Abstract
Background
Treatment options for patients with melanoma brain metastasis (MBM) have changed significantly in the last decade. Few studies have evaluated changes in outcomes and factors associated with survival in MBM patients over time. The aim of this study is to evaluate changes in clinical features and overall survival (OS) for MBM patients.
Methods
Patients diagnosed with MBMs from 1/1/2009 to 12/31/2013 (Prior Era; PE) and 1/1/2014 to 12/31/2018 (Current Era; CE) at The University of Texas MD Anderson Cancer Center were included in this retrospective analysis. The primary outcome measure was OS. Log-rank test assessed differences between groups; multivariable analyses were performed with Cox proportional hazards models and recursive partitioning analysis (RPA).
Results
A total of 791 MBM patients (PE, n = 332; CE, n = 459) were included in analysis. Median OS from MBM diagnosis was 10.3 months (95% CI, 8.9–12.4) and improved in the CE vs PE (14.4 vs 10.3 months, P < .001). Elevated serum lactate dehydrogenase (LDH) was the only factor associated with worse OS in both PE and CE patients. Factors associated with survival in CE MBM patients included patient age, primary tumor Breslow thickness, prior immunotherapy, leptomeningeal disease, symptomatic MBMs, and whole brain radiation therapy. Several factors associated with OS in the PE were not significant in the CE. RPA demonstrated that elevated serum LDH and prior immunotherapy treatment are the most important determinants of survival in CE MBM patients.
Conclusions
OS and factors associated with OS have changed for MBM patients. This information can inform contemporary patient management and clinical investigations.
Keywords: brain metastases, immunotherapy, leptomeningeal disease (LMD), melanoma
Key Points.
The overall survival of MBM patients has improved significantly over time.
Factors associated with survival in these patients have changed.
Prior immunotherapy and serum LDH are associated with shorter survival from MBM diagnosis
Importance of the Study.
Melanoma has one of the highest rates of CNS metastasis among all solid tumors. While there have been many changes in the treatments available for patients with melanoma brain metastases (MBMs) in recent years, there are limited data about if/how MBM patient outcomes have changed over this time. This retrospective analysis of a large cohort of MBM patients demonstrates that overall survival (OS) has improved significantly over time and that key factors associated with worse survival consistently identified in previous studies were not significant in the contemporary cohort. Recursive partitioning analysis (RPA) showed that immunotherapy prior to MBM diagnosis, and serum lactate dehydrogenase (LDH) at MBM diagnosis, are associated with shorter survival in contemporary MBM patients. This information can inform MBM patient management, as well as the design and interpretation MBM clinical trials. The results also highlight subsets of patients for whom new therapeutic approaches are critically needed.
Melanoma is one of the most common causes of brain metastasis. Approximately 40–60% of metastatic melanoma patients develop melanoma brain metastasis (MBM).1–3 MBMs are associated with significant morbidity and mortality, and thus are a significant problem in this disease.1,4 Historically, the primary treatments for MBMs were surgery and/or radiation. However, treatments for patients with MBMs have evolved over the last decade, including the use of immunotherapy with single-agent anti-PD1 (nivolumab and pembrolizumab; FDA approval, 2014) and combination immunotherapy with ipilimumab and nivolumab (2015). For patients with a BRAFV600 mutation (present in 40–50% of cutaneous melanomas), available targeted therapies include single-agent BRAF inhibitors, which have largely been replaced by combination regimens with MEK inhibitors (dabrafenib and trametinib, FDA approval, 2014; vemurafenib and cobimetinib, 2015; encorafenib and binimetinib, 2018). There have also been improvements in the use of CNS-directed radiation due to technical advances in stereotactic radiosurgery (SRS).5,6
Historically, the median overall survival (OS) from MBM diagnosis has been 4–6 months.3,7–11 In recognition of the impact of MBMs on prognosis, the American Joint Committee on Cancer (AJCC) 8th edition melanoma staging system refined the categorization of patients with distant metastasis by adding M1D as a new M-category to include patients with central nervous system metastasis, further stratified by whether or not the serum lactate dehydrogenase (LDH) level is elevated.12 Previous studies have identified several factors associated consistently with worse OS following MBM diagnosis, including uncontrolled extracranial disease, poor performance status, older age, greater than 3 MBM lesions, and elevated serum LDH.3,7–11 However, with the many changes in the treatment landscape relatively little is known about the contemporary outcomes of MBM patients, and particularly if/how new therapeutic approaches have impacted factors associated with patient outcomes. An improved understanding of contemporary factors that impact survival in MBM patients would enhance the counseling and management of patients and inform the design, interpretation, and prioritization of clinical trials for this population.
To address this need, we reviewed the clinical features and outcomes of a large cohort of MBM patients treated at a single institution between 2009 and 2019. Based on the timing of the aforementioned FDA approvals, we separately analyzed outcomes and factors associated with OS in patients diagnosed with MBMs before 2014 (“Prior Era”; PE) and since 2014 (“Current Era”; CE). Our analysis demonstrates improving OS and changes in factors associated with OS for MBM patients.
Methods
Study Design and Patients
Under an institutional review board-approved protocol, we conducted a single-center, retrospective cohort study of patients diagnosed with MBM from 1/1/2009 to 12/31/2018 who received their initial MBM treatment at The University of Texas MD Anderson Cancer Center. We defined PE as patients diagnosed with MBM from 1/1/2009 to 12/31/2013, and CE as patients diagnosed with MBM from 1/1/2014 to 12/31/2018. Patients were excluded if they had uveal or mucosal melanoma, concurrent non-melanoma malignancy, initial treatment for MBM at another institution, or if brain imaging studies from initial diagnosis of MBM (prior to initiation of treatment) were unavailable for review.
Data
Demographic and clinical data collected included age at MBM diagnosis, gender, primary tumor features (histologic subtype, ulceration status, mitotic rate, Breslow thickness), and BRAF mutational status. Features collected at MBM diagnosis included: serum LDH level; presence of symptoms from MBMs; presence of leptomeningeal disease (LMD); status of extracranial (EC) disease at the time of MBM diagnosis (none; present but controlled; present and uncontrolled); number and maximum size of MBM lesions; prior immune or targeted therapy treatment; and steroid use at initial treatment for MBM. Initial MBM treatment(s), and all subsequent treatment(s) received, were also collected. Initial treatment was defined as a treatment regimen initiated within 6 weeks of MBM diagnosis. The “immunotherapies” treatment category included single-agent ipilimumab, nivolumab, pembrolizumab or interleukin-2 (IL-2), and ipilimumab + nivolumab combination immunotherapy. Targeted therapies included single-agent BRAF (BRAFi), single-agent MEK (MEKi), combination BRAFi + MEKi, and c-KIT (KITi) inhibitors.
Statistical Analysis
The primary outcome measure was OS time computed from the date of MBM diagnosis to the date of last known vital status. Patients alive at the last follow-up date were censored. The Kaplan–Meier method was used to estimate OS and group differences were assessed using the log-rank test. The association between OS and groups of interest was determined using univariate and multivariable Cox proportional hazards regression models as well as Recursive Partitioning Analysis (RPA). Initial MBM treatment(s), and treatment(s) received at any time point after MBM diagnosis, were included in the Cox regression models as time-dependent covariates. In addition, the RPA was employed to determine the relative importance of clinical factors based on their discriminative ability, which was scaled from 0% to 100%. RPA was performed using R version 3.5.0. All other statistical analyses were performed using SAS 9.4 for Windows (Copyright © 2002–2012 by SAS Institute Inc., Cary, NC). All statistical tests used a significance level of 5%. No adjustments for multiple testing were made.
Results
Patient, Disease, and Treatment Characteristics
A total of 791 patients diagnosed with MBM from 2009 to 2019 were identified, including 332 in the PE and 459 in the CE. The median follow-up from MBM diagnosis was 11.7 months (range, 0.0–141.2), 10.1 months (range, 0.8–141.2), and 13.3 months (range, 0.0–82.8) for the full cohort, PE, and CE cohorts, respectively. Patient demographics, disease features, and treatments are shown in Table 1.
Table 1.
Patient, Disease and Treatment Characteristics
| Characteristic | No. (%) | P Valuec | ||
|---|---|---|---|---|
| All Patients (n = 791) |
Prior Era (n = 332)a |
Current Era (n = 459)b |
||
| Follow-up from MBM diagnosis, median (range), months | 11.7 (0.0–141.2) | 10.1 (0.8–141.2) | 13.3 (0.0–82.8) | .54d |
| Age at initial MBM diagnosis, median (range), years | 60.9 (15.6–93.6) | 58.1 (15.6–93.3) | 62.2 (16.3–93.6) | .001 d |
| Gender | ||||
| Male | 528 (67) | 218 (66) | 310 (68) | .59 |
| Female | 263 (33) | 114 (34) | 149 (32) | |
| BRAF V600 mutation | ||||
| Yes | 358 (45) | 153 (46) | 205 (45) | .005 |
| No | 321 (41) | 118 (36) | 203 (44) | |
| Unknown | 112 (14) | 61 (18) | 51 (11) | |
| Melanoma subtype | ||||
| Superficial spreading | 217 (27) | 89 (27) | 128 (28) | .45e |
| Nodular | 122 (15) | 56 (17) | 66 (14) | |
| Acral lentiginous | 39 (5) | 12 (4) | 27 (6) | |
| Lentigo maligna | 39 (5) | 17 (5) | 22 (5) | |
| In situ | 2 (<1) | 2 (1) | 0 | |
| Spindle cell | 2 (<1) | 1 (<1) | 1 (<1) | |
| Unknown | 370 (47) | 155 (47) | 215 (47) | |
| Breslow thickness, median (range), mm | 2.5 (0.0–29.0) | 2.4 (0.2–29.0) | 2.5 (0.0–25.2) | .34d |
| Primary tumor ulceration | 191 (24) | 80 (24) | 111 (24) | .94 |
| Mitotic rate, median (range)/mm2 | 5.0 (0.0–58.0) | 5.0 (1.0–54.0) | 5.5 (0.0–58.0) | .91d |
| LDH at initial MBM diagnosis | ||||
| Elevated | 261 (33) | 92 (28) | 169 (37) | .007 |
| Not elevated | 530 (67) | 240 (72) | 290 (63) | |
| Extracranial stage IV disease statusf | ||||
| Controlled | 154 (19) | 49 (15) | 105 (23) | .020 |
| Uncontrolled | 568 (72) | 250 (75) | 318 (69) | |
| No | 63 (8) | 29 (9) | 34 (7) | |
| Unknown | 6 (1) | 4 (1) | 2 (<1) | |
| Prior immunotherapy | 233 (29) | 46 (14) | 187 (41) | <.001 |
| Prior targeted therapy | 124 (16) | 47 (14) | 77 (17) | .32 |
| Number of brain metastasis at diagnosis | ||||
| Median (range) | 2 (1–300) | 2 (1–200) | 2 (1–300) | .72d |
| 1 | 330 (42) | 140 (42) | 190 (41) | .73 |
| 2 | 146 (18) | 63 (19) | 83 (18) | |
| 3 | 73 (9) | 32 (10) | 41 (9) | |
| >3 | 239 (30) | 97 (29) | 142 (31) | |
| Unknown | 3 (<1) | 0 | 3 (1) | |
| Brain metastasis maximum size, median (range), cm | 1.0 (0.1–7.5) | 1.1 (0.1–7.5) | 1.0 (0.1–7.5) | .021 d |
| Leptomeningeal disease at initial MBM diagnosis | 43 (5) | 19 (6) | 24 (5) | .68 |
| Symptomatic brain metastasis at diagnosis | 218 (28) | 80 (24) | 138 (30) | .06 |
| Concurrent steroid use at initial MBM treatment | 325 (41) | 147 (44) | 178 (39) | .11 |
| Initial MBM treatmentg | ||||
| Craniotomy | 111 (14) | 61 (18) | 50 (11) | .004 |
| SRS | 439 (55) | 198 (60) | 241 (53) | .050 |
| WBRT | 149 (19) | 64 (19) | 85 (19) | .78 |
| Systemic | 447 (57) | 152 (46) | 295 (64) | <.001 |
| Chemotherapy | 146 (18) | 87 (26) | 59 (13) | <.001 |
| Immunotherapies | 217 (27) | 39 (12) | 178 (39) | <.001 |
| Targeted therapies | 146 (18) | 47 (14) | 99 (22) | .009 |
| Total number of initial MBM treatments | ||||
| Median (range) | 1 (0–4) | 1 (0–4) | 2 (0–3) | .18 |
| <2 | 410 (52) | 184 (55) | 226 (49) | .10 |
| ≥2 | 381 (48) | 148 (45) | 233 (51) | |
| <3 | 709 (90) | 302 (91) | 407 (89) | .34 |
| ≥3 | 82 (10) | 30 (9) | 52 (11) |
Abbreviations: LDH, lactate dehydrogenase; MBM, melanoma brain metastasis; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy. The bold values represent statistical significance.
aPatients diagnosed with MBM at 2009–2013 period.
bPatients diagnosed with MBM at 2014–2018 period.
cFisher’s exact test or its generalization.
dWilcoxon rank-sum test.
eChi-square test.
fExtracranial Stage IV disease status at the initial MBM diagnosis.
gPatients could have had more than one type of therapy.
There were no significant differences in gender or primary tumor features between the patients diagnosed with MBM in the 2 eras. The prevalence of the BRAFV600 mutation was similar (PE, 46%; CE, 45%), but CE patients were less likely to have unknown mutation status (CE, 11%; PE, 18%). Several factors differed between the PE and CE at the diagnosis of MBM. Patients diagnosed with MBM in the PE were younger (median 58.1 [range, 15.6–93.3] vs 62.2 years [range, 16.3–93.6], P = .001) and had a larger MBM maximum size (median 1.1 [range, 0.1–7.5] vs 1.0 cm [range, 0.1–7.5], P = .021). CE patients were more likely to have elevated LDH (37% vs 28%, P = .007) but were also more likely to have controlled EC disease (23% vs 15%, P = .020). More CE patients (41% vs 14%, P < .001) received immunotherapy prior to MBM diagnosis, but there was no significant difference in prior targeted therapy (17% vs 14%, P = .32) (Table 1).
Approximately 50% of patients received multiple treatment modalities in the first six weeks after MBM diagnosis, which did not differ between the PE and CE. Craniotomy (18% vs 11%, P = .004) and SRS (60% vs 53%, P = .050) were both utilized more frequently as initial MBM treatment in the PE. Systemic therapy was used more frequently in the CE (64% vs 46%, P < .001), and the types of systemic therapies used as initial treatment(s) for MBMs also changed. Chemotherapy was used more frequently in the PE (26% vs 13%, P < .001), while immunotherapy (39% vs 12%, P < .001) and targeted therapy (22% vs 14%, P = .009) were more frequent in the CE (Table 1). Analysis of treatments received at any time after MBM diagnosis similarly showed increased utilization of craniotomy (34% vs 19%, P < .001), SRS (76% vs 65%, P = .001), and chemotherapy (61% vs 34%, P < .001) in the PE, and more frequent treatment with immunotherapy (70% vs 47%, P < .001) in the CE (see Supplemental Table S1). The use of whole brain radiation therapy (WBRT) as initial treatment, or as treatment at any time after MBM diagnosis, did not differ between the PE and CE.
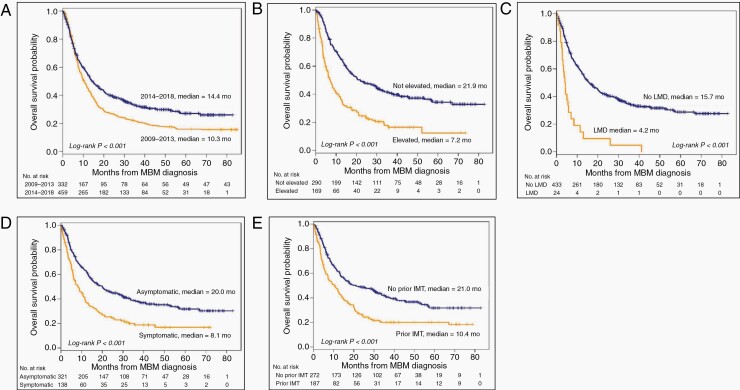
OS from MBM diagnosis improved over time. The median OS from MBM diagnosis was 10.3 months (95% CI, 8.9–12.4) for PE and 14.4 months (95% CI, 12.2–17.7) for CE patients (P < .001) (Figure 1A). One- and two-year OS rates from MBM diagnosis were 45% and 27% for PE and 56% and 39% for CE patients, respectively.
Figure 1.
Kaplan–Meier OS curves are presented by different features. (A) OS curves by prior (2009–2013, median OS 10.3 months) and current (2014–2018, median OS 14.4 months) era. (B) OS curves in the current era by LDH status. Median OS of patients with elevated and not elevated LDH were 7.2 and 21.9 months, respectively. (C) OS curves in the current era by LMD status; patients with LMD had median survival of 4.2 months vs 15.7 in those without LMD. (D) OS curves in the current era by symptom status. Symptomatic patients at MBM diagnosis had worse median survival (8.1 months) compared to asymptomatic patients (20.0 months). (E) OS curves in the current era by prior immunotherapy status. Patients who received and did not receive immunotherapy prior had median OS of 10.4 and 21.0 months, respectively. (Abbreviations: OS, overall survival; LMD, leptomeningeal disease; MBM, melanoma brain metastasis).
Univariate and Multivariable Analysis of Factors Associated with Overall Survival
Patient demographics, disease characteristics at MBM diagnosis, and initial treatment(s) for MBM(s) were assessed for associations with OS after MBM diagnosis by univariate (see Supplemental Table 2) and multivariable (Table 2) Cox proportional hazards regression models. These analyses were performed separately for the PE and CE cohorts to evaluate for changes in factors associated with OS. Factors that were significant in univariate analysis were included in multivariate models.
Table 2.
Multivariable Analysis of Overall Survival
| Category | Prior Era | Current Era | |||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Age at MBM dx | Continuous | 1.01 (1.00, 1.02) | .13 | 1.02 (1.01, 1.03) | .004 |
| LDH at MBM diagnosis | Not elevated | 1 (Reference) | NA | 1 (Reference) | NA |
| Elevated | 1.85 (1.24, 2.75) | .002 | 2.00 (1.43, 2.78) | <.001 | |
| Breslow thickness | Continuous | 0.98 (0.94, 1.02) | .31 | 1.04 (1.00, 1.08) | .039 |
| Extracranial stage IV disease | Uncontrolled | 1 (Reference) | NA | 1 (Reference) | NA |
| Controlled | 0.54 (0.34, 0.87) | .012 | 0.77 (0.51, 1.16) | .21 | |
| No | 0.20 (0.09, 0.42) | <.001 | 0.71 (0.39, 1.31) | .27 | |
| Unknown | 1.80 (0.24,13.42) | .57 | 3.20 (0.70,14.66) | .13 | |
| Prior immunotherapy | No | 1 (Reference) | NA | 1 (Reference) | NA |
| Yes | 1.08 (0.70, 1.67) | .74 | 2.03 (1.45, 2.82) | <.001 | |
| Prior targeted therapy | No | 1 (Reference) | NA | 1 (Reference) | NA |
| Yes | 2.76 (1.66, 4.59) | <.001 | 1.20 (0.76, 1.90) | .44 | |
| Number of brain metastasis at diagnosis | 1 | 1 (Reference) | NA | 1 (Reference) | NA |
| 2 | 1.13 (0.71, 1.79) | .61 | 1.10 (0.71, 1.71) | .67 | |
| 3 | 1.81 (0.97, 3.39) | .06 | 1.63 (0.96, 2.78) | .07 | |
| > 3 | 1.98 (1.22, 3.21) | .006 | 1.33 (0.87, 2.03) | .19 | |
| Unknown | NA | NA | 1.67 (0.21,13.45) | .63 | |
| Brain metastasis maximum size | Continuous | 1.23 (1.00, 1.51) | .053 | 1.02 (0.84, 1.23) | .85 |
| Leptomeningeal disease | No | 1 (Reference) | NA | 1 (Reference) | NA |
| Yes | 1.38 (0.66, 2.89) | .40 | 2.54 (1.23, 5.25) | .012 | |
| Symptoms | Asymptomatic | 1 (Reference) | NA | 1 (Reference) | NA |
| Symptomatic | 0.96 (0.58, 1.59) | .89 | 1.63 (1.11, 2.40) | .013 | |
| BRAF V600-Mutation | No | 1 (Reference) | NA | 1 (Reference) | NA |
| Yes | 0.97 (0.63, 1.50) | .88 | 1.45 (0.95, 2.22) | .08 | |
| Unknown | 1.94 (1.19, 3.16) | .008 | 1.51 (0.87, 2.60) | .14 | |
| Initial MBM treatmenta | |||||
| Craniotomy | Yes vs No | 0.52 (0.27, 1.02) | .059 | 0.86 (0.47, 1.55) | .61 |
| SRS | Yes vs No | 0.54 (0.35, 0.83) | .006 | 0.97 (0.68, 1.40) | .88 |
| WBRT | Yes vs No | 1.14 (0.64, 2.02) | .65 | 1.93 (1.16, 3.22) | .011 |
| Chemotherapy | Yes vs No | 0.67 (0.45, 1.01) | .056 | 0.92 (0.58, 1.46) | .73 |
| Immunotherapies | Yes vs No | 0.48 (0.27, 0.84) | .010 | 0.82 (0.57, 1.16) | .26 |
| Targeted therapies | Yes vs No | 0.40 (0.23, 0.68) | <.001 | 0.86 (0.54, 1.35) | .51 |
Abbreviations: HR, hazard ratio; LDH, lactate dehydrogenase; MBM, melanoma brain metastasis; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy. The bold values represent statistical significance.
aIncluded in the model as time-varying covariate.
While several features at the diagnosis of MBM were associated with OS on multivariable analysis in each cohort (Table 2), only elevated serum LDH was significant in both PE (HR, 1.8; 95% CI, 1.24–2.75; P = .002) and CE (HR, 2.0; 95% CI, 1.43–2.78; P < .001; Figure 1B) patients. Additional factors associated with worse survival in PE include prior targeted therapy (HR, 2.8; 95% CI, 1.66–4.59; P < .001) and >3 MBMs at diagnosis (HR, 2.0; 95% CI, 1.22–3.21; P = .006); Controlled (HR, 0.5; 95% CI, 0.34–0.87; P = .012) or absent (HR, 0.2; 95% CI, 0.09–0.42; P < .001) EC disease were associated with improved OS (vs Uncontrolled EC disease). In the CE, factors associated with worse OS included increased age (HR, 1.0; 95% CI, 1.01–1.03; P =.004), increased primary tumor Breslow thickness (HR, 1.0; 95% CI, 1.00–1.08; P =.039), the presence of LMD (HR, 2.5; 95% CI, 1.23–5.25; P = .012; Figure 1C), the presence of symptoms from MBMs (HR, 1.6; 95% CI, 1.11–2.40; P = .013; Figure 1D), and prior immunotherapy (HR, 2.0; 95% CI, 1.45–2.82; P < .001; Figure 1E).
Different initial treatment(s) for MBM were also associated with differences in OS (multivariable model) between the cohorts. Initial SRS was associated with improved OS in the PE (HR, 0.5; 95% CI, 0.35–0.83; P = .006) but not in the CE (P = .88). Initial WBRT was associated with worse survival in the CE (HR, 1.9; 95% CI, 1.16–3.22; P = .011) but not in the PE (P = .65). Among systemic regimens, both initial immunotherapy (HR, 0.5; 95% CI, 0.27–0.84; P = .010) and initial targeted therapy (HR, 0.4; 95% CI, 0.23–0.68; P < .0001) were associated with improved OS in the PE. Conversely, no specific initial treatment was significantly associated with improved survival in the CE on multivariable analysis.
In an alternative multivariate model including treatment(s) delivered at any time after MBM diagnosis (see Supplemental Table 3), SRS was associated with improved OS in both the PE (HR, 0.5; 95% CI, 0.31–0.69; P < .001) and CE (HR, 0.7; 95% CI, 0.51–1.00, P = .048). Treatment with immunotherapy at any time was also associated with improved survival in the PE (HR, 0.6; 95% CI, 0.43–0.87; P = .006). Treatment with chemotherapy (HR, 1.5; 95% CI, 1.09–2.14; P = .013) or WBRT (HR, 2.2; 95% CI, 1.5–3.2; P < .001) at any time was associated with worse OS in the CE cohort.
Recursive Partitioning Analysis to Evaluate Relative Importance of Factors Associated with Overall Survival
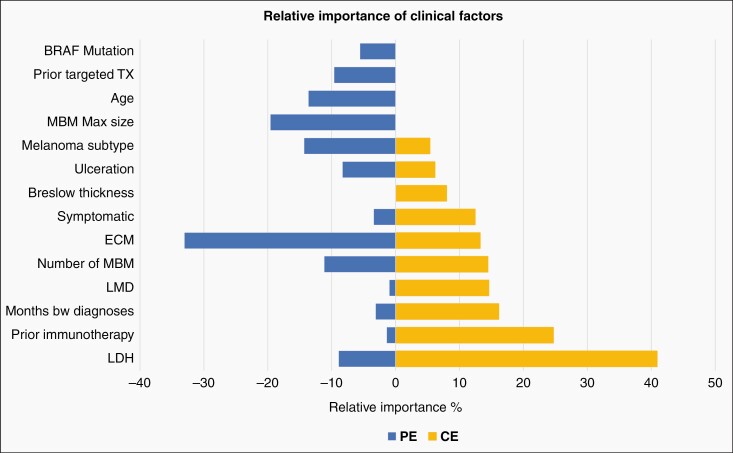
Due to the observed differences, we performed RPA to explore the relative importance of significant factors in the PE and CE (Figure 2). In the PE, the most important factors associated with worse OS were the presence of any EC disease and MBM maximum size. In the CE, the most important factors were elevated LDH and prior immunotherapy.
Figure 2.
Relative importance of factors associated with OS after MBM diagnosis. Prior era and current era prognostic factors with their relative importance bars are shown on the left panel and right panel, respectively. Only measures with relative importance >5% in either group are included in the figure. *Months btw diagnosis: months between primary diagnosis and MBM diagnosis. (Abbreviations: OS, overall survival; MBM, melanoma brain metastasis).
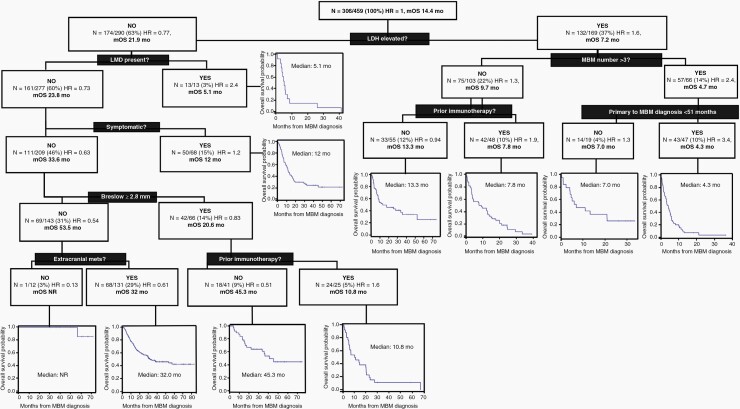
To inform contemporary MBM patient outcomes further, we performed stratification of the CE patients based on the factors identified by RPA (Figure 3). The initial split on these patients was based on LDH level at MBM diagnosis (ie the factor with the highest percentage of relative importance). For those with elevated LDH, the next discriminant was the number of MBMs. For patients with >3 MBMs, the final discriminant was months from primary melanoma to MBM diagnosis. Overall, patients with elevated LDH, >3 MBMs and <51 months from primary melanoma to MBM diagnosis exhibited the worst OS (n = 47; HR = 3.4; median OS 4.3 months). The best outcomes were observed for patients with a normal LDH; no LMD or neurological symptoms; primary melanoma Breslow thickness of <2.8 mm; and no EC disease at MBM diagnosis (n = 12; HR 0.13; median OS not reached).
Figure 3.
Recursive partitioning analysis of the factors associated with overall survival in current era MBM patients. This figure illustrates the prognostic stratification of the patients according to presence or absence of several patient, disease, or treatment related factors. (Abbreviations: N, number of patients in each group; HR, hazard ratio; mOS, median overall survival, MBM, melanoma brain metastasis).
Discussion
The treatment options for MBM patients have changed substantially over the past decade. In this study, which represents one of the largest single-center cohorts reported to date, as expected we observed an improvement in OS from MBM diagnosis over time. The reasons for this survival increase are likely multi-factorial, as we observed significant differences in both patient characteristics at MBM diagnosis and in the treatments these patients subsequently received. Importantly, the change in outcomes was accompanied by changes in factors associated with survival. Together these results provide new information to consider in the counseling and management of contemporary MBM patients.
The most important factor impacting OS in the PE in this cohort was the presence of extracranial metastatic disease, consistent with several prior studies.3,7–11 Likely reflecting the increasing effectiveness of systemic therapies, the presence of extracranial disease in the CE was not significantly associated with survival—but the extent of extracranial disease was important as serum LDH was the most significant factor associated with survival in that cohort. This observation is consistent with the worse outcomes observed in patients with elevated serum LDH in all registration studies of current FDA-approved systemic therapies for metastatic melanoma over the last decade.13–20 The relationship between LDH and clinical outcomes has been observed in some clinical trials in MBM patients,21,22 while in other studies it has not been reported.23–25 Notably, increased serum LDH was the only factor associated with survival in both the PE and CE cohorts. This observation reinforces the inclusion of LDH in the AJCC 8th edition melanoma M1D category,12 its use in decision-making and clinical trial design in contemporary MBM patients, and the continued unmet need for more effective treatments for these patients. Interestingly, Breslow thickness was associated with MBM patient survival in the CE cohort. The most recent update (8th edition) of the AJCC staging system for cutaneous melanoma includes primary tumor Breslow thickness in the staging criteria for patients with regional metastases (Stage III) based on association with melanoma-specific survival. Notably, the AJCC 8th edition did not update staging criteria for patients with distant metastases (stage IV) due to the rapid changes in treatments and outcomes for these patients. The association identified in our MBM cohort supports further investigation of Breslow thickness associations in stage IV melanoma.12
While LDH likely reflects extracranial disease burden and/or biology, we also observed significance of additional MBM features, albeit with differences between CE and PE patients. Consistent with previous studies increased number of MBMs was associated with worse OS in the PE, but it was not significant in the CE. This difference may reflect improvements in SRS that have enabled the treatment of a greater number of MBMs. The development of effective systemic therapies for MBMs may also contribute to this change, as the number of MBMs has not been associated with outcomes in MBM clinical trials.26 In contrast to the lesser importance of MBM number, the presence of neurological symptoms from MBMs and LMD were significant factors associated with survival in CE but not PE patients. Patients with symptomatic MBMs or LMD are frequently excluded from clinical trials evaluating systemic therapies for CNS disease. In the clinical trials that have included patients with symptomatic MBMs, worse outcomes have been observed with single-agent immune checkpoint inhibitors; dual checkpoint inhibition with ipilimumab and nivolumab; and combination targeted therapy with dabrafenib and trametinib.23,27–29 This association, and the results from those clinical trials, highlight the need for new therapeutic strategies for patients with symptomatic MBMs- and ideally prospective clinical trials designed specifically for that patient population instead of their continued exclusion. Furthermore, this finding supports the rationale for CNS imaging as part of surveillance in stage IV patients to facilitate discovery of asymptomatic MBMs when they are more effectively treated. Finally, the presence of LMD was associated with strikingly poor outcomes (Figure 1C; Table 2 and Supplemental Table 3). Among the aforementioned trials, only the ABC study allowed patients with LMD to participate, and it included only four such patients.22 The development of more clinical trials for melanoma patients with LMD remains a critical need, the feasibility of which is supported by recent studies of pembrolizumab and of ipilimumab and nivolumab in patients with LMD from multiple cancer types.30,31
Consistent with the changing therapeutic landscape of this disease, we observed significant differences in the initial treatments used for MBM patients in the PE and CE. Initial treatment was less likely to include craniotomy or chemotherapy, and more likely to include immunotherapy or targeted therapy in the CE. Interestingly, among the initial treatments for MBM patients in the CE only the use of WBRT was significantly associated with (worse) OS on multivariable analysis. Due to the retrospective nature of this analysis, this association may be due to other patient or disease features associated with the clinical decision to use this therapy, and this finding should not be interpreted as an assessment of the efficacy of this treatment modality In addition, CE patients who had received immunotherapy prior to MBM diagnosis had markedly worse outcomes (P < .001). Whether this is due to the emergence of or selection for resistant cancer cells, or due to the overall lack of durably effective treatments in the post-immunotherapy setting, is unknown. However, this association again highlights a novel clinical variable that previously was not linked with survival outcomes in MBM patients and is likely to become increasingly prevalent with the recent approvals of anti-PD1 immunotherapies in the adjuvant setting for stages II and III melanoma patients.32
Limitations
While our study represents one of the largest contemporary cohorts of MBM patients reported to date, it reflects a single-institution experience. Thus, associations will need to be tested in independent cohorts to determine their generalizability. In addition, this data was collected retrospectively by chart review, and due to missing data and inconsistent documentation, we could not include the patient performance status at MBM diagnosis in our analysis. Finally, we did not evaluate neurologic death specifically, which is the most immediate measure of the impact of MBM on survival.
Conclusions
Our retrospective cohort study results show significant changes in the outcome and factors associated with survival for MBM patients over the last decade. Several clinical variables associated with worse OS in PE patients (increased MBM number and extracranial disease status) were less significant in the CE. In turn, prior immunotherapy, LMD, and neurological symptoms were novel factors associated with worse OS in the CE patients with RPA demonstrating the key importance of elevated serum LDH and prior immunotherapy in contemporary patients. Several previous studies have evaluated factors associated with outcomes in MBM patients. Multivariable analysis of newly diagnosed brain metastasis patients between 1985 and 2007 resulted in the Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) scoring system. The DS-GPA for melanoma included Karnofsky performance score and number of brain metastases. This was later updated with the data from patients diagnosed with MBM from 2006 to 2015, result in the addition of age, BRAF status, and extracranial metastases. Analysis of our PE cohort, which included patients diagnosed with MBM from 1/1/2009 to 12/31/2013, similarly identified extracranial stage IV disease, number of brain metastases, and BRAF mutation status as factors significantly associated with OS. Our CE cohort includes patients diagnosed from 1/1/2014 to 12/31/2018, in part to capture the impact of changes in disease management and outcomes with contemporary systemic therapies (ie approval of ipilimumab + nivolumab in 2015), and thus is largely non-overlapping with the most recent DS-GPA. These changes in treatments, along with the inclusion of other variables that were feasible to collect from a large single-institution database, likely contributed to the identification of novel associations with OS, including prior immunotherapy. Our findings support the rationale to evaluate these factors in future multi-institutional studies.8,33–35 In addition to providing new information to guide prognostic assessment and decision-making in MBM patients, these results identify key scenarios in which new approaches and clinical trials are critically needed.
Supplementary Material
Acknowledgment
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under grant award number P50CA221703. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Contributor Information
Merve Hasanov, Department of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Denái R Milton, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Alicia Bea Davies, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Elizabeth Sirmans, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Chantal Saberian, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Eliza L Posada, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sylvia Opusunju, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jeffrey E Gershenwald, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Carlos A Torres-Cabala, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Elizabeth M Burton, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Rivka R Colen, Center for Artificial Intelligence Innovation in Medical Imaging, University of Pittsburg, Pittsburg, Pennsylvania, USA.
Jason T Huse, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Isabella C Glitza Oliva, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Caroline Chung, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Mary Frances McAleer, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Susan L McGovern, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Debra N Yeboa, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Betty Y S Kim, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sujit S Prabhu, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Ian E McCutcheon, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jeffrey S Weinberg, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Frederick F Lang, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Hussein A Tawbi, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Jing Li, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Lauren E Haydu, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Michael A Davies, Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sherise D Ferguson, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Funding
No funding source.
Conflict of Interest
The authors report the following Disclosures: JG is an advisor/consultant Merck; RC is a member, of HealthMyne Strategic Advisory Council; ICG has consulted for BMS, Array, Novartis, Sintetica, has been the PI of research funding to MD Anderson by: BMS, Merck, Pfizer; CC receives grant funding from Siemens Healthineers and RaySearch Laboratories; DNY has received research funding from the Brockman Foundation; IEM is a consultant to Merck and Vascular Technologies; FFL is a patent holder for DNAtrix, Inc., serves as a consultant for Nektar Therapeutics and Insightec and serves on the advisory board of Polypid and Cognos Therapeutics Inc; HT is supported by Bristol Myers-Squibb, Novartis, Merck, Genentech, GlaxoSmithKline, EMD Serono. Eisai, Dragonfly Therapeutics and RAPT Therapeutics. HT is a consultant for Bristol Myers-Squibb, Genentech, Novartis, Merck, Boxer Capital, Karyopharm, Iovance, Eisai, Jazz Pharmaceuticals, and Medicenna; MAD is supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the AIM at Melanoma Foundation, the NIH/NCI (1 P50 CA221703-03), the American Cancer Society and the Melanoma Research Alliance, Cancer Fighters of Houston, the Anne and John Mendelsohn Chair for Cancer Research, and philanthropic contributions to the Melanoma Moon Shots Program of MD Anderson. MAD has been a consultant to Roche/Genentech, Array, Pfizer, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex, Apexigen, Eisai, Iovance, and ABM Therapeutics, and he has been the PI of research grants to MD Anderson by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, Oncothyreon, and ABM Therapeutics; SDF has previously received research funding from Codiak. SDF is currently supported by the Brockman Foundation, Emerson Collective and the SPORE and Melanoma Moonshot Program of MD Anderson.
Author Contributions
Conception or design of the work: MH, LEH, MAD, SDF; data collection: MH, ABD, ES, CS, ELP, SO, MAD, SDF; data analysis and interpretation: MH, DRM, LEH, MAD, SDF; drafting the article: MH, DRM, LEH, MAD, SDF; critical revision of the article: MH, DRM, JEG, CAT, EMB, RC, JTH, ICG, CC, MFM, SLM, DNY, BYSK, SSP, IEM, JSW, FFL, HAT, JL, LEH, MAD, SDF; final approval of the version to be published: MH, DRM, ABD, ES, CS, ELP, SO, JEG, CAT, EMB, RC, JTH, ICG, CC, MFM, SLM, DNY, BYSK, SSP, IEM, JSW, FFL, HAT, JL, LEH, MAD, SDF.
References
- 1. Eroglu Z, Holmen SL, Chen Q, et al. Melanoma central nervous system metastases: an update to approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2019;32(3):458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abate-Daga D, Ramello MC, Smalley I, Forsyth PA, Smalley KSM. The biology and therapeutic management of melanoma brain metastases. Biochem Pharmacol. 2018;153:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glitza Oliva I, Tawbi H, Davies MA. Melanoma brain metastases: current areas of investigation and futuredirections. Cancer J. 2017;23(1):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skibber JM, Soong SJ, Austin L, Balch CM, Sawaya RE. Cranial irradiation after surgical excision of brain metastases in melanoma patients. Ann Surg Oncol. 1996;3(2):118–123. [DOI] [PubMed] [Google Scholar]
- 5. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 6. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 8. Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017;99(4):812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalmasso C, Pagès C, Chaltiel L, et al. Survival estimation of melanoma patients with brain metastasis using the Melanoma molGPA score: external validation from a French cohort. Melanoma Res. 2020;30(5):472–476. [DOI] [PubMed] [Google Scholar]
- 10. Sloot S, Chen YA, Zhao X, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer. 2018;124(2):297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bander ED, Yuan M, Carnevale JA, et al. Melanoma brain metastasis presentation, treatment, and outcomes in the age of targeted and immunotherapies. Cancer 2021;127(12):2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 14. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386(9992):444–451. [DOI] [PubMed] [Google Scholar]
- 16. Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. [DOI] [PubMed] [Google Scholar]
- 17. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372(1):30–39. [DOI] [PubMed] [Google Scholar]
- 18. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 19. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–615. [DOI] [PubMed] [Google Scholar]
- 20. Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 22. McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol. 2017;28(3):634–641. [DOI] [PubMed] [Google Scholar]
- 23. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 24. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 26. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margolin K. Ipilimumab in a Phase II trial of melanoma patients with brain metastases. Oncoimmunology. 2012;1(7):1197–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tawbi HA, Forsyth PA, Hodi S. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021;22(12):1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilmott JS, Banerjee H, Saiag P, et al. Association of baseline corticosteroid treatment with outcomes for patients (pts) with BRAF-mutant melanoma brain metastases (MBMs) in COMBI-MB treated with dabrafenib and trametinib (DT). J Clin Oncol. 2022;40(suppl 16):abstr e21546. [Google Scholar]
- 30. Brastianos PK, Strickland MR, Lee EQ, et al. Phase II study of ipilimumab and nivolumab in leptomeningeal carcinomatosis. Nat Commun. 2021;12(1):5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brastianos PK, Lee EQ, Cohen JV, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26(8):1280–1284. [DOI] [PubMed] [Google Scholar]
- 32. Luke JJ, Rutkowski P, Queirolo P, et al. ; KEYNOTE-716 Investigators. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): a randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718–1729. [DOI] [PubMed] [Google Scholar]
- 33. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiation Oncol Biol Phys. 2019;77(3): 655–661. [DOI] [PubMed] [Google Scholar]
- 35. Sperduto PW, Mesko S, Li J, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.