.94) | 5.74, p = 0.16 | 11.60, p < 0.001* | 8.43, p = 0.09 |
| ACR70 | 556 (27.74) | 275 (34.55) | 121 (21.68) | 12.87, p < 0.001* | 85 (25.84) | 75 (23.36) | 2.48, p = 0.52 | 8.71, p < 0.01* | 10.39, p < 0.05* |
| SDAI change from baseline | − 27.05 ± 19.88 | − 29.87 ± 21.58 | − 24.61 ± 17.29 | − 5.26, p < 0.001* | − 27.03 ± 19.37 | − 23.83 ± 19.05 | − 3.20, p = 0.07 | − 2.84, p = 0.07 | − 2.06, p = 0.35 |
| SDAI remission | 243 (12.13) | 122 (15.33) | 50 (8.96) | 6.37, p < 0.001* | 37 (11.25) | 34 (10.59) | 0.66, p = 0.89 | 4.08, p = 0.09 | 5.71, p = 0.08 |
| CRP value (mg/dl) change from baseline | − 1.42 ± 2.97 | − 1.64 ± 2.82 | − 1.50 ± 3.28 | − 0.14, p = 0.42 | − 1.06 ± 2.19 | − 1.09 ± 3.38 | 0.03, p = 0.88 | − 0.58, p < 0.01* | − 0.17, p = 0.56 |
| TJC out of 68 joints change from baseline | − 15.86 ± 13.68 | − 16.55 ± 13.22 | − 14.54 ± 13.00 | − 2.01, p < 0.01* | − 16.49 ± 15.27 | − 15.80 ± 14.12 | − 0.69, p = 0.55 | − 0.06, p = 0.95 | − 1.32, p = 0.31 |
| SJC out of 66 joints change from baseline | − 11.54 ± 9.68 | − 12.15 ± 9.61 | − 10.25 ± 9.07 | − 1.90, p < 0.001* | − 12.25 ± 10.87 | − 11.52 ± 9.39 | − 23.77, p = 0.36 | 0.01, p = 0.88 | − 1.16, p = 0.21 |
| HAQ-DI Score change from baseline | − 0.72 ± 0.69 | − 0.85 ± 0.70 | − 0.68 ± 0.66 | − 0.17, p < 0.001* | − 0.65 ± 0.66 | − 0.57 ± 0.66 | − 1.22, p = 0.13 | − 0.20, p < 0.001* | − 0.09, p = 0.15 |
| Patient Global Assessment change from baseline | − 31.69 ± 29.09 | − 36.54 ± 29.70 | − 28.01 ± 28.43 | − 8.53, p < 0.001* | − 30.76 ± 27.04 | − 27.02 ± 29.07 | − 3.74, p = 0.09 | − 5.78, p < 0.01* | − 4.79, p = 0.08 |
| Physician Global Assessment change from baseline | − 42.97 ± 23.53 | − 46.52 ± 23.09 | − 41.21 ± 23.63 | − 5.31, p < 0.001* | − 41.66 ± 23.09 | − 38.53 ± 23.73 | − 3.13, p = 0.09 | − 4.86, p < 0.01* | − 2.18, p = 0.33 |
| Pain visual analog scale change from baseline | − 33.51 ± 29.12 | − 37.99 ± 29.52 | − 29.26 ± 28.43 | − 8.73, p < 0.001* | − 33.25 ± 26.65 | − 30.06 ± 30.23 | − 3.19, p = 0.15 | − 4.74, p < 0.05* | − 5.54, p < 0.05* |
Data are mean ± SD using imputed follow-up data
The p values shown here were not adjusted for multiple comparisons
ABA abatacept, ACPA anti-citrullinated protein antibodies, ACR20/50/70 American College of Rheumatology 20/50/70 criteria, CI confidence interval, CRP C-reactive protein, DAS28 (CRP) Disease Activity Score 28 for RA with C-reactive protein, HAQ-DI Health Assessment Questionnaire Disability Index, RF rheumatoid factor, SD standard deviation, SDAI Simple Disease Activity Index, SJC swollen joint count, SPEAR SeroPositive Early and Active RA, TJC tender joint count
* p < 0.05
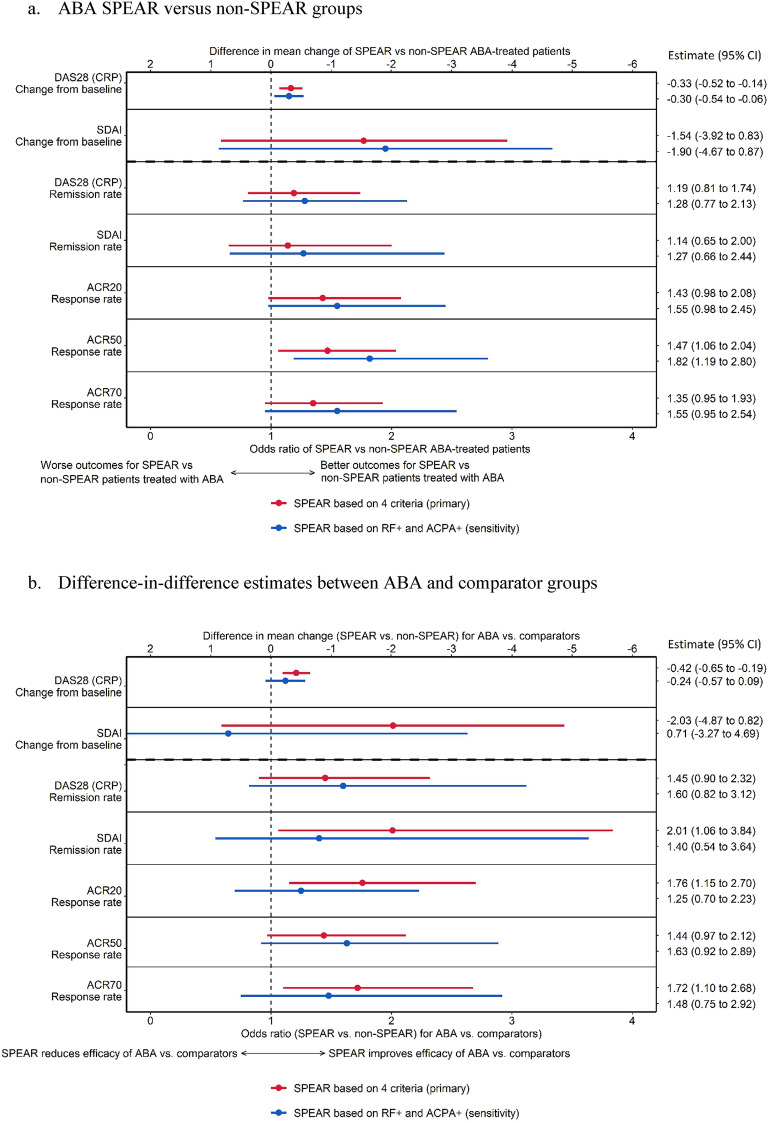
In the regression analyses among the ABA-treated population, SPEAR patients experienced statistically greater improvements in DAS28 (CRP) mean change from baseline and ACR50 relative to their non-SPEAR counterparts (Fig. 2a). SPEAR status was associated with a significant reduction in DAS28 (CRP) mean change from baseline to week 24 (− 0.33 [95% CI − 0.52 to − 0.14]) points compared to non-SPEAR patients and was associated with 19% higher odds (odds ratio [OR] 1.19 [0.81 to 1.74]) of remission based on DAS28 (CRP). Estimated odds ratios for achieving ACR20 and ACR70 response levels were from OR 1.43 (0.98 to 2.08) and OR 1.35 (0.95 to 1.93), respectively, with statistically higher odds of reaching ACR50 [OR 1.47 (1.06 to 2.04)] in favor of SPEAR patients compared to non-SPEAR patients. The sensitivity analysis (i.e., defining SPEAR based only on ACPA and RF seropositivity) produced similar results to the primary analysis (Fig. 2a).
Fig. 2.
Forest plot of analysis results for DAS28 (CRP), ACR 20/70/90, and SDAI outcomes. ABA abatacept, ACPA anti-citrullinated protein antibodies, ACR20/50/70 American College of Rheumatology 20/50/70 criteria, CI confidence interval, DAS28 (CRP) Disease Activity Score 28 for RA with C-reactive protein, RF rheumatoid factor, SDAI Simple Disease Activity Index, SPEAR SeroPositive Early and Active RA
Among the full population, the regressions indicated a statistically larger difference between SPEAR and non-SPEAR patients among the ABA-treated population compared to the comparator-treated population for DAS28 (CRP) mean change from baseline to week 24, ACR20 and ACR70 (Fig. 2b). For DAS28 (CRP) score, the estimated coefficient on the interaction was – 0.42 points (– 0.5 to – 0.19), indicating a statistically larger improvement for SPEAR patients relative to non-SPEAR patients among ABA-treated patients compared to the same difference among comparator patients. The estimated interaction for DAS28 (CRP) remission was OR 1.45 (0.9 to 2.32). The estimated coefficients for ACR20 and ACR70 indicated statistically stronger improvements of efficacy among ABA patients compared to comparator patients [OR 1.76 (1.15 to 2.70) and OR 1.72 (1.1 to 2.68), respectively]; the estimated coefficient for ACR50 was OR 1.44 (0.97 to 2.12). The sensitivity analysis produced similar results to the primary analysis.
Primary Outcomes—ACR Core Components
SPEAR patients treated with ABA experienced a larger reduction in mean scores compared to both SPEAR patients treated with comparators and compared to non-SPEAR patients treated with ABA across most ACR components at week 24 (Table 2).
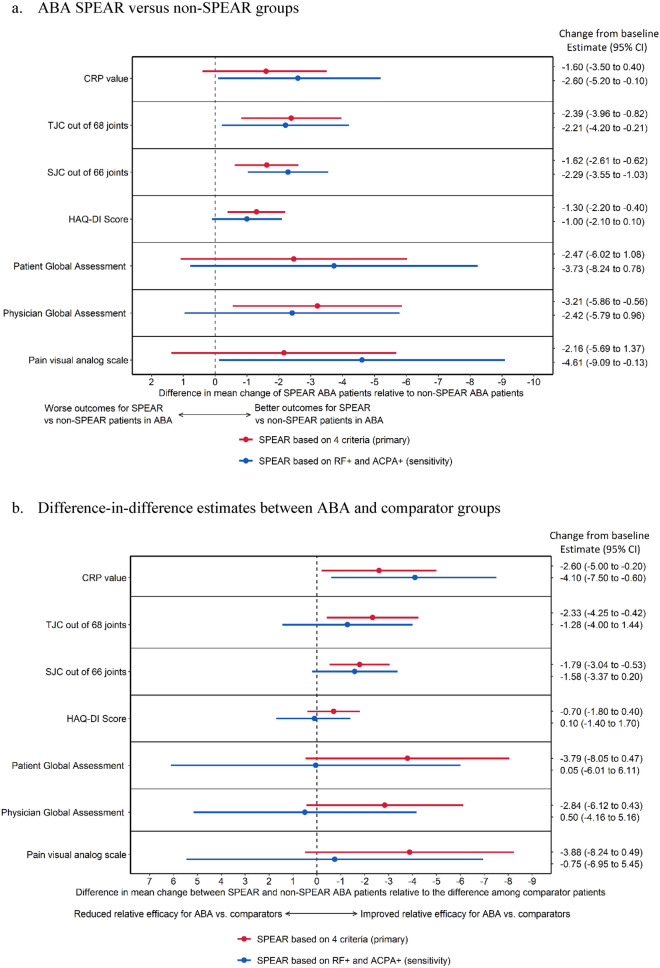
In the regression analysis of the ABA population, SPEAR status was associated with a larger reduction in mean scores across TJC, SJC, HAQ-DI, and Physician Global Assessment (Fig. 3a). The sensitivity analysis results were consistent with the primary analysis (Fig. 3a). Among the full population, the differences between SPEAR and non-SPEAR patients were larger and improved more strongly for ABA-treated patients compared to patients treated with comparators for CRP levels, TJC, and SJC (Fig. 3b). The sensitivity analyses were consistent with the primary analysis for CRP, TJC, and SJC, but resulted in wider confidence intervals (CIs) with point estimates around zero for HAQ-DI, Patient and Physician Global Assessment, and Pain visual analog scale (Fig. 3b). In descriptive analyses, SPEAR ABA patients still experienced greater reductions than SPEAR comparator patients in those outcomes (all p < 0.001, data not shown), as did non-SPEAR patients (p values of 0.06, 0.10, 0.06, and 0.14, respectively). Therefore, the movement towards a point estimate of zero does not indicate that ABA and comparator patients improved equally well, but rather that ABA patients experienced similarly larger reductions compared to comparator patients regardless of SPEAR status when using the less restrictive definition of SPEAR.
Fig. 3.
Forest plot of analysis results for ACR core component outcomes. ABA abatacept, ACPA anti-citrullinated protein antibodies, CI confidence interval, CRP C-reactive protein, HAQ-DI Health Assessment Questionnaire Disability Index, RF rheumatoid factor, SJC swollen joint count, SPEAR SeroPositive Early and Active RA, TJC tender joint count
Secondary Outcomes—SDAI
Larger improvements from baseline to week 24 were observed for SDAI mean change and remission based on SDAI for ABA-treated SPEAR patients relative to non-SPEAR patients treated with ABA, but differences were not statistically significant relative to SPEAR patients treated with comparators (Table 2).
In the regression analysis, among the ABA-treated population, the estimated difference between SPEAR and non-SPEAR patients in change in SDAI at week 24 was − 1.54 (− 3.92 to 0.83) and a difference of 14% (OR 1.19 [0.81 to 1.74]) in SDAI remission. Among the full population, the estimated interaction coefficient for SDAI change from baseline to week 24 was − 2.03 (− 4.87 to 0.82); the interaction coefficient for remission based on SDAI was statistically significant at OR 2.01 (1.06 to 3.84). The sensitivity analysis produced similar results to the primary analysis except for SDAI change from baseline among the full population, where the estimate became positive with a wide confidence interval (0.81 [− 3.27 to 4.69]) (Fig. 2a, b).
Analyses to Assess Relationship of SPEAR Criteria with SPEAR Status
The replication of the analyses for DAS28 (CRP) with only one SPEAR criterion produced numerically similar results to using the combined SPEAR status for all four criteria among both the ABA and the full population. Specifically, among the ABA population, estimates ranged from − 0.16 (DAS28 [CRP] ≥ 3.2 only, p = 0.71) to − 0.39 (RF + only, p < 0.01), and among the full population, estimates ranged from − 0.18 (RF + only, p = 0.41) to − 0.94 (DAS28 [CRP] ≥ 3.2, p = 0.09), with disease duration < 1 year also being significant (− 0.38, p < 0.01).
When regressing SPEAR status on all four SPEAR criteria, all four criteria were highly significant (p < 0.001) with odds ratios of 3.33 (CI [2.63, 4.55]) for DAS28 (CRP) ≥ 3.2, 2.33 (2.17, 2.5) for ACPA + , 2.17 (2.04, 2.38) for RF + , and 1.19 (1.16, 1.22) for disease duration < 1 year, where odds ratios > 1 indicate higher odds for SPEAR (vs. non-SPEAR). This suggests all four criteria have meaningful implications on SPEAR status.
Discussion
This study compared efficacy outcomes of SPEAR patients to those of non-SPEAR patients across four early-RA clinical trials of ABA and found a differential treatment impact of ABA across several outcomes: treatment benefits were larger for SPEAR ABA patients compared to non-SPEAR ABA patients, and for patients treated with ABA, the differences between SPEAR and non-SPEAR were larger than the respective differences in the comparator arms. The differential efficacy of ABA was robust to adjustment for baseline demographics and baseline disease status. The findings for the ACR components (secondary outcomes) further suggest that the differential treatment impact of ABA is not limited to a subset of core measures, but affects multiple dimensions, including patient-reported outcomes, laboratory values, joint counts, and Physician Global Assessment.
This post hoc study corroborates previous evidence of improved outcomes among ABA-treated SPEAR patients. While the Early AMPLE trial included only ACPA-positive patients, Rigby et al. further evaluated seropositivity of the shared epitope, which is associated with increased binding of citrullinated peptides among ACPA-positive patients with RA. In Early AMPLE, the treatment response benefit seen with ABA versus adalimumab was more pronounced in shared epitope-positive patients compared to the overall population [13]. In a recent systematic literature review and meta-analysis of 18 observational RA studies, ACPA-positive patients were 13% more likely to respond to ABA than ACPA-negative patients (risk ratio [95% CI] 1.13 [1.00 to 1.26]; based on European League Against Rheumatism [EULAR] response criteria), while ACPA positivity was associated with lower responses to tumor necrosis factor α (TNFα) inhibitors [21].
Other real-world studies have also demonstrated findings consistent with the current analysis, in particular with regard to ACPA and RF status. Indeed, in a US registry-based study by Harrold et al., treatment with ABA was associated with greater CDAI responses among ACPA-positive patients than ACPA-negative patients, with no significant difference observed with TNFα inhibitors [16]. Furthermore, ABA-treated ACPA-positive patients had higher odds of achieving low disease activity (LDA) and remission compared to ABA-treated ACPA-negative patients. Lastly, in a pooled analysis of 16 European RA registries by Courvoisier et al., an additional 1.5% and 8.1% of seropositive (i.e., ACPA- and/or RF-positive) patients achieved remission and LDA, respectively, versus seronegative (i.e., ACPA- and RF-negative) patients with ABA treatment; similar findings were observed for treatment with rituximab, but not smaller or no associations for tocilizumab and TNFα, respectively [22]. Taken together, the current study adds to the growing literature demonstrating that ABA treatment is associated with better outcomes, particularly among SPEAR patients.
The findings of this study have important clinical implications, with seropositivity demonstrating predictive value for treatment with ABA in addition to established prognostic value. ACPA and RF are commonly assessed in clinical practice, since their status is included in the ACR/EULAR classification criteria for the diagnosis of RA [2, 16]. In terms of prognostication, ACPA and RF seropositivity has been established to be associated with more aggressive joint involvement and more extra-articular manifestations [23]. Prior evidence also suggests that ACPA levels were affected differently by treatment, possibly related to each treatment’s mechanism of action, although the clinical relevance of such a reduction is not yet clear [24, 25]. Adding to this, the current study confirms and supports a differential treatment effect for co-stimulation blockade using ABA among enriched and double antibody-positive early RA patients, suggesting a potential for patient-tailored RA treatment approaches. With more than 60% of RA patients being ACPA- and/or RF-positive [23], there is potential for the use of ACPA and RF status to guide appropriate treatment selection in a large proportion of patients. Moreover, in the analysis of 16 European RA registries, Courvoisier et al. found that seropositivity was associated with longer treatment maintenance among patients treated with ABA versus TNFα inhibitors, and discontinuation of ABA was less likely in seropositive patients compared to seronegative patients [22], further adding to the clinical benefits of ABA treatment in seropositive patients. An examination of male US veterans found that anti-CCP antibodies and RF concentrations were associated with increased disease activity in RA [26]. Notably, this study was conducted with data from 2009, a time before ABA and other non-TNF inhibitors were used frequently. This suggests that access to TNF inhibitors (through the Veterans Affairs healthcare system) did not bring down the likelihood of worse RA activity and prognosis.
Important strengths of this study should be noted. This cross-trial analysis assessed the heterogeneity of the difference in efficacy outcomes between SPEAR and non-SPEAR across clinical trials of ABA in RA, which contributes to our understanding of how efficacy outcomes compare between SPEAR and non-SPEAR patients. By combining data from multiple independent trials, this study increased the effective sample size and improved statistical precision compared to analyses of individual trials. Additionally, regression analyses were adjusted for baseline characteristics to provide a robust evaluation of the outcomes despite cross-trial heterogeneity.
While the current study contributes important insight to the RA literature, certain limitations apply to the findings, including the post hoc nature of the design. Evidence was derived based on data reported from clinical trials, which may not be generalizable to real-world settings. Relatedly, since this study was limited to the data collected in the trials, extraneous factors that were not captured may have impacted the results. Finally, while the four trials included only few seronegative patients (> 90% positive for RF and ACPA), the total SPEAR- population included more than 650 patients.
Conclusions
This analysis compared clinical outcomes of patients with SPEAR versus those without SPEAR using patient-level data from four early-RA ABA trials. The findings indicate a differential treatment effect of ABA across all ACR dimensions, including patient reported outcomes, laboratory, and clinical values, at week 24 across several efficacy outcomes and found that the differential efficacy of ABA was robust to adjustment for baseline demographics and baseline disease status. The results of this study confirm prior literature demonstrating the predictive value of ACPA and RF seropositivity in ABA treatment and highlight the potential utility of serostatus to guide treatment selection.
Acknowledgements
Funding
Financial support for this research was provided by Bristol Myers Squibb. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. Bristol Myers Squibb provided funding for publication, including the journal's Rapid Service Fee. Philip Conaghan is supported in part by the UK National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Christine Tam, an employee of Analysis Group, Inc., which provided paid consulting services to Bristol Myers Squibb for the development and conduct of this study and manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors (Kaleb Michaud, Philip G Conaghan, Sang Hee Park, Karissa Lozenski, Mirko Fillbrunn, Vadim Khaychuk, Elyse Swallow, John Vaile, Henry Lane, Ha Nguyen, and Janet Pope) were involved in the conception and design, or analysis and interpretation of the data; the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Prior Presentation
Part of the material in this manuscript was presented at American College of Rheumatology Convergence 2021 held on November 1–10 as a virtual poster presentation, and at the European League Against Rheumatism European Congress of Rheumatology 2021 held on June 2–5 as a virtual poster presentation.
Disclosures
Kaleb Michaud has nothing to declare. Philip G Conaghan notes the following disclosures: Speakers Bureau: AbbVie, Amgen; consultant: AbbVie, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Galapagos, GSK, Novartis, Pfizer, UCB. Sang Hee Park, Karissa Lozenski, Vadim Khaychuk, and John Vaile are employees and shareholders of Bristol Myers Squibb. John Vaile is also a shareholder of Amgen, Novartis, and Regeneron. Elyse Swallow, Mirko Fillbrunn, and Henry Lane are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Bristol Myers Squibb, which funded the development and conduct of this study and manuscript. Ha Nguyen was an employee of Analysis Group, Inc., at the time of the study and is now a PhD student of statistics at Cornell University. Janet Pope notes the following disclosures: Speakers Bureau: AbbVie, Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Janssen, Lilly, Novartis, Pfizer, Sanofi, Sandoz; consultant: AbbVie, Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Gilead, Galapagos, Janssen, Lilly, Medexus, Merck, Novartis, Pfizer, Roche, Samsung, Sanofi, Sandoz, Teva, UCB; grant/research support: AbbVie, Bristol Myers Squibb, Merck, Pfizer, Roche, Seattle Genetics.
Compliance with Ethics Guidelines
The trials analyzed in this study (AGREE, AMPLE, AVERT, and AVERT-2) had IRB approval, patient consent and were conducted in accordance with the Helsinki Declaration. Patient IDs from AGREE, AMPLE, AVERT, and AVERT-2 were encrypted and not identified in this study. Confidentiality of patient records was maintained throughout the conduct of the study, and only aggregate data were reported. Therefore, IRB approval was not required for this specific study.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to their scope.
Footnotes
Kaleb Michaud and Philip G. Conaghan are co-first authors of this article.
Contributor Information
Kaleb Michaud, Email: kmichaud@unmc.edu.
Philip G. Conaghan, Email: p.conaghan@leeds.ac.uk
Sang Hee Park, Email: sarah.park@bms.com.
Karissa Lozenski, Email: karissa.lozenski@bms.com.
Mirko Fillbrunn, Email: mirko.fillbrunn@analysisgroup.com.
Vadim Khaychuk, Email: vadim.khaychuk@bms.com.
Elyse Swallow, Email: elyse.swallow@analysisgroup.com.
John Vaile, Email: john.vaile@bms.com.
Henry Lane, Email: henry.lane@analysisgroup.com.
Ha Nguyen, Email: hanguye97@gmail.com.
Janet Pope, Email: janet.pope@sjhc.london.on.ca.
References
- 1.Shams S, Martinez JM, Dawson JRD, et al. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol. 2021;12:680043. doi: 10.3389/fphar.2021.680043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig T, Moe RH, Kvien TK. The burden of disease in rheumatoid arthritis. Pharmacoeconomics. 2014;32(9):841–851. doi: 10.1007/s40273-014-0174-6. [DOI] [PubMed] [Google Scholar]
- 4.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35(1):10–14. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;8(4):18001. doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 7.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021;73(7):924–939. doi: 10.1002/acr.24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katchamart W, Koolvisoot A, Aromdee E, et al. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int. 2015;35(10):1693–1699. doi: 10.1007/s00296-015-3271-8. [DOI] [PubMed] [Google Scholar]
- 9.Trouw LA, Mahler M. Closing the serological gap: promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun Rev. 2012;12(2):318–322. doi: 10.1016/j.autrev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Wiik AS, van Venrooij WJ, Pruijn GJ. All you wanted to know about anti-CCP but were afraid to ask. Autoimmun Rev. 2010;10(2):90–93. doi: 10.1016/j.autrev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Sellam J, Hendel-Chavez H, Rouanet S, et al. B cell activation biomarkers as predictive factors for the response to rituximab in rheumatoid arthritis: a six-month, national, multicenter, open-label study. Arthritis Rheum. 2011;63(4):933–938. doi: 10.1002/art.30233. [DOI] [PubMed] [Google Scholar]
- 12.Visser K, Verpoort KN, van Dongen H, et al. Pretreatment serum levels of anti-cyclic citrullinated peptide antibodies are associated with the response to methotrexate in recent-onset arthritis. Ann Rheum Dis. 2008;67(8):1194–1195. doi: 10.1136/ard.2008.088070. [DOI] [PubMed] [Google Scholar]
- 13.Rigby W, Buckner JH, Louis Bridges S, Jr, et al. HLA-DRB1 risk alleles for RA are associated with differential clinical responsiveness to abatacept and adalimumab: data from a head-to-head, randomized, single-blind study in autoantibody-positive early RA. Arthritis Res Ther. 2021;23(1):245. doi: 10.1186/s13075-021-02607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huizinga TW, Connolly SE, Johnsen A, et al. Effect of anti-cyclic citrullinated peptide 2 immunoglobulin M serostatus on efficacy outcomes following treatment with abatacept plus methotrexate. 2015 ACR/ARHP Annual Meeting 2015.
- 15.Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis. 2016;75(4):709–714. doi: 10.1136/annrheumdis-2015-207942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrold LR, Litman HJ, Connolly SE, et al. Effect of anticitrullinated protein antibody status on response to abatacept or antitumor necrosis factor-alpha therapy in patients with rheumatoid arthritis: a US national observational study. J Rheumatol. 2018;45(1):32–39. doi: 10.3899/jrheum.170007. [DOI] [PubMed] [Google Scholar]
- 17.Westhovens R, Robles M, Ximenes AC, et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis. 2009;68(12):1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinblatt ME, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65(1):28–38. doi: 10.1002/art.37711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emery P, Burmester GR, Bykerk VP, et al. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis. 2015;74(1):19–26. doi: 10.1136/annrheumdis-2014-206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristol-Myers Squibb Company. Highlights of Prescribing Information ORENCIA (abatacept) 2021. https://packageinserts.bms.com/pi/pi_orencia.pdf. Accessed 13 Mar 2023.
- 21.Alemao E, Postema R, Elbez Y, et al. Presence of anti-cyclic citrullinated peptide antibodies is associated with better treatment response to abatacept but not to TNF inhibitors in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. 2020;38(3):455–466. [PubMed] [Google Scholar]
- 22.Courvoisier DS, Chatzidionysiou K, Mongin D, et al. The impact of seropositivity on the effectiveness of biologic anti-rheumatic agents: results from a collaboration of 16 registries. Rheumatology (Oxford) 2021;60(2):820–828. doi: 10.1093/rheumatology/keaa393. [DOI] [PubMed] [Google Scholar]
- 23.Sung W, Tsai W. Rethink about the role of rheumatoid factor and anti-citrullinated protein antibody in rheumatoid arthritis. Rheumatol Immunol Res. 2021;2(1):19–25. doi: 10.2478/rir-2021-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabado O, Maldonado MA, Schiff M, et al. Differential changes in ACPA fine specificity and gene expression in a randomized trial of abatacept and adalimumab in rheumatoid arthritis. Rheumatol Therapy. 2022;9(2):391–409. doi: 10.1007/s40744-021-00404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wunderlich C, Oliviera I, Figueiredo CP, et al., editors. Effects of DMARDs on citrullinated peptide autoantibody levels in RA patients—a longitudinal analysis. Seminars in arthritis and rheumatism. Amsterdam: Elsevier; 2017. [DOI] [PubMed] [Google Scholar]
- 26.Miriovsky BJ, Michaud K, Thiele GM, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292–1297. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to their scope.