Abstract
Through Pavlovian appetitive conditioning, environmental cues can become predictors of food availability. Over time, however, the food, and thus the value of the associated cues, can change based on environmental variations. This change in outcome necessitates updating of the value of the cue to appropriately alter behavioral responses to these cues. The basolateral amygdala (BLA) is critical in updating the outcomes of learned cues. However, it is unknown if the same BLA neuronal ensembles that are recruited in the initial associative memory are required when the new cue-outcome association is formed during reversal learning. The current study used the Daun02 inactivation method that enables selective targeting and disruption of activated neuronal ensembles in Fos-lacZ transgenic rats. Rats were implanted with bilateral cannulas that target the BLA and underwent appetitive discriminative conditioning in which rats had to discriminate between two auditory stimuli. One stimulus (CS+) co-terminated with food delivery, and the other stimulus was unrewarded (CS−; counterbalanced). Rats were then tested for CS+ or CS− memory retrieval and infused with either Daun02 or a vehicle solution into the BLA to inactivate either CS+ or CS− neuronal ensembles that were activated during that test. To assess if the same neuronal ensembles are necessary to update the value of the new association when the outcomes are changed, rats underwent reversal learning: the CS+ was no longer followed by food (reversal CS−, rCS−), and the CS− was now followed by food (reversal CS+; rCS+). The group that received Daun02 following CS+ session showed a decrease in conditioned responding and increased latency to the rCS− (previously CS+) during the first session of reversal learning, specifically during the first trial. This indicates that neuronal ensembles that are activated during the recall of the CS+ memory are the same neuronal ensembles needed for learning the new outcome of the same CS, now rCS−. Additionally, the group that received Daun02 following CS− session was slower to respond to the rCS+ (previously CS−) during reversal learning. This indicates that neuronal ensembles that are activated during the recall of the CS− memory are the same neuronal ensembles needed for learning the new outcome of the same CS. These results demonstrate that different neuronal ensembles within the BLA mediate memory recall of CS+ and CS− cues and reactivation of each cue-specific neuronal ensemble is necessary to update the value of that specific cue to respond appropriately during reversal learning. These results also indicate substantial plasticity within the BLA for behavioral flexibility as both groups eventually showed similar terminal levels of reversal learning.
Keywords: Discriminative Conditioning, Reversal Learning, Basolateral Amygdala, Reward, Daun02 Inactivation, Value updating
1. Introduction
Environmental cues can become strongly associated with food if they frequently occur together, and subsequent presentation of these learned cues can lead to food procurement and consumption without hunger (Birch et al., 1989; Holland and Petrovich, 2005; Petrovich, 2013; Petrovich and Gallagher, 2003; Saper et al., 2002; Weingarten, 1983). However, the outcomes, and thus the values, of associated cues are not always static and can change based on environmental variations. This change in the outcome of a learned cue requires updating the value of the cue to produce appropriate behavioral responses.
The basolateral nucleus of the amygdala (BLA) is a critical forebrain region necessary for associative conditioning and is an early processor of appetitive learning (Cole et al., 2013; Piette et al., 2012). The BLA is critically involved in appropriate behavioral responding when the values of learned appetitive cues are changed (Corbit and Balleine, 2005; Nomura et al., 2004; Schoenbaum et al., 1999; Tye et al., 2010; Fisher et al., 2020) or additional cues are incorporated to update the value of learned appetitive cues (Blundell et al., 2001; Everitt et al., 2003; Hatfield et al., 1996; Holland et al., 2002, 2001; Holland and Petrovich, 2005; Ishikawa et al., 2008; Petrovich, 2013; Setlow et al., 2002; Wassum and Izquierdo, 2015; Hoang and Sharpe, 2021). In vivo recording studies have shown that BLA neurons respond to appetitive cues, but then change their response profiles when cue outcomes are reversed (Schoenbaum et al., 1999) and when the reward is omitted (Tye et al., 2010). These studies suggest that the BLA neurons can alter their response to food predictive cues when the outcome changes. It is unknown, however, if the same or different BLA neuronal ensembles that are activated during the recall of learned cues are necessary for updating the values of these cues to form a new association when the outcomes change.
To this end, we used the Daun02 chemogenetic inactivation method (Cruz et al., 2013; Koya et al., 2009) to target BLA neuronal ensembles that are selectively activated by either the CS+ or CS− to determine if these specific neuronal ensembles are necessary to update the new value of the CSs during reversal learning. Specifically, Fos-lacZ transgenic rats underwent discriminative conditioning and were then infused with Daun02 or a vehicle solution into the BLA after presentation of either the CS+ or CS− to inactivate the responsive neuronal ensembles. Afterwards, rats underwent either one or fifteen sessions of reversal learning to observe how BLA neuronal ensemble inactivation affected conditioned responding to the initial memory recall of the CSs and during complete reversal learning, respectively. We hypothesized that separate BLA neuronal ensembles are activated during CS+ and CS− memory recall, and inactivating the neuronal ensembles that respond to a particular CS will only alter the memory of that CS and not the other CS. We also predicted that neuronal ensembles that are activated by a particular CS would be necessary to learn the new associations to the same CS when the outcome is changed during reversal learning.
2. Materials and Methods
Rats underwent surgery for implantation of bilateral cannulas aimed at the basolateral amygdala (BLA). After recovery, rats underwent ten sessions of discriminative conditioning followed by reversal learning as previously described (Cole et al., 2017; Keefer and Petrovich, 2020). Briefly, each session included 6 presentations of an auditory CS+ paired with the delivery of two food pellets (US) and 6 presentations of a separate, distinct auditory CS− presented alone. Following successful discrimination, rats underwent a brief induction session, which involved 6 presentations of either the CS+ or CS−, followed by infusion of Daun02 or vehicle ninety minutes after the beginning of the session. This induction session ensured selective activation of one CS neuronal ensemble without activating neurons recruited by the other CS or to the US. Next, rats underwent reversal learning where the outcomes of the CSs were reversed. Half of the rats were perfused 90min after the cessation of the first reversal session (R1) for histological verification of β-gal decrease in Daun02 infused rats (See Supplemental Materials and Methods). The other half received 15 reversal sessions to observe value updating after Daun02 inactivation. The primary measures of learning were the percentage of time rats expressed food cup behavior during the CSs and latency to approach the food cup during the CSs. Brain tissue was processed for double-label fluorescence immunohistochemistry for Fos and β-gal detection (see Supplemental Materials and Methods for details). Notably, we found more than 65% of β-Gal neurons were also Fos-positive, comparable to prior studies (Bossert et al., 2011, Fanous et al., 2012; Funk et al., 2016).
3. Results
3.1. Histology.
Location of injector tips were within or directly above the BLA as shown in Fig 1. Final group numbers based on proper cannula placements were CS+Daun02 (n=12 total; n=6 for Reversal Session 1 [R1]; n=6 for Reversal Session 15 [R15]), CS−Daun02 (n=11 total; n=5 for R1; n=6 for R15), and Vehicle (n=19 total; n=10 for R1; n=9 for R15). To verify Daun02 inactivation methodology, the number of β-Gal-labeled neurons was compared between drug treatment groups. Our a priori hypothesis was a decrease in the number of β-Gal-labeled neurons in rats that received the Daun02 compared to Vehicle, specifically in the groups that received only 1 session of reversal learning (Fig. 1B, C), and not 15 sessions. This was statistically confirmed (R1: t(19) = −2.459, p=0.02; R15: t(19) = −0.442, p>0.5). No difference was found between CS+ and CS− Daun02 treated groups (CS+Daun02: 59.5 ± 3.7; CS−Daun02: 51.0 ± 7.2; t(9) = 0.986, p>0.3). Additionally, no difference was found between the R15 groups, suggesting that potentially 1) additional ensembles were activated as new learning occurred to compensate for the initial neuronal inactivation or 2) the BLA became less involved in extended learning as seen in the decreased β-Gal-labeled neurons from R1 Vehicle group to R15 Vehicle group.
Figure 1.
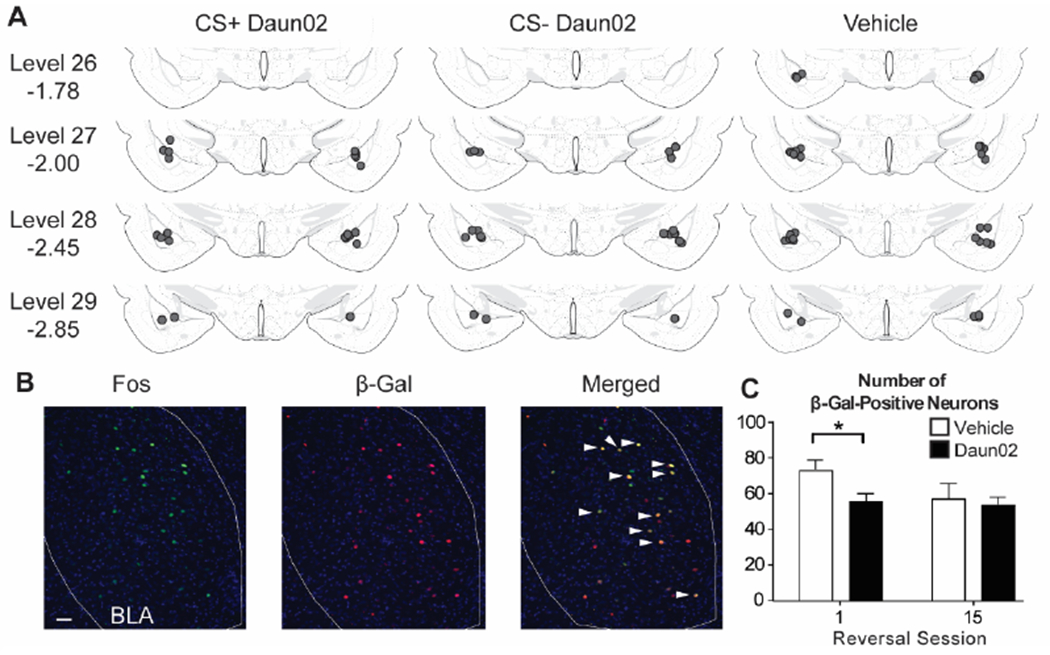
Cannula placements and Fos and β-Gal induction. (A) All cannula placements were within levels 26-29 of the BLA (−1.78 to −2.85mm from Bregma as indicated) and similar across drug groups. (B) Representative images showing Fos, β-Gal, and colocalization (white arrows). (C) There was a significant reduction in β-Gal-labeled neurons in the group that received Daun02 and were perfused after the first reversal session, but not the last reversal session. Scale bar = 25 μm. * p < 0.05.
3.2. Discriminative Conditioning and Induction Session.
All groups successfully discriminated between the CS+ and CS−, as shown by higher conditioned responding and faster latencies to the CS+ compared to the CS− during the tenth training session (Supplementary Figure 1 A–C). These results were expected since no drug was given during training, and group allocation was based on drug treatment after the induction session. A group (CS+Daun02, CS−Daun02, Vehicle) X CS (CS+, CS−) repeated measures ANOVAs showed an effect of CS Elevation on food cup behavior (F(1,39) = 331.00, p < 0.001) and an effect of CS on latency (F(1,39) = 110.85, p < 0.001), but no Group effects or interactions (F’s < 0.5). After discriminative conditioning, rats underwent an induction session with presentation of either the CS+ or CS− (not both) to induce Fos in the BLA in response to the respective CS. Conditioned responding was similar to the last conditioning session with higher responding in the CS+ induction groups compared to the CS− induction groups (Supplementary Figure 1 D–F). A Drug (Daun02, Vehicle) X CS Elevation (CS+, CS−) ANOVA during the induction session confirmed an effect of CS (F(1,38) = 61.50, p < 0.001), but no effect of drug or interaction (F’s < 2.3, p’s > 0.1). These results were expected since drug infusions occurred after the induction session and confirm similar responding between drug groups within their respective CS induction.
3.3. Reversal Learning.
3.3.1. Reversal Session 1.
The group that received Daun02 following CS+ presentations during the induction session (CS+Daun02), and thus CS+ neuronal ensemble inactivation, had lower conditioned responding to the same CS, now rCS−, during reversal session 1 (Fig. 2A). Analysis of conditioned responding with a Group X CS Elevation repeated measures ANOVA showed a significant effect of CS (F(1,39) = 273.57, p < 0.001), but no effect of Group (F = 1.80, p = 0.18) or interaction (F = 2.19, p = 0.13). We analyzed responding to each rCS separately, because of an a priori prediction that they would be differently affected based on our design. The analyses on each rCS showed a Group effect on average responding to the rCS− (F(2,39) = 4.08, p = 0.025), with lower responding in the CS+Daun02 group compared to the CS−Daun02 (p < 0.01) and Vehicle (p = 0.05) groups, and no difference between the CS−Daun02 and Vehicle groups (p > 0.1). No group differences were found in rCS+ responding (F < 0.4, p > 0.5). To evaluate the recall of the cue value following Daun02 neuronal ensemble inactivation, we analyzed each trial in the first reversal session with emphasis on the first trial. The CS+Daun02 group showed lower conditioned responding the first time the CS+, now rCS−, was presented (Fig. 2C). There was a main effect of Group (F(1,39) = 3.93, p = 0.028) with the CS+Daun02 group showing lower conditioned responding to the CS−Daun02 (p = 0.015) and Vehicle (p = 0.023) groups.
Figure 2.
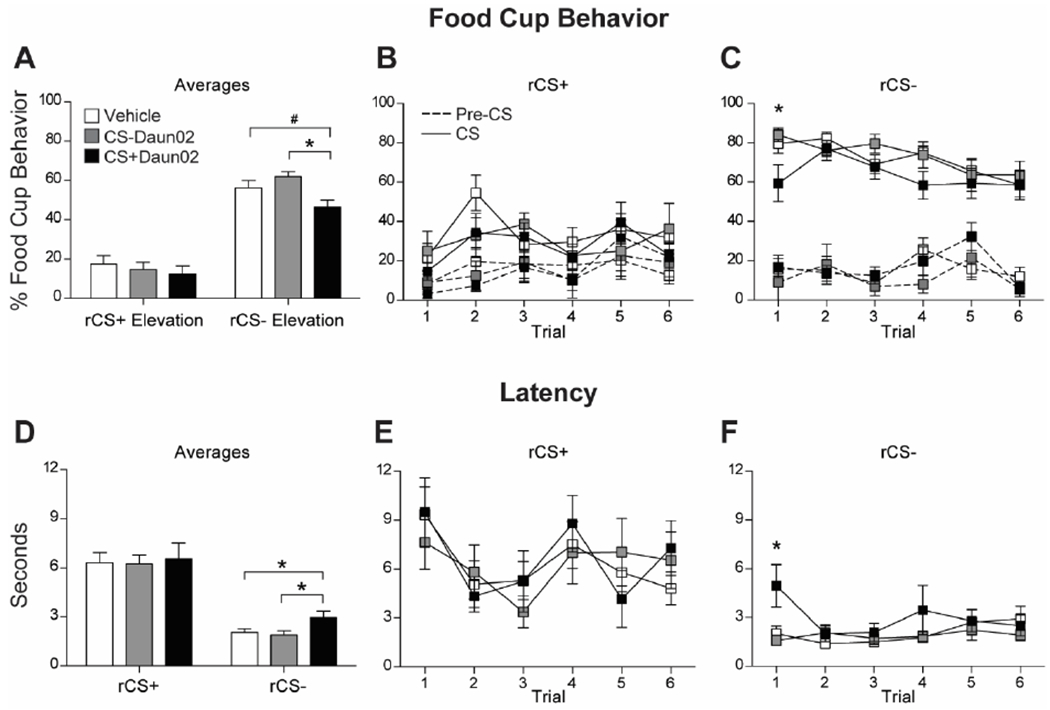
Conditioned responding during Reversal Session 1. (A) Average food cup responding (mean ± SEM) during rCS+ and rCS− during Reversal Session 1. Data shown as Elevation score (CS responding minus pre-CS [baseline] responding). (B,C) Food cup responding to each rCS+ trial (B) and rCS− trial (C) during the session. Solid line represents responding during the CS, and dashed line represents responding during the pre-CS (baseline) period. (D) Average latency to approach the food cup (mean ± SEM) during the rCS+ and rCS− during the session. (E,F) Latency responding to each rCS+ trial (E) and rCS− trial (F) during the session. # p = 0.05; * p < 0.05.
Additionally, we analyzed latency to approach the food cup after the onset of each cue and found rats approached the food cup faster during the rCS− compared to the rCS+, as expected since the rCS− was previously the CS+. A Group X CS ANOVA showed an effect of CS on overall average latency to respond to the food cup (F(1,39) = 125.36, p < 0.001; Fig. 2D) indicating rats approached the food cup faster during the rCS− compared to the rCS+, but there was no Group effect or interaction (F’s < 1, p’s < 0.5). We analyzed responding to each rCS separately, because of an a priori prediction that they would be differently affected based on our design. The analyses on each rCS showed a Group effect on latency to the rCS− (F(2,39) = 3.61, p = 0.037), with slower latencies in the CS+Daun02 group compared to the CS−Daun02 (p = 0.021) and Vehicle (p = 0.026) groups, and no difference between the CS−Daun02 and Vehicle groups (p > 0.1). No group differences were found in latency to the rCS+ (F < 0.1, p < 0.5).
Again, we analyzed the first trial during the first reversal session for latency to the rCS− and found a main effect of Group (F(2,39) = 5.27, p < 0.01) with the CS+Daun02 group showing slower latencies to the rCS− compared to the CS−Daun02 and Vehicle groups (p’s < 0.01; Fig. 2F). We found no differences for other rCS− trials or rCS+ trials (ps>0.1).
3.3.2. Reversal Learning Across Sessions.
We analyzed responding in a group of rats that underwent 15 sessions of reversal learning to determine if inactivation of specific CS BLA neuronal ensembles interfered with updating the new values of the cues during reversal learning. The group that received Daun02 following CS+ induction showed a decrease in conditioned responding to the same CS, now the rCS−, throughout reversal learning (Fig. 3B; Supplementary Figure 1 G–H). Analysis of responding to CSs with a Group X CS Elevation X Session repeated measures ANOVA showed CS Elevation X Group interaction (F(2,18) = 7.33, p < 0.01), and expected main effects of Session (F(3,54) = 5.23, p < 0.01) and CS (F(1,18) = 40.82, p < 0.001) and a Session X CS Elevation interaction (F(3,54) = 81.38, p < 0.001), but no other effects or interactions (F’s < 2, p > 0.1). Follow-up analyses confirmed a significant effect of Group on conditioned responding to the rCS− (F(2,18) = 5.08, p = 0.018) with the CS+Daun02 group showing lower conditioned responding to the rCS−, which was previously their CS+, across reversal learning compared to the CS−Daun02 (p = 0.010) and Vehicle (p = 0.014) groups. The decrease was specifically during reversal session 1 as described above and session 15 (F(2,18) = 4.71, p = 0.023), where the CS+Daun02 group had significantly lower responding compared to the Vehicle and CS−Daun02 groups (p’s < 0.015).
Figure 3.
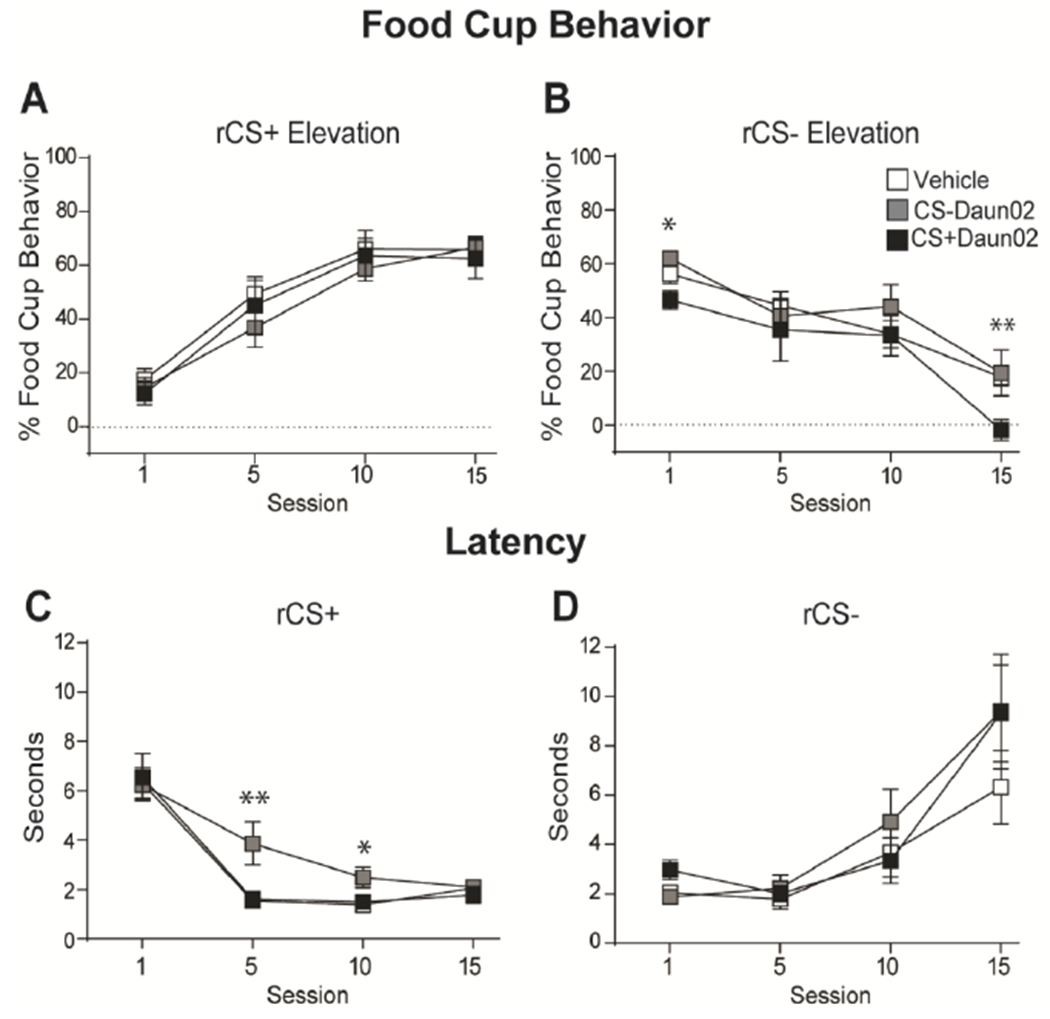
Conditioned responding throughout reversal learning. (A,B) Average food cup responding (mean ± SEM) during rCS+ (A) and rCS− (B) throughout reversal learning. Data shown as Elevation score (CS responding minus pre-CS [baseline] responding). (C,D) Average latency to approach the food cup (mean ± SEM) during the rCS+ (C) and rCS− (D) throughout reversal learning. * p < 0.05; ** p < 0.015.
The analyses on latency responding showed the group that received Daun02 following CS− induction was slower to respond to the food cup after the same cue presentation, now rCS+, during reversal learning (Fig 3C). A Group X CS X Session repeated measures ANOVA found expected main effects of Session (F(3,54) = 14.51, p < 0.001) and CS (F(1,18) = 6.85, p < 0.02), and a Session X CS interaction (F(3,54) = 40.79, p < 0.001), but no Group effects or interaction (F’s < 1.5, p’s > 0.1). Simple effect analyses showed an effect of Group on responding to the rCS+ during session 5 (F(2,18) = 7.39, p < 0.01) and session 10 (F(2,18) = 4.21, p < 0.05). The CS−Daun02 group had significantly longer latencies to the rCS+ during session 5 compared to the CS+Daun02 and Vehicle groups (p’s < 0.01) and session 10 compared to the CS+Daun02 group (p < 0.01) and Vehicle group (p = 0.037). We found no differences in latency to respond to the rCS− (p’s > 0.1).
4. Discussion
The current study examined if potentially separate BLA neuronal ensembles that are activated during CS+ and CS− memory recall are necessary for value updating when the CS contingencies are reversed. This was accomplished by testing if inactivating the neuronal ensemble that responds to a particular learned CS altered the memory of that specific CS and not the other CS. Additionally, we examined if CS-specific neuronal ensembles are necessary to learn the new associations to the same CS when the outcome is changed during reversal learning. Using the Daun02 method, we specifically inactivated BLA neuronal ensembles that were activated by either a CS that was previously associated with food (CS+) or a CS not paired with food (CS−), and then evaluated conditioned responding when the outcomes of the cues were switched during reversal learning. We found that the group that received the Daun02 following the CS+ session (CS+Daun02 group) showed a decrease in conditioned responding to the same CS, now the rCS−, during the first reversal session and specifically during the first presentation of that CS prior to any new information about the outcome. This decreased responding indicates that the original CS+ neuronal ensemble was necessary during recall of previously learned outcome of that cue and to incorporate this information during initial reversal learning. Second, we found that the group that received Daun02 following CS− induction (CS−Daun02 group) was slower to approach the food cup (i.e. longer latency) after the same cue presentation, now the rCS+, during reversal sessions 5 and 10. This slower latency suggests a decrease in motivation to attend to the cue and approach the food reward. Together, these results support our hypotheses that separate BLA neuronal ensembles mediate CS+ and CS− memory recall, and reactivation of each cue-specific neuronal ensemble is necessary for updating the value of that specific learned cue, in order to respond appropriately during reversal learning. Importantly, these results also indicate plasticity of BLA neuronal ensembles when cues’ values are altered since all rats eventually showed the same levels of conditioned responding by the end of reversal learning.
The observed impairments in conditioned responding were specific to the CS to which the neuronal ensemble was inactivated and did not cause general impairments in behavioral responding. The CS+Daun02 group was impaired on responding to rCS−, previously the CS+, and CS−Daun02 group was impaired on responding to rCS+, previously the CS−. This suggests that our preparation inactivated separate CS+ and CS− neuronal ensembles, which impaired subsequent, CS-specific reversal learning. This is in agreement with prior work that found specific effects of neuronal ensembles inactivation by Daun02 (Pfarr et al., 2015). Additionally, other studies have shown altered reward-seeking behaviors due to specific neuronal ensemble inactivation with this method (Caprioli et al., 2017; Cole et al., 2020; Cruz et al., 2014; de Guglielmo et al., 2016; Fanous et al., 2012; Funk et al., 2016; George and Hope, 2017; Koya et al., 2009; Pfarr et al., 2015; Warren et al., 2016; Whitaker et al., 2017; Xue et al., 2017; Josselyn and Frankland, 2018; Josselyn and Tonegawa, 2020).
The results of the current study are in agreement with previous studies that showed separate BLA neuronal ensembles respond to distinct learned cues. Previous studies have shown that ~60% of BLA neurons respond to a distinct learned cue during appetitive learning, and then half of these neurons alter their responding when the outcomes are switched during reversal learning in rats (Schoenbaum et al., 1999; Zhang and Li, 2018) and primates (Paton et al., 2006). Interestingly, in both studies the neurons responded selectively to specific cues prior to correct behavioral performance, indicating BLA neurons are tracking the outcome and the value of learned cues to guide behavior. Similarly, another study showed a subset of BLA neurons (~10%) respond to a well-learned reward predictive cue, but then distinctly alter their responses when food is no longer delivered during extinction (“reinforcement-omission” neurons; (Tye et al., 2010)), suggesting this subset of neurons may be tracking the outcome of the learned cues. This indicates that BLA neurons can alter their responses based on environmental changes, signifying neural plasticity.
The current results indicate that the BLA regulates the updating of the value of learned appetitive cues based on the current outcome. This was confirmed for both groups that received cue-specific neuronal ensemble inactivation by Daun02: the CS+Daun02 group had lower conditioned responding to the same CS during reversal learning (rCS−), and the CS−Daun02 group was slower to approach the food cup after presentation of the same CS during reversal learning (rCS+). Indeed, previous studies have shown an intact BLA is needed to access the value of the learned cue in order to appropriately update it when the outcome is changed and alter behavioral responding (as reviewed in (Wassum and Izquierdo, 2015)). The BLA encodes the value of the cues during learning (Cole et al., 2013; Esber and Holland, 2014; Parkes and Balleine, 2013; Piette et al., 2012; Schoenbaum et al., 1999; Tye and Janak, 2007; Uwano et al., 1995) and is involved in appetitive cue discrimination (Ambroggi et al., 2008; Ishikawa et al., 2008) and reversal learning (Churchwell et al., 2009). However, several studies have shown the BLA may not be critical for initial acquisition of cue value learning (Balleine et al., 2003; Corbit and Balleine, 2005; Hatfield et al., 1996; Holland et al., 2002; Parkinson et al., 2000), but it is critical to encode and assess the representation of the learned associations to alter subsequent behavioral motivation and learning (Blundell et al., 2001; Corbit and Balleine, 2005; Coutureau et al., 2009; Everitt et al., 2003; Galarce et al., 2010; Hatfield et al., 1996; Holland et al., 2002; Holland and Petrovich, 2005; Johnson et al., 2009; Ostlund and Balleine, 2008; Petrovich, 2013; Setlow et al., 2002; Tye and Janak, 2007; Wassum and Izquierdo, 2015; Hoang and Sharpe, 2021; Fisher et al., 2020). This suggests a specific role for the BLA in reward value representation when appetitive learning is altered, in agreement with the current findings.
5. Conclusions
The current study investigated the plasticity across a learning paradigm that requires value updating. We found that inactivation of the BLA neuronal ensemble responsive during a specific learned cue recall resulted in impaired conditioned responding to the same cue during reversal learning without interfering with responding to the other learned cue. These results show distinct neuronal ensembles within the BLA are activated during specific cue memory recall and are necessary to update the value of that cue during reversal learning.
Supplementary Material
Highlights.
Chemogenetic inactivation of BLA neuronal ensembles activated by learned CS+ orCS−
Examined if specific ensembles needed when cues’ values change in reversal learning
CS+ ensemble inactivation reduced responding to the same cue in early reversal learning
CS− ensemble inactivation slowed learning of the new value of the cue
Acknowledgements
We thank Bret Judson and the Boston College Imaging Core for infrastructure and support, and Dr. Bruce Hope and Dr. Francois Vautier for advice and protocols regarding breeding c-fos-lacZ transgenic rats. We also thank the Boston College undergraduate researchers who helped with numerous aspects of this project.
Funding sources
This work was supported by the National Institutes of Health R01DK085721 to G.D.P. and Boston College Dissertation Fellowship to S.E.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM, 2008. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron 59, 648–661. 10.1016/j.neuron.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Killcross AS, Dickinson A, 2003. The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci. Off. J. Soc. Neurosci 23, 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, Johnson S, 1989. Conditioned meal initiation in young children. Appetite 13, 105–113. 10.1016/0195-6663(89)90108-6 [DOI] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S, 2001. Lesions of the basolateral amygdala disrupt selective aspects of reinforcer representation in rats. J. Neurosci. Off. J. Soc. Neurosci 21,9018–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FRM, Cifani C, Koya E, Hope BT, Shaham Y (2011). Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse in heroin. Nat Neurosci, 14(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y, 2017. Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence. J. Neurosci. Off. J. Soc. Neurosci 37, 1014–1027. 10.1523/JNEUROSCI.3091-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP, 2009. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav. Neurosci 123, 1185–1196. 10.1037/a0017734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Keefer SE, Anderson LC, Petrovich GD, 2020. Medial Prefrontal Cortex Neural Plasticity, Orexin Receptor 1 Signaling, and Connectivity with the Lateral Hypothalamus Are Necessary in Cue-Potentiated Feeding. J. Neurosci. Off. J. Soc. Neurosci 40, 1744–1755. 10.1523/JNEUROSCI.1803-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Powell DJ, Petrovich GD, 2013. Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learn. Mem. Cold Spring Harb. N 20, 295–299. 10.1101/lm.031070.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Stone AD, Petrovich GD, 2017. The dorsomedial striatum mediates Pavlovian appetitive conditioning and food consumption. Behav. Neurosci 131,447–453. 10.1037/bne0000216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW, 2005. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J. Neurosci. Off. J. Soc. Neurosci 25, 962–970. 10.1523/JNEUROSCI.4507-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Marchand AR, Di Scala G, 2009. Goal-directed responding is sensitive to lesions to the prelimbic cortex or basolateral nucleus of the amygdala but not to their disconnection. Behav. Neurosci 123, 443–448. 10.1037/a0014818 [DOI] [PubMed] [Google Scholar]
- Cruz FC, Babin KR, Leao RM, Goldart EM, Bossert JM, Shaham Y, Hope BT, 2014. Role of nucleus accumbens shell neuronal ensembles in context-induced reinstatement of cocaine-seeking. J. Neurosci. Off. J. Soc. Neurosci 34, 7437–7446. 10.1523/JNEUROSCI.0238-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT, 2013. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat. Rev. Neurosci 14, 743–754. 10.1038/nrn3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF, George O, 2016. Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence. J. Neurosci. Off. J. Soc. Neurosci 36, 9446– 9453. 10.1523/JNEUROSCI.1395-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeln M, Bastide MF, Toulmé E, Dehay B, Bourdenx M, Doudnikoff E, Li Q, Gross CE, Boué-Grabot E, Pisani A, Bezard E, Fernagut PO. Selective Inactivation of Striatal FosB/ΔFosB-Expressing Neurons Alleviates L-DOPA-Induced Dyskinesia. Biol Psychiatry. 2016. Mar 1;79(5):354–361. doi: 10.1016/j.biopsych.2014.07.007. Epub 2014 Jul 15. [DOI] [PubMed] [Google Scholar]
- Esber GR, Holland PC, 2014. The basolateral amygdala is necessary for negative prediction errors to enhance cue salience, but not to produce conditioned inhibition. Eur. J. Neurosci 40, 3328–3337. 10.1111/ejn.12695 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW, 2003. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann. N. Y. Acad. Sci 985, 233–250. [PubMed] [Google Scholar]
- Fanous S, Goldart EM, Theberge FRM, Bossert JM, Shaham Y, Hope BT, 2012. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J. Neurosci. Off. J. Soc. Neurosci 32, 11600–11609. 10.1523/JNEUROSCI.1914-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H, Pajser A, & Pickens CL 2020. Pre-training inactivation of basolateral amygdala and mediodorsal thalamus, but not orbitofrontal cortex or prelimbic cortex, impairs devaluation in a multiple-response/multiple-reinforcer cued operant task. Behavioural brain research, 378, 112159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD, 2016. Role of Central Amygdala Neuronal Ensembles in Incubation of Nicotine Craving. J. Neurosci. Off. J. Soc. Neurosci 36, 8612–8623. 10.1523/JNEUROSCI.1505-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarce EM, McDannald MA, Holland PC, 2010. The basolateral amygdala mediates the effects of cues associated with meal interruption on feeding behavior. Brain Res. 1350, 112–122. 10.1016/j.brainres.2010.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Hope BT, 2017. Cortical and amygdalar neuronal ensembles in alcohol seeking, drinking and withdrawal. Neuropharmacology 122, 107–114. 10.1016/j.neuropharm.2017.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P, 1996. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J. Neurosci. Off. J. Soc. Neurosci 16, 5256–5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang IB & Sharpe MJ 2021. The basolateral amygdala and lateral hypothalamus bias learning towards motivationally significant events. Curr Opin Behav Sci, 41: 92–97. [Google Scholar]
- Holland PC, Hatfield T, Gallagher M, 2001. Rats with basolateral amygdala lesions show normal increases in conditioned stimulus processing but reduced conditioned potentiation of eating. Behav. Neurosci 115, 945–950. [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, 2005. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol. Behav 86, 747–761. 10.1016/j.physbeh.2005.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M, 2002. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol. Behav 76, 117–129. 10.1016/s0031-9384(02)00688-1 [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Ambroggi F, Nicola SM, Fields HL, 2008. Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience 155, 573–584. 10.1016/j.neuroscience.2008.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Gallagher M, Holland PC, 2009. The Basolateral Amygdala Is Critical to the Expression of Pavlovian and Instrumental Outcome-Specific Reinforcer Devaluation Effects. J. Neurosci 29, 696–704. 10.1523/JNEUROSCI.3758-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Frankland PW. Memory Allocation: Mechanisms and Function. Annu Rev Neurosci. 2018. Jul 8;41:389–413. doi: 10.1146/annurev-neuro-080317-061956. Epub 2018 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Tonegawa S. Memory engrams: Recalling the past and imagining the future. Science. 2020. Jan 3;367(6473):eaaw4325. doi: 10.1126/science.aaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer SE, Petrovich GD, 2020. The basolateral amygdala-medial prefrontal cortex circuitry regulates behavioral flexibility during appetitive reversal learning. Behav. Neurosci 134, 34–44. 10.1037/bne0000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez-Barber DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh SC, Mattson BJ, Hope BT, 2009. Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat. Neurosci 12, 1069–1073. 10.1038/nn.2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Izaki Y, Takita M, Tanaka J, Hori K, 2004. Extracellular level of basolateral amygdalar dopamine responding to reversal of appetitive-conditioned discrimination in young and old rats. Brain Res. 1018, 241–246. 10.1016/j.brainres.2004.05.077 [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW, 2008. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J. Neurosci. Off. J. Soc. Neurosci 28, 4398–4405. 10.1523/JNEUROSCI.5472-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes SL, Balleine BW, 2013. Incentive memory: evidence the basolateral amygdala encodes and the insular cortex retrieves outcome values to guide choice between goal-directed actions. J. Neurosci. Off. J. Soc. Neurosci 33, 8753–8763. 10.1523/JNEUROSCI.5071-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ, 2000. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur. J. Neurosci 12, 405–413. 10.1046/j.1460-9568.2000.00960.x [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD, 2006. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870. 10.1038/nature04490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, 2013. Forebrain networks and the control of feeding by environmental learned cues. Physiol. Behav 121, 10–18. 10.1016/j.physbeh.2013.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Gallagher M, 2003. Amygdala subsystems and control of feeding behavior by learned cues. Ann. N. Y. Acad. Sci 985, 251–262. 10.1111/j.1749-6632.2003.tb07086.x [DOI] [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schönig K, Bartsch D, Hope BT, Spanagel R, Sommer WH, 2015. Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J. Neurosci. Off. J. Soc. Neurosci 35, 10750–10761. 10.1523/JNEUROSCI.0684-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette CE, Baez-Santiago MA, Reid EE, Katz DB, Moran A, 2012. Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. J. Neurosci. Off. J. Soc. Neurosci 32, 9981–9991. 10.1523/JNEUROSCI.0669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santone KS, Oakes SG, Taylor SR, & Powis G (1986). Anthracycline-induced inhibition of a calcium action potential in differentiated murine neuroblastoma cells. Cancer research, 46(6), 2659–2664. [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK, 2002. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211. 10.1016/s0896-6273(02)00969-8 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M, 1999. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. Off. J. Soc. Neurosci 19, 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland PC, 2002. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning: Amygdala and motivational value. Eur. J. Neurosci 15, 1841–1853. 10.1046/j.1460-9568.2002.02010.x [DOI] [PubMed] [Google Scholar]
- Tye KM, Cone JJ, Schairer WW, Janak PH, 2010. Amygdala neural encoding of the absence of reward during extinction. J. Neurosci. Off. J. Soc. Neurosci 30, 116–125. 10.1523/JNEUROSCI.4240-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Janak PH, 2007. Amygdala neurons differentially encode motivation and reinforcement. J. Neurosci. Off. J. Soc. Neurosci 27, 3937–3945. 10.1523/JNEUROSCI.5281-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwano T, Nishijo H, Ono T, Tamura R, 1995. Neuronal responsiveness to various sensory stimuli, and associative learning in the rat amygdala. Neuroscience 68, 339–361. 10.1016/0306-4522(95)00125-3 [DOI] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, Whitaker LR, McPherson KB, Bossert JM, Shaham Y, Hope BT, 2016. Distinct Fos-Expressing Neuronal Ensembles in the Ventromedial Prefrontal Cortex Mediate Food Reward and Extinction Memories. J. Neurosci. Off. J. Soc. Neurosci 36, 6691–6703. 10.1523/JNEUROSCI.0140-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Izquierdo A, 2015. The basolateral amygdala in reward learning and addiction. Neurosci. Biobehav. Rev 57, 271–283. 10.1016/j.neubiorev.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP, 1983. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science 220, 431–433. 10.1126/science.6836286 [DOI] [PubMed] [Google Scholar]
- Whitaker LR, Warren BL, Venniro M, Harte TC, McPherson KB, Beidel J, Bossert JM, Shaham Y, Bonci A, Hope BT, 2017. Bidirectional Modulation of Intrinsic Excitability in Rat Prelimbic Cortex Neuronal Ensembles and Non-Ensembles after Operant Learning. J. Neurosci. Off. J. Soc. Neurosci 37, 8845–8856. 10.1523/JNEUROSCI.3761-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y-X, Chen Y-Y, Zhang L-B, Zhang L-Q, Huang G-D, Sun S-C, Deng J-H, Luo Y-X, Bao Y-P, Wu P, Han Y, Hope BT, Shaham Y, Shi J, Lu L, 2017. Selective Inhibition of Amygdala Neuronal Ensembles Encoding Nicotine-Associated Memories Inhibits Nicotine Preference and Relapse. Biol. Psychiatry 82, 781–793. 10.1016/j.biopsych.2017.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li B, 2018. Population coding of valence in the basolateral amygdala. Nat. Commun 9, 5195. 10.1038/s41467-018-07679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


