Abstract
Recovery of CD4-positive T lymphocyte count after initiation of antiretroviral therapy (ART) has been thoroughly examined among people with human immunodeficiency virus infection. However, immunological response after restart of ART following care interruption is less well studied. We compared CD4 cell-count trends before disengagement from care and after ART reinitiation. Data were obtained from the East Africa International Epidemiology Databases to Evaluate AIDS (IeDEA) Collaboration (2001–2011; n = 62,534). CD4 cell-count trends before disengagement, during disengagement, and after ART reinitiation were simultaneously estimated through a linear mixed model with 2 subject-specific knots placed at the times of disengagement and treatment reinitiation. We also estimated CD4 trends conditional on the baseline CD4 value. A total of 10,961 patients returned to care after disengagement from care, with the median gap in care being 2.7 (interquartile range, 2.1–5.4) months. Our model showed that CD4 cell-count increases after ART reinitiation were much slower than those before disengagement. Assuming that disengagement from care occurred 12 months after ART initiation and a 3-month treatment gap, CD4 counts measured at 3 years since ART initiation would be lower by 36.5 cells/μL than those obtained under no disengagement. Given that poorer CD4 restoration is associated with increased mortality/morbidity, specific interventions targeted at better retention in care are urgently required.
Keywords: antiretroviral therapy, CD4 cell count, disengagement from care, HIV, human immunodeficiency virus, linear mixed models, reengagement in care, treatment interruption
Abbreviations
- AIC
Akaike information criterion
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- CI
confidence interval
- HIV
human immunodeficiency virus
- IeDEA
East Africa International Epidemiology Databases to Evaluate AIDS
- LMM
linear mixed model
- PWH
people with HIV
In human immunodeficiency virus (HIV) research, various biomarkers have been used to track disease progression. Among them, the number of CD4-positive T lymphocytes is an important predictor of clinical outcomes (1), and it is monitored over time to evaluate the progress of the disease and the state of the patient’s immune system (1). In the absence of antiretroviral therapy (ART), following HIV infection the number of CD4 cells declines over time, though the patient-specific trajectories can vary substantially (2). The introduction of combination ART in 1996 has allowed long-term management of HIV, greatly improving the life expectancy and quality of life of people with HIV (PWH).
CD4 cell-count recovery after ART initiation has been thoroughly examined previously (3–5). With treatment adherence, a rapid increase in CD4 cell count occurs, followed by a slower increase thereafter. However, a substantial proportion of patients disengages from HIV care (6–8). These patients experience losses in CD4 count, are more likely to experience adverse clinical outcomes, and can transmit HIV to the community (9). A significant proportion of the patients who have disengaged from care reengage in care in cyclical patterns of disengagement from and reengagement in care (patient “churn” (10–13)).
Unlike CD4 restoration after initial use of ART, the consequences after disengagement from care and, in particular, the CD4 response after ART reinitiation have not been fully investigated. Many studies have examined the impact of planned (14–18) and unplanned (9, 19–23) ART interruptions, showing that both are associated with increased mortality and morbidity and slower CD4 recovery. Fewer studies, though, have investigated the immunological consequences of unplanned care interruption, particularly in resource-constrained settings (24). In such settings, HIV care is often combined with nutrition and general support in a holistic approach reaching beyond treatment supplementation. Given that poorer CD4 restoration may lead to increased mortality/morbidity even among those who reengage in care (22), comparison of the potential restoration of CD4 counts after reinitiation of ART with the CD4 increases observed after first initiation of ART (and before disengagement from care) is essential.
In this paper, we focus on PWH who return to care after transient disengagement from care, and we compare the CD4 cell-count trajectories observed after reinitiation of ART with the corresponding trajectories measured before disengagement from care. We hypothesized that the rate of CD4 increase would be slower after ART reinitiation than after initial use of ART, as previous studies have suggested (21). To formally examine this hypothesis, we used a unified model for CD4 cell counts during first initiation of ART, disengagement from care, and ART reinitiation. Modeling of these CD4 trajectories is critical in evaluating response to care at the individual level but also in assessing programmatic outcomes at the population level. In the era of universal treatment for PWH (“treat-all” (25)), the emphasis of current treatment protocols is on monitoring viral suppression, which minimizes onward transmission and is associated with better prognosis at the individual level, rather than on immune reconstitution; this has led to longitudinal CD4 data’s becoming more and more sparse. From this perspective, the present paper is the last best effort to address the issue of CD4 increase and, more importantly, recovery after ART reinitiation.
METHODS
Study population
Data were obtained from the East Africa International Epidemiology Databases to Evaluate AIDS (IeDEA) Collaboration (26), a network of HIV care and treatment programs in Kenya, Uganda, and Tanzania which routinely collects clinical and sociodemographic data using an electronic medical record system. Eligible for this study were persons who had initiated ART at ≥18 years of age, had disengaged from and subsequently reengaged in care, and had at least 1 observed CD4 cell-count measurement at/after (first) ART initiation from 2001 to 2011. Typically, monthly participants’ visits are scheduled with 1- to 3-month ART prescriptions, whereas CD4 cell counts are measured biannually. The date of disengagement from care was defined as the midpoint between the date of the last visit before disengagement from care and 2 months after the date of the next scheduled visit. Thus, the working definition of disengagement from care was no clinic visit for 3 months, as 3 months was the maximum amount of time patients could access ART without clinic visits. The study was approved under both the Indiana University Institutional Review Board in the United States (Indianapolis, Indiana) and the Institutional Research and Ethics Committee of the Academic Model to Provide Access to Healthcare (AMPATH) in Kenya (Moi University School of Medicine, Eldoret, Kenya).
Combined model of CD4 evolution
CD4 data before and during disengagement from care and after ART reinitiation were modeled using a linear mixed model (LMM) (27) with 2 subject-specific knots placed at the times of disengagement from care and treatment reinitiation, respectively, since CD4 trends are drastically affected by these events. Because the distribution of CD4 cell counts is heavily right-skewed, square-root transformation was applied. Let 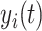 be the observed square-root–transformed CD4 cell count of the
be the observed square-root–transformed CD4 cell count of the 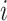 th patient at
th patient at 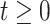 years since ART initiation. The knots are incorporated into the model by defining
years since ART initiation. The knots are incorporated into the model by defining 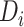 and
and 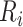 to be the subject-specific times from ART initiation to care disengagement and treatment reinitiation, respectively. Note that
to be the subject-specific times from ART initiation to care disengagement and treatment reinitiation, respectively. Note that 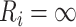 if the ith patient never restarted ART. The full structure of the model is
if the ith patient never restarted ART. The full structure of the model is
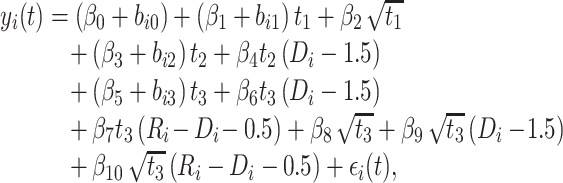 |
(1) |
where
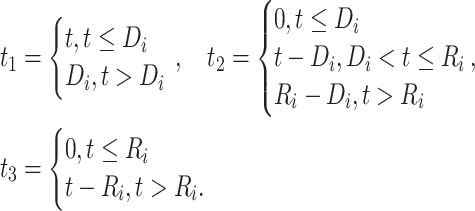 |
(2) |
The square root of t (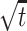 ) is included in the model to capture the nonlinear evolution of CD4 cell counts;
) is included in the model to capture the nonlinear evolution of CD4 cell counts; 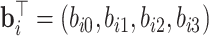 denotes the random effects (
denotes the random effects (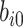 = random intercept at ART initiation and bi1, bi2, and bi3 = random slopes before care disengagement, during disengagement, and after ART reinitiation, respectively), which are assumed to follow the multivariate normal distribution with mean 0 and unstructured covariance matrix
= random intercept at ART initiation and bi1, bi2, and bi3 = random slopes before care disengagement, during disengagement, and after ART reinitiation, respectively), which are assumed to follow the multivariate normal distribution with mean 0 and unstructured covariance matrix 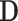 (i.e.,
(i.e., 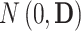 ); and
); and 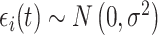 denotes the within-subject error. Thus, correlation in the data is modeled through the random effects, whereas
denotes the within-subject error. Thus, correlation in the data is modeled through the random effects, whereas  captures the variability that is unexplained by the model. The population-averaged evolution of CD4 cell count (on the square root scale),
captures the variability that is unexplained by the model. The population-averaged evolution of CD4 cell count (on the square root scale), 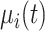 , and the corresponding rate of change before disengagement (i.e.,
, and the corresponding rate of change before disengagement (i.e., 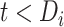 ) are equal to
) are equal to 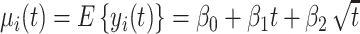 and
and 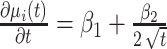 , respectively. The corresponding CD4 evolution during the period between disengagement from care and ART reinitiation (i.e.,
, respectively. The corresponding CD4 evolution during the period between disengagement from care and ART reinitiation (i.e., 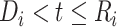 ) is equal to
) is equal to 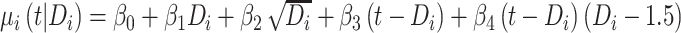 , which is linear in t, with the corresponding rate of change equal to
, which is linear in t, with the corresponding rate of change equal to 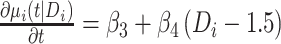 . Here, times from ART initiation to care disengagement have been centered at
. Here, times from ART initiation to care disengagement have been centered at 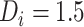 years, which is close to the corresponding mean time in our application. Note that the rate of CD4 decline during disengagement is allowed to depend on
years, which is close to the corresponding mean time in our application. Note that the rate of CD4 decline during disengagement is allowed to depend on 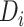 , as CD4 decline may be faster at higher CD4 counts (corresponding in general to longer times from treatment initiation to care disengagement), with
, as CD4 decline may be faster at higher CD4 counts (corresponding in general to longer times from treatment initiation to care disengagement), with 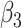 denoting the CD4 decline at 1.5 years since ART initiation (
denoting the CD4 decline at 1.5 years since ART initiation (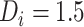 ). Because CD4 data between disengagement from care and reengagement in care are unavailable, the model for the CD4 decline is actually informed by the last CD4 measurement taken before disengagement and all available CD4 measurements taken from reengagement in care to ART reinitiation. CD4 evolution after ART reinitiation (i.e.,
). Because CD4 data between disengagement from care and reengagement in care are unavailable, the model for the CD4 decline is actually informed by the last CD4 measurement taken before disengagement and all available CD4 measurements taken from reengagement in care to ART reinitiation. CD4 evolution after ART reinitiation (i.e., 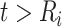 ) is of the form
) is of the form
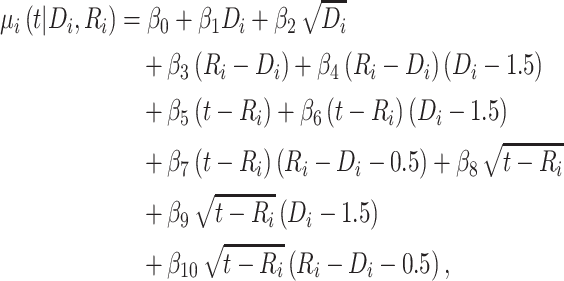 |
(3) |
since 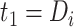 and
and 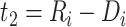 for
for 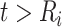 . The corresponding population-averaged slope is
. The corresponding population-averaged slope is 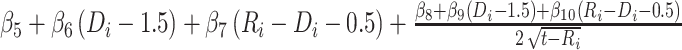 .
In the above equation, we have also centered the time between care disengagement and ART reinitiation
.
In the above equation, we have also centered the time between care disengagement and ART reinitiation 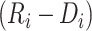 at 0.5 years, which approximates the average duration of the treatment gap in our application. Patients who never restarted ART after reinitiating care contribute to the linear model between disengagement from care and ART restart but do not contribute to the model of CD4 reconstitution after ART restart.
at 0.5 years, which approximates the average duration of the treatment gap in our application. Patients who never restarted ART after reinitiating care contribute to the linear model between disengagement from care and ART restart but do not contribute to the model of CD4 reconstitution after ART restart.
The model, including interaction terms, allows the rate of increase after ART reinitiation to depend on both 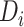 and
and 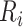 , which serves as a proxy for conditioning on CD4 counts at treatment reinitiation. In the main analysis, all available CD4 measurements at/after reengagement in care were used, ignoring any subsequent disengagement from care. As a sensitivity analysis, however, data were right-censored at the time of the second disengagement.
, which serves as a proxy for conditioning on CD4 counts at treatment reinitiation. In the main analysis, all available CD4 measurements at/after reengagement in care were used, ignoring any subsequent disengagement from care. As a sensitivity analysis, however, data were right-censored at the time of the second disengagement.
Comparing CD4 trends before care disengagement and after ART reinitiation
The rate of CD4 increase after ART (re)initiation depends heavily on the baseline CD4 value (i.e., CD4 cell count at ART (re)initiation) (4, 5). Thus, to compare the CD4 trends before care disengagement with the corresponding ones after ART restart, conditioning on the baseline CD4 value is needed.
In the literature on LMMs, the focus of inference is often on the value 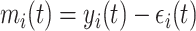 , which is the individually predicted mean (square root) CD4 cell count.
, which is the individually predicted mean (square root) CD4 cell count. 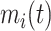 can be factorized as
can be factorized as
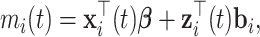 |
(4) |
where 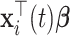 denotes the fixed-effect (i.e., population-averaged) part and
denotes the fixed-effect (i.e., population-averaged) part and 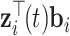 denotes the patient-specific random-effect contribution.
denotes the patient-specific random-effect contribution.
We extended the approach proposed by Harrison et al. (28) to compare the CD4 trends before care disengagement and after ART restart conditioning on the baseline CD4 intervals (categories): ≤50, >50 but ≤100, >100 but ≤200, >200 but ≤250, >250 but ≤350, >350 but ≤500, and >500 cells/μL. That is, we compared the CD4 cell-count evolution before disengagement from care (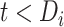 ), given a baseline CD4 category (e.g.,
), given a baseline CD4 category (e.g., 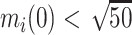 ), with the corresponding evolution after ART reinitiation, conditional on the same CD4 category at treatment reinitiation—for example,
), with the corresponding evolution after ART reinitiation, conditional on the same CD4 category at treatment reinitiation—for example,
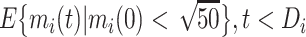 |
(5) |
and
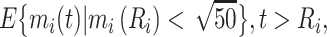 |
(6) |
respectively. It is straightforward to show that, conditional on 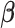 and
and 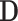 ,
, 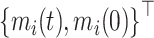 follows the bivariate normal distribution with means
follows the bivariate normal distribution with means 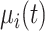 and
and 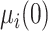 and variances
and variances 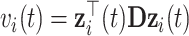 and
and 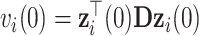 , respectively, and covariance between
, respectively, and covariance between 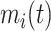 and
and 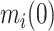 equal to
equal to 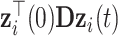 . Such expectations can be calculated using properties of the bivariate normal distribution (29); the formulas are described in detail in the Web Appendix (available at https://doi.org/10.1093/aje/kwad076). Note that this is a postestimation procedure based on the results from the LMM using all relevant data. Calculating corresponding standard errors for equations 5 and 6 is complicated. To obtain 95% confidence intervals (CIs), we relied on a Monte Carlo approach by simulating 1,000 random draws from the large-sample distribution of the estimated parameters and repeatedly applying equations 5 and 6 for all baseline CD4 strata. The 2.5% and 97.5% quantiles of the resulting empirical distribution were used to construct 95% CIs. In addition, at each time point
. Such expectations can be calculated using properties of the bivariate normal distribution (29); the formulas are described in detail in the Web Appendix (available at https://doi.org/10.1093/aje/kwad076). Note that this is a postestimation procedure based on the results from the LMM using all relevant data. Calculating corresponding standard errors for equations 5 and 6 is complicated. To obtain 95% confidence intervals (CIs), we relied on a Monte Carlo approach by simulating 1,000 random draws from the large-sample distribution of the estimated parameters and repeatedly applying equations 5 and 6 for all baseline CD4 strata. The 2.5% and 97.5% quantiles of the resulting empirical distribution were used to construct 95% CIs. In addition, at each time point 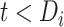 , we pointwise compared the CD4 trends from ART initiation to care disengagement with the corresponding CD4 trends after ART restart (i.e., at time
, we pointwise compared the CD4 trends from ART initiation to care disengagement with the corresponding CD4 trends after ART restart (i.e., at time 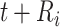 ), conditional on baseline CD4 category; for example, for the first baseline CD4 category, we estimated
), conditional on baseline CD4 category; for example, for the first baseline CD4 category, we estimated 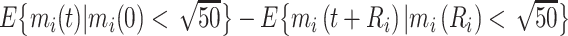 . Ninety-five percent CIs were constructed using the above-mentioned Monte Carlo approach.
. Ninety-five percent CIs were constructed using the above-mentioned Monte Carlo approach.
To further quantify the CD4 cell-count loss due to disengagement from care, we also estimated the amount of time from ART reinitiation needed to reach the CD4 levels that were present at disengagement, 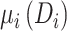 , assuming specific values for
, assuming specific values for 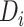 and
and 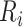 that were representative of our data. That is, we solved the equation
that were representative of our data. That is, we solved the equation 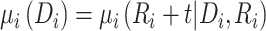 over time t and constructed 95% CIs through the aforementioned Monte Carlo approach.
over time t and constructed 95% CIs through the aforementioned Monte Carlo approach.
The appropriateness of the parametric form assumed for the CD4 evolution was assessed by refitting the LMM using a more elaborate structure involving natural splines of time with 3 internal knots at 0.5, 1, and 2 years since ART (re)initiation. The fit of the models was evaluated through the Akaike information criterion (AIC).
To investigate the robustness of our findings, we performed an additional sensitivity analysis including only individuals with at least 1 CD4 count at/after both ART initiation and ART reinitiation. Differences between CD4 increases before care disengagement and after ART restart could be partly attributed to a regression-to-the-mean effect. To investigate the potential regression-to-the-mean effect, we compared the estimated CD4 trajectories of our target population before disengagement from care with the corresponding CD4 trajectories of the whole population initiating ART.
RESULTS
Patient characteristics
In total, 62,534 patients initiated ART at ≥18 years of age and had at least 1 observed CD4 cell count after ART initiation. Of these patients, 2,647 (4.2%) died while in care, 27,744 (44.4%) were reported to have disengaged from care, and 32,143 (51.4%) were in care by the end of follow-up.
Of the 27,744 disengaged patients, 10,961 (39.5%) returned to care and comprised the target population for this study. Of those who returned to care, 8,501 (77.6%) had at least 1 observed CD4 measurement at/after return to care. The characteristics of the study population, overall and by availability of CD4 counts after reengagement in care, are provided in Table 1. The median time from ART initiation to disengagement from care was 12.5 (interquartile range, 4.5–25.1) months, and the median time from disengagement to reengagement in care was 2.7 (interquartile range, 2.1–5.4) months. Even though the duration of the gap in care was short, a significant loss in CD4 cell counts was observed, with the median CD4 count at disengagement from care declining from 297 cells/μL to 248 cells/μL at ART restart. Overall, 93.9% of returning patients restarted ART after reengagement in care. Patients who returned to care without observed CD4 measurements had a slightly shorter time from ART initiation to disengagement from care, had a longer time from disengagement to reengagement in care, and were less likely to reinitiate ART upon return to care than those who reengaged in care with observed CD4 counts (Table 1). After reengagement in care, 351 patients (3.2%) died while in care and 5,215 (47.6%) were lost to follow-up on the data-freeze date.
Table 1.
Characteristics of Human Immunodeficiency Virus Patients in the IeDEA Collaboration Who Reengaged in Antiretroviral Therapy, According to Whether Postreengagement CD4 Cell-Count Measurements Were Available, East Africa, 2001–2011a
| Availability of CD4 Cell-Count Data After Reengagement in ART | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | CD4 Data Available(n = 8,501) | CD4 Data Not Available (n = 2,460) | Total (n = 10,961) | P Value | ||||||
| No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | ||
| Sex | 0.883 | |||||||||
| Female | 5,636 | 66.3 | 1,627 | 66.1 | 7,263 | 66.3 | ||||
| Male | 2,865 | 33.7 | 833 | 33.9 | 3,698 | 33.7 | ||||
| Age at disengagement from care, years | 37.8 (32.0–44.7) | 37.6 (31.2–44.7) | 37.8 (31.9–44.7) | 0.017 | ||||||
| CD4 cell count at ART initiation, cells/μL | 139.0 (67.0–208.1) | 138.0 (58.0–217.8) | 139.0 (64.0–210.0) | 0.244 | ||||||
| CD4 cell count available at disengagement | 1,826 | 21.5 | 772 | 31.4 | 2,598 | 23.7 | ||||
| CD4 cell count at disengagement (<2 months prior to last visit before disengagement), cells/μL | 293.5 (175.0–432.8) | 314.0 (136.0–495.2) | 297.0 (166.0–452.8) | 0.040 | ||||||
| CD4 cell-count measurements from reengagement to ART restart availablea | 3,985 | 36.4 | 0 | 0.0 | 3,985 | 36.4 | ||||
| CD4 cell count from reengagement to ART restarta, cells/μL | 248.0 (126.0–412.0) | N/A | 248.0 (126.0–412.0) | |||||||
| Time from ART initiation to disengagement, months | 12.9 (4.9–25.5) | 10.8 (3.4–23.0) | 12.5 (4.5–25.1) | <0.001 | ||||||
| Time from disengagement to reengagement, months | 2.7 (2.1–5.0) | 3.2 (2.2–7.1) | 2.7 (2.1–5.4) | <0.001 | ||||||
| Restart of ART | 8,156 | 95.9 | 2,131 | 86.6 | 10,287 | 93.9 | ||||
| ART restart at reengagement in care | 7,003 | 82.4 | 2,021 | 82.2 | 9,024 | 82.3 | ||||
| Time from disengagement to ART restart, monthsb | 2.9 (2.2–6.0) | 3.5 (2.2–9.6) | 3.0 (2.2–6.6) | <0.001 | ||||||
| No. of available CD4 measurements before disengagement | 2.0 (1.0–4.0) | 2.0 (1.0–3.0) | 2.0 (1.0–4.0) | <0.001 | ||||||
| No. of available CD4 measurements after reengagement | 2.0 (1.0–3.0) | N/A | 2.0 (1.0–3.0) | |||||||
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS; IQR, interquartile range; N/A, not applicable.
a The first available CD4 cell count up to 15 days after ART restart was considered.
b Values are expressed as median survival.
Estimated CD4 trajectories
The parameter estimates from fitting of the LMM are provided in Table 2. The model indicated that CD4 counts declined quickly after treatment interruption, with losses tending to become steeper with longer initial ART duration; CD4 increases after ART reinitiation strongly depended on both the time to disengagement 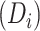 and the duration between disengagement from care and ART reinitiation
and the duration between disengagement from care and ART reinitiation 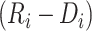 . The estimated CD4 evolution for a typical patient is presented in Figure 1, assuming
. The estimated CD4 evolution for a typical patient is presented in Figure 1, assuming 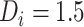 years and
years and 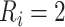 years. To better evaluate the impact of disengagement from care on long-term immunological response, we considered further reasonable scenarios regarding
years. To better evaluate the impact of disengagement from care on long-term immunological response, we considered further reasonable scenarios regarding 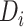 and
and 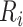 . These are presented in Figure 2. Disengagement, especially when it happened soon after ART initiation, was associated with substantially slower CD4 restoration at 3 years since ART initiation. For example, if disengagement from care occurred 12 months after ART initiation and the duration of the treatment gap was 3 months, CD4 counts at 3 years since ART initiation would be lower by 36.5 cells/μL than the ones that would have been obtained if there was no disengagement. However, a 3-month treatment gap at 3 months after ART initiation was associated with a corresponding CD4 loss at 3 years since ART initiation of 107.2 cells/μL.
. These are presented in Figure 2. Disengagement, especially when it happened soon after ART initiation, was associated with substantially slower CD4 restoration at 3 years since ART initiation. For example, if disengagement from care occurred 12 months after ART initiation and the duration of the treatment gap was 3 months, CD4 counts at 3 years since ART initiation would be lower by 36.5 cells/μL than the ones that would have been obtained if there was no disengagement. However, a 3-month treatment gap at 3 months after ART initiation was associated with a corresponding CD4 loss at 3 years since ART initiation of 107.2 cells/μL.
Table 2.
Parameter Estimates From a Linear Mixed Model Including Subject-Specific Knots at Di and Ri Fitted to the Square Root of CD4 Cell Count, Including Individuals Who Disengaged From and Subsequently Reengaged in Care, IeDEA Collaboration, East Africa, 2001–2011a
| Parameter | Estimate | 95% CI | P Value |
|---|---|---|---|
Intercept 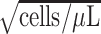
|
12.123 | 12.015, 12.230 | <0.001 |
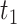 b
b
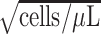 /year
/year |
−1.580 | −1.708, −1.451 | <0.001 |
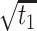
|
7.823 | 7.636, 8.010 | <0.001 |
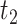 c
c
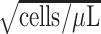 /year
/year |
−3.117 | −3.348, −2.886 | <0.001 |
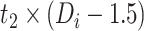
|
−0.236 | −0.435, −0.037 | 0.020 |
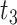 d
d
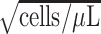 /year
/year |
−0.415 | −0.583, −0.246 | <0.001 |
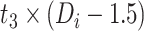
|
−0.215 | −0.358, −0.071 | 0.003 |
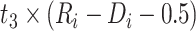
|
−0.724 | −1.059, −0.389 | <0.001 |
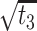
|
2.511 | 2.266, 2.756 | <0.001 |
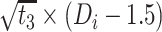
|
−0.007 | −0.204, 0.191 | 0.948 |
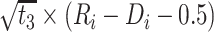
|
2.051 | 1.575, 2.527 | <0.001 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CI, confidence interval; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
a
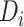 , time (years) from ART initiation to disengagement from care;
, time (years) from ART initiation to disengagement from care; 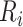 , time (years) from ART initiation to ART reinitiation.
, time (years) from ART initiation to ART reinitiation.
b
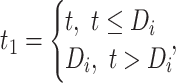 where t denotes time from ART initiation in years.
where t denotes time from ART initiation in years.
c
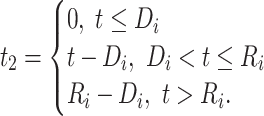
d
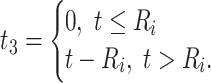
Figure 1.
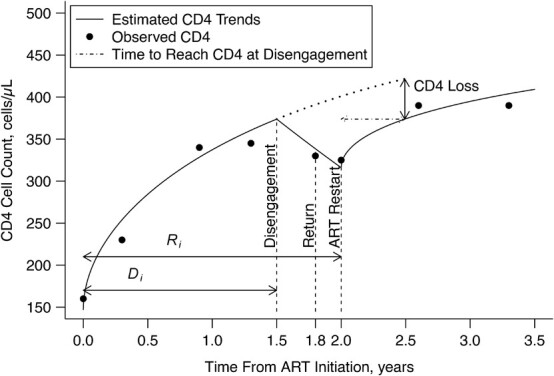
Estimated CD4 cell-count trajectory (based on a linear mixed model including subject-specific knots at time from antiretroviral therapy (ART) initiation to disengagement from care 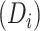 and resumption of treatment
and resumption of treatment 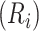 , fitted to data from the IeDEA Collaboration) among individuals who disengaged from and subsequently reengaged in care, East Africa, 2001–2011. Shown is the estimated evolution of CD4 cell count (back-transformed from the square-root scale) assuming that the time from ART initiation to disengagement is
, fitted to data from the IeDEA Collaboration) among individuals who disengaged from and subsequently reengaged in care, East Africa, 2001–2011. Shown is the estimated evolution of CD4 cell count (back-transformed from the square-root scale) assuming that the time from ART initiation to disengagement is 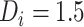 years and the time gap from disengagement from care to ART reinitiation is
years and the time gap from disengagement from care to ART reinitiation is 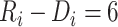 months, along with the observed CD4 cell counts (black dots). Also shown is the amount of time it takes to reach the predisengagement CD4 levels and the difference between the CD4 levels 6 months after treatment reinitiation and the CD4 levels that would have been observed had disengagement not happened by that time. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
months, along with the observed CD4 cell counts (black dots). Also shown is the amount of time it takes to reach the predisengagement CD4 levels and the difference between the CD4 levels 6 months after treatment reinitiation and the CD4 levels that would have been observed had disengagement not happened by that time. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
Figure 2.
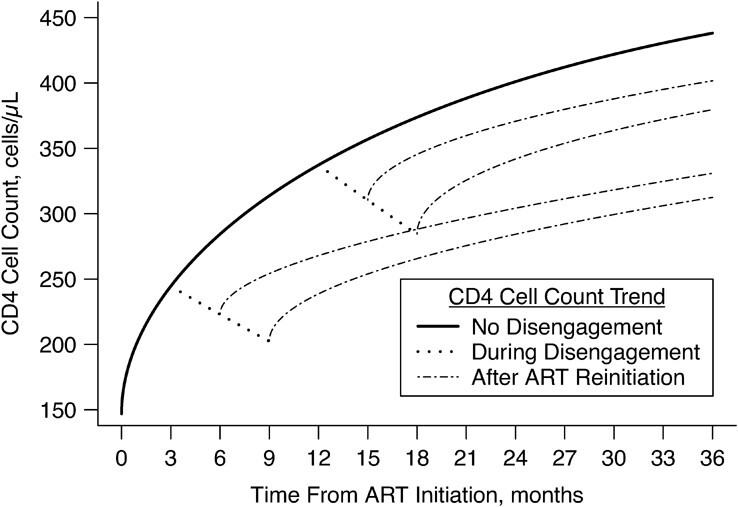
Estimated CD4 cell-count trajectory (based on a linear mixed model including subject-specific knots at time from antiretroviral therapy (ART) initiation to disengagement from care 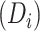 and ART reinitiation
and ART reinitiation 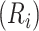 , fitted to data from the IeDEA Collaboration) among individuals who disengaged from and subsequently reengaged in care, East Africa, 2001–2011. It is estimated assuming that
, fitted to data from the IeDEA Collaboration) among individuals who disengaged from and subsequently reengaged in care, East Africa, 2001–2011. It is estimated assuming that 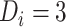 months or
months or 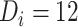 months, with the time gap from disengagement to ART reinitiation being
months, with the time gap from disengagement to ART reinitiation being 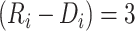 months or
months or 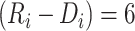 months. For comparison, the estimated CD4 evolution assuming no disengagement (solid line) is also presented. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
months. For comparison, the estimated CD4 evolution assuming no disengagement (solid line) is also presented. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
Although CD4 counts increased rapidly both after ART initiation and after reinitiation following disengagement from care, there was a trend of slower CD4 cell-count recovery after ART reinitiation. We compared the estimated CD4 evolution after the initial start of ART and before disengagement from care with the one after ART reinitiation, conditionally on being in the same baseline CD4 category. The results are presented in Figure 3. Conditionally on each baseline category, the estimated CD4 increase after initial ART initiation was substantially higher than the corresponding one observed after ART reinitiation. The corresponding results when data were right-censored at the time of the second disengagement are provided in Figure 3C. While this analysis led, unsurprisingly, to a higher CD4 cell-count trajectory, in no way did it increase the cell counts to the point of the CD4 curves seen before disengagement. The estimated differences in CD4 counts at each time point, along with the corresponding 95% CIs, are presented in Web Figure 1; most 95% CIs excluded 0.
Figure 3.
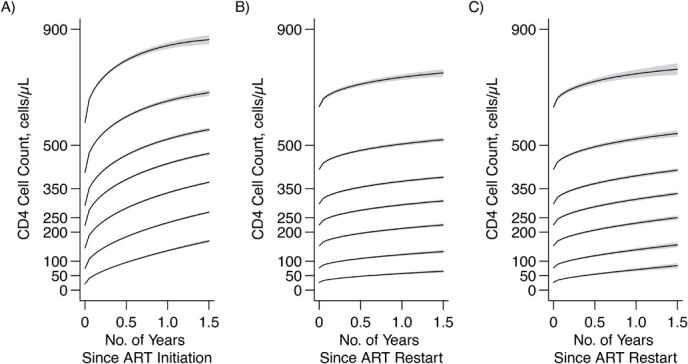
Estimated evolution of CD4 cell count since initiation of antiretroviral therapy (ART) (A) and after reinitiation of ART (B and C) among individuals who disengaged from care and subsequently reengaged in care, conditional on being on the same CD4 category at ART initiation and ART reinitiation, East Africa, 2001–2011. Data from the IeDEA Collaboration were used. B) All CD4 data at/after reengagement in care (ignoring any subsequent disengagement from care); C) censorship of CD4 data at the time of the second disengagement. Shaded regions correspond to the 95% pointwise confidence intervals of the estimated CD4 trajectories. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
Time needed to reach predisengagement CD4 levels
Figure 4 shows the predicted times from ART reinitiation to reaching the CD4 levels recorded at disengagement from care for several reasonable scenarios involving 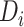 and
and 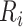 . For example, with time from ART initiation to disengagement
. For example, with time from ART initiation to disengagement 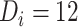 months and a treatment gap of
months and a treatment gap of 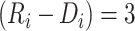 months, the estimated time needed to reach predisengagement CD4 levels was 1.8 months (95% CI: 1.5, 2.2). Despite this robust CD4 recovery after ART reinitiation, we estimated that at 1 year after treatment reinitiation, the CD4 count would be lower by 32.3 cells/μL (95% CI: 26.4, 38.0) than the one that would have been achieved if there had been no disengagement. For a given treatment gap duration (
months, the estimated time needed to reach predisengagement CD4 levels was 1.8 months (95% CI: 1.5, 2.2). Despite this robust CD4 recovery after ART reinitiation, we estimated that at 1 year after treatment reinitiation, the CD4 count would be lower by 32.3 cells/μL (95% CI: 26.4, 38.0) than the one that would have been achieved if there had been no disengagement. For a given treatment gap duration ( ), the time needed to reach the CD4 value recorded at disengagement tended to be longer with longer duration of initial ART
), the time needed to reach the CD4 value recorded at disengagement tended to be longer with longer duration of initial ART 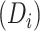 (Figure 4). For example, given a treatment gap of
(Figure 4). For example, given a treatment gap of 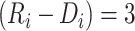 months and a time from ART initiation to disengagement
months and a time from ART initiation to disengagement 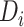 equal to 18 or 24 months, the estimated times needed to reach predisengagement CD4 levels were equal to 2 months (95% CI: 1.7, 2.4) and 2.3 months (95% CI: 1.8, 2.9), respectively. As expected, the time needed to reach CD4 levels at disengagement from care increased with longer treatment gaps; for example, when
equal to 18 or 24 months, the estimated times needed to reach predisengagement CD4 levels were equal to 2 months (95% CI: 1.7, 2.4) and 2.3 months (95% CI: 1.8, 2.9), respectively. As expected, the time needed to reach CD4 levels at disengagement from care increased with longer treatment gaps; for example, when 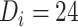 months and
months and 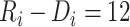 months, the duration of time from ART reinitiation to reaching disengagement CD4 levels was 24.2 months (95% CI: 16.6, 35.2).
months, the duration of time from ART reinitiation to reaching disengagement CD4 levels was 24.2 months (95% CI: 16.6, 35.2).
Figure 4.
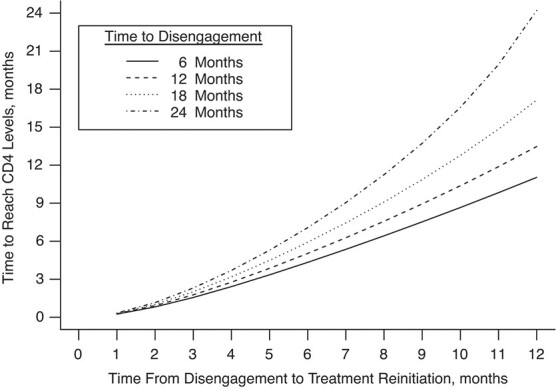
Time needed from reinitiation of antiretroviral therapy (ART) to reaching the CD4 cell count observed at disengagement from care in relation to time from ART initiation to disengagement from care (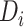 ) and time from disengagement from care to treatment reinitiation (
) and time from disengagement from care to treatment reinitiation ( ), East Africa, 2001–2011. Data from the IeDEA Collaboration were used. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
), East Africa, 2001–2011. Data from the IeDEA Collaboration were used. AIDS, acquired immunodeficiency syndrome; IeDEA, East Africa International Epidemiology Databases to Evaluate AIDS.
Sensitivity analyses
The results from the LMM with natural splines of time showed that our initial parameterization using time and the square root of time had a better fit to the data than the fit based on the natural splines approach. The AIC value obtained using the parameterization in equation 1 was 293,939.6, lower than the AIC value obtained when using natural splines (AIC = 294,658.7).
Results from the sensitivity analysis including individuals with CD4 data both at/after ART initiation and ART reinitiation are provided in Web Figure 2, with conclusions identical to those from Figure 3. A comparison of the estimated CD4 trajectories of our target population with the corresponding trajectories of the whole population initiating ART is provided in Web Figure 3. By visual inspection, it was apparent that our target population had CD4 profiles only slightly better than those of the whole population initiating ART (a 26-cells/μL CD4 difference at 3 years since ART initiation).
DISCUSSION
In this paper, we have proposed a unified approach for jointly modeling 1) CD4 trends before ART disengagement, 2) CD4 decline during disengagement, and 3) CD4 increase after ART reinitiation in a population of PWH who disengaged from and then reengaged in care, using a modified LMM with 2 subject-specific knots placed at the time of disengagement and reinitiation. The structure of the model allowed the rate of CD4 cell-count decline during disengagement to depend on the time from the initial start of treatment to disengagement from care, as CD4 declines may be faster at higher CD4 counts, which are generally expected with longer times to disengagement. Furthermore, the rate of CD4 increase after ART reinitiation was assumed to depend on both the time to disengagement and the treatment gap duration, since these 2 times are closely related to the average CD4 count at ART reinitiation.
In our study, as in many others, a rapid increase in CD4 cell count after ART initiation was observed. Similar to results from studies on treatment interruption (9, 14, 16, 24, 30, 31), we found a steep decline in CD4 counts during disengagement from care. As would be expected from past studies (20, 21), the evolution of CD4 counts after ART resumption was characterized by a very rapid initial increase, followed by a gradually less steep slope. However, there was a tendency toward a slower CD4 recovery compared with that seen after the initial start of ART and before disengagement. These differences became clearer when we conditioned on baseline CD4 levels. This is an important addition to the model, given the large variability in CD4 levels at first ART initiation and ART resumption. Failure to do so muddies the waters when trying to accurately assess the impact of treatment interruption on immune function.
Consistently with the literature (9, 20–22, 24), we showed that even short periods of disengagement from care are associated with substantially worse long-term immunological response, especially when disengagement happens soon after ART initiation. This result reflects the slower CD4 increase upon ART resumption rather than the CD4 loss that occurs during disengagement, since the latter is generally low for short treatment gaps. Moreover, in some cases, the estimated amount of time needed to reach predisengagement CD4 levels was longer than the duration of the gap itself. This is concerning, as it implies that the CD4 decline during disengagement may be steeper than the rate of CD4 gain after ART reinitiation, resulting in long periods until losses are reversed. The finding that the time needed to reach disengagement CD4 levels is longer with longer initial ART duration is attributed to the higher CD4 levels at ART reinitiation, in which cases CD4 cell-count recovery, at least the initial increase, is slower (4, 5, 21).
In this paper, we focused on proposing a unified method with which to model CD4 evolution after ART initiation, during disengagement from care, and after ART reinitiation and on comparing initial and after-ART-resumption CD4 trends conditional on baseline CD4 levels at ART (re)initiation. However, our results are also of clinical relevance, as, despite current guidelines for ART continuity, a number of PWH have had at least a temporary treatment interruption in both rich and limited-resource countries (32). Understanding the effects of such treatment interruptions on the future clinical course is very important at both the individual and population levels, because slower CD4 restoration is associated with higher risks of death and acquired immunodeficiency syndrome (AIDS). In particular, the assumption that patients who disengage from care, even only temporarily, have the same immunological reconstitution upon ART restart as others who remain in care continuously should be revised. Our results also have implications for modeling studies, where an accurate description of CD4 evolution, accounting for noncontinuous care, is important. For example, the Joint United Nations Programme on HIV/AIDS produces various projections for the HIV epidemic worldwide using discrete CD4 categories through Spectrum software (33). Our results may also contribute to current research on analytical treatment interruptions, which are a vital part of HIV cure studies (34–36), although we acknowledge that disengagement from care is clinically different from treatment interruption while in care, because in analytical treatment interruptions PWH are closely monitored.
Our study also had some limitations. First, because this study included only individuals who eventually reengaged in care, our estimate of CD4 cell-count decline during disengagement may not have been representative of the CD4 decline of a whole population disengaging from care; if the probability of reengagement is higher when the rate of CD4 decline is steeper, our model probably overestimated the rate of CD4 loss. Estimation of CD4 trajectories for the whole population initiating ART would require much more complex technical work, such as joint modeling of CD4 levels and competing risks (death while in care and disengagement from care) (37), possibly adjusting for potentially incomplete death ascertainment (38). Second, the slower CD4 recovery after ART reinitiation could have been partly attributed to intermittent adherence. However, we have no reason to believe that the level of compliance would have been substantially different between the predisengagement and postreengagement periods, as our comparison involved the same individuals. In addition, when data were right-censored at the second disengagement, results implied a slightly better CD4 restoration after ART reinitiation compared with that in the main analysis, a finding consistent with the assumption that each treatment interruption has a detrimental effect on CD4 recovery.
Regression to the mean could also partly explain the slower CD4 recovery upon ART resumption. However, in our sensitivity analysis, the difference in the estimated CD4 counts between our target population and the whole population initiating ART was very small, not supporting this hypothesis. Third, in our analysis, we included individuals who disengaged from and subsequently reengaged in care. However, disengaged individuals could have returned to care at a non-IeDEA site. In this work, we assumed that PWH who reengaged at an IeDEA site were representative of the whole population returning to care. Fourth, our estimates may have been sensitive to the limited number of available CD4 measurements taken during ART (re)initiation. Finally, some data collected after reengagement in care were censored due to death or loss to follow-up. Because we used a likelihood-based approach modeling all observed data, our results are valid under the assumption that data were missing at random—that is, the probability of dropping out of the study after return to care depended on the observed CD4 data (39). Whereas this is a reasonable assumption for loss to follow-up, deaths might have been missing “not at random,” requiring joint modeling of CD4 data and mortality. However, the proportion of individuals who died postreengagement was too small to seriously affect our results.
To conclude, this is an important and novel study, becoming increasingly difficult to perform with the decreasing availability of CD4 cell-count data during treatment. We showed that although CD4 counts increase rapidly both after ART initiation and after treatment reinitiation following disengagement, even short periods of disengagement from care and the resulting treatment interruption are associated with slower CD4 cell-count recovery, pointing to the salient advantage of maintaining continuity of care among PWH.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Hygiene, Epidemiology and Medical Statistics, Medical School, National and Kapodistrian University of Athens, Athens, Greece (Christos Thomadakis, Nikos Pantazis, Giota Touloumi); Department of Biostatistics, Fairbanks School of Public Health, Indiana University, Indianapolis, Indiana, United States (Constantin T. Yiannoutsos); Department of Medicine, College of Health Sciences, School of Medicine, Moi University, Eldoret, Kenya (Lameck Diero); Department of Behavioral Science, College of Health Sciences, School of Medicine, Moi University, Eldoret, Kenya (Ann Mwangi); Department of Biostatistics, School of Medicine, Indiana University, Indianapolis, Indiana, United States (Beverly S. Musick); and Department of Medicine, School of Medicine, Indiana University, Indianapolis, Indiana, United States (Kara Wools-Kaloustian).
This research was supported by the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Cancer Institute, and the National Institute of Mental Health in accordance with the regulatory requirements of the US National Institutes of Health, under awards U01AI069911 and R21AI145662. This research was also supported by the Lilly Endowment, Inc. (Indianapolis, Indiana) through its support for the Indiana University Pervasive Technology Institute (Bloomington, Indiana) and by the President’s Emergency Plan for AIDS Relief through the US Agency for International Development (USAID), under the terms of cooperative agreement AID-623-A-12-0001; it was made possible through the joint support of the USAID. The publication of the article in Open Access mode was financially supported by HEAL-Link (https://www.heal-link.gr/en/home-2/).
Data are available from the IeDEA Collaboration (https://www.iedea-ea.org/) and may be obtained after a concept sheet review process.
The content of this article is solely the responsibility of the authors and the Academic Model to Provide Access to Healthcare (AMPATH) and does not necessarily represent the official views of the National Institutes of Health, the USAID, or the US government.
Conflict of interest: none declared.
REFERENCES
- 1. Fahey JL, Taylor JMG, Detels R, et al. . The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322(3):166–172. [DOI] [PubMed] [Google Scholar]
- 2. Pantazis N, Porter K, Costagliola D, et al. . Temporal trends in prognostic markers of HIV-1 virulence and transmissibility: an observational cohort study. Lancet HIV. 2014;1(3):e119–e126. [DOI] [PubMed] [Google Scholar]
- 3. Balestre E, Eholié SP, Lokossue A, et al. . Effect of age on immunological response in the first year of antiretroviral therapy in HIV-1-infected adults in West Africa. AIDS. 2012;26(8):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pantazis N, Papastamopoulos V, Paparizos V, et al. . Long-term evolution of CD4+ cell count in patients under combined antiretroviral therapy. AIDS. 2019;33(10):1645–1655. [DOI] [PubMed] [Google Scholar]
- 5. Stirrup OT, Copas AJ, Phillips AN, et al. . Predictors of CD4 cell recovery following initiation of antiretroviral therapy among HIV-1 positive patients with well-estimated dates of seroconversion. HIV Med. 2018;19(3):184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kariminia A, Law M, Davies M-A, et al. . Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. J Int AIDS Soc. 2018;21(12):e25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakoyannis G, Yu M, Yiannoutsos CT. Semiparametric regression on cumulative incidence function with interval-censored competing risks data. Stat Med. 2017;36(23):3683–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderegg N, Johnson LF, Zaniewski E, et al. . All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up. AIDS. 2017;31(suppl 1):S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011;16(10):1297–1313. [DOI] [PubMed] [Google Scholar]
- 10. Rebeiro P, Althoff KN, Buchacz K, et al. . Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62(3):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakoyannis G, Diero L, Mwangi A, et al. . A semiparametric method for the analysis of outcomes during a gap in HIV care under incomplete outcome ascertainment. Stat Commun Infect Dis. 2020;12(suppl 1):20190013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H, Hogan JW, Genberg BL, et al. . A state transition framework for patient-level modeling of engagement and retention in HIV care using longitudinal cohort data. Stat Med. 2018;37(2):302–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee H, Wu XK, Genberg BL, et al. . Beyond binary retention in HIV care: predictors of the dynamic processes of patient engagement, disengagement, and re-entry into care in a US clinical cohort. AIDS. 2018;32(15):2217–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, et al. . CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296. [DOI] [PubMed] [Google Scholar]
- 15. Imaz A, Olmo M, Peñaranda M, et al. . Short-term and long-term clinical and immunological consequences of stopping antiretroviral therapy in HIV-infected patients with preserved immune function. Antivir Ther. 2013;18(1):125–130. [DOI] [PubMed] [Google Scholar]
- 16. DART Trial Team . Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/μl. AIDS. 2008;22(2):237–247. [DOI] [PubMed] [Google Scholar]
- 17. Palmisano L, Giuliano M, Bucciardini R, et al. . Determinants of virologic and immunologic outcomes in chronically HIV-infected subjects undergoing repeated treatment interruptions: the Istituto Superiore di Sanità-Pulsed Antiretroviral Therapy (ISS-PART) Study. J Acquir Immune Defic Syndr. 2007;46(1):39–47. [PubMed] [Google Scholar]
- 18. Lau JSY, Smith MZ, Lewin SR, et al. . Clinical trials of antiretroviral treatment interruption in HIV-infected individuals. AIDS. 2019;33, 773(5):–791. [DOI] [PubMed] [Google Scholar]
- 19. European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC) Study Group in EuroCoord . CD4 recovery following antiretroviral treatment interruptions in children and adolescents with HIV infection in Europe and Thailand. HIV Med. 2019;20(7):456–472. [DOI] [PubMed] [Google Scholar]
- 20. Mussini C, Touloumi G, Bakoyannis G, et al. . Magnitude and determinants of CD4 recovery after HAART resumption after 1 cycle of treatment interruption. J Acquir Immune Defic Syndr. 2009;52(5):588–594. [DOI] [PubMed] [Google Scholar]
- 21. Touloumi G, Pantazis N, Stirnadel HA, et al. . Rates and determinants of virologic and immunological response to HAART resumption after treatment interruption in HIV-1 clinical practice. J Acquir Immune Defic Syndr. 2008;49(5):492–498. [DOI] [PubMed] [Google Scholar]
- 22. Kaufmann GR, Elzi L, Weber R, et al. . Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. AIDS. 2011;25(4):441–451. [DOI] [PubMed] [Google Scholar]
- 23. Teklu AM, Yirdaw KD. Patients who restart antiretroviral medication after interruption remain at high risk of unfavorable outcomes in Ethiopia. BMC Health Serv Res. 2017;17(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahonkhai AA, Adeola J, Banigbe B, et al. . Impact of unplanned care interruption on CD4 response early after ART initiation in a Nigerian cohort. J Int Assoc Provid AIDS Care. 2016;16(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brazier E, Maruri F, Duda SN, et al. . Implementation of “treat-all” at adult HIV care and treatment sites in the Global IeDEA Consortium: results from the site assessment survey. J Int AIDS Soc. 2019;22(7):e25331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Camlin CS, Neilands TB, Odeny TA, et al. . Patient-reported factors associated with reengagement among HIV-infected patients disengaged from care in East Africa. AIDS. 2016;30(3):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 28. Harrison L, Dunn DT, Green H, et al. . Modelling the association between patient characteristics and the change over time in a disease measure using observational cohort data. Stat Med. 2009;28(26):3260–3275. [DOI] [PubMed] [Google Scholar]
- 29. Wilhelm S, Manjunath BG. tmvtnorm: Truncated Multivariate Normal and Student t Distribution. (Version 1.5). http://CRAN.R-project.org/package=tmvtnorm. Originally published 2015. Version 1.5 published March 22, 2022. Accessed June 23, 2022.
- 30. Toulson AR, Harrigan R, Heath K, et al. . Treatment interruption of highly active antiretroviral therapy in patients with nadir CD4 cell counts >200 cells/mm3. J Infect Dis. 2005;192(10):1787–1793. [DOI] [PubMed] [Google Scholar]
- 31. Touloumi G, Pantazis N, Antoniou A, et al. . Highly active antiretroviral therapy interruption: predictors and virological and immunologic consequences. J Acquir Immune Defic Syndr. 2006;42(5):554–561. [DOI] [PubMed] [Google Scholar]
- 32. Beckhoven D, Florence E, Wit S, et al. . Incidence rate, predictors and outcomes of interruption of HIV care: nationwide results from the Belgian HIV cohort. HIV Med. 2020;21(9):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stover J, Glaubius R, Mofenson L, et al. . Updates to the Spectrum/AIM model for estimating key HIV indicators at national and subnational levels. AIDS. 2019;33(suppl 3):S227–S234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Julg B, Dee L, Ananworanich J, et al. . Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. Lancet HIV. 2019;6(4):e259–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castagna A, Muccini C, Galli L, et al. . Analytical treatment interruption in chronic HIV-1 infection: time and magnitude of viral rebound in adults with 10 years of undetectable viral load and low HIV-DNA (APACHE Study). J Antimicrob Chemother. 2019;74(7):2039–2046. [DOI] [PubMed] [Google Scholar]
- 36. Stecher M, Claßen A, Klein F, et al. . Systematic review and meta-analysis of treatment interruptions in human immunodeficiency virus (HIV) type 1-infected patients receiving antiretroviral therapy: implications for future HIV cure trials. Clin Infect Dis. 2020;70(7):1406–1417. [DOI] [PubMed] [Google Scholar]
- 37. Hickey GL, Philipson P, Jorgensen A, et al. . A comparison of joint models for longitudinal and competing risks data, with application to an epilepsy drug randomized controlled trial. J R Stat Soc Ser A Stat Soc. 2018;181(4):1105–1123. [Google Scholar]
- 38. Bakoyannis G, Zhang Y, Yiannoutsos CT. Nonparametric inference for Markov processes with missing absorbing state. Stat Sin. 2019;29(4):2083–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


