Abstract
The bacterium Neisseria gonorrhoeae causes the sexually transmitted infection gonorrhoea. Although diverse clinical manifestations are associated with gonorrhoea, ranging from asymptomatic through to localized and disseminated infection, very little is known about the bacterial determinants implicated in causing such different clinical symptoms. In particular, virulence factors, although defined and investigated in particular strains, often lack comprehensive analysis of their genetic diversity and how this relates to particular disease states. This review examines the clinical manifestations of gonorrhoea and discusses them in relation to disease severity and association with expression of particular virulence factors including PorB, lipooligosaccharide (LOS) and Opa, both in terms of their mechanisms of action and inter- and intra-strain variation. Particular attention is paid to phase variation as a key mechanism of genetic variation in the gonococcus and the impact of this during infection. We describe how whole-genome-sequence-based approaches that focus on virulence factors can be employed for vaccine development and discuss whether whole-genome-sequence data can be used to predict the severity of gonococcal infection.
Keywords: genome, immune evasion, Neisseria gonorrhoeae, pathogenesis , variation
Data Summary
The Neisseria gonorrhoeae FA1090 genome was downloaded from the PubMLST database (https://pubmlst.org/). The genome was annotated using Proksee (https://proksee.ca/). Analysis of the source of gonococcal genomes was performed using the PubMLST database (https://pubmlst.org/neisseria).
Impact Statement.
In order to combat Neisseria gonorrhoeae , we need to fully understand the bacterium causing this disease, in particular how it continually eludes the immune response and is so successful at causing infection. However, despite decades of research, there is still much to learn about this versatile pathogen. Here, we provide a comprehensive review of what is known about N. gonorrhoeae disease manifestations, pathogenesis and the virulence determinants associated with this phenotype, with a particular focus on genetic variation.
Introduction
Neisseria gonorrhoeae (the gonococcus) is the causative agent of the sexually transmitted infection (STI) gonorrhoea, which affects over 86.9 million people every year [1]. The bacterium causes acute inflammation of the genitourinary tract, characterized by recruitment of phagocytic cells to the site of infection [2]. Lower genitourinary tract infection is, however, not the only site of gonococcal tropism, as the gonococcus is known to also infect the rectum, throat, eyes, upper genitourinary tract and, in rare cases, the bloodstream, which may lead to establishment of distant skin and joint colonization [3–6].
Painful inflammation of the genitourinary tract is only one consequence of infection. In some cases, gonococci ascend to infect the upper genital tract, potentially leading to infertility if untreated. This is due to scarring associated with chronic neutrophilic inflammation with untreated infection in women. Resultant pelvic inflammatory disease (PID) is associated with significant pain, and increased risk of miscarriage and extrauterine pregnancy [1, 7, 8]. In both sexes, infection can spread, leading to septicaemia, skin lesions and arthritis in a condition known as disseminated gonococcal infection (DGI) [9, 10]. In addition, gonococcal infection has been associated with an increased risk of contracting human immunodeficiency virus (HIV) – which may be due to recruitment of lymphocytes to the urogenital tract [6, 11].
Improved understanding of N. gonorrhoeae virulence and its determinants enhances our ability to identify alternative treatment strategies – potentially resulting in gonococcal-specific antimicrobials [12–14]. Furthermore, identification of new virulence factors and understanding their variation will facilitate the discovery of conserved immunogenic structures – expanding the list of potential vaccine targets [15–18]. Here, we review current understanding of gonococcal host–pathogen interactions. First, we consider the different disease phenotypes exhibited by the gonococcus and how gonococcal infection severity is classified. We then examine what is known about individual virulence factors, their functions and their intra- and inter-strain genomic variability. Finally, we describe recent advances in the methodology for identification of gonococcal virulence factor diversity, and the challenges associated with bioinformatic analyses of whole-genome-sequence data. To cause disease, a pathogen has to express determinants that permit niche establishment and immune evasion, ultimately causing damage to the host as it tries to eliminate the pathogen and maintain homeostasis. Understanding these processes is essential to developing new ways to treat gonococcal infections, especially in the light of growing antimicrobial resistance.
Gonococcal pathology is difficult to define and correlate with bacterial genetics
N. gonorrhoeae has several clinical manifestations that cannot be easily categorized in terms of severity
Gonorrhoea is characterized by infection of the lower genitourinary tract mucosa leading to urethritis in males and cervicitis in females [1, 19, 20]. Pathology includes inflammation with a neutrophilic purulent exudate [21]. This is the classical manifestation of gonorrhoea, but the severity of pathology should be robustly characterized to allow comparisons to be made on the bacterial genetic determinants implicated in each clinical scenario in terms of human pathogenesis.
Asymptomatic infection is typically recognized as the ability to recover viable gonococci from the lower reproductive tract of a patient without symptoms of pain or visible inflammatory exudate [8, 22]. It can be detected during routine STI screening. It is estimated that 50 % of gonococcal infections in women are asymptomatic compared to a much lower proportion in men (though this gender disparity has been questioned) [8, 22]. The term asymptomatic infection does not differentiate colonization from subclinical inflammation, making the issue of investigating pathogenesis challenging. Thus, asymptomatic infection has a vague pathological definition, making severity classification difficult.
In addition, there is extensive evidence indicating that asymptomatic infection is not without pathology. Firstly, the interaction of the gonococcus with other STI-causing organisms is of growing concern. N. gonorrhoeae infection is known to cause inflammation, epithelial damage and recruitment of Th17 polarized CD4+ T cells to the urogenital mucosa and, as a result, provides opportunities for HIV acquisition [23–25]. Furthermore, clinical data suggest frequent co-infection with Chlamydia trachomatis ; in a murine model of infection, co-infection was associated with a higher gonococcal burden and increased neutrophil recruitment that may translate to more severe symptoms [26, 27]. Therefore, asymptomatic infections may have an important clinical role in STI epidemiology.
Secondly, both symptomatic and asymptomatic infection can ascend if left untreated [7, 8, 28, 29]. The most common sequela of untreated gonorrhoea is PID, which is defined as bacterial infection associated with inflammation of the upper female genital tract [8]. This includes endometritis, salpingitis and oophoritis, and is most commonly associated with significant lower abdominal pain that intensifies on menstruation with serious sequelae (Table 1) [30]. Some suggest that the mechanism of ascending infection may be purely mechanical, through retrograde menstruation or sexual intercourse [8, 31]. However, attachment is vital for bacterial colonization. Differential expression of carcinoembryonic antigen-related adhesion molecule (CEACAM)1 and CEACAM5 has been shown in human female reproductive tract samples, with CEACAM1 expressed in the uterus and endocervix (upper genital tract) and CEACAM5 expressed in the ectocervix (lower genital tract) [32]. Reciprocally, OpaCEA expressing gonococci have been shown to colonize the murine uterine horn, equivalent to the human uterine body, in human CEACAM1 transgenic mice [32], indicating that expression of particular Opa alleles, perhaps by a subpopulation of gonococci, may be associated with PID development. However, data on association of gonococcal genetic variants with PID are limited, likely due to difficulties in obtaining samples from the upper genital tract. However, the recent establishment of a murine female genital tract infection model transfected with human CEACAM receptors may permit more detailed studies into PID pathology [32].
Table 1.
Summary of gonococcal disease manifestations and their severity
−, No long-term sequelae; *, minor long-term sequelae; **, significant long-term sequelae; ***, serious long-term sequelae.
|
Clinical manifestation |
Gender affected |
Mechanism |
Symptoms |
Long-term sequelae |
Reference |
|---|---|---|---|---|---|
|
Lower genital tract symptomatic infection |
Both (males higher proportion) |
Sexual transmission |
Painful inflammation of genitourinary tract; urethral stricture and pain on urination in males; increased risk of acquisition of HIV; may ascend/spread (PID, DGI) |
*/** |
[1, 19–21] |
|
Lower genital tract asymptomatic infection |
Both (females higher proportion) |
Sexual transmission |
Potentially none, but can become symptomatic or ascend/spread (PID, DGI) |
− |
|
|
Pharyngitis |
Both |
Sexual transmission |
Inflammation of pharynx (often asymptomatic) |
* |
[5, 39] |
|
Pelvic inflammatory disease (PID) |
Female |
Ascension of lower genital tract infection |
Severe abdominal pain; scarring of the reproductive tract; increased risk of extrauterine pregnancy, stillbirth and spontaneous abortion; infertility |
**/*** |
|
|
Disseminated gonococcal infection (DGI) |
Both |
Systemic infection spread from other sites |
Septic arthritis and joint pain; septic shock in rare cases |
**/*** |
[3, 9, 10] |
|
Ophthalmia neonatorum |
Both (neonates) |
Eye infection in the newborn from infected mother |
Corneal scarring, globe perforation and permanent blindness |
*** |
[40] |
|
Gonococcal conjunctivitis |
Both |
Eye infection in adults from infected urine or genital secretions |
Corneal scarring, globe perforation and permanent blindness |
*** |
[41] |
The most invasive manifestation of N. gonorrhoeae infection is DGI. Here, the bacterium disseminates to the bloodstream. Exposure to bacterial pro-inflammatory molecules can trigger life-threatening sepsis in rare cases; more common is occurrence of skin lesions or septic arthritis, which unlike other joint inflammatory conditions are non-symmetrical and difficult to predict based on patient history [3, 9, 10]. The adaptations necessary for systemic spread are serum and complement resistance; this was observed in isolates recovered from patients with DGI [33, 34]. The outer membrane PorB variant, PorB 1A, is correlated with a greater risk of developing disseminated infection. This was shown by Brunham and colleagues; in a study of 325 gonococcal isolates associated with infection, all five cases of DGI were caused by PorB 1A expressing strains, while no DGI cases were caused by PorB 1B expressing strains [7]. This was further confirmed by Illumina sequencing of gonococci from 45 cases of DGI; over 85 % of DGI isolates possessed PorB 1A alleles [35]. This may be associated with complement resistance conferred by PorB; while both variants bind C4BP (if via different mechanisms) [36, 37], PorB 1A is also able to bind factor H, a complement regulatory protein of the alternative pathway present in human plasma with binding increasing redundancy in complement degradation [38].
Finally, other manifestations of infection, such as gonococcal pharyngitis, are likely linked to the route of infection (oro-genital) [5, 39], with reports indicating that DGI could stem from isolated pharyngitis [1, 2, 39]. Another largely overlooked type of infection is ophthalmia neonatorum. It is caused by infection of the newborn during passage through the birth canal of an infected mother, and if left untreated can cause ulceration and perforation of the globe of the eye [40]. Adult gonococcal conjunctivitis caused by contact with sexual secretions is even more under-recognized; while rare, the consequences of untreated infection are the same as in neonates [41]. There is a lack of research into the receptors for adhesion and fitness of gonococci persisting on the eye, which may lead to permanent blindness [1, 2].
Thus, a number of different clinical manifestations can be induced by the gonococcus. However, are all gonococci equally able to cause PID, DGI, neonatal infections, pharyngitis and/or asymptomatic infections? Are there genetic determinants that facilitate the ability of the gonococcus to survive and persist in different anatomical sites – with variation between strains potentially pivotal in improving our understanding of the biology of this bacterium? Evidence suggests that a mix of mechanical routes of entry and genetic determinants impact establishment in different niches.
Gonococcal virulence factors are diverse, which may influence pathogenesis
The 'pathogenome' is difficult to define in Neisseria spp.
N. gonorrhoeae is a primary human pathogen colonizing the genitourinary tract [1]. This is in contrast to other human-associated Neisseria spp., which are either commensal bacteria of the nasopharynx (most of the Neisseria spp.) or opportunistic pathogens [42]. The best described is Neisseria meningitidis , which normally colonizes the nasopharynx of approximately 10 % of the adult population, but may become invasive causing life-threatening septicaemia and meningitis [43–45]. This difference in tropism and life cycle suggests that the gonococcus has diverged significantly from other Neisseria spp., which is reflected in bacterial genome content.
To understand the evolutionary history of Neisseria spp., it is vital to examine their genomic organization. N. gonorrhoeae is interesting in that it is polyploid, with three recognized plasmids (of which one is conjugative and one mobilizable), often in large copy numbers, which facilitate horizontal gene transfer [46]. The rate of genetic variation and genome reorganization is increased further by natural transformation. This capacity is enhanced for DNA originating from other gonococci distinguished by the DNA uptake sequence 5′- ATGCCGTCTGAA-3′ (underlined bases are semi-conserved between strains) [47]. To investigate lineages and evolution, several methods have been developed. Initially, meningococcal diversity was described using multilocus sequence typing (MLST), which uses seven housekeeping gene sequences to group strains into ‘clonal complexes’ often of similar biology. However, for gonococci with much higher horizontal gene transfer rates [48], MLST is insufficient to distinguish strains; strains from the same sequence type (ST) often have significantly different genome ancestry. The first directed attempt was NG-MAST, which enables identification of transmission clusters using polymorphisms in TbpB and PorB [49]. Currently, the most common method is core-genome MLST (cgMLST), which utilizes over 1600 core-genome genes to subgroup gonococcal strains into STs and lineages [50]. The division of gonococci into lineages with semi-clonal structure allows the approximation of gonococcal population dynamics [51].
Genetic variation of multiple genes in Neisseria spp. is increased by phase variation. It can be defined as a phenomenon in which genes undergo a reversible switch in their expression state. Most commonly, it is mediated by a homopolymeric tract consisting of simple repetitive sequences, such as a poly-G tract or short tandem repeat, either in the open reading frame (ORF) or the promoter region [52]. Due to the repetitiveness of the sequence, during DNA replication, the polymerase is prone to slipped-strand mispairing, resulting in either an increase or decrease in length of the track amplified. The outcome depends on the location of the homopolymeric tract. In the promoter region, an increase in the distance between promoter and ORF usually modulates the expression level of the protein; in contrast, phase variation within an ORF usually results in switching expression ON or OFF as it is brought either in or out of frame [52–54]. Alternative mechanisms of phase variation are invertible DNA sequences or altered DNA methylation states; in Neisseria spp., these are minor mechanisms and the phase variation of factors discussed in this review is mediated by slipped-strand mispairing [52].
Studies investigating the genetic lineage structure and evolution of the genus Neisseria have indicated that the two pathogenic species, N. gonorrhoeae and N. meningitidis , although evolutionarily separated, likely diverged from a common ancestor. This was followed by a change of tropism of the gonococcus to a different anatomical site, which has further driven the separation of the two species. Bennett et al. used whole-genome analysis to show that approximtely 60 % of the core-genome content is shared between commensal Neisseria lactamica and pathogenic N. meningitidis and N. gonorrhoeae , with distinct patterns of genetic variation specific to individual species suggesting little evidence of recombination and evolutionary separation [48]. Similarly, MLST approaches, either based on 51 ribosomal loci (rMLST) or 1114 core-genome loci (cgMLST) phylogeny analysis, highlight a clear separation of pathogenic Neisseria from commensal species, followed by a subsequent evolutionary separation of N. gonorrhoeae and N. meningitidis ; this can be contrasted with Neisseria polysaccharea or 'Neisseria bergeri', which consist of distinct lineages, leading to suggestion of separating N. polysaccharea into separate species [55]. This means that the majority of the core genome is shared across the genus Neisseria , making the identification of genetic determinants implicitly associated with a particular disease phenotype challenging. For example, several studies have shown that many virulence determinants are shared between both pathogenic Neisseria species and commensals; e.g. one can find the gonococcal genetic island in N. meningitidis , capsule biosynthesis (cps) loci in some commensal Neisseria ; similarly, iron transport systems like FetB or TbpB or adhesion structures such as type IV pili are present in both pathogenic Neisseria and commensals [56–59].
One exception to that rule is the recently identified meningococcal clade US_NmUC. It has a particular propensity for causing urethritis, and interestingly has acquired a unique, recombined, non-functional factor H binding protein (which in meningococci serves to protect against complement deposition by recruitment of factor H, which in turn inhibits the alternative complement pathway) [60]. It has also lost the capacity for capsule synthesis due to multi-gene deletion in the cps locus and acquired several gonococcal alleles including the anaerobic respiration cluster aniA/norB [61–64]. Firstly, this indicates that recombination of the core genome between meningococci and gonococci is possible once the anatomical barrier is removed. Interestingly, such genetic transfer events are yet to be identified in gonococcal pharyngitis, where the anatomical barrier is also absent. Second, due to its recent emergence, this clade may highlight which genes are necessary for pathogenesis at this anatomical site and should be closely monitored. Still, it is apparent that examination of the overall genome content is not sufficient to understand the different disease phenotypes exhibited by the gonococcus and meningococcus, and that alternative approaches are needed to define the gonococcal pathogenome and how it differs from commensal Neisseria and N. meningitidis .
Genetic diversity of virulence factors has been unevenly investigated
N. gonorrhoeae is a mucosal pathogen with infection characterized by efficient pathogenesis and a plethora of genetic determinants facilitating these processes (Fig. 1, Table 2). Understanding these determinants – with variation between strains being potentially pivotal for our understanding of gonococcal biology – will provide opportunities to develop new preventative and therapeutic strategies. What is known about the genetic variation of particular virulence determinants implicated in pathogenicity will be discussed here.
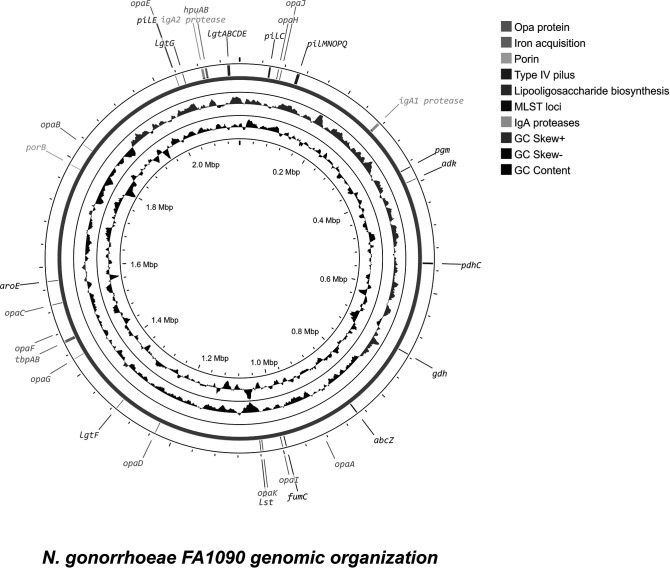
Fig. 1.
Visualization of the bacterial chromosome of N. gonorrhoeae FA1090 strain with a selection of confirmed and putative virulence factors marked. G+C content is indicated with a black wave; positive G+C skew is indicated in green, while negative G+C skew is indicated with purple waves. Key variable genes involved in pathogenesis are annotated with arrows and gene names. Colours indicate a process relevant for pathogenesis in which the gene is implicated as per the key. For reference, MLST loci are annotated with arrows and gene names in purple.
Table 2.
Nomenclature of a selection of major virulence factors in N. gonorrhoeae
The designations were accessed from the PubMLST database, which contains a complete list of gonococcal loci [169].
|
Currently used name |
Abbreviation |
Alias and historic nomenclature |
NEIS designation (gene name) |
NGO designation |
|---|---|---|---|---|
|
Porin B |
PorB |
Protein I |
NEIS2020 |
NGO1812 |
|
Lipooligosaccharide |
LOS |
Lipopolysaccharide (LPS) |
NEIS1902 (lgtA), NEIS1901 (lgtB), NEIS2154 (lgtC), NEIS2155 (lgtD), NEIS1900 (lgtE), NEIS1618 (lgtF), NEIS2011 (lgtG) |
NGO1353 (lgtF), rest undesignated |
|
Opacity protein |
Opa |
Protein II |
NEIS1403 (opaA), NEIS 1551 (opaB) and others |
Undesignated |
|
Type IV pili |
T4P |
– |
NEIS0210 (pilE), NEIS0408 (pilQ), NEIS0371 (pilC), silent pilS cassettes (unassigned) and others |
pilE and pilS cassettes – undesignated, NGO0094 (pilQ), NGO055 (pilC) and others |
|
IgA proteases 1 and 2 |
IgA1, IgA2 |
– |
NEIS1959 (iga2), NEIS0651 (IgA1 protease) |
NGO2105 (iga2), NGO0275 (IgA1 protease) |
Lipooligosaccharide (LOS) and sialylation
N. gonorrhoeae is a Gram-negative bacterium and, similarly to most Gram-negative bacteria, requires lipopolysaccharide (LPS) to maintain outer membrane integrity. In most Gram-negative bacteria, LPS consists of three main domains: lipid A, a canonical Toll-like receptor 4 (TLR4) agonist; an oligosaccharide core that is often variable within and between bacterial species; and O-antigen, which is a polysaccharide extending over several nanometres from the bacterial cell surface [65]. Neisseria are an interesting exception to this rule – they do not possess the genes required for O-antigen synthesis, rendering their LPS unusually short (‘rough’ in opposition to ‘smooth’ O-antigen containing LPS) [66]. As a result, ‘poly’ is sometimes deemed inappropriate to describe Neisseria LPS and LOS is used instead [14, 67].
LOS biosynthesis genes are mostly conserved across the genus Neisseria . The main biosynthetic genes are encoded by the lgtABCDE locus, with lgtF and lgtG located in separate locations on the chromosome (Fig. 1) [54, 68]. Conservation of locus presence across the genus does not imply nucleotide sequence homology between species or indeed between strains of a species, and four lgt genes responsible for synthesis of outer core LOS chains are phase variable. In these loci, homopolymeric tracts within the ORF elongate or shorten due to slipped-strand mispairing, resulting in a gene becoming in phase or out of phase and antigenic variation on the gonococcal cell surface (Fig. 2a) [54, 67, 69].
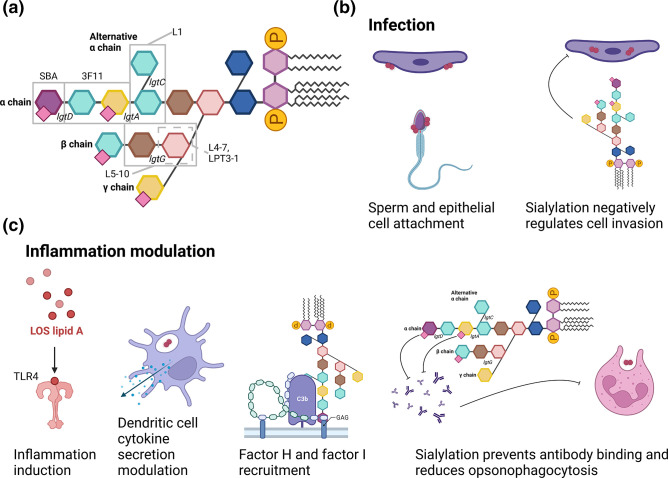
Fig. 2.
Schematic representation of roles of LOS in infection, invasion and immune evasion of N. gonorrhoeae . (a) Schematic representation of fully extended LOS molecule. Oligosaccharide chain names are indicated in bold. The grey rectangles indicate epitopes of labelled monoclonal antibodies against LOS. Names of phase variable LOS biosynthesis genes are indicated in italics next to the bond they synthesize [14, 67, 70, 71, 181]. (b) Roles of LOS in infection. Visualization of LOS-mediated sperm and epithelial cell attachment and negative impact on invasion of host epithelial cells. (c) Roles of LOS in inflammation modulation. Visualization of LOS mediated Toll-like receptor 4 (TLR4) activation, modulation of dendritic cell cytokine secretion, factor H and factor I recruitment and sialylation- and phase-variation-mediated escape from humoral immunity and opsonophagocytosis. Pink squares, sialylation sites. Created with BioRender.com.
Phase variation of LOS is interesting in terms of virulence. It is known that N. gonorrhoeae LOS core is an immunogenic structure, with antibodies developed against individual components of the molecule [70–72]. LOS oligosaccharide is not essential for gonococcal viability in vitro [54, 71, 73]. However, antigenic variation, achieved through phase variation, allows the gonococcus to escape the adaptive humoral immune response but can prevent its attachment to sperm cells and male urethral epithelial cells if lgtA phase varies OFF due to reduced engagement of asialoglycoprotein (ASGP-R) by the LOS α chain [74, 75]. This would suggest that lgtA is important for male urethral infection and transmission. Recently, phase variable lgtD has been shown to prevent the addition of terminal KDO residues in unsialylated LOS, which may have an impact on therapeutic approaches [14]. Little is known, however, on the function of lgtC and lgtG phase variation in pathogenesis. Nevertheless, it has been shown that LOS sialylation on an α-chain lactose-N-neotetraose (lgtA dependent) recruits factor H, factor I and sequesters C3b, preventing complement activation, which would be lethal for the invading bacterium [76–82]. LOS can potentially induce actin reorganization in host epithelial cells, allowing for Opa-independent invasion [83]. Thus, blockade of this molecule by neutralizing antibodies could seriously hinder gonococcal virulence.
Another aspect of gonococcal LOS impacting pathology is sialylation, a process in which α2,3 and α2,6 linked sialic acid molecules are added to gonococcal LOS by an outer membrane localized sialyltransferase [84, 85]. Sialylation is thought to induce host mimicry of human epithelial surface glycoproteins (which commonly terminate with α2,3 sialic acid residues), and binding of multiple LOS antibodies (e.g. 3F11) is completely abolished by this immune evasion mechanism [86]. Importantly, sialylation occurs in α2,3 pattern only on the lacto-N-neotetraose moiety that is lgtA dependent; other linkages are α2,6 and were not observed to reduce anti-LOS antibody binding [85, 87, 88].
With a recent article suggesting an additional ketodeoxyoctonic (KDO) transferase function for Lst, the incompleteness of our current understanding of LOS structure has been highlighted [14]. The LOS structure is also linked to skewed dendritic cell activation profile and neutrophil opsonophagocytosis susceptibility [78, 89] with sialylation significantly decreasing the propensity for cellular invasion and infection, steps vital in the development of DGI [74, 76, 90–93]. These data are consistent with LOS being an important molecule that is highly variable throughout the course of infection (Fig. 2a, b, c). All of this, combined with the abundance of the molecule in the membrane, makes it an important virulence determinant – which may have activities that are yet to be described.
Outer membrane protein PorB
N. gonorrhoeae possesses several proteins in its outer membrane, with the porin PorB the best described and most abundant [94]. It is encoded by a single gene that has two main variants, PorB 1A and PorB 1B, which differ by which portion of the protein is surface exposed and size (34–36.5 and 36–38 kDa, respectively) [94, 95]. Typically, the gonococcus expresses one variant only, which accounts for approximately 60 % of all outer membrane proteins. It forms a trimeric β-barrel porin, capable of conducting both anions and cations [96]. PorB is widely considered essential for the gonococcus, such that attempts to delete the gene have failed so far. Individual amino acid substitutions are possible in 308 of 328 amino acids of the PorB 1B allele of the FA1090 strain; the non-mutable amino acids were spread across the sequence, with the majority located within the β-barrel and some within extracellular loops [97], indicating that, despite the essential role of PorB in bacterial fitness, it can accommodate extensive non-synonymous genetic changes with unknown consequences for pathogenesis.
Nonetheless, PorB is a major gonococcal virulence factor facilitating immune evasion. Apart from serving as a decoy for antibodies, both PorB variants recruit C4 binding protein (C4BP) [77], which inhibits the classical pathway of complement activation – subverting a significant portion of innate immunity. In addition, the PorB 1A variant is more prevalent in DGI, indicating higher ‘invasiveness’, i.e. propensity to cause severe gonococcal disease compared to PorB 1B (associated with localized infection) [7, 33–35, 98].
One suggested mechanism is significantly better factor H binding to PorB 1A resulting in inactivation of over 75 % of C3b to iC3b, protecting the bacterium from complement activation [80, 88]. Furthermore, PorB can dislocate and insert into host cells; this has been associated with inhibition of neutrophil killing of the gonococcus, exocytosis of endosomes, as well as inhibition of apoptosis of infected host cells [99–104]. Overall, PorB is one of the key virulence determinants in gonococci, providing multiple avenues of immune evasion – yet, the importance of other properties, such as ability to conduce ions, is yet to be fully uncovered.
In terms of clinical practice, the abundance and essential nature of PorB makes it an attractive vaccine target, with several studies showing that monoclonal antibodies binding to PorB prevent infection in vitro [105–107]. However, PorB is highly variable [108, 109], with evidence of mosaicism and recombination between strains [110, 111]. This subverts antibody-dependent immunity and is further challenged through complex interactions of PorB with other outer membrane structures such as LOS or RmpM (protein III) [94].
Outer membrane adhesion protein Opa (protein II)
Opacity (Opa) protein describes the phenotype it induces – Opa+ colonies appear opaque on transparent medium [112]. The protein structure is quite variable with conserved, semivariable and two hypervariable regions that aid in immune evasion by antigenic variation. Gonococci possess up to 11 loci encoding Opas, which are subject to extensive phase variation. Since the frameshift events are independent of one another, the bacterium can theoretically express anything from 0 to 11 different Opa variants at any time [113, 114]. The engineering of a strain deficient for all opa genes facilitates the study of this family of proteins [115]. Still, most of the research seems to focus on the 11 phase variants of Opa. In addition, the impact of variation of individual Opa loci on pathogenesis is still unknown.
In terms of pathogenesis, Opas serve a very diverse set of functions. One that has been well described is CEACAM (CD66) binding, likely strengthening gonococcal adhesion to host cell surfaces vital for its tropism [116, 117]. Changes in Opa status may correlate with invasiveness in PID as CEACAMs are differentially expressed in the lower and upper female genital tracts [32]. Importantly, due to phase variation of Opa, an infected individual may carry bacteria expressing different Opa profiles, allowing spread of some bacteria while retaining the original infection site. This is consistent with the finding that isolates from the blood of patients with DGI did not express Opa, unlike their counterparts from the urethral or cervical tract, which were able to invade epithelial cell layers in vitro [118]. Importantly, four out of five strain pairs isolated from single individuals were not coinfections with two independent strains [118]. Secondly, Opa proteins have been implicated in immune evasion. One component is phase variation, escaping antibody immunity should it develop. However, Opa-treated dendritic cells are unable to activate T cells against their cognate antigen [in this experiment HIV peptides in HIV positive, antiretroviral therapy (ART)-treated patients] [119]. This may contribute to the difficulty of inducing a protective immune response against the gonococcus. Finally, distinct Opa variants confer further properties, such as serum resistance or host-cell killing [117, 120–123]. In general, Opa seems to be exquisitely linked to immune evasion in addition to cell attachment.
IgA proteases
IgA proteases are secreted proteins that undergo further autoproteolysis until a fully functional protein is produced [124–126]. This protein cleaves mucosal IgA1, which can prevent gonococcal cell attachment and induce opsonophagocytosis [127]. Furthermore, IgA1 protease cleaves LAMP1, disturbing lysosomal homeostasis and, thus, promoting intracellular survival and invasion [128–130].
Genetic variation of IgA protease indicates a mosaic composition mediated by horizontal gene transfer [131, 132]. Evidence regarding the essentiality of IgA1 protease for virulence is uncertain; it has a clearly defined mechanism of action, being important in vitro in epithelial cell invasion as well as being strongly associated with pathogenic but not commensal Neisseria . However, experimental infections of both female mouse models and human male volunteers show that IgA1 protease mutants can establish infection [133, 134]. It is important to note IgA2 was not investigated in these studies, and a relatively brief infection may have underestimated the importance of antibody cleavage activity. Although less studied, there is evidence to suggest that IgA2 protease undergoes phase variation, suggesting susceptibility to immune detection [59].
Outer membrane adhesion organelles: type IV pili
The gonococcus possesses characteristic structures known as type IV pili. They form long filamentous structures, recently imaged at sub-nanometre resolution using cryogenic electron microscopy [135], and are involved in cell attachment, twitching mobility, DNA transformation and microcolony formation, making them a major virulence factor [136]. These structures consist of multiple proteins: membrane spanning PilQ at the base, through which the major pilin subunit PilE in helical polymers forms the majority of the pilus. In addition, PilC is the likely tip adhesin (although its precise location is uncertain), while PilV is a fibre-like pilin subunit, both vital for adherence to epithelial cells [137–139].
The major role of the type IV pilus is attachment of the gonococcus to host cells in an Opa-independent manner; this is how Opa-negative cells retain virulence. This attachment is dependent on pilin-associated proteins PilC and PilV [139]. Once attached, type IV pilus undergoes retraction by the PilT motor subunit. This is a reversible process, and pili can be extended or retracted at any given time [140]. In addition to a role in cellular attachment and motility, this is proposed to modulate inflammation of infected cells. Some groups postulate that CD46 (complement regulatory protein that cleaves C3b and C4b) may be detached from the cells by type IV pilus retraction, modulating inflammation; this remains controversial [141]. Furthermore, CD4+ T cells have been observed to be activated by type IV pilus, resulting in secretion of anti-inflammatory IL-10 cytokine, and the pilus has been observed to bind C4BP vital for complement evasion [142, 143].
Pilin antigenic variation has been studied relatively well. The pilE major expressed subunit is only one of the many variants carried on the chromosome; non-expressed variants collectively called pilS are also carried. There are frequently several pilS loci; N. gonorrhoeae FA1090 contains 19 unique pilS genes in six separate chromosomal locations [144]. Variation results when part of or the entire pilS is transferred via a RecA-mediated recombination event into the pilE expression locus forming a new pilE variant. Both pilE and pilS consist of variable portions flanked by conserved portions; conserved sequences serve as a basis for recombination, which leads to insertion of new pilS into the pilE locus [145]. These pilE variants differ in their ability to be expressed and assembled; if they cannot be assembled into functional pili, this results in a non-piliated gonococcus (P−); the rate of variation is one of the highest in any bacterium [146]. In addition to amino acid substitutions, antigenic variation is achieved by differential ability to undergo modification by addition of phosphocholine and/or phosphoethanolamine groups [147]. Unlike phase variable proteins, which depend on slipped-strand mispairing, several DNA modification and repair mechanisms are involved in type IV pili antigenic variation, including RecA, RecQ, RecO, RecR, RecJ (RecF like pathway), RecX regulator, growth regulator RdgC, Rep helicase and Holliday junction processing enzymes RuvB and RecG [146]. The inter-strain variation in pilE and pilS remains understudied, especially in the context of correlation with pathogenesis and invasiveness; though with such a high intra-strain variability, it is difficult to predict how significant such variation would actually be.
Identification of new virulence factors – where could we look next?
There is significant scope for research into genetic variation of other, perhaps less prominent virulence factors, such as metabolic genes, which may uncover new pathogenesis mechanisms based on relative variability of proteins and regions within proteins. Genome-wide studies, such as a recent bioinformatic analysis by Bayliss and colleagues, describe the full phase variome (all phase variable genes) in gonococci and other Neisseria and may foster such discoveries [148].
The gonococcus possesses efflux pumps on its surface, with the best described the MtrCDE system with its repressor MtrR; mtrR mutations induce increased resistance to β-lactams [149, 150]. Another such system is FarAB–MtrE. Initially studied for their role in therapeutic antimicrobial efflux, they were soon found to be able to export human mucosal antimicrobial fatty acids. Both MtrCDE system and FarAB increase the minimum inhibitory concentration (MIC) of antibacterial fatty acids [150]. A study by Jerse and colleagues confirmed that a functional MtrCDE system increases bacterial survival in a female murine reproductive tract; FarAB did not confer competitive advantage in vivo [151]. On the contrary, a recent genome-wide association study (GWAS) confirmed enrichment of loss of function mutations in the MtrCDE pump components in cervical compared to urethral isolates, both for N. gonorrhoeae and urogenitally adapted N. meningitidis [152]. Still, one has to note that the gonococcus is a human-adapted pathogen; it may be that the effect of FarAB is detected only when faced with human-specific antimicrobial fatty acids. Furthermore, the pumps seem to have similar roles, and redundancy cannot be excluded.
In addition to expulsion of innate immune antimicrobials, gonococci are also able to inactivate some of these. For example, the gene katA encodes catalase, which deactivates reactive oxygen species and to some extent reactive nitrogen species – two important bacterial killing mechanisms employed by neutrophils and macrophages, respectively [153]. Another similar mechanism is ADP-ribosylation by NarE – a protein that only recently has been described in the gonococcus. Discovered to be a truncated variant of ADP-ribosyltransferase present in meningococci, it is able to ADP ribosylate human defensins HBD1, HBD2 and HBD4 [154]. However, all of the studies have only been performed with purified NarE [154]. Finally, the macrophage infectivity potentiator (Mip) protein was found in at least 20 gonococci by Starnino et al. [155]. Interestingly, the function is largely unknown except for the fact that the protein is a peptidyl-prolyl cis/trans isomerase (PPIase) that enhances macrophage but not epithelial cell infection [156].
Another interaction has been described that evades simplistic infection paradigms. Typically, pathogens subvert immune activation for their success. However, N. gonorrhoeae is associated with significant pyogenic inflammation (neutrophilic influx). Consistent with this, the gonococcus expresses lytic transglycosylases LtgA and LtgD. These enzymes break down cell wall peptidoglycan. Peptidoglycan fragments can act as cytotoxic NOD1/2 agonists, triggering inflammation, resulting in increased neutrophil influx to further disease-promoting inflammation [157]. If intentional, it suggests that immune evasion is not always in the best interest of the bacterium; some suggest that inflammation helps gonococcal spread. Even more interestingly, gonococcal LtgA and LtgD can aid survival in human neutrophils, indicating that perhaps immune overstimulation may be inhibitory [158].
Gonococcal metabolism may also have important implications in pathogenesis. Initial research begun in the second half of 20th century with the definition of gonococcal auxotypes. The nutrients used for auxotyping included ornithine, arginine, proline, uracil, hypoxanthine, leucine and methionine. Several auxotypes were defined; however, little association was found between different auxotypes and gonococcal pathogenesis [7, 159–161]. One exception to this is a correlation between DGI and the arginine, hypoxanthine, uracil (AHU)− auxotype with unknown causal mechanisms [162, 163]; another is dependence on lactate metabolism for long-term survival in human cervical epithelial cells, potentially vital for invasion and spread [164]. This is biologically plausible as an important metabolic pathway, especially that lactate is readily available in the female genital tract from commensal lactobacilli [164].
One metabolism-related mechanism vital for bacterial success is iron uptake. While the gonococcus lacks siderophores, it can obtain iron from host transferrin, haemoglobin and lactoferrin [165]. In an exemplar fashion, variability of iron-acquisition genes was analysed bioinformatically to reveal diversity of the HpuAB system in addition to previously described HmbR. In contrast to HmbR being present only in pathogenic Neisseria with gonococci possessing a non-functional hmbR gene, HpuAB is present in a wide range of Neisseria species with evidence of significant recombination and immune selection [166]. In particular, HpuA is known to be hypervariable as a consequence of phase variation [167]. TbpB, transferrin capturing protein, is also hypervariable. In contrast, TbpA has been reclassified into three major families, with several variable regions and 638 discovered alleles [59]. These data show that metabolic pathways hide previously under-appreciated insights into gonococcal genomics and pathogenesis – which could make them useful therapeutic and vaccine candidates in the future.
Concluding remarks – what are the challenges faced by gonococcal research and where should we proceed next?
N. gonorrhoeae is a pathogen that escapes many stereotypes. Because of that, development of vaccines and targeted therapeutics has stalled for decades. Virulence factors are being described in increasing detail, both the classical ones like LOS, PorB, Opa or type IV pili, as well as the non-canonical ones like iron-acquisition systems, efflux pumps or other immune modulation mechanisms.
Research into virulence factors has been improved due to the development of new research methods. Since the early 2000s, the development of whole-genome sequencing, including next-generation sequencing, has allowed rapid sequencing of whole bacterial genomes, which demonstrated significant sequence variation between bacterial strains. Several are currently available, and draft genome sequences are available for thousands of isolates in ever-expanding genomic databases such as PubMLST [168, 169]. The recent introduction of a non-polymerase-based method of Nanopore sequencing could potentially resolve difficult regions such as phase variable genes, an approach recently confirmed for yeast oligonucleotide tract repeats [170]. Affordable, reliable sequencing provides plenty of data that can be analysed to devise evolutionary patterns (e.g. as in US_NmUC meningococcal urethritis strain) and associations of particular genes or their polymorphisms with particular disease states. Similarly, whole-genome sequencing is the cornerstone of transcriptomics; the transcriptome of gonococci during mucosal infection was published recently, and similar studies may become very relevant to investigating pathogenesis in the future [171].
Furthermore, experimental approaches are needed. Here, two recent developments are key. One is the establishment of a murine female genital tract model of infection [172]. Prior to that, gonococcal studies could only be done either in vitro, from clinical patients and in male volunteers – which made them inherently limited in scope either due to the simplicity of in vitro systems or due to ethical concerns associated with human infection. The murine genital tract model has tested several putative virulence factors, novel therapeutics and vaccine candidates [173–175]. It is still limited by strict adaptation of several gonococcal virulence factors to human proteins; this was partially addressed by human transgenic mice, such as the CEACAM transgenic mice mentioned earlier [32, 176]. Another approach to this problem is the use of murine vaginal mucosa explants; they offer the benefit of investigating bacteria in association with complex 3D tissue of epithelial and innate immune cells, while being easier to manipulate than in vivo models [177, 178]. On the bacterial side, genetic studies including whole-genome sequencing have pioneered genetic modifications of the bacterium for causal and mechanistic studies into pathogenesis. Of note, Seifert and colleagues are currently developing a library of N. gonorrhoeae FA1090 deletion strains for all non-essential genes; once completed, it likely will dramatically increase the pace of research into pathogenesis [179].
However, there are some obstacles that still limit the field. The most important one is the complexity of many of the genes that are studied. Automatic genome annotation on the one hand allows huge amounts of genetic data obtained from isolate whole-genome sequencing to be processed, which is the case for the PubMLST database; at the same time, more difficult templates such as phase variable genes are often not annotated, as it is difficult to devise algorithms that work when genes have premature stop codons. Furthermore, similarity between some genes confuses annotation algorithms, and there are examples of wrongly annotated genes in the published databases. Finally, some of the templates are so complex that short-read sequencing methods such as Illumina sequencing are unable to reliably sequence them; as an example, ends of contigs commonly lie in longer poly-G tracts of phase variable genes. Combined, these make bioinformatic analyses challenging.
A second major issue is limitation in the selection of isolates that are available. Most isolates are cervical (female), urethral (male), anal or sometimes pharyngeal (both). The issue comes with acquiring DGI and PID case strains. For both of these disease manifestations, acquisition of bacterial samples from the site of infection is difficult. In the case of PID, it is very invasive to take samples from the upper genitourinary tract and almost never done. For DGI, the lesions are sometimes cultured; however, it is not uncommon to be unable to isolate viable bacteria directly from infected sites despite other methods, such as nucleic acid amplification tests (NAATs), confirming their presence [3]. Finally, ophthalmia neonatorum seems to have the least isolates classified. To show the scale of the problem, at the beginning of September 2022, out of 18 403 N. gonorrhoeae isolates published in the PubMLST database, only 17 had eye listed as their source, and only 5 of these were stated to have been isolated from children below the age of 1 – all coming from a single study based in Lisbon, Portugal [180].
Virulence factor genetic variability is an important area of study that could result in better diagnostics, therapeutics and vaccine development boost. This is more important in this bacterium, which is becoming increasingly dangerous to our communities and disproportionately affects low- and middle-income countries. Therefore, we hope that continued research efforts will help to unravel novel relationships of clinical potential in the future.
Funding information
K.K. was funded by an Amelia Jackson Senior Studentship, Exeter College, University of Oxford. O.B.H. was funded by the Wellcome Trust (214374/Z/18/Z).
Acknowledgements
We would like to thank Professor Christoph M. Tang, Professor Martin Maiden and Dr Rachel M. Exley for their insightful comments and help with reviewing of the manuscript.
Author contributions
K.K.: conceptualization (equal); data curation; investigation; visualization; writing – original draft preparation; writing – review and editing (equal). O.B.H.: conceptualization (equal); project administration; supervision; writing – review and editing (equal).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: DGI, disseminated gonococcal infection; HIV, human immunodeficiency virus; LOS, lipooligosaccharide; LPS, lipopolysaccharide; MLST, multilocus sequence typing; PID, pelvic inflammatory disease; ST, sequence type; STI, sexually transmitted infection.
References
- 1.Unemo M, Seifert HS, Hook EW, Hawkes S, Ndowa F, et al. Gonorrhoea. Nat Rev Dis Primers. 2019;5:79. doi: 10.1038/s41572-019-0128-6. [DOI] [PubMed] [Google Scholar]
- 2.Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16:226–240. doi: 10.1038/nrmicro.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkacem A, Caumes E, Ouanich J, Jarlier V, Dellion S, et al. Changing patterns of disseminated gonococcal infection in France: cross-sectional data 2009–2011. Sex Transm Infect. 2013;89:613–615. doi: 10.1136/sextrans-2013-051119. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Perez J, Hammer LA, Gallagher HC, De Jesus M, et al. Intravaginal administration of interleukin 12 during genital gonococcal infection in mice induces immunity to heterologous strains of Neisseria gonorrhoeae . mSphere. 2018;3:e00421-17. doi: 10.1128/mSphere.00421-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraus SJ. Incidence and therapy of gonococcal pharyngitis. Sex Transm Dis. 1979;6:143–147. doi: 10.1097/00007435-197904000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–543. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 7.Brunham RC, Plummer F, Slaney L, Rand F, DeWitt W. Correlation of auxotype and protein I type with expression of disease due to Neisseria gonorrhoeae . J Infect Dis. 1985;152:339–343. doi: 10.1093/infdis/152.2.339. [DOI] [PubMed] [Google Scholar]
- 8.Xu SX, Gray-Owen SD. Gonococcal pelvic inflammatory disease: placing mechanistic insights into the context of clinical and epidemiological observations. J Infect Dis. 2021;224:S56–S63. doi: 10.1093/infdis/jiab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119:597–602. doi: 10.1097/AOG.0b013e318244eda9. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Hatcher JD. Gonococcal Arthritis. Treasure Island, FL: StatPearls; 2021. [PubMed] [Google Scholar]
- 11.van de Wijgert JHHM, Morrison CS, Brown J, Kwok C, Van Der Pol B, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sex Transm Dis. 2009;36:357–364. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 12.Bettoni S, Shaughnessy J, Maziarz K, Ermert D, Gulati S, et al. C4BP-IgM protein as a therapeutic approach to treat Neisseria gonorrhoeae infections. JCI Insight. 2019;4:e131886. doi: 10.1172/jci.insight.131886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim KYL, Mullally CA, Haese EC, Kibble EA, McCluskey NR, et al. Anti-virulence therapeutic approaches for Neisseria gonorrhoeae . Antibiotics. 2021;10:103. doi: 10.3390/antibiotics10020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jen FE-C, Ketterer MR, Semchenko EA, Day CJ, Seib KL, et al. The Lst sialyltransferase of Neisseria gonorrhoeae can transfer keto-deoxyoctanoate as the terminal sugar of lipooligosaccharide: a glyco-achilles heel that provides a new strategy for vaccines to prevent gonorrhea. mBio. 2021;12:e03666-20. doi: 10.1128/mBio.03666-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semchenko EA, Everest-Dass AV, Jen FE-C, Mubaiwa TD, Day CJ, et al. Glycointeractome of Neisseria gonorrhoeae: identification of host glycans targeted by the gonococcus to facilitate adherence to cervical and urethral epithelial cells. mBio. 2019;10:e01339-19. doi: 10.1128/mBio.01339-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosini R, Nicchi S, Pizza M, Rappuoli R. Vaccines against antimicrobial resistance. Front Immunol. 2020;11:1048. doi: 10.3389/fimmu.2020.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu T, McClure R, Harrison OB, Genco C, Massari P. Integrated bioinformatic analyses and immune characterization of new Neisseria gonorrhoeae vaccine antigens expressed during natural mucosal infection. Vaccines. 2019;7:153. doi: 10.3390/vaccines7040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell MW, Jerse AE, Gray-Owen SD. Progress toward a gonococcal vaccine: the way forward. Front Immunol. 2019;10:2417. doi: 10.3389/fimmu.2019.02417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, et al. Biofilm formation by Neisseria gonorrhoeae . Infect Immun. 2005;73:1964–1970. doi: 10.1128/IAI.73.4.1964-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs MM, Sparling PF, Cohen MS, Shafer WM, Deal CD, et al. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front Microbiol. 2011;2:123. doi: 10.3389/fmicb.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JM, Dillard JP. Attention seeker: production, modification, and release of inflammatory peptidoglycan fragments in Neisseria species. J Bacteriol. 2017;199:e00354-17. doi: 10.1128/JB.00354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men – diagnosis, natural course, prevalence and significance. N Engl J Med. 2010;16:130–137. doi: 10.1056/NEJM197401172900301. [DOI] [PubMed] [Google Scholar]
- 23.Youssef DA, Peiris AN, Kelley JL, Grant WB. The possible roles of vitamin D and curcumin in treating gonorrhea. Med Hypotheses. 2013;81:131–135. doi: 10.1016/j.mehy.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Liu W, Russell MW. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol. 2014;7:165–176. doi: 10.1038/mi.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson L, Passmore J-AS, Liebenberg LJ, Werner L, Baxter C, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61:260–269. doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonck RA, Darville T, O’Connell CM, Jerse AE. Chlamydial infection increases gonococcal colonization in a novel murine coinfection model. Infect Immun. 2011;79:1566–1577. doi: 10.1128/IAI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyss SB, Kamb ML, Peterman TA, Moran JS, Newman DR, et al. Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 2003;139:178–185. doi: 10.7326/0003-4819-139-3-200308050-00007. [DOI] [PubMed] [Google Scholar]
- 28.Draper DL, James JF, Brooks GF, Sweet RL. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect Immun. 1980;27:882–888. doi: 10.1128/iai.27.3.882-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darville T. Pelvic inflammatory disease due to Neisseria gonorrhoeae and Chlamydia trachomatis: immune evasion mechanisms and pathogenic disease pathways. J Infect Dis. 2021;224:S39–S46. doi: 10.1093/infdis/jiab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Muylder X, Laga M, Thnnstedt C, Van Dyck E, Aelbers GNM, et al. The role of Neisseria gonorrhoeae and Chlamydia trachomatis in pelvic inflammatory disease and its sequelae in Zimbabwe. J Infect Dis. 1990;162:501–5058. doi: 10.1093/infdis/162.2.501. [DOI] [PubMed] [Google Scholar]
- 31.Cloud-Hansen KA, Hackett KT, Garcia DL, Dillard JP. Neisseria gonorrhoeae uses two lytic transglycosylases to produce cytotoxic peptidoglycan monomers. J Bacteriol. 2008;190:5989–5994. doi: 10.1128/JB.00506-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam EA, Anipindi VC, Francis I, Shaik-Dasthagirisaheb Y, Xu S, et al. Specific binding to differentially expressed human carcinoembryonic antigen-related cell adhesion molecules determines the outcome of Neisseria gonorrhoeae infections along the female reproductive tract. Infect Immun. 2018;86:e00092-18. doi: 10.1128/IAI.00092-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogbebor O, Mortimer TD, Fryling K, Zhang JJ, Bhanot N, et al. Disseminated gonococcal infection complicated by prosthetic joint infection: case report and genomic and phylogenetic analysis. Open Forum Infect Dis. 2021;8:ofaa632. doi: 10.1093/ofid/ofaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoolnik GK, Buchanan TM, Holmes KK. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976;58:1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raphael B, Cartee J, Joseph S, Sharpe S, Roland B. P139 A phylogenomic survey of disseminated gonococcal infection isolates in the United States (2019–2020) Sex Transm Infec. 2021;97(Suppl 1):A95. doi: 10.1136/sextrans-2021-sti.250. [DOI] [Google Scholar]
- 36.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, et al. C4bp binding to porin mediates stable serum resistance of Neisseria gonorrhoeae . Int Immunopharmacol. 2001;1:423–432. doi: 10.1016/s1567-5769(00)00037-0. [DOI] [PubMed] [Google Scholar]
- 37.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae . J Exp Med. 2001;193:281–295. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal S, Ram S, Ngampasutadol J, Gulati S, Zipfel PF, et al. Factor H facilitates adherence of Neisseria gonorrhoeae to complement receptor 3 on eukaryotic cells. J Immunol. 2010;185:4344–4353. doi: 10.4049/jimmunol.0904191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesner PJ, Tronca E, Bonin P, Pedersen AHB, Holmes KK. Clinical spectrum of pharyngeal gonococcal infection. N Engl J Med. 1973;288:181–185. doi: 10.1056/NEJM197301252880404. [DOI] [PubMed] [Google Scholar]
- 40.Fransen L, Nsanze H, Klauss V, Van der Stuyft P, D’Costa L, et al. Ophthalmia neonatorum in Nairobi Kenya: the roles of Neisseria gonorrhoeae and Chlamydia trachomatis . J Infect Dis. 1986;153:862–869. doi: 10.1093/infdis/153.5.862. [DOI] [PubMed] [Google Scholar]
- 41.Wan WL, Farkas GC, May WN, Robin JB. The clinical characteristics and course of adult gonococcal conjunctivitis. Am J Ophthalmol. 1986;102:575–583. doi: 10.1016/0002-9394(86)90527-1. [DOI] [PubMed] [Google Scholar]
- 42.Kim JJ, Mandrell RE, Griffiss JM. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2, and 3 protein epitopes. Infect Immun. 1989;57:602–608. doi: 10.1128/iai.57.2.602-608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deasy AM, Guccione E, Dale AP, Andrews N, Evans CM, et al. Nasal inoculation of the commensal Neisseria lactamica inhibits carriage of Neisseria meningitidis by young adults: a controlled human infection study. Clin Infect Dis. 2015;60:1512–1520. doi: 10.1093/cid/civ098. [DOI] [PubMed] [Google Scholar]
- 44.Caugant DA, Høiby EA, Magnus P, Scheel O, Hoel T, et al. Asymptomatic carriage of Neisseria meningitidis in a randomly sampled population. J Clin Microbiol. 1994;32:323–330. doi: 10.1128/jcm.32.2.323-330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caugant DA, Brynildsrud OB. Neisseria meningitidis: using genomics to understand diversity, evolution and pathogenesis. Nat Rev Microbiol. 2020;18:84–96. doi: 10.1038/s41579-019-0282-6. [DOI] [PubMed] [Google Scholar]
- 46.Cehovin A, Jolley KA, Maiden MCJ, Harrison OB, Tang CM. Association of Neisseria gonorrhoeae plasmids with distinct lineages and the economic status of their country of origin. J Infect Dis. 2020;222:1826–1836. doi: 10.1093/infdis/jiaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duffin PM, Seifert HS. DNA uptake sequence-mediated enhancement of transformation in Neisseria gonorrhoeae is strain dependent. J Bacteriol. 2010;192:4436–4444. doi: 10.1128/JB.00442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett JS, Bentley SD, Vernikos GS, Quail MA, Cherevach I, et al. Independent evolution of the core and accessory gene sets in the genus Neisseria: insights gained from the genome of Neisseria lactamica isolate 020-06. BMC Genomics. 2010;11:652. doi: 10.1186/1471-2164-11-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernando I, Palmer HM, Young H. Characteristics of patients infected with common Neisseria gonorrhoeae NG-MAST sequence type strains presenting at the Edinburgh genitourinary medicine clinic. Sex Transm Infect. 2009;85:443–446. doi: 10.1136/sti.2008.034538. [DOI] [PubMed] [Google Scholar]
- 50.Harrison OB, Cehovin A, Skett J, Jolley KA, Massari P, et al. Neisseria gonorrhoeae population genomics: use of the gonococcal core genome to improve surveillance of antimicrobial resistance. J Infect Dis. 2020;222:1816–1825. doi: 10.1093/infdis/jiaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison OB, Maiden MC. Recent advances in understanding and combatting Neisseria gonorrhoeae: a genomic perspective. Fac Rev. 2021;10:65. doi: 10.12703/r/10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rotman E, Seifert HS. The genetics of Neisseria species. Annu Rev Genet. 2014;48:405–431. doi: 10.1146/annurev-genet-120213-092007. [DOI] [PubMed] [Google Scholar]
- 53.Tuomanen EI, van der Ende A, Hopman CTP, Dankert J. Multiple mechanisms of phase variation of PorA in Neisseria meningitidis . Infect Immun. 2000;68:6685–6690. doi: 10.1128/IAI.68.12.6685-6690.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shafer WM, Datta A, Kumar Kolli VS, Mahbubur Rahman M, Balthazar JT, et al. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J Endotoxin Res. 2002;8:47–58. doi: 10.1177/09680519020080010501. [DOI] [PubMed] [Google Scholar]
- 55.Diallo K, MacLennan J, Harrison OB, Msefula C, Sow SO, et al. Genomic characterization of novel Neisseria species. Sci Rep. 2019;9:13742. doi: 10.1038/s41598-019-50203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder LAS, Saunders NJ. The majority of genes in the pathogenic Neisseria species are present in non-pathogenic Neisseria lactamica, including those designated as “virulence genes.”. BMC Genomics. 2006;7:128. doi: 10.1186/1471-2164-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snyder LAS, Jarvis SA, Saunders NJ. Complete and variant forms of the “gonococcal genetic island” in Neisseria meningitidis . Microbiology. 2005;151:4005–4013. doi: 10.1099/mic.0.27925-0. [DOI] [PubMed] [Google Scholar]
- 58.Marri PR, Paniscus M, Weyand NJ, Rendón MA, Calton CM, et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One. 2010;5:e11835. doi: 10.1371/journal.pone.0011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baarda BI, Zielke RA, Holm AK, Sikora AE. Comprehensive bioinformatic assessments of the variability of Neisseria gonorrhoeae vaccine candidates. mSphere. 2021;6:e00977-20. doi: 10.1128/mSphere.00977-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider MC, Exley RM, Chan H, Feavers I, Kang Y-H, et al. Functional significance of factor H binding to Neisseria meningitidis . J Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 61.Tzeng Y-L, Bazan JA, Turner AN, Wang X, Retchless AC, et al. Emergence of a new Neisseria meningitidis clonal complex 11 lineage 11.2 clade as an effective urogenital pathogen. Proc Natl Acad Sci. 2017;114:4237–4242. doi: 10.1073/pnas.1620971114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Retchless AC, Kretz CB, Chang H-Y, Bazan JA, Abrams AJ, et al. Expansion of a urethritis-associated Neisseria meningitidis clade in the United States with concurrent acquisition of N. gonorrhoeae alleles. BMC Genomics. 2018;19:176. doi: 10.1186/s12864-018-4560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tzeng YL, Giuntini S, Berman Z, Sannigrahi S, Granoff DM, et al. Neisseria meningitidis urethritis outbreak isolates express a novel factor H binding protein variant that is a potential target of group B-directed meningococcal (MenB) vaccines. Infect Immun. 2020;88:e00462-20. doi: 10.1128/IAI.00462-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bazan JA, Tzeng Y-L, Stephens DS, Carter AM, Brown MA, et al. Repeat episodes of symptomatic urethritis due to a uropathogenic meningococcal clade. Sex Transm Dis. 2020;47:e1–e4. doi: 10.1097/OLQ.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strauss J, Burnham NA, Camesano TA. Atomic force microscopy study of the role of LPS O-antigen on adhesion of E. coli . J Mol Recognit. 2009;22:347–355. doi: 10.1002/jmr.955. [DOI] [PubMed] [Google Scholar]
- 66.Kahler CM, Stephens DS. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide. Crit Rev Microbiol. 1998;24:281–334. doi: 10.1080/10408419891294216. [DOI] [PubMed] [Google Scholar]
- 67.Piekarowicz A, Stein DC. Biochemical properties of Neisseria gonorrhoeae LgtE. J Bacteriol. 2002;184:6410–6416. doi: 10.1128/JB.184.23.6410-6416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erwin AL, Haynes PA, Rice PA, Gotschlich EC. Conservation of the lipooligosaccharide synthesis locus lgt among strains of Neisseria gonorrhoeae: requirement for lgtE in synthesis of the 2C7 epitope and of the beta chain of strain 15253. J Exp Med. 1996;184:1233–1241. doi: 10.1084/jem.184.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burch CL, Danaher RJ, Stein DC. Antigenic variation in Neisseria gonorrhoeae: production of multiple lipooligosaccharides. J Bacteriol. 1997;179:982–986. doi: 10.1128/jb.179.3.982-986.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Apicella MA, Bennett KM, Hermerath CA, Roberts DE. Monoclonal antibody analysis of lipopolysaccharide from Neisseria gonorrhoeae and Neisseria meningitidis . Infect Immun. 1981;34:751–756. doi: 10.1128/iai.34.3.751-756.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gidney MAJ, Plested JS, Lacelle S, Coull PA, Wright JC, et al. Development, characterization, and functional activity of a panel of specific monoclonal antibodies to inner core lipopolysaccharide epitopes in Neisseria meningitidis . Infect Immun. 2004;72:559–569. doi: 10.1128/IAI.72.1.559-569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai CM. Molecular mimicry of host structures by lipooligosaccharides of Neisseria meningitidis: characterization of sialylated and nonsialylated lacto-N-neotetraose (Galß1-4GlcNAcß1-3Galβ1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins. Adv Exp Med Biol. 2001;491:525–542. doi: 10.1007/978-1-4615-1267-7. [DOI] [PubMed] [Google Scholar]
- 73.Zhu P, Klutch MJ, Tsai C-M. Genetic analysis of conservation and variation of lipooligosaccharide expression in two L8-immunotype strains of Neisseria meningitidis . FEMS Microbiol Lett. 2001;203:173–177. doi: 10.1111/j.1574-6968.2001.tb10837.x. [DOI] [PubMed] [Google Scholar]
- 74.Harvey HA, Porat N, Campbell CA, Jennings M, Gibson BW, et al. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol Microbiol. 2000;36:1059–1070. doi: 10.1046/j.1365-2958.2000.01938.x. [DOI] [PubMed] [Google Scholar]
- 75.Harvey HA, Swords WE, Apicella MA. The mimicry of human glycolipids and glycosphingolipids by the lipooligosaccharides of pathogenic Neisseria and Haemophilus . J Autoimmun. 2001;16:257–262. doi: 10.1006/jaut.2000.0477. [DOI] [PubMed] [Google Scholar]
- 76.Edwards JL, Apicella MA. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol. 2002;4:585–598. doi: 10.1046/j.1462-5822.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 77.Lewis LA, Shafer WM, Dutta Ray T, Ram S, Rice PA. Phosphoethanolamine residues on the lipid a moiety of Neisseria gonorrhoeae lipooligosaccharide modulate binding of complement inhibitors and resistance to complement killing. Infect Immun. 2013;81:33–42. doi: 10.1128/IAI.00751-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JJ, Zhou D, Mandrell RE, Griffiss JM. Effect of exogenous sialylation of the lipooligosaccharide of Neisseria gonorrhoeae on opsonophagocytosis. Infect Immun. 1992;60:4439–4442. doi: 10.1128/iai.60.10.4439-4442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, et al. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J Immunol. 2008;180:3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 81.Gulati S, Cox A, Lewis LA, Michael FS, Li J, et al. Enhanced factor H binding to sialylated gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in gonococci. Infect Immun. 2005;73:7390–7397. doi: 10.1128/IAI.73.11.7390-7397.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaughnessy J, Ram S, Bhattacharjee A, Pedrosa J, Tran C, et al. Molecular characterization of the interaction between sialylated Neisseria gonorrhoeae and factor H. J Biol Chem. 2011;286:22235–22242. doi: 10.1074/jbc.M111.225516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song W, Ma L, Chen R, Stein DC. Role of lipooligosaccharide in Opa-independent invasion of Neisseria gonorrhoeae into human epithelial cells. J Exp Med. 2000;191:949–960. doi: 10.1084/jem.191.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ram S, Gulati S, Lewis LA, Chakraborti S, Zheng B, et al. A novel sialylation site on Neisseria gonorrhoeae lipooligosaccharide links heptose II lactose expression with pathogenicity. Infect Immun. 2018;86:e00285-18. doi: 10.1128/IAI.00285-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis LA, Gulati S, Burrowes E, Zheng B, Ram S, et al. α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract. mBio. 2015;6:e02465-14. doi: 10.1128/mBio.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mandrell RE, Kim JJ, John CM, Gibson BW, Sugai JV, et al. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis . J Bacteriol. 1991;173:2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wakarchuk WW, Watson D, St. Michael F, Li J, Wu Y, et al. Dependence of the bi-functional nature of a sialyltransferase from Neisseria meningitidis on a single amino acid substitution. J Biol Chem. 2001;276:12785–12790. doi: 10.1074/jbc.M011293200. [DOI] [PubMed] [Google Scholar]
- 88.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, et al. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae . J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Vliet SJ, Steeghs L, Bruijns SCM, Vaezirad MM, Snijders Blok C, et al. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS Pathog. 2009;5:e1000625. doi: 10.1371/journal.ppat.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.John CM, Jarvis GA, Swanson KV, Leffler H, Cooper MD, et al. Galectin-3 binds lactosaminylated lipooligosaccharides from Neisseria gonorrhoeae and is selectively expressed by mucosal epithelial cells that are infected. Cell Microbiol. 2002;4:649–662. doi: 10.1046/j.1462-5822.2002.00219.x. [DOI] [PubMed] [Google Scholar]
- 91.Minor SY, Banerjee A, Gotschlich EC. Effect of alpha-oligosaccharide phenotype of Neisseria gonorrhoeae strain MS11 on invasion of Chang conjunctival, HEC-1-B endometrial, and ME-180 cervical cells. Infect Immun. 2000;68:6526–6534. doi: 10.1128/IAI.68.12.6526-6534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schneider H, Schmidt KA, Skillman DR, Van De Verg L, Warren RL, et al. Sialylation lessens the infectivity of Neisseria gonorrhoeae MS11mkC. J Infect Dis. 1996;173:1422–1427. doi: 10.1093/infdis/173.6.1422. [DOI] [PubMed] [Google Scholar]
- 93.van Putten JP. Phase variation of lipopolysaccharide directs interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae . EMBO J. 1993;12:4043–4051. doi: 10.1002/j.1460-2075.1993.tb06088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Judd RC. Protein I: structure, function, and genetics. Clin Microbiol Rev. 1989;2 (Suppl.):S41–S48. doi: 10.1128/CMR.2.Suppl.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barrera O, Swanson J. Proteins IA and IB exhibit different surface exposures and orientations in the outer membranes of Neisseria gonorrhoeae . Infect Immun. 1984;44:565–568. doi: 10.1128/iai.44.3.565-568.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kattner C, Zaucha J, Jaenecke F, Zachariae U, Tanabe M. Identification of a cation transport pathway in Neisseria meningitidis PorB. Proteins. 2013;81:830–840. doi: 10.1002/prot.24241. [DOI] [PubMed] [Google Scholar]
- 97.Chen A, Seifert HS. Saturating mutagenesis of an essential gene: a majority of the Neisseria gonorrhoeae major outer membrane porin (PorB) is mutable. J Bacteriol. 2014;196:540–547. doi: 10.1128/JB.01073-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cannon JG, Buchanan TM, Sparling PF. Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection. Infect Immun. 1983;40:816–819. doi: 10.1128/iai.40.2.816-819.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Putten JP, Duensing TD, Carlson J. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J Exp Med. 1998;188:941–952. doi: 10.1084/jem.188.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ayala P, Vasquez B, Wetzler L, So M. Neisseria gonorrhoeae porin P1.B induces endosome exocytosis and a redistribution of Lamp1 to the plasma membrane. Infect Immun. 2002;70:5965–5971. doi: 10.1128/IAI.70.11.5965-5971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Landig CS, Hazel A, Kellman BP, Fong JJ, Schwarz F, et al. Evolution of the exclusively human pathogen Neisseria gonorrhoeae: human-specific engagement of immunoregulatory Siglecs. Evol Appl. 2019;12:337–349. doi: 10.1111/eva.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duncan JA, Gao X, Huang MT-H, O’Connor BP, Thomas CE, et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lorenzen DR, Günther D, Pandit J, Rudel T, Brandt E, et al. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infect Immun. 2000;68:6215–6222. doi: 10.1128/IAI.68.11.6215-6222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Massari P, Ho Y, Wetzler LM. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc Natl Acad Sci. 2000;97:9070–9075. doi: 10.1073/pnas.97.16.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Virji M, Zak K, Heckels JE. Monoclonal antibodies to gonococcal outer membrane protein IB: use in investigation of the potential protective effect of antibodies directed against conserved and type-specific epitopes. J Gen Microbiol. 1986;132:1621–1629. doi: 10.1099/00221287-132-6-1621. [DOI] [PubMed] [Google Scholar]
- 106.Virji M, Fletcher JN, Zak K, Heckels JE. The potential protective effect of monoclonal antibodies to gonococcal outer membrane protein IA. J Gen Microbiol. 1987;133:2639–2646. doi: 10.1099/00221287-133-9-2639. [DOI] [PubMed] [Google Scholar]
- 107.Elkins C, Carbonetti NH, Varela VA, Stirewalt D, Klapper DG, et al. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol Microbiol. 1992;6:2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 108.Burns DL, Derrick JP, Urwin R, Suker J, Feavers IM, et al. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect Immun. 1999;67:2406–2413. doi: 10.1128/IAI.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pérez-Losada M, Viscidi RP, Demma JC, Zenilman J, Crandall KA. Population genetics of Neisseria gonorrhoeae in a high-prevalence community using a hypervariable outer membrane porB and 13 slowly evolving housekeeping genes. Mol Biol Evol. 2005;22:1887–1902. doi: 10.1093/molbev/msi184. [DOI] [PubMed] [Google Scholar]
- 110.Fudyk TC, Maclean IW, Simonsen JN, Njagi EN, Kimani J, et al. Genetic diversity and mosaicism at the por locus of Neisseria gonorrhoeae . J Bacteriol. 1999;181:5591–5599. doi: 10.1128/JB.181.18.5591-5599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carbonetti NH, Simnad VI, Seifert HS, So M, Sparling PF. Genetics of protein I of Neisseria gonorrhoeae: construction of hybrid porins. Proc Natl Acad Sci. 1988;85:6841–6845. doi: 10.1073/pnas.85.18.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swanson J. Studies on gonococcus infection. XII. Colony color and opacity variants of gonococci. Infect Immun. 1978;19:320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bhat KS, Gibbs CP, Barrera O, Morrison SG, Jähnig F, et al. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 114.Hill SA, Masters TL, Wachter J. Gonorrhea – an evolving disease of the new millennium. Microb Cell. 2016;3:371–389. doi: 10.15698/mic2016.09.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ball LM, Criss AK. Constitutively Opa-expressing and Opa-deficient Neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. J Bacteriol. 2013;195:2982–2990. doi: 10.1128/JB.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Billker O, Popp A, Gray-Owen SD, Meyer TF. The structural basis of CEACAM-receptor targeting by neisserial Opa proteins. Trends Microbiol. 2000;8:258–260. doi: 10.1016/s0966-842x(00)01771-6. [DOI] [PubMed] [Google Scholar]
- 117.Virji M, Watt SM, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae . Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 118.Wu C-T, Huang P-W, Lin C-H, Stein DC, Song W, et al. In vitro analysis of matched isolates from localized and disseminated gonococcal infections suggests that opa expression impacts clinical outcome. Pathogens. 2022;11:217. doi: 10.3390/pathogens11020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu Q, Chow EMC, McCaw SE, Hu N, Byrd D, et al. Association of Neisseria gonorrhoeae Opa(CEA) with dendritic cells suppresses their ability to elicit an HIV-1-specific T cell memory response. PLoS One. 2013;8:e56705. doi: 10.1371/journal.pone.0056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bos MP, Grunert F, Belland RJ. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae . Infect Immun. 1997;65:2353–2361. doi: 10.1128/iai.65.6.2353-2361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lambden PR, Heckels JE, James LT, Watt PJ. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae . J General Microbiol. 1979;114:305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- 122.Virji M, Everson JS. Comparative virulence of opacity variants of Neisseria gonorrhoeae strain P9. Infect Immun. 1981;31:965–970. doi: 10.1128/iai.31.3.965-970.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kupsch EM, Knepper B, Kuroki T, Heuer I, Meyer TF. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halter R, Pohlner J, Meyer TF. IgA protease of Neisseria gonorrhoeae: isolation and characterization of the gene and its extracellular product. EMBO J. 1984;3:1595–1601. doi: 10.1002/j.1460-2075.1984.tb02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pohlner J, Halter R, Beyreuther K, Meyer TF. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 126.Klauser T, Pohlner J, Meyer TF. The secretion pathway of IgA protease-type proteins in gram-negative bacteria. Bioessays. 1993;15:799–805. doi: 10.1002/bies.950151205. [DOI] [PubMed] [Google Scholar]
- 127.Plaut AG, Gilbert JV, Artenstein MS, Capra JD. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975;190:1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- 128.Lin L, Ayala P, Larson J, Mulks M, Fukuda M, et al. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 129.Ayala P, Lin L, Hopper S, Fukuda M, So M. Infection of epithelial cells by pathogenic neisseriae reduces the levels of multiple lysosomal constituents. Infect Immun. 1998;66:5001–5007. doi: 10.1128/IAI.66.10.5001-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hopper S, Vasquez B, Merz A, Clary S, Wilbur JS, et al. Effects of the immunoglobulin A1 protease on Neisseria gonorrhoeae trafficking across polarized T84 epithelial monolayers. Infect Immun. 2000;68:906–911. doi: 10.1128/IAI.68.2.906-911.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Halter R, Pohlner J, Meyer TF. Mosaic-like organization of IgA protease genes in Neisseria gonorrhoeae generated by horizontal genetic exchange in vivo. EMBO J. 1989;8:2737–2744. doi: 10.1002/j.1460-2075.1989.tb08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lomholt H, Poulsen K, Kilian M. Comparative characterization of the iga gene encoding IgA1 protease in Neisseria meningitidis, Neisseria gonorrhoeae and Haemophilus influenzae. Mol Microbiol. 1995;15:495–506. doi: 10.1111/j.1365-2958.1995.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 133.Mulks MH, Plaut AG. IgA protease production as a characteristic distinguishing pathogenic from harmless Neisseriaceae . N Engl J Med. 1978;299:973–976. doi: 10.1056/NEJM197811022991802. [DOI] [PubMed] [Google Scholar]
- 134.Johannsen DB, Johnston DM, Koymen HO, Cohen MS, Cannon JG. A Neisseria gonorrhoeae immunoglobulin A1 protease mutant is infectious in the human challenge model of urethral infection. Infect Immun. 1999;67:3009–3013. doi: 10.1128/IAI.67.6.3009-3013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang F, Coureuil M, Osinski T, Orlova A, Altindal T, et al. Cryoelectron microscopy reconstructions of the Pseudomonas aeruginosa and Neisseria gonorrhoeae type IV pili at sub-nanometer resolution. Structure. 2017;25:1423–1435. doi: 10.1016/j.str.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Higashi DL, Lee SW, Snyder A, Weyand NJ, Bakke A, et al. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect Immun. 2007;75:4743–4753. doi: 10.1128/IAI.00687-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rudel T, van Putten JPM, Gibbs CP, Haas R, Meyer TF. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol Microbiol. 1992;6:3439–3450. doi: 10.1111/j.1365-2958.1992.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 138.Rudel T, Scheurerpflug I, Meyer TF. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 139.Winther-Larsen HC, Hegge FT, Wolfgang M, Hayes SF, van Putten JP, et al. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc Natl Acad Sci. 2001;98:15276–15281. doi: 10.1073/pnas.261574998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Higashi DL, Zhang GH, Biais N, Myers LR, Weyand NJ, et al. Influence of type IV pilus retraction on the architecture of the Neisseria gonorrhoeae-infected cell cortex. Microbiology. 2009;155:4084–4092. doi: 10.1099/mic.0.032656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim WJ, Mai A, Weyand NJ, Rendón MA, Van Doorslaer K, et al. Neisseria gonorrhoeae evades autophagic killing by downregulating CD46-cyt1 and remodeling lysosomes. PLoS Pathog. 2019;15:e1007495. doi: 10.1371/journal.ppat.1007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Plant LJ, Jonsson AB. Type IV pili of Neisseria gonorrhoeae influence the activation of human CD4+ T cells. Infect Immun. 2006;74:442–448. doi: 10.1128/IAI.74.1.442-448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blom AM, Rytkönen A, Vasquez P, Lindahl G, Dahlbäck B, et al. A novel interaction between type IV pili of Neisseria gonorrhoeae and the human complement regulator C4B-binding protein. J Immunol. 2001;166:6764–6770. doi: 10.4049/jimmunol.166.11.6764. [DOI] [PubMed] [Google Scholar]
- 144.Hamrick TS, Dempsey JAF, Cohen MS, Cannon JG. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology. 2001;147:839–849. doi: 10.1099/00221287-147-4-839. [DOI] [PubMed] [Google Scholar]
- 145.Haas R, Meyer TF. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 146.Criss AK, Kline KA, Seifert HS. The frequency and rate of pilin antigenic variation in Neisseria gonorrhoeae . Mol Microbiol. 2005;58:510–519. doi: 10.1111/j.1365-2958.2005.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hegge FT, Hitchen PG, Aas FE, Kristiansen H, Løvold C, et al. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc Natl Acad Sci. 2004;101:10798–10803. doi: 10.1073/pnas.0402397101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wanford JJ, Green LR, Aidley J, Bayliss CD. Phasome analysis of pathogenic and commensal Neisseria species expands the known repertoire of phase variable genes, and highlights common adaptive strategies. PLoS One. 2018;13:e0196675. doi: 10.1371/journal.pone.0196675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Warner DM, Shafer WM, Jerse AE. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol. 2008;70:462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee EH, Shafer WM. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol Microbiol. 1999;33:839–845. doi: 10.1046/j.1365-2958.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 151.Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, et al. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun. 2003;71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ma KC, Mortimer TD, Hicks AL, Wheeler NE, Sánchez-Busó L, et al. Adaptation to the cervical environment is associated with increased antibiotic susceptibility in Neisseria gonorrhoeae . Nat Commun. 2020;11:4126. doi: 10.1038/s41467-020-17980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Spence SA, Clark VL, Isabella VM. The role of catalase in gonococcal resistance to peroxynitrite. Microbiology. 2012;158:560–570. doi: 10.1099/mic.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodas PI, Álamos-Musre AS, Álvarez FP, Escobar A, Tapia CV, et al. The NarE protein of Neisseria gonorrhoeae catalyzes ADP-ribosylation of several ADP-ribose acceptors despite an N-terminal deletion. FEMS Microbiol Lett. 2016;363:fnw181. doi: 10.1093/femsle/fnw181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Starnino S, Leuzzi R, Ghisetti V, De Francesco MA, Cusini M, et al. Molecular analysis of two novel Neisseria gonorrhoeae virulent components: the macrophage infectivity potentiator and the outer membrane protein A. New Microbiol. 2010;33:167–170. [PubMed] [Google Scholar]
- 156.Leuzzi R, Serino L, Scarselli M, Savino S, Fontana MR, et al. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol. 2005;58:669–681. doi: 10.1111/j.1365-2958.2005.04859.x. [DOI] [PubMed] [Google Scholar]
- 157.Mavrogiorgos N, Mekasha S, Yang Y, Kelliher MA, Ingalls RR. Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response. Innate Immun. 2014;20:377–389. doi: 10.1177/1753425913493453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ragland SA, Schaub RE, Hackett KT, Dillard JP, Criss AK. Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils. Cell Microbiol. 2017;19:e12662. doi: 10.1111/cmi.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Copley CG. Neisseria gonorrhoeae: subdivision of auxogroups by genetic transformation. Genitourin Med. 1987;63:153–156. doi: 10.1136/sti.63.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carifo K, Catlin BW. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973;26:223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jacques M, Turgeon PL, deRepentigny J, Mathieu LG. Antibiotic susceptibilities and auxotypes of Neisseria gonorrhoeae strains from women with pelvic inflammatory disease or uncomplicated infections. Antimicrob Agents Chemother. 1983;24:952–954. doi: 10.1128/AAC.24.6.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Knapp JS, Holmes KK. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975;132:204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- 163.Noble RC, Reyes RR, Parekh MC, Haley JV. Incidence of disseminated gonococcal infection correlated with the presence of AHU auxotype of Neisseria gonorrhoeae in a community. Sex Transm Dis. 1984;11:68–71. doi: 10.1097/00007435-198404000-00003. [DOI] [PubMed] [Google Scholar]
- 164.Chen NH, Ong C-LY, O’Sullivan J, Ibranovic I, Davey K, et al. Two distinct L-lactate dehydrogenases play a role in the survival of Neisseria gonorrhoeae in cervical epithelial cells. J Infect Dis. 2020;221:449–453. doi: 10.1093/infdis/jiz468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mickelsen PA, Sparling PF. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harrison OB, Bennett JS, Derrick JP, Maiden MCJ, Bayliss CD. Distribution and diversity of the haemoglobin–haptoglobin iron-acquisition systems in pathogenic and non-pathogenic Neisseria . Microbiology. 2013;159:1920–1930. doi: 10.1099/mic.0.068874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen CJ, Elkins C, Sparling PF. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae . Infect Immun. 1998;66:987–993. doi: 10.1128/IAI.66.3.987-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bratcher HB, Corton C, Jolley KA, Parkhill J, Maiden MCJ. A gene-by-gene population genomics platform: de novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genomics. 2014;15:1138. doi: 10.1186/1471-2164-15-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McGinty RJ, Rubinstein RG, Neil AJ, Dominska M, Kiktev D, et al. Nanopore sequencing of complex genomic rearrangements in yeast reveals mechanisms of repeat-mediated double-strand break repair. Genome Res. 2017;27:2072–2082. doi: 10.1101/gr.228148.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moreau MR, Massari P, Genco CA. The ironclad truth: how in vivo transcriptomics and in vitro mechanistic studies shape our understanding of Neisseria gonorrhoeae gene regulation during mucosal infection. Pathog Dis. 2017;75:ftx057. doi: 10.1093/femspd/ftx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, et al. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front Microbiol. 2011;2:107. doi: 10.3389/fmicb.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sikora AE, Gomez C, Le Van A, Baarda BI, Darnell S, et al. A novel gonorrhea vaccine composed of MetQ lipoprotein formulated with CpG shortens experimental murine infection. Vaccine. 2020;38:8175–8184. doi: 10.1016/j.vaccine.2020.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu Y, Hammer LA, Liu W, Hobbs MM, Zielke RA, et al. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017;10:1594–1608. doi: 10.1038/mi.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Song W, Condron S, Mocca BT, Veit SJ, Hill D, et al. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine. 2008;26:5741–5751. doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rice PA, Shafer WM, Ram S, Jerse AE. Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development. Annu Rev Microbiol. 2017;71:665–686. doi: 10.1146/annurev-micro-090816-093530. [DOI] [PubMed] [Google Scholar]
- 177.Liu Y, Islam EA, Jarvis GA, Gray-Owen SD, Russell MW. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-β-dependent mechanisms. Mucosal Immunol. 2012;5:320–331. doi: 10.1038/mi.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seifert HS. Gc Insertional Mutants. https://ngosociety.org/ngors-useful-links/ [Google Scholar]
- 180.Pinto M, Borges V, Isidro J, Rodrigues JC, Vieira L, et al. Neisseria gonorrhoeae clustering to reveal major European whole-genome-sequencing-based genogroups in association with antimicrobial resistance. Microb Genom. 2021;7:000481. doi: 10.1099/mgen.0.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zollinger WD, Mandrell RE. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]