Abstract
Evolutionarily conserved, the Hippo signaling pathway is critical in regulating organ size and tissue homeostasis. The activity of this pathway is tightly regulated under normal circumstances, since its physical function is precisely maintained to control the rate of cell proliferation. Failure of maintenance leads to a variety of tumors. Our understanding of the mechanism of Hippo dysregulation and tumorigenesis is becoming increasingly precise, relying on the emergence of upstream inhibitor or activator and the connection linking Hippo target genes, mutations, and related signaling pathways with phenotypes. In this review, we summarize recent reports on the signaling network of the Hippo pathway in tumorigenesis and progression by exploring its critical mechanisms in cancer biology and potential targeting in cancer therapy.
Keywords: Hippo, YAP, TAZ, TEAD, signal transduction, cancer, hallmark
Introduction
The Hippo signaling pathway is evolutionarily conserved. Responsible for determining cell fate, it is pivotal in regulating organ size and tissue homeostasis. The initial clues linking the Hippo pathway to tumors arose from Drosophila, where the core members were discovered based on the phenotype of massive overgrowth in genetic mosaics [ 1‒ 10] . These oncogenes or tumor suppressor genes are linked to cancer progression [ 11, 12] . The mammalian homologues of Yorkie (Yki), Yes1 associated transcriptional regulator (YAP) and WW domain-containing transcription regulator 1 (TAZ) were soon discovered [ 13‒ 15] , implying the existence of an evolutionarily conserved kinase cascade. This was confirmed by the discovery of Hippo homologue proteins in holozoans, suggesting that Hippo signaling represents a particularly conserved mechanism from unicellular organisms to mammals [16]. The dysregulation of the Hippo pathway in mammals can lead to tumorigenesis phenotypes similar to those in Drosophila. Clinical patient sample analysis has further confirmed those discoveries, making the Hippo pathway a hot target of precision medication.
The Hippo Signaling Pathway
Intensive studies have explored the multiple roles of Hippo pathway components in cell adhesion, polarity, mechanical force response, soluble factors, and stress signal transduction, expanding this kinase cascade into a complex signaling network in Drosophila and mammals. Additionally, members of this pathway are intensively involved in the occurrence and development of cancers. The core members of the Hippo pathway are a network of phosphokinase cascades, including mammalian STE20-like protein kinase 1 and 2 (MST1/2), whose counterpart is Hippo (HPO) in Drosophila, and their downstream kinases, large tumor suppressor 1 (LATS1), LATS2, their scaffold proteins Salvador homolog 1 (SAV1), and MOB kinase activator 1A (MOB1A) and MOB1B [ 1, 2, 4] . In response to upstream signals, MST/HPO proteins, facilitated by SAV1, phosphorylate and activate the kinase function of LATS1/2 and the scaffold protein MOB1A/B, and then the signal is further transmitted to the downstream transcription coactivator YAP and TAZ and, in Drosophila, Yki [ 14, 15] . YAP proteins activated by upstream phosphorylation are retained in the cytoplasm, where they are degraded or prohibited from entering the nucleus and thus lose their transcriptionally active function. When the Hippo pathway is inactivated, unphosphorylated YAP/TAZ translocates into the nucleus and binds to TEAD1, 2, 3 or 4 transcription factor proteins. The YAP-TEAD complex initiates downstream gene transcription [17] and thus regulates the related phenotypes. Competing with YAP/TAZ, vestigial-like (VGLL) family proteins could repress TEAD1-4 transcriptional function in proliferation [ 18‒ 20] .
Upstream Signals of the Hippo Signaling Pathway
To date, several upstream signals have been discovered to transmit multiple signals from stimuli to the core kinases of Hippo ( Figure 1), such as contact inhibition. At high density, cells stop growing when confluent. Sensing that, YAP is relocated to the cytoplasm from the nucleus. At a relatively lower density, YAP is located in the nucleus, where it is able to induce the transcription of downstream genes [21]. Several genes, including Moesin-Ezrin-Radixin Like Tumor Suppressor (Merlin or NF2), kidney and brain expressed protein (KIBRA) and angiomotin (AMOT), which can influence Hippo signaling pathways, play important roles in the cells sensing contact inhibitory signals, i.e., from E-cadherin (E-cad) [22]. Ectopic expression of E-cad in mesenchymal MDA-MB-231 cells prevents the nuclear localization of YAP; treatment of epithelial MCF7 cells with antibodies to E-cad allows the cytoplasmic localization of YAP in MCF7 cells [23]. As a typical tumor suppressor gene, NF2 is a well-studied Hippo upstream component. Its mutations were discovered in multiple tumors, and its inactivation is sufficient to lead to tumorigenesis. AMOT proteins function as scaffolds, facilitating MST1/2 phosphorylation of LATS1/2. Moreover, AMOT contributes to the connection between LATS1/2 and YAP, facilitating YAP activation [ 24‒ 26] . α-Catenin, a protein that contacts the intracellular region of E-cad, is present in skin cells and serves as a receptor for cell density. Its interaction with YAP can also influence the subcellular localization and level of phosphorylated YAP in keratinocytes. In the absence of 14-3-3, α-catenin can bind directly to and phosphorylate YAP1, which prevents YAP1 nuclear entry and downstream gene expression. In cells overexpressing α-catenin, YAP relocalizes to the plasma even when grown at lower density. This suggests that α-catenin can help YAP break through the regulation of nucleoplasmic localization to which it is usually subjected. It also suggests that α-catenin can act as an intermediate signal transducer in the E-cad/α-catenin/14-3-3 axis that regulates extracellular contact inhibition and intranuclear-associated protein expression [25]. As oncogenes, the activity of YAP/TAZ is strictly repressed upon phosphorylation by LATS1/2 tumor suppressors. WWC proteins (WWC1/2/3) directly interact with LATS1/2 and SAV1. SAV1 recruits MST1/2 to phosphorylate and activate LATS1/2. Hence, WWC1/2/3 is an organizer in a signaling module that mediates LATS1/2 activation by MST1/2 [27]. The Striatin-interacting phosphatases and kinases (STRIPAK) complex assists in lysophosphatidic acid-to-Hippo signaling in cultured mammalian cells [28], as well as bacteria-to-Hippo signaling in fruit flies [29]. Similarly, apicobasal cell polarity (ABCP) proteins, including the mammalian equivalent of Scribble (SCRIB), bind directly to YAP and TAZ. In addition, some G-protein-coupled receptors (GPCRs) can directly act on LATS and regulate downstream proteins by skipping MST kinases [30]. Different GPCRs can either function as activators or inhibitors of Hippo signaling, with the association of Rho-GTPase. YAP/TAZ activity can be either activated or inhibited, depending on which G protein is coupled to GPCRs and upstream signaling. Epinephrine and glucagon mediate the activation of GPCRs by increasing Lats1/2 kinase activities and inactivating YAP/TAZ in a manner dependent on protein kinase A (PKA) [31]. Hence, depending on the kind of G protein, GPCRs can differentially regulate Lats1/2 to up- or downregulate YAP activity.
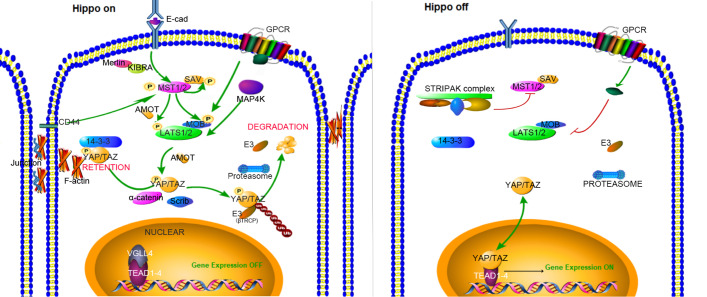
Figure 1 .
The On/Off switch of the Hippo pathway
Hippo On: In response to upstream activating signals, MST proteins, facilitated by SAV1, phosphorylates and activates the kinase function of LATS1/2 and the scaffold protein MOB1, and then the signal is further transmitted to the downstream transcription co-activator Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ). YAP/TAZ co-activators bind to TEAD1, 2, 3 or 4 transcription factor proteins and control the downstream expression. Hippo Off: The inhibitory signals, such as STRIPAK complex effects, block the MST and the LATS activities. Unphosphorylated YAP/TAZ translocates into the nucleus and binds to the TEAD1, 2, 3 or 4 transcription factor proteins. The YAP-TEAD complex promotes the downstream gene transcription.
Liquid flow, such as blood and lymph, represents the mechanical force the endothelial layer can sense. The study of osteogenesis and adipogenesis in mesenchymal stem cells (MSCs) suggests that cells can sense liquid shear forces through the Hippo pathway. In chondrocytes, increased fluid flow leads to increased YAP expression and dedifferentiation [32]. Other factors, including Integrin [33], Piezo1 [34], and plexin [35], can also act as mechanical sensor proteins that transmit signals to the Hippo pathway.
Epigenetic remodelling is essential for cellular transformation and tumorigenesis. In this process, how DNA methylation is remodelled by certain signaling pathways has not been fully demonstrated. As a direct YAP target, the 5-methylcytosine dioxygenase TET1 is an upstream regulator that coordinates the genome-wide epigenetic and transcriptional reprogramming of YAP target genes in the liver. TET1 physically interacts with TEAD to cause regional DNA demethylation, histone H3K27 acetylation and chromatin flexibility in YAP target genes to facilitate transcriptional activation. Loss of TET1 suppresses YAP-induced hepatomegaly and tumorigenesis [36].
To date, how these upstream signals coordinate the response of the Hippo pathway to a variety of foreign signals still awaits systematic research ( Figure 2). One recent finding suggests that cells can form functionally antagonizing biomolecular condensates to regulate the activity of related pathways, such as the Hippo pathway. The coalescence of these Hippo-regulating condensates is a mechanism for restricting condensate activity without condensate dissolution, suggesting a potential orchestration mechanism [37]. Glycogen accumulation is a key initiating oncogenic event during malignant liver transformation. Accumulated glycogen undergoes liquid‒liquid phase separation, which results in the assembly of the Laforin-Mst1/2 complex and consequently sequesters the Hippo kinases Mst1/2 in glycogen liquid droplets to relieve their inhibition of Yap. Moreover, deficiency of G6PC or another glycogenolysis enzyme, liver glycogen phosphorylase (PYGL), in mice results in glycogen storage disease, liver enlargement and tumorigenesis in a Yap-dependent manner [38].
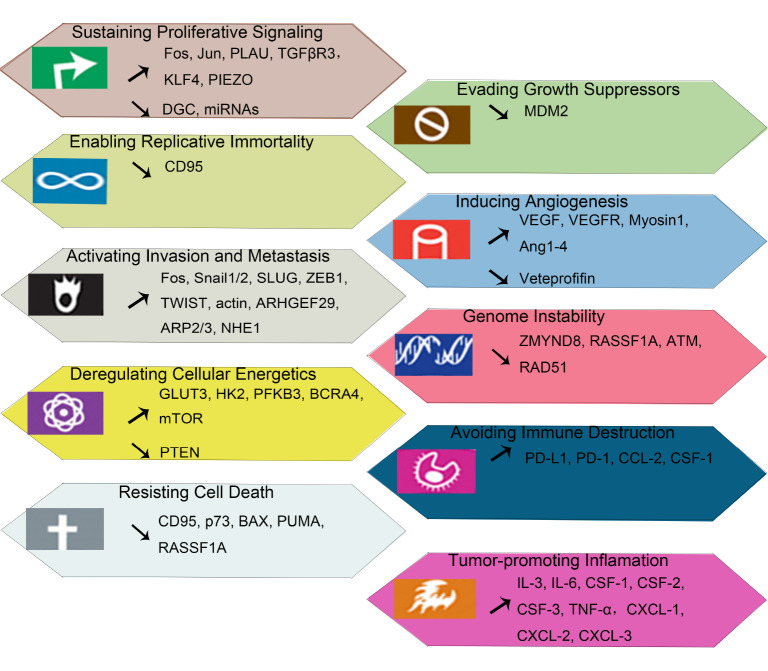
Figure 2 .
The Hippo pathway may up-regulate or down-regulate the expression or activity of the mentioned genes thus regulating the entitled hallmarks of cancer
↗: up-regulated genes; ↘: down-regulated genes.
Hippo Signaling and the Hallmarks of Cancers
Cancers are hallmarked by sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis, genome instability, reprogramming energy metabolism, and the ability to evade immune destruction, and they are capable of recruiting normal cells to contribute to the tumor microenvironment [39]. Overwhelming evidence suggests that Hippo components are dysregulated in human cancers: YAP/TAZ is commonly identified as an oncoprotein, while MST1/2 and LATS1/2 are tumor suppressors [ 40‒ 47] . With its essential roles in regulating the multiple steps of cell homeostasis, the Hippo pathway is crucial in cancer biology.
Hippo pathway regulates cell proliferation
One of the essential characteristics of a tumor is its ability to proliferate indefinitely. As a pathway regulating cell proliferation, the Hippo pathway is closely related to the occurrence and development of tumors [ 15, 30, 48, 49] . One recent study discovered the role of the Hippo pathway in cell proliferation and organ growth control from a distinct angle utilizing the model of fly eye imaginal discs and the mouse liver. They showed that the Hippo pathway did not instruct cells when to proliferate or stop proliferation during the normal development of these organs but kept YAP/TAZ/Yki in check to prevent them from triggering abnormal development [50].
Other pathways are involved in proliferation when crosstalking with the Hippo pathway. For example, YAP/TAZ can mediate AP-1 activation, directly leading to changes in the cell proliferation rate [51]. The dystrophin-glycoprotein complex (DGC) and miRNAs were further shown to inhibit cardiomyocyte proliferation by directly binding with YAP [ 52, 53] . In addition, with the association of plasminogen activator urokinase (PLAU) and transforming growth factor beta receptor 3 (TGFβR3), YAP-TEAD activation promotes cell proliferation. YAP/TAZ-TEAD transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting Krüppel-like factor (KLF)4-activated YAP-regulated genes that promote keratinocyte proliferation. In response to the signal from upstream PIEZO1 or geranylgeranylation, the Hippo pathway regulates contact-dependent cell growth and proliferation in cancer cells [ 54, 55] .
Hippo pathway helps cancer cells evade growth suppressor function
Cells are sustained in a resting state by the function of wild-type tumor suppressor genes such as p53 or RB. A screening assay based on a small hairpin RNA library targeting kinases revealed that the target genes repressed by RB were upregulated when the expression of LATS2 was partially reduced and the activity of YAP and TAZ proteins was increased, providing evidence that the Hippo pathway can antagonize tumor suppressor proteins [56]. Consistently, overexpression of LATS2 also increased the RB-mediated hyperactivity of downstream E2F target genes [56].
LATS2 can activate p53 by inhibiting the E3 ligase activity of MDM2. As a closed loop, the activation of p53 can increase LATS2 expression and enable cells to pass the G1/S checkpoint. Therefore, the alteration in Hippo pathway signaling can influence the status of tumor suppressor genes and then drive cells to escape the cell cycle checkpoint [ 57, 58] .
Hippo downstream genes in enabling replicative immortality
Inhibition of the Hippo pathway can lead to tissue enlargement, dependent on the induction of cell growth and the inhibition of the apoptosis pathway, which is essential for the tumorigenesis process, and the genes in the Hippo pathway can be considered ideal targets in controlling tumor growth. YAP overexpression prevents tumor necrosis factor’s function and inhibits CD95-induced apoptosis in cancer cells [ 59, 60] . In addition, cells that overexpress YAP tend to develop resistance to chemotherapy drugs to avoid apoptosis [ 61, 62] and acquire a growth advantage over other cells. Multiple other members of the Hippo pathway also show positive effects on cell survival.
Hippo signaling in inducing angiogenesis
In addition to growth advantages, the Hippo signaling pathway also plays roles in tumor angiogenesis. On E8.5, Yap −/− mouse embryos showed defects in the yolk sac vasculature, indicating that the Hippo pathway may contribute to angiogenesis in the gastrulation and neurulation stages [63]. Under physiological conditions, angiogenesis is tightly regulated by the vascular epithelial growth factor VEGF and its receptor VEGFR. During tumorigenesis, the lack of an energy supply system requires the generation of new blood vessels at nascent sites to ensure nutrition. This makes it necessary to upregulate the expression of VEGF and its receptor, which was reported to be regulated by the activity of YAP/TAZ [64]. In addition, VEGF/VEGFR can activate the PI3K-AKT-MEK-ERK pathway axis and Src kinase and phosphorylate MST1/2, LATS1/2, and downstream YAP/TAZ [65]. Veteprofifin, a YAP-TEAD inhibitor, inhibits the angiogenic capacity of mouse fibroblasts based on an in vivo Matrigel plug assay [66]. Myosin1C, as a downstream gene of VEGF-YAP/TAZ, can mediate the subcellular distribution of VEGFR2 and direct the latter’s function to affect angiogenesis in tumors [67]. Another important target gene of YAP is angiopoietins 1–4 (Ang1–4), which belongs to a growth factor family and acts as an autocrine factor overexpressed in epithelial cells at sites of active angiogenesis or vascular repair [ 68‒ 70] .
Hippo cascade in promoting tumor invasion and metastasis
Epithelial-to-mesenchymal transition (EMT) is considered to be one of the necessary processes before tumor cells undergo metastasis. Dissociation of intercellular junctions, deletion of the cell polarization axis, and cytoskeletal rearrangement are significant features of EMT. The Hippo pathway plays a crucial role in the EMT process. Several previous studies have shown that deletion of MST1/2 and LATS1/2 or abnormal activation of YAP and TAZ can promote cell metastasis. In contrast, the knockdown of YAP/ TAZ can reverse certain mesenchymal features of tumor cells [71]. YAP was identified as a protein that can substitute for Kirsten rat sarcoma 2 viral oncogene homolog (KRAS)’s function in KRAS-dependent colon cancer cells. The overexpression of YAP can significantly increase the activity of c-FOS, and the cells behave more like mesenchymal cells, but not epithelial cells, supported by the suppression of E-cad and occludin. Moreover, YAP overexpression is associated with the upregulation of the Snail1/2, SLUG, zinc finger E-box binding homeobox (ZEB)1, and Twist Family BHLH Transcription Factor (TWIST) genes, which drives the cells towards EMT. The expression of YAP can promote cell evasion from the primary site by altering cell morphology through regulating actin dynamics mediated by the expression of ARHGEF29 [72]. The tumor cell skeleton as well as polarity can be affected by actin activity mediated by YAP when signals are transduced to YAP from pericellular intercellular contact conditions. In circulating tumor cells, YAP activates the expression of ARHGEF29, thereby inhibiting F-actin reassembly [73] and increasing the ability of tumor cells to metastasize. Several genetic experiments support that inactivation of Hippo kinase allows the formation of metastatic protrusions in metastatic cells [73].
Mechanical forces from the surrounding fluid transduced through substrate-cell interactions have been shown to regulate cell migration behavior [74]. When investigating the effect of physiologically relevant extracellular viscosities on cell function, Bera et al. [75] discovered that breast cancer cells preexposed to elevated viscosity acquired transcriptional control of YAP localization and were dependent on TEAD2, leading to increased migration and lung colonization in mice. Increased mechanical loading enforced by elevated viscosity induces an actin-related protein 2/3 (ARP2/3)-complex-dependent dense actin network. It enhances the polarization of Na +/H + exchanger 1 (NHE1) through the actin-binding partner ezrin. The coordinated action of actin dynamics, NHE1-mediated swelling, and RHOA-based contractility facilitates enhanced motility at elevated viscosities [75].
Hippo signaling in genome instability
It has been recently shown that STRIPAK-mediated MST1/2 inactivation increases the DNA double-stranded break repair capacity of cancer cells, uncovering the role of MST1/2 in the suppression of DNA repair. Independent of classical Hippo signaling, MST1/2 directly phosphorylates zinc finger MYND type-containing 8 (ZMYND8) and results in the suppression of DNA repair in the nucleus. STRIPAK-mediated MST1/2 inactivation increases the DSB repair capacity of cancer cells and endows these cells with resistance to radio- and chemotherapy and poly(ADP-ribose) polymerase (PARP) inhibition [76]. Another group identified that the MST2 kinase localizes at the nucleoli and targets phosphorylation of H2BS14 in an ATM-dependent manner. The establishment of H2BS14p is necessary for damage-induced rDNA transcriptional shutdown and maintenance of genomic integrity [77].
Knockdown of SMG6 results in synthetic lethality in LATS2-inactivated cells. The lethality requires the nuclear translocation of YAP1 and TAZ and SMG6 in regulating telomerase reverse transcriptase (TERT) activity [78].
YAP and TAZ transcriptional activity regulates processes associated with DNA damage-related apoptosis and chromosomal instability [79]. Ras-association domain family (RASSF) 1A has a general effect on DNA damage, resulting in YAP being less pro-carcinogenic and having an increased oncogenic effect through genome instability. Metal ions or radioactive treatment can cause DNA damage and then activate ATM, which further phosphorylates RASSF1A and induces its binding to MST2, preventing the formation of an MST2 and RAF-1 dimer [ 80, 81] . In response to DNA damage, YAP leaves the TEAD conjugate, binds directly to p73, and promotes the transcription of Bcl-2-associated X-protein (BAX) and p53-upregulated modulator of apoptosis (PUMA), allowing cells to initiate the apoptotic program [82]. Overexpression of RASSF1A, followed by treatment with cisplatin, doxorubicin, and topoisomerase inhibitors, enhances the effect of drugs that target genome/DNA stability or architecture [83]. Vascular endothelial growth factor (VEGF) promotes homologous recombination (HR) in BRCA1 wild-type TNBC cells by contributing to the expression and function of RAD51, an essential enzyme in the HR pathway that mediates efficient DNA double-strand break repair. Mechanistically, VEGF-NRP2 stimulates YAP/TAZ-dependent RAD51 expression, and RAD51 is a direct YAP/TAZ-TEAD transcriptional target [84].
Hippo signaling plays multiple roles in the reprogramming of energy metabolism
In addition to mechanical forces and cell density, Hippo plays a crucial role in the perception of cell metabolites. Glucose is directly involved in the tricarboxylic acid cycle (TCA cycle) and is a central energy source for both normal and cancer cells. At the primary site of tumorigenesis, the neoplasia opts for the relatively inefficient glycolytic pathway because of the inability to construct sufficient blood vessels for oxygen supply. Thus, the demand for glucose is extraordinarily high in cancerous tissues due to the inability to sufficiently utilize glucose [85].
In addition to regulating the size of the liver, the most dominant energy-producing organ, and the regulation of metabolism-related cell proliferation, YAP can also directly regulate the glycolytic pathway through transcriptional regulation. Several studies have shown that many genes involved in glucose metabolism are downstream genes of YAP-TEAD [ 86‒ 88] . Several reported downstream genes of YAP/TAZ indicated their characteristics in the switch from aerobic to anaerobic metabolic processes. The rate of sugar utilization is significantly higher in YAP-activated cells in vitro than in YAP-silenced cells [ 89, 90] . Glucose transporter (GLUT) 3 is mainly expressed in the nervous system. Therefore, metabolic regulation by YAP may be cell specific. Because GLUT3-dependent cells are susceptible to inhibitors of YAP, YAP for GLUT3 transcriptional upregulation is important in this process [91]. Similarly, metabolism-related proteins such as hexokinase 2 (HK2) and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKB3) are also downstream of YAP [92]. YAP can upregulate metabolism-related proteins by transcribing lncRNA breast cancer anti-estrogen resistance 4 (BCRA4) to activate the hedgehog pathway [92].
YAP can regulate the ability to take up and utilize glucose and other nutrients. After in vivo hepatectomy is performed, blood glucose level decreases dramatically as the liver starts to grow, and after the liver grows to normal size, blood glucose returns to normal level [93].
The principle of glucagon action could work through stimulation of cAMP and protein kinase A (PKA) to activate LATS and inhibit YAP activity [31]. In some experiments in mice and zebrafish, simply overexpressing the activated form of YAP induces intense cell growth as well as a phenotype of hepatic hypertrophy followed by adenocarcinogenesis of the liver [94]. During this process, the gradual overdose of glycolysis becomes one of the characteristics of neoplastic tissues [95].
Stimulated by extracellular metabolism signals, such as glucose, on arterial vascular epithelial cells, the cells feedback the proliferation of the arterial epithelium through the transcriptional activation of YAP, which in turn reduces vascular stress [86]. Through binding to TEAD, YAP initiates transcription at the start region of genes to promote the production of α-ketoglutarate, which regulates glucose metabolism. Glutaminolysis processes in hepatic stellate cells during myofibroblast differentiation in liver fibrosis are very similar to some metabolic behaviors of cancer cells, most notably anaerobic metabolism [96]. The glutaminolysis phenotypes are diminished when YAP is inhibited, suggesting the critical role of YAP in fibrosis.
The mammalian target of rapamycin (mTOR) pathway is vital in sensing and transducing growth factor signaling. One of its most important roles is to control energy metabolism in response to upstream stimulation. After the initial publication reporting that YAP inhibits phosphatase and tensin homolog (PTEN) protein levels and thus promotes mTOR pathway activity [97], several teams followed up and further elucidated the crosstalk between these two pathways and their substantial impact on metabolism in tumors and related diseases [ 98‒ 102] . For tumors, abnormal lipid metabolism and excessive cholesterol synthesis are common features [103]. During this process, the excessive activation of YAP/TAZ is again prevalent in cancer [ 104, 105] . In this way, YAP/TAZ is at least one of the primary causes leading to this tumor feature. In vivo studies discovering abnormalities in lipid metabolism regulated by Yap confirmed the phenotype. In Lats2-knockout rats, the accumulation of phospholipids in their liver was remarkable. Similarly, rats with Yap/Taz overactivation also exhibited the relevant phenotype [106]. Spontaneous development of steatosis was evident in Pten-knockout rats, and this phenotype was more pronounced after additional knockout of Sav. This phenotype was utterly lost in Yap/ Taz-knockout mice [107].
Hippo facilitates cancer cells to evade immune destruction and to construct the tumor microenvironment
Cancer immunotherapy is a series of promising treatments for this deadly disease. In treating cancers such as B-cell lymphoma and melanoma, therapeutics including T cell receptor-engineered T (TCR-T), chimeric antigen receptor T (CAR-T) and natural killer T (NKT) therapies have achieved extraordinarily satisfactory clinical achievements. However, tumor cells can evade the lethal effect of cytotoxic immune cells partly by upregulating the expression of checkpoint-related proteins on the cell surface, such as cytotoxic T lymphocyte-associated antigen 4 (CTLA4), programmed cell death-ligand 1 (PD-L1), or programmed cell death 1 (PD-1).
The Hippo pathway also plays a vital role in regulating tumor immune responses. Knockout of Lats1/ 2 caused extracellular acid-rich extracellular vesicles to induce a type I interferon response through Toll-like receptor-MYD88/TRIF, activating antitumor immunity and inhibiting tumor growth in exogenous implantation [104]. Lats1/ 2 knockout, Mst1/ 2 knockout, and exogenous activation of Yap in the liver at the in vivo level all induce the production of the immunosuppressive microenvironment by releasing C-C motif chemokine ligand 2 (Ccl2) and colony stimulating factor 1 (Csf1) to recruit type II macrophages. Knockdown of Ccl2 or Csf1 prevents macrophage aggregation [108]. This emphasizes the regulatory effects of the Hippo pathway on the tumor microenvironment. YAP/TAZ-TEAD directly regulates PDL1 expression in breast cancer, non-small cell lung cancer, mesothelioma, and melanoma [ 109‒ 113] . At the same time, YAP and TEAD can also regulate a variety of cytokines, summoning myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) in the tumor microenvironment, thereby inhibiting the function of T cells or promoting apoptosis of T cells through the release of a variety of cytokines/chemokines [114], including interleukin-3, -6, CSF-1, -2, -3, tumor necrosis factor-α (TNF-α), chemokine (C-X-C motif) ligand (CXCL) 1/2, and CCL2 [ 115‒ 117] .
Interferon-γ (IFN-γ) is one major cause of immunotherapy resistance in solid tumors. In tumor cells, IFN-γ promotes nuclear translocation and phase separation of YAP after anti-PD-1 therapy. Hydrophobic interactions of the YAP coiled-coil domain mediate droplet initiation and weak interactions of the intrinsically disordered region. Inhibition of phase separation reduces tumor growth, enhances immune response, and sensitizes tumor cells to anti-PD-1 therapy. YAP activity is negatively correlated with the outcome of anti-PD-1 therapy in patients, so it can serve as a predictive biomarker and target of combination therapy [118].
Concluding Remarks
The Hippo pathway is relatively new in cancer biology compared to other classical cancer-related pathways, such as P53, RB, Ras, PI3K, and Myc. Mutations in this pathway remain to be discovered in more patient samples. In future studies, in addition to further finding evidence of mutations in core members of the pathway themselves leading to diseases, including tumors, the discovery of the pathway’s upstream regulators is also the main direction to be explored. It is believed that with the further development of high-throughput sequencing technologies, in combination with classic methods, more primary data about the pathway′s function will be better connected to cancer biology. In addition, whether there are new members in the Hippo family and what effect these members play in the pathway itself and the interaction with related pathways critical to cancers are the directions that draw the attention of researchers. Solid evidence has suggested that the Hippo pathway’s ability to regulate organ size is universal. However, the specific functions in the regulation of particular organs and direct tumorigenesis in different organs are not the same. This emphasizes the urgent need for systematic exploration of upstream molecules of this pathway. To address this question, our group has introduced some positive-genetic methods into the exploration process in this direction, such as the VBIM system [ 119, 120] . By constructing a reliable cell line expressing a selective marker mediated by the upstream sequence of CTGF, the classic TEAD target gene, to use the dead-or-alive phenotype as the reporting system, we are discovering novel regulatory genes upstream of this pathway with unbiased screening. From the perspective of several cell clones we have obtained and validated thus far, applying these methods will surely help us obtain a clearer upstream blueprint.
It is interesting that despite the well-documented role of Hippo pathway inhibition in promoting tumor growth and progression, studies have also shown that Yki/YAP/TAZ also act as tumor suppressors, inducing genome instability to promote cell apoptosis under treatment with radioactivity or chemotherapy. Similar to some other pathways or transcriptional regulators, such as Notch [121], p63 [122] or WT1 [123], it remains an open question whether a gene such as YAP or TEAD could be defined as a tumor suppressor gene or an oncogene. Hopefully, with the assistance of next-generation sequencing technologies, this question could be better answered.
In addition, drug development for this pathway has also been carried out in many laboratories. The Hippo pathway is considered a crucial targeted pathway in the design of drugs that aim to activate tissue regeneration and inhibit cancer progression. Some studies have shown that the Hippo pathway is associated with evoking stem cells in mature animals to differentiate or divide. In order to promote tissue repair and regeneration, inhibitors acting on the upstream kinase protein of Hippo have been developed [124]. Of course, as a double-edged sword, the continuous activation of this pathway leads to increased fibrosis of the organs [ 125, 126] , which is particularly significant in liver and kidney fibrosis research. This demands on the precise use of drugs to promote tissue differentiation and proliferation but not to proliferate excessively.
As mentioned earlier, the proliferation of cells caused by the Hippo pathway is its most critical feature, making it closely related to tumors. With the step-by-step, in-depth study of this pathway, YAP and TEAD downstream targets are discovered to be intimately associated with tumors. To inhibit abnormal cell proliferation, transcription inhibitors, especially those for TEAD, have been developed [127]. Compounds that can inhibit the formation of the YAP/TEAD dimer [ 128, 129] are also being tested in clinical trials. Some findings suggest that many proteins that are downstream of TEAD and YAP in vitro are not expressed in all organs. The study of the differential regulatory effects will undoubtedly enrich our understanding of this pathway and, at the same time, provide theoretical and clinical guidance for the personalized treatment of some different subtypes of tumors in clinical practice. The associated genes may appear as biomarkers in early clinical tumor screening, which will also benefit early-stage diagnosis. Some studies have shown that molecular mutations upstream of specifically related pathways, such as the Wnt and Kras pathways, can directly or indirectly affect the activity of the Hippo pathway. The chemotherapy process from the clinical discovery of the tumor to the time of surgery is the best time-frame to validate the efficacy of active inhibitors of the Hippo pathway. Observing the inhibition of tumor grade, tumor size, and biomolecular marker expression by these drugs will help us finally achieve a breakthrough in the inhibition of YAP activity, from laboratory research to successful clinical application.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Key Research and Development Program of China (No. 2019YFA0802001 to L.Z.), the National Natural Science Foundation of China (Nos. 32030025, 32293233 and 32221002 to L.Z., and No. 32270763 to N.Z.), the Shanghai Municipal Science and Technology Major Project, and the Shanghai Leading Talents Program to L.Z.
References
- 1.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation . Genes Dev. . 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 2.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase . Development. . 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 3.Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila . Development. . 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 4.Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, Hariharan IK. Salvdor promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines . Cell. . 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 5.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis . Cell. . 2003;114:457–467. doi: 10.1016/S0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 6.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis . Genes Dev. . 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantalacci S, Tapon N, Léopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila . Nat Cell Biol. . 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 8.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. . 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. . 2003;114:445–456. doi: 10.1016/S0092-8674(03)00549-X. [DOI] [PubMed] [Google Scholar]
- 10.Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. . 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 11.Sharif AAD, Hergovich A. The NDR/LATS protein kinases in immunology and cancer biology. Semin Cancer Biol. . 2018;48:104–114. doi: 10.1016/j.semcancer.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Sung V, Luo W, Qian D, Lee I, Jallal B, Gishizky M. The Ste20 kinase MST4 plays a role in prostate cancer progression. Cancer Res. 2003, 63: 3356–3363 . [PubMed]
- 13.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals . Cell. . 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the Hippo pathway. Mol Cell Biol. . 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. . 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebé-Pedrós A, Zheng Y, Ruiz-Trillo I, Pan D. Premetazoan origin of the hippo signaling pathway. Cell Rep. . 2012;1:13–20. doi: 10.1016/j.celrep.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pocaterra A, Romani P, Dupont S. YAP/TAZ functions and their regulation at a glance. J Cell Sci. . 2020;133:jcs230425. doi: 10.1242/jcs.230425. [DOI] [PubMed] [Google Scholar]
- 18.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. . 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Ma S, Tang T, Probst G, Konradi A, Jin C, Li F, Gutkind JS, et al. Transcriptional repression of estrogen receptor alpha by YAP reveals the Hippo pathway as therapeutic target for ER + breast cancer . Nat Commun. . 2022;13:1061. doi: 10.1038/s41467-022-28691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F, Han X, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. . 2014;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. . 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. . 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. . 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. . 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. . 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mana-Capelli S, McCollum D. Angiomotins stimulate LATS kinase autophosphorylation and act as scaffolds that promote Hippo signaling. J Biol Chem. . 2018;293:18230–18241. doi: 10.1074/jbc.RA118.004187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi S, Zhu Y, Liu X, Li P, Wang Y, Zeng Y, Yu A, et al. WWC proteins mediate LATS1/2 activation by Hippo kinases and imply a tumor suppression strategy. Mol Cell. . 2022;82:1850–1864.e7. doi: 10.1016/j.molcel.2022.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Xie R, Meng Z, Ma S, Guan KL. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat Cell Biol. . 2019;21:1565–1577. doi: 10.1038/s41556-019-0426-y. [DOI] [PubMed] [Google Scholar]
- 29.Liu B, Zheng Y, Yin F, Yu J, Silverman N, Pan D. Toll receptor-mediated hippo signaling controls innate immunity in drosophila. Cell. . 2016;164:406–419. doi: 10.1016/j.cell.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H, Huang C, Wu J, Li J, Hu T, Wang Z, Zhang H, et al. SCRIB promotes proliferation and metastasis by targeting Hippo/YAP signalling in colorectal cancer. Front Cell Dev Biol. . 2021;9:656359. doi: 10.3389/fcell.2021.656359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, et al. Protein kinase a activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. . 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichenbach M, Mendez P, da Silva Madaleno C, Ugorets V, Rikeit P, Boerno S, Jatzlau J, et al. Differential impact of fluid shear stress and YAP/TAZ on BMP/TGF‐β induced osteogenic target genes. Adv Biol. . 2021;5:2000051. doi: 10.1002/adbi.202000051. [DOI] [PubMed] [Google Scholar]
- 33.Lv H, Ai D. Hippo/yes-associated protein signaling functions as a mechanotransducer in regulating vascular homeostasis. J Mol Cell Cardiol. . 2022;162:158–165. doi: 10.1016/j.yjmcc.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhu B, Qian W, Han C, Bai T, Hou X. Piezo 1 activation facilitates cholangiocarcinoma metastasis via Hippo/YAP signaling axis. Mol Ther Nucleic Acids. . 2021;24:241–252. doi: 10.1016/j.omtn.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang C, Javed A, Kaiser L, Nava MM, Xu R, Brandt DT, Zhao D, et al. Mechanochemical control of epidermal stem cell divisions by B-plexins. Nat Commun. . 2021;12:1308. doi: 10.1038/s41467-021-21513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu BK, Mei SC, Chen EH, Zheng Y, Pan D. YAP induces an oncogenic transcriptional program through TET1-mediated epigenetic remodeling in liver growth and tumorigenesis. Nat Genet. . 2022;54:1202–1213. doi: 10.1038/s41588-022-01119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Choi K, Su T, Li B, Wu X, Zhang R, Driskill JH, et al. Multiphase coalescence mediates Hippo pathway activation. Cell. . 2022;185:4376–4393.e18. doi: 10.1016/j.cell.2022.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Li J, Zhang W, Xiao C, Zhang S, Nian C, Li J, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. . 2021;184:5559–5576.e19. doi: 10.1016/j.cell.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. . 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, Hilsenbeck SG, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. . 2010;70:8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver . Proc Natl Acad Sci USA. . 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia H, Qi H, Li Y, Pei J, Barton J, Blackstad M, Xu T, et al. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene. . 2002;21:1233–1241. doi: 10.1038/sj.onc.1205174. [DOI] [PubMed] [Google Scholar]
- 43.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. . 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. . 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, Tarpey P, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discovery. . 2018;8:1548–1565. doi: 10.1158/2159-8290.CD-18-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eder N, Roncaroli F, Domart MC, Horswell S, Andreiuolo F, Flynn HR, Lopes AT, et al. YAP1/TAZ drives ependymoma-like tumour formation in mice. Nat Commun. . 2020;11:2380. doi: 10.1038/s41467-020-16167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ammoun S, Hanemann CO. Emerging therapeutic targets in schwannomas and other merlin-deficient tumors. Nat Rev Neurol. . 2011;7:392–399. doi: 10.1038/nrneurol.2011.82. [DOI] [PubMed] [Google Scholar]
- 48.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. . 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Zhang M, Zheng J, Tian Y, Zhang H, Tan Y, Li Q, et al. Human umbilical cord mesenchymal stem cell-derived exosomes improve ovarian function and proliferation of premature ovarian insufficiency by regulating the hippo signaling pathway. Front Endocrinol. . 2021;12:711902. doi: 10.3389/fendo.2021.711902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kowalczyk W, Romanelli L, Atkins M, Hillen H, Bravo González-Blas C, Jacobs J, Xie J, et al. Hippo signaling instructs ectopic but not normal organ growth. Science. . 2022;378:eabg3679. doi: 10.1126/science.abg3679. [DOI] [PubMed] [Google Scholar]
- 51.Koo JH, Plouffe SW, Meng Z, Lee DH, Yang D, Lim DS, Wang CY, et al. Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. . 2020;34:72–86. doi: 10.1101/gad.331546.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morikawa Y, Heallen T, Leach J, Xiao Y, Martin JF. Dystrophin–glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature. . 2017;547:227–231. doi: 10.1038/nature22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diez-Cuñado M, Wei K, Bushway PJ, Maurya MR, Perera R, Subramaniam S, Ruiz-Lozano P, et al. miRNAs that induce human cardiomyocyte proliferation converge on the hippo pathway. Cell Rep. . 2018;23:2168–2174. doi: 10.1016/j.celrep.2018.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasegawa K, Fujii S, Matsumoto S, Tajiri Y, Kikuchi A, Kiyoshima T. YAP signaling induces PIEZO1 to promote oral squamous cell carcinoma cell proliferation . J Pathol. . 2021;253:80–93. doi: 10.1002/path.5553. [DOI] [PubMed] [Google Scholar]
- 55.Mi W, Lin Q, Childress C, Sudol M, Robishaw J, Berlot CH, Shabahang M, et al. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. . 2015;34:3095–3106. doi: 10.1038/onc.2014.251. [DOI] [PubMed] [Google Scholar]
- 56.Tschöp K, Conery AR, Litovchick L, DeCaprio JA, Settleman J, Harlow E, Dyson N. A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev. . 2011;25:814–830. doi: 10.1101/gad.2000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furth N, Aylon Y. The LATS1 and LATS2 tumor suppressors: beyond the Hippo pathway. Cell Death Differ. . 2017;24:1488–1501. doi: 10.1038/cdd.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aylon Y, Yabuta N, Besserglick H, Buganim Y, Rotter V, Nojima H, Oren M. Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation. Oncogene. . 2009;28:4469–4479. doi: 10.1038/onc.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song X, Xu H, Wang P, Wang J, Affo S, Wang H, Xu M, et al. Focal adhesion kinase (FAK) promotes cholangiocarcinoma development and progression via YAP activation. J Hepatol. . 2021;75:888–899. doi: 10.1016/j.jhep.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang B, Zhang Y, Zhang J, Liu P, Jiao B, Wang Z, Ren R. Focal adhesion kinase (FAK) inhibition synergizes with KRAS G12C inhibitors in treating cancer through the regulation of the FAK-YAP signaling. Adv Sci. . 2021;8:2100250. doi: 10.1002/advs.202100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. . 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 62.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. . 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 63.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65 . Mol Cell Biol. . 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Freire Valls A, Schermann G, Shen Y, Moya IM, Castro L, Urban S, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell. . 2017;42:462–478.e7. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Azad T, Janse van Rensburg HJ, Lightbody ED, Neveu B, Champagne A, Ghaffari A, Kay VR, et al. A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat Commun. . 2018;9:1061. doi: 10.1038/s41467-018-03278-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Kim YH, Kim J, Park DY, Bae H, Lee DH, Kim KH, et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J Clin Invest. . 2017;127:3441–3461. doi: 10.1172/JCI93825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong J, Sun X, et al. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. . 2017;108:478–487. doi: 10.1111/cas.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tiwari A, Jung JJ, Inamdar SM, Nihalani D, Choudhury A. The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling. Am J Physiol Heart Circulatory Physiol. . 2013;304:H687–H696. doi: 10.1152/ajpheart.00744.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis . Science. . 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 70.Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, Ioffe E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA. . 2006;103:15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. . 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiao Y, Chen J, Lim YB, Finch-Edmondson ML, Seshachalam VP, Qin L, Jiang T, et al. YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. . 2017;19:1495–1502. doi: 10.1016/j.celrep.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 73.Lucas EP, Khanal I, Gaspar P, Fletcher GC, Polesello C, Tapon N, Thompson BJ. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells . J Cell Biol. . 2013;201:875–885. doi: 10.1083/jcb.201210073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Follain G, Herrmann D, Harlepp S, Hyenne V, Osmani N, Warren SC, Timpson P, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer. . 2020;20:107–124. doi: 10.1038/s41568-019-0221-x. [DOI] [PubMed] [Google Scholar]
- 75.Bera K, Kiepas A, Godet I, Li Y, Mehta P, Ifemembi B, Paul CD, et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature. . 2022;611:365–373. doi: 10.1038/s41586-022-05394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.An L, Cao Z, Nie P, Zhang H, Tong Z, Chen F, Tang Y, et al. Combinatorial targeting of Hippo-STRIPAK and PARP elicits synthetic lethality in gastrointestinal cancers. J Clin Invest. . 2022;132:e155468. doi: 10.1172/JCI155468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pefani DE, Tognoli ML, Pirincci Ercan D, Gorgoulis V, O′Neill E. MST2 kinase suppresses rDNA transcription in response to DNA damage by phosphorylating nucleolar histone H2B. EMBO J. . 2018;37 doi: 10.15252/embj.201798760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki K, Tange M, Yamagishi R, Hanada H, Mukai S, Sato T, Tanaka T, et al. SMG6 regulates DNA damage and cell survival in Hippo pathway kinase LATS2-inactivated malignant mesothelioma. Cell Death Discov. . 2022;8:446. doi: 10.1038/s41420-022-01232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tóth M, Wehling L, Thiess L, Rose F, Schmitt J, Weiler SME, Sticht C, et al. Co-expression of YAP and TAZ associates with chromosomal instability in human cholangiocarcinoma. BMC Cancer. . 2021;21:1079. doi: 10.1186/s12885-021-08794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, et al. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell. . 2007;27:962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romano D, Nguyen LK, Matallanas D, Halasz M, Doherty C, Kholodenko BN, Kolch W. Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat Cell Biol. . 2014;16:673–684. doi: 10.1038/ncb2986. [DOI] [PubMed] [Google Scholar]
- 82.van der Weyden L, Papaspyropoulos A, Poulogiannis G, Rust AG, Rashid M, Adams DJ, Arends MJ, et al. Loss of rassf1a synergizes with deregulated runx2 signaling in tumorigenesis . Cancer Res. . 2012;72:3817–3827. doi: 10.1158/0008-5472.CAN-11-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yee KS, Grochola L, Hamilton G, Grawenda A, Bond EE, Taubert H, Wurl P, et al. A RASSF1A polymorphism restricts p53/p73 activation and associates with poor survival and accelerated age of onset of soft tissue sarcoma. Cancer Res. . 2012;72:2206–2217. doi: 10.1158/0008-5472.CAN-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elaimy AL, Amante JJ, Zhu LJ, Wang M, Walmsley CS, FitzGerald TJ, Goel HL, et al. The VEGF receptor neuropilin 2 promotes homologous recombination by stimulating YAP/TAZ-mediated Rad51 expression. Proc Natl Acad Sci USA. . 2019;116:14174–14180. doi: 10.1073/pnas.1821194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Altman BJ, Stine ZE, Dang CV. Erratum: From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. . 2016;16:749. doi: 10.1038/nrc.2016.114. [DOI] [PubMed] [Google Scholar]
- 86.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. . 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du K, Hyun J, Premont RT, Choi SS, Michelotti GA, Swiderska-Syn M, Dalton GD, et al. Hedgehog-YAP signaling pathway regulates glutaminolysis to control activation of hepatic stellate cells. Gastroenterology. . 2018;154:1465–1479.e13. doi: 10.1053/j.gastro.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards DN, Ngwa VM, Wang S, Shiuan E, Brantley-Sieders DM, Kim LC, Reynolds AB, et al. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci Signal. . 2017;10:eaan4667. doi: 10.1126/scisignal.aan4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plouffe SW, Meng Z, Lin KC, Lin B, Hong AW, Chun JV, Guan KL. Characterization of Hippo pathway components by gene inactivation. Mol Cell. . 2016;64:993–1008. doi: 10.1016/j.molcel.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. . 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cosset É, Ilmjärv S, Dutoit V, Elliott K, von Schalscha T, Camargo MF, Reiss A, et al. Glut3 addiction is a druggable vulnerability for a molecularly defined subpopulation of glioblastoma. Cancer Cell. . 2017;32:856–868. doi: 10.1016/j.ccell.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng X, Han H, Liu G, Ma Y, Pan R, Sang L, Li R, et al. Lnc RNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. . 2017;36:3325–3335. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang J, Rudnick DA. Elucidating the metabolic regulation of liver regeneration. Am J Pathol. . 2014;184:309–321. doi: 10.1016/j.ajpath.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cox AG, Hwang KL, Brown KK, Evason KJ, Beltz S, Tsomides A, O’Connor K, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. . 2016;18:886–896. doi: 10.1038/ncb3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. . 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. . 2012;143:1319–1329. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, et al. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. . 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. Phosphorylation of the Hippo pathway component AMOTL2 by the mTORC2 kinase promotes YAP signaling, resulting in enhanced glioblastoma growth and invasiveness. J Biol Chem. . 2015;290:19387–19401. doi: 10.1074/jbc.M115.656587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collak FK, Yagiz K, Luthringer DJ, Erkaya B, Cinar B. Threonine-120 phosphorylation regulated by Phosphoinositide-3-Kinase/Akt and mammalian target of rapamycin pathway signaling limits the antitumor activity of mammalian sterile 20-Like kinase 1. J Biol Chem. . 2012;287:23698–23709. doi: 10.1074/jbc.M112.358713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hansen CG, Ng YLD, Lam WLM, Plouffe SW, Guan KL. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. . 2015;25:1299–1313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park Y‐, Sohn BH, Johnson RL, Kang M‐, Kim SB, Shim J‐, Mangala LS, et al. Yes‐associated protein 1 and transcriptional coactivator with PDZ‐binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. . 2016;63:159–172. doi: 10.1002/hep.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sciarretta S, Zhai P, Maejima Y, Del Re DP, Nagarajan N, Yee D, Liu T, et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. . 2015;11:125–136. doi: 10.1016/j.celrep.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. . 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 104.Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, et al. The Hippo pathway kinases LATS1/2 suppress cancer immunity. Cell. . 2016;167:1525–1539.e17. doi: 10.1016/j.cell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. . 2015;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aylon Y, Gershoni A, Rotkopf R, Biton IE, Porat Z, Koh AP, Sun X, et al. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. . 2016;30:786–797. doi: 10.1101/gad.274167.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeong SH, Kim HB, Kim MC, Lee J, Lee JH, Kim JH, Kim JW, et al. Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J Clin Invest. . 2018;128:1010–1025. doi: 10.1172/JCI95802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Janse van Rensburg HJ, Azad T, Ling M, Hao Y, Snetsinger B, Khanal P, Minassian LM, et al. The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. . 2018;78:1457–1470. doi: 10.1158/0008-5472.CAN-17-3139. [DOI] [PubMed] [Google Scholar]
- 109.Yang H, Xue M, Su P, Zhou Y, Li X, Li Z, Xia Y, et al. RNF31 represses cell progression and immune evasion via YAP/PD-L1 suppression in triple negative breast cancer. J Exp Clin Cancer Res. . 2022;41:364. doi: 10.1186/s13046-022-02576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu P, Miao J, Wang Y, Zhang W, Yang Y, Wang C, Yang C, et al. Inhibition of yes‐associated protein down‐regulates PD‐L1 (CD274) expression in human malignant pleural mesothelioma. J Cell Mol Med. . 2018;22:3139–3148. doi: 10.1111/jcmm.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim MH, Kim CG, Kim SK, Shin SJ, Choe EA, Park SH, Shin EC, et al. YAP-induced PD-L1 expression drives immune evasion in BRAFi-Resistant melanoma. Cancer Immunol Res. . 2018;6:255–266. doi: 10.1158/2326-6066.CIR-17-0320. [DOI] [PubMed] [Google Scholar]
- 112.Lee BS, Park DI, Lee DH, Lee JE, Yeo M, Park YH, Lim DS, et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. . 2017;491:493–499. doi: 10.1016/j.bbrc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 113.Miao J, Hsu PC, Yang YL, Xu Z, Dai Y, Wang Y, Chan G, et al. YAP regulates PD-L1 expression in human NSCLC cells. Oncotarget. . 2017;8:114576–114587. doi: 10.18632/oncotarget.23051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. . 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, et al. Targeting YAP-Dependent MDSC infiltration impairs tumor progression. Cancer Discovery. . 2016;6:80–95. doi: 10.1158/2159-8290.CD-15-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, et al. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. . 2017;36:1232–1244. doi: 10.1038/onc.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim W, Khan SK, Liu Y, Xu R, Park O, He Y, Cha B, et al. Hepatic Hippo signaling inhibits protumoural microenvironment to suppress hepatocellular carcinoma. Gut. . 2018;67:1692–1703. doi: 10.1136/gutjnl-2017-314061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, Lin J, et al. Interferon-γ induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol Cell. . 2021;81:1216–1230.e9. doi: 10.1016/j.molcel.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 119.Zhu N, Zhang J, Du Y, Qin X, Miao R, Nan J, Chen X, et al. Loss of ZIP facilitates JAK2-STAT3 activation in tamoxifen-resistant breast cancer. Proc Natl Acad Sci USA. . 2020;117:15047–15054. doi: 10.1073/pnas.1910278117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stark GR. Forward genetics in mammaliancells: functional approaches to gene discovery. Hum Mol Genet. . 1999;8:1925–1938. doi: 10.1093/hmg/8.10.1925. [DOI] [PubMed] [Google Scholar]
- 121.Lobry C, Oh P, Mansour MR, Look AT, Aifantis I. Notch signaling: switching an oncogene to a tumor suppressor. Blood. . 2014;123:2451–2459. doi: 10.1182/blood-2013-08-355818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mills AA. p63: oncogene or tumor suppressor? Curr Opin Genet Dev. . 2006;16:38–44. doi: 10.1016/j.gde.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 123.Menke A L, et al. (1998) The Wilms’ tumor 1 gene: oncogene or tumor suppressor gene? Int Rev Cytol. 181: 151–212 . [DOI] [PubMed]
- 124.Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, Ye J, et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med. . 2016;8 doi: 10.1126/scitranslmed.aaf2304. [DOI] [PubMed] [Google Scholar]
- 125.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LFR, Hoorens A, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. . 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 126.Seo E, Kim WY, Hur J, Kim H, Nam SA, Choi A, Kim YM, et al. The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci Rep. . 2016;6:31931. doi: 10.1038/srep31931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tang TT, Konradi AW, Feng Y, Peng X, Ma M, Li J, Yu FX, et al. Small molecule inhibitors of TEAD auto-palmitoylation selectively inhibit proliferation and tumor growth of NF2-deficient mesothelioma . Mol Cancer Ther. . 2021;20:986–998. doi: 10.1158/1535-7163.MCT-20-0717. [DOI] [PubMed] [Google Scholar]
- 128.Kaneda A, et al. (2020) The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein‒protein interactions and exerts an antitumour effect on malignant pleural mesothelioma. Am J Cancer Res. 10: 4399–4415 . [PMC free article] [PubMed]
- 129.Gibault F, Sturbaut M, Coevoet M, Pugnière M, Burtscher A, Allemand F, Melnyk P, et al. Design, synthesis and evaluation of a series of 1,5‐Diaryl‐1,2,3‐triazole‐4‐carbohydrazones as inhibitors of the YAP‐TAZ/TEAD complex. ChemMedChem. . 2021;16:2823–2844. doi: 10.1002/cmdc.202100153. [DOI] [PubMed] [Google Scholar]