Abstract
Due to climate change, farmers will face more extreme weather conditions and hence will need crops that are better adapted to these challenges. The raffinose family oligosaccharides (RFOs) could play a role in the tolerance of crops towards abiotic stress. To investigate this, we determined for the first time the importance of galactinol and RFOs in the roots and leaves of common bean under drought and salt stress conditions. Initially, the physiological characteristics of common bean under agronomically relevant abiotic stress conditions were investigated by measuring the growth rate, transpiration rate, chlorophyll concentration and membrane stability, allowing to establish relevant sampling points. Subsequently, the differential gene expression profiles of the galactinol and RFO biosynthetic genes and the amount of galactinol and RFO molecules were measured in the primary leaves and roots of Phaseolus vulgaris cv. CIAP7247F at these sampling points, using RT-qPCR and HPAEC-PAD, respectively. Under drought stress, the genes galactinol synthase 1, galactinol synthase 3 and stachyose synthase were significantly upregulated in the leaves and had a high transcript level in comparison with the other galactinol and RFO biosynthetic genes. This was in accordance with the significantly higher amount of galactinol and raffinose detected in the leaves. Under salt stress, raffinose was also present in a significantly higher quantity in the leaves. In the roots, transcript levels of the RFO biosynthetic genes were generally low and no galactinol, raffinose or stachyose could be detected. These results suggest that in the leaves, both galactinol and raffinose could play a role in the protection of common bean against abiotic stresses. Especially, the isoform galactinol synthase 3 could have a specific role during drought stress and forms an interesting candidate to improve the abiotic stress resistance of common bean or other plant species.
Keywords: Abiotic stress, drought, Fabaceae, galactinol, RFO, salinity, stress tolerance
Due to climate change, farmers will face more extreme weather conditions and hence will need crops that are better adapted to these challenges. This study presents new insights into the role of raffinose family oligosaccharides (RFOs) as a resilience mechanism against environmental stress in common bean. This study particularly focuses on galactinol and RFOs in common beans exposed to drought and salt stress conditions. The findings indicate that specific RFO biosynthesis-related genes were significantly upregulated in leaves, coinciding with increased amounts of galactinol and raffinose. No similar patterns were observed in the roots. This points to a potential protective role for galactinol and raffinose in bean leaves against environmental stress. Notably, the isoform galactinol synthase 3 could play a unique role in drought stress resistance, presenting a promising candidate for enhancing climate resilience in common bean or other plant species.
Introduction
With a growing population to feed and a rapidly changing climate, our agricultural system faces great challenges (Lobell et al. 2008). In the transition towards a more sustainable agricultural system, leguminous crops such as common bean (Phaseolus vulgaris) are of key importance. The ability of common bean to fix nitrogen through symbiosis with rhizobia makes it less dependent on fertilizers, which reduces the energy consumption of our agricultural system (Herridge et al. 2008; Thilakarathna and Raizada 2018; Reinprecht et al. 2020). Furthermore, common bean is a great source of nutrients, being rich in carbohydrates, minerals, vitamins, fibre and especially proteins (Doria et al. 2012; Campos-Vega et al. 2013; Ganesan and Xu 2017). This makes it an excellent alternative source for animal-derived proteins, which are more environmentally costly (Pimentel and Pimentel 2003; Nikbakht Nasrabadi et al. 2021). Common bean is the most important grain legume for direct human consumption, especially in Latin America and East Africa, where it is the main source of carbohydrates and proteins in the diets (Broughton et al. 2003). In Western countries, however, the cultivation and consumption of common bean still have a large growth potential (Bellucci et al. 2021).
As the climate changes, crops will face more extreme weather conditions, such as drought and heat, which also result in increased soil salinity (Mukhopadhyay et al. 2021). Currently, around 20 % of the cultivated and 33 % of the irrigated arable land are already affected by salinity, with estimates stating that 50 % of the arable land will be affected by 2050 (Munns and Tester 2008; Ullah et al. 2011). Among other crops, common bean is sensitive to saline conditions and even cultivation in slightly saline soil (1 dS m−1) will already result in a yield loss of around 20 % (Chinnusamy et al. 2005). Furthermore, around 60 % of the dry beans cultivated worldwide are affected by intermittent or terminal drought stress, resulting in severe yield losses (Beebe et al. 2013). To tackle these challenges, breeders need in-depth insights into what effects these abiotic stresses have on the plant as well as finding solutions to obtain varieties that are more resilient when exposed to abiotic stress.
Raffinose family oligosaccharides (RFOs) are a family of soluble carbohydrates, derived from sucrose, which contain α-1,6-galactosyl extensions (Peterbauer and Richter 2001). Within common bean, mainly raffinose and stachyose and in lower quantities verbascose can be found in the seeds; however, no quantitative data are available regarding these oligosaccharides in vegetative tissues (Díaz-Batalla et al. 2006). The metabolic pathway for the production of RFOs is initiated by the enzyme galactinol synthase, which produces galactinol by the transfer of a galactosyl residue from uridine diphosphate galactose to myo-inositol (Peterbauer and Richter 2001). Galactinol is needed for the formation of the first RFO, raffinose, which is formed by the enzyme raffinose synthase that catalyses the transfer of a galactosyl moiety from galactinol to sucrose (Peterbauer et al. 2002a). Stachyose and subsequently verbascose are formed by the transfer of a galactosyl moiety from galactinol to raffinose or stachyose, respectively. Both reactions are catalysed by the stachyose synthase enzyme (Peterbauer et al. 2002b). The common bean genome comprises three galactinol synthase genes (PvGolS1, PvGolS2 and PvGolS3), two raffinose synthase genes (PvRS1 and PvRS2) and one stachyose synthase gene (PvSS; de Koning et al. 2021).
In plants, RFOs play a role in the transport and storage of carbon (Sengupta et al. 2015). Furthermore, RFOs accumulate during seed maturation to protect them against desiccation and provide seed longevity (Kandler and Hopf 1980; Bailly et al. 2001; Blöchl et al. 2007). They are also a source of energy and carbon during germination (Peterbauer and Richter 2001; Blöchl et al. 2008). Besides these functions under normal plant growth conditions, RFOs and their precursor galactinol could potentially play a role during abiotic stress. Taji et al. (2002) have reported that Arabidopsis thaliana plants accumulate large amounts of galactinol and raffinose, but no stachyose, under drought, cold and high salinity conditions. In tomato plants, Liu et al. (2022) have reported that galactinol synthase is upregulated during cold and heat stress. In chickpea, galactinol synthase is also upregulated during cold, heat, dehydration, salt and oxidative stress (Salvi et al. 2018). Furthermore, also the galactinol and raffinose contents are higher under these abiotic stress conditions. In Coffea arabica, dos Santos et al. (2011) have found that galactinol synthase is upregulated under heat stress, water deficit and high salinity. They have also reported an increase in the accumulation of raffinose and stachyose under these conditions. Under abiotic stress conditions, galactinol and RFOs could help the plant maintain cell turgor by acting as osmolytes, protect the plant by stabilizing proteins and membranes with hydrophilic interactions, and by scavenging reactive oxygen species (ROS) that accumulate in high amounts during these stress conditions (Hincha et al. 2003; Seki et al. 2007; Nishizawa et al. 2008; Peters 2010; Elsayed et al. 2014). From an evolutionary point of view, the RFO biosynthesis genes have likely co-evolved with the vascular development of higher plants and could have played an important role in the adaptation of plants from an aquatic to a terrestrial environment, where exposure to drought stress might be common (Yan et al. 2022).
In this study, we determined the significance of galactinol and RFOs in common bean during drought and salt stress. Initially, the physiological characteristics of common bean under agronomically relevant abiotic stress conditions were investigated by measuring the growth rate, transpiration rate, chlorophyll concentration and membrane stability, allowing to establish relevant sampling points. Subsequently, the differential gene expression of the galactinol- and RFO synthase genes and the amount of galactinol and RFO molecules were measured in the primary leaves and roots of P. vulgaris cv. CIAP7247F at these sampling points using reverse transcription quantitative real-time PCR (RT-qPCR) and high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), respectively.
Materials and Methods
Plant material
Phaseolus vulgaris cv. CIAP7247F plants were grown in a greenhouse (Brussels, Belgium) under a 16-h light and 8-h dark regime in a mixture of sand and vermiculite (ratio 2:1) in 500-mL perforated pots which were placed on individual trays to prevent water loss. The soil moisture content of all plants was measured daily and the control plants were watered in such a way as to keep the soil moisture content around 80 % of field capacity. To induce drought stress, plants were grown under well-watered conditions until they reached the V3 growth stage (18 days after sowing [DAS]), after which the irrigation was stopped (Vlasova et al. 2016). Plants were fertilized with 100 mL of full-strength Hoagland at 14 DAS (Hoagland and Arnon 1950). Leaf and root samples of well-watered control plants and plants in drought stress conditions were harvested and flash frozen with liquid nitrogen at 22 and 25 DAS between 1 p.m. and 2 p.m. to avoid variability due to the circadian rhythm and stored at −80 °C. To induce salt stress, 50 mM NaCl was added to the sand and vermiculite mixture (Chinnusamy et al. 2005). Because salt stress causes a reduction in growth rate, plants within this condition were planted 7 days before the control group to make sure the control and salt-stressed plants were in the same developmental stage upon harvesting. Salt-stressed plants were fertilized with 100 mL of full-strength Hoagland at 21 DAS. Samples of the salt-stressed condition were harvested and flash frozen at 29 DAS between 1 p.m. and 2 p.m. and stored at −80 °C.
Soil moisture content
To determine the soil moisture content at field capacity, the soil was oversaturated with water and covered to prevent evaporation. After draining the soil for 3 consecutive days, the pot containing the drained soil was weighed (Swet). The soil was then dried in an oven at 110 °C for 24 h to determine the weight of the soil in dry conditions (Sdry). Soil moisture content was calculated using the following formula:
| (1.1) |
The average of five replicates was used to determine the at field capacity. The soil moisture content in pots on a certain day was calculated using Equation (1.1) with Sdry being the weight of dry soil and pot and Swet being the current weight of the soil and pot. The weight of the developing plant was neglected, based on the fact that the average plant weighed only 3 g at growth stadium V3.
The percentage of field capacity of the soil of a certain pot was calculated by dividing that pot’s current soil moisture content by the soil moisture content at field capacity and multiplied by 100 (Equation (1.2)).
| (1.2) |
Growth rate
The growth rate was estimated by measuring the area of the primary leaves. The leaf area of five biological replicates was measured using the agronomy tool of APS Assess 2.0 (Lamari 2008). The average and standard error were calculated using Microsoft Excel (v16). IBM SPSS Statistics (v25) was used to perform independent samples t-tests to determine the significance of differences between conditions.
Transpiration rate
The stomatal conductance of the abaxial side of the primary leaves was measured using an SC-1 Leaf Porometer (Decagon Devices, Pullman, WA) around 1 p.m. For each condition, the stomatal conductance was measured of five biological replicates, and the average was calculated using Microsoft Excel (v16). IBM SPSS Statistics (v25) was used to perform independent samples t-tests to determine the significance of differences between conditions. Transpiration rates were only compared for measurements taken on the same day because the transpiration rate is highly dependent on environmental conditions and the growth stage of the plant.
Chlorophyll content
The chlorophyll content of the primary leaves was measured using a SPAD 502 chlorophyll meter 2900P (Spectrum technologies, Bridgend, UK; Richardson et al. 2002). For each condition, the SPAD values (numerical value based on the amount of light transmitted by the leaf at 940 and 650 nm) were measured of 5 biological replicates with 10 technical replicates each (measurements randomly spread across the whole leaf) around 1 p.m. and the average was calculated using Microsoft Excel (v16). The chlorophyll content could be derived from the SPAD values using a standard curve (Lichtenthaler and Wellburn 1983; Lichtenthaler 1987). This standard curve was made using leaves with 17 different SPAD values spanning the whole spectrum (from 4.5 to 38.5) and measuring their respective chlorophyll content using a spectrophotometer. To do so, the mid-veins of these 17 leaves were removed and the rest of the leaves were cut into pieces. Of each biological sample, three technical replicates of approximately 100 mg of leaf material were dissolved in 1 mL of 80 % acetone and ground in a mortar. Every sample was then vortexed and incubated in the dark for 2 h during which the samples were gently shaken to extract the chlorophyll. The samples were centrifuged for 60 s at 20 800g and the absorbance of the supernatant was measured at 646 and 663 nm with a SmartSpec Plus Spectrophotometer (Bio-Rad, Hercules, CA; Minden et al. 2017). The chlorophyll content (µg mg−1) of each technical repeat was calculated using the following formula:
| (1.3) |
A graph was made in Microsoft Excel (v16) by plotting the average chlorophyll content of the three technical replicates against the corresponding SPAD value. The standard curve was calculated using exponential regression, which was the best model to fit this dataset (y = 0.0967 e0.0736x, R2 = 0.8811; seeSupporting Information—Figure S1; Lichtenthaler and Wellburn 1983; Lichtenthaler 1987; Minden et al. 2017).
Electrolyte leakage
For each condition, three biological replicates were used to measure leaf electrolyte leakage (EL). A primary leaf was cut off at the base of the leaf. To avoid EL, paraffin was applied to the detached surface. Each leaf was gently shaken for 10 min in 100 mL demineralized water inside an individual glass container, closed with a lid to remove the surface solutes. Next, each leaf was placed in a new container containing 100 mL demineralized water and incubated for 2 h in a 35 °C water bath, after which the initial electrical conductivity (EC1) of the solution was measured using a SevenGo pro-electrical conductivity meter (Mettler Toledo, Columbus, OH) coupled to an InLab738 conductivity probe (Mettler Toledo). To measure the maximum electrical conductivity (EC2), the leaf samples were autoclaved at 121 °C for 20 min to break the cells and release all ions. After the solution was cooled down to 30 °C, the EC2 was measured. To calculate the EL, the following formula was used (Dionisio-Sese and Tobita 1998; Kodikara 2018):
| (1.4) |
To determine the EL for a certain condition, the average of three biological replicates was calculated using Microsoft Excel (v16). IBM SPSS Statistics (v25) was used to perform independent samples t-tests to determine the significance of differences between conditions.
Differential gene expression of the galactinol- and RFO synthase genes
Frozen root and leaf samples were crushed using the TissueLyser II (Qiagen, Hilden, Germany), and RNA was extracted using the Nucleospin RNA Plant and Fungi kit (Macherey-Nagel, Düren, Germany, CAT #740120.50). The quality and quantity of the samples were checked using the Quantus fluorometer (Promega, Madison, WI), and the RNA integrity was evaluated using a bleach gel electrophoresis, as described by Aranda et al. (2012). To remove genomic DNA, the RNA samples were treated with RQ1 RNase-Free DNase (Promega, CAT #M6101), after which cDNA was synthesized using the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, CAT #K1631). Primers used to amplify cDNA were as described by de Koning et al. (2021). As a reference gene, actin-11 (Act11) was used, which is stably expressed during abiotic stress conditions (Borges et al. 2012). An overview of the primers used can be found in Supporting Information—Table S1. To perform RT-qPCR, the samples were mixed with GoTaq qPCR Master Mix (Promega, CAT # A6001) and loaded on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad). The RT-qPCR settings were used as described by de Koning et al. (2021). For every condition, the gene expression of the galactinol and RFO biosynthetic genes was measured for three biological replicates, with three technical replicates each. Actin-11 was used as a reference gene to normalize the cDNA threshold cycle (Ct) values observed by RT-qPCR through Equation (1.5), using the comparative Ct method (Livak and Schmittgen 2001; Schmittgen and Livak 2008; Borges et al. 2012):
| (1.5) |
The difference in gene expression levels was calculated by comparing treatments with the control condition using Equation (1.6) (Livak and Schmittgen 2001).
| (1.6) |
IBM SPSS Statistics (v25) was used to perform independent samples t-tests to determine the significance of differences in expression.
To calculate the number of gene transcripts of a specific gene relative to the reference gene Actin-11, the ∆Ct values of Equation (1.5) were used. During RT-qPCR, the multiplication of cDNA could be calculated with Equation (1.7) (Liu and Saint 2002):
| (1.7) |
with : amount of cDNA; start amount of cDNA (or mRNA assuming perfect cDNA synthesis); primer pair efficiency; and : cycle number.
The selected primer pairs used in this analysis had an efficiency between 0.90 and 1.10 (de Koning et al. 2021). For further analysis, E was approximated by 1. Assuming this, Equation (1.7) could be written as a function of the cycle number (n):
| (1.8) |
Equation (1.8) could be substituted in Equation (1.5), resulting in Equation (1.9):
| (1.9) |
with N equal to the amount of cDNA at the threshold cycle (Ct). At the threshold cycle, the fluorescence signal surpassed a fixed threshold value and the amount of cDNA present () was approximately equal for both the reference gene and the other genes , assuming that the primer pairs amplified cDNA fragments of the same length. Using this assumption, Equation (1.9) could be written as
| (1.10) |
This could be further written as a ratio between the start amount of cDNA of a specific gene and the reference gene :
| (1.11) |
Equation (1.11) was used to calculate the number of gene transcripts of a specific gene relative to the reference gene Actin-11.
Quantification of the galactinol and RFO content using HPAEC-PAD
Frozen root and leaf samples of around 150 mg were crushed using the TissueLyser II (Qiagen), after which the exact weight of the samples was determined. To each sample, 1 mL 50 % ethanol was added, after which the samples were vortexed and incubated for 30 min at 70 °C, while the samples were gently shaken to enhance the extraction. Next, the samples were centrifuged at 20 800g for 15 min, after which the supernatant was filter-sterilized with a 0.2-µm cellulose acetate membrane (VWR, Radnor, PA). One hundred microliters of each sample was added to 900 µL of a solution consisting of 50 % acetonitrile, 49.8 % Milli-Q H2O and 0.2 % rhamnose (0.3 mM), which functioned as the internal standard. The samples were vortexed for 2 min and centrifuged at 3600g for 15 min, after which the supernatant was filtered through a 0.2 µm Millex-LG Filter (Merck, Darmstadt, Germany) and added to a high-performance liquid chromatography vial.
To quantify the amount of galactinol and RFOs in the samples, high-performance anion-exchange chromatography (HPAEC) with pulsed amperometric detection (PAD) was done, using an ICS 5000 ion chromatography system (Dionex, Sunnyvale, CA) equipped with a Carbopac PA20 column set consisting of a Microbore Guard Column (3 × 60 mm) and a Microbore Analytical Column (150 × 3 mm), which was kept at 35 °C (Kotha et al. 2020). For each sample (kept at 10 °C), 10 µL was injected. The mobile phase consisted of Milli-Q water (eluent A), 189 mM NaOH (eluent B) and 1.52 M NaOH (eluent C) and had a flow rate of 0.5 mL min−1. To quantify the compounds, the following gradient was applied: 0.0 min, 89 % A and 11 % B; 15.0 min, 100 % B; 21.0 min, 100 % C; 21.5 min, 100 % C; 30.0 min, 89 % A and 11 % B; 35.0 min, 89 % A and 11 % B. The total run time was 35 min.
Stock solutions (50 mg L−1) of galactinol (CAS: 16908-86-4, Sigma-Aldrich, St. Louis, MO), raffinose (CAS: 17629-30-0, Sigma-Aldrich) and stachyose (CAS: 54261-98-2, Sigma-Aldrich) were prepared by dissolving each compound in 50 % ethanol, after which 2-fold dilution series were made, ranging from 50 to 0.024 mg L−1. Data analysis was done using the Chromeleon Chromatography Data System software (v6.8, Thermo Fisher Scientific), and calibration curves (linearity), limits of detection (LOD) and limits of quantitation (LOQ) were calculated in Microsoft Excel (v16) following the quality guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (2005). For each condition, the quantities of three biological replicates were measured, and the average values and standard errors were calculated using Microsoft Excel (v16). IBM SPSS Statistics (v25) was used to perform independent samples t-tests to determine the quantification significance between conditions. The linearity equations, along with the corresponding LOD and LOQ values, can be found in Supporting Information—Table S2.
Results
Physiological conditions of Phaseolus vulgaris under abiotic stress
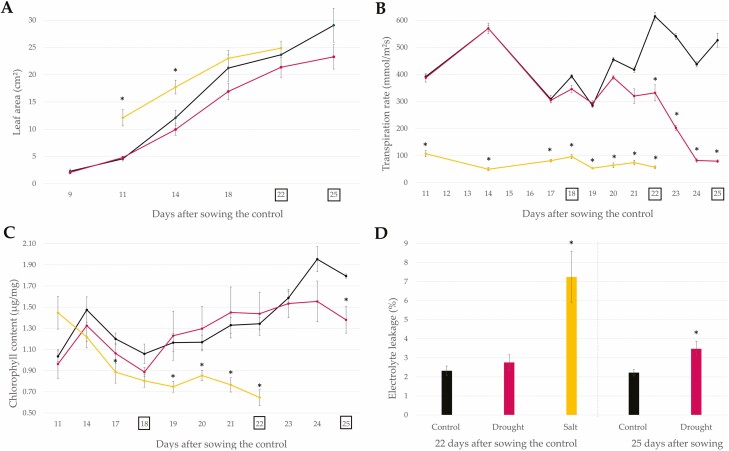
The growth rate of common bean under well-watered, drought and salt stress conditions was estimated by measuring the leaf area of the primary leaves. All plants reached a similar primary leaf area, with respective values of 23.7 cm2 (SE = 2.4) for the control plants 22 DAS, 21.4 cm2 (SE = 1.8) for the drought-stressed plants 22 DAS and 24.9 cm2 (SE = 1.4) for the salt-stressed plants 29 DAS (Fig. 1A). At this time point, the plants under well-watered, drought and salt stress conditions were also in the same V3 growth stage (Fig. 2; Vlasova et al. 2016). The plants under well-watered and drought stress conditions had a similar growth rate, even after the irrigation was stopped 18 DAS (Fig. 1A). For the drought-stressed plants, the soil moisture content dropped from 86.91 % of field capacity (SE = 1.5, n = 45) at 18 DAS to 34.2 % (SE = 0.82, n = 45) at 22 DAS and reached a value of 17.44 % (SE = 0.43, n = 45) at 25 DAS.
Figure 1.
Physiological characteristics of P. vulgaris under well-watered (black), drought (pink) and salt (yellow) stress conditions. Salt-stressed plants (50 mM NaCl) were sown 7 days prior to the control plants to compensate for the reduced growth rate. The results for the salt-stressed plants are depicted in terms of the DAS of the control plants. To initiate drought stress, irrigation was stopped at 18 DAS for the drought-stressed plants. (A) Average leaf area of the primary leaves (n = 5). (B) Average transpiration rate of the primary leaves (n = 5). (C) Average chlorophyll content of the primary leaves (n = 5). (D) Average electrolyte leakage in the primary leaves (n = 3). Time points with an asterisk (*) above represent values that are significantly different from the leaves of well-watered plants (P < 0.05). Error bars represent the standard error.
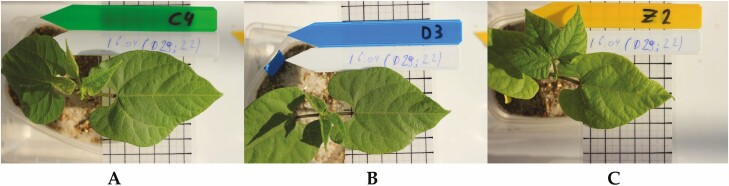
Figure 2.
Phaseolus vulgaris plants under different abiotic stress conditions in the first trifoliate leaf stage (v3). (A) Control plant 22 DAS (C4). (B) Drought-stressed plant 22 DAS (D3). (C) Salt-stressed plant 29 DAS (Z2).
The transpiration rate was estimated by measuring the stomatal conductance of the abaxial side of the primary leaves at a fixed time in the day (1 p.m.) once the first true leaves emerged (Fig. 1B). Plants that were subjected to salt stress had a significantly lower transpiration rate in comparison with the control. At 22 DAS, the average stomatal conductance of the salt-stressed plants was 57.0 mmol (m2s)−1 (SE = 4.7), whereas the control plants had a stomatal conductance of 614.9 mmol (m2s)−1 (SE = 14.8). After the irrigation was stopped 18 DAS, the first significant difference in stomatal conductance could be seen 22 DAS, where the control plants had an average stomatal conductance of 614.9 mmol (m2s)−1 (SE = 14.8) as compared to the drought-stressed plants that had an average stomatal conductance of 332.1 mmol (m2s)−1 (SE = 29.7). The stomatal conductance of the drought-stressed plants dropped in time even further, reaching its lowest point at 25 DAS with an average value of 79.4 (SE = 5.1) mmol (m2s)−1.
For each condition, the chlorophyll content of the primary leaves was calculated (Fig. 1C). For plants grown under salt stress, the first significant difference in chlorophyll content was observed at 17 DAS for the control (24 DAS for the salt-stressed plants), with the salt-stressed plants having an average chlorophyll content of 0.89 μg mg−1 (SE = 0.10) in comparison with the control that had an average chlorophyll content of 1.20 μg mg−1 (SE = 0.06). The chlorophyll content of the salt-stressed plants dropped over time, reaching its lowest value at 22 DAS the control, with an average value of 0.65 μg mg−1 (SE = 0.08). Plants under drought stress and control conditions had a similar chlorophyll content, except at 25 DAS, at which the drought-stressed plants had an average chlorophyll content of 1.38 μg mg−1 (SE = 0.13), which was significantly lower than the control plants (1.79 μg mg−1; SE = 0.02).
The membrane stability was estimated by measuring the EL in the primary leaves (Fig. 1D). Plants grown under salt stress conditions showed a significant increase in EL in comparison to the control plants 22 days after sowing the control, with an average EL of 7.25 % (SE = 1.35) in comparison with the control that had an average EL of 2.32 % (SE = 0.25). Plants grown under drought stress conditions only showed a significant increase in EL 25 DAS, with an average EL of 3.48 % (SE = 0.40) in comparison with the control that had an average EL of 2.22 % (SE = 0.17).
Differential gene expression of the galactinol and RFO synthase genes under abiotic stress conditions
For every condition, the gene expression of the galactinol and RFO biosynthetic genes was measured in the primary leaves and roots of P. vulgaris using RT-qPCR. The differential gene expression is represented as value, of which positive values represent an upregulation and negative values a downregulation of the specific gene in comparison with the control plants. values close to zero indicate no substantial change in gene expression in comparison to the control condition (Livak and Schmittgen 2001; Schmittgen and Livak 2008).
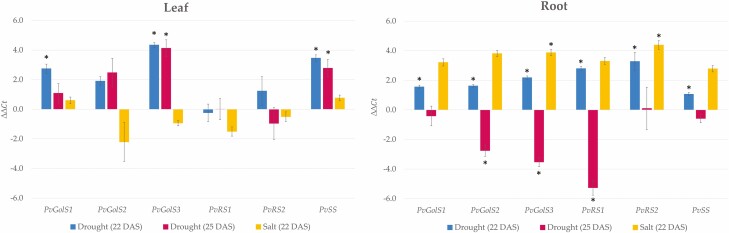
Under drought stress, common bean plants showed overexpression of galactinol synthase 1 (PvGolS1), galactinol synthase 3 (PvGolS3) and stachyose synthase (PvSS) in the primary leaves in comparison with the control (Fig. 3). During the early stages of drought stress (22 DAS), PvGolS1 was significantly upregulated having a value of 2.8 (SE = 0.3). Both PvGolS3 and PvSS were also significantly upregulated at this stage with respective values of 4.4 (SE = 0.2) and 3.5 (SE = 0.2). Under more severe drought stress (25 DAS), PvGolS3 and PvSS were still significantly upregulated with respective values of 4.1 (SE = 0.6) and 2.8 (SE = 0.6). When looking at the primary leaves of salt-stressed plants, no significant difference in the expression of the galactinol and RFO synthase genes was observed, demonstrated by values close to zero.
Figure 3.
Differential gene expression of the galactinol and RFO synthase genes in the primary leaves and roots of P. vulgaris under an early stage of drought stress (blue), more severe drought stress (red) and salt stress conditions (yellow). The differences in gene expression levels of galactinol synthase 1 (PvGolS1), galactinol synthase 2 (PvGolS2), galactinol synthase 3 (PvGolS3), raffinose synthase 1 (PvRS1), raffinose synthase 2 (PvRS2) and stachyose synthase (PvSS) are represented by values. Bars with an asterisk (*) above represent values that are significantly different from the control samples of well-watered plants (P < 0.05) and error bars represent the standard error.
In the roots, the differential gene expression of the galactinol and RFO synthase genes was more complex (Fig. 3). During the early drought stress stage (22 DAS), all galactinol and RFO synthase genes showed a significant upregulation. In contrast, under more severe drought stress (25 DAS), galactinol synthase 2 (PvGolS2), PvGolS3 and raffinose synthase 1 (PvRS1) were significantly downregulated, having respective values of −2.8 (SE = 0.4), −3.5 (SE = 0.3) and −5.3 (SE = 0.5). Under salt stress, PvGolS3 and raffinose synthase 2 (PvRS2) were significantly upregulated, with respective values of 3.9 (SE = 0.2) and 4.4 (SE = 0.3).
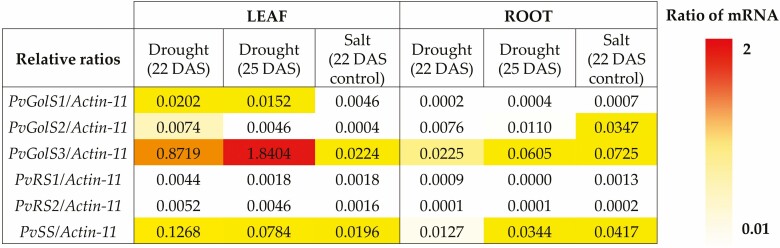
Besides the measurements of differential gene expression, the estimated number of gene transcripts of the galactinol and RFO synthase genes relative to the reference gene Actin-11 was calculated (Fig. 4). In the primary leaves of drought-stressed plants, the genes PvGolS1, PvGolS3 and PvSS showed a high relative number of gene transcripts in comparison with the other galactinol and RFO synthase genes. Especially PvGolS3, with values of 0.8719 and 1.8404, respectively, during the early stage of drought stress (22 DAS) and at the more severe stage (25 DAS). PvSS came second with values of 0.1268 at 22 DAS and 0.0784 at 25 DAS, and PvGolS1 had a relative number of gene transcripts of 0.0202 and 0.0152 at 22 and 25 DAS, respectively. Under salt stress conditions, most galactinol and RFO synthase genes had a very low relative number of gene transcripts in the primary leaves, with only PvGolS3 and PvSS having slightly higher numbers (0.0224 and 0.0196, respectively). In the roots, the relative number of gene transcripts for all galactinol and RFO synthase genes was much lower compared to the leaves. Although still low, PvGolS3 and PvSS had slightly higher values in the roots in comparison with the other galactinol and RFO synthase genes.
Figure 4.
Heat map of the estimated number of gene transcripts of the galactinol and RFO synthase genes relative to the gene transcripts of the reference gene (Actin-11) in the leaves and roots of P. vulgaris during different abiotic stress conditions. The relative number of gene transcripts is represented by a colour gradient, in which the white colour indicates a low ratio (<0.01) and the red colour a high ratio (>2).
Quantification of the galactinol and RFO content under abiotic stress conditions
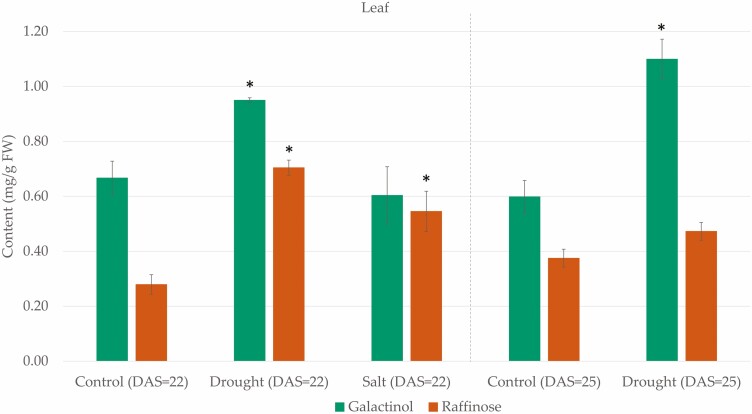
The galactinol and RFO content were measured in the primary leaves and roots of P. vulgaris under well-watered, drought and salt stress conditions using HPAEC-PAD. Under drought stress, the galactinol and raffinose content in the primary leaves both significantly increased in comparison with the control (Fig. 5). During the early stage of drought stress (22 DAS), the primary leaves of the drought-stressed plants contained 42 % more galactinol than the control plants. They contained on average 0.95 mg galactinol per g fresh weight (FW; SE = 0.01) in comparison to 0.67 mg galactinol per g FW (SE = 0.06) in the control plants. The raffinose content also significantly increased by 150 % in the primary leaves at this stage, containing on average 0.70 mg raffinose per g FW (SE = 0.03), whereas the control plants contained on average 0.28 mg raffinose per g FW (SE = 0.04). Under more severe drought stress (25 DAS), the galactinol content increased by 83 %, reaching a content of 1.10 mg galactinol per g FW (SE = 0.07) in the primary leaves, while the control plants only contained 0.60 mg galactinol per g FW (SE = 0.06). At this stage, the raffinose content also increased although not significantly. During salt stress, only a significant increase in the raffinose content was observed in the primary leaves. The salt-stressed plants contained 96 % more raffinose than the control, reaching a value of 0.55 mg raffinose per g FW (SE = 0.07) in comparison to 0.28 mg raffinose per g FW (SE = 0.04) in the control plants. In the roots, no galactinol, raffinose nor stachyose was measured above the LOD values in any condition.
Figure 5.
Quantification of the galactinol (green) and raffinose (orange) content in the primary leaves of P. vulgaris under abiotic stress conditions using HPAEC-PAD. The average content per gram of fresh weight (FW) is shown for three biological replicates and the error bars represent the standard error. Bars with an asterisk (*) above represent values that are significantly different from the well-watered control plant samples (P < 0.05).
Discussion
This study investigated the potential role that galactinol and RFOs play in the tolerance mechanism of common bean under agronomically relevant abiotic stress conditions. Gene expression and metabolite accumulation were examined in drought and salt stress conditions, making sure to compare plants in the same growth stage for accurate evaluation (Farooq et al. 2017). Moreover, the physiological characteristics of plants under stress were carefully monitored to establish relevant sampling points. During drought stress, the first physiological signs appeared 4 days after the irrigation was stopped, at 22 DAS, where for the first time, the stomatal conductance was significantly lower than for the well-watered control plants (Fig. 1B). The closure of stomata is considered an early sign of drought stress, reducing the water loss of the plant by lowering the transpiration rate (Farooq et al. 2017). This, however, also results in a reduction of carbon influx, lowering the internal concentration of CO2 in the leaves, which is considered the main limiting factor for photosynthesis during drought stress and as a consequence reduces the production of saccharides needed for plant growth (Chaves 1991; Cornic 2000; Flexas et al. 2004; Farooq et al. 2017). At 25 DAS, the first signs of more severe stress were observed. At this time point, the chlorophyll content in the primary leaves was significantly lower than the control and the EL was significantly higher(Fig. 1C and D). During abiotic stress, ROS are being formed, which initially function as signalling molecules, triggering defence mechanisms (Foyer and Noctor 2005). However, during more severe or prolonged stress, the concentration of ROS can further increase and damage the chloroplasts, reducing the chlorophyll content (Mafakheri et al. 2010). Furthermore, ROS can oxidize proteins and membrane lipids, which destabilizes the membrane and leads to EL (Gaspar et al. 2002). Both of these sampling points are indicative of the plant’s response to drought stress at an early (22 DAS) and more severe stage (25 DAS) and are good sample points to investigate the role of galactinol and RFOs. To induce agronomically relevant salt stress conditions, plants were grown from the start in saline soil of 50 mM NaCl, which corresponds with an electrical conductivity of the soil of approximately 5 dS m−1. By definition, soils are considered saline if they have an electrical conductivity of 4 dS m−1 or more (Chinnusamy et al. 2005). Twenty-two days after sowing the control, the plants were in the same V3 growth stage as the control plants (Fig. 2). At this time point, the plants experienced salt stress, as the transpiration rate was significantly lower than for the control plants (Fig. 1B). Like drought stress, plants subjected to salt stress experience osmotic stress and try to limit their water loss by closing the stomata. On top of the osmotic stress, salt-stressed plants also experience ionic stress caused by the uptake of high amounts of Na+ and Cl- ions (Tavakkoli et al. 2010). These stresses can cause a decrease in chlorophyll content and an increase in EL, which corresponded with the measurements in the salt-stressed plants of the present study (Fig. 1C and D; Tavakkoli et al. 2010). Both of these physiological traits indicate that the plants experienced more severe salt stress and demonstrate that this sample point was suitable to determine the role of galactinol and RFOs during long-term salt stress exposure (Gaspar et al. 2002; Flexas et al. 2004; Chinnusamy et al. 2005; Demidchik et al. 2014).
Previous studies have shown that there is a relationship between abiotic stress and an increase of galactinol and RFOs in plants and that these components could help the plant mitigate these stresses (Hincha et al. 2003; Seki et al. 2007; Nishizawa et al. 2008; Peters 2010; Elsayed et al. 2014). However, this has not been investigated in common bean so far. To understand the significance of these saccharides, we quantified galactinol and RFOs in leaves and roots and measured the expression of their biosynthetic genes. While interpreting the up- or downregulation of a gene, it is important to also take into account the number of gene transcripts present. A doubling of the gene expression may not be relevant if the initial amount of gene transcripts is very low. To estimate this amount, the number of gene transcripts of the galactinol and RFO synthase genes were calculated relative to the reference gene Actin-11 (Borges et al. 2012).
During early drought stress (22 DAS), the galactinol synthase genes PvGolS1 and PvGolS3 and the stachyose synthase gene PvSS were significantly upregulated in the primary leaves of common bean (Fig. 3). During more severe drought stress (25 DAS), PvGolS3 and PvSS were still significantly upregulated. Besides their upregulation, these genes also had a high relative number of gene transcripts present (Fig. 4). Especially PvGolS3 showed high values at both the early and more severe drought stress conditions. These results correspond to the significantly higher amount of galactinol observed in the primary leaves during both drought stress stages (Fig. 5). These results highlight the potential importance of isoform PvGolS3 in producing galactinol during drought stress in the leaves of common bean. Raffinose was also present in a significantly higher amount during the early stage of drought stress and slightly higher at the more severe stage. However, no overexpression of raffinose synthase was observed. It could be that due to the high concentration of galactinol, raffinose synthase can produce significantly more raffinose without the need for higher quantities of the enzyme (Vinson et al. 2020). Because PvSS showed an overexpression at both stages, we would have expected to see an increase in stachyose as well; however, no stachyose was detected. Stachyose synthase can produce, besides stachyose, also galactosyl cyclitols that have a similar chemical structure as RFOs and could potentially also provide tolerance towards abiotic stress (Obendorf 1997; Frias et al. 1999). Overexpression of PvSS might increase the production of these galactosyl cyclitols; however, the concentration of the galactosyl cyclitols was not measured in this study. The concentrations of galactinol and RFOs measured in the leaves of common bean were in correspondence with the concentrations found in the leaves of other plant species, such as Cucumis sativus and A. thaliana under abiotic stress (Sun et al. 2013; Gu et al. 2018). The roots of common bean under drought stress showed a contrasting expression pattern for the galactinol synthase genes. These genes were upregulated during early drought stress (22 DAS) but showed a downregulation at the more severe stage (25 DAS; Fig. 3). The same contrasting pattern was observed for the raffinose synthase gene, PvRS1. The genes PvRS2 and PvSS only showed a significant upregulation during early drought stress. However, these differential gene expression patterns could be less relevant because the estimated number of gene transcripts present for all these genes was very low (Fig. 4). Moreover, no galactinol nor RFOs were detected in the roots, indicating that these saccharides are either not produced, or rapidly broken down in the roots (Eom et al. 2012). The low relative abundance of gene transcripts of the RFO synthase genes further indicates that RFOs are not produced in the roots. Our results are in correspondence with previous research on common bean and on homologous genes found in other species. In common bean, an increase in raffinose was also detected in the stem and seed under drought stress conditions (Andrade et al. 2016; Herrera et al. 2019). Taji et al. (2002) have discovered in A. thaliana that stress-inducible galactinol synthase genes play an important role in the accumulation of galactinol and raffinose under drought stress conditions and could provide drought tolerance. Selvaraj et al. (2017) have shown that overexpression of such stress-inducible galactinol synthase gene (AtGolS2) of A. thaliana in transgenic rice results in plants with an elevated galactinol content in the leaves, which improves drought tolerance and grain yield in drought stress conditions. They have observed better water retention, higher photosynthesis activity and faster recovery in the overexpressing line during drought stress, which could be related to the elevated galactinol content. In C. sativus, Ma et al. (2021) have shown that overexpression of a galactinol synthase gene (CsGolS4) leads to a higher amount of galactinol and RFOs and reduced levels of ROS in the leaves, demonstrating a higher tolerance against drought and cold stress. Vinson et al. (2020) have demonstrated an increase in the drought and salt stress tolerance of A. thaliana plants overexpressing a galactinol synthase gene (AdGolS3) of Arachis duranensis. These plants had a significantly higher concentration of galactinol and raffinose in the leaves during abiotic stress, which results in better water retention, membrane stability and protection against oxidative stress.
Under salt stress, no significant up- or downregulation of the galactinol or RFO synthase genes could be seen in the primary leaves of common bean (Fig. 3). In the roots, the galactinol synthase gene PvGolS3 and the raffinose synthase gene PvRS2 were significantly upregulated (Fig. 3). However, the estimated transcript abundance of these genes was very low, questioning the significance of these upregulations (Fig. 4). Furthermore, no galactinol nor RFOs were detected in the roots. In the primary leaves, however, a significantly higher raffinose content was measured, which may indicate a possible role of raffinose in common bean plants under prolonged salt stress (Fig. 5). In other species, such as A. thaliana and C. arabica, an increase in the raffinose content together with an increase in the galactinol or stachyose content, respectively, has also been reported in plants subjected to salt stress (Taji et al. 2002; dos Santos et al. 2011). Sun et al. (2013) have discovered that the overexpression of a galactinol synthase gene (TsGolS2) from Thellungiella salsuginea in A. thaliana plants increases the amount of galactinol and raffinose in the leaves after exposure to high salinity and osmotic stress and improves the tolerance of the plants against these stresses.
During abiotic stress conditions, galactinol and RFOs could have multiple functions providing protection against osmotic and ionic stress. First, they can maintain cell turgor by acting as osmolytes (Bartels and Ramanjulu 2005). Second, they can stabilize proteins and membranes through hydrophilic interactions, ensuring normal protein functioning and preventing cell leakage (Hincha et al. 2003). Moreover, research has demonstrated that raffinose can be transported to the chloroplasts, where it can protect the thylakoids and stabilize photosystem II (Schneider and Keller 2009; Knaupp et al. 2011). Last, galactinol and RFOs can scavenge ROS, which could otherwise damage the plant (Nishizawa et al. 2008). Galactinol and raffinose have a similar capacity to scavenge ROS (Nishizawa et al. 2008). However, galactinol is energy-wise a better osmolyte than raffinose and stachyose, because only the concentration of osmolytes is important to create osmotic pressure and not the chemical properties of the solute (Seki et al. 2007). Therefore, the production of a certain concentration of solutes is less energy demanding when the plant accumulates galactinol and sucrose compared to the accumulation of raffinose or stachyose, which are trisaccharides and tetrasaccharides, respectively. From this, it could be hypothesized that the overproduction of galactinol would be the better option to provide abiotic stress resistance in plants. Besides the function of galactinol and RFOs in protecting against abiotic stress, they can also aid in plant recovery after plants underwent abiotic stress by serving as an energy source (Peters et al. 2007). In common bean or other plant species, the isoform PvGolS3 could be a promising candidate to improve the tolerance towards abiotic stress. The overexpression of this gene by inserting an extra copy of it in the genome could lead to higher levels of galactinol and raffinose under abiotic stress conditions protecting the plant. Previous research has shown that under normal plant growth conditions, PvGolS3 is mainly expressed in the vegetative tissue of common bean and no expression has been found in the seeds (de Koning et al. 2021). Therefore, an extra copy of it in the genome would not contribute to an increased amount of RFOs in the seed, because RFOs are produced de novo during seed maturation (Peterbauer and Richter 2001). Within the seeds, RFOs are among the most important anti-nutritional factors, causing flatulence and digestive problems when consumed by humans and monogastric animals, so an increase in the concentration in the seed should be avoided (Tomlin et al. 1991; Yamaguishi et al. 2009; Doria et al. 2012; Valentine et al. 2017).
Supporting Information
The following additional information is available in the online version of this article –
Figure S1. Relationship between the chlorophyll content of the leaves of 17 P. vulgaris cv. CIAP7247F plants and their corresponding SPAD values.
Table S1. Primers used for RT-qPCR.
Table S2. Analysis of the galactinol and raffinose family oligosaccharides content in root and leaf samples of P. vulgaris CIAP7247F under different abiotic stress conditions using HPAEC-PAD.
Acknowledgements
We would like to thank Prof. Dr. Harry Olde Venterink of the Functional Ecology of Plants and Ecosystems research group (VUB), for providing the SC-1 Leaf Porometer, SPAD 502 chlorophyll meter 2900P and the SevenGo pro-electrical conductivity meter. We would also want to express our gratitude towards ing. Wim Borremans of the Research Group of Industrial Microbiology and Food Biotechnology (VUB) for providing technical support for the HPAEC-PAD analyses.
Phenome, Genome and Environment. Chief Editor: Colleen Doherty
Contributor Information
Ramon de Koning, Research Group of Plant Genetics, Faculty of Sciences and Bioengineering Sciences, Vrije Universiteit Brussel, Pleinlaan 2, Brussels, Belgium.
Gertjan E Wils, Research Group of Plant Genetics, Faculty of Sciences and Bioengineering Sciences, Vrije Universiteit Brussel, Pleinlaan 2, Brussels, Belgium.
Raphaël Kiekens, Research Group of Plant Genetics, Faculty of Sciences and Bioengineering Sciences, Vrije Universiteit Brussel, Pleinlaan 2, Brussels, Belgium.
Luc De Vuyst, Research Group of Industrial Microbiology and Food Biotechnology, Faculty of Sciences and Bioengineering Sciences, Vrije Universiteit Brussel, Pleinlaan 2, Brussels, Belgium.
Geert Angenon, Research Group of Plant Genetics, Faculty of Sciences and Bioengineering Sciences, Vrije Universiteit Brussel, Pleinlaan 2, Brussels, Belgium.
Contributions by Authors
Conceptualization: R.d.K. and G.A.; methodology: R.d.K., G.E.W. and G.A.; software: R.d.K. and R.K.; validation: R.d.K., G.E.W and G.A.; formal analysis: R.d.K. and G.E.W.; investigation: R.d.K. and G.E.W.; resources: G.A. and L.D.V.; data curation: R.d.K. and G.E.W.; writing—original draft preparation: R.d.K.; writing—review and editing: R.d.K., G.E.W., G.A., R.K. and L.D.V.; visualization: R.d.K.; supervision: R.d.K. and G.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability
The datasets generated and analysed during the current study are incorporated in the manuscript and its supplementary materials. These include the tables, figures and additional data used to support the findings of this study. If further information is needed, please contact the corresponding author.
REFERENCES
- Andrade ER, Ribeiro VN, Azevedo CVG, Chiorato AF, Williams TCR, Carbonell SAM.. 2016. Biochemical indicators of drought tolerance in the common bean (Phaseolus vulgaris L.). Euphytica 210:277–289. [Google Scholar]
- Aranda PS, LaJoie DM, Jorcyk CL.. 2012. Bleach gel: a simple agarose gel for analyzing RNA quality. Electrophoresis 33:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C, Audigier C, Ladonne F, Wagner MH, Coste F, Corbineau F, Côme D.. 2001. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. Journal of Experimental Botany 52:701–708. [DOI] [PubMed] [Google Scholar]
- Bartels D, Ramanjulu S.. 2005. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences 24:23–49. [Google Scholar]
- Beebe SE, Rao IM, Blair MW, Acosta-Gallegos JA.. 2013. Phenotyping common beans for adaptation to drought. Frontiers in Physiology 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci E, Mario Aguilar O, Alseekh S, Bett K, Brezeanu C, Cook D, De la Rosa L, Delledonne M, Dostatny DF, Ferreira JJ, et al. 2021. The INCREASE project: intelligent collections of food-legume genetic resources for European agrofood systems. The Plant Journal 108:646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blöchl A, Peterbauer T, Richter A.. 2007. Inhibition of raffinose oligosaccharide breakdown delays germination of pea seeds. Journal of Plant Physiology 164:1093–1096. [DOI] [PubMed] [Google Scholar]
- Blöchl A, Peterbauer T, Hofmann J, Richter A.. 2008. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 228:99–110. [DOI] [PubMed] [Google Scholar]
- Borges A, Mui Tsai S, Caldas DGG.. 2012. Validation of reference genes for RT-qPCR normalization in common bean during biotic and abiotic stresses. Plant Cell Reports 31:827–838. [DOI] [PubMed] [Google Scholar]
- Broughton WJ, Hernández G, Blair M, Beebe S, Gepts P, Vanderleyden J.. 2003. Beans (Phaseolus spp.)-model food legumes. Plant and Soil 252:55–128. [Google Scholar]
- Campos-Vega R, Oomah BD, Loarca-Piña G, Vergara-Castañeda H.. 2013. Common beans and their non-digestible fraction: cancer inhibitory activity—an overview. Foods 2:374–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM. 1991. Effects of water deficits on carbon assimilation. Journal of Experimental Botany 42:1–16. [Google Scholar]
- Chinnusamy V, Jagendorf A, Zhu JK.. 2005. Understanding and improving salt tolerance in plants. Crop Science 45:437–448. [Google Scholar]
- Cornic G. 2000. Drought stress inhibits photosynthesis by decreasing stomatal aperture-not by affecting ATP synthesis stomata are probably mainly responsible for the decline in leaf photosynthesis during mild drought conditions. Trends in Plant Science 5:187–188. [Google Scholar]
- Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V.. 2014. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. Journal of Experimental Botany 65:1259–1270. [DOI] [PubMed] [Google Scholar]
- Díaz-Batalla L, Widholm JM, Fahey GC, Castaño-Tostado E, Paredes-López O.. 2006. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry 54:2045–2052. [DOI] [PubMed] [Google Scholar]
- Dionisio-Sese ML, Tobita S.. 1998. Antioxidant responses of rice seedlings to salinity stress. Plant Science 135:1–9. [Google Scholar]
- Doria E, Campion B, Sparvoli F, Tava A, Nielsen E.. 2012. Anti-nutrient components and metabolites with health implications in seeds of 10 common bean (Phaseolus vulgaris L. and Phaseolus lunatus L.) landraces cultivated in southern Italy. Journal of Food Composition and Analysis 26:72–80. [Google Scholar]
- dos Santos TB, Budzinski IGF, Marur CJ, Petkowicz CLO, Pereira LFP, Vieira LGE.. 2011. Expression of three galactinol synthase isoforms in Coffea arabica L. and accumulation of raffinose and stachyose in response to abiotic stresses. Plant Physiology and Biochemistry 49:441–448. [DOI] [PubMed] [Google Scholar]
- Elsayed AI, Rafudeen MS, Golldack D.. 2014. Physiological aspects of raffinose family oligosaccharides in plants: protection against abiotic stress. Plant Biology 16:1–8. [DOI] [PubMed] [Google Scholar]
- Eom JS, Choi SB, Ward JM, Jeon JS.. 2012. The mechanism of phloem loading in rice (Oryza sativa). Molecules and Cells 33:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M, Gogoi N, Barthakur S, Baroowa B, Bharadwaj N, Alghamdi SS, et al. 2017. Drought stress in grain legumes during reproduction and grain filling. Journal of Agronomy and Crop Science 203:81–102. [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD.. 2004. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biology 6:269–279. [DOI] [PubMed] [Google Scholar]
- Foyer C, Noctor G.. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias J, Bakhsh A, Jones DA, Arthur AE, Rhodes MJC, Hedley CL.. 1999. Genetic analysis of the raffinose oligosaccharide pathway in lentil seeds. Journal of Experimental Botany 50:469–476. [Google Scholar]
- Ganesan K, Xu B.. 2017. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. International Journal of Molecular Sciences 18:2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar T, Franck T, Bisbis B, Kevers C, Jouve L, Hausman JF, et al. 2002. Concepts in plant stress physiology: application to plant tissue cultures. Plant Growth Regulation 37:263–285. [Google Scholar]
- Gu H, Lu M, Zhang Z, Xu J, Cao W, Miao M.. 2018. Metabolic process of raffinose family oligosaccharides during cold stress and recovery in cucumber leaves. Journal of Plant Physiology 22:112–120. [DOI] [PubMed] [Google Scholar]
- Herrera MD, Acosta-Gallegos JA, Reynoso-Camacho R, Pérez-Ramírez IF.. 2019. Common bean seeds from plants subjected to severe drought, restricted- and full-irrigation regimes show differential phytochemical fingerprint. Food Chemistry 294:368–377. [DOI] [PubMed] [Google Scholar]
- Herridge DF, Peoples MB, Boddey RM.. 2008. Global inputs of biological nitrogen fixation in agricultural systems. Plant and Soil 311:1–18. [Google Scholar]
- Hincha DK, Zuther E, Heyer AG.. 2003. The preservation of liposomes by raffinose family oligosaccharides during drying is mediated by effects on fusion and lipid phase transitions. Biochimica et Biophysica Acta (BBA)—Biomembranes 1612:172–177. [DOI] [PubMed] [Google Scholar]
- Hoagland, D. R., and Arnon, D. I. (1950). The water-culture method for growing plants without Soil. 347th edn. Berkeley, CA: College of Agriculture, University of California, 33. [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. (2005). ICH harmonised tripartite guideline. Q2(R1): validation of analytical procedures: text and methodology. 1–17. https://database.ich.org/sites/default/files/Q2%28R1%29 Guideline.pdf [Google Scholar]
- Kandler O, Hopf H.. 1980. Carbohydrates: structure and function. Cambridge, MA: Academic Press, 221–270. [Google Scholar]
- Knaupp M, Mishra KB, Nedbal L, Heyer AG.. 2011. Evidence for a role of raffinose in stabilizing photosystem II during freeze-thaw cycles. Planta 234:477–486. [DOI] [PubMed] [Google Scholar]
- Kodikara, S. (2018). Physiology of the death of mangrove propagules, seedlings and saplings with particular emphasis on photosynthesis, transpiration, water transport and free radical accumulation [dissertation]. Brussels: Vrije Universiteit Brussel. [Google Scholar]
- de Koning R, Kiekens R, Toili MEM, Angenon G.. 2021. Identification and expression analysis of the genes involved in the raffinose family oligosaccharides pathway of Phaseolus vulgaris and Glycine max. Plants 10:1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha RR, Finley JW, Luthria DL.. 2020. Determination of soluble mono, di, and oligosaccharide content in 23 dry beans (Phaseolus vulgaris L.). Journal of Agricultural and Food Chemistry 68:6412–6419. [DOI] [PubMed] [Google Scholar]
- Lamari, L. (2008). ASSESS 2.0 image analysis software for plant disease quantification. St. Paul, MN: American Phytopathological Society. [Google Scholar]
- Lichtenthaler HK. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology 148:350–382. [Google Scholar]
- Lichtenthaler HK, Wellburn AR.. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions 11:591–592. [Google Scholar]
- Liu W, Saint DA.. 2002. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Analytical Biochemistry 302:52–59. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Ma J, Meng S, Pang C, Zhao X, Zhang H, Wang S, Xu T, He Y, et al. 2022. Application of galactinol to tomato enhances tolerance to cold and heat stresses. Horticulture, Environment and Biotechnology 63:311–323. [Google Scholar]
- Livak KJ, Schmittgen TD.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL.. 2008. Prioritizing climate change adaptation needs for food security in 2030. Science 319:607–610. [DOI] [PubMed] [Google Scholar]
- Ma S, Lv J, Li X, Ji T, Zhang Z, Gao L.. 2021. Galactinol synthase gene 4 (CsGolS4) increases cold and drought tolerance in Cucumis sativus L. by inducing RFO accumulation and ROS scavenging. Environmental and Experimental Botany 185:104406. [Google Scholar]
- Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabi Y.. 2010. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian Journal of Crop Science 4:580–585. [Google Scholar]
- Minden V, Deloy A, Volkert AM, Leonhardt SD, Pufal G.. 2017. Antibiotics impact plant traits, even at small concentrations. AoB Plants 9:plx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R, Sarkar B, Jat HS, Sharma PC, Bolan NS.. 2021. Soil salinity under climate change: challenges for sustainable agriculture and food security. Journal of Environmental Management 280:111736. [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M.. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59:651–681. [DOI] [PubMed] [Google Scholar]
- Nikbakht Nasrabadi M, Sedaghat Doost A, Mezzenga R.. 2021. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocolloids 118:106789. [Google Scholar]
- Nishizawa A, Yabuta Y, Shigeoka S.. 2008. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiology 147:1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf RL. 1997. Oligosaccharides and galactosyl cyclitols in seed desiccation tolerance. Seed Science Research 7:63–74. [Google Scholar]
- Peterbauer T, Richter A.. 2001. Biochemistry and physiology of raffinose family oligosaccharides and galactosyl cyclitols in seeds. Seed Science Research 11:185–197. [Google Scholar]
- Peterbauer T, Mach L, Mucha J, Richter A.. 2002a. Functional expression of a cDNA encoding pea (Pisum sativum L.) raffinose synthase, partial purification of the enzyme from maturing seeds, and steady-state kinetic analysis of raffinose synthesis. Planta 215:839–846. [DOI] [PubMed] [Google Scholar]
- Peterbauer T, Mucha J, Mach L, Richter A.. 2002b. Chain elongation of raffinose in pea seeds. Isolation, characterization, and molecular cloning of a multifunctional enzyme catalyzing the synthesis of stachyose and verbascose. Journal of Biological Chemistry 277:194–200. [DOI] [PubMed] [Google Scholar]
- Peters, S. W. (2010). Raffinose family oligaosaccharides (RFOs) are putative abiotic stress protectants: case studies on frost tolerance and water deficit in Ajuga reptans and Arabidopsis thaliana [dissertation]. Zürich: Universität Zürich. [Google Scholar]
- Peters S, Mundree SG, Thomson JA, Farrant JM, Keller F.. 2007. Protection mechanisms in the resurrection plant Xerophyta viscosa (Baker): both sucrose and raffinose family oligosaccharides (RFOs) accumulate in leaves in response to water deficit. Journal of Experimental Botany 58:1947–1956. [DOI] [PubMed] [Google Scholar]
- Pimentel D, Pimentel M.. 2003. Sustainability of meat-based and plant-based diets and the environment. The American Journal of Clinical Nutrition 78:660S–663S. [DOI] [PubMed] [Google Scholar]
- Reinprecht Y, Schram L, Marsolais F, Smith TH, Hill B, Pauls KP.. 2020. Effects of nitrogen application on nitrogen fixation in common bean production. Frontiers in Plant Science 11:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AD, Duigan SP, Berlyn GP.. 2002. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytologist 153:185–194. [Google Scholar]
- Salvi P, Kamble NU, Majee M.. 2018. Stress-inducible galactinol synthase of chickpea (CaGolS) is implicated in heat and oxidative stress tolerance through reducing stress-induced excessive reactive oxygen species accumulation. Plant and Cell Physiology 59:155–166. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ.. 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- Schneider T, Keller F.. 2009. Raffinose in chloroplasts is synthesized in the cytosol and transported across the chloroplast envelope. Plant and Cell Physiology 50:2174–2182. [DOI] [PubMed] [Google Scholar]
- Seki M, Umezawa T, Urano K, Shinozaki K.. 2007. Regulatory metabolic networks in drought stress responses. Current Opinion in Plant Biology 10:296–302. [DOI] [PubMed] [Google Scholar]
- Selvaraj MG, Ishizaki T, Valencia M, Ogawa S, Dedicova B, Ogata T, Yoshiwara K, Maruyama K, Kusano M, Saito K, et al. 2017. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnology Journal 15:1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Mukherjee S, Basak P, Majumder AL.. 2015. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Frontiers in Plant Science 6:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Qi X, Wang Z, Li P, Wu C, Zhang H, Zhao Y.. 2013. Overexpression of TsGOLS2, a galactinol synthase, in Arabidopsis thaliana enhances tolerance to high salinity and osmotic stresses. Plant Physiology and Biochemistry 69:82–89. [DOI] [PubMed] [Google Scholar]
- Taji T, Ohsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K.. 2002. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. The Plant Journal 29:417–426. [DOI] [PubMed] [Google Scholar]
- Tavakkoli E, Rengasamy P, McDonald GK.. 2010. High concentrations of Na+ and Cl- ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. Journal of Experimental Botany 61:4449–4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilakarathna MS, Raizada MN.. 2018. Challenges in using precision agriculture to optimize symbiotic nitrogen fixation in legumes: progress, limitations, and future improvements needed in diagnostic testing. Agronomy 8:78. [Google Scholar]
- Tomlin J, Lowis C, Read NW.. 1991. Investigation of normal flatus production in healthy volunteers. Gut 32:665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A, Bano A, Khan N.. 2011. Gene expression profiling of plants under salt stress. Critical Reviews in Plant Sciences 30:435–458. [Google Scholar]
- Valentine MF, de Tar JR, Mookkan M, Firman JD, Zhang ZJ.. 2017. Silencing of soybean raffinose synthase gene reduced raffinose family oligosaccharides and increased true metabolizable energy of poultry feed. Frontiers in Plant Science 8:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CC, Mota APZ, Porto BN, Oliveira TN, Sampaio I, Lacerda AL, Danchin EGJ, Guimaraes PM, Williams TCR, Brasileiro ACM.. 2020. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Scientific Reports 10:15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova A, Capella-Gutiérrez S, Rendón-Anaya M, Hernández-Oñate M, Minoche AE, Erb I, Câmara F, Prieto-Barja P, Corvelo A, Sanseverino W, et al. 2016. Genome and transcriptome analysis of the Mesoamerican common bean and the role of gene duplications in establishing tissue and temporal specialization of genes. Genome Biology 17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguishi CT, Sanada CT, Gouvêa PM, Pandey A, Woiciechowski AL, Parada JL, et al. 2009. Biotechnological process for producing black bean slurry without stachyose. International Food Research Journal 42:425–429. [Google Scholar]
- Yan S, Liu Q, Li W, Yan J, Fernie AR.. 2022. Raffinose family oligosaccharides: crucial regulators of plant development and stress responses. Critical Reviews in Plant Sciences 41:286–303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are incorporated in the manuscript and its supplementary materials. These include the tables, figures and additional data used to support the findings of this study. If further information is needed, please contact the corresponding author.