Abstract
Background
Endocrine deficiencies, including hypothalamic-pituitary-gonadal axis (HPGA) impairment, are common in survivors of childhood and adolescent medulloblastoma. Still, data regarding pubertal development and fecundity are limited, and few studies assessed HPGA function in males. We aimed to describe HPGA function in a large cohort of patients with medulloblastoma.
Methods
A retrospective study comprising all 62 medulloblastoma patients treated in our center between 1987 and 2021, who were at least 2 years from completion of therapy. HPGA function was assessed based on clinical data, biochemical markers, and questionnaires.
Results
Overall, 76% of female patients had clinical or biochemical evidence of HPGA dysfunction. Biochemical evidence of diminished ovarian reserve was seen in all prepubertal girls (n = 4). Among the males, 34% had clinical or biochemical evidence of gonadal dysfunction, 34% had normal function, and 29% were age-appropriately clinically and biochemically prepubertal. The difference between males and females was significant (P = .003). Cyclophosphamide-equivalent dose was significantly associated with HPGA function in females, but not in males. There was no association between HPGA dysfunction and other endocrine deficiencies, length of follow-up, weight status, and radiation treatment protocol. Two female and 2 male patients achieved successful pregnancies, resulting in 6 live births.
Conclusions
HPGA dysfunction is common after treatment for childhood medulloblastoma. This is seen more in females, likely due to damage to the ovaries from spinal radiotherapy. Our findings may assist in counseling patients and their families regarding risk to future fertility and need for fertility preservation.
Keywords: childhood cancer survivors, cyclophosphamide-equivalent dose, fertility, medulloblastoma, puberty
Key Points.
Impaired HPGA function is common after treatment for childhood medulloblastoma.
This impairment is seen more in female than male survivors.
These findings can be used when counseling patients regarding fertility preservation options.
Importance of the Study.
Endocrine deficiencies are common in survivors of childhood medulloblastoma. Previous studies assessing the hypothalmic-pituitary-gonadal axis (HPGA) in these patients reported a wide range of impaired function (4–84% of patients). Data regarding pubertal development and fecundity are limited, and few studies assessed HPGA function in males. In this study we describe HPGA function in a large cohort of 62 medulloblastoma patients, treated mostly with craniospinal irradiation and adjuvant chemotherapy, with reference to the cumulative alkylating agents doses. Our main finding was that HPGA dysfunction was significantly (P = .003) more common in female patients than in males (76% vs 34%). Furthermore, evidence of diminished ovarian reserve was seen in all prepubertal girls, while prepubertal males had normal gonadotrophin levels. Four patients achieved successful pregnancies, resulting in 6 live births. Our findings may assist in counseling patients and their families regarding risk to future fertility and need for fertility preservation.
Medulloblastoma is one of the more common malignant brain neoplasms seen in the pediatric population, accounting for 15% to 20% of pediatric brain tumors.1 Newer treatment protocols involve multimodal therapy, including surgical resection, chemotherapy, and craniospinal irradiation (CSI).2,3 Adjuvant chemotherapy, introduced in the 1980s, and risk-stratified therapy, introduced in the 1990s, are thought to be significant contributors to improved survival in these patients.1 In younger patients, radiation sparing or delaying approaches such as high-dose chemotherapy with stem cell support or the addition of intraventricular methotrexate have been employed due to the toxicity of CSI in this age group.4,5 The recent introduction of molecular genetic profiling of central nervous system (CNS) tumors allows for more accurate risk stratification and for the development and use of targeted therapies, currently within early-phase clinical trials, with the aim of further improving survival rates whilst minimizing associated morbidity.6
With increasing survival rates comes an increase in the number of patients at risk for treatment-related adverse events, including HPGA dysfunction.7 Some of these effects may manifest many years after the completion of treatment necessitating long-term follow-up.8
Impairment in gonadal function, pubertal development, and fertility, are significant amongst the complications following oncological therapies. These are important, as in addition to the impact of a shortened period of fertility or infertility on quality of life, there are medical implications including osteoporosis and increased risk of cerebrovascular accident.9 Furthermore, in children and adolescents, gonadal function is essential for the development of secondary sex characteristics, optimal linear growth, and bone mass accrual. Treatment with alkylating agents and with radiotherapy to the pelvic area, involving the ovaries, have been identified as major risk factors for gonadal failure10; cranial irradiation can also impair fertility, through damaging the hypothalamic-pituitary axis.11 The risk of gonadal damage and impaired fertility is often linked to the cumulative dosage of chemotherapeutic agents or radiotherapy.12
Medulloblastoma patients who receive both chemotherapy and CSI are at risk for damage to the hypothalamic-pituitary axis (HPGA) as well as for direct gonadal damage.13,14 Several studies assessed HPGA function in these patients. Precocious puberty (PP) was reported in 7–36% of patients, hypogonadotrophic hypogonadism (HH) in 6–60% of patients, and hypergonadotrophic hypogonadism (HH) in 4–84% of patients.7,13,15–17 These wide ranges may be attributed to small sample sizes, and differences in inclusion and exclusion criteria, length of follow-up, definitions of HPGA dysfunction, timing and protocol of endocrine evaluation, and treatment protocols. For example, patients included in older series16 did not receive adjuvant chemotherapy, likely resulting in lower rates of primary gonadal failure. Spontaneous recovery of gonadal function was reported in 2 previous studies in up to 38% of females.7,13 Only 3 previous studies reported fertility outcomes in their cohorts.8,12,15 Importantly, we found no study specifically evaluating HPGA function in males with medulloblastoma, and no study comparing the HPGA function of males vs females.
The aim of our study was to characterize gonadal function in male and female survivors of childhood and adolescent medulloblastoma and to identify risk factors for HPGA dysfunction, including gender, age at diagnosis, and disease characteristics (risk classification and treatment protocol). Our findings may allow for developing treatment recommendations and follow-up protocols and can assist in counseling patients prior to initiating treatment with regards to risk to future fertility and need for fertility preservation.
Materials and Methods
This was a single-center retrospective study, comprising all patients diagnosed with medulloblastoma treated in our center between 1987 and 2021, who were at least 2 years from completion of therapy.
Potential participants were identified from a database of patients followed in the Late Effects of Childhood Cancer Clinic in Sheba Medical Center, Ramat Gan, Israel. Most patients were referred to the endocrine clinic shortly after diagnosis, often before the commencement of therapy.
Clinical parameters were extracted from the medical charts up to the point of the most recent clinic visit for all patients, including those who had died and those lost to follow-up.
In addition, patients older than 18 years were invited to fill questionnaires regarding need for hormone replacement treatment, fertility wishes, attempts to conceive, fertility preservation, and obstetric history. Recruitment was done during clinic visits; participants eligible to answer questionnaires who were not attending follow-up were contacted and offered the option of answering a digital questionnaire online. The questionnaire was previously used in the PanCareLife study, a large pan-European study investigating fertility in female childhood cancer survivors and was translated to Hebrew for use in that study.18,19 For the current study the questionnaire was modified for use in male participants.
Gonadal function was assessed based on: 1. Clinical parameters including the spontaneous onset of puberty, pubertal progression, presence of spontaneous menstruation, and menstrual cycle regularity; 2. Laboratory parameters including luteinizing hormone (LH), follicle stimulating hormone (FSH), Estradiol, Testosterone, and Anti-Mullerian Hormone (AMH); 3. Data from questionnaires.
Abnormalities of pubertal onset and progression were determined as detailed in the supplementary material. Weight status was classified as underweight, normal, overweight, and obese according to current guidelines.20,21
The study was approved by the Institutional Review Board at the Sheba Medical Center. Written or oral informed consent was obtained from all adult participants prior to answering the questionnaires. For retrospective data, informed consent was waived, as data was de-identified.
Data Analysis
The initial analysis included estimations of mean, standard deviations, and frequency distribution. Evaluation of potential risk factors for impaired HPG function were done using ANOVA for continuous variables such as time from diagnosis, and the χ 2 test for dichotomous variables such as gender, radiation treatment protocol, and endocrine deficiencies. Logistic regression model was used to assess the association between baseline biomedical factors and the development of HPG axis dysfunction. To evaluate the impact of alkylating agent exposure on HPG dysfunction, the total alkylating agent exposure was expressed as the cyclophosphamide-equivalent dose (CED).22 The association between CED and HPG function was tested using the Kruskal-Wallis Test in male patients and the Mann-Whitney Test in female patients. Results were considered significant if the two-sided P value was <.05. Calculations were performed using SPSS Statistics 25.0 (IBM, Armonk, NY, USA), a statistical software package.
Results
Patients’ Characteristics
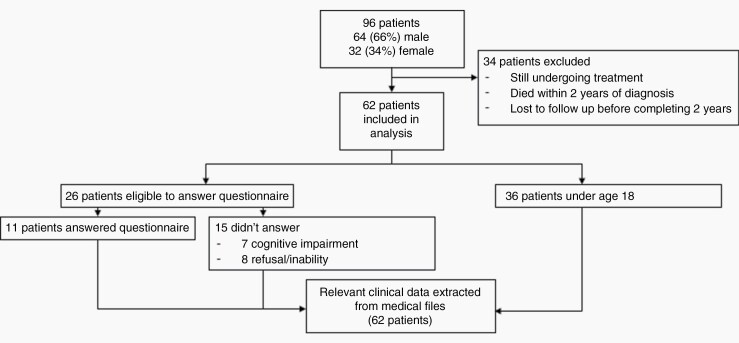
Of the 96 patients diagnosed with medulloblastoma and treated in our center during the study period, 62 patients completed at least 2 years of follow-up and were included in the final analysis. The excluded 34 patients included those that were still undergoing treatment, patients that had died or stopped attending clinic within 2 years of diagnosis and those that had stopped attending clinic before completing 2 years of follow-up after completion of therapy (Figure 1).
Figure 1.
Flow diagram for study participants.
Patients were treated from 1987 to 2021. All patients initially underwent tumor resection. Patients under age 3 years were treated with adjuvant chemotherapy only. All other patients were treated with CSI, according to risk stratification based on age, extent of surgical resection, and the presence or absence of metastases. High-risk patients received 36 Gy to the whole craniospinal axis and a boost of 18 Gy to the tumor bed in the posterior fossa; standard-risk patients received 23.4 Gy to the whole axis and a boost of 30.6 Gy to the tumor bed (Table 1). Three patients received additional radiotherapy for recurrences. Two patients were treated with proton-beam therapy, while the rest were treated with photon-beam therapy. Patients were treated with a variety of chemotherapy protocols (Table 2) according to treatment era, age, risk stratification, individual patients’ characteristics (eg, bone marrow involvement), and the treating center (4 patients had some of their treatment at other centers). Six patients were treated with more than 1 protocol because of recurrence; in 3 patients the protocol was changed mid-treatment due to revised risk stratification (n = 2) and cardiac toxicity (n = 1). Four patients received no or minimal chemotherapy due to treatment era (n = 2), toxicity (n = 1), and parental refusal (n = 1).
Table 1.
Patient Characteristics
| Total Patients 62 | ||
|---|---|---|
| Male n = 41 (66%) | Female n = 21 (34%) | |
| Median age at diagnosis years (range) | 8 (1–29) | 8 (2–20) |
| Age at last clinic visit | 19 ± 8.5 (range 9–40) | 16 ± 6.8 (range 7–28) |
| <18 y | 21 (51%) | 11 (52%) |
| >18 y | 20 (49%) | 10 (48%) |
| Radiation treatment protocol | ||
| High risk | 19 | 10 |
| Standard risk | 13 | 9 |
| Dose not available | 7 | 1 |
| No irradiation | 2 | 1 |
| Outcome | ||
| Survivors | ||
| <5 y from diagnosis | n = 5 (10%) | n = 6 (21%) |
| >5 y from diagnosis | n = 16 (40%) | n = 5 (26%) |
| >10 y from diagnosis | n = 14 (35%) | n = 9 (47%) |
| Deceased | n = 6 (15%) | n = 1 (5%) |
| Endocrine abnormalities | ||
| GH deficiency | 29 (70.7%) | 11 (52%) |
| Hypothyroidism | 22 (52%) | 10 (48%) |
| ACTH deficiency | 3 (7.3%) | 1 (4.8%) |
| Weight status | ||
| Normal | 24 (59%) | 14 (67%) |
| Underweight | 6 (15%) | 1 (5%) |
| Overweight | 4 (10%) | 3 (14%) |
| Obese | 7 (17%) | 3 (14%) |
Table 2. .
Chemotherapy Protocols Used in the Cohort and Cumulative Doses of Potentially Gonadotoxic Medications
| Protocol | n Treated | % Treated | CED mg/m2 |
Cisplatin mg/m2 |
|---|---|---|---|---|
| SJMB03 | 25 | 42.4% | 16 000 | 300 |
| COG ACNS 0331B | 15 | 25.4% | 13 200 | 450 |
| UKCCG SIOP PNET | 6 | 9.7% | 4500 | None |
| ACNS 0334A–99703* | 2 | 3.4% | 68 000 | 225 |
| POG 9631 | 2 | 3.4% | 24 000 | 270 |
| HEAD START* | 2 | 3.4% | 19 500* | 500* |
| CCG A9961 | 1 | 1.7% | 9600 | 600 |
| “Eight in one day” + Vincristin + CCNU | 1 | 1.7% | 11 000 | 180 |
| Protocol unknown | 4 | 6.4% | No data | No data |
| Minimal or no chemotherapy** | 4 | 6.4% | – | – |
CED = cyclophosphamide equivalent dose per protocol.
*The HEAD START and the ACNS 0334A–99703 were used in patients under 3 years old; Doses in the HEAD START protocol were weight based, converted here to dose/m2.
**Two patients received no chemotherapy. One patient received a single cycle of the SIOP protocol, with an estimated CED of 1500 mg/m2; one patient only received carboplatin and vincristine during radiation treatment.
Overall, including deceased patients, mean follow-up of males was 9.2 ± 7.3 years (range 3–28 years), and for females 12 ± 7.2 years, (range 3–26 years). At the time of analysis, 45 patients were under regular follow-up with a mean follow-up for males of 7 ± 8.6 years and for females 6 ± 7.5 years. Mean age at the last clinic visit for males was 19 ± 8.5 years (range 9–40) and for females 16 ± 6.8 (range 7–28) (Table 1).
Of the 62 patients included in the study (males = 41), 26 were eligible to answer the questionnaires. Of these, 7 were excluded due to cognitive impairment and 8 due to refusal or having been lost to follow-up. Thus, questionnaire data were available for 11 patients (males = 3) (Figure 1).
Forty-five patients had at least one endocrine deficiency. Forty patients (65%) had proven or suspected growth hormone (GH) deficiency; 32 patients (52%) had primary or central hypothyroidism, and 4 patients (6.5%) had Adrenocorticotropic Hormone (ACTH) deficiency. None of the patients had diabetes insipidus.
HPG Function in Male Patients
Clinical Puberty ( Table 3)
Table 3.
Clinical Puberty in the Cohort
| Male | Female | |
|---|---|---|
| Completed puberty pretreatment | 7 (17.1%) | 2 (9.5%) |
| Spontaneous puberty | 20 (48.8%) | 12 (57%) |
| Precocious | 4 (9.8%) | 0 (0%) |
| Completed | 11 (19.5%) | 6 (28.6%) |
| Arrested | 1 (2.4%) | 3 (14.3%) |
| Mid-pubertal | 4 (9.8%) | 3 (14.3%) |
| Failure of spontaneous onset of puberty | 1 (2.4%) | 2 (9.5%) |
| Pre-pubertal | 13 (31.7%) | 4 (19%) |
| Disease recurrence mid-puberty | 0 (0%) | 1 (4.8%) |
| Total | 41 | 21 |
Of the 41 males in the cohort, 7 patients had completed puberty prior to diagnosis. During the period of follow-up, 3 patients had been treated for PP, and had completed clinical puberty following cessation of treatment. One additional patient was still receiving GnRH analog for PP at the time of last evaluation. Sixteen patients entered puberty spontaneously at an appropriate age, of these 11 progressed through full puberty, 1 patient had arrested puberty and 4 patients were mid-pubertal at the time of data collection. One patient failed to enter spontaneous clinical puberty and received testosterone replacement therapy. Thirteen patients were age-appropriately prepubertal at their recent clinic visit. Of the patients who spontaneously entered and completed puberty 5 had high FSH levels suggestive of impaired spermatogenesis, as detailed below.
Biochemical Assessment
At the time of data collection, 12 patients had age-appropriate (<13 years old) gonadotrophin and testosterone levels in the prepubertal range. Two of them had died before reaching pubertal age, and 6 had been lost to follow-up prior to reaching pubertal age.
Four adult patients had gonadotrophin levels below the normal adult reference range or within the normal range with low testosterone levels, consistent with HH. In one of these patients, testosterone levels normalized over time indicating a degree of recovery of the gonadotrophs.
Eleven patients had raised FSH levels on follow-up blood tests. Of these, 2 had begun to normalize on most recent tests. All 11 patients had normal testosterone levels.
Of the remaining 13 patients with normal FSH levels, all had testosterone levels within the adult range although there was a large variation in testosterone levels across the cohort, with 6 patients (46%) having a testosterone level within the lowest quartile of the normal range.
Of the patients treated for PP, 1 had suppressed FSH levels under treatment at the time of data collection.
Fertility
One male prepubertal patient underwent an experimental fertility preservation procedure, with cryopreservation of testicular tissue prior to treatment. Of the 8 patients who answered questionnaires, 3 described themselves as being in a committed relationship, and 1 of them being married. Of these, 2 had attempted to conceive and achieved spontaneous conception, overall resulting in 3 live births.
HPG Function in Female Patients
Clinical Puberty ( Table 3)
Of the 21 female patients, 2 had completed puberty prior to diagnosis. One patient was in early puberty at diagnosis and failed to progress in puberty during and after the completion of therapy. Of the remaining patients, 12 entered spontaneous puberty following completion of therapy, of whom 6 completed clinical puberty (Tanner stage 5 with spontaneous onset of menstruation). One patient had a recurrence of the disease before the completion of puberty. Of the remaining 5, 2 had arrested puberty, and 3 were progressing through puberty but had not yet achieved menarche at the time of data collection. Six patients had not entered spontaneous puberty, of whom 2 had undergone puberty induction with estrogen treatment, and 4 were under the age of 10 years at the time of data collection.
Biochemical Assessment
At the time of data collection, 13 patients (62%) had raised FSH levels indicating ovarian failure. Of these patients, 5 had primary amenorrhea and 3 secondary amenorrhea. One patient had achieved tanner stage 2 at the age of 10 years at the most recent clinic visit. Two patients were clinically prepubertal at age 10 years.
Of these 13 patients, 1 (8%) was treated with high-dose radiotherapy only, and 1 (8%) with chemotherapy alone.
Of the 8 patients with normal FSH levels, 3 had spontaneous regular menstruation, 1 patient was oligomenorrheic and 1 patient had secondary amenorrhea. One patient, aged 16 years, with the spontaneous onset and normal progression of puberty, had not achieved menarche. One patient was taking oral contraceptive pills at the time of data collection. One patient had been lost to follow-up.
AMH, produced by developing follicles in the granulosa cells of the ovaries, is widely used as a marker of ovarian reserve and as a predictor of premature ovarian insufficiency (POI), and has been shown to be useful in predicting ovarian reserve in cancer survivors treated with gonadotoxic chemotherapy and radiation to the pelvis.23 Six of the female patients in our cohort had recorded AMH measurements. Five patients had low AMH levels, with levels increasing in 1 patient who had undergone ovarian tissue auto-transplantation. One patient had an AMH level which was in the low end of the normal range.
Fertility
Six patients underwent fertility preservation prior to treatment, 5 with ovarian tissue cryopreservation (OTC) and 1 with both OTC and oocyte cryopreservation.
Of the 6 patients who answered the questionnaires, 3 described themselves as being in a committed relationship, of whom 1 was married in the process of divorcing. Two patients were not in a relationship and 1 patient did not answer the question.
Four successful pregnancies resulting in live births were reported. One patient with spontaneous and regular menstruation had achieved 2 pregnancies following in vitro fertilization (IVF) treatment. One patient had undergone transplantation of ovarian tissue (harvested postmenarche) and IVF and had achieved 2 pregnancies. One patient with a normal endocrine profile and regular menstruation was undergoing fertility treatment for male factor infertility.
Risk Factors for Impaired Gonadal Function
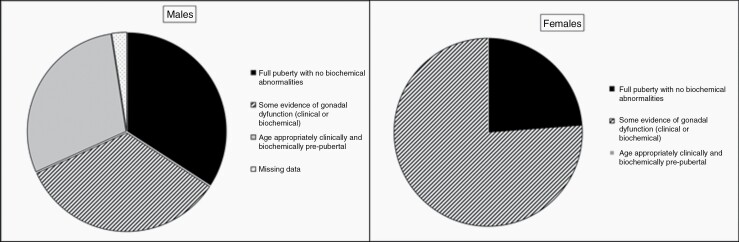
Overall, 76% of the female patients had clinical or biochemical evidence of gonadal dysfunction. Notably, biochemical evidence of diminished ovarian reserve was seen in all prepubertal girls (n = 4). Among the male patients, 34% had clinical or biochemical evidence of gonadal dysfunction, 34% had a normal gonadal function, and 29% were age-appropriately clinically and biochemically prepubertal (Figure 2). The difference between male and female patients was significant (P = .003).
Figure 2.
HPGA function in male and female study participants.
Detailed chemotherapy data, allowing for precise calculation of CED was available for 45 patients. Partial documentation allowing estimation of cumulative doses of alkylating agents was available in another 9 patients. In 4 patients there were not enough data to calculate the CED (as noted above, 4 patients received minimal or no chemotherapy). The median CED in males with normal vs abnormal HPG function were 13 800 mg/m2 (IQR 9708–16 000 m2) and 14 364 mg/m2(IQR 10 523–18 991 m2), respectively, whereas in prepubertal males the median CED was 13 200 mg/m2 (IQR 10 672–16 000 m2). The difference between the 3 groups was not significant (P = .669). Conversely, in female patients, median CED of patients with impaired HPGA function (16 000mg/m2, IQR 13 200–18 625 m2) was significantly (P = .032) higher compared to those with normal HPGA function (10 008 mg/m2, IQR 2312–14 000 m2). Notably, 2 male patients who did not receive chemotherapy had normal gonadal function. Of the 2 female patients who received minimal doses of chemotherapy, 1 had normal HPGA function while the other had ovarian failure necessitating induction of puberty.
There was no association between HPGA dysfunction and treatment with high-risk vs low-risk radiation protocol in both males (P = .428) and females (P = 1.0). As well, there was no association between HPGA dysfunction and other endocrine deficiencies, length of follow-up, and weight status. As expected, prepubertal patients were significantly younger compared to the other 2 groups (Table 4).
Table 4. .
Analyzed Risk Factors for HPGA Dysfunction
| All n = 61 |
Normal n = 30 |
Impaired n = 19 |
Prepubertal n = 12 |
PValue | |
|---|---|---|---|---|---|
| Age at diagnosis, y | 9.5 ± 6.2 | 11.3 ± 6 | 9.2 ± 7 | 5.6 ± 2.4 | .02 |
| Time from diagnosis, y | 9 ± 6.7 | 9.4 ± 7 | 10.5 ± 6.9 | 5.4 ± 2.2 | .09 |
| Endocrine deficiencies | |||||
| GH (n,%) | 39 (64) | 12 (40) | 16 (84.2) | 11 (91.7) | .363 |
| Thyroid (n,%) | 32 (52.5) | 11 (36.7) | 15 (78.9) | 6 (50) | .732 |
| ACTH (n,%) | 3 (4.9) | 1 (3.3) | 1 (5.3) | 1 (8.3) | .56 |
| BMI class (n,%) | |||||
| Normal | 37 (60.7) | 17 (56.7) | 12 (63.2) | 8 (66.7) | .757 |
| Underweight | 7 (11.5) | 2 (6.7) | 3 (15.8) | 2 (16.7) | |
| Overweight | 7 (11.5) | 4 (13.3) | 2 (10.5) | 1 (8.3) | |
| Obese | 10 (16.3) | 7 (23.3) | 2 (10.5) | 2 (16.7) |
Discussion
In the current study we found that 76% of female survivors of medulloblastoma, compared to 34% of the male patients (P = .003) had clinical or biochemical evidence of HPGA dysfunction. Furthermore, all (age-appropriately) prepubertal females, but none of the prepubertal males had biochemical evidence of gonadal dysfunction. Fourteen percent of the postpubertal males (4/28), but none of the females had HH. Four successful pregnancies resulting in live births were reported, all achieved with IVF. There was no association between HPGA dysfunction and other endocrine deficiencies or with length of follow-up, weight status, and treatment with the high-risk radiation protocol.
Therapy for medulloblastoma has evolved considerably over the past few decades. Understanding the importance of complete tumor resection and the introduction of CSI with focal boost to the tumor site resulted in an increase in survival rates from 5–10% to 30–50%, while the incorporation of chemotherapy as adjuvant therapy during the 1980s led to a further improvement in survival, making medulloblastoma curable in up to 80% of patients. By the 1990s, risk stratification according to age, extent of surgical resection, and metastatic status allowed standard-risk patients to receive reduced-dose CSI.1,24 These modifications of the treatment protocols may also impact the profile of late complications of cancer treatment. The recent introduction of proton-beam irradiation14,25 and molecular-matched targeted therapies, currently within early-phase clinical trials,26 may further impact treatment complications.
Our cohort included mainly patients treated in the 1990s and 2000s, thus most patients were treated with protocols consisting of risk-adjusted CSI and adjuvant chemotherapy. Accordingly, the current study adds novel information regarding this group of patients.
Primary Gonadal insufficiency
Among our cohort of patients, 27% of males and 62% of females showed biochemical evidence of impaired gonadal function as indicated by a raised FSH level or low AMH levels. The higher incidence of primary gonadal dysfunction among female patients can be explained by treatment with CSI to a field that includes the pelvis.
As our patients were treated with a number of chemotherapy protocols, we used the CED to estimate the cumulative gonadotoxicity of the multiple agents used in different protocols.22 Three previous studies suggested a CED exposure >8000 mg/m2 as a significant risk factor of premature ovarian failure in female survivors of childhood cancer,19,22,27 while another suggested CED ≥11 295 mg/m2 in females.28 In male survivors, a threshold of 5000 mg/m2 was associated with a decreased likelihood of siring a pregnancy.22,28 Only 10 of our patients received doses lower than these thresholds (6 patients who were treated with the UKCCG SIOP PNET protocol, and 4 patients who received no or minimal chemotherapy). Notably, as depicted in Table 2, most of our patients also received cisplatinum, which although not included in the CED calculation has been suggested to contribute to gonadal toxicity in males.29
Previous studies assessing HPGA function in survivors of medulloblastoma reported a wide range of primary gonadal insufficiency, varying from 4% to 84% of patients.7,13,15–17 As discussed above, these differences may be related to the evolution of treatment protocols over the last decades, as well as to differences in inclusion and exclusion criteria, length of follow-up, definitions of HPGA dysfunction, and timing and protocol of endocrine evaluation.
Three previous studies described cohorts of patients treated with similar protocols to ours. Despite this, results were varied, likely due to differences in the characteristics of the cohorts described, as well as differences in follow-up periods. DeWire et al. observed POI in 45% of their cohort of 23 females, with a further 38% having shown evidence of ovarian dysfunction at some stage during the follow-up with subsequent recovery.13 In the 35-patient cohort described by Uday et al., the prevalence of primary gonadal dysfunction was significantly lower (10% among females, 20% among males). Unlike our cohort, all survivors were pubertal at the time of analysis.15 Finally, Hidalgo-Santos et al. described a cohort of 23 survivors of childhood medulloblastoma, only 1 of whom had impaired gonadal function. Unlike our study, they included only patients diagnosed under the age of 14 years, and deceased patients were excluded from the analysis.17
Most studies comprising patients treated with lower doses of CSI or lower doses of chemotherapy reported, as expected, lower rates of primary gonadal failure.7,30 Within the cohort described by Xu et al. this difference was not significant, likely due to the small sample size (19 patients). The authors did not differentiate between primary and central hypogonadism. Balachandar et al.7 described a cohort of 31 female survivors, comparing outcomes between patients treated with low- and high-dose CSI with standard-dose chemotherapy, and those treated with high-dose chemotherapy with or without CSI. Of those treated with lower-dose chemotherapy (with either low- or high-dose CSI), 32% showed evidence of ovarian dysfunction over the course of the follow-up, with 40% of these having shown recovery of ovarian function by the end of the period of follow-up, which was significantly shorter than in our cohort. Within the cohort who received high-dose chemotherapy, all showed evidence of primary gonadal dysfunction. Similar to our cohort there was no correlation between risk group or age at diagnosis and risk of subsequent ovarian damage.
Several studies included patients treated with CSI alone. Two studies showed a higher incidence of gonadal damage in patients treated with CSI and high-dose chemotherapy compared to those treated with CSI alone.16,31 Similarly, in a larger, more recent study gonadal toxicity was observed only in patients who had received chemotherapy. Conversely, Brown et al. reported a high incidence of gonadal impairment (87.5%) in patients treated solely with CSI,11 and Livesey et al. described primary gonadal impairment in 40% of their cohort of 37 survivors, with a significant association between CSI and POI, and no significant increase in incidence with the addition of chemotherapy.32 In the current study, of the 2 female patients who received minimal doses of chemotherapy, 1 had normal HPGA function while the other had ovarian failure necessitating induction of puberty.
Regarding differences in risk between males and females, most previous studies did not distinguish between male and female patients,17,25,30,32–34 included only female patients,7,13 or comprised a small number of patients that did not allow for comparison.11,15,16,31 Livesey et al.32 described a cohort of 37 survivors, observing primary gonadal impairment in 40% of their patients (12 female, 3 male). Since medulloblastoma affects males more than females, we may deduce that also in this cohort, females had a higher risk of gonadal failure.
The effect of proton-beam radiation was evaluated in 2 recent studies. Lee et al. reported HPGA dysfunction in 41% of the photon therapy group compared to 66% within the proton-treated group.25 Conversely, Eaton et al. reported a significantly lower risk of hypogonadism in the cohort treated with proton-beam therapy vs photon therapy (3% vs 19%). Notably, the cohort was young, with the average age among the patients treated with proton-beam therapy of 6.2 years.14 Both studies did not distinguish between primary gonadal insufficiency and pituitary gonadotrophin deficiency.
Hypogonadotrophic Hypogonadism
In our cohort, 4 (14%) of the male survivors but none of the females had overt HH. Yet, among the 8 females with no biochemical evidence of impaired gonadal function, 4 had abnormal menstrual cycles. This may suggest a degree of HH secondary to damage to the pituitary gonadotrophs or a combination of impaired ovarian and pituitary function with an inappropriately normal FSH level in the face of ovarian insufficiency.13 Among the male patients with measurable FSH and LH levels within the adult range, there was a large variation in testosterone levels with almost 50% of the patients having levels within the lowest quartile of the normal range, which may also reflect partial damage to the pituitary gonadotrophs.
Our results are in line with the 3 previous cohorts treated with protocols similar to ours. Uday et al. reported that 12.5% of the males, but none of the female patients had HH,15 while DeWire et al. observed no cases of HH in their female patients.13 Hidalgo-Santos et al. described 3 patients (13%) as having HH, but did not specify their gender.17
Among the more heterogeneously treated cohorts, Rappaport et al. reported that 9% of female and 25% of male patients had HH,16 while in the cohort described by Spoudeas et al. none of the patients were reported as having HH.34
Precocious Puberty
Within our cohort, 4 male (9.8%) but no female survivors experienced PP, a well-recognized complication in pediatric patients who have received CSI, which can cause disruption to the HPGA with premature interruption of the pubertal inhibitory signal pathways. Due to the high incidence of ovarian damage among female survivors, the higher incidence of PP in males is unsurprising. This observation reinforces the importance of close follow-up immediately following completion of treatment, as these patients are at risk of short final height due to spinal damage from CSI and GH deficiency, and untreated PP would likely further compromise final adult height.
In contrast to our observation, DeWire et al. observed 2 cases (11%) of PP among their female cohort. The follow-up period was similar to our group.13 Uday et al. also observed 7 cases (20%) of PP in their cohort, of whom 5 were female.15
Other investigators reported PP in 8–18% of their patients, both male and female.14,16,17,25
Fertility
Survivors of medulloblastoma suffer substantial comorbidities in addition to damage to the HPGA. Of significance is cognitive damage resulting in a lower likelihood of establishing romantic relationships and attempting to conceive. This is reflected in our cohort with 38% of survivors over the age of 18 years unable to complete questionnaires due to impaired cognition, and of those who answered only 43% reported being in a stable romantic relationship. Many patients had not made any attempt to conceive during their adult lives. This makes it difficult to accurately assess the extent of fertility impairment using the data available.
Only 3 previous studies reported fertility outcomes within their cohorts, with similar observations made. Within Uday et al’s cohort only one of the female survivors reported having children, but no male survivor.15 Within Fric et al’s cohort of adult survivors, 5 (31.3%) reported having been married or in a relationship, whereas 11 (68.8%) were single. Five patients (31.3%) reported live births.8 Finally, Frange et al. described a cohort of 45 patients of whom 36 were over the age of 20 years. Of these, only 10 (27.8%) were living independently. Nine were reported as either married or in a stable relationship. Female survivors were more likely to establish romantic relationships (6/12) than male survivors (3/17). Three female survivors had 5 children, and another was pregnant at the time of the last follow-up. There were no offspring reported from male survivors.12
Study Limitations
Limitations of our study include missing information on treatment protocols in patients who received part or all of their treatment in other institutions before transferring to our center. In addition, we did not have data regarding radiation dosimetry to the hypothalamus which may be an important risk factor in the development of HH. Still, given that there was no association between the crude measure of “high-risk” vs “low-risk” radiation and the risk for HPGA dysfunction, and as only 4 patients of the whole cohort had HH, we believe that dosimetry measurements would not have significantly affected our findings. With regards to the long-term effects, data were missing in patients who did not complete 2 full years of follow-up after completion of treatment. Questionnaire data was also limited due to the inability or refusal of many eligible patients to answer the questionnaires.
Conclusion
Survivors of medulloblastoma suffer significant morbidity as a result of aggressive treatment regimens. Our study focuses on damage to the HPGA and includes fertility outcomes. As we provide detailed data regarding treatment protocols and the cumulative alkylating agent exposure, and as our study comprises patients across a wide age range with a long follow-up period, we believe our data can be used to provide more accurate information when counselling patients with a diagnosis of medulloblastoma regarding potential damage to the HPGA, and fertility preservation options. With the advent of newer treatment protocols including proton-beam therapy and molecular-matched targeted therapy there may be room for optimism regarding reduced treatment-associated morbidity, but further long-term studies are needed to be able to quantify the theoretically reduced risk.
Prior PresentationThis work was presented at The European Society for Paediatric Endocrinology annual conference, ESPE 2022.
Supplementary Material
Contributor Information
Eve Stern, Pediatric Endocrinology and Diabetes Unit, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Michal Ben-Ami, Pediatric Endocrinology and Diabetes Unit, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Noah Gruber, Pediatric Endocrinology and Diabetes Unit, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Amos Toren, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Division of Pediatric Hematology-Oncology, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel.
Shani Caspi, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Division of Pediatric Hematology-Oncology, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel.
Gadi Abebe-Campino, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Division of Pediatric Hematology-Oncology, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel.
Michal Lurye, Division of Pediatric Hematology-Oncology, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel.
Michal Yalon, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel; Division of Pediatric Hematology-Oncology, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel.
Dalit Modan-Moses, Pediatric Endocrinology and Diabetes Unit, The Edmond and Lily Safra Children’s Hospital, Sheba Medical Center, Ramat-Gan, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Funding
No funding sources were used for this work.
Conflict of Interest:
None declared.
Author contributions
The manuscript was written by ES, NG, and DMM. All authors reviewed and approved the final version of the manuscript.
References
- 1. Salloum R, Chen Y, Yasui Y, et al. Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: a report from the childhood cancer survivor study. Am J Clin Oncol. 2019; 37(9):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Venes JL, McIntosh S, O’Brien RT, Schwartz AD. Chemotherapy as an adjunct in the initial management of cerebellar medulloblastomas. A preliminary report. J Neurosurg. 1979; 50(6):721–724. [DOI] [PubMed] [Google Scholar]
- 3. Gerosa MA, di Stefano E, Olivi A, Carteri A. Multidisciplinary treatment of medulloblastoma: a 5-year experience with the SIOP trial. Child’s Brain. 1981; 8(2):107–118. [DOI] [PubMed] [Google Scholar]
- 4. Cuny A, Trivin C, Brailly-Tabard S, et al. Inhibin B and anti-Müllerian hormone as markers of gonadal function after treatment for medulloblastoma or posterior fossa ependymoma during childhood. J Pediatr. 2011; 158(6):1016–1022.e1. [DOI] [PubMed] [Google Scholar]
- 5. von Bueren AO, von Hoff K, Pietsch T, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro Oncol. 2011; 13(6):669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phipps K, Kirkman MA, Aquilina K, et al. Childhood medulloblastoma-a single institution’s historical perspective on survival and functional morbidity. Childs Nerv Syst. 2019; 35(12):2327–2338. [DOI] [PubMed] [Google Scholar]
- 7. Balachandar S, Dunkel IJ, Khakoo Y, et al. Ovarian function in survivors of childhood medulloblastoma: Impact of reduced dose craniospinal irradiation and high-dose chemotherapy with autologous stem cell rescue. Pediatric Cancer. 2015; 62(2):317–321. [DOI] [PubMed] [Google Scholar]
- 8. Frič R, Due-Tønnessen BJ, Lundar T, et al. Long-term outcome of posterior fossa medulloblastoma in patients surviving more than 20 years following primary treatment in childhood. Sci Rep. 2020; 10(1):9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Green DM, Sklar CA, Boice JD, Jr, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the childhood cancer survivor study. Am. J. Clin. Oncol. 2009; 27(14):2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gnaneswaran S, Deans R, Cohn RJ. Reproductive late effects in female survivors of childhood cancer. Obstet Gynecol Int. 2012; 2012:1564794–1564797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown IH, Lee TJ, Eden OB, Bullimore JA, Savage DC. Growth and endocrine function after treatment for medulloblastoma. Arch Dis Child. 1983; 58(9):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frange P, Alapetite C, Gaboriaud G, et al. From childhood to adulthood: long-term outcome of medulloblastoma patients. The institut curie experience (1980–2000). J Neurooncol. 2009; 95(2):271–279. [DOI] [PubMed] [Google Scholar]
- 13. DeWire M, Green DM, Sklar CA, et al. Pubertal development and primary ovarian insufficiency in female survivors of embryonal brain tumors following risk-adapted craniospinal irradiation and adjuvant chemotherapy. Pediatric Blood Cancer. 2015; 62(2):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol. 2016; 18(6):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uday S, Murray RD, Picton S, et al. Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin Endocrinol (Oxf). 2015; 83(5):663–670. [DOI] [PubMed] [Google Scholar]
- 16. Rappaport R, Brauner R, Czernichow P, et al. Effect of hypothalamic and pituitary irradiation on pubertal development in children with cranial tumors. J Clin Endocrinol Metab. 1982; 54(6):1164–1168. [DOI] [PubMed] [Google Scholar]
- 17. Hidalgo Santos AD, de Mingo Alemany MDC, Moreno Macián F, et al. Endocrinological late effects of oncologic treatment on survivors of medulloblastoma. Rev Chil Pediatr. 2019; 90(6):598–605. [DOI] [PubMed] [Google Scholar]
- 18. van den Berg M, van Dijk M, Byrne J, et al. Fertility among female survivors of childhood, adolescent, and young adult cancer: protocol for two pan-european studies (PanCareLIFE). JMIR Res Protoc. 2018; 7(9):e10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Berg MH, van Dijk M, Byrne J, et al. Treatment-related fertility impairment in long-term female childhood, adolescent and young adult cancer survivors: investigating dose-effect relationships in a European case-control study (PanCareLIFE). Hum Reprod. 2021; 36(6):1561–1573. [DOI] [PubMed] [Google Scholar]
- 20. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016; 22(7 Suppl):s176–s185. [PubMed] [Google Scholar]
- 21. Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017; 102(3):709–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014; 61(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson RA, Cameron D, Clatot F, et al. Anti-Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update. 2022; 28(3):417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hershatter BW, Halperin EC, Cox EB. Medulloblastoma: the Duke University Medical Center experience. Int J Radiat Oncol Biol Phys. 1986; 12(10):1771–1777. [DOI] [PubMed] [Google Scholar]
- 25. Lee JW, Lim DH, Sung KW, et al. Promising survival rate but high incidence of treatment-related mortality after reduced-dose craniospinal radiotherapy and tandem high-dose chemotherapy in patients with high-risk medulloblastoma. Cancer Med. 2020; 9(16):5807–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luger AL, König S, Samp PF, et al. Molecular matched targeted therapies for primary b rain tumors-a single center retrospective analysis. J Neurooncol. 2022; 159(2):243–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chemaitilly W, Li Z, Krasin MJ, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the st. jude lifetime cohort. J Clin Endocrinol Metab. 2017; 102(7):2242–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chow EJ, Stratton KL, Leisenring WM, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016; 17(5):567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian En L, Brougham MFH, Wallace WHB, Mitchell RT. Impacts of platinum-based chemotherapy on subsequent testicular function and fertility in boys with cancer. Hum Reprod Update. 2020; 26(6):874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu W, Janss A, Packer RJ, et al. Endocrine outcome in children with medulloblastoma treated with 18 Gy of craniospinal radiation therapy. J. Neurooncol. 2004; 6(2):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmed SR, Shalet SM, Campbell RH, Deakin DP. Primary gonadal damage following treatment of brain tumors in childhood. J Pediatr. 1983; 103(4):562–565. [DOI] [PubMed] [Google Scholar]
- 32. Livesey EA, Brook CG. Gonadal dysfunction after treatment of intracranial tumours. Arch Dis Child. 1988;63(5):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. González Briceño LG, Kariyawasam D, Samara-Boustani D, et al. High prevalence of early endocrine disorders after childhood brain tumors in a large cohort. J Clin Endocrinol Metab. 2022; 107(5):e2156–e2e66. [DOI] [PubMed] [Google Scholar]
- 34. Spoudeas HA, Charmandari E, Brook CG. Hypothalamo-pituitary-adrenal axis integrity after cranial irradiation for childhood posterior fossa tumours. Med Pediatr Oncol. 2003; 40(4):224–229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.