This randomized clinical trial assesses the addition of radiotherapy to immune checkpoint inhibitors compared with immune checkpoint inhibitor monotherapy in patients with advanced solid tumors.
Key Points
Question
Could stereotactic body radiotherapy (SBRT) added concurrently to immune checkpoint inhibitors (ICIs) improve progression-free survival in patients with advanced solid tumors?
Findings
In this phase 2 randomized clinical trial comparing ICIs alone or combined with SBRT among 96 patients with locally advanced or metastatic cancers, the median progression-free survival was 2.8 months in the control arm vs 4.4 months in the experimental arm, a difference that was not statistically significant.
Meaning
Albeit safe, adding subablative multisite SBRT to ICI monotherapy did not result in a clinically meaningful benefit.
Abstract
Importance
Although immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1) and PD-1 ligand 1 have improved the outcome for many cancer types, the majority of patients fails to respond to ICI monotherapy. Hypofractionated radiotherapy has the potential to improve the therapeutic ratio of ICIs.
Objective
To assess the addition of radiotherapy to ICIs compared with ICI monotherapy in patients with advanced solid tumors.
Design, Setting, and Participants
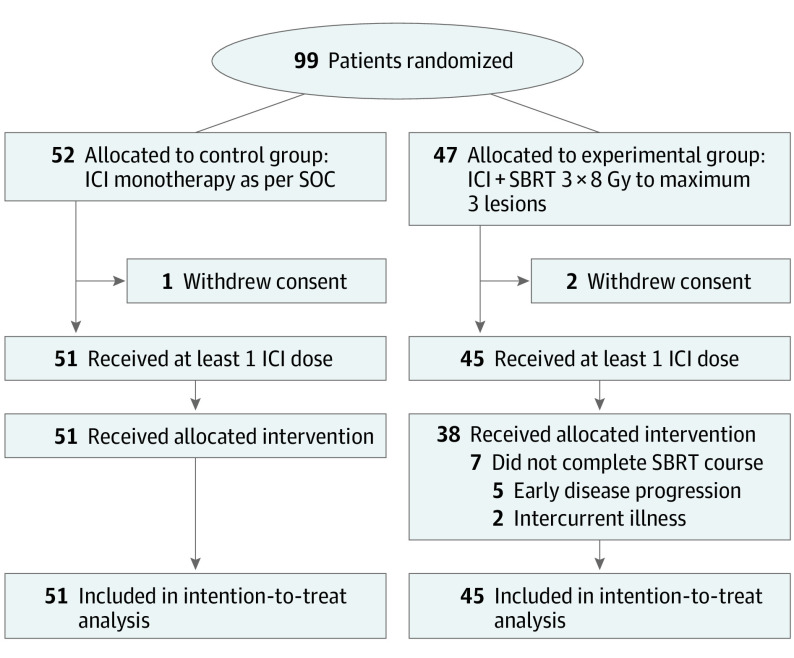
This open-label, multicenter, randomized phase 2 trial was conducted in 5 Belgian hospitals and enrolled participants between March 2018 and October 2020. Patients 18 years or older with locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, head and neck squamous cell carcinoma, or non–small cell lung carcinoma were eligible. A total of 99 patients were randomly assigned to either the control arm (n = 52) or the experimental arm (n = 47). Of those, 3 patients (1 in the control arm vs 2 in the experimental arm) withdrew consent and thus were not included in the analysis. Data analyses were performed between April 2022 and March 2023.
Interventions
Patients were randomized (1:1) to receive anti–PD-1/PD-1 ligand 1 ICIs alone as per standard of care (control arm) or combined with stereotactic body radiotherapy 3 × 8 gray to a maximum of 3 lesions prior to the second or third ICI cycle, depending on the frequency of administration (experimental arm). Randomization was stratified according to tumor histologic findings and disease burden (3 and fewer or more than 3 cancer lesions).
Main Outcomes and Measures
The primary end point was progression-free survival (PFS) as per immune Response Evaluation Criteria in Solid Tumors. Key secondary end points included overall survival (OS), objective response rate, local control rate, and toxic effects. Efficacy was assessed in the intention-to-treat population, while safety was evaluated in the as-treated population.
Results
Among 96 patients included in the analysis (mean age, 66 years; 76 [79%] female), 72 (75%) had more than 3 tumor lesions and 65 (68%) had received at least 1 previous line of systemic treatment at time of inclusion. Seven patients allocated to the experimental arm did not complete the study-prescribed radiotherapy course due to early disease progression (n = 5) or intercurrent illness (n = 2). With a median (range) follow-up of 12.5 (0.7-46.2) months, median PFS was 2.8 months in the control arm compared with 4.4 months in the experimental arm (hazard ratio, 0.95; 95% CI, 0.58-1.53; P = .82). Between the control and experimental arms, no improvement in median OS was observed (11.0 vs 14.3 months; hazard ratio, 0.82; 95% CI, 0.48-1.41; P = .47), and objective response rate was not statistically significantly different (22% vs 27%; P = .56), despite a local control rate of 75% in irradiated patients. Acute treatment-related toxic effects of any grade and grade 3 or higher occurred in 79% and 18% of patients in the control arm vs 78% and 18% in the experimental arm, respectively. No grade 5 adverse events occurred.
Conclusions and Relevance
This phase 2 randomized clinical trial demonstrated that while safe, adding subablative stereotactic radiotherapy of a limited number of metastatic lesions to ICI monotherapy failed to show improvement in PFS or OS.
Trial Registration
ClinicalTrials.gov Identifier: NCT03511391
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1) and PD-1 ligand 1 (PD-L1) have revolutionized the therapeutic landscape for various advanced solid tumors. Still, only a minority of patients (typically <20%) derives benefit from these immunotherapeutic agents in terms of durable response, particularly when they are offered as monotherapy.1 Although combinatorial approaches with other ICIs or chemotherapy in some cases have improved treatment outcomes considerably, they are often associated with a sizable increase in toxic effects.2
In recent years, stereotactic body radiotherapy (SBRT) has emerged as a safe and effective treatment option for patients with limited metastatic disease.3 In delivering high doses of radiation in a small number of fractions by means of highly conformal techniques, SBRT has the potential to neutralize active tumor sites in a noninvasive and relatively atoxic manner. Moreover, preclinical and early clinical data suggest that hypofractionated radiotherapy (particularly subablative doses of 3 × 8 gray [Gy]) could synergize with modern immunotherapy by inducing immunogenic cell death, whereby cytotoxic T lymphocytes are primed and activated due to a sudden release of tumor antigens.4 This in turn could theoretically instigate a systemic antitumor immune response—more commonly referred to as the abscopal effect—meaning the benefit of SBRT could extend beyond the so-called oligometastatic state.
Herein, we report the results of the Checkpoint Inhibition in Combination With an Immunoboost of External Beam Radiotherapy in Solid Tumors (CHEERS) study, a randomized phase 2 trial of ICIs with or without SBRT in patients with locally advanced or metastatic solid tumors, including head and neck squamous cell carcinoma (HNSCC), melanoma, non–small cell lung carcinoma (NSCLC), renal cell carcinoma (RCC), and urothelial carcinoma (UC). To our knowledge, this is the first trial to evaluate whether the addition of SBRT to immunotherapy could improve progression-free survival (PFS) in such a diverse population.
Methods
Study Design and Participants
This multicenter, phase 2 randomized clinical trial was conducted in 5 Belgian hospitals (Ghent University Hospital, GZA Hospitals, Jules Bordet Institute, AZ Sint-Lucas in Bruges, and AZ Sint-Lucas in Ghent). Patients who were at least 18 years old were eligible for enrollment if they (1) had a histologically confirmed diagnosis of (locally) advanced HNSCC, melanoma, NSCLC, RCC, or UC; (2) had a Karnofsky Performance Status score of 70 or higher; (3) qualified for anti–PD-1/PD-L1 checkpoint inhibitor monotherapy as per standard of care; and (4) had at least 1 extracranial tumor lesion amenable to radiotherapy administration. Patients were excluded if they had a known additional cancer that was progressing or required active treatment, had uncontrolled central nervous system metastases, had active autoimmune disease, or were receiving systemic treatment with corticosteroids or other immunosuppressive medication. Patients were also excluded if they had received prior anti–PD-1/PD-L1 immunotherapy. Full eligibility criteria are listed in the trial protocol (Supplement 1) and published elsewhere.5
The study protocol and all amendments were approved by the institutional review boards at each participating center. The trial was conducted in accordance with the protocol and all of its amendments, as well as with Good Clinical Practice standards. All patients provided written informed consent before enrollment. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Randomization and Procedures
Patients were randomly assigned, in a 1:1 ratio, to receive either anti–PD-1/PD-L1 ICI therapy alone as per standard of care (control arm) or combined with SBRT 3 × 8 Gy every other day to a maximum of 3 tumor lesions, administered prior to the second or third ICI cycle, depending on the frequency of administration (experimental arm). After eligibility screening and on receipt of written informed consent, patients were assigned to groups using a computer-generated randomization list with permuted blocks of variable length 2 and 4, stratified according to tumor histologic findings (HNSCC, melanoma, NSCLC, RCC, UC) and disease burden (3 and fewer or more than 3 cancer lesions). In literature, up to 3 metastatic lesions was, and still is, one of the most commonly used quantifiers for low disease burden and is recognized as a prognostic factor in patients with advanced solid tumors.6,7,8,9 Treatment allocation was carried out by the study statistician (D.R.) and communicated by email. Because this was an open-label trial, the randomization procedure and outcome were not blinded.
The choice of ICI was left to the discretion of the investigator, within the bounds of standard-of-care treatment. Allowed immunotherapeutic agents and dosing schedules were: (1) nivolumab, 240 mg, intravenously (IV) every 2 weeks; (2) nivolumab, 480 mg, IV every 4 weeks; (3) pembrolizumab, 200 mg, IV every 3 weeks; and (4) atezolizumab, 1200 mg, IV every 3 weeks. In case of immunotherapy every 2 or 3 weeks, SBRT was performed prior to the third ICI cycle, whereas for dosing every 4 weeks, SBRT was performed prior to the second cycle. Treatment was continued until disease progression, the occurrence of unacceptable toxic effects, patient withdrawal of consent, or per investigator decision. Treatment beyond progression was allowed in the presence of confirmed radiographic progression if the patient was clinically stable or clinically improved. Concerning the choice of the radiotherapy targets, lesions that were symptomatic and/or could be safely treated were prioritized. Moreover, because the goal of the experimental treatment was to boost an antitumor immune response and not to eradicate all visible disease, treatment of all disease sites in patients with up to 3 lesions was not mandatory. Additionally, palliative radiotherapy with the goal of alleviating symptoms was allowed in both study arms, with recommended doses ranging from 8 Gy in 1 fraction to 20 Gy in 5 fractions.
Outcomes
The primary end point was PFS (time from randomization to disease progression or death from any cause, whichever occurred first) in all patients who received at least 1 cycle of ICI, as assessed by computed tomography or positron emission tomographic computed tomography and defined according to immune Response Evaluation Criteria in Solid Tumors (iRECIST). Imaging was performed every 10 to 12 weeks, or earlier if clinically indicated. Key secondary end points included overall survival (OS; time from randomization to death from any cause), best overall response, local control of irradiated lesions according to iRECIST or RECIST, and safety. Objective response rate was defined as the proportion of patients experiencing an iRECIST-defined complete response (iCR) or partial response. Disease control rate comprised all patients who demonstrated iCR or iRECIST-defined partial response, as well as those achieving iRECIST-defined stable disease, according to the same criteria. The relative proportion of patients in the experimental arm showing disease control at the irradiated site(s) constituted the local control rate (LCR). Adverse events and laboratory abnormalities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. Acute toxicity was defined as any adverse event occurring after the first ICI cycle and within 3 months after the administration of SBRT (experimental arm) or an equivalent reference time point (control arm). Patients were contacted every 12 weeks to assess survival during follow-up.
Statistical Analysis
Efficacy was assessed in the intention-to-treat population, which included all of the patients who had undergone randomization. Safety was assessed in the as-treated population, which included all patients who had undergone randomization and received at least 1 dose of the assigned therapy. Those who withdrew consent were not included in either analysis. The Kaplan-Meier method was used to estimate OS and PFS. Data for patients who were lost to follow-up or alive at study closure were censored for OS at the time they were last known to be alive. Data for patients who were alive and did not have disease progression or who were lost to follow-up were censored for the analysis of PFS at the time of the last imaging assessment. The stratified log-rank test was used to assess between-group differences in OS and PFS for tumor histologic results (HNSCC, melanoma, NSCLC, RCC, UC) and disease burden (3 and fewer or more than 3 lesions). Hazard ratios (HRs) and associated 95% CIs were calculated with the use of a stratified Cox proportional hazards model. Stratum-specific treatment effects were assessed by adding stratum-treatment interactions to the stratified Cox model. P values were calculated using the likelihood-ratio test. Landmark analysis at 6 months was used to examine the association of irradiated tumor response with OS.
We determined that the study would have 80% power to detect an improvement in PFS of 3 months in the experimental arm compared with the control arm, at a 2-sided α level of .05. Median PFS in the control arm was estimated to be 3.1 months. The planned enrollment was 98 patients.
Post hoc exploratory analyses of changes in lymphocyte counts were based on comparisons of measurements at baseline and 8 weeks post-SBRT in the experimental arm and at corresponding time points in the control arm. Between-group differences were assessed using Mann-Whitney U tests. All data were analyzed using SPSS, version 24.0 (IBM), and R, version 4.2.2 (R Foundation).
Results
Patients and Treatment
Between March 2018 and October 2020, a total of 99 patients were randomized to either the control arm (n = 52) or the experimental arm (n = 47). Of those, 3 patients (1 in the control arm and 2 in the experimental arm) withdrew consent and thus were not included in the analysis (Figure 1). The baseline demographic and disease characteristics were generally well balanced between the groups (Table 1). Of note, more than 70% of patients had more than 3 tumor lesions at the time of inclusion, and about two-thirds of patients had received at least 1 line of systemic treatment prior to study inclusion (a more detailed overview of disease burden, including number and location of cancer lesions at baseline, is provided in eTable 1 in Supplement 2). Seven patients allocated to the experimental arm did not complete the study-prescribed SBRT course due to early disease progression (n = 5) or intercurrent illness (n = 2). The tumor sites selected for SBRT were primarily lung lesions and lymph nodes. Although allowed in the study protocol, only 8 patients (18%) in the experimental arm had all active tumor lesions treated. At the time of data cutoff, 7 patients (14%) in the control arm and 4 patients (9%) in the experimental arm were still receiving ICI treatment, with a median (range) follow-up of 12.5 (0.7-46.2) months.
Figure 1. CONSORT Diagram.
Gy indicates gray; ICI, immune checkpoint inhibitor; SBRT, stereotactic body radiotherapy; SOC, standard of care.
Table 1. Baseline Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Control arm (n = 51) | Experimental arm (n = 45) | |
| Age, median (IQR), y | 67 (58-74) | 67 (59-75) |
| Sex | ||
| Female | 9 (18) | 11 (24) |
| Male | 42 (82) | 34 (76) |
| Karnofsky Performance Status score | ||
| 100 | 3 (6) | 4 (9) |
| 90 | 17 (33) | 20 (44) |
| 80 | 19 (37) | 17 (38) |
| 70 | 12 (24) | 4 (9) |
| Primary tumor histologic result | ||
| Head and neck squamous cell carcinoma | 11 (22) | 9 (20) |
| Melanoma | 12 (24) | 11 (24) |
| Non–small cell lung carcinoma | 4 (8) | 3 (7) |
| Renal cell carcinoma | 8 (16) | 6 (13) |
| Urothelial carcinoma | 16 (31) | 16 (36) |
| No. of lesions | ||
| ≤3 | 14 (27) | 10 (22) |
| >3 | 37 (73) | 35 (78) |
| American Joint Committee on Cancer stage | ||
| III | 2 (4) | 6 (13) |
| IV | 49 (96) | 39 (87) |
| No. of prior systemic treatment lines | ||
| 0 | 17 (33) | 14 (31) |
| 1 | 23 (45) | 24 (53) |
| ≥2 | 11 (22) | 7 (16) |
| Prior radiotherapy | 28 (55) | 18 (40) |
| Immune checkpoint inhibitor regimen | ||
| Atezolizumab every 3 wk | 6 (12) | 5 (11) |
| Nivolumab every 2 wk | 21 (41) | 21 (47) |
| Nivolumab every 4 wk | 4 (8) | 2 (4) |
| Pembrolizumab every 3 wk | 20 (39) | 17 (38) |
| No. of SBRT-treated lesions | ||
| 1 | NA | 16 (36) |
| 2 | 13 (29) | |
| 3 | 10 (20) | |
| Location of SBRT-treated lesions (n = 69) | ||
| Lymph node | NA | 21 (30) |
| Lung | 23 (33) | |
| Liver | 4 (6) | |
| Bone | 9 (13) | |
| Other | 12 (17) | |
Abbreviations: NA, not applicable; SBRT, stereotactic body radiotherapy.
Efficacy
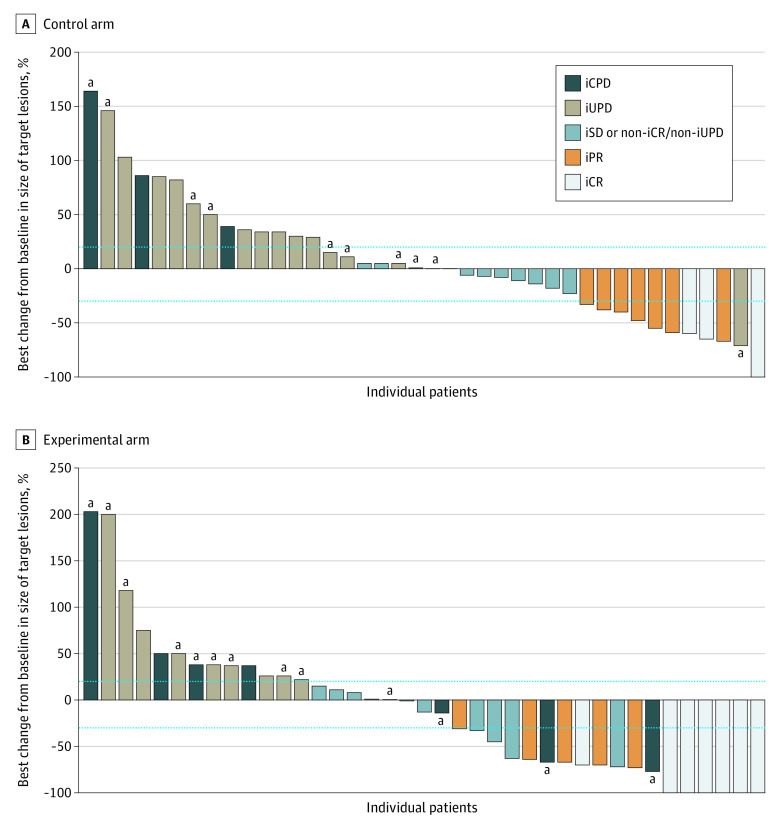
At the time of analysis, median PFS was 2.8 (95% CI, 2.5-8.4) months in the control arm compared with 4.4 (95% CI, 2.8-7.8) months in the experimental arm (Figure 2). This difference was not statistically significant (HR, 0.95; 95% CI, 0.58-1.53; P = .82), and no differential treatment effect was demonstrated between prespecified strata for disease burden or tumor type. With 57 deaths in the intention-to-treat population at data cutoff, median OS was 11.0 months (95% CI, 9.0 months to not reached) in the control arm vs 14.3 months (95% CI, 11.0 months to not reached) in the experimental arm (HR, 0.82; 95% CI, 0.48-1.41; P = .47) (Figure 2). Again, there was no evidence of a differential treatment effect between levels of disease burden or tumor histology. Objective response rate was not statistically significantly different between the control and experimental arms (22% vs 27%; P = .56). The LCR of irradiated lesions was 75% in patients in the experimental arm. Four (8%) and 7 (16%) patients achieved an iCR as best overall response in the control and experimental arm respectively (Figure 3). Notably, this subgroup of patients exclusively consisted of those with melanoma (n = 6) and UC (n = 5). A complete response of irradiated lesions was seen in 15 patients (33%) in the SBRT group.
Figure 2. Progression-Free and Overall Survival in the Intention-to-Treat Population.
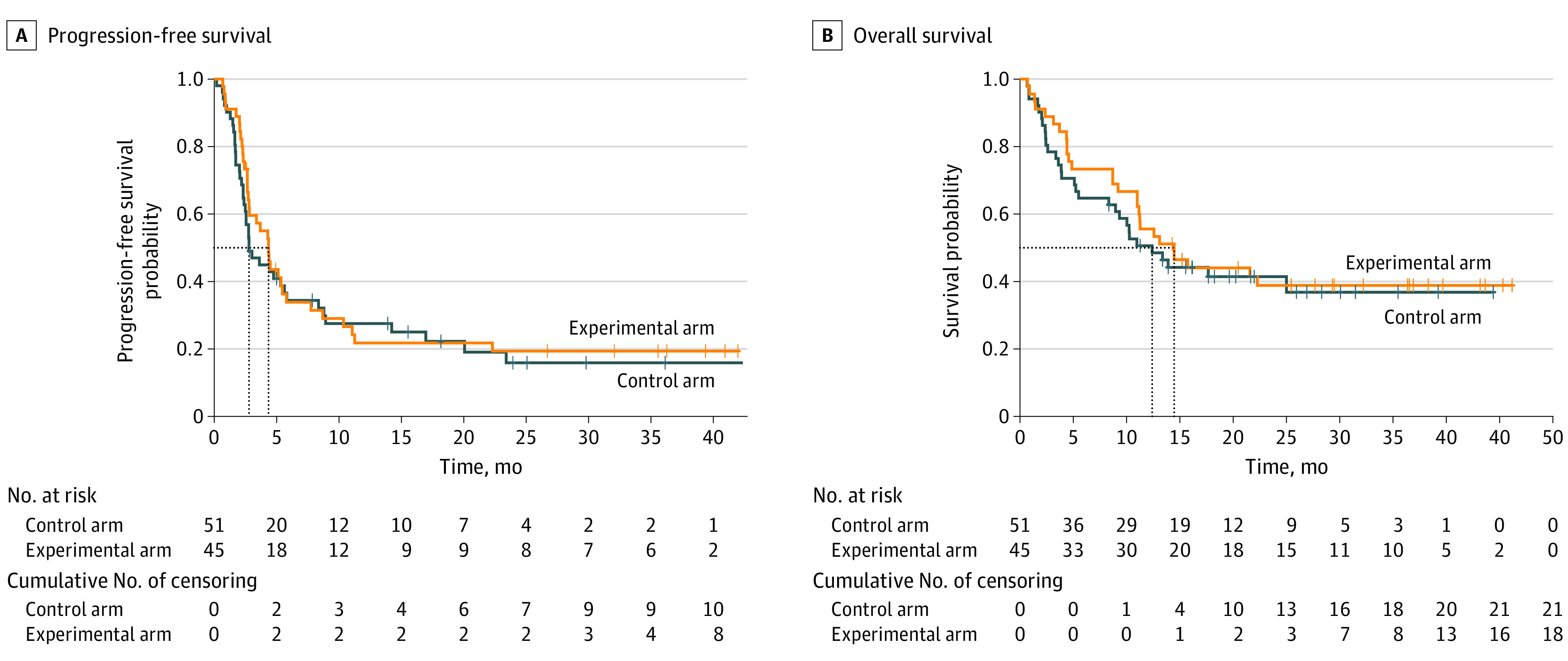
Figure 3. Best Change From Baseline in Size of Target Lesions in Evaluable Patients.
Outcomes defined according to immune Response Evaluation Criteria in Solid Tumors (iRECIST). The dotted lines indicate thresholds for the definitions of partial response and progressive disease according to iRECIST. iCPD indicates iRECIST-defined confirmed progressive disease; iCR, iRECIST-defined complete response; iPR, iRECIST-defined partial response; iSD, iRECIST-defined stable disease; iUPD, iRECIST-defined unconfirmed progressive disease.
aPatients in whom disease progression was driven by the appearance of new lesions.
Safety
All acute adverse events recorded in the trial in at least 10% of patients, regardless of their attribution, are summarized in Table 2. Acute treatment-related toxic effects of any grade and grade 3 or 4 occurred in 79% and 18% of patients in the control arm vs 78% and 18% in the experimental arm, respectively (Table 2). No grade 5 toxic effects were reported. The most common adverse events included fatigue and pruritus. All but gastrointestinal toxic effects were recorded more frequently in the experimental arm. Grade 4 lymphopenia only occurred in patients treated with SBRT (n = 2). Serious adverse events attributed to trial treatment included grade 3 anorexia (n = 2), dyspnea (n = 1), diarrhea (n = 1), rash (n = 1), dysphagia (n = 1), pneumonitis (n = 2), sarcoidosis (n = 1), acute kidney injury (n = 1), hiccups (n = 1), Stevens-Johnson syndrome (n = 1); and grade 4 colitis (n = 1). Of those, only 1 case of grade 3 pneumonitis was indicated to be SBRT related.
Table 2. Adverse Events of Any Cause in the As-Treated Population.
| Event | No. (%) | |||
|---|---|---|---|---|
| Control arm (n = 51) | Experimental arm (n = 45) | |||
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| Clinical | ||||
| Fatigue | 18 (35) | 4 (8) | 19 (42) | 2 (4) |
| Pruritus | 18 (35) | 0 | 19 (42) | 0 |
| Anorexia | 19 (37) | 1 (2) | 12 (27) | 2 (4) |
| Cough | 13 (25) | 0 | 12 (27) | 0 |
| Insomnia | 11 (22) | 1 (2) | 13 (29) | 2 (4) |
| Constipation | 13 (25) | 0 | 10 (22) | 0 |
| Dry mouth | 11 (22) | 0 | 12 (27) | 0 |
| Dyspnea | 10 (20) | 1 (2) | 13 (29) | 4 (9) |
| Nausea | 13 (25) | 0 | 10 (22) | 0 |
| Diarrhea | 11 (22) | 2 (4) | 9 (20) | 0 |
| Dysgeusia | 10 (20) | 0 | 8 (18) | 0 |
| Vomiting | 8 (16) | 1 (2) | 10 (22) | 0 |
| Edema | 7 (14) | 1 (2) | 10 (22) | 0 |
| Dyspepsia | 9 (18) | 0 | 7 (16) | 0 |
| Rash | 5 (10) | 2 (4) | 8 (18) | 0 |
| Laboratory | ||||
| Anemia | 16 (31) | 1 (2) | 18 (40) | 1 (2) |
| Lymphopenia | 14 (27) | 4 (8) | 19 (42) | 6 (13) |
| Hyponatremia | 11 (22) | 0 | 13 (29) | 0 |
| Hyperkalemia | 15 (29) | 0 | 9 (20) | 1 (2) |
| Blood LDH increased | 12 (24) | 0 | 10 (22) | 0 |
| Creatinine increased | 11 (22) | 1 (2) | 6 (13) | 1 (2) |
| AST increased | 9 (18) | 0 | 8 (18) | 0 |
| GGT increased | 8 (16) | 2 (4) | 8 (18) | 2 (4) |
| Alkaline phosphatase increased | 9 (18) | 2 (4) | 7 (16) | 1 (2) |
| ALT increased | 7 (14) | 0 | 7 (16) | 0 |
Abbreviations: LDH, lactate dehydrogenase; AST, aspartate transaminase; GGT, gamma-glutamyl transferase; ALT, alanine transaminase.
Post Hoc Analyses
Subgroup analyses for the risk of progression or death across baseline demographic and disease characteristics are presented in eFigures 1 and 2 in Supplement 2. No statistically significant differential treatment effects were identified. However, supplementary Cox regression analyses demonstrated a statistically significant association between the number of irradiated lesions and OS in the experimental arm (HR, 0.31; 95% CI, 0.15-0.65; P = .002; eTable 2 in Supplement 2). Post-hoc comparison of changes in absolute lymphocyte counts (n = 54) showed statistically significant differences between study arms (median [IQR] change, 3.0% [−8.4% to 27.2%] in the control arm vs −13.6% [−31.3% to 3.9%] in the experimental arm; P = .006; eFigure 3 in Supplement 2), which were found to be associated with the location of the irradiated target (median [IQR] change, −31.3% [−47.2% to −16.7%] inside vs −5.5% [−14.8% to 9.5%] outside the chest; P = .007; eFigure 5 in Supplement 2). No association was seen with treatment response (eFigure 4 in Supplement 2). Exploratory landmark analysis of OS according to irradiated tumor response did not show any meaningful differences in responders vs nonresponders (HR, 0.51; 95% CI, 0.12-2.16; P = .38; eFigure 6 in Supplement 2).
Discussion
In this phase 2 randomized clinical trial, we tested the hypothesis that the addition of multisite stereotactic radiotherapy (3 × 8 Gy) to a proportion of active disease would improve PFS in patients receiving standard-of-care ICIs targeting PD-1/PD-L1. We observed no benefit in terms of PFS (HR, 0.95; 95% CI, 0.58-1.53; P = .80) or OS (HR, 0.82; 95% CI, 0.48-1.41; P = .50) associated with the experimental treatment. To our knowledge, this is the largest study to date to evaluate the combination of radiotherapy and checkpoint blockade in a randomized fashion.
These results are consistent with those of previously published smaller phase 2 trials with similar designs focusing on 1 specific tumor type, including melanoma (n = 20), NSCLC (n = 76), HNSCC (n = 62), and RCC (n = 69).10,11,12,13 Notably, all of these studies used a single-site approach, whereby only 1 tumor lesion was irradiated together with anti–PD-1 immunotherapy. In contrast, the CHEERS trial allowed for multisite SBRT of up to 3 tumor lesions. Indeed, more than half of patients in the experimental arm who completed study-prescribed radiotherapy received SBRT to more than 1 tumor site. In doing so, we were able to confirm that targeting multiple disease sites with immunoradiotherapy remains safe, even when both modalities are administered concurrently. These findings could therefore be viewed as an extension of earlier evidence of a favorable tolerability profile of sequential SBRT-ICI approaches.14,15 Since then, many authors have argued that the benefit of combining radiotherapy with ICI in terms of efficacy may be proportionate to the irradiated tumor volume, and more pronounced when a variety of affected organs is targeted, by a release of a more diverse set of tumor-specific antigen.16,17 Because the majority of patients in the present study had more than 3 active tumor sites at the time of inclusion and treatment of all disease sites in patients with low disease burden was not mandatory, only a very small fraction of patients in the experimental arm had treatment for all visible cancer lesions (n = 8). Hence, it is unclear whether there still might be a rationale for subtotal irradiation of the disease but with larger volumes treated compared with this trial and perhaps avoiding specific targets, such as those located in the chest. Although exploratory and limited to a subset of the cohort with available data, post hoc analyses confirmed that a decrease in peripheral lymphocyte counts in the experimental arm compared with the control arm was indeed associated with thoracic SBRT. This effect could be explained by taking into consideration unintentional radiation doses to circulating lymphocytes passing through the radiation field.18 As so-called radiation-induced lymphopenia is recognized as a negative prognostic factor in patients with solid tumors,19 this finding may guide further investigation into appropriate target selection, in particular in the context of SBRT-ICI combinations.
As repeatedly shown in preclinical mouse models, radiation dose, fractionation, and timing play crucial roles in the immunologic effects of radiotherapy. Several years ago, Vanpouille-Box et al identified the optimal fractionation for inducing abscopal responses in combination with ICI as 3 × 8 Gy in a variety of mouse and human cancer cells.4 This exact dosing schedule has since been adopted in many immunoradiotherapy clinical trials, including this one. It is possible, however, that radiotherapy’s cytoreductive qualities may outweigh the value of its potential immunostimulatory effects in terms of clinical benefit, as previously demonstrated outside of the context of immunotherapy.3 Indeed, post hoc subgroup analyses demonstrate a statistically significant correlation between the number of irradiated lesions and improved OS, though these results should be interpreted with caution due to limited sample size and the risk of bias. Emerging data suggest that this may also be the case in patients with oligometastatic cancer when ablative radiation doses are combined with ICI.15,20 Despite the higher than expected LCR (75%) in this trial, we did not observe a difference in survival between responders and nonresponders. Thus, achieving systemic disease control may require ablation of all visible disease at radiation doses larger than 3 × 8 Gy. This strategy is currently under investigation in trials such as SABR-COMET 1021 and OligoRARE.22
Nevertheless, if boosting a systemic antitumor immune response is in fact the primary goal, perhaps the timing of radiotherapy administration is of even greater importance. We addressed the question of sequencing in a previous phase 1 trial, where we randomized 18 patients to receive SBRT either prior to the first or third ICI cycle.23 Even though not powered to compare efficacy between both arms, the only responses were observed in the latter group. For this reason, it was chosen to administer radiotherapy concurrently with ICI in the CHEERS study. Radiotherapy administration during the ICI treatment course meant, however, that a large number of patients had discontinued ICI therapy before the point in time when SBRT would have been added (12 in the control arm and 7 in the experimental arm), primarily due to rapid disease progression. These findings suggest a more aggressive approach earlier on could perhaps be more advantageous.
Limitations
An important limitation to be addressed is the heterogeneous patient population. While a multitumor basket trial design allowed for fast accrual, the resulting small sample size per stratum (eg, 7 patients with NSCLC) meant that there would be insufficient power to detect a differential treatment effect between subgroups. In addition, as more than half of patients in the control arm had already received some form of radiotherapy prior to study inclusion, this may have resulted in an underestimation of the overall benefit of the experimental treatment.24 A heavily pretreated patient population might also offer an explanation for the lower-than-anticipated median PFS in the control arm.
Conclusions
In this phase 2 randomized clinical trial, while the addition of subablative multisite stereotactic radiotherapy to ICI monotherapy failed to result in any clinically meaningful abscopal effect in a diverse and largely polymetastatic patient population, the findings suggest it is safe to combine both treatment modalities in this context and offer important insights for future trial design. Recent evidence suggests that treating all active disease sites with higher radiation doses in a selected patient population may be a more promising strategy to optimize systemic disease control. Moving forward, we aim to further elucidate the immunologic effects of radiotherapy and identify predictive biomarkers for patient selection in exploratory in silico and in vitro analyses of the CHEERS trial population.
Trial Protocol
eTable 1. Overview of Disease Burden at Baseline
eFigure 1. Subgroup Analysis of Progression-free Survival
eFigure 2. Subgroup Analysis of Overall Survival
eTable 2. Subgroup Analysis of Disease Burden and Radiation Targets in the Experimental Arm
eFigure 3. Change in Absolute Lymphocyte Count Across Both Study Arms
eFigure 4. Change in Absolute Lymphocyte Count According to Treatment Response
eFigure 5. Change in Absolute Lymphocyte Count in the Experimental Arm According to Location of Irradiated Target
eFigure 6. Landmark Analysis of Overall Survival Stratified by SBRT Response
Data Sharing Statement
References
- 1.Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi: 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Yao Z, Bai H, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22(9):1265-1274. doi: 10.1016/S1470-2045(21)00333-8 [DOI] [PubMed] [Google Scholar]
- 3.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830-2838. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaas M, Sundahl N, Hulstaert E, et al. Checkpoint inhibition in combination with an immunoboost of external beam radiotherapy in solid tumors (CHEERS): study protocol for a phase 2, open-label, randomized controlled trial. BMC Cancer. 2021;21(1):514. doi: 10.1186/s12885-021-08088-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dingemans AC, Hendriks LEL, Berghmans T, et al. Definition of synchronous oligometastatic non-small cell lung cancer—a consensus report. J Thorac Oncol. 2019;14(12):2109-2119. doi: 10.1016/j.jtho.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 7.Lievens Y, Guckenberger M, Gomez D, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157-166. doi: 10.1016/j.radonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 8.Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21(1):e18-e28. doi: 10.1016/S1470-2045(19)30718-1 [DOI] [PubMed] [Google Scholar]
- 9.Olson R, Mathews L, Liu M, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of 1-3 oligometastatic tumors (SABR-COMET-3): study protocol for a randomized phase III trial. BMC Cancer. 2020;20(1):380. doi: 10.1186/s12885-020-06876-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundahl N, Seremet T, Van Dorpe J, et al. Phase 2 trial of nivolumab combined with stereotactic body radiation therapy in patients with metastatic or locally advanced inoperable melanoma. Int J Radiat Oncol Biol Phys. 2019;104(4):828-835. doi: 10.1016/j.ijrobp.2019.03.041 [DOI] [PubMed] [Google Scholar]
- 11.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019;5(9):1276-1282. doi: 10.1001/jamaoncol.2019.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride S, Sherman E, Tsai CJ, et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol. 2021;39(1):30-37. doi: 10.1200/JCO.20.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masini C, Iotti C, De Giorgi U, et al. Nivolumab in combination with stereotactic body radiotherapy in pretreated patients with metastatic renal cell carcinoma: results of the phase II NIVES study. Eur Urol. 2022;81(3):274-282. doi: 10.1016/j.eururo.2021.09.016 [DOI] [PubMed] [Google Scholar]
- 14.Luke JJ, Lemons JM, Karrison TG, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611-1618. doi: 10.1200/JCO.2017.76.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siva S, Bressel M, Wood S, et al. Stereotactic radiotherapy and pembrolizumab for oligometastatic renal tumors: the RAPPORT trial. J Clin Oncol. 2021;39(6). doi: 10.1200/JCO.2021.39.6_suppl.277 [DOI] [Google Scholar]
- 16.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16(2):123-135. doi: 10.1038/s41571-018-0119-7 [DOI] [PubMed] [Google Scholar]
- 17.Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and immunotherapy for cancer: from “systemic” to “multisite”. Clin Cancer Res. 2020;26(12):2777-2782. doi: 10.1158/1078-0432.CCR-19-2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. 2019;105(2):346-355. doi: 10.1016/j.ijrobp.2019.05.064 [DOI] [PubMed] [Google Scholar]
- 19.Grassberger C, Ellsworth SG, Wilks MQ, Keane FK, Loeffler JS. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol. 2019;16(12):729-745. doi: 10.1038/s41571-019-0238-9 [DOI] [PubMed] [Google Scholar]
- 20.Luke JJ, Onderdonk BE, Bhave SR, et al. Improved survival associated with local tumor response following multisite radiotherapy and pembrolizumab: secondary analysis of a phase I trial. Clin Cancer Res. 2020;26(24):6437-6444. doi: 10.1158/1078-0432.CCR-20-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stereotactic ablative radiotherapy for comprehensive treatment of 4-10 oligometastatic tumors (SABR-COMET 10). ClinicalTrials.gov identifier: NCT03721341. Updated October 14, 2022. Accessed May 31, 2023. https://clinicaltrials.gov/ct2/show/NCT03721341?term=NCT03721341&draw=2&rank=1
- 22.Stereotactic body radiotherapy in patients with rare oligometastatic cancers (OligoRARE) (OligoRARE). ClinicalTrials.gov identifier: NCT04498767. Updated April 18, 2023. Accessed May 31, 2023. https://clinicaltrials.gov/ct2/show/NCT04498767?term=NCT04498767&draw=2&rank=1
- 23.Sundahl N, Vandekerkhove G, Decaestecker K, et al. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur Urol. 2019;75(5):707-711. doi: 10.1016/j.eururo.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 24.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903. doi: 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Overview of Disease Burden at Baseline
eFigure 1. Subgroup Analysis of Progression-free Survival
eFigure 2. Subgroup Analysis of Overall Survival
eTable 2. Subgroup Analysis of Disease Burden and Radiation Targets in the Experimental Arm
eFigure 3. Change in Absolute Lymphocyte Count Across Both Study Arms
eFigure 4. Change in Absolute Lymphocyte Count According to Treatment Response
eFigure 5. Change in Absolute Lymphocyte Count in the Experimental Arm According to Location of Irradiated Target
eFigure 6. Landmark Analysis of Overall Survival Stratified by SBRT Response
Data Sharing Statement