Summary
In pursuit of Universal Health Coverage (UHC) for eye health, countries must strengthen services for older adults, who experience the highest prevalence of eye conditions. This scoping review narratively summarised (i) primary eye health services for older adults in eleven high-income countries/territories (from government websites), and (ii) the evidence that eye health services reduced vision impairment and/or provided UHC (access, quality, equity, or financial protection) (from a systematic literature search). We identified 76 services, commonly comprehensive eye examinations ± refractive error correction. Of 102 included publications reporting UHC outcomes, there was no evidence to support vision screening in the absence of follow-up care. Included studies tended to report the UHC dimensions of access (n=70), equity (n=47), and/or quality (n=39), and rarely reported financial protection (n=5). Insufficient access for population subgroups was common; several examples of horizontal and vertical integration of eye health services within the health system were described.
Funding
This work was funded by Blind Low Vision New Zealand for Eye Health Aotearoa.
Keywords: Universal health coverage, Healthy ageing, Older people, Eye health service
Research in context.
Evidence before this study
In its inaugural World Report on Vision in 2019, the World Health Organization called for eye care to be included in efforts to achieve Universal Health Coverage (UHC). In 2021, the Lancet Global Health Commission on Global Eye Health supported this call, stating that UHC should not be considered “universal” without including eye health. As the prevalence of eye conditions and vision impairment increases with age, older adults are an important group to consider when strengthening eye health services.
In 2020, the government of Aotearoa New Zealand (hereafter ‘New Zealand’) proposed funding eye health checks for the population ≥65 years. This proposal aligned with New Zealand's Healthy Ageing strategy, but how this eye health check should be implemented was unclear. In July 2020, we conducted a preliminary search of the published literature within the PubMED database (using “vision screening” and “New Zealand” MeSH search tags), where we observed a paucity of evidence describing adult eye screening services in New Zealand. Widening our search internationally, we identified three systematic reviews (from Cochrane, and both the Canadian and US Preventative Task Force), each reporting insufficient evidence that community vision screening services for older people (such as the proposed “eye health check”) can reduce vision impairment in this population.
Countries such as New Zealand that are attempting to improve eye health among older adults are challenged by the lack of evidence supporting the effectiveness of primary eye health services, and the best way to structure these services within the health system to achieve UHC. We designed this review to fill this evidence gap, and therefore to better inform future eye services in New Zealand and elsewhere.
Added value of this study
This review provides the practical and theoretical evidence that can direct policy makers in high-income countries towards achieving UHC when designing new eye health services for older people. Firstly, we collated the types of primary eye health services available in New Zealand and comparable high-income countries (Australia, the devolved countries of the United Kingdom, Ireland, Singapore, Hong Kong, Canada, and the United State of America). A key finding was that community vision screening services for older adults were uncommon (we identified only one visual acuity screening service, in Singapore). All other included countries, except New Zealand, funded eye examinations (often with refractive error correction) for all older adults, or a subgroup considered most at risk of financial barriers. Secondly, we systematically scoped the literature for evidence of services that reduced population vision impairment or incorporated UHC in its design and implementation (access/coverage, equity, quality [using the Institute of Medicine (IOM) dimensions: integration, efficiency, timeliness, safety, and people-centredness], or financial protection). The identified evidence describes a need for services that prioritise underserved population groups, to avoid further widening existing inequalities (e.g. as observed in Scotland's universal eye care policy); a lack of evidence reporting financial protection for health system users; and several examples of service integration (e.g. shared care schemes) that may improve access to services via horizontal and vertical integration.
Implications of all the available evidence
Vision screening for older adults (in isolation from broader eye health services) was used in only one of the countries we assessed, and there is no evidence to suggest this strategy reduces vision impairment. Financial protection is a cornerstone of UHC and all countries except New Zealand provided this protection for at least some older adults. We identified a range of strategies that countries can consider when wishing to improve access, quality, or financial protection of primary eye health services for older adults as part of UHC initiatives. Embedding equity into new strategies by prioritising the needs of the historically underserved groups will help avoid intervention-generated inequities.
Alt-text: Unlabelled box
Introduction
In its inaugural World report on vision, the World Health Organization called for eye health to be part of efforts to achieve universal health coverage (UHC) through implementing Integrated People-centred Eye Care (IPEC) across the spectrum of promotive, preventative, curative and rehabilitative services.1 In 2020 this call was adopted by 194 countries at the 73rd World Health Assembly.2 UHC is defined as people being able to access the care they need, of sufficient quality to be effective, without suffering financial hardship.3 UHC has received increasing attention in eye health in recent years, including as a central theme of the Lancet Global Health Commission on Global Eye Health.4
Eye conditions are strongly associated with increasing age—more than three-quarters of the estimated 43 million people living with blindness and almost two-thirds of the 553 million with distance vision impairment in 2020 were aged ≥50 years.4 Older adults may face a range of barriers to accessing health services that prohibit healthy ageing.5 Therefore, when countries are planning to improve access to eye health for their population, older adults are a group in need of particular attention. In 2020, the government of Aotearoa New Zealand (hereafter called New Zealand) announced a strategy to provide eye health checks to the population ≥65 years.6 However, New Zealand had minimal research on eye health services or monitoring data to inform the government on which strategy would be most appropriate and effective to improve access to eye health services for older New Zealanders.7
The aims of this review were to summarise (i) the nature and extent of community or primary eye health services for older adults in eleven high-income countries and territories, and (ii) the extent to which eye health services in these settings reduce vision impairment and/or provide UHC of eye health services for older adults. While the lack of synthesized evidence in New Zealand provided the impetus for this review, we believe the findings are relevant for many countries wishing to improve access to eye health services in pursuit of UHC. We have synthesised our findings as a scoping review due to the broad nature of the research questions and the diversity of the included evidence.
Methods
Overview
The protocol8 was registered on the Open Science Framework on 31st October 2020, following input from a stakeholder group.
We looked for evidence in the grey and published literature by asking two complementary questions.
Question 1: Existing eye health services
What government-led community or primary eye health services for older adults are offered in New Zealand and similar countries, and how are they structured within the health system?
Question 2: Availability of evidence
What is the evidence that eye health services within the selected countries:
-
i)
reduce vision impairment? and/or
-
ii)
provide UHC of eye health for older adults?
We envisaged limited evidence reporting outcomes on vision impairment from the specific services identified in the grey literature. Therefore, we sought evidence that described services in the included countries across any of the UHC dimensions of access, quality, equity, or financial protection, with the assumption that services addressing these dimensions are contributing to strengthening eye health services.
Search strategy & selection criteria
For both Questions 1 and 2, the included evidence reported eye care services within eleven countries: New Zealand, Australia, Canada, Ireland, United Kingdom (England, Scotland, Wales and Northern Ireland), Singapore, Hong Kong, and the United States of America. The included countries were predominantly English-speaking high-income countries or territories9 with a population of at least 2 million10 in 2019 considered to have health care systems with similarities to that in New Zealand. The included countries also ranked relatively highly in their health expenditure per capita,11 and the WHO's UHC service coverage index12 (Supplementary Table 1).
We used separate but complementary inclusion criteria to address the two questions (Table 1).
Table 1.
Inclusion or exclusion criteria for evidence addressing Question 1 and 2.
| Characteristics of service/evidence | Question 1: Existing eye care services | Question 2: Evidence for the effectiveness of eye care services |
|---|---|---|
| Population | • Older adults (∼65 years and above), or adult populations where outcomes for older adults are described separately. | |
| Setting | • Services or evidence from ≥1countries most relevant to the New Zealand health system (defined as high-income countries9 with ≥2 million10 population in 2019, where English is an official language): New Zealand, Australia, Canada, Ireland, United Kingdom (England, Scotland, Wales, Northern Ireland), Singapore, Hong Kong, and the United States of America. | |
| Time period | • Available/advertised services in mid-2020 | • Evidence published between1 January 2010 and October 2020 |
| Interventions | • Community or primary eye care services that offer screening, general eye care, treatment, referral, or rehabilitation, and report on at least one of the WHO building blocks (Question 1) or UHC dimensions (Question 2) described below. | |
| • Administered at a state/provincial or national level • Wholly or partially funded by the government or other public funds Exclusion criteria: • Non patient-facing services, e.g. funding for research or equipment. • Research, pilot, time-limited, and/or non-governmental projects • Secondary or tertiary eye care services offered exclusively outside the primary care setting (e.g. hospital eye care services) |
• Evidence relating to service delivery (specific or general eye care service) and/or: • Interventions that treat any of the eye conditions commonly causing vision impairment in older adults: cataract, uncorrected refractive error, age-related macular degeneration, glaucoma, or diabetic retinopathy Exclusion criteria: • Methodological evidence (e.g. technical comparisons, diagnostic accuracy of screening equipment). |
|
| Types of evidence | • Evidence written in English with full-text available. | |
| • Information describing service structure retrieved from government web pages and relevant policy documents within, including reports, guidelines, audits, or government legislation. Exclusion criteria: • Outdated documents replaced by more recent information |
• Experimental, quasi-experimental and observational studies, systematic and scoping reviews, overviews of systematic reviews, research letters • Grey literature describing service performance identified within Question 1 Exclusion criteria: • Qualitative research including case studies, opinions, editorials • Data derived solely from computer modelling (e.g. cost-effectiveness) |
|
| Outcomes | 1. Service structure described by at least one of WHO's health system building blocks63:
|
Exclusion criteria: • Self-reported vision impairment |
For Question 1, one reviewer (LG) searched grey literature (including policy documents, reports, guidelines, audits, and general information described within government web pages) during August-October 2020, with verification by a second reviewer (SM, LK, BT). Eye care programmes were identified from the national government's website of each included country. Additional searches of state/provincial government websites were also completed, except for the USA, where the large number of states and the complexity of the health system meant only national-level services were included. Parallel programmes available within the devolved countries of the United Kingdom were included separately. General search terms, including “eye”, “vision”, “optical”, “optometry” and “ophthalmology” were used to identify eligible information within each website, and we repeated the search using the Google search engine. Relevant links within documents to other sources of information were pursued. Searching continued until retrieved results were unambiguously irrelevant.
For Question 2, an Information Specialist from Cochrane Eyes and Vision (IG) conducted a search in MEDLINE, Embase, Cochrane Library and the CRD Database (DARE, NHS EED and HTA) on 19th October 2020 (Supplementary Table 2). Grey literature from Question 1 that described programme performance were also included. Screening of retrieved publications was conducted in Covidence (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). During each round of screening, two reviewers (from LG, JB, SM, BT, SW and JR) independently screened i) each title and abstract, then ii) the full-text manuscript of each potentially relevant publication, against the eligibility criteria (Table 1). Data extraction was performed in duplicate for ∼20% of included full text publications (n=20 publications). At each stage of screening, differences of opinion were resolved by discussion. The remaining data extraction was performed by a single reviewer (LG).
Data synthesis
Primary eye care programmes included in Question 1 were described using the six WHO health system building blocks outlined in our study protocol8 and Table 1. Where relevant, programmes were assigned to multiple sub-categories within a building block. The number of programmes fulfilling each building block was calculated, and relevant examples described narratively.
For the evidence describing effectiveness of eye care programmes (Question 2), general information was extracted from each publication and included the country in which the data were collected, the study design, the year of publication, and the eye condition assessed or treated by the service. For the latter category, publications describing vision impairment in general (i.e. not a specific condition) were classified under ‘general’ eye conditions. For each publication, we summarised outcomes that described (i) change in vision impairment, and/or (ii) any of the UHC dimensions (access, equity, financial protection, or quality (integration, efficiency, timeliness, safety, and people-centredness)8 (Table 1). The number of included publications that reported each outcome was calculated, and the overall findings described narratively. All study types (i.e. observational and interventional studies) were analysed together, and evidence reporting specifically on eye care programmes identified in Question 1 was highlighted.
Changes to the protocol
Our original intention was to complete a rapid systematic review that could inform New Zealand policy.8 However, due to the heterogeneity of the included evidence, we synthesised our findings as a scoping review and omitted the quality appraisal so that we could draw conclusions from a wider range of sources.
Role of the funding source
The funders had no role in the study design, collection, analysis, or interpretation of data for this review.
Results
Question 1: Existing services for older adults
From our website search, we identified 76 eye care programmes relevant to older adults across the eleven included countries/territories. The service structure of the identified programmes across WHO's building blocks are summarised below (and in Table 2), and key examples of individual programmes are highlighted in Table 3. Most programmes from Australia and Canada were operated at a state or provincial level (12/15 and 29/31 programmes respectively), whereas included programmes from the remaining countries were nationally operated (Table 2).
Table 2.
Characteristics of eye care programmes relevant to older adults in New Zealand and similar countries described within WHO building blocks.
| SERVICE STRUCTURE | n (N=76) | % | ||
|---|---|---|---|---|
| Country | Canada | national | 2 | 2.6 |
| provincial | 29 | 38.2 | ||
| Australia | national | 3 | 3.9 | |
| state | 12 | 15.8 | ||
| UK | England | 3 | 3.9 | |
| Northern Ireland | 3 | 3.9 | ||
| Scotland | 2 | 2.6 | ||
| Wales | 4 | 5.3 | ||
| Great Britain | 1 | 1.3 | ||
| USA | 5 | 6.6 | ||
| New Zealand | 4 | 5.3 | ||
| Ireland | 3 | 3.9 | ||
| Singapore | 3 | 3.9 | ||
| Hong Kong | 2 | 2.6 | ||
| Service delivery | ||||
| Eligible populationa | Socially disadvantaged | 33 | 43.4 | |
| Condition-specific | 26 | 34.2 | ||
| Older people | 18 | 23.7 | ||
| General population | 10 | 13.2 | ||
| Medical technologies | ||||
| Assessmentsa | Eye examinations | 42 | 55.3 | |
| Diabetic retinal screening | 9 | 11.8 | ||
| Glaucoma screening | 3 | 3.9 | ||
| Visual acuity screening | 1 | 1.3 | ||
| Other screening | 1 | 1.3 | ||
| Treatmentsa | Refractive error correction | 44 | 57.9 | |
| Low vision rehabilitation | 13 | 17.1 | ||
| Prosthetic eye | 8 | 10.5 | ||
| Surgery | 8 | 10.5 | ||
| Health workforce | ||||
| Providera | Optometry clinician | 55 | 72.4 | |
| Ophthalmology clinician | 37 | 48.7 | ||
| Technician / screener / nurse | 9 | 11.8 | ||
| Specialty worker | 6 | 7.9 | ||
| General practitioner | 4 | 5.3 | ||
| Not identified | 10 | 13.2 | ||
| Locationa | Healthcare locations | 35 | 46.1 | |
| Community locations | 10 | 13.2 | ||
| Not identified | 37 | 48.7 | ||
| Health financing | ||||
| Financial protectiona | Subsidised | 55 | 72.4 | |
| No out-of-pocket payment | 20 | 26.3 | ||
| Loan | 1 | 1.3 | ||
| Management of financesa | Direct payment / reimbursement | 71 | 93.4 | |
| Voucher | 8 | 10.5 | ||
| Not applicable | 1 | 1.3 | ||
| Funding structurea | Primarily government funded | 74 | 97.4 | |
| Public-private funding | 2 | 2.6 | ||
| Leadership & governance | ||||
| Accountabilitya | Governance identified | 14 | 18.4 | |
| Stakeholders identified | 8 | 10.5 | ||
| Not identified | 59 | 77.6 | ||
| Health information | ||||
| Reportinga | Health monitoring identified | 10 | 13.2 | |
| Service performance identified | 17 | 22.4 | ||
| Not identified | 55 | 72.4 | ||
As each programme can fulfil multiple categories within each building block, the total number of programmes may sum to >76.
Table 3.
Examples of services relevant to older adults from countries of interest. Service structure is described within WHO Building Blocks and evidence from the literature across Universal Health Coverage dimensions.
| Service | Service structure (WHO health system building blocks) | Universal Health Coverage dimensions reported in the published literature |
|---|---|---|
| Victorian Aboriginal Spectacle Subsidy Scheme (Australia)66 |
|
|
| Equipment for people who are blind or have reduced vision (New Zealand)67 |
|
|
| National Health Service General Ophthalmic Services (UK)18,68, 69, 70 |
|
|
| Eye Health Examination Wales (UK)73 |
|
|
| Northern Ireland Primary Eyecare Assessment and Treatment Service (NI-PEARS) (UK)74 |
|
|
| National Health Service Diabetic Eye Screening Programme (UK)75, 76, 77, 78 |
|
|
| Welsh Low vision Service (UK)85 |
|
|
| Diabetic Retina Screen (Ireland)89 |
|
|
| Primary Care Networks (Singapore)91 |
|
|
| Elderly healthcare voucher scheme (Hong Kong)93 |
|
|
| Hospital Authority's Risk Assessment and Management Programme (Hong Kong)94 |
|
|
What is offered and who for?
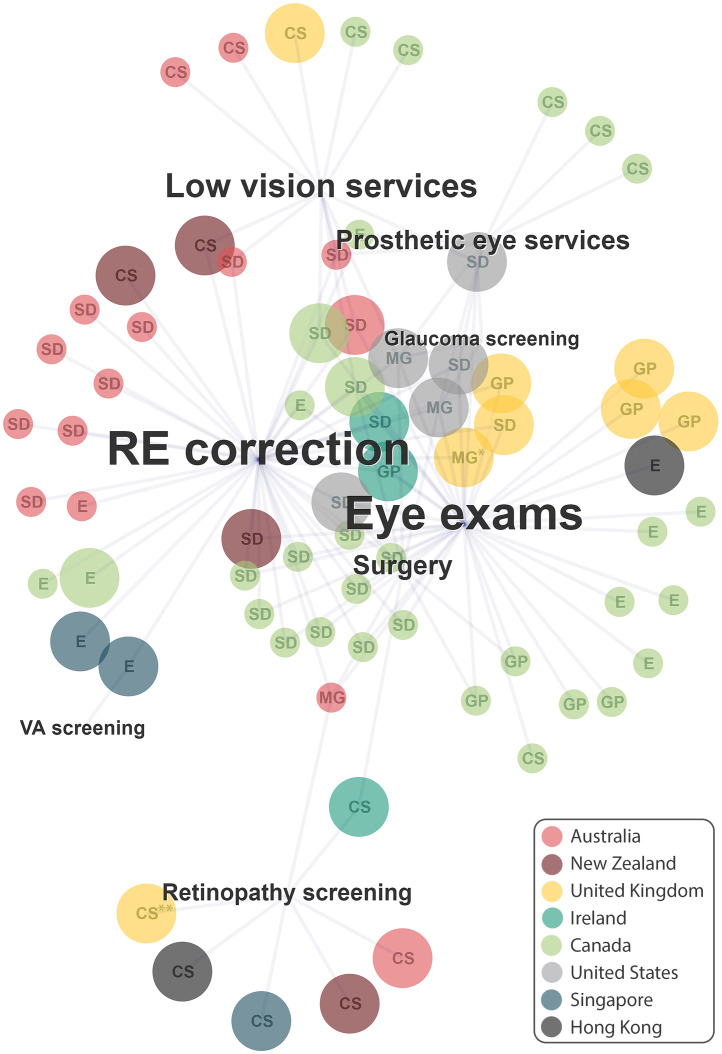
The 76 eye care programmes offered a range of assessment and treatment services. Some ran isolated services, while others offered a combination of several services (Table 2, Figure 1). In general, programmes that targeted broad population groups (e.g. socially disadvantaged people) offered comprehensive services including eye exams, refractive error correction, and surgery (shown as the core services in the centre of Figure 1), and these were mostly staffed by clinicians. Programmes offering specific services (e.g. screening or rehabilitation) were less likely to provide general eye care and were administered by technical workers (shown at the edges of Figure 1).
Figure 1.
Network diagramme illustrating the types of services offered by the included 76 eye care programmes across 11 high-income countries/territories.
Each programme is illustrated by a single circle: colour indicates the country that offers the programme (see key); size indicates the programme scope (small circles=state or provincial, large circles=national programmes; letters indicate eligible population: E=Elderly, SD=socially disadvantaged, CS=condition specific, GP=general population, MG=multiple groups). Each programme offers one or more of eight services, illustrated by text labels: size of the text represents the relative proportion of programmes offering that service. Services: Refractive error correction (RE correction; n=44), eye exams (n=42), low vision services (n=13), diabetic retinal screening (n=10), prosthetic eye services (n=8), surgery (n=8), glaucoma screening (n=3), visual acuity (VA) screening (n=1). *Indicates parallel services offered within England, Northern Ireland, and Wales; **Indicates parallel services offered within England, Northern Ireland, Scotland, and Wales.
All countries had at least one programme offering visual acuity assessment (Singapore) or eye examinations (all other countries); New Zealand was the only country without a programme that provided this care without requiring out-of-pocket payments from groups least able to pay. Of the 43 programmes offering eye examinations, 27 also included refractive error correction, and an additional 15 subsidy programmes offering refractive error correction were identified (ten of which were across eight states/territories of Australia). Fourteen screening services were identified: national-level diabetic retinal screening programmes in nine countries, three glaucoma screening services in the USA packaged within comprehensive eye care programmes, one programme in Australia that encouraged service providers to enrol people with diabetes into a reminder system to promote access to retinal screening, and one visual acuity screening service in Singapore. Two of the screening services included follow-up care within the service itself (i.e. Ireland's Diabetic RetinaScreen, and Singapore's Project Silver Screen that included some funding for refractive error correction, Table 3). Nineteen rehabilitation services were identified from five countries (New Zealand, Australia, Wales, Canada, and the USA); thirteen of these provided low vision aids (e.g. Welsh Low Vision Service, Table 3) and eight provided prosthetic eye services.
Populations eligible for each programme varied, and some programmes targeted multiple population groups (Table 2, Figure 1). Diabetic retinal screening, glaucoma screening, and low vision or prosthetic eye services were available specifically for people with these eye conditions or needs. Eighteen services targeted older people (thirteen of these exclusively), including one screening programme, twelve general eye care programmes offering eye examinations, and five subsidies for refractive error correction. The population group most commonly eligible for the services we identified were those experiencing social disadvantage, targeted by 33 services (offering eye examinations and/or refractive error correction) including General Ophthalmic Services in England, Northern Ireland and Wales, and the Victorian Aboriginal Spectacle Subsidy Scheme in Australia (Table 3). Ten services offering eye examinations were available to the general population (mostly in the UK and Canada), including the General Ophthalmic Services Scotland (Table 3).
Who provides the service and where?
The 76 eye care programmes were administered by a range of service providers, and some programmes by multiple provider types and different locations (Table 2). Clinicians working in optometry (e.g. opticians or optometrists) or ophthalmology (e.g. ophthalmic practitioners or ophthalmologists) were the most common (55 and 37 services respectively). Eight (of nine) services provided by technicians or nurses, and two (of four) services provided by GPs (e.g. Singapore's Primary Care Networks, Table 3) were screening services. The six services operated by technical workers (e.g. ocularists and low vision assessors) were low vision and prosthetic eye services. The location of the service was described for about half of the identified programmes (n=39). These services were provided at healthcare centres (including public clinics, private practices, or hospitals) and/or community locations not conventionally dedicated for healthcare (e.g. mobile clinics or community centres).
How is it funded?
Seventy-four of the 76 identified services were publicly funded (e.g. Medicare in Australia and the USA, the UK's National Health Service), while two services reported corporate funding in addition to public funding (Australia's KeepSight, and Singapore's Project SilverScreen).
What does the user pay?
Twenty services required no out-of-pocket payment by the user: nine of these provided eye examinations, six were national diabetic retinal screening services, and four were refractive error subsidies or low vision services. Most services required the user to pay an additional fee or co-payment for basic service. Payment for services were made directly to the service provider or by reimbursement to the service user, although we also identified eight services where monetary vouchers were provided to the service user, (including Hong Kong's Elderly Healthcare Voucher Scheme, Table 3). The eye examination service offered in New Zealand differed from the service offered within other countries, as it was provided as a financial loan for eye care.
Who is accountable for maintaining service quality?
Limited information was available describing the governance structure and health reporting within the included services. We identified fourteen services that reported how the service was governed, eight services that acknowledged communication with stakeholders, and ten services with published health monitoring standards. We also identified eighteen published audits of service performance (from 11 services) (outlined below in Question 2).
Question 2: Availability of evidence
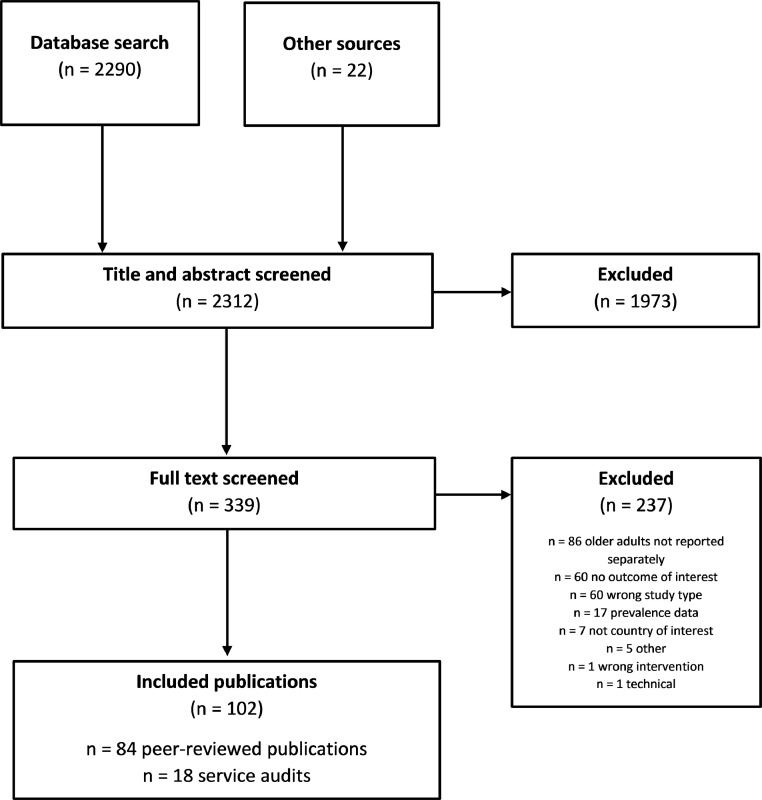
Of the 2,312 publications retrieved from our search, we examined the full-text of 339 and ultimately included 102 in our synthesis: 84 peer-reviewed studies and 18 service audits identified from Question 1 (Figure 2). Most publications reported findings from cross-sectional or cohort studies (n=73) and described eye care services in the UK (n=39), USA (n=21), or Australia (n=15); almost twice as many were published in the second half (n=68) compared to the first half (n=34) of the decade. Forty-two publications reported on diabetic retinopathy, 18 on glaucoma services, and 32 reported general eye care services not pertaining to any particular condition (Table 4).
Figure 2.
PRISMA flow diagram summarising the screening and selection of evidence to answer Question 2 of the review.
Table 4.
Characteristics of included studies reporting Universal Health Coverage outcomes from eye care services within the included countries.
| Study Characteristics | n (N=102) | % | ||
|---|---|---|---|---|
| Country | UK | 39 | 38.2 | |
| USA | 21 | 20.6 | ||
| Australia | 15 | 14.7 | ||
| Canada | 5 | 4.9 | ||
| Ireland | 6 | 5.9 | ||
| Singapore | 5 | 4.9 | ||
| Hong Kong | 4 | 3.9 | ||
| International | 5 | 4.9 | ||
| New Zealand | 1 | 1.0 | ||
| New Zealand /Australia | 1 | 1.0 | ||
| Study design | Cross-sectional or cohort | 73 | 71.6 | |
| Quasi-experiment | 15 | 14.7 | ||
| Meta-analysis | 5 | 4.9 | ||
| Mixed methods | 4 | 3.9 | ||
| Randomised Controlled Trial (RCT) | 2 | 2.0 | ||
| Survey | 2 | 2.0 | ||
| Open controlled trial | 1 | 1.0 | ||
| Year of publication | 2010-2014 | 34 | 33.3 | |
| 2015-2020 | 68 | 66.7 | ||
| Eye condition | Diabetic retinopathy | 42 | 41.2 | |
| General | 32 | 31.4 | ||
| Glaucoma | 18 | 17.6 | ||
| Cataract | 6 | 5.9 | ||
| Uncorrected refractive error | 3 | 2.9 | ||
| Age-related macular degeneration | 1 | 1.0 | ||
| Outcomea | Change in vision impairment | 7 | 6.0 | |
| Access | 70 | 68.6 | ||
| Equity | 47 | 46.1 | ||
| Financial protection | 5 | 4.9 | ||
| Quality | Integration | 39 | 38.2 | |
| Efficiency | 28 | 27.5 | ||
| Timeliness | 18 | 17.6 | ||
| Safety | 11 | 10.8 | ||
| People-centredness | 10 | 9.8 | ||
As each publication can report multiple outcomes, the total number of publications sums to >102.
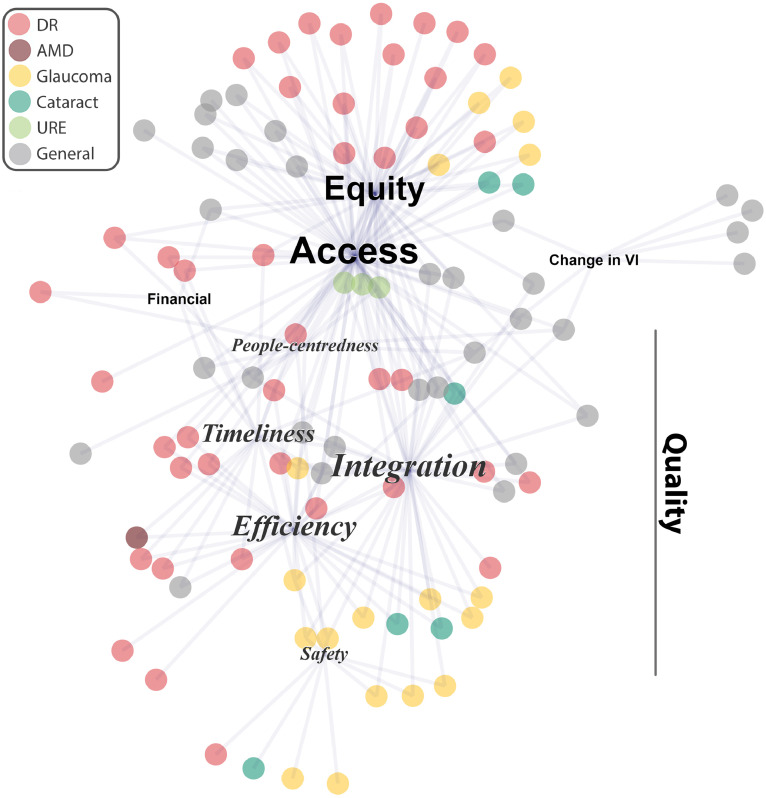
Most included publications reported more than one outcome (i.e. (i) change in vision impairment, and/or (ii) any of the UHC dimensions: median=2 per publication, interquartile range (IQR) 2-3; Table 4; Figure 3). The identified evidence is summarised in brief below across each outcome (and detailed in Supplementary Table 3), and the evidence for the specific programmes identified in Question 1 (detailed in Table 3) is highlighted.
Figure 3.
Network diagramme illustrating the Universal Health Coverage dimensions of eye health services reported by the included publications (n=102).
Each publication is illustrated by a single circle: colour indicates the vision condition that the published evidence described (see key). Each publication reported one or more UHC dimensions, illustrated by text labels, whereby the size of the text illustrates the relative proportion of publications reporting that dimension. UHC dimensions: access (n=70), equity (n=47), quality (integration (n=39), efficiency (n=28), timeliness (n=18), safety (n=11), people-centredness (n=10), change in vision impairment (n=7), financial protection (n=5); Quality dimensions are shown in italics; VI=vision impairment, DR = diabetic retinopathy, AMD=age-related macular degeneration; URE=uncorrected refractive error.
Reduction in vision impairment
The seven publications reporting changes in vision impairment described general eye care services (Figure 3). These included three systematic reviews each concluding that there is insufficient evidence to support community vision screening for vision impairment in older people.13, 14, 15, 16
Universal health coverage for eye health
Access to services was the most reported UHC dimension (n=70 publications; Table 4). Audits of UK's NHS-funded eye care services illustrated increased use of these services over time, and that people aged ≥65 years received the largest proportion of funded eye examinations (Table 3).17, 18, 19, 20 Equity was also a frequently reported UHC dimension (n=47), with substantial overlap between publications describing both access and equity (n=45 publications; Figure 3). Scotland's universal eye care policy improved access to eye care services overall, but these improvements were less apparent in population groups with lower income or lower education, suggesting that the policy may have widened socioeconomic inequities (Table 3).21 Thirty-one publications identified the socioeconomic factors associated with poorer access to eye screening or follow-up; under-served population groups included those with lower levels of education or income, non-Caucasian ethnicities, those without health insurance, and people living in areas that are remote or with high area-level deprivation (Supplementary Table 3).
Financial protection was reported infrequently (n=5), and the included evidence illustrated that even minimal costs present a barrier to accessing eye care. An RCT conducted within the Hong Kong Hospital Authority showed poorer access to diabetic retinal screening when a small co-payment was required compared to a service with no out-of-pocket costs,22 and an audit of New Zealand's low vision services illustrated that some vision aids are expensive, which may reduce their accessibility23 (Table 3).
Publications reporting quality dimensions were less connected to access, equity, or financial protection (Figure 3). Of the quality dimensions, integration (n=39) was commonly reported (Table 4). Eleven of these reported the integration (± safety dimension) of glaucoma eye care services24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 in the UK that refined referrals or allowed shared management and post-operative care of low-risk patients by community optometrists. These services reduced the burden on ophthalmologists in hospital eye care services by minimising false-positive referrals and managing patients outside the hospital system. Nine publications illustrated how eye care services (including primary diabetic screening) can be integrated into primary care via general practice (Supplementary Table 3). Eight publications from Australia illustrated the importance of well-integrated, culturally safe eye care services for Indigenous people (Supplementary Table 3), such as the Victorian Aboriginal Spectacle Subsidy Scheme35, 36, 37 (Table 3). In Hong Kong, a scheme encouraging older people to seek primary health care from private rather than public providers has had mixed results (Table 3).38
The efficiency of eye care services was also a commonly reported quality dimension (n=28), which frequently overlapped with timeliness (n=18; 12 of which also reported an efficiency outcome; Table 4, Figure 3). Data from the national diabetic eye screening programmes in England,39 Scotland,40,41 and Ireland42, 43, 44 reported long wait times for screening and a high referral rate during the initial years of the programme, however this reduced within a few years as existing conditions were detected and treated. Eight publications described how teleophthalmology can improve the efficiency of diabetic retinopathy,45, 46, 47, 48, 49 age-related macular degeneration (AMD),50 general vision screening,51 or ophthalmology52 services (Supplementary Table 3). Safety (n=11) and people-centredness (n=10) were the least reported quality dimensions (Table 4; Supplementary Table 3).
Discussion
This systematic scoping review includes a broad range of evidence describing the types of eye health services for older adults that are available within eleven high-income countries/territories. In addition to evidence for a reduction in population vision impairment, we sought evidence for the extent to which eye care services are addressing UHC dimensions. We aimed to inform a specific policy being considered in New Zealand,6 but our findings are relevant more generally as countries attempt to improve UHC for eye health.1 In general, we observed a disparity between what existing services offer (Question 1) and the research focus (Question 2): most services targeted underserved populations with general care, while research most commonly focused on diabetes services.
The initial idea proposed for improving eye health among older adults in New Zealand was visual acuity screening for everyone aged ≥65 years.6 We found no evidence to support vision screening without also providing adequate follow-up care. Indeed, we identified reviews by three separate groups that determined there was no or inconclusive evidence to support community vision screening for impaired vision in older people, including from Cochrane, and both the Canadian and US Preventative Task Force.13, 14, 15, 16 Singapore was the only country we identified with a vision screening programme specifically for older adults.53 Eye care policies from other countries favour more comprehensive eye care services. For example, Scotland replaced the NHS “sight test” with a comprehensive eye examination in 2006,54 and more recently expanded this so clinicians can perform additional follow-up tests.19 Beyond Scotland, the other included countries commonly provided a fully or partially subsidised eye examination, generally performed by an optometrist and available to a subset of the population most in need of subsidised services, which often included people aged ≥65 years. Many of the identified services also had integrated follow-up treatment, including subsidised refractive error correction. New Zealand was the only country included in this review without any subsidised eye examinations or refractive error correction for older adults.
The most common UHC dimensions reported in the literature were access and equity, followed by quality (particularly the quality dimensions of integration and efficiency); very few publications reported on financial protection. Our review provides some key considerations for decision-makers in pursuit of UHC for eye health in their country.
Achieving UHC for eye health among older adults will require decision-makers to incorporate equity into the design of services, including targeting the most underserved populations subgroups. Our included evidence commonly reported inequities in access to eye care for people aged ≥65 years particularly if they were of a non-Caucasian ethnicity, had low education or income, did not have health insurance, or lived in areas that were remote or with high deprivation. To address these disparities, we found evidence that eye care services operated by culturally safe service providers in a community setting rather than formal eye health care setting can reach more older people from minority or underserved groups,55 particularly in rural and remote locations.49 Another strategy could involve lower age thresholds for population groups with lower life expectancy to become eligible for programmes, such as Māori and Pacific people in New Zealand.56
Integration and the continuum of care across levels was another common theme in this review, evident in some but not all countries and featured in more than one-third of the literature we identified. Integration is currently a key priority in global eye health. In the World Report on Vision, WHO outlined the need for Integrated People-centred Eye Care (IPEC) to achieve UHC,1 and this was endorsed by all Member States via a World Health Assembly Resolution in 2020.2 Further, integration featured twice in the top 10 priorities following a recent Grand Challenges in global eye health prioritisation process, highlighting the need for better vertical integration between levels of eye health services, and horizontal integration with other health services.57 We found examples of both of these.
One horizontal integration example we identified was retinal screening for people with diabetes delivered via general practice, which increased retinal screening rates.58 Strategies like this that increase access to primary eye health services will increase detection of conditions requiring treatment which can lead to increased demand for tertiary care.57 We found examples of vertical integration strategies to mitigate this, including shared-care between optometrists and ophthalmologists in the UK for people with glaucoma24 and cataract.59 A common theme of studies reporting elements of service integration was the importance of good communication between providers and enabling technology—including teleophthalmology and secure electronic health information systems—to enhance efficiency and timeliness of the service.57
Refractive error correction is a condition that highlights the importance of integrated eye care services. Refractive error is the leading cause of vision impairment globally, including among older adults.60 The importance of its correction in the pursuit of UHC was confirmed by the World Health Assembly Resolution in 2021, where Member States signed up to ambitious targets to increase effective refractive error coverage (eREC).61 Of our included countries, Australia is the only one which has an estimate of eREC—in 2016 approximately 9 out of 10 non-Indigenous (eREC: 93.5%) and 8 out of 10 Indigenous Australians (eREC 82.2%) requiring refractive error correction had it and could see 6/12 (i.e. were not vision impaired).62 While this inequity between the two groups must be overcome, we believe the spectacle subsidies available in Australia make an important contribution to Australia currently having the highest eREC estimates, albeit among a small group of countries with data available.4 This can be confirmed when eREC estimates become available for other countries with spectacles subsidies, including the UK and Canada.
Comprehensive monitoring to assess effectiveness of the 76 programmes we identified in Question 1 was largely unavailable—we found reports on 11 of these services, the majority from service audits or other grey literature. While most of these reported positive outcomes, including increased use of services or improved efficiency over time, it is unclear whether these services are adequate to meet the needs of the population. Moreover, a lack of evidence within the included UHC dimensions may be a result of under-reporting, rather than a lack of adherence to eye care services within UHC principles. In pursuit of UHC for eye health, more and better evidence is required on what works, for whom, and in what circumstances—this can include better use of routinely collected information, as well as via stronger partnerships between researchers and decision-makers to answer policy-relevant questions. The scoping nature of our review and the heterogeneity of the included evidence precluded critical appraisal of the evidence we identified. As eye health researchers respond to the call made by WHO1 and the Lancet Commission4 to generate more UHC-aligned evidence in the coming years, more extensive synthesis, including critical appraisal will be possible.
Finally, we found very few studies reporting outcomes relating to financial protection and those that did highlighted the need for it rather than demonstrating ways in which providing it improved coverage of services. Out-of-pockets costs are a major barrier to eye health,4 and the urgent need to address this dearth of evidence on financial protection was recognised in the recent Grand Challenges in global eye health exercise.57
Our review must be considered in light of several limitations. First, we limited our search to English-speaking countries with health services comparable to New Zealand. While the range of programmes identified provide useful information for New Zealand and similar countries, there may be strategies to improve eye health in older adults in countries that are absent from this summary. Second, we accessed information from public-facing websites which meant the details we sought on service structure were not always available. To reduce missing information, where possible we verified our comprehensive search results with researchers familiar with the health system in that country. Third, due to the focus of our search on primary-level services for older adults, we may have missed publications that report on relevant services that are available at other levels and to a larger age range. Despite these limitations, we have found a broad range of relevant evidence that can be considered by countries wanting to improve access to eye care among older adults.
Conclusion
In most of the high-income countries included in this review, eye care examinations are available for people aged ≥65 years for little or no out-of-pocket cost. New Zealand is a notable outlier, with no subsidised general eye health services for older adults. Future eye care policies in New Zealand or elsewhere should incorporate UHC in their design, by targeting services towards underserved population groups, and integrating eye health services within the current health system. This could be achieved via horizontal integration with other primary care services and vertical integration that allows shared-care between optometrists and tertiary care providers. Future research in this area could investigate how financial protection for the service user can encourage access to services.
Contributors
The review was conceptualised by JR, JE, and LG. Supervision was provided by JR and JE, and project administration performed by LG. Grey literature searching on government websites was performed by LG with assistance from SM, LK, and BT. IG constructed the search. Screening of abstracts and full-text reports was performed by LG, JR, JB, BT, SM, and SW. Data extraction from included reports was completed by LG, and confirmed by JB, BT, SM, and SW. Data curation was performed by LG. Data synthesis was performed by LG, LH, and JR. Figures were created by LH. LG and JR drafted the manuscript, and feedback was provided by all authors.
Declaration of interests
I/we declare no competing interests.
Role of the funding source
This work was funded by Blind Low Vision New Zealand for Eye Health Aotearoa. The funder had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Acknowledgements
JR's position at the University of Auckland is funded by the Buchanan Charitable Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100560.
Appendix. Supplementary materials
References
- 1.World Health Organization . World Health Organization; 2019. World report on vision.https://www.who.int/publications/i/item/9789241516570 [cited 2021 Dec 14]. Available from: [Google Scholar]
- 2.World Health Assembly . World Health Assembly; Geneva: 2020 Aug 3. Integrated people-centred eye care, including preventable vision impairment and blindness: WHA 73.4.https://apps.who.int/gb/ebwha/pdf_files/WHA73/A73_R4-en.pdf [cited 2021 Dec 14]. Available from: [Google Scholar]
- 3.World Health Organization . World Health Organization; 2021. Universal health coverage (UHC)https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc) [cited 2021 Sep 16]. Available from: [Google Scholar]
- 4.Burton MJ, Ramke J, Marques AP, et al. The Lancet Global Health Commission on Global Eye Health: vision beyond 2020. The Lancet Global Health. 2021 Apr 1;9(4):e489–e551. doi: 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; 2015. World report on ageing and health.https://apps.who.int/iris/handle/10665/186463 [cited 2021 Dec 14]. Available from: [Google Scholar]
- 6.Clark D, Minister of Health. Free health check for SuperGold card holders. New Zealand Government; [cited 2020 Oct 13]. Available from: https://www.health.govt.nz/system/files/documents/pages/budget_initiative_1_-_sgc_health_check_-_budget_template.pdf.
- 7.Silwal P, Watene R, Cowan C, et al. Eye care in Aotearoa New Zealand 2022: Eye care situation analysis tool (ECSAT) Open Sci Framew. 2022 https://osf.io/r75zs/ [cited 2022 Apr 13]; Available from: [Google Scholar]
- 8.Goodman L, Black J, Tousignant B, et al. How should New Zealand implement the “Eye Health Check” for older adults to maximise the potential to reduce vision impairment? Protocol for a Rapid Review. Open Sci Framework. 2020 https://osf.io/rpgt2/ Available from. [Google Scholar]
- 9.World Bank . The World Bank Group. The World Bank Group; Washington, D.C.: 2019. GNI per capita, Atlas method (current US$)https://data.worldbank.org/indicator/NY.GNP.PCAP.CD?locations=XD [cited 2020 Aug 18]. Available from: [Google Scholar]
- 10.World Bank . The World Bank Group; Washington, D.C.: 2019. Population, total.https://data.worldbank.org/indicator/SP.POP.TOTL [cited 2020 Sep 15]. Available from: [Google Scholar]
- 11.World Bank . The World Bank Group; Washington, D.C.: 2022. Current health expenditure per capita (current US$)https://data.worldbank.org/indicator/SH.XPD.CHEX.PC.CD?end=2019&most_recent_value_desc=true&start=2019&view=map [cited 2022 Jul 12]. Available from: [Google Scholar]
- 12.Global Health Observatory . World Health Organization; 2022. UHC service coverage index (3.8.1)https://www.who.int/data/gho/indicator-metadata-registry/imr-details/4834 [cited 2022 Jul 11]. Available from: [Google Scholar]
- 13.EL Clarke, Evans JR, Smeeth L. Community screening for visual impairment in older people. Cochrane Database Syst Rev. 2018;2018(2) doi: 10.1002/14651858.CD001054.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson BJ, Courage S, Bacchus M, et al. Screening for impaired vision in community-dwelling adults aged 65 years and older in primary care settings. Can Med Assoc J. 2018;190(19):E588–E594. doi: 10.1503/cmaj.171430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou R, Dana T, Bougatsos C, Grusing S, Blazina I. Screening for impaired visual acuity in older adults: Updated evidence report and systematic review for the US preventive services task force. J Am Med Assoc. 2016;315(9):915–933. doi: 10.1001/jama.2016.0783. [DOI] [PubMed] [Google Scholar]
- 16.Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for impaired visual acuity in older adults: US preventive services task force recommendation statement. J Am Med Assoc. 2016;315(9):908–914. doi: 10.1001/jama.2016.0763. [DOI] [PubMed] [Google Scholar]
- 17.Northern Ireland Statistics and Research Organisation. General Ophthalmic Services Statistics for Northern Ireland, Annual Statistics 2019/20. Belfast: Business Services Organisation Family Practitioner Services Information Unit; 2020 [cited 2020 Nov 29]. Available from: https://hscbusiness.hscni.net/services/3175.htm.
- 18.Public Health Scotland. General Ophthalmic Services. Edinburgh: Public Health Scotland; 2020 [cited 2020 Oct 21]. Available from: https://www.isdscotland.org/Health-Topics/Eye-Care/General-Ophthalmic-Services/.
- 19.Community eyecare services: review. Scottish Government; Edinburgh: 2017. Population Health Directorate, Health and Social Care, Scottish Government.https://www.gov.scot/publications/community-eyecare-services-review/ [cited 2020 Nov 29]. Available from: [Google Scholar]
- 20.Statistics for Wales . Welsh Government; 2019. Sensory health (eye care and hearing statistics): April 2017 to March 2019.https://gov.wales/sensory-health-eye-care-and-hearing-statistics-april-2017-march-2019 [cited 2020 Nov 29]. Available from: [Google Scholar]
- 21.Dickey H, Ikenwilo D, Norwood P, Watson V, Zangelidis A. Utilisation of eye-care services: The effect of Scotland's free eye examination policy. Health Policy. 2012;108(2–3):286–293. doi: 10.1016/j.healthpol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Lian JX, McGhee SM, Gangwani RA, et al. Screening for diabetic retinopathy with or without a copayment in a randomized controlled trial: influence of the inverse care law. Ophthalmology. 2013;120(6):1247–1253. doi: 10.1016/j.ophtha.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Duckworth S. Ministry of Health; New Zealand Government: 2015. Stocktake and Needs Analysis of Low Vision Services in New Zealand Prepared for Ministry of Health Manatū Hauora. [Google Scholar]
- 24.Ratnarajan G, Kean J, French K, Parker M, Bourne R. The false negative rate and the role for virtual review in a nationally evaluated glaucoma referral refinement scheme. Ophthalmic Physiol Optics. 2015;35(5):577–581. doi: 10.1111/opo.12224. [DOI] [PubMed] [Google Scholar]
- 25.Bourne RRA, French KA, Chang L, Borman AD, Hingorani M, Newsom WD. Can a community optometrist-based referral refinement scheme reduce false-positive glaucoma hospital referrals without compromising quality of care? The community and hospital allied network glaucoma evaluation scheme (CHANGES) Eye. 2010;24(5):881–887. doi: 10.1038/eye.2009.190. [DOI] [PubMed] [Google Scholar]
- 26.Chawla A, Patel I, Yuen C, Fenerty C. Patterns of adherence to NICE Glaucoma Guidance in two different service delivery models. Eye. 2012;26(11):1412–1417. doi: 10.1038/eye.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trikha S, Macgregor C, Jeffery M, Kirwan J. The Portsmouth-based glaucoma refinement scheme: a role for virtual clinics in the future? Eye. 2012;26(10):1288–1294. doi: 10.1038/eye.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts HW, Rughani K, Syam P, Dhingra S, Ramirez-Florez S. The Peterborough Scheme for Community Specialist Optometrists in Glaucoma: Results of 4 Years of a Two-Tiered Community-Based Assessment and Follow-up Service. Curr Eye Res. 2015;40(7):690–696. doi: 10.3109/02713683.2014.957326. [DOI] [PubMed] [Google Scholar]
- 29.Parkins DJ, Edgar DF. Comparison of the effectiveness of two enhanced glaucoma referral schemes. Ophthalm Physiol Opt. 2011;31(4):343–352. doi: 10.1111/j.1475-1313.2011.00853.x. [DOI] [PubMed] [Google Scholar]
- 30.Devarajan N, Williams GS, Hopes M, O'Sullivan D, Jones D. The Carmarthenshire Glaucoma Referral Refinement Scheme, a safe and efficient screening service. Eye. 2011;25(1):43–49. doi: 10.1038/eye.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vernon SA, Adair A. Shared care in glaucoma: a national study of secondary care lead schemes in England. Eye. 2010;24(2):265–269. doi: 10.1038/eye.2009.118. [DOI] [PubMed] [Google Scholar]
- 32.Sii S, Nasser A, Loo CY, Croghan C, Rotchford A, Agarwal PK. The impact of SIGN glaucoma guidelines on false-positive referrals from community optometrists in Central Scotland. Br J Ophthalmol. 2019;103(3):369–373. doi: 10.1136/bjophthalmol-2017-311429. [DOI] [PubMed] [Google Scholar]
- 33.El-Assal K, Foulds J, Dobson S, Sanders R. A comparative study of glaucoma referrals in Southeast Scotland: effect of the new general ophthalmic service contract, Eyecare integration pilot programme and NICE guidelines. BMJ Open. 2015;15(1):172. doi: 10.1186/s12886-015-0161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratnarajan G, Newsom W, Vernon SA, Fenerty C, Henson D, Spencer F, et al. The effectiveness of schemes that refine referrals between primary and secondary care—the UK experience with glaucoma referrals: the Health Innovation & Education Cluster (HIEC) Glaucoma Pathways Project. BMJ Open. 2013;3(7) doi: 10.1136/bmjopen-2013-002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fricke T, Consultants M-M. Australian College of Optometry and the Victorian Government Department of Health and Human Services; Melbourne: 2016. Evaluation of the Victorian Aboriginal Spectacles Subsidy Scheme.https://www.aco.org.au/wp-content/uploads/2020/04/VASSS-evaluation-report-2017_final.pdf [cited 2020 Oct 14]. Available from: [Google Scholar]
- 36.Napper G, Fricke T, Anjou MD, Jackson AJ. Breaking down barriers to eye care for Indigenous people: a new scheme for delivery of eye care in Victoria. Clin Experiment Opt. 2015;98(5):430–434. doi: 10.1111/cxo.12325. [DOI] [PubMed] [Google Scholar]
- 37.Fricke TR, Brand C, Lovett L, Turner NW, Anjou MD, Bentley SA. Lessons learned from a subsidised spectacles scheme aiming to improve eye health in Aboriginal people in Victoria, Australia. Austr Health Rev. 2021;45(2):194. doi: 10.1071/AH20023. [DOI] [PubMed] [Google Scholar]
- 38.Food and Health Bureau, Department of Health . Government of Hong Kong Special Administrative Region; 2019. Report on the review of the elderly health care voucher scheme.https://www.hcv.gov.hk/files/pdf/Review_Report_English.pdf [cited 2020 Oct 10]. Available from: [Google Scholar]
- 39.Forster AS, Forbes A, Dodhia H, et al. Changes in Detection of Retinopathy in Type 2 Diabetes in the First 4 Years of a Population-Based Diabetic Eye Screening Program. Diabetes Care. 2013;36(9):2663–2669. doi: 10.2337/dc13-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Looker HC, Nyangoma SO, Cromie D, et al. Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia. 2012;55:2335–2342. doi: 10.1007/s00125-012-2596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looker HC, Nyangoma SO, Cromie DT, et al. Rates of referable eye disease in the Scottish National Diabetic Retinopathy Screening Programme. Br J Ophthalmol. 2014;98(6):790–795. doi: 10.1136/bjophthalmol-2013-303948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Screening Service. Diabetic RetinaScreen Programme Report 2013-2015. Ireland: Health Services Executive; 2017 [cited 2020 Oct 10]. Available from:https://www.diabeticretinascreen.ie/_fileupload/Documents/Diabetic%20RetinaScreen%20Programme%20Report%202013-15%20(FINAL%20web%202)%20(4).pdf.
- 43.National Screening Service . Health Services Executive; Ireland: 2019. Diabetic RetinaScreen Statistical Bulletin 2016-2017.https://www.diabeticretinascreen.ie/_fileupload/Documents/DRS-Statistical-Bulletin-2016-2017-FINAL-29_11_19.pdf [cited 2020 Oct 10]. Available from: [Google Scholar]
- 44.National Screening Service . Health Services Executive; Ireland: 2019. Diabetic RetinaScreen Statistical Bulletin 2018-2019.https://www.diabeticretinascreen.ie/_fileupload/Documents/DRS-Statistical-Bulletin-2016-2017-FINAL-29_11_19.pdf Available from: [Google Scholar]
- 45.Mamillapalli CK, Prentice JR, Garg AK, Hampsey SL, Bhandari R. Implementation and challenges unique to teleretinal diabetic retinal screening (TDRS) in a private practice setting in the United States. J Clin Translat Endocrinol. 2020;19 doi: 10.1016/j.jcte.2019.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daskivich LP, Vasquez C, Martinez C, Tseng CH, Mangione CM. Implementation and evaluation of a large-scale teleretinal diabetic retinopathy screening program in the los angeles county department of health services. JAMA Intern. Med. 2017;177(5):642–649. doi: 10.1001/jamainternmed.2017.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsan GL, Hoban KL, Jun W, Riedel KJ, Pedersen AL, Hayes J. Assessment of diabetic teleretinal imaging program at the Portland Department of Veterans Affairs Medical Center. J Rehabil Res Dev. 2015;52(2):193–200. doi: 10.1682/JRRD.2014.03.0077. [DOI] [PubMed] [Google Scholar]
- 48.Crossland L, Askew D, Ware R, et al. Diabetic retinopathy screening and monitoring of early stage disease in Australian general practice: tackling preventable blindness within a chronic care model. J Diabetes Res. 2016;2016:1–7. doi: 10.1155/2016/8405395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glasson NM, Crossland LJ, Larkins SL. An innovative Australian outreach model of diabetic retinopathy screening in remote communities. J Diabetes Res. 2016;2016:1–10. doi: 10.1155/2016/1267215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B, Powell AM, Hooper PL, Sheidow TG. Prospective Evaluation of Teleophthalmology in Screening and Recurrence Monitoring of Neovascular Age-Related Macular Degeneration. JAMA Ophthalmology. 2015;133(3):276–282. doi: 10.1001/jamaophthalmol.2014.5014. [DOI] [PubMed] [Google Scholar]
- 51.Maa AY, Wojciechowski B, Hunt K, Dismuke C, Janjua R, Lynch MG. Remote eye care screening for rural veterans with Technology-based Eye Care Services: a quality improvement project. Rural Remote Health. 2017;17:4045. doi: 10.22605/rrh4045. [DOI] [PubMed] [Google Scholar]
- 52.Borooah S, Grant B, Blaikie A, et al. Using electronic referral with digital imaging between primary and secondary ophthalmic services: A long term prospective analysis of regional service redesign. Eye (Basingstoke) 2013;27(3):392–397. doi: 10.1038/eye.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ministerial Committee on Ageing. Project Silver Screen: I Feel Young SG. Singapore: Ministry of Health, Singapore Government; [cited 2020 Oct 9]. Available from: https://www.moh.gov.sg/ifeelyoungsg/how-can-i-age-actively/stay-healthy/project-silver-screen.
- 54.The National Health Service (General Ophthalmic Services) (Scotland) Regulations. 2006 [cited 2020 Oct 21]. Available from:https://www.legislation.gov.uk/ssi/2006/135/contents.
- 55.Turner AW, Xie J, Arnold AL, Dunn RA, Taylor HR. Eye health service access and utilization in the National Indigenous Eye Health Survey. Clin Exp Ophthalmol. 2011;39(7):598–603. doi: 10.1111/j.1442-9071.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 56.Stats NZ Tatauranga Aotearoa . New Zealand Government; Wellington: 2021. National and subnational period life tables: 2017–2019.https://www.stats.govt.nz/information-releases/national-and-subnational-period-life-tables-2017-2019 [cited 2022 Feb 14]. Available from: [Google Scholar]
- 57.Ramke J, Evans JR, Habtamu E, et al. Grand Challenges in global eye health: a global prioritisation process using Delphi method. Lancet Healthy Longevity. 2022;3(1):e31–e41. doi: 10.1016/S2666-7568(21)00302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crossland L, Jackson C. Successfully implementing a diabetic retinopathy screening service in general practice: What does the evidence tell us? Aust Fam Physician. 2017;46:529–535. [PubMed] [Google Scholar]
- 59.Bowes OMB, Shah P, Rana M, Farrell S, Rajan MS. Quality indicators in a community optometrist led cataract shared care scheme. Ophthalmic Physiol Opt. 2018;38:183–192. doi: 10.1111/opo.12444. [DOI] [PubMed] [Google Scholar]
- 60.Bourne RRA, Steinmetz JD, Saylan M, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Global Health. 2021;9(2):e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Assembly . World Health Assembly; Geneva: 2021 Apr 19. Integrated people-centred eye care, including preventable vision impairment and blindness Global targets for 2030 Draft decision: A74/9 Add.3.https://apps.who.int/gb/ebwha/pdf_files/WHA74/A74_9Add3-en.pdf [cited 2021 Dec 14]. Available from: [Google Scholar]
- 62.Foreman J, Xie J, Keel S, Taylor HR, Dirani M. Treatment coverage rates for refractive error in the National Eye Health survey. Pan CW, editor. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Byass P. Systems thinking for health systems strengthening. Public Health. 2011;125(2):117–118. [Google Scholar]
- 64.Kieny M-P, Evans TG, Scarpetta S, et al. Delivering Quality Health Services: A Global Imperative. The World Bank Group; Washington, D.C.: 2018. Delivering Quality Health Services: A Global Imperative.https://www.worldbank.org/en/topic/universalhealthcoverage/publication/delivering-quality-health-services-a-global-imperative-for-universal-health-coverage [cited 2020 Sep 21]. Available from: [Google Scholar]
- 65.Institute of Medicine (USNational Academies Press; Washington, D.C.: 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. Committee on Quality of Health Care in America. [PubMed] [Google Scholar]
- 66.Australian College of Optometry. Victorian Eyecare Service. Melbourne: Australian College of Optometry; [cited 2020 Oct 14]. Available from: https://www.aco.org.au/victorian-eyecare-service/.
- 67.Ministry of Health, Government NZ . New Zealand Government; Wellington: 2019. Equipment for people who are blind or have reduced vision.https://www.health.govt.nz/your-health/services-and-support/disability-services/types-disability-support/hearing-and-vision-services/equipment-people-who-are-blind-or-have-reduced-vision [cited 2020 Oct 9]. Available from: [Google Scholar]
- 68.National Health Service. Free NHS eye tests and optical vouchers. National Health Service; 2019 [cited 2020 Oct 11]. Available from:https://www.nhs.uk/using-the-nhs/help-with-health-costs/free-nhs-eye-tests-and-optical-vouchers/.
- 69.NIdirect government services . NIdirect; 2020. Eye care.https://www.nidirect.gov.uk/articles/eye-care#toc-7 [cited Nov 29]. Available from. [Google Scholar]
- 70.Welsh Government . Welsh Government; 2020. Get help with NHS eye care costs.https://gov.wales/get-help-nhs-eye-care-costs [cited 2020 Nov 29]. Available from: [Google Scholar]
- 71.NHS Digital. General Ophthalmic Services Activity Statistics England, year ending 31 March 2020 - NHS Digital. 2020 [cited 2020 Nov 29]. Available from:https://digital.nhs.uk/data-and-information/publications/statistical/general-ophthalmic-services-activity-statistics/england-year-ending-31-march-2020.
- 72.Information Services Division . Public Health Scotland; 2019. General Ophthalmic Services Statistics: A National Statistics publication for Scotland.https://www.isdscotland.org/Health-Topics/Eye-Care/Publications/2019-10-29/2019-10-29-Ophthalmic-Report.pdf? [cited 2020 Nov 29]. Available from: [Google Scholar]
- 73.Eye Health Examination Wales. Eye Health Examination Wales - including urgent eye care. Rhondda Cynon Taff: Wales Eye Care Services; [cited 2020 Oct 22]. Available from: http://www.eyecare.wales.nhs.uk/eye-health-examination-wales.
- 74.Health and Social Care Board. Northern Ireland Primary Eyecare Assessment and Referral Scheme. Belfast: Health and Social Care Board; [cited 2020 Nov 29]. Available from: http://www.hscboard.hscni.net/eyes/.
- 75.Public Health England & NHS England. Population screening programmes: NHS diabetic eye screening (DES) programme - detailed information. gov.uk; [cited 2020 Oct 11]. Available from:https://www.gov.uk/topic/population-screening-programmes/diabetic-eye.
- 76.Public Health Agency. Diabetic Eye Screening Programme (also known as Diabetic Retinopathy Screening). Belfast: Public Health Agency; [cited 2020 Oct 22]. Available from:https://www.publichealth.hscni.net/directorate-public-health/service-development-and-screening/diabetic-eye-screening-programme-also-kn.
- 77.Public Health Scotland . NHSinform; 2022. Diabetic retinopathy screening (DRS) in Scotland.https://www.nhsinform.scot/healthy-living/screening/diabetic-retinopathy/diabetic-retinopathy-screening-drs#overview [cited 2020 Oct 22]Available from: [Google Scholar]
- 78.Diabetic Retinopathy Screening Service for Wales. Eye Care Wales - Diabetic Eye Screening Wales. Rhondda Cynon Taff: Wales Eye Care Services; [cited 2020 Oct 22]. Available from:http://www.eyecare.wales.nhs.uk/diabetic-retinopathy-screening-service-w.
- 79.Public Health England . gov.uk; 2021. NHS screening programmes: KPI reports 2019 to 2020.https://www.gov.uk/government/publications/nhs-screening-programmes-kpi-reports-2019-to-2020 [cited 2020 Oct 22]. Available from: [Google Scholar]
- 80.Public Health Agency . Public Health Agency; Belfast: 2017. Diabetic Eye Screening Programme Annual Report 2016-2017.https://www.publichealth.hscni.net/publications/diabetic-eye-screening-programme-annual-report-2016-2017 [cited 2020 Oct 22]. Available from: [Google Scholar]
- 81.Scottish Diabetic Retinopathy Screening Collaborative . Scottish DES Collaborative; 2019. Diabetic Retinopathy Screening Service Annual Report 2018/19.https://www.ndrs.scot.nhs.uk/wp-content/uploads/2019/11/DRS-Annual-Report-2018.pdf [cited 2020 Oct 22]. Available from: [Google Scholar]
- 82.Public Health Wales . Public Health Wales; Rhondda Cynon Taff: 2020. Diabetic Eye Screening Wales Annual Statistical Report 2018-19.https://phw.nhs.wales/services-and-teams/screening/diabetic-eye-screening-wales-desw/diabetic-eye-screening-wales-annual-statistical-report-2018-191/ [cited 2020 Oct 22]. Available from: [Google Scholar]
- 83.Public Health England . Public Health England; 2020. Screening KPI data summary factsheets: June 2020 - Issue 11.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/897785/Screening_KPI_Summary_Factsheets_June2020_Issue11.pdf [cited 2021 Jan 18]. Available from: [Google Scholar]
- 84.Looker HC, Nyangoma SO, Cromie D, Olson JA, Leese GP, Black M, et al. Diabetic retinopathy at diagnosis of type 2 diabetes in Scotland. Diabetologia. 2012;55(9):2335–2342. doi: 10.1007/s00125-012-2596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Low Vision Service Wales. Low Vision Service Wales. Rhondda Cynon Taff: Wales Eye Care Services; [cited 2020 Oct 22]. Available from: http://www.eyecare.wales.nhs.uk/low-vision-service-wales.
- 86.Ryan B, White S, Wild J, Court H, Margrain TH. The newly established primary care based Welsh Low Vision Service is effective and has improved access to low vision services in Wales. Ophthalmic Physiol Opt. 2010;30:358–364. doi: 10.1111/j.1475-1313.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 87.Court H, Ryan B, Bunce C, Margrain TH. How effective is the new community-based Welsh low vision service? Br J Ophthalmol. 2011;95:178–184. doi: 10.1136/bjo.2010.179606. [DOI] [PubMed] [Google Scholar]
- 88.Ryan B, Khadka J, Bunce C, Court H. Effectiveness of the community-based Low Vision Service Wales: a long-term outcome study. Br J Ophthalmol. 2013;97:487–491. doi: 10.1136/bjophthalmol-2012-302416. [DOI] [PubMed] [Google Scholar]
- 89.National Screening Service Ireland. Diabetic RetinaScreen - The National Diabetic Retinal Screening Programme. Health Services Executive; [cited 2020 Oct 10]. Available from: https://www.diabeticretinascreen.ie/.
- 90.Tracey M, Racine E, Riordan F, McHugh SM, Kearney PM. Understanding the uptake of a national retinopathy screening programme: An audit of people with diabetes in two large primary care centres. HRB Open Res. 2019;2:17. doi: 10.12688/hrbopenres.12926.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Primary Care Pages. Primary Care Network (PCN). Agency for Integrated Care; [cited 2020 Oct 9]. Available from: https://www.primarycarepages.sg/practice-management/primary-care-model/primary-care-network-(pcn).
- 92.Kee LLC, Chong CK, Hwee-lin W, Yean TT. Primary care network (PCN) as a model of care for GP chronic disease management. Singapore Family Phys. 2015;41(2):61–64. [Google Scholar]
- 93.The Government of Hong Kong Special Administrative Region . The Government of Hong Kong Special Administrative Region; Kowloon: 2022. Health Care Voucher.https://www.hcv.gov.hk/en/index.html [cited 2020 Oct 10]. Available from: [Google Scholar]
- 94.Hong Kong Hospital Authority. Chronic Disease Management Project: Risk Assessment and Management. Hospital Authority; [cited 2020 Nov 12]. Available from: https://www.ha.org.hk/haho/ho/hacp/RAMP_GOPC_en.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.