Abstract
Background:
There has been a worldwide surge in interventional procedures for low back pain (LBP), with studies yielding mixed results. These data support the need for identifying outcome predictors based on unique characteristics in a pragmatic setting.
Methods:
We prospectively evaluated the association between over 2 dozen demographic, clinical and technical factors on treatment outcomes for 3 procedures: epidural steroid injections (ESI) for sciatica, and sacroiliac joint (SIJ) injections and facet interventions for axial LBP. The primary outcome was change in patient-reported average pain intensity on a numerical rating scale (average NRS-PI) using linear regression. For SIJ injections and facet radiofrequency ablation, this was average LBP score at 1- and 3-months post-procedure, respectively. For ESI, it was leg pain 1-month post-injection. Secondary outcomes included a binary indicator of treatment response (success).
Results:
346 patients were enrolled at 7 hospitals. All groups experienced a decrease in average NRS-PI (p < 0.0001; mean 1.8 ± 2.6). There were no differences in change in average NRS-PI among procedural groups (p = 0.50). Lower baseline pain score (adjusted coefficient −0.32, 95% CI −0.48to −0.16, p<0.0001), depressive symptomatology (0.076, 95% CI 0.039 to 0.113, p<0.0001) and obesity (0.62, 95% CI 0.038 to 1.21, p=.037) were associated with smaller pain reductions. For procedural outcome, depression (adjusted OR 0.94, 95% CI 0.91, 0.97, p<.0001) and poorer baseline function (adjusted OR 0.59, 95% CI 0.36, 0.96, p=.034) were associated with failure. Smoking, sleep dysfunction and non-organic signs were associated with negative outcomes in univariate, but not multivariate analyses.
Conclusions:
Identifying treatment responders is a critical endeavor for the viability of procedures in LBP. Patients with greater disease burden, depression and obesity are more likely to fail interventions. Steps to address these should be considered before or concurrent with procedures as considerations dictate.
Keywords: Epidural steroid injection, low back pain, lumbar facet block, radiofrequency ablation, sacroiliac joint injection, phenotype
Introduction
Low back pain (LBP) is the leading cause of years lost to disability worldwide, and in about two-thirds of individual countries.1 In a systematic review, Hoy et al. found the point prevalence to be 18%, annual prevalence to be 38% and the lifetime prevalence to exceed 40%.2 There are no reliable treatments for LBP, with many patients receiving injections. In the U.S. fee-for-service Medicare population alone, over 5,600,000 interventional pain procedures were performed in 2018, with a majority done for LBP.3,4 The three most common procedures are epidural steroid injections (ESI), facet interventions and sacroiliac joint (SIJ) injections.3
There are wide variations in reported success rates for interventional lumbar spine procedures that depend on outcome measures, study design, the specialty of those performing the procedure and the population studied, but in general geographic non-surgical lumbar procedural volumes are strongly correlated with surgical rates.5 This strongly suggests the need for better patient selection, which would improve outcomes, reduce risks,6 and save millions of dollars for procedures unlikely to provide long-term benefit.
Personalized medicine involves the utilization of an individual’s unique clinical and psychosocial profile to make informed decisions about treatments. Instead of a biomedical approach wherein medical treatments, including procedures, are generically applied in a symptom or disease-based context (e.g. ESI for sciatica), a personalized approach operates in the framework of a biopsychosocial model, focusing on individualized aspects of the pain experience.7
The wide variations in outcomes for lumbar spine interventions signal a strong need for studies focused on identifying likely responders, which would favorably alter the risk: benefit ratio. Clinical trials may provide a window into traits associated with treatment response, but typically enroll a homogenous population and thus tend to be poorly generalizable. Yet to date, there have been few large-scale studies that sought to determine factors associated with treatment outcome, with most concentrating on clinical signs and symptoms and radiological pathology, with all focused on a single procedure.
Previously, our group published 2 articles examining the correlation between specific variables that have not been previously evaluated for their influence on LBP procedural outcomes (hypersensitivity reactions for ESI and nonorganic signs for ESI, facet interventions and SIJ injections) as pilot studies during planned interim analyses.6,13 Herein, we present the complete dataset on the effect of over two dozen clinical, psychosocial, technical, and conditionspecific variables (e.g. physical exam signs) on interventional treatment outcomes for chronic LBP. Our objectives were to determine which factors are associated with treatment outcome and quantify the magnitude of effect which would guide pain interventionalists and referring providers in selecting candidates for LBP procedures.
Patients & Methods
Permission to conduct this multi-center, cohort study was granted by the Institutional Review Boards of Johns Hopkins School of Medicine (IRB00050132), Drexel University, Walter Reed National Military Medical Center, Puget Sound Veterans Affairs (VA) Hospital, Seattle, WA, Landstuhl Regional Medical Center, Landstuhl, Germany, Naval Medical Center-San Diego and Womack Army Medical Center, Fort Bragg, NC. The study was registered on clinicaltrials.gov on 1 January 2015 (ClinicalTrials.gov Identifier: NCT02329951), with all participants providing informed consent. All enrollments, treatments and follow-up visits took place between January 13, 2015 and May 30, 2021. Detailed descriptions of the procedures have previously been published in manuscripts outlining 2 planned interim analyses.6,13
Participants and Settings
This study was performed at 2 civilian, 4 military and 1 VA pain treatment centers, 4 of which had pain medicine training programs. Selection criteria were age ≥ 18 years; > 6 weeks pain duration; lumbosacral radicular pain or mechanical LBP of presumed facetogenic or sacroiliac joint etiology;8,14 candidate for an ESI, lumbar facet block or SIJ injection and agreement to undergo that injection; and average leg or LBP pain score ≥ 4/10 over the past week for participants with radicular and non-radicular pain, respectively. Excluded from participation were individuals who had received injections within the previous 2 years; with inflammatory arthritis; prior spine surgery for patients undergoing facet blocks and ESI; hypersensitivity reaction to bupivacaine, contrast or corticosteroid; coagulopathy precluding treatment; and pregnancy.
Procedures
All procedures were performed using fluoroscopic guidance by a board-certified pain medicine physician or trainee under their direct supervision. Procedures were done in accordance with standard protocols and were previously described in detail.6,8,12,13 After a 1 mL skin wheal was created with a 25-gauge needle using lidocaine 1%, the soft tissue in the impending needle track(s) was anesthetized using up to 10 mL of lidocaine 1%, as clinically indicated.
Sacroiliac Joint Injections
SIJ injections were accomplished by inserting 22-gauge needles into the inferior one-third of the joint as previously described.6,15 If the joint capsule was not visualized, the needle was readjusted at the physician’s discretion considering the comparable prevalence of intra- and extra-articular pathology and evidence for benefit.15 Once needle position was deemed sufficient, a 3 mL solution containing 1 mL of 40 mg/mL of depo-methylprednisolone and 2 mL of 0.5% bupivacaine was injected. A positive diagnostic block was considered ≥ 50% pain reduction lasting at least 3 hours based on pain diaries with activity logs.
Facet Interventions
Medial Branch Blocks
The targets for facet interventions were based on historical and physical exam findings, pain referral patterns, and radiological imaging when available.6,8,16 22-gauge needles were inserted between the sacral ala and articular process for the L5 dorsal ramus, and just below the junction of the upper transverse process and the superior articular process for L1–4 medial branch blocks (MBB) with real-time contrast injection.6,16 At each nerve, 0.5 mL of 0.5% bupivacaine was administered. A block was designated as positive if the patient experienced ≥ 50% pain reduction lasting at least 3 hours.
Lumbar Medial Branch Radiofrequency Ablation
RFA was performed on participants who had positive diagnostic MBB, but failed to experience > 3 months pain relief in accordance with previous protocols.6,16 Procedures were performed with 18- or 20-gauge (used in some procedures before January 2019) curved radiofrequency needles with 10 mm- active tips situated near-parallel to target nerves.6,16 At each target, electrodes were adjusted to optimize sensory (ideally < 0.6 volts) stimulation and maximize paraspinal muscle contraction. After appropriate placement, 1 mL of 2% lidocaine was injected. Ablation was implemented at 90° for 135 seconds using a radiofrequency generator after which a 0.5 mL mixture containing10 mg depo-methylprednisolone and saline was administered to prevent neuritis.8
Epidural Steroid Injections
Our technique for ESI has previously been described for other trials.6,13,17 A single-level transforaminal ESI (TFESI) was performed in individuals with unilateral pain, while interlaminar ESI (ILESI) were done for bilateral symptoms. Injections were performed at the level thought to be most responsible for pathology unless anatomical considerations dictated otherwise, with real-time contrast confirmation. For transforaminal ESI, a 3 ml of a solution containing 10 mg of dexamethasone, saline and 1 ml of 0.25 % bupivacaine was administered. ILESI were performed midline in individuals with symmetrical pain or parasagitally when one side was more painful than the other. After position was confirmed, a 4 mL solution containing 40 mg depomethylprednisolone, 1 mL of 0.25% bupivacaine and 2 mL of saline was injected.
Follow-Up
No co-interventions between procedures and follow-up visits were permitted, with the exception of negative MBB (small likelihood of benefit) or short-term relief from RFA (> 1 month until 3-month follow-up; see supplemental table 1). Rescue medications provided were nonsteroidal anti-inflammatory drugs, tramadol when NSAIDs were contraindicated or ineffective, or a < 20% increase in opioid dose in participants already receiving opioids. The first follow-up visit was 1 month after the ESI, SIJ injection or lumbar medial branch RFA. Participants who experienced a positive binary outcome at their 1-month follow-up visit returned for the final 3-month follow-up visit, while those with a negative outcome exited to receive other treatments. In participants who experienced prolonged benefit (a positive categorical outcome) from the MBB, the initial data collection occurred 1-month after the MBB. In individuals with a positive 1-month facet outcome whose pain returned shortly thereafter and requested treatment, data was recorded before treatment was implemented and carried forward to 3 months.
Data Collection
At all follow-up visits, a disinterested observer collected data via in-person, telemedicine, MyChart (after March 2020) or telephone visits. Baseline data collected included demographic information; general (inciting event, type of injection, laterality) and condition-specific clinical data (e.g. exam findings); treatment-specific data (e.g. categorization of SIJ injection as intra- or extra-articular, type of ESI performed); concomitant pain conditions; number and type of analgesic medications including opioid use stratified by mean morphine equivalents per day (MME); smoking status; co-existing active psychiatric diagnoses; ongoing secondary financial gain (disability, litigation or Worker’s compensation case); average and worst back and leg (for ESI patients) pain score on a 0–10 numerical rating scale (NRS) over the past week; number and type of non-organic (Waddell) signs;6 expectations; Oswestry Disability Index version 2.1 (ODI) score; Athens Insomnia Scale (AIS) to measure sleep quality; and Quick Inventory of Depressive Symptomatology (QIDS) to measure mood. For ODI, AIS and QIDS, higher scores represent greater dysfunction.
Pre-procedure expectations were measured on a 6-point Likert scale based on the degree of improvement the participant would be satisfied with (1= not expecting pain relief, but trying anyway; 2= any improvement, 3= 30%-49% improvement, 4=50%-74% improvement, 5=75%-99% improvement, 6=only complete resolution of all symptoms). Condition-specific provocative tests evaluated included straight leg raising test for individuals receiving ESI, and Gaenslen’s and Patrick’s tests before SIJ injections. At the time of the procedure (MBB but not RFA), a subcutaneous skin wheal was created by a 1 mL lidocaine injection using a 25-gauge needle, immediately after which the pain intensity of the injection was assessed via a 0–10 verbal rating scale. After the procedure, procedure-related was measured using the same verbal rating scale. These data were collected because all are associated with LBP procedure outcomes.17–20
Outcome Measures
The primary outcome measure was change in average LBP or leg pain (for ESI) score over the past week. The primary endpoint was 1-month post-injection for MBB, ESI and SIJ injections, and 3 months after lumbar medial branch RFA (or MBB in those with prolonged pain relief). Specific scenarios for individuals with a negative MBB, prolonged relief after their MBB, or relief after RFA lasting 1 but not 3 months, are noted in supplemental table 1. Hereafter, average NRS-PI (pain intensity) refers to the primary outcome measure at the primary endpoint.
A positive categorical response for procedure success was pre-designated as a 2-point or greater decrease in the NRS-PI not attributable to starting a rescue medication in conjunction with a score of greater than 3 on a 5-point Likert scale measuring procedural satisfaction (1=very unsatisfied, 2=unsatisfied, 3=neither satisfied nor unsatisfied, 4=satisfied and 5=very satisfied). At each follow-up, along with average pain scores, secondary outcome measures recorded included worst pain scores over the past week were recorded, along with ODI 2.1, satisfaction score, and medication reduction, defined per previous studies to be > 20% decrease in opioid use or cessation of a non-opioid analgesic.13,15,16
Statistical Analysis
Sample size calculation and power analysis were performed for the binary categorical outcome because of differences in effect size for the 3 procedures.21 For the power analysis, we assumed an overall procedural success rate of 45%, that approximately 7 independent variables would be included in the final mode and that at least 10 subjects would be needed for each variable.22 In order to accrue at a minimum of 20 positive outcomes for each variable, we estimated that 311 patients would be required. Assuming a procedural success rate of 45% and a standard deviation of 2.5 points in NRS-PI in both groups, we estimated a sample size of 311 patients would be 94% powered to detect a 1.0-point minimal clinically important difference between responders and non-responders in NRS-PI at the primary endpoint, at a significance level of 0.05.23 Accounting for dropouts, we planned to enroll 346 participants.
All statistical analyses were performed in Stata/IC 16.1 (StataCorp, USA). For continuous outcomes, group means and standard deviations were reported, and analysis of variance (ANOVA) was used to compare the three procedural groups (facets, SIJ, and ESI). For categorical outcomes, percentages were reported and χ2 tests were used to compare the three procedural groups. For comparisons between two groups, a p-value less than alpha of 0.05 was considered statistically significant. For comparisons among three or more groups, a p-value less than the Bonferroni-corrected alpha of 0.05/n, where n is the number of between-group comparisons, was judged as a conservative estimate of statistical significance.
The primary outcome, average NRS-PI was analyzed using an intention-to-treat approach, with the last observation carried forward (including from baseline for those who missed 1-month visits) in the case of missing data. For each treatment group, average baseline NRS and average NRS-PI were compared using paired t-tests, with means, standard deviations, and 95% confidence intervals reported for the baseline NRS-PI, primary endpoint NRS-PI, and difference. A subset of the facet intervention patients, those who underwent RFA, underwent a similar analysis. Change in average and worst NRS-PI, as well as change in ODI were compared using three-way ANOVA.
For the categorical response of treatment outcome, demographic, patient-specific, and procedure-specific variables were reported as above and compared between the procedural failure and procedural success groups. ANOVA was used for continuous variables, and χ2 tests were used for categorical variables.
To identify potential outcome predictors, we performed two multivariate analyses. For the first analysis, change in average NRS-PI was modeled as a function of 10 candidate variables selected as most likely to influence outcome based on a combination of our univariate analyses, clinical knowledge and review of relevant literature: age, duration of pain, obesity, smoking status, opioid use, sleep disturbance (Athens Insomnia Scale), depression (QIDS), nonorganic signs (Waddell), ODI, and baseline NRS-PI. Each candidate variable was modeled individually in a simple linear regression (unadjusted), then as a multivariable linear regression model using all 10 variables (full model). To identify the variables most likely to impact the outcome of interest, the multivariable linear regression model was simplified, starting with the full model and eliminating variables using a backward stepwise approach. A two-sided p-value less than 0.05 was considered to indicate statistical significance. A nonparametric bootstrap method using 100 resamples was used to internally validate the proposed regression model. Similarly, for the second exploratory analysis, treatment outcome was modeled using logistic regression, using the same 10 candidate variables identified above. Odds ratios were reported for the unadjusted analyses, full model, and a simplified model developed using a backward stepwise approach. Again, a two-sided p-value less than 0.05 was considered to indicate statistical significance and a nonparametric bootstrap method using 100 resamples was used to internally validate the proposed model.
Results
A total of 346 patients were enrolled: 112 underwent lumbar facet blocks, 67 received SIJ injections, and 167 underwent lumbar ESI (81 transforaminal, 85 interlaminar). Of these patients, 331 were followed up at 1 month (Figure 1). For facet block patients who proceeded to RFA, 58 were followed up at 1 month, and 38 were followed 3 months after RFA.
Figure 1.
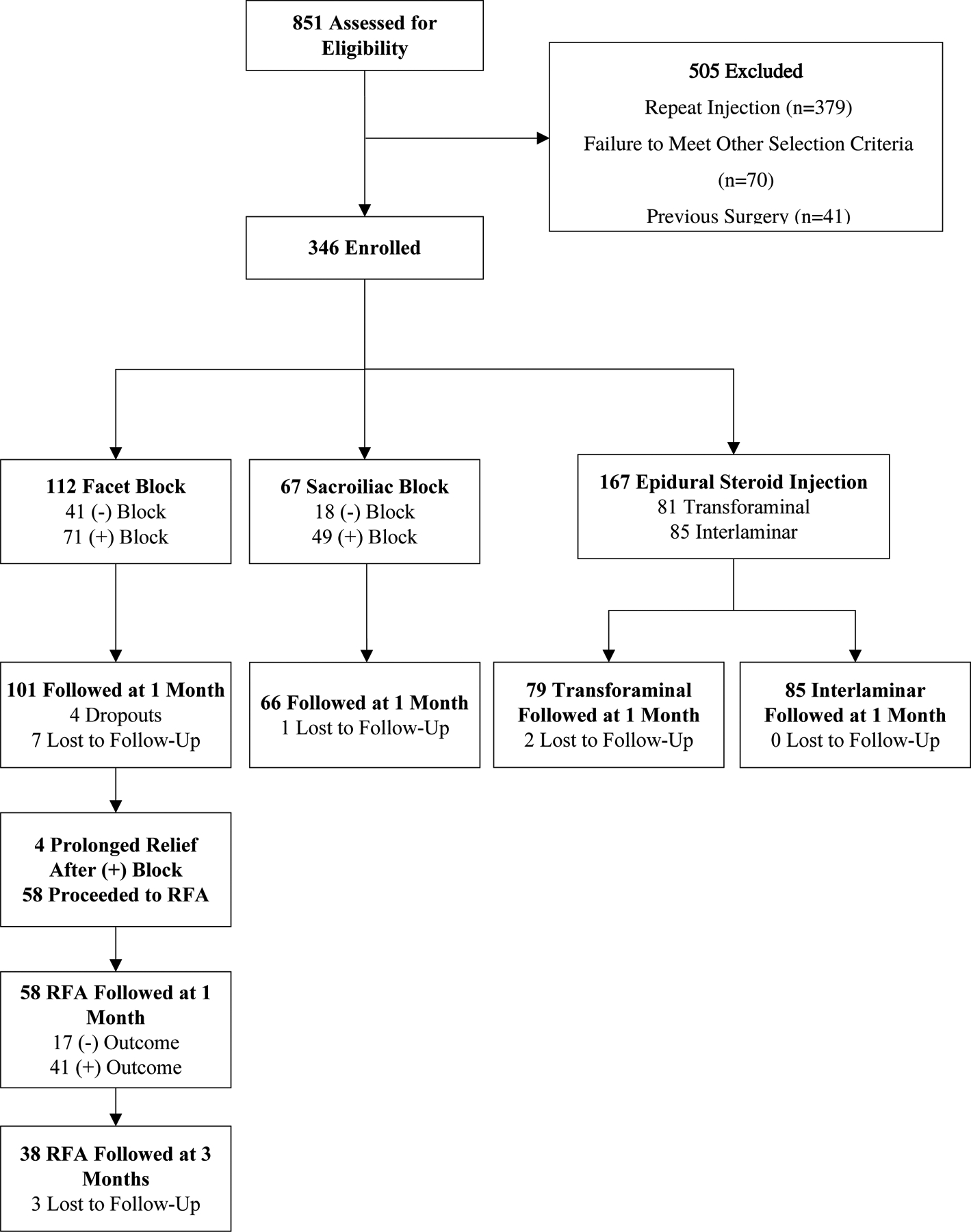
Study flow chart
Baseline Characteristics
Table 1 shows baseline demographic and clinical characteristics of the cohort. Participants in the facet group were significantly more likely to undergo bilateral procedures, have a co-existing chronic pain condition, and reported lower expectations for pain relief. The ESI group was significantly more likely to be receiving neuropathic adjuvants, less likely to be in the military, demonstrated more nonorganic signs, had slightly higher ODI scores, and reported lower mean procedure-related pain during both the skin wheal and the procedure itself. There were no significant differences in other variables.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Subjects
| Characteristic | Facet Interventions (N=112) | Sacroiliac Joint Injections (N=67) | Epidural Steroid Injection (N=167) | Total Cohort (N=346) | P-Value |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 51 ± 14 | 53 ± 16 | 52 ± 14 | 52 ± 14 | 0.68 |
| Male | 59 (52.7%) | 24 (35.8%) | 78 (46.7%) | 185 (53.5%) | |
| Duration of Pain, years (mean ± SD) | 7 ± 7 | 5 ± 6 | 5 ± 7 | 6 ± 7 | 0.057 |
| Obesity (n, %) | 54 (48.2%) | 27 (40.3%) | 74 (44.3%) | 155 (44.8%) | 0.58 |
| Smoking (n, %) | 24 (21.4%) | 10 (14.9%) | 47 (28.1%) | 81 (23.4%) | 0.081 |
| Bilateral Procedure (n, %) | 66 (60.0%) | 25 (37.9%) | 59 (35.3%) | 150 (43.7%) | < 0.001 |
| Inciting Event (n, %) | 41 (36.6%) | 31 (46.3%) | 52 (31.1%) | 124 (35.8%) | 0.091 |
| Opioid Use (n, %) | 21 (18.8%) | 13 (19.4%) | 30 (18.0%) | 64 (18.5%) | 0.96 |
| Tramadol | 16 (14.3%) | 7 (10.4%) | 26 (15.6%) | 49 (14.2%) | 0.60 |
| Active Duty (n, %) | 27 (24.1%) | 14 (20.9%) | 15 (9.6%) | 57 (16.5%) | 0.013 |
| Disability, Worker’s Compensation or Medical Board2 (n, %) | 25 (22.9%) | 16 (24.6%) | 48 (29.6%) | 89 (26.5%) | 0.44 |
| Multiple Pain Conditions | 13 (11.6%) | 9 (13.4%) | 22 (13.2%) | 44 (12.7%) | 0.91 |
| Multiple | 15 (13.4%) | 5 (7.5%) | 26 (15.6%) | 46 (13.3%) | 0.26 |
| Nonorganic Signs (mean ± SD) 5 | 0.5 ± 1.0 | 0.6 ± 1.2 | 0.9 ± 1.3 | 0.7 ± 1.2 | 0.035 |
| Expectations (mean ± SD) 6 | 3.6 ± 1.3 | 3.9 ± 1.4 | 4.0 ± 1.3 | 3.9 ± 1.3 | 0.047 |
| Athens Insomnia Scale (mean ± SD) | 9 ± 5 | 9 ± 6 | 10 ± 6 | 9 ± 5 | 0.28 |
| Quick Inventory of Depressive Symptomatology (QIDS; mean ± SD) | 11 ± 7 | 11 ± 9 | 12 ± 8 | 12 ± 8 | 0.46 |
| Oswestry Disability Score (mean ± SD) | 36 ± 15 | 37 ± 17 | 41 ± 17 | 39 ± 16 | 0.030 |
| Procedure | 5.5 ± 2.7 | 6.4 ± 2.7 | 4.9 ± 3.0 | 5.4 ± 2.9 | 0.001 |
| Average NRS Back Pain Score (mean ± SD) | 6.0 ± 1.6 | 6.2 ± 1.8 | 5.9 ± 2.3 | 6.0 ± 2.0 | 0.46 |
| Worst NRS Back Pain Score (mean ± SD) | 8.7 ± 1.3 | 8.6 ± 1.4 | 8.3 ± 2.5 | 8.5 ± 1.9 | 0.23 |
| Average NRS Leg Pain Score (mean ± SD) | 5.9 ± 2.0 | ||||
| Worst NRS Leg Pain Score (mean ± SD) | 8.5 ± 1.6 |
NRS- numerical rating scale
Neuropathic adjuvant includes anticonvulsants and antidepressants. Nonsteroidal anti-inflammatory drug also includes acetaminophen. Other non-opioid analgesic includes topical agents such as lidocaine and capsaicin.
Military equivalent of civilian disability.
Denotes active condition (i.e., being treated); other includes post-surgical pain, plantar fasciitis, post-stroke pain, and pain secondary to systemic disease (e.g., lupus, sickle cell anemia).
Denotes active condition (i.e., being treated) except for substance abuse (ongoing recovery); other includes somatic symptom disorder, personality disorder, etc.
Non-organic signs based on 5 categories of Waddell signs.
Expectations based on 6-point Likert scale (1= don’t expect any relief, 6=would only be satisfied with complete pain relief and restoration of function).
Procedural Outcomes
For all procedures (facet block, RFA, SI, and ESI), there was a significant decrease in mean pain NRS pain score at the primary pain location from baseline to the primary endpoint (1 month for facet block, SI, and ESI; 3 months for RFA) (Figure 2). Mean decrease in average NRS pain score was 1.2 ± 2.4 (95% CI 0.8 to 1.7, p < 0.0001) for facet interventions, 2.0 ± 2.5 (95% CI 1.3 to 2.6, p < 0.0001, includes all MBB and RFA) for RFA, 1.8 ± 2.5 (95% CI 1.2 to 2.4, p < 0.0001) for SI, and 1.5 ± 2.7 (95% CI 1.1 to 1.9, p < 0.0001) for ESI. Among the 139 patients with a successful outcome, the mean reductions in NRS-PI for facet interventions, SI injection, and ESI were 4.0 ± 1.5, 3.6 ± 2.0, 3.6 ± 1.9, respectively. Procedural outcomes are summarized in Table 2. There were no significant differences among the RFA, SI, and ESI groups for change in average or worst back/leg pain scores or ODI.
Figure 2.
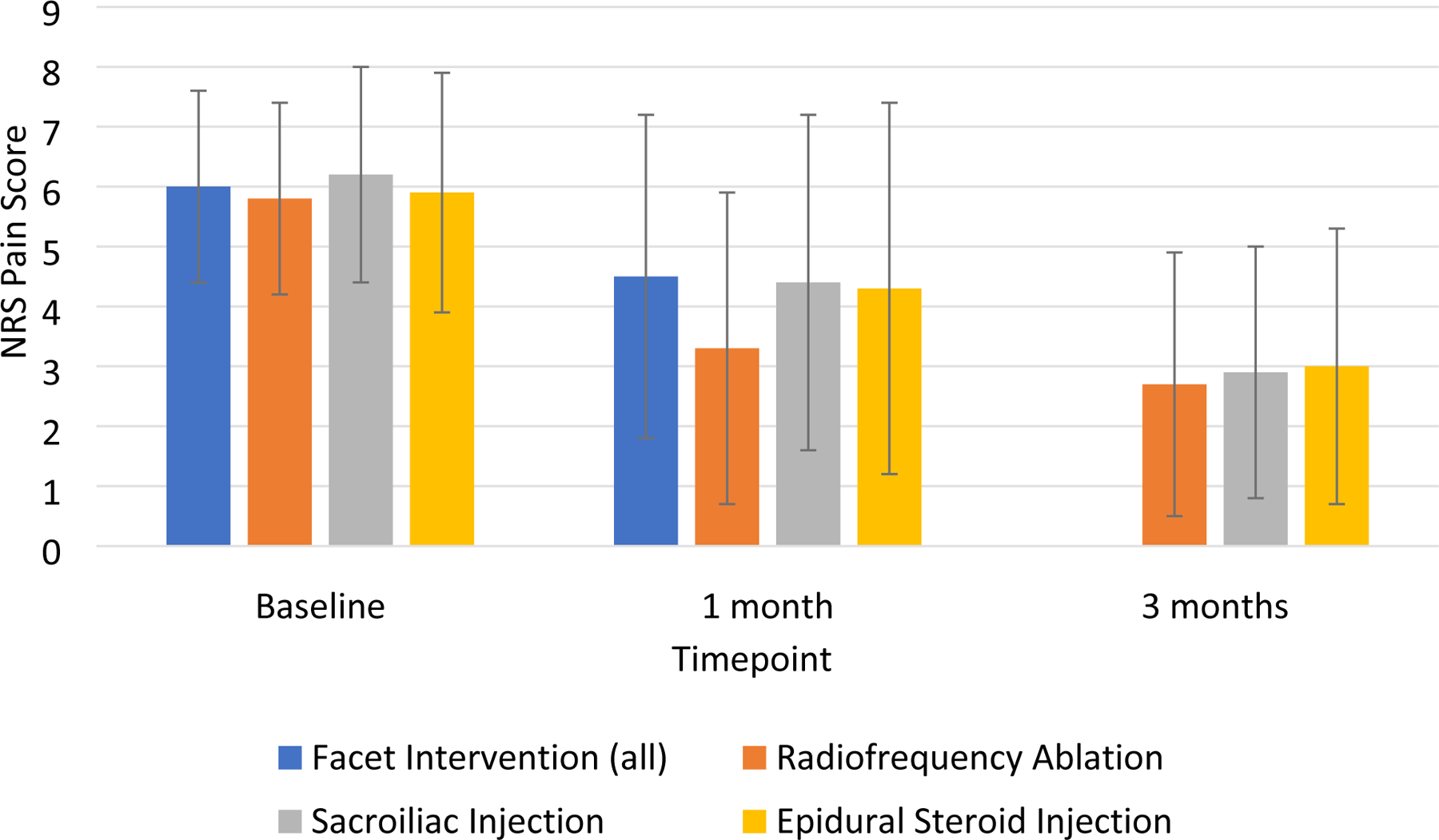
Average numerical rating scale pain score at primary pain location (low back for facet and SI, leg for ESI) over time. Note that “Facet Intervention (all)” includes both medial branch blocks (including negative blocks) and RFA patients. Patients who did not undergo RFA were not required to follow-up at 3 months.
Table 2.
Procedural Outcomes
| Outcome | Facet Interventions | Sacroiliac Joint Injections (N=67) | Epidural Steroid Injection (N=167) | P-Value2 | Total Cohort (N=346) | |
|---|---|---|---|---|---|---|
| All Facet Interventions1 (N=112) | Radiofrequency Ablation (N=58) | |||||
| Change in Average NRS Back Pain Score (mean ± SD) | −1.2 ± 2.4 | −1.9 ± 2.5 | −1.8 ± 2.5 | −1.1 ± 2.4 | 0.022 | −1.6 ± 2.5 |
| Change in Worst NRS Back Pain Score (mean ± SD) | −1.6 ± 2.5 | −2.4 ± 2.7 | −2.4 ± 2.9 | −1.7 ± 3.0 | 0.14 | −2.1 ± 2.9 |
| Change in Average NRS Leg Pain Score (mean ± SD) | −1.5 ± 2.7 | |||||
| Change in Worst NRS Leg Pain Score (mean ± SD) | −2.4 ± 3.0 | |||||
| Change in Average NRS Primary Location Pain Score (mean ± SD) 3 | −1.2 ± 2.4 | −2.0 ± 2.5 | −1.8 ± 2.5 | −1.5 ± 2.7 | 0.50 | −1.8 ± 2.6 |
| Change in Worst NRS Primary Location Pain Score (mean ± SD) 3 | −1.6 ± 2.5 | −2.4 ± 2.7 | −2.4 ± 2.9 | −2.4 ± 3.0 | 1.00 | −2.5 ± 2.9 |
| Change in Oswestry Disability Score (mean ± SD) | −4 ± 12 | −7 ± 11 | −7 ± 13 | −4 ± 15 | 0.29 | −5 ± 14 |
NRS- numerical rating scale. Differences are listed as change from baseline, with negative numbers denoting a decrease in NRS pain score.
Includes individuals with negative diagnostic medial branch blocks.
P-value is for comparison of radiofrequency ablation, sacroiliac joint injections, and epidural steroid injections (excludes medial branch blocks).
Primary location is back for facet and sacroiliac joint injections, leg for epidural steroid injections.
Factors Associated with Treatment Outcome
A comparison of clinical and demographic characteristics stratified by procedural outcome is shown in Table 3. In univariate analyses, patients having successful treatment outcomes were significantly more likely to be older, have shorter duration of pain, be receiving non-steroidal anti-inflammatory drugs, and have other chronic pain conditions (e.g. post-surgical pain, plantar fasciitis, pain secondary to systemic disease). They were significantly less likely to be obese, smoke, undergo bilateral procedures, be on disability/Worker’s compensation/medical board, have any or multiple co-existing psychiatric conditions, most prominently depression. As a group, patients with treatment success had fewer nonorganic signs, lower Athens Insomnia Scale, QIDS and ODI scores, reported less procedure-related pain, and baseline average and worst pain scores compared to patients who failed treatment. Procedure type, sex, presence of an inciting event, opioid use, co-existing chronic pain conditions, patient expectations for pain relief, and pain due to skin wheal were not associated with outcome.
Table 3.
Demographic and Clinical Factors Associated with Treatment Outcome
| Variable | Procedural Failure (N=193) | Procedural Success (N=139)1 | P-Value |
|---|---|---|---|
| Procedure (n, % of procedure type) | |||
| Facet Interventions2 | 68 (67.3%) | 33 (32.7%) | |
| Radiofrequency Ablation | 29/58 (50%) | 29/58 (50%) | |
| Sacroiliac Joint Injection | 33 (50%) | 33 (50%) | 0.0583 |
| Epidural Steroid Injection | 92 (55.8%) | 73 (44.2%) | |
| Transforaminal | 38/79 (48.1%) | 41/79 (51.9%) | |
| Interlaminar | 53/85 (62.4%) | 32/85 (37.6%) | |
| Age, years (mean ± SD) | 50 ± 13 | 54 ± 16 | 0.019 |
| Sex (n, %) | |||
| Female | 108 (56.0%) | 71 (51.1%) | 0.38 |
| Male | 85 (44.0%) | 68 (48.9%) | |
| Duration of Pain, years (mean ± SD) | 6.7 ± 7.7 | 4.7 ± 5.7 | 0.010 |
| Obesity (n, %) | 97 (50.3%) | 54 (38.8%) | 0.039 |
| Bilateral Procedure | 86 (45.3%) | 53 (38.1%) | 0.039 |
| Smoking (n, %) | 59 (30.6%) | 19 (13.7%) | < 0.001 |
| Inciting Event | 76 (39.4%) | 44 (31.7%) | 0.15 |
| Opioid Use (n, %) | 42 (21.8%) | 22 (15.8%) | 0.18 |
| Nonopioid Use 4 | |||
| Any Adjuvant | 138 (71.5%) | 112 (80.6%) | 0.059 |
| Neuropathic Adjuvant | 61 (31.6%) | 35 (25.2%) | 0.20 |
| Non-Steroidal Anti-Inflammatory Drug | 87 (45.1%) | 82 (59.0%) | 0.012 |
| Muscle Relaxant | 29 (15.0%) | 30 (21.6%) | 0.12 |
| Other Non-Opioid Analgesic | 15 (7.8%) | 14 (10.1%) | 0.46 |
| Tramadol | 32 (16.6%) | 16 (11.5%) | 0.20 |
| Active Duty (n, %) | 31 (16.1%) | 23 (16.6%) | 0.12 |
| Disability, Worker’s | |||
| Compensation or Medical Board5 (n, %) | 61 (32.4%) | 26 (19.4%) | 0.009 |
| Co-Existing Chronic Pain Conditions (n, %) 6 | |||
| Any Pain Condition | 122 (63.2%) | 80 (57.6%) | 0.30 |
| Neck Pain | 32 (16.6%) | 23 (16.5%) | 0.99 |
| Headache | 17 (8.8%) | 10 (7.2%) | 0.60 |
| Arthralgia | 73 (37.8%) | 49 (35.3%) | 0.63 |
| Neuropathy | 19 (9.8%) | 15 (10.8%) | 0.78 |
| Abdominal/Pelvic Pain | 7 (3.6%) | 1 (0.7%) | 0.088 |
| Fibromyalgia | 8 (4.1%) | 6 (4.3%) | 0.94 |
| Other Chronic Pain7 | 20 (10.4%) | 5 (3.6%) | 0.021 |
| Multiple Pain Conditions | 28 (14.5%) | 15 (10.8%) | 0.32 |
| Co-Existing Psychiatric Condition (n, %) 8 | |||
| Any Psychiatric Condition | 96 (49.7%) | 49 (35.3%) | 0.009 |
| Anxiety | 47 (24.4%) | 25 (18.0%) | 0.16 |
| Depression | 56 (29.0%) | 23 (16.5%) | 0.008 |
| Posttraumatic stress disorder | 11 (5.7%) | 3 (2.2%) | 0.11 |
| Substance abuse | 22 (11.5%) | 7 (5.1%) | 0.043 |
| Other | 12 (6.2%) | 4 (2.9%) | 0.16 |
| Multiple Psychiatric Conditions | 33 (17.1%) | 13 (9.4%) | 0.044 |
| Nonorganic Signs (mean, SD) 9 | 0.9 ± 1.3 | 0.5 ± 1.0 | 0.004 |
| Expectations (mean ± SD) 10 | 3.9 ± 1.3 | 3.8 ± 1.3 | 0.31 |
| Athens Insomnia Scale (mean ± SD) | 10 ± 5 | 8 ± 5 | < 0.001 |
| Quick Inventory of Depressive Symptomatology (QIDS; mean ± SD) | 13 ± 8 | 9 ± 7 | < 0.001 |
| Oswestry Disability Score (mean ± SD) | 42 ± 17 | 35 ± 15 | < 0.001 |
| Procedure-Related Pain (mean, SD | |||
| Skin wheal | 4.5 ± 2.7 | 4.1 ± 2.7 | 0.21 |
| Procedure | 5.9 ± 3.0 | 4.6 ± 2.6 | < 0.001 |
| Average Baseline Back Pain Score (mean ± SD) | 6.3 ± 2.0 | 5.6 ± 2.0 | 0.002 |
| Worst Baseline Back Pain Score (mean ± SD) | 8.8 ± 1.7 | 8.1 ± 2.2 | 0.001 |
| Average Baseline Leg Pain Score (mean ± SD) 11 | 6.2 ± 2.0 | 5.4 ± 1.8 | 0.006 |
| Worst Baseline Leg Pain Score (mean ± SD) 11 | 8.8 ± 1.6 | 8.1 ± 1.6 | 0.005 |
Procedural success defined as ≥ 2-point decrease in baseline low back pain score at 1-month after sacroiliac joint injection or 3-months after radiofrequency ablation (or blocks for patients with sustained relief) for lumbar facet joint pain coupled with > 3/5 on a Likert satisfaction scale.
Negative facet interventions include negative RFA outcome and negative facet blocks who did not obtain long-term relief (n=1).
Comparison among procedural success for facet RFA at 3 months, SI at 1 month, and ESI at 1 month.
Neuropathic adjuvants include anticonvulsants and antidepressants. Nonsteroidal anti-inflammatory drug also includes acetaminophen. Other non-opioid analgesic includes topical agents such as lidocaine and capsaicin.
Military equivalent of civilian disability.
Denotes active condition (i.e., being treated).
Other pain conditions include plantar fasciitis, central pain, persistent postsurgical pain and atypical chest pain.
Denotes active condition (i.e., being treated) except for substance abuse.
Non-organic signs based on 5 categories of Waddell signs.
Expectations based on a 6-point Likert scale.
Leg pain score only recorded for epidural steroid injections.
Tables 4 and 5 show procedure-specific characteristics for treatment failure versus success. For facet interventions, both ≥ 50% and ≥80% pain relief from diagnostic block were significantly associated with procedural success. For SI, ≥ 50–79% pain relief immediately following the procedure was associated with treatment success, while ≥ 80% relief was actually associated with a lower success rate (52% vs. 39%). Physical exam findings and history of prior surgery were not significantly different between SIJ injection successes and failures. Similarly, physical exam findings and spinal pathology were not significantly different between patients with procedural success versus failure in the ESI group.
Table 4.
Exploration of Factors Associated with Procedural Outcome for Therapeutic Procedures
| Variable | Unadjusted Odds Ratio (95% CI)1 | P-Value | Adjusted Odds Ratio, Full Model (95% CI)1 | P-Value | Adjusted Odds Ratio, Revised Model (95% CI)1 | P-Value |
|---|---|---|---|---|---|---|
| Age, years | 1.02 (1.00, 1.03) | 0.039 | 1.02 (1.00, 1.04) | 0.024 | ||
| Duration of Pain, years | 0.96 (0.93, 1.00) | 0.051 | 0.96 (0.93, 1.00) | 0.072 | ||
| Obesity | 0.58 (0.36, 0.92) | 0.022 | 0.62 (0.37, 1.03) | 0.066 | ||
| Smoking | 0.41 (0.23, 0.74) | 0.003 | 0.64 (0.33, 1.24) | 0.19 | ||
| Any Daily Opioid Use | 0.68 (0.42, 1.12) | 0.13 | 0.75 (0.42, 1.35) | 0.34 | ||
| Athens Insomnia Scale (mean ± SD) | 0.93 (0.89, 0.97) | 0.002 | 1.01 (0.95, 1.09) | 0.66 | ||
| Quick Inventory of Depressive Symptomatology (QIDS; mean ± SD) | 0.94 (0.91, 0.97) | <0.0001 | 0.96 (0.91, 1.00) | 0.077 | 0.94 (0.91, 0.97) | <0.0001 |
| Nonorganic Signs, number | 0.73 (0.59, 0.90) | 0.004 | 0.94 (0.72, 1.22) | 0.63 | ||
| Oswestry Disability Score (mean ± SD) | 0.97 (0.96, 0.99) | <0.0001 | 0.99 (0.97, 1.01) | 0.31 | 0.59 (0.36, 0.96) | 0.034 |
| Average Baseline NRS Primary Pain Location Score 2 | 0.86 (0.76, 0.98) | 0.019 | 0.94 (0.80, 1.10) | 0.41 |
Odds ratios are reported as likelihood of procedural success with presence (for categorical variables) or each single unit increase (for continuous variables) in the specified variable, with odds ratios greater than 1 being associated with increased likelihood of procedural success.
Primary location is back for facet and SI injections, leg for ESI.
Table 5.
Procedure-Specific Clinical Variables Associated with Treatment Outcome
| Clinical Variable | Procedural Failure (N=193) | Procedural Success (N=139)1 | P-Value |
|---|---|---|---|
| Epidural Steroid Injections | 90 | 73 | |
| Physical Exam | |||
| Straight Leg Raising Test (−) | 39 (43%) | 32 (44%) | 0.95 |
| Straight Leg Raising Test (+) | 51 (57%) | 41 (56%) | |
| Pathology | |||
| Herniated Disc | 42 (48%) | 32 (45%) | 0.13 |
| Spinal Stenosis | 31 (35%) | 28 (39%) | |
| Herniated Disc & Spinal Stenosis | 4 (5%) | 8 (11%) | |
| Spondylosis Without Compression | 11 (13%) | 3 (4%) | |
| Facet Interventions | 68 | 33 | |
| < 50% Pain Relief from Diagnostic Block | 36 (53%) | 1 (3%) | - |
| 50–79% Pain Relief from Diagnostic Block | 23 (34%) | 15 (45%) | < 0.001 1 |
| ≥ 80% Pain Relief from Diagnostic Block | 9 (13%) | 17 (52%) | < 0.001 2 |
| Sacroiliac Joint Injection | 33 | 33 | |
| Physical Exam | |||
| Gaenslen’s test (−) | 7 (21%) | 10 (30%) | 0.40 |
| Gaenslen’s test (+) | 26 (79%) | 23 (70%) | |
| Patrick’s test (−) | 9 (27%) | 15 (45%) | 0.12 |
| Patrick’s test (+) | 24 (73%) | 18 (55%) | |
| Positive Patrick’s & Gaenslen’s tests | 22 (67%) | 15 (45%) | 0.083 |
| Prior Surgery | |||
| Prior back surgery | 7 (21%) | 4 (13%) | 0.35 |
| No prior back surgery | 26 (79%) | 28 (88%) | |
| < 50% Pain Relief from Diagnostic Block | 15 (45%) | 3 (9%) | - |
| 50–79% Pain Relief from Diagnostic Block | 9 (27%) | 17 (52%) | < 0.001 1 |
| ≥ 80% Pain Relief from Diagnostic Block | 9 (27%) | 13 (39%) | 0.302 |
Comparison of procedural failure versus success based on ≥ 50% pain relief threshold as definition of positive block.
Comparison of procedural failure versus success based on ≥ 80% pain relief threshold as definition of positive block.
Coefficients for linear regressions of change for NRS-PI are shown in supplemental Table 2 as a function of unadjusted (univariate) and adjusted (full and simplified models) covariates. Procedure type was not chosen as a covariate since it was not found to be associated with outcome. The full model contained all selected variables, while the simplified model was generated by eliminating variables using a backward stepwise approach. In the simplified model, obesity, QIDS, and baseline NRS pain score were selected as the final variables. Obesity and higher QIDS score were positively associated with change in average pain score (i.e. smaller decrease in pain score), while baseline pain score was negatively associated with change in pain score. The presence of obesity was associated with a 0.62-point increase in change in NRS pain score (i.e., worse pain relief, 95% CI 0.038 to 1.21, p = 0.037; figure 3). Every 1-point increase in QIDS was associated with a 0.076-point decrease in pain score improvement (95% CI 0.039 to 0.113, p < 0.0001), while every 1-point increase in baseline NRS pain score was associated with a 0.32-point improvement in NRS pain score (95% CI 0.16 to 0.48, p < 0.0001).
Figure 3.
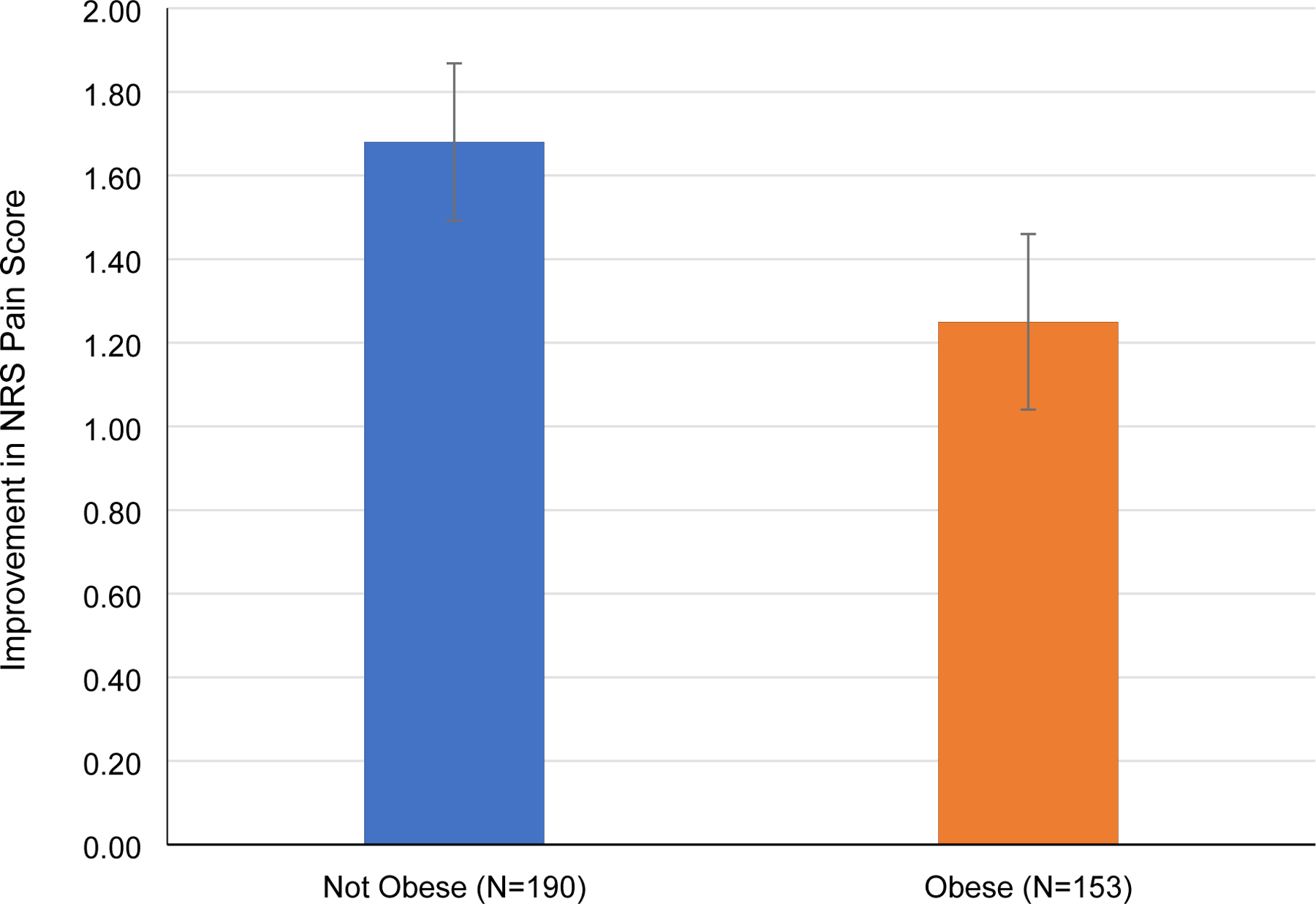
Change in Average NRS Pain Score in Non-Obese vs Obese Patients.
Higher values denote greater improvement in NRS pain score from baseline. Error bars denote standard error.
Unadjusted and adjusted odds ratios for logistic regression analyses of procedural outcome as a function of the same ten selected variables are shown in Table 4. As with linear regression, the full model contained all variables, while the simplified model was generated by eliminating variables using a backward stepwise approach. In the simplified model, baseline QIDS and ODI were selected as final variables. In this model, every 1-point increase in QIDS was associated with a 0.94-times decreased odds of procedural success (95% CI 0.91 to 0.97, p < 0.0001), while every 1-point increase in ODI was associated with a 0.59-times decreased odds of success (95% CI 0.36 to 0.96, p = 0.034). In other words, there was a 6% decrease in the odds of procedural success with a 1point increase in baseline QIDS score, and there was a 41% decrease in the odds of procedural success with each 1-point increase in baseline ODI score. Figure 4 shows the decline in procedural success rate with worsening depression, while Figure 5 shows a similar decline in procedural success rate with worsening disability.
Figure 4.
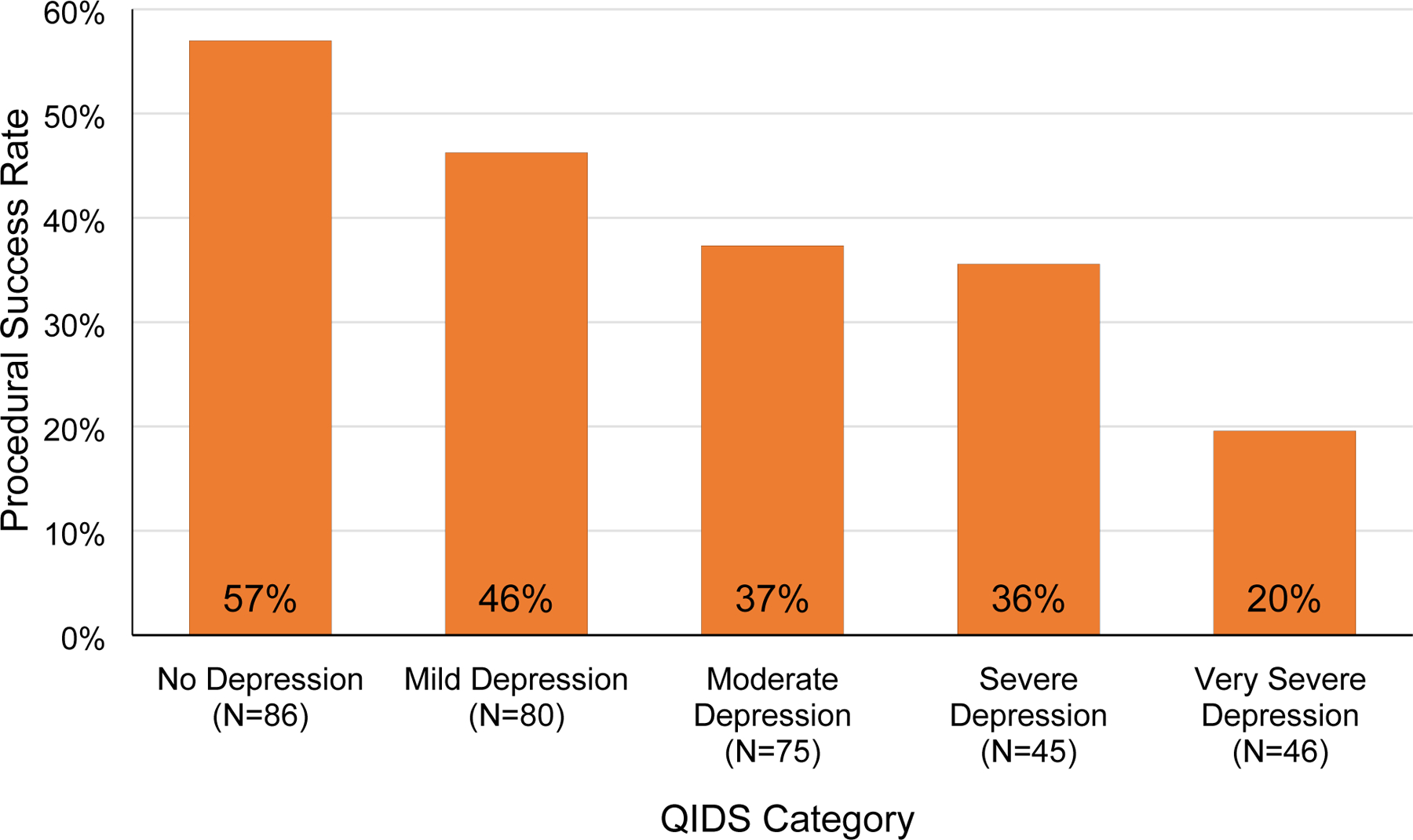
Procedural Success by stratified by Quick Inventory of Depressive Symptomatology (QIDS) score. QIDS scores range from 0 to 27. A score of 5 or lower indicates no depression, 6 to 10 mild depression, 11 to 15 moderate depression, 16 to 20 severe depression, and greater than 21 very severe depression.
Figure 5.
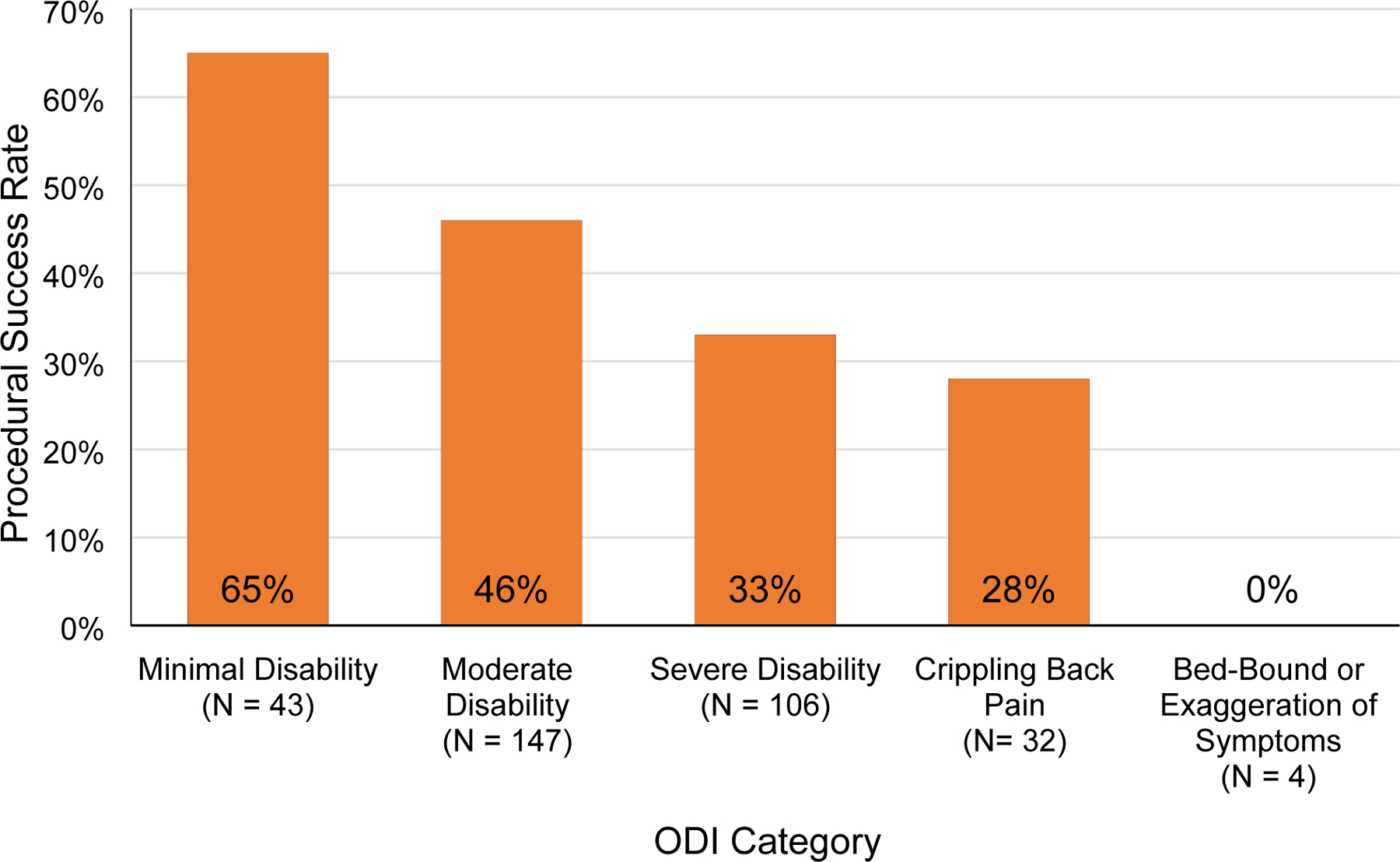
Procedural Success by Oswestry Disability Index (ODI) score. ODI scores range from 0% (no disability) to 100% (maximum possible disability). A score of 20% or lower indicates minimal disability, 21– 40% moderate disability, 41–60% severe disability, 61–80% crippling back pain, and 81% or greater indicates a patient is either bed-bound or has exaggeration of symptoms.
Complications
There were 10 complications in the ESI group (6.0%), which included inadvertent dural puncture (n=3) with one spinal headache, temporary worsening pain (n=4) or new neurological symptoms (n=2), and an accidental intradiscal injection. In the facet intervention group, there were 9 complications (8.0%) which included a panic attack, a pseudoseizure, a patient who reported insomnia for several weeks, 2 vasovagal episodes, 2 cases of neuritis, and 2 cases of new temporary pain (leg cramp) or neurological symptoms (shooting pain in leg). In the SIJ injection group, there was one case of worsening pain and one vasovagal episode for a complication rate of 3.0%.
Discussion
Personalized medicine represents one of the fastest growing areas in pain medicine, and may be particularly relevant for interventional procedures, which are characterized by high risks, costs and in some cases, rewards.24 This study is the largest to date examining the effect of dozens of factors on interventional LBP outcomes, and examined several factors never evaluated for their effects on outcomes (e.g. physical exam signs for SI joint outcomes, expectations, co-existing pain conditions, nonopioid medication usage).
Comparison to Other Studies
Some of our findings have been reported for other pain conditions. The negative effects of smoking, disease burden (duration of pain, higher pain scores, greater disability), secondary gain, sleep dysfunction, and psychiatric co-morbidities, have been shown to adversely affect LBP outcomes in both interventional and non-interventional studies.8,12,25–28 Our results demonstrating how varying degrees of depression and disability linearly correlate, in a dramatic fashion, with outcome, have not previously reported for procedural interventions and suggest that even modest treatment results in poorly controlled depression and severe disability may yield fruitful results. Yet, other findings were unexpected. We hypothesized that more chronic pain conditions, which may indicate central sensitization or somatization, would foretell treatment failure, as has been shown in other studies.29,30 However, we found no significant association between most chronic pain conditions and interventional treatment outcomes, which may reflect differences in treatment (interventional vs. conservative), methodology, and our relatively short-term follow-up. Unlike other studies, we found no meaningful relationship between pre-procedure expectations and treatment results. Whereas most studies have found a positive relationship between expectations for benefit and pain-treatment outcomes,31 our categorical outcome was tied to procedural satisfaction, and studies have also found that unrealistic outcomes correlate with dissatisfaction with treatment.32 We also found no association between the present of an inciting event and procedure success, which is consistent with a study that examined the association in individuals with sciatica, but divergent with the results of a study that found a positive correlation in individuals who underwent lumbar medial branch RFA.33,34 In contrast with a prospective study that found pain rating in response to a subcutaneous skin wheal (standardized pain stimulus) predicted less pain relief with ESI, we found no correlation between treatment outcome and pain score after a skin wheal.35 However, we did find that a higher procedure-related pain score was associated with poorer treatment outcome. This is surprising since procedure-related stimuli cannot be standardized, and most but not all prior studies on quantitative sensory testing found an inverse relationship between pain thresholds and interventional treatment outcomes.36–38 Although a standardized pain stimulus could theoretically be used when making treatment decisions, procedure-related pain cannot.
Our study also examined exam findings and immediate (i.e. diagnostic) block results on intermediate-term outcomes. For ESI, unlike previous studies, we found that neither straight leg raising test nor herniated disc were associated with a positive outcome, though individuals with non-compressive etiologies (e.g. spondylosis) fared worse.12,39 For SIJ injections, no significant association was noted for two of the most common provocative tests. Prior studies have found that while no single test is specific for identifying a painful SIJ, a battery of 3 or more positive provocative tests is associated with a positive response to diagnostic injections, and a positive diagnostic injection was present in 91% of those who experienced a positive outcome at 1-month in our study.40 Similar to recommendations in lumbar facet guidelines, we found that a higher degree of pain relief was more likely to lead to a positive outcome after RFA, but that setting the threshold for a positive diagnostic block at 80% would have led to a substantial proportion of patients who benefitted from treatment being denied access.8 For SIJ pain, unlike those who obtained between 50% and 79% pain relief, experiencing greater ≥ 80% immediate pain relief did not statistically separate on 1-month outcome from having a negative block, which suggests that many of these patients may have been placebo responders.
Limitations
There are several limitations that warrant consideration. First, because this study was designed to determine factors associated with treatment outcome in a practice setting, our inclusion criteria were less stringent than typically employed in a clinical trial. Second, there is inter-rater reliability variability regarding some of the factors we examined such as radiological findings, physical exam findings, and even what constitutes an active medical or psychiatric problem. Third, we combined 3 different LBP treatments which address different etiologies, and it is therefore possible that our study may have been underpowered to detect factors that may predict treatment outcome for one, but not the other procedures. These conditions also contain different pathophysiologies (e.g. neuropathic vs. non-neuropathic pain) and hence may be associated with different outcome predictors; however, there is extensive overlaps in outcome predictors for nearly all chronic pain conditions,12,25,28,41 and few cohort studies examining LBP outcome predictors differentiate between etiologies. Last, all of the participating institutions in this study were teaching hospitals, which may limit the generalization to private practice settings.
Future Research & Conclusions
The continued rise in interventions for LBP, with no appreciable effect on disability rates, has led to increased scrutiny from payers and regulators, and a push towards precision medicine- tailoring treatment to individual patient characteristics rather than symptoms and pathology.1,42 When employed indiscriminately, LBP interventions are not cost-effective,43 but refining selection based on our findings and those in large registries and clinical trials can favorably alter risk: benefit ratios and cost-effectiveness. However, physicians should consider that evidence-based medicine involves taking into consideration not just data from research studies, but an individual patient’s unique circumstances and goals; hence, values-based medicine, should be viewed as complementary rather than mutually exclusive with evidence-based medicine.44 Given our findings, interventions that might be considered before or concurrent with LBP interventions including psychotherapy or psychotherapeutics in individuals with depression, functional restoration in obese patients and those with high baseline disability, and referrals for smoking cessation in individuals amenable to quitting.
Supplementary Material
Acknowledgments
Funded in part by a grant from MIRROR, Uniformed Services University of the Health Sciences, U.S. Dept. of Defense, grant # HU00011920011. The sponsor did not play a role in study design or performance, or analysis or interpretation of data.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012; 64: 2028–37. [DOI] [PubMed] [Google Scholar]
- 3.Manchikanti L, Sanapati MR, Pampati V, Boswell MV, Kaye AD, Hirsch JA. Update on reversal and decline of growth of utilization of interventional techniques in managing chronic pain in the Medicare population from 2000 to 2018. Pain Physician 2019; 22: 521–36. [PubMed] [Google Scholar]
- 4.Manchikanti L, Soin A, Mann DP, Bakshi S, Pampati V, Hirsch JA. Comparative analysis of utilization of epidural procedures in managing chronic pain in the Medicare Population: Pre and post Affordable Care Act. Spine (Phila Pa 1976) 2019; 44: 22032. [DOI] [PubMed] [Google Scholar]
- 5.Friedly J, Chan L, Deyo R. Geographic variation in epidural steroid injection use in Medicare patients. J Bone Joint Surg Am 2008; 90: 1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SP, Doshi TL, Kurihara C, Dolomisiewicz E, Liu RC, Dawson TC, et al. Waddell (nonorganic) signs and their association with interventional treatment outcomes for low back pain. Anesth Analg 2021; 132: 639–51. [DOI] [PubMed] [Google Scholar]
- 7.Roth RS, Geisser ME, Williams DA. Interventional pain medicine: retreat from the biopsychosocial model of pain. Transl Behav Med 2012; 2: 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SP, Bhaskar A, Bhatia A, Buvanendran A, Deer T, Garg S, et al. Consensus practice guidelines on interventions for lumbar facet joint pain from an international, multispecialty working group. Reg Anesth Pain Med 2020; 45: 424–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra-articular sacroiliac joint injections: A systematic review. Pain Med 2015; 16: 1500–18. [DOI] [PubMed] [Google Scholar]
- 10.Şencan S, Çelenlioğlu AE, Asadov R, Gündüz OH. Predictive factors for treatment success of transforaminal epidural steroid injection in lumbar disc herniation-induced sciatica. Turk J Med Sci 2020; 50: 126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivaganesan A, Chotai S, Parker SL, Asher AL, McGirt MJ, Devin CJ. Predictors of the efficacy of epidural steroid injections for structural lumbar degenerative pathology. Spine J 2016; 16: 928–34. [DOI] [PubMed] [Google Scholar]
- 12.Cohen SP, Jamison D, Bicket M, Wilkinson I, Rathmell JN. Epidural steroids: A comprehensive, evidence-based review. Reg Anesth Pain Med 2013; 38: 175–200. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SP, Doshi TL, Dawson TC, Gupta A, Durbhakula S, Constantinescu OC, et al. The prognostic value of hypersensitivity reactions on epidural steroid injection outcomes: A phenotypic signature? A prospective cohort study. Reg Anesth Pain Med 2019; 44: 586–94. [DOI] [PubMed] [Google Scholar]
- 14.Thawrani DP, Agabegi SS, Asghar F. Diagnosing sacroiliac joint pain. J Am Acad Orthop Surg 2019; 27: 85–93. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SP, Bicket MC, Kurihara C, Griffith SR, Fowler IM, Jacobs MB, et al. Fluoroscopically-guided vs. landmark-guided sacroiliac joint injections: A randomized, controlled study. Mayo Clin Proceed 2019; 94: 628–42. [DOI] [PubMed] [Google Scholar]
- 16.Cohen SP, Doshi TL, Constantinescu OC, Zhao Z, Kurihara C, Larkin TM, et al. Effectiveness of lumbar facet joint blocks and their predictive value before radiofrequency denervation: The FACet treatment study (FACTS). Anesthesiology 2018; 129: 521–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SP, Mao J, Vu TV, Strassels SA, Gupta A, Erdek MA, et al. Does pain score in response to a standardized subcutaneous local anesthetic injection predict epidural steroid injection outcomes in patients with lumbosacral radiculopathy: A prospective correlational study. Pain Med 2013; 14:327–35. [DOI] [PubMed] [Google Scholar]
- 18.Jamison RN, VadeBoncouer T, Ferrante FM. Low back pain patients unresponsive to an epidural steroid injection: identifying predictive factors. Clin J Pain 1991; 7: 311–7. [DOI] [PubMed] [Google Scholar]
- 19.Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain 2009; 10: 354–68. [DOI] [PubMed] [Google Scholar]
- 20.Eklund A, De Carvalho D, Pagé I, Wong A, Johansson MS, Pohlman KA, et al. Expectations influence treatment outcomes in patients with low back pain. A secondary analysis of data from a randomized clinical trial. Eur J Pain 2019; 23: 1378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakobsen JC, Ovesen C, Winkel P, Hilden J, Gluud C, Wetterslev J. Power estimations for non-primary outcomes in randomised clinical trials. BMJ Open 2019; 9: e027092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC, Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol 2015; 68: 627–36. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–21. [DOI] [PubMed] [Google Scholar]
- 24.Cohen SP, Vase L, Hooten WM. Chronic Pain: An update on burden, best practices and recent advances. Lancet 2021; 397: 2082–97. [DOI] [PubMed] [Google Scholar]
- 25.Wilkens P, Scheel IB, Grundnes O, Hellum C, Storheim K. Prognostic factors of prolonged disability in patients with chronic low back pain and lumbar degeneration in primary care: a cohort study. Spine (Phila Pa 1976) 2013; 38: 65–74. [DOI] [PubMed] [Google Scholar]
- 26.Aljawadi A, Sethi G, Islam A, Elmajee M, Pillai A. Sciatica presentations and predictors of poor outcomes following surgical decompression of herniated lumbar discs: A review article. Cureus 2020; 12: e11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris I, Mulford J, Solomon M, van Gelder JM, Young J. Association between compensation status and outcome after surgery: a meta-analysis. JAMA 2005; 293: 1644–52. [DOI] [PubMed] [Google Scholar]
- 28.Pakpour AH, Yaghoubidoust M, Campbell P. Persistent and developing sleep problems: A prospective cohort study on the relationship to poor outcome in patients attending a pain clinic with chronic low back pain. Pain Pract 2018; 18: 79–86. [DOI] [PubMed] [Google Scholar]
- 29.Collados-Maestre I, Lizaur-Utrilla A, Martinez-Mendez D, Marco-Gomez L, Lopez-Prats FA. Concomitant low back pain impairs outcomes after primary total knee arthroplasty in patients over 65 years: a prospective, matched cohort study. Arch Orthop Trauma Surg. 2016; 136: 1767–71. [DOI] [PubMed] [Google Scholar]
- 30.Scherer M, Hansen H, Gensichen J, Mergenthal K, Riedel-Heller S, Weyerer S, et al. Association between multimorbidity patterns and chronic pain in elderly primary care patients: a cross-sectional observational study. BMC Fam Pract 2016; 17: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bialosky JE, Bishop MD, Cleland JA. Individual expectation: an overlooked, but pertinent, factor in the treatment of individuals experiencing musculoskeletal pain. Phys Ther 2010; 90(9): 1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafkamp FJ, Gosens T, de Vries J, den Oudsten BL. Do dissatisfied patients have unrealistic expectations? A systematic review and best-evidence synthesis in knee and hip arthroplasty patients. EFORT Open Rev 2020; 5: 226–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engle AM, Chen Y, Marascalchi B, Wilkinson I, Abrams WB, He C, et al. Precipitating events associated with lumbar radicular pain and their association with epidural steroid injection outcomes. Pain Med 2019; 20(12): 2360–70. [DOI] [PubMed] [Google Scholar]
- 34.Odonkor CA, Chen Y, Adekoya P, Marascalchi BJ, Chaudhry-Richter H, Tang T, et al. Inciting events associated with lumbar facet joint pain. Anesth Analg 2018; 126: 280–8. [DOI] [PubMed] [Google Scholar]
- 35.Cohen SP, Mao J, Vu TV, Strassels SA, Gupta A, Erdek MA, et al. Does pain score in response to a standardized subcutaneous local anesthetic injection predict epidural steroid injection outcomes in patients with lumbosacral radiculopathy: A prospective correlational study. Pain Med 2013; 14: 327–35. [DOI] [PubMed] [Google Scholar]
- 36.Campbell CM, Buenaver LF, Raja SN, Kiley KB, Swedberg LJ, Wacnik PW, et al. Dynamic Pain Phenotypes are Associated with Spinal Cord Stimulation-Induced Reduction in Pain: A Repeated Measures Observational Pilot Study. Pain Med 2015; 16(7): 1349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiff E, Eisenberg E. Can quantitative sensory testing predict the outcome of epidural steroid injections in sciatica? A preliminary study. Anesth Analg 2003; 97: 828–32. [DOI] [PubMed] [Google Scholar]
- 38.Maher DP, Ding W, Singh S, Opalacz A, Fishman C, Houghton M, et al. Thermal QST Phenotypes associated with response to lumbar epidural steroid injections: A pilot study. Pain Med 2017; 18: 1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamison RN, VadeBoncouer T, Ferrante FM. Low back pain patients unresponsive to an epidural steroid injection: identifying predictive factors. Clin J Pain 1991; 7: 311–7. [DOI] [PubMed] [Google Scholar]
- 40.Thawrani DP, Agabegi SS, Asghar F. Diagnosing sacroiliac joint pain. J Am Acad Orthop Surg 2019; 27: 85–93. [DOI] [PubMed] [Google Scholar]
- 41.Karran EL, McAuley JH, Traeger AC, Hillier SL, Grabherr L, Russek LN, Moseley GL. Can screening instruments accurately determine poor outcome risk in adults with recent onset low back pain? A systematic review and meta-analysis. BMC Med 2017; 15: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manchikanti L, Sanapati MR, Pampati V, Boswell MV, Kaye AD, Hirsch JA. Update on reversal and decline of growth of utilization of interventional techniques in managing chronic pain in the Medicare population from 2000 to 2018. Pain Physician 2019; 22: 521–36. [PubMed] [Google Scholar]
- 43.Carreon LY, Bratcher KR, Ammous F, Glassman SD. Cost-effectiveness of lumbar epidural steroid injections. Spine (Phila Pa 1976) 2018; 43: 35–40. [DOI] [PubMed] [Google Scholar]
- 44.Peile E Evidence-based medicine and values-based medicine: partners in clinical education as well as in clinical practice. BMC Med 2013; 11: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


