Abstract
Estrogen receptor alpha (ERα) plays a crucial role in reproductive function in both sexes. It also mediates cellular responses to estrogens in multiple nonreproductive organ systems, many of which regulate systemic metabolic homeostasis and inflammatory processes in mammals. The loss of estrogens and/or ERα agonism during aging is associated with the emergence of several comorbid conditions, particularly in females undergoing the menopausal transition. Emerging data also suggests that male mammals likely benefit from ERα agonism if done in a way that circumvents feminizing characteristics. This has led us, and others, to speculate that tissue-specific ERα agonism may hold therapeutic potential for curtailing aging and chronic disease burden in males and females that are at high-risk of cancer and/or cardiovascular events with traditional estrogen replacement therapies. In this mini-review, we emphasize the role of ERα in the brain and liver, summarizing recent evidence that indicates these two organs systems mediate the beneficial effects of estrogens on metabolism and inflammation during aging. We also discuss how 17α-estradiol administration elicits health benefits in an ERα-dependent manner, which provides proof-of-concept that ERα may be a druggable target for attenuating aging and age-related disease burden.
Keywords: 17α-estradiol, hypothalamus, HPG axis, liver, metabolism, neuroinflammation
INTRODUCTION
Signaling through estrogen receptor alpha (ERα) is required for normal reproductive function in mammals. ERα also mediates estrogenic cellular responses in a wide range of nonreproductive organ systems, many of which regulate systemic metabolic homeostasis and inflammatory processes that underlie chronic disease onset. For example, specific mutations and polymorphisms in Esr1, the gene that encodes ERα, have been associated with greater body mass and adiposity [1,2], in addition to infertility in both sexes [3,4]. Other Esr1 mutations have been linked to osteoporosis, breast cancer, and Alzheimer’s disease (AD) in females [5,6]. Similarly, the global ablation of ERα in mice increases adiposity and reduces insulin sensitivity in both sexes [7], which further supports the role of ERα in controlling metabolic processes. ERα possesses both genomic (nuclear hormone) and nongenomic (membrane-associated) capabilities [8], which underlies its ability to exert metabolic control in numerous organ systems. It is also noteworthy that numerous Esr1 splice variants have been identified, several of which are translated into ERα proteins with different molecular weights and functional domains [9]. However, the physiological function of these truncated ERα isoforms remain unknown, particularly with regard to regulating metabolic homeostasis and inflammatory processes. Conversely, many of the truncated ERα isoforms have been associated with tumor cell activity in a variety of cancers [10], which is outside the scope of this mini-review. Despite actions in a variety of metabolically active tissues, the goal of this mini-review is to summarize how ERα modulates metabolism and chronic disease progression through actions in the brain and liver, which we postulate is closely related to the control of systemic aging processes.
MODULATION OF THE HPG AXIS BY ERα
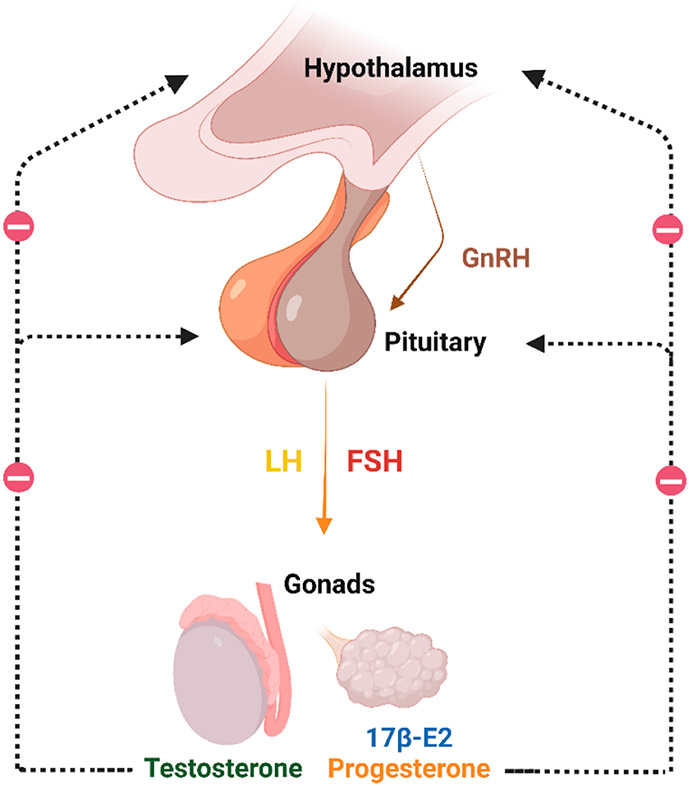
The hypothalamic-pituitary-gonadal (HPG) axis plays a vital role in controlling reproduction, metabolism, and immune function. Gonadotropin-releasing hormone (GnRH) secreted from the hypothalamus serves to stimulate the production and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary, which in turn stimulates the production and release of sex hormones from the gonads. These sex hormones, predominantly 17β-estradiol (17β-E2) and progesterone in females [11,12] and testosterone in males [13,14], signal in the hypothalamus and pituitary to suppress the production of GnRH and FSH/LH, respectively, as part of the HPG negative feedback loop (Figure 1). 17β-E2 can also signal in males to dampen gonadotropin production, most of which occurs following the aromatization of testosterone to 17β-E2 [15,16]. The aforementioned hormonal cycles regulate germ cell release in females, and germ cell creation in males, therefore the HPG axis is relevant to aging and chronic disease burden because it plays a major role in the established tradeoff effects between reproduction and longevity [17]. With advancing age, gonadal and neuroendocrine changes occur that result in declines in sex hormone production [18], and thus, declines in negative feedback within the HPG axis [19,20]. Age-related hormonal declines are more rapid in females because 17β-E2 production is directly linked to ovarian follicular depletion [21,22]. The reduction in sex hormones leads to elevated production and secretion of GnRH, LH, and FSH, which have been linked to the aging process and a variety of comorbid conditions in sex-specific manners [23-27]. In fact, after menopause, females are confronted with greater risk for numerous age-related diseases with a metabolic and/or proinflammatory underpinning [28-37], several of which rise to incidences commonly observed in males [38].
Figure 1.
Gonadotropin-releasing hormone (GnRH) is secreted by the hypothalamus and stimulates the production and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary, which in turn stimulates the production and release of 17β-estradiol (17β-E2), progesterone, and testosterone from the gonads. The release of these hormones controls the production of GnRH and FSH/LH in a negative feedback loop. This figure was created with BioRender.com.
ERα is the primary receptor involved in 17β-E2-mediated suppression of gonadotropin release in both sexes [39,40], although other receptors, including estrogen receptor beta (ERβ) and G protein-coupled estrogen receptor (GPER), have also been reported to play a role in this process) [41], but are outside the scope of this mini-review. several different neuronal populations have been implicated in the 17β-E2 negative feedback mechanism [11,42,43]. GABAergic neurons in the preoptic area (POA) are believed to provide input to the GnRH negative feedback system [42,44]. There is also evidence that ERα is expressed in a subset of GnRH neurons within the POA, which suggests the possibility of direct regulation of GnRH production by ERα [45]. Further investigation has revealed a functional hierarchy among the various possible mechanisms involved in the HPG feedback process. ERα expression in the arcuate nucleus (ARN) has been demonstrated to be crucial to maintaining reproductive function and E2-dependent negative feedback [46,47]. Although selective knockdown of ERα in kisspeptin neurons within the ARN was found to have no effect on LH secretion [48], these mice exhibited GnRH pulse activity similar to that of gonadectomized mice with high frequency, low amplitude LH pulses [49]. These results suggest 17β-E2 signaling through ERα in kisspeptin neurons in the ARN is the principal mechanism responsible for controlling GnRH pulsatility in mice. Interestingly, ERα in the pituitary has also been implicated in 17β-E2 feedback, and its ablation causes infertility in female mice [50]. Collectively, the findings outlined above indicate that ERα plays a major role in controlling HPG activity, which could conceivably make it a pharmacological target within the hypothalamus and/or pituitary for attenuating aging and chronic disease burden. For instance, agonizing ERα in a manner that curtails age-related increases in GnRH, LH, and FSH production could potentially blunt mechanisms that promote arthritis, kidney disease, obesity, metabolic dysfunction, and neuroinflammation in a sex-specific manner [23-27].
ROLE OF ERα IN NEUROINFLAMMATION
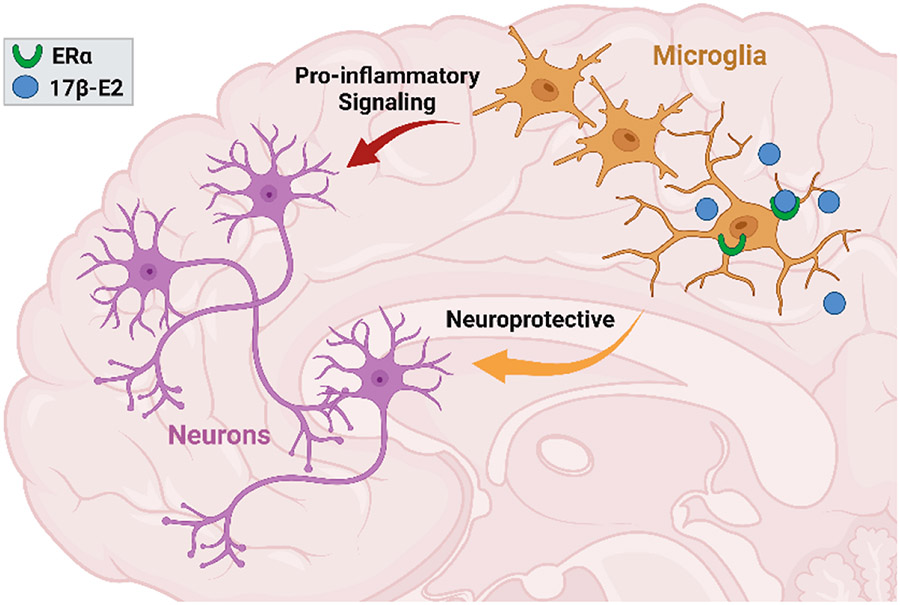
Estrogens are known to exert anti-inflammatory and neuroprotective effects by agonizing ERα. Interestingly, ERα in microglia have been shown to temper pro-inflammatory processes in both female and male rodents (Figure 2) [51-54]. Microglia are brain-resident immune cells that serve diverse functions across the lifespan, including debris clearance, synaptic pruning, and response to infectious agents [55-57]. In vivo and in vitro studies show that ERα agonism limits the transition of microglia toward pro-inflammatory phenotypes when challenged with noxious stimuli such as bacteria [58] and viruses [59]. Furthermore, synthetic ligands for ERα have also been shown to attenuate the production of tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), and macrophage inflammation protein-2 (MIP2) in primary microglial cultures [60].
Figure 2.
Prior studies show that ERα agonism on microglia, the brain-resident immune cells, limits their transition towards a pro-inflammatory phenotype which has been linked to neuroprotection. This figure was created with BioRender.com.
Interestingly, ovariectomy (OVX) increases a large number of markers associated with microglial reactivity, including the recognition of inflammatory stimuli and phagocytosis in female rodents [61]. The administration of 17β-E2 in the setting of OVX prevents microglia phenotypic switching, suggesting that ERα agonism plays a critical role in regulating microglia homeostasis [53,61-63]. ERα density within the mouse hippocampus is dramatically reduced with advancing age in female mice [64,65], suggesting that ERα in the aging brain is associated with impaired anti-inflammatory activity and microglial-mediated neurotoxicity. In support of this, global ERα knockout (ERαKO) mice display increased hippocampal expression of IL-1β, interleukin-6 (IL-6), and interleukin-12p40 (IL-12p40), all of which are linked to neurotoxicity [66]. Conversely, chronic treatment with 17β-E2 or selective estrogen receptor modulators (SERMs) in OVX females significantly reduces the number of microglial within the hippocampus [64,67]. This further implicates ERα agonism in neuroprotection during aging and disease processes. Similarly, ERα agonism suppresses microglial neuroinflammation in traumatic brain injury (TBI)-induced male mice by attenuating the decrease in neuronal ERα expression in the ischemic cortex [68]. It should be noted, however, that females generally display a greater prevalence of neurodegenerative diseases, such as AD, and are burdened with more severe pathology and greater cognitive declines than their male counterparts, which worsens following menopause [69,70]. Although definitive mechanisms underlying the aforementioned observations remain unresolved, some reports suggest that declines in ERα agonism during the menopausal transition is a major contributor to female-dominant cognitive declines [71-73], which is further supported by the fact that females receiving estrogen replacement therapies have decreased risk for onset and/or development of AD [74]. If this is indeed proven to be the case, it suggests that ERα plays a greater role in modulating female brain diseases than it does in males, which provides support for the overall goal of developing ERα agonists for treating disease burden in a sex-specific manner. An interesting caveat to the aforementioned findings is the discovery that the maintenance of hippocampal ERα expression, even in the absence of estrogen signaling, is associated with improved cognition in rodents [75,76]. Emerging evidence suggests that ligand-independent activation of ERα, potentially by insulin-like growth factor 1, can affect the transcriptional activity of ERα in a way that improves memory [77]; thereby suggesting that just maintaining ERα expression may be at least partially beneficial for neurocognitive declines.
The molecular mechanisms responsible for ERα-mediated anti-inflammatory effects in the aging brain remain unclear [55]. One potential mechanism is the ERα-mediated regulation of Toll-like receptor (TLR) signaling in myeloid-lineage cells, which has been linked to reduced inflammatory responses [78,79]. Murine and human studies demonstrate that activation of ERα inhibits TLR4 signaling in macrophages and reduces inflammation [80-82]. In addition, ERα interactions with the phosphatidylinositol 3-kinase (PI3K) p85 subunit and AP-1 promoter sites may be involved in blocking TLR4 signaling in macrophages [55]. Another potential mechanism by which ERα agonism may attenuate inflammatory cytokine production following TLR activation is through the inhibition of NF-kB, which has been shown to occur through direct and indirect mechanisms [83,84]. Recent reports have also proposed that 17β-E2 regulates the transition of macrophages into different activation states in an ERα-dependent manner [55]. For example, quantification of inflammatory cytokine production during time-lapse microscopy demonstrated that 17β-E2 inhibits IL-1β and increases interleukin-10 (IL-10) expression, the latter of which is an anti-inflammatory cytokine, during acute lipopolysaccharide exposure [85]. These effects were mediated by suppressor of cytokine signaling 3 (SOCS3), a transcription factor that is partially regulated by ERα, which provided the ability of macrophages to terminate the pro-inflammatory phase [55,86]. Collectively, ERα agonism facilitates intrinsic and extrinsic macrophage programming that allows for the resolution of inflammation. It should be noted that ERα actions in astrocytes have also been shown to provide neuroprotective effects in the brain [87], however discussion of these actions is beyond the scope of this review.
In addition to the role of ERα in modulating chronic brain inflammation, neuronal injury, and neurodegeneration through actions in the hippocampus, amygdala, and cortex [54,88-90], it also plays a major role in regulating pro-inflammatory processes in the hypothalamus [91]. The hypothalamus is one of the most important brain regions involved in the control of feeding behavior, energy expenditure, and systemic glucose homeostasis in both sexes [92]. In the setting of obesity and advancing age, microglia activation is commonly observed in the hypothalamus [93,94], which has been linked to neuronal endoplasmic reticulum stress, declines in insulin and leptin sensitivity, and faster aging in male and female mice [93,95,96]. These events promote hyperphagia and the diminished control of hepatic gluconeogenesis [97], which further exacerbates metabolic dysfunction and the aging process. ERα activity in the hypothalamus has been linked to the aforementioned decline in metabolic function and mechanisms that promote aging, which occurs through actions on both microglia and neurons [53,91,98]. These observations provide additional support for the idea that tissue-specific ERα agonism may serve as a target for delaying the aging process and chronic disease onset.
ROLE OF ERα IN METABOLIC PLASTICITY
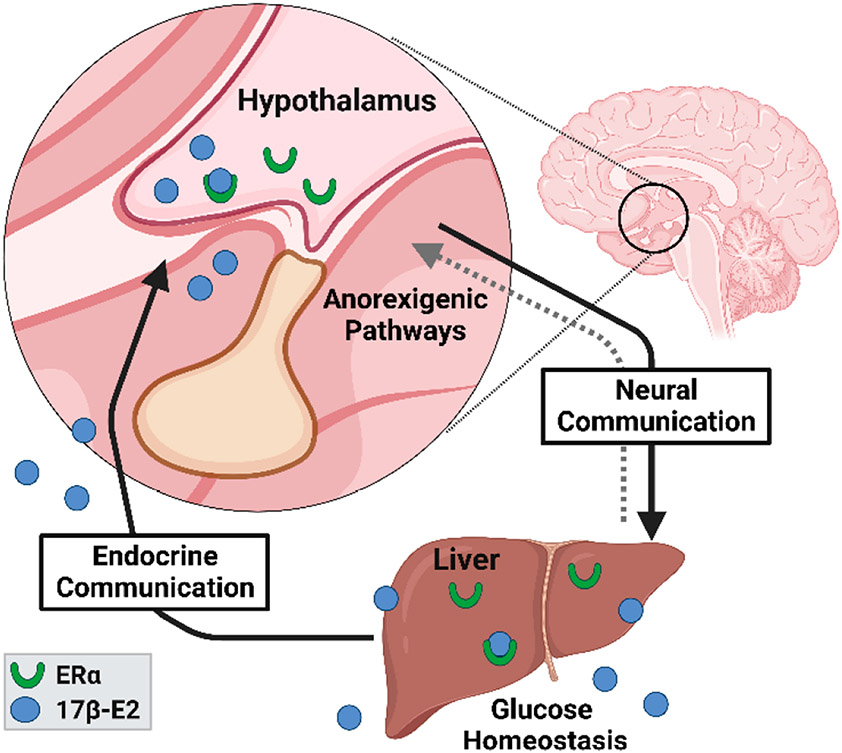
There is abundant data demonstrating that ERα is a major regulator of systemic metabolic parameters through actions in the brain and liver [99-101]. ERα has also been implicated in the control of skeletal muscle metabolism by regulating mitochondrial function and quality [102], but this is outside the scope of the current mini-review. 17β-E2 acts through ERα in brain and/or liver to regulate glucose homeostasis, lipid distribution, thermogenesis, and hypothalamic anorexigenic pathways (Figure 3) [57,99,103]. The loss of endogenous estrogen actions after menopause in humans or OVX in mice eliminates these beneficial effects and elicits metabolic perturbations [104] that are nearly identical to those seen in global ERαKO mice [7,105]. Estrogen replacement therapies in both humans and mice reverses the adverse metabolic effects associated with menopause [106,107] and OVX [108]. Most of the prior studies that have evaluated the effects of ERα on metabolic readouts have been done in female mammals, although more recent work has demonstrated that ERα also plays a critical role in modulating metabolism in male mammals. For example, Allard et al. recently reported that genomic actions of ERα regulate systemic glucose homeostasis in mice of both sexes and insulin production and release in males [109]. Other reports have also shown that hepatic steatosis, insulin sensitivity, and the control of hepatic gluconeogenesis are regulated through FOXO1 in an ERα-dependent manner in male mice [110]. Lastly, ERα ablation in hepatocytes abrogates similar estrogen-mediated metabolic benefits [111-113]. Interestingly, ligand-independent activation of ERα in human hepatocytes has been reported to modulate the expression of several cytochrome P450 genes [114], although the role this may play in modulating systemic physiological parameters remains unknown.
Figure 3.
17β-E2 acts through ERα in brain and liver to regulate systemic metabolism by controlling feeding neurocircuitry and macronutrient utilization. All the communication pathways between the two organ systems are still being elucidated. This figure was created with BioRender.com.
In the brain, a variety of hypothalamic neuronal populations are critically important for central control of feeding and energy expenditure. Prior work has shown that brain-specific ERα ablation promotes obesity in both female and male mice [115]. This observation was associated with increased food intake and decreased locomotion and energy expenditure [115]. Other studies employing mice with conditional deletion of ERα indicate that 17β-E2 actions in subsets of pro-opiomelanocortin (Pomc) and agouti-related protein/ neuropeptide Y (AgRP/NPY) neurons within the ARN play critical roles in controlling feeding behavior and energy balance [115-118]. Pomc and AgRP/NPY neurons in the ARN receive and integrate hormonal (e.g., insulin, ghrelin, leptin, cholecystokinin) and nutritional (e.g., glucose, fatty acids) signals from the peripheral circulation as well as neural signals in an effort to coordinate counterregulatory metabolic responses [92]. As mentioned above, obesity and aging are associated with impaired insulin-sensitivity, leptin-sensitivity, and nutrient-sensing in neurons within the ARC, which promotes increased food intake, hepatic gluconeogenesis, and adipocyte lipolysis [92,119]. Interestingly, 17β-E2 signaling through ERα reverses these declines and restores metabolic flexibility through what is currently believed to be direct interactions with insulin and/or leptin receptor signaling in Pomc and AgRP/NPY neurons [120-122]. Pomc- and AgRP/NPY-mediated control of hepatic gluconeogenesis is known to be regulated by sympathetic outflow to the liver [119,123-128], although recent reports suggest that brain-liver crosstalk is almost certainly a bidirectional pathway that is also controlled by nutrient-sensing within the gastrointestinal tract [129,130].
The role that ERα plays in regulating the gut-brain-liver axis remains unresolved, although the ablation of ERα in hepatocytes has been reported to adversely affect AgRP/NPY neuronal activity within the ARN of female mice [131]. It remains unclear if the change in AgRP/NPY activity in hepatocyte ERαKO mice occurs through vagal afferent signaling from the liver, or a change in metabolic substrates and/or endocrine factors being released from liver that cross the blood-brain barrier (BBB) and signal in the ARN. However, the authors did report that hypothalamic microglia in hepatocyte ERαKO mice present morphology indicative of an overt inflammatory phenotype [131], which led to speculation that changes in hepatic lipid metabolism with ERα ablation promotes the production and secretion of pro-inflammatory lipid moieties that cross the BBB and signal in the ARN. Additional studies will be needed to clearly define how hepatic ERα modulates Pomc and/or AgRP/NPY neuronal activity, but an emerging body of literature indicates that 17β-E2, likely through ERα, beneficially modulates vagal afferent signaling in the gut-brain-liver axis [132-135]; highlighting this pathway as a potential therapeutic target for mitigating aging and metabolic diseases.
HEALTH BENEFITS OF AGONIZING ERα WITH 17α-ESTRADIOL
Although estrogen replacement therapies improve a variety of comorbid conditions and likely elicit benefits on aging processes [136-139], chronic administration has been linked with greater cancer and cardiovascular risks in some female populations [140,141]. Additionally, elevated serum 17β-E2 in males is associated with stroke risk [142], prostate cancer development [143], and feminization [144]. Therefore, the challenge remains of determining how best to exploit the beneficial effects of systemic estrogen therapies while circumventing adverse biological consequences. We and others have begun to address this biological challenge through the use of 17α-estradiol (17α-E2). 17α-E2 is a naturally-occurring diastereomer of 17β-E2 [145,146] that is present in both mammalian sexes [147-149], although circulating levels are quite low. 17α-E2 is also a minor constituent of estrogen replacement therapies [150] but only possesses about 3%–4% of the binding affinity to ERα that 17β-E2 does [151]. 17α-E2 has predominantly been studied as a neuroprotective hormone with mild to moderate efficacy in both male and female models of ischemia, Alzheimer’s, and Parkinson’s diseases [147,150,152-155]. It was not until recently that the effects of 17α-E2 on systemic aging, longevity, and conditions that promote aging (e.g., obesity) were evaluated. The National Institute on Aging Interventions Testing Program has shown that 17α-E2 extends lifespan in male mice when treatment is initiated in mid-life [156,157] and late-life [158]. The magnitude of lifespan extension with 17α-E2 treatment in male mice is similar to that of calorie restriction [159] and rapamycin administration [160], which indicates 17α-E2 elicits potent effects that could conceivably be translated to men.
Our previous work has established that 17α-E2 administration reduces calorie intake and adiposity in conjunction with dramatic improvements in metabolic parameters (e.g., glucose tolerance, insulin sensitivity, ectopic lipid deposition) in obese and/or aged male mice [161-165]. We surmise these benefits underly the lifespan-extending effects of 17α-E2. Others have also reported that 17α-E2 treatment elicits benefits on glucose tolerance, mTORC2 signaling, hepatic urea cycling, markers of neuroinflammation, and sarcopenia [166-170]. Importantly, male-specific benefits occur without overt feminization of sex hormone profiles [161] or reproductive function [171]. Female mice are generally unresponsive to 17α-E2 treatment [166-170,172,173], unless subjected to chronic high-fat feeding over several months (unpublished observation) or following OVX [174]. Until recently the receptor(s) that mediate the actions of 17α-E2 were believed to be uncharacterized [146,148,154,175], although our recent report clearly demonstrated that the majority of health benefits attributed to 17α-E2 treatment are ERα-dependent [163]. This report also established that the hypothalamus and liver are the primary organ systems where 17α-E2 signals to regulate metabolic homeostasis in male rodents. Additional studies are needed to determine if 17α-E2 acts predominantly through ERα in a cell-type-specific manner in the hypothalamus and/or liver to modulate not only systemic metabolic homeostasis, but also aging and longevity. Although not definitive, the data generated thus far indicates that ERα agonism by 17α-E2 in hypothalamic neurons and/or hepatocytes may hold therapeutic potential for attenuating mechanisms that promote aging and chronic disease burden in men.
FUTURE STUDIES & CONCLUSIONS
The possibility of developing SERMs that modulate ERα for the treatment of aging and age-related diseases in a sex-specific manner is encouraging, but important knowledge gaps remain. Given the widespread expression of ERα isoforms in organs systems throughout the mammalian system, there are opportunities to mechanistically explore the effects of 17α-E2 in these tissues and how they influence metabolism, inflammatory responses, and ultimately aging. However, given the link between ERα activity and cancer in females, rigorous preclinical evaluation of newly developed SERMs and existing ligands, including 17α-E2, is required prior to clinical application. Additional studies that unravel the genomic and nongenomic actions of ERα in the context of metabolic and inflammatory processes are also needed because they could also present opportunities to develop therapies aims at treating sex-specific disease burden. Lastly, differences in ERα regulation between rodents and humans will also need to be carefully considered when attempting to translate newly developed SERMs or 17α-E2 into human studies.
ACKNOWLEDGEMENTS
Given the short nature of this report, we were unable to review a wealth of insightful literature that is related to this area of study. As such, it should be noted that this is not meant to be an exhaustive overview of the topic, but a summary of prior work that suggests additional studies focusing on the role of ERα in aging are needed. Our laboratories are supported by the National Institutes of Health (R00 AG51661 and R01 AG069742 to M.B.S. and DP5 OD033443 to S.R.O.) and the US Department of Veterans Affairs (Pilot Research Funding to M.B.S. and S.R.O.).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY
No new data was generated for the purposes of this mini-review.
REFERENCES
- 1.Okura T, Koda M, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord. 2003;27(9):1020–7. [DOI] [PubMed] [Google Scholar]
- 2.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–61. [DOI] [PubMed] [Google Scholar]
- 3.Corbo RM, Ulizzi L, Positano L, Scacchi R. Association of CYP19 and ESR1 pleiotropic genes with human longevity. J Gerontol A. 2011;66(1):51–5. [DOI] [PubMed] [Google Scholar]
- 4.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in Male Physiology. Physiol Rev. 2017;97(3):995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, et al. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer's disease. Biochem Biophys Res Commun. 1999;265(2):335–8. [DOI] [PubMed] [Google Scholar]
- 7.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, et al. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol Rev. 2017;97(3):1045–87. [DOI] [PubMed] [Google Scholar]
- 9.Saito K, Cui H. Estrogen Receptor Alpha Splice Variants, Post-Translational Modifications, and Their Physiological Functions. Cells. 2023;12(6):895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;ll(8):597–608. [DOI] [PubMed] [Google Scholar]
- 11.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19(3):302–30. [DOI] [PubMed] [Google Scholar]
- 12.Ronnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26(2):65–84. [DOI] [PubMed] [Google Scholar]
- 13.Hileman SM, Lubbers LS, Kuehl DE, Schaeffer DJ, Rhodes L, Jackson GL. Effect of inhibiting 5 alpha-reductase activity on the ability of testosterone to inhibit luteinizing hormone release in male sheep. Biol Reprod. 1994;50(6):1244–50. [DOI] [PubMed] [Google Scholar]
- 14.Scott CJ, Tilbrook AJ, Rawson JA, Clarke IJ. Gonadal steroid receptors in the regulation of GnRH secretion in farm animals. Anim Reprod Sci. 2000;60-61:313–26. [DOI] [PubMed] [Google Scholar]
- 15.Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular Biology of the Pituitary Gonadotropins. Endocr Rev. 1990;11(1):177–99. [DOI] [PubMed] [Google Scholar]
- 16.Sharma TP, Blache D, Blackberry MA, Martin GB. Role of peripheral and central aromatization in the control of gonadotrophin secretion in the male sheep. Reprod Fertil Dev. 1999;11(4-5):293–302. [DOI] [PubMed] [Google Scholar]
- 17.Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B. 1991;332(1262):15–24. [DOI] [PubMed] [Google Scholar]
- 18.Varlamov O. Western-style diet, sex steroids and metabolism. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1147–55. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16(5):837–43; discussion 55-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 2013;497(7448):211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–93. [DOI] [PubMed] [Google Scholar]
- 22.Shifren JL, Gass ML; NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21(10):1038–62. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Wu W, Kim MS, Cai DJNA. Author Correction: GnRH pulse frequency and irregularity play a role in male aging. Nat Aging. 2021;1(11):1068. [DOI] [PubMed] [Google Scholar]
- 24.Veldhuis-Vlug AG, Woods GN, Sigurdsson S, Ewing SK, Le PT, Hue TF, et al. Serum FSH Is Associated With BMD, Bone Marrow Adiposity, and Body Composition in the AGES-Reykjavik Study of Older Adults. J Clin Endocrinol Metab. 2021;106(3):e1156–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Zheng D, Lin H, Zhong F, Liu J, Wu Y, et al. High Circulating Follicle-Stimulating Hormone Level Is a Potential Risk Factor for Renal Dysfunction in Post-Menopausal Women. Front Endocrinol (Lausanne). 2021;12:627903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Qiao P, Guo Q, Liang Z, Pan J, Wu F, et al. High Follicle-Stimulating Hormone Level Associated With Risk of Rheumatoid Arthritis and Disease Activity. Front Endocrinol (Lausanne). 2022;13:862849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowers M, Crutchfield M, Bandekar R, Randolph JF, Shapiro B, Schork MA, et al. Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res. 1998;13(7):1134–40. [DOI] [PubMed] [Google Scholar]
- 29.Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology. 2016;87(7):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19(10):1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen L, Song L, Liu B, Li H, Zheng X, Zhang L, et al. Effects of early age at natural menopause on coronary heart disease and stroke in Chinese women. Int J Cardiol. 2017;241:6–11. [DOI] [PubMed] [Google Scholar]
- 32.Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. 2008;29(4):507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imtiaz B, Tuppurainen M, Rikkonen T, Kivipelto M, Soininen H, Kröger H, et al. Postmenopausal hormone therapy and Alzheimer disease: A prospective cohort study. Neurology. 2017;88(11):1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–62. [DOI] [PubMed] [Google Scholar]
- 35.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–58. [DOI] [PubMed] [Google Scholar]
- 36.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–57. [DOI] [PubMed] [Google Scholar]
- 37.Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, et al. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology. 2016;64(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62(10):1761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology. 1998;139(10):4092–101. [DOI] [PubMed] [Google Scholar]
- 40.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 41.Chimento A, Sirianni R, Casaburi I, Pezzi V. Role of estrogen receptors and g protein-coupled estrogen receptor in regulation of hypothalamus-pituitary-testis axis and spermatogenesis. Front Endocrinol (Lausanne). 2014;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2009;21(4):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gouw AM, Efe G, Barakat R, Preecha A, Mehdizadeh M, Garan SA, et al. Roles of estrogen receptor-alpha in mediating life span: the hypothalamic deregulation hypothesis. Physiol Genomics. 2017;49(2):88–95. [DOI] [PubMed] [Google Scholar]
- 44.Herbison AE. Estrogen regulation of GABA transmission in rat preoptic area. Brain Res Bull. 1997;44(4):321–6. [DOI] [PubMed] [Google Scholar]
- 45.Butler JA, Sjoberg M, Coen CW. Evidence for oestrogen receptor alpha-immunoreactivity in gonadotrophin-releasing hormone-expressing neurones. J Neuroendocrinol. 1999;11(5):331–5. [DOI] [PubMed] [Google Scholar]
- 46.Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-alpha expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155(8):2986–95. [DOI] [PubMed] [Google Scholar]
- 47.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17(6):1039–53. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Vanacker C, Burger LL, Barnes T, Shah YM, Myers MG, et al. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. Elife. 2019;8:43999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McQuillan HJ, Clarkson J, Kauff A, Han SY, Yip SH, Cheong I, et al. Definition of the estrogen negative feedback pathway controlling the GnRH pulse generator in female mice. Nat Commun. 2022;13(1):7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol Reprod. 2009;81(3):488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith JA, Das A, Butler JT, Ray SK, Banik NL. Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death. Neurochem Res. 2011;36:1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordeau P Jr, Lalancette-Hébert M, Weng YC, Kriz J. Estrogen receptors alpha mediates postischemic inflammation in chronically estrogen-deprived mice. Neurobiol Aging. 2016;40:50–60. [DOI] [PubMed] [Google Scholar]
- 53.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, et al. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100(16):9614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sárvári M, Hrabovszky E, Kalló I, Solymosi N, Tóth K, Likó I, et al. Estrogens regulate neuroinflammatory genes via estrogen receptors α and β in the frontal cortex of middle-aged female rats. J Neuroinflammation. 2011;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37(4):372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21(3):306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab. 2015;26(8):411–21. [DOI] [PubMed] [Google Scholar]
- 58.Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35(9-10):1309–16. [DOI] [PubMed] [Google Scholar]
- 59.Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174(10):6391–8. [DOI] [PubMed] [Google Scholar]
- 60.Ishihara Y, Itoh K, Ishida A, Yamazaki T. Selective estrogen-receptor modulators suppress microglial activation and neuronal cell death via an estrogen receptor-dependent pathway. J Steroid Biochem Mol Biol. 2015;145:85–93. [DOI] [PubMed] [Google Scholar]
- 61.Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology. 2012;153(6):2777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sárvári M, Hrabovszky E, Kalló I, Solymosi N, Likó I, Berchtold N, et al. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: rat and human studies identify strikingly similar changes. J Neuroinflammation. 2012;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147(5):2263–72. [DOI] [PubMed] [Google Scholar]
- 64.Lei D-L, Long J, Hengemihle J, O’Neill J, Manaye K, Ingram D, et al. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003;121(3):659–66. [DOI] [PubMed] [Google Scholar]
- 65.Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003;9(1):82–6. [DOI] [PubMed] [Google Scholar]
- 66.Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors α and β. Endocrinology. 2010;151(10):4916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suuronen T, Nuutinen T, Huuskonen J, Ojala J, Thornell A, Salminen A. Anti-inflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflamm Res. 2005;54:194–203. [DOI] [PubMed] [Google Scholar]
- 68.Lim S-W, Tt EN, Hu C-Y, Chio C-C, Wang C-C, Kuo J-R. Estrogen receptor-α is involved in tamoxifen neuroprotective effects in a traumatic brain injury male rat model. World Neurosurg. 2018;112:e278–87. [DOI] [PubMed] [Google Scholar]
- 69.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mielke MM. Sex and Gender Differences in Alzheimer's Disease Dementia. Psychiatr Times. 2018;35(11):14–7. [PMC free article] [PubMed] [Google Scholar]
- 71.Paganini-Hill A, Henderson VW. Estrogen Deficiency and Risk of Alzheimer's Disease in Women. Am J Epidemiol. 1994;140(3):256–61. [DOI] [PubMed] [Google Scholar]
- 72.Yue X, Lu M, Lancaster T, Cao P, Honda S-I, Staufenbiel M, et al. Brain estrogen deficiency accelerates Aβ plaque formation in an Alzheimer's disease animal model. Proc the Natl Acad Sci. 2005;102(52):19198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, Osorio RS, et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS One. 2017;12(10):e0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song YJ, Li SR, Li XW, Chen X, Wei ZX, Liu QS, et al. The Effect of Estrogen Replacement Therapy on Alzheimer’s Disease and Parkinson's Disease in Postmenopausal Women: A Meta-Analysis. Front Neurosci. 2020;14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Foster TC, Rani A, Kumar A, Cui L, Semple-Rowland SL. Viral vector-mediated delivery of estrogen receptor-alpha to the hippocampus improves spatial learning in estrogen receptor-alpha knockout mice. Mol Ther. 2008;16(9):1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151(3):1194–203. [DOI] [PubMed] [Google Scholar]
- 77.Grissom EM, Daniel JM. Evidence for Ligand-Independent Activation of Hippocampal Estrogen Receptor-alpha by IGF-1 in Hippocampus of Ovariectomized Rats. Endocrinology. 2016;157(8):3149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150(8):3877–84. [DOI] [PubMed] [Google Scholar]
- 79.Liu L, Zhao Y, Xie K, Sun X, Jiang L, Gao Y, et al. Estrogen inhibits LPS-induced IL-6 production in macrophages partially via the nongenomic pathway. Immunol Invest. 2014;43(7):693–704. [DOI] [PubMed] [Google Scholar]
- 80.Meng Q, Bi Y, Feng H, Ding X, Zhang S, Chen Q, et al. Activation of estrogen receptor α inhibits TLR4 signaling in macrophages and alleviates the instability of atherosclerotic plaques in the postmenopausal stage. Int Immunopharmacol. 2023;116:109825. [DOI] [PubMed] [Google Scholar]
- 81.Chen Q, Qi X, Zhang W, Zhang Y, Bi Y, Meng Q, et al. Catalpol inhibits macrophage polarization and prevents postmenopausal atherosclerosis through regulating estrogen receptor alpha. Front Pharmacol. 2021;12:655081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Q, Zhang Y, Meng Q, Wang S, Yu X, Cai D, et al. Liuwei Dihuang prevents postmenopausal atherosclerosis and endothelial cell apoptosis via inhibiting DNMT1-medicated ERα methylation. J Ethnopharmacol. 2020;252:112531. [DOI] [PubMed] [Google Scholar]
- 83.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25(8):2957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16(2):46–52. [DOI] [PubMed] [Google Scholar]
- 85.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matthews J, Almlöf T, Kietz S, Leers J, Gustafsson J-Å. Estrogen receptor-α regulates SOCS-3 expression in human breast cancer cells. Biochem Biophys Res Commun. 2005;335(1):168–74. [DOI] [PubMed] [Google Scholar]
- 87.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, et al. Neuroprotection mediated through estrogen receptor-α in astrocytes. Proc Natl Acad Sci U S A. 2011;108(21):8867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahman A, Jackson H, Hristov H, Isaacson RS, Saif N, Shetty T, et al. Sex and Gender Driven Modifiers of Alzheimer's: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Front Aging Neurosci. 2019;11:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22(4):656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014;9(2):633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Timper K, Bruning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10(6):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, et al. Hypothalamic programming of systemic ageing involving IKK-β, NF-κΒ and GnRH. Nature. 2013;497(7448):211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10(4):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pimentel GD, Ganeshan K, Carvalheira JB. Hypothalamic inflammation and the central nervous system control of energy homeostasis. Mol Cell Endocrinol. 2014;397(l-2):15–22. [DOI] [PubMed] [Google Scholar]
- 97.Tsaousidou E, Paeger L, Belgardt BF, Pal M, Wunderlich CM, Brönneke H, et al. Distinct Roles for JNK and IKK Activation in Agouti-Related Peptide Neurons in the Development of Obesity and Insulin Resistance. Cell Rep. 2014;9(4):1495–506. [DOI] [PubMed] [Google Scholar]
- 98.Debarba LK, Jayarathne HS, Miller RA, Garratt M, Sadagurski M. 17-α-Estradiol has sex-specific effects on neuroinflammation that are partly reversed by gonadectomy. J Gerontol A. 2022;77(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14(3):289–99. [DOI] [PubMed] [Google Scholar]
- 100.Xu Y, Lopez M. Central regulation of energy metabolism by estrogens. Mol Metab. 2018;15:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Della Torre S. Non-alcoholic Fatty Liver Disease as a Canonical Example of Metabolic Inflammatory-Based Liver Disease Showing a Sex-Specific Prevalence: Relevance of Estrogen Signaling. Front Endocrinol (Lausanne). 2020;11:572490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hevener AL, Ribas V, Moore TM, Zhou Z. The Impact of Skeletal Muscle ERalpha on Mitochondrial Function and Metabolic Health. Endocrinology. 2020. Feb 1;161(2):bqz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stincic TL, Ronnekleiv OK, Kelly MJ. Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons. Horm Behav. 2018;104:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stefanska A, Bergmann K, Sypniewska G. Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance. Adv Clin Chem. 2015;72:1–75. [DOI] [PubMed] [Google Scholar]
- 105.Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001;178(1-2):147–54. [DOI] [PubMed] [Google Scholar]
- 106.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, et al. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138(1):1–9. [DOI] [PubMed] [Google Scholar]
- 107.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, et al. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–87. [DOI] [PubMed] [Google Scholar]
- 108.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep. 2004;6(3):180–5. [DOI] [PubMed] [Google Scholar]
- 109.Allard C, Morford JJ, Xu B, Salwen B, Xu W, Desmoulins L, et al. Loss of Nuclear and Membrane Estrogen Receptor-alpha Differentially Impairs Insulin Secretion and Action in Male and Female Mice. Diabetes. 2019;68(3):490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes. 2019;68(2):291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guillaume M, Riant E, Fabre A, Raymond-Letron I, Buscato M, Davezac M, et al. Selective Liver Estrogen Receptor alpha Modulation Prevents Steatosis, Diabetes, and Obesity Through the Anorectic Growth Differentiation Factor 15 Hepatokine in Mice. Hepatol Commun. 2019;3(7):908–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qiu S, Vazquez JT, Boulger E, Liu H, Xue P, Hussain MA, et al. Hepatic estrogen receptor alpha is critical for regulation of gluconeogenesis and lipid metabolism in males. Sci Rep. 2017;7(1):1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meda C, Barone M, Mitro N, Lolli F, Pedretti S, Caruso D, et al. Hepatic ERalpha accounts for sex differences in the ability to cope with an excess of dietary lipids. Mol Metab. 2020;32:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Collins JM, Huo Z, Wang D. ESR1 ChIP-Seq Identifies Distinct Ligand-Free ESR1 Genomic Binding Sites in Human Hepatocytes and Liver Tissue. Int J Mol Sci. 2021;22(3):1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olofsson LE, Pierce AA, Xu AW. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proc Natl Acad Sci U S A. 2009;106(37):15932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith AW, Bosch MA, Wagner EJ, Ronnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17beta-estradiol. Am J Physiol Endocrinol Metab. 2013;305(5):E632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruud J, Steculorum SM, Bruning JC. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat Commun. 2017;8:15259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qiu J, Bosch MA, Meza C, Navarro UV, Nestor CC, Wagner EJ, et al. Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance. Endocrinology. 2018;159(2):647–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.González-García I, García-Clavé E, Cebrian-Serrano A, Le Thuc O, Contreras RE, Xu Y, et al. Estradiol regulates leptin sensitivity to control feeding via hypothalamic Cited1. Cell Metab. 2023;35(3):438–55.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stincic TL, Ronnekleiv OK, Kelly MJ. Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons. Horm Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005;1(1):53–61. [DOI] [PubMed] [Google Scholar]
- 124.Brandt C, Nolte H, Henschke S, Engstrom Ruud L, Awazawa M, Morgan DA, et al. Food Perception Primes Hepatic ER Homeostasis via Melanocortin-Dependent Control of mTOR Activation. Cell. 2018;175(5):1321–35.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang X, Yang S, Chen J, Su Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front Endocrinol (Lausanne). 2018;9:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dodd GT, Michael NJ, Lee-Young RS, Mangiafico SP, Pryor JT, Munder AC, et al. Insulin regulates POMC neuronal plasticity to control glucose metabolism. Elifee. 2018;7:e38704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–49. [DOI] [PubMed] [Google Scholar]
- 128.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434(7036):1026–31. [DOI] [PubMed] [Google Scholar]
- 129.Waise TMZ, Dranse HJ, Lam TKT. The metabolic role of vagal afferent innervation. Nat Rev Gastroenterol Hepatol. 2018;15(10):625–36. [DOI] [PubMed] [Google Scholar]
- 130.Wachsmuth HR, Weninger SN, Duca FA. Role of the gut-brain axis in energy and glucose metabolism. Exp Mol Med. 2022;54(4):377–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benedusi V, Della Torre S, Mitro N, Caruso D, Oberto A, Tronel C, et al. Liver ERalpha regulates AgRP neuronal activity in the arcuate nucleus of female mice. Sci Rep. 2017;7(1):1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang KP, Raybould HE. Estrogen and gut satiety hormones in vagus-hindbrain axis. Peptides. 2020;133:170389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiang Y, Greenwood-Van Meerveld B, Johnson AC, Travagli RA. Role of estrogen and stress on the brain-gut axis. Am J Physiol Gastrointest Liver Physiol. 2019;317(2):G203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ciriello J, Caverson MM. Effect of estrogen on vagal afferent projections to the brainstem in the female. Brain Res. 2016;1636:21–42. [DOI] [PubMed] [Google Scholar]
- 135.Vigil P, Melendez J, Petkovic G, Del Rio JP. The importance of estradiol for body weight regulation in women. Front Endocrinol (Lausanne). 2022;13:951186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A. 2009;64(12):1207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstet Gynecol. 2013;121(4):709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Benedusi V, Martini E, Kallikourdis M, Villa A, Meda C, Maggi A. Ovariectomy shortens the life span of female mice. Oncotarget. 2015;6(13):10801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Austad SN, Fischer KE. Sex Differences in Lifespan. Cell Metab. 2016;23(6):1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280(7):605–13. [DOI] [PubMed] [Google Scholar]
- 141.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- 142.Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, Masaki KH, et al. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68(8):563–8. [DOI] [PubMed] [Google Scholar]
- 143.Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011;6(3):437–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stratakis CA, Vottero A, Brodie A, Kirschner LS, DeAtkine D, Lu Q, et al. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998;83(4):1348–57. [DOI] [PubMed] [Google Scholar]
- 145.Ikeda T, Makino Y, Yamada MK. 17alpha-estradiol is generated locally in the male rat brain and can regulate GAD65 expression and anxiety. Neuropharmacology. 2015;90:9–14. [DOI] [PubMed] [Google Scholar]
- 146.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146(9):3843–50. [DOI] [PubMed] [Google Scholar]
- 147.Dykens JA, Moos WH, Howell N. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann N Y Acad Sci. 2005;1052:116–35. [DOI] [PubMed] [Google Scholar]
- 148.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–44. [DOI] [PubMed] [Google Scholar]
- 149.Courant F, Aksglaede L, Antignac JP, Monteau F, Sorensen K, Andersson AM, et al. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. J Clin Endocrinol Metab. 2010;95(1):82–92. [DOI] [PubMed] [Google Scholar]
- 150.Moos WH, Dykens JA, Nohynek D, Rubinchik E, Howell N. Review of the Effects of 17 alpha-Estradiol in Humans: A Less Feminizing Estrogen With Neuroprotective Potential. Drug Develop Res. 2009;70(1):1–21. [Google Scholar]
- 151.Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, et al. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54(1):138–53. [DOI] [PubMed] [Google Scholar]
- 152.Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005;1038(2):216–22. [DOI] [PubMed] [Google Scholar]
- 153.Ozacmak VH, Sayan H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res. 2009;58(6):909–12. [DOI] [PubMed] [Google Scholar]
- 154.Green PS, Simpkins JW. Estrogens and estrogen-like non-feminizing compounds. Their role in the prevention and treatment of Alzheimer's disease. Ann N Y Acad Sci. 2000;924:93–8. [DOI] [PubMed] [Google Scholar]
- 155.Levin-Ailerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis. 2002;4(6):449–57. [DOI] [PubMed] [Google Scholar]
- 156.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Ceil. 2014;13(2):273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harrison DE, Strong R, Reifsnyder P, Kumar N, Fernandez E, Flurkey K, et al. 17-a-estradiol late in life extends lifespan in aging UM-HET3 male mice; nicotinamide riboside and three other drugs do not affect lifespan in either sex. Aging Cell. 2021;20(5):e13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A. 1999;54(11):B492–501. [DOI] [PubMed] [Google Scholar]
- 160.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stout MB, Steyn FJ, Jurczak MJ, Camporez JG, Zhu Y, Hawse JR, et al. 17alpha-Estradiol Alleviates Age-related Metabolic and Inflammatory Dysfunction in Male Mice Without Inducing Feminization. J Gerontol A. 2017;72(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steyn FJ, Ngo ST, Chen VP, Bailey-Downs LC, Xie TY, Ghadami M, et al. 17alpha-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell. 2018;17(1):e12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mann SN, Hadad N, Nelson Holte M, Rothman AR, Sathiaseelan R, Ali Mondal S, et al. Health benefits attributed to 17alpha-estradiol, a lifespan-extending compound, are mediated through estrogen receptor alpha. Elife. 2020;9:e59616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miller BF, Pharaoh GA, Hamilton KL, Peelor FF, Kirkland JL, Freeman WM, et al. Short-term Calorie Restriction and 17alpha-Estradiol Administration Elicit Divergent Effects on Proteostatic Processes and Protein Content in Metabolically Active Tissues. J Gerontol A. 2020;75(5):849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sidhom S, Schneider A, Fang Y, McFadden S, Darcy J, Sathiaseelan R, et al. 17alpha-Estradiol Modulates IGF1 and Hepatic Gene Expression in a Sex-Specific Manner. J Gerontol A. 2021;76(5):778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16(6):1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garratt M, Lagerborg KA, Tsai YM, Galecki A, Jain M, Miller RA. Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018;17(4):e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garratt M, Leander D, Pifer K, Bower B, Herrera JJ, Day SM, et al. 17-alpha estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019;18(2):e12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Garratt M, Stout MB. Hormone actions controlling sex-specific life-extension. Aging (Albany NY). 2018;10(3):293–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Debarba LK, Jayarathne HSM, Miller RA, Garratt M, Sadagurski M. 17-alpha-Estradiol Has Sex-Specific Effects on Neuroinflammation That Are Partly Reversed by Gonadectomy. J Gerontol A. 2022;77(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Isola JVV, Veiga GB, de Brito CRC, Alvarado-Rincón JA, Garcia DN, Zanini BM, et al. 17α-estradiol does not adversely affect sperm parameters or fertility in male mice: implications for reproduction-longevity trade-offs. GeroScience. 2022. Jun 11. doi: 10.1007/s11357-022-00601-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Isola JVV, Zanini BM, Hense JD, Alvarado-Rincon JA, Garcia DN, Pereira GC, et al. Mild calorie restriction, but not 17alpha-estradiol, extends ovarian reserve and fertility in female mice. Exp Gerontol. 2022;159:111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Isola JVV, Zanini BM, Sidhom S, Kopchick JJ, Bartke A, Masternak MM, et al. 17alpha-Estradiol promotes ovarian aging in growth hormone receptor knockout mice, but not wild-type littermates. Exp Gerontol. 2020;129:110769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mann SN, Pitel KS, Nelson-Holte MH, Iwaniec UT, Turner RT, Sathiaseelan R, et al. 17α-Estradiol prevents ovariectomy-mediated obesity and bone loss. Exp Gerontol. 2020;142:111113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, et al. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22(19):8391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data was generated for the purposes of this mini-review.