To the Editor:
Lenvatinib, an oral multikinase inhibitor, was approved by the United States Food and Drug Administration (FDA) for the treatment of radiation-refractory differentiated thyroid cancer in 2015.1,2 In 2017, the phase 3 study of lenvatinib (E7080) in differentiated cancer of the thyroid (SELECT) demonstrated that lenvatinib prolongs progression-free survival in such patients.1,2 Thereafter, the REFLECT [multicenter, randomized, open-label, phase 3 trial to compare the efficacy and safety of lenvatinib (E7080) versus sorafenib in first-line treatment of subjects with unresectable hepatocellular carcinoma] trial showed that lenvatinib is non-inferior to sorafenib in terms of overall survival of patients with unresectable hepatocellular carcinoma (HCC); thus, the FDA approved lenvatinib as a first-line drug for the treatment of unresectable HCC.1,3,4 According to REFLECT and SELECT, common adverse events (AEs) for lenvatinib include hypertension and diarrhea.2,3 To our knowledge, no detailed cases of lenvatinibinduced generalized erythematous rash (GER) have led to its discontinuation. 1-4 Herein, we present the first such case reported in detail.
A 72-year-old man and a history of ichthyosis and no history of allergies underwent partial liver resection (hepatic segment VIII) for the treatment of HCC. Six months after surgery, computed tomography revealed unresectable HCC lung metastases for which he received lenvatinib.
Two weeks after the administration of lenvatinib, erythematous scales covered his entire body (Figure 1 a-c). He had been diagnosed with dermatomycosis of the skin of whole body and treated by terbinafine tablets and luliconazole cream at another hospital; nevertheless, his symptoms worsened. Two weeks after commencing dermatomycosis treatment, he presented with GER and was admitted to our hospital. We discontinued all oral drugs and applied urea 10% cream. The eruptions improved within two weeks.
Two weeks after discharge, lenvatinib was re-administered because the patient’s metastatic lung tumor enlarged, which led to a recurrence of erythema with pruritus on the upper part of patient’s body. Laboratory examination showed an increased blood eosinophil count (1449/μL). Histopathological examination of a specimen of the erythematous lesion of his thigh revealing acanthosis, slight liquefaction degeneration, slight reduction of granular cell layer, and lympholic infiltration around the blood vessels of the dermis (Figure 1 d-f). Lenvatinib was again discontinued; difluprednate ointment and urea 10% cream were prescribed. Within two weeks, the eruption improved. We performed a patch test of Lenvatinib after the informed consent, similar to a previous report of sorafenib showing positive results of patch test,5 which was positive per the standards set by the International Contact Dermatitis Research Group. Based on that result, we diagnosed the patient with lenvatinib-induced GER.
Palmer-plantar erythrodysesthesia syndrome,2 a known cutaneous AE of Lenvatinib, is also the most common AE for the multikinase inhibitor sorafenib.3 In contrast, there have been no detailed case reports of lenvatinib-induced GER. Although our patient’s history of ichthyosis and mycosis complicated his diagnosis of GER, the results of patch test by using lenvatinib confirmed the diagnosis. One limitation is that it is unclear how much his congenital ichthyosis affected on the present condition. As lenvatinib is a standard drug in cancer therapy, physicians should be aware of this potentially serious AE.
Figure 1.
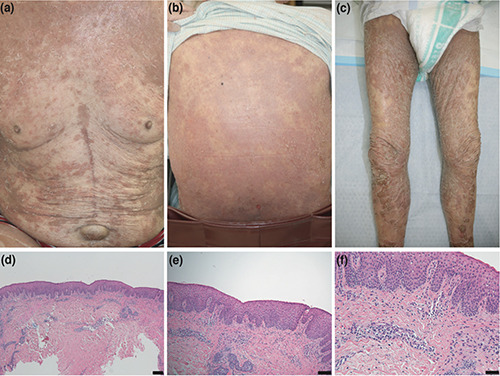
Patient’s clinical and histopathological features. a-c) A few days after hospitalization, erythema covered the body; d-f) histopathological examination of a specimen of the erythematous lesion of his thigh revealing acanthosis, slight liquefaction degeneration, slight reduction of granular cell layer, and lymphocytic infiltration around the blood vessels of the dermis (H&E staining, original magnification; d) ×40, scale bar =200 μm; e) ×100, scale bar =100 μm (f)×200, scale bar = 50 μm) .
Funding Statement
Funding: none.
References
- 1.Hao Z, Wang P. Lenvatinib in management of solid tumors. Oncologist 2020;25:e302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddad RI, Schlumberger M, Wirth LJ, et al. Incidence and timing of common adverse events in lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine 2017;56:121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto H, Suzuki H, Shimose S, et al. Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda M, Fujita T, Mii S, et al. Erythema multiforme induced by sorafenib for metastatic renal cell carcinoma. Jpn J Clin Oncol 2012;42:820-4. [DOI] [PubMed] [Google Scholar]


