Abstract
Introduction
Tofacitinib is an oral Janus kinase inhibitor for treatment of ankylosing spondylitis (AS). Using mediation modelling, we describe interrelationships between fatigue, pain, morning stiffness, C-reactive protein (CRP) and tofacitinib treatment in patients with AS.
Methods
Data from phase 2 (NCT01786668)/phase 3 (NCT03502616) studies of patients receiving tofacitinib 5 mg twice daily (BID) or placebo were used. Initial models included treatment as the independent binary variable (tofacitinib 5 mg BID versus placebo); fatigue (Functional Assessment of Chronic Illness Therapy-Fatigue [FACIT-F; model A] or Bath AS Disease Activity Index [BASDAI] Q1 [model B]) as the dependent variable; and pain (total back pain/nocturnal spinal pain [model A] or pain measured by BASDAI Q2/3 [model B]), morning stiffness (BASDAI Q5/6) and CRP as mediator variables.
Results
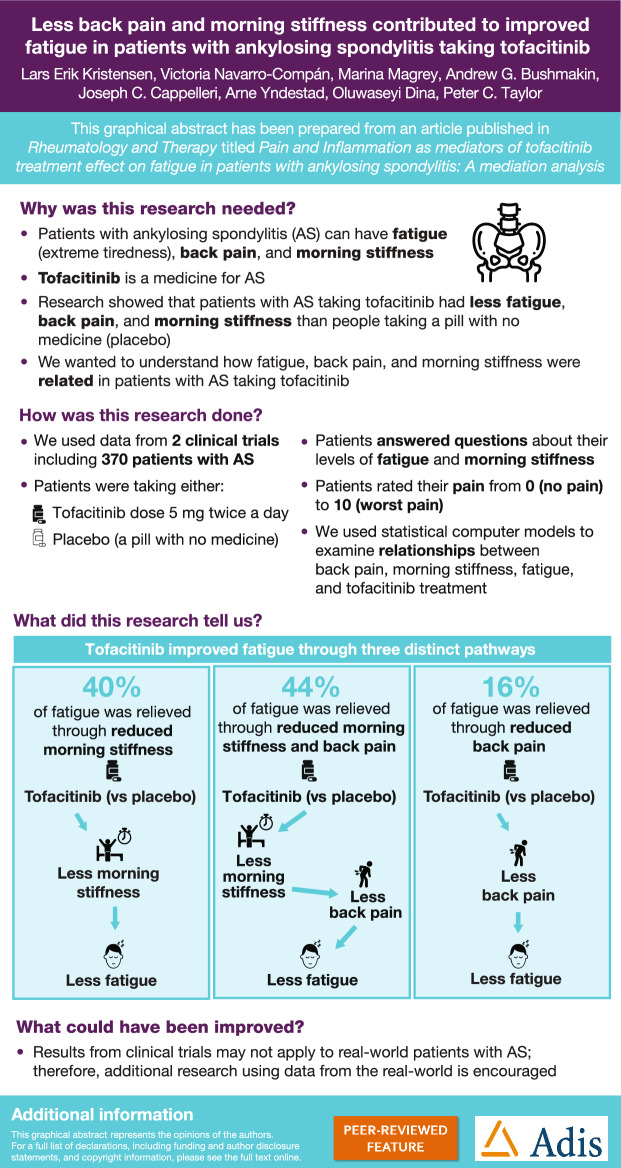
Pooled data from 370/371 patients were included in models A/B. Initial models demonstrated that tofacitinib treatment affects fatigue mainly indirectly via pain and morning stiffness. As a result, initial models were respecified to exclude direct treatment effect and the indirect effect via CRP. For respecified model A, 44.0% of the indirect effect of tofacitinib treatment on fatigue was mediated via back pain/morning stiffness, 40.0% via morning stiffness alone and 16.0% via back pain alone (all P < 0.05). For respecified model B, 80.8% of the indirect effect of tofacitinib treatment on fatigue was mediated via pain/morning stiffness and 19.2% via pain alone (both P < 0.05).
Conclusions
In tofacitinib-treated patients with AS, improvements in fatigue were collectively mediated through combined treatment effects on morning stiffness and pain.
Graphical Abstract
Keywords: Ankylosing spondylitis, C-reactive protein, Mediation modelling, Morning stiffness, Pain, Tofacitinib
Key Summary Points
| Why carry out this study? |
| Treatment of fatigue, pain and morning stiffness is a priority for patients with ankylosing spondylitis (AS) and their healthcare providers; however, the mechanisms underlying the interrelationships between these symptoms, inflammatory markers and treatment remain unclear. |
| Tofacitinib is an oral Janus kinase inhibitor for the treatment of adult patients with active AS that has been shown to reduce fatigue, back pain, morning stiffness and C-reactive protein (CRP) in clinical studies. |
| Here, we used mediation modelling to simultaneously quantify the interrelationships between fatigue, pain, morning stiffness, CRP and tofacitinib treatment in patients with AS. |
| What was learned from the study? |
| We found that in tofacitinib-treated patients with AS, improvements in fatigue were collectively mediated through combined indirect treatment effects on morning stiffness and pain. |
| Further research is needed to explore how the relief of pain and stiffness contributes to observed improvements in fatigue. |
Introduction
Ankylosing spondylitis (AS), often referred to as radiographic axial spondyloarthritis (r-axSpA), is a chronic inflammatory arthritis affecting predominantly the axial skeleton [1–3]. Key symptoms of AS include back pain, fatigue, joint stiffness and reduced spinal mobility [4–6]. Fatigue is reported by more than 70% of patients with AS [7, 8], and is associated with higher levels of disease activity and functional disability, as well as reduced health-related quality of life (HRQoL) and wellbeing [5, 9, 10]. Therefore, fatigue is considered a core domain of disease assessment in randomised controlled trials in AS [11, 12].
Treatment of fatigue, pain and morning stiffness is a priority for patients with AS and their healthcare providers; however, the mechanisms underlying the interrelationships between these symptoms, inflammatory markers (e.g. C-reactive protein [CRP] [13]) and treatment remain unclear and have not yet been quantified. Furthermore, because fatigue impacts HRQoL in patients with AS, understanding how other AS symptoms affect fatigue, and the mechanisms by which treatment can improve fatigue, could be beneficial in enhancing patient care.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of adult patients with active AS. The efficacy and safety of tofacitinib versus placebo in patients with active AS have been established in phase 2 and 3 studies [14, 15]; in these studies, greater improvements in fatigue, back pain, morning stiffness and CRP were reported in patients receiving tofacitinib versus placebo at each of the studies’ respective primary analysis time points. For example, greater improvements from baseline in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) score were observed in patients receiving tofacitinib 5 mg twice daily (BID) versus placebo at week 12 of the phase 2 study [14] and week 16 of the phase 3 study [15]. Further analysis of the phase 3 study showed greater improvements from baseline in Bath AS Disease Activity Index (BASDAI) Q1 score from week 2 to week 16 in patients receiving tofacitinib 5 mg BID versus placebo, and improvements with tofacitinib 5 mg BID continued to week 48 [16].
Mediation modelling is a statistical method used to assess the mechanisms underlying observed relationships between different independent and dependent variables, via other explanatory variables, termed mediators [17, 18]. It should be noted that mediation differs from confounding in relation to the direction of causality. Mediators and confounders both have associations with treatment and outcome, but mediators lie along the causal pathway between exposure and outcome, whereas confounding variables have a causal effect on exposure and the outcome of interest [19, 20]. Typically, randomised studies include mediators but not confounding variables [20]. The objective of mediation modelling is to determine the extent to which the effect of an independent variable on a dependent variable is indirect, via identified mediators, or direct, which captures all other effects [17, 18]. Mediation modelling has recently been used to demonstrate that itch relief is a primary mediator of tofacitinib-dependent improvements in dermatology-specific HRQoL in patients with psoriatic arthritis (PsA) [21], and to quantify the role of inflammation-associated mediators in pain reduction in tofacitinib-treated patients with PsA [22].
Here, for the first time to our knowledge, mediation modelling was used to describe the extent to which pain, morning stiffness and CRP mediate improvements in fatigue in patients with active AS receiving tofacitinib.
Methods
Study Design and Patients
This analysis used data from 16-week phase 2 (NCT01786668) [14] and 48-week phase 3 (NCT03502616) [15] randomised, double-blind, placebo-controlled trials.
In the double-blind phase 2 study, patients with a history of inadequate response (IR) to two or more oral non-steroidal anti-inflammatory drugs (NSAIDs) or intolerance to prior NSAIDs were randomised 1:1:1:1 to tofacitinib 2, 5 or 10 mg BID or placebo for 12 weeks, followed by a 4-week washout period [14].
The phase 3 study enrolled patients with a history of IR to two or more oral NSAIDs; 76.7% of patients were naïve to biologic disease-modifying antirheumatic drugs (bDMARDs) and 23.3% had IR to at least one but no more than two tumour necrosis factor inhibitors (TNFi) or prior bDMARD use (TNFi or non-TNFi) without IR. Patients were randomised to tofacitinib 5 mg BID or placebo for 16 weeks (double-blind phase), after which all patients received open-label tofacitinib 5 mg BID to week 48 [15].
Both studies were conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines [23] and the principles of the Helsinki Declaration of 1964 [24]. Ethics approval was not required by any relevant institutional review board or independent ethics committee for this post hoc analysis. All patients provided informed consent.
Mediation Modelling
For the current analysis, data were pooled from the phase 2 and the phase 3 studies; only data for patients receiving tofacitinib 5 mg BID or placebo were included. The interrelationships between fatigue, pain, morning stiffness, CRP and tofacitinib treatment were assessed using two different models, one based on FACIT-F (model A) [25, 26] and one based on BASDAI Q1 (model B) [27–29]. FACIT-F is a 13-item patient-reported measure of fatigue, whereby item responses range from 0 (‘not at all’) to 4 (‘very much’) [25, 26]. BASDAI comprises six questions pertaining to the main symptoms of AS [27, 28]. Pain and morning stiffness were selected as mediator variables as these are key symptoms experienced by patients with AS [4–6]. CRP (mg/dL) was also selected as a mediator variable as it is a marker of systemic inflammation [13, 30]. Statistical analyses were performed using the CALIS procedure (SAS v9.4).
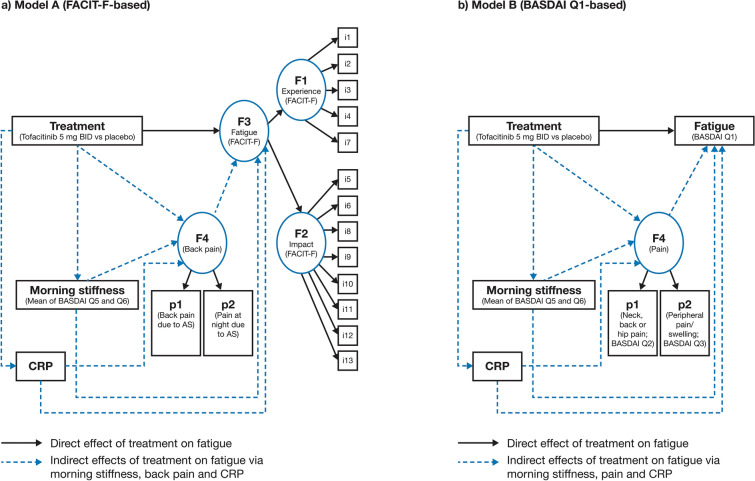
An overview of initial models A and B is shown in Fig. 1. Both models included treatment as the independent binary variable (tofacitinib 5 mg BID versus placebo), fatigue as the dependent variable, and pain, morning stiffness and CRP as mediator variables. All available post-baseline data were averaged for every observed variable at the patient level over the double-blind phase of both studies. In the initial models, the effects of tofacitinib treatment on fatigue, mediated by pain, morning stiffness and CRP, were designated as ‘indirect effects’. All other possible effects of tofacitinib treatment on fatigue not attributable to pain, morning stiffness and CRP were designated as ‘direct effects’. Pathways with P < 0.05 were considered meaningful. Model construction is dynamic; therefore, the mediation model could be respecified and refined on the basis of the results of the initial mediation model.
Fig. 1.
Initial mediation models a A (FACIT-F-based) and b B (BASDAI Q1-based). Model A: Treatment was the independent binary variable (tofacitinib 5 mg BID versus placebo). Variables i1–i13 represented items of the FACIT-F scale. Latent factor F3 represented overall fatigue; latent factors F1 and F2 represented ‘experience’ (items i1–i4 and i7 of the FACIT-F) and ‘impact’ (items i5–i6, i8–i13 of the FACIT-F) domains, respectively. Latent factor F4 represented back pain as measured by total back pain due to AS on average during last week (p1) and nocturnal spinal pain due to AS on average during last week (p2). Morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 and Q6. CRP was considered an observed variable. Model B: Treatment was the independent binary variable (tofacitinib 5 mg BID versus placebo). Observed variable fatigue was represented by BASDAI Q1. Latent factor F4 represented pain as measured by BASDAI Q2 (p1) and Q3 (p2). Morning stiffness was considered as an observed variable and was represented by the mean of BASDAI Q5 and Q6. CRP was considered an observed variable. AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BID twice daily, CRP C-reactive protein, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, Q question
In initial model A (FACIT-F-based), latent factor F3 represented overall fatigue with latent factors F1 and F2 representing ‘experience’ (items 1–4 and 7) and ‘impact’ (items 5–6 and 8–13) domains, respectively. Latent factor F4 represented back pain which was measured by the following two items: total back pain due to AS on average during the last week (p1) and nocturnal spinal pain due to AS during the last week (p2). These items were scored from 0 to 10 on a numerical rating scale (NRS), with 0 denoting ‘no pain’ and 10 denoting ‘most severe pain’. Morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 (‘How would you describe the level of morning stiffness you have had from the time you wake up?’; NRS 0–10, where 0 denotes ‘none’ and 10 denotes ‘very severe’) and Q6 (‘How long does your morning stiffness last from the time you wake up?’; NRS 0–10, where 0 denotes ‘0 h’ and 10 denotes ‘2 or more hours’). CRP (mg/dL) was considered an observed variable.
In model B, fatigue was based on BASDAI Q1 (‘How would you describe the overall level of fatigue/tiredness you have experienced?’; NRS 0–10, where 0 denotes ‘none’ and 10 denotes ‘very severe’ [observed variable]). Latent factor F4 represented pain which was measured by BASDAI Q2 (‘How would you describe the overall level of AS neck, back, or hip pain you have had?’ [p1]) and Q3 (‘How would you describe the overall level of pain/swelling you have had in joints other than neck, back, and hips?’ [p2]; NRS 0–10, where 0 denotes ‘none’ and 10 denotes ‘very severe’). As in model A, morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 and Q6 and CRP (mg/dL) was considered an observed variable.
Results
Patients
Data from 370/371 patients with AS were included in models A/B. Baseline demographics and disease characteristics for all patients have been previously published [14, 15].
Initial Models
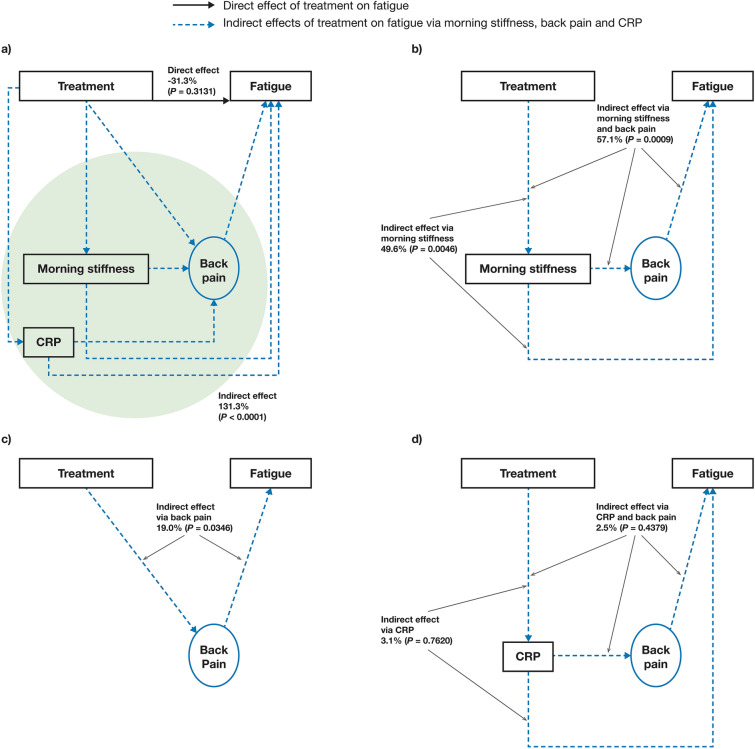
Initial model A showed that 131.3% (P < 0.0001) of the tofacitinib treatment effect on fatigue was mediated by indirect pathways (Fig. 2a), with 57.1% (P = 0.0009) mediated by the indirect path of treatment → morning stiffness → back pain → fatigue (Fig. 2b). Mediation via treatment → morning stiffness → fatigue was 49.6% (P = 0.0046; Fig. 2b) and via treatment → back pain → fatigue was 19.0% (P = 0.0346; Fig. 2c). The indirect paths of treatment → CRP → back pain → fatigue and treatment → CRP → fatigue were not considered meaningful (2.5%, P = 0.4379 and 3.1%, P = 0.7620, respectively; Fig. 2d). The effect of treatment on fatigue attributable to all other possible factors except the current set of mediators (i.e. back pain, morning stiffness and CRP) was not considered meaningful (− 31.3%, P = 0.3131; direct effect in Fig. 2a).
Fig. 2.
Effects of tofacitinib treatment on fatigue in initial mediation model A (FACIT-F-based). Estimated: a direct effect and overall indirect effect,a b indirect effect via morning stiffness and back pain or morning stiffness alone, c indirect effect via back pain alone, and d indirect effect via C-reactive protein and back pain or C-reactive protein alone. Results based on pooled data from phase 2 (NCT01786668) and phase 3 (NCT03502616) studies. Treatment was the independent binary variable (tofacitinib 5 mg BID versus placebo). Fatigue was based on FACIT-F. Back pain was represented by total back pain due to AS on average during last week and nocturnal spinal pain due to AS on average during last week. Morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 and Q6. CRP was considered an observed variable. aGreen shading denotes all indirect effects of tofacitinib on fatigue via back pain, morning stiffness and CRP. AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BID twice daily, CRP C-reactive protein, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, Q question
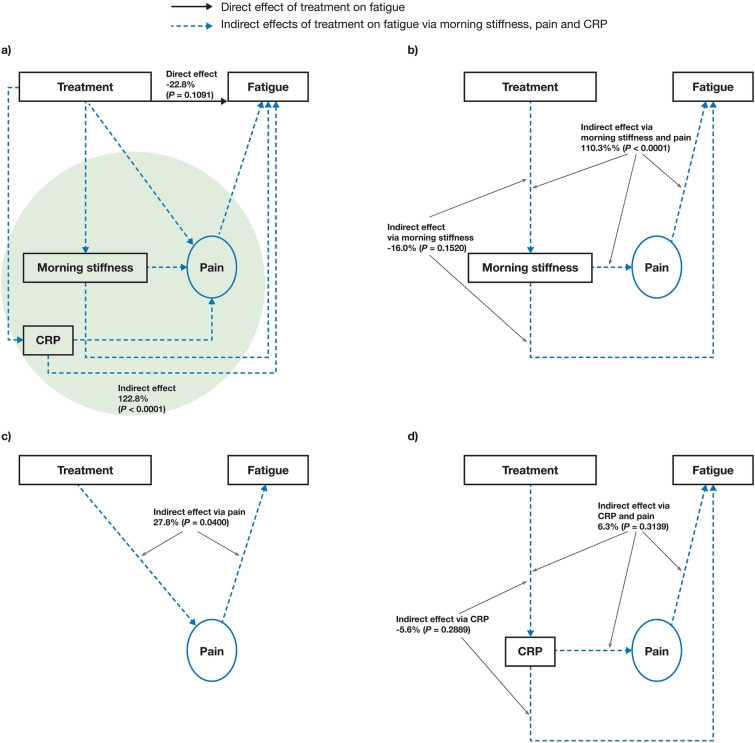
Initial model B showed 122.8% (P < 0.0001) of the tofacitinib treatment effect on fatigue was mediated by indirect pathways (Fig. 3a), with 110.3% (P < 0.0001) mediated by the indirect path of treatment → morning stiffness → pain → fatigue (Fig. 3b). The indirect path of treatment → morning stiffness → fatigue was not considered meaningful in model B (− 16.0%, P = 0.1520; Fig. 3b). The indirect path of treatment → pain → fatigue was 27.8% (P = 0.0400; Fig. 3c). As with model A, the indirect paths of treatment → CRP → pain → fatigue and treatment → CRP → fatigue were not considered meaningful (6.3%, P = 0.3139 and − 5.6%, P = 0.2889, respectively; Fig. 3d). The direct effect of treatment on fatigue, attributable to factors other than pain, morning stiffness and CRP, was not considered meaningful (− 22.8%, P = 0.1091; Fig. 3a).
Fig. 3.
Effects of tofacitinib treatment on fatigue in initial mediation model B (BASDAI Q1-based). Estimated: a direct effect and overall indirect effect,a b indirect effect via morning stiffness and pain or morning stiffness alone, c indirect effect via pain alone, and d indirect effect via C-reactive protein and pain or C-reactive protein alone. Results based on pooled data from phase 2 (NCT01786668) and phase 3 (NCT03502616) studies. Treatment was the independent binary variable (tofacitinib 5 mg BID versus placebo). Fatigue was based on BASDAI Q1 (observed variable). Pain was represented by BASDAI Q2 and Q3. Morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 and Q6. CRP was considered an observed variable. BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BID twice daily, CRP C-reactive protein, Q question
Therefore, the results of the initial models demonstrated that tofacitinib treatment affects fatigue mainly indirectly via pain and morning stiffness; and the direct effect of tofacitinib treatment on fatigue/indirect effects of tofacitinib treatment on fatigue via CRP were not considered meaningful. On the basis of these results, models A and B were respecified to exclude the direct treatment effect on fatigue and the indirect treatment effects via CRP. Additionally, the respecified model B excluded the path from morning stiffness to fatigue as the indirect effect of tofacitinib treatment on fatigue via morning stiffness was not considered meaningful in the initial model, but morning stiffness itself played an important role as part of the other indirect pathways.
Respecified Models
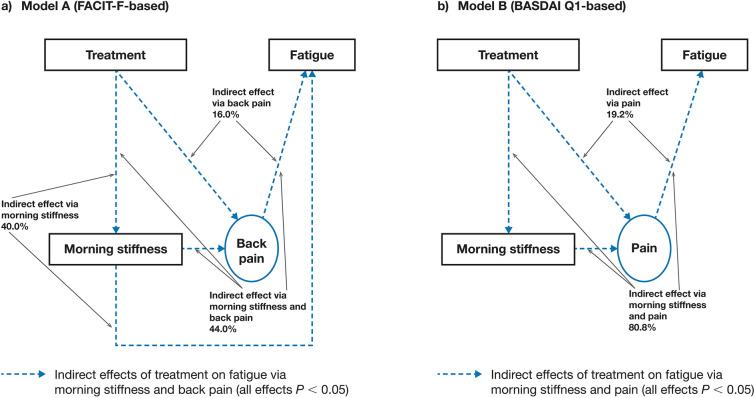
In respecified model A, 44.0% (P < 0.0001) of the indirect tofacitinib treatment effect on fatigue was mediated via treatment → morning stiffness → back pain → fatigue, 40.0% (P = 0.0005) via treatment → morning stiffness → fatigue and 16.0% (P = 0.0049) via treatment → back pain → fatigue (Fig. 4a).
Fig. 4.
Effects of tofacitinib treatment on fatigue in the respecified mediation models a A (FACIT-F-based) and b B (BASDAI Q1-based). Estimated indirect effects of morning stiffness and pain, morning stiffness alone (model A only) and pain alone. Results based on pooled data from phase 2 (NCT01786668) and phase 3 (NCT03502616) studies. Treatment was the independent binary variable (tofacitinib 5 mg BID versus placebo) in both models. In respecified model A, fatigue was based on FACIT-F. Back pain was represented by total back pain due to AS on average during last week and nocturnal spinal pain due to AS on average during last week. Morning stiffness was considered an observed variable and was represented by the mean of BASDAI Q5 and Q6. In respecified model B, fatigue was based on BASDAI Q1 (observed variable). Pain was represented by BASDAI Q2 and Q3. Morning stiffness was considered as an observed variable and was represented by the mean of BASDAI Q5 and Q6. AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BID twice daily, FACIT-F Functional Assessment of Chronic Illness Therapy-Fatigue, Q question
In respecified model B, 80.8% (P < 0.0001) of the indirect tofacitinib treatment effect on fatigue was mediated via treatment → morning stiffness → pain → fatigue and 19.2% (P = 0.0206) via treatment → pain → fatigue (Fig. 4b).
Correlations Between Outcomes in Mediation Models A and B
The correlation (Pearson coefficient) between fatigue in model A (total FACIT-F score) and fatigue in model B (BASDAI Q1) was − 0.77 (P < 0.0001). Correlations were 0.94/0.90 (both P < 0.0001) between total back pain/nocturnal spinal pain in model A and BASDAI Q2 pain in model B. Correlations were 0.73/0.71 (both P < 0.0001) between total back pain/nocturnal spinal pain in model A and BASDAI Q3 pain in model B.
Discussion
Here, for the first time to our knowledge, mediation modelling was used to describe and quantify the extent to which pain, morning stiffness and CRP mediate improvements in fatigue in patients with active AS receiving tofacitinib.
Using pooled data from phase 2 and 3 studies of tofacitinib-treated patients with AS [14, 15], we found that indirect pathways accounted for more than 80% of the tofacitinib treatment effect on fatigue: (1) tofacitinib treatment affects morning stiffness, which impacts fatigue; and (2) tofacitinib treatment affects morning stiffness, which affects pain, and ultimately pain affects fatigue. The indirect pathway via pain alone accounted for approximately 20% of the tofacitinib treatment effect on fatigue. The effect of tofacitinib treatment on fatigue due to factors other than pain and morning stiffness (i.e. the direct effect, and the indirect pathways involving CRP) were not considered meaningful.
Factors other than pain and morning stiffness can contribute to fatigue in patients with AS, including inflammation, poor functional ability, sleep disturbances and poor mental health [9, 10, 31]. These factors do not appear to participate in tofacitinib-mediated improvements in fatigue, because in the current analysis, the direct treatment effect (via all factors except pain, morning stiffness and CRP) and the indirect effect via CRP were not considered meaningful. However, further research is required to fully quantify the extent to which other individual explanatory variables may mediate improvements in fatigue with tofacitinib treatment.
Previous studies in patients with AS have shown that the effect of tofacitinib treatment on fatigue may be the result of both indirect (via back pain and morning stiffness) and direct (all other possible ways) effects [9, 32–34]. We found that the majority of the tofacitinib treatment effect on fatigue is collectively mediated indirectly by morning stiffness and pain, and these findings were consistent between both model A (FACIT-F-based) and model B (BASDAI Q1-based), which used different measures of fatigue. Inflammatory and biomechanical pain would be expected to have strong relationships to stiffness; accordingly, in patients with rheumatoid arthritis, there is a strong significant correlation between pain (visual analogue scale) and morning stiffness [35]. We can hypothesise that fatigue in patients with AS may be related to the biomechanical effects of aberrant muscular contraction in response to axial pain. Notably, one of the best ways to manage and ameliorate the fatigue caused by AS is to encourage regular movement and exercise [36]. It is likely that the ability to exercise comfortably will be optimised by pharmacological treatment, such as tofacitinib, that reduces inflammation and associated axial pain. These observations are a salutary reminder of the value of both pharmacological and non-pharmacological approaches to the management of fatigue in AS. It should be noted that the investigated mediation model is strictly for tofacitinib-treated patients, explaining and detailing the mechanism of action of tofacitinib on fatigue. For a different drug with a different mechanism of action, or for non-pharmacological approaches, the overall results of a similar mediation model would likely be distinct (with the effects of morning stiffness and pain on fatigue likely to be similar). However, this would require exploration in further studies.
Previous studies report that back pain and nocturnal back pain in patients with AS are highly associated with poorer sleep quality and fatigue [32, 33, 37, 38]. This is likely due to back pain causing sleep disturbance, which in turn could exacerbate fatigue. Accordingly, in patients with AS, pain in the pre-sleep and sleep periods is significantly correlated with daytime fatigue [39]. Additionally, in golimumab-treated patients with AS, reduced nocturnal back pain was the most significant predictor of reduced sleep disturbance [40]. In the current analysis, approximately 20% of the indirect effect of tofacitinib treatment on fatigue was mediated via pain alone. Pain was represented by total back pain/nocturnal spinal pain in model A and BASDAI Q2/3 in model B; although model A (FACIT-F-based) and model B (BASDAI Q1-based) measured pain and fatigue using different components, the correlations between these components were relatively high.
Differences in results between models A and B (e.g. the fact that the effect of morning stiffness alone on fatigue was considered meaningful in initial model A, but not in initial model B) are likely to stem from differences in how fatigue and pain were measured in the models. Fatigue was measured via the multi-item FACIT-F scale in model A and the single-item BASDAI Q1 in model B. Multi-item scales are generally considered to be more precise measures for complex outcomes (such as fatigue), as they allow for a more detailed evaluation, compared with single-item scales [41, 42]. While single-item scores may be more feasible, they are more likely to provide limited information to the physician, and may result in ambiguous score meanings when used to represent complex outcomes, such as fatigue [41]. The FACIT-F scale has been shown to be a valid and reliable tool to measure fatigue in patients with AS [43]. However, there are no clear recommendations for fatigue assessment in AS as current patient-reported outcome measures are of limited methodological quality and relevance to patients with AS [29]. This is perhaps unsurprising since fatigue in patients with AS is multifaceted and triggered by a variety of physical and mental factors [9, 10, 31, 37, 44].
The results of this analysis support earlier findings: a cross-sectional multicentre study of 2251 patients with AS reported that high fatigue scores were associated with stiffness (BASDAI Q5) and high levels of vertebral pain [34]. Furthermore, a global survey of patients with AS receiving TNFi reported that patients with high levels of pain and fatigue had a significantly higher severity and duration of morning stiffness than those with low levels of pain and fatigue [9]. Although results from model B in the current analysis showed that the indirect effect of tofacitinib on fatigue via morning stiffness alone was not considered meaningful, this may be due to how fatigue was defined in model B in comparison to model A, as previously discussed. Interestingly, indirect pathways involving CRP were not considered meaningful in either model, and so CRP appeared not to be associated with morning stiffness in this study. A lack of association between CRP and clinical signs of disease activity, including stiffness, has been reported previously in patients with AS [45, 46]. CRP also had low correlations with other outcomes in the models of the current study.
Limitations of this post hoc analysis should be considered. Data were used from clinical trials that were not designed to evaluate relationships among the various factors that our models assessed, potentially limiting the interpretation of our results. In model A, pain was solely represented by total back pain/nocturnal spinal pain, and in both models stiffness was solely represented by the average of two questions in the BASDAI, referring to morning stiffness only. Therefore, it is likely that models with different sets of mediators representing pain and stiffness would provide different results. In addition, other explanatory variables not assessed in this study may also mediate the effect of tofacitinib treatment on fatigue (e.g. sleep disturbance); however, these explanatory variables would have been encompassed by the ‘direct effect’ that captured ‘all other effects’. Furthermore, the patients with AS analysed had long mean symptom duration and therefore the results may not be applicable to the entire spectrum of the disease (e.g. non-radiographic axSpA or early axSpA). CRP may not fully capture all of the inflammatory processes occurring in patients with AS [47]. For example, some studies have reported a lack of association between levels of acute-phase reactants and signs of disease activity in patients with AS [45, 46].
Conclusions
Results from this mediation model analysis suggest that in patients with AS, improvements in fatigue during tofacitinib treatment are mediated indirectly through effects on morning stiffness and pain. Further research is needed to explore how the relief of pain and stiffness contributes to the observed improvements in fatigue.
Acknowledgements
The authors would like to thank the participants of the study.
Funding
This study was sponsored by Pfizer and the Rapid Service Fee was funded by Pfizer.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing and Editorial Assistance
Medical writing support under the direction of the authors was provided by Robyn Wilson and Emma Mitchell, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;175:1298–304).
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Lars Erik Kristensen, Victoria Navarro-Compán, Andrew G. Bushmakin, Joseph C. Cappelleri, Arne Yndestad, Oluwaseyi Dina and Peter C. Taylor contributed to the study conception/design. Andrew G. Bushmakin, Joseph C. Cappelleri, Arne Yndestad and Oluwaseyi Dina contributed to the data acquisition. Lars Erik Kristensen, Peter C. Taylor, Victoria Navarro-Compán, Andrew G. Bushmakin, Joseph C. Cappelleri, Arne Yndestad and Oluwaseyi Dina contributed to the analysis and interpretation of data.
Disclosures
Lars Erik Kristensen has received grant/research support from AbbVie, Eli Lilly, Pfizer Inc and UCB; has been a consultant for AbbVie, Biogen, Eli Lilly, Galapagos NV, Gilead Sciences, Janssen, Pfizer Inc, Sanofi and UCB; and has been a member of the speakers’ bureau for AbbVie, Biogen, Eli Lilly, Galapagos NV, Gilead Sciences, Janssen, Pfizer Inc, Sanofi and UCB. Peter C. Taylor has received grant/research support from Celgene and Galapagos; and has been a consultant for AbbVie, Bristol-Myers Squibb, Biogen, Celltrion, Eli Lilly, Fresenius, Galapagos NV, Gilead Sciences, GlaxoSmithKline, Janssen, Nordic Pharma, Pfizer Inc, Sanofi and UCB. Victoria Navarro-Compán has received grant/research support from AbbVie and Novartis; has acted as a consultant for AbbVie, Janssen, Lilly, MoonLake, MSD, Pfizer Inc and UCB; and been a member of the speakers’ bureau for AbbVie, Janssen, Lilly, MSD, Pfizer Inc and UCB. Marina Magrey has received research/grant support from AbbVie and UCB; and is a consultant for AbbVie, Eli Lilly, Janssen, Novartis, Pfizer Inc and UCB. Andrew G. Bushmakin, Joseph C. Cappelleri, Arne Yndestad and Oluwaseyi Dina are employees and stockholders of Pfizer Inc.
Compliance with Ethics Guidelines
Both primary studies (NCT01786668; NCT03502616) were conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the principles of the Helsinki Declaration of 1964. Trial protocols for the primary studies were approved by the institutional review board or independent ethics committee at each participating centre. Ethics approval was not required by any relevant institutional review board or independent ethics committee for this post hoc analysis. All patients provided informed consent.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Garcia-Montoya L, Gul H, Emery P. Recent advances in ankylosing spondylitis: understanding the disease and management. F1000Res. 2018;7(F1000 Faculty Rev):1512. doi: 10.12688/f1000research.14956.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 3.Navarro-Compán V, Sepriano A, El-Zorkany B, van der Heijde D. Axial spondyloarthritis. Ann Rheum Dis. 2021;80:1511–1521. doi: 10.1136/annrheumdis-2021-221035. [DOI] [PubMed] [Google Scholar]
- 4.Magrey MN, Danve AS, Ermann J, Walsh JA. Recognizing axial spondyloarthritis: a guide for primary care. Mayo Clin Proc. 2020;95:2499–2508. doi: 10.1016/j.mayocp.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Connolly D, Fitzpatrick C, O'Shea F. Disease activity, occupational participation, and quality of life for individuals with and without severe fatigue in ankylosing spondylitis. Occup Ther Int. 2019;2019:3027280. doi: 10.1155/2019/3027280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun J. Axial spondyloarthritis including ankylosing spondylitis. Rheumatology (Oxford) 2018;57:vi1–vi3. doi: 10.1093/rheumatology/key079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneeberger EE, Marengo MF, Dal Pra F, Maldonado Cocco JA, Citera G. Fatigue assessment and its impact in the quality of life of patients with ankylosing spondylitis. Clin Rheumatol. 2015;34:497–501. doi: 10.1007/s10067-014-2682-3. [DOI] [PubMed] [Google Scholar]
- 8.Turan Y, Duruöz MT, Bal S, Guvenc A, Cerrahoglu L, Gurgan A. Assessment of fatigue in patients with ankylosing spondylitis. Rheumatol Int. 2007;27:847–852. doi: 10.1007/s00296-007-0313-x. [DOI] [PubMed] [Google Scholar]
- 9.Strand V, Deodhar A, Alten R, et al. Pain and fatigue in patients with ankylosing spondylitis treated with tumor necrosis factor inhibitors: multinational real-world findings. J Clin Rheumatol. 2021;27:e446–e455. doi: 10.1097/RHU.0000000000001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esbensen BA, Stallknecht SE, Madsen ME, Hagelund L, Pilgaard T. Correlations of fatigue in Danish patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis. PLoS ONE. 2020;15:e0237117. doi: 10.1371/journal.pone.0237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Heijde D, van der Linden S, Dougados M, Bellamy N, Russell AS, Edmonds J. Ankylosing spondylitis: plenary discussion and results of voting on selection of domains and some specific instruments. J Rheumatol. 1999;26:1003–1005. [PubMed] [Google Scholar]
- 12.Navarro-Compán V, Boel A, Boonen A, et al. The ASAS-OMERACT core domain set for axial spondyloarthritis. Semin Arthritis Rheum. 2021;51:1342–1349. doi: 10.1016/j.semarthrit.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Poddubnyy D, Rudwaleit M, Haibel H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70:1369–1374. doi: 10.1136/ard.2010.145995. [DOI] [PubMed] [Google Scholar]
- 14.van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76:1340–1347. doi: 10.1136/annrheumdis-2016-210322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80:1004–1013. doi: 10.1136/annrheumdis-2020-219601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navarro-Compán V, Wei JCC, Van den Bosch F, et al. Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: results from a phase III, randomised, double-blind, placebo-controlled trial. RMD Open. 2022;8:e002253. doi: 10.1136/rmdopen-2022-002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cappelleri JC, Zou KH, Bushmakin AG, Alvir JMJ, Alemayehu D, Symonds T. Patient-reported outcomes: measurement, implementation and interpretation. Boca Raton: Chapman & Hall/CRC; 2013. [Google Scholar]
- 18.Agler R, De Boeck P. On the interpretation and use of mediation: multiple perspectives on mediation analysis. Front Psychol. 2017;8:1984. doi: 10.3389/fpsyg.2017.01984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 20.Mascha EJ, Dalton JE, Kurz A, Saager L. Understanding the mechanism: mediation analysis in randomized and nonrandomized studies. Anesth Analg. 2013;117:980–994. doi: 10.1213/ANE.0b013e3182a44cb9. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PC, Bushmakin AG, Cappelleri JC, et al. Itch as major mediator of effect of tofacitinib on health-related quality of life in psoriatic arthritis: a mediation analysis. J Clin Med. 2021;10:4081. doi: 10.3390/jcm10184081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor P, Mease P, Bushmakin A, et al. Understanding mediators of pain reduction in psoriatic arthritis patients treated with tofacitinib: role of inflammation. Ann Rheum Dis. 2019;78(Suppl 2):924–925. [Google Scholar]
- 23.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2) 2016 [updated November 9, 2016. Available from: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf].
- 24.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 25.Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S263–S286. doi: 10.1002/acr.20579. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32:811–819. [PubMed] [Google Scholar]
- 27.Zochling J. Measures of symptoms and disease status in ankylosing spondylitis: Ankylosing Spondylitis Disease Activity Score (ASDAS), Ankylosing Spondylitis Quality of Life Scale (ASQoL), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Global Score (BAS-G), Bath Ankylosing Spondylitis Metrology Index (BASMI), Dougados Functional Index (DFI), and Health Assessment Questionnaire for the Spondylarthropathies (HAQ-S) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S47–58. doi: 10.1002/acr.20575. [DOI] [PubMed] [Google Scholar]
- 28.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–2291. [PubMed] [Google Scholar]
- 29.Pearson NA, Packham JC, Tutton E, Parsons H, Haywood KL. Assessing fatigue in adults with axial spondyloarthritis: a systematic review of the quality and acceptability of patient-reported outcome measures. Rheumatol Adv Pract. 2018;2:rky017. doi: 10.1093/rap/rky017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzin M, Ometto F, Ortolan A, et al. An update on serum biomarkers to assess axial spondyloarthritis and to guide treatment decision. Ther Adv Musculoskelet Dis. 2020;12:1759720X20934277. doi: 10.1177/1759720X20934277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skougaard M, Jørgensen TS, Rifbjerg-Madsen S, et al. Relationship between fatigue and inflammation, disease duration, and chronic pain in psoriatic arthritis: an observational DANBIO registry study. J Rheumatol. 2020;47:548–552. doi: 10.3899/jrheum.181412. [DOI] [PubMed] [Google Scholar]
- 32.Nie A, Wang C, Song Y, Xie X, Yang H, Chen H. Prevalence and factors associated with disturbed sleep in outpatients with ankylosing spondylitis. Clin Rheumatol. 2018;37:2161–2168. doi: 10.1007/s10067-018-4190-3. [DOI] [PubMed] [Google Scholar]
- 33.Wadeley A, Clarke E, Leverment S, Sengupta R. Sleep in ankylosing spondylitis and non-radiographic axial spondyloarthritis: associations with disease activity, gender and mood. Clin Rheumatol. 2018;37:1045–1052. doi: 10.1007/s10067-018-3984-7. [DOI] [PubMed] [Google Scholar]
- 34.López-Medina C, Schiotis RE, Font-Ugalde P, et al. Assessment of fatigue in spondyloarthritis and its association with disease activity. J Rheumatol. 2016;43:751–757. doi: 10.3899/jrheum.150832. [DOI] [PubMed] [Google Scholar]
- 35.Sarzi-Puttini P, Fiorini T, Panni B, Turiel M, Cazzola M, Atzeni F. Correlation of the score for subjective pain with physical disability, clinical and radiographic scores in recent onset rheumatoid arthritis. BMC Musculoskelet Disord. 2002;3:18. doi: 10.1186/1471-2474-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pécourneau V, Degboé Y, Barnetche T, Cantagrel A, Constantin A, Ruyssen-Witrand A. Effectiveness of exercise programs in ankylosing spondylitis: a meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018;99:383–9.e1. doi: 10.1016/j.apmr.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Zhang S, Zhu J, Du X, Huang F. Sleep disturbances are associated with increased pain, disease activity, depression, and anxiety in ankylosing spondylitis: a case-control study. Arthritis Res Ther. 2012;14:R215. doi: 10.1186/ar4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammoudeh M, Zack DJ, Li W, Stewart VM, Koenig AS. Associations between inflammation, nocturnal back pain and fatigue in ankylosing spondylitis and improvements with etanercept therapy. J Int Med Res. 2013;41:1150–1159. doi: 10.1177/0300060513488501. [DOI] [PubMed] [Google Scholar]
- 39.Hultgren S, Broman JE, Gudbjörnsson B, Hetta J, Lindqvist U. Sleep disturbances in outpatients with ankylosing spondylitisa questionnaire study with gender implications. Scand J Rheumatol. 2000;29:365–369. doi: 10.1080/030097400447561. [DOI] [PubMed] [Google Scholar]
- 40.Deodhar A, Braun J, Inman RD, et al. Golimumab reduces sleep disturbance in patients with active ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Care Res (Hoboken) 2010;62:1266–1271. doi: 10.1002/acr.20233. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Martin P. Composite rating scales. J Neurol Sci. 2010;289:7–11. doi: 10.1016/j.jns.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002;77:479–487. doi: 10.1016/S0025-6196(11)62218-0. [DOI] [PubMed] [Google Scholar]
- 43.Cella D, Lenderking W, Chongpinitchai P, et al. Psychometric evaluation of Functional Assessment of Chronic Illness Therapy-Fatigue in patients with active ankylosing spondylitis [abstract] Ann Rheum Dis. 2021;80(Suppl 1):146. doi: 10.1136/annrheumdis-2021-eular.62. [DOI] [Google Scholar]
- 44.Dagfinrud H, Kjeken I, Mowinckel P, Hagen KB, Kvien TK. Impact of functional impairment in ankylosing spondylitis: impairment, activity limitation, and participation restrictions. J Rheumatol. 2005;32:516–523. [PubMed] [Google Scholar]
- 45.Spoorenberg A, van der Heijde D, de Klerk E, et al. Relative value of erythrocyte sedimentation rate and C-reactive protein in assessment of disease activity in ankylosing spondylitis. J Rheumatol. 1999;26:980–984. [PubMed] [Google Scholar]
- 46.Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis. 2002;61:iii8–18. doi: 10.1136/ard.61.suppl_3.iii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol. 1999;26:966–970. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.