Abstract
Leishmania donovani is the causative organism for visceral leishmaniasis. Although this parasite was discovered over a century ago, nothing is known about role of potassium channels in L. donovani. Potassium channels are known for their crucial roles in cellular functions in other organisms. Recently the presence of a calcium-activated potassium channel in L. donovani was reported which prompted us to look for other proteins which could be potassium channels and to investigate their possible physiological roles. Twenty sequences were identified in L. donovani genome and subjected to estimation of physio-chemical properties, motif analysis, localization prediction and transmembrane domain analysis. Structural predictions were also done. The channels were majorly α-helical and predominantly localized in cell membrane and lysosomes. The signature selectivity filter of potassium channel was present in all the sequences. In addition to the conventional potassium channel activity, they were associated with gene ontology terms for mitotic cell cycle, cell death, modulation by virus of host process, cell motility etc. The entire study indicates the presence of potassium channel families in L. donovani which may have involvement in several cellular pathways. Further investigations on these putative potassium channels are needed to elucidate their roles in Leishmania.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03692-y.
Keywords: Potassium channel, Selectivity filter, Gene ontology, Leishmania donovani, Visceral leishmaniasis
Introduction
Potassium channels have been molecules of much interest and scrutiny. Several types of potassium channels are identified and are well characterized in a wide range of organisms ranging from prokaryote Streptomyces lividans, invertebrate Drosophila melanogaster, nematode worm Caenorhabditis elegans to humans (Salkoff and Wyman 1981; Kamb et al. 1987; Murai et al. 1989; Grupe et al. 1990; Schrempf et al. 1995; Wei et al. 1996; Bargmann 1998; Frolov et al. 2012). The crystal structure of many of the potassium channels have been elucidated, the first one being the S. lividans KcsA (Doyle et al. 1998).
Potassium channels are the ion channels that conduct the selective movement of potassium ions across it (MacKinnon 2003). The process of potassium ion permeation remains in tight regulation since it is an active process. The efficient opening and closing of the potassium ion channels are achieved through diverse ‘gating’ or ‘activation’ and ‘inactivation’ process, when a stimulus is received (Choe 2002). Depending on how these channels are modulated, they may be classified as voltage-gated potassium channel- Kv, calcium-activated potassium channel- KCa, inward-rectifier potassium channel- Kir, cyclic-nucleotide gated potassium channel-KCNG, two-pore potassium channel- K2P etc. (Wei et al. 1996; Miller 2000; Kuang et al. 2015). Even though there are different types of potassium channels in prokaryotes to higher organisms, structural conservation has been seen in the selectivity filter (P-loop) in the sequence. The sequence in the other regions can be variable (Heginbotham et al. 1994; MacKinnon et al. 1998). They localize on membranes of cell and of various organelles such as mitochondria, lysosomes, Golgi bodies, nucleus, endoplasmic reticulum etc. (Checchetto et al. 2016; Capera et al. 2019).
The potassium channels are involved in a huge array of physiological roles inside the cell. For instance, they are involved in regulating membrane potential (Nelson and Quayle 1995), cell proliferation, neurotransmitter release, calcium signalling, maintaining intracellular calcium level (Kshatri et al. 2018), intercellular communication (Beagle and Lockless 2021), cell cycle regulation (Urrego et al. 2014) and apoptosis (Bachmann et al. 2020) to name a few.
Plethora of information is available about the sequence, structure, types and function of potassium channels in bacteria, fungi, humans etc. However, only a few potassium channels have been characterized till date in case of Kinetoplastid protozoan parasites such as Trypanosoma and Leishmania.
In Trypanosoma brucei (T. brucei), a potassium transporter was identified in both fly stage and mammalian stage of the parasite was identified. It was identified as a member of Trk/HKT superfamily. This is a family of monovalent cation permeases and the members were earlier only known in archaea, prokaryotes, fungal species and plants (Mosimann et al. 2010). Two T. brucei proteins-TbK1 and TbK2, were identified as members of putative calcium-activated potassium channel family. Heteromeric arrangement of these two proteins was required for generation of measurable currents and silencing of its genes led to changes in plasma membrane potential (Steinmann et al. 2015). An inward rectifier potassium channel (TbIRK) which is localized into acidocalcisomes and may be involved in osmotic stress response was also characterized from T. brucei. This TbIRK was unique in lacking the signature sequence that is generally present in potassium channels (Steinmann et al. 2017).
Presence of potassium conductive pathway in Trypanosoma cruzi (T. cruzi) was identified and possible roles in maintaining membrane, pH etc. were implicated (Jimenez et al. 2011). A plasma membrane localized calcium-activated potassium channel was later identified in T. cruzi (TcCAKC). Knocking-out of its gene led to impaired replication, growth and infectivity. Involvement of this channel was also reported in maintaining the cellular osmolarity, membrane potential, intracellular calcium ion and pH levels (Barrera et al. 2020).
From T. cruzi, T. brucei, Leishmania major (L. major), Leishmania infantum (L. infantum) and Leishmania braziliensis (L. braziliensis), several putative homologues of potassium channels were identified (Prole and Marrion 2012). A calcium-activated potassium channel from Leishmania donovani (L. donovani) has been identified by heterologous expression in bacterial system (LdKCa). It was predicted to have a plasma membrane localization, conserved signature sequence and antigenic epitopes (Paul et al. 2021).
Collectively looking at it, only a few potassium channels are known in these parasites. Also no information is available on the role of these proteins in Leishmania physiology. This is in comparison to the wealth of knowledge available on potassium channels of other organisms. Here, we have identified putative potassium channel sequences from genome of L. donovani. These sequences were analysed for different properties such as physiochemical properties, conserved domains, signature sequences, structure, localization etc. Gene Ontology terms were used to understand their possible associations and involvements in biological processes and molecular functions.
Methodology
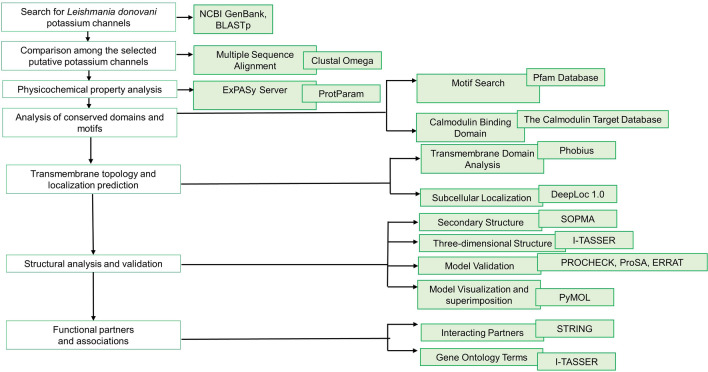
To identify the protein sequences for potassium channel in L. donovani, the putative homologues from other pathogenic protozoa were subjected to BLASTp search (Altschul et al. 1990) against the L. donovani genome (taxid-5661). The query sequences were: Plasmodium falciparum (XP_001348796 and XP_001350669); Plasmodium knowlesi (XP_002260211 and XP_002262343); Plasmodium vivax (XP_001615733 and XP_001617360); L. infantum (XP_001462697, XP_001462696, XP_001465142, XP_001464237 and XP_001464236), L. braziliensis (XP_001561516) and T. cruzi (XP_821941, XP_816151, XP_820381, XP_820126, XP_818052, XP_813982, XP_813983, XP_818073) (Prole and Marrion 2012). Twenty protein sequences from the L. donovani genome which aligned with the query sequences were analysed further. The work flow and tools used for analysis have been summarized in Fig. 1.
Fig. 1.
Schematic representation of the workflow and the tools used in the analysis of the L. donovani putative potassium channels
Multiple sequence alignment
The multiple sequence alignment (MSA) was done using the Clustal Omega 1.2.4 tool (https://www.ebi.ac.uk/Tools/msa/clustalo/) (Sievers et al. 2011). The protein sequences were used as input and the default parameters were selected for output. It was also used to obtain the phylogenetic tree.
Physiochemical properties of the channels
The physiochemical properties of proteins were analysed using the ExPasy’s ProtParam tool (http://web.expasy.org/protparam) (Gasteiger et al. 2005). It includes the parameters such as sequence length, molecular weight, isoelectric point (pI), extinction coefficient (EC), instability index (II), aliphatic index (Ai) and Grand average Hydropathicities (GRAVY).
Conserved motifs and calmodulin binding site analysis
In order to find the conserved motifs present in the sequences, MOTIF Search was used (https://www.genome.jp/tools/motif/) (Finn et al. 2014). The amino acid sequence of the protein was provided in FASTA format as input and under the database Pfam and E-value cut-off score was selected as 1.0. The Calmodulin (CaM) binding site search was done using the Calmodulin Target Database (CTDB) (http://calcium.uhnres.utoronto.ca/ctdb/) (Yap et al. 2000).
Transmembrane domain prediction
Transmembrane domains (TMDs) were analysed using a web server Phobius (https://phobius.sbc.su.se/) (Käll et al. 2004; Kall et al. 2007). With the protein sequence as input, Phobius generates a list of predicted transmembrane helices, intervening loop regions and signal peptide locations.
Prediction of cellular localization
Here, we used Deeploc 1.0 (Almagro Armenteros et al. 2017) web server (https://services.healthtech.dtu.dk/service.php?DeepLoc-1.0) which predicts subcellular localization of the eukaryotic proteins on the basis of the likelihood score.
Secondary structure
The secondary structure analysis of proteins was done using SOPMA from Network protein sequences analysis (NPS@) server (https://npsa-prabi.ibcp.fr/NPSA/npsa_sopma.html) for conformational states- alpha helix, extended strand, beta turn and random coil (Geourjon and Deléage 1995). To run the SOPMA tool, the default parameters were selected which were- its output width of 70, number of conformation state as 4, similarity threshold of 8 and window width as 17 using the amino acid sequences.
Three-dimensional structure of the channels, structure validation and GO term prediction
To generate the three-dimensional models of all the identified potassium channel proteins, I-TASSER server (https://zhanggroup.org/I-TASSER/) (Roy et al. 2010; Yang and Zhang 2015a, b) was selected as it has been reported to generate high quality models even from templates having low identity (Khor et al. 2015; Scarpati et al. 2019). For each protein sequence, five models were generated by I-TASSER. C-score denotes the confidence in each model, ranging between − 5 and 2, where a higher score means better quality model. Also, the first model out of five usually has the better quality, hence, here the first model has been considered for each protein. Template Modelling score (TM-score) assesses the similarity with template and it can range between 0–1. A TM-score above 0.5 indicates that the model topology is correct and a score less than 0.17 indicates that the similarity with the template is random. Root-Mean-Square Deviation (RMSD) shows the average distance between each residue of the model and template and it can have higher values as it is extremely sensitive to local errors even if the model has a correct global topology. The RMSD value can lie in the range of 0-30 Å (Roy et al. 2010). I-TASSER also predicted the gene ontology (GO) terms for each protein for their molecular functions (GOMF), biological processes (GOBP) and cellular components (GOCC) (Yang and Zhang 2015b).
Ramachandran plot using PROCHECK (to assess the structural stereochemical property of predicted protein structures) and ERRAT were used in SAVES v6.0-UCLA website (https://saves.mbi.ucla.edu) (Laskowski et al. 1993; Colovos and Yeates 1993). The other tool which was used to analyze protein structure is ProSA which is accessible at https://prosa.services.came.sbg.ac.at/prosa.php. The PDB file of each protein was submitted to it and z-score was obtained (Sippl 1993; Wiederstein and Sippl 2007).
The model generated for each channel was superimposed with its templates using PyMOL 0.99rc6.
Functional partner prediction
Protein–protein interaction analysis was done using STRING V 11.5. The amino acid sequence was provided and then the organism was selected as L. donovani species complex. The proteins were then annotated from L. infantum database as separate database for L. donovani is not available. Further the functional partners were predicted (Szklarczyk et al. 2019).
Results
Sequence alignment of the putative potassium channel sequences
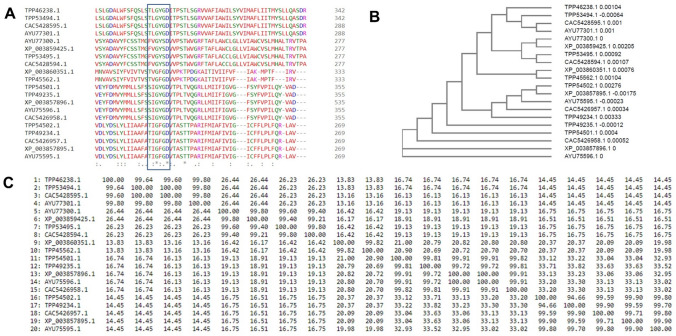
The GenBank Accession number of the twenty putative potassium channel sequences identified using BLASTp were- XP_003857896.1, AYU75596.1, CAC5426958.1, TPP54501.1, TPP49235.1, CAC5426957.1, TPP49234.1, XP_003857895.1, AYU75595.1, TPP54502.1, XP_003860351.1, TPP45562.1, AYU77300.1, TPP53495.1, CAC5428594.1, XP_003859425.1, TPP53494.1,CAC5428595.1, AYU77301.1 and TPP46238.1. The multiple sequence alignment revealed a high degree of conservation in the selectivity filter-TXGXGD among these sequences. Some of the sequences had TLGYGD, TVGFGD or TIGFGD. A few also had FVGYSD or SIGYGD as shown in Fig. 2A. The relationship among these sequences has been shown in the form of a phylogenetic tree (Fig. 2B). AYU75596.1 and XP_003857896.1 are evolutionary distant from the other sequences. The other 18 sequences arise from a common node which bifurcates into two edges. One for the protein CAC5426958.1 and the other one then forms a common node for the other 17 proteins. The percentage identity matrix in Fig. 2C shows that the sequences have varying sequence identity among themselves. The proteins- TPP46238.1, TPP53494.1, CAC5428595.1 and AYU77301.1 have ~ 99% similarity among them, but lower identity with other sequences. Similar was the cases with other sequences. Based on the respective identities and their selectivity filter sequence, the proteins have been divided into five groups- Group 1 (XP_003857896.1, AYU75596.1, CAC5426958.1, TPP54501.1 and TPP49235.1), Group 2 (CAC5426957.1, TPP49234.1, XP_003857895.1, AYU75595.1 and TPP54502.1), Group 3 (XP_003860351.1 and TPP45562.1), Group 4 (AYU77300.1, TPP53495.1, CAC5428594.1 and XP_003859425.1) and Group 5 (TPP53494.1, CAC5428595.1, AYU77301.1 and TPP46238.1) which have been shown in Table 1.
Fig. 2.
Multiple sequence alignment and phylogenetic analysis of the twenty sequences of the potassium channels. A Sequence alignment showing the signature sequence of the selectivity filter. B Phylogenetic analysis of the putative potassium channel sequences. C Percentage identity matrix showing the identities among the twenty sequences
Table 1.
Physicochemical properties of the putative potassium channel proteins of Leishmania donovani
| Group | Accession Number | Name | Sequence length | Molecular Weight (Da) | Isoelectric Point | Instability Index | Aliphatic index | GRAVY | |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | XP_003857896.1 | potassium channel subunit-like protein [Leishmania donovani] | 1346 | 146,217.25 | 6.35 | 43.23 | 99.30 | 0.154 | |
| AYU75596.1 | potassium channel subunit-like protein [Leishmania donovani] | 1168 | 127,541.39 | 5.66 | 41.79 | 99.72 | 0.136 | ||
| CAC5426958.1 | potassium_channel_subunit-like_protein|GeneDB:LmjF.01.0820 [Leishmania donovani] | 1168 | 127,550.40 | 5.68 | 41.78 | 99.72 | 0.136 | ||
| TPP54501.1 | Ion channel family protein [Leishmania donovani] | 1168 | 127,541.39 | 5.66 | 41.79 | 99.72 | 0.136 | ||
| TPP49235.1 | Ion channel family protein [Leishmania donovani] | 1068 | 118,010.92 | 5.83 | 39.47 | 102.06 | 0.161 | ||
| Group 2 | CAC5426957.1 | calcium/potassium_channel_(CAKC)_putative| GeneDB:LmjF.01.0810 [Leishmania donovani] | 1017 | 113,836.75 | 6.74 | 46.00 | 94.93 | 0.012 | |
| TPP49234.1 | Calcium-activated BK potassium channel alpha subunit family protein [Leishmania donovani] | 1136 | 126,569.40 | 7.23 | 43.27 | 94.17 | 0.018 | ||
| XP_003857895.1 | calcium/potassium channel (CAKC), putative [Leishmania donovani] | 1017 | 113,793.68 | 6.56 | 45.44 | 94.93 | 0.018 | ||
| AYU75595.1 | calcium/potassium channel (CAKC), putative [Leishmania donovani] | 1017 | 113,807.70 | 6.56 | 45.63 | 94.93 | 0.018 | ||
| TPP54502.1 | Calcium-activated BK potassium channel alpha subunit family protein [Leishmania donovani] | 1131 | 125,454.25 | 6.59 | 44.10 | 95.29 | 0.081 | ||
| Group 3 | XP_003860351.1 | hypothetical protein, conserved [Leishmania donovani] | 1110 | 127,234.48 | 7.55 | 36.92 | 89.65 | -0.041 | |
| TPP45562.1 | Calcium-activated BK potassium channel alpha subunit family protein [Leishmania donovani] | 1110 | 127,274.54 | 7.54 | 36.99 | 89.74 | -0.039 | ||
| Group 4 | AYU77300.1 | Ion channel, putative [Leishmania donovani] | 504 | 55,346.52 | 9.82 | 43.66 | 94.46 | 0.225 | |
| TPP53495.1 | Ion channel family protein [Leishmania donovani] | 504 | 55,187.35 | 9.80 | 45.25 | 94.46 | 0.232 | ||
| CAC5428594.1 | Ion_channel_putative|Pfam:PF07885 [Leishmania donovani] | 504 | 55,219.41 | 9.80 | 45.25 | 93.89 | 0.228 | ||
| XP_003859425.1 | hypothetical protein, conserved [Leishmania donovani] | 504 | 55,394.61 | 9.85 | 44.53 | 94.46 | 0.229 | ||
| Group 5 | TPP53494.1 | Ion channel family protein [Leishmania donovani] | 553 | 61,433.65 | 9.87 | 47.09 | 105.15 | 0.220 | |
| CAC5428595.1 | ion_transport_protein-like_protein|GeneDB:LmjF.14.0540 [Leishmania donovani] | 499 | 55,965.59 | 9.77 | 41.45 | 111.40 | 0.286 | ||
| AYU77301.1 | ion transport protein-like protein [Leishmania donovani] | 499 | 55,974.60 | 9.77 | 41.92 | 111.40 | 0.287 | ||
| TPP46238.1 | Ion channel family protein [Leishmania donovani] | 553 | 61,470.71 | 9.87 | 47.52 | 105.50 | 0.225 |
Physiochemical properties of the channels
The physiochemical properties of different L. donovani potassium channel proteins in various groups are tabulated in Table 1. The number of amino acids in these proteins ranged from 499 to 1346 with variable molecular weights. XP_003857896.1, which is made up of 1346 amino acid sequence was the largest protein among these with the molecular weight of 146,217.25 Dalton (Da). CAC5428595.1 and AYU77301.1 had 499 amino acids and were the smallest among all with molecular weight of 55,965.59 Da and 55,974.60 Da respectively.
The isoelectric point of these proteins ranged from 5.66 to 9.87 and at this isoelectric point, these proteins have no net charge. The instability index denotes the protein’s in vitro stability. These proteins had an instability index ranging from 36.92 to 47.52. The three proteins i.e., TPP49235.1, XP_003860351.1 and TPP45562.1 have the instability index less than 40 which means that these proteins are stable. The remaining 18 proteins have instability index more than 40 which points towards its unstable characteristics. The proteins had aliphatic index ranging from 89.65 to 111.78. The higher aliphatic index of these proteins indicates that these proteins are thermostable in wider range of temperature.
Hydropathicity (GRAVY) score of the proteins ranged from − 0.041 to 0.154. Most of them had a positive GRAVY score with the exception of XP_003860351.1 and TPP45562.1. The GRAVY score for these two proteins were − 0.041 and − 0.039 respectively. A positive GRAVY score indicates that the protein is hydrophobic in nature whereas the negative GRAVY score indicates that the protein is hydrophilic.
Motif analysis
The motifs present in each protein sequence has been summarized in Table S1. There were six different motifs present in these 20 proteins, i.e., PF07885- Ion channel, PF03493- Calcium-activated BK potassium channel alpha subunit, PF10500- Nuclear RNA-splicing-associated protein, PF00060- Ligand-gated ion channel, PF00520- Ion transport protein, PF17597- Family of unknown function (DUF5493). Ion channel motif was present in all 20 proteins. The motifs for Ion channel (PF07885) along with Calcium-activated BK potassium channel alpha subunit (PF03493) were present in 9 proteins- XP_003857896.1, AYU75596.1, CAC5426958.1, TPP54501.1, TPP49235.1, TPP49234.1, TPP54502.1, XP_003860351.1 and TPP45562.1.
Three proteins had the motifs for Ion channel (PF07885) and Calcium-activated BK potassium channel alpha subunit (PF03493) along with the motif for Nuclear RNA-splicing-associated protein (PF10500). These proteins were CAC5426957.1, XP_003857895.1 and AYU75595.1. Four proteins only had the motif for Ion channel (PF07885) which are AYU77300.1, TPP53495.1, CAC5428594.1 and XP_003859425.1. The motifs for Ion channel (PF07885) and Calcium-activated BK potassium channel alpha subunit (PF03493) and Ion transport protein (PF00520) were predicted from TPP53494.1 and TPP46238.1. Ion channel (PF07885), Ligand-gated ion channel (PF00060), Ion transport protein (PF00520) and family of unknown function (PF17597) motifs were found in CAC5428595.1 and AYU77301.1.
The calmodulin binding domains are also summarized in Table S1. The sequence ‘VLVMQLVRQLIS’ was identified as the binding site in Group 1 (Fig. S1A). In the proteins of Group 2, it is ‘PDKKAYLISWL’ (Fig. S1B). Group 3 has ‘PFLVNLVRTAWA’ as the predicted site, as shown in Fig. S1C. For Group 4, ‘VFKR’ is the sequence for calmodulin binding (Fig. S1D). ‘ARVIQLAWRL’ is predicted to be the binding site for Group 5 (Fig. S1E).
Transmembrane domain prediction of the potassium channels
A maximum of 12 TMDs were found, in XP_003857896.1. The proteins TPP53494.1, CAC5428595.1, AYU77301.1 and TPP46238.1 have 6 TMDs which is the least among all the sequences. The regions spanning the membrane are depicted in Table S2.
Prediction of cellular localization
According to Deeploc 1.0 server predictions, the proteins were found to be located in the cell membrane, plastid and lysosome/vacuole as shown in Table S3. AYU75596.1, CAC5426958.1, TPP54501.1, TPP49235.1, CAC5426957.1, TPP49234.1, XP_003857895.1, AYU75595.1, TPP54502.1, XP_003860351.1 and TPP45562.1 had a higher likelihood of being localized at the cell membrane. The proteins predicted to be localized at the lysosome/vacuole are- AYU77300.1, TPP53495.1, CAC5428594.1, XP_003859425.1, TPP53494.1, CAC5428595.1, AYU77301.1 and TPP46238.1. The cellular localization of XP_003857896.1 was predicted to be in plastids.
Structure of the channels and its validation
The secondary structure prediction was done using the NPS@ server and is tabulated in Table S4. The proteins mainly had alpha helical conformation. CAC5428595.1 has the highest percentage of amino acids i.e., 66.73% in the alpha helix conformational state. XP_003857895.1 and AYU75595.1 had the least share, 38.25% of amino acids residues as alpha-helix, as compared to the other sequences. Apart from this, all the remaining putative potassium channel proteins also had approximately ~ 21–36% of amino acids as random coil.
The three-dimensional structure of the putative potassium channels were generated using I-TASSER. The templates used in the modelling of these structures have been shown in Table S5. The C-score for the final models for each protein along with their estimated TM-scores and RMSD have been summarized in Table S6. The model for the protein TPP54502.1 (Calcium-activated BK potassium channel alpha subunit family protein) had the highest C-score of − 0.34 and estimated TM of 0.67 ± 0.13.
To validate the models, Ramachandran Plots (Residues in various regions), ERRAT Plots (Overall quality factor) and ProSA plots (Z-scores) were generated which are summarized in Table S7. The plots have also been shown for each individual protein in Supplementary Fig. 2–21 (The ERRAT Plots for AYU75596.1, XP_003857895.1, AYU75595.1, XP_003860351.1 and CAC5428595.1 could not be generated). In addition to the above, the Supplementary Fig. 2–21 contains the protein model generated and structural superimposition with their respective templates.
I-TASSER also predicted several gene ontology terms associated with the biological process, molecular function and cellular component. Apart from their conventional roles like ion channel activity, potassium channel activity and potassium ion transport, they were predicted to participate in important cellular events such as mitotic cell cycle, apoptosis, response to oxidative stress etc. which have been mentioned in Table S8–S10.
Functional partner prediction
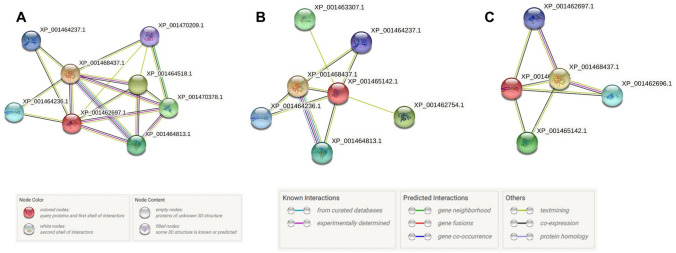
The STRING analysis of the putative potassium channels identified several possible functional partners. In its database, XP_003857896.1, AYU75596.1, CAC5426958.1, TPP54501.1 and TPP49235.1 were annotated as XP_001462697.1 (potassium channel subunit-like protein [Leishmania infantum JPCM5]). The interactions have been shown in Fig. 3A. For these proteins, the predicted functional partners were: Putative calcium channel protein; Putative ion transporter (XP_001468437.1), Conserved hypothetical protein; Uncharacterized protein (XP_001464518.1), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-1-like protein (XP_001470378.1), Hypothetical protein, unknown function (XP_001464813.1), Conserved hypothetical protein; Uncharacterized protein (XP_001464236.1), Ion transport protein-like protein (XP_001464237.1) and Sulfate transporter-like protein (XP_001470209.1) (Table S11).
Fig. 3.
Functional partners of the potassium channels from L. donovani. Protein–Protein interactions for A XP_001462697.1, potassium channel subunit-like protein (Annotation for the proteins- XP_003857896.1, AYU75596.1, CAC5426958.1, TPP54501.1 and TPP49235.1) B XP_001465142.1, conserved hypothetical protein (Annotations for XP_003860351.1 and TPP45562.1) C. XP_001464236.1, conserved hypothetical protein (Annotations for AYU77300.1, TPP53495.1, CAC5428594.1 and XP_003859425.1) and XP_001464237.1, ion transport protein-like protein (Annotations for TPP53494.1, CAC5428595.1, AYU77301.1 and TPP46238.1). [Please see Table S11 for a detailed description of each interacting partner.]
The L. donovani potassium channel proteins- CAC5426957.1, TPP49234.1, XP_003857895.1, AYU75595.1 and TPP54502.1 were annotated in the database as putative calcium/potassium channel (CAKC) [Leishmania infantum JPCM5] (XP_001462696.1). The predicted functional partners of this protein were same as for the above mentioned proteins.
XP_003860351.1 and TPP45562.1 were annotated as XP_001465142.1 (conserved hypothetical protein [Leishmania infantum JPCM5]). The proteins AYU77300.1, TPP53495.1, CAC5428594.1 and XP_003859425.1 were annotated as XP_001464236.1 (conserved hypothetical protein [Leishmania infantum JPCM5]). For TPP53494.1, CAC5428595.1, AYU77301.1 and TPP46238.1, the annotated protein was XP_001464237.1 (ion transport protein-like protein [Leishmania infantum JPCM5]). The functional partners are enlisted in Table S11 and Fig. 3B, C.
Discussion
Twenty putative potassium channel sequences were identified and analysed in the present work. The sequences which were more identical and have similar characteristics were classified into five groups.
Amongst these groups, there was varied degree of conservation in their signature sequence. It has been reported that potassium channels are homologous in their signature sequence i.e., their selectivity filter which consists of amino acids ‘TXXTXGYG’ (Heginbotham et al. 1994). In the present work, signature sequences identified in the putative potassium channels were: ‘SIGYGD’, ‘TIGFGD’, ‘TVGFGD’, ‘FYGYSD’ and ‘TLGYGD’. In literature, several variations of the selectivity filter in the pore region such as TIGYGF, TIGYGL, XXGFGD, XXGYGS and XXGYGD have been reported (So et al. 2001; Shealy et al. 2003; Prole and Marrion 2012). In Shaker voltage-activated potassium channels, ‘TMTIVGYG’ is the signature sequence (Heginbotham et al. 1994). Also, D in ‘GYGD’ motif is crucial for potassium ion selectivity (Kirsch et al. 1995).However, some potassium channels lack the classical selectivity filter, such as potassium channel (TbIRK) from T. brucei (Steinmann et al. 2017).
Calmodulin (CaM) is a calcium ion sensor protein and vital in regulating several cellular pathways. Potassium channels are also regulated by CaM such as Kv7.1 to Kv7.5 (Alaimo and Villarroel 2018). The CaM binding sequences predicted here were ‘VLVMQLVRQLIS’, ‘PDKKAYLISWL’, ‘PFLVNLVRTAWA’, ‘VFKR’ and ‘ARVIQLAWRL’. In the KCNQ family of voltage-gated potassium channels, the CaM binding motif consists of ‘IQXXXRXXXXR’ which is similar to that of the putative potassium channels in Group 5 (Yus-Nájera et al. 2002). Two CaM binding domains—‘IVFRKISD’ and ‘RRLFQRFRQQK’ are present at the C-terminal domain of Human Ether à Go-Go 1 (hEAG1) potassium channel (Ziechner and Malesevic 2006; Lörinczi et al. 2016).
In the current study, potassium channels such as TPP49235.1, XP_003860351.1 and TPP45562.1 have instability index lesser than 40, classifying them as stable. All the potassium channels analysed here have a high aliphatic index, ranging between ~ 89 to ~ 111. It indicates that these proteins are thermostable. The L. donovani calcium-activated potassium channel (LdKCa) also had a high aliphatic index of 115.18 (Paul et al. 2021). AYU77300.1, TPP53495.1, CAC5428594.1, XP_003859425.1, TPP53494.1, CAC5428595.1, AYU77301.1 and TPP46238.1 have higher GRAVY scores (0.220 to 0.287) which denote that they could be hydrophobic. GRAVY score of LdKCa was 0.381 (Paul et al. 2021).
The ‘Ion_trans_2’ (pfam07885) motif is present in all the 20 putative potassium channel sequences that have been studied here. The domain PF07885 forms a part of potassium channels (Suh and Hutter 2012). Also, this motif has been reported to be present in a Giardia lamblia potassium channel (Palomo-Ligas et al. 2019). Two-pore potassium channels in various plant species such as Arabidopsis, Oryza etc. have been found to contain the domain PF07885 (Musavizadeh et al. 2021; Dabravolski and Isayenkov 2021). T. brucei potassium channels have been reported to contain PF07885 and PF03493 (Steinmann et al. 2015). The PF07885 and PF03493 domains are also present in human KCNT1 which is a sodium-gated potassium channel involved in epilepsies (McTague et al. 2018). In Dunaliella salina, an alga which has high tolerance to osmotic stress, the domains- PF07885 and PF03493 are found in its potassium channels (Polle et al. 2020). A potassium channel member identified from a wasp Leptopilina boulardi contains PF03493 and PF07885 domains (Varaldi and Lepetit 2018). PF00520 is also present in pores of voltage-dependent potassium channels in plants (Dabravolski and Isayenkov 2021). Human voltage-gated potassium channels also contain the PF00520 domain (Hou et al. 2020).
Many of the potassium channel sequences in this study were predicted to have a high probability to be localized on the cell membrane. The previously identified LdKCa was also predicted to have a cell membrane localization (Paul et al. 2021). In T. cruzi, a calcium-activated potassium channel is also localized at the plasma membrane (Barrera et al. 2020). Plasma membrane localization was also seen in T. brucei potassium channels- TbK1 and TbK2 (Steinmann et al. 2015). The potassium channels from Plasmodium- PfKch1, localizes on the plasma membrane whereas, PfKch2 is present at the internal membranes (Molbaek et al. 2020).
Several potassium channels identified in this study were predicted to be localized at the lysosome. A human transmembrane protein TMEM175, which was identified to act as potassium channel localizes at lysosome membranes. Perturbations in this protein caused aberrations in lysosomal pH, membrane potential and autophagy (Cang et al. 2015; Jinn et al. 2017; Li et al. 2019; Oh et al. 2020). A big conductance calcium-activated potassium channel is present in lysosomes which is important for lysosomal calcium release and helps in membrane trafficking (Cao et al. 2015). In macrophages, lysosomal big-conductance potassium channels are essential for phagocytosis of large particles (Sun et al. 2020). It was fascinating to see that one of the proteins- XP_003857896.1, was predicted to be localized at the plastids. It has been reported that plastids had been present in Trypanosomatids at some point during their evolution. ∆4 desaturases, 6-phosphogluconate dehydrogenase, superoxide dismutases and triosephosphate isomerase are the earliest identified examples of such plant-like genes, which are involved in vital cellular processes such as hexose monophosphate shunt, fatty acid synthesis, protection against ROS etc. (Hannaert et al. 2003; Waller et al. 2004; Tripodi et al. 2006; Maruyama et al. 2008; Sun et al. 2008; Bodył et al. 2010).
All the putative potassium channels analysed here have α-helical structure. Similarly, LdKCa structure was mainly α-helical (Paul et al. 2021). Most other potassium channel also have a α-helical structure (Spencer and Rees 2002; Yellen 2002; MacKinnon 2003; Kuang et al. 2015, p. 1; Knyazev et al. 2018).
Several functional partners for these potassium channel proteins were identified in the present study. It has been reported that ion channels such as calcium ion channel form part of various signalling pathways, and in doing so, they interact with the transmembrane protein such as potassium channels, opioid receptors, G-Protein coupled receptors etc., in addition to other cellular signalling molecules. SK and BK channel (small conductance and big conductance calcium-activated potassium channel) activation are dependent on changes in intracellular calcium levels. The calcium ion concentrations are maintained by calcium ion channels. This kind of dependence is seen in Purkinje cells and several other types of neuronal cells etc. Cav3 T-type calcium ion channels have also been seen to interact with voltage gated potassium channels, Kv4. This interaction happens via KChIP (Kv channel-interacting proteins) binding to N-terminal of Kv4 and act as calcium sensors (Coetzee et al. 1999; Edgerton and Reinhart 2003; Wei et al. 2005; Altier and Zamponi 2008; Rudy et al. 2009; Cui et al. 2009; Anderson et al. 2010a, b; Turner et al. 2011). In network analysis of O. sativa, during amino acid biosynthesis, co-expression of potassium channels was predicted with an ATP-dependent 6-phosphofructokinase. It was indicative of potassium channel involvement in response to stimuli and other cellular processes (Musavizadeh et al. 2021).
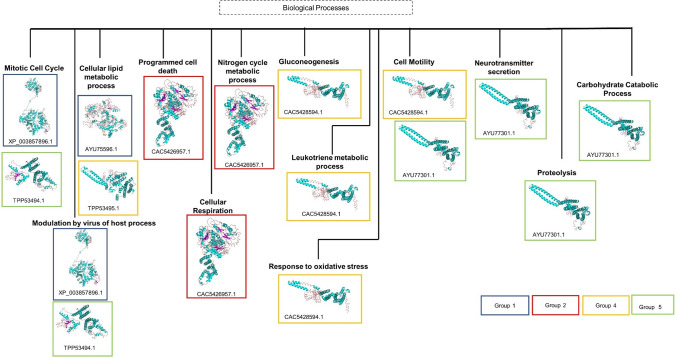
Group 1 (CAC5426958.1, TPP54501.1 and TPP49235.1), Group 2 (TPP49234.1) and Group 3 (XP_003860351.1 and TPP45562.1) channels are putatively associated with ‘inorganic cation transmembrane transport’ (GO:0015672). Group 2 proteins- CAC5426957.1, XP_003857895.1, AYU75595.1 and TPP54502.1 are associated with ‘potassium ion transport’ (GO:0006813). Apart from the conventional role of transporting potassium cations across the membrane, involvement of these channels in several other physiological processes and pathways were seen which are summarized in Fig. 4.
Fig. 4.
Summary of the unconventional associations of the L. donovani potassium channels in various biological processes. Each box colour represents the group in which the protein has been characterized into. The accession ID and the three-dimensional model have been shown for each involved protein
The putative potassium channels- XP_003857896.1 (Group 1) and TPP53494.1 (Group 5) were associated with biological process of ‘mitotic cell cycle’ (GO:0007067). Several types of potassium channels regulate the process of cell cycle and also control cell proliferation. For instance, mitosis can be inhibited by blocking of potassium channels Kv11.1 (Ouadid-Ahidouch and Ahidouch 2013; Urrego et al. 2014). Both the proteins XP_003857896.1 (Group 1) and TPP53494.1 (Group 5) are also linked with ‘modulation by virus of host process’ (GO:0019048). Two-pore domain potassium channels (K2P) of host has been reported to be involved in Bunyavirus infection (Bunyamwera virus- BUNV, negative sense single-stranded RNA virus) (Hover et al. 2016).
AYU75596.1 (Group 1) and TPP53495.1 (Group 4) are associated with the ‘cellular lipid metabolic process’ (GO:0044255). Slo1 BK channels can be activated by docosahexaenoic acid without calcium ion or voltage stimulation (Hoshi et al. 2013). A plethora of other potassium channels are modulated by fatty acids and affect lipid metabolism inside the cell (Antollini and Barrantes 2016).
It is noteworthy that protein CAC5426957.1 is associated with the process of ‘programmed cell death’ (GO:0012501). Increased potassium ion efflux causes apoptotic volume decrease and cell shrinkage which are early signs of apoptosis. Further potassium ion efflux causes caspase activation and apoptosis (Burg et al. 2006).
CAC5426957.1 is predicted to be involved in ‘cellular respiration’ (GO:0045333). A voltage-gated potassium channel Kv1.3 promotes oxidative phosphorylation along with proliferation of cell which further causes increase in reactive oxygen species inside the cell. If the reactive oxygen species is reduced, it also reduces the Kv1.3 mediated cell proliferation (Styles et al. 2021).
Also, CAC5426957.1 is associated with ‘nitrogen cycle metabolic process’ (GO:0071941). In algae Chlamydomonas reinhardtii, potassium channel KCN11, during nitrogen starvation condition, regulates osmolarity and helps in maintaining the cellular physiology. In absence of this channel, cell growth reduces (Xu and Pan 2020).
Involvement in biological process of ‘gluconeogenesis’ (GO:0006094) was predicted for the protein CAC5428594.1. Potassium channels such as KATP and Kir6.2 are involved in glucose metabolism and uptake (Miki et al. 2002). KATP channels are involved in limiting gluconeogenesis in liver (Pocai et al. 2005). CAC5428594.1 (Group 4) was also predicted to be associated with ‘leukotriene metabolic process’ (GO:0006691). Leukotrienes are inflammatory mediators and large conductance calcium-gated and voltage-gated potassium channels are reported to be activated by the action of leukotriene LTB4 (Bukiya et al. 2014). This channel is also linked to ‘response to oxidative stress’ (GO:0006979). Voltage-gated potassium channels undergo oxidative alteration and are regulated by reactive oxygen species (ROS) (Sahoo et al. 2014).
CAC5428594.1 and AYU77301.1 are related to ‘cell motility’ (GO:0048870). Various potassium channels are important mediators of cellular motility by cilia, flagella or by amoeboid movement (Schwab et al. 2008). The biological process of ‘neurotransmitter secretion’ (GO:0007269) was predicted for AYU77301.1. Various potassium channels are involved in neurotransmitter release (Wang 2008). AYU77301.1 is also associated with ‘proteolysis’ (GO:0006508). In Paramecium, a hyperpolarization- and calcium-dependent potassium channel is activated by proteolysis of a cytoplasmic inhibitory domain(Kubalski et al. 1989). Involvement of AYU77301.1 is also seen in ‘carbohydrate catabolic process’ (GO:0016052). The role of KATP channels have been reported in metabolism of carbohydrates (Murase et al. 2019).
In line with the conventional functions, the proteins- CAC5426958.1, TPP54501.1 and TPP49235.1 (Group 1), TPP49234.1 (Group 2) and XP_003860351.1 and TPP45562.1 (Group 3) are associated with ‘potassium channel activity’ (GO:0005267). Also, molecular function of ‘calcium-activated cation channel activity’ (GO:0005227) is associated with Group 1 (CAC5426958.1, TPP54501.1 and TPP49235.1), Group 2 (TPP49234.1) and Group 3 (XP_003860351.1 and TPP45562.1) channels. Group 2 (CAC5426957.1, XP_003857895.1, AYU75595.1 and TPP54502.1) was associated with calcium-activated potassium channel activity (GO:0015269). This is in addition to other similar GOMF terms such as ‘ion gated channel activity’ (GO:0022839), ion binding (GO:0043167), inorganic cation transmembrane transporter activity (GO:0022890) and ion transmembrane transporter activity (GO:0015075). Other associated GOMF terms are summarized in Fig. 5.
Fig. 5.
Summary of the unconventional molecular functions associated with the potassium channel sequences from L. donovani. Each box colour shows each group the protein belongs to. The accession ID of the proteins and their three-dimensional model are mentioned in the boxes
Putative potassium channel XP_003857896.1 may have ‘RNA binding’ (GO:0003723) function. The Cold-inducible RNA-binding protein was reported to help in translation process of KCND2 and KCND3 potassium channels and modulate transient outward potassium current in cardiac cells (Li et al. 2015). Association of these channels with GOMF terms such as adenyl ribonucleotide binding (GO:0032559), purine ribonucleoside triphosphate binding (GO:0035639) points towards the involvement of purine and pyrimidine nucleotides in modulating potassium channel function. The nucleoside triphosphate and diphosphate levels also control potassium channel activity (Beech et al. 1993; Lazdunski 1994; Reinhardt et al. 2002).
AYU77300.1 and XP_003859425.1 (Group 4) channels were predicted to be associated with ‘ATPase-coupled ion transmembrane transporter activity’ (GO:0042625) which indicates that they could also function as ion pumps like H+-K+-ATPases and Na+-K+-ATPases (Morth et al. 2011).
It is noteworthy that TPP53495.1 is associated with ‘catalytic activity’ (GO:0003824). The β-subunit of voltage-gated potassium channels (Kvβ) have been reported to perform the catalytic activity of aldo–keto reductases. They carry out NADPH-dependent reduction of aldehyde and ketones into alcohols and modulate function and localization of Kv1 and Kv4. They also participate in redox regulation (Tipparaju et al. 2008; Dwenger et al. 2018; Raph et al. 2019).
The protein CAC5428594.1 is associated with ‘magnesium ion binding’ (GO:0000287). Magnesium ions have been reported to bind with ether-à-go-go (eag) potassium channels and modulate its activation (Terlau et al. 1996; Silverman et al. 2000). This protein also is predicted to have ‘iron ion binding’ (GO:0005506). In KATP channels, regulation takes place by binding of heme at a cytoplasmic heme-binding ‘CXXHX16H’ domain (Burton et al. 2016).
TPP53494.1 may have a role in ‘vitamin binding’ (GO:0019842). Vitamin D (Calcitriol) regulates the activity of a large conductance calcium-activated potassium channel which is localized in the inner mitochondrial membrane (Olszewska et al. 2022). Involvement of vitamin C is also implicated in activation of a mitochondrial KATP channel and subsequently in cardio-protection (Hao et al. 2016). CAC5428595.1 could be associated with ‘DNA-binding transcription factor activity’ (GO:0001071). It has been studied that, changes in intracellular potassium ion concentration can affect the ability of DNA binding of transcription factors which may activate expression of apoptotic genes in neuronal cells (Yang et al. 2006).
In this analysis of the putative potassium channels from Leishmania donovani, we could see that they may have diverse characteristics. They are associated with a wide range of biological processes and molecular functions. Functional characterization of these individual channels will unveil new chapters in Leishmania biology.
Way forward
This study has focused on bioinformatic analyses of the identified putative potassium channels. These transporters have been predicted to associate with diverse roles as discussed in Sect. "Discussion" (Figs. 4, 5). In Leishmania and other parasitic protozoa, there are very few studies on these proteins. The knowledge of how these potassium channels work and what cellular functions they participate in, would give us a new perspective of on L. donovani. If these potassium channels are demonstrated to be vital for the parasite’s survival or disease pathogenesis, these proteins can also be studied as drug targets, vaccine candidates or biomarkers for diagnosis. Specific potassium channel modulators can be developed to target these ion channels and act as anti-leishmanial compounds.
In the present scenario, visceral leishmaniasis is being aimed for elimination and on the other hand it is cooccurring with other infectious diseases such as COVID-19 (Paul and Singh 2022). Anti-leishmanials are associated with drug resistance and in some cases are facilitated by transporter molecules (Leandro 2003). Functional insights into the roles of these proteins may be important in understanding and developing therapeutic strategies that can circumvent drug resistance (Gezelle et al. 2021). It is paramount to identify uncharacterized proteins such as potassium channels to fully understand this disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the SERB grant [EMR/2016/ 005152] provided by the Government of India. We thank the Director, NIPER, S.A.S. Nagar, India for research infrastructure. Anindita Paul, Shubham Sunil Chumbale, Anjana Lakra, Vijay Kumar, Dhanashri Sudam Alhat were supported by NIPER fellowship.
Author contributions
Conceptualization, formal analysis: AP, SS. Data curation, investigation, methodology: AP, SSC, AL, VK, DSA. Funding acquisition, project administration, resources, supervision: SS. roles/writing—original draft; writing—review & editing: AP, SS.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Accession number
Not Applicable.
References
- Alaimo A, Villarroel A (2018) Calmodulin: A Multitasking Protein in Kv7.2 Potassium Channel Functions. Biomolecules 8:57. 10.3390/biom8030057 [DOI] [PMC free article] [PubMed]
- Almagro Armenteros JJ, Sønderby CK, Sønderby SK, et al. DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics. 2017;33:3387–3395. doi: 10.1093/bioinformatics/btx431. [DOI] [PubMed] [Google Scholar]
- Altier C, Zamponi GW. Signaling complexes of voltage-gated calcium channels and G protein-coupled receptors. J Recept Signal Trans. 2008;28:71–81. doi: 10.1080/10799890801941947. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anderson D, Mehaffey WH, Iftinca M, et al. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. doi: 10.1038/nn.2493. [DOI] [PubMed] [Google Scholar]
- Anderson D, Rehak R, Hameed S, et al. Regulation of the K V 4.2 complex by Ca V 3.1 calcium channels. Channels. 2010;4:163–167. doi: 10.4161/chan.4.3.11955. [DOI] [PubMed] [Google Scholar]
- Antollini SS, Barrantes FJ (2016) Fatty acid regulation of voltage- and ligand-gated ion channel function. Front Physiol 7:. 10.3389/fphys.2016.00573 [DOI] [PMC free article] [PubMed]
- Bachmann M, Li W, Edwards MJ, et al (2020) Voltage-gated potassium channels as regulators of cell death. Front Cell Dev Biol 8:611853. 10.3389/fcell.2020.611853 [DOI] [PMC free article] [PubMed]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans. Genome. 1998;282:6. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Barrera P, Skorka C, Boktor M, et al. A novel calcium-activated potassium channel controls membrane potential and intracellular pH in Trypanosoma cruzi. Front Cell Infect Microbiol. 2020;9:464. doi: 10.3389/fcimb.2019.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagle SD, Lockless SW. Unappreciated roles for K+ channels in bacterial physiology. Trends Microbiol. 2021;29:942–950. doi: 10.1016/j.tim.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodył A, Mackiewicz P, Milanowski R. Did trypanosomatid parasites contain a eukaryotic alga-derived plastid in their evolutionary past? J Parasitol. 2010;96:465–475. doi: 10.1645/GE-1810.1. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, McMillan J, Liu J, et al. Activation of calcium- and voltage-gated potassium channels of large conductance by leukotriene B4. J Biol Chem. 2014;289:35314–35325. doi: 10.1074/jbc.M114.577825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg ED, Remillard CV, Yuan JX-J. K+ channels in apoptosis. J Membrane Biol. 2006;209:3–20. doi: 10.1007/s00232-005-0838-4. [DOI] [PubMed] [Google Scholar]
- Burton MJ, Kapetanaki SM, Chernova T, et al. A heme-binding domain controls regulation of ATP-dependent potassium channels. Proc Natl Acad Sci USA. 2016;113:3785–3790. doi: 10.1073/pnas.1600211113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Aranda K, Seo Y, et al. TMEM175 is an organelle K+ channel regulating lysosomal function. Cell. 2015;162:1101–1112. doi: 10.1016/j.cell.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zhong XZ, Zou Y, et al. BK channels alleviate lysosomal storage diseases by providing positive feedback regulation of lysosomal Ca2+ release. Dev Cell. 2015;33:427–441. doi: 10.1016/j.devcel.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Capera J, Serrano-Novillo C, Navarro-Pérez M, et al (2019) The potassium channel odyssey: mechanisms of traffic and membrane arrangement. Int J Mol Sci 20:. 10.3390/ijms20030734 [DOI] [PMC free article] [PubMed]
- Checchetto V, Teardo E, Carraretto L, et al (2016) Physiology of intracellular potassium channels: A unifying role as mediators of counterion fluxes? Biochimica et Biophysica Acta (BBA)—Bioenergetics 1857:1258–1266. 10.1016/j.bbabio.2016.03.011 [DOI] [PubMed]
- Choe S. Potassium channel structures. Nat Rev Neurosci. 2002;3:115–121. doi: 10.1038/nrn727. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, et al. Molecular diversity of K+ Channels. Annals NY Acad Sci. 1999;868:233–255. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Colovos C, Yeates TO. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 1993;2:1511–1519. doi: 10.1002/pro.5560020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabravolski SA, Isayenkov SV. New insights into plant TPK ion channel evolution. Plants. 2021;10:2328. doi: 10.3390/plants10112328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, et al. The structure of the potassium channel: molecular basis of K + conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Dwenger MM, Ohanyan V, Navedo MF, Nystoriak MA (2018) Coronary microvascular Kv1 channels as regulatory sensors of intracellular pyridine nucleotide redox potential. Microcirculation 25:e12426. 10.1111/micc.12426 [DOI] [PMC free article] [PubMed]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, et al. Pfam: the protein families database. Nucl Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov RV, Bagati A, Casino B, Singh S. Potassium channels in Drosophila : historical breakthroughs, significance, and perspectives. J Neurogenet. 2012;26:275–290. doi: 10.3109/01677063.2012.744990. [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, et al., editors. The proteomics protocols handbook. Totowa, NJ: Humana Press; 2005. pp. 571–607. [Google Scholar]
- Geourjon C, Deléage G. SOPMA: significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput Appl Biosci. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- Gezelle J, Saggu G, Desai SA. Promises and pitfalls of parasite patch-clamp. Trends Parasitol. 2021;37:414–429. doi: 10.1016/j.pt.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe A, Schröter KH, Ruppersberg JP, et al. Cloning and expression of a human voltage-gated potassium channel. A novel member of the RCK potassium channel family. EMBO J. 1990;9:1749–1756. doi: 10.1002/j.1460-2075.1990.tb08299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Adelman JP, et al. International union of pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- Hannaert V, Saavedra E, Duffieux F, et al. Plant-like traits associated with metabolism of Trypanosoma parasites. Proc Natl Acad Sci. 2003;100:1067–1071. doi: 10.1073/pnas.0335769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Li W-W, Du H, et al. Role of vitamin C in cardioprotection of ischemia/reperfusion injury by activation of mitochondrial KATP channel. Chem Pharm Bull. 2016;64:548–557. doi: 10.1248/cpb.c15-00693. [DOI] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Wissuwa B, Tian Y, et al. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca 2+ -dependent K + channels. Proc Natl Acad Sci USA. 2013;110:4816–4821. doi: 10.1073/pnas.1221997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou P, Kang PW, Kongmeneck AD, et al. Two-stage electro–mechanical coupling of a KV channel in voltage-dependent activation. Nat Commun. 2020;11:676. doi: 10.1038/s41467-020-14406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hover S, King B, Hall B, et al. Modulation of potassium channels inhibits bunyavirus infection. J Biol Chem. 2016;291:3411–3422. doi: 10.1074/jbc.M115.692673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez V, Henriquez M, Galanti N, Riquelme G. Electrophysiological characterization of potassium conductive pathways in Trypanosoma cruzi. J Cell Biochem. 2011;112:1093–1102. doi: 10.1002/jcb.23023. [DOI] [PubMed] [Google Scholar]
- Jinn S, Drolet RE, Cramer PE, et al. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation. Proc Natl Acad Sci USA. 2017;114:2389–2394. doi: 10.1073/pnas.1616332114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Aldrich RW, Chandy KG, et al. International Union of basic and clinical pharmacology. C. Nomenclature and properties of calcium-activated and sodium-activated potassium channels. Pharmacol Rev. 2017;69:1–11. doi: 10.1124/pr.116.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käll L, Krogh A, Sonnhammer ELL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer ELL. Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A, Iverson LE, Tanouye MA. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987;50:405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Khor BY, Tye GJ, Lim TS, Choong YS. General overview on structure prediction of twilight-zone proteins. Theor Biol Med Model. 2015;12:15. doi: 10.1186/s12976-015-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch GE, Pascual JM, Shieh CC. Functional role of a conserved aspartate in the external mouth of voltage-gated potassium channels. Biophys J. 1995;68:1804–1813. doi: 10.1016/S0006-3495(95)80357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev AN, Avrova NF, Vlasova YA, et al (2018) Evolutionary physiology and biochemistry—advances and perspectives. InTech
- Kshatri AS, Gonzalez-Hernandez A, Giraldez T (2018) Physiological roles and therapeutic potential of Ca2+ activated potassium channels in the nervous system. Front Mol Neurosci 11:. 10.3389/fnmol.2018.00258 [DOI] [PMC free article] [PubMed]
- Kuang Q, Purhonen P, Hebert H. Structure of potassium channels. Cell Mol Life Sci. 2015;72:3677–3693. doi: 10.1007/s00018-015-1948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalski A, Martinac B, Saimi Y. Proteolytic activation of a hyperpolarization- and calcium-dependent potassium channel in Paramecium. J Membrain Biol. 1989;112:91–96. doi: 10.1007/BF01871167. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. doi: 10.1107/S0021889892009944. [DOI] [Google Scholar]
- Lazdunski M. ATP-sensitive potassium channels: an overview. J Cardiovasc Pharmacol. 1994;24(Suppl 4):S1–5. [PubMed] [Google Scholar]
- Leandro C. Leishmaniasis: efflux pumps and chemoresistance. Int J Antimicrob Agents. 2003;22:352–357. doi: 10.1016/S0924-8579(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Li J, Xie D, Huang J, et al. Cold-inducible RNA-binding protein regulates cardiac repolarization by targeting transient outward potassium channels. Circ Res. 2015;116:1655–1659. doi: 10.1161/CIRCRESAHA.116.306287. [DOI] [PubMed] [Google Scholar]
- Li P, Gu M, Xu H. Lysosomal ion channels as decoders of cellular signals. Trends Biochem Sci. 2019;44:110–124. doi: 10.1016/j.tibs.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörinczi E, Helliwell M, Finch A, et al. Calmodulin regulates human ether à Go-Go 1 (hEAG1) potassium channels through interactions of the eag domain with the cyclic nucleotide binding homology domain. J Biol Chem. 2016;291:17907–17918. doi: 10.1074/jbc.M116.733576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/S0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- MacKinnon R, Cohen SL, Kuo A, et al. Structural conservation in prokaryotic and eukaryotic potassium channels. Science. 1998;280:106–109. doi: 10.1126/science.280.5360.106. [DOI] [PubMed] [Google Scholar]
- Maruyama S, Misawa K, Iseki M, et al. Origins of a cyanobacterial 6-phosphogluconate dehydrogenase in plastid-lacking eukaryotes. BMC Evol Biol. 2008;8:151. doi: 10.1186/1471-2148-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTague A, Nair U, Malhotra S, et al. Clinical and molecular characterization of KCNT1 -related severe early-onset epilepsy. Neurology. 2018;90:e55–e66. doi: 10.1212/WNL.0000000000004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Minami K, Zhang L, et al. ATP-sensitive potassium channels participate in glucose uptake in skeletal muscle and adipose tissue. Am J Physiol-Endocrinol Metab. 2002;283:E1178–E1184. doi: 10.1152/ajpendo.00313.2002. [DOI] [PubMed] [Google Scholar]
- Miller C. An overview of the potassium channel family. Genome Biol. 2000;1(reviews0004):1. doi: 10.1186/gb-2000-1-4-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molbaek K, Tejada M, Ricke CH, et al. Purification and initial characterization of Plasmodium falciparum K+ channels, PfKch1 and PfKch2 produced in Saccharomyces cerevisiae. Microb Cell Fact. 2020;19:183. doi: 10.1186/s12934-020-01437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morth JP, Pedersen BP, Buch-Pedersen MJ, et al. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat Rev Mol Cell Biol. 2011;12:60–70. doi: 10.1038/nrm3031. [DOI] [PubMed] [Google Scholar]
- Mosimann M, Goshima S, Wenzler T, et al. A Trk/HKT-Type K + Transporter from Trypanosoma brucei. Eukaryot Cell. 2010;9:539–546. doi: 10.1128/EC.00314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T, Kakizuka A, Takumi T, et al. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem Biophys Res Commun. 1989;161:176–181. doi: 10.1016/0006-291X(89)91577-5. [DOI] [PubMed] [Google Scholar]
- Murase M, Seino Y, Maekawa R, et al. Functional adenosine triphosphate-sensitive potassium channel is required in high-carbohydrate diet-induced increase in β-cell mass. J Diabetes Investig. 2019;10:238–250. doi: 10.1111/jdi.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musavizadeh Z, Najafi-Zarrini H, Kazemitabar SK, et al. Genome-wide analysis of potassium channel genes in rice: expression of the OsAKT and OsKAT genes under salt stress. Genes. 2021;12:784. doi: 10.3390/genes12050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Oh S, Paknejad N, Hite RK (2020) Gating and selectivity mechanisms for the lysosomal K+ channel TMEM175. eLife 9:e53430. 10.7554/eLife.53430 [DOI] [PMC free article] [PubMed]
- Olszewska AM, Sieradzan AK, Bednarczyk P, et al. Mitochondrial potassium channels: a novel calcitriol target. Cell Mol Biol Lett. 2022;27:3. doi: 10.1186/s11658-021-00299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Ahidouch A (2013) K+ channels and cell cycle progression in tumor cells. Front Physiol 4:. 10.3389/fphys.2013.00220 [DOI] [PMC free article] [PubMed]
- Palomo-Ligas L, Gutiérrez-Gutiérrez F, Ochoa-Maganda VY, et al (2019) Identification of a novel potassium channel (GiK) as a potential drug target in Giardia lamblia : Computational descriptions of binding sites. PeerJ 7:e6430. 10.7717/peerj.6430 [DOI] [PMC free article] [PubMed]
- Paul A, Mubashra, Singh S (2021) Identification of a novel calcium activated potassium channel from Leishmania donovani and in silico predictions of its antigenic features. Acta Tropica 220:105922. 10.1016/j.actatropica.2021.105922 [DOI] [PubMed]
- Paul A, Singh S (2020) Comparative genomics facilitates drug target selection and develops intervention strategies against leishmania infections. In: Singh S (ed) Metagenomic systems biology. Springer Singapore, Singapore, pp 75–93
- Paul A, Singh S (2022) Visceral leishmaniasis in the COVID-19 pandemic era. Transactions of The Royal Society of Tropical Medicine and Hygiene trac100. 10.1093/trstmh/trac100 [DOI] [PMC free article] [PubMed]
- Pocai A, Lam TKT, Gutierrez-Juarez R, et al. Hypothalamic KATP channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Polle JEW, Calhoun S, McKie-Krisberg Z, et al (2020) Genomic adaptations of the green alga Dunaliella salina to life under high salinity. Algal Research 50:101990. 10.1016/j.algal.2020.101990
- Prole DL, Marrion NV (2012) Identification of Putative Potassium Channel Homologues in Pathogenic Protozoa. PLoS ONE 7:e32264. 10.1371/journal.pone.0032264 [DOI] [PMC free article] [PubMed]
- Raph SM, Bhatnagar A, Nystoriak MA. Biochemical and physiological properties of K+ channel-associated AKR6A (Kvβ) proteins. Chem Biol Interact. 2019;305:21–27. doi: 10.1016/j.cbi.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt R, Manaenko A, Pissarek M, et al. Alterations of purine and pyrimidine nucleotide contents in rat corticoencephalic cell cultures following metabolic damage and treatment with openers and blockers of ATP-sensitive potassium channels. Neurochem Int. 2002;40:427–433. doi: 10.1016/S0197-0186(01)00102-4. [DOI] [PubMed] [Google Scholar]
- Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Maffie J, Amarillo Y, et al (2009) Voltage Gated Potassium Channels: Structure and Function of Kv1 to Kv9 Subfamilies. In: Encyclopedia of Neuroscience. Elsevier, pp 397–425
- Sahoo N, Hoshi T, Heinemann SH. Oxidative Modulation of Voltage-Gated Potassium Channels. Antioxid Redox Signal. 2014;21:933–952. doi: 10.1089/ars.2013.5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L, Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981;293:228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Scarpati M, Qi Y, Govind S, Singh S (2019) A combined computational strategy of sequence and structural analysis predicts the existence of a functional eicosanoid pathway in Drosophila melanogaster. PLoS ONE 14:e0211897. 10.1371/journal.pone.0211897 [DOI] [PMC free article] [PubMed]
- Schrempf H, Schmidt O, Kümmerlen R, et al. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 1995;14:5170–5178. doi: 10.1002/j.1460-2075.1995.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A, Hanley P, Fabian A, Stock C. Potassium channels keep mobile cells on the go. Physiology. 2008;23:212–220. doi: 10.1152/physiol.00003.2008. [DOI] [PubMed] [Google Scholar]
- Shealy RT, Murphy AD, Ramarathnam R, et al. Sequence-function analysis of the K+-selective family of ion channels using a comprehensive alignment and the KcsA channel structure. Biophys J. 2003;84:2929–2942. doi: 10.1016/S0006-3495(03)70020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, Tang C-Y, Mock AF, et al. Mg2+ modulates voltage-dependent activation in ether-à-Go-Go potassium channels by binding between transmembrane segments S2 and S3. J Gen Physiol. 2000;116:663–678. doi: 10.1085/jgp.116.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins: structure. Function, and Bioinformatics. 1993;17:355–362. doi: 10.1002/prot.340170404. [DOI] [PubMed] [Google Scholar]
- So I, Ashmole I, Davies NW, et al. The K+ channel signature sequence of murine Kir2.1: mutations that affect microscopic gating but not ionic selectivity. J Physiol. 2001;531:37–50. doi: 10.1111/j.1469-7793.2001.0037j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RH, Rees DC. The α-helix and the organization and gating of channels. Annu Rev Biophys Biomol Struct. 2002;31:207–233. doi: 10.1146/annurev.biophys.31.082901.134329. [DOI] [PubMed] [Google Scholar]
- Steinmann ME, González-Salgado A, Bütikofer P, et al. A heteromeric potassium channel involved in the modulation of the plasma membrane potential is essential for the survival of African trypanosomes. FASEB J. 2015;29:3228–3237. doi: 10.1096/fj.15-271353. [DOI] [PubMed] [Google Scholar]
- Steinmann ME, Schmidt RS, Bütikofer P, et al. TbIRK is a signature sequence free potassium channel from Trypanosoma brucei locating to acidocalcisomes. Sci Rep. 2017;7:656. doi: 10.1038/s41598-017-00752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles FL, Al-Owais MM, Scragg JL, et al (2021) Kv1.3 voltage-gated potassium channels link cellular respiration to proliferation through a non-conducting mechanism. Cell Death Dis 12:372. 10.1038/s41419-021-03627-6 [DOI] [PMC free article] [PubMed]
- Suh J, Hutter H (2012) A survey of putative secreted and transmembrane proteins encoded in the C. elegans genome. BMC Genomics 13:333. 10.1186/1471-2164-13-333 [DOI] [PMC free article] [PubMed]
- Sun G-L, Shen W, Wen J-F. Triosephosphate isomerase genes in two trophic modes of euglenoids (Euglenophyceae) and their phylogenetic analysis. J Eukaryot Microbiol. 2008;55:170–177. doi: 10.1111/j.1550-7408.2008.00324.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Xu M, Cao Q, et al. A lysosomal K+ channel regulates large particle phagocytosis by facilitating lysosome Ca2+ release. Sci Rep. 2020;10:1038. doi: 10.1038/s41598-020-57874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Ludwig J, Steffan R, et al. Extracellular Mg2+ regulates activation of rat eag potassium channel. Pflugers Arch - Eur J Physiol. 1996;432:301–312. doi: 10.1007/s004240050137. [DOI] [PubMed] [Google Scholar]
- Tipparaju SM, Barski OA, Srivastava S, Bhatnagar A. Catalytic mechanism and substrate specificity of the β-subunit of the voltage-gated potassium channel. Biochemistry. 2008;47:8840–8854. doi: 10.1021/bi800301b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi KEJ, Buttigliero LV, Altabe SG, Uttaro AD. Functional characterization of front-end desaturases from trypanosomatids depicts the first polyunsaturated fatty acid biosynthetic pathway from a parasitic protozoan. FEBS J. 2006;273:271–280. doi: 10.1111/j.1742-4658.2005.05049.x. [DOI] [PubMed] [Google Scholar]
- Turner RW, Anderson D, Zamponi GW. Signaling complexes of voltage-gated calcium channels. Channels. 2011;5:440–448. doi: 10.4161/chan.5.5.16473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrego D, Tomczak AP, Zahed F, et al. Potassium channels in cell cycle and cell proliferation. Phil Trans R Soc B. 2014;369:20130094. doi: 10.1098/rstb.2013.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varaldi J, Lepetit D. Deciphering the behaviour manipulation imposed by a virus on its parasitoid host: insights from a dual transcriptomic approach. Parasitology. 2018;145:1979–1989. doi: 10.1017/S0031182018000835. [DOI] [PubMed] [Google Scholar]
- Waller RF, McConville MJ, McFadden GI. More plastids in human parasites? Trends Parasitol. 2004;20:54–57. doi: 10.1016/j.pt.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Wang Z-W, editor. Molecular mechanisms of neurotransmitter release. Totowa, NJ: Humana Press; 2008. [Google Scholar]
- Wei A, Jegla T, Salkoff L. Eight potassium channel families revealed by the C. elegans genome project. Neuropharmacology. 1996;35:805–829. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- Wei AD, Gutman GA, Aldrich R, et al. International union of pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- Wiederstein M, Sippl MJ. ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Pan J. Potassium channel KCN11 is required for maintaining cellular osmolarity during nitrogen starvation to control proper cell physiology and TAG accumulation in Chlamydomonas reinhardtii. Biotechnol Biofuels. 2020;13:129. doi: 10.1186/s13068-020-01769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 2015;43:W174–W181. doi: 10.1093/nar/gkv342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Yan D, Wang Y. K+ regulates DNA binding of transcription factors to control gene expression related to neuronal apoptosis. NeuroReport. 2006;17:1199–1204. doi: 10.1097/01.wnr.0000224. [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang Y (2015b) Protein structure and function prediction using I-TASSER. Curr Protoc Bioinformatics 52:5.8.1–5.815. 10.1002/0471250953.bi0508s52 [DOI] [PMC free article] [PubMed]
- Yap KL, Kim J, Truong K, et al. Calmodulin target database. J Struct Funct Genomics. 2000;1:8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]
- Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- Yus-Nájera E, Santana-Castro I, Villarroel A. The identification and characterization of a noncontinuous calmodulin-binding site in non inactivating voltage-dependent KCNQ potassium channels. J Biol Chem. 2002;277:28545–28553. doi: 10.1074/jbc.M204130200. [DOI] [PubMed] [Google Scholar]
- Ziechner U, Malesevic M (2006) Inhibition of human ether à go-go potassium channels by Ca2+/calmodulin binding to the cytosolic N- and C-termini. FEBS Journal 13 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.