Summary
Background
China, which has the largest chronic hepatitis B virus (HBV) burden, may expand antiviral therapy to attain the World Health Organization (WHO)-2030 goal of 65% reduction in mortality. We evaluated health outcomes and cost-effectiveness of chronic HBV infection treatments based on alanine transaminase (ALT) antiviral treatment initiation thresholds and coverage in China to identify an optimal strategy.
Methods
A decision-tree Markov state-transition model evaluated the cost-effectiveness of expanded antiviral treatment for chronic HBV infection by simulating 136 scenarios by ALT treatment initiation thresholds (40 U/L, 35 U/L for males and 25 U/L for females, 30 U/L for males and 19 U/L for females, and treating HBsAg+ individuals regardless of ALT values), population age groups (18–80, 30–80, and 40–80 years), implementation durations (2023, 2028, and 2033) under and treatment coverages (20%, 40%, 60%, and 80%). Deterministic and probabilistic sensitivity analyses explored model uncertainty.
Findings
Besides the status quo, we finally simulated 135 treatment-expanding scenarios based on the cross combination of different thresholds of ALT, treatment coverages, population's age groups and implementation time. For the status quo, a cumulative incidence of 16,038–42,691 HBV-related complications and 3116–18,428 related deaths will happened between 2030 and 2050. When the treatment threshold is expanded to ‘ALT > 35 in males & ALT > 25 in females’ immediately without expanding treatment coverage, it will save 2554 HBV-related complications and 348 related deaths compared to the status quo among the whole cohort by 2030, and US$ 156 million more will be costed for gaining 2962 more QALYs. If we just expand the ALT threshold to ALT > 30 in males & ALT > 19 in females, 3247 HBV-related complications and 470 related deaths will be prevented by 2030 under the current treatment coverage of 20%, which will cost US$ 242 million, US$ 583 million or US$ 606 million more by the year of 2030, 2040 or 2050, respectively. Treatment expanded to HBsAg+ will save the largest number of HBV-related complications and death. This expanding strategy also results in large complications or death reduction when it is limited to patients older than 30 years or 40 years. Under this strategy, four scenarios (Treating HBsAg+ with coverage of 60% or 80% for patients older than 18 years or 30 years) showed the effectiveness in reaching the target before the year 2030. Among all the strategies, treatment expanded to HBsAg+ would cost the most while providing the highest total QALYs compared to other strategies with similar implementation scenarios. ALT thresholds of 30 U/L and 19 U/L for males and females, respectively, with 80% coverage for 18–80 years, can attain the goal by 2043.
Interpretation
Treating HBsAg+ individuals with 80% coverage for 18–80 years is optimal; earlier implementation of expanded antiviral treatment with a modified ALT threshold could decrease HBV-related complications and deaths to support the global target of 65% reduction in viral hepatitis B deaths.
Funding
This study was funded by Global Center for Infectious Disease and Policy Research (BMU2022XY030); Global Health and Infectious Diseases Group (BMU2022XY030); The Chinese Foundations for Hepatitis Control and Prevention (2021ZC032); National Science and Technology Project on Development Assistance for Technology, Developing China-ASEAN Public Health Research and Development Collaborating Center (KY202101004); in part by National Key R&D Program of China (2022YFC2505100).
Keywords: Chronic HBV infection, Treatment, Cost-effectiveness, Alanine transaminase
Research in context.
Evidence before this study
By 2030, the incidence of new chronic viral hepatitis B infections and mortality of viral hepatitis B should be decreased by 90% and 65%, respectively. China is the country having the highest burden of the chronic HBV infection, accounting for one-third of the global infections. Prevention of mother-to-child transmission in China has significantly prevented incident pediatric HBV infections, and prevention of HBV-related complications in adults with chronic HBV infections and improvement of patient quality of life has been prioritized. Antiviral treatment is widely used in China to reduce HBV-related complications and mortality, although the overall treatment coverage is only around 20% for all eligible patients. Alanine transaminase (ALT) is the key indicator for initiating antiviral treatment; however, the ALT treatment threshold varies worldwide. The American Association for the Study of Liver Diseases and the World Health Organization recommend ALT >2 × ULN (35 and 25 U/L) and >1 × ULN (30 and 19 U/L) for male and female patients, respectively. The European Association for the Study of the Liver, the Asia Pacific Association for the Study of the Liver, and the 2019 Chinese Guidelines for the Prevention and Treatment of Chronic Hepatitis B recommend ALT 40 U/L as the threshold. We searched PubMed, Embase, and Web of Science between January 1, 2000, and February 28, 2022, with no language restrictions using the terms “Hepatitis B”, “HBV”, “treatment”, “Alanine transaminase” or “ALT” and “cost-effectiveness” to identify published cost-effective evaluations of expanded antiviral treatment for chronic HBV infection, but we found no study of the effectiveness or cost-effectiveness of expanded treatment based on ALT thresholds for initiating antiviral treatment.
Added value of this study
According to our analysis of 136 expanded treatment strategies, treating HBsAg+ individuals with ≥60% treatment coverage for 18–80 and 30–80 years could achieve the goal of a 65% reduction in mortality by 2030. Although all expanded treatment strategies were cost-effective by 2050, treating HBsAg+ individuals with 80% coverage for 18–80 years was the most cost-effective strategy even after reductions in willingness to pay (WTP) levels. With an ALT cutoff of 30 U/L for males and 19 U/L for females and treatment coverage of 80% for 18–80 years, a 65% reduction in mortality is unattainable until 2043. However, expanded treatment coverage without adjusting the ALT threshold for initiating antiviral treatment cannot achieve this target. Delayed implementation of expanded treatment reduced fewer HBV-related complications and deaths, and the overall cost-effectiveness was reduced although the strategies remained cost-effective.
Implications of all the available evidence
Expanded treatment for chronic HBV infection with adjustments for ALT thresholds for initiating antiviral treatment and improving treatment coverage can reduce HBV-related complications and deaths cost-effectively in China, especially when implemented early. Delayed implementation of expanded treatment reduces cost-effectiveness. Expanded treatment should be implemented as soon as possible to improve current treatment coverages, reduce mortality, achieve the largest effectiveness and cost-effectiveness for chronic HBV infection treatment, and help China achieve the WHO-2030 objective of 65% reduction in mortality of viral hepatitis B as a public health threat.
Introduction
Hepatitis B virus (HBV) infection confers a large disease burden globally, with an estimated 296 million chronic infections in 2019.1 The World Health Organization (WHO) set ambitious targets to reduce the incidence of new chronic HBV infections by 90% and HBV-related mortality by 65% globally by 2030.1 With nearly one-third of the global infections and more than 300,000 HBV-related deaths each year, China has the largest chronic HBV-infected population2 and plays a major role in achieving the global target through chronic HBV prevention and treatment. The incidence of pediatric HBV infections has significantly decreased with the prevention of mother-to-child transmission in China,3 preventing adults with chronic HBV infection from progressing to HBV-related complications and improving their life quality has become the priority. Antiviral treatment has been widely used in the country to reduce HBV-related complications and mortality.4, 5, 6
Serum alanine aminotransferase (ALT), the key indicator for antiviral treatment initiation in chronic HBV infection, has various thresholds worldwide. The upper limit of normal value (ULN) recommended for treatment initiation by the American Association for the Study of Liver Diseases and the WHO is >2 × ULN (35 and 25 U/L for male and female patients)7 and >1 × ULN (30 and 19 U/L for male and female patients),8 respectively. The European Association for the Study of the Liver,9 Asia Pacific Association for the Study of the Liver,10 and Chinese Guidelines for the Prevention and Treatment of Chronic Hepatitis B (2019 Edition)11 recommend a 40-U/L ALT cutoff for both male and female patients. In China, besides serum ALT levels for patients with chronic HBV infection persistently abnormal (>ULN) and other causes of ALT elevation excluded, HBV DNA seropositive is also required for antiviral therapy.11,12
The current ALT threshold is an unsuitable indicator for antiviral treatment initiation in chronic HBV infection.13, 14, 15 The 40 U/L cutoff is suboptimal because a considerable proportion of HBV-infected patients with normal ALT levels have a high degree of liver inflammation and fibrosis.16, 17, 18 More than 80% of HBeAg-negative patients had normal ALT levels, among whom a considerable proportion developed cirrhosis and hepatocellular carcinoma (HCC).19,20 Cutoff values of 30 and 19 U/L for male and female patients, respectively, would decrease their HCC risk.21
With the availability of low-cost generic drugs, such as tenofovir and entecavir, and revised medical insurance subsidization policies in China,22,23 patients with chronic HBV infection are more likely to benefit from treatment for clinical cure.24, 25, 26 Moreover, a common understanding indicates that expanded chronic HBV infection treatment is a necessary way in getting the WHO 2030 target in time. Consolidated proofs are needed to provide before we put forward and start the implementation of the expanded treatment policy. At present, some studies have explored the cost-effectiveness of expanded antiviral treatment for chronic HBV infection.27, 28, 29 However, the effectiveness or cost-effectiveness of ALT-based cutoffs for antiviral treatment initiation has not been evaluated, and the implementation and effect of the ALT treatment initiation threshold remain unclear.
Thus, this study explored whether expanded antiviral treatment strategies with modified ALT-based treatment initiation thresholds or treatment coverage would result in a 65%-mortality reduction by 2030 in China and estimated the year by which the target would be achieved. The evaluation of the effectiveness and cost-effectiveness of these antiviral treatment strategies would provide insights to enable revision of the 2019 Chinese Guidelines for the Prevention and Treatment of Chronic Hepatitis B and offer clues on expanded HBV treatment around the world.
Methods
Study design
Decision-analytic Markov models were commonly used to evaluate the cost-effectiveness of major intervention programs.30, 31, 32, 33, 34, 35 In this study, we construct a decision-analytic Markov model to simulate HBV-related disease progression and outcome and evaluate the effectiveness and cost-effectiveness of various HBV-antiviral-treatment strategies based on four ALT thresholds for initiating antiviral treatment and coverages among three age groups in China. The model was constructed using TreeAge Pro 2020 (TreeAge Software, Williamstown, MA).
Definition of treatment strategies and scenarios
With the current chronic HBV infection treatment practices in China as the status quo (ALT > 40 U/L), 20% of eligible individuals with chronic HBV infection received treatment aged 18–80 years.1,22,31 Now that various guidelines indicate chronic HBV infection aged older than 30 or 40 years bear a largely increased risk of getting liver damage, we then classified our population cohort into three age groups. The simulation included 136 treatment strategies that comprised (1) four treatment initiating thresholds: ‘ALT > 40 U/L for both males and females’ (ALT > 40)9, 10, 11; ‘ALT > 35 for males and >25 U/L for females’ (ALT > 35/25)7; ‘ALT > 30 for male and >19 U/L for female patients’ (ALT > 30/19)8; and diagnosed with chronic HBV infection (treating HBsAg+). We consider the sex factor because the disease severity and disease progression are different between males and females despite under the same ALT threshold; (2) four treatment coverage (20%, 40%, 60%, and 80%) among the treatment-eligible population; (3) three target groups stratified by age: 18–80, 30–80, and 40–80 years; (4) implementation time set to start in 2023, 2028 (delayed for 5 years), and 2033 (delayed for 10 years).
Modeling
The model was developed to simulate the chronic HBV infection status of 100,000 people (HBsAg positive for ≥6 months) aged 18–80 years after excluding individuals acutely infected or susceptible. Since detectable HBV DNA was widely supported as a basic threshold for the antiviral treatment for chronic HBV infection, HBV DNA seronegative individuals, which were not suitable for treatment, were not included in our cohort which is also in line with the Guidelines for the Prevention and Treatment of Chronic Hepatitis B in China.11,36,37 Besides the status quo, another 135 scenarios were modeled and explored for analysis. The initial year of the model is set at 2023, and the results of model operation reaching 2030, 2035, 2040, 2045, 2050 and lifetime are analyzed, that is because the disease progression of chronic HBV infection is a chronic and long-term process. The analysis was conducted every five years until 2050, which is due to the policy may be changed before 2050. In addition, we also analyzed the effect and cost-effectiveness of the extended antiviral treatment for the population at the end of the cohort's lifetime.
A decision tree (Fig. S1) representing the four ALT treatment initiating thresholds in chronic HBV infection was developed where in each threshold had a specific progression of chronic HBV infection (Fig. S2), simplified as follows: (1) ALT > 40, including states ALT > 40 U/L and ALT ≤ 40 U/L (Fig. S2a), where ALT > 40 U/L indicates HBeAg-negative and HBeAg-positive hepatitis (according to the Chinese Guidelines for the Prevention and Treatment of Chronic Hepatitis B) and those who do not fit into any of the usual states defined in guidelines, whereas the state ALT ≤ 40 U/L comprised immune-tolerant and inactive carriers and those not belonging to any of the usual states defined in guidelines11; (2) ALT > 35/25, (including states ALT > 40 U/L, ALT ∼35–40 U/L, and ALT ≤ 35 U/L for males; ALT >40 U/L, ALT ∼25–40 U/L, and ALT ≤ 25 U/L for females) (Fig. S2b); (3) ALT > 30/19, (including states ALT > 40 U/L, ALT ∼30–40 U/L, and ALT ≤ 30 U/L for males; ALT > 40 U/L, ALT ∼19–40 U/L, and ALT ≤ 19 U/L for females) (Fig. S2c); and (4) treating HBsAg+ (treating all chronic HBV infection, including ALT ≤ 30 U/L for males and ALT ≤ 19 U/L for females). All Markov models contained states of compensated cirrhosis (CC), decompensated cirrhosis (DC), HCC, liver transplantation (LT), HBV-related death, and HBsAg loss with or without anti-HBs seroconversion (goal of a functional cure; Fig. S2).
According to the 2019 Chinese Guidelines for the Prevention and Treatment of Chronic Hepatitis B, in ALT > 40, treatment-eligible states were ALT > 40 U/L among HBV DNA-positive individuals, CC, DC, HCC, or LT.11 After expanding antiviral treatment, in ALT > 35/25, states ALT ∼35–40 U/L and ALT ∼25–40 U/L for males and females, respectively and in ALT > 30/19, states ALT ∼30–40 U/L and ALT ∼19–40 U/L for males and females, respectively could receive treatment. In treating HBsAg+, states ALT ≤ 30 U/L for males and ALT ≤ 19 U/L for males and females, respectively, also met the criteria for treatment (Fig. S1). However, some patients with chronic HBV infection who met the treatment initiation criteria did not receive treatment. The transition probabilities were likely related to the two aspects of the ALT ULN values and treatment choice. Nucleos(t)ide analogues (a combination of entecavir and tenofovir) were included in simulated therapy; interferon therapy was excluded because of its finite duration and numerous side effects and contraindications.38 The model is half-cycle corrected.
Data source
Data were collected from published medical literature and public databases, along with parameterized treatment cost, transition probabilities, and other model parameters (Table S1). The starting state of the model was a specific ALT distribution based on the serum ALT level of chronic HBV-infected people in China as described in Table S2 extracted from published studies or obtained through direct requests to the authors (Table S2).39, 40, 41, 42 Costs included the direct medical treatment cost. The annual transition probabilities and utility scores were derived from published literature (Table S1). Background age-specific mortality was ascertained from the China Population & Employment Statistics Yearbook, 2020.
Effectiveness and cost-effectiveness analyses
We estimated the number of HBV-related complications (CC, DC, and HCC), HBV-related deaths, and quality-adjusted life-years (QALYs) in each cohort. An incremental cost-effectiveness ratio (ICER) was calculated to identify the additional cost of each QALY, with an ICER <3 times the per-capita annual gross domestic product (GDP) indicating a cost-effective strategy43 in 2021, the cost-effectiveness threshold was US$ 37,653 (per-capita GDP US$ 12,551 in China).44 Strategies were depicted on the cost–benefit plane and were connected by a line to define the cost-effectiveness plane. According to the Chinese guide to cost-effectiveness analysis, a 5% (0–8%) discount rate was used when computing present values of a stream of future costs and effects. According to the roadmap from WHO 2022–2030, hepatitis B contribute much more disease burden than hepatitis C, and a 65% reduction in viral hepatitis mortality is indicated as a relative target indicator, that hepatitis B's target was set to be 62%. And chronic HBV infection brings the major burden of viral hepatitis that 86 million CHB was largely overweighted the 7 million hepatitis C patients in China, we still set the elimination goal of hepatitis B to be 65%.
Sensitivity analysis
Probabilistic sensitivity analyses (PSA) were used to characterize the combined uncertainty of all model parameters based on 10,000 Monte Carlo simulations and are presented by cost-effectiveness acceptability curves, which indicate the probability that each alternative will become the most cost-effective treatment as a dominant strategy with various the willingness to pay (WTP). One-way deterministic sensitivity analyses determined the effects of parameter uncertainties and model robustness. The findings are presented as tornado plots.
Role of the funding source
This study was funded by Global Center for Infectious Disease and Policy Research (BMU2022XY030); Global Health and Infectious Diseases Group (BMU2022XY030); The Chinese Foundations for Hepatitis Control and Prevention (2021ZC032); National Science and Technology Project on Development Assistance for Technology, Developing China-ASEAN Public Health Research and Development Collaborating Center (KY202101004); in part by National Key R&D Program of China (2022YFC2505100).
Results
Effectiveness and cost-effectiveness of current treatment threshold of ALT > 40 U/L
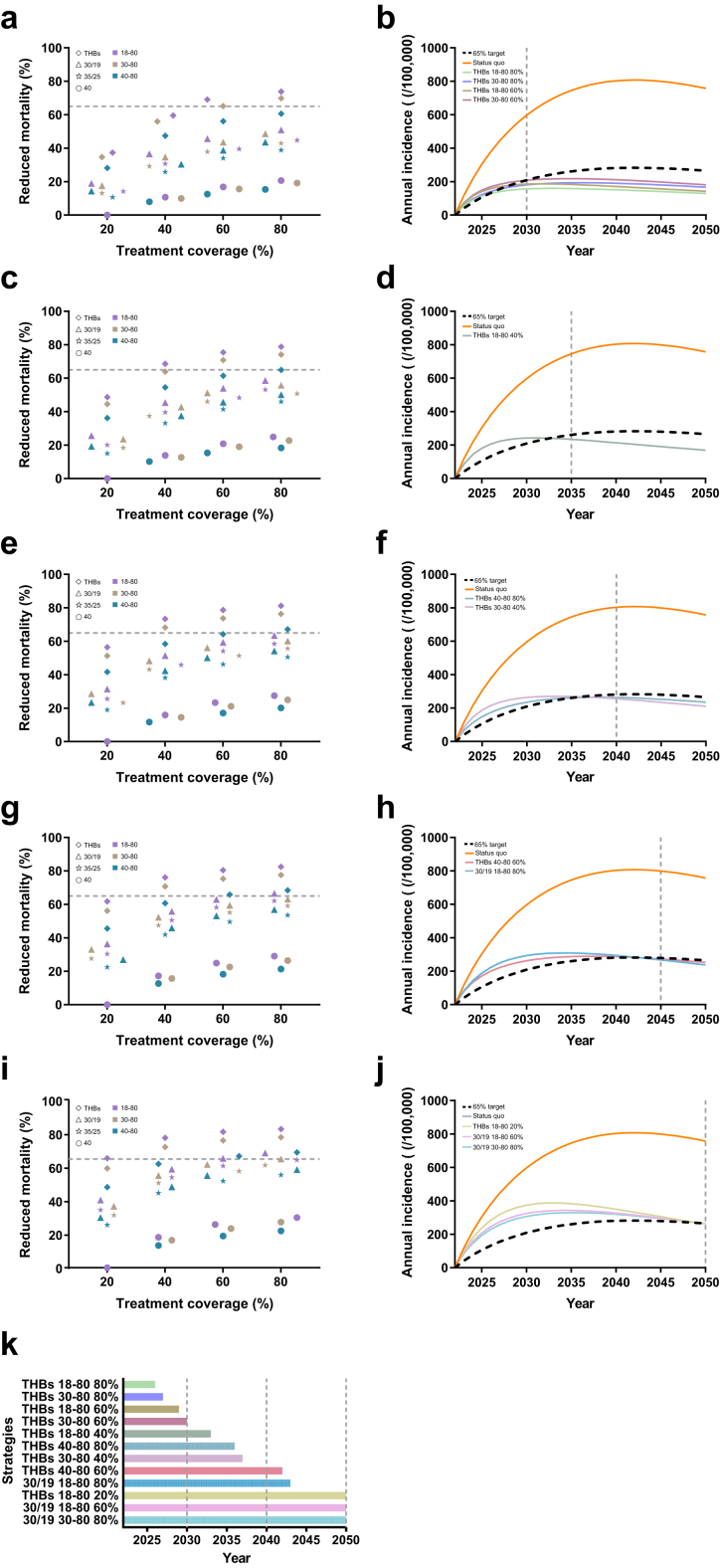
Besides the status quo, we finally simulated 135 treatment-expanding scenarios based on the cross combination of different key values of thresholds of ALT, treatment coverages, population's age groups and implementation time. The separate expanding treatment coverage under current treatment threshold of ALT > 40 U/L will lead to a significant decrease in HBV-related complications or deaths, while it would not reach the mortality reduction target even by 2050 (Fig. 1a and k).
Fig. 1.
Effect of strategies to reduce mortality attributable to CHB implemented from 2023 in China. a, c, e, g and i, reduced mortality by 2030, 2035, 2040, 2045, and 2050 respectively; b, d, f, h and j, the annual incidence of each strategy that could reach 65% mortality-reduction target by 2030, 2035, 3040, 2045, and 2050, respectively;k, the year in which each strategy achieved the goal of reduced mortality by 65%. Abbreviation: THBs, Treating HBsAg+; 30/19, ALT > 30/19; 35/25, ALT > 35/25; 40, ALT > 40; 18–80, 18–80 years; 30–80, 30–80 years; 40–80, 40–80 years.
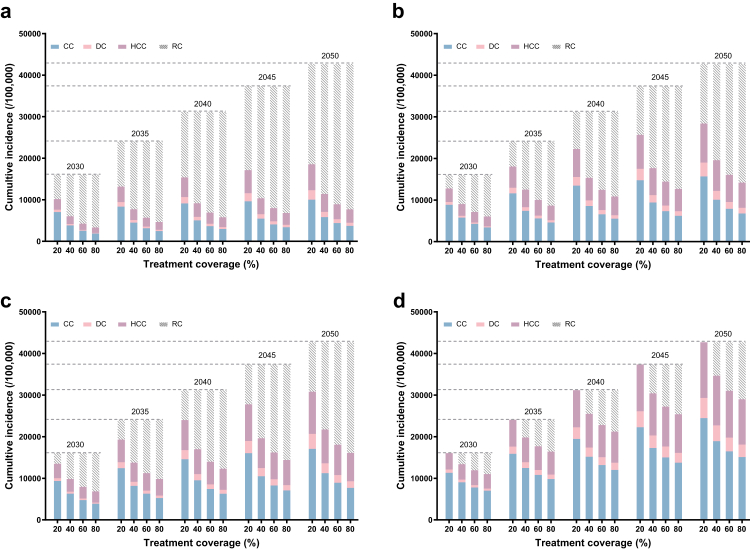
For the status quo (ALT > 40 with 20% coverage for 18–80 years), it will result in cumulative incidences of 16,038–42,691 HBV-related complications (CC, DC, and HCC) and 3116–18,428 related deaths between 2030 and 2050, respectively (Fig. 2d; Tables S3–S7).
Fig. 2.
Cumulative incidence of HBV-related complications for 18–80 years implemented from 2023. a, Treating HBsAg+; b, ALT > 30/19; c, ALT > 35/25; d, ALT > 40. Abbreviation: CC, compensated cirrhosis; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; RC, reduced complications.
The overall treatment under current guidance will cost $511 million to $1833 million among the 100,000 chronic HBV infection cohort. Current treatment strategies with ALT > 40 UI/L showed the least cost in total as well as the least number of QALY gained in each time horizon. Compared to the 20% coverage strategy, increased coverages showed a decreasing trend in ICER from US$ 114,832/QALY to US$ 130,814/QALY gained by 2030, and US$ 29,069/QALY to US$ 31,546/QALY gained by 2040, respectively. It did not make substantial differences when such a treatment strategy was limited to patients aged 30 or 40 years older. The ICERs showed significant decreases when the time horizon was set to 2050, from US$ 14,521/QALY to US$ 15,445/QALY gained (Tables S3, S5 and S7).
Effectiveness and cost-effectiveness of treatment expanded to HBsAg+
Under this strategy, four scenarios, including ‘80% coverage among all the patients’, ‘80% coverage among patients older than 30 years’, ‘60% coverage among all the patients’ and ‘60% coverage among patients older than 30 years’, could achieve the goal of 65% reduction in mortality (Fig. 1 a and b) before the year of 2030. And other scenarios (‘40% coverage among all the patients’, ‘80% coverage among patients older than 40 years’, ‘40% coverage among patients older than 30 years’, ‘60% coverage among patients older than 40 years’ and ‘80% coverage among all the patients’) could also reach the 65% reduction target by 2050 if they were implemented immediately in 2023 (Fig. 1a–k). If such an expanded strategy is implemented five years later, only the ‘80% coverage among all the patients’ scenario could permit the WHO target by the year 2050 (Fig. S3). If such an expanded strategy is implemented ten years later, no scenario could permit the WHO target even by the year 2050 (Fig. S5).
Treatment expanded to HBsAg+ will save the largest number of HBV-related complications and death. Compared to the expanded treatment coverage, treatment solely expanded for the threshold from ALT > 40 to all the HBsAg+ patients showed much more cases of complications and death saved, as 5028 complications and 534 death for only expanded treatment coverage to 80% versus 5869 complications and 918 death for only expanded treatment threshold by 2030 will be saved (Table S3). It shows more proportional decreases when the time horizon is extended to 2040 or 2050. Further, expanded treatment threshold with broadened treatment coverages, such as 40%, 60% or 80%, will largely reduce the complication cases that happened by 31,305, 33,779, or 35,007 respectively by 2050 (Fig. 2a). This expanding strategy also results in large complications or death reduction when it is limited to patients older than 30 years or 40 years. Delayed implementation will result in increases in HBV-related complications and deaths under each scenario (Figs. S4 and S6).
Among all the strategies, treatment expanded to HBsAg+ cost the most while providing the highest total QALYs compared to other strategies with similar implementation scenarios. It showed slightly higher ICERs from US$ 80,258/QALY to US$ 101,605/QALY gained by 2030, and the gap will continuously narrow in the long run compared to other expanded strategies. The ICERs of those four scenarios that could reach the elimination target by 2030 were US$ 81,051/QALY, US$ 80,582/QALY, US$ 85,040/QALY, or US$ 84,495/QALY gained, respectively, which will largely decrease to US$ 14,590/QALY, US$ 14,458/QALY, US$ 14,878/QALY, or US$ 14,743/QALY gained by 2050 (Tables S3 and S7). ICERs of other scenarios under such strategy which could achieve the goal by 2050 included ‘40% treatment coverage for all ages’, ‘80% treatment coverage limited to those aged 40 years older’, ‘40% treatment coverage limited to those aged 30 years older’, ‘60% coverage limited to 40 years older’ or ‘20% coverage for all ages’, ranged from US$ 15,469/QALY, US$ 14,364/QALY, US$ 15,325/QALY, US$ 14,653/QALY, or US$ 17,250/QALY gained, by the year of 2050, respectively (Table S7).
Effectiveness and cost-effectiveness of treatment threshold expanded to ALT > 30 in males & ALT > 19 in females
None of the scenarios under such an expanded threshold will reach the hepatitis elimination target before 2040 (Fig. 1c–f and k). If the expanded strategy is implemented in the whole cohort with over 60% treatment coverage or limited to those aged 30 years older, the target will be reached in the year 2043 or 2050, respectively (Fig. 1g–j). However, any of the scenarios under such a threshold would not reach the WHO target by 2030 if it was implemented in 2028 (Fig. S3) but ‘80% coverage among all the patients’ could reduce 65% mortality in the year 2050. If implemented in 2033, until 2050, no scenarios could reduce 65% mortality (Fig. S5).
If we just expand the ALT threshold to ALT > 30 in males & ALT > 19 in females, 14,297 HBV-related complications and 5316 related deaths will be prevented by 2050 under the current treatment coverage of 20%. With the increase in treatment coverages from 20% to 80%, the preventable complications or death increased from 28,479 or 11,017 (Fig. 2b). The age limitation to over 30 or 40 years older will avert 27,169 or 24,712 complications and 10,476 or 9423 deaths by 2050 under the treatment coverage of 80% (Table S7).
Expanding the ALT threshold to ‘ALT > 30 in males & ALT > 19 in females’ among the chronic HBV infection cohort with 20% treatment coverage will cost US$ 753 million, US$ 1680 million or US$ 2274 million by the year 2030, 2040 or 2050, respectively (Tables S3, S5, and S7). ICERs under such threshold strategy decreased significantly when the time horizon extended to the year 2040 (US$ 19,610/QALY gained), and 2050 (US$ 10,409/QALY gained) among those aged 18–80 years with 80% treatment coverage. Restrictions to patients aged 30 years older would cost less with similar total QALY by the year 2030 (with less ICER US$ 61,290/QALY gained for all patients versus US$ 61,492/QALY gained for >30 years) with 80% treatment coverage, however, the trend will be reversed from 2040 (Table S5). The ICERs of those three scenarios that could reach the 65% mortality reduction target was ‘80% treatment coverage for all ages’, ‘80% treatment coverage limited to those aged 30 years older’ or ‘60% treatment coverage for all ages’, respectively by 2050. Delayed implementation will result in significant increases in ICERs under each scenario (Tables S8–S17).
Effectiveness and cost-effectiveness of treatment threshold expanded to ALT > 35 in males & ALT > 25 in females
Under such an ALT threshold and no matter when it is implemented, none scenario will lead to a 65% mortality reduction, though 80% treatment coverage started in 2023 among the whole cohort will save 26,578 HBV-related complications and 10,063 deaths till 2050 (Fig. 2c, Figs. S3, S5 and Tables S3, S7).
When the treatment threshold is expanded to ‘ALT > 35 in males & ALT > 25 in females’ immediately without expanding treatment coverage, it will save 2554 HBV-related complications and 348 related deaths compared to the status quo among the whole cohort by 2030. When its implementation was limited to those older than 30 years or 40 years, cases of HBV-related complications or deaths were estimated to be 13,703, 14,140, or 2791, 2854 respectively. Fewer treatment coverages of 20%, 40%, or 60% mean 2768, 2354, or 2100 deaths compared to the 80% treatment coverage among the whole cohort.
By 2030, US$ 753 million will be costed for gaining 539,939 QALYs when we separately expand the ALT threshold. Increased coverages will result in US$ 918 million to US$ 1043 million while gaining 542,425–545,016 QALYs. And ICER under such strategy showed to be the least compared to other strategies. Little ICER differences were found when this strategy was limited to patients aged 30 years or older. The expanded threshold of ALT costs more compared to the current ‘ALT > 40U/L’ strategy, while it will offer 1,162,029–1,191,743 QALYs by the year 2050 with various treatment coverages from 20% to 80% (Tables S3 and S7).
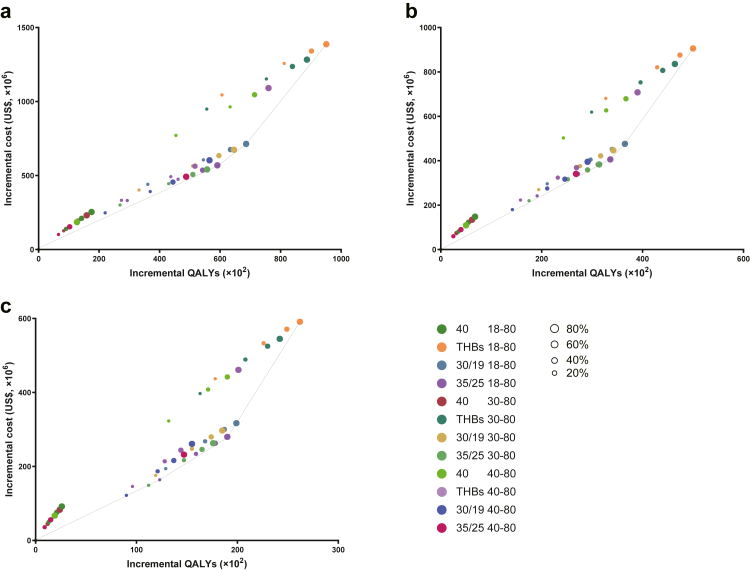
Whether implemented immediately or delayed, there were three strategies on the cost-effectiveness frontier as described in Fig. 3a. ‘Treating-HBsAg+ with 80% coverage among all the patients’ (ICER US$ 81,051/QALY to US$ 14,590/QALY gained from 2030 to 2050) was the most cost-effective strategy, followed by ALT > 30/19 with 80% coverage for 18–70 years (ICER US$ 61,290/QALY to US$ 10,409/QALY gained) and ALT > 35/25 with 80% coverage for 18–70 years (ICER US$ 56,365/QALY to US$ 9643/QALY gained). When the implementation of extended treatment was delayed, the cost-effectiveness plan had a similar trend (Fig. 3b and c).
Fig. 3.
Cost-effectiveness plane demonstrating the cost-effectiveness for all treatment strategies of the HBV treatment programs by 2050 implemented from 2023, 2028, and 2033. Strategies on the cost-effectiveness frontier dominate strategies above the frontier. Abbreviation: THBs, Treating HBsAg+; 30/19, ALT > 30/19; 35/25, ALT > 35/25; 40, ALT > 40; 18–80, 18–80 years; 30–80, 30–80 years; 40–80, 40–80 years.
Probabilistic sensitivity analysis
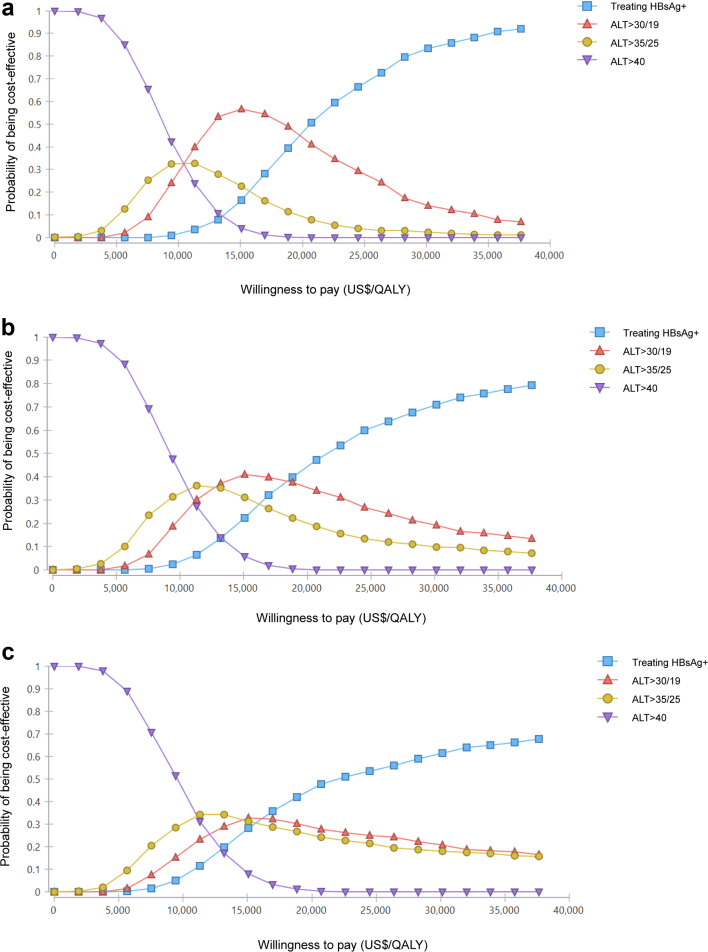
Fig. 4 depicts cost-effectiveness acceptability curves of various treatment strategies for various decision-makers WTP between zero and the per-capita GDP per additional QALY gained by 2050, with ICER much lower than the per-capita GDP. At a WTP threshold of US$ 37,653, treating HBsAg+ with 80% coverage showed a more than 90% probability of being cost-effective while outperforming other strategies for 18–80 years by 2050. After delayed treatment, the result had a similar trend but the probability of being cost-effective would be reduced.
Fig. 4.
Cost-effectiveness acceptability curves for HBV treatment strategies of the HBV treatment programs by 2050 implemented from 2023, 2028, and 2033. Strategies on the cost-effectiveness frontier dominate strategies above the frontier.
One-way sensitivity analysis
One-way sensitivity analysis showed that regardless of how the parameters change, treating HBsAg+ with 80% coverage was the most cost-effective strategy. The Discount rate had the greatest effect on ICER for 18–80 years. The other nine parameters that most affected ICER were treatment-related transition probability from ALT < 19 U/L to HBsAg loss, transition probability from ALT > 40 U/L to CC, cost of CC, transition probability from ALT > 40 U/L to ALT < 40 U/L, treatment-related transition probability from ALT < 40 U/L to HBsAg loss, treatment-related transition probability from ALT > 40 U/L to HBsAg loss, treatment-related transition probability from ALT = 30–40 U/L to HBsAg loss, transition probability from CC to LT, and utility score of CC (Fig. S7).
Discussion
Chinese guidelines recommend ALT levels over the ULN (>40 U/L) as the threshold for antiviral treatment initiation in chronic HBV infection with positivity for serum HBV DNA, which has increasingly been challenged because of histological changes in liver tissues’ low ALT levels.11,12,16,45,46 We analyzed whether the WHO-specified 65% mortality reduction by 20301 was achievable and assessed the year of achievement via several expanded strategies. Only treating HBsAg+ with more than 60% treatment coverage for 18–80 or 30–80 years can achieve the target by 2030, and high treatment coverage would enable earlier goal attainment. The HBsAg+ treatment strategy was most effective for reducing complications, followed by ALT > 30/19 and ALT > 35/25. The least reduction of complications was achieved by only expanding treatment coverage under the current strategy. Treating HBsAg+ with 80% coverage for 18–80 years, either implemented immediately or delayed, was cost-effective. With a 5-year delay in implementing expanded treatment, only treating HBsAg+ with 80% coverage for 18–80 years could achieve a 65% mortality reduction by 2050. Delayed implementation of expanded treatment decreased HBV-related complications less compared with immediate implementation but showed similar trends in preventing HBV-related complications. This is the first HBV-related economic evaluation study of modification in the ALT antiviral treatment initiation thresholds for chronic HBV infection in China.
Another key finding is that adjusting the ALT threshold for expanding antiviral therapy is the best opportunity to avoid deaths. A lower ALT treatment initiation threshold significantly decreased the number of HBV-related deaths, indicating that several strategies, including treating HBsAg+ with maximal coverage, could facilitate the achievement of the mortality reduction target. Implementation of treating HBsAg+ with more than 60% treatment coverage for 18–80 or 30–80 years can achieve this target by 2030. Treating HBsAg+ regardless of ALT levels with 80% coverage for 18–80 years achieved the goal first, followed by 80% coverage for 30–80 years, further emphasizing the need for treating HBsAg+ patients older than 30 years with chronic HBV infection and high coverage. Health literacy is important for promoting treatment coverage. With an ALT threshold of ‘30 U/L for males and 19 U/L for females’, treatment coverage of 80% for 18–80 years would not achieve a 65% reduction in mortality until 2043.
Compared with increased treatment coverage, treatment threshold modification is more valuable for preventing HBV-related complications. Only expanding treatment coverage without adjusting the ALT threshold antiviral treatment initiation cannot achieve the target. Treatment threshold modification might be a more practical strategy than increased coverage as medical treatment is more controllable than individual/crowd behavior. Compared to the current treatment strategy, treating HBsAg+ with 80% coverage for 18–80 years has the highest effectiveness in averting 82% of HBV-related complications by 2050; 56.6% of HBV-related complications will be averted by modifying the treatment threshold from ALT > 40 to treating HBsAg+ with the current treatment coverage. Only 32.1% of HBV-related complications would be averted with the current treatment threshold and increased coverage from 20% to 80% for the total population. Thus, treatment compliance among chronic HBV infections should be improved and deserve more attention. The medical service level and ability should also be improved as much as possible to ensure better compliance among chronic HBV infections. As is indicated, lack of training and financial support were cited as the major barriers to not offering HBV care. The rolling out of expanded treatment needs careful planning, and addressing the differing needs of individual provinces, which can be further limited by local government budgets, primary care networks and the manufacturing and capacity constraints of the existing health facilities. The medical testing and treating system as well as the chronic HBV infection surveillance system should also be adequately invested in, especially in those resource-limited areas in China.
Each expanded treatment strategy in China is cost-effective by 2050, and expanded treatment offers many benefits to the population's health and medical system. Because of the large effectiveness of increased treatment of chronic HBV infection, expanded treatment strategies show no cost-effectiveness before 2030, but are all cost-effective in the long run by 2050. Expanded treatment implementation requires strong governmental support initially. Treating HBsAg+ with 80% coverage for the total population is the most cost-effective strategy, despite the ICER being the highest compared to other strategies, with the same coverage because it gets the most QALYs lower than the threshold of 3 times per-capita GDP (US$ 37,653), even one time the per-capita GDP. Our sensitivity analysis indicated that the discount rate has a maximum effect on cost-effectiveness (ICER range US$ 0 to US$ 16,000, well below three times the per-capita GDP).44 Although the cost of nucleos(t)ide analogues varies within a certain range, treating HBsAg+ is the most cost-effective option, given the antiviral drugs recommended by the Chinese guidelines,11 such as five nucleoside analogs, included in the National List of Reimbursable Medicines.22 Thus, the treatment cost in China is not a major problem. Also, nucleos(t)ide analogues such as TDF seems to have a lipid-lowering effect.47 We also expect new drugs to treat chronic HVB infection.48
The result demonstrates the timelines of adjusting the threshold for expanding antiviral therapy. A 5-year delay in expanded treatment implementation prevents goal achievement by 2030, whereas treating HBsAg+ with 80% coverage for 18–80 years can achieve the goal by 2049. Commencement of expanded treatment in 2033 without treatment strategy can reduce the 65% mortality even by 2050. Delayed implementation by 5–10 years of expanded treatment reduced fewer HBV-related complications than immediate implementation. If each strategy is delayed to 2028 or 2033, the ICER would be higher than that of a strategy that is implemented immediately but still less than three times the per-capita GDP by 2050.
This study had several limitations. First, the complicated real-world effectiveness of future implementation under the current healthcare system and the compliance of chronic HBV infection were not considered nor were the resource or economic effects on the healthcare system with the progression of the expanded treatment. Second, although our target cohort was a population with chronic HBV infection, we did not consider whether this population had liver histological changes; also, we just include chronic HBV infections with detectable HBV DNA according to the latest evidence and treatment guidelines.36,37 On the one hand, chronic HBV infection without detectable HBV DNA bear much less risk of liver injury before they progressed to HBV DNA positive. In addition, the level of serum HBV DNA is not necessarily associated with liver injury, but with patients' infectivity. Thus, we acquired ALT in our models to correspond to the actual liver injury during clinical practice by recruiting chronic HBV infection with detectable HBV DNA into our cohort. Third, we neglected the occurrence of adverse effects or antiviral resistance. Fourth, the available medical literature used to construct utility scores and transition probability for our cost-effectiveness analysis was mainly from specialized tertiary centers and could not completely represent China's situation, such as the transition probability to HCC. China should establish the hepatocellular carcinoma (HCC) surveillance system to investigate the effect of curative treatment for HCC and mortality in patients.49 Fifth, the Markov model did not distinguish different HBV genotypes or HBV e antigen-positive and HBV e antigen-negative chronic HBV infection. We assessed only the direct costs involved from a health payer perspective without including indirect costs, such as loss of productivity. For policymakers, each factor is important for determining whether to implement a strategy.
Conclusion
Expanded antiviral treatment strategies based on modified ALT thresholds, including maximally lowering the threshold or treatment that was not limited by the ALT value, is a valuable and simplified indicator for expanding chronic HBV infection treatment and should be implemented earlier and treatment coverage should be improved maximally in the total population to reduce the disease burden of chronic HBV infection and HBV-related deaths cost-effectively. Expanded treatment based on ALT adjustment should be implemented as soon as possible to get the most cost-effectiveness. Expanded treatment to HBsAg positive patients and higher coverage is the guarantee for achieving the goal of a 65% reduction in mortality by 2030–2050, with cost-effectiveness. It also needs to be confirmed by real-world research and continued to track and monitor the actually expanded treatment population and health economic effects in the future.
Contributors
SHZ and CW were involved in study concept and design, data acquisition, data analysis, interpretation of data, and drafting of the manuscript. BL and QBL provided critical revision of the manuscript. JS, YHZ, JDJ, XYX, and HYR were involved in the interpretation of data and provided important guidance for this study. BFH, TSZ, LYC, MZX, and JHC were involved in the finally revision of the manuscript. FQC, HZ, and LZ were involved in study concept and design, interpretation of data, critical revision of the manuscript, and overall study supervision. SHZ, CW, JD, JZ, NHH, and YQL had access to and verified the underlying data. All authors had full access to the data and had the final responsibility for the decision to submit for publication.
Data sharing statement
All data relevant to the study are included in the Article or the online appendix.
Declaration of interests
FQC is a staff member of Chinese Foundation for hepatitis Control and prevention and has received research funding from Chinese Foundation for hepatitis Control and prevention. All other authors declare no competing interests.
Acknowledgments
FQC is funded by the Chinese Foundations for Hepatitis Control and Prevention (2021ZC032); Global Health and Infectious Diseases Group (BMU2022XY030); and National Science and Technology Project on Development Assistance for Technology, Developing China-ASEAN Public Health Research and Development Collaborating Center (KY202101004).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100738.
Contributor Information
Lei Zhang, Email: lei.zhang1@monash.edu, lei.zhang1@xjtu.edu.cn.
Hui Zhuang, Email: zhuangbmu@126.com.
Fuqiang Cui, Email: cuifuq@bjmu.edu.cn, cuifuq@126.com.
Appendix A. Supplementary data
References
- 1.Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. 2021. http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ [Google Scholar]
- 2.Authors/Task Force M, Guidelines ESCCfP, Societies ESCNC 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Cui F.Q., Shen L.P., Li L., et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Easterbrook P., Luhmann N., Newman M., Walsh N., Lesi O., Doherty M. New WHO guidance for country validation of viral hepatitis B and C elimination. Lancet Gastroenterol Hepatol. 2021;6(10):778–780. doi: 10.1016/S2468-1253(21)00267-3. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Zhang Y.G., Wang X., et al. Long-term antiviral efficacy of entecavir and liver histology improvement in Chinese patients with hepatitis B virus-related cirrhosis. World J Gastroenterol. 2015;21(25):7869–7876. doi: 10.3748/wjg.v21.i25.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcellin P., Gane E., Buti M., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 7.Terrault N.A., Lok A.S.F., McMahon B.J., et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidelines for the prevention, care and treatment of persons with chronic hepatitisB infection. World Health Organization; Geneva: 2015. [PubMed] [Google Scholar]
- 9.Liver EAftSot EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Sarin S.K., Kumar M., Lau G.K., et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinese Society of Infectious Diseases CMA, Chinese Society of Hepatology CMA The guidelines of prevention and treatment for chronic hepatitis B (2019 version)Zhonghua Gan Zang Bing Za Zhi. 2019;27(12):938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang G., Duan Z. Guidelines for prevention and treatment of chronic hepatitis B. J Clin Transl Hepatol. 2021;9(5):769–791. doi: 10.14218/JCTH.2021.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Ru G.Q., Yan R., Zhou Y., Wang M.S., Cheng M.J. Histologic disease in Chinese chronic hepatitis B patients with low viral loads and persistently normal alanine aminotransferase levels. J Clin Gastroenterol. 2016;50(9):790–796. doi: 10.1097/MCG.0000000000000544. [DOI] [PubMed] [Google Scholar]
- 14.Gui H.L., Wang H., Yang Y.H., et al. Significant histopathology in Chinese chronic hepatitis B patients with persistently high-normal alanine aminotransferase. J Viral Hepat. 2010;17:44–50. doi: 10.1111/j.1365-2893.2010.01270.x. [DOI] [PubMed] [Google Scholar]
- 15.Shim J.J., Kim J.W., Oh C.H., et al. Serum alanine aminotransferase level and liver-related mortality in patients with chronic hepatitis B: a large national cohort study. Liver Int. 2018;38(10):1751–1759. doi: 10.1111/liv.13705. [DOI] [PubMed] [Google Scholar]
- 16.Kao J.H., Hu T.H., Jia J., et al. East Asia expert opinion on treatment initiation for chronic hepatitis B. Aliment Pharmacol Ther. 2020;52(10):1540–1550. doi: 10.1111/apt.16097. [DOI] [PubMed] [Google Scholar]
- 17.Lai M., Hyatt B.J., Nasser I., Curry M., Afdhal N.H. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47(6):760–767. doi: 10.1016/j.jhep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Kumar M., Sarin S.K., Hissar S., et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134(5):1376–1384. doi: 10.1053/j.gastro.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 19.Chen C.J., Yang H.I., Su J., et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 20.Iloeje U.H., Yang H.I., Su J., et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Fung J., Cheung K.S., Wong D.K., et al. Long-term outcomes and predictive scores for hepatocellular carcinoma and hepatitis B surface antigen seroclearance after hepatitis B e-antigen seroclearance. Hepatology. 2018;68(2):462–472. doi: 10.1002/hep.29874. [DOI] [PubMed] [Google Scholar]
- 22.Chen S., Mao W., Guo L., Zhang J., Tang S. Combating hepatitis B and C by 2030: achievements, gaps, and options for actions in China. BMJ Glob Health. 2020;5(6) doi: 10.1136/bmjgh-2020-002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J., Hou J.L. Management of chronic hepatitis B: experience from China. J Viral Hepat. 2010;17(Suppl 1):10–17. doi: 10.1111/j.1365-2893.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 24.Mak L.Y., Seto W.K., Fung J., Yuen M.F. Novel developments of hepatitis B: treatment goals, agents and monitoring tools. Expert Rev Clin Pharmacol. 2019;12(2):109–120. doi: 10.1080/17512433.2019.1567327. [DOI] [PubMed] [Google Scholar]
- 25.Seto W.K., Lo Y.R., Pawlotsky J.M., Yuen M.F. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313–2324. doi: 10.1016/S0140-6736(18)31865-8. [DOI] [PubMed] [Google Scholar]
- 26.Ning Q., Wu D., Wang G.Q., et al. Roadmap to functional cure of chronic hepatitis B: an expert consensus. J Viral Hepat. 2019;26(10):1146–1155. doi: 10.1111/jvh.13126. [DOI] [PubMed] [Google Scholar]
- 27.Lee H., Jang S., Ahn S.H., Kim B.K. Cost-effectiveness of antiviral therapy in untreated compensated cirrhosis patient with serum HBV-DNA level < 2000 IU/mL. Hepatol Int. 2022;16(2):294–305. doi: 10.1007/s12072-022-10310-1. [DOI] [PubMed] [Google Scholar]
- 28.Lee H., Kim B.K., Jang S., Ahn S.H. Cost-effectiveness analysis of antiviral therapy for untreated minimally active chronic hepatitis B to prevent liver disease progression. Clin Transl Gastroenterol. 2021;12(2) doi: 10.14309/ctg.0000000000000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tordrup D., Hutin Y., Stenberg K., et al. Cost-effectiveness of testing and treatment for hepatitis B virus and hepatitis C virus infections: an analysis by scenarios, regions, and income. Value Health. 2020;23(12):1552–1560. doi: 10.1016/j.jval.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Z., Fairley C.K., Ong J.J., et al. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: a cost-effectiveness analysis. Lancet Glob Health. 2020;8(10):e1335–e1344. doi: 10.1016/S2214-109X(20)30277-1. [DOI] [PubMed] [Google Scholar]
- 31.Su S., Wong W.C., Zou Z., et al. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health. 2022;10(2):e278–e287. doi: 10.1016/S2214-109X(21)00517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Phanuphak N., Henderson K., et al. Scaling up of HIV treatment for men who have sex with men in Bangkok: a modelling and costing study. Lancet HIV. 2015;2(5):e200–e207. doi: 10.1016/S2352-3018(15)00020-X. [DOI] [PubMed] [Google Scholar]
- 33.Shen M., Zou Z., Bao H., et al. Cost-effectiveness of artificial intelligence-assisted liquid-based cytology testing for cervical cancer screening in China. Lancet Reg Health West Pac. 2023:100726. doi: 10.1016/j.lanwpc.2023.100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J., Liu M., Chai Z., et al. Projected rapid growth in diabetes disease burden and economic burden in China: a spatio-temporal study from 2020 to 2030. Lancet Reg Health West Pac. 2023;33:100700. doi: 10.1016/j.lanwpc.2023.100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L., Liu H., Zou Z., et al. Shared-care models are highly effective and cost-effective for managing chronic hepatitis B in China: Reinterpreting the primary care and specialty divide. Lancet Reg Health West Pac. 2023 doi: 10.1016/j.lanwpc.2023.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong R.J., Kaufman H.W., Niles J.K., Kapoor H., Gish R.G. Simplifying treatment criteria in chronic hepatitis B: reducing barriers to elimination. Clin Infect Dis. 2022;76(3):e791–e800. doi: 10.1093/cid/ciac385. [DOI] [PubMed] [Google Scholar]
- 37.Hepatology Branch of Chinese Medical Association Expert opinions on expanding the antiviral treatment of chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2022;20(2):131–136. doi: 10.3760/cma.j.cn501113-20220209-00060. [DOI] [PubMed] [Google Scholar]
- 38.Tang C.M., Yau T.O., Yu J. Management of chronic hepatitis B infection: current treatment guidelines, challenges, and new developments. World J Gastroenterol. 2014;20(20):6262–6278. doi: 10.3748/wjg.v20.i20.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia J., Shang J., Tang H., et al. Long-term outcomes in Chinese patients with chronic hepatitis B receiving nucleoside/nucleotide analogue therapy in real-world clinical practice: 5-year results from the EVOLVE study. Antivir Ther. 2020;25(6):293–304. doi: 10.3851/IMP3372. [DOI] [PubMed] [Google Scholar]
- 40.Huang H.Y., Xu C.Y., Liu L.H., et al. Increased protection of earlier use of immunoprophylaxis in preventing perinatal transmission of hepatitis B virus. Clin Infect Dis. 2021;73(9):e3317–e3323. doi: 10.1093/cid/ciaa898. [DOI] [PubMed] [Google Scholar]
- 41.Xu B.Y., Xu C.Y., Feng J., et al. Reduced mother-to-child transmission of hepatitis B after implementation of completely charge-free active-passive immunoprophylaxis: an observational cohort study. Expert Rev Vaccines. 2021;20(7):899–905. doi: 10.1080/14760584.2021.1927723. [DOI] [PubMed] [Google Scholar]
- 42.Huang H., Zhang X., Luo Y., et al. The optimal interval for post-vaccination serological test in infants born to mothers with positive hepatitis B surface antigen. Hum Vaccin Immunother. 2021;17(12):5585–5589. doi: 10.1080/21645515.2021.1992213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marseille E., Larson B., Kazi D.S., Kahn J.G., Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Per capita GDP exceeds the world average China’s future development depends on three factors. Chin J Clin Nutr. 2022 doi: 10.38300/n.cnki.nzgjy.2022.000589. [DOI] [Google Scholar]
- 45.Gobel T., Erhardt A., Herwig M., et al. High prevalence of significant liver fibrosis and cirrhosis in chronic hepatitis B patients with normal ALT in central Europe. J Med Virol. 2011;83(6):968–973. doi: 10.1002/jmv.22048. [DOI] [PubMed] [Google Scholar]
- 46.Yuen M.F., Yuan H.J., Wong D.K.H., et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54(11):1610–1614. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong J., Shin J.W., Jung S.W., Park E.J., Park N.H. Tenofovir alafenamide treatment may not worsen the lipid profile of chronic hepatitis B patients: a propensity score-matched analysis. Clin Mol Hepatol. 2022;28(2):254–264. doi: 10.3350/cmh.2021.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hui R.W., Mak L.Y., Seto W.K., Yuen M.F. RNA interference as a novel treatment strategy for chronic hepatitis B infection. Clin Mol Hepatol. 2022;28(3):408–424. doi: 10.3350/cmh.2022.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohn W., Kang D., Kang M., Guallar E., Cho J., Paik Y.H. Impact of nationwide hepatocellular carcinoma surveillance on the prognosis in patients with chronic liver disease. Clin Mol Hepatol. 2022;28(4):851–863. doi: 10.3350/cmh.2022.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.