Abstract
This study evaluated the antibacterial activity of cinnamaldehyde (CIN) and biogenic silver nanoparticles (BioAgNP), alone and in combination, against Escherichia coli, Salmonella Typhimurium, and Staphylococcus aureus in vitro. Their sanitation activities on fresh sweet grape tomatoes were also evaluated. CIN and BioAgNP inhibited the growth of the tested bacteria, and at low concentrations, their combinations presented a synergistic effect. In the sanitization of fresh sweet grape tomatoes, CIN (156 µg/mL) combined with BioAgNP (31.25 µM) at subinhibitory concentrations inhibited the growth of E. coli after only 5 min of contact. Exposed samples showed no growth of E. coli during their shelf life. The combination of these compounds did not change significantly (p > 0.05) the physicochemical properties of sweet grape tomatoes and showed that CIN combined with BioAgNP could represent an effective method for decontaminating fruits and vegetables. This combination has great potential for application in the prevention of foodborne diseases.
Keywords: Antibacterial, Natural compounds, Sanitizers, Silver nanoparticles, Sweet grape tomato
Introduction
Microbial contamination is a worldwide problem that causes enormous losses for the food industry and generates high healthcare costs (US$ 15.6 billion each year) (CDC 2022). Foodborne diseases, in turn, have been perceived as a serious public health problem worldwide. Centers for Disease Control and Prevention (CDC) estimates that each year one in six Americans become ill from contaminated food or beverages, and 3000 die from foodborne illness (CDC 2022). The foodborne pathogens commonly involved in food safety incidents include Escherichia coli, Staphylococcus aureus, Salmonella sp., and Listeria monocytogenes (CDC 2022). Although there are methods to control microbial growth in foods, there remains a need for novel techniques that prove effective at microbial inactivation and the maintenance of organoleptic characteristics of foods (Nile et al. 2020).
Natural compounds are promising alternative food preservatives (Batista et al. 2019). Among them, cinnamaldehyde (CIN) has been studied extensively due to its antimicrobial effect (Burt et al. 2016; Malheiro et al. 2019). CIN is the major compound in cinnamon essential oil and can be used as a food additive and flavoring agent (Barceloux 2008). Furthermore, it qualifies as ‘generally recognized as safe' (GRAS) according to the Food and Drug Administration (21 CFR 182.60) (FDA 2018). However, its strong taste and aroma limit its use; therefore, novel alternatives are needed to minimize or eliminate these undesirable organoleptic effects (Li et al. 2022).
Green nanotechnology has also received considerable attention in the scientific community due to its eco-friendly and low-cost nature (Kobayashi and Nakazato 2020). Among the engineered nanomaterials, silver nanoparticles (AgNPs) have gained increased interest due to their strong antimicrobial activities and antiviral properties (Chue-Gonçalves et al. 2021; Kobayashi and Nakazato 2020). In the food sector, silver nanoparticles have been applied to food processing, packaging, and sanitation (Nile et al. 2020). Among the commercially available nanotechnology-based disinfectants, silver nanoparticles are the most used active constituent (Nile et al. 2020). However, some works have reported microbial resistance to silver (Graves et al. 2015) and toxicity when applied directly to food (Li et al. 2022).
Compounds can be incorporated into nanoparticles, making it possible to assess the effect of several substances simultaneously (Nile et al. 2020). The combined use of nanoparticles and antimicrobials provides more potent antimicrobial activity than that of a single compound (Ghosh et al. 2013; Nile et al. 2020). Previous studies showed additive or synergistic antibacterial effects combining AgNPs with natural compounds against Gram-positive and Gram-negative bacteria (Ghosh et al. 2013; Scandorieiro et al. 2016; Dehkordi et al. 2019; Scandorieiro et al. 2022). Synergistic effect of AgNPs combined with eugenol in milk, against Staphylococcus aureus was reported by Dehkordi et al. (2019). Ghosh et al. (2013) also described a synergistic effect combining AgNPs with CIN against Clostridium perfringens and Bacillus cereus. Scandorieiro et al. (2022) combined BioAgNP and oregano essential oil and its terpenoids (carvacrol and thymol) and found an additive action against multidrug-resistant Gram-positive and negative strains.
In this way, the combination of CIN and AgNPs is a potential strategy to increase the antibacterial activity and reduce the effective concentration of both compounds, thus reducing the impact of undesirable characteristics of compounds on food (Ghosh et al. 2013; Scandorieiro et al. 2016).
To this end, we aimed to investigate the association of CIN with biogenic silver nanoparticles (BioAgNP) and their effects as a sanitizer for fresh sweet grape tomato. The first part of this study evaluated the antibacterial activity of CIN and BioAgNP alone and in combination against E. coli, Salmonella Typhimurium, and S. aureus. The second part was conducted to investigate the effect of CIN and BioAgNP alone and in combination as a sanitizer for fresh sweet grape tomato.
Materials and methods
Bacterial strains
Escherichia coli ATCC 25922, Salmonella enterica serotype Typhimurium ATCC 14028, and Staphylococcus aureus ATCC 25923 were used in this study (U.S. 2022). The strains were provided by the Laboratory of Food Microbiology, State University of Maringá, Paraná, Brazil. The cultures were maintained in Tryptic Soy Broth (TSB) (Difco, Becton Dickinson, Sparks, MD, USA) supplemented with 20% glycerol at − 20 °C.
Antimicrobial agents
Cinnamaldehyde (CIN) with 93% purity was obtained from Sigma Aldrich, Buchs, Switzerland. Biogenic silver nanoparticles (BioAgNP) were obtained from GRAL Bioativos®, Londrina, Brazil. These BioAgNP were produced from catuaba extract (Trichilia catigua) and showed an average bioAgNP diameter of 82.73 nm, zeta potential of − 23.27 mV, and polydispersity index (PI) of 0.17.
Antibacterial activity
Antibacterial activity of cinnamaldehyde and BioAgNP
The minimum inhibitory concentration (MIC) of CIN and BioAgNP were determined using the broth microdilution method following the recommendations of the Clinical and Laboratory Standards Institute (CLSI 2018). CIN was initially diluted in 0.5% dimethyl sulfoxide (DMSO), and BioAgNP was prepared in Mueller Hinton Broth (MHB, Difco, Becton Dickinson, Sparks, MD, USA). Tested concentrations of CIN and BioAgNP ranged from 19 to 5000 μg/mL and 0.97 to 500 µM, respectively. Bacterial suspensions were standardized by 0.5 McFarland scale (1.5 × 108 bacteria/mL) and diluted at 1:20, and 10 μL were inoculated in each microplate well. After 24 h of incubation at 35 °C, the MIC was defined as the lowest concentration of antimicrobial agent that inhibited visible growth. Bacterial growth control in MHB and 0.5% DMSO and a control with CIN in MHB and BioAgNP in MHB were included. All assays were carried out in triplicate and on at least three different occasions.
Antibacterial combination assay
The interaction of CIN and BioAgNP was determined by the checkerboard method (Doern 2014).
BioAgNP was added to 96-well microtiter plates with MHB and diluted along the x-axis. CIN was added and diluted along the y-axis in the same way. Bacterial suspensions with approximately 106 colony-forming units per milliliter (CFU/mL) were inoculated in each microplate well. The plates were incubated at 35 °C for 24 h. Substances were combined following calculation of the fractional inhibitory concentration (FIC) index (ƩFIC), which was determined as follows:
The interaction between CIN and BioAgNP was defined as synergistic (FICI < 0.5), additive (0.5 ≤ FICI ≤ 1), indifferent (1 < FICI ≤ 4), or antagonistic (FICI > 4) (Pillai et al. 2005). The Bliss-independent interactions were analyzed with Combenefit software (Di Veroli et al. 2016). All the experiments were repeated in triplicate.
Time-kill assay
Time-kill assays were performed according to Isenberg (2004), with modifications. Overnight cultures of E. coli ATCC 25922, S. Typhimurium ATCC 14028, and S. aureus ATCC 25923 were standardized equivalent to 1.0 McFarland (3.0 × 108 bacteria/mL) and transferred to MHB supplemented with CIN and BioAgNP, alone and in combination, to obtain a final inoculum of 6 × 105 CFU/mL. CIN was tested at 156 μg/mL (1/2 MIC) and BioAgNP at 31.25 μM (1/2 MIC), according to the checkerboard results. Aliquots of 100 μL were withdrawn at different time intervals, serially diluted, and plated on Mueller–Hinton agar (MHA) (Difco, Becton Dickinson, Sparks, MD, USA). Plates were incubated at 35 °C for 24 h, and CFUs were counted. Each test was performed in duplicate and repeated three times.
Application in fresh sweet grape tomatoes
Escherichia coli was chosen for evaluation of the antibacterial effect of CIN and BioAgNP alone and in combination as sanitizers in fresh sweet grape tomatoes. The concentrations of evaluated compounds were selected by the checkerboard method.
Microbiological quality
Samples of tomatoes not subjected to sanitizing treatments and without the artificial inoculation step were also submitted to microbiological analysis (Salmonella spp. and E. coli), following the standards required by Brazilian legislation (Brasil 2019).
For detection of Salmonella spp., 25 g of the samples were added to 225 mL of lactose broth (Difco, Becton Dickinson, Sparks, MD, USA) and incubated at 35 °C for 24 h. After the incubation period, selective enrichment was performed in selenite cystine broth (Difco, Becton Dickinson, Sparks, MD, USA) and in Rappaport–Vassiliadis medium (Difco, Becton Dickinson, Sparks, MD, USA). Subsequently, samples were plated on Hektoen agar and incubated at 35 °C for 24 h. Typical colonies were submitted to biochemical and serological tests.
Escherichia coli enumeration was performed using Petrifilm™ EC plates (3 M Company, St. Paul, MN, EUA). Aliquots of 1 mL of each sample were seeded in Petrifilm™ EC plates and incubated at 35 °C for 24 and 48 h. E. coli colonies were enumerated according to the manufacturer’s instructions.
Preparation and inoculation of sweet grape tomatoes
Sweet grape mini tomatoes (Lycopersicum esculentum Mill.) were purchased in a local market and selected during their mature phase without mechanical blemishes. The tomatoes were washed in running water, immersed in cold water with 200 ppm chloride for 10 min, washed, and dried. The sweet grape tomatoes were submerged in E. coli ATCC 25922 suspensions standardized at 108 CFU/mL in sterile 0.1% peptone water supplemented with 0.1% agar for 30 min. Afterwards, the samples were air dried for 2 h to facilitate bacterial adhesion before exposure to disinfection treatments (Choi et al. 2018).
Sanitizing treatments and shelf life
To assess the antibacterial activity of CIN and BioAgNP alone and in combination against E. coli in sweet grape tomatoes, four samples were defined: a control sample (without antimicrobials), a CIN sample at 156 μg/mL (CIN), a BioAgNP sample at 31.25 μM (BioAgNP), and a CIN + BioAgNP sample at 156 μg/mL + 31.25 μM (CIN and BioAgNP). For the treatments, the tomatoes were immersed in a solution containing sterile distilled water plus the studied antimicrobials and evaluated at 0, 5, 10, 15, 30 and 60 min. The inoculated bacteria were counted by diluting 10 g of tomato in 90 mL of sterile peptone water (1 g/L). Serial dilutions were performed, plated on Eosin Methylene Blue Agar (Difco, Becton Dickinson, Sparks, MD, USA) plates, and incubated at 35 °C for 24 h before counting. The analyses were repeated twice, and the results are expressed in log CFU/mL.
The survival of E. coli in sweet grape tomatoes treated with CIN and BioAgNP alone and in combination during the shelf life was also evaluated. The samples were treated for 5 min, air dried, and packaged in sealed bags for 7 days. The samples were stored at room temperature and analyzed on days 0, 4, and 7 of their shelf life.
Physicochemical analysis
The sweet grape tomato samples stored at room temperature were analyzed at intervals of 0, 4, and 7 days of shelf life with regard to pH, titratable acidity, and soluble solids (Instituto Adolfo Lutz 1985). The hydrogen ionic potential (pH) was determined in homogenized tomato pulp using a digital potentiometer (MCA-150, Lucadema). Soluble solids (°Brix) were determined using a drop of tomato pulp homogenized using an ABBE benchtop refractometer (AR1000S, Megabrix) calibrated at 20 °C. Total titratable acidity was determined from the titration of 5 g of homogenate pulp diluted in 100 ml of distilled water and a standard solution of 0.1 mol/L sodium hydroxide. Analyses were performed in triplicate, with three replicates.
Statistical analysis
The results were analyzed with GraphPad Prism 7.0 Software. In vitro analyses were performed in duplicate, with three replications, and sanitizer analysis was repeated twice. Results were expressed as the mean ± standard deviation. The data were analyzed by ANOVA at the 5% significance level. Post hoc comparisons were performed by Tukey's test.
Results and discussion
Antibacterial activity in vitro
Antibacterial activity of cinnamaldehyde and BioAgNP
Cinnamaldehyde and BioAgNP exhibited antimicrobial activity against E. coli, S. Typhimurium, and S. aureus. In this study, CIN presented an MIC of 624 µg/mL for all bacteria investigated. Other studies evaluating the activity of CIN against E. coli revealed MIC values between 100 and 310 μg/mL (Andrade-Ochoa et al. 2021; Ghosh et al. 2013; Ye et al. 2013). An MIC between 131 and 600 μg/mL was obtained against S. Typhimurium (Andrade-Ochoa et al. 2021; Burt et al. 2016), and MIC values between 58 and 500 μg/mL were observed for S. aureus. (Al-Bayati and Mohammed 2009; Ghosh et al. 2013; Ye et al. 2013).
BioAgNP showed MIC values of 125 µM against E. coli, S. Typhimurium, and S. aureus. Scandorieiro et al. (2016) analyzed the antimicrobial action of BioAgNP produced from fungi extract and reported MICs of 62.5, 125, and 250 µM against E. coli (ATCC 25922), S. Typhimurium (ATCC 68169), and S. aureus (ATCC 25923), respectively. Other studies revealed AgNP or BioAgNP MIC values between 3.12 and 50 μg/mL against E. coli (Al-Sharqi et al. 2019; Dalir et al. 2020; Kelkawi et al. 2016; Zarei et al. 2014) and S. Typhimurium (Dehkordi et al. 2019; Zarei et al. 2014), respectively. AgNP MIC values between 6.7 and 128 μg/mL were also found against S. aureus. (Al-Sharqi et al. 2019; Andrade et al. 2017; Dalir et al. 2020).
Synergistic effect between cinnamaldehyde and BioAgNP
The effect of the combination of CIN and BioAgNP against E. coli, S. Typhimurium, and S. aureus was synergistic, with an FIC value of 0.49. The combination of compounds reduced the MIC value of CIN two-fold (from 624 to 156 μg/mL) and that of BioAgNP (from 125 to 31.25 µM) for all bacteria tested. To our best knowledge, the only study reporting on a combination of CIN and AgNP was performed by Ghosh et al. (2013); however, those authors used a silver nanoparticle synthesized by silver nitrate. Ghosh et al. (2013) showed that the effect of the combination of CIN and BioAgNP was additive (FICI 0.53) against E. coli, Salmonella typhi, and S. aureus. Scandorieiro et al. (2016) studied the antibacterial effect of oregano essential oil (OEO) and biological silver nanoparticles (BioAgNP) on E. coli, S. Typhimurium, and S. aureus. The FICI value (0.50) indicated that OEO and BioAgNP had a synergistic effect on S. aureus. The combination of OEO and BioAgNP significantly decreased the MIC of EOE (twofold) and BioAgNP (twofold), in agreement with our results. The combination of OEO and BioAgNP showed an additive interaction when tested against E. coli and S. Typhimurium. Dehkordi et al. (2019) also showed a synergistic effect on S. aureus (FICI 0.5) by combining eugenol and colloidal silver nanoparticles.
The synergic effect revealed by FIC values was validated by the results of Bliss independence surface analysis (Fig. 1). In this way, CIN combined with BioAgNP showed a predominance of blue areas, confirming the synergism.
Fig. 1.
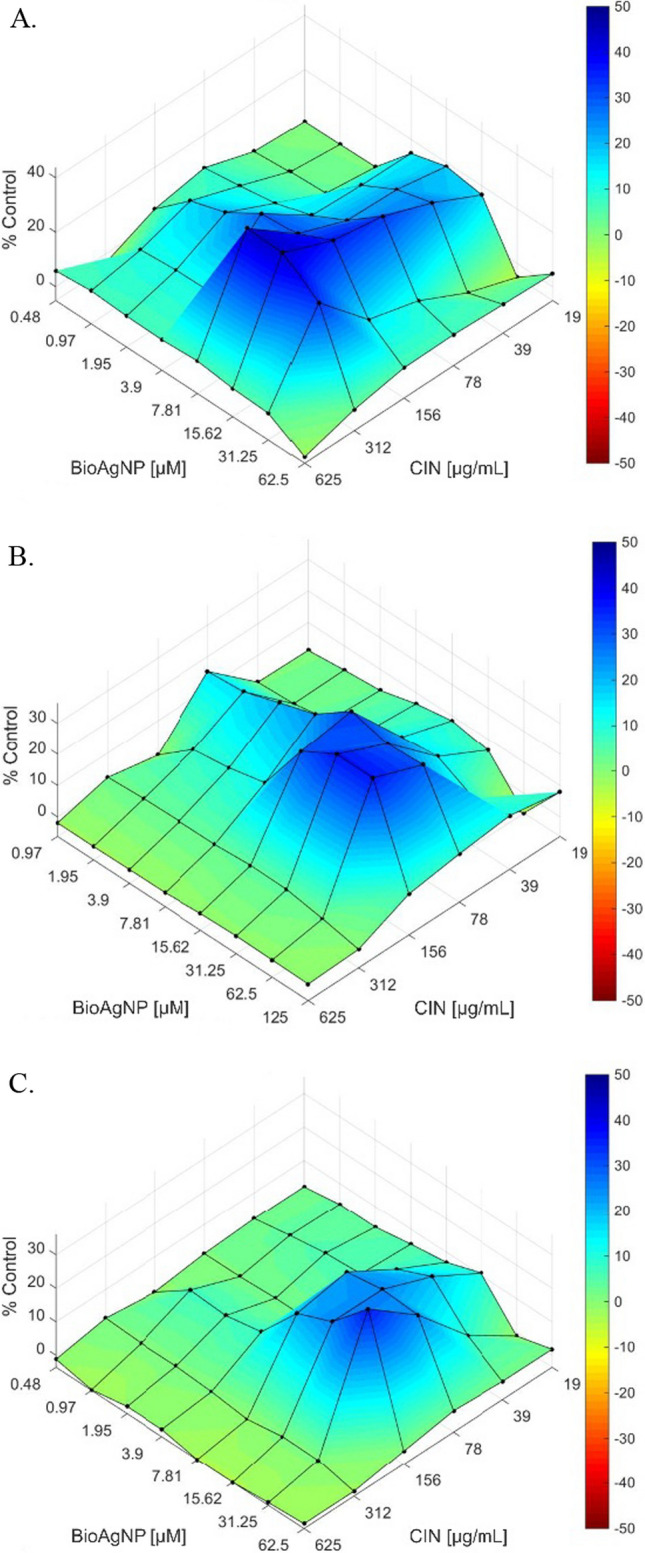
The Bliss independence surface analysis for in vitro combinations CIN and BioAgNP against A Escherichia coli ATCC 25922; B Salmonella Typhimurium ATCC 14028; C Staphylococcus aureus ATCC 25923 by Bliss independence surface analysis
Silver nanoparticles have been widely studied because of their broad-spectrum antimicrobial effect, even at low concentrations (Dalir et al. 2020; Dehkordi et al. 2019; Kelkawi et al. 2016; Scandorieiro et al. 2016). In addition, the combination of these nanoparticles with several compounds, such as plant derivatives and bacteriocins, has shown potent antimicrobial activity in different microbial species, including foodborne bacteria (Al-Sharqi et al. 2019; Dehkordi et al. 2019).
Time-kill curve
Time-kill curves were used to assess the antibacterial activity of CIN and BioAgNP alone and combined against E. coli, S. Typhimurium, and S. aureus (Fig. 2).
Fig. 2.
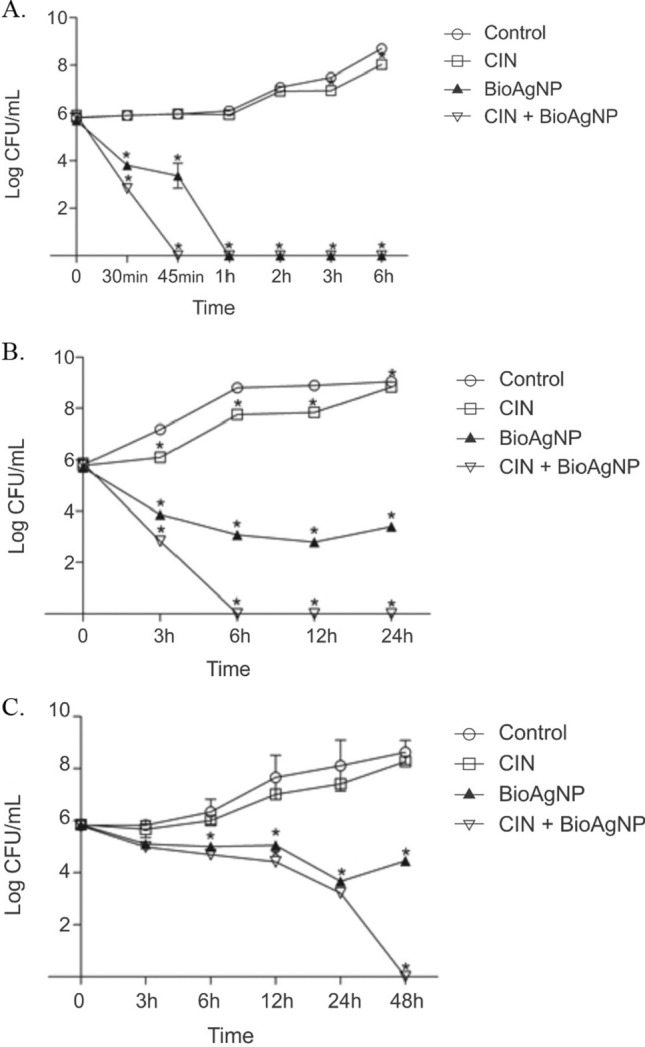
Time-kill curve assay of CIN (156 µg/mL), BioAgNP (31.25 µM) and CIN + BioAgNP (156 µg/mL + 31.25 µM). A Escherichia coli ATCC 25922; B Salmonella Typhimurium ATCC 14028; C Staphylococcus aureus ATCC 25923
Escherichia coli (control group) reached 8.7 log CFU/mL after 6 h at 35 °C. CIN at a subinhibitory concentration (156 µg/mL) did not reduce the bacterial count during 6 h of incubation. Treatment with BioAgNP alone (31.25 µM) completely inhibited bacterial growth after 1 h. The association between CIN at 1/4 MIC (156 µg/mL) and BioAgNP at 1/4 MIC (31.25 µM) inactivated E. coli after 45 min. Scandorieiro et al. (2016) also showed a 3.3 log CFU/mL reduction after 2 h, and no viable cells were detected after 4 h of incubation with BioAgNP at 62.5 µM. The effect of the combination of silver nanoparticles and OEO was also evaluated by Scandorieiro et al. (2016), indicating that OEO (298 µg/mL) and BioAgNP (15.62 µM) in combination were able to decrease 2.3 log CFU/mL within 10 min of treatment, and there were no viable cells after 20 min.
The control group of S. Typhimurium reached approximately 9.0 log CFU/mL after 24 h at 35 °C. Treatment with CIN alone (156 µg/mL) failed to reduce the bacterial population during all intervals evaluated. On the other hand, BioAgNP alone (31.25 µM) showed a reduction of approximately 2.5–3 log CFU/mL after 12 h. A mixture of CIN and BioAgNP inhibited bacterial growth, and no cells were observed after 6 h of incubation. Dehkordi et al. (2019) also found a > 2 log CFU/mL reduction in S. Typhimurium upon exposure to silver nanoparticles at 12.5 µg/mL during 3 h of treatment. These authors also evaluated the combined effects of silver nanoparticles and eugenol on the growth of S. Typhimurium and reported a ~ 6 log CFU/mL reduction 9 h after treatment with eugenol at 1250 µg/mL combined with silver nanoparticles at 6.25 µg/mL.
Staphylococcus aureus reached 8.6 log CFU/mL after 48 h of incubation at 35 °C. CIN at 156 µg/mL did not reduce the bacterial counts. BioAgNP at 31.25 µM decreased bacterial counts by approximately 2 log CFU/mL after 24 h; however, it was possible to observe partial cellular recovery with 48 h of incubation. No viable bacterial cells were observed after 48 h of treatment with CIN and BioAgNP in combination. The synergistic activity of silver nanoparticles and natural compounds on S. aureus was previously investigated (Dehkordi et al. 2019; Scandorieiro et al. 2016). Significant bactericidal activity was found for S. aureus treated with silver nanoparticles at 6.25 µg/mL and eugenol at 625 µg/mL after 6 h (Dehkordi et al. 2019). Scandorieiro et al. (2016) showed that the combination of OEO and BioAgNP at 298 µg/mL and 125 µM, respectively, against S. aureus ATCC 25923 caused a 3.48 log CFU/mL decrease in the cell population in 2 h and resulted in no viable bacterial cells after 7 h of incubation. Ghosh et al. (2013) demonstrated synergism between CIN and AgNP against Clostridium perfringens and Bacillus cereus and showed a 2 log reduction of both bacteria after 1 h of treatment. To our knowledge, there are no studies employing the time-kill assay to evaluate the antibacterial activity of CIN and BioAgNP alone and combined against E. coli, S. Typhimurium, and S. aureus.
Application in fresh sweet grape tomatoes
Microbiological quality
Sweet grape tomato samples were analyzed to evaluate their microbiological quality during their shelf life following the standards required by Brazilian legislation (Brasil 2019). Salmonella spp. was absent in all groups on the different days evaluated. Escherichia coli enumeration was < 2 log CFU/g for all days analyzed. These results demonstrate that sweet grape tomato samples complied with the current standards established by Brazilian legislation (Brasil 2019).
Efficacy of CIN and BioAgNP in the sanitization of fresh sweet grape tomatoes
Table 1 shows the efficacy of CIN and BioAgNP at subinhibitory concentrations, alone and in combination, in the sanitization of fresh sweet grape tomatoes experimentally contaminated with E. coli. In the control groups, E. coli counts ranged from 4.15 to 5.37 log CFU/g. CIN alone reduced the bacterial load by only ~ 1 log CFU/g after 60 min of treatment, and no viable cells were observed after 15 min in the treatment with BioAgNP alone. Sanitizing of sweet grape tomatoes with CIN and BioAgNP in combination was able to eradicate E. coli after 5 min.
Table 1.
Counts of Escherichia coli ATCC 25922 in sanitization of sweet grape tomatoes added from CIN and BioAgNP alone and in combination
| Time (min) | Control | CIN | BioAgNP | CIN + BioAgNP |
|---|---|---|---|---|
| 0 | 4.88 ± 0.00A | 4.85 ± 0.06A | 5.00 ± 0.00A | 4.70 ± 0.00A |
| 5 | 4.29 ± 0.07A | 3.85 ± 0.03B | 3.40 ± 0.05B | ND |
| 10 | 4.15 ± 0.21A | 3.77 ± 0.32A | 2.29 ± 0.57B | ND |
| 15 | 4.50 ± 0.04A | 3.79 ± 0.12B | ND | ND |
| 30 | 5.22 ± 0.05A | 3.58 ± 0.09B | ND | ND |
| 60 | 5.37 ± 0.03A | 3.81 ± 0.02B | ND | ND |
Values are mean log CFU/g followed by standard deviation. Means in the same line with different letters are significantly different (p < 0.05; Tukey’s test). Control (without treatment); CIN (156 µg/mL); BioAgNP (31.25 µM) and CIN + BioAgNP (156 µg/mL + 31.25 µM)
ND not detected
Gopal et al. (2010) evaluated the effect of washing shredded lettuce with water containing low concentrations of silver (0.1 ppm) and hydrogen peroxide (0.4 ppm). A less than 1 log CFU/g reduction in Pseudomonas sp. counts and an approximately 1.5 log CFU/g reduction in Enterobactericeae were observed following treatment with silver and hydrogen peroxide in combination after 7 days of storage at 12 °C. Combinations of plant-derived antimicrobials and hydrogen peroxide reduced L. monocytogenes to undetectable levels in cantaloupes after a 10-min wash treatment (Upadhyay et al. 2014). To our best knowledge, this is the first study reporting the antimicrobial action of CIN and silver nanoparticles applied in combination as a sanitizer in sweet grape tomatoes.
Sanitization of vegetables is one of the important steps designed to reduce or eliminate microbial hazards in fresh vegetables (Ssemanda et al. 2018). In this process, contact time with sanitizers is important to guarantee microbial and chemical safety and acceptability for consumption (Ssemanda et al. 2018). In our work, only 5 min of contact with CIN + BioAgNP was sufficient to verify microbial control in fresh grape tomatoes. Bermúdez-Aguirre et al. (2013) showed total inactivation of E. coli in tomatoes after 15 min using 200 ppm of chlorine (8.06 log), which is considered the standard sanitizer in the decontamination of vegetables (BRASIL 2004).
Shelf life of fresh sweet grape tomatoes treated with CIN and BioAgNP
The effects of CIN and BioAgNP alone and in combination in the sanitization of fresh sweet grape tomatoes contaminated by E. coli, on different days of shelf life are shown in Table 2. In control groups, E. coli counts were maintaining during storage and reached approximately 6.12 log CFU/g on the seventh day. E. coli counts in tomatoes treated with CIN were reduced less than 1 log CFU/g after 7 days of storage. The samples sanitized with BioAgNP alone and in association with CIN showed no growth of E. coli during the shelf life.
Table 2.
Counts of Escherichia coli ATCC 25922 in shelf life of sweet grape tomatoes added from CIN and BioAgNP alone and in combination
| Time (days) | Control | CIN | BioAgNP | CIN + BioAgNP |
|---|---|---|---|---|
| 1 | 5.14 ± 0.04A | 3.71 ± 0.01B | ND | ND |
| 2 | 6.31 ± 0.02A | 2.89 ± 0.04B | ND | ND |
| 3 | 6.21 ± 0.04A | 2.36 ± 0.06B | ND | ND |
| 5 | 6.27 ± 0.17A | 2.28 ± 0.02B | ND | ND |
| 7 | 6.12 ± 0.03A | 2.84 ± 0.02B | ND | ND |
Values are mean log CFU/g followed by standard deviation. Means in the same line with different letters are significantly different (p < 0.05; Tukey’s test). Control (without treatment); CIN (156 µg/mL); BioAgNP (31.25 µM) and CIN + BioAgNP (156 µg/mL + 31.25 µM)
ND not detected
Upadhyay et al. (2014) reported on the reduction of L. monocytogenes on artificially contaminated cantaloupes. Combinations of plant-derived antimicrobials + hydrogen peroxide reduced L. monocytogenes to undetectable levels in cantaloupes after a 10-min wash treatment, and no bacterial cells were recovered after 7 days of storage.
Our study showed that BioAgNP alone presented antimicrobial activity; however, the prolonged use of silver nanoparticles can be toxic to humans (Li et al. 2022). Furthermore, resistance to silver nanoparticles has already been reported (Graves et al. 2015). By contrast, natural compounds used as sanitizers are biodegradable and environmentally friendly and pose a lower risk to human health (Cacciatore et al. 2020). In addition to antimicrobial activity, CIN presents anti-inflammatory and antioxidant properties (Barceloux 2008); however, the highly volatile nature of CIN can cause organoleptic changes in foods (Li et al. 2022).
Therefore, the combination of antimicrobials is a potential strategy to minimize the undesirable effects of substances, since their combined use reduces the concentration of the compounds and the probability of selecting resistant bacteria (Nile et al. 2020).
Physicochemical analysis
Sweet grape tomato samples sanitized with CIN, BioAgNP, and CIN + BioAgNP were subjected to physicochemical analysis for evaluation of pH, soluble solids, and titratable acidity during the shelf life (Table 3). No significant difference (p > 0.05) in pH was found between the control and treatment groups on the analyzed days. Sweet grape tomato samples presented pH values ranging from 4.16 to 4.26. According to the Center for Food Safety and Applied Nutrition (CFSAN, 2003), tomato pH levels should range between 4.3 and 4.9. In addition, a tomato pH below 4.3 prevents microorganism proliferation (CFSAN 2003).
Table 3.
Physicochemical analysis of sweet grape tomatoes added from CIN and BioAgNP alone and in combination
| Group | pH | Soluble solids (°Brix) | Titratable acidity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 0 | 4 | 7 | 0 | 4 | 7 | |
| Control | 4.19 ± 0.01A | 4.36 ± 0.01A | 4.19 ± 0.01A | 8.50 ± 0.00A | 8.30 ± 0.00A | 7.90 ± 0.00A | 9.26 ± 0.15A | 7.95 ± 0.00A | 9.22 ± 0.11A |
| CIN | 4.16 ± 0.01A | 4.33 ± 0.02A | 4.23 ± 0.02A | 8.50 ± 0.00A | 8.30 ± 0.00A | 7.90 ± 0.00A | 9.37 ± 0.17A | 8.60 ± 0.14A | 8.62 ± 0.15A |
| BioAgNP | 4.18 ± 0.01A | 4.34 ± 0.00A | 4.28 ± 0.01A | 8.50 ± 0.00A | 8.30 ± 0.00A | 7.90 ± 0.00A | 9.04 ± 0.17A | 8.36 ± 0.01A | 8.55 ± 0.21A |
| CIN + BioAgNP | 4.16 ± 0.01A | 4.33 ± 0.01A | 4.20 ± 0.02A | 8.50 ± 0.00A | 8.30 ± 0.00A | 7.90 ± 0.00A | 9.19 ± 0.05A | 9.17 ± 0.00A | 8.75 ± 0.01A |
Values are mean log CFU/g followed by standard deviation. Means in the same column with different letters are significantly different (p < 0.05; Tukey’s test). Control (without treatment); CIN (156 µg/mL); BioAgNP (31.25 µM) and CIN + BioAgNP (156 µg/mL + 31.25 µM)
ND not detected
There were no significant differences (p > 0.05) in soluble solids in any sample during the 7 days of storage. Their rates ranged from 7.9 °Brix to 8.5 °Brix. These values are in agreement with Ribeiro et al. (2010), who found soluble solid values between 7.63 and 8.5 °Brix in sweet grape tomatoes treated with coatings containing phenolic compounds. Sweet grape tomato is sweeter than traditional tomato and can reach 9 °Brix (between 4 and 6 °Brix) (Onoda 2010).
No significant difference (p > 0.05) was observed in titratable acidity for all samples analyzed. The titratable acidity rates varied between 7.95% and 9.37% in the 7 days of storage. Acidity is an important determinant of tomato quality. According to Kader et al. (Kader et al. 1978), tomatoes that present a titratable acidity greater than 0.32% are considered of good quality.
Conclusion
From this study, it is clear that CIN and BioAgNP in combination at subinhibitory concentrations effectively inhibited the in vitro growth of foodborne pathogenic bacteria. The use of a sanitizer based on CIN and BioAgNP combined inhibited the growth of E. coli in fresh sweet grape tomatoes after 5 min of treatment. The antibacterial activity of the compounds in combination in sweet grape tomatoes was maintained during their shelf life and did not change the physicochemical properties tomatoes. Further researches could contribute to elucidating the mechanism of action of the antimicrobials, as well as their toxicity when applied directly to food, to better characterize their antibacterial activity. The disinfectant activity of plant-derived compounds combined with AgNPs could pave the way for a new generation of disinfection products to control and prevent further disease outbreaks.
Acknowledgements
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES for a scholarship granted to the first author.
Authors' contributions
Conceptualization: AFPB, JMGM; Methodology: AFPB, JMGM, JSP; Formal analysis and investigation: AFPB, JSP, LCMR; Writing: AFPB; Original draft preparation: AFPB, AFS, JMGM; review and editing: BAAF, JVV, JMGM; Funding acquisition: JMGM; Resources: RKTK, GN, JMGM; Supervision: RKTK, GN, JMGM; All the authors viewed and approved the final version of the manuscript. AFPB, RKTK, GN and JMGM contributed equally to this study.
Funding
Not applicable.
Data availability
The data supporting the findings of this study will be available on request from the corresponding author. Due to privacy or ethical restrictions, the data are not publicly available.
Declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical approval
Ethics approval was not required for this research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Al-Bayati FA, Mohammed MJ. Isolation, identification, and purification of cinnamaldehyde from Cinnamomum zeylanicum bark oil. An Antibacterial Study Pharmaceutical Biology. 2009;47(1):61–66. doi: 10.1080/13880200802430607. [DOI] [Google Scholar]
- Al-Sharqi A, Apun K, Vincent M, Kanakaraju D, Bilung LM. Enhancement of the antibacterial efficiency of silver nanoparticles against gram-positive and gram-negative bacteria using blue laser light. Int J Photoenergy. 2019 doi: 10.1155/2019/2528490. [DOI] [Google Scholar]
- Andrade PF, Nakazato G, Durán N. Additive interaction of carbon dots extracted from soluble coffee and biogenic silver nanoparticles against bacteria. J Phys: Conf Ser. 2017;838:012028. doi: 10.1088/1742-6596/838/1/012028. [DOI] [Google Scholar]
- Andrade-Ochoa S, Chacón-Vargas KF, Sánchez-Torres LE, Rivera-Chavira BE, Nogueda-Torres B, Nevárez-Moorillón GV. Differential antimicrobial effect of essential oils and their main components: insights based on the cell membrane and external structure. Membranes. 2021;2021(11):405. doi: 10.3390/membranes11060405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barceloux DG. Medical toxicology of natural substances: foods, fungi, medicinal herbs, toxic plants, and venomous animals. Hoboken, NJ: John Wiley & Sons; 2008. pp. 39–43. [Google Scholar]
- Batista AFP, dos Santos AR, da Silva AF, Trevisan DAC, Ribeiro LH, Campanerut-Sá PAZ, et al. Inhibition of Salmonella enterica serovar Typhimurium by combined carvacrol and potassium sorbate in vitro and in tomato paste. LWT Food Sci Technol. 2019;100:92–98. doi: 10.1016/j.lwt.2018.10.006. [DOI] [Google Scholar]
- Bermúdez-Aguirre D, Barbosa-Cánovas GV. Disinfection of selected vegetables under nonthermal treatments: chlorine, acid citric, ultraviolet light and ozone. Food Control. 2013;29:82e90. doi: 10.1016/j.foodcont.2012.05.073. [DOI] [Google Scholar]
- Brasil (2004) Ministério da saúde, Agência Nacional de Vigilância Sanitária - ANVISA. Resolução – RDC Nº 216, de 15 de Setembro de 2004. Estabelece procedimentos de boas práticas para serviço de alimentação, garantindo as condições higiênico-sanitárias do alimento preparado. Diário Oficial da União, Brasília
- Brasil (2019) Ministério da saúde, Agência Nacional de Vigilância Sanitária - ANVISA. Instrução normativa n° 60, de 23 de dezembro de 2019. Listas de padrões microbiológicos para alimentos. Diário Oficial da União. Brasília
- Burt SA, Adolfse SJM, Ahad DSA, Tersteeg-Zijderveld MHG, Jongerius-Gortemaker BGM, Post JA, et al. Cinnamaldehyde, carvacrol and organic acids affect gene expression of selected oxidative stress and inflammation markers in IPEC J2Cells exposed to Salmonella Typhimurium. Phytother Res. 2016;30:1988–2000. doi: 10.1002/ptr.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciatore FA, Brandelli A, Malheiros PS. Combining natural antimicrobials and nanotechnology for disinfecting food surfaces and control microbial biofilm formation. Crit Rev Food Sci Nutr. 2020 doi: 10.1080/10408398.2020.1806782. [DOI] [PubMed] [Google Scholar]
- Center for Food Safety and Applied Nutrition (CFSAN) (2003) Approximate pH of foods and food products. https://www.webpal.org/SAFE/aaarecovery/2_food_storage/Processing/lacf-phs.htm. Accessed date: August 2022
- Centers for Disease Control and Prevention (CDC) (2022) Department of health & human services, national center for emerging and zoonotic infectious diseases, division of foodborne, waterborne, and environmental diseases [online]. Available: https://www.cdc.gov/ Accessed date: August 2022
- Choi JI, Chae SJ, Kim JM, Choi JC, Park SJ, Choi HJ, Bae H, Park HJ. Potential silver nanoparticles migration from commercially available polymeric baby products into food simulants. Food Addit Contam Part A. 2018;35(5):996–1005. doi: 10.1080/19440049.2017.1411611. [DOI] [PubMed] [Google Scholar]
- Chue-Gonçalves M, Pereira GN, Faccin-Galhardi LC, Kobayashi RKT, Nakazato G. Metal nanoparticles against viruses: possibilities to fight SARS-CoV-2. Nanomaterials. 2021;11:3118. doi: 10.3390/nano11113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard CLSI document M07–A10. 10. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- Dalir SJB, Djahaniani H, Nabati F, Hekmati M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon. 2020;6:e03624. doi: 10.1016/j.heliyon.2020.e03624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehkordi NH, Tajik H, Moradi M, Kousheh SA, Molaei R. Antibacterial interactions of colloid nanosilver with eugenol and food ingredients. J Food Prot. 2019;82:1783–1792. doi: 10.4315/0362-028X.JFP-19-174. [DOI] [PubMed] [Google Scholar]
- Di Veroli GY, Fornari C, Wang D, Mollard S, Bramhall JL, Richards FM, Jodrell DI. Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinf. 2016;32:2866–2868. doi: 10.1093/bioinformatics/btw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern CD. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol. 2014;52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA/Food and Drug Administration (2018) Food additives permitted for direct addition to food for human consumption. U.S. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=172.515, Accessed date: May 2022
- Ghosh IN, Deepak Patil S, Sharma TK, Srivastava SK, Pathania R, Navani NK. Synergistic action of cinnamaldehyde with silver nanoparticles against spore-forming bacteria: a case for judicious use of silver nanoparticles for antibacterial applications. Int J Nanomed. 2013;2013(8):4721–4731. doi: 10.2147/IJN.S49649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal A, Coventry J, Wan J, Roginski H, Ajlouni S. Alternative disinfection techniques to extend the shelf life of minimally processed iceberg lettuce. Food Microbiol. 2010;27(2):210–219. doi: 10.1016/j.fm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Graves JL, Jr, Tajkarimi M, Cunningham Q, Campbell A, Nonga H, Harrison SH, Barrick JE. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front Genet. 2015;17(6):42. doi: 10.3389/fgene.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Adolfo Lutz (1985). Normas Analíticas do Instituto Adolfo Lutz. v. 1: Métodos químicos e físicos para análise de alimentos. 3rd edn. IMESP, São Paulo, p 181–182
- Isenberg HD (2004) Clinical Microbiology procedures handbook. Washington: ASM Press 488, p 5.10.2
- Kader AA, Morris MA, Stevens MA, Albright-Holton M. Composition and flavor quality of fresh market as influenced by some postharvest handing procedures. J Am Soc Hortic Sci. Alexandria. 1978;103(1):6–11. doi: 10.21273/JASHS.103.1.6. [DOI] [Google Scholar]
- Kelkawi AHA, Kajani AA, Bordbar A. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2016;11:370–376. doi: 10.1049/iet-nbt.2016.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi RKT, Nakazato G. Editorial: nanotechnology for antimicrobials. Front Microbiol. 2020;11:1421. doi: 10.3389/fmicb.2020.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ren T, Perkins P. The development and application of nanocomposites with pH-sensitive “gates” to control the release of active agents: extending the shelf-life of fresh wheat noodles. Food Control. 2022;132:108563. doi: 10.1016/j.foodcont.2021.108563. [DOI] [Google Scholar]
- Malheiro JF, Maillard JY, Borges F, Simões M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Int Biodeterior Biodegradation. 2019;141:71–78. doi: 10.1016/j.ibiod.2018.06.003. [DOI] [Google Scholar]
- Nile SH, Baskar V, Selvaraj D, Nile A, Xiao J, Kai G. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano-Micro Lett. 2020;12:45. doi: 10.1007/s40820-020-0383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda SM (2010) Pesquisa Qualitativa para o Tomate Sweet Hearts. Trabalho de Conclusão do Curso de MBA em Marketing, da Fundação Instituto de Administração - FIA, Universidade de São Paulo
- Pillai SK, Moellering RC, Eliopoulos GM. Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 5. Philadelphia: Lippincott Williams & Wilkins Co; 2005. pp. 365–440. [Google Scholar]
- Scandorieiro S, Camargo LC, Lancheros CAC, Yamada-Ogatta SF, Nakamura CV, Oliveira AG, Andrade CGTJ, Duran N, Nakazato G, Kobayashi RKT. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front Microbiol. 2016;7:760. doi: 10.3389/fmicb.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandorieiro S, Rodrigues BCD, Nishio EK, Panagio LA, de Oliveira AG, Durán N, Nakazato G, Kobayashi RKT. Biogenic silver nanoparticles strategically combined with origanum vulgare derivatives: antibacterial mechanism of action and effect on multidrug-resistant strains. Front Microbiol. 2022;7:760. doi: 10.3389/fmicb.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssemanda JN, Joosten H, Bagabe MC, Zwietering MH, Reij MW. Reduction of microbial counts during kitchen scale washing and sanitization of salad vegetables. Food Control. 2018;85:495–503. doi: 10.1016/j.foodcont.2017.10.004. [DOI] [Google Scholar]
- U.S. (2022) American Type Culture Collection. The ATCC trademark and trade name, and any other trademarks listed in this publication are trademarks owned by the American Type Culture Collection unless indicated otherwise. University Boulevard Manassas, Virginia, 20110–2209
- Upadhyay A, Upadhyay I, Mooyottu S, Kollanoor-Johny A, Venkitanarayanan K. Efficacy of plant-derived compounds combined with hydrogen peroxide as antimicrobial wash and coating treatment for reducing Listeria monocytogenes on cantaloupes. Food Microbiol. 2014;44:47–53. doi: 10.1016/j.fm.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Ye H, Shen S, Xu J, Lin S, Yuan Y, Jones G. Synergistic interactions of cinnamaldehyde in combination with carvacrol against food-borne bacteria. Food Control. 2013;34:619–623. doi: 10.1016/j.foodcont.2013.05.032. [DOI] [Google Scholar]
- Zarei M, Jamnejad A, Khajehali E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J Microbiol. 2014;7(1):e8720. doi: 10.5812/jjm.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study will be available on request from the corresponding author. Due to privacy or ethical restrictions, the data are not publicly available.


