Extract
Optimal characterisation of early structural abnormalities has been highlighted as critical to future efforts to detect and prevent cystic fibrosis (CF) lung disease progression [1]. Computed tomography (CT) is currently the gold standard, detecting structural disease at an earlier stage than radiography or lung function testing, with predictive value for subsequent progression and later forced expiratory volume in 1 s (FEV1) decline [1]. Compared to other emerging radiological modalities for early disease detection, such as magnetic resonance imaging (MRI), CT is more widely accessible and MRI at present is not able to directly quantify the extent of bronchiectasis due to its limitations in spatial resolution. Current US CF Foundation clinical guidelines state that CT should be “considered every 2–3 years, using the lowest possible radiation dose” [2]. Early lung disease is identified on CT by air trapping, increase in airway wall thickness and airway diameter, and potentially irreversible bronchiectasis [3]. Spirometer-directed CT has standardised lung volume acquisition and outlined the importance of both inspiratory and expiratory image acquisition [4]. Consensus recommendations for “low dose” CT scanning were published in 2016 [5], but radiation exposure remains a concern, given the six-fold increase in CT use and ongoing increase in cumulative radiation exposure for CF patients [6]. Imaging requires optimal structural information at the lowest possible radiation dose [5]. Given the increased vulnerability of children to ionising radiation, “low dose” CT protocols have already been incorporated in paediatric clinical trials using CT-based primary outcome measures [7], and use of ultra-low dose (ULD) CT has emerged in adult CF research and in isolated paediatric patients [6]. However, formal validation of the effects on quantitative measures of structural disease has not been performed. Low dose (LD) CT shows limitations compared to standard dose CT because of known drifts in CT lung density dependent on dose [8]. We hypothesised that ULD implementation would not detrimentally affect structural disease detection in early CF lung disease, and specifically targeted an age range to explore this where volume variation during spirometry-directed CT could be minimised between imaging modalities. Thus, the primary aims in this study were to 1) implement spirometer-directed novel ULD CT in school-aged children with CF, and 2) validate its outcomes against same-session LD CT using visual as well as fully automated quantitative CT analysis for structural airway disease.
Tweetable abstract
Early cystic fibrosis lung disease requires urgent sensitive outcome measures. Ultra-low dose CT provides comparable quantitative structural insight into bronchiectasis compared to conventional low dose CT, but higher rates of air trapping. https://bit.ly/3Wi8MLr
To the Editor:
Optimal characterisation of early structural abnormalities has been highlighted as critical to future efforts to detect and prevent cystic fibrosis (CF) lung disease progression [1]. Computed tomography (CT) is currently the gold standard, detecting structural disease at an earlier stage than radiography or lung function testing, with predictive value for subsequent progression and later forced expiratory volume in 1 s (FEV1) decline [1]. Compared to other emerging radiological modalities for early disease detection, such as magnetic resonance imaging (MRI), CT is more widely accessible and MRI at present is not able to directly quantify the extent of bronchiectasis due to its limitations in spatial resolution. Current US CF Foundation clinical guidelines state that CT should be “considered every 2–3 years, using the lowest possible radiation dose” [2]. Early lung disease is identified on CT by air trapping, increase in airway wall thickness and airway diameter, and potentially irreversible bronchiectasis [3]. Spirometer-directed CT has standardised lung volume acquisition and outlined the importance of both inspiratory and expiratory image acquisition [4]. Consensus recommendations for “low dose” CT scanning were published in 2016 [5], but radiation exposure remains a concern, given the six-fold increase in CT use and ongoing increase in cumulative radiation exposure for CF patients [6]. Imaging requires optimal structural information at the lowest possible radiation dose [5]. Given the increased vulnerability of children to ionising radiation, “low dose” CT protocols have already been incorporated in paediatric clinical trials using CT-based primary outcome measures [7], and use of ultra-low dose (ULD) CT has emerged in adult CF research and in isolated paediatric patients [6]. However, formal validation of the effects on quantitative measures of structural disease has not been performed. Low dose (LD) CT shows limitations compared to standard dose CT because of known drifts in CT lung density dependent on dose [8]. We hypothesised that ULD implementation would not detrimentally affect structural disease detection in early CF lung disease, and specifically targeted an age range to explore this where volume variation during spirometry-directed CT could be minimised between imaging modalities. Thus, the primary aims in this study were to 1) implement spirometer-directed novel ULD CT in school-aged children with CF, and 2) validate its outcomes against same-session LD CT using visual as well as fully automated quantitative CT analysis for structural airway disease.
Participants were recruited from a single centre between May 2018 and September 2020. Inclusion criteria were: 1) confirmed CF diagnosis from genotype, 2) age 5–18 years, 3) FEV1 ≥70% predicted, 4) exacerbation free, and 5) indication for routine surveillance CT by local standards. Ethics committee approval was granted (18/SCHN/469) and written informed consent/assent was obtained from all parents/guardians and children.
Consecutive paired inspiratory–expiratory chest CT scans were performed on a third-generation dual-source scanner (SOMATOM Force, Siemens Healthineers AG, Germany) at LD and ULD in the same session using spirometry direction [9]. LD CT settings complied with current consensus recommendations [5]. ULD CT adjusted mAs values to achieve target CT dose index (CTDI) per age (0.09 mGy for 5 years and 0.13 mGy for 10 years). Each scan was iteratively reconstructed at Kernel Br49d\3 with a slice thickness of 0.6 mm (and 50% or 0.3 mm overlap), which was selected following an in-house analysis of the performance of six reconstruction settings on an initial cohort of 10 inspiratory–expiratory scan pairs in both LD and ULD.
Fully automatic software (YACTA v2.9.1.12) analysed for the primary outcomes of interest, air trapping (parameter A1) [10] and bronchiectasis (bronchiectasis index [11]), plus additional outcomes tracheal air density, mean lung density, total airway diameter, lumen area, airway wall thickness, wall area percentage, and number of airways segments detected. Bronchiectasis index and air trapping were examined as both continuous and categorical variables with the latter defined as normal/abnormal based on thresholds of >1 for the presence of bronchiectasis and >5% for the presence of air trapping. This latter threshold was based on updated analysis of a school-aged healthy paediatric cohort (n=10) from within a previously published study [12, 13], and agrees with recent MRI-based recommendations [14].
As the fully automated software is not widely available, a blinded radiologist (H. Issa) also performed a visual assessment across all 50 scan pairs to assess intra-observer agreement for detection of findings. In a subset of 10 participants, interobserver agreement was assessed against a second radiologist (R. Goetti). The scoring system assessed the presence (yes/no) of eight different criteria for each lobe. Statistical analyses were performed using GraphPad Prism (version 8.4.3, GraphPad Software, USA) and R (version 4.0.2, R Foundation for Statistical Computing, Austria).
From 57 subjects, 50 were included in the final analysis: seven were excluded due to 1) CT export error (n=4), 2) no suitable LD and ULD scans to compare (n=2), and 3) incorrect spirometer-directed technique (n=1). Effective radiation dose [15] for paired inspiratory and expiratory CT was 78% lower with ULD (median (range) LD 0.66 (0.33–2.0) versus ULD 0.15 (0.06–0.34) mSv; p<0.0001) with lower CTDI for both inspiratory (LD 0.70 (0.24–2.08) versus ULD 0.11 (0.04–0.14) mGy; p<0.0001) and expiratory scans (LD 0.37 (0.18–0.82) versus ULD 0.12 (0.04–0.29) mGy; p<0.0001) and lower size-specific dose estimates for both inspiratory (LD 1.12 (0.38–3.31) versus ULD 0.17 (0.07–0.22) mGy; p<0.0001) and expiratory scans (LD 0.58 (0.15–1.62) versus ULD 0.19 (0.08–0.48) mGy; p<0.0001). Spirometry technical quality was good/excellent in 92% of inspiratory and 100% of expiratory scans, with almost identical LD and ULD CT lung volumes between corresponding inspiratory and expiratory scans.
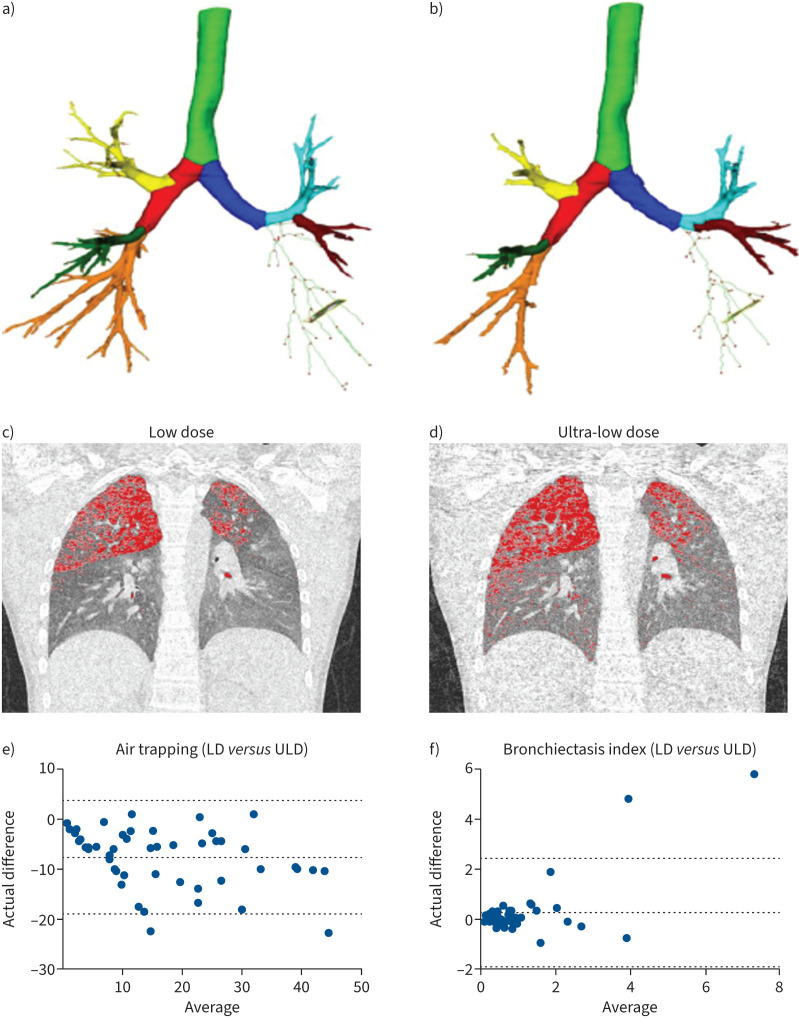
Concordance in classification for bronchiectasis was 94% (κ=0.95). Bronchiectasis was detected in 24% and 26% of LD and ULD CTs (p=1.00), with strong correlation (r=0.79, p<0.001) and no difference in bronchiectasis index values (0.57 (0.01–10.3) versus 0.63 (0.06–4.46); p=0.38). Two subjects (4%) were false-positives for bronchiectasis on ULD, using LD CT as the gold standard, with one subject (2%) a false-negative. Concordance in classification for air trapping was lower at 70% (κ=0.32). Abnormal air trapping was detected in 58% and 88%, respectively (p<0.0001) with very strong correlation (r=0.91, p<0.001) but a systematic difference in air trapping values (9.3% (0.2–38.4%) versus 17.1% (1.2–56.1%); p<0.0001). 15 subjects (30%) were false-positives for air trapping on ULD, using LD CT as the gold standard, with no false-negatives. Quantitative outputs from a representative subject and Bland–Altmann analyses of agreement between LD and ULD CT for bronchiectasis index and air trapping across the entire cohort are shown in figure 1. In general correlations were strong for airway and parenchyma quantitative indices (r=0.82–0.99, p<0.0001), and just moderate for airway wall thickness (r=0.65 (0.46–0.79), p<0.0001). Segmented airway number was reduced for ULD (94 (32–202) versus 133(33–424); p<0.0001) (figure 1), with significant differences from generation 5 onwards and increasing magnitude of difference with increasing airway generation. Visual reporting revealed substantial intra-observer inter-method agreement for detection of bronchial wall thickening (κ=0.75), bronchiectasis (κ=0.63), mucous plugging (κ=0.75), atelectasis (κ=0.63), ground-glass opacities (κ=0.66) and features of bronchiolitis (κ=0.80) and was almost perfect for consolidation (κ=0.84) and air trapping (κ=0.85) [16]. Inter-observer agreement was substantial for both LD (κ=0.76) and ULD (κ=0.72) groups.
FIGURE 1.
Quantitative analysis software output (YACTA) for a representative participant in the study for the low dose (LD) computed tomography (CT) scan (a and c) and the corresponding ultra-low dose (ULD) CT scan (b and d). The top panels show the YACTA airway segmentation (a and b) and the middle panels show the air trapping (A1) identification: LD 15.7% (c) and ULD 18.2% (d). Radiation dose was reduced in ULD compared to LD (0.0846 mSv versus 0.2538 mSv). The bottom panels summarise Bland–Altman analysis of LD and ULD comparison for air trapping (e) and bronchiectasis index (f). The representative subject is a 12-year-old male with cystic fibrosis. Demographics: height 153 cm (−0.33z), weight 43.6 kg (0.36z), forced expiratory volume in 1 s (FEV1) 91% pred (−0.52z), forced vital capacity (FVC) 92% pred (−0.51z), FEV1/FVC 85%. Lung volume during the spirometry-directed CT scans were similar between LD and ULD for inspiration and expiration (3847.9 versus 3648.2 and 1378.1 versus 1451.7 mL). e and f) Dotted horizontal lines indicate mean and 95% limits of agreement for the difference between LD and ULD CT based values of the index.
This is the first study to perform and compare same-session spirometer-controlled LD and ULD CT in school-aged CF children. A fully quantitative analysis software was specifically chosen to enhance resolution to detect small differences compared to semi-quantitative analysis software available (e.g. PRAGMA-CF [7]). ULD CT was highly feasible and significantly reduced effective radiation dose by 78% whilst balancing image quality with diagnostic acceptability, as called for in the literature [6], for bronchiectasis. However, higher rates of air trapping compared to LD CT were observed, although it was reassuring to see that strong (or near perfect) agreement for visual reporting between LD and ULD across all indices was achieved. Decreased peripheral airway number on ULD may reflect increased noise on ULD, as standard deviation in attenuation (in Hounsfield units; SD HU) of tracheal air was also increased versus LD. The appearance of unsegmented peripheral airways as lower density regions may partially explain larger amounts of air trapping detected. There are two important areas required before clinical implementation can be recommended. Firstly, using this unique dataset we will train artificial intelligence and neural networks for quantitative CT analyses in the future to address concerns that wider implementation of ULD imaging may falsely increase the degree of air trapping present [17]. Secondly, ability to detect change over time in longitudinal data is required. The results presented here highlight the potential utility of ULD CT to reduce radiation burden in this susceptible population, by outlining its ability to accurately detect bronchiectasis. Further work is required to achieve this for air trapping but is underway by our group. Given that “low dose” protocols are already being used in intervention studies [7] and in clinical care (as evidenced by our site's use of them), our data serve as an important reminder that further efforts to reduce radiation with CT imaging will need to be assessed for detrimental effects on diagnostic sensitivity and the ability to apply advanced image analysis techniques to quantify structural lung disease.
Shareable PDF
Acknowledgements
Authors would like to thank the children and families who took part in this study, staff of the Cystic Fibrosis clinic and Respiratory Function Unit Radiology Department at CHW who assisted during the study and the institutional statistician involved throughout this project (Liz Barnes, University of Sydney).
Footnotes
Author contributions: Conception and design: K.J. Bayfield, O. Weinheimer, M.O. Wielpütz, L. Yu, T.E. Robinson, B. Bartholmai, D. Fitzgerald, H. Selvadurai and P.D. Robinson. Acquisition, analysis and interpretation of data: K.J. Bayfield, O. Weinheimer, C. Boyton, R. Fitzpatrick, A. Middleton, B. Kennedy, A. Blaxland, G. Jayasuriya, N. Caplain, H. Issa, R. Goetti, M.O. Wielpütz, L. Yu, C.J. Galban, T.E. Robinson, B. Bartholmai and P.D. Robinson. Writing the manuscript or revising it critically for important intellectual content: all authors.
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Support statement: A 2018 Australian Cystic Fibrosis Research Trust innovation grant funded this study. This study was also supported by grants from the German Federal Ministry of Education and Research to O. Weinheimer and M.O. Wielpütz (82DZL004A1). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Bayfield KJ, Douglas TA, Rosenow T, et al. . Time to get serious about the detection and monitoring of early lung disease in cystic fibrosis. Thorax 2021; 76: 1255–1265. doi: 10.1136/thoraxjnl-2020-216085 [DOI] [PubMed] [Google Scholar]
- 2. Cystic Fibrosis Foundation. Preschool-Aged Care Clinical Care Guidelines. Date last accessed: 31 January 2023. www.cff.org/medical-professionals/preschool-aged-care-clinical-care-guidelines.
- 3.Turkovic L, Caudri D, Rosenow T, et al. . Structural determinants of long-term functional outcomes in young children with cystic fibrosis. Eur Respir J 2020; 55: 1900748. doi: 10.1183/13993003.00748-201932139454 [DOI] [PubMed] [Google Scholar]
- 4.Kongstad T, Buchvald FF, Green K, et al. . Improved air trapping evaluation in chest computed tomography in children with cystic fibrosis using real-time spirometric monitoring and biofeedback. J Cyst Fibros 2013; 12: 559–566. doi: 10.1016/j.jcf.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 5.Kuo W, Kemner-van de Corput MP, Perez-Rovira A, et al. . Multicentre chest computed tomography standardisation in children and adolescents with cystic fibrosis: the way forward. Eur Respir J 2016; 47: 1706–1717. doi: 10.1183/13993003.01601-2015 [DOI] [PubMed] [Google Scholar]
- 6.Joyce S, Carey BW, Moore N, et al. . Computed tomography in cystic fibrosis lung disease: a focus on radiation exposure. Pediatr Radiol 2021; 51: 544–553. doi: 10.1007/s00247-020-04706-0 [DOI] [PubMed] [Google Scholar]
- 7.Tiddens H, Chen Y, Andrinopoulou ER, et al. . The effect of inhaled hypertonic saline on lung structure in children aged 3–6 years with cystic fibrosis (SHIP-CT): a multicentre, randomised, double-blind, controlled trial. Lancet Respir Med 2022; 10: 669–678. doi: 10.1016/S2213-2600(21)00546-4 [DOI] [PubMed] [Google Scholar]
- 8.Sieren JP, Hoffman EA, Fuld MK Jr, et al. . Sinogram Affirmed Iterative Reconstruction (SAFIRE) versus weighted filtered back projection (WFBP) effects on quantitative measure in the COPDGene 2 test object. Med Phys 2014; 41: 091910. doi: 10.1118/1.4893498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salamon E, Lever S, Kuo W, et al. . Spirometer guided chest imaging in children: it is worth the effort! Pediatr Pulmonol 2017; 52: 48–56. doi: 10.1002/ppul.23490 [DOI] [PubMed] [Google Scholar]
- 10.Robinson TE, Goris ML, Moss RB, et al. . Mucus plugging, air trapping, and bronchiectasis are important outcome measures in assessing progressive childhood cystic fibrosis lung disease. Pediatr Pulmonol 2020; 55: 929–938. doi: 10.1002/ppul.24646 [DOI] [PubMed] [Google Scholar]
- 11.Weinheimer O, Wielpütz M, Konietzke P, et al. . Fully automated lobe-based airway taper index calculation in a low dose MDCT CF study over 4 time-points. SPIE Medical Imaging 2017; 10133: 10133OU. [Google Scholar]
- 12.Bonnel AS, Song SM, Kesavarju K, et al. . Quantitative air-trapping analysis in children with mild cystic fibrosis lung disease. Pediatr Pulmonol 2004; 38: 396–405. doi: 10.1002/ppul.20091 [DOI] [PubMed] [Google Scholar]
- 13.Ram S, Hoff BA, Bell AJ, et al. . Improved detection of air trapping on expiratory computed tomography using deep learning. PLoS One 2021; 16: e0248902. doi: 10.1371/journal.pone.0248902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischer S, Kraus MS, Gatidis S, et al. . New severity assessment in cystic fibrosis: signal intensity and lung volume compared to LCI and FEV1: preliminary results. Eur Radiol 2020; 30: 1350–1358. doi: 10.1007/s00330-019-06462-8 [DOI] [PubMed] [Google Scholar]
- 15.The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP 2007; 37: 1–332. doi: 10.1016/j.icrp.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 16.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012; 22: 276–282. doi: 10.11613/BM.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller SM, Ram S, Bayfield KJ, et al. . Deep learning-based air trapping quantification in children with cystic fibrosis using paired inspiratory-expiratory ultra-low dose CT. European Congress of Radiology, 2022. Abstract Poster Presentation. Available from: https://www.myesti.org/project/page/7/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-00286-2023.Shareable (535.9KB, pdf)