Abstract
Immunotherapy emerged as a paradigm shift in cancer treatments, which can effectively inhibit cancer progression by activating the immune system. Remarkable clinical outcomes have been achieved through recent advances in cancer immunotherapy, including checkpoint blockades, adoptive cellular therapy, cancer vaccine, and tumor microenvironment modulation. However, extending the application of immunotherapy in cancer patients has been limited by the low response rate and side effects such as autoimmune toxicities. With great progress being made in nanotechnology, nanomedicine has been exploited to overcome biological barriers for drug delivery. Given the spatiotemporal control, light-responsive nanomedicine is of great interest in designing precise modality for cancer immunotherapy. Herein, we summarized current research utilizing light-responsive nanoplatforms to enhance checkpoint blockade immunotherapy, facilitate targeted delivery of cancer vaccines, activate immune cell functions, and modulate tumor microenvironment. The clinical translation potential of those designs is highlighted and challenges for the next breakthrough in cancer immunotherapy are discussed.
Key words: Light-responsive nanoparticles, Photodynamic therapy, Photothermal therapy, Nanomedicine, Cancer therapy, Immunotherapy, Photopharmacology, Light-triggered drug release
Graphical abstract
This review provides an overview of recent research utilizing light-responsive nanomedicine to enhance checkpoint blockade immunotherapy, activate adoptive cellular therapy, facilitate precise delivery of cancer vaccines, and modulate tumor microenvironment.

1. Introduction
Great progress has been made in the fight against cancer over the past decades. Hailed as a revolution in the treatment of cancer, immunotherapy attempts to boost the body's immune system to destroy tumors with enhanced efficacy and fewer side effects than conventional cancer therapies, such as chemotherapy and radiotherapy1, 2, 3. In 2018, pioneering cancer immunotherapy studies were recognized with a Nobel Prize in Medicine or Physiology for achieving effective and long-lasting therapeutic responses for numerous patients. Meanwhile, multiple immunotherapy drugs have been approved by the US Food and Drug Administration (FDA) and used in cancer patients as first-line treatments. To date, there are more than 20 types of immunotherapy drugs in the market4. However, most of them were originally developed for hematological cancers and limited options are available for solid tumors5. Accumulating evidence has pinpointed tumor microenvironment in solid tumors as one of the major obstacles against cancer immunotherapy, posing barriers to the delivery of therapeutic agents6.
In recent years, nanomedicine has been developed to overcome the limitations of anti-cancer drugs and regulate tumor microenvironment. By exploiting the leaky vasculature, nano-sized particles can transport cargos across biological barriers and specifically accumulate in tumor area based on the well-known enhanced permeation and retention (EPR) effect7. In particular, tremendous efforts have been made in developing advanced nano-platforms for immunotherapy including liposomes8, polymeric nanoparticles9, nanoscale metal-organic frameworks (nMOFs)10 and cell-derived exosomes11. For decades it has been thought that nanomedicine can achieve tumor targeting based on the EPR effect. However, vascularization and blood flow are not homogenous across tumors, resulting in non-uniform EPR effect and leading to uneven drug distribution within the tumors12,13. To further enhance the targeting effect, active targeting strategies have been exploited taking advantage of the ligand–receptor interaction to increase the accumulation of nanoparticles in tumors. However, it cannot provide precise control of drug release or drug activation in most cases14. Therefore, efficient targeting delivery strategies with a precise control are needed.
Biomaterials with stimuli-responsiveness have been considered as one of the most promising tools for advanced drug delivery. Stimuli-responsive materials are sensitive to certain triggers, including temperature, light, electrical or magnetic fields, and chemicals, and undergo conformational and chemical changes15. Nano drug delivery systems made of stimuli-responsive materials are capable of releasing drugs on receiving certain signals and realising precise targeting delivery. Among the currently applied stimuli, light is a safe source for controlled drug delivery with its unparalleled advantages, such as high precision and minimal invasiveness16,17. The function of light-controlled drug delivery systems such as drug release can be achieved in a precise and spatiotemporal way by adjusting the irradiance, time and position of light source. Some light-controlled therapies have already been approved for clinical use. For example, photodynamic therapy (PDT) is used for the management of a variety of cancers and benign diseases by selectively destroying unwanted cells in a minimally invasive manner15; while photothermal therapy (PTT) is applied to convert the light energy to heat that leads to thermal burns on tumors18. More importantly, light-responsive nanomedicine provides unique opportunities for precise spatiotemporal control of delivery process, which is of great significance in improving the efficacy of cancer immunotherapy and minimising “off-target” delivery.
As a potential modality, light is of great interest in filling the gap in current cancer immunotherapy modalities, such as off-target effects, immunosuppressive tumor microenvironment and limited T cell filtration. Light-controlled nanosystems have been developed for the delivery of immunotherapeutic agents selectively in tumor area. Additionally, PDT or PTT-induced cell death was reported to enhance antigen presentation synergy, resulting in a systemic antitumor immune response to control residual tumor cells at the treatment site and distant metastases19. In this review, we provide an overview of the most widely used types of cancer immunotherapy, their mechanisms and clinical status. Four widely used immunotherapies combined with light-responsive nanomedicine will be highlighted (Fig. 1). Further, light-controlled nanoplatforms for improving the efficacy of immunotherapeutics and PDT/PTT-related immunotherapy based on nanomedicine are summarized and discussed, with particular focus on recent advanced designs in the field. Lastly, we provide insights into photoresponsive nanomedicine development for cancer immunotherapy and the challenges for their clinical translation.
Figure 1.
Light-responsive strategies for cancer immunotherapy.
2. Major types of immunotherapies for cancer
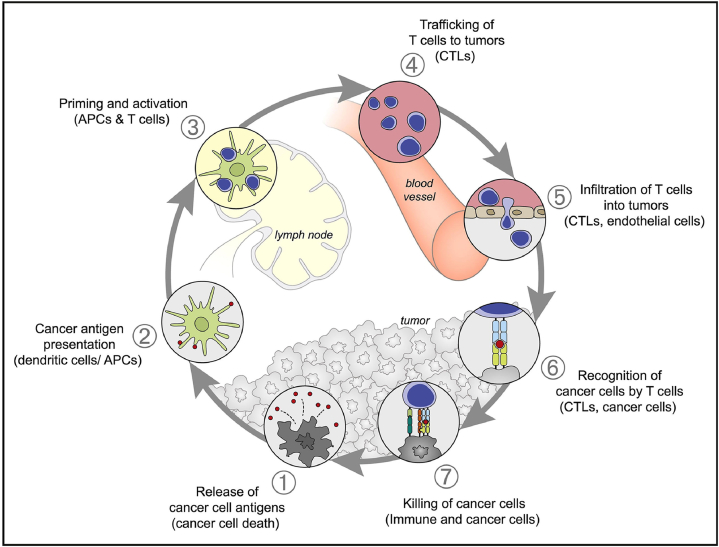
To exploit anticancer immune responses, a 7-step cancer-immunity cycle needs to be fully elucidated (Fig. 2)20. The cancer-immunity cycle starts with neoantigen capture (1), in which antigen-presenting cells (APCs) (such as dendritic cells) recognize the antigens created and released during oncogenesis and capture them for later processing. APCs then present the captured antigens on major histocompatibility complex class I (MHCI) and II (MHCII) molecules to naïve T cells (2), to activate effector T cell responses against such cancer-specific antigens (3). Next, the activated effector T cells (cytotoxic T cells) circulate to tumor area (4) and infiltrate into tumors (5). They then identify and bind to tumor cells via specific interaction between the T cell receptor and its cognate antigen (6), leading to cytotoxic effects on the target cancer cells (7). Tumor-associated neoantigens release upon cancer cell death, which then initiate the cancer-immunity cycle again. However, cancer cells often create an immunosuppressive tumor microenvironment to interfere the cancer-immunity cycle, either by ‘masking’ the tumor-associated antigens, or hindering the infiltration of T cells, or suppressing effector T cell activities21.
Figure 2.
A schematic illustration of the seven major steps involved in the generation of an immune response against cancer. APCs, antigen-presenting cells; CTLs, cytotoxic T lymphocytes. Reprinted with permission from Ref. 20. Copyright © 2013 Elsevier.
To restore the cancer-immunity cycle in cancer patients, a number of evolutionary immunotherapy modalities have been developed, including cytokines, checkpoint blockades, immune system modulators, oncolytic virus therapy, adoptive cellular therapy and cancer vaccine. This review focuses on the most widely used types, namely, checkpoint blockades, adoptive cellular therapy, cancer vaccine, and tumor microenvironment modulation.
2.1. Checkpoint blockades
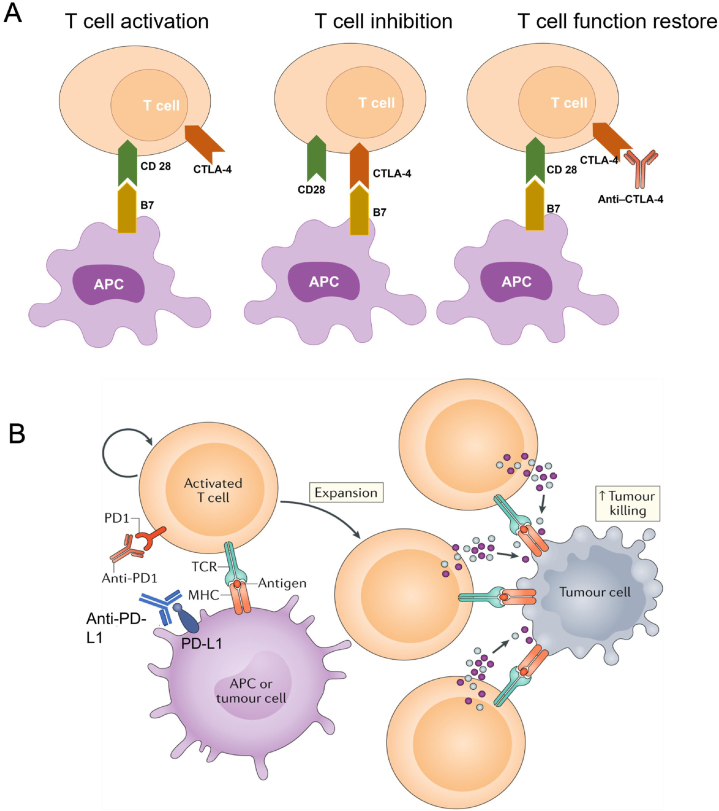
Checkpoint blockades have been predominantly applied in clinical practice, with cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 (PD1) being the most potent examples of T cell immune checkpoint molecules. CTLA-4 has structural and biochemical similarities to cell surface protein cluster of differentiation 28 (CD28), but greater affinity than CD28 to its ligand B722. The up-regulated CTLA-4 on activated T cells competes with CD28 for B7 ligation, and therefore inhibits further T cell activation. Inhibition of CTLA-4 on T cell surface frees B7 on APCs and restores its ligation with CD28. Therefore, T cell responses to tumor-associated neoantigens can be enhanced, leading to the priming and activation of effector T cells (Fig. 3A)23. Ipilimumab, a monoclonal antibody against CTLA-4, has been used in clinic for melanoma treatment since 201124. Another CTLA-4 blockade tremelimumab is still seeking FDA's approval25. Meanwhile, antibodies against PD1 on T cells and its ligand (PD-L1) on tumor cells have been used in cancer patients with multiple formulations in the market since 201425. Both PD1 and PD-L1 are highly targetable. Inhibition of the PD1/PD-L1 axis reverses the immune checkpoint and releases the brake on T cells by enhancing their effector functions and the formation of memory cells (Fig. 3B)23. As a result, PD1 and PD-L1 blockade can enhance immunogenic killing effects in tumors and limit metastasis26.
Figure 3.
Mechanisms of CTLA-4 and PD1/PD-L1 blockades. (A) CTLA-4 competes with CD28 to bind to B7, inhibiting T-cell function. Anti-CTLA-4 antibodies block CTLA-4 and B7 ligation and prevent inhibition of T cell function. (B) Programmed cell death 1 (PD1) on activated T cells binds to its ligand (PD-L1) on the antigen-presenting cell (APC) or tumor cell surface to inhibit T cell activation. Blocking of the PD1/PD-L1 axis via anti-PD1 or anti-PD-L1 antibody prevents this inhibitory interaction and increases T cell activation and proliferation, enhancing tumor killing effect eventually. Reprinted with permission from Ref. 26. Copyright © 2020 Springer Nature Limited.
Though CTLA-4 and PD1/PD-L1 blockades are the only types that are FDA-approved and currently used in clinic, the discovery of new immune checkpoint molecules continuously inspires the development of corresponding checkpoint blockades. Lymphocyte-activation gene 3 (LAG3) is one of the most promising targets. It has a similar structure to cell surface protein cluster of differentiation 4 (CD4) and can competitively bind to MHC-II, α-synuclein fibrils and lectins galectin-327. LAG3 inhibits the activation of its host cells, such as activated T cells and regulatory T cells, contributing to suppressive immune responses28. Promising results of using LAG3 blockade alone or in combination with PD1 blockades have been demonstrated in animal models and clinical trials in recent years29. However, mechanisms involved in LAG3-related inhibition of immune cell activation need to be fully elucidated to promote the clinical application of LAG3 blockades.
In addition, transmembrane proteins B7-H3 and H4 have been identified as important immune suppressors. They were found to be overexpressed in various solid tumors and immune cells, and involved in suppressing T cell activation, proliferation, and cytokine secretion30. Antibodies targeting B7-H3 and H4, namely FPA150 and MGC018, are currently under investigation in clinical trials31,32. Despite the satisfactory anti-tumor results achieved, severe adverse effects, such as lymphopenia and hypertension, have been reported33, 34, 35. Moreover, studies focusing on innate immune checkpoint on myeloid cells have demonstrated promising clinical outcomes with mild levels of toxicity. Anti-CD47 antibody is one of the most successful examples. By blocking the interaction between CD47 on tumor cells and the inhibitory receptor SIRPα on myeloid cells (macrophages, red blood cells, etc.), anti-CD47 antibody can promote the macrophages-induced destruction of cancer cells and tumor-specific cytotoxic T cell responses36,37. Encouraging clinical responses have been reported in trials of combinational use of CD20 antibody rituximab and CD47–SIRPα inhibition38.
Another immunoregulatory protein TIGIT (also called WUCAM, Vstm3, VSIG9) is a hot immunotherapy target. As a receptor of Ig superfamily, TIGIT plays an important role in regulating adaptive and innate immunity39,40. In solid tumors, TIGIT is co-expressed with other inhibitory receptors like PD1 on TILs and tumor antigen-specific CD8+ T cells41. TIGIT is also highly expressed by regulatory T cells (Tregs) in peripheral blood mononuclear cells of cancer patients and further upregulated in the tumor microenvironment. The combination of TIGIT with other immune checkpoint inhibitors represents as a promising treatment tactic42. In one clinical trial, dual PD-L1/TIGIT blockade (atezolizumab/tiragolumab) exhibits superior clinical outcomes in comparison with PD-L1 blockade alone for patients with PD-L1-positive non-small cell lung cancers43.
Checkpoint blockades immunotherapy is now a clinical reality and remarkable successes have been achieved. However, blocking a central immune checkpoint may lead to severe immune-related adverse effects, such as autoimmune toxicities44. A major challenge lies in safely engaging these checkpoint blockades at the right time and place. Therefore, precise spatiotemporal delivery, such as light-controlled delivery, is of great need to expand the application of checkpoint blockade immunotherapy.
2.2. Adoptive cellular therapy
Adoptive cellular therapy by infusing autologous or allogeneic T cells into patients, is another potential alternative to modulate the immune system in cancer patients. Presently, adoptive cellular therapies can be categorized into three types, tumor-infiltrating lymphocytes (TILs), T cell receptor (TCR)-engineered T cells and chimeric antigen receptor T (CAR-T) cells. TIL therapy was initially introduced in 1986 for metastatic melanomas, using ex vivo expanded autologous TILs with interleukin 2 (IL-2) following transferring back into patients45. TIL therapy has consistently yielded durable clinical responses in patients with metastatic melanoma, but not yet in other solid tumors46. Furthermore, TCR-based therapy is a potent anti-tumor treatment in various cancer types, in which tumor antigen recognition is achieved by the introduction of a novel T cell receptor into T cells. Promising outcomes were shown in TCR-based therapy clinical trials, using targeting antigen NY-ESO-1 for the treatment of solid tumors, such as neuroblastoma and sarcomas47,48. More recently, the success of preclinical and clinical trials has brought CAR-T cell therapy into the spotlight. CAR-T cell therapy starts with harvesting T cells from patients, followed by engineering them with chimeric antigen receptors that can bypass MHC restriction and directly target tumor cells. Thereafter, the resulting CAR-T cells are infused back to the body for activation of immune response49. As a revolutionary paradigm, 5 CAR-T cell therapies have been approved by FDA since 2017 with many more in clinical trials50.
Despite that CAR-T therapy has displayed good effectiveness in hematological tumor treatment, the effectiveness to treat solid tumors is still lacking due to limited infiltration of T cells51. The same problems occur in CAR-NK therapy, which refers to applying CAR strategy to NK cells based on the broad cytotoxicity and rapid killing ability of NK cells52. Tumor-associated macrophages (TAMs) can infiltrate solid tumors with a high infiltration rate and then interact with other cells within the tumor microenvironment including tumors cells and a variety of immune cells like T cells, NK cells, DCs, etc. Therefore, endeavours are made to modify macrophages with CAR against solid tumor53. CAR-M therapy refers to the edit of designed specific CAR gene (improve phagocytic activity and antigen presentation) into patient derived-macrophages to equip them with the capability to bind to the tumor cell surface via specific antigen identification, subsequently activating macrophage activity against tumor cells54. Currently, two clinical trials based on the CAR-M strategy have been approved by the FDA while considerations need to be addressed like fitness between CAR structure and macrophages55, safety issues, effectiveness in human body, etc. Combination of CAR-M therapy with other immunotherapies is also a potential attempt.
Although adoptive cellular therapies have produced remarkably effective responses, limited availability of specific antigens significantly hinders the application of adoptive cellular therapies in more intractable cancer types. In addition, as the antigens explored up until now are not solely expressed by tumors, the associated toxicities can be life-threatening in some cases due to systemic cytokine release and severe immune cell cross-activation56. Other challenges to promote adoptive cellular therapies are the immunosuppressive microenvironment and the limited immune cell infiltration in solid tumors. Therefore, it is necessary to develop delivery technologies that can enhance the transport of engineered immune cells to target sites, to minimise the toxicities of adoptive cellular therapies while enhancing the efficacy in solid tumors.
2.3. Cancer vaccine
The other intriguing strategy to defeat cancers is to elicit a specific immune response against tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) by using cancer vaccines. Most of cancer vaccines are developed based on the activation of APCs and the consequent stimulation of cytotoxic T cell-mediated immune response57. In theory, patients who received vaccination could mount an immune response towards tumor cells to keep them under constant restraint and eventually eliminate tumor cells, delay tumor recurrence, and prolong survival. Several types of techniques have been developed to compensate weak innate immune response against TSAs and TAAs, including dendritic cell-based vaccine58, protein/peptide vaccines59, and nucleic acid vaccines (DNA and RNA)60. Among them, nucleic acid vaccines have been particularly promising. Upon the delivery of DNA/RNA to APCs, the information they carry are then translated to induce specific antigen expression, which triggers T cell activation. To date, numerous clinical trials have demonstrated promising therapeutic results with cancer vaccines61. Besides vaccines for virus-related cancers (i.e., HPV vaccine), two cancer vaccines have been approved by FDA62. Screening neoantigens that can elicit strong antitumor responses still face great challenges. Vaccine development for the neoantigens requires a considerable amount of time and financial support.
At present, limited delivery platforms are available for transporting nucleic acids across cellular and nuclear barriers. Furthermore, delivering RNA-based vaccines is particularly challenging due to their poor cellular internalisation and low stability towards nucleases. Advanced delivery systems which can protect nucleic acid vaccines from degradation and effectively transport them to target tissues and cells would greatly benefit further clinical application of nucleic acid vaccines.
2.4. Tumor microenvironment modulation
Tumor microenvironment is a milieu of tumor cells, immune cells, stroma cells, extracellular matrix, cytokines, and other signalling molecules. The components work individually and in combination to influence the immunogenicity of the body. Numerous studies have reported that tumor microenvironment is responsible for limited T cell filtration, reduced activity of TILs and the down-regulated expression of immune checkpoint molecules63, 64, 65. Therefore, modulating the immunosuppressive tumor microenvironment is essential to produce robust systemic immune responses and improve the efficacy of other immunotherapies, such as adoptive cellular therapy and checkpoint blockade.
Cytokines are important immune regulators that have pleiotropic effects in tumor microenvironment. Each cytokine controls different types of the immune cell to either support tumor growth or trigger anti-tumor responses. Cytokines, including interferon, interleukin, and granulocyte-macrophage colony-stimulating factor (GM-CSF), have been clinically used as mono-immunotherapy or combinational therapy with other treatments66. Interferon-γ has been approved for several types of cancer, as it plays a vital role in the maturation of dendritic cells (DCs) and activation of effector T cells67. Besides, interleukins promote innate and adaptive immune responses via the activation of CD4+ T cells and CD8+ T cells66, while GM-CSF promotes the expansion and activation of myeloid cells such as DCs and macrophages.
An alternative strategy to restore the immune surveillance in tumor microenvironment is by targeting immunosuppressive cells such as TAMs and myeloid-derived suppressor cells (MDSCs). Recent efforts have been directed to the inhibition of colony-stimulating factor 1 receptor (CSF1R) and Janus kinase (JAK)-signal transducer that are expressed on TAMs and MDSCs contributing to the immunosuppressive environment68,69. Several inhibitors and antibodies have been developed with encouraging therapeutic effects in animal models while clinical trials in cancer patients are still ongoing68,70,71.
Besides, the physiochemical properties such as acidity, hypoxia, abnormal vasculature, rigid extracellular matrix, and irregular enzyme level of tumor microenvironment also contribute to immunosuppressive conditions and hinder drug delivery72. Diverse strategies of nanomedicine have been exploited to overcome the barrier by regulating tumor microenvironment73. Employing light to deliver modulators is a potential modality to realize the spatiotemporal control of drug release with less off-target effect.
Although cytokines and inhibitors can effectively modulate the immunosuppressive tumor microenvironment, their side effects are not negligible in clinical use. Due to the short half-life of cytokines, repetitive doses are required for satisfactory therapeutic efficacy, which may lead to cytokine release syndrome66. Additionally, immune regulators such as CSF1R and JAK are involved in normal cell functioning, their inhibition could influence normal cells and lead to severe side effects68,74. Therefore, delivery systems that can realise precise tumor targeting with a high drug loading capacity are needed to fully validate the clinical value of tumor microenvironment modulators.
3. Light-responsive nanomedicine for immunotherapy
Although the immunotherapies described above have demonstrated promising results, challenges remain. Clinical translations of immunotherapies face multiple delivery barriers, either cellular or microenvironmental. The success of checkpoint blockades relies on their interaction with the right target proteins at desirable time. The application of adoptive cellular therapy is hindered by limited T cell infiltration in tumor microenvironment, while cytokines and cancer vaccines require systems to protect them from degradation. Further, a major challenge of cancer immunotherapy is the potential immune cell cross-activation, leading to autoimmunity and severe toxicity. Therefore, it is essential to develop strategies to realize targeted and precisely controlled cargo delivery or activation of immune systems for cancer immunotherapy.
For decades, the EPR effect has been considered as the gold standard in nanomedicine that allows nanoparticles to accumulate in tumor area following intravenous administration. However, conventional nanomedicine solely based on EPR effect is not able to overcome the heterogeneity of tumors and vasculature in clinical practice75. Despite endogenous stimuli can be applied to enhance the targeting ability of nanomedicine spatially based on specific condition of tumor microenvironment, such stimuli-responsive nanoplatforms lack capability to deliver drugs in a temporal fashion76. Strategies to realize spatiotemporal control of drug release are needed to further improve the efficacy of nanomedicine for cancer therapy.
Light surpasses in controlling biological systems in a spatiotemporal resolution by virtue of easy adjustability of irradiation conditions like time, power and site77. A branch of light technology applied in health and medicine can be traced back to the middle 2000s78. The past two decades witnessed the rapidly growing influence of light in controlling biological systems via synthesis and modification of photoresponsive small molecules, proteins and nucleic acids79. In the late 2000s, emerging research employed light technology on nanomedicine, which pioneered a new route to therapeutics, in vitro diagnostics, and medical devices80. The transfer from small molecule-based optochemical-controlled synthetic molecules to polymer-based light-responsive drug delivery systems diversified nanomedicine with intelligence and personalization. Moreover, the concept of “near-infrared (NIR) window”, which refers to light between 650 and 900 nm, was brought up81. The light with wavelengths within the range of the NIR window stands as a desirable tool to regulate biological activities in vivo by virtue of higher penetration ability and less absorbance by tissue chromophores.
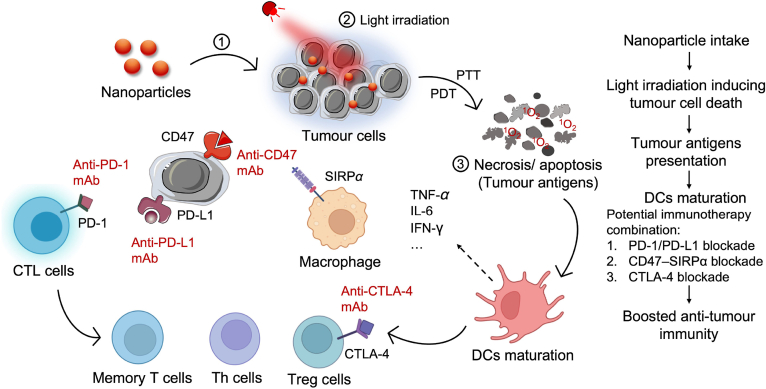
To exploit nanomedicine for cancer immunotherapy, light-controlled delivery nanoplatforms have been designed and demonstrated promising results with clinical application potentials. Furthermore, clinical-approved phototherapies, such as PDT and PTT, are able to precisely induce immunogenic cell death (ICD) and elicit the immune system (Fig. 4). PDT employs the application of nontoxic photosensitizers and localized external light irradiation, to generate reactive oxygen species (ROS) in tumor area. The resulting ROS can generate tumor cell destruction and produce dying tumor cell debris to send a danger signal to the innate immune system, resulting in the enhanced antigen presentation and activation of T cells82. PTT utilises hyperthermia induced by light to kill cancer cells. The tumor cells undergo apoptosis or necrosis and release tumor-associated antigens that can be recognized by APCs, then activate immune cells83. Combining phototherapy with immunotherapy has become a promising strategy for cancer treatments as it has satisfactory synergistic effects for primary and metastatic cancer treatments. In the section, we discussed four different strategies and corresponding delivery systems to advance light-responsive nanomedicine for immunotherapy (Table 1)84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110.
Figure 4.
Schematic demonstration of PTT/PDT-induced immunogenic cell death and succeeding immune responses.
Table 1.
Four different strategies and corresponding delivery systems on light-responsive nanomedicine for immunotherapy were summarized.
| Strategy | Delivery system | Short description | Immunotherapeutic agent | Significance | Ref. |
|---|---|---|---|---|---|
| Light-responsive strategies to enhance checkpoint blockade immunotherapy | Self-assembled nanoparticles | 820 nm light-responsive nanoparticles co-delivered a PD-L1 agonist and chemo drug docetaxel (DTX) | CF27, a PD-L1 agonist peptide | Combination of photosensitiser, chemotherapeutic and immune checkpoint blockade inhibitor | 84 |
| Self-assembled nanoparticles | Near-infrared (NIR) light-responsive hydrogels co-delivered NIR dye, TLR-7 and TLR-9 agonist | CPG and R848, immune stimulators | Combination of photothermal therapy (PTT) with toll-like receptor agonist | 85 | |
| Self-assembled nanoparticles | NIR light-responsive nanoparticles co-delivered photosensitizer and chemo drug doxorubicin (DOX) | Anti-PD-L1 antibody | Photodynamic therapy (PDT) induced immunogenic cell death (ICD) to elevate the immune response | 86 | |
| Self-assembled nanoparticles | NIR light-responsive nanoparticles were self-assembled by a prodrug (photosensitizer verteporfin linking with doxorubicin via a cathepin B-cleavable peptide) | Anti-PD-L1 antibody | PDT induced ICD to elevate immune response | 87 | |
| Polymeric nanoparticles | NIR light-responsive polymeric nanoparticles generated 1O2 to release tumor-associated antigens and delivered immunostimulatory agents | NLG919, IDO-1 inhibitor | Combination of IDO-1 pathway inhibition and tumor-associated antigen presentation to achieve enhanced immune responses | 88 | |
| Prodrug | Blue light responsive-prodrug selectively activated immune responses | BMS-1, inducing PD-L1 dimerization | The first photocaged prodrug for immunotherapy | 89 | |
| Light-controlled modulation of immune cells | Optical dimerizer pair | Blue light-responsive CAR-T systems | CAR-T cells | Reversible light-controlled immunotherapy | 90 |
| Silica-coated upconversion nanoparticles (UCNPs) | NIR light-responsive CAR-T systems were realized by incorporating hexagonal-shaped UCNPs | CAR-T cells | A nano-optogenetic engineering strategy with robust efficacy and potential for clinical translation | 91 | |
| PLGA nanoparticles | Simultaneous injection of PLGA nanoparticles loaded ICG and CAR-T cells | CAR-T cells | Combination of PTT with CAR-T therapy | 92 | |
| Light-directed delivery of cancer vaccine | Cationic PAMAM dendrimer | UV-responsive PAMAM-based nanosystems delivered antigen ovalbumin (OVA) | Antigen OVA | Endosomal escape of OVA and elevated tumor necrosis factor α (TNF-α) secretion from macrophages | 93 |
| PLGA nanoparticles | NIR light-responsive copper sulfide nanoparticles delivered antigen OVA | Antigen OVA | Escalated cytokines and activation of cytotoxic T cells were achieved after NIR light irradiation | 94 | |
| PheoA-PEI nanoparticles | NIR light-responsive nanoparticles co-assembled by a photosensitizer pheophorbide A grafted polyethyleneimine and OVA | Antigen OVA | Enhanced CD8+ T cell proliferation and tumor inhibitory effect were found in mice injected with dendritic cells and the nanoparticles after light irradiation | 95 | |
| Self-assembled nanoparticles | Nanoparticles co-assembled by OVA and photosensitizer ICG | Antigen OVA | Maturation of DCs and elevated excretion of cytokines | 96 | |
| Polymeric nanoparticles | PLGA-ICG polymeric nanoparticles as the PTT core and coated with CMV-OMV fused membrane | Fusing melanoma cytomembrane vesicles (CMVs, antigen) and attenuated Salmonella outer membrane vesicles (OMVs, adjuvant) | Bacterial OMVs can stimulate immunity cytokines and melanoma CMVs can trigger antitumor immunity due to the specific antigens expressed on the membrane. The immune responses were further boosted by the PTT-induced ICD effect | 97 | |
| Photosynthetic microorganism | NIR light-responsive synthetic microorganism PCC 7942 delivered adjuvants and achieves a synergistic effect with PTT | PCC7942, adjuvants | PCC7942 served as both PTT agent and adjuvant to stimulate immune responses | 98 | |
| UCNPs | NIR light-responsive UCNPs delivered adjuvant oligonucleotides | CpG oligonucleotides, adjuvants | NIR-light irradiation activated adjuvant CpG, resulting in a substantially increased proportion of tumor-infiltrating T cells for improved antitumor efficacy | 99 | |
| Manganese-porphyrin metal-organic framework (MOF) | MOF modified with AS1411 aptamer to load vorinostat and photosensitizer TCPP | Manganese ions (Mn2+); tumor-associated antigens | Combination of PDT-induced ICD and Mn2+ mediated STING pathway activation | 100 | |
| Polymeric nanoparticles | NIR light-responsive polymeric nanoparticles co-delivered immunostimulatory agents and photosensitizers | Toll-like receptor agonist; tumor-associated antigens | Combination of PDT-induced ICD and Toll-like receptor activation | 101 | |
| Light-responsive strategies to modulate tumour microenvironment to enhance immunotherapy | Polydopamine nanoparticles coated with macrophage membrane | Polydopamine acted as a PTT agent and the macrophage membrane exhibited targeted ability | TMP195, TAMs repolarization agent | Combination of PTT with macrophage polarization | 102 |
| UCNPs NaYF4:Yb | Coated UCNPs with photosensitizer and tumor-associated macrophage membrane (TAMM) | TAMM | Combination of PDT with macrophage polarization | 103 | |
| Polymeric nanoparticles | Poly (styrene-co-maleic anhydride) (PSMA) co-assembled with polymer poly [2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (PPV) with photosensitivity | PSMA-composed nanoparticles, TAMs repolarization agent | Combination of PDT with macrophage polarization | 104 | |
| Liposomes | Macrophages served as carriers to load NIR light-responsive nanoparticles | Tumor-associated antigens; anti-PD-L1 antibody | Combination of PDT, chemotherapy, and macrophage polarization with PD-L1 blockade | 105 | |
| Self-assembled nanoparticles | Ce6 self-assembled with tyrosine kinase inhibitor axitinib, indoleamine 2,3-dioxygenase (IDO) inhibitor dextro-1-methyl tryptophan and human serum albumin | Axitinib, reversing immunosuppressive microenvironment by regulating the aberrant vasculature; IDO inhibitor dextro-1-methyl tryptophan | Combination of PDT with vascular endothelial growth factor receptors (VEGFR) inhibitor and IDO inhibitor | 106 | |
| Chemically modified cytokines | 370 nm light-controlled galactosylceramides | Cytokines | The first example of modifying cytokines to activate T cells and immune responses | 107 | |
| Photoactivatable chemokine C–X–C motif receptor 4 (PA-CXCR4) | 505 nm light-controlled T-cell infiltration | Chemokine C–X–C motif receptor 4 (PA-CXCR4) | The first example of modifying chemokines | 108 | |
| Photosynthetic microcapsules modified with UCNPs | NIR light-controlled oxygen generation | Hypoxia relief; anti-PD-1 antibody | Reversed tumor hypoxic and immunosuppressive microenvironment | 109 | |
| Iron clusters-porphyrin metal-organic framework (MOF) | NIR light-controlled oxygen generation | Hypoxia relief; anti-PD-L1 antibody; tumor-associated antigens | Reversed tumor hypoxic and immunosuppressive microenvironment | 110 |
3.1. Light-responsive strategies to enhance checkpoint blockade immunotherapy
Though checkpoint blockade immunotherapy has been used as a first-line treatment for several cancer types, patients’ response varies due to tumor heterogenicity and the immunosuppressive microenvironment111,112. Increasing the injection dose will increase the risk of side effects as many of checkpoint blockades are not cancer specific. Therefore, controlled release of checkpoint blockades at the target site and eliciting antitumor immunity are desired for improving the efficacy of checkpoint blockade immunotherapy. Light-responsive nanomedicine-based strategies have been explored for reversing the immune-suppressive microenvironment to potentiate checkpoint blockade immunotherapy or delivering checkpoint agonist peptides specifically in tumor area.
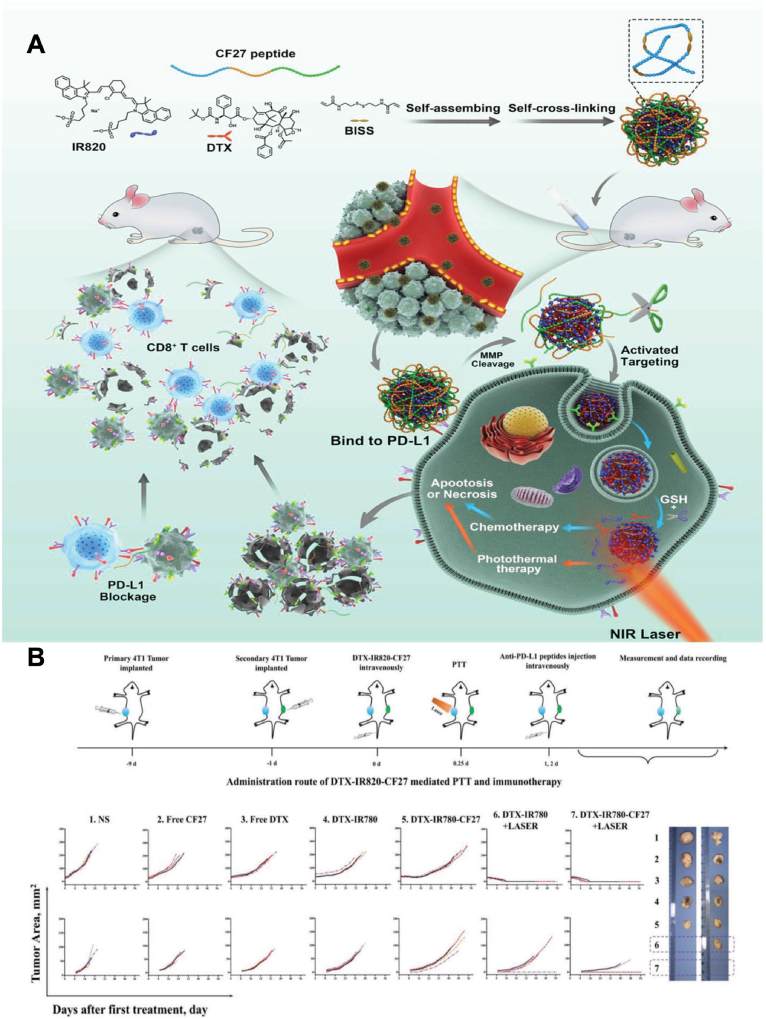
Combinational use of photosensitisers and checkpoint blockades have been explored in recent years to generate synergistic cytotoxicity and immunogenicity. Peng et al.84 designed a nanosystem to simultaneously deliver photosensitiser, PD-L1 agonist and chemotherapeutic agent. In this design, NIR dye IR820 self-assembled with chemotherapeutic docetaxel (DTX) and N′-bis(acryloyl)cystamine (BISS) into nanoassemblies (DTX/BISS-IR820). CF27, a PD-L1 agonist peptide with a matrix metalloproteinase (MMP)-responsive sequence, was cross-linked with BISS and further formed a nanosystem (DTX-IR820-CF27) (Fig. 5)84. DTX-IR820-CF27 showed significant tumor-specific release and accumulation of CF27 in tumor microenvironment to bind to PD-L1. In the meantime, DTX and IR820 underwent endocytosis and generated chemotherapy and photothermal effect upon light irradiation which further strengthened tumor cell apoptosis and necrosis. In a breast cancer mouse model, primary tumors were eliminated with systemic injection of DTX-IR820-CF27 plus light irradiation at tumor sites, while the growth of secondary tumors without light irradiation was also drastically impeded with an increase in the relative proportion of infiltrated effector T cells (CD3+CD8+ T cells). This study presented an example of the combinational use of photosensitiser, chemotherapeutic drug, and checkpoint blockade in one system, and the applicability of using NIR dye to enhance the immunotherapy efficacy of checkpoint inhibitors. Similar design has been explored in another study. Nanoparticles were formed by self-assembly of NIR dye indocyanine green (ICG), toll-like receptor (TLR)-7/8 agonist resiquimod (R848), and TLR-9 agonist CPG85. ICG can generate a strong photothermal effect, while CPG and R848 work as important immune stimulators. The nanoparticles were then loaded in a thermosensitive hydrogel to realise NIR light-controlled release of TLR-7/8 agonist and TLR-9 agonist. By in situ injection, hydrogel-treated tumor-bearing mice had the photothermal ablation, together with a strong immune response to inhibit lung metastasis and postoperative tumor recurrence.
Figure 5.
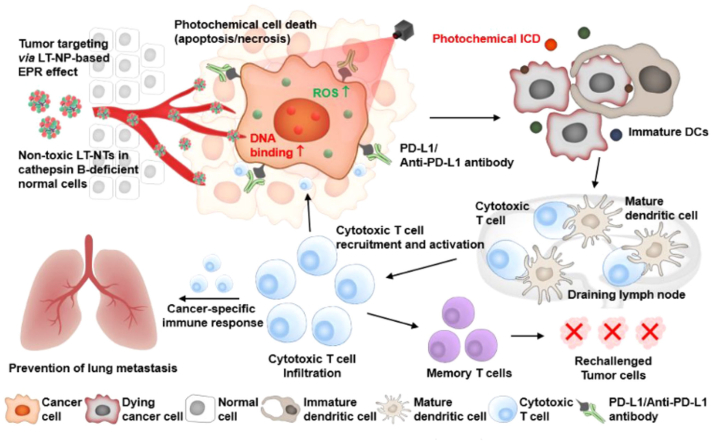
Illustration of self-assembly of NIR dye/drug/peptide hybrid nanosystem DTX-IR820-CF27. (A) Scheme of DTX-IR820-CF27 nanoparticles that can simultaneously deliver photosensitiser IR820, PD-L1 agonist CF27 and chemotherapeutic DTX, achieving combinatorial therapies. (B) The administration route of DTX-IR820-CF27-mediated PTT/chemotherapy/immunotherapy and anti-tumor effect in breast cancer mice model. Reprinted with permission from Ref. 84. Copyright © 2019 John Wiley and Sons.
In addition, eliciting immune response in the immunosuppressive microenvironment in solid tumors is a formidable challenge to potentiate checkpoint blockade immunotherapy. Light-responsive nanomedicine has also been used to enhance the efficacy of successively delivered checkpoint blockades. Photosensitisers have been loaded in several types of nanoparticles, such as liposomes113, inorganic114 and organic nanoparticles97, to induce ICD and elevated immune response for the following injection of checkpoint blockades. Hu et al.86 developed a drug delivery system by combining photosensitiser chlorin e6 (Ce6) with a ROS-sensitive hydrophobic core to achieve light-controlled release of chemotherapeutic doxorubicin at tumor sites. The combinational use of photosensitiser and chemotherapeutic agent induced immunogenic cell death, subsequently promoted the maturation of dendritic cells and increased T cell infiltration to tumor tissues. As a result, successive anti-PD-L1 antibody injection generated an abscopal effect in mouse models, simultaneously inhibited primary and distant tumor growth. An additional example presented a light-responsive prodrug nanosystem to potentiate a following PD-L1 blockade immunotherapy87. The authors developed prodrugs consisting of doxorubicin and photosensitizer (verteporfin) which were then self-assembled into nanoparticles (LT-NPs) (Fig. 6)87. LT-NPs accumulated in tumor area and generated immunogenic cell death upon light irradiation which led to dendritic cell recruitment, maturation, migration, and cytotoxic T cell activation. These results further amplified immune response for successive PD-L1 blockade immunotherapy. Drastically delayed tumor growth, tumor recurrence, and lung metastasis were observed in colon tumor models and metastatic lung cancer models owing to the enhanced immune response. Apart from PD1/PD-L1 blockades, other immunoregulatory molecules can be targets as well. Li's group88 developed a NIR light-activatable cancer vaccine by attaching immunostimulatory IDO-1 inhibitor NLG919 and singlet oxygen (1O2)-responsive cleavable linker on a semiconducting polymer nanoparticle core. NIR-light excitation enabled the generation of heat and 1O2 and the subsequent release of NLG919 and tumor-associated antigens, inducing a combined antitumor immunity.
Figure 6.
Schematic illustration of prodrug nanoparticles (LT-NPs) generating immunogenic cell death (ICD) at tumor sites upon light irradiation to potentiate PD-L1 checkpoint blockade cancer immunotherapy. LT-NPs accumulate in tumor area via the EPR effect and generate cytotoxicity and reactive oxygen species (ROS) upon light irradiation, then result in an effective ICD effect for dendritic cell (DC) recruitment, maturation, migration, and cytotoxic T cell activation. PD-L1 blockades induce a cancer-specific immune response and efficiently inhibit primary tumor progression and distant pulmonary metastatic tumors. Eventually, immunological memory established by the use of LT-NPs with PD-L1 blockade prevents tumor recurrence. Reprinted with permission from Ref. 87. Copyright © 2021 American Chemical Society.
Though light-responsive nanomedicine has been able to enhance the efficacy of checkpoint blockades, multiple injections might compromise patient compliance which could impede its clinical translation. The ideal strategy to integrate all components (photosensitisers, chemotherapeutics and checkpoint blockades) into one delivery system has not yet been reported. Existing light-responsive immunotherapeutic strategies for enhanced checkpoint blockades focused on combinational delivery of photosensitiser and checkpoint blockades. Recently, Liu et al.89 developed a photocaging strategy that modified PD-1/PD-L1 inhibitor BMS-1 with a photo-removable molecule, (diethylamino)coumarin-4-yl]methyl (DEACM). The photocleavable prodrug showed effective PD-1/PD-L1 axis inhibition upon 420 nm light irradiation. The prodrug can be encapsulated into nanocarriers or form nanoassemblies by virtue of its hydrophobicity. Such direct modification (e.g., photocaging) of checkpoint blockades to facilitate light controlling of T cell functions remains a relatively unexplored and promising field which is worthy of further research.
Overall, several immune checkpoint blockades combined with photosensitizers and chemotherapeutics to enhance anti-tumor efficacy have been reported. However, current studies limited the use of light to trigger PDT/PTT to elevate anti-tumor immunity with immune checkpoint blockades. In the future, light-controlled precise drug delivery at the right time and place can be a direction to avoid unspecific immune system activation and resulting side effects like inflammation, autoimmune responses, etc. Precise modulation of immune checkpoint blockades by light can greatly enhance the treatment efficacy, thus reduce multiple injections and undesired side effects, which can greatly expand the application of immune checkpoint blockades for clinical applications.
3.2. Light-controlled modulation of immune cells
Adoptive cellular therapies such as CAR-T therapy are becoming the game-changing approach in treating cancers with promising clinical outcomes for hematologic cancers. However, personalized dosing remains the biggest challenge, as the immune response varies in individual patients and overdosing of T cell infusion can cause cytokine release syndrome and tumor lysis syndrome115,116. Hence, there is a great need to modulate immune cells with high precision in both space and time. Additionally, limited T infiltration in tumor microenvironment often hinders the application of adoptive cellular therapies. To this end, light-responsive delivery strategies can be utilized for the spatiotemporal control while phototherapies PDT and PTT can boost T infiltration in tumor area.
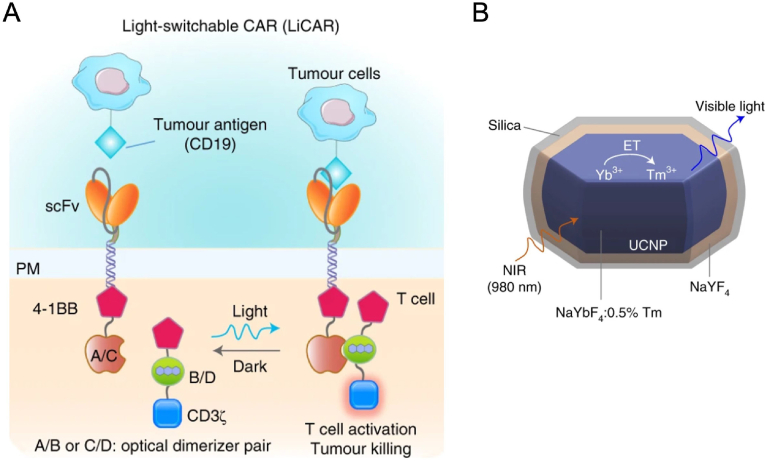
Light-switchable CAR-T cells have been developed to address the critical issue of “on-target, off-tumor” in CAR-T immunotherapy117. An interesting light-inducible gene activation system has been developed by combining CRY2-CIB1 (arabidopsis cryptochrome 2- cryptochrome-interacting basic-helix-loop-helix 1) dimerization with light-inducible nuclear translocation90. Upon blue light stimulation, the helix in the system unfolded to expose the NLS peptide which directed the translocation into the nucleus. Simultaneously, CRY2 can bind to CIB1 in the nucleus to trigger the reporter gene expression. The authors genetically encoded this system to T cells and achieved precise control with blue light in a mouse model. However, the wavelength of blue light limited its penetration depth, therefore hampering clinical translation of the reported system. Nguyen and colleagues91 have solved this problem using upconversion nanoparticles. Firstly, CAR-T cells were engineered with photoresponsive modules in two separate domains (LiCAR T cells), respectively. Upon blue light irradiation, the two domains assembled and activated T cells to react with tumor antigen CD19 (Fig. 7A)91. To facilitate in vivo application of LiCAR T cells, a hexagonal-shaped upconversion nanoplate (β-NaYbF4:0.5% Tm@NaYF¬4 core–shell UCNPs) (Fig. 7B)91 was injected together with LiCAR T cells to covert NIR light at 808 nm to blue light. Desirable therapeutic effects were achieved in hematologic and solid tumor mouse models with the combinational treatment. Comprehensive biosafety evaluation was carried out, LiCAR T cells treatment showed attenuated side effects including reduced B cell aplasia and cytokine storm syndrome. This study provided a nano-optogenetic engineering strategy with robust efficacy and reduced side effects.
Figure 7.
Graphic illustration of light-switchable CAR (LiCAR) T cells and upconversion nanoparticle. (A) Engineered CAR-T cells can only be switched on in the presence of tumor antigen (CD19) and blue light. (B) Core–shell structure of silica-coated upconversion nanoparticles which can NIR light to visible light. Reprinted with permission from Ref. 91. Copyright © 2022 Springer Nature Limited.
To tackle the challenge of limited T cell infiltration in tumor microenvironment, nanoparticles were used to deliver photosensitisers to generate hyperthermia in tumor tissues. The hyperthermia in tumor can change its compact structure, reduce interstitial fluid pressure and promote the perfusion of T cells118. Chen et al.92 developed a poly (lactic-co-glycolic) acid (PLGA) nanoparticle loaded with photosensitiser ICG to potentiate CAR-T therapy (Fig. 8)92. T lymphocytes obtained from healthy donors were engineered to express the chondroitin sulfate proteoglycan-4 (CSPG4). CAR. CSPG4+ T cells were then selected to exploit the overexpressed CSPG4 in melanoma. The PLGA nanoparticles were intratumorally injected into melanoma-bearing mice to induce PTT. CSPG4+ T cells were intravenously injected to the mice 2 h after PTT. As a result, CSPG4+ T cells was found to accumulate in tumors in PTT-treated mice due to the increased tumor perfusion and hypoxia relief induced by PTT. The PTT-CAR T cell combinational treatment significantly inhibited the tumor growth in melanoma-bearing mice with 2 out of 6 mice being completely cured. This study provided a potential platform to enhance the efficacy of CAR-T therapy in solid tumors by combining PTT and CAR-T therapy, which could inspire more combinational use of phototherapy and immunotherapy.
Figure 8.
Example study of utilizing PTT to potentiate CAR-T therapy. (A) Schematic illustration of the PTT induced by nanomedicine and promoting successive CAR-T cell infiltration in the tumor and cytokine release. (B) Representative images and tumor growth kinetics of tumor-bearing mice with different treatments, namely PTT, CAR-T and their combinational therapy. Reprinted with permission from Ref. 92. Copyright © 2019 John Wiley and Sons.
To conclude, light can not only induce PDT/PTT to enhance immune cell infiltration but also active CAR-T function in a spatiotemporal solution. Light-controlled CAR-T function can realize personalized dosing and minimize systemic cytokine release-associated toxicities. In this way, both two challenges can be addressed by exploiting light-responsive nanomedicine for adoptive cellular therapies. Elevated immune responses and precise drug delivery hold great promise to concurrently combat solid tumors. Future work can be focused on developing more longer-wavelength light-responsive delivery systems and applying them in adoptive cellular therapies for other cell types like CAR-NK and CAR-M therapies.
3.3. Light-directed delivery of cancer vaccine
Apart from T cells, APCs, including dendritic cells and tumor-associated macrophages, are playing central roles in the activation of adaptive immune response and can be targets for vaccine delivery to enhance cancer immunotherapy. Most of nanomedicine are designed to avoid opsonization and uptake by phagocytes during blood circulation which then provides sufficient accumulation at target sites via the EPR effect. Unlike conventional nanomedicine, uptake by APCs in circulating blood is advantageous for cancer vaccine delivery, namely extending antigen exposure to APCs119. However, the endosomal escape of antigens plays a key role in activation of immune responses. Apart from light-controlled cargo release strategies, photosensitisers also can disrupt the endosome and promote the escape of antigens upon light irradiation, owing to the production of ROS. Therefore, co-delivery of tumor antigens and photosensitisers by nanoparticles can be a promising strategy for cancer immunotherapy.
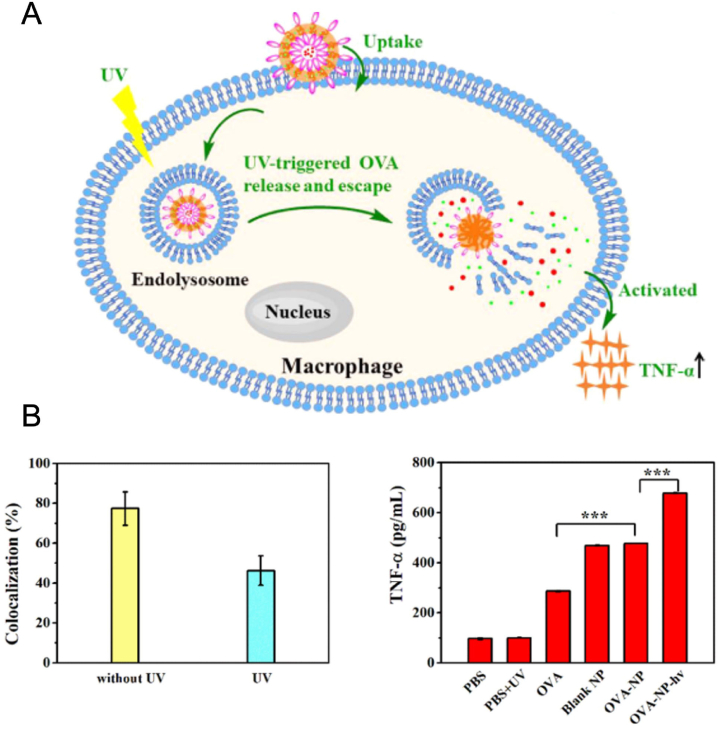
Cationic polymers are one of the priority options for delivering proteins, owing to their positive surface charge and excellent drug loading capacity. In one example, UV-responsive azido-terminated polypeptide was conjugated to glycosylated poly (amidoamine), introducing photo-responsiveness to the cationic polymer (Fig. 9)93. The synthesized polymers were then self-assembled with model antigen ovalbumin (OVA) in an aqueous solution to form antigen-loaded polymersomes. Under UV light irradiation, the polymersomes transformed into micellar aggregates at pH 7.4 and underwent complete disassembling at pH 5. As a result, an accelerated OVA release was observed in endosomal acidic pH after UV irradiation. Besides, endosomal escape of OVA and elevated tumor necrosis factor α (TNF-α) secretion from macrophages were also observed as results upon UV light irradiation. However, the UV light-triggered cytosolic OVA release and endosome escape were only demonstrated in macrophage cell model. Though this study provided a light-responsive polymersome platform for cancer vaccine delivery, its in vivo applicability needs to be further investigated.
Figure 9.
(A) Cellular uptake of OVA-loaded light-responsive polymersomes. UV light triggers OVA release and escape from endosomes, resulting in macrophage activation and TNF-α secretion. (B) Co-localization of OVA-loaded polymersomes and endolysosomes in macrophages and TNF-α secretion with/without UV light irradiation. OVA, antigen ovalbumin; TNF-α, tumor necrosis factor α. Reprinted with permission from Ref. 93. Copyright © 2020 American Chemical Society.
Researchers have also investigated combining metal nanoparticles with OVA in polymer nanocarriers to achieve accelerated OVA release. Copper sulfide nanoparticles were used as photosensitizers for ROS production and PLGA was used as the matrix to form nanoparticles94. Escalated cytokines and activation of cytotoxic T cells were achieved after NIR light irradiation. The activated immune response also inhibited distant metastatic tumor growth in a mouse model. In addition, Zhang et al.95 developed a hydrophobic photosensitizer pheophorbide A (PheoA) grafted polyethyleneimine (PheoA-PEI) to deliver OVA. OVA was complexed with PheoA-PEI to form nanoparticles which produced ROS to promote antigen endosomal escape and subsequent cytosolic antigen release after light irradiation. In tumor-bearing mice, enhanced CD8+ T cell proliferation and tumor inhibitory effect were found in mice injected with dendritic cells and the nanoparticles after irradiation with 670 nm laser at the tumor sites. Furthermore, OVA itself has been utilized as a carrier to deliver photosensitiser ICG to achieve combinational therapeutic effects. Pan and colleagues96 have developed a multifunctional cancer vaccine by simple mixing of OVA and ICG (Fig. 10)96. The OVA-ICG nanovaccine has demonstrated desirable stability with an average size of 14.7 nm and a high antigen loading efficiency of 80.8%. It was observed that OVA-ICG nanovaccine promoted the maturity of an immature dendritic cell line, DC2.4 cells, characterized by elevated secretion of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and increased expression of dendritic cell maturation markers, MHC-II, CD 80, and CD 83. Melanoma-bearing mice were intratumorally injected with PBS, ICG, and OVA–ICG nanovaccine, separately, followed by 808 nm laser irradiation to trigger PTT after injection. Owing to the fluorescence of ICG, the nanovaccine can be easily detected in vivo, providing a sensitive tracking of dendritic cells. Significant fluorescence enhancement in tumor regions was observed after injecting OVA–ICG nanovaccine. Besides, OVA-ICG nanovaccine and laser irradiation-treated group demonstrated significantly delayed tumor growth, in comparison with other groups. Tumor prevention was observed in mice immunized with OVA-ICG nanovaccine with light irradiation in tumor rechallenging experiment, together with increased CD8+ cytotoxic T cells in tumors and elevated level of the anti-OVA immunoglobulin G (IgG) in serum. This study presented a robust nanovaccine with scaling-up potential which demonstrated the enhanced therapeutic efficacy by combining PTT and immunotherapy.
Figure 10.
Nanovaccine fabricated with OVA and photosensitiser ICG to achieve photothermal immunotherapy against tumor. (A) Graphic illustration of the fabrication of OVA and ICG to form nanovaccine and the anti-tumor mechanism. (B) Protocol of in vivo anti-tumor experiments and tumor growth monitoring after different treatments. (C) Tumor prevention assay design and tumor growth curves of the mice treated with PBS or OVA–ICG. OVA, ovalbumin; ICG, indocyanine green. Reprinted with permission from Ref. 96. Copyright © 2019 John Wiley and Sons.
More interestingly, biomimetic platforms have also been exploited in the delivery of light-responsive cancer vaccine. Chen et al.97 designed a tumor-specific antigenic nanoplatform with self-adjuvants activities by fusing melanoma cytomembrane vesicles (CMVs) and attenuated Salmonella outer membrane vesicles (OMVs) (Fig. 11)97. Bacterial OMVs provide the system with a rigid structure and ability to stimulate immunity cytokines, while melanoma CMVs can trigger antitumor immunity due to the specific antigens expressed on the membrane. The authors used a well-researched polymeric nanoparticle PLGA-ICG as the PTT core, and then coated with CMV-OMV fused membrane (PI@EPV). PI@EPV was found to have ability to stimulate dendritic cell maturation and T cell proliferation, which was further boosted by laser irradiation due to the PTT-induced ICD effect. In comparison with monovesicle-camouflaged formulations, PI@EPV demonstrated superiority in preventing tumorigenesis in mice challenged with B16F10 melanoma tumors, with 78.57% of tumor suppression rate. Moreover, the PI@EPV vaccination showed no noticeable tumor suppression in mice challenged with 4T1 breast cancer cells, indicating the specificity of anti-tumor immunity of melanoma cell vesicle-derived vaccine. Additionally, increased tumor specific cytotoxic T lymphocytes (CD3+ CD8+ CD107a) in spleens and enhanced induction of effector memory T cells (CD3+ CD8+ CD44+ CD62L‒) were found in mice vaccinated with PI@EPV. Furthermore, PI@EPV plus laser irradiation also demonstrated great photothermal-immunotherapy synergy, with 80% of the treated mice becoming tumor free and reaching 60 d survival.
Figure 11.
Tumor-specific biomimetic nanovaccine with PTT inducer in the core. (A) Preparation of eukaryotic–prokaryotic membrane-coated polymeric ICG nanoparticle, PI@EPV. (B) Tumor growth curves of B16F10 tumors and 4T1 tumors with different treatments. (C) Survival rate of tumor-bearing mice after nanovaccine injection with or without laser irradiation to trigger PTT. Reprinted with permission from Ref. 97. Copyright © 2019 John Wiley and Sons.
In another study, a photosynthetic microorganism PCC 7942 was utilized as photosensitiser and drug delivery platform98. Under light irradiation at 660 nm, PCC 7942 released pathogen-associated molecular patterns to act as adjuvants, which then activated dendritic cells and promoted the proliferation of antitumor CTLs. At the meantime, heat was generated by PCC 7942 to induce PTT effect. As a result, local injection of PCC 7942 in 4T1 tumors enhanced local tumor killing and antitumor immune response in tumor-bearing mice. This study has proved that microorganism is a promising light-controlled platform for cancer vaccine delivery. Nevertheless, comprehensive biosafety evaluation is needed prior to its clinical translation. A NIR light-controlled drug delivery system to boost immune response was developed by Chu and co-workers99 via linking a UV light-responsive CpG oligonucleotides (ONDs) adjuvant with UCNPs. NIR-light irradiation can liberate ONDs from the nanoparticles, resulting in enhanced immune responses to combat solid tumors, since a substantially increased proportion of tumor-infiltrating T cells were observed in tumor microenvironment from in vivo experiment.
Apart from delivering antigens and adjuvants, ROS and heat-induced ICD served as internal antigens, which can be combined with other immunotherapies. Recently, Zhao and co-workers100 designed an intracellular self-assembly-driven nucleus-targeted photo-immune stimulator. The system comprised of vorinostat (SAHA)-loaded manganese-porphyrin metal-organic framework (Mn(III)-TCPP MOF) modified with AS1411 aptamer. Intracellular glutathione-controlled release of AS1411 can self-assemble with photosensitizer TCPP120, which further facilitated nucleus-targeted delivery of TCPP. The released manganese ions (Mn2+) further enhanced the cytosolic DNA/cyclic GMP-AMP synthase-stimulator of interferon gene (STING) pathway-mediated innate immunity. The encapsulated SAHA can induce chromatin decompaction, synergistically promoting TCPP-mediated PDT. PDT-induced immunogenic cell death led to the co-activation of robust adaptive and innate anti-tumor immunity. In another research, a charge-reversal enhanced nucleus-targeting drug delivery system to improve immunotherapy was fabricated by Fang et al.101 NIR light-responsive nanoparticles were utilized to co-deliver Toll-like receptor (TLR2) agonist Poly (I:C)121 and photosensitizer chlorin e6 (Ce6). Light irradiation can not only trigger PDT-induced ICD but also turn the surface charge of nanoparticles from positive to negative, which led to the dissociation of the nanoparticles. Poly (I:C) release can cooperatively function with ICD to boost anti-tumor immunity and inhibit tumor growth as a result.
Light-responsive cancer vaccines are still at the early stage of development and current research are mainly focused on protein-based antigen delivery. More advanced light-responsive delivery systems to facilitate nucleus targeting are needed for the development of RNA/DNA vaccination. Detailed in vitro and in vivo studies are necessary to demonstrate their clinical potential. Moreover, most of studies focus on co-deliver tumor antigens and photosensitizers to achieve synergistic anti-tumor effects. The heat or ROS generated by photosensitizers can destroy the endosome and facilitate the escape of antigens upon light illumination. More novel light-responsive delivery strategies can be exploited to co-deliver antigens, photosensitizers along with adjuvants to realize boosted immunity with maximum treatment efficacy.
3.4. Light-responsive strategies to modulate tumor microenvironment to enhance immunotherapy
Immunotherapy has undergone rapid development in recent years with well-established modalities being clinically used daily, including checkpoint blockades and CAR-T cell therapies. However, the number of patients who showed resistance to immunotherapies is not negligible. Scientists have paid much attention to the immunosuppressive microenvironment surrounding tumors and identified the factors that contribute to the hampered immunity in cancer patients. A variety of strategies aiming to enhance patients’ response to immunotherapies are based on alterations of the complex immunosuppressive tumor microenvironment like regulating functions of cells within TME including immune cells and tumor cells, or modulating physiochemical properties including hypoxia, acidity, high levels of ROS, dense extracellular matrix, and abnormal vasculature. Light-responsive nanomedicine is particularly promising in the modulation of tumor microenvironment, due to their properties of specific targeting, spatiotemporally controlled drug release and desirable drug loading capacity.
Macrophages, one of the major cell types in the tumor microenvironment, can be divided into M1 macrophages with antitumor functions, and M2 macrophages that promote tumor progression122. In the immunosuppressive microenvironment, M2 macrophages are the dominant phenotype associated with poor clinical outcomes in several cancer types123. As TAMs consist of M2 macrophages and a small population of M1 macrophages, repolarizing the TAMs to immune-activating phenotype is with copious potential124,125. A variety of strategies have been developed to repolarize TAMs, including using cytokines, microRNAs and phototherapies122. PDT and PTT can produce ROS under light irradiation to induce ICD, release damage-associated molecular patterns (DAMPs), and polarize macrophages from the immunosuppressive M2 phenotype to the antitumor M1 phenotype126, 127, 128.
Biomimetic nanomedicine, such as cell membrane or extracellular vesicle-coated nanoparticles, have been extensively investigated to target macrophages and release therapeutics for repolarization. Yue et al.102 developed a polydopamine nanoparticle loaded with the TAMs repolarization agent TMP195. The nanoparticles were then coated with macrophage membrane (P/T@MM NPs) Polydopamine is a photothermal transduction agent, which can effectively convert NIR light to heat and induce ICD and the secretion of inflammatory cytokines. The macrophage membrane on P/T@MM NPs surface provides excellent targeting ability to the TAMs, which are enriched in the post-PTT tumor microenvironment. As a result, in a breast tumor model, P/T@MM NPs exhibited preferential accumulation in tumor area and significantly elevated the level of M1-like TAMs (CD80+ in F4/80+ cells) after PTT. Additionally, after PTT, tumor-infiltrating immune cells, including CD3+ CD8+ CTLs, were significantly increased while the immunosuppressive cells such as MDSCs and Tregs dramatically decreased. TAMs repolarization and alterations in tumor microenvironment eventually led to a tumor-elimination rate of 60%. Similar design has been exploited in another study by coating upconversion nanoparticles conjugated with photosensitizer with tumor-associated macrophage membrane (TAMM) derived from the primary tumor (NPR@TAMMs) (Fig. 12)103. The upconversion nanoparticles NaYF4:Yb,Er@NaYF4 conjugated with photosensitiser Rose Bengal were able to respond to NIR light at 980 nm facilitating treatment for deep-seated tumors. TAMM on the surface endows the system with unique antigen-homing affinity and immune compatibility. Notably, TAMM can bind with CSF1 which is a key regulator of macrophage differentiation. By combining PDT and TAMs repolarization, NPR@TAMMs demonstrated superior tumor inhibition efficacy and antitumor immunity efficiency in an orthotopic mouse model with primary and distant breast tumors.
Figure 12.
Tumor-associated macrophage membrane (TAMM)-coated upconversion nanoparticles NPR@TAMMs for enhanced photodynamic immunotherapy. (A) Preparation of upconversion nanoparticle conjugated with photosensitiser rose bengal (RB) (I), and coated with cell membrane extracted from primary tumor-associated macrophages (TAMs) (II). (B) Primary and distant tumors were significantly suppressed by combining PDT and TAMs repolarization using NPR@TAMMs. Reprinted with permission from Ref. 103. Copyright © 2021, American Chemical Society.
Furthermore, polymeric nanoparticles have also been utilized to inhibit tumor growth by repolarizing TAMs into the tumoricidal M1 phenotype104. The polymeric nanoparticles (PPV-PSMA-NPs) were co-assembled by poly (styrene-co-maleic anhydride) (PSMA) and conjugated polymer poly [2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (PPV) with photosensitivity. In vitro results showed that PPV-PSMA-NPs were co-localized with lysosomal tracker inside macrophages (RAW 264.7) for 3 days, allowing modulations to alter the biological function of macrophages. Macrophage repolarization was then elucidated by immunofluorescence staining of tumor slices from the nanoparticles-treated tumor-bearing mice. Up-regulation of M1-related markers CD80, inducible nitric oxide synthase (iNOS) and inflammatory factor TNFα were observed, along with down-regulated M2 markers CD206 and CD163. PDT triggered by light irradiation was able to accelerate tumor cell death by ROS generation in cell models, but not yet tested in animal models. Besides, white light was used in this study which raises concerns about possible phototoxicity. Furthermore, light-responsive nanoparticles have been exploited to activate neutrophils129 and natural killer cells130, therefore potentiating antitumor immune response.
An engineered macrophage that can load nanoparticles to enhance tumor tropism capacity was developed by Huang and co-workers105 to achieve enhanced chemo/photo/immunotherapy. Oxaliplatin prodrug and photosensitizer Zinc phthalocyanine are designed as NIR light-responsive drug vectors and concurrently loaded in the lipid bilayer. The drug encapsulated polarized macrophages to the anti-tumor M1 phenotype, providing macrophages the ability to exert anti-tumor effects themselves. The combination of PD-L1 blockade with PDT/chemotherapy displayed desired anti-tumor efficacy in primary and bone metastatic breast cancer.
Other than immunosuppressive cells, the aberrant vasculature with dysfunctional blood flow is another major reason for the immunosuppressive environment. Zhou et al.106 designed a photosensitizer-based nanoplatform to achieve enhanced photodynamic immunotherapy by promoting vascular normalization in tumor microenvironment (Fig. 13)106. The nanoplatform (CAM NP) was self-assembled by photosensitizer Ce6, tyrosine kinase inhibitor axitinib and indoleamine 2,3-dioxygenase (IDO) inhibitor dextro-1-methyl tryptophan with the help of human serum albumin. Axitinib can inhibit vascular endothelial growth factor receptors (VEGFR), thus promoting the normalization of blood vessels131. On the other hand, IDO inhibitors can suppress the conversion of tryptophan to kynurenine, thereby enhancing the immune response132. CAM NPs exhibited excellent in vitro cytotoxicity on melanoma cell line B16F10 after light irradiation and increased apoptosis rate (63.4%) with peripheral blood mononuclear cells incubation. In vivo evaluation elucidated that CAM NPs could accumulate in tumor area and demonstrated significant inhibition of tumor growth with the aid of laser irradiation. Furthermore, in comparison to the control group, the blood vessel density in CAM NPs treated tumors was significantly increased based on anti-CD31 immunohistochemistry (IHC) staining. A remarkably higher proportion of CTLs and reduced population immunosuppressive TAMs were observed in both primary and abscopal tumors, in comparison with those in other groups (blank, Ce6 and single inhibitor-treated groups). Thereby, modulation of the tumor microenvironment was achieved by normalization of tumor vessels and IDO inhibition, resulting in enhanced immune response and amplified PDT effect. In addition, light-responsive strategies have been used in selective activation of cytokines in the tumor microenvironment with high spatiotemporal precision. Upon irradiation, chemically modified cytokines undergo changes in conformation or polarity, resulting in a difference in binding affinity to the target receptor and reducing off-target effects. Photoswitchable galactosylceramides have been developed to activate natural killer T cells under 370 nm light, to produce immunomodulatory cytokines such as IFN-γ107. Moreover, photoactivatable chemokine C–X–C motif receptor 4 (PA-CXCR4) has been developed to transmit intracellular signals in response to 505 nm light108. Increased T-cell infiltration in tumors has been observed in melanoma-bearing mice, resulting in enhanced immune responses and optimal outcomes in the later adoptive cellular therapy. However, the combinational use of nanomedicine with photoswitchable cytokines remains unexplored and yet with potential in modulation of tumor microenvironment.
Figure 13.
Photosensitizer-based nanoplatform to achieve enhanced photodynamic immunotherapy. (A) Graphic illustration of the fabrication of the nanoplatform CAM NP and mechanisms of enhanced PDT and anti-tumor immune responses. (B) Tumor targeting effect of CAM NPs based on biodistribution compared to free Ce6. (C) Increased T cell infiltration in tumors treated with CAM NPs, in comparison with free drug-treated groups. Reprinted with permission from Ref. 106. Copyright © The Royal Society of Chemistry 2021.
Besides modulating abnormal vasculature, tumor immunosuppressive environment can be reversed by creating a hyperoxic tumor microenvironment. A photosynthetic microcapsule was developed by encapsulating cyanobacteria and upconverting nanoparticles in alginate microcapsules109. The microcapsules can convert laser light into red-wavelength radiation, which triggers stable cyanobacterial photosynthesis, increasing the oxygen level in the tumor. The developed system was applied to combine with anti-PD-1 therapy. The improved anti-tumor efficacy was confirmed in orthotopic breast cancer mouse model and transplanted hepatocarcinoma in rabbit model. In another research, a nanoscale metal−organic framework, Fe-TBP made of Fe3O clusters and porphyrin ligands was prepared to overcome tumor hypoxia110. Upon light irradiation, a cascade reaction in which intracellular H2O2 was decomposed by the Fe3O clusters to produce oxygen through a Fenton-like reaction whereas the generated oxygen was converted to cytotoxic singlet oxygen (1O2) by photoexcited porphyrins. The efficacy of PDT can be greatly enhanced by generated oxygen. Furthermore, PDT-induced anti-tumor immunity concurrently worked with anti-PD-L1 immune checkpoint blockade, resulting in effective suppressed growth of both primary and distant tumors.
Light-responsive nanomedicine surpasses in its ability to modulate tumor microenvironment by virtue of its characteristics of precisely controlled drug release and specific targeting. Existing examples focus on repolarizing tumor-associated macrophages and normalizing tumor microenvironment. More advanced light-responsive drug delivery system can be developed to regulate the functions of other types of immune cells, fibroblasts and etc., which also play a crucial role in tumor microenvironment.
4. Challenges and future directions
Cancer immunotherapy research has begun to bear fruit only in recent years, principally in the form of checkpoint blockades, CAR-T cell therapies and cancer vaccines, while new approaches, such as modulation of tumor microenvironment, are being developed rapidly. With the same momentum, a growing number of advanced nanomedicines for cancer immunotherapy are being investigated in pre-clinical and clinical stages. Given the ease of spatiotemporal control, light-responsive nanomedicine has attracted increasing attention in research on cancer immunotherapy. In the review, the combination of light-responsive nanomedicine with four types of widely used immunotherapies was illustrated with representative examples. The mechanism, virtues and limitations of each modality were discussed as well.
Despite the potential achievements summarized in this review, light-responsive nanomedicine for immunotherapy is still at its early stage with preclinical studies. More endeavours are still needed to tackle the unresolved issues to advance its clinical translation potential. Further progresses can be expected particularly in the following aspects: (1) Comprehensive evaluation of biosafety studies. Comprehensive biosafety studies are needed to characterize the toxicity profiles of nanomedicine in cancer immunotherapy. Light-responsive nanomedicine has been demonstrated to have greater selectivity and higher efficacy in activating adoptive immune response, but it is unclear whether the autoimmune side effects will be increased. (2) Mass production and manufacturing cost. Nanomedicine and cancer immunotherapy are generally more expensive than other existing treatments even in the optimistic setting. For example, in CAR-T therapy cell generation and administration processes can cost $373,000–475,000133. Introducing light-responsiveness may lead to an increase in complexity and duration for manufacturing. Therefore, simple formulation design with scaling-up capability will greatly accelerate the clinical translation of light-responsive nanomedicine in cancer immunotherapy. (3) Development of advanced light source. A homogeneous illumination from light source is a key factor for the success of light-responsive nanomedicine. In addition, the penetration of light in vivo is also a major concern to realize desired treatment efficacy. Despite that the light within the NIR window displays relatively better tissue penetration, the precise light delivery at the diseased regimen remains a current challenge. One study has applied optical fibres in treating esophageal carcinoma via introducing optical fibres through the biopsy channel of the endoscope to improve light delivery precision and overcome the limitation of delivery distance134. For tumors deep in the human body such as liver cancer, portable and insertable micro-LEDs with feasibility to match with targeting tissues serve as a potential modality to overcome the limited penetration of light source.
Furthermore, a variety of strategies can be exploited to improve the treatment efficacy of light-responsive nanomedicine for immunotherapy. Firstly, four types of immunotherapies can work cooperatively to achieve boosted immunity. Combination of different immunotherapies represents as a bellwether for clinical use in the future. Secondly, the internal stimuli, such as enzymes, redox, hypoxia and pH, can be employed with light irradiation for dual-responsive drug delivery to further improve precise targeting and reduce off-target drug release135.
Light-responsive nanomedicine for cancer immunotherapy is at the cutting edge of cancer therapy. The precise and controllable nature of light-responsive nanomedicine fits the need for cancer immunotherapy. With continuous advancements in light-responsive nanotechnology and increasing knowledge of cancer immunology and biology, it is anticipated that light-responsive nanomedicine will move from the laboratory to the clinic.
Acknowledgments
This study was supported by Hong Kong Research Grants Council, University Grants Committee (No. 2711522, Hong Kong, China), and Ming Wai Lau Centre for Reparative Medicine (Associate Member Programme, Hong Kong, China).
Author contributions
Weirong Kang and Weiping Wang conceptualized and designed this article. Weirong Kang and Yuwei Liu wrote and revised the manuscript. Weiping Wang revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quesada J.R., Hersh E.M., Manning J., Reuben J., Keating M., Schnipper E., et al. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. 1986;68:493–497. [PubMed] [Google Scholar]
- 3.Rosenberg S.A. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 4.Jin Y., Li H.T., Zhang P., Yu M., Zhang H., Li X. The regulatory approvals of immune checkpoint inhibitors in China and the United States: a cross-national comparison study. Int J Cancer. 2023;152:2351–2361. doi: 10.1002/ijc.34427. [DOI] [PubMed] [Google Scholar]
- 5.Tawfik E.A., Aldrak N.A., Albrahim S.H., Alzahrani D.A., Alfassam H.A., Alkoblan S.M., et al. Immunotherapy in hematological malignancies: recent advances and open questions. Immunotherapy. 2021;13:1215–1229. doi: 10.2217/imt-2021-0065. [DOI] [PubMed] [Google Scholar]
- 6.Pires A., Burnell S., Gallimore A. Exploiting ECM remodelling to promote immune-mediated tumour destruction. Curr Opin Immunol. 2022;74:32–38. doi: 10.1016/j.coi.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Ishida O., Maruyama K., Sasaki K., Iwatsuru M. Size-dependent extravasation and interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. Int J Pharm. 1999;190:49–56. doi: 10.1016/s0378-5173(99)00256-2. [DOI] [PubMed] [Google Scholar]
- 8.Zahednezhad F., Saadat M., Valizadeh H., Zakeri-Milani P., Baradaran B. Liposome and immune system interplay: challenges and potentials. J Control Release. 2019;305:194–209. doi: 10.1016/j.jconrel.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Liaw K., Reddy R., Sharma A., Li J., Chang M., Sharma R., et al. Targeted systemic dendrimer delivery of CSF-1R inhibitor to tumor-associated macrophages improves outcomes in orthotopic glioblastoma. Bioeng Transl Med. 2021;6 doi: 10.1002/btm2.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong X.F., Sun X. Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy. Acta Pharmacol Sin. 2020;41:928–935. doi: 10.1038/s41401-020-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Song J., Lou L., Qi X.J., Zhao L., Fan B., et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng Transl Med. 2021;6 doi: 10.1002/btm2.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F., et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:1–12. [Google Scholar]
- 13.Mitchell M.J., Jain R.K., Langer R. Engineering and physical sciences in oncology: challenges and opportunities. Nat Rev Cancer. 2017;17:659–675. doi: 10.1038/nrc.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlett D.W., Su H., Hildebrandt I.J., Weber W.A., Davis M.E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 16.Liu J.Z., Kang W.R., Wang W.P. Photocleavage-based photoresponsive drug delivery. Photochem Photobiol. 2022;98:288–302. doi: 10.1111/php.13570. [DOI] [PubMed] [Google Scholar]
- 17.Rwei A.Y., Wang W., Kohane D.S. Photoresponsive nanoparticles for drug delivery. Nano Today. 2015;10:451–467. doi: 10.1016/j.nantod.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhi D.F., Yang T., O'Hagan J., Zhang S.B., Donnelly R.F. Photothermal therapy. J Control Release. 2020;325:52–71. doi: 10.1016/j.jconrel.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Hou X.Y., Tao Y.K., Pang Y.Y., Li X.X., Jiang G., Liu Y.Q. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143:3050–3060. doi: 10.1002/ijc.31717. [DOI] [PubMed] [Google Scholar]
- 20.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Labani-Motlagh A., Ashja-Mahdavi M., Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. doi: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz J.M., Lenardo M.J. Development of immune checkpoint therapy for cancer. J Exp Med. 2019;216:1244–1254. doi: 10.1084/jem.20182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchbinder E., Hodi F.S. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125:3377–3383. doi: 10.1172/JCI80012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schadendorf D., Hodi F.S., Robert C., Weber J.S., Margolin K., Hamid O., et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribas A., Kefford R., Marshall M.A., Punt C.J., Haanen J.B., Marmol M., et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graydon C.G., Mohideen S., Fowke K.R. LAG3's enigmatic mechanism of action. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.615317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruhashi T., Sugiura D., Okazaki I.M., Okazaki T. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang A.C., Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23:660–670. doi: 10.1038/s41590-022-01141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang S., Wei W., Zhao Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int J Biol Sci. 2020;16:1767–1773. doi: 10.7150/ijbs.41105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang S., Powderly J.D., Spira A.I., Bakkacha O., Loo D., Bohac G.C., et al. vol. 39. 15_suppl; 2021. p. 2631. (Phase 1 dose escalation study of MGC018, an anti-B7-H3 antibody-drug conjugate (ADC), in patients with advanced solid tumors). [Google Scholar]
- 32.Sachdev J.C., Bauer T.M., Chawla S.P., Pant S., Patnaik A., Wainberg Z.A., et al. vol. 37. 15_suppl; 2019. p. 2529. (Phase 1a/1b study of first-in-class B7-H4 antibody, FPA150, as monotherapy in patients with advanced solid tumors). [Google Scholar]
- 33.Gauzy-Lazo L., Sassoon I., Brun M.P. Advances in antibody–drug conjugate design: current clinical landscape and future innovations. SLAS Discov. 2020;25:843–868. doi: 10.1177/2472555220912955. [DOI] [PubMed] [Google Scholar]
- 34.Kaushik I., Ramachandran S., Zabel C., Gaikwad S., Srivastava S.K. The evolutionary legacy of immune checkpoint inhibitors. Semin Cancer Biol. 2022;86:491‒8. doi: 10.1016/j.semcancer.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Hafeez U., Parakh S., Gan H.K., Scott A.M. Antibody–drug conjugates for cancer therapy. Molecules. 2020;25:4764. doi: 10.3390/molecules25204764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matlung H.L., Szilagyi K., Barclay N.A., van den Berg T.K. The CD47–SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145–164. doi: 10.1111/imr.12527. [DOI] [PubMed] [Google Scholar]
- 37.van den Berg T.K., Valerius T. Myeloid immune-checkpoint inhibition enters the clinical stage. Nat Rev Clin Oncol. 2019;16:275–276. doi: 10.1038/s41571-018-0155-3. [DOI] [PubMed] [Google Scholar]
- 38.Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanietsky N., Simic H., Arapovic J., Toporik A., Levy O., Novik A., et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chauvin J.M., Pagliano O., Fourcade J., Sun Z., Wang H., Sander C., et al. TIGIT and PD-1 impair tumor antigen-specific CD8⁺ T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inozume T., Yaguchi T., Furuta J., Harada K., Kawakami Y., Shimada S. Melanoma cells control antimelanoma CTL responses via interaction between TIGIT and CD155 in the effector phase. J Invest Dermatol. 2016;136:255–263. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Abreu D., Johnson M.L., Hussein M.A., Cobo M., Patel A.J., Secen N.M., et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE) J Clin Oncol. 2020;38(15_suppl):9503. [Google Scholar]
- 44.Michot J.M., Bigenwald C., Champiat S., Collins M., Carbonnel F., Postel-Vinay S., et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg S.A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233:1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- 46.Radvanyi L.G. Tumor-infiltrating lymphocyte therapy: addressing prevailing questions. Cancer J. 2015;21:450–464. doi: 10.1097/PPO.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 47.Robbins P.F., Morgan R.A., Feldman S.A., Yang J.C., Sherry R.M., Dudley M.E., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oba-Shinjo S.M., Caballero O.L., Jungbluth A.A., Rosemberg S., Old L.J., Simpson A.J., et al. Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun. 2008;8:7. [PMC free article] [PubMed] [Google Scholar]
- 49.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.U.S. Food and Drug Administration. Approved cellular and gene therapy products. Availble from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
- 51.Martinez M., Moon E.K. CAR T cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rezvani K., Rouce R., Liu E., Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y.Z., Yu Z.Y., Tan X.W., Jiang H.F., Xu Z., Fang Y.L., et al. CAR-macrophage: a new immunotherapy candidate against solid tumors. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111605. [DOI] [PubMed] [Google Scholar]
- 54.Villanueva M.T. Macrophages get a CAR. Nat Rev Immunol. 2020;20:273. doi: 10.1038/s41577-020-0302-9. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C., Oberoi P., Oelsner S., Waldmann A., Lindner A., Tonn T., et al. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front Immunol. 2017;8:533. doi: 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Igarashi Y., Sasada T. Cancer vaccines: toward the next breakthrough in cancer immunotherapy. J Immunol Res. 2020;2020 doi: 10.1155/2020/5825401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santos P.M., Butterfield L.H. Dendritic cell-based cancer vaccines. J Immunol. 2018;200:443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W.S., Tang H.C., Li L.F., Wang X.Y., Yu Z.J., Li J.P. Peptide-based therapeutic cancer vaccine: current trends in clinical application. Cell Prolif. 2021;54 doi: 10.1111/cpr.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan C.M., Qu H.K., Wang X., Sobhani N., Wang L., Liu S.L., et al. Cancer/testis antigens: from serology to mRNA cancer vaccine. Semin Cancer Biol. 2021;76:218–231. doi: 10.1016/j.semcancer.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Berzofsky J.A., Terabe M., Trepel J.B., Pastan I., Stroncek D.F., Morris J.C., et al. Cancer vaccine strategies: translation from mice to human clinical trials. Cancer Immunol Immunother. 2018;67:1863–1869. doi: 10.1007/s00262-017-2084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallerla S., Abdul A., Comeau J., Jois S. Cancer vaccines, treatment of the future: with emphasis on HER2-positive breast cancer. Int J Mol Sci. 2021;22:779–794. doi: 10.3390/ijms22020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murciano-Goroff Y.R., Warner A.B., Wolchok J.D. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Looi C.K., Chung F.F., Leong C.O., Wong S.F., Rosli R., Mai C.W. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38:162. doi: 10.1186/s13046-019-1153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu C., Rong D.W., Zhang B., Zheng W.B., Wang X.H., Chen Z.Y., et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. doi: 10.1186/s12943-019-1047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berraondo P., Sanmamed M.F., Ochoa M.C., Etxeberria I., Aznar M.A., Pérez-Gracia J.L., et al. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hervas-Stubbs S., Perez-Gracia J.L., Rouzaut A., Sanmamed M.F., Le Bon A., Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 68.Cannarile M.A., Weisser M., Jacob W., Jegg A.M., Ries C.H., Rüttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5:53. doi: 10.1186/s40425-017-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seif F., Khoshmirsafa M., Aazami H., Mohsenzadegan M., Sedighi G., Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Candido J.B., Morton J.P., Bailey P., Campbell A.D., Karim S.A., Jamieson T., et al. CSF1R(+) macrophages sustain pancreatic tumor growth through T cell suppression and maintenance of key gene programs that define the squamous subtype. Cell Rep. 2018;23:1448–1460. doi: 10.1016/j.celrep.2018.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McLornan D.P., Pope J.E., Gotlib J., Harrison C.N. Current and future status of JAK inhibitors. Lancet. 2021;398:803–816. doi: 10.1016/S0140-6736(21)00438-4. [DOI] [PubMed] [Google Scholar]
- 72.Zhou Y., Chen X.C., Cao J., Gao H.L. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B. 2020;8:6765–6781. doi: 10.1039/d0tb00649a. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y.P., Yu J., Luo Z.J., Shi Q.K., Liu G.L., Wu F., et al. Engineering endogenous tumor-associated macrophage-targeted biomimetic Nano-RBC to reprogram tumor immunosuppressive microenvironment for enhanced chemo-immunotherapy. Adv Mater. 2021;33 doi: 10.1002/adma.202103497. [DOI] [PubMed] [Google Scholar]
- 74.Zhao H.K., Wu L., Yan G.F., Chen Y., Zhou M.Y., Wu Y.Z., et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Targeted Ther. 2021;6:263. doi: 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riley R.S., June C.H., Langer R., Mitchell M.J. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luan X.W., Pan Y.C., Gao Y.F., Song Y.J. Recent near-infrared light-activated nanomedicine toward precision cancer therapy. J Mater Chem B. 2021;9:7076–7099. doi: 10.1039/d1tb00671a. [DOI] [PubMed] [Google Scholar]
- 77.Hüll K., Morstein J., Trauner D. In vivo photopharmacology. Chem Rev. 2018;118:10710–10747. doi: 10.1021/acs.chemrev.8b00037. [DOI] [PubMed] [Google Scholar]
- 78.Pecot M.Y., Malhotra V. Golgi membranes remain segregated from the endoplasmic reticulum during mitosis in mammalian cells. Cell. 2004;116:99–107. doi: 10.1016/s0092-8674(03)01068-7. [DOI] [PubMed] [Google Scholar]
- 79.Ankenbruck N., Courtney T., Naro Y., Deiters A. Optochemical control of biological processes in cells and animals. Angew Chem Int Ed Engl. 2018;57:2768–2798. doi: 10.1002/anie.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weinstain R., Slanina T., Kand D., Klán P. Visible-to-NIR-light activated release: from small molecules to nanomaterials. Chem Rev. 2020;120:13135–13272. doi: 10.1021/acs.chemrev.0c00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 82.Maeding N., Verwanger T., Krammer B. Boosting tumor-specific immunity using PDT. Cancers. 2016;8:91–104. doi: 10.3390/cancers8100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zou J.H., Li L., Yang Z., Chen X.Y. Phototherapy meets immunotherapy: a win–win strategy to fight against cancer. Nanophotonics. 2021;10:3229–3245. [Google Scholar]
- 84.Peng J.R., Yang Q., Xiao Y., Shi K., Liu Q.G., Hao Y., et al. Tumor microenvironment responsive drug‒dye‒peptide nanoassembly for enhanced tumor—targeting, penetration, and photo‒chemo‒immunotherapy. Adv Funct Mater. 2019;29 [Google Scholar]
- 85.Jia Y.P., Shi K., Yang F., Liao J.F., Han R.X., Yuan L.P., et al. Multifunctional nanoparticle loaded injectable thermoresponsive hydrogel as NIR controlled release platform for local photothermal immunotherapy to prevent breast cancer postoperative recurrence and metastases. Adv Funct Mater. 2020;30 [Google Scholar]
- 86.Hu L.Q., Cao Z.Y., Ma L.L., Liu Z.Q., Liao G.C., Wang J.X., et al. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials. 2019;223 doi: 10.1016/j.biomaterials.2019.119469. [DOI] [PubMed] [Google Scholar]
- 87.Choi J., Shim M.K., Yang S., Hwang H.S., Cho H., Kim J., et al. Visible-light-triggered prodrug nanoparticles combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. ACS Nano. 2021;15:12086–12098. doi: 10.1021/acsnano.1c03416. [DOI] [PubMed] [Google Scholar]
- 88.Li J.C., Cui D., Huang J.Q., He S.S., Yang Z.B., Zhang Y., et al. Organic semiconducting pro-nanostimulants for near-infrared photoactivatable cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58:12680–12687. doi: 10.1002/anie.201906288. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y.W., Long K.Q., Kang W.E., Wang T.Y., Wang W.P. Optochemical control of immune checkpoint blockade via light-triggered PD-L1 dimerization. Adv NanoBiomed Res. 2022;2 [Google Scholar]
- 90.Huang Z.L., Wu Y.Q., Allen M.E., Pan Y.J., Kyriakakis P., Lu S.Y., et al. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen N.T., Huang K., Zeng H., Jing J., Wang R., Fang S., et al. Nano-optogenetic engineering of CAR T cells for precision immunotherapy with enhanced safety. Nat Nanotechnol. 2021;16:1424–1434. doi: 10.1038/s41565-021-00982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Q., Hu Q.Y., Dukhovlinova E., Chen G.J., Ahn S., Wang C., et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31 doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song Y.Y., Chen Y.Z., Li P., Dong C.M. Photoresponsive polypeptide-glycosylated dendron amphiphiles: UV-triggered polymersomes, OVA release, and in vitro enhanced uptake and immune response. Biomacromolecules. 2020;21:5345–5357. doi: 10.1021/acs.biomac.0c01465. [DOI] [PubMed] [Google Scholar]
- 94.Chen Z.Z., Zhang Q., Zeng L.J., Zhang J.L., Liu Z.H., Zhang M.X., et al. Light-triggered OVA release based on CuS@poly(lactide-co-glycolide acid) nanoparticles for synergistic photothermal-immunotherapy of tumor. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104902. [DOI] [PubMed] [Google Scholar]
- 95.Zhang C.N., Zhang J., Shi G.N., Song H.J., Shi S.B., Zhang X.Y., et al. A light responsive nanoparticle-based delivery system using pheophorbide a graft polyethylenimine for dendritic cell-based cancer immunotherapy. Mol Pharm. 2017;14:1760–1770. doi: 10.1021/acs.molpharmaceut.7b00015. [DOI] [PubMed] [Google Scholar]
- 96.Pan J.B., Wang Y.Q., Zhang C., Wang X.Y., Wang H.Y., Wang J.J., et al. Antigen-directed fabrication of a multifunctional nanovaccine with ultrahigh antigen loading efficiency for tumor photothermal-immunotherapy. Adv Mater. 2018;30 doi: 10.1002/adma.201704408. [DOI] [PubMed] [Google Scholar]
- 97.Chen Q., Huang G.J., Wu W.T., Wang J.W., Hu J.W., Mao J.M., et al. A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020;32 doi: 10.1002/adma.201908185. [DOI] [PubMed] [Google Scholar]
- 98.Wang H.R., Guo Y.F., Gan S.J., Liu H.H., Chen Q., Yuan A., et al. Photosynthetic microorganisms-based biophotothermal therapy with enhanced immune response. Small. 2021;17 doi: 10.1002/smll.202007734. [DOI] [PubMed] [Google Scholar]
- 99.Chu H.Q., Zhao J., Mi Y.S., Di Z.H., Li L.L. NIR-light-mediated spatially selective triggering of anti-tumor immunity via upconversion nanoparticle-based immunodevices. Nat Commun. 2019;10:2839. doi: 10.1038/s41467-019-10847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao X., Zhang K.X., Wang Y.Y., Jiang W.X., Cheng H., Wang Q.G., et al. Intracellular self-assembly driven nucleus-targeted photo-immune stimulator with chromatin decompaction function for robust innate and adaptive antitumor immunity. Adv Funct Mater. 2022;32 [Google Scholar]
- 101.Fang L., Zhao Z.T., Wang J., Xiao P., Sun X.S., Ding Y.P., et al. Light-controllable charge-reversal nanoparticles with polyinosinic-polycytidylic acid for enhancing immunotherapy of triple negative breast cancer. Acta Pharm Sin B. 2022;12:353–363. doi: 10.1016/j.apsb.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yue Y.L., Li F.F., Li Y., Wang Y.Z., Guo X.J., Cheng Z.X., et al. Biomimetic nanoparticles carrying a repolarization agent of tumor-associated macrophages for remodeling of the inflammatory microenvironment following photothermal therapy. ACS Nano. 2021;15:15166–15179. doi: 10.1021/acsnano.1c05618. [DOI] [PubMed] [Google Scholar]
- 103.Chen C.L., Song M.Y., Du Y.Y., Yu Y., Li C.G., Han Y., et al. Tumor-associated-macrophage-membrane-coated nanoparticles for improved photodynamic immunotherapy. Nano Lett. 2021;21:5522–5531. doi: 10.1021/acs.nanolett.1c00818. [DOI] [PubMed] [Google Scholar]
- 104.Fu X.C., Yu J.M., Yuan A.R., Liu L.B., Zhao H., Huang Y.M., et al. Polymer nanoparticles regulate macrophage repolarization for antitumor treatment. Chem Commun. 2021;57:6919–6922. doi: 10.1039/d1cc02678j. [DOI] [PubMed] [Google Scholar]
- 105.Huang Y.J., Guan Z.L., Dai X.L., Shen Y.F., Wei Q., Ren L.L., et al. Engineered macrophages as near-infrared light activated drug vectors for chemo-photodynamic therapy of primary and bone metastatic breast cancer. Nat Commun. 2021;12:4310. doi: 10.1038/s41467-021-24564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Y.X., Ren X.M., Hou Z.S., Wang N.N., Jiang Y., Luan Y.X. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horiz. 2021;6:120–131. doi: 10.1039/d0nh00480d. [DOI] [PubMed] [Google Scholar]
- 107.Hartrampf N., Seki T., Baumann A., Watson P., Vepřek N.A., Hetzler B.E., et al. Optical control of cytokine production using photoswitchable galactosylceramides. Chemistry. 2020;26:4476–4479. doi: 10.1002/chem.201905279. [DOI] [PubMed] [Google Scholar]
- 108.Xu Y., Hyun Y.M., Lim K., Lee H., Cummings R.J., Gerber S.A., et al. Optogenetic control of chemokine receptor signal and T-cell migration. Proc Natl Acad Sci U S A. 2014;111:6371–6376. doi: 10.1073/pnas.1319296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang W.L., Zheng H.Z., Jiang J., Li Z., Jiang D.P., Shi X.R., et al. Engineering micro oxygen factories to slow tumor progression via hyperoxic microenvironments. Nat Commun. 2022;13:4495. doi: 10.1038/s41467-022-32066-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lan G.X., Ni K.Y., Xu Z.W., Veroneau S.S., Song Y., Lin W.B. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc. 2018;140:5670–5673. doi: 10.1021/jacs.8b01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swoboda A., Nanda R. Immune checkpoint blockade for breast cancer. Cancer Treat Res. 2018;173:155–165. doi: 10.1007/978-3-319-70197-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tun A.M., Thein K.Z., Thein W.L., Guevara E. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA. 2019;5:Fso421. doi: 10.2144/fsoa-2019-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang T.Y., Huang G.L., Zhang C.Y., Zhuang B.W., Liu B.X., Su L.Y., et al. Supramolecular photothermal nanomedicine mediated distant tumor inhibition via PD-1 and TIM-3 blockage. Front Chem. 2020;8:1. doi: 10.3389/fchem.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang F., Lu G.H., Wen X.L., Li F., Ji X.Y., Li Q.Q., et al. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J Control Release. 2020;326:131–139. doi: 10.1016/j.jconrel.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 115.Miao L.L., Zhang Z.C., Ren Z.J., Li Y.M. Reactions related to CAR-T cell therapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.663201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramos C.A., Savoldo B., Dotti G. CD19-CAR trials. Cancer J. 2014;20:112–118. doi: 10.1097/PPO.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stapleton S., Dunne M., Milosevic M., Tran C.W., Gold M.J., Vedadi A., et al. Radiation and heat improve the delivery and efficacy of nanotherapeutics by modulating intratumoral fluid dynamics. ACS Nano. 2018;12:7583–7600. doi: 10.1021/acsnano.7b06301. [DOI] [PubMed] [Google Scholar]
- 119.Mi Y., Hagan CTt, Vincent B.G., Wang A.Z. Emerging nano-/microapproaches for cancer immunotherapy. Adv Sci. 2019;6 doi: 10.1002/advs.201801847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi J.J., Nie W.M., Zhao X., Yang X.Y., Cheng H., Zhou T.H., et al. An intracellular self-assembly-driven uninterrupted ROS generator augments 5-aminolevulinic-acid-based tumor therapy. Adv Mater. 2022;34 doi: 10.1002/adma.202201049. [DOI] [PubMed] [Google Scholar]
- 121.Du X.Q., Hou Y.Y., Huang J., Pang Y., Ruan C.L., Wu W., et al. Cytosolic delivery of the immunological adjuvant Poly I:C and cytotoxic drug crystals via a carrier-free strategy significantly amplifies immune response. Acta Pharm Sin B. 2021;11:3272–3285. doi: 10.1016/j.apsb.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kumari N., Choi S.H. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022;41:68. doi: 10.1186/s13046-022-02272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruffell B., Coussens L.M. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumor-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.An J.Y., Liu M.Y., Zhao L., Lu W.X., Wu S.X., Zhang K.X., et al. Boosting tumor immunotherapy by bioactive nanoparticles via Ca2+ interference mediated TME reprogramming and specific PD-L1 depletion. Adv Funct Mater. 2022;32 [Google Scholar]
- 126.Tan H.Y., Wang N., Li S., Hong M., Wang X., Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Y., Que K.T., Zhang Z., Yi Z.J., Zhao P.X., You Y., et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018;7:4012–4022. doi: 10.1002/cam4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He W., Kapate N., Shields CWt, Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv Drug Deliv Rev. 2020;165–166:15–40. doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Qiu Q.J., Li C., Yan X.Y., Zhang H.X., Luo X., Gao X., et al. Photodynamic/photothermal therapy enhances neutrophil-mediated ibrutinib tumor delivery for potent tumor immunotherapy: more than one plus one? Biomaterials. 2021;269 doi: 10.1016/j.biomaterials.2021.120652. [DOI] [PubMed] [Google Scholar]
- 130.Deng G.J., Sun Z.H., Li S.P., Peng X.H., Li W.J., Zhou L.H., et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 131.Du Four S., Maenhout S.K., De Pierre K., Renmans D., Niclou S.P., Thielemans K., et al. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2014.998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Löb S., Königsrainer A., Rammensee H.G., Opelz G., Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 133.Geethakumari P.R., Ramasamy D.P., Dholaria B., Berdeja J., Kansagra A. Balancing quality, cost, and access during delivery of newer cellular and immunotherapy treatments. Curr Hematol Malig Rep. 2021;16:345–356. doi: 10.1007/s11899-021-00635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reginato E., Lindenmann J., Langner C., Schweintzger N., Bambach I., Smolle-Juettner F., et al. Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochem Photobiol Sci. 2014;13:1281–1289. doi: 10.1039/c4pp00186a. [DOI] [PubMed] [Google Scholar]
- 135.Jia W.F., Liu R., Wang Y.S., Hu C., Yu W.Q., Zhou Y., et al. Dual-responsive nanoparticles with transformable shape and reversible charge for amplified chemo-photodynamic therapy of breast cancer. Acta Pharm Sin B. 2022;12:3354–3366. doi: 10.1016/j.apsb.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]