Abstract
The functional capacity of organisms declines in the process of aging. In the case of breast tissue, abnormal mammary gland development can lead to dysfunction in milk secretion, a primary function, as well as the onset of various diseases, such as breast cancer. In the process of aging, the terminal duct lobular units (TDLUs) within the breast undergo gradual degeneration, while the proportion of adipose tissue in the breast continues to increase and hormonal levels in the breast change accordingly. Here, we review changes in morphology, internal structure, and cellular composition that occur in the mammary gland during aging. We also explore the emerging mechanisms of breast aging and the relationship between changes during aging and breast-related diseases, as well as potential interventions for delaying mammary gland aging and preventing breast disease.
Keywords: aging, mammary gland, breast disease, breast cancer
Introduction
Mammary glands are epidermal attachments that may originate from the apocrine glands [1]. The main function of the mammary gland is to secrete milk for offspring, and abnormal development can result in hypolactation and the occurrence of breast disease.
Aging is a noteworthy factor in human breast development and related diseases. There has been a definitive trend towards delayed childbirth and breastfeeding in reproductive-aged women, with an increase in the birthrates of women aged 35–39, 40–44, and 45–49 of 30%, 47%, and 190%, respectively, from 1990 to 2001 [2]. This increase in the age of breastfeeding women may impact human mammary gland development, resulting in a higher risk of hypolactation and breast-related diseases.
In addition, age is the most significant risk factor for breast cancer, with the incidence of breast cancer gradually increasing with age. Studies have found that more than 80% of breast cancers occur after the age of 50 [3]. Therefore, studying the changes that occur in breast development during aging is necessary to understand the functional defects and diseases of aged mammary glands.
As women age, their breasts undergo a series of biological changes, including regression of terminal duct lobular units (TDLUs), increased breast density and fat pads, hormonal changes, and cellular transformation. These changes are often associated with the occurrence of breast-related diseases. Hormonal fluctuations in the mammary gland during aging can also decrease the incidence of benign breast diseases such as fibroadenoma (FA) [ 4– 6] . Furthermore, normal TDLU degeneration during aging reduces the incidence of breast cancer, while abnormal degeneration and pro-inflammatory factor secretion can lead to an increase in the incidence of breast cancer [ 7, 8] . In addition, overall breast density decreases during aging, which is a strong risk factor for breast cancer [9].
As the world’s population continues to age, economic challenges and social burdens will become increasingly severe, highlighting the importance of aging-related research [10]. Breast tissue exhibits accelerated aging compared to other organs, such as the spleen, skin, adipose tissue, ovary, lung, lymph nodes, and bladder [ 11, 12] . This review focuses on the histological, cellular, and molecular changes that occur in the mammary gland during aging, as well as the relationship between these changes and the occurrence and pathogenesis of breast diseases.
Structural and Histological Changes of Breast During Aging
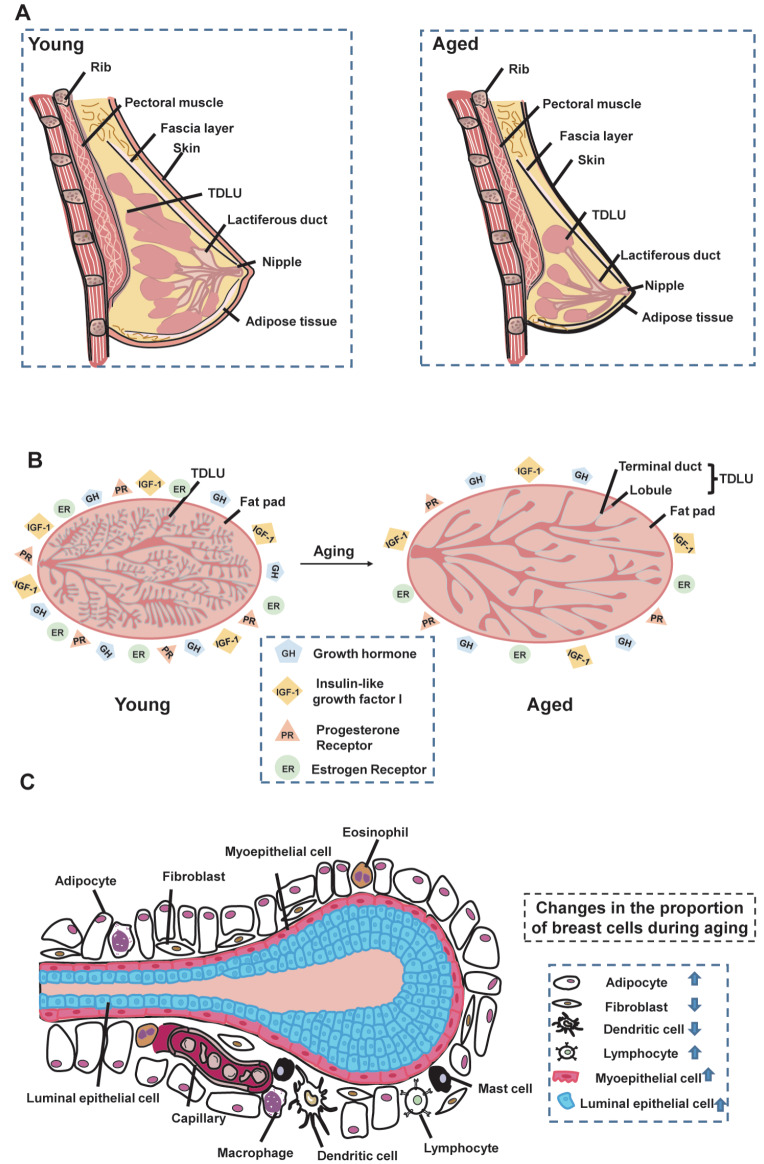
The female breast is composed of outer skin tissue and inner breast tissue [13]. The skin layer consists of the epidermis and dermis, which covers the entire surface of the mammary gland, including the nipple and areola ( Figure 1A). Under the mammary epidermis lies the dermis, which primarily consists of elastic fibers and collagen and connects the epidermis to the fibro-adipose tissue [14]. The internal structure of the mammary gland is composed of a fascial layer, fibro-adipose pocket, and fibroglandular tissue [13]. The fascial layer covers the surface of the mammary glandular tissue [ 15, 16] . Underneath this layer, the fibro-adipose pocket is composed of adipose and fibrous tissues, which play an essential role in morphological support. The mammary glandular tissue is composed of glandular lobes located in a central location throughout the mammary gland [ 17, 18] . These lobes consist of ducts with lobules for transporting milk to the nipple.
Figure 1 .
Mammary gland structure and changes during aging
(A) Overall structure of mammary gland. Mammary gland is primarily composed of outer epidermal tissue and inner adipose and glandular tissues. Changes in mammary gland morphology during aging. With increasing age, the epidermis of the female breast continues to thin, the elasticity of the mammary gland decreases, and the mammary gland matrix becomes soft and undergoes ptosis as it is replaced by fatty tissue. (B) Changes in mammary gland structure during aging. Changes in TDLU, fat pads, hormones, and overall density of the mammary gland during aging. Left, image of a young mammary gland; right, image of an older mammary gland, with arrows indicating the aging process. Breasts in aged women have reduced TDLU and increased proportion of fat pads compared to young breasts. Overall breast density can be expressed by the ratio of glandular tissue to adipose tissue. With the reduction in glandular tissue, the ratio decreases and overall breast density decreases. Growth hormone (GH) and insulin-like growth factor 1 (IGF-1) decline with age. (C) Changes in breast cells during aging. Breast cells are composed of epithelial cells (including luminal and myoepithelial cells) and stromal cells (including adipocytes, fibroblasts, macrophages, immune cells, vascular cells). Compared with normal young women, the proportion of adipocytes and lymphocytes in the breasts of older women are increased, and the proportions of fibroblasts and dendritic cells are decreased. Arrows indicate increasing and decreasing proportions of cells.
Female mammary gland elasticity begins to decline around the age of 25, and the mammary epidermis begins to thin around the age of 40 ( Figure 1A), eventually leading to changes in breast morphology [13]. During breast involution, the breast matrix is replaced by fat, causing the breast to become soft and ptotic [19]. As a woman ages, the breast undergoes various structural and histological changes.
Breast TDLU degradation during aging
Human mammary gland tissue is composed of 12–15 main lobes, each containing many TDLUs [20]. TDLUs represent the functional units of the mammary gland, responsible for milk production during lactation and considered the primary sites for the development of breast cancer [21]. TDLUs experience a series of changes during mammary gland development ( Figure 1B).
Mammary gland development begins in the embryonic period, then further develops under the stimulation of hormones such as growth hormone (GH), insulin-like growth factor 1 (IGF-1), and estrogen during puberty, before reaching full maturity during lactation and forming mammary glands in the fat pad. TDLUs gradually degenerate with the completion of childbearing and increasing age [22].
Involution of TDLUs is a physiological aging process of the breast tissue, characterized by a decline in the epithelial component [20] and related to the complexity and extent of the ductal epithelium [23]. In the human mammary gland, TDLUs degenerate with age, leading to a reduction in TDLU size, total TDLU number, and acini number per TDLU [24]. The reduction in acini and shorter TDLU spans ultimately lead to reduced TDLUs [25]. Lobular degeneration resulting from aging differs from post-lactation degeneration, which is characterized by marked apoptosis and morphological changes. The less extensive changes in age-dependent TDLU involution are negatively correlated with the risk of breast cancer [26].
In the process of aging, several canonical senescence pathways regulate the degradation of TDLUs. Among these pathways, the insulin and IGF-1 signaling (IIS) pathway is associated with dysregulated nutrient sensing, which is a hallmark of aging. The IIS pathway is one of the most evolutionarily conserved aging control pathway and its multiple targeting sequences include the FOXO family of transcription factors and the mTOR complex [ 27– 29] . Mechanistically, the IIS pathway can activate PI3K/AKT, leading to FOXO inhibition and mTOR activation. The downstream target genes of FOXO and mTOR, such as cyclin B, GADD45, 4E-BP1 and S6K, regulate the cell cycle, metabolism, apoptosis, and cell stress response [ 30, 31] . Decreased IIS is characteristic of both physiological and accelerated aging [30]. The IIS pathway plays a role not only in body aging, but also in the regulation of TDLU degradation. Notably, increased circulating IGF factors are closely related to the degradation and reduction of TDLUs, which can induce breast disease during aging [25].
Changes in mammary adipose tissue during aging
The mammary gland is predominantly composed of glandular and adipose tissues. Adipose tissue in the mammary gland includes white, pink, and brown adipose tissue (WAT, PAT, and BAT, respectively). Glandular and adipose tissues can exhibit mutual transition under certain circumstances. For example, WAT can differentiate into mammary glandular tissue and WAT and PAT (responsible for lactation) can transition into one another during pregnancy [32]. BAT, the primary source of energy for infants, is gradually replaced by WAT with increasing age [33], a process closely related to the incidence of breast cancer [34].
Mammary adipose tissue can secrete many factors, including adipokines, cytokines, chemokines, and growth factors, which control various cellular processes involved in breast cancer development [35]. Among these factors, resistin plays an essential role in controlling TLR-4 expression, leading to NF-κB and STAT3 pathway activation, mitochondrial dysfunction, cell apoptosis, cell proliferation, and eventually cell stemness and breast cancer progression [36]. Overactivation of NF-κB is a transcriptomic hallmark of aging, with the application of NF-κB inhibitors on the skin surface of aged transgenic mice shown to result in a younger phenotype and expression pattern [37]. As the proportion of breast adipose tissue increases during aging, there is an apparent corresponding increase in resistin expression, which, in turn, activates the TLR4/NF-κB/STAT3 signaling pathway and promotes breast cancer.
Adipose tissue within the breast increases with body mass and breast volume, as well as with advancing age [38]. Systemic lipid accumulation occurs during aging, resulting in a marked expansion of the mammary fat pad ( Figure 1B). Moreover, menopause is regarded as a critical point for many age-related changes, with several studies showing that postmenopausal breast adipose tissue increases more rapidly with age, accompanied by a decrease in parenchymal components [39]. The underlying causes of age-related mammary fat gain are complex and variable but may include decreased lipase activity for fat breakdown. Baldwin et al. [40] reported that adipose lipase activity is higher in young populations of rats, guinea pigs, and cows than in older populations. In addition, loss of the lipid-membrane-scaffolding protein metacaveolin-1 (Cav-1) in the mammary gland can result in disrupted lipid accumulation and reduction in the mammary fat pad, manifesting as a decrease in adipocyte diameter and the formation of poorly differentiated white adipose parenchyma [41]. The loss of lipid related protein Caveolin-1 in fibroblasts also promotes the progression of breast cancer via TGF-β/Smad signaling pathway [42].
Hormonal changes in breast during aging
Hormones in the mammary gland are predominantly secreted from the pituitary gland and ovaries, both of which undergo age-related degeneration [ 43, 44] . These secreted hormones, such as estrogen, flavonoids, and GH, are transported to the mammary gland via the circulatory system and play vital roles in mammary gland development, particularly morphological development of mammary ducts in puberty [45]. In mice, mammary gland development is slow before puberty, but is rapidly accelerated under GH, IGF-1, and estrogen stimulation, with subsequent formation of a complete vessel tree within the fat pad under progesterone and prolactin stimulation [46].
Various hormones that decline with age are important components of lifespan pathways ( Figure 1B). The mammary gland is affected by a series of changes in hormone levels during aging, likely attributed to the decline in ovarian function [47]. In particular, GH and IGF-1 levels decline with age, potentially influenced by changes in sex hormones after menopause [48]. Certain hormones in the mammary gland also participate in the regulation of age-related pathways involving histone methylation. Histone methylation is a hallmark of invertebrate aging [30], and loss of histone methylation complexes can lead to increased lifespan in worms and flies [ 49, 50] . Histone demethylases can modulate longevity by targeting components of key longevity pathways, such as insulin/IGF-1 signaling [51].
Age-dependent changes in breast hormones may also be related to the development and progression of breast cancer, which increases with age. Ovarian-secreted estrogen and flavonoids promote both normal mammary gland development and tumor development, and thus longterm high levels of estrogen and flavonoids are associated with increased breast cancer risk [52]. Hormonal changes occur around menopause, most notably with decreased levels of estrogen and progesterone. Although breast cancer-related hormones decrease with age, these hormones may be converted by other hormones during aging, leading to an increase in breast cancer incidence [53].
Mammographic density decreases with age
Mammographic density (MD) is a ratio of mammary gland stroma reflected by X-ray images and is considered a strong risk factor for breast cancer [54]. MD is measured based on both dense breast area and percentage density (percentage of dense breast area divided by total breast area) [40]. Adipose tissue is radiologically transparent, while epithelial and connective tissue is dense and appears bright in X-ray. Therefore, MD is mainly determined by the relative proportion of fat and structural tissues in the breast [55], with greater MD associated with a larger nuclear area of epithelial and non-epithelial cells and higher collagen ratio [56].
Many factors affect MD, such as parity, body mass index (BMI), menopausal status, hormone levels, and age [ 57– 59] . Female parity and menopausal status are negatively correlated with MD. BMI is also significantly negatively correlated with MD, which may be due to increased adipose tissue reducing the fibro-glandular component or stimulating stromal cell differentiation to produce adipocytes instead of collagen [58]. Serum levels of IGF-1 and oxytocin are correlated with MD in postmenopausal women [ 60, 61] and high levels of circulating IGF-1 and its connexin IGFBP3 are associated with increased MD [62].
MD decreases with age and is generally lower in women who have given birth compared with nulliparous women [63]. Age-dependent lobular involution begins before menopause and continues afterwards and is associated with reduced MD [57]. Lokate et al. [64] found that the percentage of MD decreases continuously and more slowly with age. The age-related decrease in MD may be due to mammary involution, in which epithelial and stromal cells continue to decline while adipose tissue increases [65]. Breast density can also differ among populations, with studies showing that breast density is higher in Asian populations than in Western populations [ 66, 67] .
Cellular Changes in Aged Mammary Gland
The breast is a complex tissue composed of different types of cells, including epithelial and stromal cells that are distributed throughout the gland to ensure proper function. Over a woman’s lifetime, the mammary gland undergoes dynamic changes in cell composition and gene expression [68]. Epithelial cells in the mammary gland include myoepithelial, glandular epithelial, and sensory epithelial cells, while stromal cells include adipocytes, immune cells, fibroblasts, lymphocytes, and vascular cells ( Figure 1C). These cells undergo a series of changes during aging, which are closely related to the occurrence and progression of breast-related diseases.
Changes in mammary epithelial cells during aging
Mammary epithelial cells are primarily distributed in the mammary ducts, which consist of an outer layer of myoepithelial cells and an inner layer of hormone-sensing (HS) and secretory alveolar (AV) cells. HS cells can respond to endocrine stimuli, such as estrogen, progesterone, and prolactin, while the central role of AV cells is lactation.
Li et al. [69] found that the proportion of epithelial cells increases from 45% to 82%, while the proportion of stromal cells decreases from 55% to 18% during mammary aging in mice, with the AV cell subtype increasing from 26% to 69% and the HS cell subtype decreasing from 53% to 9% ( Table 1). Thus, these results demonstrate that the proportion of epithelial cells continues to increase, and the proportion of stromal cells continues to decline in the process of aging.
Table 1 Changes in the proportion of breast cells during aging
|
Cell types |
Proportions of youth |
Proportions of aged |
|
Epithelial cells |
45% |
82% |
|
Stromal cells |
55% |
18% |
|
Alveolar cells |
26% |
69% |
|
Hormone-sensing cells |
53% |
9% |
The gene expression profile of myoepithelial cells in the mammary gland changes significantly during aging ( Table 2). Bioinformatics analysis shows that these changes are mainly related to cytokines/growth factors, oxidative phosphorylation, extracellular matrix (ECM), and cytoskeleton/contractility genes [69], which are directly connected to tumor progression [ 70– 73] . In HS cells, most altered genes are regulated by hormone receptors, indicating hormonal changes during aging. Of note, two genes are specifically upregulated in HS cells during aging, namely Tph1 and Arg1 [69]. Increased Tph1 is activated during lactation and can promote serotonin biosynthesis [74]. Serotonin then binds to its receptor, activates the downstream PI3K/Akt signaling pathway, promotes the expression of vascular endothelial growth factor (VEGF), and induces migration and invasion of breast cancer cells [75]. Arg1 inhibits the proliferation of T cells and natural killer (NK) cells by downregulating L-arginine in the mammary microenvironment [76] and is usually expressed in immunosuppressive or tumor cells.
Table 2 Breast cells gene changes and related pathways during aging
|
Cell types |
Gene changes during aging |
Gene related pathways |
|
Myoepithelial cells |
Immune-related genes: Cxcl1, Cxcl2, Cxcl16, Csf1, and Csf3 Oxidative phosphorylation genes: Ndufa3, Ndufa5, Ndufa7, Ndufa8, Ndufa13, Ndufb3, Ndufb9, Ndufb10, Ndufc1, Ndufv3, Atp5j, Etfb, Uqcr10, and Uqcr11 Growth factor genes: Tgfb1, Jag1, and Vegfa ECM-related genes: Dcn, Col4a1, Col4a2, Serpinh1, Sparc, Emid1, Dag, and Spon2 Actomyosin-related genes: Acta2, Actg2, Mylk, Myl6, Myl9, Myh11, and Krt15 |
Cytokines or growth factors, oxidative phosphorylation, extracellular matrix (ECM), and cytoskeleton/contractility genes. |
|
Alveolar cells |
Csn1s1, Csn1s2a, Csn2, and Spp1 |
Milk biosynthesis. |
|
Hormone-sensing cells |
Tph1 and Arg1 |
Hormone and immune. |
|
Fibroblasts |
Upregulated genes: Hspa1a, Sqstm1, Ubc, Cebpb, and Gadd45b Downregulated genes: Col5a3, Col6a3, Fn1, and Mmp23 |
Upregulated genes are related to cellular stress. Downregulated genes are related to ECM. |
|
Vascular endothelial cells |
Upregulated genes: Csf3, Cxcl1, Cxcl16, and Il-6 Downregulated genes: Ctnnb1, Jup, Pvrl2, Cdh5, llt4, Cldn5, and F11r |
Upregulated genes are related to immune microenvironment. Downregulated genes are related to cell-cell junctions. |
|
M b macrophages |
Cytokines Ccl5, Cxcl2, and Gdf15 |
– |
|
Lymphoid cells |
Cd274 and Lilrb4 |
– |
Age-dependent changes in mammary gland epithelial cells are related to the incidence of benign breast diseases such as FA, which is relatively common among women and increases with age before menopause. The proportion of breast epithelial cells increases with age, and Tgfb1 expression in these cells is upregulated during aging ( Table 2), thereby promoting the incidence of FA. Notably, Pilichowska et al. [77] found that Tgfb1 is highly expressed in FA and breast cancer and its overexpression in breast epithelial cells during aging can lead to an increase in the incidence of both diseases. In addition, the expression of some inflammatory factors from breast epithelial cells increases during aging, providing an inflammatory microenvironment and promoting the occurrence of mastitis. Mammary epithelial cells, the proportion of which increases with age, are the first cells to produce an immune response to bacteria. The NF-κB pathway is activated when bacteria invade mammary epithelial cells, which increases inflammatory factor release, ultimately leading to an increase in the incidence of mastitis [78]. Thus, the higher proportion of breast epithelial cells and inflammatory factors during aging may increase the frequency of mastitis.
The increase in the proportion of epithelial cells during aging is closely related to the occurrence and development of breast cancer. Specifically, the proportion of AV cells increases during aging, which is closely connected to the occurrence of breast cancer [79]. The expression of VEGF-A in breast epithelial cells also increases during aging, which is related to an increase in the incidence of FA and breast cancer [80]. Furthermore, upregulation of TGF-β in aging breast epithelium may function to promote the growth and metastasis of breast cancer, and its expression can also be used to evaluate breast cancer malignancy [ 81, 82] . Consequently, the cellular changes in breast epithelial cells during aging are related to the increase of the incidence of FA, mastitis, and breast cancer.
Changes in mammary stromal cells during aging
Among the different types of stromal cells in the mammary gland, adipocytes are predominantly located in the fat pad and are involved in lactation metabolism [ 83, 84] , as well as endocrine functions such as the secretion of VEGF to regulate mammary angiogenesis [85]. Mammary fibroblasts are mainly distributed near the basal side of the epithelial branching tree in the mammary fat pad [86] and can communicate with epithelial cells through secreted factors and proteases [4]. Immune cells in the breast are mainly myeloid cells, such as dendritic cells and macrophages, and lymphocytes, such as T cells, B cells, and NK cells [69]. These immune cells exhibit multiple functions in mammary gland development, such as branching [6], apoptosis initiation, and adipocyte regeneration [87].
Mammary gland stromal cells and expression of their genes change with aging ( Table 2). Notably, the proportion of fibroblasts declines with increasing age. Stress-related genes, such as Hspa1a, Sqstm1, Ubc, and Cebpb, are upregulated in aged fibroblasts, while ECM-related genes, such as Col5a3, Col6a3, and Fn1, are downregulated [69]. The ratio of myeloid cells in breast tissue also decreases with age, whereas the percentages of lymphocytes and T cells continue to increase, as does the expression of replicative aging markers. In addition, the expression levels of CD274 and LILRB4, two immunosuppressive ligands targeting T cells and NK cells, are reportedly upregulated in the tissues of aged mice, potentially promoting an immunosuppressive microenvironment [69].
Changes in mammary gland stromal cells during aging can promote the development of breast diseases, including cancer. The proportion of breast adipocytes increases with age, leading to aromatase secretion, breast epithelial cell proliferation, and increased risk of breast cancer [88]. Accumulated adipose tissue in mammary gland with age is close to breast cancer tissue, which can foster cancer cells by secreting factors and nutrient substances and promote tumor metastasis [89]. The proportion of mammary gland M a macrophages, which play an important role in the immune response, decreases with age, thereby inducing immune response inactivation and breast disease development, such as mastitis, FA, and breast cancer [69].
Marked age-related changes in breast fibroblast genes, such as Hspa1a, Sqstm1, Ubc, Cebpb, and Gadd45b ( Table 2), can influence the development and progression of breast cancer. Hspa1a expression increases in fibroblasts with age and its upregulation can promote breast cancer resistance to radiotherapy through stress response signaling pathways [ 90, 91] . The autophagy adapter protein SQSTM1/p62, an important regulator of breast cancer metastasis, also increases in breast fibroblasts during aging. Inhibition of SQSTM1/p62 can restrain tumor growth and metastasis, mediated by cell cycle arrest and tumor microenvironment regulation [92]. The CEBPB/glycolysis pathways can help maintain a tumor immunosuppressive environment by inhibiting the expression of LAP, thereby preventing immune cells from killing tumor cells [93]. Breast vascular endothelial cell genes that are upregulated during aging include Csf3, Cxcl1, Cxcl16, and Il-6 ( Table 2). Chemokine CXCL1 can regulate the NF-κB and VEGF pathways and promote tumor chemotherapy resistance [94]. Cytokine IL-6 and its downstream gene Stat3 constitute a key carcinogenic signaling pathway, which can promote ER + breast cancer metastasis [95]. As a multi-effect cytokine with anti-inflammatory and pro-inflammatory properties, IL-6 can directly act on breast cancer to promote its proliferation and survival [96]. Therefore, dysregulated genes in aging breast stromal cells are associated with breast cancer progression.
Changes in Molecular Signaling Pathways in Aged Mammary Gland
Aging is also associated with changes in various molecular signaling pathways, including cyclin D1, STAT3, RANK/RANKL, and Slug/Snail. These pathways play critical roles in the regulation of cell proliferation, growth, differentiation, and the senescence-related secretory phenotype (SASP), which are key processes involved in the development of breast diseases. Dysregulation of cyclin D1, STAT3, RANK/RANKL, and Slug/Snail signaling during aging is related to breast epithelial cell and fibroblast senescence, and thus the incidence of breast-related diseases such as cancer.
Cyclin D1
The cell cycle machinery drives cell proliferation in mammals. Critical components in this process include cyclin family proteins, which can activate cyclin-dependent kinases (CDKs) [97]. Cyclin-CDK complexes can phosphorylate intracellular proteins and drive cell cycle progression [98]. Cyclin D1 can bind with CDK4 and CDK6 to form cyclin D1-CDK4 and cyclin D1-CDK6 complexes, the activation of which can phosphorylate retinoblastomas (Rb) to activate E2F transcription factors [97]. E2F induces various target genes to enable the cell cycle to enter the S phase ( Figure 2C), thus promoting cell proliferation. Irreversible cell cycle arrest occurs in senescent cells, which represses the activation of cyclin-CDK complexes [ 99, 100] and inhibits cell proliferation.
Figure 2 .
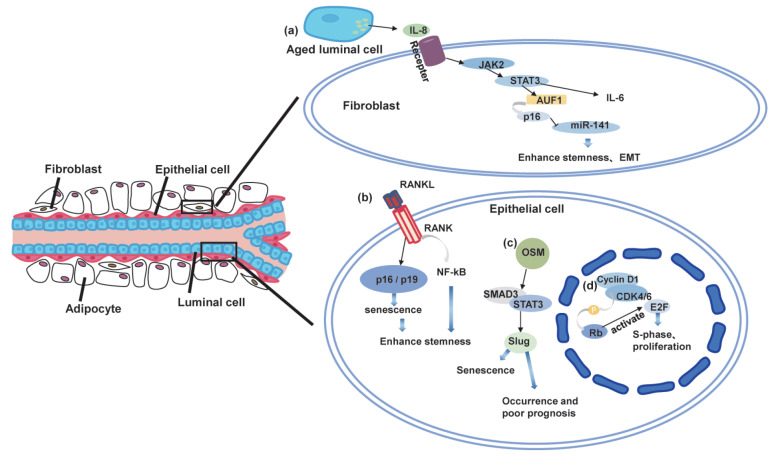
Senescence-associated signaling pathway changes in mammary gland
(A) STAT3 signaling pathway during mammary gland aging. Aged luminal cells activate the JAK2/STAT3 signaling pathway in fibroblasts through paracrine IL-8, and then activate downstream targets IL-6, AUF1, and AUF1 to further activate P16 and inhibit miR-141 expression, which can enhance breast cancer cell expression. (B) Relationship between RANK signaling pathway and mammary epithelial cells and breast cancer cells. RANKL and its receptor RANK activate NF-κB signaling, which can promote the stemness of breast cancer cells or mammary epithelial cells; RANKL/RANK signaling pathway also induces mammary epithelial cell senescence in a P16/P19-dependent manner, ultimately promoting mammary epithelial cells. (C) Slug signaling pathway and breast epithelial cell senescence. In mammary epithelial cells, OSM induces slug expression through STAT3/SMAD3-signaling, which then inhibits the execution of DDR signaling, ultimately inducing mammary epithelial cell senescence. (D) Cyclin D1 signaling pathway and breast epithelial cell senescence and proliferation. Cyclin D1 binds to CDK4/6 to activate retinoblastomas. Phosphorylated retinoblastomas further activate E2F, which targets its downstream genes to enter the S phase of the cell cycle, ultimately promoting proliferation of mammary epithelial cells. Cyclin D1 deletion can lead to mammary epithelial cell senescence.
Cyclin D1 is a well-established oncogene that is highly expressed in various cancers and is associated with tumor progression and metastasis. Studies have shown that cyclin D1 ablation in ErbB2-overexpressing breast cancer can cause cancer cell senescence and breast cancer delay [97]. Cyclin D-CDK kinase can protect cells against senescence by regulating downstream genes, such as Rb proteins [ 101, 102] and FOXM1 [103]. Inactivation of hypo-phosphorylated Rb and FOXM1 proteins can enhance the senescent state by permanently silencing E2F target genes, such as cyclin E1, cyclin A2, and Dhfr, thereby inhibiting cancer progression [97].
The expression of cyclin D1 in mammary epithelial cells increases with age [104], likely due to the activation of various upstream factors. For example, age-related nuclear translocation of EGFR can promote cyclin D1 expression and increase the proliferation of mammary epithelial cells [104]. In addition, the expression of chemokine C-C motif ligand 5 (CCL5) in fibroblasts significantly increases during aging, and its overexpression can activate the ERK1/2/cyclin D1 signaling pathway, thereby promoting breast cancer cell proliferation [105]. Given its role in the cell cycle process, cyclin D1 is clearly related to cellular senescence and its knockdown can cause cell cycle arrest by preventing the formation of cyclin D1-CDK4/CDK6 complexes. Therefore, targeting cyclin D1 and its upstream regulators may be a promising strategy for inhibiting breast cancer.
STAT3
STATs are cytoplasmic transcription factor family proteins [106]. Mammalian STAT family proteins include STAT1, 2, 3, 4, 5a, 5b, and 6, which mediate various intracellular signaling pathways [107]. STAT3 is an important regulator of SASP factors [108] and plays a vital role in body aging and cellular senescence. Studies have found that altered STAT3 signaling can trigger immune and non-immune cell senescence partly by activating the p53 pathway, thereby accelerating the aging process in organisms [109].
Cellular senescence can be triggered by various factors, including aging and external stress. Senescent cells can secrete SASP factors that can promote breast cancer development, mediated by the STAT3 signaling pathway. Activation of STAT3 can promote malignant tumor metastasis by promoting cell cycle progression, inhibiting apoptosis, and enabling tumor immune escape. Targeting STAT3 in breast cancer cells can induce cellular senescence and enhance immunotherapy, thus limiting breast cancer cell growth and metastasis [110]. Normal aging luminal cells in the breast also secrete SASP factors, including IL-6 and IL-8 [111]. In the context of cellular senescence, IL-8 can activate the JAK2/STAT3 pathway and its downstream target genes, including IL-6 ( Figure 2A), thus promoting the expression of genes related to stemness and epithelial-mesenchymal transition (EMT) ( e.g., CD44, OCT-4, vimentin, and ZEB-1) and increasing breast cancer cell proliferation and metastasis. Senescence-induced IL-8 can also downregulate miR-141 expression via STAT3/AUF1/p16 signaling. miR-141 has been shown to negatively regulate ZEB1 and ZEB2, which are associated with EMT and stemness [ 112, 113] . Senescent fibroblasts can promote the oncogenicity, EMT, and stemness of breast cancer cells through the STAT3 pathway [114]. Moreover, ionizing radiation-induced senescent cells can produce bystander effects to promote the migration and invasion of adjacent non-senescent tumor cells through the release of CSF2 and subsequent activation of the JAK2/STAT3 and AKT pathways [115]. Therefore, inhibiting STAT3 can induce cellular senescence in the mammary gland, with the STAT3 pathway playing an essential role in promoting breast cancer development due to the secretion of SASP factors by aging cells.
RANK/RANKL
RANKL is important for mouse mammary gland development and the proliferation of mammary epithelial cells, which stimulate ductal side-branching and alveologenesis [ 116, 117] . RANKL/RANK/OPG signaling plays an essential role in cell death, proliferation, inflammation, and immune processes [ 118, 119] , and is associated with postmenopausal hormone-related diseases of the mammary gland in elderly women [120]. Moreover, the RANKL/RANK/OPG pathway can activate NF-κB and its downstream genes [121], playing a vital role in mammary duct morphogenesis [122]. The RANK pathway not only plays a crucial role in mammary gland development, but also acts as an important regulator in breast aging and associated diseases.
RANKL mRNA expression increases with age [123], which may promote breast cancer development. Overexpression of RANK or treatment with RANKL activators induces senescence in mammary epithelial cells via p16/p19 [124]. In oncogene-induced mouse models (Neu and PyMT), RANK in the mammary epithelium causes cellular senescence, thereby delaying and reducing tumor initiation and incidence [124].
RANK signaling can also regulate normal mammary gland development. RANKL overexpression in transgenic mouse models results in prematurely developed mammary glands in young mice, with excessive growth of ductal side-branching and alveologenesis. These morphological changes are due to RANKL-induced mammary epithelial cell proliferation via activation of NF-κB and cyclin D1 expression. Therefore, prolonged exposure to RANKL can cause limited mammary epithelial hyperplasia with increasing age [116]. The promotion of both cell proliferation and senescence by RANK appears to be a contradiction. One explanation is that RANK promotes normal epithelial cell division, accelerating its approach to the Hayflick limit, then prematurely activates the cellular senescence pathway. However, further studies are needed to demonstrate the role of RANK in breast epithelial development and breast cancer during aging.
Hormones constantly change in the body aging process, regulating the RANK pathway and breast disease development. Gonadotropin-releasing hormone (GnRH) negatively regulates RANKL expression in breast cancer cells and decreases continuously with age [125]. Thus, the decline in GnRH level during aging facilitates the RANK pathway and promotes breast cancer development. The expression levels of RANK and RANKL are also regulated by estrogen, follicle-stimulating hormone, and dehydroepiandrosterone (DHA) in different cells [ 126– 128] . Hormone-driven activation of the RANK signaling pathway with age can enhance the stemness of human and murine mammary epithelial cells and mediate breast cancer initiation and invasiveness [124].
Slug/Snail
The SNAI family of zinc finger proteins includes three main members, namely SNAI1 (Snail), SNAI2 (Slug), and SNAI3 (Smuc) [129]. These proteins are known to regulate apoptosis, EMT, stemness, differentiation, and DNA damage response [130]. Slug and Snail play critical roles in the senescence of normal mammary epithelial cells and the development of breast cancer during aging by regulating EMT molecules, including E-cadherin and vimentin, and participating in tissue degeneration [131]. Slug acts as a direct transcriptional repressor of senescence marker p16 Ink4a, and thus inhibits cellular senescence [132]. Deletion of Slug can lead to DNA damage, which triggers breast epithelial cell senescence. Deletion of Slug decreases the phosphorylation level of RPA32 and CHK1, leading to the recruitment of damaged RAD51 to DNA damage sites and impairment of the normal DNA damage response (DDR). As a result, DNA damage continues to accumulate, ultimately accelerating premature aging of mammary epithelium [133]. Snail is also reported to be an indispensable mediator of oncostatin M (OSM)-induced senescence, which promotes MYC expression and increases breast cancer resistance to palbociclib treatment [ 134, 135] .
Hallmarks in Breast Aging and Diseases
Aging is an inevitable, progressive, and ultimately degenerative process, accompanied by tissue stem cell depletion, tissue inflammation, matrix alterations, and metabolic dysfunction [30]. This process is influenced by genetic and environmental factors and is characterized by genomic instability, epigenetic modifications, mitochondrial dysfunction, cellular senescence, telomere attrition, proteostasis loss, nutrient sensing dysregulation, stem cell exhaustion, and changes in intercellular communication [30]. Many of these aging characteristics are also associated with breast aging and related diseases ( Figure 3).
Figure 3 .
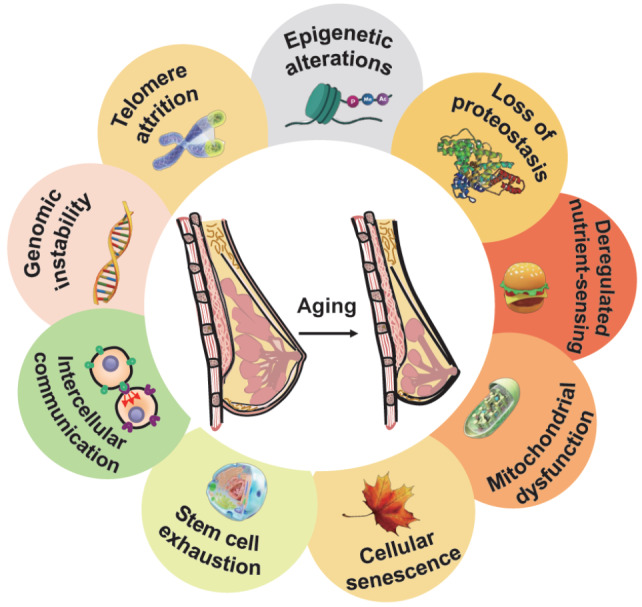
Aging hallmarks in breast aging
Breast aging includes several markers, such as genomic instability, epigenetic modification, mitochondrial dysfunction, and cellular senescence.
Genomic instability
Genomic instability refers to the frequency at which cells acquire genomic changes, including site-specific structural variations involving mutations of base pairs and insertions and deletions of fragments in chromosomes [136]. Evidence indicates that aging is accompanied by genomic instability, with artificially induced genomic instability leading to accelerated aging [30]. Moreover, genomic instability is considered an essential factor for cells to accumulate multiple mutations and induce senescence [137].
Genomic instability is also closely associated with the occurrence and development of breast cancer. Based on copy number alterations (CNAs), breast cancer can be characterized into three distinct genetic modes. The first mode, simplex, involves the gain or loss of an entire chromosome arm and is typically associated with ER + breast cancer. The second mode, amplifier, is related to the Luminal B and ERBB2 subtypes of breast cancer and involves the amplification of sites that regulate the cell cycle and nucleic acid metabolism, such as 8p12 (FGFR1), 8q24 (MYC), 11q13 (CCND1), 12q15 (MDM2), 17q12 (ERBB2), and 20q13 (ZNF217). The third mode, sawtooth, is mainly related to triple-negative (TNBC) or basal-like breast cancer [138].
Epigenetic alterations
Aging stimulates epigenetic changes that not only affect biological cells and tissues [30] but are also recognized as important contributors to cancer development. Common epigenetic changes in cancer include histone modification, expression level changes in non-coding RNAs, and abnormal DNA methylation, which is the most extensively studied epigenetic change in cancer [139].
Gene hypermethylation is a common occurrence in the early stages of many cancers, including breast cancer [140]. The promoter regions of tumor suppressor genes, such as RASSF1A, APC, and cyclin D2, are frequently methylated in human cancers. In different human breast cancer cell lines, the promoter region and CpG island of the RASSF1A gene are fully methylated, inhibiting the transcription process and activating cyclin D1 and cell proliferation [141]. In addition, compared with noncancerous adjacent tissues, the promoter region CpG island of the APC gene in breast cancer tissues is hypermethylated, promoting downstream WNT signaling and cell stemness [142]. These findings indicate a close association between abnormal methylation of the promoter region of tumor suppressor genes and breast cancer.
Mitochondrial dysfunction
Mitochondrial dysfunction has a profound impact on the aging process in mammals [30], as well as the occurrence of cancer. This dysfunction is primarily characterized by impaired respiratory chain efficiency, increased electron leakage, reduced adenosine triphosphate (ATP) production, and enhanced senescence [143]. Mitochondrial dysfunction is also associated with increased levels of reactive oxygen species (ROS), resulting in oxidative damage to lipids, proteins, and DNA, as well as the onset of various diseases, such as diabetes, neurodegeneration, inflammation, and cancer [144].
Mitochondrial dysfunction is caused by mutations in mitochondrial DNA, deletions of mitochondrial enzymes, and alterations in oncogenes [145]. Mitochondrial dysregulation in cancer-related fibroblasts can significantly promote the occurrence and development of breast cancer, which occurs through the production of high-energy mitochondrial fuel [146]. Compared with other cells, TNBC cells exhibit profound metabolic changes, including decreased mitochondrial respiration and increased glycolysis [147]. In basal-like breast cancer cells, serious mitochondrial functional defects occur, leading to the destruction of redox balance and ultimately the function and regulation of cell metabolism [148].
Cellular senescence
Cellular senescence is a complex stress response in which cells irreversibly lose the ability to proliferate, accompanied by changes in gene expression [149]. Cellular senescence includes replicative senescence, oncogene-induced senescence (OIS) and therapy-induced senescence (TIS) [150]. Senescent cells, which accumulate with age and contribute to body aging [30], produce various SASP factors, including inflammatory cytokines, chemokines, growth factor matrix metalloproteinases (MMPs), and other paracrine factors [151]. These SASP factors participate in the aging process of the body.
Cellular senescence inhibits the proliferation of cancer cells through irreversible growth retardation [ 152– 154] . Although cellular aging can act as an inhibitory barrier to the occurrence of human tumors, the secretion of SASP factors by aging cells can promote the development of cancer [155]. For example, TIS-induced senescent epithelial cells can secrete SASP factors that promote the invasion of breast cancer cells via the CXCL11/AKT/ERK pathway [156]. Furthermore, in xenotransplantation models, the co-culture of aging and breast cancer cells can promote cell proliferation and tumor formation [ 156– 158] .
Other aging hallmarks
Oxidative stress occurs due to an imbalance between ROS and antioxidant defense. Oxidative stress can increase with age, leading to abnormal tissue function [159] and potentially to aging-related diseases and cancer progression. Systemic oxidative stress during aging not only leads to ROS accumulation but also to epigenetic damage of the DNA structure [160]. Oxidative stress accumulation during aging is also influenced by various factors, such as inflammation, intracellular factors, and environmental factors, which contribute to the occurrence and development of age-related diseases, including cancer [161]. ROS is a critical regulatory factor of growth factor receptor signaling, which is involved in the pathogenesis of estrogen-induced breast cancer. Furthermore, activated mitogenic pathways in malignant breast cancer epithelial cells can increase intracellular ROS levels [162].
Autophagy is an evolutionarily conserved process, responsible for degrading intracellular proteins and organelles [163]. Autophagy continues to decline during aging and plays an important role in cellular senescence and aging-related diseases such as breast cancer [164]. Autophagy not only promotes breast cancer proliferation but also contributes to chemotherapy resistance. Several factors that regulate breast disease development and treatment are mediated by autophagy and autophagy-induced senescence. The stress-induced kinase PKCη can promote autophagy induced by ER and oxidative stress, as well as autophagy-induced senescence, leading to chemotherapy resistance in breast cancer cells [165]. The protein-coupled estrogen receptor (GPR30/GPER) is associated with tamoxifen (TAM) resistance. TAM treatment activates GPR30, thereby promoting the expression of HMGB1, activating MEK/ERK signaling, increasing autophagy of breast cancer cells, and inducing TAM-treated cellular senescence and survival [166]. circ-Dnmt1 can also promote the proliferation of breast cancer cells, which is dependent on autophagy-induced senescence [167]. Thus, autophagy is not only closely related to body aging, but also involved in treatment-induced breast cancer cell senescence.
Aging and Breast Disease
Breast diseases can be categorized as either benign or malignant. Benign breast diseases are markedly more common than malignant tumors and include mastitis, FA, lipoma, adenoma, hamartoma, and granular cell tumor [ 168, 169] . Breast cancer, which primarily belongs to the category of malignant breast diseases, increases with age [170]. In addition to breast diseases, milk production deficiency also increases with age.
FA during aging
FA is a common hormone-dependent benign breast disease but is associated with an increased risk of breast cancer. FA originates from stromal and epithelial connective tissue cells that exhibit high expression of estrogen and flavonoid receptors. The incidence of FA increases with age but decreases significantly after menopause [6]. FA originates from the TDLUs, and its size is influenced by increased levels of estrogen, progesterone, and prolactin [171]. FA is more prevalent in women before the age of 30 [172], when mammary glands undergo rapid development under hormonal stimulation, including during pregnancy and lactation.
Compared with normal breast tissue, FA is characterized by higher expression of the prolactin receptor ( PRLR) gene [ 173– 175] . In a study of 74 FA patients and 170 normal controls, Roman et al. [176] identified four FA patients with mutations in exon 6 of the PRLR. Mutations in PRLR can lead to PRL-independent tyrosine phosphorylation of PRLR and activation of the STAT5 signaling pathway, ultimately leading to evasion of cell death and a proliferative phenotype.
Research has shown that approximately 65% of FA patients carry mutations in mediator complex subunit 12 ( MED12) gene, most commonly in exon 2 [ 7, 8, 177] . As the mediator complex interacts with ERα and β [115], MED12 mutations can disrupt estrogen signaling in the mammary gland and contribute to FA pathogenesis [171]. Furthermore, as MED12 can activate CDK8 by binding with cyclin C, MED12 mutations can lead to inactivation of the cyclin C-CDK8/CDK19 pathway, thereby increasing the incidence of FA [178].
Mastitis and related diseases during aging
Mastitis, a form of breast inflammation, can occur at all ages [179]. Mastitis is generally categorized as non-lactational or lactational. Non-lactational mastitis includes periductal and idiopathic granulomatous mastitis [180]. Lactating mastitis, which is the most common type, is typically caused by bacteria that live on the skin, such as Staphylococcus aureus [180].
Although the relationship between mastitis and age has been less studied in humans and mice, recent research on dairy cows suggests that the risk and incidence of mastitis increase with age [ 181, 182] . Furthermore, the high incidence of latent mastitis in older multiparous cows may be associated with increased nipple patency and frequency of previous exposure [183], as well as a potential decline in immunity [184].
Mastitis is often caused by suboptimal milk removal from the mammary gland, resulting in milk retention and the promotion of a microenvironment favorable for microbial overgrowth. As breast cancer can rise from inflammatory or infected sites, patients with mastitis have a higher risk of developing breast cancer [185], as also reported for women with non-lactational mastitis [180]. Bacterial infections, which are common in mastitis, can also trigger tumorigenesis, with recent studies showing that tumor-resident intracellular microbiota may be involved in promoting breast cancer metastasis [186].
Insufficient lactation during aging
Milk is a complex liquid mixture developed to meet the nutritional needs of infants [187]. Decreased lactation is generally considered to be the result of a decrease in the ability of epithelial cells to produce milk [ 188, 189] . Mammalian lactation decreases with age [190], which results from cumulative cellular damage caused by endogenous oxygen free radicals during aging [191]. Decreased lactation can also occur due to cellular senescence, which is a marker of aging [ 181, 192] . Although continued milk removal from the mammary gland prolongs lactation and prevents mammary gland involution, milk synthesis declines over time during prolonged lactation, and the aged status of lactating cells may accelerate reduced milk production. This is partly attributed to changes in mammary mitochondrial oxidation markers during the extended lactation cycle [181]. Insufficient lactation is also associated with the incidence of breast cancer [193]. Notably, breastfeeding can reduce the incidence of breast cancer by about 20%, and prolonged lactation can significantly reduce the incidence of breast cancer [194].
Breast cancer during aging
The prevalence of various malignancies increases with age, suggesting that aging is a key factor in the development of cancer [ 195, 196] . Breast cancer, a malignancy that commonly affects women, also increases with age, with over 80% of cases occurring in women older than 50 years [ 53, 197] . The aging process initiates a series of changes in the breast, including regression of TDLUs, increase in adipose tissue, and decrease in overall breast density, which can promote the occurrence of breast cancer.
Decreased TDLU degeneration leads to increased breast cancer
As the functional unit of normal tissue in the mammary gland, TDLUs are responsible for milk production during lactation. Invasive lobular carcinomas (ILCs) are frequently derived from TDLU cells [198], with higher and lower age-related TDLU involution associated with reduced and increased breast cancer risk, respectively [ 22, 26] .
The mechanism underlying delayed TDLU degeneration is not well understood. A standard approach for detecting TDLU degradation is to measure TDLU counts, median TDLU span, and median acini counts per TDLU. Zeina et al. [26] investigated the effects of hormones on TDLU degeneration in normal tissue and found that high levels of oxytocin in premenopausal women are associated with higher TDLU counts, while high levels of progesterone are significantly associated with lower TDLU counts. In contrast, in postmenopausal women, high levels of estradiol and testosterone are associated with high TDLU counts. Oh et al. [199] studied the relationship between circulating IGF and TDLU involution in African and Caucasian American women and revealed that postmenopausal IGFBP-3 (IGF receptor) is negatively correlated with TDLU count, with higher IGF-1:IGFBP-3 ratio resulting in a higher concentration of TDLUs in premenopausal women. Several studies have found that highly expressed pro-inflammatory markers, such as TNF-α, COX-2, IL-6, CRP, leptin, SAA1, IL-8, and IL-10, are negatively correlated with the degree of lobular degeneration. Thus, high expression of inflammatory markers in the mammary gland is associated with reduced lobular degeneration, which may increase the risk of breast cancer [200].
Increased breast fat increases breast cancer incidence
Mammary adipose tissue is the primary endocrine system of the breast, secreting a variety of growth factors and enzymes [35]. The proportion of this tissue increases with age and is significantly associated with the incidence of breast cancer, with mammary adipocytes involved in the initiation, progression, invasion, and metastasis of breast cancer [89]. Moreover, interactions between adipocytes and surrounding cancer cells can modify the tumor microenvironment in favor of breast cancer development [35].
Breast adipose tissue contains special crown-like structures (CLSs), which may explain, at least in part, the positive age-related correlation between this tissue and breast cancer incidence. These CLSs are formed by macrophages surrounding dead or dying adipocytes [201], which are rich in aromatase, an enzyme that converts androgens to estrogens [202]. Estrogens play a substantial role in promoting the occurrence and development of breast cancer. In postmenopausal women, most endogenous estrogens are converted from androgens, a process dependent on aromatase, with higher circulating estrogen levels associated with increased breast cancer incidence [ 203, 204] . The occurrence of CLSs in breast adipose tissue in breast cancer patients is associated with an increased estrogen to androgen ratio in breast adipose tissue and serum [205]. In older women, increased CLS number and density are associated with increased BMI and adipocyte size [ 206, 207] . Thus, female breast adipose tissue and CLS formation will continue to increase with increasing age, eventually leading to an increase in the incidence of breast cancer. Therefore, aromatase inhibitors alone or in combination with TAM may significantly reduce tumor progression of hormone receptor-positive breast cancers in postmenopausal women [208].
As women age, PAT is transformed into WAT in the mammary gland, which contributes to an increase in the risk of breast cancer. This transdifferentiation process is mediated by PPARγ, a crucial factor for preventing age-related dysfunction in many organs and tissues [209], and occurs during mammary gland involution after lactation [32]. Downregulation of PPARγ creates a favorable tumorigenic microenvironment for breast cancer due to inflammatory signaling pathways [210], suggesting that transition from PAT to WAT is necessary for mammary involution, with reduced involution associated with increased breast cancer risk.
Breast hormonal changes and breast cancer during aging
In the process of aging, numerous hormones in the breast decline, with a corresponding increase in the prevalence of hormone-dependent breast cancer. GH and IGF-1 signaling decreases during aging, associated with reductions in postmenopausal sex hormones [48]. Although postmenopausal women have lower levels of estrogen, older women experience a higher rate of ERα-positive breast cancer [211]. Studies have found that the incidence of hormone-dependent breast cancer increases with age, including both ER + breast cancer [212] and PR + breast cancer [48].
The expression levels of various hormone-related enzymes, including aromatase, sulfatase, and 17β-hydroxy-steroid dehydrogenase-1, increase with age, allowing mammary epithelial cells to maintain proliferation at lower circulating estrogen levels [213]. These changes lead to enhanced hormone sensitivity in mammary epithelial cells [214], thereby fostering the development and progression of breast cancer.
Overall breast density and breast cancer
High overall breast density, the third strongest risk factor for breast cancer after age and BRCA1/ 2 mutations [9], can be affected by many factors, such as TDLU degeneration and age. Research has shown that TDLU degeneration during aging is associated with MD, while delayed TDLU degeneration is associated with increased overall breast density [215]. Overall breast density is also positively correlated with collagen, epithelial, and non-epithelial cells, and negatively correlated with fat [9], consistent with the increased proportion of mammary adipose tissue during aging.
Certain hormones, including progesterone and IGFs, can also affect overall breast density [ 25, 57, 216] . Postmenopausal hormone therapy (HT) can lead to an increase in MD, especially under combined estrogen/progestin treatment [217]. Studies have shown that the use of HT, particularly estrogen and progestin, slows the transition from dense to more fatty patterns in the mammary gland, which typically develops with age [218]. Therefore, HT may slow breast involution and increase breast cancer incidence.
Changes in breast immune cells during aging and their relationship with breast cancer
In the aging process, the proportion of different immune cells in the mammary gland changes markedly. Studies have shown that NK and CD8 + T cells are significantly increased (by 2-fold and 8-fold, respectively) in the mammary tissue of aged mice [9] and the serum levels of NK cells are significantly higher in the elderly groups than in the young groups [219].
During aging, immune cells in the breast and blood undergo changes that may contribute to the incidence of breast cancer. The relationship between immune cells and breast cancer is complex but may be partly attributed to changes in immune cell populations. Carman et al. [69] demonstrated that Cd274 (also known as Pd-l1) and Lilrb4 (also known as Ilt3) are upregulated in aged mouse mammary glands and may act as immunosuppressive ligands that target T cells and NK cells, thereby promoting an immunosuppressive microenvironment. It has also been reported that the number of CD56 −CD16 + NK cells is significantly increased in elderly individuals. This increase in immunosuppressive CD56 −CD16 + NK cells can lead to a decrease in the expression of immune regulators, such as granzymes A, B, and NKG2A [219], resulting in a decrease in anti-tumor immunoreactions.
Accumulative mutations during aging and breast cancer
Gene mutations occur in almost all organisms, and continue to accumulate in the aging process, with about 40 new mutations developing in the body every year [220]. Somatic cells also continue to mutate throughout life. While most of these mutations do not harm the body, some can change key functions of cells, including proliferation and differentiation [ 221, 222] , which play an important role in the development of aging diseases and tumors. Generally, cancer is caused by mutations that have accumulated continuously in the aging process in individuals [ 223, 224] .
While mutation accumulation is closely related to breast cancer occurrence and development, it is not the only factor responsible for the disease. Rather, breast cancer can be considered a multi-step process, with each step related to the mutation of essential regulatory genes. Clinical diagnosis of breast cancer generally reveals 4 to 6 major regulatory gene mutations distributed on different chromosomes of breast cancer cells, which play crucial roles in maintaining cell proliferation, apoptosis, and differentiation [225]. Various genes are prone to mutations in breast cancer during aging, including BRCA1, BRCA2, CHEK2, TP53, and CYP1A1 [ 225– 228] . However, age-related mutation accumulation cannot explain the increased incidence of breast cancer with age [229]. Notably, while the incidence of many types of cancer increases exponentially with age, the incidence of breast cancer does not follow the same pattern, instead increasing slowly after the age of 50 [230].
Interventions for Delaying Mammary Gland Aging and Preventing Breast Disease
Breast aging is associated with an increased risk of breast cancer and other related diseases. As such, delaying the aging process could potentially reduce the incidence of these diseases. As an effective and widely used anti-aging endocrine therapy, HT can increase breast volume by the administration of different hormones, including GH, androgen, estrogen, and progesterone [231]. Both GH and IGF-1 levels decline with age. Studies on female rhesus monkeys have shown that treatment with IGF-1, GH, or GH+IGF-1 can result in progressively greater breast volume enlargement, suggesting that the GH/IGF-1 axis may stimulate the proliferation of mammary glands in primates via activation of downstream proteins C-MYC, CDKs, ZO-1, and VEGF [232]. Although HT has anti-aging benefits, it may also increase the risk of breast cancer. Biological hormone replacement therapy (BHRT), a new type of HT, is an effective anti-aging and aging-related disease treatment strategy [233].
Breast cancer can be resulted from multiple factors, including genetic and non-genetic risk factors. Non-genetic risk factors include obesity, alcohol intake, and poor exercise. Studies suggest that reducing BMI, limiting alcohol intake, and increasing physical exercise can help prevent breast cancer [234]. Notably, regular exercise has been shown to reduce aging T cells, increase the proliferation of T cells, and enhance the killing capacity of NK cells to prevent immune aging [235]. Furthermore, moderate regular exercise can partially restore mitochondrial dysfunction and other dysfunctions caused by aging or lack of exercise, with caloric restriction potentially able to extend lifespan [236]. Regular exercise may also help prevent breast aging as well as the occurrence and development of breast-related diseases. Obesity can also promote aging and age-related metabolic abnormalities similar to those caused by normal aging [237]. Hence, reducing obesity may help delay both aging and breast disease. Epigenetic changes also play a role in the occurrence and development of breast cancer. Histone deacetylase and DNA methyltransferase inhibitors, which modulate epigenetic activity, have attracted increasing attention in cancer and aging research [238]. Caloric restriction also exerts epigenetic regulation activity in a variety of aging-related diseases and cancers [238].
During aging, nutrient sensing is an evolutionarily conserved process that involves various signaling pathways, such as mTOR. These pathways are closely related to the occurrence and development of breast cancer. Targeting important molecules in these pathways may be an effective strategy for preventing breast cancer. Temsirolimus, an mTOR inhibitor, has been shown to be effective in treating advanced hormone receptor-positive breast cancers [239]. Targeting cancer-related fibroblasts that secrete SASP factors may also inhibit breast cancer progression. Researchers have reported that the mTOR inhibitor rapamycin effectively reduces SASP MMP-2 activity and TGF-β1 expression after 14 days of treatment [ 239, 240] . Moreover, while SASP factors secreted by senescent epithelial cells can promote the proliferation, migration, and invasion of breast cancer cells, targeting the SASP factor CXCL11 can eliminate the promotion of senescent cells and proliferation of breast cancer cells [240].
Interventions that target aged molecules have been shown to effectively inhibit breast cancer. Both CDK4 and CDK6 promote the transition of cells from the G1 phase to the S phase, and thus CDK4/6 inhibitors can block the proliferation of cancer cells and promote cellular senescence [241]. The combination of CDK4/6 inhibitors and other therapies has been widely applied in clinical treatment, resulting in cell sensitization to palbociclib resistance by enhancing breast cancer cell aging [242]. The RANK signaling pathway plays an important role in breast cancer progression. Overexpression of RANK or exposure to RANKL in breast cancer cells can induce aging, which is dependent on p16/p19. RANK-induced aging is essential due to its induced stemness and tumor-promoting effects. RANKL intervention is an effective treatment for proliferative breast diseases [ 125, 243] . PAK4 can prevent breast cancer cells from aging, leading to malignant proliferation, while inhibition of PAK4 can promote the aging of breast cancer cells [244]. Targeting promyelocytic leukemia protein (PML), which is significantly upregulated in TNBC cells and regulates the initiation of breast cancer, can lead to significant growth inhibition and MYC and PIM1 kinase reduction, thereby triggering CDKN1B (p27) accumulation and initiation of aging [245]. CXCR2 can promote the anti-aging, anti-apoptosis, and EMT processes of breast cancer cells, ultimately leading to tumor metastasis and enhanced chemoresistance. Thus, CXCR2 antagonists can be used to treat breast cancer patients [246].
Conclusions and Perspectives
This review provides an overview of the various changes that occur in the mammary gland during aging, including regression of mammary TDLUs, enhanced mammary gland density, increased adipose tissue deposition, changes in hormone levels, and changes in cellular composition. These modifications are closely connected to the development of mammary gland-related diseases. Delayed regression of TDLUs during aging is associated with an increase in the incidence of breast cancer and may be related to hormonal imbalances in the breast, such as aberrant levels of estradiol, testosterone, circulating IGF, and pro-inflammatory cytokines [ 26, 199, 200] . Although age-related changes in TDLUs have been confirmed in several studies, the underlying mechanism and related factors require further study to elucidate the roles of aged TDLUs in breast pathologies.
The abovementioned age-related changes in the mammary gland stimulate breast diseases through complex mechanisms. The high proportion of adipose tissue in the mammary gland during aging is implicated in increased breast cancer risk, which may be due to adipocytes secreting cytokines that alter the tumor microenvironment [35]. In addition, excess adipose tissue forms specific structures [ 35, 206, 207] that can accelerate the conversion of androgens to estrogens, thereby initiating the potential development of breast cancer. Hormones in the mammary gland arise from both systemic circulation and local synthesis. Although breast cancer-related hormones, such as estrogen, decline in postmenopausal women [211], breast cells become more sensitive to estrogen [ 48, 214] , thereby increasing the incidence of breast cancer.
In the aging process, the proportion of mammary epithelial cells continuously increases, while the proportion of stromal cells decreases [69]. Breast cancer can originate from breast epithelial cells, and the increasing proportion of epithelial cells during aging is implicated in breast cancer incidence. Upregulation of genes such as TGFB1 and VEGFA in aging epithelial cells is also associated with breast-related diseases, such as FA and breast cancer. Stromal cells decline with age, and changes in certain stromal cell factors, such as HSPA1A, SQSTM, CEBPB, CXCL1/2, and IL-6, may not only promote the occurrence and development of breast cancer, but also participate in the process of chemoresistance. Changes in the proportion of breast cells and corresponding gene expression during aging are closely related to the increased risk of breast cancer. However, further research is needed to investigate the relationship between aging-related breast diseases and changes in breast cells.
Several important regulatory pathways, including cyclin D1, STAT3, RANK/RANKL and Slug/Snail, undergo changes during aging and participate in the aging process of breast epithelial cells, tumor fibroblasts, and tumor cells. Cyclin D1 is highly expressed in elderly breast cancer patients [247] and targeting the cyclin D1-CDK4/CDK6 complexes and their downstream genes can inhibit the development of breast cancer. STAT3 also contributes to the regulation of body aging and cellular senescence. STAT3 signaling can promote tumor growth, while its inhibition can lead to breast cancer cell senescence [110]. The RANK/RANKL pathway regulates mammary gland development and promotes breast epithelial cell proliferation. Overexpression of RANK can cause breast cancer hyperplasia, as well as breast cancer cell senescence. The Slug/Snail signaling pathway is related to DNA damage response, and deletion of Slug can lead to breast cancer cell senescence. These regulatory pathways are essential to aging and breast cancer cell senescence and could potentially be targeted for clinical applications.
Aging is a gradual and time-dependent functional decline that affects most organisms and is characterized by loss of physiological integrity, impaired function, and greater susceptibility to death [30]. Aging exhibits several characteristics in cellular and molecular biology, including genomic instability, telomere attrition, epigenetic modification, proteostasis loss, nutrient sensing dysregulation, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and changes in intercellular communication [30]. Here, we primarily focused on the relationship between breast-related diseases and aging hallmarks, including genomic instability, epigenetic modifications, mitochondrial dysfunction, cellular senescence, oxidative stress, and autophagy. Although oxidative stress and autophagy are linked to breast cancer cell senescence, their functions during breast aging development remain poorly studied. Given their inevitability during aging, it would be worthwhile studying the contribution and mechanisms of oxidative stress and autophagy to breast diseases during breast aging.
The mammary gland undergoes various changes in the aging process, many of which are associated with breast-related diseases, especially cancer. In this review, we discussed changes in the process of breast aging, including decreased TDLUs and overall density, increased adipose tissue, and alterations in hormone levels. These changes in mammary gland development during aging can facilitate the progression of breast diseases, such as FA, mastitis, hypolactation, and cancer. Understanding the relationship between breast changes during aging and breast diseases, as well as the relationship between these diseases, will provide guidance for the prevention and treatment of breast-related disorders.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Key R&D Program of China (No. 2020YFA0112300), the National Natural Science Foundation of China (Nos. 82260488, 32200679, 81830087, and U2102203), China Postdoctoral Science Foundation (Nos. 2021TQ0137 and 2021M701544), the Yunnan Applied Basic Research Key Projects (Nos. 202001AT070102, 202001AT070104, 2019FB047, and 202101AS070050), the Jiangxi Provincial Natural Science Foundation (Nos. 20224BAB205014 and 20224BAB216071), the Natural Science Foundation of Chongqing (No. CSTB2022NSCQ-MSX056), the Postgraduate Innovation Special Fund Project of Jiangxi (No. YC2022-s047), the College Students’ Innovative Entrepreneurial Training Plan Program of Nanchang University (Nos. 2022CX024 and 2022CX158), and the Scientific Research Training Program of Nanchang University (2022).
References
- 1.Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia. . 2002;7:253–266. doi: 10.1023/A:1022848632125. [DOI] [PubMed] [Google Scholar]
- 2.Blickstein I. Motherhood at or beyond the edge of reproductive age. Int J Fertil Womens Med. 2003, 48: 17–24 . [PubMed]
- 3.Aytekin A, Karatas F, Sahin S, Erdem GU, Altundag K. Clinicopathological features of patients with breast cancer aged 70 years or over. J BUON. 2017, 22: 200–207 . [PubMed]
- 4.Howard BA, Lu P. Stromal regulation of embryonic and postnatal mammary epithelial development and differentiation. Semin Cell Dev Biol. . 2014;25-26:43–51. doi: 10.1016/j.semcdb.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Betterman KL, Paquet-Fifield S, Asselin-Labat ML, Visvader JE, Butler LM, Stacker SA, Achen MG, et al. Remodeling of the lymphatic vasculature during mouse mammary gland morphogenesis is mediated via epithelial-derived lymphangiogenic stimuli. Am J Pathol. . 2012;181:2225–2238. doi: 10.1016/j.ajpath.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. . 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 7.Pfarr N, Kriegsmann M, Sinn P, Klauschen F, Endris V, Herpel E, Muckenhuber A, et al. Distribution of MED12 mutations in fibroadenomas and phyllodes tumors of the breast-implications for tumor biology and pathological diagnosis . Genes Chromosomes Cancer. . 2015;54:444–452. doi: 10.1002/gcc.22256. [DOI] [PubMed] [Google Scholar]
- 8.Piscuoglio S, Murray M, Fusco N, Marchiò C, Loo FL, Martelotto LG, Schultheis AM, et al. MED12 somatic mutations in fibroadenomas and phyllodes tumours of the breast . Histopathology. . 2015;67:719–729. doi: 10.1111/his.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginsburg OM, Martin LJ, Boyd NF. Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer. . 2008;99:1369–1374. doi: 10.1038/sj.bjc.6604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarti D, LaBella KA, DePinho RA. Telomeres: history, health, and hallmarks of aging. Cell. . 2021;184:306–322. doi: 10.1016/j.cell.2020.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. . 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle JR, Lin N, Liu J, Storniolo AMV, Shendre A, Hou L, Horvath S, et al. Estimating breast tissue-specific DNA methylation age using next-generation sequencing data. Clin Epigenet. . 2020;12:45. doi: 10.1186/s13148-020-00834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGhee DE, Steele JR. Breast biomechanics: what do we really know? Physiology. . 2020;35:144–156. doi: 10.1152/physiol.00024.2019. [DOI] [PubMed] [Google Scholar]
- 14.Langton AK, Sherratt MJ, Griffiths CEM, Watson REB. A new wrinkle on old skin: the role of elastic fibres in skin ageing. Int J Cosmet Sci. . 2010;32:330–339. doi: 10.1111/j.1468-2494.2010.00574.x. [DOI] [PubMed] [Google Scholar]
- 15.Gaskin KM, Peoples GE, McGhee DE. The fibro‐adipose structure of the female breast: a dissection study. Clin Anat. . 2020;33:146–155. doi: 10.1002/ca.23505. [DOI] [PubMed] [Google Scholar]
- 16.Rehnke RD, Groening RM, Van Buskirk ER, Clarke JM. Anatomy of the superficial fascia system of the breast. Plast Reconstr Surg. . 2018;142:1135–1144. doi: 10.1097/PRS.0000000000004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassiotou F, Geddes D. Anatomy of the human mammary gland: current status of knowledge. Clin Anat. . 2013;26:29–48. doi: 10.1002/ca.22165. [DOI] [PubMed] [Google Scholar]
- 18.Huang SY, Boone JM, Yang K, Packard NJ, McKenney SE, Prionas ND, Lindfors KK, et al. The characterization of breast anatomical metrics using dedicated breast CT. Med Phys. . 2011;38:2180–2191. doi: 10.1118/1.3567147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon JM, Mansel RE. ABC of breast diseases: congenital problems and aberrations of normal breast development and involution. BMJ. . 1994;309:797–800. doi: 10.1136/bmj.309.6957.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo C, Sung H, Zheng S, Guida J, Li E, Li J, Hu N, et al. Age-related terminal duct lobular unit involution in benign tissues from Chinese breast cancer patients with luminal and triple-negative tumors. Breast Cancer Res. . 2017;19:61. doi: 10.1186/s13058-017-0850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975, 55: 231–273 . [PubMed]
- 22.Figueroa JD, Pfeiffer RM, Patel DA, Linville L, Brinton LA, Gierach GL, Yang XR, et al. Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Institute. . 2014;106:dju286. doi: 10.1093/jnci/dju286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radisky DC, Hartmann LC. Mammary involution and breast cancer risk: transgenic models and clinical studies. J Mammary Gland Biol Neoplasia. . 2009;14:181–191. doi: 10.1007/s10911-009-9123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueroa JD, Pfeiffer RM, Brinton LA, Palakal MM, Degnim AC, Radisky D, Hartmann LC, et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: a nested case–control study. Breast Cancer Res Treat. . 2016;159:163–172. doi: 10.1007/s10549-016-3908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horne HN, Sherman ME, Pfeiffer RM, Figueroa JD, Khodr ZG, Falk RT, Pollak M, et al. Circulating insulin-like growth factor-I, insulin-like growth factor binding protein-3 and terminal duct lobular unit involution of the breast: a cross-sectional study of women with benign breast disease. Breast Cancer Res. . 2016;18:24. doi: 10.1186/s13058-016-0678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khodr ZG, Sherman ME, Pfeiffer RM, Gierach GL, Brinton LA, Falk RT, Patel DA, et al. Circulating sex hormones and terminal duct lobular unit involution of the normal breast. Cancer Epidemiol Biomarkers Prev. . 2014;23:2765–2773. doi: 10.1158/1055-9965.EPI-14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. . 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. . 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenyon CJ. The genetics of ageing. Nature. . 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 30.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. . 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slack C, Giannakou ME, Foley A, Goss M, Partridge L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila . Aging Cell. . 2011;10:735–748. doi: 10.1111/j.1474-9726.2011.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. . 2014;170:R159–R171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 33.Cannon B, Nedergaard J. Studies of thermogenesis and mitochondrial function in adipose tissues. Methods Mol Biol. 2008, 456: 109–121 . [DOI] [PubMed]
- 34.Bellanger D, Dziagwa C, Guimaraes C, Pinault M, Dumas JF, Brisson L. Adipocytes promote breast cancer cell survival and migration through autophagy activation. Cancers. . 2021;13:3917. doi: 10.3390/cancers13153917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kothari C, Diorio C, Durocher F. The importance of breast adipose tissue in breast cancer. Int J Mol Sci. . 2020;21:5760. doi: 10.3390/ijms21165760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC, Tsai CH, et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. . 2018;37:589–600. doi: 10.1038/onc.2017.357. [DOI] [PubMed] [Google Scholar]
- 37.Adler AS, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-κB activity. Genes Dev. . 2007;21:3244–3257. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lejour M. Evaluation of fat in breast tissue removed by vertical mammaplasty. Plast Reconstr Surg. . 1997;99:386–393. doi: 10.1097/00006534-199702000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. . 2000;5:119–137. doi: 10.1023/A:1026487120779. [DOI] [PubMed] [Google Scholar]
- 40.Baldwin RL, Reichl JR, Louis S, Smith NE, Yang YT, Osborne E. Effects of age, pregnancy, and lactation on rat, guinea pig, and cow adipose enzyme activities and cow adipose metabolism. J Dairy Sci. . 1973;56:340–349. doi: 10.3168/jds.S0022-0302(73)85177-X. [DOI] [PubMed] [Google Scholar]
- 41.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. . 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 42.Huang Q, Wu L, Wang Y, Kong X, Xiao X, Huang Q, Li M, et al. Caveolin-1-deficient fibroblasts promote migration, invasion, and stemness via activating the TGF-β/Smad signaling pathway in breast cancer cells. Acta Biochim Biophys Sin. . 2022;54:1587–1598. doi: 10.3724/abbs.2022150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bédard K, Bédard J, Rocheleau G, Ferland G, Gaudreau P. Aging and diets regulate the rat anterior pituitary and hypothalamic transcriptome. Neuroendocrinology. . 2013;97:146–159. doi: 10.1159/000338411. [DOI] [PubMed] [Google Scholar]
- 44.Magaña-Schwencke N, Ekert B. Biochemical analysis of damage induced in yeast by formaldehyde. II. Induction of cross-links between DNA and protein. Mutat Res. . 1978;51:11–19. doi: 10.1016/0027-5107(78)90003-9. [DOI] [PubMed] [Google Scholar]
- 45.LaMarca HL, Rosen JM. Hormones and mammary cell fate—what will I become when I grow up? Endocrinology. . 2008;149:4317–4321. doi: 10.1210/en.2008-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. . 2012;1:533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russo J, Calaf G, Roi L, Russo IH. Influence of age and gland topography on cell kinetics of normal human breast tissue. J Natl Cancer Inst. 1987, 78: 413–418 . [PubMed]
- 48.Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. . 2002;80:137–148. doi: 10.1016/S0960-0760(01)00182-0. [DOI] [PubMed] [Google Scholar]
- 49.Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. . 2010;466:383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siebold AP, Banerjee R, Tie F, Kiss DL, Moskowitz J, Harte PJ. Polycomb Repressive Complex 2 and Trithorax modulate Drosophila longevity and stress resistance . Proc Natl Acad Sci USA. . 2010;107:169–174. doi: 10.1073/pnas.0907739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin C, Li J, Green CD, Yu X, Tang X, Han D, Xian B, et al. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. . 2011;14:161–172. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. . 2005;12:483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 53.Walker RA, Martin CV. The aged breast. J Pathol. . 2007;211:232–240. doi: 10.1002/path.2079. [DOI] [PubMed] [Google Scholar]
- 54.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. . 2006;15:1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 55.Reeves KW, Stone RA, Modugno F, Ness RB, Vogel VG, Weissfeld JL, Habel LA, et al. Longitudinal association of anthropometry with mammographic breast density in the Study of Women’s Health Across the Nation. Int J Cancer. . 2009;124:1169–1177. doi: 10.1002/ijc.23996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, et al. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. . 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 57.Mathur A, Taurin S. What influence does mammographic density have on breast cancer occurrence? Expert Rev Anticancer Ther. . 2022;22:445–447. doi: 10.1080/14737140.2022.2065985. [DOI] [PubMed] [Google Scholar]
- 58.Sherratt MJ, McConnell JC, Streuli CH. Raised mammographic density: causative mechanisms and biological consequences. Breast Cancer Res. . 2016;18:45. doi: 10.1186/s13058-016-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greendale GA. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Ann Intern Med. . 1999;130:262. doi: 10.7326/0003-4819-130-4_Part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 60.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000, 60: 3744–3748 . [PubMed]
- 61.Pan B, Ren H, Ma Y, Liu D, Yu B, Ji L, Pan L, et al. High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int J Cancer. . 2012;131:70–82. doi: 10.1002/ijc.26341. [DOI] [PubMed] [Google Scholar]
- 62.Rinaldi S, Biessy C, Hernandez M, Lesueur F, dos-Santos-Silva I, Rice MS, Lajous M, et al. Circulating concentrations of insulin-like growth factor-I, insulin-like growth factor-binding protein-3, genetic polymorphisms and mammographic density in premenopausal Mexican women: Results from the ESMaestras cohort. Int J Cancer. . 2014;134:1436–1444. doi: 10.1002/ijc.28469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grove JS, Goodman MJ, Gilbert Jr. FI, Mi MP. Factors associated with mammographic pattern. Br J Radiol. . 1985;58:21–25. doi: 10.1259/0007-1285-58-685-21. [DOI] [PubMed] [Google Scholar]
- 64.Lokate M, Stellato RK, Veldhuis WB, Peeters PHM, van Gils CH. Age-related changes in mammographic density and breast cancer risk. Am J Epidemiol. . 2013;178:101–109. doi: 10.1093/aje/kws446. [DOI] [PubMed] [Google Scholar]
- 65.Henson DE, Tarone RE. Involution and the etiology of breast cancer. Cancer. . 1994;74:424–429. doi: 10.1002/cncr.2820741330. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida R, Yamauchi T, Akashi-Tanaka S, Matsuyanagi M, Taruno K, Sawada T, Kokaze A, et al. Optimal breast density characterization using a three-dimensional automated breast densitometry system. Curr Oncol. . 2021;28:5384–5394. doi: 10.3390/curroncol28060448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leong SPL, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, Sandelin K, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. . 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. . 2015;142:1028–1042. doi: 10.1242/dev.087643. [DOI] [PubMed] [Google Scholar]
- 69.Li CMC, Shapiro H, Tsiobikas C, Selfors LM, Chen H, Rosenbluth J, Moore K, et al. Aging-associated alterations in mammary epithelia and stroma revealed by single-cell RNA sequencing. Cell Rep. . 2020;33:108566. doi: 10.1016/j.celrep.2020.108566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rasha F, Ramalingam L, Gollahon L, Rahman RL, Rahman SM, Menikdiwela K, Moustaid-Moussa N. Mechanisms linking the renin-angiotensin system, obesity, and breast cancer. Endocr Relat Cancer. . 2019;26:R653–R672. doi: 10.1530/ERC-19-0314. [DOI] [PubMed] [Google Scholar]
- 71.Evans KW, Yuca E, Scott SS, Zhao M, Paez Arango N, Cruz Pico CX, Saridogan T, et al. Oxidative phosphorylation is a metabolic vulnerability in chemotherapy-resistant triple-negative breast cancer. Cancer Res. . 2021;81:5572–5581. doi: 10.1158/0008-5472.CAN-20-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Insua-Rodríguez J, Oskarsson T. The extracellular matrix in breast cancer. Adv Drug Deliver Rev. . 2016;97:41–55. doi: 10.1016/j.addr.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 73.Bockhorn J, Yee K, Chang YF, Prat A, Huo D, Nwachukwu C, Dalton R, et al. MicroRNA-30c targets cytoskeleton genes involved in breast cancer cell invasion. Breast Cancer Res Treat. . 2013;137:373–382. doi: 10.1007/s10549-012-2346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laporta J, Keil KP, Vezina CM, Hernandez LL. Peripheral serotonin regulates maternal calcium trafficking in mammary epithelial cells during lactation in mice. PLoS One. . 2014;9:e110190. doi: 10.1371/journal.pone.0110190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gautam J, Banskota S, Regmi SC, Ahn S, Jeon YH, Jeong H, Kim SJ, et al. Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. . 2016;15:75. doi: 10.1186/s12943-016-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. . 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 77.Pilichowska M, Kimura N, Fujiwara H, Nagura H. Immunohistochemical study of TGF-alpha, TGF-beta1, EGFR, and IGF-1 expression in human breast carcinoma. Mod Pathol. 1997, 10: 969–975 . [PubMed]
- 78.Xiao HB, Wang CR, Liu ZK, Wang JY. LPS induces pro-inflammatory response in mastitis mice and mammary epithelial cells: possible involvement of NF-κB signaling and OPN. Pathologie Biologie. . 2015;63:11–16. doi: 10.1016/j.patbio.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Gray GK, Li CMC, Rosenbluth JM, Selfors LM, Girnius N, Lin JR, Schackmann RCJ, et al. A human breast atlas integrating single-cell proteomics and transcriptomics. Dev Cell. . 2022;57:1400–1420.e7. doi: 10.1016/j.devcel.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Autenshlyus AI, Arkhipov SA, Kunts TA, Marinkin IO, Mikhailova ES, Karpukhina XV, Varaksin NA. Cytokine profiles of tumor supernatants in invasive ductal cancer and fibroadenoma of the breast and its relationship with VEGF-A expression in the tumors. Int J Immunopathol Pharmacol. . 2017;30:83–88. doi: 10.1177/0394632016681306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lebrecht A, Grimm C, Euller G, Ludwig E, Ulbrich E, Lantzsch T, Hefler L, et al. Transforming growth factor beta 1 serum levels in patients with preinvasive and invasive lesions of the breast. Int J Biol Markers. . 2004;19:236–239. doi: 10.1177/172460080401900309. [DOI] [PubMed] [Google Scholar]
- 82.Sheen-Chen SM. Serum levels of transforming growth factor β1 in patients with breast cancer. Arch Surg. . 2001;136:937. doi: 10.1001/archsurg.136.8.937. [DOI] [PubMed] [Google Scholar]
- 83.Gregor MF, Misch ES, Yang L, Hummasti S, Inouye KE, Lee AH, Bierie B, et al. The role of adipocyte XBP1 in metabolic regulation during lactation. Cell Rep. . 2013;3:1430–1439. doi: 10.1016/j.celrep.2013.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia. . 2010;15:279–290. doi: 10.1007/s10911-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hovey RC, Goldhar AS, Baffi J, Vonderhaar BK. Transcriptional regulation of vascular endothelial growth factor expression in epithelial and stromal cells during mouse mammary gland development. Mol Endocrinol. . 2001;15:819–831. doi: 10.1210/mend.15.5.0635. [DOI] [PubMed] [Google Scholar]
- 86.Muschler J, Streuli CH. Cell-matrix interactions in mammary gland development and breast cancer. Cold Spring Harbor Perspectives Biol. . 2010;2:a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. . 2012;139:269–275. doi: 10.1242/dev.071696. [DOI] [PubMed] [Google Scholar]
- 88.Bulun SE, Simpson ER. Regulation of aromatase expression in human tissues. Breast Cancer Res Tr. . 1994;30:19–29. doi: 10.1007/BF00682738. [DOI] [PubMed] [Google Scholar]
- 89.Choi J, Cha YJ, Koo JS. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog Lipid Res. . 2018;69:11–20. doi: 10.1016/j.plipres.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 90.Nibbering PH, Zomerdijk TPL, van Haastert PJM, van Furth R. A competition binding assay for determination of the inositol (1,4,5)-trisphosphate content of human leucocytes. Biochem Biophys Res Commun. . 1990;170:755–762. doi: 10.1016/0006-291X(90)92155-S. [DOI] [PubMed] [Google Scholar]
- 91.Zhang S, Wang B, Xiao H, Dong J, Li Y, Zhu C, Jin Y, et al. LncRNA HOTAIR enhances breast cancer radioresistance through facilitating HSPA1A expression via sequestering miR-449b‐5p. Thorac Cancer. . 2020;11:1801–1816. doi: 10.1111/1759-7714.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qi JL, He JR, Liu CB, Jin SM, Yang X, Bai HM, Ma YB. SQSTM1/p62 regulate breast cancer progression and metastasis by inducing cell cycle arrest and regulating immune cell infiltration. Genes Dis. . 2022;9:1332–1344. doi: 10.1016/j.gendis.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, Wei S, et al. Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab. . 2018;28:87–103.e6. doi: 10.1016/j.cmet.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. . 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siersbæk R, Scabia V, Nagarajan S, Chernukhin I, Papachristou EK, Broome R, Johnston SJ, et al. IL6/STAT3 signaling hijacks estrogen receptor α enhancers to drive breast cancer metastasis. Cancer Cell. . 2020;38:412–423.e9. doi: 10.1016/j.ccell.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Miyahira AK, Simons DL, Lu X, Chang AY, Wang C, Suni MA, et al. IL6 signaling in peripheral blood T cells predicts clinical outcome in breast cancer. Cancer Res. . 2017;77:1119–1126. doi: 10.1158/0008-5472.CAN-16-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. . 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. . 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 99.Dulić V, Beney GE, Frebourg G, Drullinger LF, Stein GH. Uncoupling between phenotypic senescence and cell cycle arrest in aging p21-deficient fibroblasts. Mol Cell Biol. . 2000;20:6741–6754. doi: 10.1128/MCB.20.18.6741-6754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wong H, Riabowol K. Differential CDK-inhibitor gene expression in aging human diploid fibroblasts. Exp Gerontol. . 1996;31:311–325. doi: 10.1016/0531-5565(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 101.Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. . 2010;29:4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 102.Ruas M, Gregory F, Jones R, Poolman R, Starborg M, Rowe J, Brookes S, et al. CDK4 and CDK6 Delay Senescence by Kinase-Dependent and p16 INK4a-Independent Mechanisms . Mol Cell Biol. . 2007;27:4273–4282. doi: 10.1128/MCB.02286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. . 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu Y, Zhang S, Wu Q, Xu S, Cui Y, Yang Z, Zhao X, et al. Differential expression of decorin, EGFR and cyclin D1 during mammary gland carcinogenesis in TA2 mice with spontaneous breast cancer. J Exp Clin Cancer Res. . 2010;29:6. doi: 10.1186/1756-9966-29-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarsour EH, Goswami M, Kalen AL, Lafin JT, Goswami PC. Hydroxytyrosol inhibits chemokine C-C motif ligand 5 mediated aged quiescent fibroblast-induced stimulation of breast cancer cell proliferation. Age. . 2014;36:9645. doi: 10.1007/s11357-014-9645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. . 2020;19:145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Darnell Jr JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. . 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 108.Yang D, Guo Q, Liang Y, Zhao Y, Tian X, Ye Y, Tian J, et al. Wogonin induces cellular senescence in breast cancer via suppressing TXNRD2 expression. Arch Toxicol. . 2020;94:3433–3447. doi: 10.1007/s00204-020-02842-y. [DOI] [PubMed] [Google Scholar]
- 109.Salminen A, Kaarniranta K, Kauppinen A. Insulin/IGF-1 signaling promotes immunosuppression via the STAT3 pathway: impact on the aging process and age-related diseases. Inflamm Res. . 2021;70:1043–1061. doi: 10.1007/s00011-021-01498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tkach M, Coria L, Rosemblit C, Rivas MA, Proietti CJ, Díaz Flaqué MC, Beguelin W, et al. Targeting Stat3 induces senescence in tumor cells and elicits prophylactic and therapeutic immune responses against breast cancer growth mediated by NK cells and CD4+ T cells. J Immunol. . 2012;189:1162–1172. doi: 10.4049/jimmunol.1102538. [DOI] [PubMed] [Google Scholar]
- 111.Al-Khalaf HH, Ghebeh H, Inass R, Aboussekhra A. Senescent breast luminal cells promote carcinogenesis through interleukin-8-dependent activation of stromal fibroblasts. Mol Cell Biol. . 2019;39:e00359-18. doi: 10.1128/MCB.00359-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. . 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gao Y, Feng B, Han S, Zhang K, Chen J, Li C, Wang R, et al. The roles of microRNA-141 in human cancers: from diagnosis to treatment. Cell Physiol Biochem. . 2016;38:427–448. doi: 10.1159/000438641. [DOI] [PubMed] [Google Scholar]
- 114.Li H, Qiu L, Liu Q, Ma Z, Xie X, Luo Y, Wu X. Senescent fibroblasts generate a CAF phenotype through the Stat3 pathway. Genes. . 2022;13:1579. doi: 10.3390/genes13091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang YH, Yang PM, Chuah QY, Lee YJ, Hsieh YF, Peng CW, Chiu SJ. Autophagy promotes radiation-induced senescence but inhibits bystander effects in human breast cancer cells. Autophagy. . 2014;10:1212–1228. doi: 10.4161/auto.28772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernandez-Valdivia R, Mukherjee A, Ying Y, Li J, Paquet M, DeMayo FJ, Lydon JP. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol. . 2009;328:127–139. doi: 10.1016/j.ydbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 117.Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKα provides an essential link between rank signaling and cyclin D1 expression during mammary gland development. Cell. . 2001;107:763–775. doi: 10.1016/S0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- 118.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. . 2007;9:S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM. RANKL/RANK—beyond bones. J Mol Med. . 2011;89:647–656. doi: 10.1007/s00109-011-0749-z. [DOI] [PubMed] [Google Scholar]
- 120.Duan P, Wang ZM, Liu J, Wang LN, Yang Z, Tu P. Gene polymorphisms in RANKL/RANK/OPG pathway are associated with ages at menarche and natural menopause in Chinese women. BMC Womens Health. . 2015;15:32. doi: 10.1186/s12905-015-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanada R, Hanada T, Penninger JM. Physiology and pathophysiology of the RANKL/RANK system. Biol Chem. . 2010;391:1365. doi: 10.1515/bc.2010.149. [DOI] [PubMed] [Google Scholar]
- 122.Voutilainen M, Lindfors PH, Lefebvre S, Ahtiainen L, Fliniaux I, Rysti E, Murtoniemi M, et al. Ectodysplasin regulates hormone-independent mammary ductal morphogenesis via NF-κB. Proc Natl Acad Sci USA. . 2012;109:5744–5749. doi: 10.1073/pnas.1110627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao J, Venton L, Sakata T, Halloran BP. Expression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice. J Bone Miner Res. . 2003;18:270–277. doi: 10.1359/jbmr.2003.18.2.270. [DOI] [PubMed] [Google Scholar]
- 124.Benítez S, Cordero A, Santamaría PG, Redondo-Pedraza J, Rocha AS, Collado-Solé A, Jimenez M, et al. RANK links senescence to stemness in the mammary epithelia, delaying tumor onset but increasing tumor aggressiveness. Dev Cell. . 2021;56:1727–1741.e7. doi: 10.1016/j.devcel.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schubert A, Schulz H, Emons G, Gründker C. Expression of osteoprotegerin and receptor activator of nuclear factor-κB ligand (RANKL) in HCC70 breast cancer cells and effects of treatment with gonadotropin-releasing hormone on RANKL expression. Gynecol endocrinol. . 2008;24:331–338. doi: 10.1080/09513590802095845. [DOI] [PubMed] [Google Scholar]
- 126.Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. . 2003;32:136–141. doi: 10.1016/S8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- 127.Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. . 2011;53:141–144. doi: 10.1016/j.cyto.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang YD, Tao MF, Wang L, Cheng WW, Wan XP. Selective regulation of osteoblastic OPG and RANKL by dehydroepiandrosterone through activation of the estrogen receptor β-mediated MAPK signaling pathway. Horm Metab Res. . 2012;44:494–500. doi: 10.1055/s-0032-1311567. [DOI] [PubMed] [Google Scholar]
- 129.Razmara E, Bitaraf A, Karimi B, Babashah S. Functions of the SNAI family in chondrocyte-to-osteocyte development. Ann NY Acad Sci. . 2021;1503:5–22. doi: 10.1111/nyas.14668. [DOI] [PubMed] [Google Scholar]
- 130.Wu Y, Zhou BP. Snail: more than EMT. Cell Adh Migr. . 2010;4:199–203. doi: 10.4161/cam.4.2.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Imran SAM, Yazid MD, Idrus RBH, Maarof M, Nordin A, Razali RA, Lokanathan Y. Is there an interconnection between epithelial-mesenchymal transition (EMT) and telomere shortening in aging? Int J Mol Sci. . 2021;22:3888. doi: 10.3390/ijms22083888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu P, Zhang C, Gao Y, Wu F, Zhou Y, Wu WS. The transcription factor Slug represses p16Ink4a and regulates murine muscle stem cell aging. Nat Commun. . 2019;10:2568. doi: 10.1038/s41467-019-10479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gross KM, Zhou W, Breindel JL, Ouyang J, Jin DX, Sokol ES, Gupta PB, et al. Loss of slug compromises dna damage repair and accelerates stem cell aging in mammary epithelium. Cell Rep. . 2019;28:394–407.e6. doi: 10.1016/j.celrep.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 134.Bryson BL, Tamagno I, Taylor SE, Parameswaran N, Chernosky NM, Balasubramaniam N, Jackson MW. Aberrant induction of a mesenchymal/stem cell program engages senescence in normal mammary epithelial cells. Mol Cancer Res. . 2021;19:651–666. doi: 10.1158/1541-7786.MCR-19-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Doherty MR, Parvani JG, Tamagno I, Junk DJ, Bryson BL, Cheon HJ, Stark GR, et al. The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer. Breast Cancer Res. . 2019;21:54. doi: 10.1186/s13058-019-1136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duijf PHG, Nanayakkara D, Nones K, Srihari S, Kalimutho M, Khanna KK. Mechanisms of genomic instability in breast cancer. Trends Mol Med. . 2019;25:595–611. doi: 10.1016/j.molmed.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 137.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001, 61: 3230–3239 . [PubMed]
- 138.Kwei KA, Kung Y, Salari K, Holcomb IN, Pollack JR. Genomic instability in breast cancer: pathogenesis and clinical implications. Mol Oncol. . 2010;4:255–266. doi: 10.1016/j.molonc.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Okugawa Y, Grady WM, Goel A. Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology. . 2015;149:1204–1225.e12. doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dulaimi E, Hillinck J, de Caceres II, Al-Saleem T, Cairns P. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res. . 2004;10:6189–6193. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- 141.Dammann R, Yang G, Pfeifer GP. Hypermethylation of the cpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001, 61: 3105–3109 . [PubMed]
- 142.Jin Z, Tamura G, Tsuchiya T, Sakata K, Kashiwaba M, Osakabe M, Motoyama T. Adenomatous polyposis coli (APC) gene promoter hypermethylation in primary breast cancers. Br J Cancer. . 2001;85:69–73. doi: 10.1054/bjoc.2001.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. . 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pizzorno J. Mitochondria—fundamental to life and health. Integr Med (Encinitas) 2014, 13: 8-15 . [PMC free article] [PubMed]
- 145.Hsu CC, Tseng LM, Lee HC. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood) . 2016;241:1281–1295. doi: 10.1177/1535370216641787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sanchez-Alvarez R, Martinez-Outschoorn UE, Lamb R, Hulit J, Howell A, Gandara R, Sartini M, et al. Mitochondrial dysfunction in breast cancer cells prevents tumor growth. Cell Cycle. . 2013;12:172–182. doi: 10.4161/cc.23058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pelicano H, Zhang W, Liu J, Hammoudi N, Dai J, Xu RH, Pusztai L, et al. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast Cancer Res. . 2014;16:434. doi: 10.1186/s13058-014-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lunetti P, Di Giacomo M, Vergara D, De Domenico S, Maffia M, Zara V, Capobianco L, et al. Metabolic reprogramming in breast cancer results in distinct mitochondrial bioenergetics between luminal and basal subtypes. FEBS J. . 2019;286:688–709. doi: 10.1111/febs.14756. [DOI] [PubMed] [Google Scholar]
- 149.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. . 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Birch J, Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. . 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ruscetti M, Morris Iv JP, Mezzadra R, Russell J, Leibold J, Romesser PB, Simon J, et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. . 2020;181:424–441.e21. doi: 10.1016/j.cell.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sirinian C, Peroukidis S, Kriegsmann K, Chaniotis D, Koutras A, Kriegsmann M, Papanastasiou AD. Cellular senescence in normal mammary gland and breast cancer. Implications for cancer therapy. Genes. . 2022;13:994. doi: 10.3390/genes13060994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguría A, et al. Tumour biology: senescence in premalignant tumours. Nature. . 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 154.Demaria M, O′Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. . 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu S, Uppal H, Demaria M, Desprez PY, Campisi J, Kapahi P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci Rep. . 2015;5:17895. doi: 10.1038/srep17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hwang HJ, Lee YR, Kang D, Lee HC, Seo HR, Ryu JK, Kim YN, et al. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. . 2020;490:100–110. doi: 10.1016/j.canlet.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 157.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. . 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. . 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 159.Cabello-Verrugio C, Simon F, Trollet C, Santibañez JF. Oxidative stress in disease and aging: mechanisms and therapies 2016. Oxid Med Cell Longev. . 2017;2017:1–2. doi: 10.1155/2017/4310469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shaw PX, Werstuck G, Chen Y. Oxidative stress and aging diseases. Oxid Med Cell Longev. 2014, 2014: 569146 . [DOI] [PMC free article] [PubMed]
- 161.Barnes RP, Fouquerel E, Opresko PL. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. . 2019;177:37–45. doi: 10.1016/j.mad.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Benz CC, Yau C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat Rev Cancer. . 2008;8:875–879. doi: 10.1038/nrc2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Escobar KA, Cole NH, Mermier CM, VanDusseldorp TA. Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell. . 2019;18:e12876. doi: 10.1111/acel.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yessenkyzy A, Saliev T, Zhanaliyeva M, Masoud AR, Umbayev B, Sergazy S, Krivykh E, et al. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients. . 2020;12:1344. doi: 10.3390/nu12051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rotem-Dai N, Muraleedharan A, Livneh E. PKCeta promotes stress-induced autophagy and senescence in breast cancer cells, presenting a target for therapy. Pharmaceutics. . 2022;14:1704. doi: 10.3390/pharmaceutics14081704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu L, Liu S, Luo H, Chen C, Zhang X, He L, Tu G. GPR30-mediated HMGB1 upregulation in CAFs induces autophagy and tamoxifen resistance in ERα-positive breast cancer cells. Aging. . 2021;13:16178–16197. doi: 10.18632/aging.203145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Du WW, Yang W, Li X, Awan FM, Yang Z, Fang L, Lyu J, et al. A circular RNA circ-DNMT1 enhances breast cancer progression by activating autophagy. Oncogene. . 2018;37:5829–5842. doi: 10.1038/s41388-018-0369-y. [DOI] [PubMed] [Google Scholar]
- 168.Sangma MB, Panda K, Dasiah S. A clinico-pathological study on benign breast diseases. J Clin Diagn Res. 2013, 7: 503–506 . [DOI] [PMC free article] [PubMed]
- 169.Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist. . 2006;11:435–449. doi: 10.1634/theoncologist.11-5-435. [DOI] [PubMed] [Google Scholar]
- 170.Hill A, Gutierrez E, Liu J, Sammons S, Kimmick G, Sedrak MS. The evolving complexity of treating hormone receptor-positive, human epidermal growth factor receptor-2 (her2)-negative breast cancer: special considerations in older breast cancer patients-part II: metastatic disease. Drugs Aging. . 2020;37:349–358. doi: 10.1007/s40266-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Loke BN, Md Nasir ND, Thike AA, Lee JYH, Lee CS, Teh BT, Tan PH. Genetics and genomics of breast fibroadenomas. J Clin Pathol. . 2018;71:381–387. doi: 10.1136/jclinpath-2017-204838. [DOI] [PubMed] [Google Scholar]
- 172.Weinstein D, Strano S, Cohen P, Fields S, Gomori JM, Degani H. Breast fibroadenoma: mapping of pathophysiologic features with three-time-point, contrast-enhanced MR imaging—pilot study. Radiology. . 1999;210:233–240. doi: 10.1148/radiology.210.1.r99ja18233. [DOI] [PubMed] [Google Scholar]
- 173.Touraine P, Martini JF, Zafrani B, Durand JC, Labaille F, Malet C, Nicolas Á, et al. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. . 1998;83:667–674. doi: 10.1210/jcem.83.2.4564. [DOI] [PubMed] [Google Scholar]
- 174.Mertani HC, Garcia-Caballero T, Lambert A, Gérard F, Palayer C, Boutin JM, Vonderhaar BK, et al. Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer. . 1998;79:202–211. doi: 10.1002/(SICI)1097-0215(19980417)79:2<202::AID-IJC17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 175.Gill S, Peston D, Vonderhaar BK, Shousha S. Expression of prolactin receptors in normal, benign, and malignant breast tissue: an immunohistological study. J Clin Pathol. . 2001;54:956–960. doi: 10.1136/jcp.54.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bogorad RL, Courtillot C, Mestayer C, Bernichtein S, Harutyunyan L, Jomain JB, Bachelot A, et al. Identification of a gain-of-function mutation of the prolactin receptor in women with benign breast tumors. Proc Natl Acad Sci USA. . 2008;105:14533–14538. doi: 10.1073/pnas.0800685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lien HC, Huang CS, Yang YW, Jeng YM. Mutational analysis of MED12 exon 2 in a spectrum of fibroepithelial tumours of the breast: implications for pathogenesis and histogenesis . Histopathology. . 2016;68:433–441. doi: 10.1111/his.12764. [DOI] [PubMed] [Google Scholar]
- 178.Turunen M, Spaeth JM, Keskitalo S, Park MJ, Kivioja T, Clark AD, Mäkinen N, et al. Uterine leiomyoma-linked MED12 mutations disrupt mediator-associated CDK activity. Cell Rep. . 2014;7:654–660. doi: 10.1016/j.celrep.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kämpjärvi K, Park MJ, Mehine M, Kim NH, Clark AD, Bützow R, Böhling T, et al. Mutations in exon 1 highlight the role of MED12 in uterine leiomyomas . Hum Mutat. . 2014;35:1136–1141. doi: 10.1002/humu.22612. [DOI] [PubMed] [Google Scholar]
- 180.Chang CM, Lin MC, Yin WY. Risk of breast cancer in women with non-lactational mastitis. Sci Rep. . 2019;9:15587. doi: 10.1038/s41598-019-52046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hadsell D, Torres D, George J, Capuco A, Ellis S, Fiorotto M. Changes in secretory cell turnover, and mitochondrial oxidative damage in the mouse mammary gland during a single prolonged lactation cycle suggest the possibility of accelerated cellular aging. Exp Gerontol. . 2006;41:271–281. doi: 10.1016/j.exger.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 182.Ali T, Rahman A, Qureshi MS, Hussain MT, Khan MS, Uddin S, Iqbal M, et al. Effect of management practices and animal age on incidence of mastitis in Nili Ravi buffaloes. Trop Anim Health Prod. . 2014;46:1279–1285. doi: 10.1007/s11250-014-0641-2. [DOI] [PubMed] [Google Scholar]
- 183.Alemu AA, Fikiru H, Alemante MS, Aster Y. Prevalence of subclinical mastitis in lactating cows in selected commercial dairy farms of Holeta district. Journal of Veterinary Medicine and Animal Health. 2013, 5: 67–72
- 184.Kurjogi MM, Kaliwal BB. Epidemiology of bovine mastitis in cows of dharwad district. Int Scholarly Res Not. . 2014;2014:1–9. doi: 10.1155/2014/968076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lambe M, Johansson ALV, Altman D, Eloranta S. Mastitis and the risk of breast cancer. Epidemiology. . 2009;20:747–751. doi: 10.1097/EDE.0b013e3181adbb1e. [DOI] [PubMed] [Google Scholar]
- 186.Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, Li H, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. . 2022;185:1356–1372.e26. doi: 10.1016/j.cell.2022.02.027. [DOI] [PubMed] [Google Scholar]
- 187.Witkowska-Zimny M, Kaminska-El-Hassan E. Cells of human breast milk. Cell Mol Biol Lett. . 2017;22:11. doi: 10.1186/s11658-017-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Capuco AV, Akers RM. Mammary involution in dairy animals. J Mammary Gland Biol Neoplasia. . 1999;4:137–144. doi: 10.1023/A:1018769022990. [DOI] [PubMed] [Google Scholar]
- 189.Wilde CJ, Knight CH. Metabolic adaptations in mammary gland during the declining phase of lactation. J Dairy Sci. . 1989;72:1679–1692. doi: 10.3168/jds.S0022-0302(89)79279-1. [DOI] [PubMed] [Google Scholar]
- 190.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. . 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 191.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontology. . 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 192.Schmeer C, Kretz A, Wengerodt D, Stojiljkovic, Witte OW. Dissecting aging and senescence-current concepts and open lessons. Cells. . 2019;8:1446. doi: 10.3390/cells8111446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Freudenheim JL, Marshall JR, Vena JE, Moysich KB, Muti P, Laughlin R, Nemoto T, et al. Lactation history and breast cancer risk. Am J Epidemiol. . 1997;146:932–938. doi: 10.1093/oxfordjournals.aje.a009219. [DOI] [PubMed] [Google Scholar]
- 194.Newcomb PA. Lactation and breast cancer risk. J Mammary Gland Biol Neoplasia. . 1997;2:311–318. doi: 10.1023/A:1026344707161. [DOI] [PubMed] [Google Scholar]
- 195.Bailur JK, Derhovanessian E, Gueckel B, Pawelec G. Prognostic impact of circulating Her-2-reactive T-cells producing pro- and/or anti-inflammatory cytokines in elderly breast cancer patients. J Immunother Cancer. . 2015;3:45. doi: 10.1186/s40425-015-0090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bahcecioglu G, Yue X, Howe E, Guldner I, Stack MS, Nakshatri H, Zhang S, et al. Aged breast extracellular matrix drives mammary epithelial cells to an invasive and cancer-like phenotype. Adv Sci. . 2021;8:2100128. doi: 10.1002/advs.202100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Garbe JC, Pepin F, Pelissier FA, Sputova K, Fridriksdottir AJ, Guo DE, Villadsen R, et al. Accumulation of multipotent progenitors with a basal differentiation bias during aging of human mammary epithelia. Cancer Res. . 2012;72:3687–3701. doi: 10.1158/0008-5472.CAN-12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975, 55: 231–273 . [PubMed]
- 199.Oh H, Pfeiffer RM, Falk RT, Horne HN, Xiang J, Pollak M, Brinton LA, et al. Serum insulin-like growth factor (IGF)-I and IGF binding protein-3 in relation to terminal duct lobular unit involution of the normal breast in Caucasian and African American women: The Susan G. Komen Tissue Bank. Int J Cancer. . 2018;143:496–507. doi: 10.1002/ijc.31333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hanna M, Dumas I, Orain M, Jacob S, Têtu B, Sanschagrin F, et al. Association between local inflammation and breast tissue age-related lobular involution among premenopausal and postmenopausal breast cancer patients. PLoS One. 2017, 12: e0183579 . [DOI] [PMC free article] [PubMed]
- 201.Maliniak ML, Miller-Kleinhenz J, Cronin-Fenton DP, Lash TL, Gogineni K, Janssen EAM, McCullough LE. Crown-like structures in breast adipose tissue: early evidence and current issues in breast cancer. Cancers. . 2021;13:2222. doi: 10.3390/cancers13092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, Radisky DC, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. . 2017;19:8. doi: 10.1186/s13058-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brown SB, Hankinson SE. Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. . 2015;99:8–10. doi: 10.1016/j.steroids.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 204.Yaghjyan L, Colditz GA. Estrogens in the breast tissue: a systematic review. Cancer Causes Control. . 2011;22:529–540. doi: 10.1007/s10552-011-9729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sung H, Guo C, Li E, Li J, Pfeiffer RM, Guida JL, Cora R, et al. The relationship between terminal duct lobular unit features and mammographic density among Chinese breast cancer patients. Int J Cancer. . 2019;145:70–77. doi: 10.1002/ijc.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, Falcone DJ, et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev Res. . 2015;8:349–358. doi: 10.1158/1940-6207.CAPR-14-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, Morrow M, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. . 2016;22:2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. . 2016;375:209–219. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ulrich-Lai YM, Ryan KK. PPARγ and stress: implications for aging. Exp Gerontol. . 2013;48:671–676. doi: 10.1016/j.exger.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Apostoli AJ, Skelhorne‐Gross GEA, Rubino RE, Peterson NT, Di Lena MA, Schneider MM, SenGupta SK, et al. Loss of PPARγ expression in mammary secretory epithelial cells creates a pro‐breast tumorigenic environment. Int J Cancer. . 2014;134:1055–1066. doi: 10.1002/ijc.28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jenkins EC, Chattopadhyay M, Germain D. Are the estrogen receptor and SIRT3 axes of the mitochondrial UPR key regulators of breast cancer subtype determination according to age? Aging Cancer. . 2021;2:75–81. doi: 10.1002/aac2.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vrbanec D, Petricević B. Estrogen and progesterone receptor status in primary breast cancer—a study of 11,273 patients from the year 1990 to 2002. Coll Antropol. 2007, 31: 535–540 . [PubMed]
- 213.Srkalovic G, Cai RZ, Schally AV. Evaluation of receptors for somatostatin in various tumors using different analogs. J Clin Endocrinol Metab. . 1990;70:661–669. doi: 10.1210/jcem-70-3-661. [DOI] [PubMed] [Google Scholar]
- 214.Lodi M, Scheer L, Reix N, Heitz D, Carin AJ, Thiébaut N, Neuberger K, et al. Breast cancer in elderly women and altered clinico-pathological characteristics: a systematic review. Breast Cancer Res Treat. . 2017;166:657–668. doi: 10.1007/s10549-017-4448-5. [DOI] [PubMed] [Google Scholar]
- 215.Mullooly M, Puvanesarajah S, Fan S, Pfeiffer RM, Olsson LT, Hada M, Kirk EL, et al. Using digital pathology to understand epithelial characteristics of benign breast disease among women undergoing diagnostic image-guided breast biopsy. Cancer Prevention Res. . 2019;12:861–870. doi: 10.1158/1940-6207.CAPR-19-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hada M, Oh H, Fan S, Falk RT, Geller B, Vacek P, Weaver D, et al. Relationship of serum progesterone and progesterone metabolites with mammographic breast density and terminal ductal lobular unit involution among women undergoing diagnostic breast biopsy. J Clin Med. . 2020;9:245. doi: 10.3390/jcm9010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. . 2003;95:30–37. doi: 10.1093/jnci/95.1.30. [DOI] [PubMed] [Google Scholar]
- 218.van Duijnhoven FJB, Peeters PHM, Warren RML, Bingham SA, van Noord PAH, Monninkhof EM, Grobbee DE, et al. Postmenopausal hormone therapy and changes in mammographic density. J Clin Oncol. . 2007;25:1323–1328. doi: 10.1200/JCO.2005.04.7332. [DOI] [PubMed] [Google Scholar]
- 219.Campos C, Pera A, Sanchez-Correa B, Alonso C, Lopez-Fernandez I, Morgado S, Tarazona R, et al. Effect of age and CMV on NK cell subpopulations. Exp Gerontol. . 2014;54:130–137. doi: 10.1016/j.exger.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 220.Blokzijl F, de Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, Huch M, et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. . 2016;538:260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Erratum for the Review ″Somatic mutation in cancer and normal cells″ by I. Martincorena and P. J. Campbell. Science. 2016, 351: aaf5401 . [DOI] [PubMed]
- 222.Olopade OI, Pichert G. Cancer genetics in oncology practice. Ann Oncol. . 2001;12:895–908. doi: 10.1023/A:1011176107455. [DOI] [PubMed] [Google Scholar]
- 223.Balon K, Sheriff A, Jacków J, Łaczmański Ł. Targeting cancer with CRISPR/Cas9-based therapy. Int J Mol Sci. . 2022;23:573. doi: 10.3390/ijms23010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jain S, Mazaheri B, Raviv N, Bruck J. Glioblastoma signature in the DNA of blood-derived cells. PLoS One. . 2021;16:e0256831. doi: 10.1371/journal.pone.0256831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kenemans P, Verstraeten RA, Verheijen RHM. Reprint of Oncogenic pathways in hereditary and sporadic breast cancer. Maturitas. . 2008;61:141–150. doi: 10.1016/j.maturitas.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 226.Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF. A systematic review of genetic polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999, 8: 843–854 . [PubMed]
- 227.Stenmark-Askmalm M, Gentile M, Wingren S, Ståhl O. Protein accumulation and gene mutation of p53 in bilateral breast cancer. Acta Oncol. . 2001;40:56–62. doi: 10.1080/028418601750071064. [DOI] [PubMed] [Google Scholar]
- 228.Hobbs EA, Litton JK, Yap TA. Development of the PARP inhibitor talazoparib for the treatment of advanced BRCA1 and BRCA2 mutated breast cancer . Expert Opin Pharmacother. . 2021;22:1825–1837. doi: 10.1080/14656566.2021.1952181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.LaBarge MA, Mora-Blanco EL, Samson S, Miyano M. Breast cancer beyond the age of mutation. Gerontology. . 2016;62:434–442. doi: 10.1159/000441030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. . 2014;106:dju165. doi: 10.1093/jnci/dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bao Q, Pan J, Qi H, Wang L, Qian H, Jiang F, Shao Z, et al. Aging and age-related diseases – From endocrine therapy to target therapy. Mol Cell Endocrinol. . 2014;394:115–118. doi: 10.1016/j.mce.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 232.Ng ST, Zhou J, Adesanya OO, Wang J, Leroith D, Bondy CA. Growth hormone treatment induces mammary gland hyperplasia in aging primates. Nat Med. . 1997;3:1141–1144. doi: 10.1038/nm1097-1141. [DOI] [PubMed] [Google Scholar]
- 233.Fishman JR, Flatt MA, Settersten Jr RA. Bioidentical hormones, menopausal women, and the lure of the “natural” in U.S. anti-aging medicine. Soc Sci Med. . 2015;132:79–87. doi: 10.1016/j.socscimed.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. . 2020;20:417–436. doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 235.Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev. . 2012;11:404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 236.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, et al. Endurance exercise as a countermeasure for aging. Diabetes. . 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Santos AL, Sinha S. Obesity and aging: molecular mechanisms and therapeutic approaches. Ageing Res Rev. . 2021;67:101268. doi: 10.1016/j.arr.2021.101268. [DOI] [PubMed] [Google Scholar]
- 238.Khan S, Shukla S, Sinha S, Meeran SM. Epigenetic targets in cancer and aging: dietary and therapeutic interventions. Expert Opin Ther Targets. . 2016;20:689–703. doi: 10.1517/14728222.2016.1132702. [DOI] [PubMed] [Google Scholar]
- 239.Aunan JR, Cho WC, Søreide K. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. . 2017;8:628. doi: 10.14336/AD.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. . 2006;45:786–796. doi: 10.1016/j.jhep.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 241.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. . 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pandey K, Park N, Park KS, Hur J, Cho YB, Kang M, An HJ, et al. Combined CDK2 and CDK4/6 inhibition overcomes palbociclib resistance in breast cancer by enhancing senescence. Cancers. . 2020;12:3566. doi: 10.3390/cancers12123566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. . 2010;468:103–107. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 244.Costa TDF, Zhuang T, Lorent J, Turco E, Olofsson H, Masia-Balague M, Zhao M, et al. PAK4 suppresses RELB to prevent senescence-like growth arrest in breast cancer. Nat Commun. . 2019;10:3589. doi: 10.1038/s41467-019-11510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arreal L, Piva M, Fernández S, Revandkar A, Schaub- Clerigué A, Villanueva J, Zabala-Letona A, et al. Targeting PML in triple negative breast cancer elicits growth suppression and senescence. Cell Death Differ. . 2020;27:1186–1199. doi: 10.1038/s41418-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xu H, Lin F, Wang Z, Yang L, Meng J, Ou Z, Shao Z, et al. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. . 2018;412:69–80. doi: 10.1016/j.canlet.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 247.Rinaldi C, Malara NM, D′Angelo R, Sidoti A, Leotta A, Lio S, Caparello B, et al. Age dependent switching role of cyclin D1 in breast cancer. Anal Cell Pathol. . 2012;35:179–185. doi: 10.1155/2012/820906. [DOI] [PMC free article] [PubMed] [Google Scholar]