Abstract
EVs are membranous subcellular structures originating from various cells, including platelets which consist of biomolecules that can modify the target cell’s pathophysiological functions including inflammation, cell communication, coagulation, and metastasis. EVs, which are known to allow the transmission of a wide range of molecules between cells, are gaining popularity in the fields of subcellular treatment, regenerative medicine, and drug delivery. PEVs are the most abundant EVs in circulation, being produced by platelet activation, and are considered to have a significant role in coagulation. PEV cargo is extremely diverse, containing lipids, proteins, nucleic acids, and organelles depending on the condition that induced their release and can regulate a wide range of biological activities. PEVs, unlike platelets, can overcome tissue barriers, allowing platelet-derived contents to be transferred to target cells and organs that platelets cannot reach. Their isolation, characterization, and therapeutic efficacy, on the other hand, are poorly understood. This review summarizes the technical elements of PEV isolation and characterization methods as well as the pathophysiological role of PEVs, including therapeutic potential and translational possibility in diverse disciplines.
Keywords: platelets, PEVs, hemostasis, inflammation, angiogenesis, wound healing, carcinogenesis, therapy
1. Introduction
EVs are membranous subcellular structures originating from various cells, including platelets through a wide range of biomechanism [1]. EVs are divided into subpopulations based on morphology, size, content, cellular origin, and the functions they perform [2]. The EVs can bundle active cargo such as proteins, nucleic acids, and lipids and convey it to a recipient cell, whether close or far away, and thus, they can modify the destination cell’s pathophysiological functions including inflammation, cell communication, coagulation, and metastasis in the process [3]. EVs have enormous promise for the advancement of innovative biological treatments. Recently, EVs are being engineered as a disease-modifying biotherapy for age-related degeneration and as medication delivery vehicles for cancer, immunological, and inflammatory illnesses.
The most common type of EVs in circulation are PEVs, which are released upon activation of platelets by various factors [4]. PEVs have capabilities comparable to platelets and are thus thought to have an impact on a variety of biological processes such as coagulation, wound healing, and inflammation. PEVs have been shown to stimulate cellular differentiation, hence improving musculoskeletal or neurological regeneration. Unlike platelets, PEVs can pass across tissue barriers, extending their capabilities outside of the blood [5]. PEVs do not have legal and safety issues, can be obtained as a byproduct from whole-blood donations, bring no concerns regarding contamination or immunological reactions, and are unable to multiply because they lack a functioning nucleus. Therefore, using PEVs instead can have the desirable advantage of boosting the benefits of their clinical application. However, PEVs are poorly understood in terms of standardization, heterogeneity, repeatability, and storage conditions. It is unknown how PEVs package their machinery, transport it to other cells, and communicate between the cells in order to alter the pathophysiology of the target cells.
In this review, we summarize the technical features of PEV isolation and characterization approaches including the pathophysiological role of PEVs. In addition, this review will also look at the advantages and limitations of therapeutic applications of PEVs to grasp the fundamental needs for their clinical translation.
2. Extracellular Vesicles
EVs are lipid bilayer-delimited particles that are generated by nearly all types of cells in a normal manner but are unable to proliferate like cells. EVs are a tool for intercellular communication, facilitating the interchange of a wide variety of chemicals between nearby or far-away cells. EVs usually contain lipids, nucleic acids, and proteins, particularly those connected to the cell membrane, the cytosol, and those involved in lipid metabolism [6,7]. The diversity of cell types and functional states, as well as the various biogenetic pathways, all contribute to the variability of EVs. Exosomes, microvesicles, and apoptotic bodies are the three primary subtypes of EVs, distinguished by their biogenesis, release mechanisms, size, composition, and function [8]. As a brief explanation, exosomes are encased in a single outer membrane, typically ranging from 30 to 150 nm, released by all types of the cell through the endosomal pathway, and present in various kinds of body fluids [6,7]. Exosomal vesicles specifically originate by inward budding of early endosomes’ limiting membranes, which develop into multivesicular bodies (MVBs) in the process [9]. Exosomes are engaged in the cell’s endocytic and material trafficking processes, playing a part in protein sorting, recycling, storage, transport, and release, specifically [10]. Alix, TSG101, HSC70, and HSP90 are expected to be expressed by exosomes and can be used as “exosomal marker proteins” [11,12]. Exosomes frequently contain the tetraspanin proteins including CD63, CD9, and CD81. Exosomes have a role in cell-to-cell communication, cell maintenance, tumor progression, and cellular waste management, and they behave as antigen-presenting vesicles and promote immunological responses [13].
Microvesicles are EVs that develop from the cell membrane by directly outward budding, or pinching, and typically have diameters between 100 nm and 1000 nm. Microvesicles mostly contain cytosolic and cell membrane-associated proteins, such as tetraspanins. Integrins, heat shock proteins, cytoskeletal proteins, and proteins with post-translational modifications including glycosylation and phosphorylation are other proteins that are frequently found in microvesicles [14]. Initially, it was believed that microvesicles were a cellular dumping or maintenance process, similarly to exosomes, by which the cell would get rid of waste [9]. However, microvesicles are now recognized to play a role in cell–cell communication between nearby and distant cells. Likewise, dying cells discharge apoptotic bodies into the extracellular environment. According to reports, they can be as little as 50 nm or as large as 5000 nm in diameter, with most apoptotic bodies being on the larger side [15]. During cell contraction, increasing hydrostatic pressure causes the cell membrane to separate from the cytoskeleton, resulting in the generation of these apoptotic bodies [16]. Apoptotic bodies, as opposed to exosomes and microvesicles, include intact organelles, chromatin, and modest levels of glycosylated proteins [16]. So, it makes sense to anticipate seeing larger quantities of proteins associated with the nucleus, mitochondria, Golgi apparatus, and endoplasmic reticulum (such as histones), among other structures.
3. Origin of PEVs
Platelets are anucleated, discoid cells that originate from megakaryocytes in the bone marrow and circulate in the bloodstream with a physiological count of 150,000 to 450,000 platelets/µL of blood and a lifespan of 8–10 days. The resting platelet consists of alpha granules, dense granules, lysosomal granules, and glycogen granules, which contain various kinds of proteins, growth factors, angiogenic factors, chemokines, immune mediators, etc. that are involved in various pathophysiological activities of the platelet (Table 1) [17]. The activation of platelets leads to the development of hemostasis and thrombosis. Upon vascular injury, von Willebrand Factor (vWF) and collagen become exposed to the extracellular matrix which binds with their respective receptors present in the platelet and activates the platelet. The activated platelet releases adenosine diphosphate (ADP) and generates thromboxane A2, which further recruits the circulating platelet in the bloodstream, activates them, and forms the hemostatic plug by converting fibrinogen to fibrin in the presence of thrombin.
Under normal circumstances, the circulating microvesicles found in the plasma come mostly from megakaryocytes. However, in pathological conditions, microvesicles are produced by activated platelets. Upon activation of platelets by a variety of agonists, platelets readily generate EVs as well (cellular plasma membrane (microvesicles) or endosomal compartment (exosomes)) that remain circulating in the bloodstream. Chargaff and West initially documented PEVs as coagulant lipoproteins that were separated from platelets by differential centrifugation [18]. Shortly after, electron microscopic analyses of α-granule release from platelets also imaged small vesicles being released, which were referred to as exosomes [19,20]. Data from various electron microscopy demonstrated that depending on the type of agonist stimulation, there are two types of PEVs released: small vesicles (exosomes) with a diameter of ~40 to 100 nm that expose CD63 and are undetectable by flow cytometry, and larger vesicles (microvesicles) with a diameter of 100 to 1000 nm that expose annexin-V and express αIIb-β3 and β1, GP1bα, and P-selectin, which enables Factor X and prothrombin [14,21]. It is widely acknowledged that megakaryocytes and platelets are the main sources of EVs in blood circulation [22,23]. Another research demonstrated the first proof that PEVs may have both pro- and anti-coagulatory effects, even though PEVs had previously only been linked to procoagulant activity [24]. Now, PEV cargo is considered to be highly diverse consisting of proteins, growth factors, nucleic acids, and organelles that are present in the platelet itself (as shown in Table 1) and are engaged in diverse biological activities in different cell types. PEVs can infiltrate into various organs and tissues where they contribute to more distant cellular communication. This makes it possible to deliver platelet-derived material to cells and organs that platelets cannot reach.
Table 1.
Various chemical modulators present in platelets.
| Location | Type | Chemical Modulators | References |
|---|---|---|---|
| α-granules | Adhesive proteins | P-selectin Fibrinogen Von Willebrand factor Fibronectin Thrombospondin-1 Thrombospondin-2 Laminin-8 Vitronectin |
[25,26,27,28,29,30,31] |
| Growth factors | EGF IGF-1 HGF TGF-β PDGF |
[32,33,34,35,36] | |
| Angiogenic factors | VEGF PDGF FGF |
[37,38,39] | |
| Chemokines | CXCL1/2/5/6/7/8/12 CCL2/3/5/7 (RANTES) IL1β CD40L Proteases |
[40,41,42,43,44,45,46,47,48,49,50] | |
| Coagulation factors | Factor V Protein S Factor XI Factor XIII Kininogens Plasminogen |
[51,52,53,54,55,56] | |
| Integral membrane proteins | Integrin αIIbβ3 GPIba-IX-V GPVI TLT-1 P-selectin |
[25,57,58,59,60] | |
| Immune mediators | Complement C3/C4 precursor Factor D/H C1 inhibitor Immunoglobulins |
[61,62,63,64,65] | |
| Protease inhibitors | α2-antiplasmin PAI-1 α2-antitrypsin α2-macroglobulin TFPI C1-inhibitor |
[64,66,67,68,69,70] | |
| Proteoglycans | MMP2, MMP9 | [71] | |
| Dense granules | Amines | Serotonin Histamine |
[72,73] |
| Bivalent cations | Ca2+ Mg2+ |
||
| Nucleotides Polyphosphates |
ATP ADP GTP GDP |
||
EGF: epidermal growth factor; IGF-1: insulin-like growth factor 1; HGF: hepatocyte growth factor; TGF-β: transforming growth factor-β; PDGF: platelet-derived growth factor; VEGF: vascular-endothelial growth factor; FGF: fibroblast growth factor; PAI-1: plasminogen activator inhibitor-1; TFPI: tissue factor inhibitor; MMP: matrix metalloprotease.
4. Isolation and Detection of Platelet-Derived Extracellular Vesicles
While there are several PEV isolation techniques that have been invented, the lack of consistent and optimum techniques is a significant barrier to introducing exosomes in the clinical and experimental field. Most of the PEV isolation methods are divided into platelet isolation and PEV isolation. Platelets must be very pure at this time, so not only simple centrifugation methods but also a 10–17% iodixanol gradient or leukocyte reduction filtration by PVC-citrate storage bag are used to isolate completely pure platelets [23,74]. Additionally, PEVs can be isolated from platelet lysate [75,76], platelet derivatives, and plasma [77]. After that, platelets can be completely activated not only with platelet agonists including collagen, thrombin, collagen-related peptide (CRP), ADP, and thrombin receptor-activating peptide (TRAP)-6, but also with lipopolysaccharide (LPS), Ca2+ ionophore, and LPS-binding protein [23]. The most commonly known method is the centrifugation technique, which obtains PEVs from the supernatant from which platelets and cell debris have been removed through centrifugation. This technique can also separate various types of EVs such as microvesicles and exosomes by adding more steps of centrifugation. However, it has been known that high-speed centrifugation should be used with precaution because it can affect the concentration of EVs, their size, or their biochemical composition via the generation of EV aggregates [78]. In addition, gel-filtration via size-exclusion chromatography, immunoaffinity chromatography using disk with anti-human CD61 antibody [77], and/or anti-CD-41, CD63, CD9, and CD81 antibody-covered beads can be applied to further separate the desired PEVs [74,75,79,80]. Although it is not currently applied to PEV isolation, precipitation is known to be used as a technique for EV isolation and purification in several other cells [81]. Detailed PEV preparation conditions that can be applied are mentioned in Table 2.
Table 2.
| Method of Isolation | Approaches | Advantages | Disadvantages |
|---|---|---|---|
| Platelet activation |
Activation for 30 min - Thrombin 1 U/mL - Collagen 10 µg/mL - CRP-XL 1 µg/mL - ADP 60 µM - TRAP-6 10 µM - Thrombin 1 U/mL + collagen 10 µg/mL - Ca2+ ionophore 10 µM Activation for 3 h - LPS 100 ng/mL - LBP 100 ng/mL - CD14 100 ng/mL Activation by CaCl2 |
- Characteristically different types of PEVs can be produced - Enhances PEVs release |
- Lower procoagulant activity - Expensive |
| Centrifugation |
PEV preparation - 800–5000× g for 5 min–30 min Purification - 20,000× g for 60 min Microvesicle pellet preparation - 2500–12,000× g for 15 min–60 min Exosome pellet preparation - 20,000–120,000× g 40 min–18 h |
- Cost efficient - Pure preparation |
- Low reproducibility - Possibility of exosomes damage |
| Membrane filtration |
PEV preparation - 0.2 µm pore membrane filtration PEV purification - 0.8 µm pore membrane filtration |
- Simple procedure - Process many samples at the same time - Pure preparation |
- Deformation of vesicles (less exosomal proteins) |
| Gel filtration (size exclusion chromatography) |
Further isolation - Isolating 0.5 mL of 26 fraction, harvest fraction 9–12 - Isolating 24–30 fraction |
- High reproducibility - Pure preparation - Preserves vesicle integrity - Prevent PEV aggregation |
- Need specialized equipment - Expensive |
| Immunoaffinity chromatography |
Further isolation - Filtering sample with the disk with anti-human CD61 antibody at a flow rate of 0.5 mL/min repeated five times. |
- Fast and easy - Enrichment of hundred to thousand-fold |
- Expensive |
| Iodixanol density gradient |
Further isolation - Collect the band from the 30% and 10% interface |
- Pure preparations without viral particles | - Sample loss - Unable to separate large particles with similar sedimentation rates |
| Immuno-bead capturing |
Further isolation - Incubate sample with Anti-CD63, CD9, and CD81 antibody covered beads |
- High reproducibility - Pure preparation |
- Not suitable for large-volume samples |
5. Analysis and Detection of Platelet Extracellular Vesicles
After the preparation of the PEVs, several analyses are applied for confirmation and characterization of them. One of the most commonly used methods is nanoparticle tracking analysis (NTA). This method gives the position, vesicle concentration, and size of particles suspended in a fluid by detecting the light they scatter [85]. It can detect nanoparticles ranging from 10 nm to 2000 nm. However, particles smaller than 50 nm are not well identified, and contaminants in the sample such as protein aggregates and cell fragments can be detected. In this case, utilizing 0.02 µm filtration or immuno-labeling the particle can specify the target particles [86]. Dynamic light scattering (DLS), a method similar to NTA, estimates scattering intensity from bulk samples, unlike NTA. The main advantage of DLS is that it can measure particles ranging from 1 nm to 6 µm. However, purification is still required because the data can only be trusted if just there is the presence of one sort of particle [87]. Flow cytometry, a method of detecting, counting, and sorting single-file passage targets using a laser beam, is another approach often employed in EV analysis [88]. This approach has the advantage of determining the absolute number of particles, although it only detects particles over 200 nm and frequently detects many vesicles as one when concentrations are high (swarming effect) [89]. Furthermore, electron microscopes are utilized for PEV detection, and not only the size of the vesicle but also its exact form can be viewed through direct measurement, but structural damage may be occurred through high surface tension during evaporation of water [90]. There are many additional approaches, such as tunable resistive pulse sensing, atomic force microscopy, and mass spectrometry, but it is important to evaluate based on the purpose and size of the target.
6. PEVs in Health and Diseases
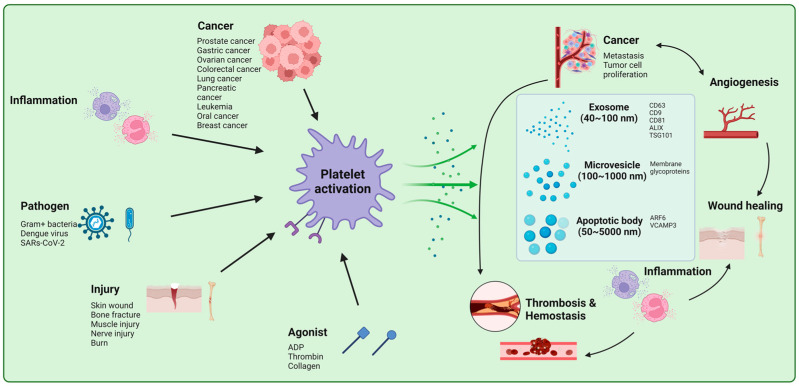
PEVs have been associated with both noninfectious chronic inflammatory diseases (e.g., atherosclerosis, diabetes, coronary artery disease, and hypertension) and infectious diseases (e.g., influenza and COVID-19) as well as other pathophysiology. The diverse roles of PEVs in various fields are shown in Figure 1 and are summarized below:
Figure 1.
The effect of PEVs in diverse pathophysiological processes. Activation of platelet by various stimuli including cancer, inflammation, pathogens, and injury leads to the release of EVs: exosomes, microvesicles, and apoptotic body The EVs can bundle active cargo such as proteins, nucleic acids, and lipids and convey it to a recipient cell, whether close or far away; thus, they can modify the destination cell’s pathophysiological functions including inflammation, cell communication, angiogenesis, coagulation, and metastasis in the process. “Created with BioRender.com”.
6.1. PEVs in Hemostasis, Coagulation, and Hemorrhagic Shock
Worldwide, trauma is responsible for more than 500,000 deaths each year, and severe hemorrhage leading to trauma-induced coagulopathy (TIC) characterizes the majority of these cases [91]. Platelet and plasma therapy have been shown to decrease hemorrhage-associated mortality in TIC patients [92]. One of the known primary activities assigned to platelet EVs is coagulation [4,18]. The surface of circulating PEVs has been shown to be 50–100-fold more procoagulant than activated platelets [93]. Negatively charged phosphatidylserine and tissue factor (TF) exposed to the surface of PEVs have been substantially attributed to their pro-coagulant activities. PEVs express binding sites for coagulation factors such as activated factor V and factor VIII as well as thrombin [93,94,95]. EV clearance was recently demonstrated to be aided by coating PS with lactadherin, which reduced coagulopathy and improved survival in a traumatic brain injury mouse model [96]. PEVs produce activated protein C, which is known to be involved coagulation process [24]. Following severe trauma, treatment with PEVs has been shown to enhance hemostasis, stop blood loss, and slow the development of hemorrhagic shock [85]. Since platelets cannot be kept and must be utilized within five days of withdrawal, PEV preparation clearly outperforms platelet transfusion in terms of storage. Investigating plasma proteins, blood cells, and the endothelium is important to have a thorough grasp of the hemodynamic physiology of PEVs. Taken together, although platelets and PEVs have numerous functional similarities, including a strong procoagulant capacity, PEVs may be a better option for hemostasis. However, before these encouraging results can be applied to clinical practice, more study is required to characterize the PEV isolates in greater detail. Investigating the interaction between proteins and cells present in the blood, and the endothelium is important to have a thorough grasp of hemodynamic physiology. By doing so, we can explore the dynamics of the biomarkers and have the opportunity to gauge the treatment’s immediate impact on coagulation.
6.2. PEVs in Immune Response and Inflammation
PEVs have a significant effect on the pathophysiology of the immune system by increasing in quantity or changing granule contents. PEV is released by platelet activation mediated by platelet agonists in a physiologic state and inflammation and/or infection in a pathologic state [97,98]. Various viruses trigger the release of PEV, of which dengue virus induces PEV release through c-type lectin (CLEC)-2 of the platelet [99]. Additionally, COVID-19 has recently been found to induce PEV release through CLEC-2 [100] and elevate PF4+ and HMGF1+ PEVs, which are elevated in sepsis [101,102]. It is known that several Gram-positive bacteria increase the release of EVs, but this has not been elucidated in PEVs [103]. Generated PEVs are involved in a variety of immune-related pathways, and they interact with mononuclear cells more than other inflammatory cells. PEVs can deposit inflammatory mediators such as CCL5 onto the endothelium surface, resulting in the recruitment of mononuclear cells during rolling [104]. Additionally, PEVs increased the adhesion of monocytes to endothelial cells by arachidonic acid and protein kinase C activation in a time- and dose-dependent manner [105]. In addition, PEVs bind to monocytes via p-selectin-p-selectin glycoprotein ligands-1, and this is sustained by phosphatidylserine binding, at which GPIbα transfers to the monocyte, which is recruited into the blood vessel and stimulate angiogenesis [106]. Additionally, PEVs have the ability to change the distribution of monocyte subsets towards intermediate CD14+ CD16+ monocytes with inflammatory properties [107]. PEVs also can significantly increase the expression of MMP 9, hydrogen peroxide, and pro-inflammatory factors, including C5a and tumor necrosis factor (TNF) [108]. PEV is also known to interact with several other inflammatory cells. Recently, PEVs have been known to interact with T-lymphocyte [109]. PEVs induced the differentiation of naive CD4+ T Cells into Foxp3+ regulatory T cells by TGF-β, and they induced immunosuppressive response by decreasing the release of IFNr, TNFα, and IL-6 [110]. Furthermore, unlike platelets, PEVs circulate in lymph and express MHC-1 to perform co-activation with lymphocytes via CD40L and OX40L [111]. However, the role of PEVs in T-lymphocyte, including proliferation, differentiation, and cytokine production, needs further research [109]. Additionally, it is known that PEVs can enhance inflammatory response by capturing and activating neutrophils and endothelial cells to promote interaction [112]. In particular, PEVs worsen the symptoms by activating neutrophils through heterocomplexes of TLR2 and CLEC5a [99], and they increase the activity of EV-TF [113]. PEVs eventually induce a number of diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). PEVs in RA enter the lymphatic system and influence joint vascular leakage via the fibrinogen receptor αIIbβ3 and serotonin [114]. A high number of influxed microvesicles, the majority of which carried the platelet marker CD41a, were found in synovial fluid from an RA patient and stimulated monocyte adhesion to the endothelium, thus increasing the ICAM-1 in monocytes [115,116]. In addition, presence of intra-vesicular arachidonic acid in PEVs increases monocyte adhesion to endothelial cells, which further transfers the lipids and lipid metabolism in cells, eventually inducing atherosclerosis and inflammation [117]. Furthermore, PEVs promote inflammation through serotonin and IL-1 in SLE an autoimmune disease, as well as RA [118,119]. PEVs also can spread infection by delivering functional viral RNA from cell to cell in several viral infections [120,121]. As a result, PEVs are intimately linked to immunity, including release by immune-mediated disease, interaction with inflammatory cells, and expression of immune-mediated disease symptoms.
6.3. PEVs in Angiogenesis and Wound Healing
PEVs can indirectly increase angiogenesis in the inflammatory induction and the subsequent hypoxic condition in the vascular injury [122]. However, PEVs further induce angiogenesis through various mechanisms. PEVs are known to secrete various angiogenic growth factors including lipid growth factors (sphingosine-1-phosphate) [123] RANTES [124] and several growth factors (VEGF, FGF-2, bFBF, PDGF, TFG-beta, EGF, hybridoma growth factor, MMP-2, MMP-9) [123,125,126,127,128]. Increased VEGF, FGF-2, and lipid growth factors induce endothelial progenitor cells differentiation, endothelial proliferation, chemotaxis, tube formation, and stimulating resident mature endothelial cells via PTX-sensitive G protein, extracellular signal-regulated kinase, phosphoinositide 3-kinases, AKT, and Src kinase activation [123,125,126,127,129]. Furthermore, PEVs amplify the vaso-regenerative potential of endothelial progenitor cells (EPCs) and support the maintenance of vascular integrity after arterial injury through recruitment, migration, and differentiation via CXCR4 sensitization and by providing CD31, vWF, and lectin phenotype of EPCs [129,130]. PEVs also contain angiogenic microRNAs (miRNAs), including miR-320, miR-25, and miR-126. Among these, miR-126 has the ability to down-regulate vascular cell adhesion molecule-1 upon the VEGF, thereby contributing to endothelial migration and proliferation [131]. The angiogenic effect of PEV changes in several disease conditions. PEVs in patients with pulmonary arterial hypertension induced more transcription and translation of VEGF-A, and FGF, further promoting endothelial cell activation through escape lysosomal degradation [132]. In addition, the PEVs improve the process of revascularization in ischemic myocardium [125]. On the other hand, Nitric oxide (NO) and bacterial elements can trigger the PEV release and further induce caspase-3 activation and apoptosis of target endothelial cells through active ROS/RNS generation by NADPH oxidase and NO synthase via redox-signaling pathway type II in sepsis [133,134]. In addition, PEVs secreted from PM2.5 (fine dust)-exposed platelets significantly reduced the proliferation of vascular endothelium by changing miRNAs level, decreasing the effective angiogenic factors and increasing proinflammatory factors (ICAM-1, IL-6, and TNF-a), ROS level, and apoptosis (up-regulation of cytochrome-C, BAX, and cleaved caspase-3, and down-regulation of Bcl-2) [135]. As a result, PEVs can be an effective therapeutic target in a certain disease state, and they can also be a candidate for investigating delayed or augmented angiogenesis in numerous diseases with unknown angiogenesis mechanisms.
6.4. PEVs in Carcinogenesis
PEVs are influenced by tumor cells and, in turn, influence tumor cells by various mechanisms in different tumor cell types. It is known that PEVs are increased by several types of tumors including tumor including prostate cancer [136,137], gastric cancer [138], ovarian cancer [139], colorectal cancer [140,141,142], lung cancer [143], pancreatic cancer [141], acute lymphoblastic leukemia, oral squamous cell carcinoma [144], and breast cancer [145]. Increased PEVs raise the level of factors, such as VEGF, IL-6, RANTET, fibrinogen, and TNF-α, which increases metastasis and cancer grade [138,144], and eventually, this significantly reduces median survival time [136]. This increase in PEVs is especially pronounced in the presence of a large tumor, distant metastases, or invasiveness [145]. In addition, the increase in D-dimer may have come from an increase in PEVs, which promotes thrombosis [137,139,140]. All of these tumor-induced PEV alterations are also seen in proteomics. Increased HLA and PSMD2 levels in PEVs derived from colorectal cancer patients promote immune response, and an increase in HLA elevates platelet activity, resulting in accelerated carcinogenesis [142]. However, further research is needed to determine whether the increase in PEVs number is the result of chemotherapy [136,143,146,147].
On the other hand, PEVs also affect tumor cells. PEVs enhance tumor cell invasion by stimulating MMP-2 synthesis and secretion [148]. PEVs transfer CD41 to lung cancer and induce the phosphorylation of mitogen-activated protein kinase (MAPK) p42/44, serine/threonine kinases, and membrane type 1-matrix metalloproteinase (MT1-MMP), which stimulate proliferation, upregulate cyclin D2 expression, and increase trans-Matrigel chemo-invasion [149]. Furthermore, PEVs stimulate mRNA expression of angiogenic factors, including MMP-9, VEGF, IL-8, and HGF, which promote lung cancer metastasis. Additionally, miR-939 in PEVs increases the migration, proliferation, and expression of molecules associated with epithelial–mesenchymal transition in epithelial ovarian cancer mediated by sPLA2-IIa [150]. Tropomyosin 3 (TPM3) mRNA, which has been associated with metastasis in breast cancer, significantly increases the platelet of breast cancer patients, which transmit TPM3 mRNA to breast cancer through PEVs, giving breast cancer a migrative phenotype [151]. Moreover, PEVs aggregate breast cancer cells, bind and internalize to the breast cancer cell, and stimulate migration and invasion via phosphorylating p38 MAPK and myosin light chain [152]. PEVs from colorectal cancer patients accelerate metastasis by increasing EMT markers TWIST1 and VIM in the colorectal cancer cell line, as well as enhancing COX2 and TxA2 generation to promote cancer development [142]. In addition, colorectal cancer cell line acquired the capacity to produce 12-HETE from PEVs generated from platelet type 12-LOX, which is detected in adenoma or adenocarcinoma patients [153]. Furthermore, EMT genes expression are suppressed by 12-LOX inhibitors, suggesting that they can be utilized as a treatment for cancer. However, PEVs can suppress the growth of lung and colon carcinomas by miR-24 by inhibiting mitochondrial noncoding small nucleolar RNA mt-Nd2 and Snora75 [154]. PEVs also inhibit the expression of the EMT marker CDH1 [142]. Not only the characteristics of each tumor by PEVs but also the contradictory findings of PEVs on tumor growth should be thoroughly investigated in order to identify the mechanisms and efficient treatment of the tumor in the future.
7. Therapeutic Applications and Future Perspectives of PEVs
PEVs are bioproduct that have recently been used in regenerative medicine through their functions including inflammation, hemostasis, angiogenesis, and cell proliferation, and it is gaining attention dues to their efficiency more than platelets [155]. In addition, the research has not been thoroughly conducted, and thus, possibilities in various fields are expected. In breast cancer cell lines (MDA-MB-231, SKBR3, and BT474 but not MCF-7 cells), PEVs efficiently interact with all except MCF-7 [156]. Similarly, in rheumatic arthritis, platelet exosome displays antigens that are detected by rheumatic arthritis-specific autoantibodies [157]. In the case of a COVID-19 patient, proteins linked to cardiovascular disease and pro-thrombotic/endothelial damage factors were elevated in EVs from severe cases. However, in moderate cases, levels of TF, CD163, and EN-RAGE were lower compared to severe cases [157]. Thus, if proteomic and genomic profiling of PEVs is completed, it can serve as a biomarker. Furthermore, PEVs can bind with integrin and membrane glycoproteins such as GPIIbIIIa (CD41/CD61 or integrin αIIbβ3), GPIaIIa (CD49b/CD29), GPIba (CD42b), P-selectin (CD62P), platelet endothelial cell adhesion molecule-1 (CD31), and GP53, allowing us to employ it in drug delivery. Additionally, since cells inhibit apoptosis by exporting their intracellular caspase-3 through EVs to perform waste management, platelet homeostasis research can be undertaken by controlling PEV release [158]. Moreover, it has been known that cells secrete viral DNA and RNA via EV in a viral infection state, and thus, it will be available as an identifying mechanism for spreading and as a new virucidal target [159,160]. In addition, PEVs are taken up by cancer cells, causing them to produce corresponding substances by transferring molecules (as discussed earlier) [153]. Beyond just drug delivery, this mechanism can provide a new approach to preventing or improving various pathological conditions. However, there are many hurdles to using these PEVs in clinical practice. PEVs derived from optimized preparation from blood without blood-related infectious diseases should be treated with appropriate treatment methods and should be kept for a long time without degradation. Additionally, we should know exactly in which diseases PEVs should not be utilized.
Abbreviations
| EV | Extracellular vesicle |
| PEV | Platelet-derived extracellular vesicle |
| vWF | Von Willebrand factor |
| ADP | Adenosine diphosphate |
| EGF | Epidermal growth factor |
| IGF-1 | Insulin-like growth factor-1 |
| HGF | Hepatocyte growth factor |
| TGF-β | Transforming growth factor-β |
| PDGF | Platelet-derived growth factor |
| VEGF | Vascular-endothelium growth factor |
| FGF | Fibroblast growth factor |
| PAI-1 | Plasminogen activator inhibitor-1 |
| TFPI | Tissue factor inhibitor |
| MMP | Matrix metalloprotease |
| CRP | Collagen-related protein |
| TRAP | Thrombin receptor-activating protein |
| LPS | Lipopolysaccharide |
| NTA | Nanoparticle Tracking Analysis |
| TIC | Trauma-induced coagulopathy |
| TF | Tissue factor |
| CLEC | C-type lectin |
| RA | Rheumatoid arthritis |
| SLE | Systematic lupus erythematosus |
| EPC | Endothelial progenitor cell |
| miRNAs | Micro RNAs |
| NO | Nitric Oxide |
| MAPK | Mitogen-activated kinase |
Author Contributions
Conceptualization, S.K. (Soochong Kim), P.K.C. and S.K. (Sanggu Kim); validation, S.K. (Soochong Kim); investigation, P.K.C. and S.K. (Sanggu Kim); writing―original draft, P.K.C. and S.K. (Sanggu Kim); writing―review and editing, S.K. (Soochong Kim); supervision, S.K. (Soochong Kim). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Research Foundation of Korea (NRF-2022R1A2C1003638), the Basic Research Lab Program (2022R1A4A1025557) through the NRF of Korea funded by the Ministry of Science and ICT, the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Convergence Technologies Program for Educating Creative Global Leader, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA; 320005-4), and the National IT Industry Promotion Agency (NIPA) of Korea grant funded by the Korea government (MSIT) (S2005-22-1031).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Buzas E.I., György B., Nagy G., Falus A., Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 2.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boilard E., Duchez A.-C., Brisson A. The diversity of platelet microparticles. Curr. Opin. Hematol. 2015;22:437–444. doi: 10.1097/MOH.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 4.Berckmans R.J., Nieuwland R., Böing A.N., Romijn F.P., Hack C.E., Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001;85:639–649. [PubMed] [Google Scholar]
- 5.Puhm F., Boilard E., Machlus K.R. Platelet extracellular vesicles: Beyond the blood. Atertio. Thromb. Vasc. Biol. 2021;41:87–96. doi: 10.1161/ATVBAHA.120.314644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaborowski M.P., Balaj L., Breakefield X.O., Lai C.P. Extracellular vesicles: Composition, biological relevance, and methods of study. Bioscience. 2015;65:783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bebelman M.P., Smit M.J., Pegtel D.M., Baglio S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018;188:1–11. doi: 10.1016/j.pharmthera.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Veziroglu E.M., Mias G.I. Characterizing extracellular vesicles and their diverse RNA contents. Front. Genet. 2020;11:700. doi: 10.3389/fgene.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yáñez-Mó M., Siljander P.R.-M., Andreu Z., Bedina Zavec A., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra K., Ventura M. Bioética, imigração e assistência à saúde: Tensões e convergências sobre o direito humano à saúde no Brasil na integração regional dos países. Cad. Saúde Colet. 2017;25:123–129. doi: 10.1590/1414-462x201700010185. [DOI] [Google Scholar]
- 11.Morita E., Sandrin V., Chung H.Y., Morham S.G., Gygi S.P., Rodesch C.K., Sundquist W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Géminard C., De Gassart A., Blanc L., Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5:181–193. doi: 10.1111/j.1600-0854.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- 13.Chaput N., Théry C. Exosomes: Immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 14.Heijnen H.F., Schiel A.E., Fijnheer R., Geuze H.J., Sixma J.J. Activated Platelets Release Two Types of Membrane Vesicles: Microvesicles by Surface Shedding and Exosomes Derived from Exocytosis of Multivesicular Bodies and α-Granules. Blood J. Am. Soc. Hematol. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 15.Borges F.T., Reis L., Schor N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013;46:824–830. doi: 10.1590/1414-431X20132964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickman G., Julian L., Olson M. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012;19:735–742. doi: 10.1038/cdd.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senzel L., Gnatenko D.V., Bahou W.F. The platelet proteome. Curr. Opin. Hematol. 2009;16:329–333. doi: 10.1097/MOH.0b013e32832e9dc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chargaff E., West R. The biological significance of the thromboplastic protein of Wood. J. Biol. Chem. 1946;166:189–197. doi: 10.1016/S0021-9258(17)34997-9. [DOI] [PubMed] [Google Scholar]
- 19.Webber A.J., Johnson S.A. Platelet participation in blood coagulation aspects of hemostasis. Am. J. Pathol. 1970;60:19. [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br. J. Haematol. 1971;21:53–69. doi: 10.1111/j.1365-2141.1971.tb03416.x. [DOI] [PubMed] [Google Scholar]
- 21.Sandberg H., Bode A.P., Dombrose F.A., Hoechli M., Lentz B.R. Expression of coagulant activity in human platelets: Release of membranous vesicles providing platelet factor 1 and platelet factor 3. Thromb. Res. 1985;39:63–79. doi: 10.1016/0049-3848(85)90122-7. [DOI] [PubMed] [Google Scholar]
- 22.Flaumenhaft R., Dilks J.R., Richardson J., Alden E., Patel-Hett S.R., Battinelli E., Klement G.L., Sola-Visner M., Italiano J.E., Jr. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. J. Am. So. Hematol. 2009;113:1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aatonen M.T., Öhman T., Nyman T.A., Laitinen S., Grönholm M., Siljander P.R.-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles. 2014;3:24692. doi: 10.3402/jev.v3.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tans G., Rosing J., Thomassen M.C., Heeb M.J., Zwaal R., Griffin J.H. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77:2641–2648. doi: 10.1182/blood.V77.12.2641.2641. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi S.-i., Watanabe N., Nakazawa K., Suzuki J., Tsushima K., Tamatani T., Sakamoto S., Isobe M. Roles of P-selectin in inflammation, neointimal formation, and vascular remodeling in balloon-injured rat carotid arteries. Circ. J. 2000;102:1710–1717. doi: 10.1161/01.CIR.102.14.1710. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen B., Tang M., Larsen O.H., Laursen P.N., Fenger-Eriksen C., Rea C.J. The role of fibrinogen: A new paradigm in the treatment of coagulopathic bleeding. Thromb. Res. 2011;128:S13–S16. doi: 10.1016/S0049-3848(12)70004-X. [DOI] [PubMed] [Google Scholar]
- 27.Peyvandi F., Garagiola I., Baronciani L. Role of von Willebrand factor in the haemostasis. Blood Transfus. 2011;9:s3–s8. doi: 10.2450/2011.002S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huveneers S., Truong H., Fässler R., Sonnenberg A., Danen E.H. Binding of soluble fibronectin to integrin α5β1–link to focal adhesion redistribution and contractile shape. J. Cell Sci. 2008;121:2452–2462. doi: 10.1242/jcs.033001. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K., Li M., Yin L., Fu G., Liu Z. Role of thrombospondin-1 and thrombospondin-2 in cardiovascular diseases. Int. J. Mol. Med. 2020;45:1275–1293. doi: 10.3892/ijmm.2020.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara H., Kikkawa Y., Sanzen N., Sekiguchi K. Purification and Characterization of Human Laminin-8: Laminin-8 Stimulates Cell Adhesion and Migration through A3β1 and A6β1integrins. J. Biol. Chem. 2001;276:17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 31.Madsen C.D., Ferraris G.M.S., Andolfo A., Cunningham O., Sidenius N. uPAR-induced cell adhesion and migration: Vitronectin provides the key. J. Cell Biol. 2007;177:927–939. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong R.W.C., Guillaud L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 2004;15:147–156. doi: 10.1016/j.cytogfr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Clemmons D.R., Snyder P., Martin K. Physiology of Insulin-Like Growth Factor 1. Up to Date Website. 2014. [(accessed on 21 April 2023)]. Available online: https://www.uptodate.com/contents/physiology-of-insulin-like-growth-factor-1.
- 34.Oliveira A.G., Araújo T.G., Carvalho B.d.M., Rocha G.Z., Santos A., Saad M.J. The role of hepatocyte growth factor (HGF) in insulin resistance and diabetes. Front. Endocrinol. 2018;9:503. doi: 10.3389/fendo.2018.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagaraj N.S., Datta P.K. Targeting the transforming growth factor-β signaling pathway in human cancer. Expert Opin. Investig. Drugs. 2010;19:77–91. doi: 10.1517/13543780903382609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kardas G., Daszyńska-Kardas A., Marynowski M., Brząkalska O., Kuna P., Panek M. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front. Pharmacol. 2020;11:47. doi: 10.3389/fphar.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niu G., Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets. 2010;11:1000–1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raica M., Cimpean A.M. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals. 2010;3:572–599. doi: 10.3390/ph3030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia T., Jacquet T., Dalonneau F., Coudert P., Vaganay E., Exbrayat-Héritier C., Vollaire J., Josserand V., Ruggiero F., Coll J.-L. FGF-2 promotes angiogenesis through a SRSF1/SRSF3/SRPK1-dependent axis that controls VEGFR1 splicing in endothelial cells. BMC Biol. 2021;19:173. doi: 10.1186/s12915-021-01103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernhard S., Hug S., Stratmann A.E.P., Erber M., Vidoni L., Knapp C.L., Thomaß B.D., Fauler M., Nilsson B., Ekdahl K.N. Interleukin 8 elicits rapid physiological changes in neutrophils that are altered by inflammatory conditions. J. Innate. Immun. 2021;13:225–241. doi: 10.1159/000514885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown A.J., Sepuru K.M., Sawant K.V., Rajarathnam K. Platelet-derived chemokine CXCL7 dimer preferentially exists in the glycosaminoglycan-bound form: Implications for neutrophil–platelet crosstalk. Front. Immunol. 2017;8:1248. doi: 10.3389/fimmu.2017.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawant K.V., Poluri K.M., Dutta A.K., Sepuru K.M., Troshkina A., Garofalo R.P., Rajarathnam K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016;6:33123. doi: 10.1038/srep33123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Disteldorf E.M., Krebs C.F., Paust H.-J., Turner J.-E., Nouailles G., Tittel A., Meyer-Schwesinger C., Stege G., Brix S., Velden J. CXCL5 drives neutrophil recruitment in TH17-mediated GN. Clin. J. Am. Soc. Nephrol. 2015;26:55–66. doi: 10.1681/ASN.2013101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang G., Huang W., Wang S., Wang J., Cui W., Zhang W., Lou A., Geng S., Li X. Macrophagic Extracellular Vesicle CXCL2 Recruits and Activates the Neutrophil CXCR2/PKC/NOX4 Axis in Sepsis. J. Immunol. Res. 2021;207:2118–2128. doi: 10.4049/jimmunol.2100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jovic S., Linge H., Shikhagaie M., Olin A., Lannefors L., Erjefält J., Mörgelin M., Egesten A. The neutrophil-recruiting chemokine GCP-2/CXCL6 is expressed in cystic fibrosis airways and retains its functional properties after binding to extracellular DNA. Mucosal Immunol. 2016;9:112–123. doi: 10.1038/mi.2015.43. [DOI] [PubMed] [Google Scholar]
- 46.Isles H.M., Herman K.D., Robertson A.L., Loynes C.A., Prince L.R., Elks P.M., Renshaw S.A. The CXCL12/CXCR4 signaling axis retains neutrophils at inflammatory sites in zebrafish. Front. Immunol. 2019;10:1784. doi: 10.3389/fimmu.2019.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aldinucci D., Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;2014:292376. doi: 10.1155/2014/292376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue M.L., Thakur A., Cole N., Lloyd A., Stapleton F., Wakefield D., Willcox M.D. A critical role for CCL2 and CCL3 chemokines in the regulation of polymorphonuclear neutrophils recruitment during corneal infection in mice. Immunol. Cell Biol. 2007;85:525–531. doi: 10.1038/sj.icb.7100082. [DOI] [PubMed] [Google Scholar]
- 49.Ford J., Hughson A., Lim K., Bardina S.V., Lu W., Charo I.F., Lim J.K., Fowell D.J. CCL7 is a negative regulator of cutaneous inflammation following Leishmania major infection. Front. Immunol. 2019;9:3063. doi: 10.3389/fimmu.2018.03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39:1–16. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam W., Moosavi L. Physiology, Factor V. StatPearls Publishing; Treasure Island, FL, USA: 2019. [PubMed] [Google Scholar]
- 52.Pilli V., Plautz W., Majumder R. The journey of protein S from an anticoagulant to a signaling molecule. JSM Biochem. Mol. Biol. 2016;3:1014. [PMC free article] [PubMed] [Google Scholar]
- 53.Emsley J., McEwan P.A., Gailani D. Structure and function of factor XI. Blood J. Am. Soc. Hematol. 2010;115:2569–2577. doi: 10.1182/blood-2009-09-199182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaz B.H., Hillyer C.D. Transfusion Medicine and Hemostasis: Clinical and Laboratory Aspects. Newnes; Oxford, UK: 2013. [Google Scholar]
- 55.Wu Y. Contact pathway of coagulation and inflammation. Thromb. J. 2015;13:17. doi: 10.1186/s12959-015-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baker S.K., Strickland S. A critical role for plasminogen in inflammation. J. Exp. 2020;217:e20191865. doi: 10.1084/jem.20191865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albert F., Christopher N.F. The platelet fibrinogen receptor: From megakaryocyte to the mortuary. JRSM Cardiovasc. Dis. 2012;1:cvd-2012. doi: 10.1258/cvd.2012.012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrews R.K., Gardiner E.E., Shen Y., Whisstock J.C., Berndt M.C. Glycoprotein Ib–IX–V. Int. J. Biochem. Cell Biol. 2003;35:1170–1174. doi: 10.1016/S1357-2725(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 59.Moroi M., Jung S.M. Platelet glycoprotein VI: Its structure and function. Thromb. Res. 2004;114:221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 60.Schmoker A.M., Pearson L.M.P., Cruz C., Flores L.G.C., Branfeild S., Torres F.D.P., Fonseca K., Cantres Y.M., Ramirez C.A.S., Melendez L.M. Defining the TLT-1 interactome from resting and activated human platelets. J. Proteom. 2020;215:103638. doi: 10.1016/j.jprot.2020.103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Girardi G., Lingo J.J., Fleming S.D., Regal J.F. Essential role of complement in pregnancy: From implantation to parturition and beyond. Front. Immunol. 2020;11:1681. doi: 10.3389/fimmu.2020.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biesma D.H., Hannema A.J., van Velzen-Blad H., Mulder L., van Zwieten R., Kluijt I., Roos D. A family with complement factor D deficiency. J. Clin. Investig. 2001;108:233–240. doi: 10.1172/JCI200112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreira V.P., Pangburn M.K., Cortés C. Complement control protein factor H: The good, the bad, and the inadequate. Mol. Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmaier A.H., Smith P.M., Colman R.W. Platelet C1-inhibitor. A secreted alpha-granule protein. J. Clin. Investig. 1985;75:242–250. doi: 10.1172/JCI111680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schroeder Jr H.W., Cavacini L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maron B.A., Loscalzo J. Platelets. Elsevier; Amsterdam, The Netherlands: 2007. The role of platelets in fibrinolysis; pp. 415–430. [Google Scholar]
- 67.Damare J., Brandal S., Fortenberry Y.M. Inhibition of PAI-1 antiproteolytic activity against tPA by RNA aptamers. Nucleic Acid Ther. 2014;24:239–249. doi: 10.1089/nat.2013.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bergin D.A., Hurley K., McElvaney N.G., Reeves E.P. Alpha-1 antitrypsin: A potent anti-inflammatory and potential novel therapeutic agent. Arch. Immunol. Ther. Exp. 2012;60:81–97. doi: 10.1007/s00005-012-0162-5. [DOI] [PubMed] [Google Scholar]
- 69.Borth W. α2 “Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 70.Kato H. Tissue factor pathway inhibitor; its structure, function and clinical significance. Pol. J. Pharmacol. 1996;48:67–72. [PubMed] [Google Scholar]
- 71.Cancemi P., Aiello A., Accardi G., Caldarella R., Candore G., Caruso C., Ciaccio M., Cristaldi L., Di Gaudio F., Siino V. The role of matrix metalloproteinases (MMP-2 and MMP-9) in ageing and longevity: Focus on sicilian long-living individuals (LLIs) Mediat. Inflamm. 2020;2020:8635158. doi: 10.1155/2020/8635158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duerschmied D., Bode C. The role of serotonin in haemostasis. Hämostaseologie. 2009;29:356–359. [PubMed] [Google Scholar]
- 73.Mannaioni P., Di Bello M., Raspanti S., Gambassi F., Mugnai L., Masini E. Platelet histamine: Characterization of the proaggregatory effect of histamine in human platelets. Int. Arch. Allergy. Immunol. 1992;99:394–396. doi: 10.1159/000236294. [DOI] [PubMed] [Google Scholar]
- 74.Boing A.N., van der Pol E., Grootemaat A.E., Coumans F.A., Sturk A., Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nyam-Erdene A., Nebie O., Delila L., Buee L., Devos D., Chou S.Y., Blum D., Burnouf T. Characterization and Chromatographic Isolation of Platelet Extracellular Vesicles from Human Platelet Lysates for Applications in Neuroregenerative Medicine. ACS Biomater. Sci. Eng. 2021;7:5823–5835. doi: 10.1021/acsbiomaterials.1c01226. [DOI] [PubMed] [Google Scholar]
- 76.Antich-Rossello M., Forteza-Genestra M.A., Calvo J., Gaya A., Monjo M., Ramis J.M. Platelet-derived extracellular vesicles promote osteoinduction of mesenchymal stromal cells. Bone Joint Res. 2020;9:667–674. doi: 10.1302/2046-3758.910.BJR-2020-0111.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Multia E., Tear C.J.Y., Palviainen M., Siljander P., Riekkola M.L. Fast isolation of highly specific population of platelet-derived extracellular vesicles from blood plasma by affinity monolithic column, immobilized with anti-human CD61 antibody. Anal. Chim. Acta. 2019;1091:160–168. doi: 10.1016/j.aca.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 78.Linares R., Tan S., Gounou C., Arraud N., Brisson A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles. 2015;4:29509. doi: 10.3402/jev.v4.29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aatonen M., Valkonen S., Boing A., Yuana Y., Nieuwland R., Siljander P. Isolation of Platelet-Derived Extracellular Vesicles. Methods Mol. Biol. 2017;1545:177–188. doi: 10.1007/978-1-4939-6728-5_12. [DOI] [PubMed] [Google Scholar]
- 80.Vajen T., Benedikter B.J., Heinzmann A.C.A., Vasina E.M., Henskens Y., Parsons M., Maguire P.B., Stassen F.R., Heemskerk J.W.M., Schurgers L.J., et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell. Vesicles. 2017;6:1322454. doi: 10.1080/20013078.2017.1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles. 2014;3:24858. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karimi N., Dalirfardouei R., Dias T., Lotvall J., Lasser C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma—Contributions of platelet extracellular vesicles in plasma samples. J. Extracell. Vesicles. 2022;11:e12213. doi: 10.1002/jev2.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Preusser C., Hung L.H., Schneider T., Schreiner S., Hardt M., Moebus A., Santoso S., Bindereif A. Selective release of circRNAs in platelet-derived extracellular vesicles. J. Extracell. Vesicles. 2018;7:1424473. doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez E., Srivastava A.K., Burchfield J., Wang Y.W., Cardenas J.C., Togarrati P.P., Miyazawa B., Gonzalez E., Holcomb J.B., Pati S., et al. Platelet-derived- Extracellular Vesicles Promote Hemostasis and Prevent the Development of Hemorrhagic Shock. Sci. Rep. 2019;9:17676. doi: 10.1038/s41598-019-53724-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boyd R.D., Pichaimuthu S.K., Cuenat A. New approach to inter-technique comparisons for nanoparticle size measurements; using atomic force microscopy, nanoparticle tracking analysis and dynamic light scattering. Colloids Surf. A. Physicochem. 2011;387:35–42. doi: 10.1016/j.colsurfa.2011.07.020. [DOI] [Google Scholar]
- 86.Comfort N., Cai K., Bloomquist T.R., Strait M.D., Ferrante A.W., Jr., Baccarelli A.A. Nanoparticle Tracking Analysis for the Quantification and Size Determination of Extracellular Vesicles. J. Vis. Exp. 2021;169:e62447. doi: 10.3791/62447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szatanek R., Baj-Krzyworzeka M., Zimoch J., Lekka M., Siedlar M., Baran J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017;18:1153. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van der Pol E., Coumans F.A., Grootemaat A.E., Gardiner C., Sargent I.L., Harrison P., Sturk A., van Leeuwen T.G., Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014;12:1182–1192. doi: 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
- 89.Van Der Pol E., Van Gemert M., Sturk A., Nieuwland R., Van Leeuwen T. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 2012;10:919–930. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 90.Malenica M., Vukomanovic M., Kurtjak M., Masciotti V., Dal Zilio S., Greco S., Lazzarino M., Krusic V., Percic M., Jelovica Badovinac I., et al. Perspectives of Microscopy Methods for Morphology Characterisation of Extracellular Vesicles from Human Biofluids. Biomedicines. 2021;9:603. doi: 10.3390/biomedicines9060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thetford C., Myers L. Establishing a Regional Trauma Preventable/Potentially Preventable Death Rate. Ann. Surg. 2018:1–8. doi: 10.1097/SLA.0000000000002999. [DOI] [PubMed] [Google Scholar]
- 92.Holcomb J.B., Tilley B.C., Baraniuk S., Fox E.E., Wade C.E., Podbielski J.M., del Junco D.J., Brasel K.J., Bulger E.M., Callcut R.A. Transfusion of plasma, platelets, and red blood cells in a 1: 1: 1 vs a 1: 1: 2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. Jama. 2015;313:471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sinauridze E.I., Kireev D.A., Popenko N.Y., Pichugin A.V., Panteleev M.A., Krymskaya O.V., Ataullakhanov F.I. Platelet microparticle membranes have 50-to 100-fold higher specific procoagulant activity than activated platelets. J. Thromb. Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- 94.Kerris E.W., Hoptay C., Calderon T., Freishtat R.J. Platelets and platelet extracellular vesicles in hemostasis and sepsis. J. Investig. Med. 2020;68:813–820. doi: 10.1136/jim-2019-001195. [DOI] [PubMed] [Google Scholar]
- 95.Gardiner C., Harrison P., Belting M., Böing A., Campello E., Carter B.S., Collier M.E., Coumans F., Ettelaie C., van Es N. Extracellular vesicles, tissue factor, cancer and thrombosis–discussion themes of the ISEV 2014 Educational Day. J. Extracell. Vesicles. 2015;4:26901. doi: 10.3402/jev.v4.26901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Y., Cai W., Zhao Z., Hilton T., Wang M., Yeon J., Liu W., Zhang F., Shi F.-D., Wu X. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. J. Am. Soc. Hematol. 2018;131:563–572. doi: 10.1182/blood-2017-08-801738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Raeven P., Zipperle J., Drechsler S. Extracellular vesicles as markers and mediators in sepsis. Theranostics. 2018;8:3348–3365. doi: 10.7150/thno.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cappellano G., Raineri D., Rolla R., Giordano M., Puricelli C., Vilardo B., Manfredi M., Cantaluppi V., Sainaghi P.P., Castello L., et al. Circulating Platelet-Derived Extracellular Vesicles Are a Hallmark of SARS-CoV-2 Infection. Cells. 2021;10:85. doi: 10.3390/cells10010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sung P.S., Huang T.F., Hsieh S.L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 2019;10:2402. doi: 10.1038/s41467-019-10360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sung P.S., Hsieh S.L. C-type lectins and extracellular vesicles in virus-induced NETosis. J. Biomed. Sci. 2021;28:46. doi: 10.1186/s12929-021-00741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sartori M.T., Zurlo C., Bon M., Bertomoro A., Bendo R., Bertozzi I., Radu C.M., Campello E., Simioni P., Fabris F. Platelet-Derived Microparticles Bearing PF4 and Anti-GAGS Immunoglobulins in Patients with Sepsis. Diagnostics. 2020;10:627. doi: 10.3390/diagnostics10090627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maugeri N., De Lorenzo R., Clementi N., Antonia Diotti R., Criscuolo E., Godino C., Tresoldi C., Angels for COVID-Bio B.S.G.B., Bonini C., Clementi M., et al. Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 2022;20:434–448. doi: 10.1111/jth.15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bose S., Aggarwal S., Singh D.V., Acharya N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microb. Cell. 2020;7:312–322. doi: 10.15698/mic2020.12.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mause S.F., von Hundelshausen P., Zernecke A., Koenen R.R., Weber C. Platelet microparticles: A transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 105.Barry O.P., Pratico D., Savani R.C., FitzGerald G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Investig. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chimen M., Evryviadou A., Box C.L., Harrison M.J., Hazeldine J., Dib L.H., Kuravi S.J., Payne H., Price J.M.J., Kavanagh D., et al. Appropriation of GPIbalpha from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica. 2020;105:1248–1261. doi: 10.3324/haematol.2018.215145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S.J., Yoon B.R., Kim H.Y., Yoo S.J., Kang S.W., Lee W.W. Activated Platelets Convert CD14(+)CD16(-) Into CD14(+)CD16(+) Monocytes with Enhanced FcgammaR-Mediated Phagocytosis and Skewed M2 Polarization. Front. Immunol. 2020;11:611133. doi: 10.3389/fimmu.2020.611133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vasina E.M., Cauwenberghs S., Feijge M.A., Heemskerk J.W., Weber C., Koenen R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011;2:e211. doi: 10.1038/cddis.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zmigrodzka M., Witkowska-Pilaszewicz O., Pingwara R., Winnicka A. Platelet Extracellular Vesicles Are Taken up by Canine T Lymphocytes but Do Not Play a Role in Their Proliferation, Differentiation and Cytokine Production In Vitro. Int. J. Mol. Sci. 2022;23:5504. doi: 10.3390/ijms23105504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sadallah S., Amicarella F., Eken C., Iezzi G., Schifferli J.A. Ectosomes released by platelets induce differentiation of CD4+T cells into T regulatory cells. Thromb. Haemost. 2014;112:1219–1229. doi: 10.1160/TH14-03-0281. [DOI] [PubMed] [Google Scholar]
- 111.Marcoux G., Laroche A., Hasse S., Tamagne M., Tessandier N., Becker Y.L.C., Zufferey A., Levesque T., Allaeys I., Karakeussian-Rimbaud A., et al. Platelet-Derived Extracellular Vesicles Actively Process Proteins and Present Antigen via MHC Class I [abstract] Res. Pract. Thromb. Haemost. 2020;4:3011–3023. [Google Scholar]
- 112.Kuravi S.J., Harrison P., Rainger G.E., Nash G.B. Ability of Platelet-Derived Extracellular Vesicles to Promote Neutrophil-Endothelial Cell Interactions. Inflammation. 2019;42:290–305. doi: 10.1007/s10753-018-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pao C.R.R., Righy C., Franco S., Souza T.M.L., Kurtz P., et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136:1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tessandier N., Melki I., Cloutier N., Allaeys I., Miszta A., Tan S., Milasan A., Michel S., Benmoussa A., Levesque T., et al. Platelets Disseminate Extracellular Vesicles in Lymph in Rheumatoid Arthritis. Arterioscler. Thromb. Vasc. Biol. 2020;40:929–942. doi: 10.1161/ATVBAHA.119.313698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boilard E., Nigrovic P.A., Larabee K., Watts G.F., Coblyn J.S., Weinblatt M.E., Massarotti E.M., Remold-O’Donnell E., Farndale R.W., Ware J., et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rautou P.E., Leroyer A.S., Ramkhelawon B., Devue C., Duflaut D., Vion A.C., Nalbone G., Castier Y., Leseche G., Lehoux S., et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 2011;108:335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 117.Bui K.L., Nyberg A., Maltais F., Saey D. Functional Tests in Chronic Obstructive Pulmonary Disease, Part 1: Clinical Relevance and Links to the International Classification of Functioning, Disability, and Health. Ann. Am. Thorac. Soc. 2017;14:778–784. doi: 10.1513/AnnalsATS.201609-733AS. [DOI] [PubMed] [Google Scholar]
- 118.Knijff-Dutmer E.A., Koerts J., Nieuwland R., Kalsbeek-Batenburg E.M., van de Laar M.A. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum. 2002;46:1498–1503. doi: 10.1002/art.10312. [DOI] [PubMed] [Google Scholar]
- 119.Burbano C., Villar-Vesga J., Orejuela J., Munoz C., Vanegas A., Vasquez G., Rojas M., Castano D. Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus. Front. Immunol. 2018;9:322. doi: 10.3389/fimmu.2018.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 121.Bello-Morales R., Ripa I., Lopez-Guerrero J.A. Extracellular Vesicles in Viral Spread and Antiviral Response. Viruses. 2020;12:623. doi: 10.3390/v12060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Angelo L.S., Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin. Cancer Res. 2007;13:2825–2830. doi: 10.1158/1078-0432.CCR-06-2416. [DOI] [PubMed] [Google Scholar]
- 123.Kim H.K., Song K.S., Chung J.H., Lee K.R., Lee S.N. Platelet microparticles induce angiogenesis in vitro. Br. J. Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 124.Ohtsuka M., Sasaki K., Ueno T., Seki R., Nakayoshi T., Koiwaya H., Toyama Y., Yokoyama S., Mitsutake Y., Chibana H., et al. Platelet-derived microparticles augment the adhesion and neovascularization capacities of circulating angiogenic cells obtained from atherosclerotic patients. Atherosclerosis. 2013;227:275–282. doi: 10.1016/j.atherosclerosis.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 125.Brill A., Dashevsky O., Rivo J., Gozal Y., Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005;67:30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 126.Guo S.C., Tao S.C., Yin W.J., Qi X., Yuan T., Zhang C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81–96. doi: 10.7150/thno.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tao S.C., Yuan T., Rui B.Y., Zhu Z.Z., Guo S.C., Zhang C.Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7:733–750. doi: 10.7150/thno.17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sun C., Feng S.B., Cao Z.W., Bei J.J., Chen Q., Zhao W.B., Xu X.J., Zhou Z., Yu Z.P., Hu H.Y. Up-Regulated Expression of Matrix Metalloproteinases in Endothelial Cells Mediates Platelet Microvesicle-Induced Angiogenesis. Cell Physiol. Biochem. 2017;41:2319–2332. doi: 10.1159/000475651. [DOI] [PubMed] [Google Scholar]
- 129.Mause S.F., Ritzel E., Liehn E.A., Hristov M., Bidzhekov K., Muller-Newen G., Soehnlein O., Weber C. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 130.Prokopi M., Pula G., Mayr U., Devue C., Gallagher J., Xiao Q., Boulanger C.M., Westwood N., Urbich C., Willeit J., et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 131.Bordin A., Chirivi M., Pagano F., Milan M., Iuliano M., Scaccia E., Fortunato O., Mangino G., Dhori X., De Marinis E., et al. Human platelet lysate-derived extracellular vesicles enhance angiogenesis through miR-126. Cell Prolif. 2022;55:e13312. doi: 10.1111/cpr.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khandagale A., Aberg M., Wikstrom G., Bergstrom Lind S., Shevchenko G., Bjorklund E., Siegbahn A., Christersson C. Role of Extracellular Vesicles in Pulmonary Arterial Hypertension: Modulation of Pulmonary Endothelial Function and Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2020;40:2293–2309. doi: 10.1161/ATVBAHA.120.314152. [DOI] [PubMed] [Google Scholar]
- 133.Janiszewski M., Do Carmo A.O., Pedro M.A., Silva E., Knobel E., Laurindo F.R. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: A novel vascular redox pathway. Crit. Care Med. 2004;32:818–825. doi: 10.1097/01.CCM.0000114829.17746.19. [DOI] [PubMed] [Google Scholar]
- 134.Gambim M.H., do Carmo Ade O., Marti L., Verissimo-Filho S., Lopes L.R., Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: Experimental evidence for a novel mechanism of septic vascular dysfunction. Crit. Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kong L., Li K., Gao L., Yin A., Zhou L., Teng G., Huang P. Mediating effects of platelet-derived extracellular vesicles on PM(2.5)-induced vascular endothelial injury. Ecotoxicol. Environ. Saf. 2020;198:110652. doi: 10.1016/j.ecoenv.2020.110652. [DOI] [PubMed] [Google Scholar]
- 136.Helley D., Banu E., Bouziane A., Banu A., Scotte F., Fischer A.M., Oudard S. Platelet microparticles: A potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur. Urol. 2009;56:479–484. doi: 10.1016/j.eururo.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 137.Haubold K., Rink M., Spath B., Friedrich M., Chun F.K., Marx G., Amirkhosravi A., Francis J.L., Bokemeyer C., Eifrig B., et al. Tissue factor procoagulant activity of plasma microparticles is increased in patients with early-stage prostate cancer. Thromb. Haemost. 2009;101:1147–1155. [PubMed] [Google Scholar]
- 138.Kim H.K., Song K.S., Park Y.S., Kang Y.H., Lee Y.J., Lee K.R., Kim H.K., Ryu K.W., Bae J.M., Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: Possible role of a metastasis predictor. Eur. J. Cancer. 2003;39:184–191. doi: 10.1016/S0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 139.Rank A., Liebhardt S., Zwirner J., Burges A., Nieuwland R., Toth B. Circulating microparticles in patients with benign and malignant ovarian tumors. Anticancer Res. 2012;32:2009–2014. [PubMed] [Google Scholar]
- 140.Hron G., Kollars M., Weber H., Sagaster V., Quehenberger P., Eichinger S., Kyrle P.A., Weltermann A. Tissue factor-positive microparticles: Cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb. Haemost. 2007;97:119–123. doi: 10.1160/TH06-03-0141. [DOI] [PubMed] [Google Scholar]
- 141.Mege D., Panicot-Dubois L., Ouaissi M., Robert S., Sielezneff I., Sastre B., Dignat-George F., Dubois C. The origin and concentration of circulating microparticles differ according to cancer type and evolution: A prospective single-center study. Int. J. Cancer. 2016;138:939–948. doi: 10.1002/ijc.29837. [DOI] [PubMed] [Google Scholar]
- 142.Contursi A., Fullone R., Szklanna-Koszalinska P., Marcone S., Lanuti P., Taus F., Meneguzzi A., Turri G., Dovizio M., Bruno A. Tumor-Educated Platelet Extracellular Vesicles: Proteomic Profiling and Crosstalk with Colorectal Cancer Cells. Cancers. 2023;15:350. doi: 10.3390/cancers15020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nomura S., Yasunaga K. Influence of platelet-derived microparticles on coagulation in a lung cancer patient receiving chemotherapy. Chest. 1993;103:979–980. doi: 10.1378/chest.103.3.979a. [DOI] [PubMed] [Google Scholar]
- 144.Ren J.G., Man Q.W., Zhang W., Li C., Xiong X.P., Zhu J.Y., Wang W.M., Sun Z.J., Jia J., Zhang W.F., et al. Elevated Level of Circulating Platelet-derived Microparticles in Oral Cancer. J. Dent. Res. 2016;95:87–93. doi: 10.1177/0022034515592593. [DOI] [PubMed] [Google Scholar]
- 145.Toth B., Liebhardt S., Steinig K., Ditsch N., Rank A., Bauerfeind I., Spannagl M., Friese K., Reininger A.J. Platelet-derived microparticles and coagulation activation in breast cancer patients. Thromb. Haemost. 2008;100:663–669. doi: 10.1160/TH07-10-0602. [DOI] [PubMed] [Google Scholar]
- 146.Trappenburg M.C., van Schilfgaarde M., Bredewold E.O., van Aalderen M.C., Spronk H.M., Ten Cate H., Leyte A., Terpstra W.E. Elevated numbers and altered subsets of procoagulant microparticles in breast cancer patients using endocrine therapy. Thromb. Res. 2011;127:363–369. doi: 10.1016/j.thromres.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 147.Yenigurbuz F.D., Kizmazoglu D., Ates H., Erdem M., Tufekci O., Yilmaz S., Oren H. Analysis of apoptotic, platelet-derived, endothelial-derived, and tissue factor-positive microparticles of children with acute lymphoblastic leukemia during induction therapy. Blood Coagul. Fibrinolysis. 2019;30:149–155. doi: 10.1097/MBC.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 148.Dashevsky O., Varon D., Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int. J. Cancer. 2009;124:1773–1777. doi: 10.1002/ijc.24016. [DOI] [PubMed] [Google Scholar]
- 149.Janowska-Wieczorek A., Wysoczynski M., Kijowski J., Marquez-Curtis L., Machalinski B., Ratajczak J., Ratajczak M.Z. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int. J. Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 150.Tang M., Jiang L., Lin Y., Wu X., Wang K., He Q., Wang X., Li W. Platelet microparticle-mediated transfer of miR-939 to epithelial ovarian cancer cells promotes epithelial to mesenchymal transition. Oncotarget. 2017;8:97464–97475. doi: 10.18632/oncotarget.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yao B., Qu S., Hu R., Gao W., Jin S., Ju J., Zhao Q. Delivery of platelet TPM3 mRNA into breast cancer cells via microvesicles enhances metastasis. FEBS Open Bio. 2019;9:2159–2169. doi: 10.1002/2211-5463.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zara M., Guidetti G.F., Boselli D., Villa C., Canobbio I., Seppi C., Visconte C., Canino J., Torti M. Release of Prometastatic Platelet-Derived Microparticles Induced by Breast Cancer Cells: A Novel Positive Feedback Mechanism for Metastasis. TH Open. 2017;1:e155–e163. doi: 10.1055/s-0037-1613674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Contursi A., Schiavone S., Dovizio M., Hinz C., Fullone R., Tacconelli S., Tyrrell V.J., Grande R., Lanuti P., Marchisio M. Platelets induce free and phospholipid-esterified 12-hydroxyeicosatetraenoic acid generation in colon cancer cells by delivering 12-lipoxygenase. J. Lipid Res. 2021;62:100109. doi: 10.1016/j.jlr.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Michael J.V., Wurtzel J.G.T., Mao G.F., Rao A.K., Kolpakov M.A., Sabri A., Hoffman N.E., Rajan S., Tomar D., Madesh M., et al. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood. 2017;130:567–580. doi: 10.1182/blood-2016-11-751099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Widyaningrum R., Wu Y.W., Delila L., Lee D.Y., Wang T.J., Burnouf T. In vitro evaluation of platelet extracellular vesicles (PEVs) for corneal endothelial regeneration. Platelets. 2022;33:1237–1250. doi: 10.1080/09537104.2022.2105829. [DOI] [PubMed] [Google Scholar]
- 156.Vismara M., Zara M., Negri S., Canino J., Canobbio I., Barbieri S.S., Moccia F., Torti M., Guidetti G.F. Platelet-derived extracellular vesicles regulate cell cycle progression and cell migration in breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:118886. doi: 10.1016/j.bbamcr.2020.118886. [DOI] [PubMed] [Google Scholar]
- 157.Cloutier N., Tan S., Boudreau L.H., Cramb C., Subbaiah R., Lahey L., Albert A., Shnayder R., Gobezie R., Nigrovic P.A., et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol. Med. 2013;5:235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Szabo-Taylor K., Ryan B., Osteikoetxea X., Szabo T.G., Sodar B., Holub M., Nemeth A., Paloczi K., Pallinger E., Winyard P., et al. Oxidative and other posttranslational modifications in extracellular vesicle biology. Semin. Cell Dev. Biol. 2015;40:8–16. doi: 10.1016/j.semcdb.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 159.Takahashi A., Okada R., Nagao K., Kawamata Y., Hanyu A., Yoshimoto S., Takasugi M., Watanabe S., Kanemaki M.T., Obuse C., et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017;8:15287. doi: 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baglio S.R., van Eijndhoven M.A., Koppers-Lalic D., Berenguer J., Lougheed S.M., Gibbs S., Leveille N., Rinkel R.N., Hopmans E.S., Swaminathan S., et al. Sensing of latent EBV infection through exosomal transfer of 5’pppRNA. Proc. Natl. Acad. Sci. USA. 2016;113:E587–E596. doi: 10.1073/pnas.1518130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.