Abstract
Cancer immunotherapy has emerged as a promising approach for treating various malignancies. In this study, we investigated the combined therapeutic effects of mesenchymal stem cells expressing cytosine deaminase (MSC/CD) and 5-fluorocytosine (5-FC) with α-galactosylceramide (α-GalCer) in a colon cancer model. Our findings demonstrated that the combination of MSC/CD, 5-FC, and α-GalCer resulted in enhanced antitumor activity compared to the individual treatments. This was evidenced by increased infiltration of immune cells, such as natural killer T (NKT) cells, antigen-presenting cells (APCs), T cells, and natural killer (NK) cells, in the tumor microenvironment, as well as elevated expression of proinflammatory cytokines and chemokines. Furthermore, we observed no significant hepatotoxicity following the combined treatment. Our study highlights the potential therapeutic benefits of combining MSC/CD, 5-FC, and α-GalCer for colon cancer treatment and contributes valuable insights to the field of cancer immunotherapy. Future research should focus on elucidating the underlying mechanisms and exploring the applicability of these findings to other cancer types and immunotherapy strategies.
Keywords: Mesenchymal stem cells, cytosine deaminase, α-galactosylceramide, colon cancer, immunotherapy, tumor microenvironment, immune cells
Introduction
Colorectal cancer (CRC) is a major global health concern, ranking as the third most common cancer and the second leading cause of cancer-related deaths worldwide [1]. Despite significant advances in diagnostic techniques and therapeutic interventions, CRC remains a significant clinical challenge, with 50-60% of patients diagnosed with malignant tumors [2,3]. The development of innovative and effective treatments is essential to improve patient outcomes and reduce the burden of this disease.
Cancer immunotherapy has emerged as a promising approach to harness the power of the immune system to fight cancer [4]. Recent studies have focused on understanding the complex interactions between immune cells and cancer cells in the tumor microenvironment, aiming to develop therapeutic strategies that modulate these interactions to enhance antitumor responses [5]. Among the various immunotherapeutic approaches, mesenchymal stem cells (MSCs) have gained considerable attention due to their inherent tumor-homing ability [6] and potential to deliver anticancer agents directly to the tumor site [7,8].
In this study, we investigate the anticancer effects of the combined administration of mesenchymal stem cells expressing cytosine deaminase (MSC/CD), 5-fluorocytosine (5-FC), and α-galactosylceramide (α-GalCer) in a colon cancer model. MSC/CD-based therapy works by utilizing the ability of MSCs to selectively migrate to tumor sites, where they express the prodrug-converting enzyme cytosine deaminase (CD) and convert the non-toxic 5-FC into the highly cytotoxic 5-fluorouracil (5-FU), leading to localized cancer cell death [9,10]. However, its efficacy can be limited by various factors, including the immunosuppressive nature of the tumor microenvironment [11-13]. α-GalCer, a glycolipid antigen, has been shown to activate natural killer T (NKT) cells and promote antitumor immunity [14-16]. We hypothesize that the combination of MSC/CD with 5-FC and α-GalCer will enhance the anticancer effects by modulating immune cell populations in the tumor microenvironment.
To test our hypothesis, we employed a colon cancer model and analyzed the effects of the combined therapy on tumor growth, immune cell populations, and cytokine levels in the tumor microenvironment. Our results demonstrate that the multi-modal regimen combining α-GalCer and MSC/CD with 5-FC induced DNA damage, induced immune cells, and further inhibited tumor cell proliferation. In addition, killing tumor cells with MSC/CD and 5-FC increases the sensitivity of α-GalCer-induced immune cells to tumors, suppressing tumor cell regrowth following 5-FU depletion. Therefore, based on preclinical evidence, intratumoral administration of MSC/CD and α-GalCer and oral administration of 5-FC during the postoperative period may suppress the growth of residual tumor cells after surgical resection and activate the immune response to solid tumors, significantly improving patient prognosis.
This study contributes new knowledge to the field of cancer immunotherapy and advances our understanding of immune cell modulation in the tumor microenvironment, paving the way for further advancements in cancer management.
Materials and methods
Cell culture and maintenance
The CT26 murine colon carcinoma cell line was purchased from Korean Cell Line Bank (Seoul, Korea). The Lenti-X 293T cell line was obtained from Takara Bio Inc. (Shiga, Japan). All experiments involving human MSCs were approved by the Institutional Review Board of Ajou University Medical Center (Suwon, South Korea). The MSCs used in this study were sourced and engineered to express the CD transgene as detailed in our previous work [9,17,18]. In those studies, we demonstrated the level of CD transgene expression and the efficacy of these MSCs in converting 5-FC to 5-FU. The MSC/CD system used in this study is currently being evaluated in clinical trials for brain tumor treatment (NCT04657315), which indicates its preclinically secured level of safety. The MSCs were utilized under conditions that minimize the risk of tumorigenicity.
To label CT26 cells with green fluorescent protein (GFP), we transduced them with a lentivirus vector expressing GFP. GFP-expressing CT26 cells were selected using a BD FACSAria III cell sorter (BD, Franklin Lakes, NJ, USA). We maintained the CT26, CT26-GFP, Lenti-X 293T, and MSC/CD cell lines in high glucose Dulbecco’s Modified Eagle Medium (Welgene, Gyeongsan, South Korea), supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Gibco). The MSC/CD culture was further supplemented with 20 ng/mL bFGF (Peprotech, Cranbury, NJ, USA). All cells were incubated with 5% CO2 at 37°C.
Reagent preparation
5-Fluorouracil (5-FU) was purchased from Sigma-Aldrich (St. Louis, MO, USA) for in vitro experiments, while 5-FC was obtained from Nantong Jinghua Pharmaceutical Co., Ltd. (Nantong, Jiangsu, China) for in vivo experiments. α-GalCer was acquired from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). A stock solution of α-GalCer (1 mg/mL) was prepared by dissolving it in 100% dimethyl sulfoxide (DMSO, Duksan, Ansan, South Korea) and the solution was heated at 80°C for 2 hours while shaking.
5-FU sensitivity test
CT26 and CT26-GFP were seeded in 24-well plates at 5 × 103 cells per well. After 24 hours, the indicated 5-FU concentrations were added and replaced with fresh growth medium containing 5-FU every other day. Viable cells were measured for cytotoxicity using the MTT assay. Briefly, a 5 mg/mL MTT solution is diluted to 10% in medium, 500 μL is added to each well and reacted for 4 hours. After adding 100% DMSO and reacting sufficiently, O.D. was measured at 540 nm.
Bystander effect test
Two bystander effect tests were performed. In the first, MSC/CD and CT26-GFP were co-cultured in a 1:1 ratio (with 1 × 104 MSC/CD cells per well) in a 12-well plate. After 24 hours, the indicated 5-FC concentration was added and replaced with fresh growth medium containing 5-FC every other day. The surviving cells were lysed in passive lysis buffer (Promega, Madison, WI, USA), and cell lysate fluorescence was measured at 485 nm/535 nm using a SpectraMax iD3 fluorescence meter (Molecular Devices, San Jose, CA, USA). In the second test, MSC/CD and CT26-GFP were co-cultured at the indicated ratio in a 12-well plate. After 24 hours, 1 mM 5-FC was added and replaced with fresh growth medium containing 5-FC every other day. CT26-GFP cell images were acquired on the 2nd and 4th days using an inverted IX71 microscope (Olympus, Tokyo, Japan). The surviving cells were measured in the same way as the first test, using passive lysis buffer.
Tumor model and evaluation of therapeutic efficacy in vivo
Male BALB/c mice aged 7-8 weeks were purchased from Orient Bio (Seongnam, South Korea) and housed in a temperature- and humidity-controlled room with a 12-hour light/dark cycle. All animal care and experiments were conducted following the guidelines of the Animal Care Committee of Ajou University School of Medicine.
To establish the tumor model, CT26 cells (2 × 106) were implanted subcutaneously on the left side of flank containing 20% Matrigel (Corning, Corning, NY, USA). When tumors reached 80 to 120 mm3 (usually 7-8 days post-implantation), mice were randomly grouped so that the average tumor size was similar, and treatment commenced.
For the administration of MSCs/CD, we employed intratumoral injection. While it is known that MSCs/CD can migrate to tumor sites in vivo, direct intratumoral injection was chosen to ensure the presence of a high concentration of therapeutic cells at the tumor site from the onset of treatment. This is particularly relevant given the heterogeneity of tumor microenvironments and potential barriers to cell migration.
The first group, the control group, was treated with intratumoral injections of PBS, along with oral administration of 2% methylcellulose as a placebo. The second group received intratumoral injections of α-GalCer (120 μg/kg) on Day 7 and additional α-GalCer was administered on Days 11 and 13. They also received oral administration of 2% methylcellulose as a placebo. The third group was treated with intratumoral injections of MSC/CD cells (1 × 106) on Day 7, and from the next day, these mice received oral administration of 5-FC (900 mg/kg) twice a day for three days at 6-9 hour intervals. The fourth group was treated similarly to the third group, with the addition of α-GalCer (120 μg/kg) on Day 7, and additional α-GalCer was administered on Days 11 and 13.
Tumor size in mice was measured every 2 to 3 days, and tumor volume was calculated using the formula: V = π × (Length × Width2)/6. Mice were considered dead when the tumor size exceeded 3,000 mm3.
Measurement of in vivo cytokines
For cytokine analysis, tumors were collected from the mice on days 8 (prior to 5-FC administration) and 12. The tumors were rapidly frozen on dry ice and stored at -80°C. Prior to homogenization, tumors were thawed and homogenized with extraction buffer (100 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100, 0.5% Sodium deoxycholate, Protease inhibitor cocktail, 1 mM PMSF) at a concentration of 60 μL/mg. The samples were shaken constantly at 4°C for 2 hours and then centrifuged at 4°C at 18,000 g for 20 minutes. The supernatant was placed into a refrigerated tube and stored at -80°C. Cytokine analysis was performed on LABISKOMA (Seoul, South Korea) using a Luminex system (Luminex, Austin, TX, USA). Intratumoral cytokines were assessed in 4-6 mice, and statistical analysis was conducted.
Measurement of in vivo immune cells
Lymph nodes and tumors were harvested from the mice on days 8 (prior to 5-FC administration) and 12. The harvested tissues were placed in MACS tissue storage solution (Miltenyi Biotec, Bergisch Gladbach, NRW, Germany) and stored at 4°C. Lymph nodes (axillary and inguinal) were isolated from both the tumor-drained (dLN) and non-drained (NdLN) sides of the mouse. The lymph nodes were crushed using homogenizing pestles, passed through a 40 μm cell strainer, and washed before immune cell analysis. Tumors were isolated, trimmed of surrounding fat and skin, and then cut into 2-4 mm3 pieces. A tumor dissociation kit for mice (Miltenyi) was used to dissociate the tumors into single cells using a GentleMACS™ Dissociator (Miltenyi).
Immunophenotypic characterization of the isolated immune cells was performed using an Attune NxT Flow Cytometer (Thermo Fisher Scientific, Waltham, MA, USA). The dissociated cells were washed with flow cytometry staining (FCS) buffer (Thermo Fisher Scientific) and resuspended in 100 μL of FCS buffer. Fc receptors were blocked using CD16/CD32 (Thermo Fisher Scientific) for 10 min at 4°C. The immune cells were then stained with indicated antibody for 30 min in the dark at room temperature. Data were analyzed using Attune NxT software version 3.1.2 (Thermo Fisher Scientific). Markers for each immune cell type are listed in Supplementary Table 2, and antibodies are listed in Supplementary Table 3. Gating for FACS analysis was performed according to Supplementary Figure 4. Immunophenotypic characterization was carried out in a sample of 3-4 mice, and followed by statistical analysis.
Quantification and statistical analysis
Data management and analysis activities were carried out in SigmaPlot software version 12.0 (SYSTAT software Inc., Palo Alto, CA, USA). In vitro results were expressed as mean ± standard deviation (SD), and in vivo results were expressed as mean ± standard error of the mean (SEM). Normal distribution data obtained from the two groups of experiments and immune cell and cytokine experiments were compared by t-test, and non-normal distribution data were analyzed by the Mann-Whitney Rank Sum Test. Tumor size and survival data of different groups were analyzed by one-way analysis of variance (ANOVA) test followed by the Bonferroni test. Statistical significance was assigned at the P<0.05 level.
Result
MSC/CD with 5-FC demonstrates moderate anticancer effects in a colon cancer model
We assessed the bystander effect of MSC/CD on colon cancer cells using a co-culture method, where CT26-GFP cells were prepared using lentivirus expressing GFP. The IC50 value for 5-FU in CT26-GFP cells was found to be similar to that of naïve CT26 cells (Figure 1A), indicating that GFP expression does not alter CT26 cells’ sensitivity to 5-FU. The results demonstrated that 5-FC displayed a concentration-dependent killing of CT26-GFP cells in the MSC/CD bystander effect against CT26-GFP (IC50 value for 5-FC = 30.42 ± 1.17 μM) (Figure 1B). Additionally, the bystander effect of MSC/CD was confirmed by examining the ratio of MSC/CD to CT26-GFP, and the survival rate of CT26-GFP was inversely proportional to the number of MSC/CD cells (Figure 1C, 1D). These findings suggest that the MSC/CD bystander effect relies on the MSC/CD dose, 5-FC concentration, and 5-FC treatment duration.
Figure 1.
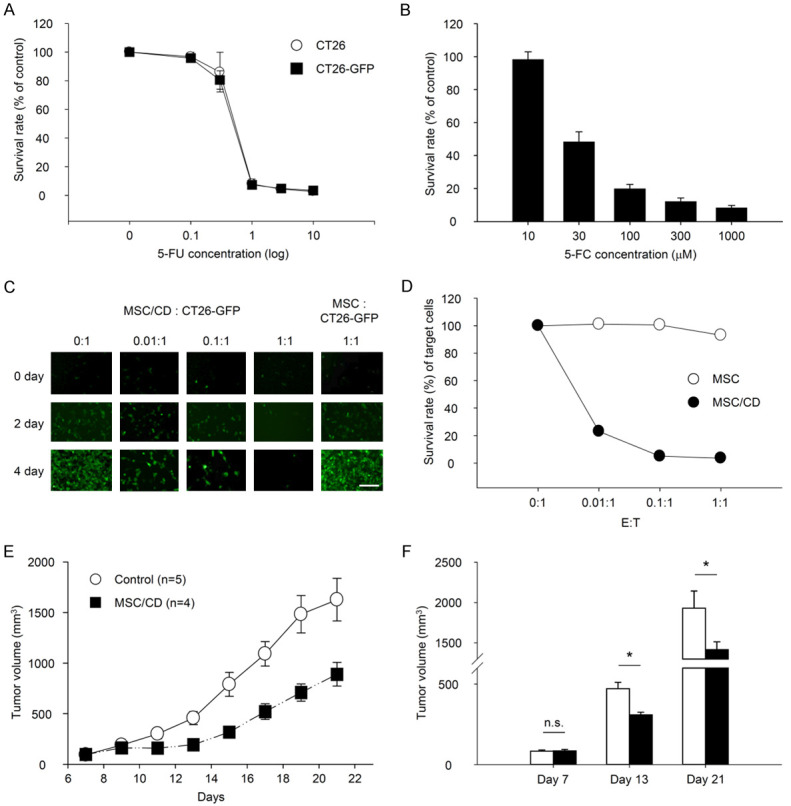
Antitumor effects of MSC/CD and 5-FC in CT26 colon cancer cells in vitro and in vivo. A. CT26 and CT26-GFP cells cultured with various concentrations of 5-FU. B-D. Bystander effect of MSC/CD. B. CT26-GFP co-cultured with MSC/CD and treated with different concentrations of 5-FC. C, D. MSC/CD (E: Effector cells) and CT26-GFP (T: Target cells) co-cultured at various E:T ratios and treated with 5-FC. E, F. CT26 cells inoculated into the left flank of BALB/c mice. After tumor formation, MSC/CD was injected intratumorally, and 5-FC was orally administered. In the control group, PBS was injected instead of MSC/CD, and 2% methylcellulose was orally administered instead of 5-FC. E. Tumor volume over time; F. Tumor volume on days 7, 13, and 21. *P<0.05.
In an in vivo study, a cancer model was created by subcutaneously injecting CT26 cells into mice. MSC/CD with 5-FC administration resulted in weak differences in tumor size compared to controls on days 13 and 21 (Figure 1E, 1F). Additionally, changes in immune cells in the tumor tissue were observed following MSC/CD and 5-FC treatment, leading to a reduction of CD4 T cells and CD8 T cells compared to the control group (data not shown).
Combination of MSC/CD with 5-FC and α-GalCer exhibits enhanced anticancer effects in a colon cancer model
To increase the immune cells in tumors killed by 5-FU, we co-administered MSC/CD containing 5-FC and α-GalCer to a colorectal cancer model (Figure 2A). The combined treatment significantly reduced tumor size and increased survival rate in the colorectal cancer model compared to the control group (Figure 2B-D). This result indicates that the combined administration of 5-FC-containing MSC/CD and α-GalCer has more effective anticancer effects than 5-FC-containing MSC/CD alone. On day 12, a decrease in leukocytes and an increase in neutrophils were observed in the blood of tumor-bearing mice compared to normal mice. When MSC/CD and α-GalCer were co-administered, the number of leukocytes in the blood of tumor-bearing mice increased to levels similar to those in normal mice, and the number of neutrophils also decreased to levels comparable to those in normal mice (Supplementary Table 1).
Figure 2.
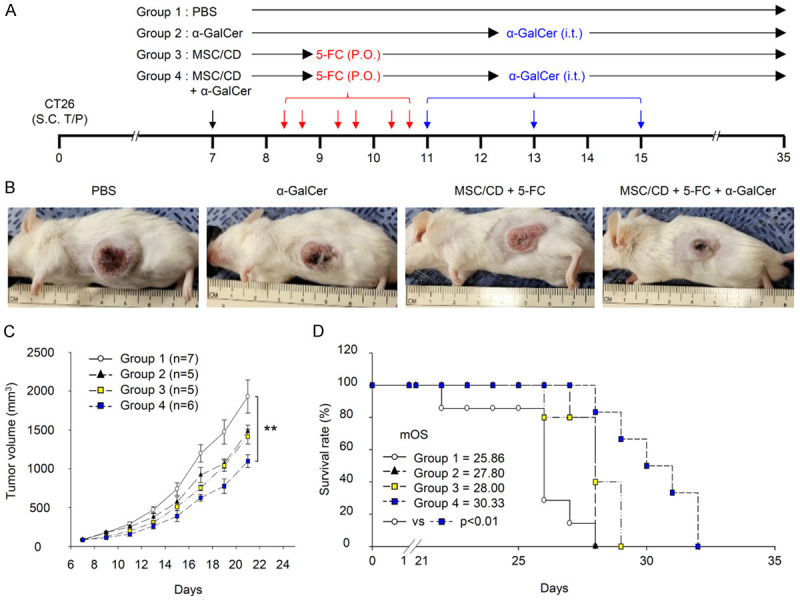
Antitumor effect of combined treatment with MSC/CD, 5-FC, and α-GalCer in CT26 colon cancer model. BALB/c mice with CT26 tumors were randomly divided into four groups (n = 5-7). Group 1 (Control) received PBS and 2% methylcellulose; Group 2 (α-GalCer) received α-GalCer and 2% methylcellulose; Group 3 (MSC/CD + 5-FC) received MSC/CD and 5-FC; Group 4 (Combined) received MSC/CD, 5-FC, and α-GalCer. Treatment schedule is illustrated in (A). A representative image of a tumor-bearing mouse on day 12 is depicted in (B). The change in tumor volume over time is shown in (C), with distinct markers for each treatment group. Kaplan-Meier survival plots are displayed in (D), again with separate lines for each group. **P<0.01. Notably, some mice were sacrificed on day 8 (before 5-FC administration) and day 12 for histological analysis.
Administration of MSC/CD and α-GalCer affects NKT cells and intratumoral cytokines
We investigated the effect of MSC/CD and/or α-GalCer administration on NKT cells in draining lymph nodes and tumors at different times (Figure 3A). It’s important to note that on Day 8, before the administration of 5-FC, the effects observed in Groups 3 (MSC/CD) and 4 (MSC/CD + α-GalCer) were primarily due to the presence of naive MSC or MSC + α-GalCer. The distinct response in Group 4 can be attributed to the synergistic effect of MSC/CD and α-GalCer, which enhances the activation of NKT cells even before the administration of 5-FC. On day 12, following the administration of 5-FC, Group 4 showed an increase in NKT cells in the drained lymph nodes compared to the other groups (Figure 3B). Within the tumor, Group 4 displayed a significantly increased percentage of NKT cells compared to Group 1 on day 8. On day 12, NKT cells decreased in Group 3, where MSC/CD was administered alone; however, this decrease was recovered by the co-administration of α-GalCer in Group 4 (Figure 3C). Intratumoral cytokine levels, specifically interferon-gamma (IFN-γ) and interleukin-2 (IL-2), exhibited varying patterns among the groups. Notably, Group 4 demonstrated higher levels of IFN-γ on day 12 compared to the other groups (Figure 3D, 3E). Interleukin-4 (IL-4) was increased by administration of MSC/CD and 5-FC alone in Group 3 and decreased when α-GalCer was administered together in Group 4, but there was no statistical significance (Figure 3F).
Figure 3.
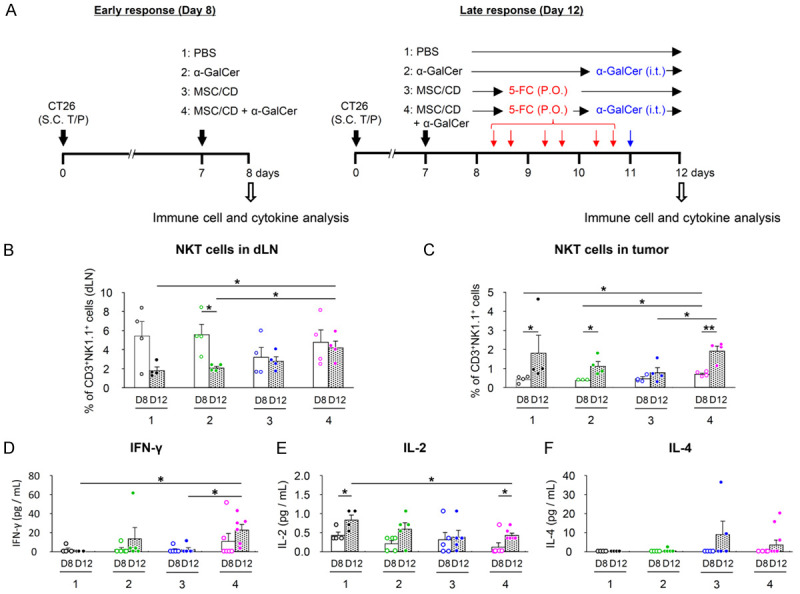
NKT cells and cytokine levels in combined treatment with MSC/CD, 5-FC, and α-GalCer in CT26 colon cancer model. After tumor formation, Groups 1-4 received PBS, α-GalCer, MSC/CD, and MSC/CD with α-GalCer intratumoral injection, respectively. Mice were sacrificed for Day 8 (before 5-FC administration; D8) and Day 12 (D12) analysis. A. In vivo analysis scheme. The percentage of NKT cells in each tissue was analyzed by FACS, gated on CD3+NK1.1+. B. Drained lymph nodes; C. NKT cells in tumors; D-F. Cytokine levels in tumors. Statistical analyses were performed on days 8 and 12, and between days 8 and 12 for each group. *P<0.05, **P<0.01.
Effects of MSC/CD and/or α-GalCer administration on intratumoral antigen-presenting cells (APCs) and cytokine levels
We further explored whether the increase in NKT cells induced by MSC/CD and/or α-GalCer administration was accompanied by an increase in intratumoral APCs, by analyzing the B cells, macrophages, and dendritic cells (DCs) in tumors. On day 8, the B cell ratio tended to increase in Group 2 compared to the control group, but the difference was not statistically significant (Figure 4A). Intratumoral macrophages increased in all groups on day 12, and Group 4 increased significantly more than Groups 2 and 3 (Figure 4B). Within the tumor, Group 4 showed a significant increase in DCs compared to the other groups on day 12 (Figure 4C). Among the intratumoral cytokines analyzed, GM-CSF showed the highest level in Group 2, followed by Group 4 (Figure 4D). Interleukin-12 (IL-12) showed different patterns between groups (Figure 4E).
Figure 4.
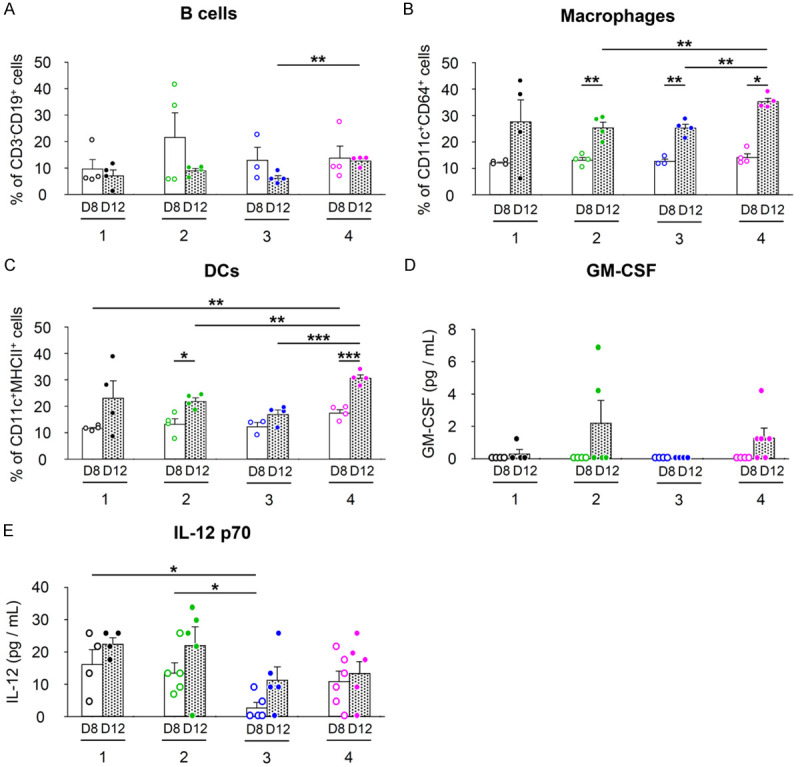
Antigen-presenting cells and cytokine levels in combined treatment with MSC/CD, 5-FC, and α-GalCer in CT26 colon cancer model (Refer to Figure 3A for group treatments and experimental scheme). A-C. Intratumoral APCs measured by FACS. A. CD3-CD19+ B cells; B. CD3-CD11c+CD64+ macrophages; C. CD3-CD11c+MHCII+ DCs. D, E. Intratumoral cytokine levels. D. GM-CSF; E. IL-12 p70. Statistical analyses were performed on D8 and D12, respectively, and between D8 and D12 for each group. *P<0.05, **P<0.01, ***P<0.001.
Effects of MSC/CD and α-GalCer administration on CD4 T cell and CD8 T cell counts and cytotoxicity of these cells
In our study, we confirmed that the administration of MSC/CD and α-GalCer resulted in an increase in NKT cells and APCs. Following this, we investigated the effects on CD4 T cells, CD8 T cells, and their cytotoxicity. In the drained lymph nodes, at day 12, Group 4 showed an increased CD4 T cells compared to the control group (Figure 5A). In the tumor, CD4 T cells in Groups 2 and 3, which were treated with α-GalCer and MSC/CD alone, were significantly reduced compared to Group 1. However, it was restored by co-administration of α-GalCer and MSC/CD (Figure 5B). CD8 T cells in drained lymph nodes were similar for all groups on both days 8 and 12 (Figure 5C). Within the tumor, Group 4 had an increase compared to the other groups on day 8. On day 12, Group 3 treated with MSC/CD had a significant decrease in CD8 T cells, but this decrease was reversed by the combined administration of α-GalCer (Figure 5D).
Figure 5.
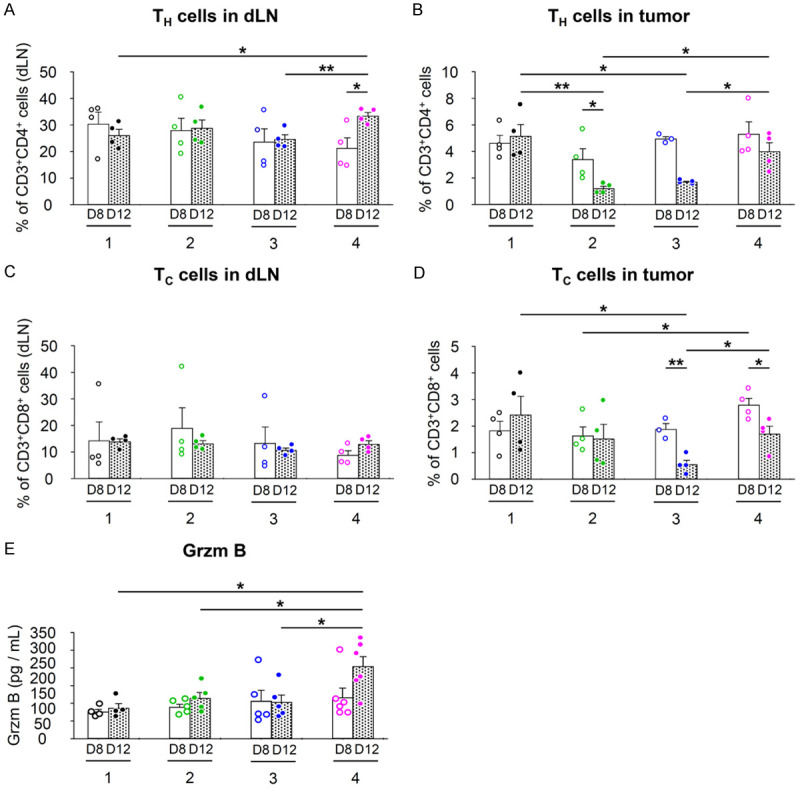
CD4+ and CD8+ T cell response in combined treatment with MSC/CD, 5-FC, and α-GalCer in CT26 colon cancer model (Refer to Figure 3A for group treatments and experimental scheme). T cells in tumors and dLNs were measured by FACS with each corresponding gating. A, C. Drained lymph node analysis; B, D. Tumor analysis. A, B. CD3+CD4+ T cells; C, D. CD3+CD8+ T cells. E. Intratumoral Granzyme B (Grzm B) levels. Statistical analyses were performed on D8 and D12, respectively, and between D8 and D12 for each group. *P<0.05, **P<0.01.
Additionally, the effect on natural killer (NK) cells, which are cytotoxic to tumor cells, was investigated. In drained lymph nodes, Group 4 tended to increase significantly both on day 8 and day 12 (Supplementary Figure 2A). Within the tumor, Group 4 showed a statistically significant increase in NK cells on day 8 compared to Groups 1 and 2. On day 12, Group 2, which received α-GalCer alone, decreased compared to the control group, and this decrease was restored by the combined administration of MSC/CD (Supplementary Figure 2B). Intratumoral Granzyme B levels were significantly higher in Group 4 than in the other groups at day 12 (Figure 5E).
Effects of MSC/CD and α-GalCer administration on immunosuppressive cells and cytokine levels
We investigated the effect of MSC/CD and/or α-GalCer administration on the immunosuppressive cells, regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs), in tumors. The results showed that at day 12, Group 2 tended to decrease the proportion of Tregs within the tumor compared to the control group. Group 4 receiving the combination treatment also showed a tendency to decrease, but the difference was not statistically significant (Figure 6A). On day 12, intratumoral MDSCs tended to increase significantly in Group 2 compared to the control group. In Group 4, it was decreased again by the combined administration of MSC/CD, but higher than the control group (Figure 6B). Intratumoral interleukin-10 (IL-10) and interleukin-13 (IL-13) tended to increase significantly with tumor growth in the control group. However, Groups 2 and 4 did not have increased cytokine levels (Figure 6C, 6D).
Figure 6.
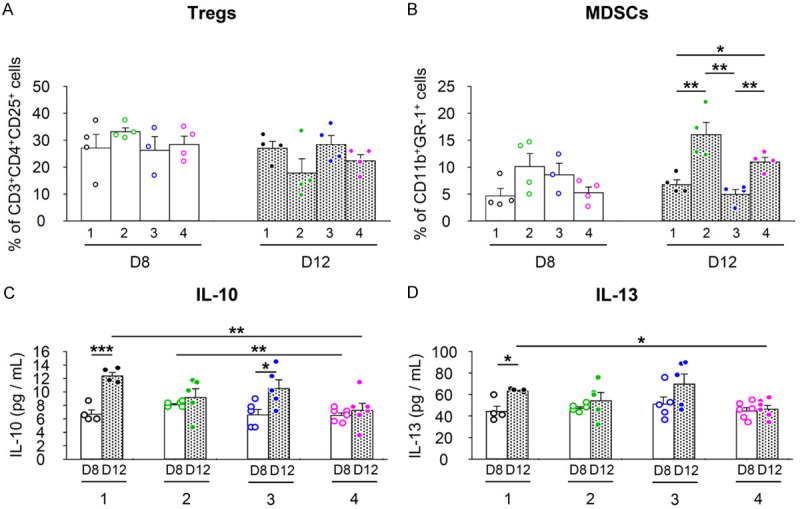
Immunosuppressive cell response and cytokine levels in combined treatment with MSC/CD, 5-FC, and α-GalCer in CT26 colon cancer model (Refer to Figure 3A for group treatments and experimental scheme). A, B. Immunosuppressive cells in tumors were analyzed using FACS. A. CD3+CD4+CD25+ Tregs; B. CD11b+Gr-1+ MDSCs. C, D. Cytokine levels in tumors. C. IL-10; D. IL-13. Statistical analyses were performed on D8 and D12, respectively, and between D8 and D12 for each group. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In this study, we aimed to investigate the anticancer effects of the combined administration of MSC/CD, α-GalCer, and 5-FC in a colon cancer model. Our results demonstrate that combination therapy enhances the anticancer effects by modulating immune cell populations in the tumor microenvironment. The findings contribute new knowledge to the field and have implications for the development of novel therapeutic strategies for colon cancer.
One point of divergence observed in our study is that the combination of MSC/CD and 5-FC treatment was less effective in the colon cancer model compared to the glioma model reported in previous research [9]. This difference may be due to the distinct immune environments of the brain and other tissues, which could affect immune cell infiltration and activation [19]. This highlights the importance of further investigating the role of the immune environment in the therapeutic efficacy of MSC/CD and 5-FC.
Another contrasting finding is the decrease in T cells following the administration of MSC/CD and 5-FC in our study. Previous studies have reported that 5-FU can induce T cells by killing MDSCs [20,21]. However, in our approach, the MSC/CD converts 5-FC into 5-FU within the tumor microenvironment, leading to a local high concentration of 5-FU, which may have different impacts on T cells. These differences could include variations in the mode of 5-FU administration, dosages, or time points examined. A deeper understanding of these differences is crucial for fully comprehending the overall anticancer activity of combination therapy.
Reflecting on our findings, we observed a significant effect of MSC/CD, 5-FC, and α-GalCer on CD4 T cells, CD8 T cells, and NK cells within the tumor microenvironment. Importantly, we observed significantly higher intratumoral granzyme B levels in Group 4 compared to other groups on day 12. This finding might appear to contrast with the numbers of NK and CD8+ T cells in Group 4, which on day 12 were comparable to, or even lower than, those in Group 1. However, it’s important to note that Group 4 had an increase in both CD8 T cells and NK cells compared to the other groups on day 8.
The observed increase in granzyme B on day 12 might therefore be a result of the heightened presence of these cytotoxic cells earlier in the treatment. Granzyme B is a key effector molecule of cytotoxic cells that can remain in the tissue after the cells have decreased in number. As such, the elevated levels of granzyme B on day 12 could be a residual effect of the increased CD8 T cells and NK cells observed on day 8. This hypothesis aligns with the known characteristics of immune responses, which often involve temporal shifts in cellular composition and activity. Further studies are needed to verify this hypothesis and to fully understand the dynamics of immune cells and their effector molecules in response to the combination treatment.
Interestingly, we observed that while there was a significant increase in %CD3+NK1.1+ cells and dendritic cells (DCs) in Group 4 (MSC/CD + α-GalCer) on day 8, this difference was no longer apparent on day 12. Although at first glance this may appear contradictory to the enhanced anti-tumor effects of the combination treatment, it is important to note that the function of immune cells is not solely dictated by their quantity. For instance, even if the number of these cells decreased by day 12, those present earlier may have initiated a robust immune response, leading to enhanced production of effector molecules such as INF-γ. This aligns with our observation of significantly increased INF-γ in Group 4 compared to Group 1 on day 12, despite similar %CD3+NK1.1+ cell levels. Furthermore, the presence of immune cells in the tumor microenvironment can also influence other immune cell types and modulate the overall immune response, which may explain the observed anti-tumor effects despite similar %CD3+NK1.1+ cell and DC counts in Group 4 and Group 1 on day 12. This underscores the complexity and dynamism of immune responses in the tumor microenvironment and warrants further investigation.
To enhance the infiltration of immune cells into tumors eradicated by 5-FU, α-GalCer, an NKT cell augmenting agent, was administered. Previous studies have reported hepatotoxicity associated with α-GalCer [22,23]. Consequently, the potential hepatotoxicity of α-GalCer, based on the intratumoral (i.t.) injection route employed in this study, was investigated. Hepatic fibrosis assessment via trichrome staining and liver function markers, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), revealed no hepatotoxic effects following i.t. injection (Supplementary Figure 1). Thus, concerning α-GalCer’s adverse effects, i.t. administration may be regarded as a more favorable route of administration.
We observed that the administration of α-GalCer alone did not result in increased NKT cells, which is in contrast to what has been reported in previous studies [24,25]. These differences may be due to the immunosuppressive state within the tumors observed in our study [26-28]. Further investigation of the underlying mechanisms responsible for these differences and their potential implications for cancer immunotherapy is essential.
Furthermore, our study observed that co-administration of α-GalCer with MSC/CD and 5-FC led to increased infiltration of immune cells such as NKT cells, APCs, T cells, and NK cells in the tumor microenvironment. This observation aligns with previous studies highlighting the immunostimulatory effects of α-GalCer [25,29]. The increased immune cell infiltration in our study resulted in enhanced therapeutic effects in the colon cancer model, suggesting that the combination of MSC/CD, 5-FC, and α-GalCer could potentially improve the outcomes of colon cancer treatment.
On day 12, a decrease in leukocytes and an increase in neutrophils were observed in the blood of tumor-bearing mice compared to normal mice (Supplementary Table 1). Interestingly, when MSC/CD and α-GalCer were co-administered, the number of leukocytes in the blood of tumor-bearing mice increased to levels similar to those in normal mice, and the number of neutrophils also decreased to levels comparable to those in normal mice. The increase in neutrophils observed in tumor-bearing mice may be indicative of an inflammatory response and the presence of a tumor-promoting environment [30]. In the context of cancer, neutrophils have been reported to exhibit pro-tumorigenic properties, including promoting angiogenesis, tissue remodeling, and suppression of antitumor immunity [31,32]. The observed reduction in neutrophil numbers following the combined treatment suggests a shift towards a more favorable immune environment, which may contribute to the enhanced therapeutic efficacy of the combination therapy. Further investigation into the specific roles of neutrophils in the tumor microenvironment and their modulation by the combined treatment could provide valuable insights for optimizing cancer immunotherapy strategies.
To provide a more comprehensive understanding of our findings, we also examined the expression of various cytokines and chemokines, including IFN-γ, tumor necrosis factor-alpha (TNF-α), and IL-12 in serum. Our results showed that co-treatment with MSC/CD, 5-FC, and α-GalCer increased the expression of cytokines and chemokines related to tumor-promoting immunity as well as antitumor immune response (Supplementary Figure 3). This observation is a result of excessive activation of the immune response of NKT cells and peripheral immune cells [33]. This suggests that immunotherapy can have lethal outcomes, but the absence of these side effects may raise questions about the efficacy of treatment and tumor cell ablation. In addition, our results are advantageous in terms of the stability of immunotherapy, with short symptoms that occurred on day 8 and were not observed on day 12.
Additionally, we evaluated the potential side effects of the combined treatment on healthy tissues and organs, particularly the liver. Our findings suggested that the combined treatment did not induce significant hepatotoxicity, as indicated by the absence of any noticeable histological changes or elevations in serum markers of liver damage.
In summary, our study demonstrated the potential therapeutic benefits of combining MSC/CD and 5-FC with α-GalCer in a colon cancer model. While our findings are consistent with certain aspects of existing literature, there are discrepancies that warrant further investigation. Our study contributes new knowledge to the field of cancer immunotherapy, particularly regarding the immune environment and its impact on therapeutic efficacy. Moreover, our findings emphasize the importance of understanding the role of immune cells in the tumor microenvironment and the underlying mechanisms that govern their activity.
Future studies should aim to elucidate the precise mechanisms behind the discrepancies observed between our results and previous reports, as well as the potential implications of these findings for cancer immunotherapy. Furthermore, additional research is needed to explore the generalizability of our results to other cancer types and the potential applications of our findings in other immunotherapy strategies.
By providing a comprehensive analysis of our results in relation to the originally stated problem, hypotheses, and methods, this discussion allows for a more in-depth understanding of the strengths and limitations of our study. This critical evaluation is an essential step towards advancing the science and art of environmental management and cancer immunotherapy.
Acknowledgements
This research was supported by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (RS-2022-00165974).
Disclosure of conflict of interest
J.J.H., D.Y.C., N.B., and H.S.K. are employees of and stock and/or option holders in Cell&Brain Co., Ltd. The other authors have no potential conflicts of interest to disclose.
Supporting Information
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Silen W. Hepatic resection for metastases from colorectal carcinoma is of dubious value. Arch Surg. 1989;124:1021–1022. doi: 10.1001/archsurg.1989.01410090027004. [DOI] [PubMed] [Google Scholar]
- 3.Tandan VR, Harmantas A, Gallinger S. Long-term survival after hepatic cryosurgery versus surgical resection for metastatic colorectal carcinoma: a critical review of the literature. Can J Surg. 1997;40:175–181. [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS, Sung YC. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011;18:488–495. doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossignoli F, Spano C, Grisendi G, Foppiani EM, Golinelli G, Mastrolia I, Bestagno M, Candini O, Petrachi T, Recchia A, Miselli F, Rovesti G, Orsi G, Veronesi E, Medici G, Petocchi B, Pinelli M, Horwitz EM, Conte P, Dominici M. MSC-delivered soluble TRAIL and paclitaxel as novel combinatory treatment for pancreatic adenocarcinoma. Theranostics. 2019;9:436–448. doi: 10.7150/thno.27576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang DY, Yoo SW, Hong Y, Kim S, Kim SJ, Yoon SH, Cho KG, Paek SH, Lee YD, Kim SS, Suh-Kim H. The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer. 2010;127:1975–1983. doi: 10.1002/ijc.25383. [DOI] [PubMed] [Google Scholar]
- 10.Chang DY, Jung JH, Kim AA, Marasini S, Lee YJ, Paek SH, Kim SS, Suh-Kim H. Combined effects of mesenchymal stem cells carrying cytosine deaminase gene with 5-fluorocytosine and temozolomide in orthotopic glioma model. Am J Cancer Res. 2020;10:1429–1441. [PMC free article] [PubMed] [Google Scholar]
- 11.Baitsch L, Fuertes-Marraco SA, Legat A, Meyer C, Speiser DE. The three main stumbling blocks for anticancer T cells. Trends Immunol. 2012;33:364–372. doi: 10.1016/j.it.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Mayer A, Vaupel P. Hypoxia, lactate accumulation, and acidosis: siblings or accomplices driving tumor progression and resistance to therapy? Adv Exp Med Biol. 2013;789:203–209. doi: 10.1007/978-1-4614-7411-1_28. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa Y, Godfrey DI, Smyth MJ. Alpha-galactosylceramide: potential immunomodulatory activity and future application. Curr Med Chem. 2004;11:241–252. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa Y, Rovero S, Forni G, Smyth MJ. Alpha-galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:9464–9469. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 17.Jung JH, Kim AA, Chang DY, Park YR, Suh-Kim H, Kim SS. Three-dimensional assessment of bystander effects of mesenchymal stem cells carrying a cytosine deaminase gene on glioma cells. Am J Cancer Res. 2015;5:2686–2696. [PMC free article] [PubMed] [Google Scholar]
- 18.Park JS, Chang DY, Kim JH, Jung JH, Park J, Kim SH, Lee YD, Kim SS, Suh-Kim H. Retrovirus-mediated transduction of a cytosine deaminase gene preserves the stemness of mesenchymal stem cells. Exp Mol Med. 2013;45:e10. doi: 10.1038/emm.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Zhang M, Zhang Y, Liu K, Lu C. 5-fluorouracil suppresses colon tumor through activating the p53-fas pathway to sensitize myeloid-derived suppressor cells to FasL(+) cytotoxic T lymphocyte cytotoxicity. Cancers (Basel) 2023;15:1563. doi: 10.3390/cancers15051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biburger M, Tiegs G. Alpha-galactosylceramide-induced liver injury in mice is mediated by TNF-alpha but independent of Kupffer cells. J Immunol. 2005;175:1540–1550. doi: 10.4049/jimmunol.175.3.1540. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa H, Yamashita K, Otsubo D, Kakeji Y. Liver injury after invariant NKT cell activation by free alpha-galactosylceramide and alpha-galactosylceramide-loaded dendritic cells. Anticancer Res. 2016;36:3667–3672. [PubMed] [Google Scholar]
- 24.Nagaraj S, Ziske C, Strehl J, Messmer D, Sauerbruch T, Schmidt-Wolf IG. Dendritic cells pulsed with alpha-galactosylceramide induce anti-tumor immunity against pancreatic cancer in vivo. Int Immunol. 2006;18:1279–1283. doi: 10.1093/intimm/dxl059. [DOI] [PubMed] [Google Scholar]
- 25.Bae EA, Seo H, Kim IK, Jeon I, Kang CY. Roles of NKT cells in cancer immunotherapy. Arch Pharm Res. 2019;42:543–548. doi: 10.1007/s12272-019-01139-8. [DOI] [PubMed] [Google Scholar]
- 26.Wittke F, Hoffmann R, Buer J, Dallmann I, Oevermann K, Sel S, Wandert T, Ganser A, Atzpodien J. Interleukin 10 (IL-10): an immunosuppressive factor and independent predictor in patients with metastatic renal cell carcinoma. Br J Cancer. 1999;79:1182–1184. doi: 10.1038/sj.bjc.6690189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Song X, Traub B, Luxenhofer M, Kornmann M. Involvement of IL-4, IL-13 and their receptors in pancreatic cancer. Int J Mol Sci. 2021;22:2998. doi: 10.3390/ijms22062998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirlekar B. Tumor promoting roles of IL-10, TGF-beta, IL-4, and IL-35: its implications in cancer immunotherapy. SAGE Open Med. 2022;10:20503121211069012. doi: 10.1177/20503121211069012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King LA, Lameris R, de Gruijl TD, van der Vliet HJ. CD1d-invariant natural killer T cell-based cancer immunotherapy: alpha-galactosylceramide and beyond. Front Immunol. 2018;9:1519. doi: 10.3389/fimmu.2018.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 31.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 32.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


