Abstract
Background & aims
Apolipoproteins and lipoprotein(a) are associated with various cardiometabolic diseases, including insulin resistance, diabetes mellitus, hypertension, dyslipidemia, among others. This systematic review and meta-analysis was conducted to evaluate the association of these markers with metabolic syndrome (MetS).
Methods
We ran a systematic search through PubMed, Scopus, Embase, Ovid/Medline, and Web of Science on March 15, 2023. No language or date restrictions were applied. The only synthesised effect measure reported was the odds ratio (OR) with its corresponding 95% confidence interval (95% CI). We utilised the random-effects model for the quantitative synthesis.
Results
We analysed 50 studies (n = 150 519) with different definitions for MetS. Increased ApoB values were associated with MetS (OR = 2.8; 95% CI: 2.44–3.22; p < 0.01, I2 = 99%). Decreased ApoA1 values were associated with MetS (OR = 0.42; 95% CI: 0.38–0.47; p < 0.01, I2 = 99%). Increased values of the ApoB/ApoA1 ratio were associated with MetS (OR = 4.97; 95% CI: 3.83–6.44; p < 0.01, I2 = 97%). Decreased values of Lp(a) were associated with MetS (OR = 0.89; 95% CI: 0.82–0.96; p < 0.01; I2 = 92%).
Conclusions
Increased values of ApoB and ApoB/ApoA1 ratio are associated with MetS, while decreased values of ApoA1 and Lp(a) are associated with MetS. These findings suggest that these lipid markers may serve as potential indicators for identifying subjects at risk of developing MetS. However, further research is required to elucidate the underlying mechanisms of these associations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-023-01860-w.
Keyword: Metabolic syndrome, Apolipoproteins, Lipoprotein(a)
Introduction
Metabolic syndrome (MetS) is associated with a fivefold increase in the risk of diabetes mellitus, a twofold higher risk of cardiovascular events, and a 1.5-fold higher risk of all-cause mortality [1, 2]. Consequently, the increase in its prevalence is a public health concern. Indeed, although estimates of the prevalence of MetS vary according to the criteria used for its definition, certain studies indicate an increase in its prevalence in some countries. In the United States, the prevalence of MetS increased from 28.23% to 37.09% between 1999 and 2018 [3], and in Mexico increased from 40.2% to 56.31% between 2006 and 2018, respectively [4].
Apolipoproteins are constituents of high-density lipoproteins (HDL) and triglyceride-rich lipoproteins [5]. Due to their potential effects and prominence in different pathologies, apolipoproteins have been extensively investigated as predictors of clinical outcomes [6, 7]. For example, in the case of Apolipoprotein A1 (ApoA1), some studies found that alteration in its levels was associated with cardiovascular outcomes and it has also been evaluated as a diagnostic and prognostic marker for some cancers [8, 9]. In addition to these, other studies have associated apolipoprotein values with the development of some metabolic diseases [10].
Circulating apolipoprotein levels reflect the number of lipoprotein particles, rather than the concentration of cholesterol [11]. In this regard, the level of Apolipoprotein B (Apo B) reflects the number of triglyceride-rich Very Low Density Lipoprotein (VLDL) particles and the number of Low Density Lipoprotein (LDL) particles [11]. Therefore, it places more emphasis on the number of small and dense LDL particles than the usual measurement of LDL cholesterol [11]. Similarly, the level of ApoA1 corresponds to the quantity of HDL particles; therefore, apolipoproteins taken individually or the ratio of ApoB and ApoA1 (ApoB/A1 ratio) would theoretically serve as optimal markers of lipid abnormalities associated with insulin resistance and MetS [11]. Several studies have found an independent association between ApoA1, ApoB and ApoB/A1 ratio values with this syndrome [12–15]. However, to the best of our knowledge, there has been no systematic review of the available evidence regarding these associations. Therefore, the objective of this study was to conduct a systematic review and meta-analysis to synthesise the evidence on the association between ApoA1, ApoB, ApoB/A1 ratio and lipoprotein(a) [Lp(a)] values with MetS.
Methods
Registration and reporting
In the development of this systematic review, we adhered to guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [16]. A version of this systematic review’s protocol has been uploaded to the International Prospective Register of Systematic Reviews (PROSPERO) [CRD42023416427].
Search strategy and databases
The search strategy was designed using the Peer Review of Electronic Search Strategies Checklist [17]. There were no limitations regarding language or date. We performed a systematic search of various databases including PubMed, Scopus, Embase, Ovid/Medline, and Web of Science on March 15, 2023. We also reviewed the reference list of the selected studies and manually searched preprint databases. Additionally, the reference lists of the included studies were thoroughly examined and we conducted a manual search of preprint databases. For details of the complete search strategy, please refer to Supplementary Material (Table S1).
Study selection and data extraction
We selected studies with the following characteristics: cohort/case–control/cross-sectional studies that evaluated the association between ApoB, ApoA1, ApoB/ApoA1 ratio and Lp (a), and MetS in adult patients (> 18 years). We excluded: duplicated studies, scoping reviews, systematic reviews, narrative reviews and conference abstracts.
The studies retrieved from the systematic search were uploaded to the data management software Rayyan QCRI. After removing duplicated studies, four authors (J.R.U-B, M.A.H-C, X.L.C-T and R.A.S-A) independently assessed the title/abstract of each study according to the selection criteria. Once the relevant literature was identified, two reviewers (M.A.H-C and X.L.C-T) independently assessed the full text of each article. The studies that did not comply with the entire selection criteria were excluded from the review. In case of missing information, we contacted the authors. Any discrepancies were resolved through discussion and consensus between the two reviewers.
For extracting data, we employed a standardised data collection sheet created in Google Sheets©. The data were gathered independently by two authors (E.A.H-B and E.A.A-B) from each study and included the following details: title, study location, first author, publication date, study design sample size, age, sex, definition of MetS, ApoB levels(mg/dL), ApoA1 levels(mg/dL), ApoB/ApoA1 ratio levels, Lp(a) levels(mg/dL) and assay technique. For articles published in a language other than English, we proceeded to translate them using online translation tools.
Risk of bias and publication bias
Two authors (J.R.U-B and E.A.A-B) independently performed the risk of bias assessment. The Newcastle–Ottawa Scale (NOS) was used for cohort and case-controls studies, whereas an adjusted version of the NOS for cross-sectional studies (NOS-CS) was applied to this type of study. A score of ≥ 7 stars was considered indicative of a low risk of bias, while a score of < 7 stars was considered indicative of a high risk of bias. To assess publication bias, we employed funnel plots and the Begg test.
Data synthesis
The statistical analysis was performed in STATA 17.0 © and Review Manager v.5.4 (The Cochrane Collaboration, Copenhagen, Denmark). For all meta-analyses, we employed a random-effects model (Restricted Maximum Likelihood). All the effect measures were reported as the odds ratios (OR) with their corresponding 95% confidence intervals (CI). Any other effect measure was transformed into OR. Using Hozo's method, we converted median values and their interquartile ranges were converted into means and their corresponding standard deviations (SD) [18]. We also transformed standard mean differences into the natural logarithm of the OR (lnOR) and its standard error using Chinn's method [19]. We used the Cochran's Q test and the I2 statistic to assess between-study heterogeneity, in which I2 ≥ 60% for the I2 test and a p-value < 0.05 for the Cochran's Q test indicated high heterogeneity. Subgroup analyses were conducted according to continent, sex, assay method, and MetS diagnostic criteria. In the sensitivity analysis, we excluded studies with a high risk of bias.
Results
Eligible studies
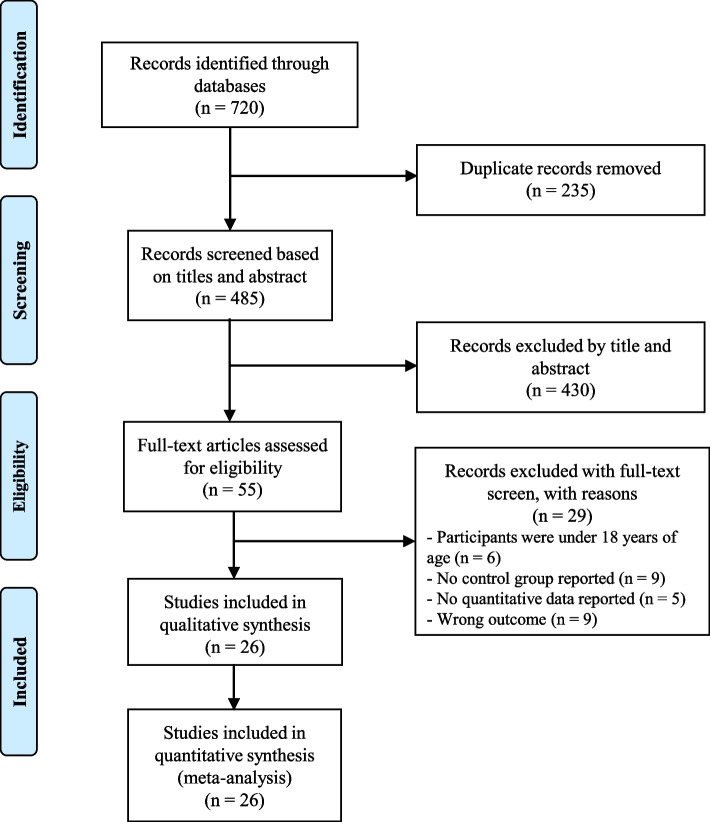
In the search strategy, a total of 2262 studies were identified. After removing duplicates and reviewing titles and abstracts, 153 studies met the selection criteria and advanced to the full-text reading phase. Upon reading the full text of each article, 50 studies were deemed eligible for inclusion in the qualitative synthesis and meta-analysis [11, 13, 20–67]. Figure 1 represents the flow chart of the selection process.
Fig. 1.
PRISMA flow diagram of study selection process
Study characteristics and risk of bias assessment
A total of 150 519 participants were included, of which 62 083 were female and 77 958 were male. Six studies (n = 10,486) did not report the sex of the included participants. Ultimately, fifty studies were included, of which 40 had a cross-sectional design, 7 were case–control studies, and 3 were cohort studies. Six studies [21, 33, 49, 50, 52, 66] analysed the association between two different participant groups, resulting in a total of 56 included studies. Characteristics from all included studies are summarised in Table 1.
Table 1.
Characteristics of the included studies
| Author | Year | Country | Median/mean/Range age (IQR/SD) | Participants (male/female) | Comorbidities (n) | MetS definition criteria | Marker analyzed | Marker mean (SD) in patients with MetS | Marker mean (SD) in patients without MetS | Assay Method | Odds Ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obesity | CHD | HT | DM | |||||||||||
| Bonora E et al | 2003 | Italy | MetS: 60.7(11.6) Control: 58.5(11.4) | 888(446/442) | NR | NR | 551 | 201 | NCEP-ATP III | ApoA1 | 150 (30) | 170(30) | IN | NR |
| ApoB | 150 (40) | 120(30) | IN | NR | ||||||||||
| Muntner P et al | 2004 | United States of America | ≥ 20 | 7347(NR/NR) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | NR | NR | IN | 2.27 (1.30–3.96) |
| ApoB | NR | NR | IN | 2.97 (2.03–4.34) | ||||||||||
| Lp(a) | NR | NR | IN | 0.78 (0.54–1.12) | ||||||||||
| Panagiotakos D et al | 2004 | Greece | MetS: 55(13) Control: 45(13) | 2282(1126/1154) | 466 | NR | 897 | NR | NCEP-ATP III | ApoA1 | 146.45 (71.77) | 158.04(26.1) | IN | NR |
| ApoB | 123.95 (28.42) | 105.35(41.27) | IN | NR | ||||||||||
| Lp(a) | 17.65 (22.07) | 19.84(28.95) | IN | NR | ||||||||||
| Blatter M et al | 2005 | Switzerland | MetS: 62.1(8.1) Control: 59.6(9.6) | 773(558/215) | NR | 606 | NR | 145 | WHO 1999 | ApoA1 | 91 (17) | 97 (22) | IN | NR |
| ApoB | 98 (21) | 96 (23) | IN | NR | ||||||||||
| Guven A et al | 2005 | Turkey | MetS:38(25–48) Control:35(26–48) | 101(49/52) | NR | NR | NR | NR | NCEP-ATP III | Lp(a) | 51.1 (85.48) | 25.25 (42.07) | IN | NR |
| Lind L et al | 2005 | Sweden | 50–70 | 1826(1826/0) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | 126(19) | 146(25) | RIA | NR |
| ApoB | 137(28) | 122(27) | RIA | NR | ||||||||||
| ApoB/ApoA1 ratio | 1.11(0.27) | 0.86(0.24) | - | NR | ||||||||||
| Cankurtaran M et al | 2006 | Turkey | 71.8(6.3) | 1255(466/789) | 574 | 427 | 1117 | 732 | NCEP-ATP III | ApoA1 | NR | NR | IN | 0.997 (0.992–1.002) |
| ApoB | NR | NR | IN | 1.005 (1–1.01) | ||||||||||
| Lp(a) | NR | NR | IN | 1 (0.992–1.007) | ||||||||||
| Sierra-Johnson J et al | 2006 | United States of America | 46.8(19) | 2954 (1516/1448) | NR | NR | NR | NR | NCEP-ATP III | ApoB/ApoA1 ratio | 0.91 (0.2) | 0.69 (0.2) | – | NR |
| Al-Daghri N et al | 2007 | Saudi Arabia | MetS: 48.82(12.22) Control: 41.05(10.15) | 581(294/287) | NR | 154 | NR | 186 | IDF | ApoA1 | 106 (144) | 83 (26) | IT | NR |
| Pei W et al | 2007 | China | ≥ 20 | 560(268/292) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | NR | NR | IT | 0.951 (0.937–0.965) |
| ApoB | NR | NR | IT | 1.064 (1.048–1.080) | ||||||||||
| De Souza J et al | 2008 | France | MetS: 53(10) Control: 46(12) | 23(23/0) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | 136(23) | 159(16) | IN | NR |
| ApoB | 133(24) | 85(19) | IN | NR | ||||||||||
| Hye J et al. (A) | 2008 | South Korea | 20–78 | 1671(1671/0) | NR | NR | NR | NR | NCEP-ATP III | ApoB | 99.7 (22.6) | 86.5(23.5) | IT | NR |
| Hye J et al. (B) | 2008 | South Korea | 20–78 | 1664(0/1664) | NR | NR | NR | NR | NCEP-ATP III | ApoB | 100.1 (23.5) | 77.4(24.9) | IT | NR |
| Kotani K et al | 2008 | Japan |
MetS: 71(6.6) Control: 73.2(7.6) |
182(62/120) | NR | NR | NR | NR | NCEP-ATP III | Lp(a) | NR | NR | IT | 1.01 (0.99–1.03) |
| Onat A et al | 2008 | Turkey | 56.8(11.3) | 1309(608/701) | NR | NR | NR | NR | NCEP-ATP III | Lp(a) | NR | NR | IN | 0.62(0.47–0.81) |
| Pitsavos C et al | 2008 | Greece | MetS: 51(13) Control: 43(13) | 3042(1518/1524) | 438 | NR | 622 | NR | NCEP-ATP III | ApoA1 | NR | NR | IN | 0.94 (0.90–0.98) |
| ApoB | NR | NR | IN | 1.09(1–1.18) | ||||||||||
| ApoB/ApoA1 ratio | NR | NR | – | 2.3(1.65–3.2) | ||||||||||
| Dullaart R et al | 2009 | Netherlands | MetS: 59(10) Control: 55(9) | 79(43/36) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | 130 (24) | 147 (20) | IT | NR |
| ApoB | 99 (22) | 93 (25) | IT | NR | ||||||||||
| Boumaiza I et al | 2010 | Tunisia | MetS: 62.6(9.3) Control: 59.7(10.2) | 192(NR/NR) | NR | 113 | 91 | 72 | IDF | ApoA1 | 125 (4.2) | 154 (39) | IN | NR |
| ApoB | NR | NR | IN | 2.80 (1.50–5.21) | ||||||||||
| ApoB/ApoA1 ratio | 1.58 (0.61) | 0.97 (0.41) | – | NR | ||||||||||
| Mattsson N et al | 2010 | Finland | 24–39 | 2183(NR/NR) | NR | NR | NR | NR | IDF | ApoA1 | 138 (22) | 150 (25) | IT | NR |
| ApoB | 131 (26) | 101 (23) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.97 (0.21) | 0.69(0.2) | – | NR | ||||||||||
| Park J et al | 2010 | South Korea | MetS: 54.64(10.84) Control: 53.99(11.28) | 658(327/331) | NR | NR | NR | NR | NCEP-ATP III | ApoB/ApoA1 ratio | 0.75 (0.25) | 0.69 (0.41) | – | NR |
| Riediger N et al | 2010 | Canada | ≥ 18 | 475(NR/NR) | NR | NR | 201 | 140 | NCEP-ATP III | ApoA1 | 113 (17) | 120 (18) | IN | NR |
| ApoB | 102 (26) | 80 (23) | IN | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.9 (0.22) | 0.65 (0.2) | – | NR | ||||||||||
| Belfki H et al | 2011 | Tunisia | MetS: 54.9(11) Control: 50.4(13.2) | 330(94/236) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | 149 (28) | 167(31) | IT | NR |
| ApoB | 97 (23) | 80(19) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.67 (0.19) | 0.49(0.13) | – | NR | ||||||||||
| Hee C et al. (A) | 2012 | South Korea | 51.8(10.9) | 7867(7867/0) | NR | 297 | 2438 | 902 | NCEP-ATP III | ApoB/ApoA1 ratio | NR | NR | – | 2.43(2.17–2.72) |
| Hee C et al. (B) | 2012 | South Korea | 51.9(9) | 3073(0/3073) | NR | 108 | 724 | 179 | NCEP-ATP III | ApoB/ApoA1 ratio | NR | NR | – | 3.84(3.13–4.71) |
| Won D et al | 2012 | South Korea | 52.9(8.2) | 244(159/85) | NR | NR | 100 | 76 | NCEP-ATP III | ApoA1 | 128 (20.3) | 137.4(18.5) | IT | NR |
| ApoB | 101.7 (24.2) | 88.9(20.4) | IT | NR | ||||||||||
| Li Y et al | 2013 | China | MetS: 27.36(4.79) Control: 26.68(4.18) | 185(0/185) | NR | NR | NR | NR | IDF | ApoA1 | 101 (20) | 125(30) | IT | NR |
| ApoB | 93 (16) | 72(17) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.89 (0.21) | 0.6(0.18) | – | NR | ||||||||||
| Sung K et al | 2013 | South Korea | 42.42(6.91) | 14,283(12,031/2252) | NR | 102 | 1704 | 539 | HDM | Lp(a) | NR | NR | IT | 0.96(0.84–1.09) |
| Won S et al. (A) | 2013 | South Korea | MetS: 51.6(9.2) Control: 50.5(9.4) | 23,010(23,010/0) | NR | NR | NR | NR | HDM | ApoA1 | 132.2(24.1) | 139.2(23.4) | IT | NR |
| ApoB | 102.7(21.5) | 93(20.9) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.8(0.21) | 0.69(0.2) | – | NR | ||||||||||
| Won S et al. (B) | 2013 | South Korea | MetS: 56.9(8.9) Control: 49.2(8.9) | 18,811(0/18811) | NR | NR | NR | NR | HDM | ApoA1 | 137.9(23.3) | 152.6(25) | IT | NR |
| ApoB | 103.8(22.3) | 85.8(21.3) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.77(0.21) | 0.58(0.18) | – | NR | ||||||||||
| Jing F et al | 2014 | China | MetS: 55.7(12.68) Control: 49.27(15.14) | 8120(3781/4339) | NR | 179 | 2469 | 600 | IDF | ApoB/ApoA1 ratio | NR | NR | – | 4.3 (3.48–5.31) |
| Makaridze Z et al. (A) | 2014 | Georgia | 18–80 | 869(869/0) | NR | NR | NR | NR | NCEP-ATP III | ApoB/ApoA1 ratio | NR | NR | – | 1.18(0.58–2.4) |
| Makaridze Z et al. (B) | 2014 | Georgia | 18–80 | 653(0/653) | NR | NR | NR | NR | NCEP-ATP III | ApoB/ApoA1 ratio | NR | NR | – | 1.75(0.78—3.9) |
| Prasad M et al | 2014 | India | 43(10) | 1000(701/299) | NR | 24 | 307 | 163 | HDM | Lp(a) | NR | NR | IT | 0.7(0.5–1) |
| Savinova O et al | 2014 | United States of America | 44.96(12.3) | 70(42/28) | NR | NR | 14 | NR | NCEP-ATP III | ApoA1 | 98.86(19.1) | 117.76(14.9) | Electrophoresis | NR |
| ApoB | 87.96(23.2) | 69.86(17.4) | Electrophoresis | NR | ||||||||||
| Chou YL et al. (A) | 2015 | China | 39.8(15.61) | 1531(1531/0) | 192 | NR | 201 | 57 | HDM | ApoB/ApoA1 ratio | NR | NR | – | 2.86 (1.6–5.1) |
| Chou YL et al. (B) | 2015 | China | 39.8(15.61) | 1811(0/1811) | 155 | NR | 126 | 65 | HDM | ApoB/ApoA1 ratio | NR | NR | – | 2.01 (1.67–2.41) |
| Lim Y et al | 2015 | South Korea | MetS: 58.6(12.8) Control: 58.1(13.4) | 912(516/396) | NR | NR | NR | 912 | NCEP-ATP III | ApoB | 104.5 (53.3) | 87.7(33.7) | IN | NR |
| Barkas F et al | 2016 | Greece | MetS: 57(50–64) Control: 52(43–61) | 738(314/424) | NR | NR | NR | NR | HDM | ApoA1 | 141 (30) | 155(30) | IN | NR |
| ApoB | 122 (25) | 123(31) | IN | NR | ||||||||||
| Lp(a) | 10.65 (9.92) | 14.97(14.59) | IN | NR | ||||||||||
| Gentile M et al | 2016 | Italy | MetS: 64.1(7.4) Control: 62.5(8.7) | 222(0/222) | NR | NR | NR | NR | AHA/NLBI | ApoB | 110 (20) | 100 (20) | IT | NR |
| Lp(a) | 19.1 (22.1) | 27.9 (29.7) | ELISA | NR | ||||||||||
| Borja M et al | 2017 | United States of America | MetS: 47(10) Control: 45(12) | 74(44/30) | NR | NR | 13 | NR | HDM | ApoA1 | 98 (19) | 116 (14) | Electrophoresis | NR |
| Sreckovic B et al | 2017 | Serbia | 30–75 | 76(NR/NR) | NR | NR | NR | NR | NCEP-ATP III | ApoB | 108 (29) | 91 (25) | IT | NR |
| Vaverková H et al | 2017 | Czech Republic | 45.6(14) | 607(295/312) | NR | NR | NR | NR | HDM | Lp(a) | NR | NR | IT | 0.309 (0.184–0.516) |
| Andrea G et al | 2018 | India | MetS: 55.6(5.66) Control: 52.98(6.76) | 100(53/47) | NR | NR | NR | NR | AHA/NLBI | ApoA1 | 89.06 (24.1) | 173.13(24.11) | IN | NR |
| ApoB/ApoA1 ratio | 1.93 (1.18) | 0.48(0.13) | – | NR | ||||||||||
| Mokhsin A et al. (A) | 2018 | Malaysia | MetS: 49.53(11.7) Control: 40(14.69) | 1177(465/712) | NR | NR | 1246 | 98 | IDF | Lp(a) | 6 (4) | 6 (4) | IT | NR |
| Mokhsin A et al. (B) | 2018 | Malaysia | Mets: 30.56(11.1) Control: 31.29(11) | 150(79/71) | NR | NR | 86 | 3 | IDF | Lp(a) | 23 (26) | 22 (22) | IT | NR |
| Boiko A et al | 2019 | Russia | 35(25.5–42.5) | 53(27/26) | NR | NR | NR | NR | IDF | ApoA1 | 48.69 (16.07) | 56.3 (24.25) | IT | NR |
| Du R et al | 2019 | China | 58.5(9.7) | 10,340(3940/6400) | NR | NR | NR | NR | NCEP-ATP III | ApoB | NR | NR | CLIA | 1.49 (1.43–1.55) |
| Jun J et al | 2019 | South Korea | 51.6(NR) | 10,150(6141/4009) | NR | NR | NR | NR | NCEP-ATP III | Lp(a) | NR | NR | IT | 0.63(0.49–0.80) |
| Reynoso-Villalpando G et al | 2019 | Spain | MetS: 65(9.94) Control: 68(1.3) | 100(65/35) | 50 | NR | 80 | 100 | NCEP-ATP III | ApoA1 | 132.45 (15.91) | 145.96(17.5) | IT | NR |
| ApoB | 84.84 (20.2) | 75.65(19.03) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 0.61 (0.16) | 0.5(0.16) | – | NR | ||||||||||
| Lp(a) | 28.99 (36) | 33.88(40.11) | IT | NR | ||||||||||
| Wu X et al | 2019 | China | ≥ 40 | 10,336(3944/6392) | NR | 1270 | 6259 | 1488 | NCEP-ATP III | Lp(a) | NR | NR | IT | 1.67(1.52–1.83) |
| Cardoso-Saldaña G et al | 2020 | Mexico | MetS: 54.1(8.8) Control: 52.7(9.6) | 953(481/472) | NR | NR | NR | NR | NCEP-ATP III | Lp(a) | 4.7 (5.11) | 6.85 (7.85) | IN | NR |
| Nurtazina A et al | 2020 | Kazakhstan | 25–75 | 704(314/390) | NR | 158 | 408 | NR | IDF | ApoB/ApoA1 ratio | NR | NR | – | 4.73 (3.01–7.43) |
| Rohit A et al | 2020 | India | 21–80 | 150(90/60) | NR | NR | NR | NR | AHA/NLBI | ApoA1 | 113.91 (24.22) | 146.94(12.82) | IN | NR |
| ApoB | 126.09 (37.02) | 85.65(18.85) | IN | NR | ||||||||||
| Lp(a) | 19.33 (7.64) | 19.58(4.75) | IN | NR | ||||||||||
| He H et al | 2021 | China | 27.25(3) | 957(0/957) | NR | NR | NR | NR | IDF | ApoB/ApoA1 ratio | NR | NR | – | 8.7 (6.1–12.4) |
| Sharan H et al | 2022 | Nepal | MetS: 54.89(8.93) Control: 54.63(9.44) | 213(NR/NR) | NR | NR | NR | NR | NCEP-ATP III | ApoA1 | 101.36 (11.75) | 116.52(12.22) | IT | NR |
| ApoB | 115.22 (25.03) | 81.77(16.13) | IT | NR | ||||||||||
| ApoB/ApoA1 ratio | 1.14 (0.27) | 0.7 (0.15) | – | NR | ||||||||||
| Wang W et al | 2022 | China | 53.4(7.5) | 605(304/301) | NR | NR | 215 | 605 | CDS | ApoA1 | 96 (19) | 114 (20) | IT | NR |
AHA/NHLBI American Heart Association/National Heart, Lung, and Blood Institute, ApoB Apolipoprotein B, ApoA1 Apolipoprotein A1, CDS Chinese Diabetes Society, HDM Harmonized Definition of MetS, MetS Metabolic Syndrome, NCEP-ATP III National Cholesterol Education ProgramAdult Treatment Panel III, Lp(a) Lipoprotein(a), CLIA Chemiluminescence immunoassay, IT Immunoturbidimetric, IN Immunonephelometry, ELISA Enzyme-linked immune-sorbent assay, IDF International Diabetes Federation, NR Not Reported, RIA Radioimmunoassay, WHO World Health Organization
The distribution of MetS definitions used in the studies was as follows: 32 studies used the National Cholesterol Education Programme-Adult Treatment Panel III (NCEP-ATP III) criteria [68], 10 studies used the International Diabetes Federation criteria [69], 9 studies met the Harmonised Definition of MetS (HDM) criteria [70], 3 studies met the American Heart Association/National Heart Lung and Blood Institute (AHA/NHLBI) criteria [71], 1 study fulfilled the Chinese Diabetes Society (CDS) criteria [72], and 1 study met the World Health Organization (WHO 1999) criteria [73]. The definitions of MetS according to each criterion are detailed in Supplementary Table S2. Regarding the risk of bias assessment, a total of 46 studies were classified as having a low risk of bias, while 10 studies were classified as having a high risk of bias (Supplementary Table S3).
Association between ApoB levels and the presence of MetS
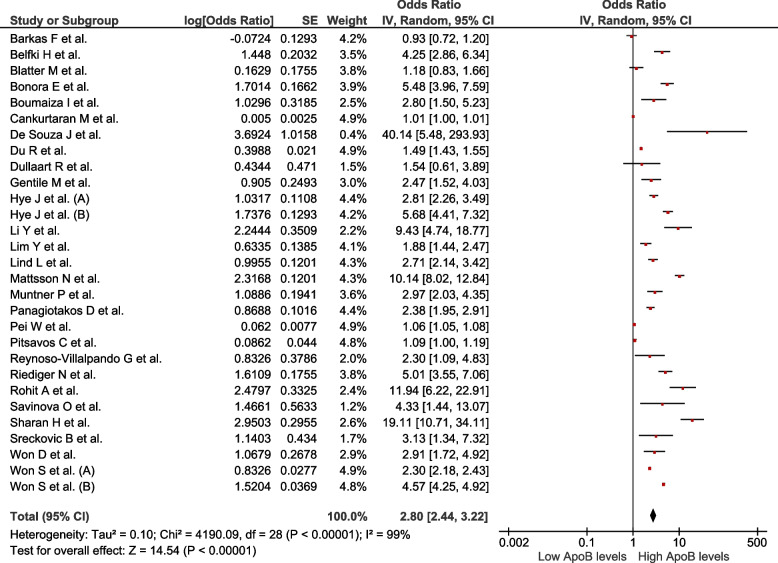
Twenty-nine studies assessed this association (n = 79,661). Increased ApoB values were associated with MetS (OR = 2.8; 95% CI: 2.44–3.22; p < 0.01, I2 = 99%) (Fig. 2). Subgroup analysis was performed according to assay method (Supplementary Figure S1), MetS diagnostic criteria (Supplementary Figure S2), sex (Supplementary Figure S3), continent (Supplementary Figure S4), and study design (Supplementary Figure S5). High heterogeneity was observed in all subgroups, indicating that the association persisted across various subgropus. In the sensitivity analysis (Supplementary Figure S6), after eliminating studies at high risk of bias, the association persisted with high heterogeneity (OR = 3.29; 95% CI: 2.63–4.13; p < 0.01, I2 = 98%).
Fig. 2.
Association between ApoB and MetS
Association between ApoA1 levels and the presence of MetS
Twenty-eight studies assessed this association (n = 66 189). Reduced ApoA1 values were associated with MetS (OR = 0.42; 95% CI: 0.38–0.47; p < 0.01, I2 = 99%) (Fig. 3). Subgroup analysis was performed according to assay method (Supplementary Figure S7), MetS diagnostic criteria (Supplementary Figure S8), sex (Supplementary Figure S9), continent (Supplementary Figure S10), and study design (Supplementary Figure S11). In all subgroups, except for the subgroup of American studies, the association remained significant despite the presence of high heterogeneity. In the sensitivity analysis (Supplementary Figure S12), after eliminating studies at high risk of bias, the association persisted with high heterogeneity (OR = 0.26; 95% CI: 0.18–0.38; p < 0.01, I2 = 97%).
Fig. 3.
Association between ApoA1 and MetS
Association between ApoB/ApoA1 ratio levels and the presence of MetS
Twenty-three studies assessed this association (n = 79 664). Increased values of the ApoB/ApoA1 ratio were associated with MetS (OR = 4.97; 95% CI: 3.83–6.44; p < 0.01, I2 = 97%) (Fig. 4). The subgroup analysis was performed according to MetS diagnostic criteria (Supplementary Figure S13), sex (Supplementary Figure S14), continent (Supplementary Figure S15), and study design (Supplementary Figure S16). In all subgroups, the association remained with high heterogeneity. In the sensitivity analysis (Supplementary Figure S17), the association continued to be observed despite the exclusion of studies considered to have a high risk of bias. However, high heterogeneity remained a characteristic of the association (OR = 5.42; 95% CI: 3.88–7.56; p < 0.01, I2 = 96%).
Fig. 4.
Association between ApoB/ApoA1 ratio and MetS
Association between Lp(a) levels and the presence of MetS
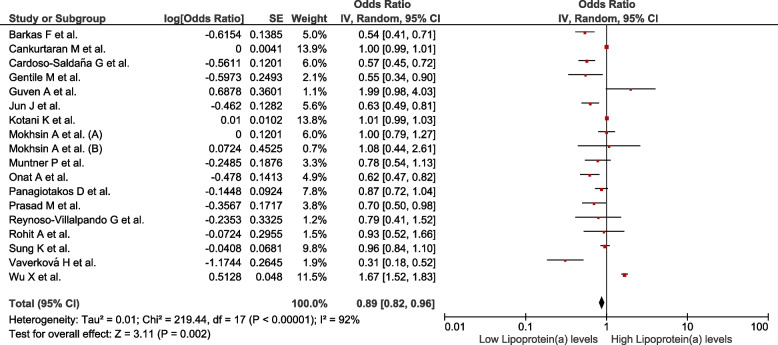
Eighteen studies assessed this association (n = 52 342). Decreased Lp (a) values were associated with MetS (OR = 0.89; 95% CI: 0.82–0.96; p < 0.01; I2 = 92%) (Fig. 5). The subgroup analysis was performed according to assay method (Supplementary Figure S18), MetS diagnostic criteria (Supplementary Figure S19), continent (Supplementary Figure S20), and study design (Supplementary Figure S21). The studies conducted in Europe (OR = 0.71; 95% CI: 0.55–0.93; p = 0.01; I2 = 89%), those using immunonephelometry to measure Lp(a) values (OR = 0.78; 95% CI: 0.63–0.98; p = 0.03; I2 = 87%), and those diagnosing MetS using HDM criteria (OR = 0.6; 95% CI: 0.39–0.93; p = 0.02; I2 = 90%) maintained a significant association with high heterogeneity. In the sensitivity analysis (Supplementary Figure S22), after eliminating studies at high risk of bias, the association was found to not remain (OR = 0.91; 95% CI: 0.76–1.1; p < 0.01, I2 = 93%).
Fig. 5.
Association between lipoprotein(a) and MetS
Publication bias
No asymmetry was found in the funnel plot (Supplementary Figures S23, 24, 25 and 26). No evidence of publication bias was found in any of the associations examined (Begg test > 0.1).
Discussion
The primary objective of this systematic review and meta-analysis was to provide a comprehensive synthesis of the existing evidence regarding the relationship between ApoA1, ApoB, ApoB/A1 ratio and Lp(a), and MetS. The main findings of our investigation demonstrate evidence that high ApoB levels, low ApoA1 levels, and elevated ApoB/ApoA1 ratio are significantly associated with MetS. Despite the implementation of sensitivity analyses, our findings continue to demonstrate significant heterogeneity across the included studies.
Apolipoproteins are proteins synthesised in the liver that play a crucial role in the transport and redistribution of lipids [74, 75]. ApoA1, found in HDL, facilitates the reverse transport of peripheral cholesterol to the liver, thereby exerting an anti-atherogenic effect [74]. In contrast, ApoB, is responsible for transporting cholesterol to peripheral cells and may enhance atherothrombosis [75]. Thus, some studies have shown that increasing ApoA1 and decreasing ApoB have cardiovascular benefits, similar to our results. For example, the association between high ApoA1 levels and premature coronary heart disease is well known [76]. Additionally, individuals with smaller apolipoprotein A isoforms exhibit an approximately 2 times higher risk of developing coronary heart disease or experiencing ischaemic stroke than those with larger isoforms [9]. In contrast, a systematic review revealed that both statin and non-statin therapies effectively reduced cardiovascular risk by lowering ApoB levels [77]. Considering these findings, it is not surprising that the ratio of both lipoproteins has emerged as a cardiovascular marker. Thus, in the case of the ApoB/ApoA1 ratio, another systematic review demonstrated that elevated levels can enhance risk prediction of cardiovascular events, even after accounting for traditional risk factors, particularly in high-risk populations [78]. Although the explanation for the association between lipoprotein levels and cardiovascular risk is multifactorial [74, 75], it is plausible that some of these factors may also contribute to our observed associations between the levels of these markers and MetS.
The pathophysiology of MetS involves multiple mechanisms that are not yet not fully understood. There is ongoing debate regarding the individual components of MetS, in addition to genetic and epigenetic factors, represent distinct pathologies or are interconnected within a common broader pathogenic process [79]. Regardless of these mechanisms, they result in three major processes: hormonal activation, chronic inflammation and insulin resistance [79], and there is evidence of an association between apolipoprotein alterations and some of these mechanisms. In this regard, the well-established association between apolipoproteins, inflammation and insulin is widely recognised. For instance, ApoA1 has anti-inflammatory properties as evidenced by a study that identified 33 significant correlations between ApoA1 and urinary cytokine levels [80]. The strongest associations were observed for interleukin-1 alpha, spondin2, advanced glycation end-product receptor, protease-activated receptor-1, TNF-related apoptosis-inducing ligand receptor 2, interleukin-4 receptor alpha and stem cell factor [80]. Another study conducted on overweight and obese postmenopausal women showed that ApoB is the main predictor of inflammatory markers as it was an independent predictor of interindividual variation in IL-6, orosomucoid, haptoglobin and alpha 1-antitrypsin [81]. Regarding insulin resistance, numerous studies have established correlation between ApoA1, Apo B and ApoA1/ApoB ratio with insulin resistance in both diabetic and normo-glycemic patients [82–84]. However, the association between lipoproteins and insulin resistance appears to be a two-way relationship. This is due to the suggestion that under conditions of insulin resistance, the inhibitory effect of insulin on lipase activity is diminished, resulting in increased free fatty acids release through lipolysis. This, in turn, can lead to modifications in atherogenic lipoproteins, including the overproduction of ApoB [82].
It is worth emphasising that some authors consider the ApoB/ApoA1 ratio as the most accurate marker for assessing the balance between atherogenic and anti-atherogenic lipoproteins. They argue that this ratio serves as a superior predictor of cardiovascular risk associated with cardiovascular lipoproteins than traditional lipid indices [11, 85]. Similarly, it is hypothesised that it would be an ideal marker for lipid alterations associated with insulin resistance and MetS, as it captures the key characteristics of dyslipidemia associated with insulin resistance and MetS, including low HDL levels and elevated levels of VLDL and small dense LDL [11].
Despite the observed associations, the internal validity of our results is limited by their high heterogeneity, which can be attributed to inherent variations in the studies included in our study. Although sensitivity analysis was performed according to sex, diagnostic criteria for MetS, method of assessment of apolipoproteins and according to the continent in which the studies were conducted, the heterogeneity was still high, which means that other variables that can potentially affect these associations were not considered. One of these may be related to the prevalence of MetS, as it has been suggested that associations between ApoB and various cardiometabolic disorders are limited to populations with a relatively high prevalence of MetS [14]. Furthermore, it has been suggested that this association may not only vary based on the diagnosis of MetS but also by the number of diagnostic criteria utilised [83]. Similarly, this heterogeneity may be influenced by the cut-off point used to assess, for example, the ApoB/ApoA1 ratio, as some studies define it by numerical values and others by quartiles [50]. Likewise, although an elevated ApoB/ApoA1 ratio may imply high ApoB levels or low ApoA1 levels per se, its association with MetS may also reflect other factors associated with an elevated ApoB/ApoAI ratio that were not measured in the present study as suggested by its association with myocardial infarction [11]. This heterogeneity had a notable impact on the results concerning the association between Lp (a) and MetS, as after excluding studies with high risk of bias, the association was not maintained and high heterogeneity persisted in the remaining studies. Lp(a) primarily comprises LDL particles bound to apolipoprotein(a), is elevated in up to 20% of the general population and is associated with an elevated risk of atherothrombosis [86, 87]. Because mechanisms such as insulin resistance affect its concentration, it has been associated with the development of MetS, although the results of these associations remain controversial. Although our study does not resolve this controversy, it highlights the need for conducting more rigorous investigations to elucidate this association.
Limitations and strenghts
Our study should be interpreted considering its limitations. First, it is important to note that the high heterogeneity observed between studies did not diminish even after conducting subgroup analysis and sensitivity analysis. This implies that there is high clinical and methodological variability among these studies, so it would be desirable that future studies take into account more variables that may influence the values of these lipoproteins (e.g. sociodemographic, comorbidities, and lifestyles). Second, due to the limited information in the studies, the sensitivity, specificity and optimal cut-off point of these markers for estimating the risk of developing MetS were not determined, which would be important to evaluate in future studies. Third, since the majority of the included studies were of cross-sectional design, there is a risk of reverse causality. Despite these limitations, our study has several strengths. A large number of studies were included, resulting in a substantial number of participants, thereby ensuring adequate statistical power. Furthermore, a thorough search was conducted across multiple databases, ensuring a comprehensive inclusion of the available evidence. Likewise, subgroup analysis was performed according to assay method, MetS diagnostic criteria, continents and study design. To the best of our knowledge, this systematic review and meta-analysis represents the first comprehensive synthesis of available studies examining the association between apolipoproteins and Lp(a) levels in patients with MetS.
Conclusion
Increased values of ApoB and ApoB/ApoA1 ratio and reduced values of ApoA1 and Lp(a) are associated with the presence of MetS. These findings suggest that these lipid markers may serve as potential indicators for identifying subjects at risk of developing MetS. However, additional studies are warranted to gain a deeper understanding of the underlying mechanisms driving these associations. In addition, further clinical research and longitudinal studies are recommended to better understand the causal relationship between these lipid markers and MetS, as well as to explore their potential utility in clinical practice for early detection and management of MetS.
Supplementary Information
Additional file 1: Table S1. Search strategies. Table S2. Criteria for clinical diagnosis of metabolic syndrome used in the included studies. Table S3. Quality assessment of included studies. Figure S1. Subgroup analysis according to assay method of the association between ApoB levels and MetS. Figure S2. Subgroup analysis according to diagnostic criteria of the association between ApoB levels and MetS. Figure S3. Subgroup analysis according to sex of the association between ApoB levels and MetS. Figure S4. Subgroup analysis according to continents of the association between ApoB levels and MetS. Figure S5. Subgroup analysis according to study design of the association between ApoB levels and MetS. Figure S6. Sensitivity analysis according to risk of bias of the association between ApoB levels and MetS. Figure S7. Subgroup analysis according to assay method of the association between ApoA1 levels and MetS. Figure S8. Subgroup analysis according to diagnostic criteria of the association between ApoA1 levels and MetS. Figure S9. Subgroup analysis according to sex of the association between ApoA1 levels and MetS. Figure S10. Subgroup analysis according to continents of the association between ApoA1 levels and MetS. Figure S11. Subgroup analysis according to study design of the association between ApoA1 levels and MetS. Figure S12. Sensitivity analysis according to risk of bias of the association between ApoA1 levels and MetS. Figure S13. Subgroup analysis according to diagnostic criteria of the association between ApoB/ApoA1 ratio levels and MetS. Figure S14. Subgroup analysis according to gender of the association between ApoB/ApoA1 ratio levels and MetS. Figure S15. Subgroup analysis according to continents of the association between ApoB/ApoA1 ratio levels and MetS. Figure S16. Subgroup analysis according to study design of the association between ApoB/ApoA1 ratio levels and MetS. Figure S17. Sensitivity analysis according to risk of bias of the association between ApoB/ApoA1 ratio levels and MetS. Figure S18. Subgroup analysis according to assay method of the association between lipoprotein(a) levels and MetS. Figure S19. Subgroup analysis according to diagnostic criteria of the association between lipoprotein(a) levels and MetS. Figure S20. Subgroup analysis according to continents of the association between lipoprotein(a) levels and MetS. Figure S21. Subgroup analysis according to study design of the association between lipoprotein(a) levels and MetS. Figure S22. Sensitivity analysis according to risk of bias of the association between lipoprotein(a) levels and MetS. Figure S23. Funnel Plot of the studies that evaluated the association between ApoB and MetS. Figure S24. Funnel Plot of the studies that evaluated the association between ApoA1 and MetS. Figure S25. Funnel Plot of the studies that evaluated the association between ApoB/ApoA1 ratio and MetS. Figure S26. Funnel Plot of the studies that evaluated the association between Lipoprotein(a) and MetS.
Acknowledgements
None.
Abbreviations
- ApoA1
Apolipoprotein A1
- ApoB
Apolipoprotein B
- Hcy
Homocysteine
- LDL
Low Density Lipoprotein
- Lp(a)
Lipoprotein(a)
- MetS
Metabolic syndrome
- VLDL
Very Low-Density Lipoprotein
Author’s contributions
Conceptualization: J.R.U-B and V.A.B.-Z; Data curation: J.R.U-B, E.A.A-B, E.A.H-B, A.A-C, X.L.C-T, R.A.S-A and M.A.H-C; Formal analysis: J.R.U-B, E.A.A-B., and V.A.B.-Z.; Methodology: J.R.U-B, E.A.A-B, E.A.H-B and V.A.B.-Z.; Writing—original draft: J.R.U-B, E.A.A-B, E.A.H-B, X.L.C-T, R.A.S-A, M.A.H-C, A.A-C, P.H.-A and V.A.B-Z.; Writing review & editing: J.R.U-B, A.A-C, P.H-A and V.A.B.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Availability of data and materials
All data generated or analysed during the current study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1–23. doi: 10.1016/j.ecl.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Yang C, Jia X, Wang Y, Fan J, Zhao C, Yang Y, et al. Trends and influence factors in the prevalence, intervention, and control of metabolic syndrome among US adults, 1999–2018. BMC Geriatr. 2022;22(1):979. doi: 10.1186/s12877-022-03672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas-Martínez R, Aguilar-Salinas CA, Romero-Martínez M, Castro-Porras L, Gómez-Velasco D, Mehta R. Trends in the prevalence of metabolic syndrome and its components in Mexican adults, 2006–2018. Salud Publica Mex. 2021;63(6):713–24. doi: 10.21149/12835. [DOI] [PubMed] [Google Scholar]
- 5.Gursky O. Apolipoprotein structure and dynamics. Curr Opin Lipidol. 2005;16(3):287–294. doi: 10.1097/01.mol.0000169348.61191.ac. [DOI] [PubMed] [Google Scholar]
- 6.Ulloque-Badaracco JR, Mosquera-Rojas MD, Hernandez-Bustamante EA, Alarcón-Braga EA, Ulloque-Badaracco RR, Al-Kassab-Córdova A, et al. Association between lipid profile and apolipoproteins with risk of diabetic foot ulcer: a systematic review and meta-analysis. Int J Clin Pract. 2022;2022:1–15. doi: 10.1155/2022/5450173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulloque-Badaracco JR, Hernandez-Bustamante EA, Herrera-Añazco P, Benites-Zapata VA. Prognostic value of apolipoproteins in COVID-19 patients: A systematic review and meta-analysis. Travel Med Infect Dis. 2021;44:102200. doi: 10.1016/j.tmaid.2021.102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dardeer KT, Mohammed KA, Hussein TD, Elsheemy MS. Apolipoprotein A1 as a novel urinary biomarker for diagnosis of bladder cancer: a systematic review and meta-analysis. Indian J Urol. 2021;37(3):217–225. doi: 10.4103/iju.iju_69_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55(19):2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 10.Dominiczak MH, Caslake MJ. Apolipoproteins: metabolic role and clinical biochemistry applications. Ann Clin Biochem. 2011;48(Pt 6):498–515. doi: 10.1258/acb.2011.011111. [DOI] [PubMed] [Google Scholar]
- 11.Lind L, Vessby B, Sundström J. The apolipoprotein B/AI ratio and the metabolic syndrome independently predict risk for myocardial infarction in middle-aged men. Arterioscler Thromb Vasc Biol. 2006;26(2):406–410. doi: 10.1161/01.atv.0000197827.12431.d0. [DOI] [PubMed] [Google Scholar]
- 12.Wallenfeldt K, Bokemark L, Wikstrand J, Hulthe J, Fagerberg B. Apolipoprotein B/apolipoprotein A-I in relation to the metabolic syndrome and change in carotid artery intima-media thickness during 3 years in middle-aged men. Stroke. 2004;35(10):2248–2252. doi: 10.1161/01.str.0000140629.65145.3c. [DOI] [PubMed] [Google Scholar]
- 13.Boiko AS, Mednova IA, Kornetova EG, Semke AV, Bokhan NA, Loonen AJM, et al. Apolipoprotein serum levels related to metabolic syndrome in patients with schizophrenia. Heliyon. 2019;5(7):e02033. doi: 10.1016/j.heliyon.2019.e02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onat A, Can G, Hergenç G, Yazici M, Karabulut A, Albayrak S. Serum apolipoprotein B predicts dyslipidemia, metabolic syndrome and in women, hypertension and diabetes, independent of markers of central obesity and inflammation. Int J Obes (Lond) 2007;31(7):1119–1125. doi: 10.1038/sj.ijo.0803552. [DOI] [PubMed] [Google Scholar]
- 15.Ryoo J-H, Park SK. Association of apolipoprotein B and incidence of metabolic syndrome in Korean men: a 5-years' follow-up study. Atherosclerosis. 2012;226(2):496–501. doi: 10.1016/j.atherosclerosis.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::AID-SIM784>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Savinova OV, Fillaus K, Jing L, Harris WS, Shearer GC. Reduced apolipoprotein glycosylation in patients with the metabolic syndrome. PLOS ONE. 2014;9(8):e104833. doi: 10.1371/journal.pone.0104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SW, Jee JH, Kim HJ, Jin SM, Suh S, Bae JC, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168(3):2678–2683. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 22.de Souza JA, Vindis C, Hansel B, Nègre-Salvayre A, Therond P, Serrano CV, Jr, et al. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis. 2008;197(1):84–94. doi: 10.1016/j.atherosclerosis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Pitsavos C, Panagiotakos DB, Skoumas J, Papadimitriou L, Stefanadis C. Risk stratification of apolipoprotein B, apolipoprotein A1, and apolipoprotein B/AI ratio on the prevalence of the metabolic syndrome: the ATTICA study. Angiology. 2008;59(3):335–341. doi: 10.1177/0003319707307273. [DOI] [PubMed] [Google Scholar]
- 24.Jung CH, Hwang JY, Yu JH, Shin MS, Bae SJ, Park JY, et al. The value of apolipoprotein B/A1 ratio in the diagnosis of metabolic syndrome in a Korean population. Clin Endocrinol (Oxf) 2012;77(5):699–706. doi: 10.1111/j.1365-2265.2012.04329.x. [DOI] [PubMed] [Google Scholar]
- 25.Lim Y, Yoo S, Lee SA, Chin SO, Heo D, Moon JC, et al. Apolipoprotein B is related to metabolic syndrome independently of low density lipoprotein cholesterol in patients with type 2 diabetes. Endocrinol Metab (Seoul) 2015;30(2):208–15. doi: 10.3803/EnM.2015.30.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dullaart RPF, Plomgaard P, de Vries R, Dahlbäck B, Nielsen LB. Plasma apolipoprotein M is reduced in metabolic syndrome but does not predict intima media thickness. Clin Chim Acta. 2009;406(1):129–133. doi: 10.1016/j.cca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Sreckovic B, Sreckovic VD, Soldatovic I, Colak E, Sumarac-Dumanovic M, Janeski H, et al. Homocysteine is a marker for metabolic syndrome and atherosclerosis. Diabetes Metab Syndr. 2017;11(3):179–182. doi: 10.1016/j.dsx.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Mattsson N, Magnussen CG, Rönnemaa T, Mallat Z, Benessiano J, Jula A, et al. Metabolic syndrome and carotid intima-media thickness in young adults: roles of apolipoprotein B, apolipoprotein A-I, C-reactive protein, and secretory phospholipase A2: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol. 2010;30(9):1861–1866. doi: 10.1161/atvbaha.110.204669. [DOI] [PubMed] [Google Scholar]
- 29.Nurtazina A, Kozhakhmetova D, Dautov D, Shakhanova A, Chattu VK. Apolipoprotein B/A1 ratio as a diagnostic alternative to triglycerides and HDL-Cholesterol for the prediction of metabolic syndrome among hypertensives in Kazakhstan. Diagnostics (Basel) 2020;10(8):510. doi: 10.3390/diagnostics10080510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belfki H, Ben Ali S, Bougatef S, Ben Ahmed D, Haddad N, Jmal A, et al. The Apolipoprotein B/Apolipoprotein A 1 ratio in relation to metabolic syndrome and its components in a sample of the Tunisian population. Exp Mol Pathol. 2011;91(2):622–625. doi: 10.1016/j.yexmp.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Yi DW, Jeong DW, Lee SY, Son SM, Kang YH. The Association between Apolipoprotein A-II and metabolic syndrome in Korean adults: a comparison study of Apolipoprotein A-I and Apolipoprotein B. Diabetes Metab J. 2012;36(1):56–63. doi: 10.4093/dmj.2012.36.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du R, Wu X, Peng K, Lin L, Li M, Xu Y, et al. Serum apolipoprotein B is associated with increased risk of metabolic syndrome among middle-aged and elderly Chinese: a cross-sectional and prospective cohort study. J Diabetes. 2019;11(9):752–760. doi: 10.1111/1753-0407.12904. [DOI] [PubMed] [Google Scholar]
- 33.Han JH, Park HS, Kim JA, Kim SM. Apolipoprotein B is a better marker than non-HDL-cholesterol for the metabolic syndrome in Koreans. Atherosclerosis. 2008;197(1):333–338. doi: 10.1016/j.atherosclerosis.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Pei WD, Sun YH, Lu B, Liu Q, Zhang CY, Zhang J, et al. Apolipoprotein B is associated with metabolic syndrome in Chinese families with familial combined hyperlipidemia, familial hypertriglyceridemia and familial hypercholesterolemia. Int J Cardiol. 2007;116(2):194–200. doi: 10.1016/j.ijcard.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 35.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, et al. Metabolic syndrome: epidemiology and more extensive phenotypic description. Cross-sectional data from the Bruneck Study. Int J Obes Relat Metab Disord. 2003;27(10):1283–9. doi: 10.1038/sj.ijo.0802381. [DOI] [PubMed] [Google Scholar]
- 36.Cankurtaran M, Halil M, Yavuz BB, Dagli N, Oyan B, Ariogul S. Prevalence and correlates of metabolic syndrome (MS) in older adults. Arch Gerontol Geriatr. 2006;42(1):35–45. doi: 10.1016/j.archger.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III) Ann Epidemiol. 2004;14(9):686–695. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Tousoulis D, Toutouza M, et al. Impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J. 2004;147(1):106–112. doi: 10.1016/s0002-8703(03)00442-3. [DOI] [PubMed] [Google Scholar]
- 39.Rohit A, Haridas N. Studies on apolipoproteins (Apo A1 & Apo B) and lipoprotein (a) along with lipid profile in the patients of type ii diabetes mellitus and metabolic syndrome. Int J Med Biomed Stud. 2020;4(2). 10.32553/ijmbs.v4i2.933.
- 40.Barkas F, Elisaf M, Liberopoulos E, Liontos A, Rizos EC. High triglyceride levels alter the correlation of apolipoprotein B with low- and non-high-density lipoprotein cholesterol mostly in individuals with diabetes or metabolic syndrome. Atherosclerosis. 2016;247:58–63. doi: 10.1016/j.atherosclerosis.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Reynoso-Villalpando GL, Sevillano-Collantes C, Valle Y, Moreno-Ruiz I, Padilla-Gutiérrez JR, Del Cañizo-Gómez FJ. ApoB/ApoA1 ratio and non-HDL-cholesterol/HDL-cholesterol ratio are associated to metabolic syndrome in patients with type 2 diabetes mellitus subjects and to ischemic cardiomyopathy in diabetic women. Endocrinol Diabetes Nutr (Engl Ed) 2019;66(8):502–511. doi: 10.1016/j.endinu.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Li Y-t, He Y-l, Zhu H-l. The diagnostic significance of apoB/apoAI for metabolic syndrome in patients with polycysticovary syndrome. Med J Chin People's Lib Army. 2013;38(6). Available from: http://www.plamj.org/index.php/plamj/article/view/727.
- 43.War GA, Raina S, Jain R, Kant S. Correlation of Apolipoprotein B and Apolipoprotein A1 with metabolic syndrome – single centre experience from Delhi. JIACM. 2018;19(3):191–194. [Google Scholar]
- 44.Makaju HS, Sharma VK, Yadav BK, Tuladhar ET, Bhandari R, Bhattarai A, et al. Association of Apolipoprotein B /Apolipoprotein A-I Ratio With Cardiometabolic Risk Biomarkers in Type 2 Diabetes Mellitus. Research Square. 2022. 10.21203/rs.3.rs-1474761/v1.
- 45.Garin MC, Kalix B, Morabia A, James RW. Small, dense lipoprotein particles and reduced paraoxonase-1 in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90(4):2264–2269. doi: 10.1210/jc.2004-1295. [DOI] [PubMed] [Google Scholar]
- 46.Boumaiza I, Omezzine A, Rejeb J, Rebhi L, Kalboussi N, Ben Rejeb N, et al. Apolipoprotein B and non-high-density lipoprotein cholesterol are better risk markers for coronary artery disease than low-density lipoprotein cholesterol in hypertriglyceridemic metabolic syndrome patients. Metab Syndr Relat Disord. 2010;8(6):515–522. doi: 10.1089/met.2010.0006. [DOI] [PubMed] [Google Scholar]
- 47.Jing F, Mao Y, Guo J, Zhang Z, Li Y, Ye Z, et al. The value of Apolipoprotein B/Apolipoprotein A1 ratio for metabolic syndrome diagnosis in a Chinese population: a cross-sectional study. Lipids Health Dis. 2014;13:81. doi: 10.1186/1476-511x-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sierra-Johnson J, Somers VK, Kuniyoshi FH, Garza CA, Isley WL, Gami AS, et al. Comparison of apolipoprotein-B/apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98(10):1369–1373. doi: 10.1016/j.amjcard.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 49.Chou YC, Kuan JC, Bai CH, Yang T, Chou WY, Hsieh PC, et al. Predictive value of serum apolipoprotein B/apolipoprotein A-I ratio in metabolic syndrome risk: a Chinese cohort study. Endocrine. 2015;49(2):404–414. doi: 10.1007/s12020-014-0447-z. [DOI] [PubMed] [Google Scholar]
- 50.He H, Feng J, Zhang S, Wang Y, Li J, Gao J, et al. The Apolipoprotein B/A1 ratio is associated with metabolic syndrome components, insulin resistance, androgen hormones, and liver enzymes in women with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2021;12:773781. doi: 10.3389/fendo.2021.773781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JS, Cho MH, Nam JS, Yoo JS, Lee YB, Roh JM, et al. Adiponectin is independently associated with apolipoprotein B to A-1 ratio in Koreans. Metabolism. 2010;59(5):677–682. doi: 10.1016/j.metabol.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Makaridzea Z, Asatianib K. The optimal cutoff value ofApolipoproteinB/ApolipoproteinA-I ratio for thediagnosis of metabolic syndrome and insulinresistance inthe population of Georgia. IJSBAR. 2014;17(2):224–235. [Google Scholar]
- 53.Borja MS, Hammerson B, Tang C, Savinova OV, Shearer GC, Oda MN. Apolipoprotein A-I exchange is impaired in metabolic syndrome patients asymptomatic for diabetes and cardiovascular disease. PLOS ONE. 2017;12(8):e0182217. doi: 10.1371/journal.pone.0182217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Daghri NM. Hyperhomocysteinemia, coronary heart disease, and diabetes mellitus as predicted by various definitions for metabolic syndrome in a hypertensive Saudi population. Saudi Med J. 2007;28(3):339–46. Available from: https://pubmed.ncbi.nlm.nih.gov/17334456/. [PubMed]
- 55.Wang W, Chen ZY, Guo XL, Tu M. Monocyte to high-density lipoprotein and Apolipoprotein A1 ratios: novel indicators for metabolic syndrome in Chinese newly diagnosed type 2 diabetes. Front Endocrinol (Lausanne) 2022;13:935776. doi: 10.3389/fendo.2022.935776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guven A, Inanc F, Kilinc M, Ekerbicer H. Plasma homocysteine and lipoprotein (a) levels in Turkish patients with metabolic syndrome. Heart Vessels. 2005;20(6):290–295. doi: 10.1007/s00380-004-0822-4. [DOI] [PubMed] [Google Scholar]
- 57.Wu XY, Lin L, Qi HY, Du R, Hu CY, Ma LN, et al. Association between lipoprotein (a) levels and metabolic syndrome in a middle-aged and elderly Chinese cohort. Biomed Environ Sci. 2019;32(7):477–485. doi: 10.3967/bes2019.065. [DOI] [PubMed] [Google Scholar]
- 58.Cardoso-Saldaña GC, González-Salazar MDC, Posadas-Sánchez R, Vargas-Alarcón G. Síndrome metabólico, lipoproteína (a) y aterosclerosis subclínica en población mexicana. Arch Cardiol Méx. 2021;91(3):307. doi: 10.24875/acm.20000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jun JE, Hwang Y-C, Ahn KJ, Chung HY, Jeong I-K, Bae JC, et al. The association between lipoprotein(a) and metabolic syndrome. Endocrine Abstracts. 2019;63(P156). 10.1530/endoabs.63.P156.
- 60.Onat A, Hergenç G, Ozhan H, Kaya Z, Bulur S, Ayhan E, et al. Lipoprotein(a) is associated with coronary heart disease independent of metabolic syndrome. Coron Artery Dis. 2008;19(3):125–131. doi: 10.1097/mca.0b013e3282f399cf. [DOI] [PubMed] [Google Scholar]
- 61.Gentile M, Iannuzzo G, Mattiello A, Marotta G, Iannuzzi A, Panico S, et al. Association between Lp (a) and atherosclerosis in menopausal women without metabolic syndrome. Biomark Med. 2016;10(4):397–402. doi: 10.2217/bmm.16.4. [DOI] [PubMed] [Google Scholar]
- 62.Sung KC, Wild SH, Byrne CD. Lipoprotein (a), metabolic syndrome and coronary calcium score in a large occupational cohort. Nutr Metab Cardiovasc Dis. 2013;23(12):1239–1246. doi: 10.1016/j.numecd.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Vaverková H, Karásek D, Halenka M, Cibíčková L, Kubíčková V. Inverse association of lipoprotein (a) with markers of insulin resistance in dyslipidemic subjects. Physiol Res. 2017;66(1):113–20. doi: 10.33549/physiolres.933583. [DOI] [PubMed] [Google Scholar]
- 64.Prasad M, Flowers E, Divakaruni M, Molina C, Mathur A, Assimes TL, et al. The relationship of lipoprotein(a), C-reactive protein and homocysteine with metabolic syndrome in South Asians. J Indian Coll Cardiol. 2014;4(4):208–213. doi: 10.1016/j.jicc.2014.08.004. [DOI] [Google Scholar]
- 65.Kotani K, Shimohiro H, Adachi S, Sakane N. Relationship between lipoprotein(a), metabolic syndrome, and carotid atherosclerosis in older Japanese people. Gerontology. 2008;54(6):361–364. doi: 10.1159/000145541. [DOI] [PubMed] [Google Scholar]
- 66.Mokhsin A, Mokhtar SS, Mohd Ismail A, Nor FM, Shaari SA, Nawawi H, et al. Observational study of the status of coronary risk biomarkers among Negritos with metabolic syndrome in the east coast of Malaysia. BMJ Open. 2018;8(12):e021580. doi: 10.1136/bmjopen-2018-021580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riediger ND, Bruce SG, Young TK. Cardiovascular risk according to plasma apolipoprotein and lipid profiles in a Canadian First Nation. Chronic Dis Can. 2010;31(1):33–8. Available from: https://pubmed.ncbi.nlm.nih.gov/21213617/. [PubMed]
- 68.Expert Panel on Detection Evaluation aToHBCiA Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 69.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 70.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/circulationaha.109.192644. [DOI] [PubMed] [Google Scholar]
- 71.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart association/National heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/circulationaha.105.169404. [DOI] [PubMed] [Google Scholar]
- 72.Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev. 2019;35(6):e3158. doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 73.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(sici)1096-9136(199807)15:7<539::aid-dia668>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 74.Su X, Peng D. The exchangeable apolipoproteins in lipid metabolism and obesity. Clin Chim Acta. 2020;503:128–135. doi: 10.1016/j.cca.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 75.Morita SY. Metabolism and modification of Apolipoprotein B-containing lipoproteins involved in Dyslipidemia and Atherosclerosis. Biol Pharm Bull. 2016;39(1):1–24. doi: 10.1248/bpb.b15-00716. [DOI] [PubMed] [Google Scholar]
- 76.Haji Aghajani M, MadaniNeishaboori A, Ahmadzadeh K, Toloui A, Yousefifard M. The association between apolipoprotein A-1 plasma level and premature coronary artery disease: a systematic review and meta-analysis. Int J Clin Pract. 2021;75(11):e14578. doi: 10.1111/ijcp.14578. [DOI] [PubMed] [Google Scholar]
- 77.Khan SU, Khan MU, Valavoor S, Khan MS, Okunrintemi V, Mamas MA, et al. Association of lowering apolipoprotein B with cardiovascular outcomes across various lipid-lowering therapies: systematic review and meta-analysis of trials. Eur J Prev Cardiol. 2020;27(12):1255–1268. doi: 10.1177/2047487319871733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandhu PK, Musaad SM, Remaley AT, Buehler SS, Strider S, Derzon JH, et al. Lipoprotein biomarkers and risk of cardiovascular disease: a Laboratory Medicine Best Practices (LMBP) systematic review. J Appl Lab Med. 2016;1(2):214–229. doi: 10.1373/jalm.2016.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fahed G, Aoun L, BouZerdan M, Allam S, BouZerdan M, Bouferraa Y, et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786. doi: 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fellström B, Helmersson-Karlqvist J, Lind L, Soveri I, Wu PH, Thulin M, et al. Associations between Apolipoprotein A1, high-density lipoprotein cholesterol, and urinary cytokine levels in elderly males and females. J Interferon Cytokine Res. 2020;40(2):71–74. doi: 10.1089/jir.2019.0074. [DOI] [PubMed] [Google Scholar]
- 81.Faraj M, Messier L, Bastard JP, Tardif A, Godbout A, Prud'homme D, et al. Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia. 2006;49(7):1637–1646. doi: 10.1007/s00125-006-0259-7. [DOI] [PubMed] [Google Scholar]
- 82.Sniderman AD, Faraj M. Apolipoprotein B, apolipoprotein A-I, insulin resistance and the metabolic syndrome. Curr Opin Lipidol. 2007;18(6):633–637. doi: 10.1097/mol.0b013e3282f0dd33. [DOI] [PubMed] [Google Scholar]
- 83.Sung KC, Hwang ST. Association between insulin resistance and apolipoprotein B in normoglycemic Koreans. Atherosclerosis. 2005;180(1):161–169. doi: 10.1016/j.atherosclerosis.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 84.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Walldius G, Hamsten A, et al. ApoB/apoA-I ratio: an independent predictor of insulin resistance in US non-diabetic subjects. Eur Heart J. 2007;28(21):2637–2643. doi: 10.1093/eurheartj/ehm360. [DOI] [PubMed] [Google Scholar]
- 85.Ulloque-Badaracco JR, Hernandez-Bustamante EA, Alarcon-Braga EA, Mosquera-Rojas MD, Campos-Aspajo A, Salazar-Valdivia FE, et al. Atherogenic index of plasma and coronary artery disease: a systematic review. Open Med. 2022;17(1):1915–1926. doi: 10.1515/med-2022-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchmann N, Ittermann T, Demuth I, Markus MRP, Völzke H, Dörr M, et al. Lipoprotein (a) and metabolic syndrome. Dtsch Arztebl Int. 2022;119(15):270–6. doi: 10.3238/arztebl.m2022.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cremonini AL, Pasta A, Carbone F, Visconti L, Casula M, Elia E, et al. Lipoprotein(a) modulates carotid atherosclerosis in metabolic syndrome. Front Mol Biosci. 2022;9:854624. doi: 10.3389/fmolb.2022.854624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategies. Table S2. Criteria for clinical diagnosis of metabolic syndrome used in the included studies. Table S3. Quality assessment of included studies. Figure S1. Subgroup analysis according to assay method of the association between ApoB levels and MetS. Figure S2. Subgroup analysis according to diagnostic criteria of the association between ApoB levels and MetS. Figure S3. Subgroup analysis according to sex of the association between ApoB levels and MetS. Figure S4. Subgroup analysis according to continents of the association between ApoB levels and MetS. Figure S5. Subgroup analysis according to study design of the association between ApoB levels and MetS. Figure S6. Sensitivity analysis according to risk of bias of the association between ApoB levels and MetS. Figure S7. Subgroup analysis according to assay method of the association between ApoA1 levels and MetS. Figure S8. Subgroup analysis according to diagnostic criteria of the association between ApoA1 levels and MetS. Figure S9. Subgroup analysis according to sex of the association between ApoA1 levels and MetS. Figure S10. Subgroup analysis according to continents of the association between ApoA1 levels and MetS. Figure S11. Subgroup analysis according to study design of the association between ApoA1 levels and MetS. Figure S12. Sensitivity analysis according to risk of bias of the association between ApoA1 levels and MetS. Figure S13. Subgroup analysis according to diagnostic criteria of the association between ApoB/ApoA1 ratio levels and MetS. Figure S14. Subgroup analysis according to gender of the association between ApoB/ApoA1 ratio levels and MetS. Figure S15. Subgroup analysis according to continents of the association between ApoB/ApoA1 ratio levels and MetS. Figure S16. Subgroup analysis according to study design of the association between ApoB/ApoA1 ratio levels and MetS. Figure S17. Sensitivity analysis according to risk of bias of the association between ApoB/ApoA1 ratio levels and MetS. Figure S18. Subgroup analysis according to assay method of the association between lipoprotein(a) levels and MetS. Figure S19. Subgroup analysis according to diagnostic criteria of the association between lipoprotein(a) levels and MetS. Figure S20. Subgroup analysis according to continents of the association between lipoprotein(a) levels and MetS. Figure S21. Subgroup analysis according to study design of the association between lipoprotein(a) levels and MetS. Figure S22. Sensitivity analysis according to risk of bias of the association between lipoprotein(a) levels and MetS. Figure S23. Funnel Plot of the studies that evaluated the association between ApoB and MetS. Figure S24. Funnel Plot of the studies that evaluated the association between ApoA1 and MetS. Figure S25. Funnel Plot of the studies that evaluated the association between ApoB/ApoA1 ratio and MetS. Figure S26. Funnel Plot of the studies that evaluated the association between Lipoprotein(a) and MetS.
Data Availability Statement
All data generated or analysed during the current study are included in this published article and its supplementary information files.